Introduction
Alport syndrome (AS) is a heredity disease caused by mutations in the COL4A3/4/5. The disease involves multiple organs, including the kidney, eye, ear, etc. A recent study showed that the predicted pathogenic mutation frequency of COL4A3/4 was about 1/106 and was 1/2320 in COL4A5, suggesting the underestimated prevalence of AS.[1] To improve monitoring and treatment, the Alport Classification Working Group recommended that diseases caused by COL4A3/4/5 mutations be uniformly classified as AS, and the designation of TBMN and COL4A5 “mutation carriers” be removed.[2] High-throughput sequencing further broadens the disease spectrum corresponding to COL4 mutation. It can also be seen in thin basement membrane nephropathy, focal segmental glomerulosclerosis, IgA nephropathy, and diabetic kidney disease.[3,4,5]
Hemizygous male and COL4A3/4 recessive inheritance patients have a 100% risk of progression to ESRD. Heterozygous female subjects have a risk to ESRD of approximately 25%. Renin-angiotensin-aldosterone system inhibitor has been the only standard medication for treating AS patients. The poor prognosis of this disease urgently needs more treatment.[2] As a terminally differentiated cell, persistent podocyte damage results in irreversible kidney impairment. Since the podocyte is primarily responsible for synthesizing the COL4A3/4/5 trimer in glomerular basement membrane (GBM), it plays a major role in developing this disease.[6] Until now, no specific drugs targeting podocytes have been developed since the pathogenesis of podocyte injury remains unclear. Here, we summarized the progress on mechanism research of podocytes in AS and the prospect for new therapeutic targets.
Glomerular basement membrane defection
Podocytes synthesize and secrete COL4A3/4/5 trimers predominantly in the kidney. Impaired trimer formation and GBM defection are two of the fundamental mechanisms contributing to AS progression.[7] To address this issue, editing mutated genes or introducing normal genes using the CRISPR/Cas9 technique or Antisense-oligonucleotides (ASO) has been conducted to prevent or slow disease progression.
Daga et al. have successfully corrected Col4A3 and Col4A5 mutations in urine-derived podocytes using dual-plasmid CRISPR/Cas9 gene editing. However, this approach still needs to be verified in future in vivo studies.[8] Another study showed an exon-skipping therapy using ASO to reduce disease severity in male X-linked AS.[9] This therapy selectively skipped a targeted exon containing a premature termination codon mutation and corrected the open reading frame. As a result, exon-skipping therapy can re-express the COL4α5 chain on glomerular and tubular basement membranes, significantly reducing proteinuria and prolonging survival in AS mice. In summary, CRISPR/Cas9 gene editing could completely correct all kinds of mutation in podocyte and therefore synthesize normal COL4α3/4/5 trimers, but concerns exist about off-target effect and lack of in vivo validation. ASO therapy to skip the exon where the mutation is located, was used in truncated COL4α3/4/5 resulting from nonsense or splicing mutations. Although this is not a complete recovery, it can significantly improve the patients’ prognosis by changing the mutation types.
In some cases, COL4A3/4/5 are truncated due to nonsense variants or premature termination codons (PTCs). Drug-induced PTC readthrough is a promising therapeutic strategy and has been well studied in other genetic diseases like cystic fibrosis and duchenne muscular dystrophy.[10,11] Excitingly, a study showed that 11 nonsense variants from AS patients were highly sensitive to aminoglycoside-mediated PTC readthrough, suggesting a fraction of patients could benefit from such therapy.[12] ELX-02 is a small-molecule eukaryotic ribosomal selective glycoside acting to induce read-through of PTCs. A Phase 2 open label pilot study to evaluate the safety and efficacy of ELX-02 in patients with X-linked or autosomal recessive AS with Col4A3/4/5 nonsense mutation is currently recruiting patients.
Studies have shown that injecting healthy mouse or human embryonic stem cells into COL4 α 3 knockout mice promoted the re-synthesis of the COL4 α 3 chain, reshaped the GBM structure, and eventually improved the renal function of AS mice.[13] Our group demonstrated that intravenous injection of human umbilical cord mesenchymal stem cells into COL4A3 mutant mice attenuated proteinuria and renal pathological damages, and the expression of the COL4A3 chains was also restored to some extent.[14] Stem cell therapy might be a promising treatment option for AS patients in the future.
Unfold protein reaction and endoplasmic reticulum stress
Unfold protein reaction (UPR) promotes the synthesis of molecular chaperones involving protein folding that prevents cells from stress at an early stage. However, the mutations resulting in persistent UPR could become maladaptive and cytotoxic.[15] Endoplasmic reticulum stress (ERS) has been discussed in many kidney diseases, including diabetic nephropathy, acute kidney injury, and chronic kidney disease.[15,16] COL4A3/4/5 mutations cause abnormal or defective proteins that fail to fold correctly. These misfolded proteins accumulate in the ER, leading to UPR and ERS.[17,18] One of our previous studies also indicated that MG132 (a proteasome inhibitor) intervention improved ERS-related apoptosis in podocytes with truncated COL4A3 mutation.[19] A study in fibroblast cell lines of men with XLAS further showed Sodium 4-phenylbutyrate enhanced COL4α5 transcript levels, reduced ERS, and possibly facilitated COL4A5 extracellular transport.[20] Thus, some molecular chaperone molecules that are able to alleviate ER stress in podocytes might be a promising therapeutic approach for delaying AS progression.
Cell cycle reentry
The cell cycle is composed of Gap 1 (G1), DNA synthesis (S), Gap 2 (G2), and mitosis (M). The progression of cells through different phases of the cell cycle is mediated by cyclins and cyclin - dependent kinases (CDKs). Manipulating CDKs like CDK4/6 inhibitors has been used in halting tumor growth.[21] However, these approaches have not been thoroughly studied in glomerular disease. Research has revealed that increasing detachment of podocytes was detected in AS patients’ urine. Ding et al. reported that the podocyte detachment rate increased 11-fold in the mutant compared to the control condition, and glomeruli lost an average of 26 podocytes per year in AS versus the controls, which equals to 2.3 podocytes per year.[22] After loss of podocytes in glomeruli, remaining podocytes reenter the cell cycle to become hypertrophy, which may help to cover more area on the GMB as compensation.[23] Using proteomic analysis, Frank et al. found an increasing fraction of podocytes in G1 or later cell cycle stages in XLAS mice. The podocyte becomes hypertrophic with the transition from G0 to G1.[24] Another study showed a significant decrease in cyclin kinase inhibitors (p27, P21) expression in the kidneys of children with AS.[25] Therefore, cell cycle dysregulation may contribute to the pathogenesis of AS.
Lipid metabolism
Numerous studies proposed that cholesterol deposition plays a vital role in Alport nephropathy. Wright et al. showed that Col4a3 knockout mice exhibited significantly elevated cholesterol accumulation in glomeruli and decreased expression of ABCA1, a gene related to cholesterol efflux and transport.[26] Non-selective cholesterol scavenger hydroxypropyl-β-cyclodextrin (HPβCD) mitigates renal cortex cholesterol accumulation, reduces proteinuria, and alleviates renal tissue inflammation and fibrosis.[26] Sterol-O-acyltransferase-1 (SOAT1) can convert free cholesterol to cholesteryl esters. Inhibition of SOAT1 reduces cholesterol esters in podocytes and promotes free cholesterol efflux through upregulation of ABCA1, thereby attenuating renal damage in AS mice.[27] Another study also found that COL1-mediated discoid domain receptor 1 (DDR1) activation in Col4α3 knockout mice promoted fatty acid uptake by podocytes via CD36 and triggered cellular lipo-toxicity. [28] Thus, drugs that reduce serum cholesterol level such as Ezetimibe, Atorvastatin or small molecules that target ABCA1, SOAT1, and CD36 could be potential approach to treat AS. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) empagliflozin has shown its potential in treating AS mice model via switching energy substrates from glucose to fatty acids in podocytes, leading to downregulated lipo-toxicity and improved kidney.[29] Excitingly, a phase 3 clinical trial with Dapagliflozin (NCT05944016) is starting to recruit AS in children and young adults at the early stages of the disease. Altogether, these data indicate that designing drugs targeting lipid metabolism and mitochondria is a meaningful direction for AS treatment.
Abnormal cell signaling
Furthermore, COL4α3/4/5 defects resulted in sustained expression of the COL4α1/1/2 trimer in GBM, which exists only in the developmental stages of the kidney.[7] Abnormal collagen deposition causes podocyte injury by activating collagen receptors, like DDR1 and integrin α2β. Integrin α2β1 deletion attenuated glomerulosclerosis and interstitial fibrosis in AS mice.[30] Loss of collagen-receptor DDR1 delayed renal fibrosis and reduced inflammation of AS mice.[31] However, selective DDR1 or Integrinα2β1 antagonist has not been developed.
Additionally, endothelin-1 (ET-1) produced by glomerular endothelial cells binds to endothelin A receptors expressed in mesangial cells, causing Laminin α2β1γ1 secretion and deposition in GBM. Ectopic Laminin α2 deposition in GBM activates nuclear factor ϰB (NF-ϰB), stimulates podocyte focal adhesion kinase, and increases the expression of matrix metalloproteinases and proinflammatory factors.[32,33] A Phase 2 clinical trial (AFFINITY) has been initiated to assess the efficacy and safety of Atrasentan, a selective endothelin receptor antagonist, in patients with proteinuric glomerular disease, including 20 patients with AS.
Oxidative stress and inflammation
Finally, oxidative stress and subsequent activation of inflammation are also important factors contributing to the development of podocyte injuries in Alport syndrome. Nuclear factor erythroid2-related factor2 (Nrf2) has recently been regarded as a major role in regulating cellular oxidative stress in podocytes. Under stress conditions, Keap1 separated from Nrf2, and Nrf2 transferred to the nucleus, promoting the expression of genes carrying antioxidant response elements and initiating antioxidation.[34,35] Nrf2 also suppresses transcription of inflammation-related genes by inhibiting NK-ϰB activation.[35] A Phase 3 clinical trial (CARDINAL) has been conducted to investigate the safety and efficacy of bardoxolone in treating patients with AS. However, the trial’s outcomes were unsatisfactory.[36] NOX4 is the major renal reactive oxygen species (ROS) source and is abundantly expressed in podocytes.[37] Our earliest research found that NOX4 and ROS were significantly upregulated in patients with ADAS, and increased MMP-2 and podocyte apoptosis could be rescued by NOX4 inhibition in vivo and in vitro.[38] A recent study demonstrated that Mineralocorticoid receptor antagonist finerenone protected Col4a3-/- mice by suppressing the residual interstitial inflammation and fibrosis,[39] further studies are required to pinpoint finerenone’s precise mechanism on podocytes. We speculate that drugs targeting NF-kB activation and reducing ROS production in podocytes will effectively improve patients’ prognosis.
Conclusion and perspective
To summarize, the podocyte injury in AS is primarily caused by ERS, cell cycle dysregulation, lipid toxicity, inflammation, oxidation stress, and abnormal downstream cell signaling activation (Figure 1). Enabling podocytes to express the correct COL4A3/4/5 through gene editing or transferring the corrected COL4A3/4/5 gene into podocytes might be the most effective way to cure the disease. Delaying the onset of the disease and reducing the disease progression are also essential for management of this disease. Preventing the generation of truncated proteins by exon skipping or PTCs readthrough therapy, Reducing ERS by molecular chaperone, decreasing cell detachment by modification of cell cycle, promoting lipid metabolism or cholesterol efflux in podocyte, and inhibiting inflammatory response and oxidative stress are all potential therapeutic approaches to ameliorate podocyte injury in AS.
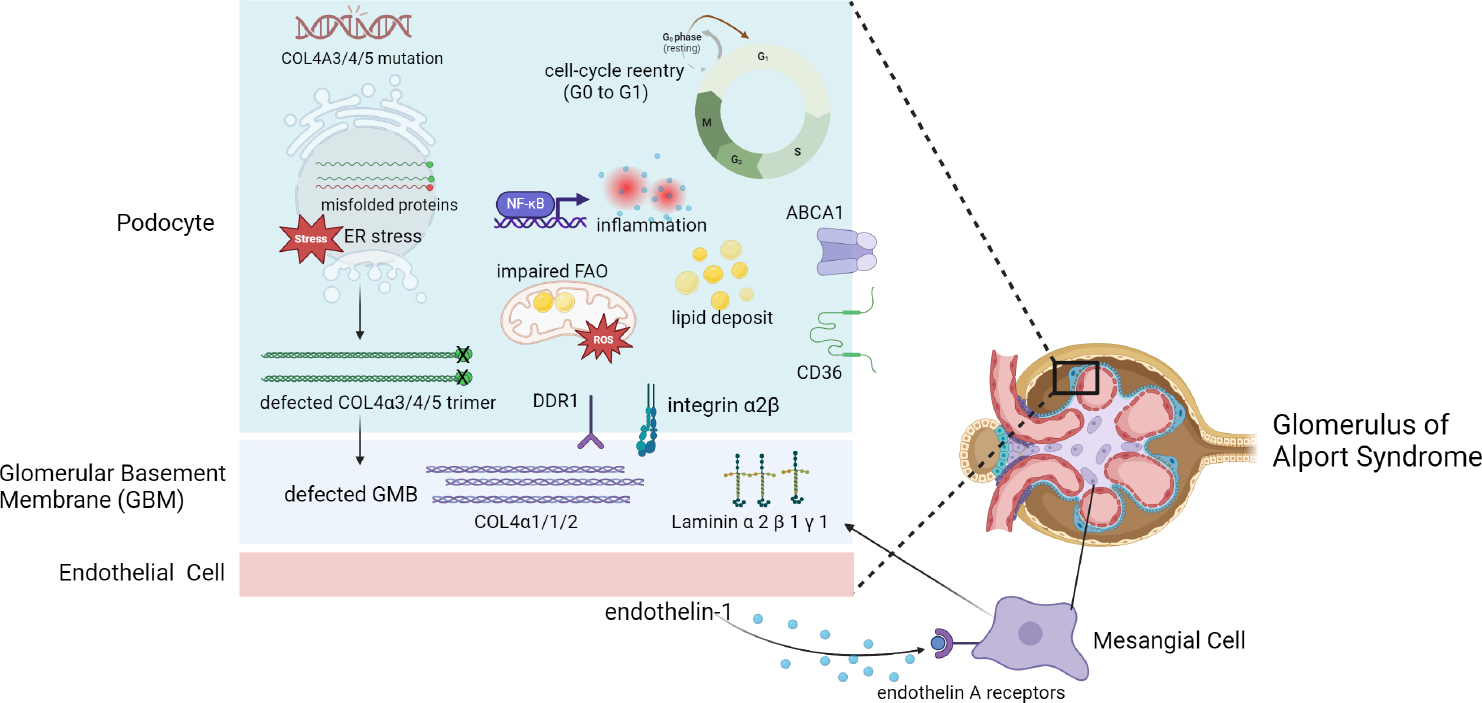
Pathogenic mechanisms of intrinsic cells and basement membrane in glomerulus of Alport syndrome. Created with BioRender.
Funding statement: This work was supported by grants from the Major International (Regional) Joint Research Program of National Natural Science Foundation of China (No: 82120108007), the National Natural Science Foundation of China (No: 81870460, 81570598, 81370015), “Excellent Academic Leader” by Shanghai Science and Technology Commission (No: 21XD1402000), Science and Technology Innovation Action Plan of Shanghai Science and Technology Committee (No: 22140904000, 17441902200), Shanghai Municipal Education Commission, Gaofeng Clinical Medicine Grant (No: 20152207), Shanghai Shenkang Hospital Development Center “Three-year Action Plan for Promoting Clinical Skills and Clinical Innovation in Municipal Hospitals”(No: SHDC2020CR6017), Shanghai Science and Technology Innovation Action Plan Biopharmaceutical Technology Support Particular Project (No. 23S11900500), Shanghai Jiao Tong University School of Medicine, 2022 Integrated Traditional Chinese and Western Medicine Research Platform (No. 2022zxy003).
-
Author Contributions
Qimin Zheng: Writing—Original draft preparation and Editing. Xiangchen Gu: Conceptualization, Writing—Reviewing and Editing. John Cijiang He: Writing—Reviewing and Editing. Jingyuan Xie: Conceptualization, Supervision, Writing—Reviewing and Editing.
-
Conflict of Interest None declared.
References
1 Gibson J, Fieldhouse R, Chan MMY, Sadeghi-Alavijeh O, Burnett L, Izzi V, et al. Prevalence Estimates of Predicted Pathogenic COL4A3-COL4A5 Variants in a Population Sequencing Database and Their Implications for Alport Syndrome. J Am Soc Nephrol 2021;32:2273–2290.10.1681/ASN.2020071065Search in Google Scholar PubMed PubMed Central
2 Kashtan CE, Ding J, Garosi G, Heidet L, Massella L, Nakanishi K, et al. Alport syndrome: a unified classification of genetic disorders of collagen IV α345: a position paper of the Alport Syndrome Classification Working Group. Kidney Int 2018;93:1045–1051.10.1016/j.kint.2017.12.018Search in Google Scholar PubMed
3 Li Y, Groopman EE, D’Agati V, Prakash S, Zhang J, Mizerska-Wasiak M, et al. Type IV Collagen Mutations in Familial IgA Nephropathy. Kidney Int Rep 2020;5:1075–1078.10.1016/j.ekir.2020.04.011Search in Google Scholar PubMed PubMed Central
4 Xie J, Wu X, Ren H, Wang W, Wang Z, Pan X, et al. COL4A3 mutations cause focal segmental glomerulosclerosis. J Mol Cell Biol 2014;6:498–505.10.1093/jmcb/mju040Search in Google Scholar PubMed
5 Wang Y, Zhang J, Zhao Y, Wang S, Zhang J, Han Q, et al. COL4A3 Gene Variants and Diabetic Kidney Disease in MODY. Clin J Am Soc Nephrol 2018;13:1162–1171.10.2215/CJN.09100817Search in Google Scholar PubMed PubMed Central
6 Abrahamson DR, Hudson BG, Stroganova L, Borza DB, St John PL. Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol 2009;20:1471–1479.10.1681/ASN.2008101086Search in Google Scholar PubMed PubMed Central
7 Kalluri R, Shield CF, Todd P, Hudson BG, Neilson EG. Isoform switching of type IV collagen is developmentally arrested in X-linked Alport syndrome leading to increased susceptibility of renal basement membranes to endoproteolysis. J Clin Invest 1997;99:2470–2478.10.1172/JCI119431Search in Google Scholar PubMed PubMed Central
8 Daga S, Donati F, Capitani K, Croci S, Tita R, Giliberti A, et al. New frontiers to cure Alport syndrome: COL4A3 and COL4A5 gene editing in podocyte-lineage cells. Eur J Hum Genet 2020;28:480–490.10.1038/s41431-019-0537-8Search in Google Scholar PubMed PubMed Central
9 Yamamura T, Horinouchi T, Adachi T, Terakawa M, Takaoka Y, Omachi K, et al. Development of an exon skipping therapy for X-linked Alport syndrome with truncating variants in COL4A5. Nat Commun 2020;11:2777.10.1038/s41467-020-16605-xSearch in Google Scholar PubMed PubMed Central
10 Crawford DK, Mullenders J, Pott J, Boj SF, Landskroner-Eiger S, Goddeeris MM. Targeting G542X CFTR nonsense alleles with ELX-02 restores CFTR function in human-derived intestinal organoids. J Cyst Fibros 2021;20:436–442.10.1016/j.jcf.2021.01.009Search in Google Scholar PubMed
11 Crawford DK, Alroy I, Sharpe N, Goddeeris MM, Williams G. ELX-02 Generates Protein via Premature Stop Codon Read-Through without Inducing Native Stop Codon Read-Through Proteins. J Pharmacol Exp Ther 2020;374:264–272.10.1124/jpet.120.265595Search in Google Scholar PubMed
12 Omachi K, Kai H, Roberge M, Miner JH. NanoLuc reporters identify COL4A5 nonsense mutations susceptible to drug-induced stop codon readthrough. iScience 2022;25:103891.10.1016/j.isci.2022.103891Search in Google Scholar PubMed PubMed Central
13 LeBleu V, Sugimoto H, Mundel TM, Gerami-Naini B, Finan E, Miller CA, et al. Stem cell therapies benefit Alport syndrome. J Am Soc Nephrol 2009;20:2359–2370.10.1681/ASN.2009010123Search in Google Scholar PubMed PubMed Central
14 Shi Y, Xie J, Yang M, Ma J, Ren H. Transplantation of umbilical cord mesenchymal stem cells into mice with focal segmental glomerulosclerosis delayed disease manifestation. Ann Transl Med 2019;7:383.10.21037/atm.2019.07.71Search in Google Scholar PubMed PubMed Central
15 Cybulsky AV. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol 2017;13:681–696.10.1038/nrneph.2017.129Search in Google Scholar PubMed
16 Luan Y, Huang E, Huang J, Yang Z, Zhou Z, Liu Y, et al. Serum myoglobin modulates kidney injury via inducing ferroptosis after exertional heatstroke. J Transl Int Med 2023;11:178–188.10.2478/jtim-2023-0092Search in Google Scholar PubMed PubMed Central
17 Pieri M, Stefanou C, Zaravinos A, Erguler K, Stylianou K, Lapathitis G, et al. Evidence for activation of the unfolded protein response in collagen IV nephropathies. J Am Soc Nephrol 2014;25:260–275.10.1681/ASN.2012121217Search in Google Scholar PubMed PubMed Central
18 Wang C, Liang S, Xing S, Xu K, Xiao H, Deng H, et al. Endoplasmic Reticulum Stress Activation in Alport Syndrome Varies Between Genotype and Cell Type. Front Genet 2020;11:36.10.3389/fgene.2020.00036Search in Google Scholar PubMed PubMed Central
19 Zhang HD, Huang JN, Liu YZ, Ren H, Xie JY, Chen N. Endoplasmic reticulum stress and proteasome pathway involvement in human podocyte injury with a truncated COL4A3 mutation. Chin Med J (Engl) 2019;132:1823–1832.10.1097/CM9.0000000000000294Search in Google Scholar PubMed PubMed Central
20 Wang D, Mohammad M, Wang Y, Tan R, Murray LS, Ricardo S, et al. The Chemical Chaperone, PBA, Reduces ER Stress and Autophagy and Increases Collagen IV α5 Expression in Cultured Fibroblasts From Men With X-Linked Alport Syndrome and Missense Mutations. Kidney Int Rep 2017;2:739–748.10.1016/j.ekir.2017.03.004Search in Google Scholar PubMed PubMed Central
21 Suski JM, Braun M, Strmiska V, Sicinski P. Targeting cell-cycle machinery in cancer. Cancer Cell 2021;39:759–778.10.1016/j.ccell.2021.03.010Search in Google Scholar PubMed PubMed Central
22 Ding F, Wickman L, Wang SQ, Zhang Y, Wang F, Afshinnia F, et al. Accelerated podocyte detachment and progressive podocyte loss from glomeruli with age in Alport Syndrome. Kidney Int 2017;92:1515–1525.10.1016/j.kint.2017.05.017Search in Google Scholar PubMed PubMed Central
23 Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, et al. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol 2005;16:2953–2966.10.1681/ASN.2005050488Search in Google Scholar PubMed
24 Frank CN, Hou X, Petrosyan A, Villani V, Zhao R, Hansen JR, et al. Effect of disease progression on the podocyte cell cycle in Alport Syndrome. Kidney Int 2022;101:106–118.10.1016/j.kint.2021.08.026Search in Google Scholar PubMed
25 Srivastava T, Garola RE, Whiting JM, Alon US. Cell-cycle regulatory proteins in podocyte cell in idiopathic nephrotic syndrome of childhood. Kidney Int 2003;63:1374–1381.10.1046/j.1523-1755.2003.00877.xSearch in Google Scholar PubMed
26 Wright MB, Varona Santos J, Kemmer C, Maugeais C, Carralot JP, Roever S, et al. Compounds targeting OSBPL7 increase ABCA1-dependent cholesterol efflux preserving kidney function in two models of kidney disease. Nat Commun 2021;12:4662.10.1038/s41467-021-24890-3Search in Google Scholar PubMed PubMed Central
27 Liu X, Ducasa GM, Mallela SK, Kim JJ, Molina J, Mitrofanova A, et al. Sterol-O-acyltransferase-1 has a role in kidney disease associated with diabetes and Alport syndrome. Kidney Int 2020;98:1275–1285.10.1016/j.kint.2020.06.040Search in Google Scholar PubMed PubMed Central
28 Kim JJ, David JM, Wilbon SS, Santos JV, Patel DM, Ahmad A, et al. Discoidin domain receptor 1 activation links extracellular matrix to podocyte lipotoxicity in Alport syndrome. EBioMedicine 2021;63:103162.10.1016/j.ebiom.2020.103162Search in Google Scholar PubMed PubMed Central
29 Ge M, Molina J, Kim JJ, Mallela SK, Ahmad A, Varona Santos J, et al. Empagliflozin reduces podocyte lipotoxicity in experimental Alport syndrome. Elife 2023;12:e83353.10.7554/eLife.83353Search in Google Scholar PubMed PubMed Central
30 Rubel D, Frese J, Martin M, Leibnitz A, Girgert R, Miosge N, et al. Collagen receptors integrin alpha2beta1 and discoidin domain receptor 1 regulate maturation of the glomerular basement membrane and loss of integrin alpha2beta1 delays kidney fibrosis in COL4A3 knockout mice. Matrix Biol 2014;34:13–21.10.1016/j.matbio.2014.01.006Search in Google Scholar PubMed
31 Gross O, Girgert R, Beirowski B, Kretzler M, Kang HG, Kruegel J, et al. Loss of collagen-receptor DDR1 delays renal fibrosis in hereditary type IV collagen disease. Matrix Biol 2010;29:346–356.10.1016/j.matbio.2010.03.002Search in Google Scholar PubMed
32 Abrahamson DR, Isom K, Roach E, Stroganova L, Zelenchuk A, Miner JH, et al. Laminin compensation in collagen alpha3(IV) knockout (Al-port) glomeruli contributes to permeability defects. J Am Soc Nephrol 2007;18:2465–2472.10.1681/ASN.2007030328Search in Google Scholar PubMed
33 Delimont D, Dufek BM, Meehan DT, Zallocchi M, Gratton MA, Phillips G, et al. Laminin α2-mediated focal adhesion kinase activation triggers Alport glomerular pathogenesis. PLoS One 2014;9:e99083.10.1371/journal.pone.0099083Search in Google Scholar PubMed PubMed Central
34 Aranda-Rivera AK, Cruz-Gregorio A, Pedraza-Chaverri J, Scholze A. Nrf2 Activation in Chronic Kidney Disease: Promises and Pitfalls. Antioxidants (Basel) 2022;11:1112.10.3390/antiox11061112Search in Google Scholar PubMed PubMed Central
35 Stenvinkel P, Chertow GM, Devarajan P, Levin A, Andreoli SP, Bangalore S, et al. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int Rep 2021;6:1775–1787.10.1016/j.ekir.2021.04.023Search in Google Scholar PubMed PubMed Central
36 Warady BA, Pergola PE, Agarwal R, Andreoli S, Appel GB, Bangalore S, et al. Effects of Bardoxolone Methyl in Alport Syndrome. Clin J Am Soc Nephrol 2022;17:1763–1774.10.2215/CJN.02400222Search in Google Scholar PubMed PubMed Central
37 Yang Q, Wu FR, Wang JN, Gao L, Jiang L, Li HD, et al. Nox4 in renal diseases: An update. Free Radic Biol Med 2018;124:466–472.10.1016/j.freeradbiomed.2018.06.042Search in Google Scholar PubMed
38 Tong J, Zheng Q, Gu X, Weng Q, Yu S, Fang Z, et al. COL4A3 Mutation Induced Podocyte Apoptosis by Dysregulation of NADPH Oxidase 4 and MMP-2. Kidney Int Rep 2023;8:1864–1874.10.1016/j.ekir.2023.06.007Search in Google Scholar PubMed PubMed Central
39 Zhu Z, Rosenkranz KAT, Kusunoki Y, Li C, Klaus M, Gross O, et al. Finerenone Added to RAS/SGLT2 Blockade for CKD in Alport Syndrome. Results of a Randomized Controlled Trial with Col4a3-/-Mice. J Am Soc Nephrol 2023;34:1513–1520.10.1681/ASN.0000000000000186Search in Google Scholar PubMed PubMed Central
© 2024 Qimin Zheng, Xiangchen Gu, John Cijiang He, Jingyuan Xie, published by De Gruyter on behalf of Scholar Media Publishing
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Irbesartan ameliorates diabetic kidney injury in db/db mice by restoring circadian rhythm and cell cycle
- Commentary
- Biomarker development perspective: Exploring comorbid chronic pain in depression through deep transcranial magnetic stimulation
- Perspective
- Progress in therapeutic targets on podocyte for Alport syndrome
- Review Article
- Latest advances on new promising molecular-based therapeutic approaches for Huntington’s disease
- Original Article
- Insufficiency of quality of life as the treatment endpoint for balloon pulmonary angioplasty in inoperable chronic thromboembolic pulmonary hypertension
- Irbesartan ameliorates diabetic kidney injury in db/db mice by restoring circadian rhythm and cell cycle
- Microarray analysis of microrna expression in peripheral blood mononuclear cells of patients with polymyositis and dermatomyositis
- Development and status quo of digestive endoscopy in China: An analysis based on the national census in 2013 and 2020
- Comparison of admission glycemic variability and glycosylated hemoglobin in predicting major adverse cardiac events among type 2 diabetes patients with heart failure following acute ST-segment elevation myocardial infarction
- Impact of the Alberta Stroke Program CT Score subregions on long-term functional outcomes in acute ischemic stroke: Results from two multicenter studies in China
- Letter to Editor
- EV-Call 120: A new-generation emergency medical service system in China
- Acute esophageal necrosis syndrome as a rare complication of diabetic ketoacidosis
Articles in the same Issue
- Irbesartan ameliorates diabetic kidney injury in db/db mice by restoring circadian rhythm and cell cycle
- Commentary
- Biomarker development perspective: Exploring comorbid chronic pain in depression through deep transcranial magnetic stimulation
- Perspective
- Progress in therapeutic targets on podocyte for Alport syndrome
- Review Article
- Latest advances on new promising molecular-based therapeutic approaches for Huntington’s disease
- Original Article
- Insufficiency of quality of life as the treatment endpoint for balloon pulmonary angioplasty in inoperable chronic thromboembolic pulmonary hypertension
- Irbesartan ameliorates diabetic kidney injury in db/db mice by restoring circadian rhythm and cell cycle
- Microarray analysis of microrna expression in peripheral blood mononuclear cells of patients with polymyositis and dermatomyositis
- Development and status quo of digestive endoscopy in China: An analysis based on the national census in 2013 and 2020
- Comparison of admission glycemic variability and glycosylated hemoglobin in predicting major adverse cardiac events among type 2 diabetes patients with heart failure following acute ST-segment elevation myocardial infarction
- Impact of the Alberta Stroke Program CT Score subregions on long-term functional outcomes in acute ischemic stroke: Results from two multicenter studies in China
- Letter to Editor
- EV-Call 120: A new-generation emergency medical service system in China
- Acute esophageal necrosis syndrome as a rare complication of diabetic ketoacidosis

