Abstract
This report provides a description of an efficient and simple procedure for the synthesis of 2-azetidinones via a one-pot reaction of imines and carboxylic acids in the presence of silicaphosphine at room temperature. The reagent is cheap and stable. The yields are good to excellent, and the reaction conditions are mild.
Introduction
β-Lactam antibiotics, such as penicillins, cephalosporins, carbapenems, and aztreonam, serve as effective agents against bacterial infections [1–3]. Their activity is due to the presence of a 2-azetidinone ring [4]. Some monocyclic β-lactam (2-azetidinone) derivatives possess a wide variety of pharmacological activities [5–7]. For example, ezetimibe is used clinically for its cholesterol absorption inhibitory property [8, 9]. Use of 2-azetidinones in the synthesis of many classes of compounds is well established [10–13] including the semi-synthesis of taxol derivatives [14].
β-Lactam ring formation is a crucial step in the synthesis of new β-lactams. As such, new synthetic methods for the preparation of the β-lactam ring have been developed [15–20]. The Staudinger reaction [21] (ketene-imine cycloaddition) is undoubtedly the most widely used route to 2-azetidinones [22–28]. Ketenes are commonly generated by reaction of acyl halides with tertiary amines [29–31], however, the use of acyl halides generally is not an easy or safe task. The preparation of ketenes from carboxylic acids is a more practical process [32–46]. A common approach to the synthesis of β-lactams involves treatment of the acid with activators (i.e., triphenylphosphine dibromide [47], POCl3 [48], and some other phosphorus reagents [49–52]) to form an activated intermediate, which can be treated with a base to form a ketene in situ [32–46]. However, in addition to tedious reaction conditions, some acid activators are quite expensive, and separation of the byproducts is difficult. Especially difficult is the removal of phosphine oxide derivatives, which is a disadvantage in the use of phosphorus reagents in the aforementioned reactions. To this end, we realized that use of supported reagents may offer practical advantages [53].
Silicaphosphine (silphos), [P(Cl)3-n(SiO2)n], is easily prepared by the reaction of silica gel and PCl3 [54]. Silphos has been applied for the conversion of alcohols and thiols to alkyl bromides and iodides [54], acetylation and formylation of alcohols and amines with ethyl formate and acetate [55], deoxygenation of sulfoxides to thioethers, reductive coupling of sulfonyl chlorides, conversion of sodium sulfinates and thiosulfonates to their corresponding disulfides [56], regioselective synthesis of vic-haloalcohols [57], conversion of oximes to nitriles and amides or carbonyl compounds [58], as well as the Beckmann rearrangement of ketoximes and dehydration of aldoximes [59]. To our best knowledge, there are no reports on the use of silphos in β-lactam ring formation. Herein, we report the practical application of this heterogeneous reagent in the synthesis of 2-azetidinones.
Results and discussion
The reaction of phenoxyacetic acid and N-(4-chlorobenzylidene)-4-ethoxyaniline with PCl3 in the presence of triethylamine in dry dichloromethane at room temperature did not afford a β-lactam product. When the reaction was performed at low temperature (-12°C), the desired 2-azetidinone 3a was formed in a 6% yield.
Then it was decided to attempt to generate the β-lactam ring in the presence of Silphos [54–59]. Silphos was prepared as a white solid as described [54]. The reaction of phenoxyacetic acid and N-(4-chlorobenzylidene)-4-ethoxyaniline in dry dichloromethane at room temperature in the presence of triethylamine and silphos afforded 2-azetidinone 3a in 57% yield after crystallization from EtOAc (Table 1, entry 3).
Reaction condition in the synthesis of 3a.
| Entry | Solvent | Temp (°C) | Reagent (mmol) | Yield (%) |
|---|---|---|---|---|
| 1 | CH2Cl2 | rt | PCl3 (1.0) | – |
| 2 | CH2Cl2 | -12 | PCl3 (1.0) | 6 |
| 3 | CH2Cl2 | rt | Silphos (1.0) | 57 |
| 4 | Toluene | rt | Silphos (1.0) | 43 |
| 5 | DMF | rt | Silphos (1.0) | 25 |
| 6 | CH2Cl2 | 0 | Silphos (1.0) | 55 |
| 7 | CH2Cl2 | rt | Silphos (1.2) | 83 |
| 8 | CH2Cl2 | rt | Silphos (1.3) | 88 |
| 9 | CH2Cl2 | rt | Silphos (1.5) | 87 |
Based on this successful result, we tried to optimize the effects of different solvents, temperatures, and the amounts of silphos. As shown in Table 1, dry dichloromethane is the best solvent for this reaction. The highest yield of 3a is obtained when 1.0 mmol of Schiff base undergoes a reaction with 1.3 mmol of phenoxyacetic acid in the presence of 1.3 mmol of silphos in dry dichloromethane at room temperature (Table 1, entry 8).
The generality of this strategy is shown in Scheme 1 and Table 2. The synthesis was extended to several types of monocyclic β-lactams bearing diverse substituents. β-Lactams 3a–g and 3k–m were purified by recrystallization from EtOAc and β-lactams 3h–j were purified by short-column chromatography on silica gel (hexane/EtOAc=9:1).
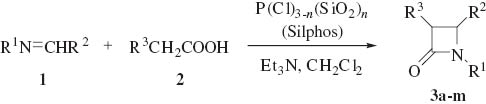
Synthesis of 2-azetidinones 3a–m using Silphos.
| Entry | R1 | R2 | R3 | cis/trans | Product |
|---|---|---|---|---|---|
| 1 | 4-EtOC6H4 | 4-ClC6H4 | PhO | cis | 3a |
| 2 | 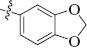 | 4-MeOC6H4 | PhO | cis | 3b |
| 3 | Ph | 4-NO2C6H4 | MeO | cis | 3c |
| 4 | 4-MeOC6H4 | 4-NO2C6H4 | 2,4-Cl2C6H3O | cis | 3d |
| 5 | 4-EtC6H4 | 4-(Me2N)C6H4 | 4-ClC6H4O | cis | 3e |
| 6 | Ph | 4-NO2C6H4 | 2-NaphthO | cis | 3f |
| 7 | 4-EtOC6H4 | 4-MeOC6H4 | PhthN | trans | 3g |
| 8 | 4-MeOC6H4 | 4-ClC6H4 | 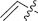 | trans | 3h |
| 9 | 4-EtOC6H4 | C6H5 | N3 | cis | 3i |
| 10 | Ph | 4-ClC6H4 | 4-MeC6H4SO2 | trans | 3j |
| 11 | Bn | 4-ClC6H4 | PhO | cis | 3k |
| 12 | 4-MeOC6H4CH2 | 4-NO2C6H4 | 2,4-Cl2C6H3O | cis | 3l |
| 13 | Ph | 4-ClC6H4 | 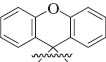 | – | 3m |
The structure of new product 3b was confirmed by 1H NMR, 13C NMR, IR, and elemental analysis. The 1H NMR spectrum of 3b shows the characteristic AB pattern of the β-lactam ring protons H-4 at δ 4.7 and δ 5.3 of the proton H-3. The coupling constant of 4.5 Hz indicates cis stereochemistry. In general, the coupling constant smaller than 3 Hz is indicative of trans stereochemistry. The remaining products have been described previously, and their spectral data are virtually identical with those reported.
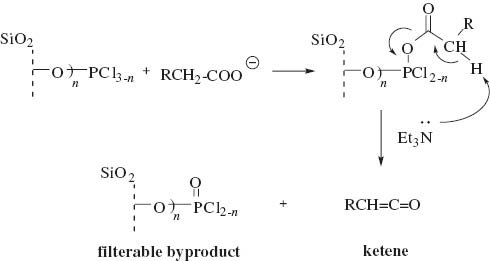
According to the accepted mechanism of the Staudinger reaction [60–62], it is suggested that the reaction involves the formation of an activated ester (Scheme 2) and a ketene. Then the intermediate ketene undergoes a reaction with an imine to produce the β-lactam [60–62].
Experimental
General
IR spectra were run on a Shimadzu FT-IR 8300 spectrophotometer. 1H NMR and 13C NMR spectra were recorded in CDCl3 using a Bruker Avance DPX instrument. Elemental analyses were run on a Thermo Finnigan Flash EA-1112 series instrument. Melting points were determined in open capillaries with Buchi 510 melting point apparatus. Thin-layer chromatography was carried out on silica gel 254 analytical sheets obtained from Fluka. Column chromatography was performed on Merck Kiesel gel (230–270 mesh).
General procedure for synthesis of 2-azetidinones 3a–m
To a solution of an imine (1.0 mmol), carboxylic acid (1.3 mmol), and dry Et3N (5.0 mmol) in dry CH2Cl2 (10 mL) was added silphos (1.0 g, 1.3 mmol) at room temperature, and the resulting mixture was stirred overnight. Then the mixture was filtered and the filtrate was washed successively with saturated NaHCO3 (10 mL) and brine (10 mL). The organic layer was dried (Na2SO4) and filtered, and the solvent was removed to give the crude product. β-Lactams 3a–g and 3k–m were purified by crystallization from EtOAc and β-lactams 3h–j were purified by short-column chromatography on silica gel (hexane/EtOAc, 9:1).
4-(4-Chlorophenyl)-1-(4-ethoxyphenyl)-3-phenoxyazetidin-2-one (3a) [37]:
Yield 88%.
1-(Benzo[d][1,3]dioxol-5-yl)-4-(4-methoxyphenyl)-3-phenoxyazetidin-2-one (3b):
Yield 90%; mp 159–161°C; IR (KBr): 1749 cm-1 (CO, β-lactam); 1H NMR: δ 3.78 (OMe, s, 3H), 5.11 (H-4, d, 1H, J = 4.5 Hz), 5.37 (H-3, d, 1H, J = 4.5 Hz), 5.85 (OCH2O, s, 2H), 6.77–7.82 (ArH, m, 12H); 13C NMR: δ 56.0 (OMe), 61.8 (C-4), 82.4 (C-3), 111.6 (OCH2O), 108.6, 113.1, 119.1, 122.0, 122.9, 125.7, 128.3, 129.5, 130.2, 131.8, 134.1, 142.6, 149.4, 158.1 (aromatic carbons), 162.9 (CO, β-lactam). Anal. Calcd for C23H19NO5: C, 70.94; H, 4.92; N, 3.60. Found: C, 71.05; H, 5.04; N, 3.54.
3-Methoxy-4-(4-nitrophenyl)-1-phenylazetidin-2-one (3c) [62]:
Yield 85%.
3-(2,4-Dichlorophenoxy)-1-(4-methoxyphenyl)-4-(4-nitro-phenyl)-azetidin-2-one (3d) [61]:
Yield 91%.
3-(4-Chlorophenoxy)-4-(4-(dimethylamino)phenyl)-1-(4-ethylphenyl)azetidin-2-one (3e) [16]:
Yield 91%.
3-(Naphthalen-2-yloxy)-4-(4-nitrophenyl)-1-phenylazetidin-2-one (3f) [33]:
Yield 93%.
2-(1-(4-Ethoxyphenyl)-2-(4-methoxyphenyl)-4-oxoazetidin-3-yl)isoindoline-1,3-dione (3g) [35]:
Yield 83%.
4-(4-Chlorophenyl)-1-(4-methoxyphenyl)-3-vinylazetidin-2-one (3h) [35]:
Yield 67%. yield.
3-Azido-1-(4-ethoxyphenyl)-4-phenylazetidin-2-one (3i) [38]:
Yield 59%.
4-(4-Chlorophenyl)-1-phenyl-3-tosylazetidin-2-one (3j) [33]:
Yield 52%.
1-Benzyl-4-(4-chlorophenyl)-3-phenoxyazetidin-2-one (3k) [61]:
Yield 89%.
3-(2,4-Dichlorophenoxy)-1-(4-methoxybenzyl)-4-(4-nitrophenyl)-azetidin-2-one (3l) [40]:
Yield 92%.
2-(4-Chlorophenyl)-1-phenylspiro[azetidine-3,9′-xanthen]-4-one (3m) [33]:
Yield 83%.
Acknowledgments
The authors thank the Shiraz University Research Council for financial support (Grant No. 92-GR-SC-23).
References
[1] Long, T. E.; Turos, E. N-Thiolated β-lactams. Curr. Med. Chem. Anti-Infect. Agents 2002, 1, 251–268.10.2174/1568012023354820Suche in Google Scholar
[2] Hwu, J. R.; Ethiraj, S. K.; Hakimelahi, G. H. Biological activity of some monocyclic- and bicyclic β-lactams with specified functional groups. Mini-Rev. Med. Chem. 2003, 3, 305–313.Suche in Google Scholar
[3] Ceric, H.; Sindler-Kulyk, M.; Kovacevic, M.; Peric, M.; Zivkovic, A. Azetidinone-isothiazolidinones: stereoselective synthesis and antibacterial evaluation of new monocyclic beta-lactams. Bioorg. Med. Chem. 2010, 18, 3053–3058.Suche in Google Scholar
[4] Morin, R. B.; Gorman, M. Chemistry and Biology of β-Lactam Antibiotics; Academic Press: New York, 1982.Suche in Google Scholar
[5] Mehta, P. D.; Sengar, N. P. S.; Pathak, A. K. 2-Azetidinone – a new profile of various pharmacological activities. Eur. J. Med. Chem. 2010, 45, 5541–5560.Suche in Google Scholar
[6] Veinberg, G.; Vorona, M.; Shestakova, I.; Kanepe, I.; Lukevics, E. Design of β-lactams with mechanism based nonantibacterial activities. Curr. Med. Chem. 2003, 10, 1741–1757.Suche in Google Scholar
[7] Jarrahpour, A.; Ebrahimi, E.; Sinou, V.; Latour, C.; Brunel, J.-H. Diastereoselective synthesis of potent antimalarial cis-β-lactam agents through a [2+2] cycloaddition of chiral imines with a chiral ketene. Eur. J. Med. Chem. 2014, 87, 364–371.Suche in Google Scholar
[8] Xu, X.; Fu, R.; Chen, J.; Chen, S.; Bai, S. Ezetimibe analogs with a reorganized azetidinone ring: design, synthesis, and evaluation of cholesterol absorption inhibitions. Bioorg. Med. Chem. Lett. 2007, 17, 101–104.Suche in Google Scholar
[9] Li, S.; Liu, G.; Jia, J.; Li, X.; Yu, C. Liquid chromatography-negative ion electrospray tandem mass spectrometry method for the quantification of ezetimibe in human plasma. J. Pharm. Biomed.Anal. 2006, 40, 987–992.Suche in Google Scholar
[10] Mehra, V.; Kumar, V. Single pot diastereoselective synthesis of six membered cyclic (E)-endo-aldonitrones via intramolecular cyclization of ω-alkenyl oximes. Tetrahedron Lett. 2014, 55, 845–848.Suche in Google Scholar
[11] Alcaide, B.; Almendros, P.; Aragoncillo, C. β-Lactams: versatile building blocks for the stereoselective synthesis of non-β-lactam products. Chem. Rev. 2007,107, 4437–4492.Suche in Google Scholar
[12] Ojima, I.; Delaloge, F. Asymmetric synthesis of building-blocks for peptides and peptidomimetics by means of the b-lactam synthon method. Chem. Soc. Rev. 1997, 26, 377–386.Suche in Google Scholar
[13] Palomo, C.; Oiarbide, M. β-Lactam Ring Opening: A Useful Entry to Amino Acids and Relevant Nitrogen-Containing Compounds. In Topics Heterocycl. Chem. Banik, B. K., Ed. Springer-Verlag: Berlin, 2010; Vol. 22, pp 211–259.Suche in Google Scholar
[14] Suffness, M. Taxol Science and Applications; CRC Press: Boca Raton, FL, 1995.Suche in Google Scholar
[15] Li, W.; Liu, C.; Zhang, H.; Ye, K.; Zhang, G.; Zhang, W.; Duan, Z.; You, S.; Lei, A. Palladium-catalyzed oxidative carbonylation of N-allylamines for the synthesis of β-lactams.Angew. Chem. Int. Ed. 2014, 53, 2443–2446.Suche in Google Scholar
[16] Darvishi, A.; Zarei, M.; Akhgar, M. R. A simple and highly efficient procedure for one-pot synthesis of 2-azetidinones using 3,5-dinitrobenzoyl chloride. Lett. Org. Chem. 2013, 10, 645–650.Suche in Google Scholar
[17] Alcaide, B.; Almendros, P. Four-Membered Ring Systems. In Progress in Heterocyclic Chemistry. Gribble, G. W., Joule, J. A., Eds. Elsevier: Oxford, UK, 2011; Vol. 22, pp 85–107.10.1016/S0959-6380(11)22004-XSuche in Google Scholar
[18] Aguilar H.; Banik, B. K. Stereoselectivity of 3,3-disubstituted β-lactam formation via Staudinger reaction. Heterocycl. Commun. 2009, 15, 365–368.Suche in Google Scholar
[19] Fu, N.; Tidwell, T. T. Preparation of β-lactams by [2+2] cycloaddition of ketenes and imines. Tetrahedron 2008, 64, 10465–10496.10.1016/j.tet.2008.08.028Suche in Google Scholar
[20] Georg, G. I. The Organic Chemistry of β-Lactams; Verlag Chemie: New York, 1993.Suche in Google Scholar
[21] Staudinger, H. Zur Kenntniss der Ketene. Diphenylketen. Liebigs Ann. Chem. 1907, 356, 51–123.Suche in Google Scholar
[22] Zarei, M. An efficient and green method for the synthesis of 2-azetidinones mediated by propylphosphonic anhydride (T3P). Monatsh. Chem. 2014, 145, 1495–1499.Suche in Google Scholar
[23] Zarei, M.; Karimi-Jaberi, Z.; Movahedi, A. Synthesis of β-lactams from acids and imines using thiocarbonyldiimidazole.Synth. Commun. 2013, 43, 728–734.Suche in Google Scholar
[24] Jarrahpour, A.; Motamedifar, M.; Zarei, M.; Mimouni, M. Synthesis of new N-sulfonyl monocyclic β-lactams and the investigation of their antibacterial activities. Phosphorus Sulfur Silicon 2010, 185, 287–297.Suche in Google Scholar
[25] Banik, B. K.; Banik, I.; Becker, F. F. Asymmetric synthesis of anticancer b-lactams via Staudinger reaction: utilization of chiral ketene from carbohydrate. Eur. J. Med. Chem. 2010, 45, 846–850.Suche in Google Scholar
[26] Jarrahpour, A.; Fadavi, A.; Zarei, M. Synthesis of structurally diverse 2-azetidinones via Staudinger reaction on a solid support. Bull. Chem. Soc. Jpn. 2011, 84, 320–327.Suche in Google Scholar
[27] Keri, R. S.; Hosamani, K. M.; Shingalapur, R. V.; Reddy, H. R. S. 2-Azetidinone derivatives: design, synthesis, in vitro anti-microbial, cytotoxic activities and DNA cleavage study. Eur. J. Med. Chem. 2009, 44, 5123–5130.Suche in Google Scholar
[28] Zarei, M.; Jarrahpour, A. A mild and efficient route to 2-azetidinones using the cyanuric chloride-DMF complex. Synlett 2001, 22, 2572–2576.10.1055/s-0030-1289517Suche in Google Scholar
[29] Jarrahpour, A.; Zarei, M. Synthesis of novel N-sulfonyl monocyclic β-lactams as potential antibacterial agents. Molecules 2006, 11, 49–58.10.3390/11010049Suche in Google Scholar PubMed PubMed Central
[30] Tidwell, T. T. Ketenes II; John Wiley & Sons: Hoboken, NJ, 2006, pp 55–192.10.1002/0471767670Suche in Google Scholar
[31] Khadsan, R. E.; Kadu, M. V. Synthesis and biological evaluation of some novel N-substitutedphenyl-4-(3′,4′-methylenedioxyphenyl)-3-chloro-2-azetidinone derivatives. Heterocycl. Commun. 2005, 11, 455–458.Suche in Google Scholar
[32] Zarei, M. A straightforward approach to 2-azetidinones from imines and carboxylic acids using dimethyl sulfoxide and acetic anhydride. Tetrahedron Lett. 2014, 55, 5354–5357.Suche in Google Scholar
[33] Zarei, M. A facile and effective synthesis of 2-azetidinones via phosphonitrilic chloride. Tetrahedron 2013, 69, 6620–6626.10.1016/j.tet.2013.05.121Suche in Google Scholar
[34] Jarrahpour, A.; Zarei, M. DMF-dimethyl sulfate as a new reagent for the synthesis of β-lactams. Tetrahedron Lett. 2009, 50, 1568–1570.Suche in Google Scholar
[35] Jarrahpour, A.; Zarei, M. The Vilsmeier reagent: a useful and versatile reagent for the synthesis of 2-azetidinones. Tetrahedron 2009, 65, 2927–2934.10.1016/j.tet.2009.02.005Suche in Google Scholar
[36] Nahmany, M.; Melman, A. Simple approach to β-lactam derivatives from N-acylimidazoles. J. Org. Chem. 2006, 71, 5804–5806.Suche in Google Scholar
[37] Jarrahpour, A.; Zarei, M. Synthesis of novel N-(4-ethoxyphenyl) azetidin-2-ones and their oxidative N-deprotection by ceric ammonium nitrate. Molecules 2007, 12, 2364–2379.10.3390/12102364Suche in Google Scholar PubMed PubMed Central
[38] Zarei, M. A convenient synthesis of 2-azetidinones via 2-fluoro-1-methylpyridinium p-toluenesulfonate. Monatsh. Chem. 2013, 144, 1021–1025.Suche in Google Scholar
[39] Jarrahpour, A.; Zarei, M. The Vilsmeier reagent as an efficient acid activator for the synthesis of β-lactams. Tetrahedron Lett. 2007, 48, 8712–8714.Suche in Google Scholar
[40] Zarei, M. One-step synthesis of β-lactams using cyanuric fluoride. J. Chem. Res. 2013, 37, 25–27.Suche in Google Scholar
[41] Matsui, S.; Hashimoto, Y.; Saigo, K. Application of erythro-2-amino-1,2-diphenylethanol as a highly efficient chiral auxiliary. Highly stereoselective Staudinger-type β-lactam synthesis using a 2-chloro-1-methylpyridinium salt as the dehydrating agent. Synthesis 1998, 1161–1166.10.1055/s-1998-2117Suche in Google Scholar
[42] Bandyopadhyay, D.; Xavier, M.; Banik, B. K. Highly stereoselective β-lactam synthesis via the Staudinger reaction using polyaromatic imines. Heterocycl. Commun. 2009, 15, 229–232.Suche in Google Scholar
[43] Palomo, C.; Aizpurua, J. M.; Urchegui, R.; Iturburu, M.; de Retana, A. O.; Cuevas, C. A convenient method for β-lactam formation from β-amino acids using phenyl phosphorodichloridate reagent. J. Org. Chem. 1991, 56, 2244–2247.Suche in Google Scholar
[44] Unsworth, W. P.; Kitsiou, C.; Taylor, R. J. K. Direct imine acylation: rapid access to diverse heterocyclic scaffolds. Org. Lett. 2013, 15, 258–261.Suche in Google Scholar
[45] Jarrahpour, A.; Ebrahimi, E.; Khalifeh, K.; Sharghi, H.; Sahraei, M.; Sinou, V.; Latour, C.; Brunel, J. M. Synthesis of novel b-lactams bearing an anthraquinone moiety, and evaluation of their antimalarial activities. Tetrahedron 2012, 68, 4740–4744.Suche in Google Scholar
[46] Zarei, M.; Mohamadzadeh, M. 3-Thiolated 2-azetidinones: synthesis and in vitro antibacterial and antifungal activities. Tetrahedron 2011, 67, 5832–5840.10.1016/j.tet.2011.05.043Suche in Google Scholar
[47] Cossio, F. P.; Ganboa, I.; Palomo, C. Triphenylphosphine dibromide and dimethylsulfide dibromide as versatile reagents for beta-lactam synthesis. Tetrahedron Lett. 1985, 26, 3041–3044.Suche in Google Scholar
[48] Sharma, S. D.; Gupta, P. K. A new method for the synthesis of α-amino-β-lactams. Tetrahedron Lett. 1978, 46, 4587–4590.Suche in Google Scholar
[49] Zarei, M. One-pot sequence synthesis of azetidin-2-one using diethyl chlorophosphate. J. Chem. Res. 2012, 36, 118–120.Suche in Google Scholar
[50] Kanwar, S.; Sharma, S. D. Use of 2,2′-dibenzothiazolyl disulfide-triphenylphosphine and Lawesson’s reagent in the cyclization of β-amino acids Bull. Chem. Soc. Jpn. 2006, 79, 1748–1752.Suche in Google Scholar
[51] Bernardi, L.; Bonini, B. F.; Comes-Franchini, M.; Dessole, C.; Fochi, M.; Ricci, A. One-pot synthesis of novel enantiomerically pure and racemic 4-ferrocenyl-β-lactams and their reactivity in acidic media. Eur. J. Org. Chem. 2005, 3326–3333.10.1002/ejoc.200500170Suche in Google Scholar
[52] Sharma, S. D.; Kanwar S. 2,2′-Dibenzothiazolyl disulfide: a versatile reagent for the synthesis of 2-azetidinones. Synlett 2004, 15, 2824–2826.Suche in Google Scholar
[53] Yadav, V. K.; Kapoor, K. K. Al2O3 supported KF: an efficient mediator in the epoxidation of electron deficient alkenes with t-BuOOH.Tetrahedron Lett 1994,35, 9481–4984.Suche in Google Scholar
[54] Iranpoor, N.; Firouzabadi, H.; Jamalian, A.; Kazemi, F. Silicaphosphine (silphos): a filterable reagent for the conversion of alcohols and thiols to alkyl bromides and iodides Tetrahedron 2005, 61, 5699–5704.Suche in Google Scholar
[55] Iranpoor, N.; Firouzabadi, H.; Jamalian, A. Silphos [PCl3-n(SiO2)n]: a heterogeneous phosphine reagent for formylation and acetylation of alcohols and amines with ethyl formate and acetate. Tetrahedron Lett. 2005, 46, 7963–7966.Suche in Google Scholar
[56] Iranpoor, N.; Firouzabadi, H.; Jamalian, A. Deoxygenation of sulfoxides and reductive coupling of sulfonyl chlorides, sulfinates and thiosulfonates using silphos [PCl3-n (SiO2)n] as a heterogeneous phosphine reagent. Synlett 2005, 16, 1447–1449.Suche in Google Scholar
[57] Iranpoor, N.; Firouzabadi, H.; Jamalian, A. Silphos [PCl3-n (SiO2)n]: a heterogeneous phosphine reagent for the regioselective synthesis of vic-haloalcohols. Phosphorus Sulfur Silicon 2006, 181, 2615–2621.Suche in Google Scholar
[58] Iranpoor, N.; Firouzabadi, H.; Jamalian, A.; Tamami, M. Silphos [PCl3-n (SiO2)n], a heterogeneous phosphine reagent mediated the conversion of oximes to nitriles and amides or carbonyl compounds. Lett. Org. Chem. 2006, 3, 267–270.Suche in Google Scholar
[59] Li, Z.; Lu, Z.; Zhu, A.; Feng, X.; Liu, J.; Tian, G. Silica-supported phosphorus chloride: an efficient and recyclable catalyst for beckmann rearrangement of ketoximes and dehydration of aldoximes under microwave irradiation. Catal. Lett. 2008, 120, 100–105.Suche in Google Scholar
[60] Wang, Y.; Liang, Y.; Jiao, L.; Du, D.-M.; Xu, J. Do reaction conditions affect the stereoselectivity in the Staudinger reaction? J. Org. Chem. 2006, 71, 6983–6990.Suche in Google Scholar
[61] Jarrahpour, A.; Zarei, M. Efficient one-pot synthesis of 2-azetidinones from acetic acid derivatives and imines using methoxymethylene-N,N-dimethyliminiumsalt. Tetrahedron 2010, 66, 5017–5023.10.1016/j.tet.2010.05.009Suche in Google Scholar
[62] Zarei, M. Utilization of DMF-PhCOCl adduct as an acid activator in a new and convenient method for preparation of β-lactams. Bull. Chem. Soc. Jpn. 2012, 85, 360–368.Suche in Google Scholar
©2014 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Review
- Application of dimethyl N-cyanodithioiminocarbonate in synthesis of fused heterocycles and in biological chemistry
- Research Articles
- Synthesis of novel glycosyl 1,3,4-oxadiazole derivatives
- Synthesis of the new heterocyclic system 7,8-dihydro-6H-benzotetrazolothiadiazine and derivatives
- Synthesis and antimicrobial assessment of new substituted 10H-phenothiazines, their sulfone derivatives, and ribofuranosides
- Reaction of hydrazones derived from electron-deficient ketones with Vilsmeier-Haack reagent
- Silphos as an efficient heterogeneous reagent for the synthesis of 2-azetidinones
- Regioselective one-pot synthesis of 1,4-disubstituted 1,2,3-triazole derivatives
Artikel in diesem Heft
- Frontmatter
- Review
- Application of dimethyl N-cyanodithioiminocarbonate in synthesis of fused heterocycles and in biological chemistry
- Research Articles
- Synthesis of novel glycosyl 1,3,4-oxadiazole derivatives
- Synthesis of the new heterocyclic system 7,8-dihydro-6H-benzotetrazolothiadiazine and derivatives
- Synthesis and antimicrobial assessment of new substituted 10H-phenothiazines, their sulfone derivatives, and ribofuranosides
- Reaction of hydrazones derived from electron-deficient ketones with Vilsmeier-Haack reagent
- Silphos as an efficient heterogeneous reagent for the synthesis of 2-azetidinones
- Regioselective one-pot synthesis of 1,4-disubstituted 1,2,3-triazole derivatives

