The Role of medicinal herbs in treatment of insulin resistance in patients with Polycystic Ovary Syndrome: A literature review
-
Fatemeh Ashkar
Abstract
Background
Polycystic Ovary Syndrome (PCOS) is one of the most common endocrine abnormalities in women. Due to the side effects of drugs, the tendency to use natural antioxidants and anti-inflammatory agents to regulate metabolism, hyperinsulinemia, and hyperlipidemia in PCOS patients has been increased. This review aimed to investigate the role of herbal substances on the treatment of PCOS.
Methods
The present review was carried out using keywords such as polycystic ovary syndrome and/or PCOS and/or herb. Databases including Web of Science, PubMed, and Science Direct were used to collect all related articles published from 1990 to 2019. We excluded studies unrelated to the PCOS and medical herbs.
Results
Overall, 361 records were identified through database searching. After primary screening and the full-texts assessment, 323 records were excluded, and 38 articles were finally included. The results indicate that some medicinal herbs may have a key role in treating PCOS. The compounds in these medical herbs can affect lipid profiles (Aloe vera, chamomile, and cinnamon), insulin resistance (cinnamon, chamomile, Aloe vera, and Camellia sinensis), blood glucose (Aloe vera, cinnamon, and Camellia sinensis), hormones (Aloe vera, silymarin, chamomile, fenugreek, Camellia sinensis, Heracleum persicum, Potentilla, Mentha spicata, Foeniculum vulgar, licorice, and Marrubium), and ovarian tissue (Aloe vera, chamomile, Camellia sinensis, Mentha spicata, and silymarin).
Conclusion
Natural substances such as Aloe vera, cinnamon, green tea, fenugreek, and silymarin can be used as a new supportive care for PCOS. Further clinical trials are warranted to confirm their benefits and safety.
Introduction
Polycystic ovary syndrome (PCOS) is an endocrine disorder related to elevated androgens (male hormones) in females in reproductive age. PCOS is associated with various clinical symptoms such as irregular menstruation, infertility, androgen growth, hirsutism, insulin resistance, acne, weight gain, and ovarian cyst [1]. The prevalence of PCOS has been reported to be 2.2–26% in different societies [2].
Some factors appear to play an important in PCOS development including hypothalamicpituitary dysfunction, ovarian dysfunction, and increased insulin level [3]. PCOS is a disorder characterized by abnormal gonadotropin secretion including Luteinizing hormone (LH) and Follicle-stimulating hormone (FSH), and increased secretion of ovarian steroids that may be associated with insulin resistance [4]. Insulin stimulates androgens synthesis and increases LH function. The ovaries produce too much testosterone androstenedione and dehydroepiandrosterone in PCOS patients [3, 4].
Excess adrenal precursor androgen secretion is demonstrated in PCOS women [5]. Hyperandrogenism may play a major pathological role in the development of the severe endocrine and metabolic disturbances associated with PCOS. Sex hormone-binding globulin (SHBG) is a glycoprotein that can regulate the bioavailability of sex steroid hormone and SHBG levels are correlated with the risk of PCOS. PCOS women with low SHBG levels were more likely to have hyperandrogenism, diabetes type 2, insulin resistance, glucose intolerance, obesity, infertility, and cardiovascular disease (CVD) [6] due to existed insulin resistance [7]. PCOS has been divided into 3 phenotypes that include Classic PCOS (Phenotypes A and B), Ovulatory PCOS (Phenotype C), and Nonhyperandrogenic PCOS (Phenotype D) [8]. Treatment should be tailored to each patient’s phenotype and expectations, such as a desire for pregnancy [9].
Different treatment strategies have been tried for patients with PCOS, such as lifestyle modification, ovulation induction, high testosterone therapy, insulin sensitizer, supplementation with myoinositol, folic acid, and vitamin D, assisted reproductive technology therapy, and surgical treatment [10, 11]. The main treatment for insulin resistance and glucose intolerance of women with PCOS is metformin [7, 11, 12], but a study reported that pioglitazone improved the menstrual cycle and ovulation of PCOS patients better than metformin [13]. Another drug prescribed is Clomiphene Citrate (CC). CC is a non-steroidal selective estrogen receptor modulator, and although it has efficient stimulation on ovulation, but the pregnancy rate is not satisfactory [14]. However, because of the side effects of drugs, the tendency to use of herbal drugs as the natural antioxidants and anti-inflammatory agents to regulate metabolism and control of hypertension and hyperlipidemia has increased [13, 14]. Medical herbs may be an important role in PCOS treatment. These medical herbs have a steroidogenic response and express estrogen receptor protein, reduce androgens, increases glucose uptake and improve the conditions in PCOS patient [15, 16].
This study aimed to assess the effects of natural antioxidants and herbal substances on PCOS-IR by a systematic review in in patients with PCOS.
Methods
Search Strategy
The present research reviews the studies that focus on PCOS, herb, medical herbs, antioxidant, and nutrition by searching international databases of PubMed, Google Scholar, ISI, Embase (Elsevier) from 1990 to 2019. The current research was performed using the terms of medical subject headings and combinations of the keywords using the following search strategy: “polycystic ovary syndrome” or “PCOS” and “medical herbs” or “herb” or “antioxidant” or “nutrition” or “ovarian cysts” or “hyperandrogenism” or “hirsutism” or “botanical medicine”, or “insulin resistance”. All articles collected in the electronic search process, as well as the references used in these articles, were reviewed. Irrelevant, non-English, and inappropriate articles were excluded from the review process. Studies that have quantitatively investigated the association between polycystic ovary syndrome and herb or medical herbs were included in our review.
Assessment of methodological rigor
At this stage, studies conducted to investigate the association between medicinal herbs, insulin resistance, and polycystic ovary syndrome were selected. The quality of the studies was independently assessed by 2 persons (SD and MGh), and if two assessors do not agree with each other, the assessment was completed as a discussion with a third person (SAMJ). The irrelevant articles were excluded from the intended articles. The full text of the articles known as appropriate in this study was investigated. Figure 1 reported the selection process of articles. In the initial search, 361 articles were collected. After investigating the title and abstract, 221 articles were excluded from the study. Additionally, 102 articles were excluded after studying the full text of the articles, and finally 38 eligible articles were identified and included in the review study. The main characteristics of the studies are presented in table 1.
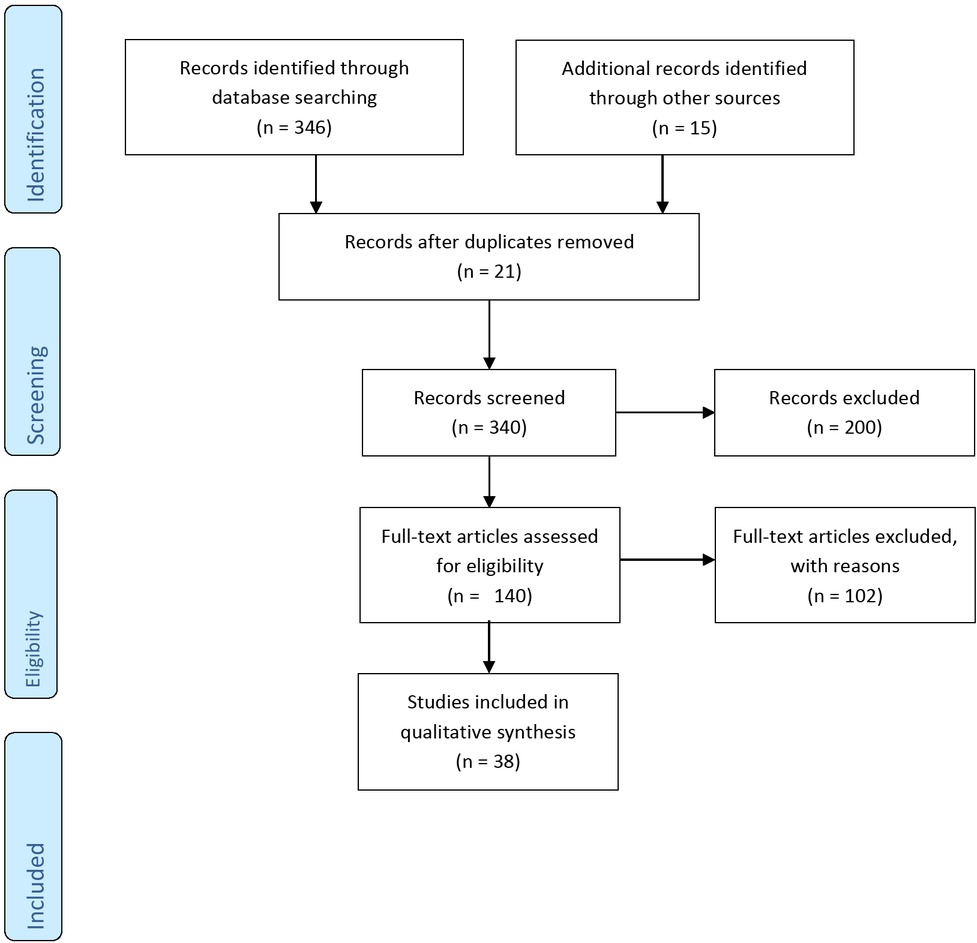
The number of records identified, included and excluded through the different phases of the review.
| Author and year | Country | Type of intervention | Time of experiment, and number | Study design and tested material | Significant outcomes | Adverse effect | Influence on PCOS |
|---|---|---|---|---|---|---|---|
| Aloe barbadensis Mill. or Aloe vera | |||||||
| Desai BN et al, 201114 | India | AVG formulation (1 ml (10 mg/day) orally | 30 days + The carboxymethyl cellulose and AVG control groups had 6 to 8 animals and PCOS group had 32 animals | Animal | Aloe vera reduced TG and LDL-C levels, increase HDL-C, improved glucose intolerance and lipid metabolizing enzyme activities. The Aloe vera derivates have anti-hyperlipidemic effects. | None | Aloe vera exert influence on PCOS. It can manage PCOS and dyslipidemia. |
| Maharjan et al, 201416 | India | Aloe vera gel (1 ml dose daily for 45 days) | 45 days + 8 control and 8 PCOS group that received Aloe vera gel | Animal | Aloe vera gel formulation exerts have a protective effect in PCOS and high dose Aloe treatment decrease atretic follicles and reverting ovary to normalcy. Restoring the ovarian steroid status, by modulation of key steroidogenic enzymes activities. | None | Aloe vera had exerted influence on PCOS. |
| Radha et al, 201417 | India | Aloe vera gel (5 mg, 10 mg, 15 mg of dry weight for 60 days each group) | 60 days + 20 rats were divided into 2 groups | Animal | It was found that more effective doses are 10 mg/kg and 15 mg/kg and all doses could improve glucose tolerance. Serum insulin levels (P < 0.001) and insulin resistance (HOMA IR < 3) were significantly decreased in all groups and the doses of 10 and 15 mg had a significant decrease in testosterone levels. | None | Aloe vera had exerted influence on PCOS. |
| Moniruzaman 201218 | Malaysia | five groups: (1) water (WC), (2) libenclamide, (3) concentrated gel extract (Gel-C), (4) ethanol (80%) gel extract (Gel-Et), and (5) ethanol (80%) skin extract of Aloe vera (Skin-Et) | 4 weeks + 34 female rats were divided into 5 groups: group 1 (n = 7), WC rats; group 2 (n = 7), libenclamide-treated rats; group 3 (n = 6), concentrated gel extract ; group 4 (n = 7), ethanol (80%) gel extract ; and group 5 (n = 7), ethanol (80%) skin extract of Aloe vera | Animal | The rats treated with Gel-C, Gel-Et and Skin-Et had a significant reduction in fasting serum glucose levels, total cholesterol levels and LDL cholesterol. | None | Aloe vera had exerted influence on PCOS. |
| Aloe barbadensis Mill. or Aloe vera | |||||||
| Ho-Chun Choi 201219 | South Korea | 700 mg capsule containing processed aloe vera gel( 147 mg/cap), aloesin powder (3 mg/cap), yeast chromone (125 mg/cap), and excipients (soy bean oil, yellow beeswax, and lecithin) (425 mg/cap) The control group received 700 mg soft capsule containing natural pigment (4.2 mg/cap) and excipients (soybean oil, yellow beeswax, and lecithin) (695.8 mg/cap). | 8 weeks + 6 subjects in the control group and 8 in the intervention group | People with obese prediabetes and early non-treated diabetic patients | Body weight (P = 0.02), body fat mass (p = 0.03) and insulin resistance were significantly lower in the intervention group. FBG reduced in the intervention group (P = 0.02). | None | Aloe vera had exerted influence on PCOS. |
| Kim et al, 201821 | Republic of Korea | Aloe Vera (100 mg/g) or metformin (100 mg/g) for 3 weeks | 3 weeks + rats divided in to 2 group (group1 received (100 mg/g Aloe vera and group 2 received metformin | Animal | After oral supplementation with Aloe vera fasting blood glucose decrease in mice. Aloe vera is insulin sensitizer that influence on pancreatic β-cells. | None | Aloe vera had exerted influence on PCOS. |
| Cinnamon | |||||||
| Wang et al, 200722 | Colombia | 3 cinnamon capsules (each one contained 333 mg cinnamon) or placebo daily | 8 weeks + 15 women with PCOS | Women with PCOS | Cinnamon supplementation improved significant reduction in fasting glucose and insulin resistance. | None | Cinnamon had exerted influence on PCOS. |
| Kort et al 201423 | USA | 1.5 g/d cinnamon supplements | 6 months + 45 women with PCOS receive cinnamon supplements or placebo for 6 months. | Women with PCOS | During 6 month intervention, menstrual cycles were more frequent in patients received cinnamon compared with patients received placebo. Luteal phase progesterone concentration confirmed ovulatory menses. Insulin resistance and serum androgen did not change. | None | Cinnamon had exerted influence on PCOS. |
| Cinnamon | |||||||
| Hajimonfared- nejad 201725 | Iran | cinnamon powder capsules 1.5 g/day in 3 divided doses for 12 weeks | 12 weeks + 66 women participated in the clinical trial 33 women receive cinnamon and 33 women received placebo | Women with PCOS | Fasting insulin, LDL-C, and insulin resistance were reduced after 12 weeks in intervention group compared with control group. | There was Observed of rash and itchiness in one patient. | Cinnamon had exerted influence on PCOS. |
| Borzoei 201724 | Iran | 3 cinnamon capsules (each one contained 500 mg cinnamon) or placebo daily | 8 weeks + 84 overweight or obese PCOS patients (42 subjects in cinnamon and 42 subjects in placebo groups) | Overweight or obese PCOS patients | Serum total antioxidant capacity significantly increased and malondialdehyde decreased in intervention group. Cinnamon supplementation significantly improved serum level of total cholesterol, LDL, and HDL-C, and decreased serum fasting blood glucose, insulin, and homeostatic model assessment for insulin resistance. | None | Cinnamon had exerted influence on PCOS. |
| Wiweko 201726 | Indonesia | 1500 mg metformin divided into two doses or 100 mg DLBS3233 (including cinnamon) for 6 months | 6 months + 20 received metformin, and 18 patients received | Women with PCOS | Decrease in anti- Müllerian hormone (AMH) level was higher in the metformin group compared to the Cinnamon group. AMH can reduced follicle sensitivity to FSH. | More side effects were observed in the metformin group compare with cinnamon group. | Cinnamon had exerted influence on PCOS. |
| Dou et al, 201828 | The control group received 0.1 ml sesame oil and 100 μL 0.5% methylcellulose+ the DHEA group received 6 mg/100 g body weight dissolved in 0.1 ml of sesame oil and the DHEA + cinnamon group received 10 mg/100 g body weight mixed in 100 μL 0.5% methylcellulose | 20 days + the animal randomly divided into 3 groups (control group (n=10), DHEA group (n=25), DHEA + cinnamon group (n=25)) | Animal | Cinnamon down- regulate serum testosterone and insulin level, reduced insulin-like growth factor-1 and increase IGF-binding protein 1 level in plasma as well as in the ovary in PCOS mice. | None | Cinnamon had exerted influence on PCOS. | |
| Camellia sinensis (Green Tea) | |||||||
| Chan et al, 200629 | China | Green tea capsules or placebo for 3 months | 3 months+ 34 obese women with PCOS | Women with PCOS | The body weight in intervention group decreased, whereas the body weight, BMI, and body fat in the control group were significantly higher after 3 months. There were no differences in any of the hormone levels measured in either group. The triglyceride level in intervention group was increased. | None | Green tea does not exert influence on PCOS |
| Allahdadian et al, 201530 | Iran | 500 mg green tea twice a week for 12 weeks or placebo | 12 weeks + 60 obese women with PCOS | Women with PCOS | After 12 weeks intervention with green tea weight loss decreased fasting insulin levels and testosterone concentration were significantly higher in the intervention group than in the control group. The green tea combinations inhibited the production of the base and stimulated testosterone. | None | Freen tea exerts influence on PCOS. |
| Tomatis 201531 | United States | Tablets providing 2093 mg green tea , catechins and 220 mg chlorogenic acids/day | 16 weeks + women divided in to 2 group | Women with PCOS | The nitric oxide production did not improve, but the intervention reduced waist circumference, changed eicosanoid profile, and reduced diastolic blood pressure, total cholstrol, and LDL cholesterol. | None | Green tea exerts influence on PCOS. |
| Camellia sinensis (Green Tea) | |||||||
| Ghafurniyan 201432 | Iran | 50, 100 & 200 mg/kg green tea extract | 10 days + 96 mature Wistar rats | Wistar rats | Significant changes in the number of follicles and theca layer thickness. Reduction in LH serum level, body and ovarian weight between the green tea extract treated-groups. Reduction in insulin resistance index was seen in the treatment groups related to PCOS. | None | Green tea exerts influence on PCOS. |
| Gasemi Tehrani 201733 | Iran | Green tea in tablet produced by DINEH IRAN | 12 weeks + 60 overweight women suffering from PCOS (30 received green tea and 30 placebo) | Women with PCOS | Weight lost, decrease in fasting insulin, and level of free testosterone. | None | Green tea exerts influence on PCOS. |
| Mombaini 200734 | Iran | The four tablets of green tea containing 500 mg green tea and placebo group received the same number of placebo tablets contain (corn starch) | 45 days + women randomly allocated into two groups receiving green tea tablets or placebo | Women with PCOS | Green tea tablets intake did not change inflammation biomarkers in PCOS women but it may be effective as a complementary treatment for weighting control. | None | Green tea exerts influence on PCOS. |
| Fenugreek (Trigonella foenum-graecum L) | |||||||
| Bashtian et al, 201235 | Iran | Three tablets of metformin 500 mg and 2 tablets containing 500 mg foenum and placebo group received 3 tablets of metformin 500 mg and 2 tablets of placebo for 2 months. | 8 weeks + 58 women with PCOS (30 women in intervention group and 28 women in control group) | Women with PCOS | The fasting glucose, insulin sensitivity and hormonal concentrations were not significantly different between two groups. Improved the sonographic results and menstrual cyclicity. | None | Fenugreek exerts influence on PCOS. |
| Swaroop 201536 | USA | Trigonella foenum- graecum seed extract 500 mg/day | 90 days + 50 premenopausal women with PCOS | Women with PCOS | The LH and FSH levels increased and ovarian volume, cyst size, and the number of ovarian cysts decreased. | None | Fenugreek exerts influence on PCOS. |
| Silymarin | |||||||
| Kayedpoor 201737 | Iran | 100 & 200 mg/kg silymarin | 14 consecutive days + 144 adult female Wistar | Animal | Different doses of silymarin caused a significant decrease in the levels of estradiol, testosterone, LH and significant increase in the levels of progesterone and FSH, reduced body weight and abdominal fat, decreased follicular sheath thickness, and increased granulosa cells due to the appearance of corpus luteum in the silymarin-treated ovaries. | None | Silymarin exerts influence on PCOS. |
| Nabiuni et al, 201538 | Iran | Doses of 20 mg/kg, 50 mg/kg, 100 mg/kg, 200 mg/kg, and 300 mg/kg | 60 days + 144 adult female Wistar | Animal | Silymarin has anti-angiogenesis effects, reducing the proliferation and thickness of the follicular sheath layer, thereby reducing the production of testosterone. | None | Silymarin exerts influence on PCOS |
| Chamomile | |||||||
| Rafraf et al, 201541 | Iran | The intervention group received chamomile tea (3 g/150 mL water) three times per day | 60 days + 64 individuals with T2DM (males and females) | Patients with T2DM | Chamomile tea significantly decreased HbA1C and serum insulin levels (p < 0.001), homeostatic no significant changes were reported in serum HDL. | None | Chamomile exerts influence on PCOS |
| Zafari Zangeneh 201043 | Iran | 25, 50, 75 mg/kg of Chamomile alcoholic extract | 10 days + 30 rats were divided into 4 groups: one group is control and other three groups consume doses (25, 50 and 75 mg/kg) of chamomile | Animal | Decrease the signs of PCOS in the ovarian tissue and help LH secretion. | None | Chamomile exerts influence on PCOS |
| Chamomile | |||||||
| Heidary 201842 | Iran | 4 tablets of chamomile containing 370 mg | 3 months + 80 women (40 patients in each group) | Women with PCOS | Testosterone level was decrease in the intervention group who received chamomile capsules. Changes in low‐density lipoprotein cholesterol level, high‐density lipoprotein cholesterol, and triglycerides were not significant. | None | Chamomile exerts influence on PCOS |
| Heracleum persicum (Persian Hogweed or Golpar) | |||||||
| Alizadeh 201551 | Iran | 200 mg, 400 mg/kg and 800 mg Heracleum persicum extract | 10 days + 30 rat | Animal | Heracleum persicum reduced LH, estradiol, and testosterone. The high dose of Heracleum persicum increase FSH levels. | None | Heracleum persicum exerts influence on PCOS |
| Haj-Husein 201650 | Iran | The intervention group receive marjoram tea and placebo tea twice daily for 1 month | 4 weeks + 25 patients with PCOS participate in study (intervention group: n = 14; placebo group: n = 11) | Patients with PCOS | Furanocoumarin inhibit nitric oxide syntheses that lead to reduced releasing LH level and estradiol. The estradiol reduction helps to natural process of human reproduction. | None | Heracleum persicum exerts influence on PCOS |
| Mentha spicata (Spearmint) | |||||||
| Sadeghi Ataabadi 201755 | Iran | 50 mg/kg spearmint oil or 300 mg/kg spearmint oil | 20 days + Group 1: control, Group 2: consume letrozole; Group 3: received letrozole; Group 4: received letrozole and spearmint oil (300 mg/kg); Group 5: consume letrozole and sesame oil; Group 6: consume 150 mg/kg spearmint oil; Group 7: spearmint oil; and Group 8: received sesame oil | Animal | Menta oil reduced body weight, testosterone concentration, ovarian cysts, and atretic follicles in PCOS rats. | None | Mentha exerts influence on PCOS |
| Akdoan 200753 | Turkey | 5 g of dried mentha leaves in 250 mL of boiling water | 5 days + 21 female hirsute patients, 12 with PCOS and 9 with idiopathic hirsutism | Women with PCOS and idiopathic hirsutism | Spearmint teas did not change testosterone and increase luteinizing hormone, follicle stimulating hormone, and estradiol. | None | Mentha exerts influence on PCOS |
| Foeniculum vulgare (fennel) | |||||||
| Fozalaee 201559 | Iran | Capsule containing fennel (150 mg/kg) (100 mg/kg) and metformin (100 mg/kg) | 63 days + 40 female rats were divided into five (group 1: control; Group 2: estradiol valerate; Group 3: PCOS + fennel received 150 mg/kg; Group 4: PCOS + fennel 2 group received fennel (100 mg/kg); Group 5: PCOS + metformin consumed metformin (100 mg/kg) | Animal | Rats treated with Foeniculum vulgare at doses of 150 mg/kg and 100 mg/kg had decreased in urea levels. | None | Fennel exerts influence on PCOS |
| Karampoor 201458 | Iran | Capsule containing 250, 500, and 1000 mg/kg fennel extract | 10 days + 30 rats case and 6 rats were considered as control | Animal | Treatment groups have been increased serum concentrations of FSH, decrease LH and testosterone in treatment groups. The FSH hormone (dose of 500 and 1000 mg/kg levels) and testosterone (dose 1000 mg/kg) have reported statistically significant differences compared to control groups. | None | Fennel exerts influence on PCOS |
| Sadrefozalayi 201257 | Iran | Intervention group received various doses of 100 mg/kg and 150 mg/kg | 4 weeks + 40 female rats (n = 8 in each group) Group 1: control, Group 2: Foeniculum vulgare (150 mg/kg ). Group 3: received 4 mg in 0.2 mL of sesame oil Group 4: 150 mg/kg Group 5: 100 mg/kg | Animal | There was a significant decrease in serum progesterone level in the low dose of Foeniculum vulgar in the treatment group compared with a high dose of Foeniculum vulgare . The mean serum estrogen concentration in the treatment group with a high dose of Foeniculum vulgare and metformin shows a significant increase. | None | Fennel exerts influence on PCOS |
| Mokaberinejad et at 201960 | Iran | Intervention group received fennel tea and control received metformin | 6 month + 61 patients with oligomenorrhoea divided in two group (Group 1: fennel infusion plus dry cupping and Group 2: treatment with metformin) | Patients with oligomenor- rhoea | This study reported that the fennel tea plus dry cupping decreased the days between two menstrual cycles and pain of dysmenorrhea in PCOS patients. | None | Fennel exerts influence on PCOS |
| Potentilla | |||||||
| Jelodar 201741 | Iran | Capsule containing 365 mg/kg Vitex extract | 30 days + 28 mice | Animal | Potentilla treatment did not significantly change the number of offspring. | None | Potentilla exerts influence on PCOS |
| Licorice or Glycyrrhiza glabra | |||||||
| Faghihi 201567 | Iran | Licorice gel plus alexandrite laser | 24 weeks + 90 female subjects with hirsutism (Subjects were divided into two groups: Group 1: alexandrite laser plus 15% licorice gel and Group 2: control) | Women with hirsutism | A treatment of idiopathic hirsutism with licorice gel plus laser is more effective than laser only. | Nno serious adverse reactions | Licorice with laser exerts influence on hirsutism |
| Armanini 200668 | Italy | Group (1) received 100 mg spironolactone and (group 2) spironolactone plus 3.5 g of licorice a day | 60 days +32 woman( 16 woman consume 100 mg spironolactone and 16 subjects received spironolactone plus 3.5 g of licorice) | Woman with PCOS | The blood pressure was significantly reduced in spironolactone treatment group, while it was not significant change in women receiving spironolactone plus licorice. | None | Licorice does not exert influence on PCOS |
| Yang et al, 201869 | South Korea | Licorice (300 mg/kg) | 2 weeks + 18 rats (3 groups n = 6 rats in each group) | Animal | licorice extract inhibits the symptoms of PCOS by regulating controlling levels of serum FSH, LH/FSH ratio, and irregular ovarian follicles. | None | Licorice exerts influence on PCOS |
| Marrubium vulgare (White Horehound) | |||||||
| Mokhtae70 | Iran | Experimental group orally received doses of 500 mg/kg and 1000 mg/kg for 21 days. | 21 days + 48 adult female rats | Animal | LH hormone significantly decreased in dose 1000 mg/kg and estradiol and progesterone decreased in doses 500 mg/kg and 1000 mg/kg and testosterone decreased in dose 1000 mg/kg. | none | White Horehound exerts influence on PCOS |
Aloe vera
Aloe vera is a medicinal plant with hypoglycemic effects [13, 15, 16]. Aloe vera is rich in fiber which accelerates gastrointestinal transit, absorption, and modulation of hemostasis [15, 16, 17]
Aloe vera contains many compounds with different potential biological activities. Phytosterols in the Aloe vera can alter the steroidogenic response and express estrogen receptor protein, reduce androgens, increase estrogens, and ultimately improve the conditions of the PCOS. Aloe vera phytosterols such as sitosterol reduce serum cholesterol levels and normalized (3β-Hydroxysteroid dehydrogenase) 3βHSD activity in PCOS rats [15, 17, 18].
One study by Radha et al. was conducted to investigate the effect of Aloe vera gel on rats with PCOS. In this study, Aloe vera was administered to rats of each group at different doses (5 mg/kg, 10 mg/kg, and 15 mg/kg) for 60 days, and it was found that Aloe vera can improve glucose tolerance in a dose-dependent manner. Although all dosages of Aloe vera may cause changes in the structure of the ovary, high dose treatment decreases atretic follicles and 3βHSD and 17β-. Hydroxysteroid dehydrogenase (17βHSD) activates. Serum insulin levels and insulin resistance were significantly decreased in all groups and the doses of 10 and 15 mg significantly decreased the testosterone levels [17].
A clinical trial examined the endocrine effects of 1 mL of Aloe vera gel for 45 days in rats; as a result, Aloe vera did not change the biomarker enzymes and weight but improved the insulin sensitivity and 3βHSD and 17βHSD activity [18]. Some studies reported that Aloe vera can reduce Triglyceride (TG) and low-density lipoprotein (LDL-C) levels, decrease atretic follicles, and improve glucose intolerance and lipid metabolizing enzyme activities [15, 18, 19].
Faisal [20] reported a significant reduction in plasma glucose, insulin, and TG to high-density lipoprotein (HDL) ratio after oral supplementation with Aloe vera in mice. Aloe vera is an insulin sensitizer and influence on pancreatic β-cells [21].
Cinnamon
Cinnamomum is an herbaceous plant belonging to the Lauraceae family. Cinnamomum grows in tropical Southern India and Sri Lanka. Many studies have reported that cinnamon acts as an insulin sensitizer [22]. Cinnamon includes different flavonoids and polyphenols that have free radical scavenging and antioxidant activities [23].
Some studies reported that Type-A polymers and procyanidine polyphenols in the cinnamon extract enhance insulin signaling at the post-receptor level, increase the activity of Phosphoinositide 3 (PI3) kinase, increase the glucose uptake via enhancing the GLUT4 glucose transporter, inhibit the glycogen synthesis, and enhance glycogen synthesis and hypoglycemic effects [22, 23]. A study by Wang et al. investigated the effect of cinnamon extract on insulin resistance in patients with PCOS. In this study, the control group received 3 meals and 1 capsule of placebo for each meal and the intervention group received capsules containing 333 mg of cinnamon extract per serving 3 times a day. The intervention group had a significant decrease in fasting blood sugar (FBS) and insulin resistance. The cinnamon improved insulin sensitivity and reduced oral glucose tolerance test in this study [22].
In another study by Kort et al. the intervention group received the cinnamon supplement (1.5 g) for 6 months and the control group received a placebo. The regular menstrual cycle in the intervention group confirmed the progesterone secretion in the luteal phase of the menstrual cycle. But the androgen levels and the insulin resistance had no significant changes between the two groups [23].
Borzoei et al. reported that using 500 mg cinnamon 3 days for 8 weeks improved FBS, insulin, and total cholesterol in patients with PCOS [24]. Another study carried out on 66 women that were diagnosed as PCOS. Participants were randomly allocated to two groups. The intervention group was treated by cinnamon powder capsules 1.5 g/day in 3 divided doses for 3 months and the control group received a placebo. It was concluded that cinnamon significantly decreased insulin resistance and fasting insulin levels in women with PCOS [25]. Another study reported that the metformin group had a lower anti-Müllerian hormone level, which is related to PCOS and reduces follicle sensitivity to FSH, compared to the cinnamon group. [26]. However, more side effects were observed in the metformin group compared to the cinnamon group. The ginger and cinnamon supplementation increase catalase, glutathione peroxidase, and superoxide dismutase levels [27]. Dou et al. reported that Cinnamon supplementation decreased insulin resistance and improved the health status of patients with PCOS [28]. It is possible that cinnamon down-regulates serum testosterone and insulin level reduces insulin-like growth factor-1 and increases Insulin-like growth factor 1 (IGF) binding protein level in plasma as well as in the ovary in PCOS. Cinnamon is a potential therapeutic agent for the PCOS [28].
Camellia sinensis
The scientific name of green tea is Camellia sinesis. Green tea is one of the richest sources of flavonoids and is used as a medicinal plant. Studies have indicated that green tea consumption might decrease FBS levels in diabetic patients and reduces the risk of CVD, cancer, and metabolic syndrome. Catechin inhibits catechol-O-methyltransferase (COMT) which is responsible for reduction of norepinephrine. Norepinephrine has a long-term effect on lipid metabolism. In humans, green tea supplementation increases energy consumption, fat oxidation, and reduces weight up to 4.6% in obese subjects for 3 months. Green tea contains caffeine that increases metabolic rate even at small doses (such as 100 mg/day) [31, 32, 33, 34, 35, 36, 37, 38, 39].
The green tea extract enhanced lipolysis and reduced hypertrophy of the follicular theca layer and reduced the thickness of this layer in PCOS rats. Due to this reduction, the level of steroid hormones and androgens produced by the follicular theca layer will decrease. Green tea extract enhanced the follicles and corpus luteum, and reduced cystic follicles in the ovary [32]. A study by Chan et al. in 2006 in Hong Kong aimed to investigate the effects of Chinese green tea on weight and biochemical and hormonal profiles in obese patients with PCOS. In this study, 340 Chinese obese women with PCOS were randomly divided into the intervention and placebo groups. The intervention group received green tea capsules at a dose of 540 mg (6 capsules 3 a day times for 3 months) and the control group received a placebo. At the end of the study, it was concluded that there was no significant change in BMI, weight, waist-hip ratio, and skin folds between the intervention and placebo groups. However, the level of triglyceride in the intervention group increased significantly [29].
Dadian et al. investigated the effect of green tea consumption on weight loss and hormonal changes in obese patients with PCOS. Weight loss decreased fasting insulin levels and testosterone concentrations were significantly higher in the intervention group than the control group after 12 weeks of the intervention [30]. Green tea inhibited testosterone production and stimulation, reduced LH level, diastolic blood pressure, body and ovarian weight. However, some other studies indicated that green tea increases testosterone levels and it cannot be recommended to all women with PCOS, only those who do not have elevated levels [29, 30, 31, 32, 33, 34].
Fenugreek (Trigonella foenum-graecum L)
Fenugreek (Trigonella foenum-graecum L) is an annual plant and a traditional spice crop which is cultivated in Asia. Its crust contains 10–20 yellow seeds with appetizing aroma. Fenugreek has anti-diabetic and cholesterol-lowering effects and decreases insulin resistance in women with PCOS [35]. Fenugreek extracts have soluble fibers which decrease blood sugar by reducing enzymatic digestion and absorption of carbohydrates, thus decreasing post-prandial glucose levels [35, 36]. Fenugreek has hypoglycemic effects through stimulating insulin synthesis, insulin secretion from beta-pancreatic cells, and inhibiting alpha-amylase and sucrose [35, 36].
Hassanzadeh et al. investigated the effect of fenugreek seed extract on insulin resistance in women with PCOS. The intervention group received 3 tablets of 500 mg metformin and 2 tablets of 500 mg foenum, and the control group received 3 tablets of 500 mg metformin and 2 tablets of placebo for 2 months. A significant reduction was seen in ovarian cysts after 2 months. There was no change in fasting glucose, insulin sensitivity, and hormonal concentrations between the two groups [35]. Another study on premenopausal women with a similar methodology reported 46% reduction in cyst size and 71% of women reported the return of regular menstrual cycle after completion of the treatment [36]. However, fenugreek should be recommended to women with irregular periods and polycystic ovarian ultrasound, but not necessarily to those with impaired glucose tolerance [35, 36].
Silymarin
The flavonoid silymarin is extracted from the milk thistle (Silybum marianum L. Gaernt.). Silymarin has been reported to possess various pharmacological properties with hepatoprotective, anti-oxidant, anti-inflammatory, anti-cancer, and cardioprotective activities.
It is a strong inhibitor of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kb) activation. It helps to eliminate free radicals in the body and prevents peroxidation of lipids by increasing cellular glutathione [37, 38].
Silymarin has anti-angiogenesis effects which reduce proliferation of follicular cells, thereby reducing the production of testosterone, and increases in corpus luteum due to increasing progesterone hormone [37]. Silymarin lowers testosterone level but also acts as a hepatoprotective factor and can increase SHBG protein synthesis, and inhibit cyclooxygenase (COX) and inflammation by reducing cysts [37, 38]. Silymarin influences glucose 6-phosphatase and inhibits gluconeogenesis, reduces blood glucose level, and thereby decreases the symptoms of the PCOS. Reduction of oxidative stress is a beneficial effect in reducing blood glucose levels by silymarin, and silymarin reduces inflammation in the PCOS by inhibiting cyclooxygenase-2 (COX-2) and lipoxygenase [37, 38].
A study by Nebuni et al. in 2014 investigated the effect of silymarin on PCOS induced by estradiol valerate in rats. In this study, silymarin was administered to rats at doses of 20 mg/kg, 50 mg/kg, 100 mg/kg, 200 mg/kg, and 300 mg/kg for 14 days [38]. It was reported that in the group treated with silymarin, body weight, abdominal size, number, and size of cysts were decreased. They reported no cyst at high doses (300 mg/kg), which could be due to anti-inflammatory properties of silymarin. Different doses of silymarin had positive effects such as a decrease in estradiol, testosterone, and LH and a significant increase in FSH and progesterone hormones due to the appearance of corpus luteum cysts in the ovary. Silymarin reduced inflammation and collagen in the follicular sheath and eventually reduced the layer thickness [37, 38].
Toch et al. in a meta-analysis reported the effects of a fixed combination of Berberis aristata and Silybum marianum on sugar and lipid profile. Silybum marianum decreased low-density lipoprotein, cholesterol, and plasma glucose levels [39]. Another study reported that expression levels of the insulin receptor in the Alzheimer’s group were significantly down-regulated compared with the healthy group, and silibinin (polyphenolic flavonoid extracted of Silybum marianum) supplementation decreased down-regulation the insulin receptor expression level. This result suggests that silibinin improves the brain’s insulin signaling pathways [40].
Chamomile
Chamomile is a medicinal herb which is native to Western Europe and North Africa. The main derivatives of chamomile are amino acids, polysaccharides, fatty acids, essential fatty acid, minerals, flavonoids, and phytoestrogens that have anti-inflammatory, antispasmodic, and antioxidant effects [41]. Antispasmodic effect of chamomile makes the menstrual cramps easier and reduce premature births [42, 43]. Apigenin is one of the major flavonoid chamomile components which inhibit the binding of flunitrazepam (benzodiazepine derivatives) [42]. Benzodiazepine joined to gamma aminobutyric acid (GABA) is a natural neurotransmitter amino acid in brain and reduces the secretion of LH [44]. Chamomile contains phytoestrogen which can decrease the menstrual disorder through changes in hormone positive estrogen feedback [45].
Rafraf et al. reported that chamomile significantly decreased hemoglobin A1c (HbA1c), the insulin levels, total cholesterol, TG, and LDL-C [41].
A study by Zanganeh et al. investigated the effects of chamomile extract on biochemical and clinical parameters in PCOS rats. The intervention group received chamomile extract in different doses of 25 mg/kg, 50 mg/kg, and 75 mg/kg. In rats treated with a dose of 50 mg/kg, cysts were disappeared, the number of follicles was increased, and the level of estradiol levels, gonadotropins, LH, and FSH was significantly decreased [43]. In another study, subjects received 370 mg of oral capsules of chamomile for 3 months; the level of testosterone decreased. In addition, phytoestrogens inhibit progesterone metabolizing enzyme, 20-alpha-hydroxysteroid dehydrogenase and increase progesterone hormone. Some phytoestrogen compounds that control this enzyme include 3- and 7-dihydroxyflavone and flavones. An increase in progesterone leads to an increase in basal metabolism rate and may be the cause of weight loss. Finally, the sterols found in chamomile can reduce cholesterol absorption. Phytosterols in chamomile extracts increase the dehydroepiandrosterone, which is produced in the liver. Hydroalcoholic extract of chamomile also contains ascorbic acid to prevent weight gain and reduce cholesterol levels [46].
Heracleum persicum (Persian Hogweed or Golpar)
Heracleum persicum is a perennial herb that commonly used in the preparation of food and medicine in Iran, Iraq, and Turkey [47]. Heracleum persicum contains alkaloids, terpenoids, terpene, and steroids. Hydroalcoholic extract of Heracleum persicum contain furocoumarins such as sphondin. Heracleum persicum inhibited cyclooxygenase- 2 and decreased inflammation [48].
Heracleum persicum is used as an anti-inflammatory, antiseptic, anti-diabetic, and anti-bacterial in traditional medicine [47]. Heracleum persicum extract probably decrease plasma testosterone, body and testis weight, and thus can help to treat sexual dysfunction in males [49].
Moreover, the hydroalcoholic extract of Heracleum persicum changes plasma sex hormone levels, inhibits folliculogenesis, and affects sexuality in women [50]. Furanocoumarins such as sphondin, xanthotoxin, and pimpinellin in the Heracleum persicum inhibits nitric oxide (NO) syntheses that reduced LH levels and estradiol release. The estradiol reduction helps the natural process of human reproduction [49, 50]. Haj Hosseini et al. investigated the effect of Heracleum persicum tea on hormone profile in PCOS women. The intervention group received 2 cups daily (containing 250 ml of herbal tea for 4 week) and the control group received a placebo. Heracleum persicum caused a significant decrease in fasting insulin, DHEA-S (dehydroepiandrosterone sulfate) levels, and a significant improvement in HOMA-IR index. Also, Heracleum persicum reduces androgens, especially adrenal androgens. Another study reported that Heracleum persicum reduced LH, estradiol, and testosterone, while increased FSH in PCOS rats [51]. Alkan et al, found that Heracleum persicum extract decreased plasma glucose and HbAlc in diabetic groups and increased insulin and c-peptide levels [52].
Mentha
Mentha (peppermint) is a medicinal plant of the Lamiaceae family. The mentha is native to East India and Asia. Essential oils of mentha are used in the food and beverage industries. Mentha has strong inhibitory effects which induce cytochrome P450 3A4 (CYP3A4) that leads to a change in the concentration of steroid hormones and androgen and reduce free testosterone levels due to increased SHBG. Peppermint tea can increase the level of LH, FSH, and estradiol due to physiological changes in the menstrual cycle. Peppermint tea can replace anti-androgenic treatments for hirsutism [53, 54, 55].
One study by Mehmet Akdogan et al. in Turkey investigated the effect of peppermint tea on the level of androgen in women with hirsutism. In this study, the intervention group received a cup of peppermint tea, containing 5 grams of dried mentha leaves in 250 ml of boiling water (5 days, twice a day) during the follicular phase of menstrual period. The intervention group had a significant decrease in the level of free testosterone, triglyceride, and significant increase in the levels of LH, FSH, and Prostaglandin E2 (PGE2). However, the level of DHEA and total testosterone did not decrease substantially [54]. In another clinical trial, the intervention group received peppermint tea or chamomile tea twice a day for 30 days and covered one complete menstrual cycle. The results of the studies showed that peppermint tea caused a significant decrease in testosterone levels and an increase in LH and FSH levels. Similarly, the degree of hirsutism is reduced [55]. Another study investigated the effect of herbal mixture supplements including menthe, zingiber, and Cinnamomum with and without CC in PCOS women. They found that these supplements have important effects on the antioxidants levels, glycemic control, menstrual regulation, and pregnancy rate [27].
Foeniculum vulgare (fennel)
Foeniculum vulgare (fennel) is used in traditional medicine to treat hormonal and metabolic disorders in women with PCOS. Fennel is regarded as phytoestrogenand have protective effects against oxidative stress and kidney disease. The essential oil of Foeniculum vulgar has antimicrobial and antioxidant effects [57, 58].
The chemical analysis of the extract of fennel showed that linoleic acid (54.9 %), palmitic acid (5.4 %) and oleic acid (5.4 %) were major components of fennel. The palmitic acid β-oxidation has anti-androgenic effects. This compound also exerts an anti-androgenic effect by inhibiting the formation of the dihydrotestosterone receptor complex and reducing testosterone levels. Foeniculum vulgare may increase the aromatase enzyme activity and reduce testosterone levels [57]. Long-term use of the Foeniculum vulgare has a negative feedback effect on LH and testosterone levels. Reducing the androgen levels lead to reducing LH which can be a natural menstrual cycle in women with PCOS. Foeniculum vulga extract does not change creatinine level but decreases urea. Kerempour et al. in Iran investigated the effect of hydro-alcoholic extract of Foeniculum vulgare seeds on the serum levels of sex hormones in rats with PCOS. In this study, Foeniculum vulgare was injected intraperitoneally in different doses of 250 mg/kg, 500 mg/kg, and 1000 mg/kg for 10 days. The intervention group received 500 mg/kg and 1000 mg/kg. FSH significantly increased, and testosterone and LH levels were decreased in the group treated with 1000 mg/kg dose [58]. In line with this study, Fozalaee reported that the rats received a low dose of Foeniculum vulgare had a lower progesterone level than the control group [59].
Another study indicated that Foeniculum vulgare, as well as metformin, decreased the days between two menstrual cycles and pain of dysmenorrhea in PCOS patients [60].
Potentilla
Potentilla is used to treat menstrual irregularities, regulate sex hormones and improve fertility. Recent studies reported the non-estrogenic effects of Potentilla [61]. Different phytochemicals derivatives including tannins, phenolic acid, and triterpenoids have hypoglycemic, hypolipidemic, and anti-inflammatory activities. These compounds can decrease fasting blood glucose level, glycated serum protein, malondialdehyde, and NO through inhibition of glycogen phosphorylase activity [62, 63].
Potentillas increases the number of follicles and reduces the number of ovarian cysts. Phytoestrogens that have anti estrogenic effects are found in this plant. Vitex and lactone in Potentilla extract bind to the Dopamine receptor D2 (D2 R) of dopamine in the hypothalamus and glandular pituitary, thereby inhibiting prolactin secretion and reducing fibrocystic mastopathy [61, 64].
Jaldar et al. investigated the effects of the ethanolic root extract of Potentilla on ovarian tissue changes in rats with PCOS. The intervention group received 365 mg/kg dose for 30 days. Potentilla treatment did not significantly change the number of offspring [64].
Wang et al. investigated network pharmacology-based analysis on Potentilla derivates and found that Potentilla compounds may have important effects on glucose uptake [65].
Licorice
Licorice is a member of the Leguminosae family, a native plant that is growing in Spain, Italy, Turkey, Iran, Iraq, Central Asia, and Northeast China. Licorice may have estrogen-like activity and mild inhibitory effects on the metabolism of endogenous hormones [66]. Licorice inhibits the activity of 17-hydroxyl esterase dehydrogenase and 17,20-lyase activity, stimulates aromatase activity, affects α5 and β5 reductase, and is used for the treatment of menopause due to estrogen-like effects. Licorice reduces excess hair growth due to enzymatic effects on the melatonin production cycle and possibly inhibits tyrosinase activity [66, 67]. Also, licorice reduces serum hormones level by damaging the activity of 11β- hydroxysteroids dehydrogenase and increasing the aromatase activity or by progesterone-like activity [66, 67, 68]. Faghihi et al. reported that combination therapy of licorice gel and laser are much more effective than laser alone [67]. Another study concluded that treatment with licorice and spironolactone reduced the activity of the renin-angiotensin system but did not affect the blood pressure in the treatment group [68]. Yang et al. found that licorice extract inhibits the symptoms of PCOS by regulating controlling levels of serum FSH, LH/FSH ratio, and irregular ovarian follicles [69]
Marrubium vulgare (White Horehound)
Marrubium vulgare is a flowering plant in the mint family, a native plant that is growing in Europe, northern Africa, and Asia [44]. Marrubium vulgare contains polyphenols and flavonoids that produced hypoglycemic effects, reduced cholesterol, triglyceride, and oxidative stress. Some flavonoids, such as apigenin, competitively inhibit the binding of flunitrazpam, thereby reduce the secretion of LH [44, 70, 71]. Also, the β-testosterone in the extract of Marrubium vulgare reduces LH. β-sitosterol reduces testosterone synthesis by lowering cholesterol. β-sitosterol reduces estradiol levels by decreasing aromatase enzyme activity, thereby preventing conversion testosterone to estrogen. Also, apigenin and ursolic acid of white horehound extract, inhibit cytochrome P450 and inhibit the conversion of cholesterol to pregnenolone, and thus reduce the synthesis of steroid hormones such as progesterone [70, 71].
A study by Mokhtari et al. was conducted to investigate the effect of bleach extract on hormonal parameters in rats with PCOS. In this study, the experimental group orally received doses of 500 mg/kg and 1000 mg/kg for 21 days. LH hormone was significantly decreased in 1000 mg/kg dose [70].
Conclusion
This study reported that herbal medicines may have beneficial effects on PCOS. The compounds of herbal medicine can affect lipid profiles, insulin resistance, blood glucose, the serum levels of hormones, and the ovarian tissue. Therefore, these plants can be considered as a new approach to treatment or/and controlling PCOS. Nonetheless, due to the inadequacy of studies and contradictory results, further investigations are needed in this regard in the future.
Conflict of interest: Authors state no conflict of interest
References
1 Howe E. Polycystic ovarian syndrome. PCOS; 2015.Suche in Google Scholar
2 Tehrani FR, Simbar M, Tohidi M, Hosseinpanah F, Azizi F. The prevalence of polycystic ovary syndrome in a community sample of Iranian population: iranian PCOS prevalence study. Reprod Biol Endocrinol. 2011 Mar;9(1):39.10.1186/1477-7827-9-39Suche in Google Scholar PubMed PubMed Central
3 Mohseni Kouchesfahani H, Nabyooni M, Adham H. Investigating the therapeutic effect of eee venom on polycystic ovarian syndrome in rats. Shahid Beheshti University of Medical Sciences. 2010;15(1):1–6.Suche in Google Scholar
4 Marx TL, Mehta AE. Polycystic ovary syndrome: pathogenesis and treatment over the short and long term. Cleve Clin J Med. 2003 Jan;70(1):31–3.10.3949/ccjm.70.1.31Suche in Google Scholar PubMed
5 Goodarzi MO, Carmina E, Azziz R. DHEA, DHEAS and PCOS. J Steroid Biochem Mol Biol. 2015 Jan;145:213–25.10.1016/j.jsbmb.2014.06.003Suche in Google Scholar PubMed
6 Deswal R, Yadav A, Dang AS. Sex hormone binding globulin - an important biomarker for predicting PCOS risk: A systematic review and meta-analysis. Syst Biol Reprod Med. 2018 Feb;64(1):12–24.10.1080/19396368.2017.1410591Suche in Google Scholar PubMed
7 Hopkinson ZE, Sattar N, Fleming R, Greer IA. Polycystic ovarian syndrome: the metabolic syndrome comes to gynaecology. BMJ. 1998 Aug;317(7154):329–32.10.1136/bmj.317.7154.329Suche in Google Scholar PubMed PubMed Central
8 Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016 Jul;106(1):6–15.10.1016/j.fertnstert.2016.05.003Suche in Google Scholar PubMed
9 Lua AC, How CH, King TF. Managing polycystic ovary syndrome in primary care. Singapore Med J. 2018 Nov;59(11):567–71.10.11622/smedj.2018135Suche in Google Scholar PubMed PubMed Central
10 Jin P, Xie Y. Treatment strategies for women with polycystic ovary syndrome. Gynecol Endocrinol. 2018 Apr;34(4):272–7.10.1080/09513590.2017.1395841Suche in Google Scholar PubMed
11 Regidor PA, Schindler AE, Lesoine B, Druckman R. Management of women with PCOS using myo-inositol and folic acid. New clinical data and review of the literature. Horm Mol Biol Clin Investig. 2018 Mar;34(2):/j/hmbci.2018.34.issue-2/hmbci-2017-0067/hmbci-2017-0067.xml.10.1515/hmbci-2017-0067Suche in Google Scholar PubMed
12 Xu Y, Wu Y, Huang Q. Comparison of the effect between pioglitazone and metformin in treating patients with PCOS:a meta-analysis. Arch Gynecol Obstet. 2017 Oct;296(4):661–77.10.1007/s00404-017-4480-zSuche in Google Scholar PubMed PubMed Central
13 Ma QW, Tan Y. Effectiveness of co-treatment with traditional Chinese medicine and letrozole for polycystic ovary syndrome: a meta-analysis. J Integr Med. 2017 Mar;15(2):95–101.10.1016/S2095-4964(17)60320-0Suche in Google Scholar
14 Desai BN, Maharjan RH, Nampoothiri LP. Aloe barbadensis Mill. formulation restores lipid profile to normal in a letrozole-induced polycystic ovarian syndrome rat model. Pharmacognosy Res. 2012 Apr;4(2):109–15.10.4103/0974-8490.94736Suche in Google Scholar PubMed PubMed Central
15 Doaei S, Hajiesmaeil M, Aminifard A, Mosavi-Jarrahi SA, Akbari ME, Gholamalizadeh M. Effects of gene polymorphisms of metabolic enzymes on the association between red and processed meat consumption and the development of colon cancer; a literature review. J Nutr Sci. 2018 Oct;7:e26.10.1017/jns.2018.17Suche in Google Scholar PubMed PubMed Central
16 Gholamalizadeh M, Doaei S, Akbari ME, Rezaei S, Jarrahi AM. Influence of fat mass-and obesity-associated genotype, body mass index, and dietary intake on effects of iroquois-related homeobox 3 gene on body weight. Chin Med J (Engl). 2018 Sep;131(17):2112–3.10.4103/0366-6999.239309Suche in Google Scholar PubMed PubMed Central
17 Radha M, Padamnabhi N, Laxmipriya N. Evaluation of Aloe barbadensis mill. Gel on letrozole induced polycystic ovarian syndrome (pcos) rat model-a dose dependent study. Int J Pharm Sci Res. 2014;5(12):5293.Suche in Google Scholar
18 Moniruzzaman M, Rokeya B, Ahmed S, Bhowmik A, Khalil MI, Gan SH. In vitro antioxidant effects of Aloe barbadensis Miller extracts and the potential role of these extracts as antidiabetic and antilipidemic agents on streptozotocin-induced type 2 diabetic model rats. Molecules. 2012 Nov;17(11):12851–67.10.3390/molecules171112851Suche in Google Scholar PubMed PubMed Central
19 Choi HC, Kim SJ, Son KY, Oh BJ, Cho BL. Metabolic effects of aloe vera gel complex in obese prediabetes and early non-treated diabetic patients: randomized controlled trial. Nutrition. 2013 Sep;29(9):1110–4.10.1016/j.nut.2013.02.015Suche in Google Scholar PubMed
20 Rizwan Faisal, Effect of Aloe vera Whole Leaf Extract on Blood Glucose, yperinsulinemia, and Insulin Resistance in Streptozotocin Induced Type 2 Diabetic Rats. December 2015, Medical Forum Monthly 26(12).Suche in Google Scholar
21 Kim K, Chung MH, Park S, Cha J, Baek JH, Lee SY, et al. ER stress attenuation by Aloe-derived polysaccharides in the protection of pancreatic β-cells from free fatty acid-induced lipotoxicity. Biochem Biophys Res Commun. 2018 Jun;500(3):797–803.10.1016/j.bbrc.2018.04.162Suche in Google Scholar PubMed
22 Wang JG, Anderson RA, Graham GM 3rd, Chu MC, Sauer MV, Guarnaccia MM, et al. The effect of cinnamon extract on insulin resistance parameters in polycystic ovary syndrome: a pilot study. Fertil Steril. 2007 Jul;88(1):240–3.10.1016/j.fertnstert.2006.11.082Suche in Google Scholar PubMed
23 Kort DH, Lobo RA. Preliminary evidence that cinnamon improves menstrual cyclicity in women with polycystic ovary syndrome: a randomized controlled trial. American journal of obstetrics and gynecology. 2014;211(5):487. e1-. e6. https://doi.org/10.1016/j.ajog.2014.05.00910.1097/01.ogx.0000461902.16853.84Suche in Google Scholar
24 Borzoei A, Rafraf M, Asghari-Jafarabadi M. Cinnamon improves metabolic factors without detectable effects on adiponectin in women with polycystic ovary syndrome. Asia Pac J Clin Nutr. 2018;27(3):556–63.Suche in Google Scholar
25 Hajimonfarednejad M, Nimrouzi M, Heydari M, Zarshenas MM, Raee MJ, Jahromi BN. Insulin resistance improvement by cinnamon powder in polycystic ovary syndrome: A randomized double-blind placebo controlled clinical trial. Phytother Res. 2018 Feb;32(2):276–83.10.1002/ptr.5970Suche in Google Scholar PubMed
26 Wiweko B, Susanto CA. The effect of metformin and cinnamon on serum anti-mullerian hormone in women having PCOS: A Double-blind, randomized, controlled trial. J Hum Reprod Sci. 2017 Jan-Mar;10(1):31–6.Suche in Google Scholar
27 Ainehchi N, Khaki A, Farshbaf-Khalili A, Hammadeh M, Ouladsahebmadarek E. The Effectiveness of Herbal Mixture Supplements with and without Clomiphene Citrate in Comparison to Clomiphene Citrate on Serum Antioxidants and Glycemic Biomarkers in Women with Polycystic Ovary Syndrome Willing to be Pregnant: A Randomized Clinical Trial. Biomolecules. 2019 Jun;9(6):215.10.3390/biom9060215Suche in Google Scholar PubMed PubMed Central
28 Dou L, Zheng Y, Li L, Gui X, Chen Y, Yu M, et al. The effect of cinnamon on polycystic ovary syndrome in a mouse model. Reprod Biol Endocrinol. 2018 Oct;16(1):99.10.1186/s12958-018-0418-ySuche in Google Scholar PubMed PubMed Central
29 Chan CC, Koo MW, Ng EH, Tang OS, Yeung WS, Ho PC. Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome—a randomized placebo-controlled trial. J Soc Gynecol Investig. 2006 Jan;13(1):63–8.10.1016/j.jsgi.2005.10.006Suche in Google Scholar PubMed
30 Allahdadian M, Ranjbar H, Ghasemi H, Janighorban M, Dadkhah A, Allahdadian F, et al. Exploring the effect of green tea on weight loss and serum hormone levels in overweight and obese patients with polycystic ovary syndrome. Avicenna Journal of Clinical Medicine. 2015 Jun;22(1):16–22.Suche in Google Scholar
31 Tomatis V, Wassell S, Venables M, Walker C, Ray S, Siervo M, Griffin J, Bluck L. Effects of Green Tea and Coffee Polyphenols on Cardiometabolic Function in Women with Polycystic Ovary Syndrome. The FASEB Journal. 2015 Apr;29(1_ supplement):LB271.10.1096/fasebj.29.1_supplement.lb271Suche in Google Scholar
32 Ghafurniyan H, Azarnia M, Nabiuni M, Karimzadeh L. The effect of green tea extract on reproductive improvement in estradiol valerate-induced polycystic ovarian syndrome in rat. Iranian journal of pharmaceutical research. Iran J Pharm Res. 2015;14(4):1215–33.Suche in Google Scholar
33 Tehrani HG, Allahdadian M, Zarre F, Ranjbar H, Allahdadian F. Effect of green tea on metabolic and hormonal aspect of polycystic ovarian syndrome in overweight and obese women suffering from polycystic ovarian syndrome: A clinical trial. J Educ Health Promot. 2017 May;6(1):36.10.4103/jehp.jehp_67_15Suche in Google Scholar PubMed PubMed Central
34 Mombaini E, Jafarirad S, Husain D, Haghighizadeh MH, Padfar P. The impact of green tea supplementation on anthropometric indices and inflammatory cytokines in women with Polycystic Ovary Syndrome. Phytother Res. 2017 May;31(5):747–54.10.1002/ptr.5795Suche in Google Scholar PubMed
35 Hassanzadeh Bashtian M, Emami SA, Mousavifar N, Esmaily HA, Mahmoudi M, Mohammad Poor AH. Evaluation of Fenugreek (Trigonella foenum-graceum L.), Effects Seeds Extract on Insulin Resistance in Women with Polycystic Ovarian Syndrome. Iran J Pharm Res. 2013;12(2):475–81.Suche in Google Scholar
36 Swaroop A, Jaipuriar AS, Gupta SK, Bagchi M, Kumar P, Preuss HG, et al. Efficacy of a novel fenugreek seed extract (Trigonella foenum-graecum, FurocystTM) in polycystic ovary syndrome (PCOS). Int J Med Sci. 2015 Oct;12(10):825–31.10.7150/ijms.13024Suche in Google Scholar PubMed PubMed Central
37 Kayedpoor P, Mohamadi S, Karimzadeh-Bardei L, Nabiuni M. Anti-inflammatory Effect of Silymarin on Ovarian Immunohistochemical Localization of TNF-α Associated with Systemic Inflammation in Polycystic Ovarian Syndrome. Int J Morphol. 2017 Jun;35(2). https://doi.org/10.4067/S0717-9502201700020005410.4067/S0717-95022017000200054Suche in Google Scholar
38 Nabiuni M, Kayedpoor P, Mohammadi S, Karimzadeh L. Effect of silymarin on estradiol valerate- induced polycystic ovary syndrome. MEDICAL SCIENCES. 2015;25(1):16-26 URL: http://tmuj.iautmu.ac.ir/article-1-900-fa.htmlSuche in Google Scholar
39 Tóth B, Németh D, Soós A, Hegyi P, Pham-Dobor G, Varga O, et al. The Effects of a Fixed Combination of Berberis aristata and Silybum marianum on Dyslipidaemia–A Meta-analysis and Systematic Review. Planta Med. 2019 Nov.10.1055/a-1063-1649Suche in Google Scholar PubMed
40 Liu P, Cui L, Liu B, Liu W, Hayashi T, Mizuno K, et al. Silibinin ameliorates STZ-induced impairment of memory and learning by up- regulating insulin signaling pathway and attenuating apoptosis. Physiol Behav. 2020 Jan;213:112689.10.1016/j.physbeh.2019.112689Suche in Google Scholar PubMed
41 Rafraf M, Zemestani M, Asghari-Jafarabadi M. Effectiveness of chamomile tea on glycemic control and serum lipid profile in patients with type 2 diabetes. J Endocrinol Invest. 2015 Feb;38(2):163–70.10.1007/s40618-014-0170-xSuche in Google Scholar
42 Adib-Hajbaghery M, Mousavi SN. The effects of chamomile extract on sleep quality among elderly people: A clinical trial. Complement Ther Med. 2017 Dec;35:109–14.10.1016/j.ctim.2017.09.010Suche in Google Scholar
43 Farideh ZZ, Bagher M, Ashraf A, Akram A, Kazem M. Effects of chamomile extract on biochemical and clinical parameters in a rat model of polycystic ovary syndrome. J Reprod Infertil. 2010 Oct;11(3):169–74.Suche in Google Scholar
44 Bouterfas K, Mehdadi Z, Elaoufi M, Latreche A, Benchiha W, editors. Antioxidant activity and total phenolic and flavonoids content variations of leaves extracts of white Horehound (Marrubium vulgare Linné) from three geographical origins. Annales pharmaceutiques francaises. Elsevier; 2016. https://doi.org/10.1016/j.pharma.2016.07.00210.1016/j.pharma.2016.07.002Suche in Google Scholar
45 Johari HA, Sharifi ES, Mardan MA, Kafilzadeh FA, Hemayatkhah VA, Kargar HO, et al. The effects of a hydroalcoholic extract of Matricaria chamomilla flower on the pituitary-gonadal axis and ovaries of rats. Int J Endocrinol Metab 2011;9:33010.5812/kowsar.1726913X.1822Suche in Google Scholar
46 Heidary M, Yazdanpanahi Z, Dabbaghmanesh MH, Parsanezhad ME, Emamghoreishi M, Akbarzadeh M. Effect of chamomile capsule on lipid-and hormonal-related parameters among women of reproductive age with polycystic ovary syndrome. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences. 2018;23.10.4103/jrms.JRMS_90_17Suche in Google Scholar
47 Yang LL, Liang YC, Chang CW, Lee WS, Kuo CT, Wang CC, et al. Effects of sphondin, isolated from Heracleum laciniatum, on IL-1beta-induced cyclooxygenase-2 expression in human pulmonary epithelial cells. Life Sci. 2002 Nov;72(2):199–213.10.1016/S0024-3205(02)02173-2Suche in Google Scholar
48 Barzegari Firouzabadi F, Mirhosseini M. Effect of Persian hogweed (Heracleum persicum) on the morphological changes in mice testes and the level of hormone testosterone. RJMS. 2012;19(99):18–24.Suche in Google Scholar
49 Hajhashemi V, Sajjadi SE, Heshmati M. Anti-inflammatory and analgesic properties of Heracleum persicum essential oil and hydroalcoholic extract in animal models. J Ethnopharmacol. 2009 Jul;124(3):475–80.10.1016/j.jep.2009.05.012Suche in Google Scholar PubMed
50 Haj-Husein I, Tukan S, Alkazaleh F. The effect of marjoram (Origanum majorana) tea on the hormonal profile of women with polycystic ovary syndrome: a randomised controlled pilot study. J Hum Nutr Diet. 2015.10.1111/jhn.12290Suche in Google Scholar PubMed
51 Alizadeh F, Azarnia M, Mirabolghasemi G, Karampoor P. Effect of Fruit Heracleum Persicum Extract on Changes in Serum Levels of Sex Hormones in Rats with Polcystic Ovary Syndrome (PCOS). Armaghane danesh. 2015;20(1):31-42Suche in Google Scholar
52 Alkan EE, Celik I. The therapeutics effects and toxic risk of Heracleum persicum Desf. extract on streptozotocin-induced diabetic rats. Toxicol Rep. 2018 Aug;5:919–26.10.1016/j.toxrep.2018.08.004Suche in Google Scholar PubMed PubMed Central
53 Akdoğan M, Tamer MN, Cüre E, Cüre MC, Köroğlu BK, Delibaş N. Effect of spearmint (Mentha spicata Labiatae) teas on androgen levels in women with hirsutism. Phytother Res. 2007 May;21(5):444–7.10.1002/ptr.2074Suche in Google Scholar PubMed
54 Grant P. Spearmint herbal tea has significant anti-androgen effects in polycystic ovarian syndrome. A randomized controlled trial. Phytother Res. 2010 Feb;24(2):186–8.10.1002/ptr.2900Suche in Google Scholar
55 Sadeghi Ataabadi M, Alaee S, Bagheri MJ, Bahmanpoor S. Role of Essential Oil of Mentha Spicata (Spearmint) in Addressing Reverse Hormonal and Folliculogenesis Disturbances in a Polycystic Ovarian Syndrome in a Rat Model. Adv Pharm Bull. 2017 Dec;7(4):651–4.10.15171/apb.2017.078Suche in Google Scholar
56 Ainehchi N, Khaki A, Farshbaf-Khalili A, Hammadeh M, Ouladsahebmadarek E. The Effectiveness of Herbal Mixture Supplements with and without Clomiphene Citrate in Comparison to Clomiphene Citrate on Serum Antioxidants and Glycemic Biomarkers in Women with Polycystic Ovary Syndrome Willing to be Pregnant: A Randomized Clinical Trial. Biomolecules. 2019 Jun;9(6):215.10.3390/biom9060215Suche in Google Scholar
57 Sadrefozalayi S, Farokhi F. Effect of the aqueous extract of Foeniculum vulgare (fennel) on the kidney in experimental PCOS female rats. Avicenna J Phytomed. 2014 Mar;4(2):110–7.Suche in Google Scholar
58 Karampoor P, Azarnia M, Mirabolghasemi G, Alizadeh F. The effect of hydroalcoholic extract of fennel (foeniculum vulgare) seed on serum levels of sexual hormones in female wistar rats with polycystic ovarian syndrome (PCOS). J Arak Univ Med Sci. 2014 Aug;17(5):70–8.Suche in Google Scholar
59 Fozalaee SS, Farokhi F. The Effect of Metformin and Aqueous Extract Foeniculum vulgare (Fennel) on Endometrial Histomorphometry and the Level of Steroid Hormones in Rats with Polycystic Ovary Syndrome. Qom University of Medical Sciences Journal. 2015;8(5).Suche in Google Scholar
60 Mokaberinejad R, Rampisheh Z, Aliasl J, Akhtari E. The comparison of fennel infusion plus dry cupping versus metformin in management of oligomenorrhoea in patients with polycystic ovary syndrome: a randomised clinical trial. J Obstet Gynaecol. 2019 Jul;39(5):652–8.10.1080/01443615.2018.1541232Suche in Google Scholar
61 Mazurek S, Fecka I, Węglińska M, Szostak R. Quantification of active ingredients in Potentilla tormentilla by Raman and infrared spectroscopy. Talanta. 2018 Nov;189:308–14.10.1016/j.talanta.2018.07.012Suche in Google Scholar
62 Vickers NJ. Animal Communication: When I’m Calling You, Will You Answer Too? Curr Biol. 2017 Jul;27(14):R713–5.10.1016/j.cub.2017.05.064Suche in Google Scholar
63 Syiem D, Syngai G, Khup PZ, Khongwir BS, Kharbuli B, Kayang H. Hypoglycemic effects of Potentilla fulgens L in normal and alloxan-induced diabetic mice. J Ethnopharmacol. 2002 Nov;83(1-2):55–61.10.1016/S0378-8741(02)00190-3Suche in Google Scholar
64 Jelodar G, Askari K. Effect of hydroalcoholic extract of Vitex agnus-castus fruit on fertility and estrous cycle in letrozole-induced polycystic ovary (PCOS) in rat. RJMS. 2017;24(156):42– 8.Suche in Google Scholar
65 Wang N, Zhu F, Shen M, Qiu L, Tang M, Xia H, et al. Network pharmacology-based analysis on bioactive anti-diabetic compounds in Potentilla discolor bunge. J Ethnopharmacol. 2019 Sep;241:111905.10.1016/j.jep.2019.111905Suche in Google Scholar PubMed
66 Nazari S, Rameshrad M, Hosseinzadeh H. Toxicological effects of Glycyrrhiza glabra (licorice): a review. Phytother Res. 2017 Nov;31(11):1635–50.10.1002/ptr.5893Suche in Google Scholar PubMed
67 Faghihi G, Iraji F, Abtahi-Naeini B, Saffar B, Saffaei A, Pourazizi M, et al. Complementary Therapies for Idiopathic Hirsutism: Topical Licorice as Promising Option. Evidence-Based Complementary and Alternative Medicine. 2015;2015.10.1155/2015/659041Suche in Google Scholar PubMed PubMed Central
68 Armanini D, Castello R, Scaroni C, Bonanni G, Faccini G, Pellati D, et al. Treatment of polycystic ovary syndrome with spironolactone plus licorice. Eur J Obstet Gynecol Reprod Biol. 2007 Mar;131(1):61–7.10.1016/j.ejogrb.2006.10.013Suche in Google Scholar PubMed
69 Yang H, Kim HJ, Pyun BJ, Lee HW. Licorice ethanol extract improves symptoms of polycytic ovary syndrome in Letrozole-induced female rats. Integr Med Res. 2018 Sep;7(3):264–70.10.1016/j.imr.2018.05.003Suche in Google Scholar PubMed PubMed Central
70 Mokhtari M, Ebrahimpoor MR, Harfsheno S. The effects of alcoholic extract of Marrubum vulgare on hormonal parameters in female rat model of polycystic ovarian syndrome. MEDICAL SCIENCES. 2014;24(2):74–80.Suche in Google Scholar
71 Gavarić A, Vladić J, Ambrus R, Jokić S, Szabó-Révész P, Tomić M, et al. Spray Drying of a Subcritical Extract Using Marrubium vulgare as a Method of Choice for Obtaining High Quality Powder. Pharmaceutics. 2019 Oct;11(10):523.10.3390/pharmaceutics11100523Suche in Google Scholar PubMed PubMed Central
© 2020 Fatemeh Ashkar et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Article
- A new biological definition of life
- Letter to the Editor
- Murburn concept: a paradigm shift in cellular metabolism and physiology
- Mini-Review
- Are the biomedical sciences ready for synthetic biology?
- Research Article
- Acute toxicity of cyanide in aerobic respiration: Theoretical and experimental support for murburn explanation
- The Role of medicinal herbs in treatment of insulin resistance in patients with Polycystic Ovary Syndrome: A literature review
- Regulation of Interferon-γ receptor (IFN-γR) expression in macrophages during Mycobacterium tuberculosis infection
- Chemical composition, antioxidant, anti-inflammatory and antiproliferative activities of the essential oil of Cymbopogon nardus, a plant used in traditional medicine
- Association of TNF-α-308G/A and IL-18 Polymorphisms with risk of HPV infection among sexually active women in Burkina Faso
- Three-dimensional reconstruction of individual helical nano-filament structures from atomic force microscopy topographs
- Polymorphism of MMP1 and MMP3 promoter regions and HR-HPV infection in women from Burkina Faso and Côte d‘Ivoire
- Genotypic distribution of human oncogenic papillomaviruses in sexually active women in Burkina Faso: Central, Central-Eastern and Hauts-Bassins regions
- Insights into Endothelin-3 and Multiple Sclerosis
- A Novel Conceptual Model for the Dual Role of FOF1-ATP Synthase in Cell Life and Cell Death
- Mass spectrometry-based glycomic profiling of the total IgG and total proteome N-glycomes isolated from follicular fluid
- Influence of photobiomodulation and vitamin D on osteoblastic differentiation of human periodontal ligament stem cells and bone-like tissue formation through enzymatic activity and gene expression
- Review Article
- Graphene Oxide: A Promising Material for Regenerative Medicine and Tissue Engineering
- Mini Review
- Alzheimer‘s disease: exploring nature’s ‘medicinal chest’ for new therapeutic agents
- Research Article
- Role of phase partitioning in coordinating DNA damage response: focus on the Apurinic Apyrimidinic Endonuclease 1 interactome
- Dysregulation of epigenetic related genes in Diabetic Trigger finger Patients; preliminary analysis of Patient-Derived Samples
- Calcium Dynamics Regulate Protective Responses and Growth of Staphylococcus aureus in Macrophages
- Erratum
- Erratum to “Polymorphism of MMP1 and MMP3 promoter regions and HR-HPV infection in women from Burkina Faso and Côte d‘Ivoire”
Artikel in diesem Heft
- Research Article
- A new biological definition of life
- Letter to the Editor
- Murburn concept: a paradigm shift in cellular metabolism and physiology
- Mini-Review
- Are the biomedical sciences ready for synthetic biology?
- Research Article
- Acute toxicity of cyanide in aerobic respiration: Theoretical and experimental support for murburn explanation
- The Role of medicinal herbs in treatment of insulin resistance in patients with Polycystic Ovary Syndrome: A literature review
- Regulation of Interferon-γ receptor (IFN-γR) expression in macrophages during Mycobacterium tuberculosis infection
- Chemical composition, antioxidant, anti-inflammatory and antiproliferative activities of the essential oil of Cymbopogon nardus, a plant used in traditional medicine
- Association of TNF-α-308G/A and IL-18 Polymorphisms with risk of HPV infection among sexually active women in Burkina Faso
- Three-dimensional reconstruction of individual helical nano-filament structures from atomic force microscopy topographs
- Polymorphism of MMP1 and MMP3 promoter regions and HR-HPV infection in women from Burkina Faso and Côte d‘Ivoire
- Genotypic distribution of human oncogenic papillomaviruses in sexually active women in Burkina Faso: Central, Central-Eastern and Hauts-Bassins regions
- Insights into Endothelin-3 and Multiple Sclerosis
- A Novel Conceptual Model for the Dual Role of FOF1-ATP Synthase in Cell Life and Cell Death
- Mass spectrometry-based glycomic profiling of the total IgG and total proteome N-glycomes isolated from follicular fluid
- Influence of photobiomodulation and vitamin D on osteoblastic differentiation of human periodontal ligament stem cells and bone-like tissue formation through enzymatic activity and gene expression
- Review Article
- Graphene Oxide: A Promising Material for Regenerative Medicine and Tissue Engineering
- Mini Review
- Alzheimer‘s disease: exploring nature’s ‘medicinal chest’ for new therapeutic agents
- Research Article
- Role of phase partitioning in coordinating DNA damage response: focus on the Apurinic Apyrimidinic Endonuclease 1 interactome
- Dysregulation of epigenetic related genes in Diabetic Trigger finger Patients; preliminary analysis of Patient-Derived Samples
- Calcium Dynamics Regulate Protective Responses and Growth of Staphylococcus aureus in Macrophages
- Erratum
- Erratum to “Polymorphism of MMP1 and MMP3 promoter regions and HR-HPV infection in women from Burkina Faso and Côte d‘Ivoire”


