The underestimated issue of non-reproducible cardiac troponin I and T results: case series and systematic review of the literature
-
Julien Favresse
, Jean-Louis Bayart
Abstract
Cardiac troponins (cTn) are the preferred biomarkers for the evaluation of myocardial injury and play a key role in the diagnosis of acute myocardial infarction (MI). Pre-analytical or analytical issues and interferences affecting troponin T and I assays are therefore of major concern given the risk of misdiagnosis. False positive troponin results have been related to various interferences including anti-troponin antibodies, heterophilic antibodies, or elevated alkaline phosphatase level. On the other hand, false negative results have been reported in the case of a large biotin intake. These interferences are characterized with erroneous but reproducible troponin results. Of interest, non-reproducible results have also been reported in the literature. In other words, if the sample is reanalyzed a second time, a significant difference in troponin results will be observed. These interferences have been named “fliers” or “outliers”. Compared to the biotin interference that received major attention in the literature, troponin outliers are also able to induce harmful clinical consequences for the patient. Moreover, the prevalence of outliers in recent studies was found to be higher (0.28–0.57%) compared to the biotin interference. The aim of this systematic review is to warn clinicians about these non-reproducible results that may alter their clinical judgment. Four case reports that occurred in the Clinique of Saint-Luc Bouge are presented to attest this point. Moreover, we aimed at identifying the nature of these non-reproducible troponin results, determining their occurrence, and describing the best way for their identification.
Introduction
Cardiac troponins (cTn) are the preferred biomarkers for the evaluation of myocardial injury and play a key role in the diagnosis of acute myocardial infarction (MI) [1]. Some pre-analytical of analytical issues and interferences leading to false positive or false negative could therefore have harmful clinical consequences for the patient.
Case report 1
On May 2019, an 80-year-old female went to her general practitioner (GP) to get a blood test for diabetes control. The patient is known with heart failure, hypertension, hypercholesterolemia, and hypertriglyceridemia and complained about chronical retrosternal pain since several months. The patient was sent to our emergency department (ED) based on an increased high-sensitivity cardiac troponin T (hs-cTnT) concentration (102.0 ng/L; 99th upper reference limit [RL] = 14.0 ng/L; Roche Diagnostics, Basel, Switzerland). Creatine kinase-MB isoenzyme (CK-MB, immunological; Roche Diagnostics) and N-terminal pro B-type natriuretic peptide (NT-proBNP; Roche Diagnostics) were 3.4 μg/L (RL <4.9 μg/L) and 557 pg/mL (RL <486 pg/mL), respectively. Fifteen years ago, the patient was diagnosed with transient ischemic attack. Her familial history revealed fatal MI by her father, five uncles and aunts. Her brother also died from stroke. A new blood sample was drawn in the ED 5 h after the first one, and a significant hs-cTnT drop was observed (15.1 ng/L). Based on this large difference, the first sample drawn by the GP was re-assayed in our laboratory and near normal hs-cTnT result was obtained (14.4 ng/L). The sample drawn in the ED was also re-assayed and gave somewhat similar results (14.0 ng/L). Electrocardiogram (ECG) was performed and was characterized with left bundle branch block. The patient was admitted to the cardiology service where a coronary angiography was performed and a pharmacological stent was placed. The first result (102.0 ng/L) was considered to be a false positive and non-reproducible result.
Case report 2
On December 2019, a 53-year-old man was seen by his GP for a follow-up visit, owing to slight chest discomfort. The patient was known with hypercholesterolemia and hypertension. Based on the clinical presentation, the GP ordered a cTn test. A few hours later, our laboratory called the GP because of the elevated hs-cTnT (84.0 ng/L, 99th percentile of the assay = 14.0 ng/L). Although creatine kinase (CK) and CK-MB were both elevated (301 U/L; RL <190 and 7.7 μg/L; RL <4.9 μg/L, respectively), the CK-MB index (CK-MB*100/CK) was negative (2.6; RL <3.5). In light of this information, the GP decided to refer the patient to the ED. There, a new sample was taken and a normal hs-cTnT result was obtained (6.8 ng/L). CK-MB was still increased (8.6 μg/L) and the CK-MB index was still negative (2.7). The first sample was subsequently measured again, which returned a normal hs-cTnT result (5.3 ng/L). The patient’s ECG, echocardiography, and stress test were all normal. For this patient, hs-cTnT was, thus, falsely elevated. Fortunately, the incorrect result did not lead to any unneeded invasive procedures [2].
Case report 3
On December 2019, a 65-year-old man with known chronic obstructive pulmonary disease was hospitalized for a respiratory tract infection. C-Reactive Protein (CRP) and neutrophils count were both elevated (224 mg/L; RL <5 mg/L and 11.0×103/µL; reference interval 2.0–7.5×103/µL, respectively). The patient’s hs-cTnT was also substantially elevated (393.0 ng/L). Three hours later, a new sample was taken, and normal results were obtained (10.3 ng/L). Both samples were measured again, which returned normal results (12.0 and 13.0 ng/L, respectively). CK-MB and NT-proBNP were both normal, and the ECG revealed no abnormalities. For this patient, hs-cTnT was thus again falsely elevated. Fortunately, the incorrect result did not lead to any unneeded invasive procedures [2].
Case report 4
On September 2020, a 43-year-old man was seen by his GP for intermittent chest pain. The patient is known with Behçet’s disease and takes ciclosporin. The patient was sent to our ED based on an increased hs-cTnT concentration (42.3 ng/L) performed at our laboratory. However, ECG and echocardiography investigations were both normal in this patient. A new blood sample was drawn in the ED 4 h after the first one, and a significant drop in the measured hs-cTnT level was observed (10.8 ng/L). Based on this large difference, the first sample drawn by the GP was re-assayed in our laboratory and a normal hs-cTnT result was obtained (11.8 ng/L). The sample drawn in ED was also re-assayed and gave similar results (12.0 ng/L).
Based on the observation of these discordant results, we checked the precision of our hs-cTnT assay at several occasions. Using patient pools, coefficient of variations near the decision level (i.e., 14 ng/L) ranged from 2.3 to 3.9%. Roche Diagnostics also checked our analytical process and did not identify any failure. Of note, the four cases abovementioned occurred on three different reagent lots.
Interferences in troponin assays
False positive cTn results have been related to the presence of anti-cTn antibodies or immunocomplexes [3], [4], heterophilic antibodies [5], elevated alkaline phosphatase level [6], contrast media [7], instrument malfunction [8], or carry-over [9]. False negative results with cTn assay may also be observed in the case of a large biotin intake [10], [11]. All these interferences are characterized with erroneous but reproducible cTn results: in other words, if the sample is reanalyzed a second time, the same erroneous result will occur [12], [13]. Non-reproducible (also named irregular) interferences have also been reported in the literature [14]: in this case, if the sample is reanalyzed a second time, a significant difference in cTn results will be observed. These interferences have been described as “fliers” or “outliers” [14]. In this review, we decided to use the term “outlier”. It is well known that the irregular analytical errors due to interferences in immunoassay systems are not only variable and sporadic but also clinically misleading because they can lead to both false-positive and false-negative results [14]. The aim of this review is to warn clinicians and medical laboratory scientists about these non-reproducible results that may alter the clinical judgment. Moreover, we aimed at identifying the nature of these non-reproducible cTn results, determining their occurrence, and describing the best way for their identification.
Systematic review of the literature
For systematic analysis of the literature about non-reproducible cardiac cTn results (cTnI, cTnT, hs-cTnI, hs-cTnT), we carried out a systematic electronic search on PubMed and Scopus without date restriction. The following keywords were used: “outlier” or “flier” or “flyer”, or “non-reproducible”, in combination with “troponin”. The electronic search according to the established criteria identified 39 items on PubMed and 44 items on Scopus. Abstract and/or full texts not directly reporting data about non-reproducible cTn results on both searchable databases were excluded. Eleven articles have been included. The bibliographic references of the selected articles published in English and French were reviewed for additional relevant studies and 15 additional studies were included. All the articles identified according to these search criteria were systematically assessed by two authors (JF and JLB). Overall, 26 studies published between 1997 and 2020 were finally included for review.
Nature of these outliers
It has been suggested that the nature of these non-reproducible results could be related to the presence of fibrin in serum or plasma. The proposed mechanism is that fibrin may cross-link the assay antibodies to produce false positive cTn results [15]. Under optimal clotting conditions, the serum does not contain fibrinogen, fibrin, or blood cells [16]. In case of latent or incomplete removal during centrifugation, residual fibrin may interfere with the assay [17]. Of note, the plasma still contains fibrinogen because clot formation has been prevented by the presence of anticoagulants during blood collection [16]. The main advantage of using plasma is the reduced turn-around time (TAT) since the samples can be centrifuged without any delay, as compared to serum [16]. However, appropriate mixing of the blood tube after phlebotomy is mandatory to ensure that the anticoagulant has been adequately mixed [18]. In case of ineffective mixing, or in plasma samples stored at 2–8 °C during more than 24 h, fibrin is formed from fibrinogen and may also interfere with the assay [15], [19]. However, the fact that fibrin is the source of such non-reproducible results is mostly speculative. Only Sheehan et al. [20] and Dimeski et al. [15], [21] proved that fibrin caused a positive interference on a limited number of plasma samples. Kazmierczak et al. showed that fibrin was visible in seven serum samples out of 24 for which non-reproducible results were noted [22]. Up to now, the clear nature of these outliers are not known [23], [24], even if some pre-analytical variables cannot be excluded. Therefore, the nature of outliers is categorized as unknown in several studies [3], [12], [13], [24], [25], [26], [27], [28], [29]. Recently, the nature of some non-reproducible cTn results was found to be due to magnetic/paramagnetic particles contamination, which occurred during the reagent filling process by the manufacturer and was specific to a particular reagent lot [2].
Definition of an outlier
There is no unique way to identify an outlier using statistical procedures. Nevertheless, three main statistical approaches have been suggested in the literature. First, results are identified as outliers when the difference between the two results exceeded a specified z score value (result 1 – result 2)/√(SD12 + SD22), with a very high probability (i.e., p=0.0005 for z=3.48) [23]. Second, when the difference exceeded a critical difference (CD) defined by the formula CD = z × √2 × SDAnalytical [12], [13], [24]. SDA equivalent to a fixed 10% CV or derived from internal quality controls were both used. Third, critical number of outliers has also been used. A critical outlier is a false value (identified using the CD formula) potentially affecting the clinical decision (i.e., the first hs-cTnT result > 14.0 ng/L, while the second hs-cTnT result < 14.0 ng/L) [13], [24]. The detection of a critical outlier will therefore more likely lead to harmful clinical consequences compared to a non-critical outlier. Since the second result is an analytical repeat on the same analyzer, no biological or preanalytical variation is needed to be included in the calculation [12]. Less widely used outlier definition included a difference of two SD [30], a difference of 30% [31], or 45% [22]. Some authors did not disclose a clear outlier definition [19], [20], [32], [33], [34].
Procedures to identify outliers
Two methods have been used to identify non-reproducible cTn results in the literature: (1) recentrifuging the samples or (2) repeat testing without recentrifuging the sample.
Identification through recentrifugation
Studies that used the recentrifugation method to identify outliers are present in Table 1. Grossly, the outlier rate varied from 0.17 to 28.57% in serum and from 0.091 to 4.0% in plasma. Outlier definition varied widely between studies. For example, Li et al. described that a difference of 30% was sufficient [31], while a difference of two SD was required for Fleming et al. [30]. Some studies did not report a clear outlier definition [3], [19], [20]. Hejl et al. defined that an original result above 100 ng/L and below 100 ng/L after recentrifugation was a false positive result [32]. However, this approach does not take into account assay imprecision, and so the number of false positive samples may be grossly overestimated [33]. The integration of assay imprecision in the outlier definition is therefore, in our opinion, critical. Inclusion criteria were also different across studies. Fleming et al. only included samples with cTnI >100 ng/L along with normal CK [30], while Beyne et al. included all range of cTnI concentrations [34] and Warner et al. [3] samples with a discordance with another platform (Ortho Vitros). Noteworthy, Kazmierczak et al. noticed that the occurrence of these non-reproducible results was not specific to a particular reagent lot [22]. Sheenhan et al. also found that two distinct reagent lots were both impacted by outliers [20].
Summary of studies having identify outliers by recentrifugation.
| Reference | Sample type | Troponin | Platform | Included samples | Outlier definition | Outlier rate, % | Nature | Comment |
|---|---|---|---|---|---|---|---|---|
| Roberts et al. 1997 [46] | Serum | cTnI | Stratus II (Dade International) | 1,200 | Clear difference, normal CK-MB and ECG. No previous cTnI increase | 2/1,200 (0.17%) | Fibrina | Use of plasma eliminated these outliers |
| Nosanchuk et al. 1999 [47] | Serum | cTnI | AxSYM (Abbott Diagnostics) | 8 | Clear difference and consistent with plasma results | NA | Fibrina | Repeat testing not useful |
| Beyne et al. 2000 [34] | Serum | cTnI | Access (Beckman Coulter) | 64 | Clear difference | 6/64 (9.38%) | Fibrin/microparticlesa | |
| Fleming et al. 2002 [30] | Serum | cTnI | Access (Beckman Coulter) | 56 (cTnI > 100 ng/L, Normal CK) | Difference of ±2 SD | 16/56 (28.57%) | Fibrin/microparticlesa | |
| Sheehan et al. 2002 [20] | Plasma | cTnI | ACS:180 (Bayer Diagnostics) | 20 | Clear difference | NA | Fibrin | Fibrin added in samples |
| Kazmierczak et al. 2005 [22] | Serum | cTnI | AxSYM (Abbott Diagnostics) | 307 (cTnI > 2,000 ng/L and/or visible fibrin/microparticles) | Difference of 45% | 24/307 (7.82%) | Visible fibrin (n=7) | Use of plasma eliminated these outliers |
| Er et al. 2006 [48] | Serum | cTnI | Access (Beckman Coulter) | 6 | Clear difference and consistent with plasma results | NA | Fibrina | |
| Hejl et al. 2008 [32] | Plasma | cTnI | DxI 800 (Beckman Coulter) | 478 (cTnI > 100 ng/L) | Below < 100 ng/L after recentrifugation | 17/478 (3.56%) | Fibrina | Recentrifuged at 2,300 g, 15 min |
| Plasma | cTnI | DxI 800 (Beckman Coulter) | 1,695 (cTnI > 100 ng/L) | Below < 100 ng/L after recentrifugation | 19/1,695 (1.12%) | Fibrina | Recentrifuged at 6,700 g, 5 min | |
| Dimeski et al. 2011 [15] | Plasma | cTnI | AccuTnI (Beckman Coulter) | Two (one patient and one pool of two patients) | Clear difference | NA | Fibrin | Insoluble fibrin in samples |
| Strathmann et al. 2011 [19] | Plasma | cTnI | DxI 800 (Beckman Coulter) | Not clear (cTnI > 400 ng/L) | Clear difference and consistent with plasma results | 2.27%b | Microparticlesa | |
| Li et al. 2014 [31] | Serum | cTnI | AxSYM (Abbott Diagnostics) | 132 (cTnI > 40 ng/L, normal CK-MB and myoglobin) | Difference of 30% | 18/132 (13.64%) | Fibrin/microparticlesa | |
| Dimeski et al. 2015 [21] | Plasma | cTnI | Access 2 and DxI 800 (Beckman Coulter) | One (pool) | Clear difference | NA | Fibrin | Insoluble fibrin in samples. AccuTnI+3 |
| Lee et al. 2016 [27] | Plasma | cTnI | Architect i2000 SR (Abbott Diagnostics) | 1,239 | Critical outlierc | 12/1,239 (0.97%) | Unknown | |
| Plasma | Hs-cTnI | Architect i2000 SR (Abbott Diagnostics) | 1,239 | Critical outlierc | 1/1,239 (0.091%) | Unknown | ||
| Warner et al. 2016 [3] | Plasma | Hs-cTnI | Architect i4000SR (Abbott Diagnostics) | 50 (hs-cTnI > cutoff but < 12.0 ng/L or 2× times higher on Vitros) | Clear difference | 2/50 (4.00%) | Unknown | |
| Karon et al. 2018 [26] | Serum | cTnT | Roche e411 (Roche Diagnostics) | 3,008 | Critical outlierc | 10/3,008 (0.33%) | Unknown | Within 24 h of initial analysis, samples were aliquoted before re-centrifugation and duplicate analysis |
| Serum | cTnI | Architecti2000 SR (Abbott Diagnostics) | 3,008 | Critical outlierc | 110/3,008 (3.66%) | Unknown | Within 24 h of initial analysis, samples were aliquoted before re-centrifugation and duplicate analysis | |
| Serum | Hs-cTnI | Architecti2000 SR (Abbott Diagnostics) | 3,008 | Critical outlierc | 14/3,008 (0.47%) | Unknown | Within 24 h of initial analysis, samples were aliquoted before re-centrifugation and duplicate analysis |
aHypothesis. bNumber of total samples not known. cOutlier that could potentially have affected the clinical decision (i.e., first cTn result > cut-off and second cTn result < cut-off). Units have been harmonized (ng/L) for clarity. CK-MB, creatine kinase-MB isoenzyme; ECG, electrocardiogram.
Considering the large difference in analytical performances and measured results among hs-cTnI and hs-cTnT methods [35], [36], it is not surprising that a large dishomogeneity in outlier frequency have been reported in the literature. Of interest, Lee et al. identified a higher outlier rate with a cTnI assay (0.97%) compared to a hs-cTnI assay (0.09%) [27]. More recently, Karon et al. confirmed that a hs-cTnI assay had a lower outlier rate (0.47%) compared to cTnI assay (3.66%). However, the outlier rate of the hs-cTnI assay was not statistically different compared to a cTnT assay [26]. The use of fixed or sex-specific cut-offs modified the number of outliers [26]. If we focus our analysis on studies published since 2010 that used one of the three main outlier definitions, the outlier prevalence is overall lower in high-sensitive assays compared to previous generation assays (Table 3).
Identification through repeat testing
Studies that used the repeat testing method (i.e., without recentrifugation) to identify outliers are presented in Table 2. Grossly, the outlier rate varied from 0.0 to 34.48% in serum and from 0.10 to 2.0% in plasma. Included numbers of samples were far higher as compared to studies having used recentrifugation. Favresse et al. included up to 1,243 samples [2], Pretorius et al. 2,391 samples [23], Pretorius et al. 4,336 samples [29], Ryan et al. up to 4,009 samples [24], Sheehan et al. 4,850 samples [20], Ungerer et al. up to 5,265 samples [12], and Sawyer et al. up to 7,089 [13].
Summary of studies having identify outliers by repeat testing.
| Reference | Sample type | Troponin | Platform | Included samples | Outlier definition | Outlier rate, % | Nature | Comment |
|---|---|---|---|---|---|---|---|---|
| Sheehan et al. 2002 [20] | Plasma | cTnI | ACS:180 (Bayer Diagnostics) | 4,850 | CV > 20% and absolute difference > 160 ng/L | 19/4,850 (0.39%) | Visible fibrin/microparticles/clot sample (n=6) | |
| Ungerer et al. 2010 [12] | Serum and plasma | cTnI | Access 2 and DxI 800 (Beckman Coulter) | 5,265 (cTnI > 40 ng/L) | Critical differenceb | 102/5,265 (1.94%) | Unknown | First evaluation (six months) |
| Serum | cTnI | Access 2 and DxI 800 (Beckman Coulter) | 419 | Critical differenceb | 6/419 (1.43%) | Unknown | Second evaluation (one month) | |
| Plasma | cTnI | Access 2 and DxI 800 (Beckman Coulter) | 462 | Critical differenceb | 5/462 (1.08%) | Unknown | Second evaluation (one month) | |
| Dimeski et al. 2011 [15] | Plasma | cTnI | AccuTnI (Beckman Coulter) | Two (including one pool of two patients) | Clear difference | NA | Fibrin | Insoluble fibrin in samples |
| Pretorius et al. 2011 [23] | Serum | cTnI | Access 2 (Beckman Coulter) | 2,391 | z valuec > 3.48 with (a) IQC SD, (b) duplicate SD, (c) constant SD equivalent to 10% CV | (a) 21 (0.88%), (b) 30 (1.25%), (c) 19/2,391 (0.79%) | Fibrina | |
| Serum | cTnI | ADVIA Centaur XP (Siemens) | 2,391 | z valuec > 3.48 with (a) IQC SD, (b) duplicate SD, (c) constant SD equivalent to 10% CV | (a) 5 (0.21%), (b) 35 (1.46%), (c) 11/2,391 (0.46%) | Fibrina | ||
| Serum | cTnI | Architect i2000SR (Abbott Diagnostics) | 2,391 | z valuec > 3.48 with (a) IQC SD, (b) duplicate SD, (c) constant SD equivalent to 10% CV | (a) 5 (0.21%), (b) 6 (0.25%), (c) 4/2,391 (0.17%) | Fibrina | ||
| Serum | Hs-cTnT | Cobas e601 (Roche Diagnostics) | 2,391 | z valuec > 3.48 with (a) IQC SD, (b) duplicate SD, (c) constant SD equivalent to 10% CV | (a) 3 (0.13%), (b) 11 (0.46%), (c) 1/2,391 (0.04%) | Fibrina | ||
| Kavsak et al. 2014 [37] | Plasma | cTnI | Architect (Abbott Diagnostics) | One (low patient pool) | Clear difference | NA | Reagent pack (following an inactivity period) | From 100-test reagent pack to 500 |
| Ryan et al. 2014 [24] | Plasma | cTnI | Architect i2000SR (Abbott Diagnostics) | 4,009 (cTnI 30–300.0 ng/L) | (a) Critical differenceb and (b) critical outlierd | (a) 9/4,009 (0.22%) and (b) 4/4,009 (0.10%) | Unknown | |
| Plasma | Hs-cTnI | Architect i2000SR (Abbott Diagnostics) | 3,878 (hs-cTnI 16–300.0 ng/L) | (a) Critical differenceb and (b) critical outlierd | (a) 7/3,878 (0.18%) and (b) 5/3,878 (0.13%) | Unknown | ||
| Sawyer et al. 2014 [13] | Serum | cTnI | Architect i2000SR (Abbott Diagnostics) | 7,011 | (a) Critical differenceb and (b) critical outlierd | (a) 137 (1.95%) and (b) 36/7,011 (0.51%) | Unknown | Before full maintenance |
| Serum | cTnI | Architect i2000SR (Abbott Diagnostics) | 7,089 | (a) Critical differenceb and (b) critical outlierd | (a) 34 (0.48%) and (b) 26/7,089 (0.37%) | Unknown | After full maintenance | |
| Serum | Hs-cTnI | Architect i2000SR (Abbott Diagnostics) | 1,522 | (a) Critical differenceb and (b) critical outlierd | (a) 9 (0.59%) and (b) 0/1,522 (0%) | Unknown | ||
| Dimeski et al. 2015 [21] | Plasma | cTnI | Access 2 and DxI 800 (Beckman Coulter) | One (pool) | Clear difference | NA | Fibrin | Insoluble fibrin in samples. AccuTnI+3 |
| Greene et al. 2015 [25] | Serum | cTnI | Access 2 (Beckman Coulter) | 29 (cTnI > 30.0 ng/L) | Clear difference | 10/29 (34.48%) | Unknown | Healthy volunteers |
| Loh et al. 2016 [28] | Plasma | cTnI | Vitros TnI ES (Ortho Clinical Diagnostics) | 50 | >3.5 (z value) x 20.5x(CVA2+CVP2) = 23.1% | 1/50 (2.0%) | Unknown | Samples enriched with native full-length cTnI protein |
| Pretorius et al. 2018 [29] | Serum | Hs-cTnI | Access (Beckman Coulter) | 4,336 | z valuec > 3.5 | 2/4,336 (0.046%) | Unknown | |
| Wockenfus et al. 2019 [49] | Plasma | cTnT | Cobas 411 (Roche Diagnostics) | 17,154e | (a) Initial and repeat values differing by >±5 ng/L (cTnT <100 ng/L) or ±5% (cTnT ≥100 ng/L) and (b) if (a) and abnormal/normal to normal/abnormal | (a) 19 (0.11%) and (b) 8//17,154 (0.05%) | Unknown | |
| Favresse et al. 2020 [2] | Serum | Hs-cTnT | Cobas e801 (Roche Diagnostics) | 1,243 (hs-cTnT >14.0 ng/L) | (a) Critical differenceb and (b) critical outlierd | (a) 40 (3.22%) and (b) 17/1,253 (1.37%) | Contamination with magnetic/paramagnetic microparticles | Reagent lot 1 [2] |
| Serum | Hs-cTnT | Cobas e801 (Roche Diagnostics) | 1,160 (hs-cTnT >14.0 ng/L) | (a) Critical differenceb and (b) critical outlierd | (a) 0 (0%) and (b) 0/1,160 (0%) | NA | Reagent lot 2 [2] | |
| Serum | Hs-cTnT | Cobas e801 (Roche Diagnostics) | 999 (hs-cTnT >14.0 ng/L) | (a) Critical differenceb and (b) critical outlierd | (a) 2 (0.20%) and (b) 1/999 (0.1%) | Contamination? | Reagent lot 2 (second part) | |
| Serum | Hs-cTnT | Cobas e801 (Roche Diagnostics) | 1,148 (hs-cTnT >14.0 ng/L) | (a) Critical differenceb and (b) critical outlierd | (a) 2 (0.17%) and (b) 1/1,148 (0.09%) | Contamination? | Reagent lot 3 | |
| Serum | Hs-cTnT | Cobas e801 (Roche Diagnostics) | 290 (hs-cTnT >14.0 ng/L) | (a) Critical differenceb and (b) critical outlierd | (a) 2 (0.69%) and (b) 2/290 (0.69%) | Contamination? | Reagent lot 3 | |
| Serum | Hs-cTnT | Cobas e801 (Roche Diagnostics) | 1,748 (hs-cTnT >14.0 ng/L) | (a) Critical differenceb and (b) critical outlierd | (a) 2 (0.11%) and (b) 2/1,748 (0.11%) | Contamination? | Reagent lot 4 |
aHypothesis. bz value (result 1 – result 2)/√(SD12 + SD22). cCritical difference (z × √2 × SDAnalytical). dOutlier that could potentially have affected the clinical decision (i.e., first cTn result > cut-off and second cTn result < cut-off). CVP, coefficient of variation of pipetting procedure. Units have been harmonized (ng/L) for clarity. eNot clear if representing the total number of samples analyzed in duplicate or not.
Inclusion criteria and outlier definition varied also greatly across studies. The z value definition was used in the study of Pretorius et al. [23], [29], while Ungerer et al. [12], Favresse et al. [2], Ryan et al. [24], and Sawyer et al. [13] used the CD definition. Saywer et al. found that the hs-cTnI assay was less affected than contemporary cTnI assays for critical outliers [13]. Using the hs-cTnT method, Pretorius et al. found two outliers above the 99th percentile cut-off out of 2,391 duplicate samples (outlier rate = 0.08%; z>3.48) [23]. This outlier rate was significantly lower compared to previous generation assays [23]. However, Ryan et al. found that the outlier rate was not different between the hs-cTnI assay and the contemporary cTnI assay they previously used [24]. In studies published since 2010 and using one of the three main outlier definitions, the outlier prevalence is overall lower in high-sensitive assays compared to previous generation assays (Table 3). Interestingly, Sawyer et al. showed that a lower outlier rate was observed following a full maintenance of their platform [13]. Additionally, Kavsak et al. suggested that other factors contributing to variability may include periods of inactivity of the analyzer as well as the reagent pack sizes [37]. No outlier was classified as a critical outlier in their study.
The mean outlier rate calculated from studies published since 2010 according to the type of cTn assay and to the method to identify outlier.
| Repeat testing | Recentrifugation | |||
|---|---|---|---|---|
| Critical difference or z value | Critical outlier | Critical difference or z value | Critical outlier | |
| cTnI and cTnT | 1.05% | 0.33% | NA | 1.65% |
| hs-cTnI and hs-cTnI | 0.57% | 0.34% | NA | 0.28% |
In our recent report, we showed that the outlier rate varied among two reagent lots used (hs-cTnT). An outlier rate of 3.22 and of 0% was observed on two different and consecutive reagent lots (429178 and 460113). The high outlier rate observed on the first reagent lot was due to contamination with magnetic/paramagnetic particles [2]. Further using the lot 460113 between March 28 and June 6, 2020 (1,998 samples requests for cTn), we encountered two outliers (19.6 to 48.5 and 67.7 to 4.4 ng/L) out of 999 samples analyzed in duplicate. Therefore, the updated outlier rate was 0.093% (4/2,159) and the release of the new reagent lot drastically decreases the occurrence of outliers (Figure 1). On June 7, we installed a new reagent lot (470034). Between June 7 and September 23, we analyzed 1,438 cTn samples in duplicate and four outliers were observed (17.0 to 4.0, 39.8 to 22.7, 42.3 to 10.8, and 28.0 to 3.7 ng/L), leading to an outlier rate of 0.28% (Figure 1). On October 13, we installed a new reagent lot (474941). Between October 13 and December 16, we analyzed 1,748 cTn samples in duplicate and two outliers were observed (25.6 to 6.4 and 15.8 to 6.5 ng/L), leading to an outlier rate of 0.11% (Figure 1). These latter outlier rates were close to the one published by Pretorius et al. using a hs-cTnT assay [23].
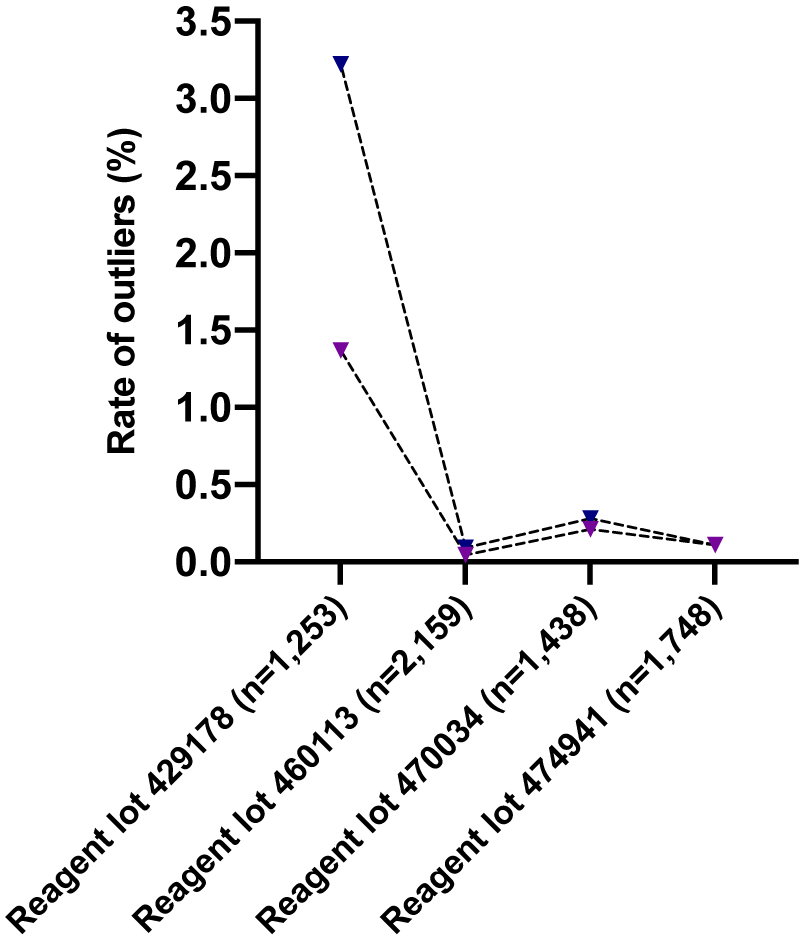
Rates of outliers observed in Clinique Saint-Luc Bouge using four different hs-cTnT reagent lots.
Dark blue = rate of outliers calculated by the CD formula; purple = rate of critical outliers (i.e., first cTn result > cut-off and second cTn result < cut-off).
Discussion
Outliers have been described in all cTn methods and it is strictly related to assay performance. Outliers occur randomly and can produce both false positive and negative results (14). The outlier rate vary according to the outlier definition used (i.e., critical outlier, z value [z=3.29, 3.48, …]), to the samples included (i.e., random samples, positive cTn results, samples with negative CK-MB results, serum vs. plasma, storage conditions, delay before reanalysis), to the procedure used to identify outliers (i.e., repeat testing or recentrifugation) and to the platform considered (including reagent lots). The direct comparison of studies is therefore difficult. The use of a repeat testing strategy in clinical practice is more convenient than recentrifugation (extra manipulation and TAT). Furthermore, recentrifugation might have an impact on the measurand as warned by the Clinical and Laboratory Standard Institute. Indeed, Canovi et al. showed a median reduction of 10.0 ng/L following recentrifugation [38]. The stability of cTn during a second centrifugation process and a possible increased adherence to the container cannot be excluded [33]. Third, some studies show that recentrifugation had no added value compared to repeat testing to identify outliers [12], [23].
We also focused our analysis on recent studies (i.e., published since 2010) to assess the outlier prevalence of previous and new generation assays (i.e., high-sensitive assays). The outlier definitions included were the most used ones (exceeding a z score value, CD or critical outlier). The outlier prevalence is overall lower in high-sensitive assays (0.28–0.57%) compared to previous generation assays (0.33–1.65%) (Table 3). In contrast to outliers, the biotin interference in cTn assays has received much more attention in the literature since the U.S. Food and Drug Administration notified a death attributable to a false rule-out of MI based on a falsely decreased cTn result. However, the prevalence of biotin >20.0 ng/mL in real-life studies was found to be 0% (0/572) and 0.13% (1/797) [39], [40]. Furthermore, the misclassification risk due to biotin interference was even lower (0.025, 0.0064, 0.00048%, and <0.00001% at 0, 1, 3, and 6 h) [40]. The prevalence of cTn outliers is therefore higher (Table 3). To limit the impact of biotin in cTn assays, manufacturers have added blockers that successfully overcame the interference up to 1,200 ng/mL [11]. The prevalence of heterophilic antibodies in cTn assays has been found to range from 0.07 to 3.0% [21], [41] and manufacturers also add blockers to limit interference due to heterophilic antibodies [5]. The identification of outliers is however more problematic.
A pragmatic solution would be to test every elevated cTn in duplicate to identify outliers. However, such a solution would be costly and significantly increases the TAT. In our laboratory (Saint-Luc Bouge) the prevalence of positive cTn results is about 50%. Testing in duplicate would hence double the reagent cost. In the same way, we suggest that a 1-h (or 3-h) cTn result significantly lower (>30% difference [42]) than the 0-h result (0-h/1-h [or 3-h] algorithm) could be investigated for a potential outlier in patients presenting with symptoms suggestive of acute MI (including acute chest pain and angina pectoris) [43], [44]. Automatic comments and repeat testing could therefore be generated in the laboratory information system in case of particular cTn kinetics. Continuous discussion between clinicians and the laboratory is fundamental to identify these erroneous and non-reproducible results. We further recommend that manufacturers continuously improve the robustness of their procedures to avoid erroneous cTn results that may potentially lead to harmful consequences for the patient. Given the growing data on the subject, in addition to precision and trueness, the frequency of analytical outliers should be systematically evaluated before the implementation of a new cTn assay. We therefore also encourage manufacturers, in collaboration with clinical laboratories and regulatory bodies, to conduct large studies to assess the occurrence of these outliers and to provide the probability of outliers using their assays. These evaluations could be performed for cTn but also for other measurands for which outliers have also been identified [45].
Conclusions
The outliers described in the literature for cTn measurement occurred randomly, sometimes frequently, were non-reproducible, and could not be explained by imprecision of the analyzer. Simple repeat testing of the original sample represents the best way to identify these outliers. Clinicians need to have an ongoing dialog with laboratory professionals in case of discordance with the clinical presentation to prevent harmful clinical consequences. We further recommend that manufacturers continuously improve the robustness of their procedures to avoid erroneous cTn results that may potentially lead to harmful consequences for the patient.
Research funding: None declared.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
References
1. Thygesen, K, Alpert, JS, Jaffe, AS, Chaitman, BR, Bax, JJ, Morrow, DA, et al.. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237–69. https://doi.org/10.1093/eurheartj/ehy856.Suche in Google Scholar PubMed
2. Favresse, J, Cadrobbi, J, Eucher, C, Laffineur, K, Rosseels, C, Pieters, D, et al.. Non-reproducible cardiac troponin results occurring with a particular reagent lot. Clin Chem Lab Med 2020;59:e9–e12. https://doi.org/10.1002/jmv.26669.Suche in Google Scholar PubMed PubMed Central
3. Warner, JV, Marshall, GA. High incidence of macrotroponin I with a high-sensitivity troponin I assay. Clin Chem Lab Med 2016;54:1821–9. https://doi.org/10.1515/cclm-2015-1276.Suche in Google Scholar PubMed
4. Dalle Carbonare, L, Pizzini, M, Micheletti, V, Lo Cascio, C, Bovo, C, Girelli, D, et al.. Interference from immunocomplexes on a high-sensitivity cardiac troponin T immunoassay. Clin Chem Lab Med 2020;58:e225–7. https://doi.org/10.1515/cclm-2020-0028.Suche in Google Scholar PubMed
5. Lippi, G, Aloe, R, Meschi, T, Borghi, L, Cervellin, G. Interference from heterophilic antibodies in troponin testing. Case report and systematic review of the literature. Clin Chim Acta 2013;426:79–84. https://doi.org/10.1016/j.cca.2013.09.004.Suche in Google Scholar PubMed
6. Herman, DS, Ranjitkar, P, Yamaguchi, D, Grenache, DG, Greene, DN. Endogenous alkaline phosphatase interference in cardiac troponin I and other sensitive chemiluminescence immunoassays that use alkaline phosphatase activity for signal amplification. Clin Biochem 2016;49:1118–21. https://doi.org/10.1016/j.clinbiochem.2016.06.006.Suche in Google Scholar PubMed
7. Lin, CT, Lee, HC, Voon, WC, Yen, HW, Tang, MH, Chin, TT, et al.. Positive interference from contrast media in cardiac troponin I immunoassays. Kaohsiung J Med Sci 2006;22:107–13. https://doi.org/10.1016/s1607-551x(09)70229-3.Suche in Google Scholar PubMed
8. Galambos, C, Brink, DS, Ritter, D, Chung, HD, Creer, MH. False-positive plasma troponin I with the AxSYM analyzer. Clin Chem 2000;46:1014–5. https://doi.org/10.1093/clinchem/46.7.1014.Suche in Google Scholar
9. Wilgen, U, Pretorius, CJ, Gould, MJ, Ungerer, JP. Cardiac Troponin I carryover by very high patient samples still causes false-positive results on the Beckman Coulter AccuTnI + 3. Ann Clin Biochem 2016;53:177–9. https://doi.org/10.1177/0004563215606739.Suche in Google Scholar PubMed
10. Clerico, A, Plebani, M. Biotin interference on immunoassay methods: sporadic cases or hidden epidemic? Clin Chem Lab Med 2017;55:777–9. https://doi.org/10.1515/cclm-2017-0070.Suche in Google Scholar PubMed
11. Mzougui, S, Favresse, J, Soleimani, R, Fillee, C, Gruson, D. Biotin interference: evaluation of a new generation of electrochemiluminescent immunoassays for high-sensitive troponin T and thyroid-stimulating hormone testing. Clin Chem Lab Med 2020;58:2037–45. https://doi.org/10.1515/cclm-2020-0214.Suche in Google Scholar PubMed
12. Ungerer, JP, Pretorius, CJ, Dimeski, G, O’Rourke, PK, Tyack, SA. Falsely elevated troponin I results due to outliers indicate a lack of analytical robustness. Ann Clin Biochem 2010;47:242–7. https://doi.org/10.1258/acb.2010.010012.Suche in Google Scholar PubMed
13. Sawyer, N, Blennerhassett, J, Lambert, R, Sheehan, P, Vasikaran, SD. Outliers affecting cardiac troponin I measurement: comparison of a new high sensitivity assay with a contemporary assay on the Abbott ARCHITECT analyser. Ann Clin Biochem 2014;51:476–84. https://doi.org/10.1177/0004563213499737.Suche in Google Scholar PubMed
14. Clerico, A, Belloni, L, Carrozza, C, Correale, M, Dittadi, R, Dotti, C, et al.. A Black Swan in clinical laboratory practice: the analytical error due to interferences in immunoassay methods. Clin Chem Lab Med 2018;56:397–402. https://doi.org/10.1515/cclm-2017-0881.Suche in Google Scholar PubMed
15. Dimeski, G. Evidence on the cause of false positive troponin I results with the Beckman AccuTnI method. Clin Chem Lab Med 2011;49:1079–80. https://doi.org/10.1515/cclm.2011.163.Suche in Google Scholar PubMed
16. Lima-Oliveira, G, Monneret, D, Guerber, F, Guidi, GC. Sample management for clinical biochemistry assays: are serum and plasma interchangeable specimens? Crit Rev Clin Lab Sci 2018;55:480–500. https://doi.org/10.1080/10408363.2018.1499708.Suche in Google Scholar PubMed
17. Dimeski, G, Masci, PP, Trabi, M, Lavin, MF, de Jersey, J. Evaluation of the Becton-Dickinson rapid serum tube: does it provide a suitable alternative to lithium heparin plasma tubes? Clin Chem Lab Med 2010;48:651–7. https://doi.org/10.1515/cclm.2010.141.Suche in Google Scholar PubMed
18. Simundic, AM, Bolenius, K, Cadamuro, J, Church, S, Cornes, MP, van Dongen-Lases, EC, et al.. Joint EFLM-COLABIOCLI Recommendation for venous blood sampling. Clin Chem Lab Med 2018;56:2015–38. https://doi.org/10.1515/cclm-2018-0602.Suche in Google Scholar PubMed
19. Strathmann, FG, Ka, MM, Rainey, PM, Baird, GS. Use of the BD vacutainer rapid serum tube reduces false-positive results for selected beckman coulter Unicel DxI immunoassays. Am J Clin Pathol 2011;136:325–9. https://doi.org/10.1309/ajcpzofj7kx5qmrw.Suche in Google Scholar PubMed
20. Sheehan, P, Blennerhassett, J, Vasikaran, SD. Decision limit for troponin I and assay performance. Ann Clin Biochem 2002;39:231–6. https://doi.org/10.1258/0004563021902161.Suche in Google Scholar PubMed
21. Dimeski, G, Coogan, M, Jones, B, Brown, N. Is the new Beckman AccuTnI+3 assay capable of producing false-positive troponin I results? Clin Chem Lab Med 2015;53:e101–3. https://doi.org/10.1515/cclm-2014-0616.Suche in Google Scholar PubMed
22. Kazmierczak, SC, Sekhon, H, Richards, C. False-positive troponin I measured with the Abbott AxSYM attributed to fibrin interference. Int J Cardiol 2005;101:27–31. https://doi.org/10.1016/j.ijcard.2004.03.008.Suche in Google Scholar PubMed
23. Pretorius, CJ, Dimeski, G, O’Rourke, PK, Marquart, L, Tyack, SA, Wilgen, U, et al.. Outliers as a cause of false cardiac troponin results: investigating the robustness of 4 contemporary assays. Clin Chem 2011;57:710–8. https://doi.org/10.1373/clinchem.2010.159830.Suche in Google Scholar PubMed
24. Ryan, JB, Southby, SJ, Stuart, LA, Mackay, R, Florkowski, CM, George, PM. Comparison of cardiac TnI outliers using a contemporary and a high-sensitivity assay on the Abbott Architect platform. Ann Clin Biochem 2014;51:507–11. https://doi.org/10.1177/0004563214534637.Suche in Google Scholar PubMed
25. Greene, DN, Holmes, DT, Liang, J, Kwong, SL, Lorey, TS, Petrie, MS. Challenges in harmonizing integrated healthcare network laboratories: multi-center evaluation of the AccuTnI+3 troponin assay. Clin Biochem 2015;48:268–74. https://doi.org/10.1016/j.clinbiochem.2014.11.009.Suche in Google Scholar PubMed
26. Karon, BS, Wockenfus, AM, Hartung, KJ, Scott, RJ, Carter, SD, Jaffe, AS. Comparing analytical outliers and the percent of emergency department patients with results above the 99th percentile upper reference limit for 2 conventional and one high sensitivity troponin assay. Clin Biochem 2018;53:104–9. https://doi.org/10.1016/j.clinbiochem.2018.01.001.Suche in Google Scholar PubMed
27. Lee, GR, Browne, TC, Guest, B, Khan, I, Murphy, E, McGorrian, C, et al.. Transitioning high sensitivity cardiac troponin I (hs-cTnI) into routine diagnostic use: more than just a sensitivity issue. Pract Lab Med 2016;4:62–75. https://doi.org/10.1016/j.plabm.2016.01.001.Suche in Google Scholar PubMed PubMed Central
28. Loh, TP, Lim, XC, Kieu, K, Sajiir, H, Neo, SF, Cheng, WL, et al.. Recovery of spiked troponin I in four routine assays. Biochem Med 2016;26:233–9. https://doi.org/10.11613/bm.2016.025.Suche in Google Scholar
29. Pretorius, CJ, Tate, JR, Wilgen, U, Cullen, L, Ungerer, JPJ. A critical evaluation of the Beckman Coulter Access hsTnI: analytical performance, reference interval and concordance. Clin Biochem 2018;55:49–55. https://doi.org/10.1016/j.clinbiochem.2018.03.003.Suche in Google Scholar PubMed
30. Fleming, SM, O’Byrne, L, Finn, J, Grimes, H, Daly, KM. False-positive cardiac troponin I in a routine clinical population. Am J Cardiol 2002;89:1212–5. https://doi.org/10.1016/s0002-9149(02)02309-3.Suche in Google Scholar PubMed
31. Li, Y, Wang, X, Xu, L, Wen, X. Rapid identification of falsely elevated serum cardiac troponin I values in a stat laboratory. Lab Med 2014;45:82–5. https://doi.org/10.1309/lmo7hli8eodnazsg.Suche in Google Scholar PubMed
32. Hejl, CG, Astier, HT, Ramirez, JM. Prevention of preanalytical false-positive increases of cardiac troponin I on the Unicel DxI 800 analyzer. Clin Chem Lab Med 2008;46:1789–90. https://doi.org/10.1515/cclm.2008.345.Suche in Google Scholar
33. Pfafflin, A. Doubt on prevention of false-positive results of cardiac troponin I by recentrifugation. Clin Chem Lab Med 2009;47:892–3. https://doi.org/10.1515/cclm.2009.204.Suche in Google Scholar PubMed
34. Beyne, P, Vigier, JP, Bourgoin, P, Vidaud, M. Comparison of single and repeat centrifugation of blood specimens collected in BD evacuated blood collection tubes containing a clot activator for cardiac troponin I assay on the ACCESS analyzer. Clin Chem 2000;46:1869–70. https://doi.org/10.1093/clinchem/46.11.1869.Suche in Google Scholar
35. Clerico, A, Zaninotto, M, Padoan, A, Masotti, S, Musetti, V, Prontera, C, et al.. Evaluation of analytical performance of immunoassay methods for cTnI and cTnT: from theory to practice. Adv Clin Chem 2019;93:239–62. https://doi.org/10.1016/bs.acc.2019.07.005.Suche in Google Scholar PubMed
36. Clerico, A, Ripoli, A, Zaninotto, M, Masotti, S, Musetti, V, Ciaccio, M, et al.. Head-to-head comparison of plasma cTnI concentration values measured with three high-sensitivity methods in a large Italian population of healthy volunteers and patients admitted to emergency department with acute coronary syndrome: a multi-center study. Clin Chim Acta 2019;496:25–34. https://doi.org/10.1016/j.cca.2019.06.012.Suche in Google Scholar PubMed
37. Kavsak, PA, Clark, L, Lancaster, S, Don-Wauchope, AC. Within-run precision and outlier detection for the Abbott ARCHITECT cardiac troponin I assay. Ann Clin Biochem 2014;51:512–4. https://doi.org/10.1177/0004563214534400.Suche in Google Scholar PubMed
38. Canovi, S, Campioli, D, Marcheselli, L. Specimen recentrifugation and elevated troponin I levels. Lab Med 2015;46:47–50. https://doi.org/10.1309/lmpc95el4pyewwbr.Suche in Google Scholar
39. Vroemen, WHM, van Doorn, W, Kimenai, DM, Wodzig, W, de Boer, D, Bekers, O, et al.. Biotin interference in high-sensitivity cardiac troponin T testing: a real-world evaluation in acute cardiac care. Cardiovasc Res 2019;115:1950–1. https://doi.org/10.1093/cvr/cvz277.Suche in Google Scholar PubMed PubMed Central
40. Mumma, B, Diercks, D, Twerenbold, R, Valcour, A, Ziegler, A, Schützenmeister, A, et al.. Clinical risk assessment of biotin interference with a high-sensitivity cardiac troponin T assay. Clin Chem Lab Med 2020;58:1931–40. https://doi.org/10.1515/cclm-2019-0962.Suche in Google Scholar PubMed
41. Herman, DS, Kavsak, PA, Greene, DN. Variability and error in cardiac troponin testing: an ACLPS critical review. Am J Clin Pathol 2017;148:281–95. https://doi.org/10.1093/ajcp/aqx066.Suche in Google Scholar PubMed
42. Clerico, A, Padoan, A, Zaninotto, M, Passino, C, Plebani, M. Clinical relevance of biological variation of cardiac troponins. Clin Chem Lab Med 2020;59:641–52. https://doi.org/10.1515/cclm-2020-1433.Suche in Google Scholar PubMed
43. Mueller, C, Giannitsis, E, Christ, M, Ordonez-Llanos, J, deFilippi, C, McCord, J, et al.. Multicenter evaluation of a 0-hour/1-hour algorithm in the diagnosis of myocardial infarction with high-sensitivity cardiac troponin T. Ann Emerg Med 2016;68:76–87.e4. https://doi.org/10.1016/j.annemergmed.2015.11.013.Suche in Google Scholar PubMed
44. Neumann, JT, Sorensen, NA, Schwemer, T, Ojeda, F, Bourry, R, Sciacca, V, et al.. Diagnosis of myocardial infarction using a high-sensitivity troponin I 1-hour algorithm. JAMA Cardiol 2016;1:397–404. https://doi.org/10.1001/jamacardio.2016.0695.Suche in Google Scholar PubMed
45. Neubig, S, Grotevendt, A, Kallner, A, Nauck, M, Petersmann, A. Analytical robustness of nine common assays: frequency of outliers and extreme differences identified by a large number of duplicate measurements. Biochem Med 2017;27:192–8. https://doi.org/10.11613/bm.2017.021.Suche in Google Scholar
46. Roberts, WL, Calcote, CB, De, BK, Holmstrom, V, Narlock, C, Apple, FS. Prevention of analytical false-positive increases of cardiac troponin I on the Stratus II analyzer. Clin Chem 1997;43:860–1. https://doi.org/10.1093/clinchem/43.5.860.Suche in Google Scholar
47. Nosanchuk, JS, Combs, B, Abbott, G. False increases of troponin I attributable to incomplete separation of serum. Clin Chem 1999;45:714. https://doi.org/10.1093/clinchem/45.5.714.Suche in Google Scholar
48. Er, TK, Tsai, LY, Jong, YJ, Chen, BH. Falsely elevated troponin I attributed to inadequate centrifugation using the Access immunoassay analyzer. Clin Chem Lab Med 2006;44:908–9. https://doi.org/10.1515/cclm.2006.158.Suche in Google Scholar
49. Wockenfus, A, Hartung, K, Kelley, B, Katzman, B, Donato, L, Jaffe, A, et al.. Real-time detection of analytical outliers on the Roche troponin T generation 5 assay. Am J Clin Pathol 2019;152:S83. https://doi.org/10.1093/ajcp/aqz116.007.Suche in Google Scholar
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Editorial
- Machine learning and coagulation testing: the next big thing in hemostasis investigations?
- Reviews
- Updates on liquid biopsy: current trends and future perspectives for clinical application in solid tumors
- The underestimated issue of non-reproducible cardiac troponin I and T results: case series and systematic review of the literature
- Opinion Paper
- Benefits, limitations and controversies on patient-based real-time quality control (PBRTQC) and the evidence behind the practice
- Genetics and Molecular Diagnostics
- ctDNA from body fluids is an adequate source for EGFR biomarker testing in advanced lung adenocarcinoma
- General Clinical Chemistry and Laboratory Medicine
- Incidence, characteristics and outcomes among inpatient, outpatient and emergency department with reported high critical serum potassium values
- Clinical usefulness of drug-laboratory test interaction alerts: a multicentre survey
- Integrating quality assurance in autoimmunity: the changing face of the automated ANA IIF test
- Plasma thiol/disulphide homeostasis changes in patients with restless legs syndrome
- Reference Values and Biological Variations
- High-resolution pediatric reference intervals for 15 biochemical analytes described using fractional polynomials
- Continuous reference intervals for leukocyte telomere length in children: the method matters
- Hematology and Coagulation
- Using machine learning to identify clotted specimens in coagulation testing
- Cardiovascular Diseases
- Long term pronostic value of suPAR in chronic heart failure: reclassification of patients with low MAGGIC score
- Infectious Diseases
- Monocyte distribution width (MDW) parameter as a sepsis indicator in intensive care units
- A low level of CD16pos monocytes in SARS-CoV-2 infected patients is a marker of severity
- Thrombin generation in patients with COVID-19 with and without thromboprophylaxis
- Corrigendum
- Applying the concept of uncertainty to the sFlt-1/PlGF cut-offs for diagnosis and prognosis of preeclampsia
- Letters to the Editors
- Additional approaches for identifying non-reproducible cardiac troponin results
- Paediatric reference intervals for ionised calcium – a data mining approach
- A case of interference in testosterone, DHEA-S and progesterone measurements by second generation immunoassays
- Lack of cross-reactivity between anti-A IgG isoagglutinins and anti-SARS-CoV-2 IgG antibodies
- Artefactual bands on urine protein immunofixation gels
- A case of methaemoglobinaemia interference on the WDF channel on Sysmex XN-Series analysers
- Soluble fms-like tyrosine kinase-1: a potential early predictor of respiratory failure in COVID-19 patients
- Serendipitous detection of α1-antitrypsin deficiency: a single institution’s experience over a 32 month period
- The activated partial thromboplastin time may not reveal even severe fibrinogen deficiency
- Influence of C-reactive protein on thrombin generation assay
- Inappropriate extrapolations abound in fecal microbiota research
Artikel in diesem Heft
- Frontmatter
- Editorial
- Machine learning and coagulation testing: the next big thing in hemostasis investigations?
- Reviews
- Updates on liquid biopsy: current trends and future perspectives for clinical application in solid tumors
- The underestimated issue of non-reproducible cardiac troponin I and T results: case series and systematic review of the literature
- Opinion Paper
- Benefits, limitations and controversies on patient-based real-time quality control (PBRTQC) and the evidence behind the practice
- Genetics and Molecular Diagnostics
- ctDNA from body fluids is an adequate source for EGFR biomarker testing in advanced lung adenocarcinoma
- General Clinical Chemistry and Laboratory Medicine
- Incidence, characteristics and outcomes among inpatient, outpatient and emergency department with reported high critical serum potassium values
- Clinical usefulness of drug-laboratory test interaction alerts: a multicentre survey
- Integrating quality assurance in autoimmunity: the changing face of the automated ANA IIF test
- Plasma thiol/disulphide homeostasis changes in patients with restless legs syndrome
- Reference Values and Biological Variations
- High-resolution pediatric reference intervals for 15 biochemical analytes described using fractional polynomials
- Continuous reference intervals for leukocyte telomere length in children: the method matters
- Hematology and Coagulation
- Using machine learning to identify clotted specimens in coagulation testing
- Cardiovascular Diseases
- Long term pronostic value of suPAR in chronic heart failure: reclassification of patients with low MAGGIC score
- Infectious Diseases
- Monocyte distribution width (MDW) parameter as a sepsis indicator in intensive care units
- A low level of CD16pos monocytes in SARS-CoV-2 infected patients is a marker of severity
- Thrombin generation in patients with COVID-19 with and without thromboprophylaxis
- Corrigendum
- Applying the concept of uncertainty to the sFlt-1/PlGF cut-offs for diagnosis and prognosis of preeclampsia
- Letters to the Editors
- Additional approaches for identifying non-reproducible cardiac troponin results
- Paediatric reference intervals for ionised calcium – a data mining approach
- A case of interference in testosterone, DHEA-S and progesterone measurements by second generation immunoassays
- Lack of cross-reactivity between anti-A IgG isoagglutinins and anti-SARS-CoV-2 IgG antibodies
- Artefactual bands on urine protein immunofixation gels
- A case of methaemoglobinaemia interference on the WDF channel on Sysmex XN-Series analysers
- Soluble fms-like tyrosine kinase-1: a potential early predictor of respiratory failure in COVID-19 patients
- Serendipitous detection of α1-antitrypsin deficiency: a single institution’s experience over a 32 month period
- The activated partial thromboplastin time may not reveal even severe fibrinogen deficiency
- Influence of C-reactive protein on thrombin generation assay
- Inappropriate extrapolations abound in fecal microbiota research


