Abstract
Objectives
This study aims to develop a multimodal deep learning-based algorithm for detecting specific fetal heart rate (FHR) events, to enhance automatic monitoring and intelligent assessment of fetal well-being.
Methods
We analyzed FHR and uterine contraction signals by combining various feature extraction techniques, including morphological features, heart rate variability features, and nonlinear domain features, with deep learning algorithms. This approach enabled us to classify four specific FHR events (bradycardia, tachycardia, acceleration, and deceleration) as well as four distinct deceleration patterns (early, late, variable, and prolonged deceleration). We proposed a multi-model deep neural network and a pre-fusion deep learning model to accurately classify the multimodal parameters derived from Cardiotocography signals.
Results
These accuracy metrics were calculated based on expert-labeled data. The algorithm achieved a classification accuracy of 96.2 % for acceleration, 94.4 % for deceleration, 90.9 % for tachycardia, and 85.8 % for bradycardia. Additionally, it achieved 67.0 % accuracy in classifying the four distinct deceleration patterns, with 80.9 % accuracy for late deceleration and 98.9 % for prolonged deceleration.
Conclusions
The proposed multimodal deep learning algorithm serves as a reliable decision support tool for clinicians, significantly improving the detection and assessment of specific FHR events, which are crucial for fetal health monitoring.
Introduction
Cardiotocography (CTG) is widely used to assess fetal well-being in utero [1]. By monitoring fetal heart rate (FHR) and uterine contraction (UC), CTG provides critical physiological and pathological information through heart rate and UC pressure curves. This enables clinicians to intervene promptly before severe or permanent damage occurs, thereby reducing the incidence of neonatal asphyxia and improving neonatal outcomes and well-being [2], [3], [4].
However, the evaluation of CTG largely relies on the clinician’s experience, and it is challenging for doctors to monitor continuously, leading to variability and potential inaccuracies in CTG analysis. Incorrect diagnoses can result in unnecessary cesarean sections [5], 6]. To enable continuous monitoring and reduce misdiagnosis, many CTG analysis algorithms have been developed [7], [8], [9], [10], [11], providing decision support for clinicians.
Existing CTG analysis algorithms typically use the neonatal umbilical artery pH value as a marker to differentiate hypoxic samples after the newborn is delivered [12], 13]. CTG signal classification methods can be divided into traditional manual feature extraction algorithms and artificial intelligence algorithms. Researchers have extracted multiple time-domain, frequency-domain, and nonlinear features from CTG signals and employed various algorithms for feature dimensionality reduction and classification [14], [15], [16], [17], [18]. However, the accuracy of these algorithms largely depends on the precision and comprehensiveness of the selected features, and the manual feature extraction process is cumbersome.
To improve classification performance, many scholars have utilized machine learning and artificial neural network classification algorithms [19], [20], [21], [22]. These algorithms aim to explore the correlation between pre-labor CTG data and post-labor fetal pH values and to enhance classification accuracy through continuous algorithm improvement. Liu et al. [23] proposed a hybrid CNN-BiLSTM network model based on attention mechanisms combined with discrete wavelet transform (DWT) features for the classification of fetal acidosis, achieving an accuracy of 75.23 % and a quality index of 72.29 %. Liang et al. [24] introduced a model based on CNN and a weighted voting mechanism for automatic classification of FHR signals, achieving an accuracy of 89.3 % and a specificity of 90.68 %. O’Sullivan et al. [25] employed autoregressive moving average (ARMA) model features combined with a machine learning model for FHR signal classification to detect fetal compromise, achieving an accuracy of approximately 75 %. Fei et al. [21] proposed a multimodal bidirectional gated recurrent unit (MBiGRU) network for the classification of antenatal fetal monitoring signals, achieving an accuracy of 86.45 % and an F1-score of 86.14 %.
Fetal hypoxia can be classified into acute, subacute, developing, and chronic categories [26]. In acute cases such as umbilical cord prolapse, uterine rupture, or acute umbilical cord compression, CTG may only reflect changes during the event, and the specific locations of abnormalities on the CTG trace may remain unidentified [27]. As a result, unless fetal hypoxia is chronic and persistent throughout the entire recording, labeling the entire CTG record as hypoxic could introduce noise and lead to misclassification. Furthermore, some instances where fetal distress was managed promptly and effectively might still result in normal postnatal pH values [28]. These factors can undermine the reliability of using postnatal pH values as classification labels.
A theoretically more reliable labeling method involves annotation by clinical experts. Consequently, an obstetrics expert manually annotated the CTB-UHB database, and this annotation is publicly available to supplement the original database [29]. This annotation includes events of bradycardia, tachycardia, acceleration, and deceleration in the FHR signal, and it reports the classification of each deceleration associated with uterine contractions as early, late, variable, or prolonged.
The baseline of the FHR refers to the average level of the signal when it is stable, with no acceleration or deceleration occurring [30]. The normal FHR baseline ranges from 110 to 160 beats per minute (bpm). A baseline exceeding 160 bpm for more than 10 min indicates fetal tachycardia, while a baseline below 110 bpm for more than 10 min indicates fetal bradycardia. Acceleration is defined as an increase in FHR above the baseline by more than 15 bpm, lasting over 15 s. It is a sign of fetal well-being, often caused by natural fetal movements during pregnancy. Deceleration, which reflects the degree of fetal hypoxia and is related to uterine contractions, is defined as a decrease in FHR below the baseline by more than 15 bpm, lasting over 15 s [31], 32].
Decelerations are classified as early, variable, late, or prolonged. Early decelerations occur simultaneously with uterine contractions, with the nadir of the FHR coinciding with the peak of the contraction, generally posing no harm to the fetus. Variable decelerations show no fixed relationship with contractions, have varying waveforms, and can differ in duration and severity. Mild variable decelerations typically do not significantly affect the fetus, but frequent or prolonged occurrences may require monitoring and intervention. Late decelerations begin after the peak of the contraction and may indicate fetal distress, necessitating close monitoring and intervention to ensure fetal health. Prolonged decelerations, lasting over 3 min, may result from severe umbilical cord compression or other complications, requiring prompt action to prevent fetal hypoxia and harm.
Accurately and rapidly identifying various specific FHR events is crucial for improving diagnostic efficiency, reducing misdiagnosis rates, and achieving intelligent monitoring. This study successfully identified four specific FHR events and four deceleration patterns by integrating feature extraction techniques with deep learning algorithms. This approach not only enables automatic monitoring of CTG signals and intelligent evaluation of specific FHR events but also supports intelligent home monitoring systems. Additionally, it provides automated alerts to doctors, reducing their workload and the need for continuous monitoring, thereby helping obstetricians assess fetal status objectively, accurately, and with ease.
This study presents three key innovations. Firstly, unlike most studies that use weak labels such as pH values as classification criteria, this study uses precise labels to classify all specific FHR events. This greatly enhances the accuracy and intelligence of detecting specific events, making it easier for pregnant women to monitor themselves. Secondly, this article comprehensively extracts 110 features, including morphological characteristics, heart rate variability (HRV), and nonlinear domain features. By integrating two-dimensional signals from time-frequency domains, bradycardia, tachycardia, acceleration, and deceleration are classified using a multi-model deep neural network algorithm. Lastly, for the classification of the four types of deceleration, this study extracts multimodal parameters from CTG signals by combining FHR and UC data and constructs a pre-fusion deep learning model for comprehensive signal analysis and classification.
Signal processing methods
Data set and annotation source
The dataset used in this study is derived from the publicly available CTG database of the Czech Technical University-University Hospital in Brno (CTU-UHB) [33]. This dataset contains 552 original signal recordings, each lasting up to 90 min, and includes FHR time series, UC signals, as well as detailed clinical information on the mother, labor, and fetus. All signals were sampled at a frequency of 4 Hz.
The annotations used in this study were obtained by a gynecological expert with the support of a CTG analyzer [29]. The annotations include the start and end points of specific CTG events detected in the FHR and UC signals. The annotated events in the FHR signal are bradycardia, tachycardia, acceleration, and deceleration, represented by BC, TC, ACC, and DEC, respectively. The UC annotations indicate uterine contractions. The dataset also reports the classification of each deceleration associated with uterine contractions as early, late, variable, or prolonged, represented by E, L, V, and P, respectively.
Data preprocessing
The first step in data preprocessing is to verify that the length of the annotation files matches the source files. We found that 9 out of 552 files had mismatched lengths between the annotation files and the source files; these were deemed unsuitable for annotation and were thus excluded to ensure accurate subsequent processing.
During actual clinical monitoring, the CTG signals often encounter various noise interferences due to fetal movements, poor probe fixation, or changes in the maternal position [34], 35]. Interference noise in FHR signals typically manifests as spiky artifacts and missing values [36].
First, the zero-value data in the FHR signals were removed. Next, the absolute difference between adjacent points was calculated, and unstable points with a difference greater than 10 were corrected using linear interpolation, which effectively smooths small-scale irregularities by utilizing the values of stable points before and after these points. For extreme values exceeding 200 or below 50, corrections were made using the Hermite spline interpolation method. Segments with extreme values lasting longer than 2 s were removed directly to maintain the authenticity and accuracy of the data. Finally, a median filter was applied to smooth the data, reducing noise within the signal. The smoothing window length was set to five sampling points. The effectiveness of this preprocessing scheme is illustrated in Figure 1.
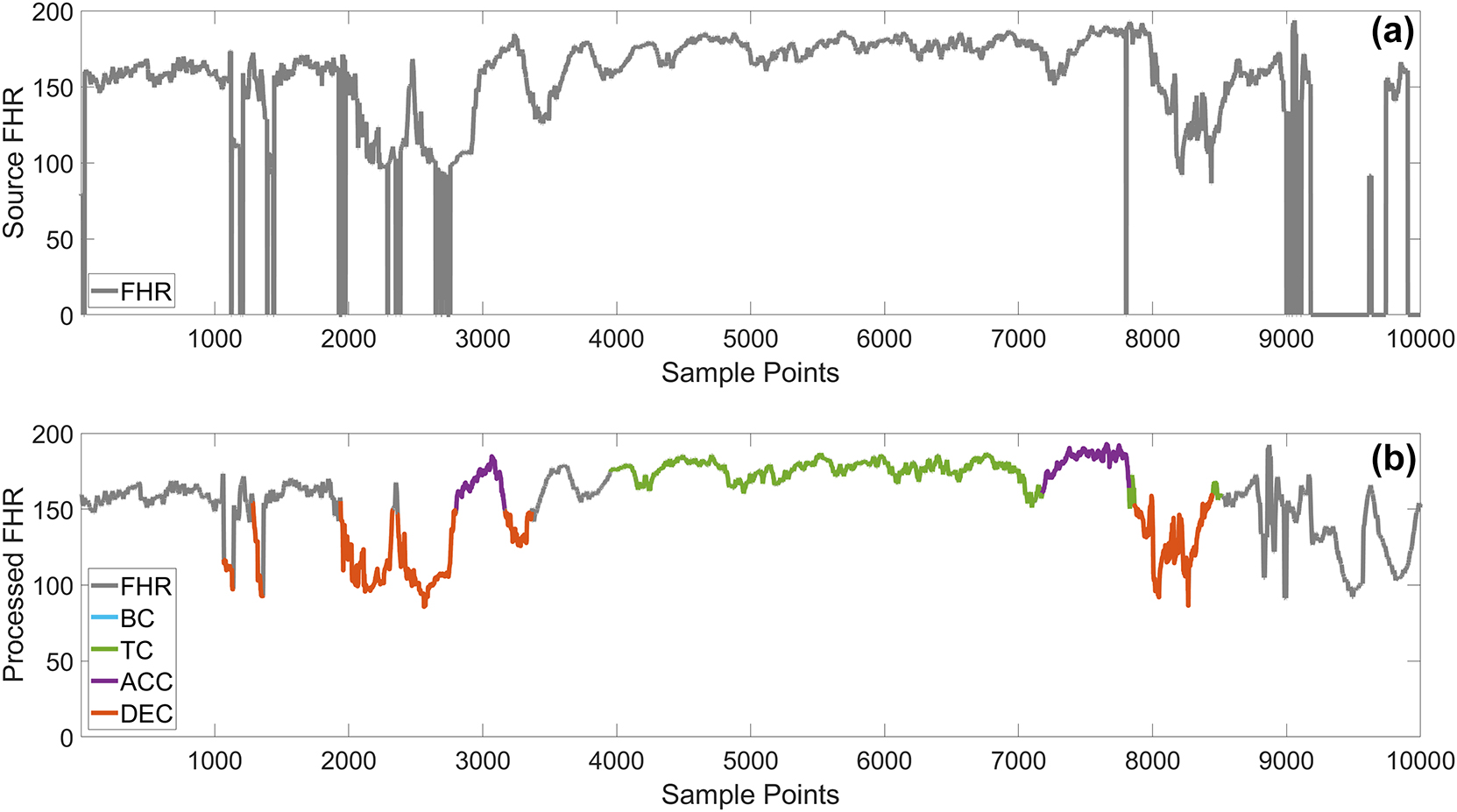
Comparison of raw and preprocessed data. The (a) graph shows the raw data, while the (b) graph shows the preprocessed data, with different colored lines representing various specific FHR events.
FHR signal feature extraction
This study aims to identify specific CTG events by extracting multidimensional features from FHR signals. This section describes all the extracted features and their calculation methods, categorized into basic statistical features, morphological features, HRV time-frequency domain features, and nonlinear features [37], [38], [39], [40], [41], [42].
Basic statistical features
Basic statistical features include the maximum, minimum, mean, median, standard deviation, variance, skewness, kurtosis, and the number of local maxima and minima of the signal [43], [44], [45].
Morphological features
The upper and lower envelopes of the signal were calculated using local maxima and minima. We then computed features of the envelopes, including the area between the envelopes, as well as basic statistics of the upper and lower envelopes, envelope width, and the slopes of the trend lines of the envelopes. The envelope width refers to the difference between the upper and lower envelopes, and the envelope area is the integral of the envelope width.
HRV time-domain features
HRV reflects fetal hypoxia and acidosis, performing well in identifying fetuses at risk [45], [46], [47]. Time-domain HRV features were calculated based on RR intervals, obtained by converting FHR into heart beat intervals. We extracted the following HRV indicators:
Maximum, minimum, mean, and median of RR intervals
SDNN: Standard deviation of RR intervals
RMSSD: Root mean square of successive differences of RR intervals
NN50: Number of adjacent RR intervals differing by more than 50 ms
pNN50: Percentage of NN50 over the total RR intervals
SDANN: Standard deviation of the average of RR intervals calculated across segments of 200 data points
SDNNi: Mean of the standard deviations of RR intervals within segments of 200 data points
Additionally, we extracted SD1 and SD2 features from Poincaré plots and TRI and TINN features from the RR interval histogram. The Poincaré plot is a scatterplot where each point represents the relationship between two consecutive RR intervals [48], [49], [50]. The RR interval histogram assesses the overall distribution of HRV.
SD1 (short-term variability): Reflects the dispersion of points perpendicular to the 45-degree diagonal line, indicating short-term HRV.
SD2 (long-term variability): Reflects the dispersion of points along the 45-degree diagonal line, indicating long-term HRV.
TRI (Triangular Index): Total number of RR intervals divided by the number of intervals in the highest bin, indicating overall distribution density.
TINN (Triangular Interpolation of NN Interval Histogram): Width of the base of the RR interval histogram, indicating the span of RR interval distribution.
Frequency-domain features
Using the Welch method, we calculated the power spectral density (PSD) of RR intervals and extracted the power and proportion of different frequency bands [51]:
VLF Power (0–0.03 Hz)
LF Power (0.03–0.15 Hz)
MF Power (0.15–0.5 Hz)
HF Power (0.5–1.0 Hz)
Total Power: Sum of power in all frequency bands
Normalized Power: Power of each band divided by total power
Percentage Power: Power of each band as a percentage of total power
LF/HF Ratio: Ratio of LF power to HF power
Nonlinear parameters
Nonlinear methods can reveal clinical information related to FHR that conventional time series analysis cannot, improving the accuracy of fetal status assessment [52], [53], [54]. We calculated sample entropy and the Lyapunov exponent to evaluate the signal’s entropy and complexity [55].
Through the above feature extraction, we can comprehensively assess the dynamic characteristics of FHR signals, thereby providing effective features for the classification of specific FHR events.
Time-frequency domain feature extraction
Studies have shown that transforming signals into images for processing is more effective in predicting fetal hypoxia than processing time-domain signals alone [21], 52], 56]. Wavelet transform is a signal processing technique that decomposes signals into different frequency components and analyzes them at various time scales, which is extremely useful in medical and biological signal processing [57]. In this study, we applied wavelet transform to convert time-domain signals into time-frequency domain signals, normalized them to a uniform size, and finally used deep learning models for learning and classification [58].
Recognition of four specific FHR events
Data composition
Before using deep neural networks to learn and classify different specific events, it is necessary to extract or segment the specific FHR data. The average durations of BC, TC, ACC, and DEC events were calculated as 2,787, 5,196, 289, and 259 samples, respectively. Consequently, different extraction and segmentation methods were applied for each category. For BC and TC data, which represent bradycardia and tachycardia respectively, a 50 s window (200 samples at a 4 Hz sampling rate) is sufficient to reflect these physiological states. Therefore, a sliding window approach with a window size of 200 samples was used to segment these data. For ACC and DEC categories, the data were segmented based on the annotations and unified to a length of 200 samples.
After processing, we obtained 2192 DEC data segments, 795 ACC data segments, 3046 BC data segments, and 5103 TC data segments. To ensure data balance during classification, the same amount of data without specific events was selected as a comparison. Figure 2 illustrates the four types of specific data within a segment of FHR signals.
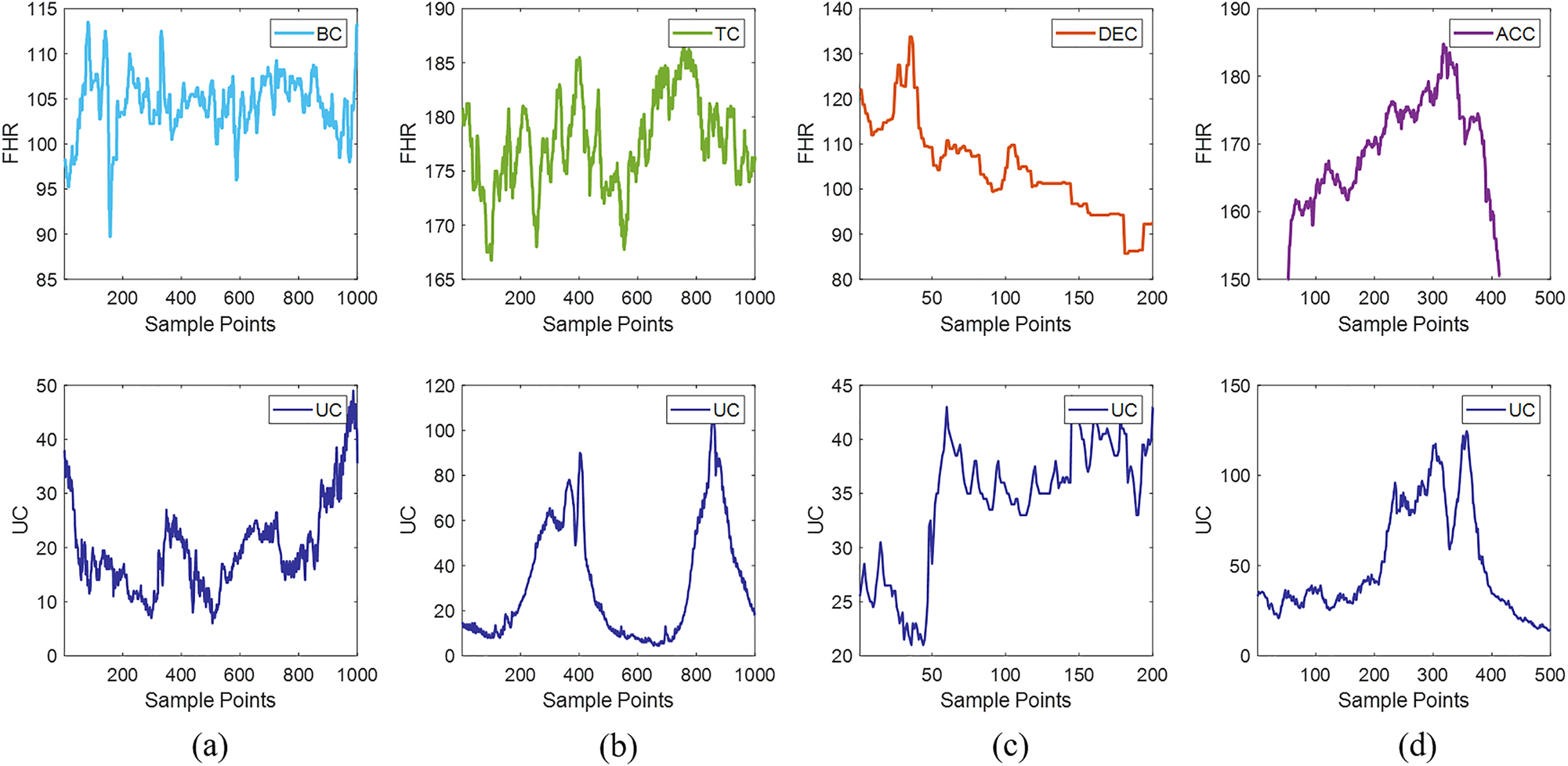
The four types of specific data within a segment of FHR signals. (a) Shows a bradycardia event, (b) shows a tachycardia event, (c) depicts a deceleration event, and (d) displays an acceleration event. The top part represents FHR, and the bottom part represents UC.
Model fusion method
Following the feature extraction process, 110 features were calculated for each data segment. However, some features may not be as informative as expected, and there might be overlapping information among features. Therefore, feature selection algorithms are required to identify the most valuable and informative features. This step enhances computational efficiency, improves model performance, and increases model interpretability [59].
We utilized statistical testing methods for feature selection, specifically using the independent samples t-test to assess the differences between positive and negative sample data. Features were selected based on their p-values, retaining only those with p-values less than 0.05, indicating significant differences [60]. Through this process, we identified 62 significant features for ACC classification, 83 for BC classification, 90 for DEC classification, and 91 for TC classification.
In the four categories (ACC, BC, TC, DEC), the following feature numbers were found to exhibit significant differences: 1–5, 7, 9, 20–26, 28–30, 32–36, 39, 40, 52, 53, 62–65, 72–76, 78, 84, 86, 89, 90, 93, 95, 96, 99, 102–105. Among these, numbers 1 to 9 represent basic statistical features such as maximum value, minimum value, mean, median, standard deviation, skewness, and the number of peaks. Numbers 20 to 40 encompass features related to signal envelopes, including the number of peaks and troughs in the envelope width, as well as the maximum, minimum, mean, standard deviation, and kurtosis of both the upper and lower envelopes. Numbers 52 and 53 correspond to the maximum and minimum slope of the lower envelope trend line. Numbers 62 to 65 represent baseline statistical features, including maximum, minimum, mean, and median values. Finally, numbers 72 to 105 cover HRV parameters, including the maximum, minimum, mean, and standard deviation of RR intervals, low-frequency power, very low-frequency power, total power, and the ratio of low-frequency to high-frequency power. Figure 3 presents the box plot differences of four specific features between normal FHR and special events.
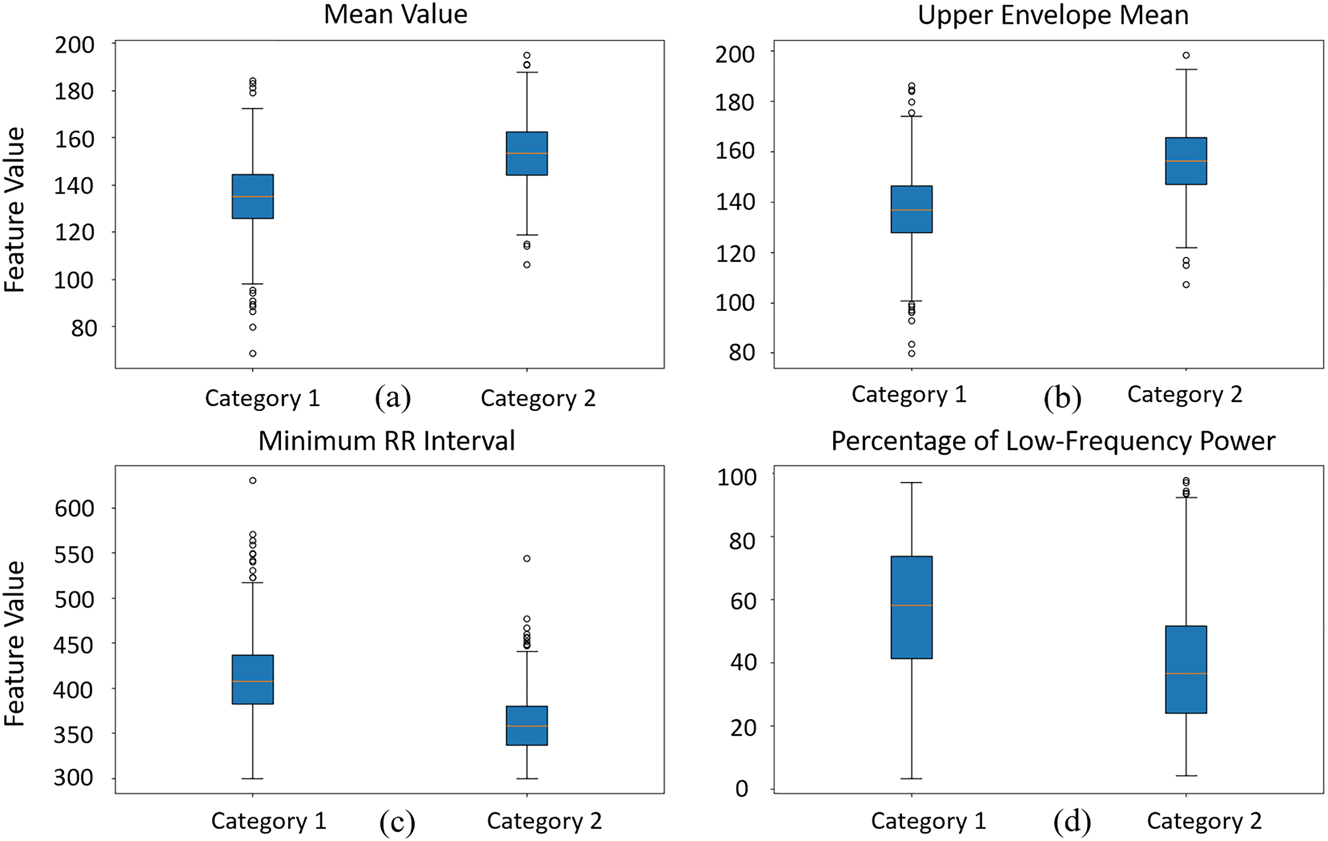
The distribution of four selected features across basal FHR and special events (using ACC as the representative event) using box plots.
This article proposes a novel deep feature fusion network for evaluating specific FHR events. This network takes as input the extracted data features and the time-frequency domain FHR signals obtained through wavelet transform. We designed and integrated several deep learning models, including ResNet1D, ModifiedResNet, and MultiAttentionModel, for feature extraction and fusion from different data representations.
The ResNet1D model employs one-dimensional convolutional neural networks (1D-CNNs) to extract features from signals such as feature values, effectively capturing relevant information. The model comprises an initial convolutional layer, multiple residual blocks, and fully connected layers. Each residual block consists of two convolutional layers, their corresponding batch normalization layers, and nonlinear activation functions.
The ModifiedResNet model is derived from a pretrained ResNet34 model, which enhances feature extraction efficiency through the use of pretrained weights via transfer learning. The initial convolutional layer in this model has been modified to accommodate single-channel input with a kernel size of 7 and stride of 2. The final fully connected layer has been replaced with a layer tailored to the specific classification task, aligning the number of output neurons with the target classes.
Additionally, the MultiAttentionModel fuses the outputs of ResNet1D and ModifiedResNet using a multihead attention mechanism, which captures complex correlations between different features for improved classification accuracy. Finally, the classification task is completed through fully connected layers. The specific network model is illustrated in Figure 4.
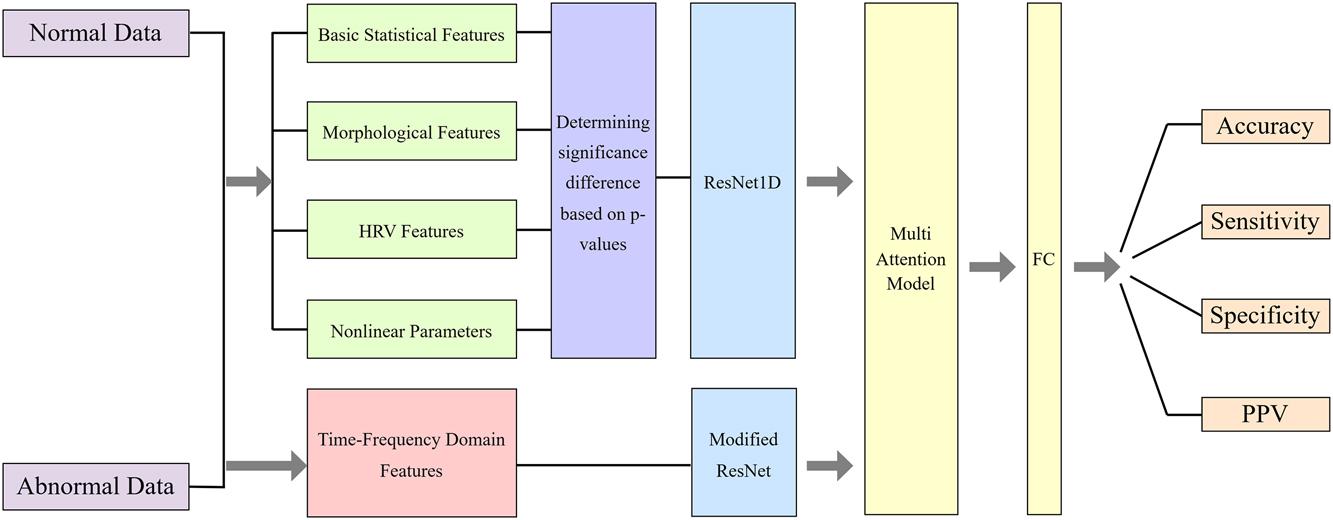
Multi-feature fusion network model.
Results
In this study, we proposed a deep feature fusion network that effectively integrates useful features from morphological, HRV, and nonlinear one-dimensional signals, as well as time-frequency domain two-dimensional signals. This integration is achieved through multi-model joint learning and the introduction of attention mechanisms, improving the accuracy and robustness of specific FHR event evaluation. The performance of the model was comprehensively evaluated using accuracy, sensitivity, specificity, positive predictive value (PPV), and F1 score on the validation set. The definitions and equations for these metrics are as follows:
where TP, TN, FP, and FN represent true positives, true negatives, false positives, and false negatives, respectively.
The experimental results indicate that our method demonstrates high detection accuracy and stability in practical applications. Table 1 shows the network results, comparing the accuracy of using single features vs. fused features for classifying ACC, DEC, TC, and BC events. As observed in Table 1, the model using fused features significantly outperforms the single-feature model across all classification tasks. For ACC event classification, the accuracy with fused features reaches 96.2 %, which is 10.2 percentage points higher than the 86.0 % accuracy obtained with single features. Similarly, for DEC, TC, and BC events, the model with fused features demonstrates superior sensitivity, specificity, PPV, and F1 score. These results further validate the superiority of multimodal data fusion and deep learning models in complex signal classification tasks, indicating that combining multiple features can significantly enhance the classification performance and stability of the model.
Network results comparison.
| Types | Accuracy | Sensitivity | Specificity | PPV | F1 score | |
|---|---|---|---|---|---|---|
| Fused features | ACC | 96.2 | 96.7 | 95.9 | 95.4 | 96.0 |
| DEC | 94.4 | 93.3 | 95.5 | 95.3 | 94.3 | |
| TC | 90.9 | 87.0 | 94.7 | 94.2 | 90.5 | |
| BC | 85.8 | 86.4 | 85.1 | 86.0 | 86.2 | |
| Single feature | ACC | 86.0 | 87.8 | 87.1 | 87.3 | 87.5 |
| DEC | 86.5 | 87.5 | 88.8 | 88.6 | 88.0 | |
| TC | 89.8 | 87.8 | 92.8 | 92.5 | 90.0 | |
| BC | 85.2 | 85.0 | 86.7 | 86.5 | 85.7 | |
| Single time-frequency | ACC | 77.0 | 58.0 | 98.6 | 98 | 72.9 |
| DEC | 92.7 | 95.9 | 89.6 | 90.1 | 92.9 | |
| TC | 91.3 | 91.1 | 91.5 | 91.4 | 91.2 | |
| BC | 86.1 | 88.3 | 83.6 | 85.1 | 86.7 |
Recognition of four deceleration-specific event patterns
Data composition
pAmong the four types of specific FHR events, deceleration (DEC) is particularly important, and we focus primarily on this specific event pattern. From the source files, we accurately extracted DEC data, and the next step is to accurately identify specific deceleration patterns within DEC. According to the annotation files, decelerations are categorized into early, late, variable, and prolonged, denoted as E, L, V, and P, respectively. From all source files, we obtained 995 E-type, 181 L-type, 1012 V-type, and 43 P-type data segments.
The data volumes for the four categories vary significantly, necessitating the use of data augmentation techniques to mitigate class imbalance [61], 62]. We employed the sliding window technique to augment the data. For L and P-type data, different strides were used to generate more samples, and the data within the window were resized to a fixed size to ensure consistent input data shapes. Subsequently, we applied wavelet transform to the CTG data for feature extraction. After these operations, we obtained 995 E-type, 1086 L-type, 1012 V-type, and 946 P-type data segments, providing a balanced and rich dataset for the subsequent training of deep learning models. Figure 5 illustrates the four types of deceleration-specific events, with the four graphs from left to right representing early, late, variable, and prolonged decelerations.
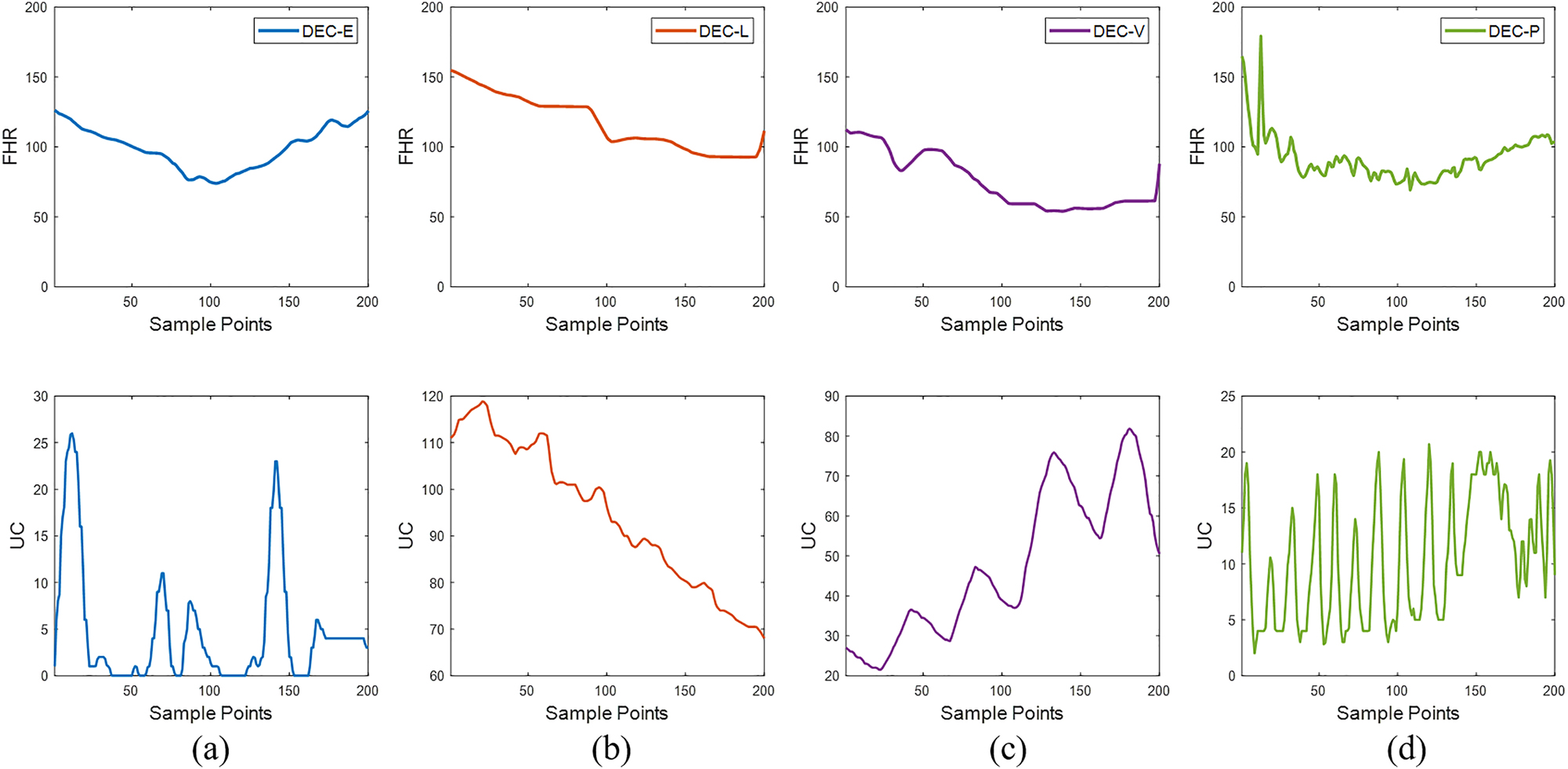
The four types of deceleration-specific events, with (a) representing early decelerations, (b) representing late decelerations, (c) representing variable decelerations, and (d) representing prolonged decelerations. The top part represents FHR, and the bottom part represents UC.
Multimodal signal processing method
Identifying the four types of DEC requires using both FHR and UC modalities, primarily because the determination of different DEC types relies on the characteristics of UC data. The combination of these two modalities can accurately reflect different deceleration patterns [63]. We designed and implemented the ModifiedResNet model [64], which is based on the pretrained ResNet34. This model adjusts the input channels of the initial convolutional layer to accommodate dual-channel input data and modifies the final fully connected layer to align with the classification task. Specifically, the initial convolutional layer in the ModifiedResNet model uses a convolutional kernel size of 7 and a stride of 2, accepting both FHR and UC signals as inputs by concatenating them along the channel dimension. By leveraging transfer learning, we utilize the pretrained model’s weights to enhance feature extraction capabilities and further improve classification performance. The architecture of the network model is illustrated in Figure 6.
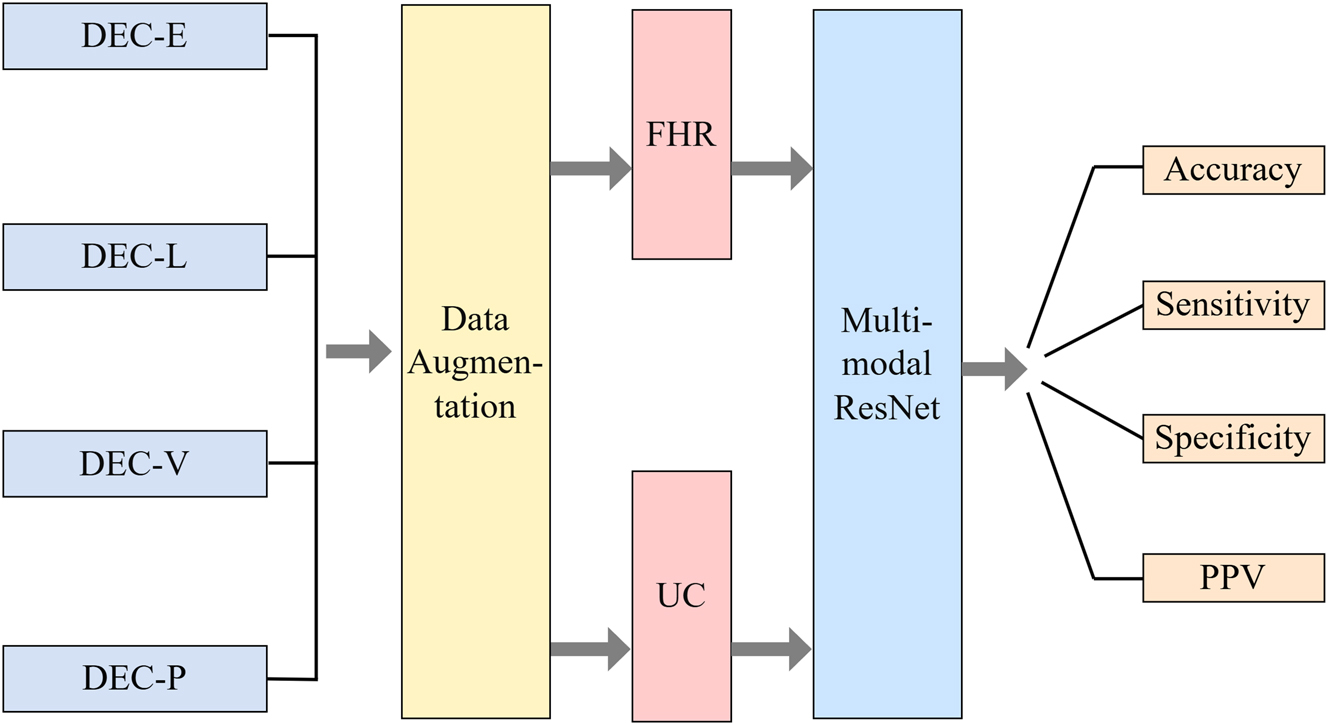
Dual-modal deep neural network architecture.
Results
Experimental results demonstrate that the ModifiedResNet model, through the joint processing of FHR and UC signals and deep feature extraction, exhibits high classification accuracy and robustness in the task of specific FHR event evaluation. Table 2 presents the four-class classification results of the multimodal deep neural network. Additionally, since late deceleration and prolonged deceleration are closely associated with fetal distress, indicating potentially adverse fetal conditions, they warrant significant attention. As the most critical monitoring indicators, we calculated the accuracy of identifying late deceleration and prolonged deceleration from all DEC-specific events. These results are also reflected in Table 2.
Results of the multimodal deep neural network.
| Accuracy | Sensitivity | Specificity | PPV | F1 score | |
|---|---|---|---|---|---|
| Four DEC-specific events | 67.0 | 80.5 | 74.4 | 79.3 | 80.0 |
| Late deceleration | 80.9 | 78.9 | 82.9 | 81.6 | 80.2 |
| Prolonged deceleration | 98.9 | 99.8 | 98.0 | 97.8 | 98.9 |
In practical applications, the ModifiedResNet model can effectively identify and classify different deceleration patterns (E, L, V, P) in FHR signals. This research outcome offers robust support for real-time monitoring and evaluation of fetal health status in clinical settings, facilitating early detection and intervention for potential fetal distress, thereby reducing perinatal risks.
Discussion
This study presents a deep feature fusion network that integrates morphological, HRV, and time-frequency domain signals for the accurate classification of specific FHR events. In the first experiment, the model achieved superior performance in classifying ACC, DEC, BC, and TC events, with ACC classification accuracy reaching 96.2 % and DEC accuracy at 94.4 %. Compared to Agostinelli et al. [65], who utilized a computerized approach to characterize ACC and DEC in FHR signals, Agostinelli’s work primarily relied on traditional methods for characterizing these events, which may lack the sensitivity and adaptability needed in more complex, real-world datasets. Furthermore, Rao et al. [66] implemented a multi-scale LSTM network for the automatic classification of FHR signals, achieving a classification accuracy of 85.73 % for ACC and DEC events. Our model’s ability to achieve higher accuracy and handle multiple FHR events simultaneously demonstrates the advantages of using deep feature fusion combined with attention mechanisms, as well as its potential for real-time clinical applications.
The second experiment focused on detecting different deceleration patterns (early, late, variable, and prolonged). The ModifiedResNet model successfully distinguished these patterns with high accuracy, especially for late and prolonged decelerations, which are critical indicators of fetal distress. Although the dataset was annotated by a single expert, the high classification accuracy observed suggests the model’s strong generalizability. Future work can address potential annotation biases by involving multiple experts and performing consistency analysis.
Conclusions
The innovation of this study lies in the integration of multimodal data fusion, the application of deep learning models, and the effective combination of feature selection techniques, providing an advanced and reliable method for the automated evaluation of specific FHR events. This research not only enriches the theoretical knowledge system in the field of fetal monitoring but also offers a highly efficient decision support tool for clinicians, aiding in the early detection and intervention of potential fetal distress.
Future research will focus on further optimizing the model architecture, exploring more data augmentation techniques, and feature extraction methods to enhance the model’s generalization ability in different application scenarios. Additionally, we plan to apply this method to larger-scale and multicenter datasets to validate its applicability and robustness in real clinical environments. Through continuous improvement and refinement, we aim to widely apply this deep feature fusion network in fetal monitoring systems, providing more reliable protection for maternal and infant health.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Zhuya Huang contributed to the conception and design of the study, data collection, analysis and interpretation of data, and drafting of the manuscript. Junsheng Yu provided critical revisions and guidance throughout the study. Ying Shan assisted in data analysis and interpretation. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: We used Large Language Models (LLM) specifically to improve the language of the manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: All data used in this study can be downloaded from https://physionet.org/content/ctu-uhb-ctgdb/1.0.0/. The annotation dataset of the cardiotocographic recordings constituting the “CTU-CHB intra-partum CTG database” can be downloaded from https://www.sciencedirect.com/science/article/pii/S2352340920305849?via%3Dihub.
References
1. Alfirevic, Z, Gyte, GM, Cuthbert, A, Devane, D. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev 2017. https://doi.org/10.1002/14651858.cd006066.pub3.Suche in Google Scholar PubMed PubMed Central
2. National Institute of Child Health and Human Development Research Planning Workshop. Electronic fetal heart rate monitoring: research guidelines for interpretation. J Obstet Gynecol Neonatal Nurs 1997;26:635–40.10.1111/j.1552-6909.1997.tb02737.xSuche in Google Scholar PubMed
3. Murray, H. Antenatal foetal heart monitoring. Best Pract Res Clin Obstet Gynaecol 2017;38:2–11. https://doi.org/10.1016/j.bpobgyn.2016.10.008.Suche in Google Scholar PubMed
4. Liang, H, Lu, Y. A CNN-RNN unified framework for intrapartum cardiotocograph classification. Comput Methods Progr Biomed 2023;229:107300. https://doi.org/10.1016/j.cmpb.2022.107300.Suche in Google Scholar PubMed
5. Abdulhay, EW, Oweis, RJ, Alhaddad, AM, Sublaban, FN, Radwan, MA, Almasaeed, HM. Monitoring techniques. Biomed Sci 2014;2:53–67.Suche in Google Scholar
6. Marques, JAL, Cortez, PC, Madeiro, JPDV, Fong, SJ, Schlindwein, FS, De Albuquerque, VHC. Automatic cardiotocography diagnostic system based on Hilbert transform and adaptive threshold technique. IEEE Access 2019;7:73085–94. https://doi.org/10.1109/access.2018.2877933.Suche in Google Scholar
7. Ayres-de-Campos, D, Bernardes, J, Garrido, A, Marques-de-Sa, J, Pereira-Leite, L. SisPorto 2.0: a program for automated analysis of cardiotocograms. J Matern Fetal Med 2000;9:311–8. https://doi.org/10.1002/1520-6661(200009/10)9:5<311::aid-mfm12>3.0.co;2-9.10.1002/1520-6661(200009/10)9:5<311::AID-MFM12>3.0.CO;2-9Suche in Google Scholar
8. Czabanski, R, Jezewski, J, Matonia, A, Jezewski, M. Computerized analysis of fetal heart rate signals as the predictor of neonatal acidemia. Expert Syst Appl 2012;39:11846–60. https://doi.org/10.1016/j.eswa.2012.01.196.Suche in Google Scholar
9. Pardey, J, Moulden, M, Redman, CW. A computer system for the numerical analysis of nonstress tests. Am J Obstet Gynecol 2002;186:1095–103. https://doi.org/10.1067/mob.2002.122447.Suche in Google Scholar
10. Romano, M, Bifulco, P, Ruffo, M, Improta, G, Clemente, F, Cesarelli, M. Software for computerised analysis of cardiotocographic traces. Comput Methods Progr Biomed 2016;124:121–37. https://doi.org/10.1016/j.cmpb.2015.10.008.Suche in Google Scholar
11. Ocak, H, Ertunc, HM. Prediction of fetal state from the cardiotocogram recordings using adaptive neuro-fuzzy inference systems. Neural Comput Appl 2013;23:1583–9. https://doi.org/10.1007/s00521-012-1110-3.Suche in Google Scholar
12. Balayla, J, Shrem, G. Use of artificial intelligence (AI) in the interpretation of intrapartum fetal heart rate (FHR) tracings: a systematic review and meta-analysis. Arch Gynecol Obstet 2019;300:7–14. https://doi.org/10.1007/s00404-019-05151-7.Suche in Google Scholar PubMed
13. Chudáček, V, Spilka, J, Burša, M, Janků, P, Hruban, L, Huptych, M, et al.. Open access intrapartum CTG database. BMC Pregnancy Childbirth 2014;14:1–12. https://doi.org/10.1186/1471-2393-14-16.Suche in Google Scholar PubMed PubMed Central
14. Georgoulas, GG, Stylios, CD, Nokas, G, Groumpos, PP. Classification of fetal heart rate during labour using hidden Markov models. IEEE Int Joint Conf Neural Networks 2004;3:2471–5. https://doi.org/10.1109/ijcnn.2004.1381017.Suche in Google Scholar
15. Spilka, J, Chudáček, V, Koucký, M, Lhotská, L, Huptych, M, Janků, P, et al.. Using nonlinear features for fetal heart rate classification. Biomed Signal Process Control 2012;7:350–7. https://doi.org/10.1016/j.bspc.2011.06.008.Suche in Google Scholar
16. Krupa, N, Ma, MA, Zahedi, E, Ahmed, S, Hassan, FM. Antepartum fetal heart rate feature extraction and classification using empirical mode decomposition and support vector machine. Biomed Eng Online 2011;10:1–15. https://doi.org/10.1186/1475-925x-10-6.Suche in Google Scholar PubMed PubMed Central
17. Ponsiglione, AM, Cosentino, C, Cesarelli, G, Amato, F, Romano, M. A comprehensive review of techniques for processing and analyzing fetal heart rate signals. Sensors 2021;21:6136. https://doi.org/10.3390/s21186136.Suche in Google Scholar PubMed PubMed Central
18. Doret, M, Spilka, J, Chudáček, V, Gonçalves, P, Abry, P. Fractal analysis and Hurst parameter for intrapartum fetal heart rate variability analysis: a versatile alternative to frequency bands and LF/HF ratio. PLoS One 2015;10. https://doi.org/10.1371/journal.pone.0136661.Suche in Google Scholar PubMed PubMed Central
19. Gao, W, Lu, Y. Fetal heart baseline extraction and classification based on deep learning. In: 2019 International Conference on Information Technology and Computer Application (ITCA). Guangzhou: IEEE; 2019:211–6 pp.10.1109/ITCA49981.2019.00053Suche in Google Scholar
20. Muhammad, HN, Rehman, AU, Othman, MTB, Zafar, J, Zafar, H, Hamam, H. Accessing artificial intelligence for fetus health status using hybrid deep learning algorithm (AlexNet-SVM) on cardiotocographic data. Sensors 2022;22:5103. https://doi.org/10.3390/s22145103.Suche in Google Scholar PubMed PubMed Central
21. Fei, Y, Chen, F, He, L, Chen, J, Hao, Y, Li, X, et al.. Intelligent classification of antenatal cardiotocography signals via multimodal bidirectional gated recurrent units. Biomed Signal Process Control 2022;78:104008. https://doi.org/10.1016/j.bspc.2022.104008.Suche in Google Scholar
22. Fergus, P, Chalmers, C, Montanez, CC, Reilly, D, Lisboa, P, Pineles, B. Modelling segmented cardiotocography time-series signals using one-dimensional convolutional neural networks for the early detection of abnormal birth outcomes. IEEE Trans Emerg Top Comput Intell 2020;5:882–92. https://doi.org/10.1109/tetci.2020.3020061.Suche in Google Scholar
23. Liu, M, Lu, Y, Long, S, Bai, J, Lian, W. An attention-based CNN-BiLSTM hybrid neural network enhanced with features of discrete wavelet transformation for fetal acidosis classification. Expert Syst Appl 2021;186:115714. https://doi.org/10.1016/j.eswa.2021.115714.Suche in Google Scholar
24. Liang, S, Li, Q. Automatic evaluation of fetal heart rate based on deep learning. In: 2021 2nd information communication technologies conference (ICTC). Nanjing: IEEE; 2021:235–40 pp.10.1109/ICTC51749.2021.9441583Suche in Google Scholar
25. O’Sullivan, M, Gabruseva, T, Boylan, GB, O’Riordan, M, Lightbody, G, Marnane, W. Classification of fetal compromise during labour: signal processing and feature engineering of the cardiotocograph. In: 2021 29th European signal processing conference (EUSIPCO). Dublin: IEEE; 2021:1331–5 pp.10.23919/EUSIPCO54536.2021.9616289Suche in Google Scholar
26. Yatham, SS, Whelehan, V, Archer, A, Chandraharan, E. Types of intrapartum hypoxia on the cardiotocograph (CTG): do they have any relationship with the type of brain injury in the MRI scan in term babies? J Obstet Gynaecol 2020;40:688–93. https://doi.org/10.1080/01443615.2019.1652576.Suche in Google Scholar PubMed
27. Pinas, A, Chandraharan, E. Continuous cardiotocography during labour: analysis, classification and management. Best Pract Res Clin Obstet Gynaecol 2016;30:33–47. https://doi.org/10.1016/j.bpobgyn.2015.03.022.Suche in Google Scholar PubMed
28. Cheong-See, F, Allotey, J, Marlin, N, Mol, BW, Schuit, E, Ter Riet, G, et al.. Prediction models in obstetrics: understanding the treatment paradox and potential solutions to the threat it poses. BJOG 2016;123:1060–4. https://doi.org/10.1111/1471-0528.13859.Suche in Google Scholar PubMed
29. Romagnoli, S, Sbrollini, A, Burattini, L, Marcantoni, I, Morettini, M, Burattini, L. Annotation dataset of the cardiotocographic recordings constituting the “CTU-CHB intra-partum CTG database”. Data Brief 2020;31:105690. https://doi.org/10.1016/j.dib.2020.105690.Suche in Google Scholar PubMed PubMed Central
30. Ayres-de-Campos, D, Spong, CY, Chandraharan, E. FIGO consensus guidelines on intrapartum fetal monitoring: cardiotocography. Int J Gynaecol Obstet 2015;131:13–24. https://doi.org/10.1016/j.ijgo.2015.06.020.Suche in Google Scholar PubMed
31. Maner, WL, Garfield, RE, Maul, H, Olson, G, Saade, G. Predicting term and preterm delivery with transabdominal uterine electromyography. Obstet Gynecol 2003;101:1254–60. https://doi.org/10.1097/00006250-200306000-00020.Suche in Google Scholar
32. Pinto, P, Bernardes, J, Costa-Santos, C, Amorim-Costa, C, Silva, M, Ayres-de-Campos, D. Development and evaluation of an algorithm for computer analysis of maternal heart rate during labor. Comput Biol Med 2014;49:30–5. https://doi.org/10.1016/j.compbiomed.2014.03.007.Suche in Google Scholar PubMed
33. Goldberger, A, Amaral, L, Glass, L, Hausdorff, J, Ivanov, PC, Mark, R, et al.. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 2000;101. https://doi.org/10.1161/01.cir.101.23.e215.Suche in Google Scholar PubMed
34. Zhou, Z, Zhao, Z, Zhang, X, Zhang, X, Jiao, P, Ye, X. Identifying fetal status with fetal heart rate: deep learning approach based on long convolution. Comput Biol Med 2023;159:106970. https://doi.org/10.1016/j.compbiomed.2023.106970.Suche in Google Scholar PubMed
35. Nageotte, MP. Fetal heart rate monitoring. Semin Fetal Neonatal Med 2015;20:144–8. https://doi.org/10.1016/j.siny.2015.02.002.Suche in Google Scholar PubMed
36. Chudáček, V, Huptych, M, Koucký, M, Spilka, J, Bauer, L, Lhotska, L. Fetal heart rate data pre-processing and annotation. In: 2009 9th International Conference on Information Technology and Applications in Biomedicine. Larnaka: IEEE; 2009:1–4 pp.10.1109/ITAB.2009.5394441Suche in Google Scholar
37. Spilka, J, Frecon, J, Leonarduzzi, R, Pustelnik, N, Abry, P, Doret, M. Sparse support vector machine for intrapartum fetal heart rate classification. IEEE J Biomed Health Inform 2016;21:664–71. https://doi.org/10.1109/jbhi.2016.2546312.Suche in Google Scholar PubMed
38. Georgoulas, G, Stylios, D, Groumpos, P. Predicting the risk of metabolic acidosis for newborns based on fetal heart rate signal classification using support vector machines. IEEE Trans Biomed Eng 2006;53:875–84. https://doi.org/10.1109/tbme.2006.872814.Suche in Google Scholar
39. Fergus, P, Hussain, A, Al-Jumeily, D, Huang, DS, Bouguila, N. Classification of caesarean section and normal vaginal deliveries using foetal heart rate signals and advanced machine learning algorithms. Biomed Eng Online 2017;16:1–26. https://doi.org/10.1186/s12938-017-0378-z.Suche in Google Scholar PubMed PubMed Central
40. Sahin, H, Subasi, A. Classification of the cardiotocogram data for anticipation of fetal risks using machine learning techniques. Appl Soft Comput 2015;33:231–8. https://doi.org/10.1016/j.asoc.2015.04.038.Suche in Google Scholar
41. Ricciardi, C, Improta, G, Amato, F, Cesarelli, G, Romano, M. Classifying the type of delivery from cardiotocographic signals: a machine learning approach. Comput Methods Progr Biomed 2020;196:105712. https://doi.org/10.1016/j.cmpb.2020.105712.Suche in Google Scholar PubMed
42. Binkowski, K, He, P, Kordzakhia, N, Shevchenko, P. On the parameter estimation in the schwartz-smith’s two-factor model. In: Research school on statistics and data science. Springer, Singapore; 2019:226–37 pp.10.1007/978-981-15-1960-4_16Suche in Google Scholar
43. Cömert, Z, Kocamaz, AF, Güngör, S. Cardiotocography signals with artificial neural network and extreme learning machine. In: 2016 24th signal processing and communication application conference (SIU). Zonguldak: IEEE; 2016:1493–6 pp.10.1109/SIU.2016.7496034Suche in Google Scholar
44. Geng, X, Ji, L, Wang, F, Zhao, Y, Gong, P. Statistical volume analysis: a new endmember extraction method for multi/hyperspectral imagery. IEEE Trans Geosci Rem Sens 2016;54:6100–9. https://doi.org/10.1109/tgrs.2016.2581180.Suche in Google Scholar
45. Huang, ML, Hsu, YY. Fetal distress prediction using discriminant analysis, decision tree, and artificial neural network. J Biomed Sci Eng 2012;5:8. https://doi.org/10.4236/jbise.2012.59065.Suche in Google Scholar
46. Jovic, A, Bogunovic, N. Electrocardiogram analysis using a combination of statistical, geometric, and nonlinear heart rate variability features. Artif Intell Med 2011;51:175–86. https://doi.org/10.1016/j.artmed.2010.09.005.Suche in Google Scholar PubMed
47. Sampson, MB, Mudaliar, NA, Lele, AS. Fetal heart rate variability: as an indicator of fetal status. Postgrad Med 1980;67:207–15. https://doi.org/10.1080/00325481.1980.11715459.Suche in Google Scholar PubMed
48. Schiermeier, S, Van Leeuwen, P, Lange, S, Geue, D, Daumer, M, Reinhard, J, et al.. Fetal heart rate variation in magnetocardiography and cardiotocography--a direct comparison of the two methods. Z Geburtsh Neonatol 2007;211:179–84. https://doi.org/10.1055/s-2007-981254.Suche in Google Scholar PubMed
49. Gaitán Carrasco, MJ, González, R, Yánez, SO, Yánez, O. Correlation among Poincaré plot indexes and time and frequency domain measures of heart rate variability. J Med Eng Technol 2001;25:240–8. https://doi.org/10.1080/03091900110086651.Suche in Google Scholar PubMed
50. Karmakar, CK, Khandoker, AH, Gubbi, J, Palaniswami, M. Complex correlation measure: a novel descriptor for Poincaré plot. Biomed Eng Online 2009;8:1–12. https://doi.org/10.1186/1475-925x-8-17.Suche in Google Scholar
51. Signorini, MG, Magenes, G, Cerutti, S, Arduini, D. Linear and nonlinear parameters for the analysis of fetal heart rate signal from cardiotocographic recordings. IEEE Trans Biomed Eng 2003;50:365–74. https://doi.org/10.1109/tbme.2003.808824.Suche in Google Scholar
52. Zhao, Z, Deng, Y, Zhang, Y, Zhang, Y, Zhang, X, Shao, L. DeepFHR: intelligent prediction of fetal acidemia using fetal heart rate signals based on convolutional neural network. BMC Med Inform Decis Mak 2019;19:1–15. https://doi.org/10.1186/s12911-019-1007-5.Suche in Google Scholar PubMed PubMed Central
53. Usha Sri, A, Malini, M, Chandana, G. Feature extraction of cardiotocography signal. In: Advances in decision sciences, image processing, security and computer vision: international conference on emerging trends in engineering (ICETE). Hyderabad: Springer International Publishing; 2020, 1:74–81 pp.10.1007/978-3-030-24322-7_10Suche in Google Scholar
54. Marques, JAL, Cortez, PC, Madeiro, JP, de Albuquerque, VHC, Fong, SJ, Schlindwein, FS. Nonlinear characterization and complexity analysis of cardiotocographic examinations using entropy measures. J Supercomput 2020;76:1305–20. https://doi.org/10.1007/s11227-018-2570-8.Suche in Google Scholar
55. Monteiro-Santos, J, Goncalves, H, Bernardes, J, Antunes, L, Nozari, M, Costa-Santos, C. Entropy and compression capture different complexity features: the case of fetal heart rate. Entropy 2017;19:688. https://doi.org/10.3390/e19120688.Suche in Google Scholar
56. Ogasawara, J, Ikenoue, S, Yamamoto, H, Sato, M, Kasuga, Y, Mitsukura, Y, et al.. Deep neural network-based classification of cardiotocograms outperformed conventional algorithms. Sci Rep 2021;11:13367. https://doi.org/10.1038/s41598-021-92805-9.Suche in Google Scholar PubMed PubMed Central
57. Spyridou, KK, Hadjileontiadis, LJ. Analysis of fetal heart rate in healthy and pathological pregnancies using wavelet-based features. In: 2007 29th annual international conference of the ieee engineering in medicine and biology society. Lyon: IEEE; 2007:1908–11 pp.10.1109/IEMBS.2007.4352689Suche in Google Scholar PubMed
58. Bursa, M, Lhotska, L. The use of convolutional neural networks in biomedical data processing. In: Information technology in bio- and medical informatics: 8th international conference, ITBAM 2017, Lyon, France, August 28–31, 2017, proceedings. Lyon: Springer International Publishing; 2017, 8:100–19 pp.10.1007/978-3-319-64265-9_9Suche in Google Scholar
59. Guyon, I, Elisseeff, A. An introduction to variable and feature selection. J Mach Learn Res 2003;3:1157–82.Suche in Google Scholar
60. De Winter, JC, Dodou, D. Five-point Likert items: t test versus Mann-Whitney-Wilcoxon. Pract Assess Res Eval 2010;15:1–12.Suche in Google Scholar
61. Sharma, S, Gosain, A, Jain, S. A review of the oversampling techniques in class imbalance problem. In: International conference on innovative computing and communications: proceedings of ICICC 2021, vol 1. Springer, Singapore; 2022:459–72 pp.10.1007/978-981-16-2594-7_38Suche in Google Scholar
62. Gosain, A, Sardana, S. Handling class imbalance problem using oversampling techniques: a review. In: 2017 international conference on advances in computing, communications and informatics (ICACCI). Udupi: IEEE; 2017:79–85 pp.10.1109/ICACCI.2017.8125820Suche in Google Scholar
63. Lear, CA, Galinsky, R, Wassink, G, Yamaguchi, K, Davidson, JO, Westgate, JA, et al.. The myths and physiology surrounding intrapartum decelerations: the critical role of the peripheral chemoreflex. J Physiol 2016;594:4711–25. https://doi.org/10.1113/jp271205.Suche in Google Scholar
64. Petrozziello, A, Redman, CW, Papageorghiou, AT, Jordanov, I, Georgieva, A. Multimodal convolutional neural networks to detect fetal compromise during labor and delivery. IEEE Access 2019;7:112026–36. https://doi.org/10.1109/access.2019.2933368.Suche in Google Scholar
65. Agostinelli, A, Belgiovine, G, Fiorentino, MC, Turri, G, Sbrollini, A, Burattini, L, et al.. Association between accelerations and decelerations of fetal heart rate. In: EMBEC & NBC 2017: joint conference of the European medical and biological engineering conference (EMBEC) and the Nordic-Baltic conference on biomedical engineering and medical physics (NBC), Tampere, Finland, June 2017. Singapore: Springer; 2018:1125–8 pp.10.1007/978-981-10-5122-7_281Suche in Google Scholar
66. Rao, L, Lu, J, Wu, HR, Zhao, S, Lu, BC, Li, H. Automatic classification of fetal heart rate based on a multi-scale LSTM network. Front Physiol 2024;15:1398735. https://doi.org/10.3389/fphys.2024.1398735.Suche in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Review
- Hydrogel promotes bone regeneration through various mechanisms: a review
- Research Articles
- Wear investigation of implant-supported upper removable prothesis with electroplated gold or PEKK secondary crowns
- Straight and helical plating with locking plates for proximal humeral shaft fractures – a biomechanical comparison under physiological load conditions
- Integration of neuromuscular control for multidirectional horizontal planar reaching movements in a portable upper limb exoskeleton for enhanced stroke rehabilitation
- Recognition analysis of spiral and straight-line drawings in tremor assessment
- Combination of edge enhancement and cold diffusion model for low dose CT image denoising
- High-performance breast cancer diagnosis method using hybrid feature selection method
- A multimodal deep learning-based algorithm for specific fetal heart rate events detection
Artikel in diesem Heft
- Frontmatter
- Review
- Hydrogel promotes bone regeneration through various mechanisms: a review
- Research Articles
- Wear investigation of implant-supported upper removable prothesis with electroplated gold or PEKK secondary crowns
- Straight and helical plating with locking plates for proximal humeral shaft fractures – a biomechanical comparison under physiological load conditions
- Integration of neuromuscular control for multidirectional horizontal planar reaching movements in a portable upper limb exoskeleton for enhanced stroke rehabilitation
- Recognition analysis of spiral and straight-line drawings in tremor assessment
- Combination of edge enhancement and cold diffusion model for low dose CT image denoising
- High-performance breast cancer diagnosis method using hybrid feature selection method
- A multimodal deep learning-based algorithm for specific fetal heart rate events detection


