Understanding Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and the Emerging Osteopathic Approach: A Narrative Review
-
Christopher Larrimore
Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating syndrome of unknown origin, characterized by profound postexertional malaise and fatigue, unrefreshing sleep, cognitive impairments, immune dysfunction, pain, autonomic dysfunction, and neuroendocrine symptoms. Although ME/CFS is well documented within the medical literature, it remains difficult to diagnosis and manage. Some of the current challenges include an absence of diagnostic markers, differing diagnostic criteria, and an overall lack of awareness within the medical community. As a result, patients are often frustrated by the difficulties in acquiring a diagnosis and from the overall lack of available treatments. In an effort to increase awareness, this review discusses disease pathophysiology, clinical presentation, and treatment options, while also highlighting the benefits of an osteopathic approach.
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex disease characterized by multiple symptoms that can include postexertional malaise and fatigue, unrefreshing sleep, cognitive impairments, immune dysfunction, pain, autonomic dysfunction, and neuroendocrine symptoms.1-4 On average, 10% of patients receive a diagnosis within 5 years.5 This delay in diagnosis is primarily attributed to the absence of specific diagnostic tests and an overall lack of clinical awareness. Although documented clinical cases span more than 2 centuries, universally accepted diagnostic criteria do not exist. Instead, there are nearly 20 clinical definitions.1-8 Multiple name changes add further confusion. The most notable names include neurasthenia, chronic fatigue immune disorder syndrome (CFIDS), chronic fatigue syndrome (CFS), myalgic encephalomyelitis (ME), and, more recently, systemic exertion intolerance disease (SEID).
With so much variation in defining the diagnostic criteria and naming the disease, confusion and doubt persist within the medical community. In a recent effort to unify definitions and focus on the primary features of the disease, the National Academy of Medicine, formerly the Institute of Medicine, suggested new diagnostic criteria and the change of name to SEID.1 However, SEID's diagnostic criteria are less specific and do not exclude psychiatric disorders. In particular, the new diagnostic criteria do not exclude individuals with major depressive disorder, which alters interpretations of epidemiology, etiology, and treatment research. Without more specific diagnostic criteria, limitations in research remain, and a better understanding within medical communities is hindered. Often causing patients to be bedridden, ME/CFS can be severely disabling and, as such, quicker diagnosis and appropriate treatment is needed. Further adding to the burden of the disease is the stigma associated with ME/CFS. Without a greater consensus among clinicians (physicians, physician assistants, and nurse practitioners) about the validity of this disease, patients will continue to feel alienated.
In an effort to reduce the confusion surrounding ME/CFS, this review provides a comprehensive summary of current ME/CFS understanding and highlights the benefits of the osteopathic approach for this condition. Using Pubmed and UpToDate, articles were selected on the basis of journal quality and content. Journal quality was determined using the journal impact factor, the publishing institute, and the number of times an article had been referenced. Content focused on current pathophysiology and treatments, osteopathic ME/CFS research, and methods for diagnosis.
Societal Impact
A population-based study9 on ME/CFS prevalence was conducted from 1995 to 1998 with a sample size of 18,675 people. The study revealed an approximated prevalence of 420 cases per 100,000 individuals. Rates were higher among minorities than whites and higher among low-income populations than high-income populations. In the United States, the National Academy of Medicine reports the number of people with the disease to be between 836,000 to 2.5 million.1 However, the accuracy of these estimates is debated because prevalence rates are largely dependent on the diagnostic criteria used.
Similar to prevalence rates, the economic loss is challenging to accurately estimate. In one study,10 the annual economic cost was estimated to be between $17 to $24 billion. In other studies,11,12 the estimated individual economic loss was between $11,780 to $20,000 annually. These amounts are similar to a previous UK study13 that found a mean cost to patients of £3,515 over a 3-month span. Variances in estimations may be attributed to study populations. Unemployment among patients with CFS is estimated to be approximately 50% to 54%.14,15 Costs related to loss of employment owing to chronic fatigue account for 61% of patients’ total economic cost.16
ME/CFS Pathophysiology
Myalgic encephalomyelitis/chronic fatigue syndrome is a heterogeneous disorder with an unknown origin. Several proposed hypotheses include microbial triggers, immune dysregulation, mitochondrial dysfunction, oxidative stress, and endocrine abnormalities in genetically susceptible people.
Microbial Triggers
Reports17-19 describing disease outbreaks that have resembled ME/CFS in “closed populations” such as hospitals and convents can be traced back more than 60 years. Because these outbreaks occurred among persons in close proximity, it has been suggested that the ME/CFS is either infectious or results from an infectious agent. To support this hypothesis, prospective studies have found the development of ME/CFS in 11% of people after serious infections with the Epstein Barr virus (EBV), non–EBV-associated glandular fever, Ross River virus, Giardia lamblia, parvovirus B19, and Q fever infections.20-23 Despite differences in the acute phase of infection by EBV, Ross River virus, and Q fever, severity of the acute illness was shown to be predictive of the development of a postinfective fatigue disease.20 The frequency of ME/CFS was 8 times higher in patients with confirmed Giardia infection when compared with the general population, and fatigue symptoms were still present up to 5 years after infection in some patients.21-22 Other infectious agents correlated to the development of ME/CFS include Borna virus,24,25 enterovirus,26 and human herpesviruses 6 and 7.27-29
The host microbiome has also been implicated as a source of dysregulation leading to ME/CFS. Individuals with ME/CFS present with alterations in their oral and intestinal microbiomes when compared with healthy controls.30-32 These alterations, coupled with dysfunction of the intestinal mucosal barrier, can precipitate an inappropriate immune response. Translocations of enteric bacteria into systemic circulation during periods of high activity may contribute to the postexertional malaise.33 Investigation into the role of microbial triggers continues; however, it is likely to be the primary cause of this disease. Given that each virus, bacteria, and parasite is uniquely different, the resulting infection is more likely due to an underlying dysfunction in the patient's immune system and not the microbe itself.
Immune Dysregulation
Studies have revealed reduced natural killer cell function in patients with ME/CFS, which supports the findings of immune dysregulation.34-37 A primary role of natural killer cells is to destroy virally infected cells. A defect in this response would increase susceptibility to viral infections. Additional supporting evidence is the observation of increased immune cell activation markers (CD38, HLA-DR) on CD8 cells using flow cytometry.38 Activated CD8 cells, also called cytotoxic T cells, target damaged host cells and virally infected cells. Overactivation of CD8 cells has been linked to autoimmune diseases and supports the theory of immune dysregulation. An elevation in interleukin-1β (IL-1β), IL-12, IL-8, IL-10, and IL-13 after moderate-intensity exercise has also been observed,39 with sustained increase in plasma tumor necrosis factor-α, in patients with ME/CFS compared with healthy controls.39 Tumor necrosis factor-α and IL-1β are known inducers of acute-phase reactants and not typically elevated after exercise. In addition, a more exaggerated response in complement and an apparent alteration in immune cell gene expression after physical exertion, most notably toll-like receptor 4, has been uncovered.40 Overall, these studies point to a likely involvement in immune dysregulation, resulting in secondary infections, altering the microbial trigger narrative.
Mitochondrial Dysfunction
Patients with ME/CFS have decreased intracellular pH and lower adenosine triphosphate (ATP) production after exercise, impaired oxidative phosphorylation, and mitochondrial damage.41-44 A study42 showed that carnitine, an essential compound correlated to functional capacity of the mitochondria, was decreased in patients with ME/CFS. Using an “ATP Profile” test, the study further revealed the degree of mitochondrial dysfunction in neutrophils correlated with severity of the patients’ illness.42 Postexertional fatigue, a common symptom, is also exhibited in patients with mitochondrial disease. In another study,45 citrate synthase, a critical enzyme needed for the tricarboxylic cycle, was found at reduced levels in patients with ME/CFS when compared with healthy controls. Additionally, other mitochondrial transmembrane enzyme complexes, including succinate reductase and cytochrome-C oxidase, were reduced in patients with ME/CFS.46 The mitochondrial hypothesis is attractive because it could explain symptoms unrelated to fatigue given that mitochondrial dysfunction in specific organs would affect organ function and could explain comorbidities associated with the disease.
Genetic Predisposition
The possibility of a genetic link or predisposition is another area of interest. Genealogy data from 3 generations in the Utah Population Database showed an increased relative risk among first-degree relatives (2.70), second-degree relatives (2.34), and third-degree relatives (1.93). In a twin study, the concordance rate was found to be higher in monozygotic twins (55%) than dizygotic twins (19%).49 The expression of major histocompatibility complex class II antigens HLA-DQA1*01 and HLA-DR4 may also be a potential risk factor for developing ME/CFS.50 Additionally, single nucleotide polymorphism in the tumor necrosis factor-α and interferon-γ genes may provide a genetic link to explain the dysregulation of inflammatory cytokine production in patients with ME/CFS.50 Single nucleotide polymorphisms were also identified in the glutamate ionotropic receptor kainate type subunit 3 gene, which codes for transmembrane subunits of neuroexcitatory receptors. In addition, a single nucleotide polymorphism in the neuronal PAS domain protein 2 gene, which is a circadian clock gene, was found in an ME/CFS patient cohort.50
Oxidative Stress and Endocrine Abnormalities
Oxidative stress and hypothalamic-pituitary-adrenal axis abnormalities are 2 additional areas of investigation. In a study51 evaluating oxidative stress in patients with ME/CFS compared with a control group, results revealed a greater increase in oxidative stress markers after exertion compared with the control group. Biomarkers evaluated included thiobarbituric acid reactive substance and reduced ascorbic acid. Because the accumulation of reactive oxygen species is known to modify mitochondrial proteins and lipids, oxidative stress may be influencing mitochondrial function.
Hypoactivity with the hypothalamic-pituitary-adrenal (HPA) axis has also been observed. In particular, patients with ME/CFS have been found to have higher adrenocorticotropic hormone (ACTH) autoantibodies, blunted dehydroepiandrosterone (DHEA) response to ACTH injections, and increased prolactin in response to buspirone.52 An increase in ACTH autoantibodies would disrupt the HPA axis, ultimately resulting in depressed cortisol production and psychologic disturbances. Decreased DHEA levels would not only influence androgen production, but also cognitive function and inflammation. The hormone DHEA is a neurotrophin that promotes nerve cell survival and is also an uncompetitive inhibitor of glucose-6-phosphate dehydrogenase, reducing free radical production. An increase in prolactin would suppress gonadotropin-releasing hormone, further altering the HPA axis.
ME/CFS Diagnostic Criteria and Comorbidities
An estimated 10% of patients with ME/CFS obtain a diagnosis within 5 years, which leaves a vast majority of patients untreated.5 The delay in diagnosis can be attributed to a present lack of known biologic markers, varying diagnostic criteria, and inadequate education about ME/CFS among clinicians. The 2 most commonly used diagnostic criteria, both in clinical practice and research, are the 2015 Institute of Medicine criteria1 and the more commonly used 2003 Canadian Consensus Criteria4 (Figure 1). For coding purposes, ME/CFS in the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) is G04.81.
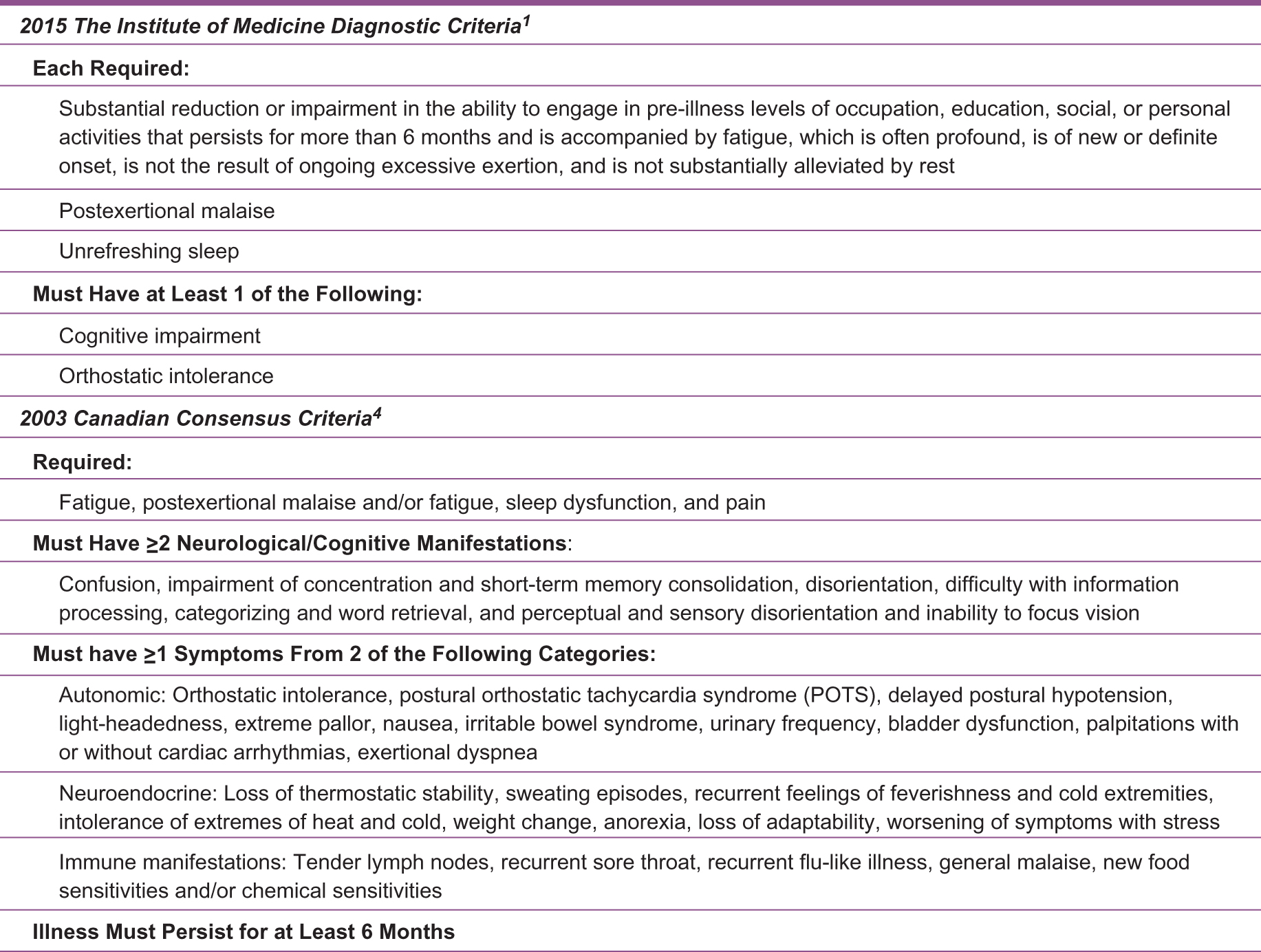
Descriptions of the 2 most commonly used myalgic encephalomyelitis/chronic fatigue syndrome diagnostic criteria.
Treating a patient requires taking a thorough patient history, satisfying the clinical case definition criteria, and ruling out other conditions. A complete laboratory workup should also be obtained to provide a more detailed picture of a patient's condition and to discover any comorbidities that may be present (Figure 2). Recognizing a comorbidity is important, as it may sometimes be the clue that leads to a ME/CFS diagnosis and alters the treatment approach. Routine laboratory tests that include thyroid function, liver function, complete blood cell count, and autoimmune testing are necessary for developing a treatment approach. Although no known ME/CFS biological markers are currently known, 2 available methods to reach diagnosis are the 2-day cardiopulmonary exercise test (CPET) and an osteopathic evaluation.
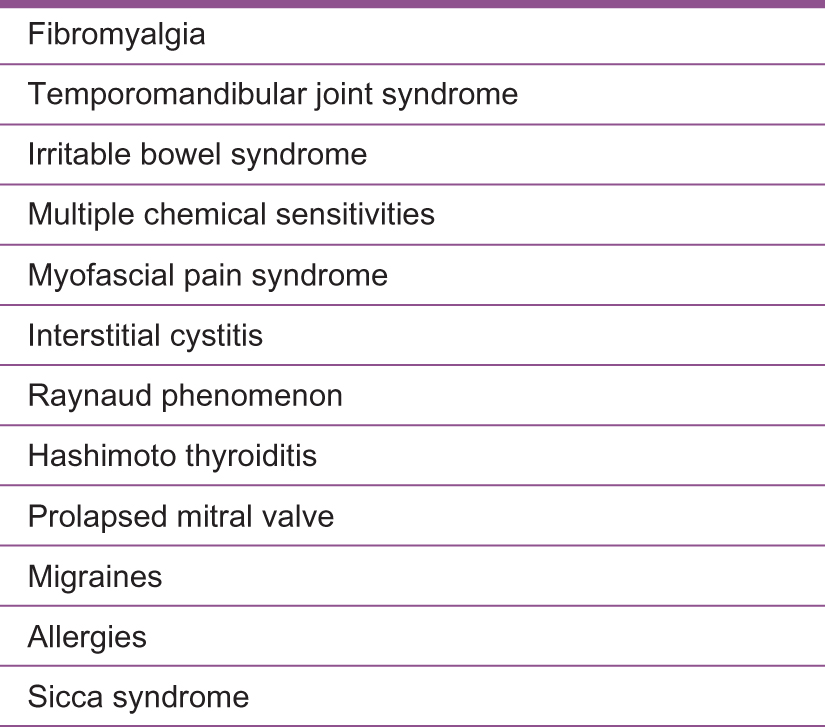
List of comorbid conditions with myalgic encephalomyelitis/chronic fatigue syndrome.
Postexertional malaise is the worsening of a patient's symptoms and function after physical or cognitive activity that was easily endured premorbidly. While fatigue in general can be considered a subjective complaint, accurate energy consumption can be measured using a CPET. The CPET is used to measure exercise capacity in an individual and provides both objective and reproducible measures of functional capacities for cardiovascular and pulmonary disease. Aerobic/oxidative metabolism is the primary pathway for energy production in activities lasting longer than 90 seconds. In patients with ME/CFS, aerobic metabolism may be impaired, thereby causing activity performed for longer than 90 seconds to rely on anaerobic metabolism. Studies show that despite maximal effort on 2 consecutive days, patients show a decrease in functional capacity.52-54 While this test objectively measures the hallmark symptom of postexertional fatigue, it is not commonly used in clinical practice because of limited availability and patient tolerance.
Treatment Approaches
As part of an individualized treatment plan, clinicians should educate patients about the disease and encourage a healthy lifestyle. Clinicians should address energy pacing, symptom management, and management of comorbidities. The main focus should include the use of integrated modalities to enable patients to better adapt and manage their chronic disease. A more holistic approach through osteopathic medicine can be used to complement more traditional methods described in this section. Specialists should also be consulted to build a more collaborative treatment plan that is beneficial in managing comorbidities. For example, a cardiologist should diagnose and manage postural orthostatic tachycardia syndrome, which is a common comorbidity of ME/CFS and a form of dysautonomia that affects blood flow through the body and often causes a person to become dizzy on standing.
Education
Patient education is imperative for the understanding and management of care. However, in our opinion, it is more important to educate clinicians how to diagnose and manage ME/CFS. Because most patients often report feelings of isolation and frustration resulting from negative experiences with clinicians who lack knowledge about ME/CFS, educating clinicians about the disease would improve patient care. Clinicians knowledgeable about ME/CFS would be able to recognize the disease, initiate treatment, and improve patient outcomes earlier than those without an understanding of ME/CFS.
Informed clinicians would result in early disease recognition, timely treatments, and better patient outcomes. Through increased understanding, clinicians can set realistic expectations and achieve improved treatment compliance.
Energy Management
A key diagnostic criterion is profound fatigue absent of ongoing exertion. With energy deficits being a major feature of the disease, educating patients in energy management techniques is helpful. Pacing is one energy management technique that can be taught. The goal of pacing is for a patient to live within his or her individual “energy envelope.” Patients learn to listen to their bodies and stop activities before their symptoms relapse. They learn to divide their daily activities into manageable portions alternating with rest periods throughout the day to avoid overexertion. Decreasing stress can also be effective in maintaining an energy envelope and influencing overall health. Techniques for stress reduction can include meditation, yoga, and tai chi. However, before engaging in alternative methods for stress reduction, an individual's energy tolerance should be considered.
Sleep Disorders
Unrefreshing sleep is a diagnostic criterion of ME/CFS. If not treated, cognitive function, memory, and pain perception can all be affected. In developing a treatment plan, actions to improve sleep hygiene should be taken. Improving sleep quality has the potential to decrease symptom severity. As such, it is important to manage sleep disorders. If not successful, medications can then be prescribed (Figure 3).
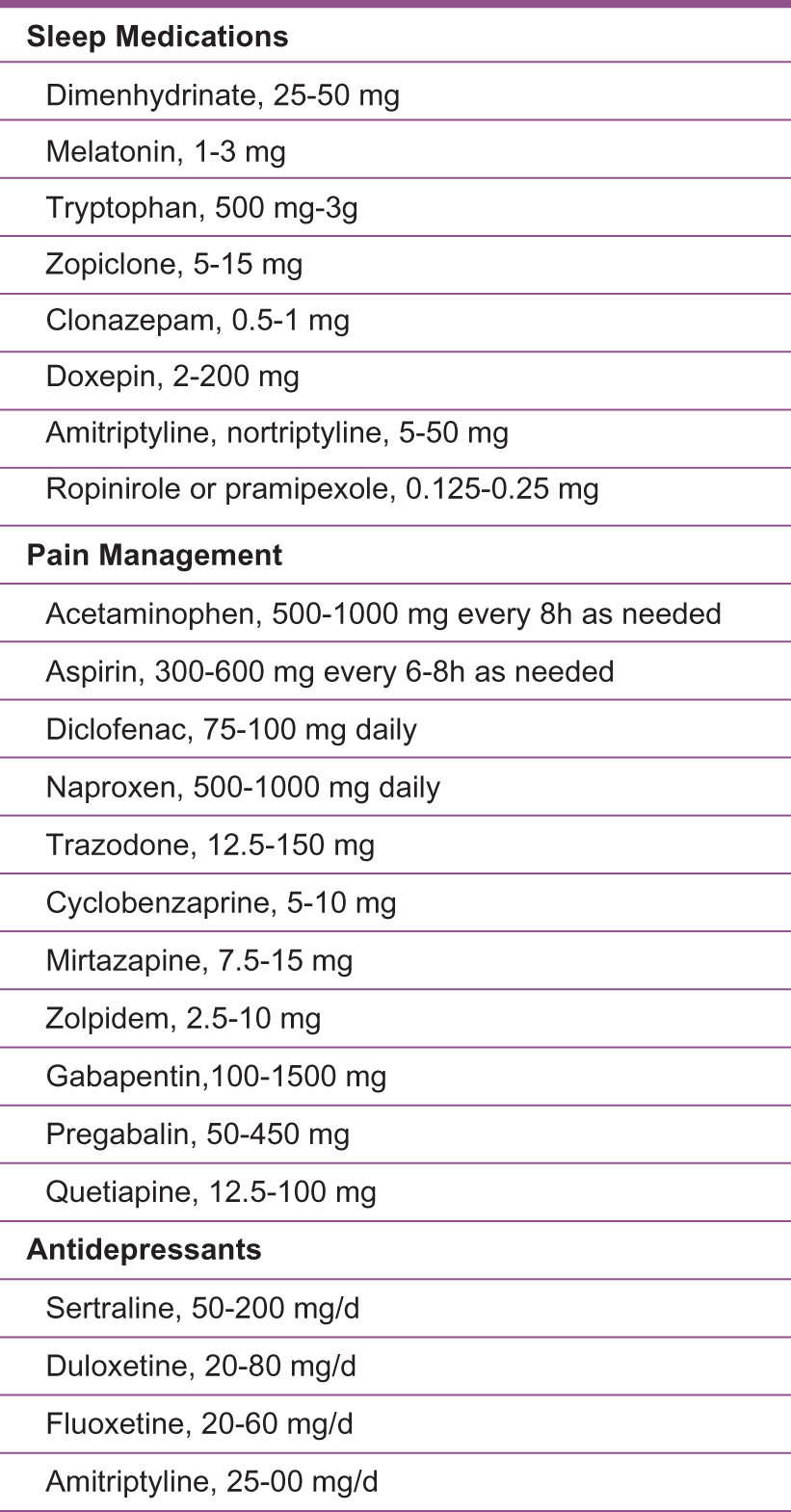
List of medications and dosages that are helpful in managing myalgic encephalomyelitis/chronic fatigue syndrome.
Diet
For many patients with ME/CFS, maintaining a balanced diet can be challenging because of lack of money to buy food, lack of energy to purchase food, and lack of energy to prepare food. With these restraints present, diet management becomes an important component of treatment. Multivitamin supplementation should be encouraged to offset vitamin and mineral deficiencies and promote better energy metabolism.
Supplements of particular interest include zinc, essential fatty acids, coenzyme Q10 (CoQ10), and vitamin B12.52 Zinc deficiency has been linked to reduced natural killer cell function, a known immune impairment of patients with ME/CFS.55,56 Essential fatty acid supplementation has been shown to improve some patient symptoms, as has CoQ10 supplementation.57,58 Plasma CoQ10 concentrations are typically low in many patients with ME/CFS because CoQ10 is an important coenzyme in the electron transport chain of the mitochondria, supplementing 100 to 400 mg daily may help.51 Vitamin B12 can be beneficial. Low levels of vitamin B12 have been found in CSF samples from patients with ME/CFS.59 In a 2015 study, frequent vitamin B12 injections combined with daily doses of oral folic acid was shown to improve energy levels among patients.60
Intestinal Dysbiosis
Altered intestinal bacteria may influence the processing of emotions. A connection between intestinal bacteria and the central nervous system, via vagal sensory nerve fibers and the peripheral immune system, is becoming an area of increasing interest.61,62 In a 2009 study,63 small amounts of microbes were found to be capable of influencing the paraventricular hypothalamus, the amygdala, and the bed nucleus of the stria terminalis. Because these regions are important for emotion and mood processing, altered intestinal flora may contribute to ME/CFS symptoms. After supplementation with Lactobacillus casei strain Shirota for 8 weeks, reduction in anxiety was found.63
Acute presentation of D-lactic acidosis, a result of intestinal Streptococcus species overgrowth, has overlapping features with ME/CFS. In a study where intestinal Streptococcus was reduced using oral erythromycin, ME/CFS patient outcomes revealed improved quality of sleep and cognition; however, no mood or fatigue symptoms were improved.64 While antibiotic therapy is not recommended for ME/CFS management, the alteration of gut bacteria, either by supplementation or removal, seems to have an effect on patient health.
Antidepressants
Since a curative treatment has not been found, the management of comorbidities in patients with ME/CFS is crucial. In addition to fatigue, patients often experience degrees of pain, depression, and anxiety.1,2,6-9 While there is stronger evidence for the efficacy of antidepressants in conditions such as fibromyalgia, antidepressants can also work to alleviate depression brought on by dealing with a chronic medical condition.65 In a double-blind crossover study,66 60 mg/day of nortriptyline was shown to relieve depressive symptoms and fatigue (as measured by the Beck Depression Inventory and Chronic Fatigue Symptom Checklist). In addition, a case report65 has also shown benefit following a regimen of amitriptyline and doxepin (25-50 mg at night). Cognitive behavioral therapy can also be beneficial when used in conjunction with antidepressants.64
Osteopathic Medicine
The unique benefits of the osteopathic approach, which recognizes the person as a unit of body, mind, and spirit, provide an individualized approach to care and may provide temporary relief for some patients. The combination of osteopathic manipulation, thorough treatment discussions, and clarity of information were each reported by patients to be a unique benefit provided from the osteopathic approach.67
The use of osteopathic manipulation, whether provided by US-trained osteopathic physicians or foreign-trained osteopaths, has shown measurable improvements with muscle function, pain reduction, and emotional distress.68/76 In a pilot study by Perrin et al,68 postexercise muscle function was found to improve after osteopathic manipulation. Considering that postexertional fatigue is a key symptom of ME/CFS, osteopathic manipulation could be a powerful treatment option. In particular, cervical and thoracic muscle energy techniques, while not directly cited in ME/CFS research, are techniques that can be used to reduce the muscle strain that is often present in these patients. Lymphatic techniques such as pedal pump, thoracic pump, and effleurage can also be used to decrease lymphatic congestion that may result from a sedentary history. Additionally, osteopathic manipulation has been shown to influence the components of pain throughout the course of chronic illnesses. Osteopathic manipulation can modify neuronal connections, increase lymphatic drainage, improve musculoskeletal dysfunctions, and, ultimately, alter a patient's perception of pain.70
Although osteopathic ME/CFS research is limited, enough is known about OMM to support its application for patients with ME/CFS. Treatments have been shown to decrease muscle pain, cortical potentials, and muscle spasms.71 Osteopathic manipulation can increase anti-inflammatory cytokine IL-10 and decrease proinflammatory cytokine IL-1β.72 Repetitive treatments have shown to reduce chronic pain.72,73 Postural orthostatic tachycardia syndrome, a common comorbidity in which heart rate increases with the change of position, can also be managed better with OMM.74 Osteopathic manipulation has been shown to alter changes in the sensorimotor cortical areas and alter autonomic nervous system activity.75
Conclusion
Myalgic encephalomyelitis/chronic fatigue syndrome does not originate from a single cause; it results from a multitude of factors that likely include biological and environmental factors. Although ME/CFS research is advancing, a clear need for more progress remains. Currently, there are no known biomarkers. Many clinicians remain uneducated about the disease, and available treatments do not directly target a root cause. To fill these gaps, research needs to expand and uncover diagnostic biomarkers that can speed up recovery. An increase in awareness is also needed to inform both medical communities and the public. Increased awareness would alleviate emotional strain that patients can experience from persons in doubt of the disease's existence and open doors for new research. Including osteopathic medicine in a patient's treatment regimen could provide temporary relief. Yet despite the many obstacles faced, current research is providing a better understanding of ME/CFS, which we believe will lead to better care for patients.
References
1. Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Washington, DC: National Academies Press; 2015. https://www.ncbi.nlm.nih.gov/books/NBK274235/. Accessed June 4, 2019. Suche in Google Scholar
2. Fukuda K , StrausSE, HickieI, SharpeMC, DobbinsJG, KomaroffA. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121(12):953-959.10.7326/0003-4819-121-12-199412150-00009Suche in Google Scholar PubMed
3. Carruthers BM , JainAK, De MeirleirKL, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatments protocols. J Chronic Fatigue Syndr. 2003;11(1):7-115. doi:10.1300/J092v11n01_02Suche in Google Scholar
4. Cairns R , HotopfM. A systematic review describing the prognosis of chronic fatigue syndrome. Occup Med (Lond). 2005;55(1):20-31.10.1093/occmed/kqi013Suche in Google Scholar PubMed
5. Holmes GP , KaplanJE, GantzNM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med. 1998;108:387-389.10.7326/0003-4819-108-3-387Suche in Google Scholar PubMed
6. Carruthers BM , van de SandeMI, De MeirleirKL, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. 2011;270(4):327-338. doi:10.1111/j.13652796.2011.02428.xSuche in Google Scholar
7. Sharpe MC , ArchardLC, BanatvalaJE, et al. A report— chronic fatigue syndrome: guidelines for research. J Royal Society Med. 1991;84(2):118-121.10.1177/014107689108400224Suche in Google Scholar
8. Green CR , CowanP, ElkR, O'Neil KM, Rasmussen AL. National Institutes of Health Pathways to Prevention Workshop: advancing the research on myalgic encephalomyelitis/chronic fatigue syndrome. Ann Intern Med. 2015;162(12):860-865. doi:10.7326/M15-0338Suche in Google Scholar PubMed
9. Jason LA , RichmanJA, RademakerAW, et al. A community-based study of chronic fatigue syndrome. Arch Intern Med. 1999;159(18):2129-2137. doi:10.1001/archinte.159.18.2129Suche in Google Scholar PubMed
10. Jason LA , BentonMC, ValentineL, JohnsonA, Torres-HardingS. The economic impact of ME/CFS: individual and societal costs. Dyn Med. 2008;7(6):1-8. doi:10.1186/1476-5918-7-6Suche in Google Scholar PubMed PubMed Central
11. Reynolds KJ , VernonSD, BoucheryE, ReevesWC. The economic impact of chronic fatigue syndrome. Cost Eff Resour Alloc. 2004;2(1):4. doi:10.1186/1478-7547-2-4Suche in Google Scholar PubMed PubMed Central
12. Lin JM , ReschSC, BrimmerDJ, et al. The economic impact of chronic fatigue syndrome in Georgia: direct and indirect costs. Cost Eff Resour Alloc. 2011;9(1):1.10.1186/1478-7547-9-1Suche in Google Scholar
13. McCrone P , DarbishireL, RidsdaleL, SeedP. The economic cost of chronic fatigue and chronic fatigue syndrome in UK primary care. Psychol Med. 2003;33(2):253-261.10.1017/S0033291702006980Suche in Google Scholar
14. Ross SD , EstokRP, FrameD, StoneLR, LudenskyV, LevineCB. Disability and chronic fatigue syndrome: a focus on function.Arch Intern Med.2004;164:1098-1107.10.1001/archinte.164.10.1098Suche in Google Scholar
15. Collin SM , CrawleyE, MayMT, SterneJA, HollingworthW. The impact of CFS/ME on employment and productivity in the UK: a cross-sectional study based on the CFS/ME national outcomes database. BMC Health Serv Res. 2011;11:217.10.1186/1472-6963-11-217Suche in Google Scholar
16. Sabes-Figuera R , McCroneP, HurleyM, KingM, DonaldsonAN, RidsdaleL. The hidden cost of chronic fatigue to patients and their families. BMC Health Serv Res. 2011;10:56.10.1186/1472-6963-10-56Suche in Google Scholar
17. Acheson D . A new clinical entity?Lancet.1956;(3):789-790.10.1016/S0140-6736(56)91252-1Suche in Google Scholar
18. Albrecht RM , OliverVL, PoskanzerDC. Epidemic neuromyasthenia. outbreak in a convent in New York state.JAMA.1964;187:904-907.10.1001/jama.1964.03060250022005Suche in Google Scholar
19. Shelokov A , HabelK, VerderE, WelshW. Epidemic neuromyasthenia; an outbreak of poliomyelitis like illness in student nurses. N Engl J Med. 1957;257(8):345-355. doi:10.1056/NEJM195708222570801Suche in Google Scholar PubMed
20. Hickie I , DavenportT, WakefieldD, et al. Infection outcomes study. postinfective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333(7568):575. doi:10.1136/bmj.38933.585764.AESuche in Google Scholar PubMed PubMed Central
21. Naess H , NylandM, HauskenT, FollestadI, NylandH. Chronic fatigue syndrome after giardia enteritis: clinical characteristics, disability and long-term sickness absence. BMC Gastroenterol. 2012;12:13.10.1186/1471-230X-12-13Suche in Google Scholar PubMed PubMed Central
22. Mørch K , HanevikK, RivenesAC, et al. Chronic fatigue syndrome 5 years after giardiasis: differential diagnoses, characteristics and natural course. BMC Gastroenterolog. 2013;13(1):28.10.1186/1471-230X-13-28Suche in Google Scholar PubMed PubMed Central
23. Katz BZ , ShiraishiY, MearsCJ, BinnsHJ, TaylorR.Chronic fatigue syndrome following infectious mononucleosis in adolescents.Pediatrics. 2009;124(1):189-193. doi:10.1542/peds.2008-1879Suche in Google Scholar PubMed PubMed Central
24. Nakaya T , KuratsuneH, KitaniT, IkutaK. Demonstration on Borna disease virus in patients with chronic fatigue syndrome [in Japanese]. Nippon Rinsho. 1997;55(11):3064-3071.Suche in Google Scholar
25. Li YJ , WangDX, BaiXL, et al. Clinical characteristics of patients with chronic fatigue syndrome: analysis of 82 cases. Zhonghua Yi Xue Za Zhi. 2005;85(10):701-704.Suche in Google Scholar
26. Chia JKS . The role of enterovirus in chronic fatigue syndrome. J Clin Pathol. 2005;58(11):1126-1132. doi:10.1136/jcp.2004.020255Suche in Google Scholar PubMed PubMed Central
27. Kondo K . Human herpesvirus latency and fatigue [review]. Uirusu. 2005;55(1):9-17. doi:10.2222/jsv.55.9Suche in Google Scholar PubMed
28. Komaroff AL . Chronic fatigue syndromes: a preliminary overview [review].Can Dis Wkly Rep. 1991;17(suppl 1E):23-28.Suche in Google Scholar
29. Ablashi DV . Viral studies of chronic fatigue syndrome.Clin Infect Dis.1994;18(suppl 1):S130-S133.10.1093/clinids/18.Supplement_1.S130Suche in Google Scholar PubMed
30. Wang T , YuL, XuC, et al. Chronic fatigue syndrome patients have alterations in their oral microbiome composition and function. PLoS ONE. 2018;13(9):e0203503.10.1371/journal.pone.0203503Suche in Google Scholar PubMed PubMed Central
31. Giloteaux L , GoodrichJK, WaltersWA, LevineSM, LeyRE, MaureenR. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2016;4(1):30. doi:10.1186/s40168-016-0171-4Suche in Google Scholar PubMed PubMed Central
32. Nagy-Szakal D , WilliamsBL, MishraN, et al. Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2017;5(1):44.10.1186/s40168-017-0261-ySuche in Google Scholar PubMed PubMed Central
33. Shukla SK , CookD, MeyerJ, et al. Changes in gut and plasma microbiome following exercise challenge in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). PLoS ONE. 2015;10(12):e0145453. doi:10.1371/journal.pone.0145453Suche in Google Scholar PubMed PubMed Central
34. Cerwenka A , LanierLL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol. 2016;16(2):112-123.10.1038/nri.2015.9Suche in Google Scholar PubMed
35. Fletcher MA , ZengXR, MaherK, et al. Biomarkers in chronic fatigue syndrome: evaluation of natural killer cell function and dipeptidyl peptidase IV/CD26. PLoS ONE. 2010;5(5):e10817.10.1371/journal.pone.0010817Suche in Google Scholar
36. Brenu EW , van DrielML, StainesDR, et al. Longitudinal investigation of natural killer cells and cytokines in chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med. 2012;10:88.10.1186/1479-5876-10-88Suche in Google Scholar
37. Caligiuri M , MurrayC, BuchwaldD, et al. Phenotypic and functional deficiency of natural killer cells in patients with chronic fatigue syndrome. J Immunol. 1987;139(10):3306-3313.10.4049/jimmunol.139.10.3306Suche in Google Scholar
38. Landay AL , JessopC, LennetteET, LevyJA. Chronic fatigue syndrome: a clinical condition associated with immune activation. Lancet. 1991;338(8769):707-712.10.1016/0140-6736(91)91440-6Suche in Google Scholar
39. White PD , NyeKE, PinchingAJ, et al. Immunological changes after both exercise and activity in chronic fatigue syndrome: a pilot study. J Chronic Fatigue Syndr. 2004;12(2):51-66. doi:10.1300/J092v12n02_06Suche in Google Scholar
40. Nijs J , NeesA, De KooningM, et al.Altered immune response to exercise in patients with chronic fatigue syndrome/ myalgic encephalomyelitis: a systematic review.Exerc Immunol Rev.2014;20:94-116.Suche in Google Scholar
41. Filler K , LyonD, BennettJ, et al. Association of mitochondrial dysfunction and fatigue: a review of the literature. BBA Clin. 2014;1:12-23. doi:10.1016/j.bbacli.2014.04.001Suche in Google Scholar PubMed PubMed Central
42. Myhill S , BoothNE, McLaren-HowardJ. Chronic fatigue syndrome and mitochondrial dysfunction. Int J Clin Exp Med. 2009;2(1):1-16.Suche in Google Scholar
43. Morris G , MaesM. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab Brain Dis. 2014;29(1):19-36. doi:10.1007/s11011-013-9435-xSuche in Google Scholar PubMed
44. VanNess JM , StevensSR, BatemanL, StilesTL, SnellCR. Postexertional malaise in women with chronic fatigue syndrome. J Womens Health (Larchmt). 2010;19(2):239-244.10.1089/jwh.2009.1507Suche in Google Scholar PubMed
45. Smits B , van den HeuvelL, KnoopH, et al. Mitochondrial enzymes discriminate between mitochondrial disorders and chronic fatigue syndrome. Mitochondrion. 2011;11(5):735-738.10.1016/j.mito.2011.05.005Suche in Google Scholar PubMed
46. McArdle A , McArdleF, JacksonMJ, PageSF, FahalI, EdwardsRH. Investigation by polymerase chain reaction of enteroviral infection in patients with chronic fatigue syndrome. Clin Sci (Lond). 1996;90(4):295-300.10.1042/cs0900295Suche in Google Scholar PubMed
47. Albright F , LightK, LightA, BatemanL, Cannon-AlbrightLA. Evidence for a heritable predisposition to chronic fatigue syndrome. BMC Neurology. 2011;11:62. doi:10.1186/1471-2377-11-62Suche in Google Scholar PubMed PubMed Central
48. Underhill R , O’GormanR. Prevalence of chronic fatigue syndrome and chronic fatigue among family members of CFS patients. J Chronic Fatigue Syndr. 2006;13(1):3-13. doi:10.1300/J092v13n01_02Suche in Google Scholar
49. Buchwald D , HerrellR, AshtonS, et al. A twin study of chronic fatigue. Psychosom Med. 2001;63(6):936-943.10.1097/00006842-200111000-00012Suche in Google Scholar PubMed
50. Schlauch KA , KhaiboullinaSF, DeMeirleir, et al. Genome-wide association analysis identifies genetic variations in subjects with myalgic encephalomyelitis/chronic fatigue syndrome. Transl Psychiatry. 2016;6:e730. doi:10.1038/tp.2015.208Suche in Google Scholar PubMed PubMed Central
51. Jammes Y , SteinbergJG, GuieuR, DelliauxS. Chronic fatigue syndrome with history of severe infection combined altered blood oxidant status, and reduced potassium efflux and muscle excitability at exercise. Open J Intern Med. 2013;3(3):98-105. doi:10.4236/ojim.2013.33023Suche in Google Scholar
52. Bested AC , MarshallLM. Review of myalgic encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians.Rev Environ Health.2015; 30(4):223-249.Suche in Google Scholar
53. Davenport TE , StevensSR, BaroniK, Van NessM, SnellCR. Diagnostic accuracy of symptoms characterising chronic fatigue syndrome. Disabil Rehabil. 2011;33(19-20):1768-1775. doi:10.3109/09638288.2010.546936Suche in Google Scholar PubMed
54. Snell C , StevensS, Davenport. T, VanNess M.Discriminative validity of metabolic and workload measurements to identify individuals with chronic fatigue syndrome.Phys Ther.2013;93(11).10.2522/ptj.20110368Suche in Google Scholar PubMed
55. Brenu EW , van DrielML, StainesDR, et al. Longitudinal investigation of natural killer cells and cytokines in chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med. 2012;10:88.10.1186/1479-5876-10-88Suche in Google Scholar PubMed PubMed Central
56. Puri BK . The use of eicosapentaenoic acid in the treatment of chronic fatigue syndrome. Prostaglandins Leukot Essent Fatty Acids. 2004;70(4):399-401. doi:10.1016/j.plefa.2003.12.015Suche in Google Scholar PubMed
57. Puri BK . Long-chain polyunsaturated fatty acids and the pathophysiology of myalgic encephalomyelitis (chronic fatigue syndrome). J Clin Pathol. 2007;60(2):122-124. doi:10.1136/jcp.2006.042424Suche in Google Scholar PubMed PubMed Central
58. Regland B , AnderssonM, AbrahamssonL, BagbyJ, DyrehagLE, GottfriesCG. Increased concentrations of homocysteine in the cerebro-spinal fluid in patients with fibromyalgia and chronic fatigue syndrome. Scand J Rheumatol. 1997;26(4):301-307.10.3109/03009749709105320Suche in Google Scholar PubMed
59. Regland B , ForsmarkS, HalaouateL, et al.Response to vitamin B12 and folic acid in myalgic encephalomyelitis and fibromyalgia.PLoS One. 2015;10(4): e0124648. doi:10.1371/journal.pone.0124648Suche in Google Scholar
60. Lyte M , VarcoeJJ, BaileyMT. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation.Physiol Behav.1998;65:63-68.10.1016/S0031-9384(98)00145-0Suche in Google Scholar
61. Goehler LF , LyteM, GaykemaRP. Infection-induced viscerosensory signals from the gut enhance anxiety: implications for psychoneuroimmunology. Brain Behav Immun. 2007;21(6):721-726.10.1016/j.bbi.2007.02.005Suche in Google Scholar
62. Rao AV , BestedAC, BeaulneTM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1(1):6.10.1186/1757-4749-1-6Suche in Google Scholar
63. Wallis A , BallM, ButtH, et al. Open-label pilot for treatment targeting guy dysbiosis in myalgic encephalomyelitis/chronic fatigue syndrome: neuropsychological symptoms and sex comparisons. J Transl Med. 2018;16(1):24. doi:10.1186/s12967-018-1392-zSuche in Google Scholar
64. Pae C , MarksDM, PatkarAA, MasandPS, LuytenP, SerrettiA. Pharmacological treatment of chronic fatigue syndrome focusing on the roles of antidepressants. Expert Opin. Pharmacother. 2009;10(10):1561-1570. doi:10.1517/14656560902988510Suche in Google Scholar
65. Delbanco TL , DaleyJ, HartmanEE. A 56-year-old woman with chronic fatigue syndrome, 1 year later.JAMA.1998;280(4):372.10.1001/jama.280.4.372Suche in Google Scholar
66. Gracious B , WisnerKL. Nortriptyline in chronic fatigue syndrome: a double blind, placebo-controlled single case study. Biol Psychiatry. 1991;30(4):405-408.10.1016/0006-3223(91)90297-YSuche in Google Scholar
67. What patients expect from their osteopath. General Osteopathic Council website. https://www.osteopathy.org.uk/news-and-resources/document-library/research-and-surveys/what-patients-expect-from-their-osteopath/. Accessed April 3, 2018.Suche in Google Scholar
68. Perrin RN , RichardsJD, PentreathV, PercyDF. Muscle fatigue in chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) and its response to a manual therapeutic approach: a pilot study. Int J Osteopath Med. 2011;14(3):96-105. doi:10.1016/j.ijosm.2010.12.002Suche in Google Scholar
69. Pelletier R , BourbonnaisD, HigginsJ. Nociception, pain, neuroplasticity and the practice of osteopathic manipulative medicine. Int J Osteopath Med. 2018;27:34-44. doi:10.1016/j.ijosm.2017.08.001Suche in Google Scholar
70. Zhu Y , HaldemanS, HsiehCY, WuP, StarrA.Do cerebral potentials to magnetic stimulation of paraspinal muscles reflect changes in palpable muscle spasm, low back pain, and activity scores?J Manipulative Physiol Ther.2000;23(7):458-464.10.1067/mmt.2000.108821Suche in Google Scholar
71. Song XJ , HuangZJ, SongWB, et al. Attenuation effect of spinal manipulation on neuropathic and postoperative pain through activating endogenous anti-inflammatory cytokine interleukin 10 in rat spinal cord. J Manipulative Physiol Ther. 2016;39(1):42-53.10.1016/j.jmpt.2015.12.004Suche in Google Scholar
72. Fryer G , CarubJ, McIverS. The effect of manipulation and mobilisation on pressure pain thresholds in the thoracic spine. J Osteopath Med. 2004;7(1):8-14. doi:10.1016/S1443-8461(04)80003-0Suche in Google Scholar
73. Goodkin M , BellewL. Osteopathic manipulative treatment for postural orthostatic tachycardia syndrome. J Am Osteopath Assoc. 2014;114(11):874-877. doi:10.7556/jaoa.2014.173Suche in Google Scholar PubMed
74. Pelletier R , BourbonnaisD, HigginsJ. Nociception, pain, neuroplasticity and the practice of osteopathic manipulative medicine. Int J Osteopath Med. 2018;27:34-44. doi:10.1016/j.ijosm.2017.08.001Suche in Google Scholar
© 2019 American Osteopathic Association
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Artikel in diesem Heft
- SURF
- OMT for the Prevention and Management of Chronic Constipation and Distal Intestinal Obstructive Syndrome in Cystic Fibrosis: A Pilot Study
- OMT MINUTE
- Mesenteric Lift for Constipation in Cystic Fibrosis
- AOA COMMUNICATION
- Official Call: 2019 Annual Business Meeting of the American Osteopathic Association
- AOA COMMUNICATION (REPRINT)
- Proposed Amendments to the AOA Constitution and Bylaws
- ORIGINAL CONTRIBUTION
- Accuracy of Canine Scent Detection of Non–Small Cell Lung Cancer in Blood Serum
- Comparison of Lumbar Fusion for Back Pain and Opioid Use at County and Managed Care Hospitals
- BRIEF REPORT
- Evaluation of Clinical Outcomes in Hospitalized Patients With Exertional Rhabdomyolysis
- Conceptual Framework to Evaluate Health Care Professionals’ Satisfaction in Utilizing Telemedicine
- REVIEW
- Understanding Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and the Emerging Osteopathic Approach: A Narrative Review
- JAOA/AACOM MEDICAL EDUCATION
- WVSOM Anatomy Lab Tour Program: An Osteopathic Medicine Pipeline With Student Teaching Opportunities
- CASE REPORT
- Suction Decompression of the Carpal Tunnel
- CLINICAL IMAGES
- Chrononutrition and the Diabetic Patient
Artikel in diesem Heft
- SURF
- OMT for the Prevention and Management of Chronic Constipation and Distal Intestinal Obstructive Syndrome in Cystic Fibrosis: A Pilot Study
- OMT MINUTE
- Mesenteric Lift for Constipation in Cystic Fibrosis
- AOA COMMUNICATION
- Official Call: 2019 Annual Business Meeting of the American Osteopathic Association
- AOA COMMUNICATION (REPRINT)
- Proposed Amendments to the AOA Constitution and Bylaws
- ORIGINAL CONTRIBUTION
- Accuracy of Canine Scent Detection of Non–Small Cell Lung Cancer in Blood Serum
- Comparison of Lumbar Fusion for Back Pain and Opioid Use at County and Managed Care Hospitals
- BRIEF REPORT
- Evaluation of Clinical Outcomes in Hospitalized Patients With Exertional Rhabdomyolysis
- Conceptual Framework to Evaluate Health Care Professionals’ Satisfaction in Utilizing Telemedicine
- REVIEW
- Understanding Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and the Emerging Osteopathic Approach: A Narrative Review
- JAOA/AACOM MEDICAL EDUCATION
- WVSOM Anatomy Lab Tour Program: An Osteopathic Medicine Pipeline With Student Teaching Opportunities
- CASE REPORT
- Suction Decompression of the Carpal Tunnel
- CLINICAL IMAGES
- Chrononutrition and the Diabetic Patient

