5-(2,2-Dimethyl-4H-1,3-benzodioxin)methanol: the synthetic precursor to o-formyl-m-hydroxycinnamic acid, the most oxidized salicylaldehyde-type phytotoxin isolated from rice blast fungus, Magnaporthe grisea
-
Akihito Saito
Abstract
o-Formyl-m-hydroxycinnamic acid, the most oxidized salicylaldehyde-type phytotoxin isolated from rice blast fungus, Magnaporthe grisea, was synthesized for the first time using 5-(2,2-dimethyl-4H-1,3-benzodioxin)methanol as the starting material, and the proposed structure was confirmed.
Introduction
Rice blast disease, caused by infection of rice blast fungus, Magnaporthe grisea (Hebert) Barr, is one of the most harmful diseases for rice [1]. Several salicylaldehyde derivatives, such as pyriculol (2) [2], pyriculariol (3) [3], pyriculone (4) [4], and pyricuol (5) [5], have been isolated from the fungus as suspicious compounds responsible for the disease; they induce dark necrotic spot, when being applied to wounded rice leaves. In addition, o-formyl-m-hydroxycinnamic acid (6), probably further oxidized compound derived from 4, has also been found in the culture extract of the fungus [6] (Scheme 1). We have reported the synthesis of the derivatives 1–3, 5 using a common intermediate, 5-(2,2-dimethyl-4H-1,3-benzodioxin)methanol (7) (Scheme 2) [7–10]. In continuation of our synthetic studies of these compounds [7–13], the most oxidized derivative 6 was prepared for the first time from 7. Isolation and synthesis of o-carboxy-m-hydroxycinnamic acid, the related phytotoxin from other sources, has been reported [14–16]. Details of the synthesis are described in this report.
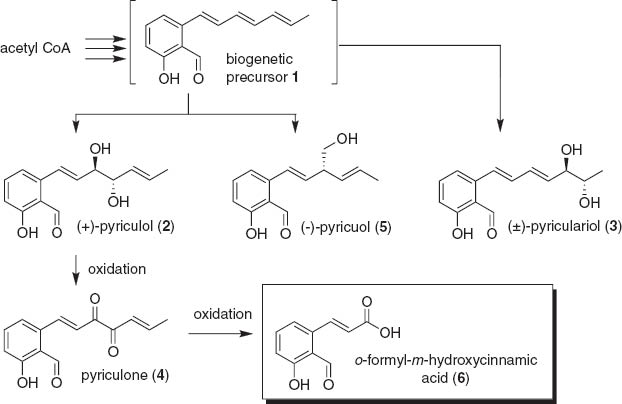
Biogenetic pathways of salicylaldehyde-type phytotoxin isolated from rice blast fungus.
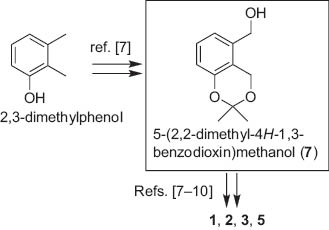
5-(2,2-Dimethyl-4H-1,3-benzodioxin)methanol (7) as the key synthetic intermediate for the synthesis of the phytotoxins.
Results and discussion
We have already reported the preparation of compounds with the same carbon skeleton as 6, as intermediates towards the synthesis of pyricuol (5) [9, 10]. Thus, we chose the intermediate 7 as the starting material. Partial oxidation of 7 and the Horner-Wadsworth-Emmons reaction afforded ester 8, which was then reduced to give aldehyde 9 according to our procedure [10] (Scheme 3). At first, the aldehyde 9 was oxidized with Jones reagent to give acid 10, and then the acetonide protecting group was removed under acidic conditions. However, the resulting dihydroxy acid 11 could barely be purified because of its high hydrophilicity. Thus, we restarted the synthesis from the ester 8, and the acetonide group was removed under acidic conditions. The desired diol 12 was obtained as a colorless oil after silica gel purification in 45% yield. Then, the alcoholic hydroxy group was oxidized using MnO2 in DMSO/CHCl3 [10] to give aldehyde 13 in 97% yield. The alkaline hydrolysis of the ester group was examined. The use of K2 CO3 or KOH in EtOH/H2 O resulted in a complex mixture. Finally, we found that LiOH in EtOH/H2 O was the best choice that afforded the target compound 6 as colorless needles (mp 132–133°C) in 59% yield. The overall yield was 26% from 8. The 1H NMR spectra of the natural product 6 and synthetic compound 6 were virtually identical.
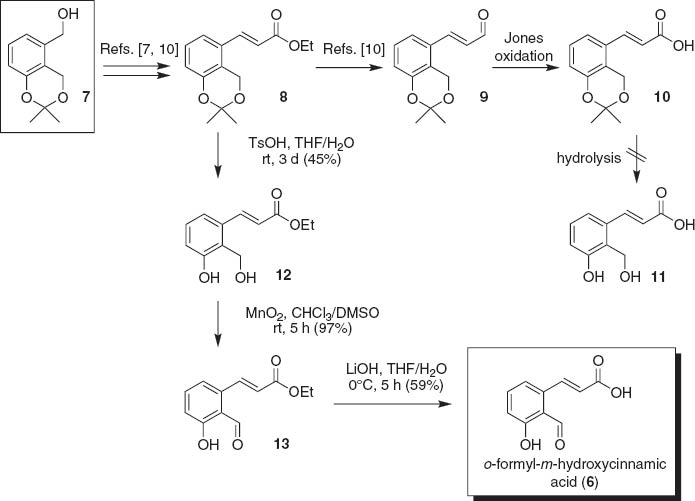
Synthesis of o-formyl-m-hydroxycinnamic acid (6).
Conclusion
o-Formyl-m-hydroxycinnamic acid, the most oxidized salicylaldehyde-type phytotoxin isolated from rice blast fungus, Magnaporthe grisea, was successfully synthesized for the first time using 5-(2,2-dimethyl-4H-1,3-benzodioxin)methanol as the starting material.
Experimental
General
Melting point was measured on a Yanako MP-J3 instrument and is uncorrected. FT-IR spectra were recorded as films by a Jasco 4100 spectrometer (ATR, Zn-Se). 1H NMR spectra were recorded with a Varian 400 MR (400 MHz) spectrometer in CDCl3 with CHCl3 (δH 7.26 ppm) or CD3 OD with CD3 OH (δH 3.30 ppm) as internal standard. Mass spectra were recorded with a Jeol JMS-700 spectrometer. Merck silica gel 60 (70–230 mesh) was used for column chromatography. Merck silica gel 60 F254 (0.25 mm thickness) was used for TLC analysis.
Ethyl (E)-3-(3′-hydroxy-2′-hydroxymethylphenyl)ethenoate (12)
A solution of 8 [9, 10] (82.0 mg, 0.31 mmol) and p-TsOH×H2 O (21.0 mg, mmol) in THF/H2 O (ca. 1 mL) was stirred at room temperature for 3 days and then treated with a saturated aqueous solution of NaHCO3. The resulting mixture was extracted with EtOAc. The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo. The residue was chromatographed on silica gel (hexane/EtOAc, 3:1) to give 12 (31.1 mg, 0.14 mmol, 45%) as a colorless oil; Rf = 0.16 (hexane/EtOAc, 1:1); IR: ν 3450 (br. s, O–H), 2924 (m), 2854 (w), 1701 (w, C=O), 1640 (w), 1019 (s, C–O), 953 (m) cm-1; 1H NMR (CDCl3, 400 MHz): δ 7.90 (1H, d, J = 15.6 Hz, H-3), 7.70 (1H, s, ArOH), 7.22 (1H, pseudo t, J = 8.0 Hz, H-5′), 7.08 (1H, d, J = 8 Hz), 6.93 (1H, d, J = 8 Hz), 6.30 (1H, d, J = 15.6 Hz, H-2), 5.08 (2H, d, J = 5 Hz, CH2 OH), 4.26 (2H, q, J = 7 Hz, CH2 CH3), 2.24 (1H, br., CH2 OH), 1.34 (3H, t, J = 7 Hz, CH2 CH3). HR-FABMS. Calcd for C12 H12 O4 Na ([M+Na]+): m/z 245.0789. Found: m/z 245.0792.
Ethyl (E)-3-(2′-formyl-3′-hydroxyphenyl)ethenoate (13)
A suspension of 12 (31.1 mg, 0.14 mmol) and MnO2 (500 mg) in DMSO/CHCl3 (7:3, 10 mL) was stirred at room temperature for 5 h. The mixture was filtered through a Celite pad and the filtrate was concentrated in vacuo. The residue was chromatographed on silica gel (hexane/EtOAc, 2:1) to give 13 (30.0 mg, 0.14 mmol, 97%) as a pale yellow oil; Rf = 0.69 (hexane/EtOAc, 1:1); IR: ν 2982 (w), 2957 (w), 2925 (w), 1717 (s, C=O), 1651 (s), 1456 (m), 1335 (m), 1265 (m), 1184 (m), 1161 (m) cm-1; 1H NMR (CDCl3, 400 MHz): δ 11.92 (1H, s, HC=O), 10.38 (1H, s, OH), 8.22 (1H, d, J = 15.8 Hz, H-3′), 7.52 (1H, t, J = 8.0 Hz, H-5′), 7.06 (1H, d, J = 7.5 Hz), 7.02 (1H, d, J = 8.8 Hz), 6.37 (1H, d, J = 15.8 Hz), 4.26 (2H, q, J = 7.2 Hz, CH2 CH3), 1.34 (3H, t, J = 7.2 Hz, CH2 CH3). HR-EIMS. Calcd for C12 H12 O4 (M+.): m/z 220.0736. Found: m/z 220.0736.
(E)-3-(2′-Formyl-3′-hydroxyphenyl)ethenoic acid [(E)-o-formyl-m-hydroxycinnnamic acid] 5
A solution of 13 (20.0 mg, 0.091 mmol) and LiOH×H2 O (40.8 mg, 0.972 mmol) in THF/H2 O (3:1, 2 mL) was stirred at 0°C for 5 h. The solution was neutralized with citric acid and the mixture extracted with CH2 Cl2. The combined organic layer was concentrated in vacuo. The residue was chromatographed on silica gel (CHCl3/MeOH, 15:1) and crystallized from hexane/EtOAc to give 6 (10.2 mg, 0.053 mmol, 59%) as colorless needles; mp 132–133°C; Rf = 0.18 (CHCl3/MeOH, 15:1); IR: ν 3400 (br. s, O–H), 2948 (m), 2833 (w), 1653 (w), 1449 (w), 1021 (s) cm-1; 1H NMR (CD3 OD, 400 MHz): δ 10.46 (1H, s, HC=O), 8.31 (1H, d, J = 15.7 Hz, H-2), 7.54 (1H, t, J = 8 Hz, H-5′), 7.17 (1H, d, J = 8 Hz), 6.99 (1H, d, J = 8 Hz), 6.39 (1H, d, J = 15.7 Hz, H-3); 1H NMR (CDCl3, 400 MHz): δ 11.92 (1H, s, HC=O), 10.39 (1H, s, OH), 8.31 (1H, d, J = 15.8 Hz, H-3′), 7.54 (1H, t, J = 8 Hz, H-5′), 7.09 (1H, d, J = 8 Hz), 7.05 (1H, d, J = 8 Hz), 6.40 (1H, d, J = 15.7 Hz). HR-FABMS. Calcd for C10 H7 O4 ([M–H]–): m/z 191.0344. Found: m/z 191.0341.
Acknowledgments
Financial support by grant-in-aid from JSPS KAKENHI (numbers 17580092, 19580120, 22560112, and 25450144), the Agricultural Chemical Research Foundation, Intelligent Cosmos Foundation, and the Naito Foundation are gratefully acknowledged.
References
[1] Umetsu, N.; Kaji, J.; Tamari, K. Investigation on the toxin production by several blast fungus strains and isolation of tenuazonic acid as a novel toxin. Agric. Biol. Chem. 1972, 36, 859–866 (and references cited therein).Suche in Google Scholar
[2] Iwasaki, S.; Nozoe, S.; Okuda, S.; Sato, Z.; Kozaka, T. Isolation and structural elucidation of a pyhtotoxic substance produced by Pyricularia oryzae Cavara. Tetrahedron Lett. 1969, 45, 3977–3980.Suche in Google Scholar
[3] Nukina, M.; Ikeda, M.; Umezawa, T.; Tasaki, H. Pyriculariol, a new phytotoxic metabolite of Pyricularia oryzae Cavara. Agric. Biol. Chem. 1981, 45, 2161–2162.Suche in Google Scholar
[4] Nukina, M.; Otuki, T.; Kurebayashi, T.; Hosokawa, K.; Sekine, M.; Ito, S.; Suenaga, M.; Sato, A.; Sassa, T. New phytotoxic metabolites produced by the blast disease fungi and microbial conversion of aromatic and aliphatic hydrocarbons as well as carbonyls by them. Abstract paper of 38th Symposium on the Chemistry of Natural Products, 1996, 391–396 (CAS No. 126: 16550).Suche in Google Scholar
[5] Kim, J.-C.; Min, J.-Y.; Kim, H.-T.; Cho, K.-Y.; Yu, S.-H. Pyricuol, a new phytotoxin from Magnaporthe grisea. Biosci. Biotechnol. Biochem. 1998, 62, 173–174.Suche in Google Scholar
[6] Nukina, M. Secondary metabolites produced by the blast disease fungi–chemotaxonomical classification based on them. Kagaku Seibutsu 1998, 36, 582–588 (in Japanese). The spectral data for 5 were not published.10.1271/kagakutoseibutsu1962.36.582Suche in Google Scholar
[7] Suzuki, M.; Sugiyama, T.; Watanabe, M.; Murayama, T.; Yamashita, K. Synthesis and absolute configuration of pyriculol. Agr. Biol. Chem. 1987, 51, 1121–1127.Suche in Google Scholar
[8] Kiyota, H.; Sasaki, A.; Tanaka, K.; Nakamura, Y.; Ueda, R.; Suzuki, Y.; Kuwahara, S.; Nukina, M. Synthetic studies of salicylaldehyde-type phytotoxins isolated from rice blast fungus. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 2011, 53, 661–666.Suche in Google Scholar
[9] Kiyota, H.; Ueda, R.; Oritani, T.; Kuwahara, S. First synthesis of (±)-pyricuol, a plant pathogen isolated from rice blast disease fungus Magnaporthe grisea. Synlett 2003, 2, 219–220.Suche in Google Scholar
[10] Tanaka, K.; Nakamura, Y.; Sasaki, A.; Ueda, R.; Suzuki, Y.; Kuwahara, S.; Kiyota, H. Synthesis and plant growth inhibitory activity of both enantiomers of pyricuol, a phytotoxin isolated from rice blast disease fungus Magnaporthe grisea. Tetrahedron 2009, 65, 6115–6122.Suche in Google Scholar
[11] Tanaka, K.; Sasaki, A.; Cao, H.-Q.; Yamada, T.; Igarashi, M.; Komine, I.; Nakahigashi, H.; Minami, N.; Kuwahara, S.; Nukina, M.; et al. Biotransformation of plausible biosynthetic intermediates of salicylaldehyde-type phytotoxins of rice blast fungus, Magnaporthe grisea. Eur. J. Org. Chem. 2011, 6276–6280.10.1002/ejoc.201100771Suche in Google Scholar
[12] Nakamura, Y.; Kiyota, H.; Ueda, R.; Kuwahara, S. Synthesis to determine the absolute configuration of (–)-pyricuol, a phytotoxin isolated from rice blast disease fungus, Magnaporthe grisea. Tetrahedron Lett. 2005, 46, 7107–7109.Suche in Google Scholar
[13] Sasaki, A.; Tanaka, K.; Sato, Y.; Kuwahara, S.; Kiyota, H. First synthesis and absolute configuration of (–)-pyriculariol, a phytotoxin isolated from rice blast fungus, Magnaporthe grisea. Use of microwave irradiation to control Stille coupling reaction products. Tetrahedron Lett. 2009, 50, 4637–4638.Suche in Google Scholar
[14] Stierle, A. A.; Upadhyay, R.; Hershenhorn, J.; Strobel, G. A.; Molina, G. The phytotoxins of Mycosphaerella fijiensis, the causative agent of Black Sigatoka disease of bananas and plantains. Experientia 1991, 47, 853–859.Suche in Google Scholar
[15] Trost, B. M.; Rivers, G. T.; Gold, J. M. Regiocontrolled synthesis of hydroxyphthalides. Synthesis of (±)-isoochracinic acid and a zealeranone intermediate. J. Org. Chem. 1980, 45, 1835–1838.Suche in Google Scholar
[16] Yeola, S. N.; Mali, R. S. A convenient total synthesis of (±)-isoochracinic acid, a phthalide from Alternaria kikuchiana. Indian J. Chem. 1986, 25B, 804–806.Suche in Google Scholar
©2014 by Walter de Gruyter Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Review
- Synthesis and reactivity of heterocyclic hydroxylamine-O-sulfonates
- Preliminary Communication
- Synthesis of pyridine derivatives as potential antagonists of chemokine receptor type 4
- Research Articles
- Design and synthesis of novel active phosphonate esters and their application in preparation of ceftriaxone
- Synthesis of novel oxepine-containing polycyclic-fused quinoline systems
- Crystal structure of 2-aminobenzothiazolinium nitrate and theoretical study of the amino-imino tautomerism of 2-aminobenzothiazole
- Short and efficient synthesis of 5-aminothiazole-4-carboxamide
- The use of diethylene glycol in the synthesis of 2,2′-bibenzimidazole from o-phenylenediamine and oxalic acid
- Facile one-pot synthesis of chromeno[4,3-b]quinoline derivatives catalyzed by Cu(II)-Schiff base/SBA-15
- 5-(2,2-Dimethyl-4H-1,3-benzodioxin)methanol: the synthetic precursor to o-formyl-m-hydroxycinnamic acid, the most oxidized salicylaldehyde-type phytotoxin isolated from rice blast fungus, Magnaporthe grisea
Artikel in diesem Heft
- Frontmatter
- Review
- Synthesis and reactivity of heterocyclic hydroxylamine-O-sulfonates
- Preliminary Communication
- Synthesis of pyridine derivatives as potential antagonists of chemokine receptor type 4
- Research Articles
- Design and synthesis of novel active phosphonate esters and their application in preparation of ceftriaxone
- Synthesis of novel oxepine-containing polycyclic-fused quinoline systems
- Crystal structure of 2-aminobenzothiazolinium nitrate and theoretical study of the amino-imino tautomerism of 2-aminobenzothiazole
- Short and efficient synthesis of 5-aminothiazole-4-carboxamide
- The use of diethylene glycol in the synthesis of 2,2′-bibenzimidazole from o-phenylenediamine and oxalic acid
- Facile one-pot synthesis of chromeno[4,3-b]quinoline derivatives catalyzed by Cu(II)-Schiff base/SBA-15
- 5-(2,2-Dimethyl-4H-1,3-benzodioxin)methanol: the synthetic precursor to o-formyl-m-hydroxycinnamic acid, the most oxidized salicylaldehyde-type phytotoxin isolated from rice blast fungus, Magnaporthe grisea


