Stretch Receptor and Somatic Dysfunction: A Narrative Review
-
Mark A.W. Andrews
Abstract
From its founding by Andrew Taylor Still, MD, DO, through the work of many contributors, one of the cornerstones of osteopathic medicine has been its ability to aid health by promoting neuromuscular homeostasis. As part of the understanding of osteopathic medicine since the time of Still, the proper functioning of stretch receptor organs (SROs) of skeletal muscle have been recognized as having a central role in this homeostasis. In doing so, the complexities of these numerous and vital sensors are described, including recent findings regarding their structure, function, and the nature of their neural connections. In their homeostatic role, SROs conduct information centrally for integration in proprioceptive and autonomic reflexes. By virtue of their integral role in muscle reflexes, they are putatively involved in somatic dysfunction and segmental facilitation. In reviewing some well-established knowledge regarding the SRO and introducing more recent scientific findings, an attempt is made to offer insights on how this knowledge may be applied to better understand somatic dysfunction.
Osteopathic medicine was founded on the premise that homeostasis in structure and function plays a central role in health and disease. It is also a premise of osteopathic medicine that the proper functioning of the neuromuscular system can be aided through the use of osteopathic manipulative therapy (OMT).1 Being the most numerous sensors in the body,2 stretch receptor organs (SROs), with their complex internal structure and complex neural connections, are well recognized as playing a central role in neuromuscular homeostasis. As such, SROs are essential for encoding the effects of external mechanical perturbations to the body and changes in muscle length.3 This review is directed to communicate updates of major concepts regarding the function of SROs and their possible clinical involvement with somatic dysfunction. In so doing, I have fully reviewed information from classic publications, dating back to 1888, through current literature describing functional aspects of the SRO, and have proposed logical links of recent breakthroughs to the bases of somatic dysfunction.
Being the basis of the monosynaptic myotatic reflex and the Hoffmann reflex, the microscopic proprioceptive SROs are sensitive to absolute stretch as well as to the rate of stretch of a muscle. Such sensitivity, coupled with their efferent connections and contractile capacity to self-adjust their length, makes SROs the essential component of any skeletal muscle reflex and allows coordinated movement with their constant bidirectional communication with the central nervous system (CNS). However, as discussed by Korr,4 the constant setting-resetting of SROs, as they work in series with the extrafusal (typical skeletal) muscle fibers, combined with their complex communication with the CNS, most likely causes significant input-output mismatching. This mismatching has been recognized since the founding of osteopathic medicine by Andrew Taylor Still, MD, DO.1 Still referred to such mismatching as osteopathic lesions, and these lesions became recognized in the concept of somatic dysfunction.4
Since the foundational work of Korr and Denslow on somatic dysfunction, segmental facilitation, and the role of proprioceptors,4-8 the understanding of proprioception and SROs has advanced significantly, as will be discussed, though few of these advances have been considered in the osteopathic literature. Although there have been alternate explanations regarding the bases of somatic dysfunction and the mechanism of action of osteopathic manipulative therapies,1,9-12 SROs remain at the center of many theories and rightfully maintain the attention of osteopathic medicine. The more complexity that is revealed in the function of SROs, the more likely it appears that they are involved in somatic dysfunction. However, the scientific basis of such dysfunctions and mechanisms of their amelioration remain to be fully elucidated. This review revisits classic publications and introduces recent scientific advances regarding SROs in order to update the understanding of the relationship between SROs and somatic dysfunction.
The history of the osteopathic lesion, as described by Still and further expounded on by other osteopathic practitioners and scientists is reviewed in detail elsewhere.1 Concerning the current review of related concepts, it can simply be stated that Still had a very general definition of lesion in mind, holding a mechanistic view of health and disease when he spoke of lesions. To him, lesions were obstructions to the correct function of the body, ie, anything that restricted normal function of the bodily systems, including blood flow, lymphatic flow, or neural activation. This view was very much in keeping with the ideas of his time regarding the maintenance of a stable state within the body, first stated by Claude Bernard, MD.13 This concept was further refined by Walter Cannon, MD,14 as he coined the term “homeostasis” and proposed the essential character of this condition. Along this line of thought, Still stated that through use of OMT, the osteopathic physician can make “adjustments” to solve such lesion and allow the body to use its inherent capacities to regain and maintain health.
While there is increasing evidence of the treatment effects of OMT,15-18 the mechanisms underlying the benefits of osteopathic manipulation, along with neurophysiological mechanisms that may underlie somatic dysfunction, still remain to be fully elucidated.19-21 The possibility for a central role of the SRO in somatic dysfunction was first intimated by the work of Denslow et al,6 in their groundbreaking scientific investigations of the osteopathic lesion, but many years passed before Korr4 fully addressed the importance of these proprioceptors in proposing a theory of segmental facilitation and somatic dysfunction involving SROs. Since that time, many discussions of somatic dysfunction have rightly involved the SRO; however, further knowledge has always depended on deeper comprehension of the complex functioning of proprioceptors, including SROs. Research and publications over the past few decades have greatly enhanced our knowledge of the structure and function of SROs and now allows for more in-depth discussions of the involvement of SROs in somatic dysfunction. While this review is by no means all-encompassing, it attempts to open these discussions and touch on the main points of a highly complex topic, hopefully piquing the curiosity of others to follow.
Historical Perspective
There has been significant historical interest in kinesthesia and proprioception through the years.22 In recent decades, SROs have rightly been recognized as one of the most complex sensory organ systems of the body.3,23 While seemingly less complex than the eye and ear, SROs are far more numerous and have both afferent and efferent connections, illustrating their complexity as sensory organs. The term kinesthesia, first introduced by Bastian,24 was used to refer to sensations of limb position and movement. Sherrington25 later coined the term proprioception, literally meaning "sense of self," with the muscles “acting as a stimulus to its own receptors.” While information from all proprioceptive organs provides input to the CNS, with integration at the spinal and higher levels, it is mainly input from approximately 44,000 SROs26 that allow the CNS to construct a conscious representation of body position and movement, while allowing muscular adaptation via efferent signaling. While kinesthesia and proprioception were topics of interest in Still's lifetime, the existence of SROs was unrealized; nonetheless, the description of the osteopathic lesion was an amazingly accurate understanding of what might occur in the body under stress.1
Shortly after the foundational work of Adrian and Zotterman27 recognized the highly complex nature of SRO signaling while conducting some of the first functional studies of action potential conduction in neurons. Recording the electrical response of muscle stretch on SRO output, they found short-term and longer- duration components of the signals now recognized as corresponding to the tonic and dynamic responses of the intrafusal fibers of the SROs. They also proposed that SRO sensitivity was modified by the CNS and noted the possible involvement of the autonomic nervous system (ANS). In consequent research, Matthews28 recorded the firing of multiple types of afferents from muscle corresponding to group Ia, group II, and an additional group Ib afferents later determined to correspond to signaling of the Golgi tendon organs.
Structure and Function of the SRO
The complexity of the SROs, which brought Korr4 to infer their role in somatic dysfunction, is quite evident on investigation (Figure). The SROs, which encompass the intrafusal fibers and their neural attachments, are 4 to 10 mm in length and 80 to 250 µm in diameter, with the wide range in sizes due to the varying number of fibers in each spindle.3,29,30 The entire SRO is enveloped in an external capsule, similar to a perimysium that attaches into the perimysium of extrafusal fibers.31 While there are numerous intrafusal fibers in each SRO, much of the volume is due to the specialized intracapsular fluid. This fluid has been compared to lymph, but in addition it includes a high concentration of hyaluronan, the polysaccharide found in synovial fluid.32 While hyaluronan may aid cellular homeostasis and the exchange of nutrients and wastes,33 it also has the capacity to greatly enhance the fluid viscosity and help generate a low friction environment in which intrafusal fibers can sense and respond to changes in muscle length. The fluid also allows clear separation among the intrafusal fibers and reduces the problem of the intrafusal fibers being compressed by the contraction of extrafusal fibers squeezing the SRO.
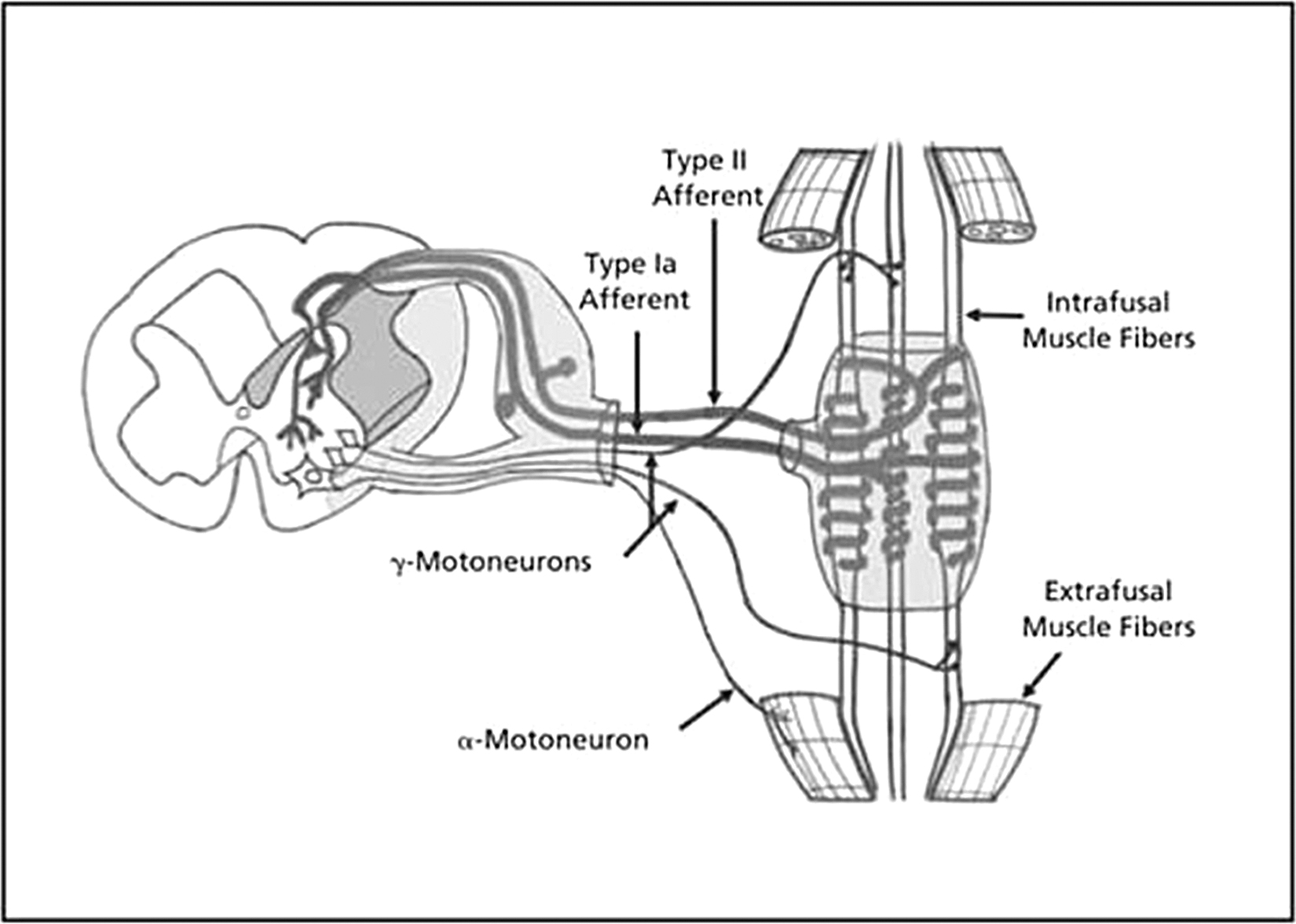
Anatomic and neural pathways of the muscle spindle. Reprinted from: Clark BC, Thomas JS, Walkowski SA, Howell JN. The biology of manual therapies. J Am Osteopath Assoc. 2012;112(9):617-629.
The SROs lie parallel among extrafusal muscle fibers in the central areas of whole muscles.3 Due to this parallel arrangement and the low-friction intrafusal environment, SROs are exquisitely sensitive to changes in muscle length. However, while all muscles contain SROs, these receptors are not equally distributed in muscles, with the highest SRO density found in muscles of fine motion with over 100 SROs per gram of muscle (eg, extraocular and lumbricals) and those helping maintain posture (eg, soleus). The lowest density of SROs is found in muscles initiating gross movement or responsible for great force generation (eg, gastrocnemius).34,35 Embryonically, intrafusal fibers have the same mesodermal origins as extrafusal fibers, being multinucleated and arising from fusion of myotubes.31 Being skeletal muscle fibers, the intrafusal fibers share characteristics with extrafusal fibers. Each fiber is covered by an internal capsule similar to the endomysium of extrafusal fibers but are generally thinner, averaging 10 µm in diameter compared with the typical extrafusal fiber diameter of 60 to 80 µm and are much shorter at 4 to 7 mm.36,37
The fibers within the SRO are of 2 general anatomic types as illustrated in the Figure and first described by Boyd36: slender, cylindrical cells referred to as nuclear chain fibers, which have their nuclei arranged in a straight “chain” in their equatorial region; and nuclear bag fibers, out pouched in their equatorial regions, thus appearing as a bags with their numerous nuclei in a dense equatorial grouping. Being sensory in these equatorial regions, intrafusal fibers also have contractile capacity in their polar regions, allowing them to shorten in tandem with extrafusal fibers so that they do not go slack when extrafusal fibers are shortened. The fiber type of the polar regions of intrafusal fibers is much more diverse than the fiber types (slow-, intermediate-, and fast-twitch) of extrafusal fibers,30,37 and their sarcoplasmic reticular calcium-ATPase isoforms are also diverse.38
While there is some variability, afferent innervation of each SRO generally includes a single “primary” group Ia neuron that coils around and innervates the equatorial regions of all intrafusal fibers, forming the annulospiral endings and multiple smaller secondary group II neurons innervating the nuclear chain fibers in “flower-spray” receptor endings.39,40 Both afferents have a baseline signal frequency that increases as they are stretched. Such increased firing is due to the increase in the dual sodium-calcium activation current of the mechanosensitive terminals.41 However, this afferent signaling is complex and has both tonic and dynamic components.3 The nuclear chain fibers are classified as tonic sensors, with their afferent signal frequency related to their absolute length. On the other hand, nuclear bag fibers, thought for many years to function solely as dynamic sensors, are now recognized to be of the following functional types: bag1 fibers, which signal dynamic changes in length which is then signaled to the CNS by the primary group Ia afferents, and bag2 fibers which, like nuclear chain fibers, signal absolute length.42-46 Likewise, as the muscle shortens, the firing rate of both afferents decrease as the fibers return to their original length.36 However, the more that is known, the more complexities are revealed, and it is recognized that some SROs lack bag1 fibers and are still capable of generating a dynamic response.47 Although this area requires further investigation for clarification, it implies that the bag1 fibers are not essential to the dynamic response of primary endings and adds complication to the understanding of the signaling process.
The afferent response of SROs to muscle stretch is well recognized as playing an essential feedback role in regulating the state of extrafusal fibers. At the most basic level, stretching of intrafusal fibers initiates the monosynaptic myotatic reflex, generating the afferent component of the reflex, which will then cause coactivation of the efferent alpha- and gamma-motoneurons to activate the extrafusal and intrafusal fibers of the same, ie, homonomous, muscle. This homeostatic reflex helps extrafusal muscle fibers resist rapid, strong, and damaging stretch, but because of the complexity of the reflex, there are points within the reflex at which malfunctions could happen. The alpha-gamma coactivation ensures that intrafusal fibers shorten in tandem with the extrafusal fibers of the homonomous muscle, shortening so that the SROs do not go slack and are thus able to retain their sensitivities and respond to consequent stretches.45 The tonic intrafusal fibers and dynamic bag1 fibers are specifically activated by gamma-static and gamma-dynamic motoneurons, respectively.48
Osteopathic Relevance of the SRO
Given the known complexity of the SRO, it is easy to appreciate that malfunction of the SRO may be linked to somatic dysfunction and segmental facilitation. While some researchers5-8 have made no reference to the proprioceptors in their initial studies, Korr4 later presented the initial insight into SRO malfunction in the gamma-loop hypothesis. In this hypothesis, he proposed that altered proprioception via malfunctioning SROs can lead to an increased gamma-motoneuron drive because of an enhanced “gain” in the SRO system. In Korr's understanding, such altered gamma-motoneuron activity might result from “minor accidents or trauma of daily life,” which would initially unload the SROs and erroneously decrease gamma-motoneuron activity. However, due to the loss of afferent signal from the SROs, there could be an enhanced gamma-motoneuron drive to shorten the intrafusal fibers to regain the afferent signaling from the SROs. Such enhanced central drive, because of alpha-gamma coactivation, might lead to an overworking of the extrafusal fibers and increase extrafusal contractile force, generating the tissue texture change and tenderness noted in a somatic dysfunction and facilitated segment.5-8,49 Therefore, from a minor trauma there could arise a significant malfunction of the SROs, which could lead to excessive gamma- and alpha-motoneuron stimulation. Without intervention, this stimulation might generate long-term effects and pathologic changes to the muscle and to the viscera via neuronal reflexes, ie, somatovisceral changes. Alternatively, malfunctions generated in the viscera might lead to changes in the somatic tissue, ie, viscerosomatic changes. While evidence has increased over the years, the hypothesis has neither been fully confirmed nor disproven, and future research in this area should prove to be very informative.
As can be understood, with such trauma and with their complex second-to-second activity, SROs are continually adjusting as the extrafusal fiber lengths change. As a result it is likely that malfunctions, as hypothesized by Korr4,7 and Denslow8 may occur. Unlike controlled laboratory conditions, where signals from a single Group Ia afferent are typically reproducible, as are responses from different SRO preparations to the same stimulus,50 in vivo conditions are not as rigorously controlled nor controllable. Also since the SROs are not directly linked to the extrafusals in a syncytial arrangement, in vivo there is no assurance that the extrafusal fibers and the SROs are always working in “lock-step,” or that they would fire correctly at all times. In fact, being a biological system with linkage between alpha- and gamma-motoneurons requiring CNS involvement, activation together and in “lock-step” at all times is unlikely and in agreement with the gamma-loop hypothesis.
The likelihood of SROs malfunctioning is even more reasonable when it is recognized that, even under highly controlled experimental conditions, SROs have been recorded to malfunction.50,51 Experiments have shown that the SROs require a conditioning prestretch or oscillation to return to their initial control state for reliable results to be generated.50,51 Such results indicate that if intrafusal fibers are not returned to their control state, with a periodic stretch or oscillation, there may be phase advance and distortion in the afferent output that would affect the efferent response. It has been proposed that this phase advance may cause a nonlinear firing relationship between the dynamic and static afferent signals of the SRO, possibly due to the bag1 and bag2 intrafusal fibers functioning differently but signaling along the same Ia afferent.50 Being found in vivo, it is rather likely and it is proposed here that such malfunctioning, such as phase advance of SRO output, can occur in vivo, with OMT functioning as the periodic resetting mechanism. In a recent study involving simulated spinal manipulation in cats, the unloading of the SROs silenced their afferent firing for 1.3 ± 0.6 seconds (range, 0.1-4.3 seconds),52 a very physiologically relevant duration that would be more than sufficient to reset SROs and possibly initiate a functional change in gamma-motoneuron activity.
Afferent malfunction has also been shown to occur in humans, where application of a high frequency vibration to muscles can alter the proprioceptive perception of participants.53 In addition, illustrating other possible malfunctioning of the SRO, it has been shown that SRO afferents fire more action potentials during the dynamic response of an initial experimental trial than in consequent trials.54 The reduced response was also reflected in the associated activity recorded from alpha-motoneurons, showing that the change occurs throughout the stretch reflex,54 again indicating possible CNS involvement in SRO activity.
As concerns efferent signaling, results by Ellaway et al,55 illustrate that gamma-motoneuron firing is more complex than responding with a simple alpha-gamma coactivation. Instead, the gamma-static and gamma-dynamic motoneurons have been recorded firing at various rates seemingly dependent not only on feedback from the SRO, but from input from the CNS and what is expected to be sensed. In such a way, it is hypothesized that altered afferent function, or more central or viscerosomatic input, may alter gamma- and alpha-motoneuron firing and cause segmental facilitation and concomitant muscle hypertonicity as described by Denslow and Clough,5 Denslow et al,6 Korr,7 and Denslo.8 If such enhanced spinal segmental motoneuron excitability occurs, it could be the result of SRO malfunctions or involve the CNS. Additionally, such noted complex behavior of the gamma-static and gamma-dynamic motoneurons is of further interest because their functions, and the reason for having multiple classes of gamma-motoneurons, is yet to be fully elucidated.
It is also of interest to note that muscle from elderly persons is known to have altered SRO activity, leading to decreased proprioceptive acuity and increased passive stiffness.56,57 While such changes are a clinically important link to geriatric balance and falls, they may also be linked to the enhanced incidence of somatic dysfunction empirically reported in elderly persons. Such decreased proprioception may, similar to the gamma-loop hypothesis, relate to decreased afferent signaling, which in turn could lead to enhanced gamma-motoneuron drive to shorten the intrafusal fibers and enhance afferent feedback. The enhanced gamma-motoneuron activity might then concomitantly co-activate the extrafusal fibers and generate the stiffened condition that is noted in these individuals. In addition the level of dehydration often found in this age group could affect the fluid content of the SRO described previously in other studies. Such dehydration may enhance frictional forces within the SROs as the intrafusal fibers are brought closer together and also make them more apt to be affected by extracapsular pressures.
In addition to changes in afferent and efferent signaling, material properties of muscle may also be involved in SRO malfunction. It was recently found that in vivo muscle exhibits the property of thixotropy. This property is expressed by stating that the current viscosity of a material is decreased by prior activity, eg, shaking a bottle of ketchup to decrease its viscosity and enhance its flow. In muscle, thixotropy involves an initial stiffness that yields to more fluidity in movement as motion is continued. Such thixotropy in extrafusal muscle fibers appears to maintain crossbridge connections in resting muscle, causing a slower contraction at the initiation of muscular movement.55 Thixotropy could possibly aid in postural stability against short-lived external perturbations. However, if it occurs in the polar regions of intrafusal fibers, it could pose a problem: intrafusal fibers may not be able to respond as quickly or take up the “slack” when extrafusal fibers activate, resulting in a hindrance in their ability to track along with extrafusal fibers. As studies of thixotropy in muscle are limited, further research is needed to fully understand the implications in SRO function.
While the recent scientific findings presented here are of significant interest and shed some light on the relationship between SROs and somatic dysfunction, there is a significant caveat when discussing research on SROs: most of the research has been accomplished in distal limb muscles, with minimal research having been done on more proximal and axial muscle. This lack of research is primarily due to the difficulty in dissecting proximal and spinal muscles, but in recent years, new techniques and surgical procedures have been developed to better investigate spinal muscles. Results from related experiments59 indicate that SROs from feline longissimus lumborum muscles may differ functionally from those found in the limbs. This makes sense, as the specific function of spinal muscles, being supportive, differs from that of distal limb muscles, although further research is needed to determine the reasons and extent of these differences.
Regarding somatovisceral and viscerosomatic reflexes, while the ANS seems to be the appropriate center for this response, there is still much to be learned and understood concerning the ANS and its connection to the proprioceptive senses. The lack of a complete knowledge of the ANS is illustrated by research indicating the sympathetic innervation of SROs60-62 that is in agreement with the results of Strauss et al.63 In that study, an induced injury to the distal portion of the sciatic nerve of rat hindlimbs caused atrophy and altered the morphology of the anococcygeal muscles, smooth muscle far removed from the induced injury but innervated via the ANS at the same segmental level. My thought concerning those results was that the alteration in the proprioceptive input led to CNS adaptations that affected the output of the ANS to the smooth muscle. It is also proposed here that, along with altered input to the smooth muscle, the heightened sympathetic influence can also cause an increase in the blood flow to the skeletal muscle with concomitant reduction in the flow to the smooth muscle, with possible generation of a facilitated segment. This hypothesis remains to be tested.
Of the information presented herein concerning SROs, while primarily addressed to foundational scientific findings of Korr and Denslow, none of the information I presented downplays the possibility of central sensitization and habituation64,65 or other effects involving the CNS. Neither does it downplay the involvement of Golgi tendon organs, the other proprioceptive organs of muscle and tendon. Although less complex, these organs may also play a significant role in somatic dysfunction. As noted above, the more that is known concerning proprioception and spinal reflexes, the more it appears that higher neural centers are involved.66 In addition, the misfiring of neurons involved in phantom pain, which can be viewed as a viscerosomatic reflex, are of growing significance because of the increased incidence of battle-based amputations.67 While phantom pain differs from viscerosomatic and somatovisceral reflexes, it is similar in complexity as concerns the involvement with the CNS.
Conclusion
Proprioception and SROs have come to be recognized as being more complex than could have been realized only a few decades ago. It is highly likely that such functional complexity, with various afferent and efferent connections, may lead to malfunctions. In all, while research has led to significant gains in knowledge regarding SRO activity, there remains much yet to be discovered. Further research will be necessary to come to a more full understanding of the working of the SROs, their interconnections with the CNS concerning somatovisceral and viscerosomatic interactions, the exact mechanisms of the malfunctions, and the ameliorative mechanisms of OMT. I hope that this thorough review of pertinent information, including recent discoveries, may contribute to a better understanding of SROs and somatic dysfunction and help lead to studies that yield definitive statements regarding the role of SROs in somatic dysfunction.
References
1. Liem T. A.T. Still's osteopathic lesion theory and evidence-based models supporting the emerged concept of somatic dysfunction. J Am Osteopath Assoc. 2016;116(10):654-661.Search in Google Scholar
2. Proske U , GandeviaSC. The kinaesthetic senses.J Physiol. 2009;587(pt17):4139-4146. doi:10.1113/jphysiol.2009.175372Search in Google Scholar PubMed PubMed Central
3. Matthews PB . Muscle spindles and their motor control.Physiol Rev.1964;44:219-288.10.1152/physrev.1964.44.2.219Search in Google Scholar PubMed
4. Korr IM . Proprioceptors and somatic dysfunction.J Am Osteopath Assoc.1975;74(7):638-650.Search in Google Scholar
5. Denslow JS , CloughGH. Reflex activity in the spinal extensors.J Neurophysiol.1941;4:430-437.10.1152/jn.1941.4.6.430Search in Google Scholar
6. Denslow JS , KorrIM, KremsAD. Quantitative studies of chronic facilitation in human motoneuron pools. Am J Physiol. 1947;150:229-238.10.1152/ajplegacy.1947.150.2.229Search in Google Scholar PubMed
7. Korr IM . The neural basis of the osteopathic lesion.J Am Osteopath Assoc.1947;47(4):191-198.Search in Google Scholar
8. Denslow JS . Neural basis of the somatic component in health and disease and its clinical management.J Am Osteopath Assoc.1972;72(2):149-156.Search in Google Scholar
9. Patterson MM , SteinmetzJE. Long-lasting alterations of spinal reflexes: a potential basis for somatic dysfunction.Man Med1986;2:38-42.Search in Google Scholar
10. van Buskirk RL . Nociceptive reflexes and the somatic dysfunction: a model.J Am Osteopath Assoc.1990;90(9):792-809.10.1515/jom-1990-900916Search in Google Scholar
11. Fryer G. Somatic dysfunction: an osteopathic conundrum. Int J Osteopath Med. 2016;22:52-63.10.1016/j.ijosm.2016.02.002Search in Google Scholar
12. Zein-Hammoud M , StandleyPR. Modeled osteopathic manipulative treatments: a review of their in vitro effects on fibroblast tissue preparations.J Am Osteopath Assoc.2015;115(8):490-502. doi:10.7556/jaoa.2015.103Search in Google Scholar PubMed
13. Olmsted JMD , HarrisE.Claude Bernard and the Experimental Method in Medicine.New York, NY: Henry Schuman; 1952.Search in Google Scholar
14. Cannon WB . The Wisdom of the Body.New York, NY: WW Norton & Company Inc;1932.Search in Google Scholar
15. Saggio G , DocimoS, PilcJ, NortonJ, GilliarW.Impact of osteopathic manipulative treatment on secretory immunoglobulin A levels in a stressed population.J Am Osteopath Assoc.2011;111(3):143-147.Search in Google Scholar
16. Licciardone JC , KearnsCM, MinottiDE. Outcomes of osteopathic manual treatment for chronic low back pain according to baseline pain severity: results from the OSTEOPATHIC Trial.Man Ther.2013;18(6):533-540. doi:10.1016/j.math.2013.05.006Search in Google Scholar PubMed
17. Hensel KL , BuchananS, BrownSK, RodriguezM, CruserDA. Pregnancy research on osteopathic manipulation optimizing treatment effects: the PROMOTE study.Am J Obstet Gynecol. 2015;212(1):108.e1-e9. doi:10.1016/j.ajog.2014.07.043Search in Google Scholar PubMed PubMed Central
18. Cerritelli F , PizzolorussoG, RenzettiC, et al.A multicenter, randomized, controlled trial of osteopathic manipulative treatment on preterms.PLoS One.2015;10(5):e0127370. doi:10.1371/journal.pone.0127370Search in Google Scholar PubMed PubMed Central
19. Korr IM . Somatic dysfunction, osteopathic manipulative treatment, and the nervous system: a few facts, some theories, many questions.J Am Osteopath Assoc.1986;86(2):109.10.1515/jom-1986-860217Search in Google Scholar
20. Ruhlen RL , SinghVK, PazdernikVK, et al. Changes in rat spinal cord gene expression after inflammatory hyperalgesia of the joint and manual therapy. J Am Osteopath Assoc. 2014;114(10):768-776. 10.7556/jaoa.2014.151Search in Google Scholar PubMed
21. Shanahan LKT , RainesSGM, CogginsRL, MooreT, CarnesM, GriffinL.Osteopathic manipulative treatment in the management of Isaacs syndrome.J Am Osteopath Assoc.2017;117(3):194-198. doi:10.7556/jaoa.2017.035Search in Google Scholar PubMed
22. Hulliger M. The mammalian muscle spindle and its central control. In:Reviews of Physiology, Biochemistry and Pharmacology.Berlin, Heidelberg: Springer;1984:1-110.10.1007/BFb0027694Search in Google Scholar PubMed
23. Suslak TJ , JarmanAP. Stretching the imagination beyond muscle spindles - stretch-sensitive mechanisms in arthropods.J Anat.2015;227(2):237-242. doi:10.1111/joa.12329Search in Google Scholar PubMed PubMed Central
24. Bastian HC . The “muscular sense”; its nature and localization.Brain.1888;10:1-36.10.1093/brain/10.1.1Search in Google Scholar
25. Sherrington C. On the proprio-ceptive system, especially in its reflex aspects. Brain . 1906;29:467-482.10.1093/brain/29.4.467Search in Google Scholar
26. Banks RW . The innervation of the muscle spindle.J Anat.2015;227(2):115-135. doi:10.1111/joa.12297Search in Google Scholar PubMed PubMed Central
27. Adrian ED , ZottermanY.The impulses produced by sensory nerve-endings, part II: the response of a single end-organ.J. Physiol.1926;61(2):151-171.10.1113/jphysiol.1926.sp002281Search in Google Scholar PubMed PubMed Central
28. Matthews BH . Nerve endings in mammalian muscle.J Physiol.1933;78:1-53.10.1113/jphysiol.1933.sp002984Search in Google Scholar PubMed PubMed Central
29. Kucera J , WalroJM. Sequences of intrafusal fiber formation are muscle-dependent in rat hindlimbs. Anat Embryol (Berl). 1994;190(3):273-286.Search in Google Scholar
30. Eriksson PO , Butler-BrowneGS, ThornellLE. Immunohistochemical characterization of human masseter muscle spindles. Muscle Nerve. 1994;17(1):31-41.10.1002/mus.880170105Search in Google Scholar
31. Kucera J , WalroJM. Origin of intrafusal muscle fibers in the rat.Histochemistry.1990;93:567-580.10.1007/BF00272199Search in Google Scholar
32. Pedrosa-Domellöf F , HellströmS, ThornellL-E.Hyaluronan in human and rat muscle spindles.Histochem Cell Biol.1998;110:179-182.10.1007/s004180050279Search in Google Scholar
33. Hunt LC , GormanC, KintakasC, McCullochDR, MackieEJ, WhiteJD. Hyaluronan synthesis and myogenesis: a requirement for hyaluronan synthesis during myogenic differentiation independent of pericellular matrix formation.J Biol Chem.2013;288(18):13006-13021.10.1074/jbc.M113.453209Search in Google Scholar
34. Botterman B , BinderMC, StuartDG. Functional anatomy of the association between motor units and muscle receptors.Integr Comp Biol. 1978;18(1):135-152. doi:10.1093/icb/18.1.135Search in Google Scholar
35. Barker D. The morphology of muscle receptors. In: HungCC, ed.Handbook of Sensory Physiology.New York, NY: Springer-Verlag;1974:90-95.10.1007/978-3-642-65945-4_1Search in Google Scholar
36. Boyd IA . The structure and innervation of the nuclear bag muscle fibre system and the nuclear chain muscle fibre system in mammalian muscle spindles.Philos Trans Royal Soc B.1962;245:81-136. doi:10.1098/rstb.1962.0007Search in Google Scholar
37. Österlund C , LiuJX, ThornellLE, ErikssonPO. Intrafusal myosin heavy chain expression of human masseter and biceps muscles at young age shows fundamental similarities but also marked differences. Histochem Cell Biol.2013;39(6):895-907. doi:10.1007/s00418-012-1072-7Search in Google Scholar
38. Liu JX , ThornellLE, Pedrosa-DomellöfF. Distribution of SERCA isoforms in human intrafusal fibers. Histochem Cell Biol.2003;120(4):299-306.10.1007/s00418-003-0569-5Search in Google Scholar
39. Walro JM , KuceraJ. Sharing of sensory terminals between the dynamic bag1 and static bag2 fibers in the rat muscle spindle.Brain Res.1987;425:311-318.10.1016/0006-8993(87)90514-2Search in Google Scholar
40. Kucera J. Histological study of motor innervation of nuclear bag1 intrafusal muscle fibers in the cat. J Comp Neurol . 1985;232(3):331-346.10.1002/cne.902320306Search in Google Scholar
41. Matthews PB . The response of de-efferented muscle spindle receptors to stretching at different velocities.J Physiol.1963;168:660-678. doi:10.1113/jphysiol.1963.sp007214Search in Google Scholar
42. Ovalle WK , SmithRS. Histochemical identification of three types of intrafusal muscle fibers in the cat and monkey based on the myosin ATPase reaction.Can J Physiol Pharmacol.1972;50(3):195-202.10.1139/y72-030Search in Google Scholar
43. Barker D , BanksRW, HarkerDW, MilburnA, StaceyMJ. Studies of the histochemistry, ultrastructure, motor innervation and reinnervation of mammalian intrafusal muscle fibers.Prog Brain Res.1976;44:67-88.10.1016/S0079-6123(08)60724-4Search in Google Scholar
44. Saito M , TomonagaM, HirayamaK, NarabayashiH.Histochemical study of normal muscle spindles. histochemical classification of intrafusal muscle fibers and intrafusal nerve endings.J Neurol. 1977;216(2):79-89.10.1007/BF00312942Search in Google Scholar
45. Boyd, IA . The isolated mammalian muscle spindle.Trends Neurosci.1980;3(11):258-265.10.1016/0166-2236(80)90096-XSearch in Google Scholar
46. Banks RW , HarkerDW, StaceyMJ. A study of mammalian intrafusal muscle fibres using a combined histochemical and ultrastructural technique.J Anat.1977;123(pt 3):783-796.Search in Google Scholar
47. Scott JJ . Responses of Ia afferent axons from muscle spindles lacking a bag1 intrafusal muscle fiber.Brain Res.1991;543(1):97-101. doi:10.1016/0006-8993(91)91052-3Search in Google Scholar
48. Emonet-Dénand F , LaporteY, MatthewsPB, PetitJ.On the subdivision of static and dynamic fusimotor actions on the primary ending of the cat muscle spindle.J Physiol. 1977;268(3):827-861.10.1113/jphysiol.1977.sp011884Search in Google Scholar PubMed PubMed Central
49. Denslow JS . Neural basis of the somatic component in health and disease and its clinical management.J Am Osteopath Assoc.1972;72(2):149-156.Search in Google Scholar
50. Banks RW , HulligerM, ScheepstraKA, OttenE.Pacemaker activity in a sensory ending with multiple encoding sites: the cat muscle spindle primary ending.J Physiol.1997;498(pt 1):177-199. doi:10.1113/jphysiol.1997.sp021850Search in Google Scholar PubMed PubMed Central
51. Morgan DL , ProchazkaA, ProskeU.The after-effects of stretch and fusimotor stimulation on the responses of primary endings of cat muscle spindles.J Physiol.1984;356:465-477.10.1113/jphysiol.1984.sp015477Search in Google Scholar PubMed PubMed Central
52. Pickar JG , WheelerJD. Response of muscle proprioceptors to spinal manipulative-like loads in the anesthetized cat.J Manipulative Physiol Ther.2001;24:2-11.10.1067/mmt.2001.112017Search in Google Scholar
53. Haftel VK , BichlerEK, NicholsTR, PinterMJ, CopeTC. Movement reduces the dynamic response of muscle spindle afferents and motoneuron synaptic potentials in rat. J Neurophysiol. 2004;91(5):2164-2171.10.1152/jn.01147.2003Search in Google Scholar
54. Goodwin GM , McCloskeyDI, MatthewsPB. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents.Brain.1972;95(4):705-748. doi:10.1093/brain/95.4.705Search in Google Scholar
55. Ellaway PH , TaylorA, DurbabaR.Muscle spindle and fusimotor activity in locomotion.J Anat.2015;227(2):157-166. doi:10.1111/joa.12299Search in Google Scholar
56. Pai YC , RymerWZ, ChangRW, SharmaL.Effect of age and osteoarthritis on knee proprioception.Arthritis Rheum.1997;40(12):2260-2265.10.1002/art.1780401223Search in Google Scholar
57. Rosant C , NagelMD, PérotC.Aging affects passive stiffness and spindle function of the rat soleus muscle.Exp Gerontol.2007;42(4):301-308. doi:10.1016/j.exger.2006.10.007Search in Google Scholar
58. Altman D , MinozzoFC, RassierDE. Thixotropy and rheopexy of muscle fibers probed using sinusoidal oscillations.PLoS ONE.2015;10(4):e0121726. doi:10.1371/journal.pone.0121726Search in Google Scholar
59. Durbaba R , TaylorA, EllawayPH, RawlinsonS.Classification of longissimus lumborum muscle spindle afferents in the anaesthetized cat.J Physiol.2006;571(pt 2):489-498.10.1113/jphysiol.2005.102731Search in Google Scholar
60. Banker B , GirvinJ.The ultrastructural features of the mammalian muscle spindle.J Neuropath Exp Neurol.1971;30:155-195.10.1097/00005072-197104000-00001Search in Google Scholar
61. Santini M , IbataY. The fine structure of thin myelinated axons within muscle spindles. Brain Res. 1971;33:289-302.10.1016/0006-8993(71)90104-1Search in Google Scholar
62. Radovanovic D , PeikertK, LindströmM, DomellöfFP. Sympathetic innervation of human muscle spindles.J Anat.2015;226(6):542-548. doi:10.1111/joa.12309Search in Google Scholar PubMed PubMed Central
63. Strauss JD , PetrizzoAM, AndrewsMAW. Alterations of neural input to rat hindlimb muscle results in atrophy of visceral smooth muscle: a somatovisceral reflex [abstract]?J Am Osteopath Assoc.1998;98:452.Search in Google Scholar
64. Patterson MM . A model mechanism for spinal segmental facilitation.J Am Osteopath Assoc.1976;76(1):62-72.Search in Google Scholar
65. Patterson MM , SteinmetzJE. Long-lasting alterations of spinal reflexes: a potential basis for somatic dysfunction.Man Med.1986;2:38-42.Search in Google Scholar
66. Proske U , GandeviaSC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force.Physiol Rev.2012;92:1651-1697. doi:10.1152/physrev.00048.2011Search in Google Scholar PubMed
67. Weeks SR , Anderson-BarnesVC, TsaoJW. Phantom limb pain: theories and therapies.Neurologist.2010;16(5):277-286. doi:10.1097/NRL.0b013e3181edf128Search in Google Scholar PubMed
© 2019 American Osteopathic Association
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- LETTERS TO THE EDITOR
- Response
- Contemporary Routes of Cannabis Consumption
- ORIGINAL CONTRIBUTION
- Using the Multitheory Model to Predict Initiation and Sustenance of Physical Activity Behavior Among Osteopathic Medical Students
- Influence of Osteopathic Medical Students’ Personal Health on Attitudes Toward Counseling Obese Pediatric Patients
- Empathy in Medicine Osteopathic and Allopathic Physician Interpersonal Manner, Empathy, and Communication Style and Clinical Status of Their Patients: A Pain Registry–Based Study
- REVIEW
- Stretch Receptor and Somatic Dysfunction: A Narrative Review
- JAOA/AACOM MEDICAL EDUCATION
- Empathy in Medicine National Norms for the Jefferson Scale of Empathy: A Nationwide Project in Osteopathic Medical Education and Empathy (POMEE)
- Novel Approach to Introducing an Ultrasonography Curriculum With Limited Instructor Resources
- CLINICAL IMAGES
- Barium Contrast Aspiration
Articles in the same Issue
- LETTERS TO THE EDITOR
- Response
- Contemporary Routes of Cannabis Consumption
- ORIGINAL CONTRIBUTION
- Using the Multitheory Model to Predict Initiation and Sustenance of Physical Activity Behavior Among Osteopathic Medical Students
- Influence of Osteopathic Medical Students’ Personal Health on Attitudes Toward Counseling Obese Pediatric Patients
- Empathy in Medicine Osteopathic and Allopathic Physician Interpersonal Manner, Empathy, and Communication Style and Clinical Status of Their Patients: A Pain Registry–Based Study
- REVIEW
- Stretch Receptor and Somatic Dysfunction: A Narrative Review
- JAOA/AACOM MEDICAL EDUCATION
- Empathy in Medicine National Norms for the Jefferson Scale of Empathy: A Nationwide Project in Osteopathic Medical Education and Empathy (POMEE)
- Novel Approach to Introducing an Ultrasonography Curriculum With Limited Instructor Resources
- CLINICAL IMAGES
- Barium Contrast Aspiration

