Abstract
Objectives
The present study challenges chronic Whiplash Associated Disorders (WAD)-subjects to a pharmacological intravenous (i.v.) test with morphine, ketamine, and active placebo (midazolam). The aim was to describe the short-term responses to drugs and the assumed heterogeneity in the patterns of responses. We related the different responder groups to the results from psychometric tests.
Methods
The study includes 95 patients, all with chronic WAD and referred to our departments. They answered a questionnaire including the following psychometric instruments relevant for chronic pain: Beck Depression Inventory, Coping Strategies Questionnaire, Multidimensional Pain Inventory, Life Satisfaction Checklist, SF36 and EuroQol. The subjects also went through sessions with separate infusions of morphine (0.3 mg/kg), ketamine (0.3 mg/kg) and midazolam (0.05 mg/kg). Infusion time was 30 min followed by a 2-h post-infusion assessment. Assessments were made using a Visual Analogue Scale (VAS) for pain intensity and unpleasantness and by statements of per cent pain relieved. A categorical pain rating scale was also used. A positive response was defined as ≥50% decrease of the VAS-level on two consecutive assessment points during the test sessions, anything less was a non response. The placebo responders were defined as those with a positive response to the active placebo infusion.
Results
The tests were completed by 94 subjects and 26% of these were placebo responders. Among the placebo non responders, 47% responded to morphine, 41% to ketamine, 25% to both drugs and 37% to neither morphine nor ketamine (pain intensity assessments). Similar proportions were found in the assessments of pain unpleasantness and per cent pain relieved. Approximately one in four subjects (27%, pain intensity assessment) did not respond to any of the drugs tested. This relatively high proportion of non responders seemed to be worst cases in some aspects of the psychometric tests. Generally, this non responder group had a trend to score worse for most items in the psychometric tests with some reaching significance in a univariate analysis. This result was confirmed in a multivariate context, although the results indicated only small differences between the groups. All three substances showed significant pain relief compared to baseline on all assessment points. On most variables, morphine and ketamine were significantly more effective compared to the active placebo.
Conclusions
There are different subgroups among subjects with chronic WAD with variations in responses to i.v. morphine, ketamine, and midazolam (active placebo). Subjects with chronic WAD who did not respond to any of the drugs tested scored badly in some aspects of the psychometric instruments.
Implications
The present study confirms one aspect of the heterogeneity in the population with chronic WAD. The study does not elucidate precise pain mechanisms but taken together with other studies exploring other aspects, it stresses the importance of individualizing the assessment and treatment of subjects with chronic WAD. A common clinical experience is that depression, anxiety and maladaptive coping strategies often are obstacles for successful medical treatment of chronic pain. The present study supports this experience and emphasizes the need for assessment of psychometric variables when planning the treatment of chronic WAD.
1 Introduction
A sudden acceleration or deceleration due to impact can lead to a whiplash trauma and cause acute symptoms (pain and stiffness in the neck, often described as acute Whiplash Associated Disorder, WAD) and a significant subgroup develops chronic pain (chronic WAD) [1,2,3].
Apart from cases where the trauma causes verifiable lesions in the musculoskeletal or neural structures, most patients with chronic WAD do not present with such signs. In the majority of patients with chronic WAD, the pathogenesis of the persistent pain is poorly understood as is evident by the different views presented in the literature [4,5,6].
When describing and analysing chronic WAD according to a biopsychosocial model [7,8], different studies put various weight on different parts along the “bio”, “psycho”, and “social” axes. For example, emphasising the “bio” side, some researchers stress lesions in the zygapophyseal joints [9]; emphasising the “psycho” and “social” sides, some researchers stress factors such as anxiety, coping, and insurance issues, often as interconnected factors [10].
A possible approach is to view patients with chronic WAD as a heterogeneous group even though the basic concept is the bio-psycho-social model. Support for this can be found in studies where responses to pharmacological interventions have been investigated [11]. Studies focusing on the “psychosocial side” of the model also show heterogeneity among patients with chronic WAD [12,13,14,15].
One way to investigate chronic pain is to study responses to pharmacological agents with known targets. This approach has been done for different patient groups including patients with chronic WAD [11]. Most studies deal with opioid agents and/or ketamine and assume the former acts on the central pain processing via the μ-receptor and the latter acts as an antagonist on the NMDA-receptor. Clearly, NMDA-receptors play an important role in central sensitization [16,17]. Several studies conclude that central hyperexcitability (sensitization) and/or disinhibiton of the somatosensory system may play a part in the pathogenesis of pain in chronic WAD [18,19,20,21,22], a conclusion that might explain why patients without detectable or minimal nociceptive input still perceive debilitating pain.
However, the pharmacological studies so far have been relatively small. Most studies incorporate a test with a placebo agent, commonly physiological saline, but sometimes benzodiazepines have been used as an active placebo [23,24] in order to simulate the sedative effects of other agents presuming no analgesic activity.
In the present study patients with chronic WAD were examined regarding the responses to different i.v. pharmacological challenges: morphine, ketamine, and “active” placebo (midazolam). The patients also answered a questionnaire including several psychometric instruments relevant for chronic pain.
The aim of the study was to describe the short term responses to drugs and the assumed heterogeneity in response patterns. Furthermore, we analysed whether the outcomes of the pharmacological challenges correlated with the psychometric results.
2 Patients and methods
2.1 Patients
Between May 2001 and October 2008, 95 subjects with chronic WAD were recruited from patients referred to the Pain Unit, Operation and Intensive Care Clinic, County Hospital Ryhov, Jönköping, Sweden and to the Pain and Rehabilitation Centre, University Hospital, Linköping, Sweden. For background data see Table 1. The study was conducted in accordance with the Declaration of Helsiniki and approved by the local Ethics Committee (00-283). All participants gave written informed consent.
Background data of the recruited subjects (n = 95).
| Area | Variables | |
|---|---|---|
| Gender/Age | Male (n (%)) | 39 (41%) |
| Mean age (years (SD)) | 36.8 (9.8) | |
| Female (n (%)) | 56 (59%) | |
| Mean age (years (SD)) | 35.4 (9.5) | |
| All subjects (n) | 95 | |
| Mean age (years (SD)) | 36.0 (9.6) | |
| Time from impact (mean (SD)) | Months | 28 (15) |
| Type of impact (n (%)) | From the rear | 41 (43%) |
| Obliquely rear | 4 (4%) | |
| From the side | 6 (6%) | |
| Other | 44 (46%) | |
| Patient position at impact (n (%)) | Driver | 69 (73%) |
| Passenger front seat | 13 (14%) | |
| Passenger back seat | 7 (7%) | |
| Other type of vehicle | 6 (6%) | |
| Time to symptoms (n (%)) | Immediately | 36 (38%) |
| First 24 h | 44 (46%) | |
| First week | 14 (15%) | |
| Use of analgesics (n (%)) | ||
| NSAID/Acetaminophen | None | 19 (20%) |
| Sporadic | 22 (23%) | |
| Daily | 42 (44%) | |
| Weak opioids | None | 40 (42%) |
| Sporadic | 17 (18%) | |
| Daily | 26 (27%) | |
| Strong opioids | None | 83 (87%) |
| Missing data | 12 (13%) | |
2.1.1 Inclusion criteria
The subjects had a well-documented whiplash trauma or a whiplash-like accident with a minimum of six months and maximum of five years before inclusion (i.e., WAD grades II–III). They had a persistent pain in the neck – with or without spread of the pain to the head, shoulder, and arm regions – with a pain intensity of ≥40 mm on a 100 mm VAS. The minimum age was set to 18 years.
2.1.2 Exclusion criteria
All subjects were given a MRI of the cervical spine. If affections of medulla and/or nerve roots corresponding to neurological signs in the periphery were discovered, these subjects were excluded from the study. If the subjects had neuroorthopedic surgery done on the cervical spine, they were excluded. Subjects were excluded if they had a significant chronic pain problem before the trauma. Subjects with generalized pain after the trauma were also excluded. That is, subjects were only included if their pain was mainly localized to the neck with or without spread of the pain to the head, shoulder, or arm regions. Subjects were excluded if they had drug addiction problems, exhibited psychotic behaviour, or were pregnant.
2.1.3 Baseline screening
All subjects answered a comprehensive questionnaire including psychometric instruments relevant for chronic pain. The questionnaire asked the subjects whether they had been using analgesics the previous six months. If they answered “yes”, they were asked for type of analgesic and whether they were using it sporadically or daily (Table 1).
2.1.4 Study context
The present study is a part of a larger project that explores the frequency of cervical zygapophyseal joints as a source for the persistent pain in subjects with chronic WAD. This larger project also tested the efficacy of radiofrequency neurotomy of the innervation of the joints.
2.2 Methods
2.2.1 Pharmacological challenge
2.2.1.1 Procedure
Before the test sessions, subjects were instructed not to take any analgesics at least 8 h before the test. The subjects were placed in a silent room and arrangements were made to achieve a relaxed supine position in a bed. An intravenous cannula was inserted, usually on the back of a hand. Using a syringe pump (Braun Perfusor®, Germany), we infused the actual drug (morphine, ketamine, or active placebo). Infusion time was 30 min. Assessments were made before and after placement of the i.v. cannula as well as at 10, 20, and 30 min after the infusion started and 10, 20, 30, 45, 60, and 120 min after the infusion stopped.
The subjects marked a 100-mm Visual Analogue Scale (VAS) with the endpoints labelled “no pain” and “worst imaginable pain” to indicate intensity of the local pain in the neck. In addition, the subjects were asked to rate the unpleasantness of the pain on a VAS. The subjects also rated their pain on a categorical scale (0 = no pain, 1 = light pain, 2 = moderate pain, 3 = serious pain, 4 = unbearable pain). Finally, the subjects stated in percentage the perceived degree of pain relief in relation to the baseline assessment (see below) at the different time points.
2.2.1.2 Drugs and dosage
All subjects received all three substances in three different sessions separated by a minimum of one week. The substances were delivered in a randomized sequence prepared by the hospital pharmacy. A person not involved in delivering the infusion or making the assessments prepared the syringe with the actual drug according to the randomization. The subject and the nurse responsible for the infusion and the assessments were unaware of the actual drug.
The drugs administered during a 30 min infusion time were as follows:
morphine hydrochloride (0.3 mg/kg, Morfin®, Meda);
ketamine hydrochloride (0.3 mg/kg, Ketalar®, Pfizer);
midazolam hydrochloride (active placebo, 0.05 mg/kg, Midazolam®, Actavis).
Dosages were chosen with references to earlier studies [11,24,25,26].
2.2.1.3 Side-effects
At all assessment points, the subjects were asked to note any side-effects and to classify them in the following categories: (1) sedation/tiredness, (2) dreams/hallucinations, (3) dizziness, (4) nausea/vomiting, (5) itching, (6) paresthesias/numbness, and (7) other. They were also asked to estimate the intensity of any side-effects on a 100 mm VAS with endpoints “no side-effect” and “worst imaginable side-effect”.
2.2.1.4 Monitoring
For safety reasons, heart rate, blood pressure, respiratory rates, and pulse oxymetry were continuously monitored during the test sessions. The drug delivery and all the assessments were made by anaesthetic nurses who were welltrained in resuscitation methods.
2.2.2 Psychometric instruments
The study used the validated Swedish versions of the instruments listed below.
2.2.2.1 BDI – Beck Depression Inventory
The Beck Depression Inventory evaluates 21 different aspects of depressive symptoms into a scale ranging between 0 and 63 [27]. For psychiatric patients a total score less than 14 indicates minimal or no depression, 14–19 a mild depression, 20–28 a moderate, and >28 a severe depression [28]. A screening cut-off point of 10 has been used for medical patients [12]. The BDI is considered as an established and well-researched scale [27,28,29].
2.2.2.2 CSQ – Coping Strategies Questionnaire
CSQ measures the way patients cope with pain. The original version included eight types of coping strategies: diverting attention, re-interpreting pain sensations, coping self-statements, ignoring pain sensations, praying and hoping, catastrophizing, increased behavioural activities, and pain behaviour. Each strategy is measured according to its frequency of use ranging between never (0) and always (6) with a maximum score of 36 for each strategy. Two additional questions assess the perceived control of and ability to minimize pain [30]. The Swedish version, which was used in this study, excludes the last type of strategy (pain behaviour) [31].
2.2.2.3 MPI – Multidimensional Pain Inventory
The West Haven-Yale Multidimensional Pain Inventory – (WHY)MPI – is a 61-item self-report questionnaire measuring psychosocial, cognitive, and behavioural effects of chronic pain [32]. It has three sections. Part 1 consists of five scales: Pain severity; Interference (pain related interference in everyday life); Perceived Life Control; Affective Distress; and Social Support (perceived support from a spouse or significant others). Part 2 assesses the perception of responses from significant others to displays of pain and suffering and consists of three scales: Punishing Responses, Solicitous Responses, and Distracting Responses. Part 3 measures the extent to which patients engage in various activities and these four scales are combined in a composite scale labelled General Activity index. We only used the General Activity index of the items in Section 3. We used the Swedish Language Version (MPI-S) [33].
2.2.2.4 Life Satisfaction Checklist according to Fugl-Meyer et al. (LiSat-11)
LiSat-11 estimates life satisfaction in general as well as in eight specific domains: vocational situation, financial situation, leisure situation, contact with friends and acquaintances, sexual life, Activities of Daily Life, family life, and partnership. Two additional variables estimate the satisfaction with physical and mental health [34]. Each item has six possible answers ranging from 1 (very dissatisfying) to 6 (very satisfying).
2.2.2.5 The SF-36 Health Survey (Swedish version)
SF-36 measures different dimensions of health, including levels of well-being and personal evaluation of health. The instrument consists of 36 questions covering eight items or dimensions: Physical functioning; Role limitations due to physical pain; Bodily pain; General health; Vitality; Social functioning; Role limitations due to emotional problems; and Mental health. Each item score is coded, summed, and transformed to a standardized scale calculated from a specific score algorithm ranging from 0 to 100, worst and best possible health state, respectively [35].
2.2.2.6 EuroQol
The EuroQol instrument measures the subject’s perceived state of health [36,37] using five dimensions – mobility, self-care, usual activities, pain/discomfort, and anxiety/depression – coded 1–3 (no problems, some problems, and severe problems). The second part of the instrument concerns a self-estimation of today’s health according to a 100-point vertical scale, a “thermometer”-style scale (EQ-VAS). The endpoints are defined and high values indicate good health and low values indicate bad health.
2.3 Definitions, calculations and statistics
2.3.1 Pharmacological challenges
The mean of the first two assessments (i.e., before and after insertion of the i.v. cannula but before start of the infusion) was considered the baseline assessment. We classified the subjects as placebo responders, responders, and non responders:
Placebo response criterion: ≥50% VAS decrease (compared to base-line) of the local pain intensity on two consecutive assessment points during the placebo test session.
Response criteria: not a placebo responder and ≥50% VAS decrease (compared to baseline) of the local pain intensity on two consecutive assessment points during test session of active drug.
Non response criteria: none of the above.
Similar classifications were made regarding the assessments of pain unpleasantness and percentage pain relief.
A global response was defined as a response to both morphine and ketamine and a global non response as a response to neither morphine nor ketamine. Placebo responders and subjects with incomplete data for deciding a placebo response were excluded when calculating the proportions for morphine, ketamine, global responders, and global non responders.
The following variables were also determined to compare the three substances:
Area under curve (i.e., the VAS × time (mm min)) for the rating of local pain intensity and unpleasantness.
Mean pain intensity and unpleasantness decreases (the difference between baseline and the mean of all other assessment points).
Mean percentage pain relieve (the mean of all values except the two assessments before start of infusion (i.e., baseline)).
Efficacy (maximum difference between baseline and a single assessment point) for the rating of local pain intensity, unpleasantness and pain on the categorical scale.
Efficacy regarding the rating of percentage pain relief (maximum value on a single assessment point).
2.3.2 Psychometric tests
The mean values for the different scales of all the psychometric instruments used were calculated.
2.3.3 Correlations pharmacological challenges and psychometric tests
The responses to the pharmacological challenges were coded into three groups according to the assessments of pain intensity during the test sessions. A positive response was defined as ≥50% VAS decrease (compared to baseline) of pain intensity on two consecutive assessment points:
Placebo responders – positive responders to the placebo infusion;
Active responders – not a placebo responder and positive responder to morphine and/or ketamine;
Non responders – neither a placebo responder nor an active responder.
The mean values of the different scales of psychometric instruments were calculated for the different responder groups and then compared.
2.3.4 Statistical tests
Analyses were made using SPSS for Windows (version 19.0 SPSS Inc. Chicago, Illinois, USA). Multivariate analyses were performed using the SIMCA-P+, version 12.0 (Umetrics Inc.). Results in the text and tables are generally given as mean values ± one standard deviation (SD). Analysis of variance for repeated measures (Friedman’s test) was used followed by two-tailed comparisons (Wilcoxon signed ranks test) to determine which time points differed from the baseline or which drugs differed from the placebo. The Wilcoxon signed ranks test was used for other paired comparisons. Kruskal–Wallis and The Mann–Whitney U test were used for non-paired group comparisons.
When investigating the correlations between the different variables, the Principal component analysis (PCA) and Partial least squares or projection to latent structures (PLS) were applied. Principal component analysis (PCA) using SIMCA-P+ was used to extract and display systemic variation in the data matrix. PCA can be viewed as a multivariate correlation analysis. Variables loading on the same component are correlated and variables with high loadings but with different signs are negatively correlated. A component can be considered as a group of intercorrelated variables. Variables with high absolute loadings and that had a 95% confidence interval not equal to zero were considered significant. Significant variables with high loadings (positive or negative) are more important for the component under consideration than variables with lower absolute loading. A component consists of a vector of numerical values between −1 and 1 (referred to as loadings) and obtained significant components are uncorrelated. Variables that have high loadings (with positive or negative sign) on the same component are intercorrelated. Items with high loadings (ignoring the sign) are considered to be of large or moderate importance for the component under consideration. A cross validation technique was used to identify nontrivial components (p). This method keeps part of the data out from the model development to assess the predictive power of the model and was used to test the significance of the components. The obtained components are, per definition, not correlated and are arranged in decreasing order with respect to explained variation. R2 describes the goodness of fit - the fraction of sum of squares of all the variables explained by a principal component.
Partial least squares or projection to latent structures (PLS) was used for the multivariate regression analysis of group membership - placebo responders, active responders, and non responders (i.e., PLS-discriminant analysis; PLS-DA) - using the psychological instruments and pain-related variables as regressors. The VIP variable (variable influence on projection) indicates the relevance of each X-variable pooled over all dimensions and Y-variables – the group of variables that best explain Y. VIP ≥0.8 was considered significant. Coefficients (PLS scaled and centred regression coefficients) were used to note the direction of the relationship (positive or negative). Multiple linear regression (MLR) could have been an alternative when regressing group membership, but it assumes that the regressor (X) variables are independent. If such multi-colinearity occurs among the X-variables, the regression coefficients become unstable and their interpretability breaks down. MLR also assumes that a high subject-to-variables ratio is present (5–10). Such requirements are not required for PCA or PLS; in fact, PLS can handle ratios lower than 1.0. In contrast to MLR, PLS also can handle several Y-variables simultaneously.
Outliers were identified using the two powerful methods available in SIMCA-P+: (1) score plots in combination with Hotelling’s T2 (identifies strong outliers) and (2) distance to model in X-space (identifies moderate outliers).
A p-value ≤ 0.05 was considered to be statistically significant in all tests.
3 Results
3.1 Pharmacological challenges
3.1.1 Drop-outs
One subject withdrew from the study (the informed consent) before the tests were carried out and is not included in the analysis.
3.1.2 Proportions of placebo responders, responders, and non responders
3.1.2.1 Placebo responders
Depending on the outcome/assessment variable selected (local pain intensity, pain unpleasantness, or percentage pain relief), the proportion of placebo responders varied between 26 and 31% (Table 2). Among the 24 placebo responders (according to the assessment of pain intensity), 15 (63%) also responded to morphine, 17 (71%) responded to ketamine, and 11 (46%) responded to both drugs. Only two subjects selectively responded to the placebo.
Number (n) and percentage (%) of responders to placebo, morphine, and ketamine together with global responders and global non responders for three different assessment modalities (local pain intensity, pain unpleasantness and percentage pain relief).
| Assessment | Response | Placebo | Morphine | Ketamine | Globalresponders | Global non responders | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| n | % | n | %[a] | n | %[a] | n | %[a] | n | %[a] | ||
| Local pain intensity | Responder | 24 | 26 | 32 | 47 | 28 | 41 | 17 | 25 | ||
| Non responder | 69 | 74 | 36 | 53 | 41 | 59 | 25 | 37 | |||
| Incomplete data | 1 | 1 | 1 | ||||||||
| Pain unpleasantness | Responder | 29 | 31 | 29 | 46 | 28 | 44 | 15 | 23 | ||
| Non responder | 64 | 69 | 34 | 54 | 36 | 56 | 21 | 33 | |||
| Incomplete data | 1 | 1 | 1 | ||||||||
| Per cent pain relief | Responder | 25 | 27 | 27 | 41 | 30 | 45 | 17 | 25 | ||
| Non responder | 67 | 73 | 39 | 59 | 37 | 55 | 26 | 39 | |||
| Incomplete data | 2 | 1 | 1 | ||||||||
3.1.2.2 Responders and non responders to morphine and ketamine
Among the placebo non responders, morphine responders varied between 41 and 47% and ketamine responders between 41 and 45% depending on the assessment variable chosen. Global responders varied between 23 and 25% and global non responders varied between 33 and 39% (Table 2). 15–22% responded only to morphine and 16–20% only to ketamine.
To summarize according to the assessments of pain intensity, there were 24 placebo responders (26%), 43 active responders (responding to morphine and/or ketamine but not to placebo, 47%) and 25 non responders (not responding to any drug, 27%). Three subjects had incomplete data, so they were not included in these calculations.
3.1.3 Assessment of pain intensity, unpleasantness, and per cent pain relief over time for the different drugs
There were no differences in the means of the baseline assessments (i.e., before the drug administration) of pain intensity and unpleasantness between the three sessions (Table 3). The non responders had a significantly higher baseline assessment regarding pain unpleasantness (p = 0.047) than the other responder groups. A similar tendency (non-significant; p = 0.080) was noted for pain intensity.
Pain intensity and unpleasantness at baseline in patients given different drugs.
| Drug | Pain intensity VAS Mean (SD) | Pain unpleasantness VAS Mean (SD) |
|---|---|---|
| Morphine | 51.9 (21.3) | 48.2 (21.1) |
| Ketamine | 50.9 (22.9) | 50.4 (22.7) |
| Placebo | 52.6 (21.0) | 48.9 (23.1) |
All three substances significantly reduced pain intensity and pain unpleasantness compared to baseline on all assessment points during the test sessions (data not shown). In the initial parts of the test sessions, ketamine reduced pain intensity significantly more than both the placebo and morphine. Morphine was more effective than the placebo on most of the assessment points and in the later parts of the test sessions morphine was more effective than ketamine. A similar pattern was noted for the assessments of percentage pain relief.
Morphine was significantly more effective than the placebo regarding VAS-area under curve for pain intensity and unpleasantness and regarding mean decrease in pain intensity and in mean per cent pain relief. Morphine also showed a tendency (p = 0.055) to be better than the placebo regarding mean decrease in pain unpleasantness (Table 4).
Mean values (±one SD) of VAS-area under curve and mean decrease (baseline vs. the mean of all assessments during the test session) in pain intensity and in unpleasantness together with mean per cent pain relief for the three drugs.
| Drug | VAS area under curve (mm min) | Mean decrease | Mean per cent pain relief Mean (SD) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Pain intensity Mean (SD) | Unpleasantness Mean (SD) | Pain intensity Mean (SD) | Unpleasantness Mean (SD) | ||
| Morphine | 5105[**] (3827) | 4624[*] (3704) | 16.6[**] (17.2) | 15.4 (17.5) | 36.1[***] (32.3) |
| Ketamine | 5863 (3386) | 5073 (3370) | 13.9 (17.1) | 16.9[**] (19.1) | 33.7[***] (28.0) |
| Placebo | 6228 (2901) | 5417 (3035) | 10.6 (12.2) | 11.7 (14.7) | 20.8 (21.5) |
Ketamine was significantly more effective than the placebo for mean decrease in pain unpleasantness and in mean per cent pain relief. For the VAS-area under curve and mean decrease in pain intensity, ketamine showed no difference compared to the placebo (Table 4).
Morphine showed significance versus ketamine for VAS-area under curve for pain intensity (p = 0.018) and a tendency to significance for VAS-area under curve for pain unpleasantness (p = 0.062). There was also a tendency for morphine to be more effective than ketamine regarding mean VAS decrease for pain intensity (p = 0.068).
3.1.4 Efficacy
Morphine and ketamine showed significant differences in efficacy when compared to placebo except for morphine’s ability to decrease pain unpleasantness (Table 5). Ketamine was significantly more effective than morphine regarding the efficacy for pain unpleasantness (p = 0.049) and per cent pain relief (p = 0.037).
Efficacy (mean values (±one SD)) in pain intensity, unpleasantness, categorical rating of pain and percentage pain relief.
| Pain intensity Mean (SD) | Unpleasantness Mean (SD) | Categorical rating Mean (SD) | Per cent pain relief Mean (SD) | |
|---|---|---|---|---|
| Morphine | 26.5[**] (19.7) | 25.8 (20.1) | 0.96[*] (0.84) | 48.5[**] (38.8) |
| Ketamine | 28.4[**] (21.2) | 31.3[***] (22.7) | 1.16[***] (0.92) | 58.5[***] (38.2) |
| Placebo | 20.6 (17.6) | 23.1 (19.5) | 0.69 (0.74) | 35.5 (32.6) |
3.1.5 Side-effects
Sedation/tiredness (all three drugs, most prominent for midazolam), dizziness (morphine and ketamine), nausea/vomiting (morphine), and paresthesias/numbness (ketamine) dominated among reported side-effects (Table 6). In two subjects who received morphine and in four subjects who received ketamine, the infusions were discontinued because of side effects.
Number and percentage (%) of subjects with registered side-effects and with an intensity of more than 50mm on a 100mm VAS.
| Side-effect | Drug | ||
|---|---|---|---|
|
|
|||
| Morphine n (%) | Ketamine n (%) | Placebo (midazolam) n (%) | |
| Sedation/tiredness | 33 (35) | 21 (22) | 45 (48) |
| Dreams/hallucinations | 1 (1) | 8 (9) | 1 (1) |
| Dizziness | 25 (27) | 46 (49) | 4 (4) |
| Nausea/vomiting | 23 (24) | 8 (9) | 0 (0) |
| Itching | 11 (12) | 2 (2) | 1 (1) |
| Paresthesias/numbness | 10 (11) | 20 (21) | 0 (0) |
3.1.6 Influence of pre-study use of analgesics
Some data were missing (13%, Table 1) regarding the pre-study use of analgesics.
3.1.6.1 NSAID (Non-Steroid Anti-infiammatory Drug)/acetaminophen
There were no differences in the proportions of the responder groups depending on variations in the pre-study use of NSAID and/or acetaminophen. Gender proportions, age, duration of symptoms, and baseline assessments of pain intensity and unpleasantness did not significantly variate with the use of these drugs (data not shown).
Similarly, there were no significant influences on the effect of morphine, ketamine, and midazolam regarding VAS-area under curve and efficacy (data not shown). With respect to the mean VAS decrease during the test sessions, those reporting no pre-study use of NSAID/acetaminophen showed a significantly lower value for morphine (p = 0.044). For ketamine and midazolam, there were no differences.
3.1.6.2 Weak opioids (tramadol, dextropropoxyphen, codeine)
There were no differences in the proportions of the responder groups depending on variations in the pre-study use of weak opioids. Similarly, there were no influence on gender, age, duration of symptoms and baseline assessments of pain unpleasantness (data not shown). Those reporting daily use of weak opioids had a significantly higher baseline assessment of pain intensity (p = 0.028).
The pre-study use of weak opioids did not affect the mean VAS decrease or efficacy of the three substances used in the pharmacological tests. Those reporting daily use of weak opioids had a larger VAS area under curve in the ketamine test (p = 0.033) but not in the morphine or midazolam tests.
3.1.6.3 Strong opioids
No subject in the study sample reported any pre-study use of strong opioids.
3.1.7 Influence of the sequence of infusions
We compared the groups where the placebo infusions were given in the first session (n = 32), second session (n = 30), and third session (n = 31). The analysis showed no significant differences with respect to gender, age, duration of symptoms, and baseline assessments of pain intensity and unpleasantness (data not shown). VAS area under curve, mean VAS decrease, efficacy, and mean and maximum per cent pain relief for all three substances did not significantly depend on where the placebo infusions were placed in the sequence. There were no significant impacts of the placebo sequence on the proportions of the responder groups (data not shown).
Similar analyses were made for morphine and ketamine where we grouped the whole sample according to where the drugs were placed in the sequence of infusions. As with the placebo infusion, morphine and ketamine did not significantly influence the main results of the study (data not shown).
3.2 Group belonging in the pharmacological challenges versus pain intensities, pain unpleasantness, and psychometric tests
There were few and relatively small differences between the three groups of subjects (i.e., placebo responders, active responders, and non responders). We found significant group differences for the following variables:
Pain unpleasantness at baseline (Placebo: 44 ± 22; Active responders: 46 ± 17; Non responders: 58 ± 23; p = 0.047);
CSQ – Coping self statements (Placebo: 19 ± 5.6; Active responders: 18.7 ± 6.6; Non responders: 14.9 ± 6.9; p = 0.045);
CSQ – Increased activities (Placebo 14.2 ± 4.8; Active responders: 14.3 ± 6.1; Non responders: 10.2 ± 5.0; p = 0.009);
CSQ – Ability to decrease pain (Placebo: 3.1 ± 0.9; Active responders: 2.7 ± 0.9; Non responders: 2.2 ± 0.9; p = 0.006);
General activity index of MPI (Placebo: 3.1 ± 0.8; Active responders: 2.6 ± 0.6; Non responders: 2.3 ± 0.8; p = 0.003); and
LiSAT-11 – Life as a whole (Placebo: 4.1 ± 1.5; Active responders: 3.8 ± 1.3; Non responders: 3.3 ± 1.1; p = 0.046).
Hence, the non responder group displayed the worse situation for these significant aspects. Generally, similar trends were noted for most items of the non-significant variables (data not shown). Hence, no significant group differences were found for BDI, for six of nine subscales of the CSQ, for eight of nine subscales of the MPI, and for ten of eleven items in the LiSAT-11. None of the subscales of SF36 and EuroQol showed any significant differences.
A PCA was made to confirm the significant results and the trends mentioned above. One multivariate outlier was identified and excluded from the subsequent analysis. Placebo response, Active response, and Non response were coded as dummy variables (1 = fulfilled the criteria and 0 = did not fulfil the criteria) and were included in the analysis together with pain-related variables and the different psychometric instruments. PCA identified four significant components (p1–4) (groups of intercorrelated variables) that together explained 44% of the variation in the data matrix (R2 = 0.44). Two components (p1 and p2) included one of the three dummy variables (Fig. 1a and b). According to p1 (Fig. 1a), pain intensity and unpleasantness, CSQ-catastrophizing, BDI, and the majority of the variables of the first section of MPI and EQ5D (except the health scale) were positively intercorrelated (in Fig. 1a with negative loadings). In addition, the Non response dummy variable intercorrelated positively weakly and significantly to this group of variables. This group of variables correlated negatively with the absolute majority of variables of LiSAT-11 and SF36. In other words, non response was (weakly) associated with high pain intensity and other symptoms and with low life satisfaction and health. Hence, the patterns of loadings in Fig. 1a confirmed the non-significant trends observed when separately scrutinizing each item of the pain variables and the psychometric instruments.
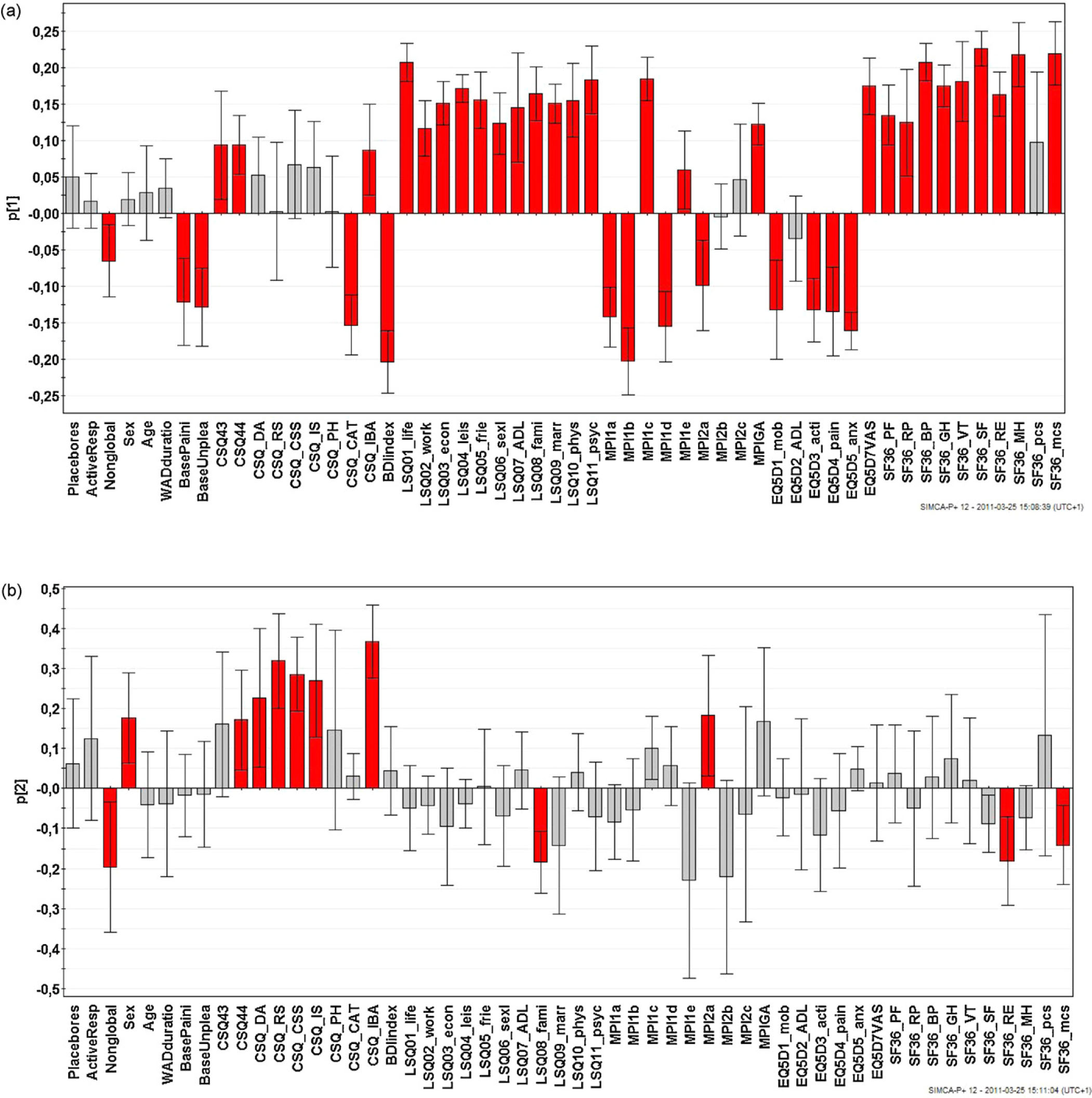
a: Loadings of the first component. The columns of variables with significant loadings are red. The non response dummy variable (labelled as Nonglobal), pain intensity and unpleasantness, CSQ-catastrophizing, BDI and the majority of the variables of the first section of MPI and of EQ5D were positively intercorrelated (all had negative loadings). These variables correlated negatively with the absolute majority of variables of LiSAT-11 and SF36 (i.e., these had positive loadings). Hence, non response was associated with high pain intensity and other symptoms and with low life satisfaction and health. For abbreviations, see Appendix A. b: Loadings of the second component. The columns of variables with significant loadings are red. The non response dummy variable (labelled as Nonglobal) mainly showed a negative intercorrelation (i.e., different signs) with certain aspects of CSQ (but not CSQ-catastrophizing; see Fig. 1a). For abbreviations, see Appendix A.
The Non response dummy variable showed a higher absolute loading on the second component p2 (Fig. 1b). This component mainly showed a negative intercorrelation between certain aspects of CSQ and Non response, generally confirming in a multivariate context the significant differences reported from the univariate analyses above.
It was not possible to predict group belonging (Placebo responders, Active responders, and Non responders) using PLS-DA. Hence, the above mentioned significant differences were relatively small.
4 Discussion
4.1 The main results
This study confirms the heterogeneity of a group of subjects with chronic WAD when it comes to responses to pharmacological challenges with morphine, ketamine, and midazolam (active placebo).
The study displays a large group of subjects not responding to any of the drugs used in the tests (Non responders, 27%).
The Non responder group seems to be the worst cases on a group level in some aspects of the psychometric instruments used in the study.
4.2 The relevance of pharmacological challenge of chronic pain states
4.2.1 Different purposes of intravenous pharmacological tests
Our study displays heterogeneity in a group of subjects with chronic WAD. With the method and definitions used, we are able to draw some conclusions about the heterogeneity, but we can neither make any predictions on the outcome of long-term therapy with the drugs tested nor conclusions regarding specific pain processing mechanisms.
Since the early 1990s, the literature has seen an expansion of reports concerning intravenous (i.v.) pharmacological tests including a multitude of drugs and different pain states. The purposes of the tests differ. Some studies try to elucidate whether results from an i.v. drug test can predict the outcome of a long-term drug therapy [23,38,39,40,41,42]. Other studies use pharmacological tests to predict the outcome of surgical or other invasive procedures [43,44]. A third focus is to use i.v. drug tests, presuming known targets of the drugs, to analyse pain pathophysiology in experimental pain [24,45,46,47,48] as well as in clinical pain states [25,49,50,51,52] and in combinations (induced experimental pain in subjects with a chronic pain state) [11,26,53].
A systematic review found weak or no evidence for the utility of i.v. infusion tests [54]. However, this review focused on evidence for the tests ability to predict the outcome of a long-term drug therapy. The shortcomings of these studies were related to more than just methodological fiaws. For instance, some drugs used for i.v. tests do not have an analogous available drug for long-term therapy or there might be side-effects not seen in the i.v. test but seen after some time during the long-term treatment.
4.2.2 Cutoff points
One aspect for consideration is where to set the cutoff point for the dichotomy response and non response to a pharmacological challenge. We chose the 50% paradigm, i.e., a response was defined as a ≥50% pain decrease on two consecutive assessments during the test session compared to baseline; anything less was considered a non response. Farrar et al. studied the subject by reanalysing former clinical trials [55,56]. They tried to define the degree of change in absolute pain rating scales (e.g., an 11-point pain intensity rating scale, NRS) that best corresponded to significant clinical improvement estimated by some global assessment (by the patient). They found, on average, that a reduction of approximately two points (on an 11-point NRS) or a reduction of approximately 30% represented a significant clinical difference. However, these studies concerned the cutoff point for significant clinical improvement in long-term treatment of chronic pain. Our study has another target: trying to display different patterns of responses to an i.v. pharmacological challenge in a group with a specified chronic pain state. Cohen et al. designed a cutoff point to a 67% pain relief, which gave the best prediction by an i.v. ketamine test for the outcome of an oral dextromethorphan treatment in neuropathic pain [39]. They used the same cutoff point in similar studies regarding fibromyalgia patients [40] and in opioid-exposed patients with persistent pain [41]. Again, this cutoff point was designed for another purpose than what is relevant for our study. To our knowledge, no similar datadriven studies exist that define the cutoff points for i.v. drug tests to elucidate patterns of responses on a group level. We find it reasonable to believe that the 30% cutoff point might be too weak and the 67% cutoff too strong for our purpose. Hence, we chose the 50% paradigm although it is an arbitrary designation to some extent.
4.2.3 Pain intensity and pain unpleasantness
In our study, there were small differences (on a group level) between the two assessment modalities – pain intensity and pain unpleasantness – both regarding the baseline assessments and the proportions of different responses to the substances. Hence, it can be questioned whether it is possible to distinguish between the intensity/sensory and the unpleasantness/affective factors in the perception of pain. An alternative is that these two factors are separable but strongly correlated. Traditionally, the pain experience has been analysed in the following three ways: sensory/discriminative, affective/motivational, and cognitive/evaluative. Fields argues that there are primary and secondary unpleasantnesses [57]. The first is stimulus bound and hence should be analysed as a sensory/discriminative component of pain. In our understanding, one could, for example, ask whether there is any pain that is not unpleasant. The secondary unpleasantness “[...] is a higher level process to which contextual features contribute powerfully resulting in an emotional experience[...]” [57]. The question can be raised regarding what kind of unpleasantness we measure, especially in a pharmacological short-term drug test. We also used a placebo substance (benzodiazepine) that could have influenced the affective pain component.
4.2.4 The possible influence of pre-study use of analgesics
Unfortunately, data were missing regarding the use of pre-study analgesics. However, we did not find any significant influence on the major results of the study. No subject in the sample reported any pre-study use of strong opioids, which, of course, would have influenced the analysis.
We find it difficult to explain why those reporting no use of NSAID and/or acetaminophen have a significant lower mean VAS decrease during the morphine test (p = 0.044). Without any rationale we interpret this as a random result.
Those subjects reporting a daily pre-study use of weak opioids had a significantly higher baseline assessment of pain intensity (p = 0.028). As the subjects were instructed not to take any analgesics for at least 8 h before the test sessions, this result might refiect the absence of their daily used analgesic at the baseline assessment. Together with the short-acting pharmacokinetic profile of ketamine, this could also explain why subjects who used weak opioids on a daily basis had a larger VAS area under curve during the ketamine infusion.
4.2.5 Alternatives in study design
There are some possible alternatives when designing a pharmacological study like the present one. For instance, a study can consider the pharmacokinetic profiles of the drugs and adjust the timetable for assessments of effect accordingly. For the sake of simplicity and for optimizing the blinding procedure, we chose a design with a fixed timetable and fixed points of time for assessment of effect, a strategy that covered most of the elimination half life for the drugs tested. The differences between pharmacokinetic profiles of the drugs were refiected in the results with the short acting ketamine proving more effective in reducing pain in the beginning of the test sessions and morphine proving more effective in the later parts. Morphine was also more prominent in reducing the VAS area under curve, whereas ketamine seemed to be more prominent in efficacy parameters.
4.3 The placebo effect/response
4.3.1 The placebo effect per se
The placebo effect is indeed a complex phenomenon. Recent years have seen an expansion of research into the mechanisms of the placebo effect, especially regarding pain (experimental and clinical). One of the main results of this research is the knowledge that a placebo response is a real psychobiological phenomenon where the central nervous system is involved not only on the psychological level but also on a physiological level. It represents a link between a complex mental activity and the body [58]. There is not one specific mechanism responsible for the placebo effect and there is not a single placebo effect, but many. There are different mechanisms for different medical conditions and interventions [58]. When it comes to pain, data indicate that the placebo effect works in part through the opioid-related endogenous pain modulatory descending circuits and in part through the dopamine-related circuits for reward [59,60]. The placebo effect regarding pain is robust and strong. In one study, the placebo was as effective as a hidden i.v. injection of 8 mg morphine [61]. Several studies have found the magnitude of the placebo analgesic effect to be around 2 out of 10 on a visual analogue scale. When viewing the placebo responders selectively, the effect is even more impressive: 3.3–5 out of 10 [60]. Some data indicate that the placebo effect working through the endogenous opioid system also has the capability to be selectively directed to local parts of the body; i.e., it works under a somatotopical structure [62]. Other data indicate that the placebo might work on a spinal as well as a supra-spinal level with impact on the mechanisms we think are responsible for central sensitization; i.e., the placebo effect might mimic the effect we assign to ketamine [63].
This knowledge about the placebo effect raises the following question: How should we interpret the placebo response in a pharmacological drug test? In addition to an active placebo (midazolam), we tested morphine and ketamine and the placebo effect might mimic them both. However, we find it reasonable to exclude the placebo responders when analysing the proportions of the other responder groups, but bearing in mind that among the placebo responders there might be some with “true” morphine-responsiveness, some with “true” ketamine-responsiveness, and some with a combination of the two. In the present study, only two out of 24 placebo responders (pain intensity) selectively responded to the placebo substance.
4.3.2 Midazolam
The present study obtained a higher proportion of placebo responders (26%, pain intensity) compared to an earlier study of WAD-subjects, which had a proportion of approximately 10% when saline was used as a placebo [11]. On the other hand, the frequency of side-effects, especially sedation and tiredness, indicates that our placebo infusions to some extent mimicked the infusions of the other substances, which was why we used midazolam as a placebo substance. The frequency of sedation and tiredness was more prominent for midazolam infusion than for morphine and ketamine (Table 6), indicating that the dosage of midazolam was too high. This finding suggests that future studies may want to consider reducing the dosage.
A possible confounding factor in the analysis of the placebo response could be where the placebo infusions were placed in the sequence – first, second, or third infusion. However, we found that where the infusions were administered in the sequence had no major impact.
We used midazolam as an active placebo substance assuming that it has no inherent analgesic activity. However, the absence of analgesic effects of benzodiazepines (BZ) is not a straightforward matter. It is fairly well documented that BZ has analgesic effects when administered intrathecally [64,65], whereas the effects with systemic administration have been more controversial. Several clinical reports indicate analgesic effects in chronic pain syndromes such as cancer pain, phantom limb pain, and myofascial pain [66]. Knabl et al. [67] speculate that the controversy regarding systemic BZ might be due to studies not distinguishing between acute pain and chronic pain states with hyperalgesia. They state that intrathecal BZ can have antihyperalgesic effect in the absence of any antinociceptive effect on acute pain [68].
4.4 The different responder groups
4.4.1 Morphine
Morphine is the prototypical µ-receptor agonist presumed to exert its main analgesic effect through a blockade or inhibition/modulation of an on-going nociceptive input to the pain transmission system [69]. Clinically, it is well known that there is a high degree of inter-individual variation of sensitivity for the analgesic effect as well as for side-effects. This variation might be due to genetic variations in the expression and distribution of receptor subtypes [70].
In the present study, 32 of 69 (47%, pain intensity, placebo responders excluded) responded to the morphine infusion according to the assessments of pain intensity. This result is similar to what a smaller study of chronic WAD subjects found [11]. We find it reasonable to interpret these results taken together as showing that at least a subgroup of subjects with chronic WAD has opioid sensitive pain.
4.4.2 Ketamine
Ketamine exerts its analgesic effect mainly through a non-competitive blockade of the NMDA-receptor in the central nervous system even though at higher doses it may interact with µ-opioid receptors and suppress sodium channels [71]. The importance of the NMDA-receptor for a nociceptive or neuropathic input to initiate central sensitization is well documented [17,72,73]. It is also well documented that at least a subgroup of patients with chronic WAD shows signs of a central sensitization [20,21,22].
We used a subanaesthetic low-dose ketamine infusion (0.3 mg/kg), which presumably would rule out any significant analgesic effect other than the NMDA-receptor blockade. Of 69 subjects, 28 (41%, pain intensity, placebo responders excluded) responded to ketamine. This result might indicate the presence of a central sensitization in a subgroup of patients with chronic WAD.
4.4.3 Global responders
Of 69 subjects, 17 (25%, pain intensity, placebo responders excluded) responded to both morphine and ketamine.
Some studies indicate the importance of a peripheral nociceptive input for initiating and maintaining a central sensitization in chronic pain [74,75,76]. It is reasonable to believe that there are variations in the degree of on-going nociceptive input as well as in the degree of central hypersensitivity among subjects with pain such as chronic WAD. In the present study some individuals responded primarily to morphine, some to ketamine, and some to both drugs.
4.4.4 Non responders
An intriguing result of our pharmacological tests is the relatively high frequency of non responders (25 out of 69, 37%, placebo responders excluded). This finding is in line with a smaller study of subjects with chronic WAD that showed a frequency of global non responders of 33% [11]. These figures for global non responders concerning chronic WAD are higher than in comparable studies regarding other pain states: one study on patients with fibromyalgia syndrome showed a frequency of 17% [25] and one study on low-back pain showed 25% global non responders [43].
Hence, one of four subjects in a relatively large group of subjects with chronic WAD did not reach 50% pain relief when challenged with an active placebo infusion, an infusion of morphine (0.3 mg/kg), or an infusion of ketamine (0.3 mg/kg), otherwise documented to have a potency for a high analgesic effect. So far we can only speculate about this result:
We tested three substances. There might be other targets in the pain processing system not reached by our drugs but relevant, e.g., for central hyperexcitability (sensitization).
A subgroup of patients with chronic WAD might be extremely biased to the psychosocial side of the biopsychosocial pain model, making these patients out of reach for any significant pain relief provided by analgesic drugs.
Assessment considerations must be addressed. We placed subjects with a chronic pain state in a rather odd experimental situation, compared to daily life, and asked them for a minute-by-minute estimation of pain intensity, unpleasantness, and per cent pain relief. All this was done during a drug infusion with side-effects probably obscuring the capability of correct estimates to some extent.
The non responders had a significantly higher baseline assessment of their pain unpleasantness compared to the other groups (p = 0.047). There was also a weak trend (p = 0.080) for higher base-line in pain intensity in the non responder group.
In addition to the tendency for the non responder group subjects to rate their baseline pain higher than the other responder groups, the non responders seem to be the worst cases in at least some aspects of the psychometric tests used in this study. This finding could be in line with clinical experience when it comes to treating chronic pain: patients with scores indicating depression, anxiety, maladaptive coping strategies, etc. seem less disposed to respond positively to a single medical intervention such as a drug treatment.
We can only speculate about the reasons why non responders seem to score badly in the psychometric tests. The biopsychosocial model is now widely accepted as a model for understanding chronic pain disorders. The underlying neuromatrix for pain is a complex neurophysiological system mediating all the factors inherent in the model, leading to the final pain experience and behaviour. The ascending somatosensory input from the periphery is modulated in the matrix and descending pathways from the matrix have the potential to facilitate or inhibit the peripheral input at different levels of the central nervous system. The complexity increases when considering the plasticity of the neuromatrix, i.e., an on-going nociceptive or neuropathic input in the system tends to change the matrix in different ways. Furthermore, we have the question of the reversibility of these plastic changes. Finally, we can consider possible genetic variations in how the matrix performs the pain processing mechanisms. In the context of this complexity we have to consider the i.v. infusion of a pain-reducing drug as a rather coarse intervention. It is understandable that some individuals with a chronic pain disorder have other dominating factors in the matrix than those targeted by the drug, leaving the pain experience more or less unaffected by the drug intervention. For some individuals, some of these other factors might be refiected in the psychometric tests.
4.5 Methodological aspects
In addition to the uncertainties mentioned in other parts of this discussion, this study may have other limitations that need to be considered.
4.5.1 Sample bias
The sample in the study was recruited from patients referred to a second or third level of health care institutions for management of chronic pain. Hence, those managed in primary care were excluded. This could put into question how representative the sample is for the whole population of chronic WAD. The estimate of the population itself is poorly defined in the literature: different studies show different results regarding incidence and prevalence of chronic WAD as well as regarding the recovery rate from acute WAD [2,3,77]. This context of what is considered to be chronic WAD adds further uncertainty regarding the relevance of a single sample.
4.5.2 Inclusion/exclusion criteria
Some chronic WAD patients develop a generalized musculoskeletal disorder including spontaneous pain in all four quadrants of the body. Studies trying to reveal a relationship between chronic WAD and fibromyalgia show contradictory results [78,79,80]. We decided to exclude from the study those with generalized pain and restricted the sample to those with localized pain in the neck with possible referred pain to the head and/or upper extremities. The reason for this exclusion criteria was that we planned to evaluate the effect of diagnostic blocks of zygapophyseal joints and the efficacy of radiofrequency neurotomy of the innervation of these joints. The results of this will be considered in future papers. However, this exclusion criteria might imply a further restriction in the relevance of the sample in relation to the population of chronic WAD.
4.5.3 Pain mechanisms
In recent years, pain research has focused on genetic variability. Genetic variations are considered to have an impact on the disposition to develop a chronic pain state and how the neuromatrix responsible for processing a nociceptive or neuropathic input is working. Genetic variability is also believed to be of importance when it comes to responses to medical interventions such as analgesic drugs. Hence, this variability is a factor to consider when trying to understand precise pain mechanisms and their treatment in the individual patient as well as in defined groups of chronic pain states. In an ideal world, we know these precise pain mechanisms when meeting the individual patient or when dealing with a group of patients with a defined chronic pain state. We can target this known mechanism in our treatment and restrict our samples for clinical research to those with pain mechanisms apt to respond to the intervention we are investigating. Today, in the clinic as well as in clinical research, we seldom have this precise knowledge of pain mechanisms. Most often we deal with syndromes with poorly defined mechanisms for chronic pain: failed back surgery, low back pain, general myofascial pain, fibromyalgia, temporo-mandibular disorders, CRPS, etc. The underlying variability in pain syndromes leads to poor treatment results. Some impact of the variability can be reduced in clinical research by expanding sample sizes, but treatments are sometimes probably ruled out simply because of poorly defined pain mechanisms in the sample.
Chronic WAD is a pain syndrome with poorly defined pain mechanisms, presumably involving a great deal of variability. When challenging patients with an i.v. infusion of morphine, ketamine, and midazolam (active placebo), we do not know whether the variations in responses reflect variations inherent in the concept of chronic WAD or simply reflect genetically determined dispositions. However, we do know that at least some of the subjects with chronic WAD have signs of central sensitization in their pain processing system [18,21,22]. We also know that the NMDA-receptor, the main target for ketamine, is of major importance in the development of central sensitization [16,17]. We do have data that suggests that morphine and ketamine have different targets for their pain reducing effects [46] andwehave data displaying synergistic effects of morphine and ketamine [24,47,48], which also implies that there are different targets for the substances. Hence, the present study does not reveal precise pain mechanisms but does reveal one aspect of the heterogeneity in the population of subjects suffering from chronic WAD.
4.6 Conclusions
The population with chronic WAD contain subgroups with variations in pain-reducing effects of morphine, ketamine, and midazolam (active placebo) when studied with an i.v. pharmacological challenge. That is, our study confirms the heterogeneity inherent in a group of subjects with chronic WAD. The study revealed that many subjects did not respond to any of the drugs used. This non responder group proved to be the worst cases in some aspects of the psychometric instruments used.
DOI of refers to article: 10.1016/j.sjpain.2012.02.008.
-
Conflict of interest
Conflict of interest statement: None.
Acknowledgements
This study was supported by the Medical Research Council of Southeast Sweden. We wish to express our thanks to the pain nurses, study nurses, and assistant nurses and to the logistic personnel in our institutions – without them there had been no study.
Appendix A. Abbreviations of Fig. 1a and b
| Placebores | Placebo responders |
| ActiveResp | Active responders |
| Nonglobal | Non responders |
| WADduratio | Duration of pain |
| BasePaini | Baseline pain intensity |
| BaseUnplea | Baseline pain unpleasantness |
| CSQ43 | Coping Strategies Questionnaire/perceived control of pain |
| CSQ44 | CSQ/ability to minimize pain |
| CSQDA | CSQ Diverting attention |
| CSQRS | CSQ Reinterpreting pain sensations |
| CSQ_CSS | CSQ Coping self-statements |
| CSQ_IS | CSQ Ignoring pain sensations |
| CSQ_PH | CSQ Praying and hoping |
| CSQ_CAT | CSQ Catastrophizing |
| CSQJBA | CSQ Increased Behavioural activities |
| BDIindex | Beck Depression Inventory index |
| LSQ01_life | Life Satisfaction Checklist (LiSat-11) Satisfaction with life as a whole |
| LSQ02_work | LiSat-11 Satisfaction with vocational situation |
| LSQP3_econ | LiSat-11 Satisfaction with financial situation |
| LSQ04_leis | LiSat-11 Satisfaction with leisure situation |
| LSQ05_frie | LiSat-11 Satisfaction with contacts with friends and acquaintances |
| LSQ06_sexl | LiSat-11 Satisfaction with sexual life |
| LSQ07_ADL | LiSat-11 Satisfaction with Activities of Daily Life |
| LSQ08_fami | LiSat-11 Satisfaction with family life |
| LSQ09_marr | LiSat-11 Satisfaction with partnership |
| LSQ10_phys | LiSat-11 Satisfaction with physical health |
| LSQ11_psyc | LiSat-11 Satisfaction with mental health |
| MPI1a | Multidimensional Pain Inventory (MPI) Pain severity |
| MPI1b | MPI Interference - pain related interference in everyday life |
| MPI1c | MPI Perceived Life Control |
| MPI1d | MPI Affective Distress |
| MPI1e | MPI Social Support - perceived support from spouse of significant others |
| MPI2a | MPI Punishing Responses |
| MPI2b | MPI Solicitous Responses |
| MPI2c | MPI Distracting Responses |
| MPIGA | MPI General Activity Index |
| EQ5D1_mob | EuroQol mobility |
| EQ5D2_ADL | EuroQol self-care |
| EQ5D3_acti | EuroQol usual activities |
| EQ5D4_pain | EuroQol pain/discomfort |
| EQ5D5_anx | EuroQol anxiety/depression |
| EQ5D7VAS | EuroQol VAS - self estimation of perceived health |
| SF36_PF | SF36 Physical functioning |
| SF36_RP | SF36 Role limitations due to physical pain |
| SF36_BP | SF36 Bodily pain |
| SF36_GH | SF36 General Health |
| SF36_VT | SF36 Vitality |
| SF36_SF | SF36 Social functioning |
| SF36_RE | SF36 Role limitations due to emotional problems |
| SF36_MH | SF36 Mental health |
| SF36_pcs | SF36 Physical score |
| SF36_mcs | SF36 Mental score |
References
[1] Barnsley L, Lord S, Bogduk N. Whiplash injury. Pain 1994;58:283–307.Suche in Google Scholar
[2] Sterner Y, Gerdle B. Acute and chronic whiplash disorders—a review. J Rehabil Med 2004;36:193–209.Suche in Google Scholar
[3] Holm LW, Carroll LJ, Cassidy JD, Hogg-Johnson S, Cote P, Guzman J, Peloso P, Nordin M, Hurwitz E, van der Velde G, Carragee E, Haldeman S. The burden and determinants of neck pain in whiplash-associated disorders after traffic collisions: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976) 2008;33:S52–9.Suche in Google Scholar
[4] Obelieniene D, Schrader H, Bovim G, Miseviciene I, Sand T. Pain after whiplash: a prospective controlled inception cohort study. J Neurol Neurosurg Psychiatry 1999;66:279–83.Suche in Google Scholar
[5] Ferrari R, Russell AS, Carroll LJ, Cassidy JD. A re-examination of the whiplash associated disorders (WAD) as a systemic illness. Ann Rheum Dis 2005;64:1337–42.Suche in Google Scholar
[6] Bogduk N, Teasell R. Whiplash: the evidence for an organic etiology. Arch Neurol 2000;57:590–1.Suche in Google Scholar
[7] Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007;133:581–624.Suche in Google Scholar
[8] Gatchel RJ, Turk DC. Criticisms of the biopsychosocial model in spine care: creating and then attacking a straw person. Spine (Phila Pa 1976) 2008;33:2831–6.Suche in Google Scholar
[9] Barnsley L, Lord SM, Wallis BJ, Bogduk N. The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine 1995;20:20–5.Suche in Google Scholar
[10] Ferrari R, Russell AS. Epidemiology of whiplash: an international dilemma. Ann Rheum Dis 1999;58:1–5.Suche in Google Scholar
[11] Lemming D, Sörensen J, Graven-Nielsen T, Arendt-Nielsen L, Gerdle B. The responses to pharmacological challenges and experimental pain in patients with chronic whiplash-associated pain. Clin J Pain 2005;21: 412–21.Suche in Google Scholar
[12] Peolsson M, Gerdle B. Coping in patients with chronic whiplash-associated disorders: a descriptive study. J Rehabil Med 2003;35:28–35.Suche in Google Scholar
[13] Söderlund A, Lindberg P. Whiplash-associated disorders—predicting disability from a process-oriented perspective of coping. Clin Rehabil 2003;17: 101–7.Suche in Google Scholar
[14] Söderlund A, Denison E. Classification of patients with whiplash associated disorders (WAD): reliable and valid subgroups based on the Multidimensional Pain Inventory (MPI-S). Eur J Pain 2006;10:113–9.Suche in Google Scholar
[15] Tenenbaum A, Rivano-Fischer M, Tjell C, Edblom M, Sunnerhagen KS. The Quebec classification and a new Swedish classification for whiplash-associated disorders in relation to life satisfaction in patients at high risk of chronic functional impairment and disability. J Rehabil Med 2002;34:114–8.Suche in Google Scholar
[16] Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology 1987;26:1235–8.Suche in Google Scholar
[17] Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-d-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain 1991;44:293–9.Suche in Google Scholar
[18] Koelbaek Johansen M, Graven-Nielsen T, Schou Olesen A, Arendt-Nielsen L. Generalised muscular hyperalgesia in chronic whiplash syndrome. Pain 1999;83:229–34.Suche in Google Scholar
[19] Petersen-Felix S, Arendt-Nielsen L, Curatolo M. Chronic pain after whiplash injury – evidence for altered central sensory processing. J Whiplash Relat Disord 2003;2:5–16.Suche in Google Scholar
[20] Sterling M, Jull G, Vicenzino B, Kenardy J. Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. Pain 2003;104:509–17.Suche in Google Scholar
[21] Curatolo M, Arendt-Nielsen L, Petersen-Felix S. Evidence, mechanisms, and clinical implications of central hypersensitivity in chronic pain after whiplash injury. Clin J Pain 2004;29:469–76.Suche in Google Scholar
[22] Banic B, Petersen-Felix S, Andersen OK, Radanov BP, Villiger PM, Arendt-Nielsen L, Curatolo M. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain 2004;107:7–15.Suche in Google Scholar
[23] Dellemijn PL, Vanneste JA. Randomised double-blind active-placebo-controlled crossover trial of intravenous fentanyl in neuropathic pain. Lancet 1997;349:753–8.Suche in Google Scholar
[24] Schulte H, Graven-Nielsen T, Sollevi A, Jansson Y, Arendt-Nielsen L, Segerdahl M. Pharmacological modulation of experimental phasic and tonic muscle pain by morphine, alfentanil and ketamine in healthy volunteers. Acta Anaesthesiol Scand 2003;47:1020–30.Suche in Google Scholar
[25] Sörensen J, Bengtsson A, Ahlner J, Henriksson KG, Ekselius L, Bengtsson M. Fibromyalgia—are there different mechanisms in the processing of pain? A double blind crossover comparison of analgesic drugs. J Rheumatol 1997;24:1615–21.Suche in Google Scholar
[26] Graven-Nielsen T, Aspegren Kendall S, Henriksson KG, Bengtsson M, Sörensen J, Johnson A, Gerdle B, Arendt-Nielsen L. Ketamine reduces muscle pain, temporal summation, and referred pain in fibromyalgia patients. Pain 2000;85:483–91.Suche in Google Scholar
[27] Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988;8:77–100.Suche in Google Scholar
[28] Furukawa TA. Assessment of mood: guides for clinicians. J Psychosom Res 2010;68:581–9.Suche in Google Scholar
[29] Beck AT, Steer RA. Beck depression inventory. Manual. Svensk version (Swedish version). Fagernes, Norway: Psykologiförlaget AB; 1996.Suche in Google Scholar
[30] Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain 1983;17:33–44.Suche in Google Scholar
[31] Jensen IB, Linton SJ. Coping Strategies Questionnaire (CSQ): reliability of the Swedish version of the CSQ. Scand J Behav Ther 1993;22:139–45.Suche in Google Scholar
[32] Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985;23:345–56.Suche in Google Scholar
[33] Bergström G, Jensen IB, Bodin L, Linton SJ, Nygren AL, Carlsson SG. Reliability and factor structure of the Multidimensional Pain Inventory—Swedish Language Version (MPI-S). Pain 1998;75:101–10.Suche in Google Scholar
[34] Fugl-Meyer AR, Melin R, Fugl-Meyer KS. Life satisfaction in 18-to 64-year-old Swedes: in relation to gender, age, partner and immigrant status. J Rehabil Med 2002;34:239–46.Suche in Google Scholar
[35] Sullivan M, Karlsson J, Ware Jr JE. The Swedish SF-36 Health Survey—I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med 1995;41:1349–58.Suche in Google Scholar
[36] Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53–72.Suche in Google Scholar
[37] Burström K, Johannesson M, Diderichsen F. Swedish population health-related quality of life results using the EQ-5D. Qual Life Res 2001;10:621–35.Suche in Google Scholar
[38] Dellemijn PL, van Duijn H, Vanneste JA. Prolonged treatment with transdermal fentanyl in neuropathic pain. J Pain Symptom Manage 1998;16:220–9.Suche in Google Scholar
[39] Cohen SP, Chang AS, Larkin T, Mao J. The intravenous ketamine test: a predictive response tool for oral dextromethorphan treatment in neuropathic pain. Anesth Analg 2004;99:1753–9, table of contents.Suche in Google Scholar
[40] Cohen SP, Verdolin MH, Chang AS, Kurihara C, Morlando BJ, Mao J. The intravenous ketamine test predicts subsequent response to an oral dextromethorphan treatment regimen in fibromyalgia patients. J Pain 2006;7:391–8.Suche in Google Scholar
[41] Cohen SP, Wang S, Chen L, Kurihara C, McKnight G, Marcuson M, Mao J. An intravenous ketamine test as a predictive response tool in opioid-exposed patients with persistent pain. J Pain Symptom Manage 2009;37:698–708.Suche in Google Scholar
[42] Gustorff B. Intravenous opioid testing in patients with chronic non-cancer pain. Eur J Pain 2005;9:123–5.Suche in Google Scholar
[43] Sörensen J, Aaro S, Bengtsson M, Kalman S, Reigo T, Tropp H. Can a pharmaco-logical pain analysis in patients with chronic low back pain predict the outcome of lumbar fusion? Preliminary report. Eur Spine J 1996;5:326–31.Suche in Google Scholar
[44] Arner S. Intravenous phentolamine test: diagnostic and prognostic use in refiex sympathetic dystrophy. Pain 1991;46:17–22.Suche in Google Scholar
[45] Arendt-Nielsen L, Petersen-Felix S, Fischer M, Bak P, Bjerring P, Zbinden AM. The effect of N-methyl-d-aspartate antagonist (ketamine) on single and repeated nociceptive stimuli: a placebo-controlled experimental human study. Anesth Analg 1995;81:63–8.Suche in Google Scholar
[46] Warncke T, Stubhaug A, Jorum E. Ketamine, an NMDA receptor antagonist, suppresses spatial and temporal properties of burn-induced secondary hyperalgesia in man: a double-blind, cross-over comparison with morphine and placebo. Pain 1997;72:99–106.Suche in Google Scholar
[47] Bossard AE, Guirimand F, Fletcher D, Gaude-Joindreau V, Chauvin M, Bouhassira D. Interaction of a combination of morphine and ketamine on the nociceptive fiexion refiex in human volunteers. Pain 2002;98:47–57.Suche in Google Scholar
[48] Luginbuhl M, Gerber A, Schnider TW, Petersen-Felix S, Arendt-Nielsen L, Curatolo M. Modulation of remifentanil-induced analgesia, hyperalgesia, and tolerance by small-dose ketamine in humans. Anesth Analg 2003;96:726–32, table of contents.Suche in Google Scholar
[49] Felsby S, Nielsen J, Arendt-Nielsen L, Jensen TS. NMDA receptor blockade in chronic neuropathic pain: a comparison of ketamine and magnesium chloride. Pain 1996;64:283–91.Suche in Google Scholar
[50] Kvarnström A, Karlsten R, Quiding H, Emanuelsson BM, Gordh T. The effectiveness of intravenous ketamine and lidocaine on peripheral neuropathic pain. Acta Anaesthesiol Scand 2003;47:868–77.Suche in Google Scholar
[51] Kvarnström A, Karlsten R, Quiding H, Gordh T. The analgesic effect of intravenous ketamine and lidocaine on pain after spinal cord injury. Acta Anaesthesiol Scand 2004;48:498–506.Suche in Google Scholar
[52] Sörensen J, Bengtsson A, Backman E, Henriksson KG, Bengtsson M. Pain analysis in patients with fibromyalgia. Effects of intravenous morphine, lidocaine, and ketamine. Scand J Rheumatol 1995;24:360–5.Suche in Google Scholar
[53] Lemming D, Sörensen J, Graven-Nielsen T, Lauber R, Arendt-Nielsen L, Gerdle B. Managing chronic whiplash associated pain with a combination of low-dose opioid (remifentanil) and NMDA-antagonist (ketamine). Eur J Pain 2007;11:719–32.Suche in Google Scholar
[54] Cohen SP, Kapoor SG, Rathmell JP. Intravenous infusion tests have limited utility for selecting long-term drug therapy in patients with chronic pain: a systematic review. Anesthesiology 2009;111:416–31.Suche in Google Scholar
[55] Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain 2000;88:287–94.Suche in Google Scholar
[56] Farrar JT, Young Jr JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58.Suche in Google Scholar
[57] Fields HL. Pain: an unpleasant topic. Pain 1999;Suppl. 6:S61–9.Suche in Google Scholar
[58] Benedetti F. Placebo effects: understanding the mechanisms in health and disease. Oxford; New York: Oxford University Press; 2009.Suche in Google Scholar
[59] Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia—imaging a shared neuronal network. Science 2002;295:1737–40.Suche in Google Scholar
[60] Koshi EB, Short CA. Placebo theory and its implications for research and clinical practice: a review of the recent literature. Pain Pract 2007;7:4–20.Suche in Google Scholar
[61] Levine JD, Gordon NC. Influence of the method of drug administration on analgesic response. Nature 1984;312:755–6.Suche in Google Scholar
[62] Benedetti F, Arduino C, Amanzio M. Somatotopic activation of opioid systems by target-directed expectations of analgesia. J Neurosci 1999;19:3639–48.Suche in Google Scholar
[63] Matre D, Casey KL, Knardahl S. Placebo-induced changes in spinal cord pain processing. J Neurosci 2006;26:559–63.Suche in Google Scholar
[64] Nishiyama T. Analgesic effects of systemic midazolam: comparison with intrathecal administration. Can J Anaesth 2006;53:1004–9.Suche in Google Scholar
[65] Ho KM, Ismail H. Use of intrathecal midazolam to improve perioperative analgesia: a meta-analysis. Anaesth Intensive Care 2008;36:365–73.Suche in Google Scholar
[66] Jasmin L, Wu MV, Ohara PT. GABA puts a stop to pain. Curr Drug Targets CNS Neurol Disord 2004;3:487–505.Suche in Google Scholar
[67] Knabl J, Zeilhofer UB, Crestani F, Rudolph U, Zeilhofer HU. Genuine antihyperalgesia by systemic diazepam revealed by experiments in GABAA receptor point-mutated mice. Pain 2009;141:233–8.Suche in Google Scholar
[68] Knabl J, Witschi R, Hosl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy JM, Rudolph U, Mohler H, Zeilhofer HU. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature 2008;451:330–4.Suche in Google Scholar
[69] Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician 2008;11:S133–53.Suche in Google Scholar
[70] Han W, Ide S, Sora I, Yamamoto H, Ikeda K. A possible genetic mechanism underlying individual and interstrain differences in opioid actions: focus on the mu opioid receptor gene. Ann N Y Acad Sci 2004;1025:370–5.Suche in Google Scholar
[71] Annetta MG, Iemma D, Garisto C, Tafani C, Proietti R. Ketamine: new indications for an old drug. Curr Drug Targets 2005;6:789–94.Suche in Google Scholar
[72] Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000;288:1765–9.Suche in Google Scholar
[73] Eide PK. Wind-up and the NMDA receptor complex from a clinical perspective. Eur J Pain 2000;4:5–15.Suche in Google Scholar
[74] Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J Pain 2000;4:229–38.Suche in Google Scholar
[75] Schneider GM, Smith AD, Hooper A, Stratford P, Schneider KJ, Westaway MD, Frizzell B, Olson L. Minimizing the source of nociception and its concurrent effect on sensory hypersensitivity: an exploratory study in chronic whiplash patients. BMC Musculoskelet Disord 2010;11:29.Suche in Google Scholar
[76] Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain 2009;145:96–104.Suche in Google Scholar
[77] Carroll LJ, Holm LW, Hogg-Johnson S, Cote P, Cassidy JD, Haldeman S, Nordin M, Hurwitz EL, Carragee EJ, van der Velde G, Peloso PM, Guzman J. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD): results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976) 2008;33:S83–92.Suche in Google Scholar
[78] Buskila D, Neumann L, Vaisberg G, Alkalay D, Wolfe F. Increased rates of fibromyalgia following cervical spine injury. A controlled study of 161 cases of traumatic injury. Arthritis Rheum 1997;40:446–52.Suche in Google Scholar
[79] Tishler M, Levy O, Maslakov I, Bar-Chaim S, Amit-Vazina M. Neck injury and fibromyalgia—are they really associated? J Rheumatol 2006;33: 1183–5.Suche in Google Scholar
[80] Robinson JP, Theodore BR, Wilson HD, Waldo PG, Turk DC. Determination of fibromyalgia syndrome after whiplash injuries: methodologic issues. Pain 2011;152:1311–6.Suche in Google Scholar
© 2012 Scandinavian Association for the Study of Pain
Artikel in diesem Heft
- Editorial comment
- Spontaneous pain is reduced by conditioning pain modulation in peripheral neuropathy but not in fibromyalgia—Implications for different pain mechanisms
- Clinical pain research
- Differential pain modulation in patients with peripheral neuropathic pain and fibromyalgia
- Editorial comment
- Pulsed radiofrequency—Time for a clinical pause and more science
- Clinical pain research
- Pulsed radiofrequency in peripheral posttraumatic neuropathic pain: A double blind sham controlled randomized clinical trial
- Editorial comment
- Phantom pains and sensations – how does it feel? Only the patient really knows
- Clinical pain research
- Phantom phenomena – Their perceived qualities and consequences from the patient’s perspective
- Editorial comment
- Impact of mental stressor on conditioned pain modulation
- Original experimental
- The effect of a mental stressor on conditioned pain modulation in healthy subjects
- Editorial comment
- Pharmacological modulation of chronic pain after whiplash injury
- Clinical pain research
- Whiplash Associated Disorders (WAD): Responses to pharmacological challenges and psychometric tests
- Editorial comment
- Why are autonomic responses to pressure pain different from those to heat pain and ischaemic pain?
- Original experimental
- Cardiovascular responses to and modulation of pressure pain sensitivity in normotensive, pain-free women
- Correspondence
- Piriformis muscle injection guided by sciatic nerve stimulation: Quick, simple, and safe technique
- Correspondence
- Musculus piriformis syndrome: Localization and injection therapy—Comment to letter from Mayo-Moldes M et al. [1]
- Abstracts
- The “pain matrix” reloaded
- Abstracts
- Endpoints in animal pain models
- Abstracts
- Evaluating pain-related behavior in spinal cord injury
- Abstracts
- The role of the amygdala in sensory and emotional-like pain behavior in neuropathic animals
- Abstracts
- Peripheral and central pain mechanisms—From animal models to clinical research
- Abstracts
- Human experimental models of central sensitization—Do they bridge the gap between animal models and clinical observations?
- Abstracts
- Assessment of central sensitization in the clinic. Is it possible?
- Abstracts
- Migraine neurobiology and treatment
- Abstracts
- Chronic headaches–Goals and obstacles
- Abstracts
- Trigeminal neuralgia and other cranial neuralgias
- Abstracts
- Temporomandibular disorders: Pathophysiology and diagnosis
- Abstracts
- HIV-associated painful polyneuropathy
- Abstracts
- Keynote: Neuronal and glial signalling in pain neuroplasticity
- Abstracts
- Neuropathic pain—From guidelines to clinical practice
- Abstracts
- Postoperative pain treatment. What’s the evidence—And how to use it?
- Abstracts
- NSAIDs in postoperative pain
- Abstracts
- How should we prevent persistent postoperative pain?
- Abstracts
- Opioids: Genetics and receptors
- Abstracts
- Chronic pain and sleep disorders
- Abstracts
- Population-based studies on chronic pain: The role of opioids
- Abstracts
- Living beyond pain: Acceptance and commitment therapy
- Abstracts
- Modality specific alterations of esophageal sensitivity caused by longstanding diabetes mellitus
- Abstracts
- Validation of a porcine behavioural model of UVB induced inflammatory pain
- Abstracts
- Recovery after a lumbar disc herniation is dependent on a gender and OPRM1 Asn40Asp genotype interaction
- Abstracts
- Pain sensitivity changes in chronic pain patients with and without spinal cord stimulation assessed by nociceptive withdrawal reflex thresholds and electrical pain thresholds
- Abstracts
- Acceptance and commitment therapy for fibromyalgia: A randomized controlled trial
- Abstracts
- Sortilins in neuropathic pain
- Abstracts
- Systematic review of neuropathic component in persistent post-surgical pain
- Abstracts
- Pain prevalence in a university hospital in Iceland
- Abstracts
- The effect of tail-docking neonate piglets on ATF-3 and NR2B immunoreactivity in coccygeal dorsal root ganglia and spinal cord dorsal horn neurons: Preliminary data
- Abstracts
- Na+/K+-ATPase dependent regulation of astrocyte Ca2+ signalling: A novel mechanism for modulation of long-term pain?
- Abstracts
- Glutamate attenuates nitric oxide release from isolated trigeminal ganglion satellite glial cells
- Abstracts
- Acute behavioural responses to tail docking in piglets – Effects of increasing docking length?
- Abstracts
- Dose and administration-period play a key role in the effect of ceftriaxone on neuropathic pain in CCI-operated rats
- Abstracts
- Translational aspects of rectal evoked potentials: A comparative study in rats and humans
- Abstracts
- Time-course of analgesic effects of botulinum neurotoxin type A (BoNTA) on human experimental model of pain induced by injection of glutamate into temporalis muscle
- Abstracts
- The effect of nerve compression and capsaicin on contact heat evoked potentials (CHEPs) related to Aδ and C fibers
- Abstracts
- Effect of specific trapezius exercises vs. coordination training on corticomotor control of neck muscles
- Abstracts
- SNP in TNFα T308G is predictive for persistent postoperative pain following inguinal hernia surgery
- Abstracts
- Chronic pain in thoracotomy
- Abstracts
- The variability in thermal threshold-assessments in post-thoracotomy pain syndrome
- Abstracts
- Persistent pain, sensory disturbances and functional impairment after adjuvant chemotherapy for breast cancer
- Abstracts
- Neuroplastic alterations in brain responses to painful visceral stimulations reflects individual neuropathic symptoms in diabetes mellitus patients
- Abstracts
- Exercise and conditioned pain modulation have different effects on cuff pressure pain tolerance in humans
- Abstracts
- Hyperalgesia in human skin and deep-tissues inside and outside of a UVB irradiated area
- Abstracts
- Effect of experimental jaw muscle pain on bite force during mastication
- Abstracts
- Reflex threshold assessment methodology for evaluation of central sensitisation is vulnerable to EMG crosstalk
- Abstracts
- Cognitive modulation of experimental pain at spinal and cortical levels
- Abstracts
- Influence of emotionally loaded visual and gustatory stimuli on pain perception
- Abstracts
- Modulating pain with augmented reality
- Abstracts
- Offset analgesia: A reproducibility study
- Abstracts
- Visualization of painful process in peripheral tissue using positron emission tomography and [11C]-D-deprenyl
- Abstracts
- Mirror-image sensory dysfunction in the post-thoracotomy pain syndrome
- Abstracts
- Genetic variation in opioid receptor genes and sensitivity to experimental pain in male and female healthy volunteers
- Abstracts
- Mechanical sensitivity in migraine patients during attack, remission, and pain-free periods:A preliminary study
- Abstracts
- Multivariate pattern analysis of evoked brain potentials by temporal matching pursuit and support vector machine
- Abstracts
- Pain following stroke: A prospective study
- Abstracts
- Chronic thoracic pain in children after cardiac surgery
- Abstracts
- Chronic pain after breast augmentation is associated with both signs of peripheral nerve injury and central nervous mechanisms
- Abstracts
- Sensory phenotypes in patients with peripheral neuropathic pain evaluated with quantitative sensory testing
- Abstracts
- Is health related quality of life related to the pattern of chronic pain?
- Abstracts
- Comparison between ropivacaine local infiltration analgesia with ketorolac or placebo for total knee replacement surgery
- Abstracts
- Treatment with topical capsaicin: Experience from a pain clinic
- Abstracts
- Distribution of concussion related symptoms after whiplash injury in risk strata
- Abstracts
- HIV/AIDS in different cultures
- Abstracts
- Pain perception is altered in patients with medication-overuse headache but can improve after detoxification
- Abstracts
- Detoxification in a structured programme is effective for medication-overuse headache
Artikel in diesem Heft
- Editorial comment
- Spontaneous pain is reduced by conditioning pain modulation in peripheral neuropathy but not in fibromyalgia—Implications for different pain mechanisms
- Clinical pain research
- Differential pain modulation in patients with peripheral neuropathic pain and fibromyalgia
- Editorial comment
- Pulsed radiofrequency—Time for a clinical pause and more science
- Clinical pain research
- Pulsed radiofrequency in peripheral posttraumatic neuropathic pain: A double blind sham controlled randomized clinical trial
- Editorial comment
- Phantom pains and sensations – how does it feel? Only the patient really knows
- Clinical pain research
- Phantom phenomena – Their perceived qualities and consequences from the patient’s perspective
- Editorial comment
- Impact of mental stressor on conditioned pain modulation
- Original experimental
- The effect of a mental stressor on conditioned pain modulation in healthy subjects
- Editorial comment
- Pharmacological modulation of chronic pain after whiplash injury
- Clinical pain research
- Whiplash Associated Disorders (WAD): Responses to pharmacological challenges and psychometric tests
- Editorial comment
- Why are autonomic responses to pressure pain different from those to heat pain and ischaemic pain?
- Original experimental
- Cardiovascular responses to and modulation of pressure pain sensitivity in normotensive, pain-free women
- Correspondence
- Piriformis muscle injection guided by sciatic nerve stimulation: Quick, simple, and safe technique
- Correspondence
- Musculus piriformis syndrome: Localization and injection therapy—Comment to letter from Mayo-Moldes M et al. [1]
- Abstracts
- The “pain matrix” reloaded
- Abstracts
- Endpoints in animal pain models
- Abstracts
- Evaluating pain-related behavior in spinal cord injury
- Abstracts
- The role of the amygdala in sensory and emotional-like pain behavior in neuropathic animals
- Abstracts
- Peripheral and central pain mechanisms—From animal models to clinical research
- Abstracts
- Human experimental models of central sensitization—Do they bridge the gap between animal models and clinical observations?
- Abstracts
- Assessment of central sensitization in the clinic. Is it possible?
- Abstracts
- Migraine neurobiology and treatment
- Abstracts
- Chronic headaches–Goals and obstacles
- Abstracts
- Trigeminal neuralgia and other cranial neuralgias
- Abstracts
- Temporomandibular disorders: Pathophysiology and diagnosis
- Abstracts
- HIV-associated painful polyneuropathy
- Abstracts
- Keynote: Neuronal and glial signalling in pain neuroplasticity
- Abstracts
- Neuropathic pain—From guidelines to clinical practice
- Abstracts
- Postoperative pain treatment. What’s the evidence—And how to use it?
- Abstracts
- NSAIDs in postoperative pain
- Abstracts
- How should we prevent persistent postoperative pain?
- Abstracts
- Opioids: Genetics and receptors
- Abstracts
- Chronic pain and sleep disorders
- Abstracts
- Population-based studies on chronic pain: The role of opioids
- Abstracts
- Living beyond pain: Acceptance and commitment therapy
- Abstracts
- Modality specific alterations of esophageal sensitivity caused by longstanding diabetes mellitus
- Abstracts
- Validation of a porcine behavioural model of UVB induced inflammatory pain
- Abstracts
- Recovery after a lumbar disc herniation is dependent on a gender and OPRM1 Asn40Asp genotype interaction
- Abstracts
- Pain sensitivity changes in chronic pain patients with and without spinal cord stimulation assessed by nociceptive withdrawal reflex thresholds and electrical pain thresholds
- Abstracts
- Acceptance and commitment therapy for fibromyalgia: A randomized controlled trial
- Abstracts
- Sortilins in neuropathic pain
- Abstracts
- Systematic review of neuropathic component in persistent post-surgical pain
- Abstracts
- Pain prevalence in a university hospital in Iceland
- Abstracts
- The effect of tail-docking neonate piglets on ATF-3 and NR2B immunoreactivity in coccygeal dorsal root ganglia and spinal cord dorsal horn neurons: Preliminary data
- Abstracts
- Na+/K+-ATPase dependent regulation of astrocyte Ca2+ signalling: A novel mechanism for modulation of long-term pain?
- Abstracts
- Glutamate attenuates nitric oxide release from isolated trigeminal ganglion satellite glial cells
- Abstracts
- Acute behavioural responses to tail docking in piglets – Effects of increasing docking length?
- Abstracts
- Dose and administration-period play a key role in the effect of ceftriaxone on neuropathic pain in CCI-operated rats
- Abstracts
- Translational aspects of rectal evoked potentials: A comparative study in rats and humans
- Abstracts
- Time-course of analgesic effects of botulinum neurotoxin type A (BoNTA) on human experimental model of pain induced by injection of glutamate into temporalis muscle
- Abstracts
- The effect of nerve compression and capsaicin on contact heat evoked potentials (CHEPs) related to Aδ and C fibers
- Abstracts
- Effect of specific trapezius exercises vs. coordination training on corticomotor control of neck muscles
- Abstracts
- SNP in TNFα T308G is predictive for persistent postoperative pain following inguinal hernia surgery
- Abstracts
- Chronic pain in thoracotomy
- Abstracts
- The variability in thermal threshold-assessments in post-thoracotomy pain syndrome
- Abstracts
- Persistent pain, sensory disturbances and functional impairment after adjuvant chemotherapy for breast cancer
- Abstracts
- Neuroplastic alterations in brain responses to painful visceral stimulations reflects individual neuropathic symptoms in diabetes mellitus patients
- Abstracts
- Exercise and conditioned pain modulation have different effects on cuff pressure pain tolerance in humans
- Abstracts
- Hyperalgesia in human skin and deep-tissues inside and outside of a UVB irradiated area
- Abstracts
- Effect of experimental jaw muscle pain on bite force during mastication
- Abstracts
- Reflex threshold assessment methodology for evaluation of central sensitisation is vulnerable to EMG crosstalk
- Abstracts
- Cognitive modulation of experimental pain at spinal and cortical levels
- Abstracts
- Influence of emotionally loaded visual and gustatory stimuli on pain perception
- Abstracts
- Modulating pain with augmented reality
- Abstracts
- Offset analgesia: A reproducibility study
- Abstracts
- Visualization of painful process in peripheral tissue using positron emission tomography and [11C]-D-deprenyl
- Abstracts
- Mirror-image sensory dysfunction in the post-thoracotomy pain syndrome
- Abstracts
- Genetic variation in opioid receptor genes and sensitivity to experimental pain in male and female healthy volunteers
- Abstracts
- Mechanical sensitivity in migraine patients during attack, remission, and pain-free periods:A preliminary study
- Abstracts
- Multivariate pattern analysis of evoked brain potentials by temporal matching pursuit and support vector machine
- Abstracts
- Pain following stroke: A prospective study
- Abstracts
- Chronic thoracic pain in children after cardiac surgery
- Abstracts
- Chronic pain after breast augmentation is associated with both signs of peripheral nerve injury and central nervous mechanisms
- Abstracts
- Sensory phenotypes in patients with peripheral neuropathic pain evaluated with quantitative sensory testing
- Abstracts
- Is health related quality of life related to the pattern of chronic pain?
- Abstracts
- Comparison between ropivacaine local infiltration analgesia with ketorolac or placebo for total knee replacement surgery
- Abstracts
- Treatment with topical capsaicin: Experience from a pain clinic
- Abstracts
- Distribution of concussion related symptoms after whiplash injury in risk strata
- Abstracts
- HIV/AIDS in different cultures
- Abstracts
- Pain perception is altered in patients with medication-overuse headache but can improve after detoxification
- Abstracts
- Detoxification in a structured programme is effective for medication-overuse headache

