Abstract
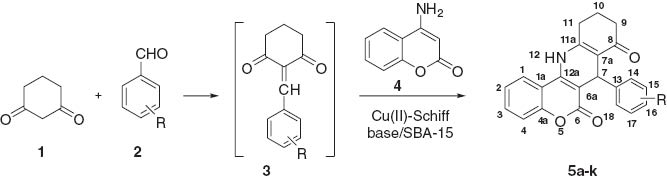
A novel, one-pot, simple, environmentally benign and efficient protocol has been employed for the synthesis of chromeno[4,3-b]quinoline derivatives in the presence of catalytic amounts of Cu(II)-Schiff base/SBA-15 under solvent-free conditions in excellent yields and rates.
Introduction
1,4-Dihydropyridines (1,4-DHPs), particularly 4-aryl-substituted 1,4-dihydropridines, are well known as calcium channel modulators and have emerged as one of the most important class of drugs for the treatment of cardiovascular disease [1, 2]. They also possess a variety of other biological activities [3]. 1,4-DHPs can be obtained by classical Hantzsch method, which involves cyclocondensation of an aldehyde, a 1,3-diketone or β-ketoester and an amine either in acetic acid or in ethanol under reflux [4]. Recently, several modifications to this classical method have been reported, including the use of catalysts such as Bronsted acid [5], lithium iodide [6], scandium triflate [7], cerium ammonium nitrate [8], magnesium perchlorate [9], and copper(II) oxide [10]. We have recently synthesized a new class of coumarin-fused dihydropyridines 5a–k (Table 1) in moderate yields under solvent-free conditions [11–14]. In this paper, we report a greatly improved synthesis of compounds 5a–k in the presence of a catalyst Cu(II)-Schiff base/SBA-15 (structure in Figure 1). This work is part of our research efforts to improve conditions for the Hantzsch reaction [15–19], in particular by using silica gel-supported catalysts [20–28].
Synthesis of 7-aryl-8,9,10,12-tetrahydro-7H-chromeno[4,3-b]quinoline-6,8-diones 5a–k catalyzed by Cu(II)-Schiff base/SBA-15.
 | |||
|---|---|---|---|
| Compound | R | mpa | Yieldb,c (%) |
| 5a | 2-nitro | >300 (>300) | 75 (35) |
| 5b | 4-nitro | 260 (260–262) | 70 (45) |
| 5c | 2-chloro | >310 (>310) | 90 (50) |
| 5d | 3-chloro | 207 (208–210) | 93 (50) |
| 5e | 4-chloro | 214 (214–216) | 74 (55) |
| 5f | 3-bromo | 265 (267–269) | 71 (60) |
| 5g | 4-bromo | 295 (294–296) | 77 (65) |
| 5h | 2-methyl | 190 (188–190) | 86 (45) |
| 5i | 3-methyl | >310 (310) | 78 (74) |
| 5j | 2-methoxy | >310 (>310) | 89 (75) |
| 5k | 4-methoxy | 275 (276–278) | 80 (75) |
aThe numbers in parentheses refer to melting points in the literature [11].
bIsolated yields of pure compounds.
cThe numbers in parentheses refer to yields in the literature [11].
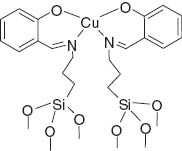
Structure of catalyst Cu(II)-Schiff base/SBA-15.
There are a few literature reports regarding the application of silica supported Schiff base cobalt(II) (Co/SBA-15) and copper(II) (Cu/SBA-15) complexes as catalysts in organic synthesis [29–34].
Herein, we describe a one-pot, simple, new, and efficient protocol for the synthesis of chromeno[4,3-b]quinolines, using the catalytic Cu(II)-Schiff base/SBA-15. To the best of our knowledge, there is no report on the use of this type of catalyst in the synthesis of 1,4-DHPs. The catalyst is prepared by anchoring the copper complex to mesoporous silica SBA-15 [33, 34] (Figure 1).
The synthetic pathway to the products 5a–k by the reaction of 1,3-cyclohexanedione (1), a benzaldehyde 2, and 4-aminocoumarin (4) is outlined in Table 1. The reaction with 4-nitrobenzaldehyde was chosen as a model for optimization. In the first step, substrates 1 and 2 were allowed to undergo Knoevenagel condensation under solvent-free conditions at 100°C for 10 min to yield the intermediate product 3. This reaction was monitored by TLC analysis.
For the subsequent Michael addition, the intermediate product 3, without isolation, was treated with 4-aminocoumarin (4) and the catalyst. This second step was conducted at 200°C for 5 min, at which time the intermediate Michael adduct underwent intramolecular cyclization to the desired product 5b. It should be noted that this second step requires heating for 1 h to 200°C in the absence of the catalyst [11]. The yield of 5b is low for the reaction conducted in the absence of the catalyst. To analyze the scope and limitations of this procedure, a number of products 5 were obtained by using the optimized conditions (Table 1). All products were obtained quickly and in high yields in the presence of the catalyst.
The reusability of the Cu(II)/SBA-15 catalyst was investigated. After the first catalytic cycle, the catalyst was removed from the mixture by filtration and successfully used in 10 subsequent runs, without any significant loss in catalytic activity. No pretreatment step was required, although the recovered catalyst was washed with 5 mL of MeOH to remove traces of the previous reaction mixture and dried before the next cycle.
The mechanism for the formation of 5a–k in the absence of catalyst has been suggested [11]. Coordination of the carbonyl groups in the intermediate products by the Cu2+ cation of the catalyst may increase electrophilicity of the carbonyl derivatives in both the Knoevenagel condensation and the Michael addition step.
In summary, a one-pot, convenient, environmentally benign, and safe synthetic method for chromeno[4,3-b]quinoline was developed. The simplicity of the method, including mild reaction conditions and the ease of product isolation, will make it attractive for use on an industrial scale. Most important, the purification procedure only involves filtration, washing, and drying.
Experimental
Preparation of the catalyst Cu(II)-Schiff base/SBA-15
The Cu(II)-Schiff base complex was prepared using the literature procedure [33] with the following modification. Activated silica gel SBA-15 (1.5 g) was suspended in a methanol solution of the Schiff base complex, and the mixture was stirred at room temperature for 24 h. The solvent was removed using a rotary evaporator, and the resulting green solid was dried at 80°C overnight. The final product was washed with MeOH and deionized water until the washings were colorless to ensure that the noncovalently grafted complex and physically adsorbed metal species were removed. Further drying was carried out in an oven at 80°C for 8 h. To measure the amount of copper loaded into SBA-15, the catalyst (0.1 g) was digested with HNO3 by stirring at room temperature for a week. Then the mixture was filtered, and the total amount of copper in SBA-15 in the colorless sample was determined as 0.14 mmol/g by atomic absorption spectroscopy.
General procedure for synthesis of chromeno[4,3-b]quinoline-6,8-diones 5a–k
A mixture of an aromatic aldehyde (1, 1.0 mmol) and 1,3-cyclohexadione (2, 1.0 mmol) were thoroughly mixed in a beaker using a spatula. Then the beaker was placed in an autoclave at 100°C for 10 min to complete the condensation reaction to 3, as monitored by TLC. After addition of 4-aminocoumarin (1.0 mmol) and the catalyst (0.01 g, 0.0014 mmol), the mixture was thoroughly mixed and placed in the autoclave at 200°C for 5 min. After cooling, the mixture was washed with acetone (50 mL) and the catalyst was removed by filtration, rinsed twice with MeOH, and then dried at 80°C for 60 min for subsequent reuse. Analytically pure product 5a–k was obtained by evaporation of the solvent. The yields and melting points are shown in Table 1. The products were identified by comparison with the original samples [11].
Acknowledgments
The authors are thankful to the University of Payame Noor for financial support.
References
[1] Bossert, F.; Meyer, H.; Wehinger, E. 4-Aryldihydropyridines, a new class of highly active calcium antagonists. Angew. Chem. Int. Ed. Engl. 1981, 20, 762–769.Suche in Google Scholar
[2] Nakayama, H.; Kasoaka, Y. Chemical identification of binding sites of calcium channel antagonists. Heterocycles 1996, 42, 901–909.10.3987/REV-95-SR4Suche in Google Scholar
[3] Morshed, S. R.; Hashimoto, K.; Murotani, Y.; Kawase, M. Tumor-specific cytotoxicity of 3,5-dibenzoyl-1,4-dihydropyridines. Anticancer Res. 2005, 25, 2033–2038.Suche in Google Scholar
[4] Hantzch, J. L. Hantzsch pyridine synthesis. Ann. Chem. 1882, 215, 1–82.Suche in Google Scholar
[5] Moreau, J.; Duboc, A.; Hubert, C.; Hurvois, J. P.; Renaud, J. L. Metal-free Brønsted acids catalyzed synthesis of functional 1,4-dihydropyridines. Tetrahedron Lett. 2007, 48, 8647–8650.Suche in Google Scholar
[6] Geirsson, J. K. F.; Johannesdottir, J. F. Convenient synthesis of N-benzyl-1,4-dihydropyridines, cyclohexenones, and bicyclo[3.3.1]nonan-3-one derivatives from 1-aza-1,3-butadienes. J. Org. Chem. 1996, 61, 7320–7325.Suche in Google Scholar
[7] Carranco, I.; Diaz, L. J.; Jimenez, O.; Lavilla, R. Dihydropyridine-based MCRs. New reaction pathways in the interaction with ethyl glyoxalate and non-aromatic amines. Tetrahedron Lett. 2003, 44, 8449–8452.Suche in Google Scholar
[8] Sridharan, V.; Perumal, P. T.; Avendano, C.; Menendez, C. A new three-component domino synthesis of 1,4-dihydropyridines. Tetrahedron 2007, 63, 4407–4413.Suche in Google Scholar
[9] Bartoli, G.; Babiuch, K.; Bosco, M.; Carlone, A.; Galzerano, P.; Melchiorre, P.; Sambri, L. Magnesium perchlorate as efficient Lewis acid: a simple and convenient route to 1,4-dihydropyridines. Synlett 2007, 18, 2897–2901.Suche in Google Scholar
[10] Kantam, M. L.; Ramani, T.; Chakrapani, L.; Choudary, B. M. Synthesis of 1,4-dihydropyridine derivatives using nanocrystalline copper(II) oxide catalyst. Catal. Commun. 2009, 10, 370–372.Suche in Google Scholar
[11] Miri, R.; Motamedi, R.; Rezaei, M. R.; Firuzi, O.; Javidnia, A.; Shafiee, A. Design, synthesis and evaluation of cytotoxicity of novel chromeno[4,3 b]quinoline derivatives. Arch. Pharm. Chem. Life Sci. 2011, 344, 111–118.Suche in Google Scholar
[12] Tanka, K.; Toda, F. Solvent-free organic synthesis. Chem. Rev. 2000, 100, 1025–1074.Suche in Google Scholar
[13] Walsh, P. J.; Li, H.; De Parrodi, C. A. A green chemistry approach to asymmetric catalysis: solvent-free and highly concentrated reactions. Chem. Rev. 2007, 107, 2503–2545.Suche in Google Scholar
[14] Garay, A. L.; Pichon, A.; James, S. L. Solvent-free synthesis of metal complexes. Chem. Soc. Rev. 2007, 6, 846–855.Suche in Google Scholar
[15] Heravi, M. M.; Motamedi, R.; Seifi, N.; Bamoharram, F. Catalytic synthesis of 6-aryl-1H-pyrazolo[3,4-d]pyrimidin-4(5H)-ones by heteropolyacid: H14[NaP5 W30 O110] and H3 PW12 O40. J. Mol. Catal. 2006, 249, 1–3.Suche in Google Scholar
[16] Heravi, M. M.; Motamedi, R. S.; Bamoharram, N. F. A catalytic method for synthesis of 6-aryl-1H-pyrazolo[3,4-d]pyrimidin-4[5H]-ones by heteropolyacid: H14[NaP5 W29 MoO110] and H3 PMo12 O40. Catal. Commun. 2007, 8, 1467–1471.Suche in Google Scholar
[17] Motamedi, R.; Heravi, M. M.; Bamoharram, F. F.; Haeriyan, A. Facile routes to 1,2,4-triazolo-[3,4-b][1,3,4]thiadiazines and 1,2,4-triazino-[3,4-b][1,3,4]thiadiazine by heteropolyacides. J. Heterocycl. Chem. 2008, 45, 1211–1214.Suche in Google Scholar
[18] Motamedi, R.; Monfared, A.; Ghahghai Nezamabadi, Z.; Bamoharram, F. F. Facile, one-pot synthesis of [1,2,4]triazolo[3,4-b][1,3,4]thiadiazines and 3,7-dimethyl-4H-[1,2,4]triazino[3,4-b][1,3,4]thiadiazin-6-one using heteropolyacid catalysts. J. Heterocycl. Chem. 2010, 48, 604–607.Suche in Google Scholar
[19] Motamedi, R.; Baghbani, S.; Bamoharram, F. F. A catalytic method for synthesis of benzopyrano[3,2-c]chromene-6,8-dione derivatives by heteropolyacids. Synth. Commun. 2012, 42, 1–9.Suche in Google Scholar
[20] Kresge, C. T.; Leonowicz, M. E.; Roth, W. J.; Vartuli, J. C.; Beck, J. S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712.Suche in Google Scholar
[21] Wight, A. P.; Davis, M. E. Design and preparation of organic–inorganic hybrid catalysts. Chem. Rev. 2002, 102, 3589–3614.Suche in Google Scholar
[22] Corma, A.; Garcia, H. Lewis acids as catalysts in oxidation reactions: from homogeneous to heterogeneous systems. Chem. Rev. 2002, 102, 3837–3892.Suche in Google Scholar
[23] Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 2004, 104, 3037–3058.Suche in Google Scholar
[24] Lu, Z. L.; Lindner, E.; Mayer, H. A. Applications of sol-gel-processed interphase catalysts. Chem. Rev. 2002, 102, 3543–3578.Suche in Google Scholar
[25] Baleizao, C.; Garcia, H. Chiral Salen complexes: an overview to recoverable and reusable homogeneous and heterogeneous catalysts. Chem. Rev. 2006, 106, 3987–4043.Suche in Google Scholar
[26] De Vos, D. E.; Dams, M.; Sels, B. F.; Jacobs, P. A. Ordered mesoporous and microporous molecular sieves functionalized with transition metal complexes as catalysts for selective organic transformations. Chem. Rev. 2002, 102, 3615–3640.Suche in Google Scholar
[27] Taguchi, A.; Schuth, F. Ordered mesoporous materials in catalysis. Micropor. Mesopor. Mater. 2005, 77, 1–45.Suche in Google Scholar
[28] Meynen, V.; Cool, P.; Vansant, E. F. Verified syntheses of mesoporous materials. Micropor. Mesopor. Mater. 2009, 125, 170–223.10.1016/j.micromeso.2009.03.046Suche in Google Scholar
[29] Rajabi, F. A heterogeneous cobalt (II) Salen complex as an efficient and reusable catalyst for acetylation of alcohols and phenols. Tetrahedron Lett. 2009, 50, 395–397.Suche in Google Scholar
[30] Rajabi, F. A highly efficient and reusable mesoporous supported Co (II) catalyst for chemoselective deprotection of aryl acetates. Tetrahedron Lett. 2009, 50, 7256–7258.Suche in Google Scholar
[31] Rezanejade Bardajee, G.; Malakooti, R.; Jami, F.; Parsaei, Z.; Atashin, H. Covalent anchoring of copper-Schiff base complex into SBA-15 as a heterogeneous catalyst for the synthesis of pyridopyrazine and quinoxaline derivatives. Catal. Commun. 2012, 27, 49–53.Suche in Google Scholar
[32] Chisem, I. C.; Rafelt, J.; Shieh, M. T.; Chisem, J.; Clark, J. H.; Jachuck, R.; Macquarrie, D. J.; Ramshaw, C.; Scott, K. Catalytic oxidation of alkyl aromatics using a novel silica supported Schiff base complex. Chem. Commun. 1998, 1949–1950.10.1039/a805420gSuche in Google Scholar
[33] Rajabi, F.; Karimi, B. Efficient aerobic oxidation of alcohols using a novel combination N-hydroxy phthalimide (NHPI) and a recyclable heterogeneous cobalt complex. J. Mol. Catal. A 2005, 232, 95–99.10.1016/j.molcata.2005.01.016Suche in Google Scholar
[34] Masteri-Farahani, M.; Farzaneh, F.; Ghandi, M. Synthesis and characterization of a new epoxidation catalyst by grafting cis-MoO2 (salpr) complex to functionalized MCM-41. J. Mol. Catal. A Chem. 2006, 243, 170–175.Suche in Google Scholar
©2014 by Walter de Gruyter Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Review
- Synthesis and reactivity of heterocyclic hydroxylamine-O-sulfonates
- Preliminary Communication
- Synthesis of pyridine derivatives as potential antagonists of chemokine receptor type 4
- Research Articles
- Design and synthesis of novel active phosphonate esters and their application in preparation of ceftriaxone
- Synthesis of novel oxepine-containing polycyclic-fused quinoline systems
- Crystal structure of 2-aminobenzothiazolinium nitrate and theoretical study of the amino-imino tautomerism of 2-aminobenzothiazole
- Short and efficient synthesis of 5-aminothiazole-4-carboxamide
- The use of diethylene glycol in the synthesis of 2,2′-bibenzimidazole from o-phenylenediamine and oxalic acid
- Facile one-pot synthesis of chromeno[4,3-b]quinoline derivatives catalyzed by Cu(II)-Schiff base/SBA-15
- 5-(2,2-Dimethyl-4H-1,3-benzodioxin)methanol: the synthetic precursor to o-formyl-m-hydroxycinnamic acid, the most oxidized salicylaldehyde-type phytotoxin isolated from rice blast fungus, Magnaporthe grisea
Artikel in diesem Heft
- Frontmatter
- Review
- Synthesis and reactivity of heterocyclic hydroxylamine-O-sulfonates
- Preliminary Communication
- Synthesis of pyridine derivatives as potential antagonists of chemokine receptor type 4
- Research Articles
- Design and synthesis of novel active phosphonate esters and their application in preparation of ceftriaxone
- Synthesis of novel oxepine-containing polycyclic-fused quinoline systems
- Crystal structure of 2-aminobenzothiazolinium nitrate and theoretical study of the amino-imino tautomerism of 2-aminobenzothiazole
- Short and efficient synthesis of 5-aminothiazole-4-carboxamide
- The use of diethylene glycol in the synthesis of 2,2′-bibenzimidazole from o-phenylenediamine and oxalic acid
- Facile one-pot synthesis of chromeno[4,3-b]quinoline derivatives catalyzed by Cu(II)-Schiff base/SBA-15
- 5-(2,2-Dimethyl-4H-1,3-benzodioxin)methanol: the synthetic precursor to o-formyl-m-hydroxycinnamic acid, the most oxidized salicylaldehyde-type phytotoxin isolated from rice blast fungus, Magnaporthe grisea


