Abstract
Atrial myxoma (AM) is the most common primary cardiac tumor. Its clinical presentation can be highly heterogeneous and can be characterized by many constitutional manifestations and development of rheumatologic symptoms.We report the case of a patient presenting with a seronegative arthritis characterized by articular and enthesis involvement and purpuric cutaneous lesions that was refractory to conventional treatments and that was later diag- nosed with an AM as first cause of the manifestations. AM can present with different symptoms; among them, it is able to cause some rheumatological manifestation as it is able to secrete proinflammatory cytokines, as interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), and interferon γ (IFN-γ). The present case is of particular interest as it presents an AM as the cause of an inflammatory arthropathy with articular and enthesis involvement. A paraneoplastic screening is always relevant in rheumatology, especially when encountering a refractory disease.
Introduction
Atrial myxoma (AM) is a rare, but the most common primary cardiac tumor. The clinical presentation depends on the dimension and localization of the mass and includes embolism, intracardiac obstruction (episodes of dyspnea, orthopnea), and constitutional systemic manifestations (fever, arthralgias, weight loss).[1] The release of inflammatory cytokines by the tumor can lead to paraneoplastic syndromes and, in some cases, to rheumatologic manifestations. Blood tests may show anemia and increase in inflammatory indices such as erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP).[2] Diagnosis is usually suggested by transthoracic (TTE) or transesophageal (TEE) echocardiography that also allows determination of the location, size, shape, invasiveness, and mobility of the tumor preoperatively.[3] Biopsy is usually not performed since AM has a high risk of embolization. Treatment is surgical with a high success rate.[4]
We present a case of an AM that presented as a seronegative enthesoarthritis with purpuric cutaneous lesions as it is fundamental, for rheumatologists, to keep in mind the possible differential diagnosis and necessity of performing paraneoplastic screening when facing a rheumatic disease of new onset, especially when it is scarcely responsive to standard treatments.
A 31-year-old woman was admitted becayse of relapse of seronegative enthesoarthritis. The patient complained of arthralgias at metatarsophalangeal joints (MTP) bilaterally and pain on the plantar surface of the feet for 1 year, which showed a partial response to anti-inflammatory drugs. After 2 months from the onset of the articular symptoms, the patient developed purpuric lesions on the plantar surface bilaterally (Figure 1, left). No improvement happened after being treated with prednisone 50mg/d (rapidly tapered). Blood tests showed increased levels of inflammatory indices (CRP 12 mg/dL, and ESR 22 mm/h), negative for rheumatoid factor (RF), and anticitrullinated peptide autoantibodies (aCCP). Musculoskeletal ultrasound showed a bilateral plantar fascia edema (compatible with plantar fasciitis) and synovitis of right metacarpophalangeal joints (MTP) (Figure 1, right). Diagnosis of seronegative “early arthritis” was made, and the patient started treatment with methylprednisolone 4 mg/d and methotrexate (MTX) 7.5 mg/week, without improvement. The purpuric lesions and arthralgias worsened with onset of moderate exertional dyspnea and episodes of palpitations. No swollen joints was found during physical examination but diffuse MTP tenderness and pain at pressure at the plantar fascia bilaterally. Purpuric lesions were found on the plantar surface bilaterally. No heart murmur was detected. She underwent a blood test with evidence of increased inflammatory indices (ESR 52 mm/h, CRP 17 mg/dL), negative for antinuclear autoantibodies (ANA), extractable nuclear antigens (ENA), anti-dsDNA, anti-neutrophil cytoplasmic antibodies, antiphospholipid antibodies, cryoglobulins, and serology for HBV, HCV, tuberculosis, and syphilis. A nail videocapillaroscopy was in normal pattern. To better characterize the dyspnea, a TTE was performed which revealed a pedunculated mass in the left atrium.
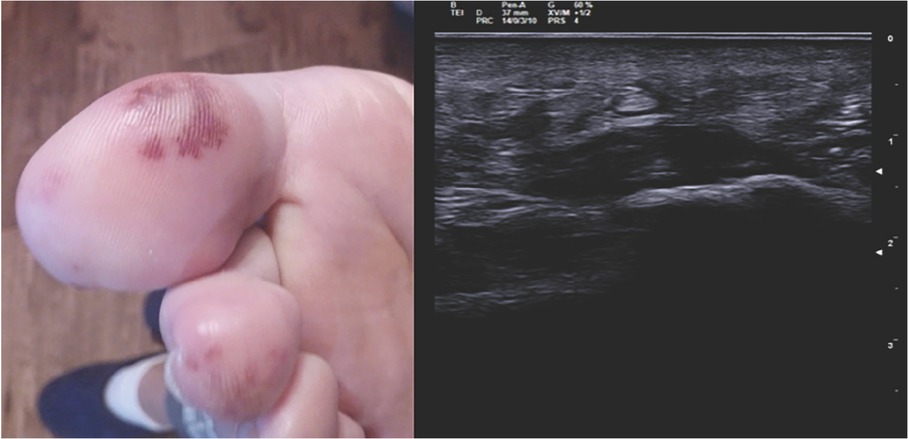
Purpuric lesions on the plantar side of the right foot (left). Echographic image of plantar fascia edema of the left foot (transverse section) (right).
The mass was 4223 mm in diameter compatible with an AM (Figure 2). The patient was operated with the mass removed. After surgery, the patient reported a complete resolution of the articular and cutaneous symptoms and methylprednisolone and MTX were withdrawn.
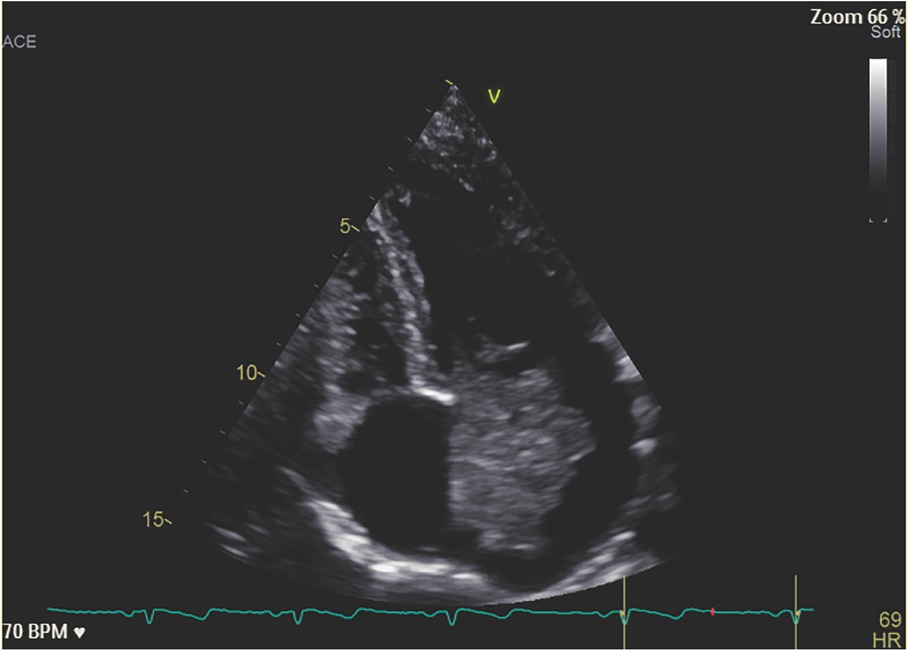
Echocardiographic image of the AM occupying almost all the left atrium and mitral valve. AM, atrial myxoma.
Discussion
As reported in the literature, cutaneous and articular symptoms may be possible paraneoplastic manifestations.[5] Paraneoplastic arthritis (PA) usually do not respond to corticosteroids or conventional antirheumatic drugs, but encounter complete resolution after oncologic treatment.[6] Usually, PA presents as abrupt onset of symptoms with elevated acute phase reactants, and negative for RF, aCCP, and ANA. AM is a rare cardiac tumor that can produce inflammatory cytokines (e.g., interleukin 1 [IL-1], interleukin 6 [IL-6], tumor necrosis factor α [TNF-α], interferon γ [IFN-γ])[7] fostering the growth of the tumor and the development of inflammatory symptoms (e.g., low grade fever, weight loss, arthralgia). Constitutional symptoms are mainly attributed to the increased release of IL-6.[8] Usually, IL-6 is involved in the increase of CRP, in tumor growth and recurrence and development of autoimmune features.[7] The release of inflammatory cytokines is also related to the development of manifestations as Raynaud’s phenomenon, malar rash, and cutaneous vasculitis.[9] However, this is the first case report to describe an enthesoarthritis as a manifestation of an AM. These features usually disappeared after AM removal but in some patients persistent elevation of the ANA titer was observed.[9] Following excision, the IL-6 levels return to normal, and the systemic symptoms eventually disappear with normalization of IL-6.[10] Cutaneous manifestations are seen in one-third of cases and are usually either due to embolization of the tumor mass or development of autoimmune features due to interleukins’ secretion. Embolization often leads to purpuric lesions on the palmar/plantar surfaces, telangiectasia, petechiae, or livedo reticularis.[9]
Conclusions
The present case is of particular interest as it presents an AM as the cause of an inflammatory arthropathy with articular and enthesis involvement. Moreover, it highlights the importance of performing a thorough paraneoplastic screening when encountering a new onset arthritis, especially when it occurs in young patients and respond poorly to conventional treatments in patients of young age.
Funding statement: This research received no external funding.
-
Conflict of Interest Matucci Cerinic Marco is an Associate Editor-in-Chief of the journal. The article was subject to the journal’s standard procedures, with peer review handled independently of the editor and the affiliated research groups.
-
Ethical Statement None declared.
-
Informed Consent Written informed consent has been obtained from the patient to publish this paper.
-
Author Contributions All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
-
Data Availability Statement No additional data are available.
References
[1] Abu Abeeleh M, Saleh S, Alhaddad E, et al. Cardiac Myxoma: Clinical Characteristics, Surgical Intervention, Intra-Operative Challenges and Outcome. Perfusion. 2017;32:686–690.10.1177/0267659117722596Search in Google Scholar PubMed
[2] MacGowan SW, Sidhu P, Aherne T, et al. Atrial Myxoma: National Incidence, Diagnosis and Surgical Management. Ir J Med Sci. 1993;162:223–226.10.1007/BF02945200Search in Google Scholar PubMed
[3] Aggarwal SK, Barik R, Sarma TCSR, et al. Clinical Presentation and Investigation Findings in Cardiac Myxomas: New Insights from the Developing World. Am Heart J. 2007;154: 1102–1107.10.1016/j.ahj.2007.07.032Search in Google Scholar PubMed
[4] Li H, Guo H, Xiong H, et al. Clinical Features and Surgical Results of Right Atrial Myxoma. J Card Surg. 2016;31:15–17.10.1111/jocs.12663Search in Google Scholar PubMed
[5] Parperis K, Constantinidou A, Panos G. Paraneoplastic Arthritides: Insights to Pathogenesis, Diagnostic Approach, and Treatment. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. 2021 ;27:e505–e509.10.1097/RHU.0000000000001202Search in Google Scholar PubMed
[6] Manger B, Schett G. Paraneoplastic Syndromes in Rheumatology. Nat Rev Rheumatol. 2014;10:662–670.10.1038/nrrheum.2014.138Search in Google Scholar PubMed
[7] Ajiro Y. Cardiac Myxoma as Cytokine Producing Tumour: A review. Interv Cardiol. 2021;13:58–63.Search in Google Scholar
[8] Thyagarajan B, Kumar MP, Patel S, et al. Extracardiac Manifestations of Atrial Myxomas. J Saudi Heart Assoc. 2017;29:37–43.10.1016/j.jsha.2016.07.003Search in Google Scholar PubMed PubMed Central
[9] Lee HJ, Park JY, Kim YS, et al. Cardiac Myxoma Diagnosed by Signs of Purpuric Macules on Both Palms and Soles. Ann Dermatol. 2012;24:337–340.10.5021/ad.2012.24.3.337Search in Google Scholar PubMed PubMed Central
[10] Yokomuro H, Yoshihara K, Watanabe Y, et al. The Variations in the Immunologic Features and Interleukin-6 Levels for the Surgical Treatment of Cardiac Myxomas. Surg Today. 2007;37:750–753.10.1007/s00595-006-3448-6Search in Google Scholar PubMed
© 2023 Bonomi Francesco, Orlandi Martina, Conforti Maria Letizia, Guiducci Serena, Matucci Cerinic Marco, published by De Gruyter on behalf of NCRC-DID.
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Editorial
- Lupus clinical trials and the promise of future therapies
- Guideline and Recommendations
- 2022 Chinese guideline for the management of pregnancy and reproduction in systemic lupus erythematosus
- Review
- Peptide-based immunotherapy in lupus: Where are we now?
- Low-dose interleukin-2 therapy in systemic lupus erythematosus
- Original Article
- East-Asian lupus nephritis in the Hopkins Lupus Cohort
- Special Article
- Improve quality of patient care for systemic lupus erythematosus in China by enhancing the construction of Centers of Excellence
- Case Report
- Seronegative enthesoarthritis as the first presentation of the atrial myxoma
- Image
- Active pattern on nailfold capillaroscopy in a patient with systemic sclerosis
- Letter to the Editor
- A rare condition that mimic myopathy: Late-onset glutaric acidaemia type II
Articles in the same Issue
- Editorial
- Lupus clinical trials and the promise of future therapies
- Guideline and Recommendations
- 2022 Chinese guideline for the management of pregnancy and reproduction in systemic lupus erythematosus
- Review
- Peptide-based immunotherapy in lupus: Where are we now?
- Low-dose interleukin-2 therapy in systemic lupus erythematosus
- Original Article
- East-Asian lupus nephritis in the Hopkins Lupus Cohort
- Special Article
- Improve quality of patient care for systemic lupus erythematosus in China by enhancing the construction of Centers of Excellence
- Case Report
- Seronegative enthesoarthritis as the first presentation of the atrial myxoma
- Image
- Active pattern on nailfold capillaroscopy in a patient with systemic sclerosis
- Letter to the Editor
- A rare condition that mimic myopathy: Late-onset glutaric acidaemia type II

