Abstract
In the present investigation, the incorporation of the oxepine ring into a polycyclic-fused quinoline system leading to a series of structurally novel hybrids benzo[h]areno[6,7]oxepino[3,4-b]quinolin-8(14H)-ones has been achieved through a simple, and economical two-step procedure that involves the one-pot synthesis of 2-(aryloxymethyl)benzo[h]quinoline-3-carboxylic acids followed by intramolecular Friedel-Crafts acylation reaction using Eaton’s reagent.
Introduction
Polycyclic-fused quinolines are an important group of compounds in medicinal chemistry [1–3] and are ubiquitous substructures associated with biologically active natural products such as Luotomins (1, Figure 1) [4] and Calothrixin B (2, Figure 1) [5]. Studies have shown that planar polycyclic-fused quinoline systems can cleave DNA [6] and exhibit significant biological properties such as antitumoral [7], anti-cancer [8], anti-inflammatory [9], antibacterial, and antituberculosis activities [10]. For example, owing to their aromatic character and planar structure, indenoquinoline derivatives (3, Figure 1) show potent antiproliferative activities against breast (MCF-7), lung epithelial (A-549), and cervical (HeLa) adenocarcinoma cells [3]. Accordingly, attention has been increasingly focused on the construction of various polycyclic-fused quinoline systems. Most reports in the literature describe a common heteroaromatic system, such as indene [3], pyrazole [11], pyran [12], indole [13], benzofuran [14], and benzothienoquinoline [15], fused to a quinoline system, and synthetic analogs thereof. However, to the best of our knowledge, there are very few reports on a medium-sized seven-membered oxepin ring fused to a quinoline unit [16].

Structures of natural products and synthetic compounds 1–6.
The seven-membered oxepine system is a pharmaceutically important structural unit of some natural products such as tournefolic acid B (4, Figure 1) [17] and bauhiniastatin 1 (5, Figure 1) [18], and has found wide application in the design and discovery of novel bioactive molecules and drugs. Some compounds with the oxepine ring have been reported to exhibit potent biological activities such as antifungal [19], anxiolytic [20], cyclooxygenase inhibitory [21], and aminopeptidase N (APN) inhibitory activities [22]. Given the importance of the oxepine core as a pharmacophore, extensive synthetic efforts have been devoted to the design and synthesis of oxepine-containing heterocycles [23–26]. A recent review concerning the synthesis of oxepines has been reported [27].
It is well known that the development of hybrid compounds through the combination of different chromophores or pharmacophores in a molecular framework is an important goal of synthetic organic chemistry and plays a prominent role in modern drug discovery [28]. In light of these findings, it would be of synthetic importance to incorporate the oxepine nucleus into planar polycyclic-fused quinoline systems leading to new prototypes, as possible drug candidates for pharmacological studies. Thus, in the context of our ongoing work on the synthesis of fused quinolines [29–32], we would like to report herein the synthesis of novel oxepine-containing polycyclic-fused quinoline systems, wherein the oxepine ring is fused at its 2,3-position to the b-position of the quinoline system to give a compact structure exemplified by compounds 6 shown in Figure 1. To the best of our knowledge, this simple polycyclic-fused system has never been previously described in the literature.
Results and discussion
Recently, we have reported a direct and efficient synthesis of ethyl 2-(chloromethyl)benzo[h]quinoline-3-carboxylate (8 in Scheme 1) and its application in the one-pot synthesis of 2-(benzofuran-2-yl)benzo[h]quinoline-3-carboxylic acid derivatives [29]. The inspiring results obtained prompted us to further study the synthetic application of 8 associated with the construction of a polycyclic-fused oxepine-containing quinoline system. Our synthetic plan, as shown in Scheme 1, was to use the 2-chloromethyl group of 8 to generate the aryloxymethyl moiety via the Williamson reaction with substituted phenols followed by the transformation of the ethyl ester at its 3-position into corresponding quinoline-3-carboxylic acids to afford intermediates 7. Subsequently, the intramolecular Friedel-Crafts acylation reaction of 7 would result in the formation of the oxepine ring by the generation of a new C-C bond.
![Scheme 1 Synthetic route to benzo[h]areno[6,7]oxepino[3,4-b]quinolin-8(14H)-ones 6a–h.](/document/doi/10.1515/hc-2013-0227/asset/graphic/hc-2013-0227_scheme1.jpg)
Synthetic route to benzo[h]areno[6,7]oxepino[3,4-b]quinolin-8(14H)-ones 6a–h.
Ethyl 2-(chloromethyl)benzo[h]quinoline-3-carboxylate (8) was first subjected to the Williamson reaction with substituted phenols in the presence of NaOEt as base in refluxing anhydrous EtOH. In this reaction, the NaOEt/EtOH base system was selected because the formation of a precipitate provided a convenient visual indicator of the reaction progress. Because the formed aryloxymethyl ether from the Williamson reaction did not interfere with further ester hydrolysis reaction, purification at this stage was unnecessary. Thus, we simply added 80% ethanolic potassium hydroxide solution to the reaction mixture and continued the reflux for an additional 2 h. After the reaction was complete followed by an acidic workup, the corresponding free quinoline-3-carboxylic acids 7a–f were obtained in good overall yields of 78–85%. Owing to the simplicity and efficiency of the one-pot synthesis, we further attempted the reaction of 8 with 1-naphthol and 2-naphthol. Interestingly, these substrates were equally amenable to the one-pot reaction sequence and gave rise to the corresponding 2-[(naphthalen-1-yloxy)methyl]benzo[h]quinoline-3-carboxylic acid (7g) and 2-[(naphthalen-2-yloxy)methyl]benzo[h]quinoline-3-carboxylic acid (7h) in 75% and 77% yields, respectively. Products 7a–h are new compounds and their structures were confirmed by both spectral data and elemental analyses.
Having in hand a series of the newly synthesized intermediates 7a–h, our attention was transferred to their intramolecular Friedel-Crafts acylation reaction for building the desired oxepino-fused quinoline systems. At this stage, we investigated the reaction using POCl3 according to the method recently reported by Meesala and Nagarajan [33]. However, in the present case, these conditions failed to efficiently promote the reaction and none of the expected products were detected. We also attempted to conduct the reaction using polyphosphoric acid (PPA) as the cyclization agent [30, 31]. Unfortunately, the reaction did not occur at temperatures lower than 150°C even after 12 h. At the temperature above 150°C, black tarry byproducts were formed, which significantly reduced the yield of the desired products. Despite numerous trials under different conditions, the yield of this reaction could not be optimized. After these fruitless attempts, it was decided to focus on the use of Eaton’s reagent (phosphorus pentoxide in methanesulfonic acid) [31, 32]. To our delight, with Eaton’s reagent the intramolecular cyclization reaction of 7a proceeded smoothly at 80°C with advantages in ease of handling, low reaction temperature, and good yield (76%). The ease of isolation of the product was notable. After simple aqueous workup and crystallization from ethanol, the corresponding product was isolated in an analytically pure form.
Encouraged by the successful synthesis of 7a, we tested this protocol to the preparation of other substrates 7b–h. With the exception of 7f, good yields of the remaining products in the range of 69–80% were obtained. The intramolecular Friedel-Crafts acylation reaction of 7f failed to give the desired cyclized product, even after raising the reaction temperature and prolonging the reaction time. This negative result can be explained in terms of the presence of the strongly electron-withdrawing NO2 group, which renders the benzene ring highly electron-deficient, thereby retarding the cyclization process.
We would also like to comment on the transformation of 7h to the cyclized product 6h. In principle, the intramolecular cyclization reaction of 7h might produce two possible structural isomers 6h and 6h′ because of the existence of two vacant ortho sites, the positions 1- and 3-, as indicated in Scheme 2. However, the cyclization is regioselective occurring at the more electron-rich position 1 to give 6h as the sole product as evidenced by analysis of the 1H NMR spectrum. In particular, the signal of the H-4 proton is a doublet at 8.52 ppm with a coupling constant J = 8.4 Hz, which is a typical value for ortho-protons coupling. If the cyclization reaction occurred at the 3-position affording the corresponding product 6h′, the H-4 proton signal in the 1H NMR spectrum should have been a singlet. A similar observation was also reported by Wang et al. for the intramolecular cyclization reaction of 2-naphthyl-substituted benzoic acid [34].
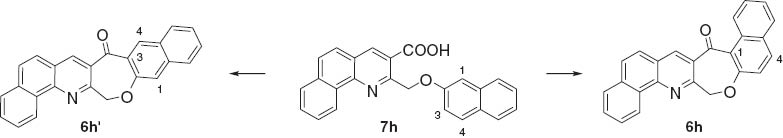
Possible intramolecular cyclization reactions of 7h.
Conclusions
A series of structurally new oxepine-containing polycyclic-fused quinoline systems, benzo[h]areno[6,7]oxepino[3,4-b] quinolin-8(14H)-ones, via the intramolecular Friedel-Crafts acylation reaction of 2-(aryloxymethyl)benzo[h]quinoline-3-carboxylic acids was synthesized. Biological activities of the newly synthesized compounds are currently being studied.
Experimental
The chemicals used in this work were obtained from Fluka and were used without purification. Melting points (uncorrected) were determined by using WRS-1B melting points apparatus. 1H NMR (600 MHz) and 13C NMR (150 MHz) spectra were recorded on a Brucker AVANCE NMR spectrometer. Elemental analyses were performed for C, H, and N using an Elementar Vario EL-III element analyzer. The progress of reactions was monitored by thin layer chromatography (TLC) on silica gel GF254 using ethyl acetate/petroleum ether (1:6) as eluent. Anhydrous ethanol was used in the synthesis work.
General procedure for the synthesis of 2-(aryloxymethyl)benzo[h]quinoline-3-carboxylic acids 7a–h
To a suspension of ethyl 2-(chloromethyl)benzo[h]quinoline-3-carboxylate (8) (0.3 g, 1 mmol) in sodium ethoxide solution [prepared by adding elemental sodium (0.025 g, 1.1 mmol) to absolute ethanol (8 mL)] was added the corresponding phenol (1 mmol). The resulting mixture was heated under reflux for 2 h, during which time a white precipitate was formed. After the reaction was complete as monitored by TLC, a solution of KOH (0.56 g, 10 mmol) in 80% ethanol (10 mL) was added and the mixture was heated under reflux for an additional 2 h, then cooled and acidified with 1 M HCl solution to pH 4–5. The resultant precipitate was collected by filtration and then washed with 25% NH4 OH solution and water. The crude product was crystalized from ethanol to afford compound 7a–h.
2-(Phenoxymethyl)benzo[h]quinoline-3-carboxylic acid (7a)
Colorless crystals; reaction time 2 h; yield 83%; mp 189–190°C; 1H NMR (CDCl3): δ 13.44 (s, 1H), 9.24 (d, 1H, 8.8 Hz), 8.75 (s, 1H), 7.90 (d, 1H, J = 8.8 Hz), 7.82 (d, 1H, J = 8.4 Hz), 7.71–7.74 (m, 3H), 7.23–7.30 (m, 3H), 7.09 (d, 1H, J = 8.4 Hz), 6.92 (t, 1H, J = 7.2 Hz), 5.84 (s, 2H); 13C NMR (DMSO-d6): δ 168.7, 159.1, 154.4, 144.8, 138.1, 137.6, 134.7, 133.8, 130.4, 129.4, 128.7, 128.1, 127.9, 127.2, 125.6, 124.9, 124.2, 121.3, 120.4, 114.8, 69.9. Anal. Calcd for C21 H15 NO3: C, 76.58; H, 4.59; N, 4.25. Found: C, 76.88; H, 4.76; N, 4.51.
2-((o-Tolyloxy)methyl)benzo[h]quinoline-3-carboxylic acid (7b)
Yellow solid; reaction time 2 h; yield 79%; mp 172–173°C; 1H NMR (DMSO-d6): δ 13.49 (s, 1H), 9.07 (d, 1H, J = 8.4 Hz), 8.96 (s, 1H), 8.08 (d, 1H, J = 7.8 Hz), 7.98–8.03 (m, 2H), 7.81 (t, 1H, J = 7.4 Hz), 7.76 (t, 1H, J = 7.4 Hz), 7.12 (d, 1H, J = 7.8), 7.00 (d, 1H, J = 7.8), 6.89 (d, 1H, J = 7.7 Hz), 6.85 (d, 1H, J = 7.7 Hz), 5.75 (s, 2H), 2.44 (s, 3H); 13C NMR (DMSO-d6): δ 167.4, 158.9, 154.5, 145.7, 139.2, 138.9, 134.2, 130.2, 129.3, 129.2, 128.5, 128.2, 127.5, 125.5, 125.4, 124.8, 124.4, 121.5, 115.5, 111.7, 70.2, 21.2. Anal. Calcd for C22 H17 NO3: C, 76.95; H, 4.99; N, 4.08. Found: C, 76.72; H, 5.14; N, 4.32.
2-((2-Methoxyphenoxy)methyl)benzo[h]quinoline-3-carboxylic acid (7c)
White solid; reaction time 2 h; yield 85%; mp 192–193°C; 1H NMR (DMSO-d6): δ 13.49 (s, 1H), 9.07 (d, 1H, J = 7.6 Hz), 8.96 (s, 1H), 8.08 (d, 1H, J = 8.2 Hz), 7.81 (t, 1H, J = 7.5 Hz), 7.76 (t, 1H, J = 7.5 Hz), 7.12 (d, 2H, J = 7.8 Hz), 7.00 (d, 2H, J = 7.8 Hz), 6.89 (t, 1H, J = 7.8 Hz), 6.78 (d, 1H, J = 8.2 Hz, 1H), 5.75 (s, 2H), 3.77 (s, 3H); 13C NMR (150 MHz, DMSO-d6): δ 167.3, 154.6, 149.2, 148.5, 145.8, 139.4, 134.2, 130.2, 129.3, 128.5, 128.2, 127.5, 125.5, 125.2, 124.8, 124.4, 121.1, 120.8, 113.8, 112.6, 70.8, 55.7. Anal. Calcd for C22 H17 NO4: C, 73.53; H, 4.77; N, 3.90. Found: C, 73.78; H, 4.85; N, 4.13.
2-((4-(tert-Butyl)phenoxy)methyl)benzo[h]quinoline-3-carboxylic acid (7d)
White crystals; reaction time 2 h; yield 82%; mp 186–188°C; 1H NMR (CDCl3): δ 13.46 (s, 1H), 9.23 (d, 1H, J = 8.7 Hz), 8.87 (s, 1H), 7.93 (d, 1H, J = 7.3 Hz), 7.88 (d, 1H, J = 8.7 Hz), 7.80–7.67 (m, 3H), 7.31 (d, 2H, J = 8.8 Hz), 7.04 (d, 2H, J = 8.8 Hz), 5.82 (s, 2H), 1.29 (s, 9H); 13C NMR (CDCl3): δ 163.4, 161.4, 156.7, 155.1, 153.9, 148.2, 147.5, 143.7, 140.3, 134.7, 131.0, 129.4, 129.0, 127.9, 127.5, 126.2, 125.4, 125.0, 124.8, 114.5, 71.1, 34.1, 31.5. Anal. Calcd for C25 H23 NO3: C, 77.90; H, 6.01; N, 3.63. Found: C, 78.06; H, 5.82; N, 3.71.
2-((4-Chlorophenoxy)methyl)benzo[h]quinoline-3-carboxylic acid (7e)
White solid; reaction time 2 h; yield 80%; mp 172–173°C; 1H NMR (DMSO-d6): δ 13.52 (s, 1H), 9.02 (s, 1H), 8.83 (d, 1H, J = 8.2 Hz), 8.22 (d, 2H, J = 9.2 Hz), 8.10–7.97 (m, 3H), 7.78 (t, 1H, J = 7.6 Hz), 7.68 (t, 1H, J = 7.6 Hz), 7.30 (d, 2H, J = 9.2 Hz), 5.99 (s, 2H); 13C NMR (DMSO-d6): δ 167.3, 166.1, 158.9, 157.8, 154.2, 149.4, 145.8, 139.4, 134.2, 130.1, 129.4, 129.2, 128.5, 128.2, 127.5, 125.5, 124.9, 124.7, 124.3, 116.6, 70.4. Anal. Calcd for C21 H14 ClNO3: C, 69.33; H, 3.88; N, 3.85. Found: C, 69.14; H, 4.02; N, 4.01.
2-((4-Nitrophenoxy)methyl)benzo[h]quinoline-3-carboxylic acid (7f)
Yellow solid; reaction time 2 h; yield 78%; mp 196–197°C; 1H NMR (DMSO-d6): δ 13.62 (s, 1H), 9.02 (s, 1H), 8.83 (d, 1H, J = 8.4 Hz), 8.22 (d, 2H, J = 9.2 Hz), 8.06 (t, 1H, J = 7.6 Hz), 8.02 (d, 2H, J = 9.2 Hz), 7.78 (t, 1H, J = 7.6 Hz), 7.68 (t, 1H, J = 7.6 Hz), 7.58–7.62 (m, 2H), 5.99 (s, 2H); 13C NMR (DMSO-d6): δ 167.1, 164.5, 153.6, 151.4, 145.8, 141.2, 140.8, 139.8, 134.3, 130.1, 129.4, 128.6, 128.2, 127.5, 125.8, 125.5, 124.7, 124.3, 124.2, 115.5, 70.5. Anal. Calcd for C21 H14 N2 O5: C, 67.38; H, 3.77; N, 7.48. Found: C, 67.15; H, 3.57; N, 7.25.
2-[(Naphthalen-1-yloxy)methyl]benzo[h]quinoline-3-carboxylic acid (7g)
White solid; reaction time 2 h; yield 75%; mp 216–217°C; 1H NMR (DMSO-d6): δ 13.44 (s, 1H), 9.36 (d, 1H, J = 8.8 Hz), 9.04 (d, 1H, J = 8.1 Hz), 8.98 (s, 1H), 8.94 (s, 1H), 8.27 (d, 1H, J = 8.1 Hz), 7.90 (d, 1H, J = 8.1 Hz), 7.85–7.76 (m, 4H), 7.43 (t, 1H, J = 7.6 Hz), 7.18 (d, 1H, J = 7.6 Hz), 5.97 (s, 2H), 5.18 (s, 2H); 13C NMR (CDCl3): δ 169.0, 154.8, 152.7, 145.9, 144.2, 137.1, 134.2, 131.6, 130.5, 130.1, 129.4, 128.9, 128.3, 127.6, 127.0, 126.8, 126.2, 125.4, 125.0, 124.5, 124.0, 123.6, 112.4, 107.7, 70.3. Anal. Calcd for C25 H17 NO3: C, 79.14; H, 4.52; N, 3.69. Found: C, 79.28; H, 4.79; N, 3.85.
2-[(Naphthalen-2-yloxy)methyl]benzo[h]quinoline-3-carboxylic acid (7h)
White solid; reaction time 2 h; yield 77%; mp 232–233°C; 1H NMR (DMSO-d6): δ 13.52 (s, 1H), 9.09 (d, 1H, J = 7.8 Hz), 8.98 (s, 1H), 8.07 (d, 1H, J = 7.8 Hz), 8.01–8.05 (m, 2H), 7.82–7.86 (m, 2H), 7.79 (t, 2H, J = 8.3 Hz), 7.73 (t, 1H, J = 7.6 Hz), 7.52 (d, 1H, J = 7.6 Hz), 7.44 (t, 1H, J = 7.0 Hz), 7.33 (t, 1H, J = 7.6 Hz), 7.29 (m, 1H), 5.89 (s, 2H); 13C NMR (DMSO-d6): δ 167.3, 156.8, 154.3, 145.7, 139.3, 139.1, 134.3, 134.2, 130.1, 129.4, 129.3, 128.7, 128.5, 128.2, 127.5, 127.5, 126.7, 126.4, 125.5, 124.8, 124.3, 123.6, 118.7, 107.4, 70.3. Anal. Calcd for C25 H17 NO3: C, 79.14; H, 4.52; N, 3.69. Found: C, 78.97; H, 4.79; N, 3.55.
General procedure for the synthesis of benzo[h]areno[6,7]oxepino[3,4-b]quinolin-8(14H)-ones 6a–h
2-(Aryloxymethyl)benzo[h]quinoline-3-carboxylic acid 7a–h (0.5 mmol) and Eaton’s reagent (5 mL) were added to round flask (10 mL) and the mixture was stirred at 80°C for 2–4 h. The conversion was monitored by TLC. After the reaction was complete, the mixture was poured slowly with stirring into cold water to induce precipitation followed by neutralization with NaHCO3 solution. The crude product 6a–h obtained after filtration and washing with water was crystallized from ethanol.
Benzo[h]benzo[6,7]oxepino[3,4-b]quinolin-8(14H)-one (6a)
White solid from 7a; reaction time 2.5 h; yield 76%; mp 197–198°C; 1H NMR (DMSO-d6): δ 9.26 (d, 1H, J = 9.0 Hz), 8.97 (s, 1H), 8.23 (d, 1H, J = 8.0 Hz), 8.13–8.10 (m, 1H), 8.08 (q, 2H, J = 9.0 Hz), 7.87–7.84 (m, 2H), 7.68 (t, 1H, J = 7.6 Hz), 7.30 (t, 1H, J = 7.6 Hz), 7.24 (d, 1H, J = 8.0 Hz), 5.68 (s, 2H). 13C NMR (CDCl3): δ 188.6, 161.5, 153.6, 148.0, 138.8, 135.7, 134.7, 133.8, 132.8, 132.1, 130.9, 129.4, 128.9, 128.0, 127.5, 126.0, 125.5, 125.3, 122.8, 121.1, 76.7. Anal. Calcd for C21 H13 NO2: C, 81.01; H, 4.21; N, 4.50. Found: C, 81.14; H, 4.39; N, 4.27.
12-Methylbenzo[h]benzo[6,7]oxepino[3,4-b]quinolin-8(14H)-one (6b)
White solid from 7b; reaction time 2 h; yield 72%; mp 218–219°C; 1H NMR (CDCl3): δ 9.37 (d, 1H, J = 8.8 Hz), 8.82 (s, 1H), 8.17 (d, 1H, J = 8.0 Hz), 7.94 (m, 1H), 7.87 (d, 1H, J = 8.8 Hz), 7.82–7.74 (m, 3H), 7.44 (d, 1H, J = 7.6 Hz), 7.10 (t, 1H, J = 7.6 Hz), 5.65 (s, 2H), 2.36 (s, 3H); 13C NMR (CDCl3): δ 189.1, 159.8, 153.8, 148.0, 138.7, 136.7, 134.6, 132.9, 131.0, 130.0, 129.9, 129.4, 128.8, 128.0, 127.4, 126.1, 126.0, 125.5, 125.2, 122.3, 76.8, 16.6. Anal. Calcd for C22 H15 NO2: C, 81.21; H, 4.65; N, 4.30. Found: C, 81.32; H, 4.79; N, 4.54.
10-Methoxybenzo[h]benzo[6,7]oxepino[3,4-b]quinolin-8(14H)-one (6c)
Yellow solid from 7c; reaction time 2 h; yield 80%; mp 197–198°C; 1H NMR (CDCl3): δ 9.37 (d, 1H, J = 9.3 Hz), 8.83 (s, 1H), 7.95 (m, 1H), 7.91 (dd, 1H, J = 7.0 and 2.8 Hz), 7.88 (d, 1H, J = 8.8 Hz), 7.82 (s, 1H), 7.79 (m, 2H), 7.16 (m, 2H), 5.72 (s, 2H), 3.96 (s, 3H); 13C NMR (DMSO-d6): δ 188.8, 153.4, 151.6, 151.1, 148.1, 138.7, 134.6, 132.6, 131.0, 129.4, 128.8, 128.0, 127.5, 127.3, 125.9, 125.5, 125.3, 123.0, 122.3, 116.8, 76.8, 56.6. Anal. Calcd for C22 H15 NO3: C, 77.41; H, 4.43; N, 4.10. Found: C, 77.24; H, 4.21; N, 4.22.
10-(tert-Butyl)benzo[h]benzo[6,7]oxepino[3,4-b]quinolin-8(14H)-one (6d)
Yellow solid from 7c; reaction time 3 h; yield 71%; mp 197–198°C; 1H NMR (CDCl3): δ 9.26 (d, 1H, J = 9.0 Hz), 8.97 (s, 1H), 8.23 (d, 1H, J = 7.8 Hz), 8.10 (m, 2H), 8.06 (d, 1H, J = 9.0 Hz), 7.86 (m, 1H), 7.68 (t, 1H, J = 7.8 Hz), 7.29 (d, 1H, J = 7.8 Hz), 7.24 (d, 1H, J = 8.4 Hz), 5.68 (s, 2H), 1.35 (s, 9H); 13C NMR (DMSO-d6): δ 189.1, 159.8, 157.4, 149.7, 148.2, 147.5, 144.6, 144.4, 143.6, 142.5, 140.0, 139.6, 138.7, 137.4, 135.7, 135.3, 134.7, 124.3, 124.2, 115.5, 76.7, 34.6, 31.7. Anal. Calcd for C25 H21 NO2: C, 81.72; H, 5.76; N, 3.81. Found: C, 81.64; H, 5.91; N, 3.62.
10-Chlorobenzo[h]benzo[6,7]oxepino[3,4-b]quinolin-8(14H)-one (6e)
Yellow solid from 7c; reaction time 4 h; yield 74%; mp 225–226°C; 1H NMR (DMSO-d6): δ 9.84 (s, 1H), 9.11 (d, 1H, J = 8.4 Hz), 8.31 (d, 1H, J = 7.8 Hz), 8.21 (m, 2H), 8.13 (t, 1H, J = 7.8 Hz), 7.74 (d, 1H, J = 8.4 Hz), 7.31 (d, 1H, J = 8.4 Hz), 6.06 (s, 2H); 13C NMR (150 MHz, DMSO-d6): δ 189.1, 162.1, 151.5, 140.6, 139.4, 136.4, 133.4, 132.5, 131.3, 126.3, 125.1, 124.5, 123.7, 120.7, 119.5, 117.6, 116.1, 115.7, 114.2, 113.9, 72.7. Anal. Calcd for C21 H12 ClNO2: C, 72.94; H, 3.50; N, 4.05. Found: C, 73.13; H, 3.31; N, 4.22.
Benzo[h]naphtho[2′,1′:6,7]oxepino[3,4-b]quinolin-8(16H)-one (6g)
Yellow solid from 7g; reaction time 3.5 h; yield 69%; mp 231–232°C; 1H NMR (CDCl3): δ 9.40 (d, 1H, J = 7.6 Hz), 8.83 (s, 1H), 8.52 (d, 1H, J = 8.4 Hz), 8.33 (d, 1H, J = 8.8 Hz), 7.93 (d, 1H, J = 7.6 Hz), 7.87 (d, 1H, J = 8.8 Hz), 7.84–7.76 (m, 4H), 7.64 (t, 1H, J = 7.6 Hz), 7.55–7.59 (m, 2H), 5.84 (s, 2H); 13C NMR (150 MHz, CDCl3): δ 188.4, 160.0, 158.3, 152.9, 148.0, 138.7, 137.4, 134.6, 133.2, 130.9, 129.7, 129.4, 128.9, 128.0, 127.5, 126.9, 126.5, 126.3, 126.2, 125.5, 125.2, 124.2, 122.3, 120.3, 76.8. Anal. Calcd for C25 H15 NO2: C, 83.09; H, 4.18; N, 3.88. Found: C, 82.94; H, 4.09; N, 3.73.
Benzo[h]naphtho[1′,2′:6,7]oxepino[3,4-b]quinolin-17(8H)-one (6h)
Yellow solid from 7h; reaction time 3.5 h; yield 71%; mp 199–201°C; 1H NMR (CDCl3): δ 9.23 (m, 1H), 8.91 (s, 1H), 8.52 (d, 1H, J = 8.4 Hz), 7.93 (d, 1H, J = 8.4 Hz), 7.86 (m, 1H), 7.79 (d, 1H, J = 8.4 Hz), 7.77 (d, 1H, J = 8.4 Hz), 7.75 (d, 1H, J = 8.8 Hz), 7.68 (m, 2H), 7.54 (m, 1H, J = 7.8 Hz), 7.42 (t, 1H, J = 7.2 Hz), 7.30 (d, 1H, J = 8.8 Hz), 5.64 (s, 2H); 13C NMR (DMSO-d6): δ 191.7, 159.1, 157.7, 155.5, 147.8, 138.6, 135.3, 134.7, 132.6, 132.0, 131.3, 130.7, 129.4, 128.6, 128.5, 128.4, 128.0, 127.4, 125.8, 125.6, 125.5, 125.4, 125.2, 120.9, 76.7. Anal. Calcd for C25 H15 NO2: C, 83.09; H, 4.18; N, 3.88. Found: C, 83.28; H, 4.33; N, 4.05.
Acknowledgments
The authors would like to thank the financial support from the Scientific Research Foundation of the Education Department of Liaoning Province (Grant No. L2013428).
References
[1] Tseng, C. H.; Lin, R. W.; Chen, Y. L.; Wang, G. J.; Ho, M. L.; Tzeng, C. C. Discovery of indeno[1,2-c]quinoline derivatives as inhibitors of osteoclastogenesis induced by receptor activator of NF-κB Ligand (RANKL). J. Med. Chem. 2011, 54, 3103–3107.Suche in Google Scholar
[2] Wright, C. W.; Addae-Kyereme, J.; Breen, A. G.; Brown, J. E.; Cox, M. F.; Croft, S. L.; Gökçek, Y.; Kendrick, H.; Phillips, R. M.; Pollet, P. L. Synthesis and evaluation of cryptolepine analogues for their potential as new antimalarial agents. J. Med. Chem. 2001, 44, 3187–3194.Suche in Google Scholar
[3] Chakrabarty, S.; Croft, M. S.; Marko, M. G.; Moyna, G. Synthesis and evaluation as potential anticancer agents of novel tetracyclic indenoquinoline derivatives. Bioorg. Med. Chem. 2013, 21, 1143–1149.Suche in Google Scholar
[4] Ma, Z. Z.; Hano, Y.; Nomura, T.; Chen, Y. J. Two new pyrroloquinazolinoquinoline alkaloids from Peganum nigellastrum. Heterocycles 1997, 46, 541–546.Suche in Google Scholar
[5] Rickards, R. W.; Rothschild, J. M.; Willis, A. C.; de Chazal, N. M.; Kirk, J.; Kirk, K.; Saliba, K. J.; Smith, G. D. Calothrixins A and B, novel pentacyclic metabolites from Calothrix cyanobacteria with potent activity against malaria parasites and human cancer cells. Tetrahedron 1999, 55, 13513–13520.Suche in Google Scholar
[6] Gatto, B.; Capranico, G.; Palumbo, M. Drugs acting on DNA topoisomerases: recent advances and future perspectives. Curr. Pharm. Design 1999, 5, 195–215.Suche in Google Scholar
[7] Faidallah, H. M.; Rostom, S. A. F. Synthesis, in vitro antitumor evaluation and DNA-binding study of novel tetrahydroquinolines and some derived tricyclic and tetracyclic ring systems. Eur. J. Med. Chem. 2013, 63, 133–143.Suche in Google Scholar
[8] Beauchard, A.; Jaunet, A.; Murillo, L.; Baldeyrou, B.; Lansiaux, A.; Chérouvrier, J. R.; Domon, L.; Picot, L.; Bailly, C.; Besson, T.; et al. Synthesis and antitumoral activity of novel thiazolobenzotriazole, thiazoloindolo[3,2-c]quinoline and quinolinoquinoline derivatives. Eur. J. Med. Chem. 2009, 44, 3858–3865.Suche in Google Scholar
[9] Chen, Y. L.; Chen, I. L.; Lu, C. M.; Tzeng, C. C.; Tsao, L. T.; Wang, J. P. Synthesis and anti-inflammatory evaluation of 4-anilinofuro[2,3-b]quinoline and 4-phenoxyfuro[2,3-b] quinoline derivatives. Part 3. Bioorg. Med. Chem. 2004, 12, 387–392.Suche in Google Scholar
[10] Eswaran, S.; Adhikari, A. V.; Kumar, R. A. New 1,3-oxazolo[4,5-c]quinoline derivatives: synthesis and evaluation of antibacterial and antituberculosis properties. Eur. J. Med. Chem. 2010, 45, 957–966.Suche in Google Scholar
[11] Mali, J. R.; Pratap, U. R.; Jawale, D. V.; Mane, R. A. Water-mediated one-pot synthetic route for pyrazolo[3,4-b]quinolines. Tetrahedron Lett. 2010, 51, 3980–3982.Suche in Google Scholar
[12] Kalita, P. K.; Baruah, B.; Bhuyan, P. J. Synthesis of novel pyrano[2,3-b]quinolines from simple acetanilides via intramolecular 1,3-dipolar cycloaddition. Tetrahedron Lett. 2006, 47, 7779–7782.Suche in Google Scholar
[13] Chen, Y. L.; Hung, H. M.; Lu, C. M.; Li, K. C.; Tzeng, C. C. Synthesis and anticancer evaluation of certain indolo[2,3-b]quinoline derivatives. Bioorg. Med. Chem. 2004, 12, 6539–6546.Suche in Google Scholar
[14] Yang, C. L.; Tseng, C. H.; Chen, Y. L.; Lu, C. M.; Kao, C. L.; Wu, M. H.; Tzeng, C. C. Identification of benzofuro[2,3-b]quinoline derivatives as a new class of antituberculosis agents. Eur. J. Med. Chem. 2010, 45, 602–607.Suche in Google Scholar
[15] David, E.; Pellet-Rostaing, S.; Lemaire, M. Heck-like coupling and Pictet-Spengler reaction for the synthesis of benzothieno[3,2-c]quinolines. Tetrahedron 2007, 63, 8999–9006.Suche in Google Scholar
[16] Bera, R.; Dhananjaya, G.; Singh, S. N.; Ramu, B.; Kiran, S. U.; Kumar, P. R.; Mukkanti, K.; Pal, M. Revisiting the reaction of β-chloroacroleins with 2-aminophenol: a new observation. Tetrahedron 2008, 64, 582–589.Suche in Google Scholar
[17] Lin, Y. L.; Chang, Y. Y.; Kuo, Y. H.; Shiao, M. S. Anti-lipid-peroxidative principles from Tournefortia sarmentosa. J. Nat. Prod. 2002, 65, 745–747.Suche in Google Scholar
[18] Pettit, G. R.; Numata, A.; Iwamoto, C.; Usami, Y.; Yamada, T.; Ohishi, H.; Cragg, G. M. Antineoplastic agents. 551. Isolation and structures of bauhiniastatins 1–4 from Bauhinia purpurea. J. Nat. Prod. 2006, 69, 323–327.Suche in Google Scholar
[19] Kahnberg, P.; Sterner, O. Synthesis of the antifungal 1-benzoxepinpterulone. Tetrahedron 2001, 57, 7181–7184.10.1016/S0040-4020(01)00640-8Suche in Google Scholar
[20] Trabanco, A. A.; Alonso, J. M.; Cid, J. M.; Font, L. M.; Megens, A. Synthesis of 2-N,N-dimethylaminomethyl-2,3,3a,12b-tetrahydrodibenzo[b,f]furo[2,3-d]oxepine derivatives as potential anxiolytic agents. Part 2: substitutions by methyl groups on the tetrahydrofuran ring. Il Farmaco 2005, 60, 241–248.Suche in Google Scholar
[21] Liu, J. H.; Steigel, A.; Reininger, E.; Bauer, R. Two new prenylated 3-benzoxepin derivatives as cyclooxygenase inhibitors from Perilla frutescens var. acuta. J. Nat. Prod. 2000, 63, 403–405.Suche in Google Scholar
[22] Roux, L.; Charrier, C.; Salomon, E.; Ilhan, M.; Bisseret, P.; Tarnus, C. An innovative strategy for the synthesis of a new series of potent aminopeptidase (APN or CD13) inhibitors derived from the oxepin-4-one family. Tetrahedron Lett. 2011, 52, 2586–2589.Suche in Google Scholar
[23] Olivera, R.; SanMartin, R.; Churruca, F.; Domínguez, E. Revisiting the Ullmann-Ether reaction: a concise and amenable synthesis of novel dibenzoxepino[4,5-d] pyrazoles by intramolecular etheration of 4,5-(o,o-halohydroxy)arylpyrazoles. J. Org. Chem. 2002, 67, 7215–7225.Suche in Google Scholar
[24] Lee, S. H.; Van, H. T. M.; Yang, S. H.; Lee, K. T.; Kwon, Y.; Cho, W. J. Molecular design, synthesis and docking study of benz[b]oxepines and 12-oxobenzo[c]phenanthridinones as topoisomerase 1 inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 2444–2447.Suche in Google Scholar
[25] Raju, B. C.; Saidachary, G.; Kumar, J. A. Wittig homologation of 2-(chloromethyl)-2H-chromen-2-ol derivatives: a new facile synthesis of substituted 2,3-dihydrobenzoxepine-4-carboxylates. Tetrahedron 2012, 68, 6289–6297.Suche in Google Scholar
[26] Choi, Y. L.; Lim, H. S.; Lim, H. J.; Heo, J. N. One-pot transition-metal-free synthesis of dibenzo[b,f]oxepins from 2-halobenzaldehydes. Org. Lett. 2012, 14, 5102–5105.Suche in Google Scholar
[27] Snyder, N. L.; Haines, H. M.; Peczuh, M. W. Recent developments in the synthesis of oxepines. Tetrahedron 2006, 62, 9301–9320.Suche in Google Scholar
[28] Dolle, R. E.; Nelson, K. H., Jr. Comprehensive survey of combinatorial library synthesis: 1998. J. Comb. Chem. 1999, 1, 235–282.Suche in Google Scholar
[29] Gao, W. T.; Jiang, Y.; Li, Y.; Li, F.; Yan, Y. A novel and facile synthesis of 2-(benzofuran-2-yl)benzo[h]quinoline-3-carboxylic acid derivatives. Chin. J. Chem. 2012, 30, 822–826.Suche in Google Scholar
[30] Gao, W. T.; Zhao, Z. G.; Li, Y.; Yan, Y.; Li, F. Synthesis and cyclization of 8-formyl-2-(phenoxymethyl)quinoline-3-carboxylic acids. Chin. J. Chem. 2012, 30, 1127–1132.Suche in Google Scholar
[31] Gao, W. T.; Lin, G.; Li, Y.; Tao, X.; Liu, R.; Sun, L. An efficient access to the synthesis of novel 12-phenylbenzo[6,7]oxepino[3,4-b]quinolin-13(6H)-one derivatives. Beilstein J. Org. Chem. 2012, 8, 1849–1857.Suche in Google Scholar
[32] Li, Y.; Zhang, C. H.; Sun, M. C.; Gao, W. T. Facile synthesis of 10-tert-butyl[1] benzoxepino[3,4-b][1,3]dioxolo[4,5-g]quinolin-12(6H)-ones. J. Heterocycl. Chem. 2009, 46, 1190–1194.Suche in Google Scholar
[33] Meesala, R.; Nagarajan, R. A short route to the synthesis of pyrroloacridines via Ullmann-Goldberg condensation. Tetrahedron Lett. 2010, 51, 422–424.Suche in Google Scholar
[34] Wang, Y.; Gulevich, A. V.; Gevorgyan, V. General and practical carboxyl-group-directed remote C-H oxygenation reactions of arenes. Chem. Eur. J. 2013, 19, 15836–15840.Suche in Google Scholar
©2014 by Walter de Gruyter Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Review
- Synthesis and reactivity of heterocyclic hydroxylamine-O-sulfonates
- Preliminary Communication
- Synthesis of pyridine derivatives as potential antagonists of chemokine receptor type 4
- Research Articles
- Design and synthesis of novel active phosphonate esters and their application in preparation of ceftriaxone
- Synthesis of novel oxepine-containing polycyclic-fused quinoline systems
- Crystal structure of 2-aminobenzothiazolinium nitrate and theoretical study of the amino-imino tautomerism of 2-aminobenzothiazole
- Short and efficient synthesis of 5-aminothiazole-4-carboxamide
- The use of diethylene glycol in the synthesis of 2,2′-bibenzimidazole from o-phenylenediamine and oxalic acid
- Facile one-pot synthesis of chromeno[4,3-b]quinoline derivatives catalyzed by Cu(II)-Schiff base/SBA-15
- 5-(2,2-Dimethyl-4H-1,3-benzodioxin)methanol: the synthetic precursor to o-formyl-m-hydroxycinnamic acid, the most oxidized salicylaldehyde-type phytotoxin isolated from rice blast fungus, Magnaporthe grisea
Artikel in diesem Heft
- Frontmatter
- Review
- Synthesis and reactivity of heterocyclic hydroxylamine-O-sulfonates
- Preliminary Communication
- Synthesis of pyridine derivatives as potential antagonists of chemokine receptor type 4
- Research Articles
- Design and synthesis of novel active phosphonate esters and their application in preparation of ceftriaxone
- Synthesis of novel oxepine-containing polycyclic-fused quinoline systems
- Crystal structure of 2-aminobenzothiazolinium nitrate and theoretical study of the amino-imino tautomerism of 2-aminobenzothiazole
- Short and efficient synthesis of 5-aminothiazole-4-carboxamide
- The use of diethylene glycol in the synthesis of 2,2′-bibenzimidazole from o-phenylenediamine and oxalic acid
- Facile one-pot synthesis of chromeno[4,3-b]quinoline derivatives catalyzed by Cu(II)-Schiff base/SBA-15
- 5-(2,2-Dimethyl-4H-1,3-benzodioxin)methanol: the synthetic precursor to o-formyl-m-hydroxycinnamic acid, the most oxidized salicylaldehyde-type phytotoxin isolated from rice blast fungus, Magnaporthe grisea

