Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic heterogeneous disease characterized by persistent airflow obstruction and variable clinical presentations.[1,2] A lack of understanding regarding the molecular mechanisms underlying COPD makes the identification of critical molecules involved in COPD crucial for the development of novel diagnostic measures and therapeutic strategies. In recent decades, wide-ranging profiling methods such as microarrays and next-generation sequencing have made it easier to identify RNA transcripts that do not encode proteins, referred to as noncoding RNAs (ncRNAs).[3] NcRNAs comprise a diverse range of RNA species, characterized according to their length, shape, and location. Many ncRNAs are involved in epigenetic and posttranscriptional gene regulation, including microRNAs (miRNAs), tRNA-derived small RNAs (tsRNAs) and PIWI-interacting RNAs (piRNAs).[4] Long noncoding RNAs (lncRNAs) and circular RNAs (circRNAs) can fold into complex secondary structures that facilitate their interactions with DNA, RNA, and protein.[4] Additionally, lncRNAs and circRNAs can bind to miRNAs in a competitive endogenous RNA (ceRNA) network that prevents targeted mRNA degradation.[5,6] Recent studies have shown that ncRNAs play crucial roles in multiple pathophysiological processes associated with COPD.[5,7,8] A better understanding of the role of ncRNAs in COPD could contribute to the detection of biomarkers and the identification of new therapeutic targets. Here, we summarize the current findings regarding the potential role of ncRNAs, especially miRNAs, lncRNAs, and circRNAs. Additionally, we propose considerations regarding present and future research in this area.
Ncrna Dysregulation Contributes to Copd Progression
Emerging evidence suggests that differentially expressed ncRNAs participate in the regulation of proliferation, apoptosis, invasion, epithelial–mesenchymal transition (EMT), and inflammation in multiple relevant cell types, contributing to the pathophysiological changes in COPD (Figure 1). Supplementary Table 1 summarizes the list of miRNAs, circRNAs, and lncRNAs with their targets and functions in COPD. Notably, in the lncRNA/ circRNA-miRNA-mRNA networks, lncRNAs and circRNAs could sponge miRNAs as ceRNAs, inhibit miRNA expression, and enhance the translation of target mRNA (Figure 2). For instance, lncRNA cancer susceptibility candidate 2 (CASC2)[9]and circRNA HECT domain and ankyrin repeat containing E3 ubiquitin protein ligase 1 (circHACE1) [10] bind to miR-18a-5p and miR-485-3p, respectively, to participate in human bronchial epithelial (16HBE) cell apoptosis and inflammation in response to cigarette smoking extract (CSE). Furthermore, Sundar et al.[11] discovered a small number of differentially expressed piRNAs and tsRNAs among nonsmokers, smokers, and COPD patients from RNA sequencing data. However, the functional role of piRNAs and tsRNAs in COPD remains unclear.
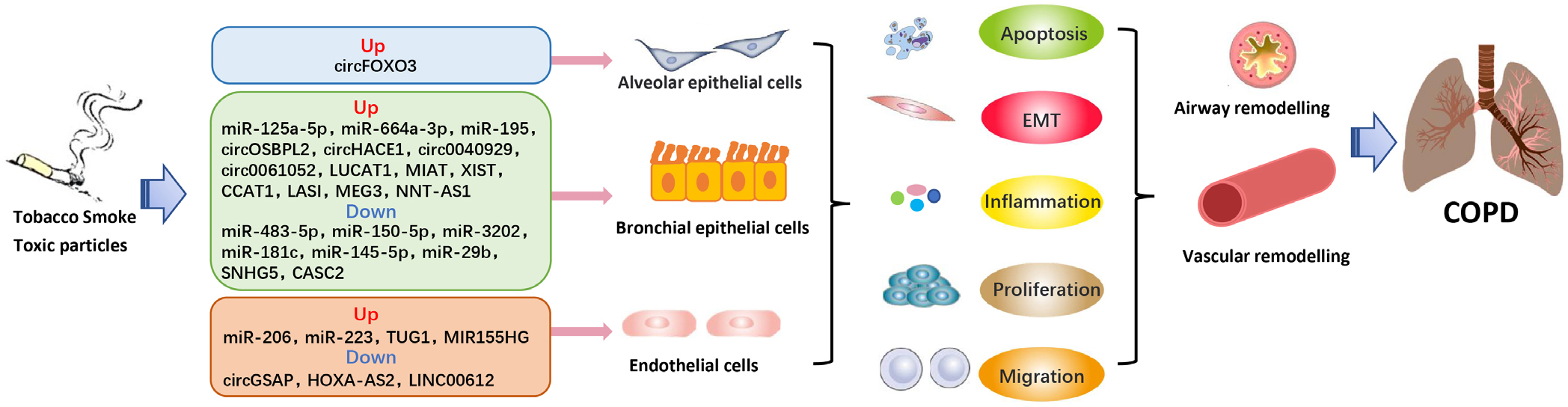
NcRNAs play a crucial role as regulators in the pathophysiological processes of COPD. COPD: chronic obstructive pulmonary diseae; EMT: epithelial mesenchymal transformation.
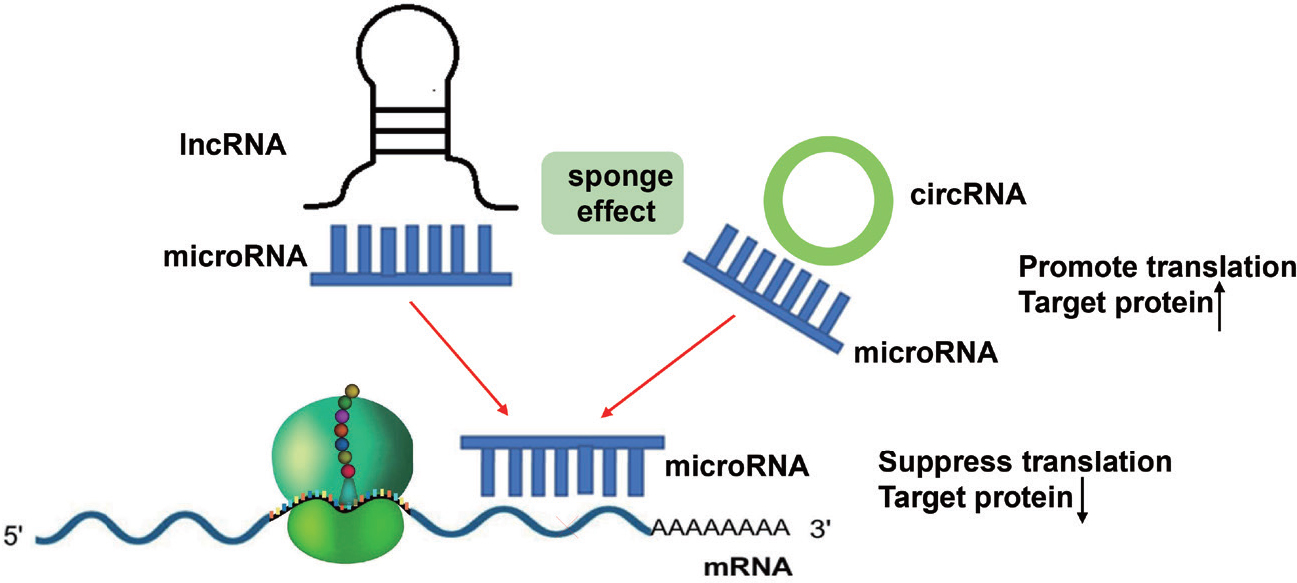
LncRNAs and circRNAs can act as sponges for microRNAS. By binding to these microRNAs, they prevent microRNAs from binding to their target mRNAs, thereby abolishing post-transcriptional regulation.
In summary, given the wide range of regulatory mechanisms and the diversity of downstream pathways affected, many ncRNAs and their crosstalk have been found through in vivo and in vitro experiments to be important contributors to COPD progression and regarded as possible clinical relevance.
Ncrnas Can Be Used as Adjunct Biomarkersfor Copd Diagnosis and Prognosis
It has been found that differentially expressed ncRNAs can be detected in human samples such as sputum, plasma, or serum, and these may serve as adjunct biomarkers for COPD diagnosis and prognosis. For example, circ0040929,[12] circRNA oxysterol binding protein like 2 (circOSBPL2),[13] lncRNA lung cancer associated transcript 1 (LUCAT1),[14] and miR-125a-5p[15] show a more sensitive expression pattern in smokers with COPD than in those without COPD. LUCAT1 [16] expression levels are correlated with inflammation in COPD patients, and circRNA0001859,[17] hsa-miR-664a-3p,[18] lncRNA small nucleolar RNA host gene 5 (SNHG5),[19] and CASC2[9] are correlated with airflow limitation severity. Furthermore, lncRNA antisense non-coding RNA at the INK4 locus (ANRIL)[20] expression is decreased in COPD patients, especially those with acute exacerbations (AECOPD), while lncRNA nuclear-enriched abundant transcript 1 (NEAT1)[21] expression is increased. The area under the curve (AUC) indicates that lncRNAs ANRIL and NEAT1 can distinguish stable COPD patients from AECOPD patients, as well as predict COPD susceptibility and acute exacerbation risk.[20,21] Moreover, the expression levels of the lncRNAs ANRIL and NEAT1 are both correlated with inflammatory cytokines (tumor necrosis factor α [TNF-α], interlenkin [IL]-1β, and IL-17A) and Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage in COPD patients,[20,21] suggesting that they are potential biomarkers of COPD progression.
In addition, noncoding RNA (ncRNA) is useful in diagnosing comorbidities of COPD. For instance, the lower level of circRNA0001859 in serum can be used to identify patients with lung cancer from COPD patients.[17] Low plasma circRNA-gamma-secretase-activating protein (circGSAP) levels might be a promising diagnostic and prognostic indicator for COPD-pulmonary arterial hypertension (PAH).[22] Serum miR-1233 and miR-134 both have higher diagnostic accuracy for AECOPD with acute pulmonary embolism (APE) than D dimers.[23] In addition, the role of certain ncRNAs in indicating COPD comorbidities is unspecified and paradoxical. A recent review discusses that lncRNA maternally expressed gene 3 (MEG3), lncRNA OPA-interacting protein 5 antisense transcript 1 (OIP5-AS1), and ln-cRNA taurine upregulated gene 1 (TUG1) are involved in asthma and COPD pathogenesis by sponging different miRNAs.[5] Further research into the ncRNAs mentioned above as biomarkers for the diagnosis of COPD overlapping with asthma is warranted. Wang et al.[24] previously demonstrated that TUG1 was significantly upregulated in patients with PAH and that TUG1 knockdown significantly prevented the development of PAH in vivo, suggesting that TUG1 may also be a novel and promising biomarker for COPD complicated with PAH. Moreover, MiR-223[16] and NEAT1[25] both have promoting roles for lung cancer carcinogenesis and COPD, and whether they are also involved in COPD complicated with lung cancer remains to be determined. It is worth mentioning that the blood levels of MEG3 are higher in COPD patients but lower in non-small-cell lung cancer patients.[26,27] Thus, the role of MEG3 in COPD with lung cancer requires further research and validation.
As noted above, in COPD, most biomarker studies related to ncRNAs (especially lncRNAs, miRNAs, and circRNAs) have focused on distinguishing COPD patients from non-COPD patients or predicting disease severity and comorbidities. It is well known that COPD is a chronic airway disease with high heterogeneity. The role of ncRNAs in determining the phenotype and endotype of COPD and in predicting the response to specific treatments (e.g., glucocorticoids) needs further investigation. In addition, no single ncRNA or ncRNA panel has passed the test as an analytically validated and clinically useful biomarker. Attempts have been made through meta-analysis to assess the diagnostic accuracy of various ncRNAs in COPD.
The Therapeutic Potential of Ncrnas for Copd
NcRNAs have been demonstrated to play a crucial role as regulators in the pathophysiological processes of COPD. The pharmacological action of ncRNA-based therapies has been demonstrated to target proliferation, apoptosis, inflammation, and migration as potential therapeutics for COPD, including small interfering RNAs, short hairpin RNAs, miRNA mimics, and anti-microRNAs. In COPD mouse models, intranasal administration of anti-miR-195 lentiviruses or miR-181c mimics attenuates neutrophil and macrophage infiltration, lung parenchymal destruction, and levels of proinflammatory factors in bronchoalveolar lavage fluid (BALF),[28] while intranasal delivery of short hairpin RNA (shRNA) lentivirus against TUG1 blocks cigarette smoking (CS)-induced inflammation and remodeling.[29] CS-induced increases in neutrophils, macrophages, and BALF cells could also be significantly reduced with lentivirus-based knockdown of circRNA forkhead box O3 (circFOXO3) in vivo.[30] in vitro studies have shown that miR-206 antagomirs or miR-483-5p mimics enhance vascular remodeling and fibrosis,[31,32] whereas miR-27-3p antagomirs or miR-3202 mimics inhibit inflammation in response to CSE.[33,34] Using small interfering RNA (siRNA) to knockdown MIR155 host gene (MIR155HG) results in a switch from the M1 to M2 macrophage phenotype along with reduced proin-flammatory cytokines in CSE-treated human pulmonary microvascular endothelial cells (HPMECs).[35] Currently available small RNA high-throughput sequencing technology can detect tsRNAs and piRNAs; however, few have been functionally characterized in COPD models for further validation of potential therapeutic targets.
To the best of our knowledge, ncRNAs as therapeutic targets (e.g., MRX34, a miR-34a mimic in advanced solid tumors) for cancer have been investigated and tested in clinical trials.[36] However, advances concerning the roles of ncRNAs in COPD have only been studied in cell lines or animal models, and further effort is needed to explore the feasibility of their clinical application.[37]
Conclusion and Perspectives
NcRNAs, including miRNAs, lncRNAs, and circRNAs, can be detected in the serum, plasma, sputum, or urine of COPD patients and may serve as diagnostic biomarkers or prognostic indicators. Notably, the abnormal expression of these ncRNAs has been linked to the various pathophysiological processes of COPD, underscoring their feasibility as a novel therapeutic modality for COPD. In addition, there are many other less studied ncRNA classes, such as piRNAs and tsRNAs, that may warrant further exploration of their precise function and mechanism in COPD. In the past several years, studies on the crosstalk of lncRNA/circRNA-miRNA-mRNA in the pathogenesis of many diseases, including COPD, have received increasing attention; this will likely open a new horizon for the identification of therapeutic targets for COPD.
There are several challenges to overcome regarding the clinical application of ncRNAs. First, a large number of investigations have discovered various ncRNAs for identifying COPD, acute exacerbations, and comorbidities. The screening and development of the optimal ncRNA will require further studies involving larger sample sizes. Second, the development of diagnostic biomarkers found in urine, sputum, or blood would avoid the need for invasive procedures associated with tissue collection. Given that ncRNAs are often expressed at lower levels than protein-coding genes, more research is needed to identify candidates that are highly stable and easily detected in body fluids. Third, regulation of the pharmacological action of ncRNA in the COPD model is mainly dependent on small interfering RNAs, short hairpin RNAs, miRNA mimics, and anti-microRNAs. Antisense oligonucleotides (ASOs) or custered regularly interspaced short palindromic repeats (CRISPR) may also be worth exploring for the treatment of COPD. However, safety issues associated with random mutations of target sites should be assessed. If there are abnormal changes such as large fragment loss and chromosome rearrangement, the safety issues will be too risky. In addition, ncRNA drugs are usually given intravenously for cancer patients, while in COPD, direct delivery to the lungs by inhalation is the most effective way to reduce systemic adverse effects. However, the stability and economic benefits of ncRNAs must be considered and optimized. Improvements in oligonucleotide chemistry, editing efficiency and accuracy of target sites, and delivery methods should continuously be pursued in future studies to mitigate these issues. Finally, ncRNAs can regulate multiple genes simultaneously; therefore, special precautions to minimize off-target adverse effects must be made.
To conclude, there is growing evidence that ncRNAs may be useful for diagnosing and treating COPD, and a large ncRNA network is being established to explore their possible mechanisms of action in the disease. Although our understanding of ncRNAs in COPD is still at an early stage, the discovery of ncRNAs has opened a new chapter in the history of medicine, one that promises to improve the way that COPD is diagnosed and treated. It is expected that genetic diagnosis and therapeutics based on ncRNA will be widely available in the future.
Funding statement: This study was supported by the National Natural Science Foundation (Project Number: 82270046).
-
Supplementary Materials
Supplementary materials mentioned in this article are online available at the journal's official site only.
-
Author Contributions
Qiao X, Yin Y, Ding YX, and Altawil A: Conceptualization, Writing—Original draft preparation, Writing—Reviewing and Editing. Yin Y: Conceptualization, Supervision. Wang W, Wang QY, and Kang J: Supervision, Project administration.
-
Ethics Approval
Not applicable.
-
Conflict of Interest
Jian Kang is an Honorary Editor-in-Chief of the journal. Wei Wang is an Editorial Board Member of the journal. The article was subject to the journal’s standard procedures, with peer review handled independently of the editor and the affiliated research groups.
References
1 Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017;195:557–82.10.1164/rccm.201701-0218PPSearch in Google Scholar PubMed
2 Sun L, Chen Y. Interpretation of the key issues of expert consensus on immunomodulatory therapies for chronic obstructive pulmonary disease. J Transl Intern Med 2022; 10: 277–80.10.2478/jtim-2022-0069Search in Google Scholar PubMed PubMed Central
3 Xue C, Gu X, Bao Z, Su Y, Lu J, Li L. The Mechanism Underlying the ncRNA Dysregulation Pattern in Hepatocellular Carcinoma and Its Tumor Microenvironment. Front Immunol 2022;13:847728.10.3389/fimmu.2022.847728Search in Google Scholar PubMed PubMed Central
4 Yan H, Bu P. Non-coding RNA in cancer. Essays Biochem 2021;65:625–39.10.1042/EBC20200032Search in Google Scholar PubMed PubMed Central
5 Qiao X, Hou G, He YL, Song DF, An Y, Altawil A, et al The Novel Regulatory Role of the lncRNA-miRNA-mRNA Axis in Chronic Inflammatory Airway Diseases. Front Mol Biosci 2022;9:927549.10.3389/fmolb.2022.927549Search in Google Scholar PubMed PubMed Central
6 Li Y, Lu X, Li W, Shi Z, Du W, Xu H, et al The circRERE/miR-144-3p/TLR2/MMP9 signaling axis in COPD pulmonary monocytes promotes the EMT of pulmonary epithelial cells. Biochem Biophys Res Commun 2022;625:1–8.10.1016/j.bbrc.2022.07.119Search in Google Scholar PubMed
7 Soni DK, Biswas R. Role of Non-Coding RNAs in Post-Transcriptional Regulation of Lung Diseases. Front Genet 2021;12:767348.10.3389/fgene.2021.767348Search in Google Scholar PubMed PubMed Central
8 Liu P, Wang Y, Zhang N, Zhao X, Li R, Wang Y, et al Comprehensive identification of RNA transcripts and construction of RNA network in chronic obstructive pulmonary disease. Respir Res 2022;23:154.10.1186/s12931-022-02069-8Search in Google Scholar PubMed PubMed Central
9 Liu P, Zhang H, Zeng H, Meng Y, Gao H, Zhang M, et al LncRNA CASC2 is involved in the development of chronic obstructive pulmonary disease via targeting miR-18a-5p/IGF1 axis. Ther Adv Respir Dis 2021;15:17534666211028072.10.1177/17534666211028072Search in Google Scholar PubMed PubMed Central
10 Zhou F, Cao C, Chai H, Hong J, Zhu M. Circ-HACE1 Aggravates Cigarette Smoke Extract-Induced Injury in Human Bronchial Epithelial Cells via Regulating Toll-Like Receptor 4 by Sponging miR-485-3p. Int J Chron Obstruct Pulmon Dis 2021;16:1535–47.10.2147/COPD.S304859Search in Google Scholar PubMed PubMed Central
11 Sundar IK, Li D, Rahman I. Small RNA-sequence analysis of plasma-derived extracellular vesicle miRNAs in smokers and patients with chronic obstructive pulmonary disease as circulating biomarkers. J Extracell Vesicles 2019;8:1684816.10.1080/20013078.2019.1684816Search in Google Scholar PubMed PubMed Central
12 Miao Y, Wu J, Wu R, Wang E, Wang J. Circ_0040929 Serves as Promising Biomarker and Potential Target for Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis 2022;17:2079–92.10.2147/COPD.S364553Search in Google Scholar PubMed PubMed Central
13 Zheng C, Zhang Y, Zhao Y, Duan Y, Mu Q, Wang X. Circ-OSBPL2 Contributes to Smoke-Related Chronic Obstructive Pulmonary Disease by Targeting miR-193a-5p/BRD4 Axis. Int J Chron Obstruct Pulmon Dis 2021;16:919–31.10.2147/COPD.S298465Search in Google Scholar PubMed PubMed Central
14 Zhao S, Lin C, Yang T, Qian X, Lu J, Cheng J. Expression of long non-coding RNA LUCAT1 in patients with chronic obstructive pulmonary disease and its potential functions in regulating cigarette smoke extract-induced 16HBE cell proliferation and apoptosis. J Clin Lab Anal 2021;35:e23823.10.1002/jcla.23823Search in Google Scholar PubMed PubMed Central
15 Wu H, Ma H, Wang L, Zhang H, Lu L, Xiao T, et al Regulation of lung epithelial cell senescence in smoking-induced COPD/emphysema by microR-125a-5p via Sp1 mediation of SIRT1/HIF-1a. Int J Biol Sci 2022;18:661–74.10.7150/ijbs.65861Search in Google Scholar PubMed PubMed Central
16 Li S, Feng Y, Huang Y, Liu Y, Wang Y, Liang Y, et al MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB. Open Life Sci 2020;15(1):389–99.10.1515/biol-2020-0040Search in Google Scholar PubMed PubMed Central
17 Chen S, Yao Y, Lu S, Chen J, Yang G, Tu L, et al CircRNA0001859, a new diagnostic and prognostic biomarkers for COPD and AECOPD. BMC Pulm Med 2020;20:311.10.1186/s12890-020-01333-1Search in Google Scholar PubMed PubMed Central
18 Zhong S, Chen C, Liu N, Yang L, Hu Z, Duan P, et al Overexpression Of hsa-miR-664a-3p Is Associated With Cigarette Smoke-Induced Chronic Obstructive Pulmonary Disease Via Targeting FHL1. Int J Chron Obstruct Pulmon Dis 2019;14:2319–29.10.2147/COPD.S224763Search in Google Scholar PubMed PubMed Central
19 Shen Q, Zheng J, Wang X, Hu W, Jiang Y, Jiang Y. LncRNA SNHG5 regulates cell apoptosis and inflammation by miR-132/PTEN axis in COPD. Biomed Pharmacother 2020;126:110016.10.1016/j.biopha.2020.110016Search in Google Scholar PubMed
20 Ge J, Geng S, Jiang H. Long noncoding RNAs antisense noncoding RNA in the INK4 locus (ANRIL) correlates with lower acute exacerbation risk, decreased inflammatory cytokines, and mild GOLD stage in patients with chronic obstructive pulmonary disease. J Clin Lab Anal 2019;33:e22678.10.1002/jcla.22678Search in Google Scholar PubMed PubMed Central
21 Ming XY, Duan WZ, Yi W. Long non-coding RNA NEAT1 predicts elevated chronic obstructive pulmonary disease (COPD) susceptibility and acute exacerbation risk, and correlates with higher disease severity, inflammation, and lower miR-193a in COPD patients. Int J Clin Exp Pathol 2019;12:2837–2848.Search in Google Scholar
22 Sun Y, Wu W, Zhao Q, Jiang R, Li J, Wang L, et al CircGSAP regulates the cell cycle of pulmonary microvascular endothelial cells via the miR-942-5p sponge in pulmonary hypertension. Front Cell Dev Biol 2022;10:967708.10.3389/fcell.2022.967708Search in Google Scholar PubMed PubMed Central
23 Peng L, Han L, Li XN, Miao YF, Xue F, Zhou C. The Predictive Value of microRNA-134 and microRNA-1233 for the Early Diagnosis of Acute Exacerbation of Chronic Obstructive Pulmonary Disease with Acute Pulmonary Embolism. Int J Chron Obstruct Pulmon Dis 2020;15:2495–503.10.2147/COPD.S266021Search in Google Scholar PubMed PubMed Central
24 Wang S, Cao W, Gao S, Nie X, Zheng X, Xing Y, et al TUG1 Regulates Pulmonary Arterial Smooth Muscle Cell Proliferation in Pulmonary Arterial Hypertension. Can J Cardiol 2019;35:1534–45.10.1016/j.cjca.2019.07.630Search in Google Scholar PubMed
25 Ma F, Lei YY, Ding MG, Luo LH, Xie YC, Liu XL. LncRNA NEAT1 Interacted With DNMT1 to Regulate Malignant Phenotype of Cancer Cell and Cytotoxic T Cell Infiltration via Epigenetic Inhibition of p53, cGAS, and STING in Lung Cancer. Front Genet 2020;11:250.10.3389/fgene.2020.00250Search in Google Scholar PubMed PubMed Central
26 Lv D, Bi Q, Li Y, Deng J, Wu N, Hao S, et al Long non‑coding RNA MEG3 inhibits cell migration and invasion of non‑small cell lung cancer cells by regulating the miR‑21‑5p/PTEN axis. Mol Med Rep 2021;23:191.10.3892/mmr.2021.11830Search in Google Scholar PubMed PubMed Central
27 Zhao Y, Zhu Z, Shi S, Wang J, Li N. Long non-coding RNA MEG3 regulates migration and invasion of lung cancer stem cells via miR-650/SLC34A2 axis. Biomed Pharmacother 2019;120:109457.10.1016/j.biopha.2019.109457Search in Google Scholar PubMed
28 Mei D, Tan WSD, Tay Y, Mukhopadhyay A, Wong WSF. Therapeutic RNA Strategies for Chronic Obstructive Pulmonary Disease. Trends Pharmacol Sci 2020;41:475–86.10.1016/j.tips.2020.04.007Search in Google Scholar PubMed
29 Gu W, Yuan Y, Wang L, Yang H, Li S, Tang Z, et al Long non-coding RNA TUG1 promotes airway remodelling by suppressing the miR-145-5p/DUSP6 axis in cigarette smoke-induced COPD. J Cell Mol Med 2019;23:7200–9.10.1111/jcmm.14389Search in Google Scholar PubMed PubMed Central
30 Zhou L, Wu B, Yang J, Wang B, Pan J, Xu D, et al Knockdown of circ-FOXO3 ameliorates cigarette smoke-induced lung injury in mice. Respir Res 2021;22:294.10.1186/s12931-021-01883-wSearch in Google Scholar PubMed PubMed Central
31 Sun Y, An N, Li J, Xia J, Tian Y, Zhao P, et al miRNA-206 regulates human pulmonary microvascular endothelial cell apoptosis via targeting in chronic obstructive pulmonary disease. J Cell Biochem 2019;120:6223–36.10.1002/jcb.27910Search in Google Scholar PubMed
32 Shen ZY, Tang WX, Guo J, Sun SH. miR-483-5p plays a protective role in chronic obstructive pulmonary disease. Int J Mol Med 2017;40:193–200.10.3892/ijmm.2017.2996Search in Google Scholar PubMed
33 Shen W, Liu J, Fan M, Wang S, Zhang Y, Wen L, et al MiR-3202 protects smokers from chronic obstructive pulmonary disease through inhibiting FAIM2: An in vivo and in vitro study. Exp Cell Res 2018;362:370–7.10.1016/j.yexcr.2017.11.038Search in Google Scholar PubMed
34 Wang D, He S, Liu B, Liu C. MiR-27-3p regulates TLR2/4-dependent mouse alveolar macrophage activation by targetting PPARγ. Clin Sci(Lond) 2018;132:943–58.10.1042/CS20180083Search in Google Scholar PubMed
35 Li N, Liu Y, Cai J. LncRNA MIR155HG regulates M1/M2 macrophage polarization in chronic obstructive pulmonary disease. Biomed Pharmacother 2019;117:109015.10.1016/j.biopha.2019.109015Search in Google Scholar PubMed
36 Hong DS, Kang YK, Borad M, Sachdev J, Ejadi S, Lim HY, et al Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer 2020;122:1630–7.10.1038/s41416-020-0802-1Search in Google Scholar PubMed PubMed Central
37 Zhang J, Han Y, Hua J, He B. Inhaled antibiotics and airway bacterial decolonization for patients with chronic obstructive pulmonary disease: The rationale and future. J Transl Intern Med 2022;10:181–4.10.2478/jtim-2022-0005Search in Google Scholar PubMed PubMed Central
© 2023 Xin Qiao, Yuxiao Ding, Abdullah Altawil, Yan Yin, Qiuyue Wang, Wei Wang, Jian Kang, published by De Gruyter on behalf of the SMP
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Perspective
- A cycloruthenated complex, ruthenium (II) Z (RuZ) overcomes in vitro and in vivo multidrug resistance in cancer cells: A pivotal breakthrough
- Protocols for Traditional Chinese Medicine guidelines for acute primary headache
- Timing of anticoagulation for the management of portal vein thrombosis in liver cirrhosis
- Roles of noncoding RNAs in chronic obstructive pulmonary disease
- Suggestions on home quarantine and recovery of novel coronavirus patients
- Review Article
- Therapeutic potential and mechanisms of sacral nerve stimulation for gastrointestinal diseases
- The evolution of folate supplementation – from one size for all to personalized, precision, poly-paths
- Original Article
- A generalized deep learning model for heart failure diagnosis using dynamic and static ultrasound
- A new ferroptosis-related signature model including messenger RNAs and long non-coding RNAs predicts the prognosis of gastric cancer patients
- A newly-synthesized compound CP-07 alleviates microglia-mediated neuroinflammation and ischemic brain injury via inhibiting STAT3 phosphorylation
- Adrenomedullin induces cisplatin chemoresistance in ovarian cancer through reprogramming of glucose metabolism
- Serum myoglobin modulates kidney injury via inducing ferroptosis after exertional heatstroke
- Letter to Editor
- Gas embolism caused by gas-forming pyogenic liver abscess
Articles in the same Issue
- Perspective
- A cycloruthenated complex, ruthenium (II) Z (RuZ) overcomes in vitro and in vivo multidrug resistance in cancer cells: A pivotal breakthrough
- Protocols for Traditional Chinese Medicine guidelines for acute primary headache
- Timing of anticoagulation for the management of portal vein thrombosis in liver cirrhosis
- Roles of noncoding RNAs in chronic obstructive pulmonary disease
- Suggestions on home quarantine and recovery of novel coronavirus patients
- Review Article
- Therapeutic potential and mechanisms of sacral nerve stimulation for gastrointestinal diseases
- The evolution of folate supplementation – from one size for all to personalized, precision, poly-paths
- Original Article
- A generalized deep learning model for heart failure diagnosis using dynamic and static ultrasound
- A new ferroptosis-related signature model including messenger RNAs and long non-coding RNAs predicts the prognosis of gastric cancer patients
- A newly-synthesized compound CP-07 alleviates microglia-mediated neuroinflammation and ischemic brain injury via inhibiting STAT3 phosphorylation
- Adrenomedullin induces cisplatin chemoresistance in ovarian cancer through reprogramming of glucose metabolism
- Serum myoglobin modulates kidney injury via inducing ferroptosis after exertional heatstroke
- Letter to Editor
- Gas embolism caused by gas-forming pyogenic liver abscess

