Abstract
Background and Objectives
RING finger protein 187 (RNF187) belongs to RING domain-containing E3 ligases family, which was recently reported to be involved in oncogenesis and development of several cancers. This research aims to clarify the role of RNF187 in colorectal cancer (CRC) development.
Methods
The expression of RNF187 and miR-144-4p were determined by quantitative real-time polymerase chain reaction (qRT-PCR). The levels of RNF187 protein were assessed by western blot analysis. Cell Counting Kit-8 (CCK8) assay, clonogenic assay, cell scratch test and transwell assay were used to determine the proliferation, migration and invasion of CRC cells in vitro. The binding of miR-144-5p and RNF197 mRNA was validated by luciferase reporter assays. Tumor-bearing nude mice were used to determine CRC cells growth in vivo.
Results
RNF187 expression significantly increased in CRC specimens and cell lines compared to normal colon tissues and normal colonic mucosa cell line, respectively. Upregulation of RNF187 expression was inversely correlated to poor prognosis in CRC patients. In addition, knockdown of RNF187 expression inhibited the proliferation, migration, and invasion but promoted the apoptosis of CRC lines Caco-2 and SW480 cells. Further studies validated that RNF187 was the direct target of miR-144-5p. The expression of miR-144-5p was downregulated in CRC tissues, which was negatively correlated to the expression of RNF187. Restoration of miR-144-5p significantly inhibited the progression of CRC cells and its anti-tumor effects could be abrogated by overexpression of RNF187.
Conclusion
Our findings demonstrate the deregulation of miR-144-5p/ RNF187 axis in CRC, as well as its role in regulation of the tumor progression, thus providing a novel therapeutic strategy for CRC treatment.
Introduction
Colorectal cancer (CRC) is one of the most prevalent malignant tumours, comprising 11% of all cancer diagnoses in 2018.[1] The current management of CRC patients includes surgery, radiotherapy, chemotherapy, targeted therapy and the combined therapies. However, most CRC patients become resistant to these treatments, leading to local recurrence of CRC. One of the main mechanisms of resistance is tumour heterogeneity.[2] Although great advancements have been made in understanding the biology of CRC, the outcome of advanced CRC remains extremely poor.[3,4] With accelerated elucidation of the precise molecular regulatory mechanisms in the oncogenesis of CRC, it is of pivotal importance to develop novel diagnostic, prognostic and therapeutic strategies for CRC, especially for late-stage patients.
MicroRNAs (miRNAs) are a group of short, conserved, non-coding RNAs which regulate gene expression through directly binding to 3ʹ-untranslated region (3ʹ-UTR) of target genes.[5,6] In humans, miRNAs are involved in the regulation of approximately 60% of protein coding genes[7] functioning extensively in various cellular processes such as apoptosis, stem cell differentiation,[8] inflammation[9,10] and carcinogenesis.[11] Mounting evidence has shown the critical regulatory roles of miRNAs in malignant neoplasms such as CRC. For example, miR-4449 targets the suppressor of cytokine signalling-3 (SOCS3) to activate signal transducer and activator of transcription 3 (STAT3) pathways, accelerating the proliferation of CRC cells.[12] In addition, our previous study has demonstrated that miR-31 expression is increased in CRC cells, and that downregulation of miR-31 inhibits the proliferation of CRC cells by targeting the tumour suppressor RhoTBT1.[13] miRNAs also play a key role in the chemo- and radio-sensitivity of CRC cells via modulating DNA repair genes.[14,15] Thus, approaches targeting miRNAs form a potential strategy for CRC prevention.
RING finger protein 187 (RNF187), also known as RACO-1, belongs to the RING domain-containing protein family. It functions as an E3 ligase to modulate various cellular processes involved in malignant neoplasms including lung cancer,[16] hepatocellular carcinoma,[17] osteosarcoma[18]and breast cancer.[19] RNF187 forms a homodimer in nuclear specifically interacting with c-Jun, which is required for c-Jun/activator protein 1 (AP-1)-activated gene transcription.[20,21] Zhang et al.[17] uncovered that RNF187 expression is driven by Notch1 at the transcriptional level, contributing to the pro-tumorigenic effects of Notch1 signalling in hepatocellular carcinoma. Besides, RNF187 is reported to negatively regulate Hippo signalling activation by targeting YAP for degradation, thus playing a role in the progression of breast cancer and oesophageal squamous cell carcinoma.[19,22] To date, the role of RNF187 in CRC progression still remains elusive.
In this study, our results showed that RNF187 expression was upregulated in CRC tissues and was correlated to a poor prognosis. Knockdown of RNF187 expression inhibited the proliferation, migration and invasion, but promoted the apoptosis of CRC cells. We also demonstrated that RNF187 was a direct target of miR-144-5p. miR-144-5p significantly inhibited the progression of CRC cells, which was abrogated by RNF187. These findings revealed the pro-tumorigenic effects of RNF187 in CRC, which is counteracted by miR-144-5p, highlighting that the miR-144-5p/RNF187 axis is a novel alternative therapeutic target for CRC treatment.
Materials and methods
Clinical CRC tissues
CRC and adjacent normal colon tissues were obtained by surgical dissection from patients with primary CRC. All the patients (N = 83) signed the informed consent at the Endoscopy Center, China-Japan Union Hospital of Jilin University (Changchun, China), according to the procedures approved by the hospital’s Ethics Review Board (2021-KYYS-005). The study was conducted in accordance with the Declaration of Helsinki.
Cell culture
Human embryonic kidney 293T (HEK-293T) cells were purchased from the Shanghai Cell Collection (Shanghai, China). The colonic mucosa cell line NCM460 cells and CRC cell lines (Caco-2, SW480, HT29 and HCT-116) were purchased from the American Type Culture Collection (Manassas, VA, USA). These cells were maintained in Dulbecco’s modified Eagle medium (DMEM; Gibco) supplemented with 10% foetal bovine serum (FBS; Gibco) and kept in a 37°C incubator with 5% CO2.
Oligonucleotides transfection and lentivirus infection
The miR-144-5p mimic and scrambled control miRNA, as well as RNF187 siRNA and Mock siRNA were purchased from Genepharma Co. (Shanghai, China) and transfected into cells using Lipofectamine™ 3000 (Invitrogen, USA), according to the manufacturer’s protocol. The RNF187-encoding gene was amplified by polymerase chain reaction (PCR) from human cDNA library using PCR overexpression and cloned into pCDH-CMV-MCS-EF1a-Puro. The plasmids were co-transfected with pMD2.G and psPAX2 into HEK293T cells. Forty-eight hours after transfection, the supernatant was collected and used to infect CRC cells with 10 multiplicities of infection (MOI). After incubation for 2 days, the cells were selected by puromycin (Invitrogen) to establish the cell lines stably expressing RNF187.
Cell Counting Kit-8 (CCK8) assay
Caco-2 or SW480 cells transfected with oligonucleotides were seeded into a 96-well plate. At the indicated time points, 10 μL of WST-8 solution (Beyotime Biotech, China) was added into the media and the cells were incubated for 2 h. Then, the cell spectrophotometric absorbance at 450 nm was determined using the Synergy H1 hybrid multi-mode microplate reader (BioTek, USA). Each experiment was performed in triplicate.
Clonogenic assay
The clonogenic assay was performed as described before.[23] Briefly, Caco-2 or SW480 cells after transfection were seeded into six-well plates and maintained at 37°C in a 5% CO2 incubator for 10 days. Then, the cell clones were stained with 0.5% crystal violet solution. The images were taken by a HICC-B automatic colony counter (Wanshen Inc., China) and the number of spheres was counted.
Immunoblotting
Cells and tissues were lysed using a Tris buffer (50 mmol/L Tris, 150 mmol/L NaCl) with 1% Triton X-100. The samples were centrifuged at 14,000 g for 10 min and the supernatant was transferred into a new tube, followed by heating at 95°C with 5× SDS loading buffer for 10 min. The samples were resolved by 10% SDS-PAGE for 90 min with a voltage of 80 V. The proteins in SDS-PAGE were then transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, USA). The membranes were blocked using 5% non-fat milk for 30 min, followed by incubation with primary antibodies at the indicated dilutions: RNF187 (Abcam, 1:3000) and GAPDH (Abcam, 1:3000). After washing for three times, the membranes were incubated with HRP-labelled secondary antibodies and the signals were taken and analysed by Tanon 5200 Chemiluminescent Imaging System.
Quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from cells or homogenised tissues using Trizol reagent (Invitrogen, USA) and used as a template for cDNA synthesis using the MonScript™ RTIII All-in-One Mix with dsDNase (Monad Biotech, China). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the MonAmp™Fast SYBR® Green qPCR Mix (Monad Biotech) on a CFX connect system (Bio-Rad, USA). The primer sequences for RNF187 and GAPDH were as follows:
RNF187-F, 5’-GTGATGGACCGTAGGAAGAAGG-3’; RNF187-R, 5’-GTGACCTGAACCGCTCAGTG-3’; GAPDH-F, 5’-GTCTCCTCTGACTTCAACAGCG-3’ and
GAPDH-R, 5’-ACCACCCTGTTGCTGTAGCCAA-3’.
The primers for miR-144-5p and the internal reference U6 were purchased from Genepharma Co. The relative gene expression was calculated using the 2-ΔΔCt method.
Cell scratch test
The cell scratch test was performed as described before.[23] Briefly, Caco-2 or SW480 cells after transfection with miRNAs were cultured in six-well plates and incubated until the confluency reached about 90%. The cell monolayer was scraped in a straight line using a p200 pipette tip. Serum-free medium was added and the cells were placed back in a 37°C incubator. Two and 24 h after scratch, images were taken using an Olympus microscope. The wound healing rate was calculated by the following formula: % wound healing = (0 h distance – 24 h distance)/24 h distance × 100.
Transwell assay
To determine the migration and invasion of cells in vitro, a Transwell assay was performed as described before.[24] Briefly, 2 × 104 cells were planted into the upper chamber of Corning Transwell Inserts (Sigma, USA) in serum-free DMEM. Then, the upper chambers were inserted into a 24-well plate with 10% FBS containing DMEM in the lower chamber. After maintaining in a 37°C incubator for 24 h, the membrane was removed and stained using 0.5% crystal violet solution. Images were taken by a microscope (Olympus, Tokyo, Japan) and the number of migrated cells was quantified by counting six random fields. The procedure of invasion assay was performed as the migration assay, except that the membrane of the upper chamber was coated with Matrigel (Corning).
Animal experiments
The procedures of the animal experiment were approved by the hospital’s Ethics Review Board of China-Japan Union Hospital of Jilin University, according to the Institutional Animal Care and Use Committee of the Model Animal Research Center. Briefly, 4-week-old male NOD-SCID mice were injected subcutaneously into the left flank with a mixture of Caco-2 cells (2 × 106) and Matrigel. Seven days after injection, the mice were treated with an intratumoral injection of oligonucleotides (1 nmol for each mouse) or an equivalent amount of corresponding negative control (Genepharma) every 3 days for a total of 21 days (seven times). The tumour burden was monitored every 3 days by measurement of tumour volume. Twenty-one days after transplantation, the mice were sacrificed and the xenograft tumours were stripped for weight measurement.
Immunohistochemistry
Immunohistological staining was performed on the collected CRC tissues to detect RNF187 expression as described before.[25] Briefly, the tissues were fixed using 10% formalin, following which they were embedded in paraffin blocks. The specimens were then sliced as 4-μm sections. The samples were heated to retrieve the antigen. Subsequently, the samples were incubated with a specific anti-RNF187 antibody (1:100; Abcam, USA) at 4°C overnight, followed by incubation with an HRP-conjugated secondary antibody (1:3000) at room temperature for 2 h. After washes, the samples were stained using 3,3′-diaminobenzidine (DAB) according to the standard protocol. The images were photographed with an Olympus light microscope (Olympus, USA).
Luciferase assay
We screened the potential miRNAs targeting RNF187 by the online database TargetScan (http://www.targetscan.org/). miR-144-5p was predicted to bind to the 3ʹ-UTR of RNF187. In order to validate if RNF187 is a bona fide target of miR-144-5p, the RNF187 3ʹ-UTR containing the putative miR-144-5p–interacting site (UGAUAUC) was inserted into the pmirGLO vector (Promega, USA), termed as hsa-RNF187-wt. pMIR-REPORT vector harbouring a mutated miR-144-5p–interacting site (CAUGUC) was set as a control (hsa-RNF187-mut). The indicated luciferase reporter was co-transfected into HEK-293T cells with miR-144-5p mimic or scrambled control using Lipo3000 (Invitrogen). Forty-eight hours after transfection, the cells were lysed and luciferase activity in the supernatant was determined using a Dual-Luciferase® Reporter Assay kit (Promega).
Statistical analysis
Student’s test (two tailed) was applied for analysis of statistical significance between two groups using SPSS 15.0. The analysis of differences between groups was performed using one-way analysis of variance (ANOVA). Spearman’s correlation coefficients were applied for correlation analysis. P < 0.05 was considered statistically significant.
Results
Upregulated expression of RNF187 in CRC is associated with a poor outcome
RNF187 expression in CRC tumour and adjacent normal tissues was determined using qPCR and the results showed that the RNF187 expression levels in the tumourous tissues were significantly higher than those in the normal tissues (Figure 1A). Consistent with this observation, the protein levels of RNF187 remarkably increased in CRC tissues, compared with normal tissues (Figure 1B). By staining with a RNF187-specific antibody, we observed that RNF187 was diffusely distributed in the nucleus and cytoplasm of CRC cells, with a stronger staining intensity in CRC tissue than in normaI tissues (Figure 1C). In addition, the mRNA expression of RNF187 (Figure 1D), as well as the protein levels (Figure 1E) significantly increased in CRC cell lines than in normal colonic mucosa cell line NCM460. The results suggest that RNF187 is aberrantly expressed both in CRC tissue and CRC cell lines. Importantly, by analysing the The Cancer Genome Atlas (TCGA) database (http://cancergenome.nih.gov/), we found that patients with higher expression of RNF187 had a shorter overall survival (OS), compared to patients with lower RNF187 expression (Figure 1F), indicating that RNF187 expression in CRC tissue predicts a poor prognosis for CRC patients.

RNF187 expression in CRC. (A) The expression levels of RNF187 in CRC specimens (N = 83) as well as the paired adjacent colon tissues were analysed by qRT-PCR. (B) The protein levels of RNF187 were determined in CRC specimens and normal colon tissues using a RNF187-specific antibody. (C) The distribution of RNF187 was analysed by immunostaining. (D and E) RNF187 mRNA expression and protein levels in NCM460 cells and CRC cell lines (Caco-2, SW480, H29 and HCT-116). Data are expressed as the mean±standard deviation of three separate experiments. (F) Kaplan–Meier curves depicting overall survival according to RNF187 expression in TCGA database (n = 597, P = 0.0193). **P < 0.01. RNF187: RING finger protein 187; CRC: colorectal cancer; TCGA: The Cancer Genome Atlas.
Knockdown of RNF187 inhibits growth of CRC cells in vitro and in vivo
The upregulation of RNF187 expression in CRC suggests that RNF187 may play an important role in the progression of CRC. Therefore, we knocked down RNF187 expression in Caco-2 and SW480 cells with two different single RNF187 siRNAs, in parallel with mock siRNA, following which we conducted cell viability analysis with CCK8. In comparison to the mock siRNA, both the RNF187 siRNAs could significantly reduce the protein levels of RNF187 in CRC cell lines (Figure 2A). Knockdown of RNF187 significantly decreased the viability of CRC cells in a time-dependent manner in both Caco-2 and SW480 cells (Figure 2B). To confirm the observation that knockdown of RNF187 results in loss of CRC cell viability, we assessed the effects of RNF187 on Caco-2 and SW480 cells in an in vitro colony formation assay. In comparison to the mock control, knockdown of RNF187 expression reduced the colony number by approximately 40% in Caco-2 and SW480 cells (Figure 2C).

Silencing miR-144-5p suppressed proliferation of CRC cells. (A) Caco-2 and SW480 cells were transfected with 100 nmol/L of either the RNF187 siRNA or mock control for 24 h. Cell viability was detected by the CCK8 assay at 24, 48 and 72 h time points. (B) Caco-2 and SW480 cells were transfected with RNF187 siRNA or mock control for 48 h, followed by colony formation. Ten days after the cells were seeded, quantitative analysis of the relative colony numbers to the blank control was performed. (C) For xenograft mouse model establishment, mice were injected subcutaneously into the left flank with a mixture of Caco-2 cells (2 × 106) and Matrigel. Seven days after injection, the mice were treated with an intratumoral injection of RNF187 siRNA (1 nmol for each mouse) or an equivalent amount of mock siRNA every 3 days for a total of 21 days (seven times). Twenty-eight days after transplantation, the mice were sacrificed and the xenograft tumours were stripped for weight measurement (D). Also, the tumour burden was monitored every 3 days by measurement of tumour volume (E). Data are representative images or expressed as the mean±standard deviation. **P < 0.01. CRC: colorectal cancer; RNF187: RING finger protein 187; CCK8: Cell Counting Kit-8.
We also treated Caco-2 cell xenograft mice with intratumoral injection of mock and RNF187 siRNA. Compared with the mock controls, RNF187 siRNA-treated mice had smaller tumour weights (Figure 2D). Consistently, RNF187 siRNA treatment greatly suppressed the tumour volume of Caco-2 tumour xenografts (Figure 2E).
Knockdown of RNF187 reduces the motility of CRC cells
To better understand the biological functions of RNF187 in the progression of CRC, the effects of RNF187 knockdown on the migration and invasion abilities of CRC cells were subsequently determined. The cell scratch test results indicated that the repair rates of the scratches in Caco-2 or SW480 cells transfected with the miR-144-5p mimic were lower, compared to the cells transfected with scrambled miRNA (Figure 3A and B). Meanwhile, the miR-144-5p mimic significantly decreased the numbers of migrated (Figure 3C) and invaded (Figure 3D) Caco-2 or SW480 cells, as assessed by Transwell assays. These results showed that the knockdown of RNF187 expression could inhibit the migration and invasion abilities of CRC cells in vitro.

Silencing RNF187 inhibited the motility of CRC cells. Caco-2 and SW480 cells were transfected with either RNF187 siRNA or mock control for 24 h. (A) and (B) Wound healing was detected by cell scratch tests. (C) Cell migration was assessed by a Transwell migration assay. (D) Cell invasion was determined by a Transwell Matrigel invasion assay. Data are representative images or expressed as the mean±standard deviation. **P < 0.01. CRC: colorectal cancer; RNF187: RING finger protein 187.
RNF187 is a direct target of miR-144-5p
The expression of RNF187 is upregulated in various types of cancers, yet the mechanism of its deregulation is still unknown. Given the fact that miRNAs function as common negative regulators of gene expression, we hypothesised that the expression of RNF187 is regulated by microRNA(s) in CRC cells. By online database analysis using TargetScan prediction algorithm, we found that the RNF187 mRNA 3ʹ-UTR contains a potential miR-144-5p seed sequence (Figure 4A). The luciferase assay showed that miR-144-5p transfection resulted in the reduction of luciferase activity in HEK-293 cells transfected with RNF187-3ʹ-UTR-wild type (wt), but not RNF187-3ʹ-UTR-mutant (mut) (Figure 4A). Furthermore, miR-144-5p transfection decreased the RNF187 protein (Figure 4B) and mRNA (Figure 4C) levels in Caco-2 and SW480 cells. Consistent with these in vitro results, further analysis of the expression of miR-144-5p by qRT-PCR in clinical samples showed that miR-144-5p expression was significantly lower in CRC tissues than in the adjacent non-tumourous tissues (Figure 4D), which was inversely correlated to RNF187 expression (Figure 4E). These findings indicate that the downregulated expression of miR-144-5p contributes to the ectopic RNF187 expression.

RNF187 is targeted by miR-144-5p in CRC. (A) The predicted binding site of miR-144-5p at the RNF187 mRNA 3ʹ-UTR and confirmation by a luciferase reporter assay. The pmirGLO vector (empty vector), pmirGLO-RNF187-3'UTR-wt or pmirGLO-RNF187-3ʹUTR-muts was separately co-transfected into HEK293 cells with miR-144-5p mimic or scrambled miRNA. (B) Western blot analysis of RNF187 protein in Caco-2 and SW480 cells transfected with the miR-144-5p mimic or scrambled miRNA for 48 h. (C) qRT-PCR analysis of RNF187 mRNA in Caco-2 and SW480 cells transfected with the miR-144-5p mimic or scrambled miRNA for 48 h. (D) The expression of miR-144-5p in CRC specimens (N = 83) and paired non-tumorous colon tissues was analysed by qRT-PCR. (E) RNF187 mRNA level is negatively correlated with miR-144-5p expression in CRC specimens (R2 = 0.521, P < 0.01). Data are representative images or expressed as the mean±standard deviation. **P < 0.01. RNF187: RING finger protein 187; CRC: colorectal cancer.
Exogenous miR-144-5p expression dampens the proliferation and growth of CRC cells
To evaluate the role of miR-144-5p in CRC, we performed CCK8 assay in CRC cell lines transfected with miR-144-5p or its scrambled control. As indicated in Figure 5A, miR-144-5p transfection obviously decreased the viability in a time-dependent manner in both Caco-2 and SW480 cells, in comparison to the scrambled control. Furthermore, enforced miR-144-5p expression reduced the colony number by approximately 50% in CRC cells (Figure 5B). To determine the anti-proliferation effects of miR-144-5p in vivo, Caco-2 cells xenograft mice were treated with agomir-144-5p or agomir-negative control (agomir-NC), which are synthetic RNA duplexes that stimulate in vivo miRNA activity. As shown in Figure 5C and D, treatment with agomir-144-5p significantly reduced the tumour weight and volume in vivo, suggesting the inhibitory role of miR-144-5p in CRC cells’ growth.

miR-144-5p inhibited the growth of CRC cells. Caco-2 and SW480 cells were transfected with either miR-144-5p mimics or scrambled control for 48 h. Cell viability was determined by the CCK8 assay at the indicated time points (A) and cell survival was tested by a colony formation assay (B). Mice were injected subcutaneously into the left flank with Caco-2 cells. Seven days after injection, the mice were treated with an intratumoral injection of agomiR-144-5p (1 nmol for each mouse) or an equivalent amount of agomiR-NC every 3 days for a total of 21 days (seven times). Twenty-eight days after transplantation, the mice were sacrificed; the xenograft tumours were stripped for weight measurement (C) and the tumour burden was monitored every 3 days by measurement of tumour volume (D). Data are representative images or expressed as the mean±standard deviation. *P < 0.05, **P < 0.01. CRC: colorectal cancer.
Exogenous miR-144-5p expression suppressed the migration and invasion of CRC cells
The effects of miR-144-5p on the migration and invasion of CRC cells were also determined. As shown in Figure 6A, cells exogenously expressing miR-144-5p showed a lower repair rate than those expressing scrambled microRNA. Similar to the RNF187 knockdown, miR-144-5p overexpression significantly reduced the migration (Figure 6B) and invasion (Figure 6C) of CRC cells.
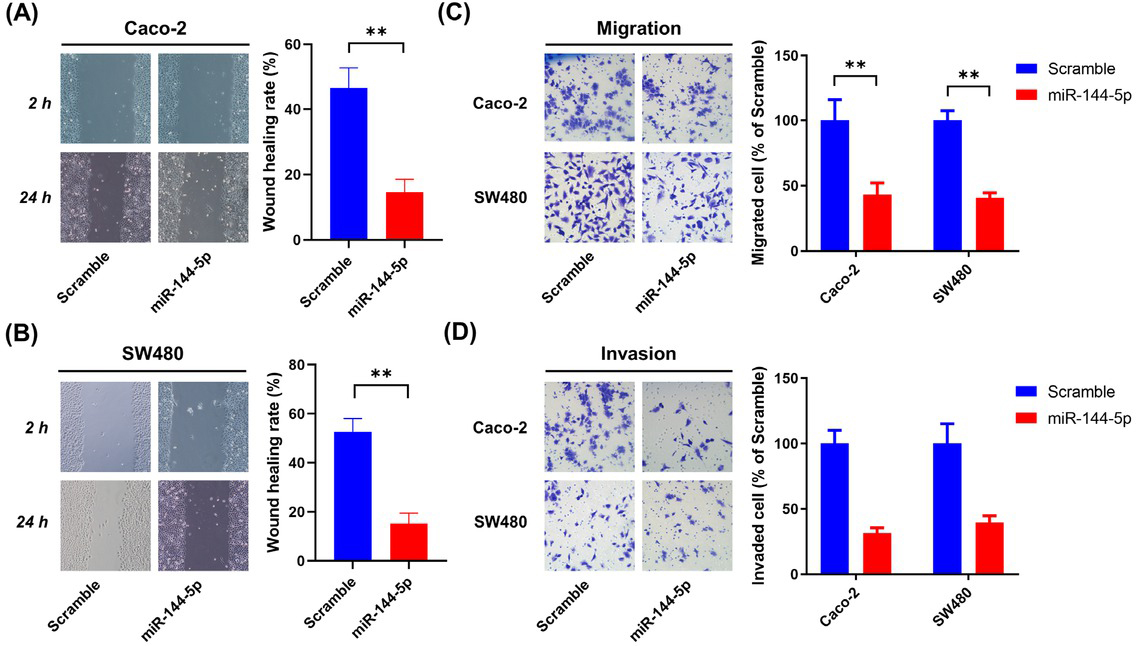
miR-144-5p restrained the migration and invasion of CRC cells. Caco-2 and SW480 cells were transfected with either miR-144-5p mimics or scrambled control for 48 h. (A and B) Wound healing was detected by cell scratch tests. (C) Cell migration was assessed by the Transwell migration assay. (D) Cell invasion was determined by the Transwell Matrigel invasion assay. Data are representative images or expressed as the mean±standard deviation. **P < 0.01. CRC: colorectal cancer
Restoration of RNF187 expression abrogates the effects of miR-144-5p on progression of CRC cells
To validate the role of RNF187 in the inhibitory effects of miR-144-5p in CRC, we restored RNF187 expression in miR-144-5p–treated Caco-2 cells with a lentivirus overexpressing RNF187. As shown in Figure 7A, RNF187 expression in Caco-2 cells stably expressing RNF187 is extremely higher than in cells infected with empty vector particles. RNF187 restoration rescued the miR-144-5p– induced defect in proliferation and clonogenic capacity of Caco-2 cells in vitro (Figure 7B and C). Furthermore, overexpression of RNF187 significantly blocked miR-144-5p–mediated inhibition of the tumour weight (Figure 7D) and volume (Figure 7E) of the Caco-2 cell tumour xenografts. Further studies showed that RNF187 overexpression abolished the inhibitory effects of miR-144-5p on the migration (Figure 7F) and invasion (Figure 7G) of Caco-2 cells.
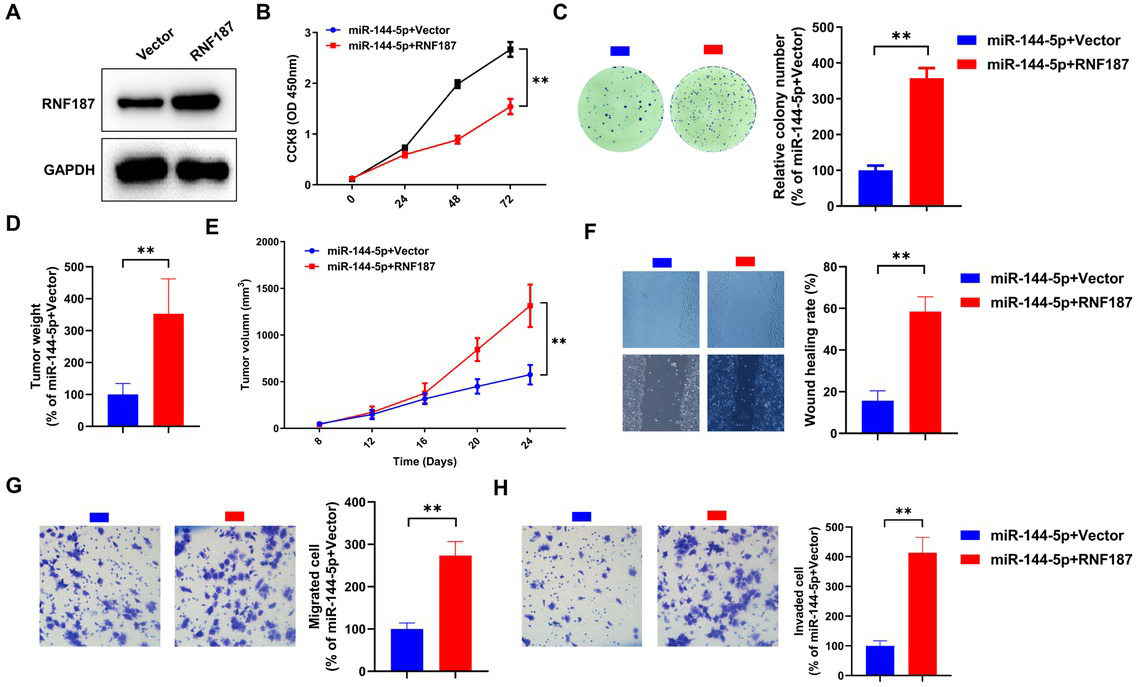
RNF187 overexpression abolished miR-144-5p–mediated suppression of Caco-2 cells. (A) The protein levels of RNF187 in Caco-2 cells infected with RNF187 lentiviral particles (RNF187) or empty vector particles (Vector) were examined by immunoblotting. Caco-2 cells stably expressing RNF187 or empty vector were transfected with the miR-144 mimic. At 48 h after transfection, cell viability (B) and survival (C) were assessed by the CCK8 assay and clonogenic assay, respectively. Caco-2 cells stably expressing RNF187 or vector were inoculated into mice (n = 6), and the tumour weight (D) and volume (E) were determined after agomir-144-5p treatment. After miR-144-5p mimic transfection, motility in cells with or without RNF187 reintroduction was determined using the cell scratch test (F) and the Transwell assay (G and H). Data are representative images or expressed as the mean±standard deviation. **P < 0.01. RNF187: RING finger protein 187.
Discussion
The role of RNF187 in different tumours remains controversial. While RNF187 exhibits tumour-suppressive activities in oesophageal squamous cell carcinoma[22] and triple-negative breast cancer,[19] ectopic RNF187 expression predicts a poor outcome and contributes to tumour progression in lung cancer,[16] hepatocellular carcinoma,[17] osteosarcoma[26] and ovarian carcinoma.[27] This discrepancy may be due to the differential substrates targeted by RNF187 in different cell types. The interaction of RNF187 with c-Jun is both necessary and sufficient for c-Jun/AP-1 activation, which promotes the cells’ proliferation and tumorigenesis.[20,21,28] However, two independent studies reported that RNF187 catalyses a K48 poly-ubiquitination in YAP for degradation, thus inhibiting the migration and invasion in oesophageal and breast cancer.[19,22] The role of RNF187 in progression of CRC is still unknown, despite transgenic overexpression of RACO‑1 coordinating K-Ras to cause hyperproliferation of colonic epithelium.[28] The present study revealed that the expression of RNF187 is significantly upregulated in CRC, which predicts a poor prognosis in CRC patients. Knockdown of RNF187 inhibited proliferation and suppressed the migration and invasion abilities of CRC cells, indicating that it plays a pro-tumorigenic role in CRC.
The mechanism underlying regulation of RNF187 in cancer cells remains elusive. Arginine methylation of RNF187 enhances its Lys63-linked ubiquitination by tripartite motif containing 7 (Trim7), leading to stabilisation of RNF187 protein.[20,21] Zhang et al.[17]reported that Notch1 directly activates RNF187 promoter to increase RNF187 expression at the transcriptional level. miRNA is a commonly conserved small RNA regulating gene expression at the post-transcriptional level. In this study, we identified RNF187 to be a direct target of microRNA-144-5p (miR-144-5p). Exogenously expressed miR-144-5p decreases both the protein and mRNA levels of RNF187 in CRC cells, providing a novel insight into the post-transcriptional regulatory mechanism of RNF187 expression.
Recent studies have indicated that miR-144-5p can serve as an effective biomarker in diseases. For instance, Satoh et al.[29] reported that the expression of miR-144-5p and 26 other miRNAs was deregulated in blood samples of patients with Alzheimer’s disease compared to healthy people, which may be involved in neuronal synaptic functions. Plasma miR-144-5p levels in the depression/anxiety patients were significantly lower compared with healthy controls and were inversely related to the depression scores.[30] Ectopic miR-144-5p expression was also found in various types of tumours including thyroid cancer,[31] oesophageal carcinoma,[32] breast cancer[33] and gastric cancer.[34] However, the expression in CRC is still unknown. In this study, we found that the levels of miR-144-5p expression in CRC were significantly downregulated. In addition, we verified that miR-144-5p expression is negatively correlated to the RNF187 level in CRC specimens, supporting the regulatory role of miR-144-5p on RNF187 expression in CRC. Thus, these findings indicate that the upregulation of RNF187 expression at least partially attributes to the decreased expression of miR-144-5p.
Processing of the pre-miRNA through Dicer1 generates an miRNA duplex consisting of a guide strand and a passenger strand.[35,36] Usually, the passenger strand miRNAs have no regulatory activity. Intriguingly, whereas the tumour-suppressive role of the passenger strand miR-144-3p has been well documented in CRC,[37, 38, 39, 40, 41] the functions of miR-144-5p, the guide strand from pre-miR-144, remain poorly understood. It has been reported that miR-144-5p possesses a tumour-suppressive role in bladder cancer by directly targeting CCNE1/2.[42] Song et al.[43] have reported that miR-144-5p is downregulated in lung cancer cells in response to radiotherapy, and gain-of-function of miR-144-5p restored the radiosensitivity of lung cancer cells. Recently, Fu et al. demonstrated that miR-144-5p suppresses the growth of cholangiocarcinoma cells through targeting mRNA of ST8SIA4 for degradation.[44] Herein, we identified a novel role of miR-144-5p in CRC. Our results demonstrated that restoration of miR-144-5p expression not only inhibited the growth of CRC cells both in vitro and in vivo, but also reduced CRC cell migration and invasion abilities, suggesting that miR-144-5p functions as a tumour suppressor in CRC akin to miR-144-3p. Intriguingly, we found that overexpression of RNF187 could rescue the growth arrest and metastasis inhibition mediated by miR-144-5p in CRC cells. Taken together, our findings prove that downregulation of RNF187 is involved in miR-144-5p–mediated tumour suppression in CRC cells.
In summary, our study reveals that RNF187 expression correlates with the clinical features of CRC and plays a pro-tumorigenic role in CRC. We also demonstrate that miR-144-5p directly targets RNF187 to form an axis of which the deregulation contributes to progression of CRC (Figure 8), providing a novel biomarker and therapeutic targets for CRC prevention.
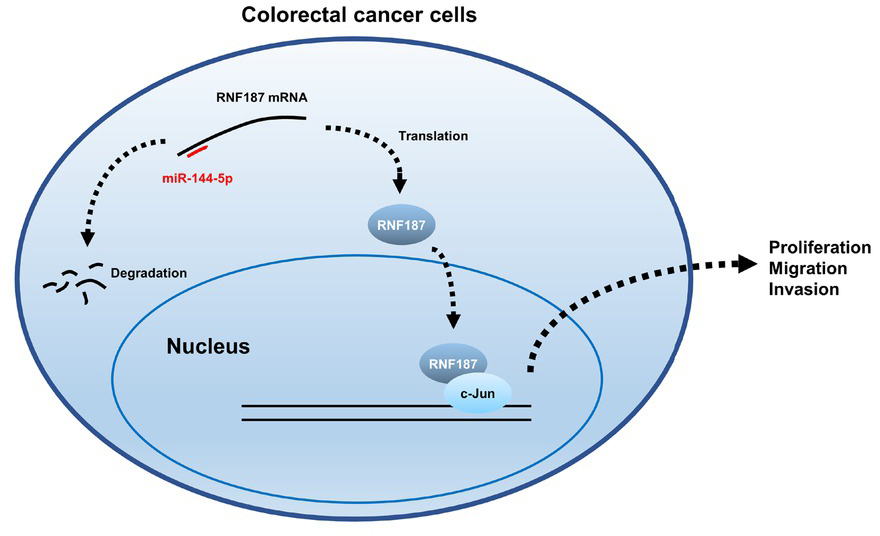
Schematic representation of dysregulation of miR-144-5p/RNF187 axis. miR-144-5p targets RNF187 mRNA for degradation, thus reducing RNF187 protein levels. In CRC cells, the downregulated expression of miR-144-5p leads to an increased level of RNF187, which interacts with c-Jun to regulate the expression of cancer-related genes involved in proliferation, invasion and migration. CRC: colorectal cancer.
-
Conflict of Interest
The authors declare no conflicts of interest.
-
Ethics Approval and Consent to Participate
This study was approved by the Ethics Review Board of China-Japan Union Hospital of Jilin University (2021-KYYS-005). The patients provided informed consents to participate in the study.
References
1 Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394424.10.3322/caac.21492Suche in Google Scholar PubMed
2 Buikhuisen JY, Torang A, Medema JP. Exploring and modelling colon cancer inter-tumour heterogeneity: opportunities and challenges. Oncogenesis 2020;9:66.10.1038/s41389-020-00250-6Suche in Google Scholar PubMed PubMed Central
3 Xu Z, Becerra AZ, Fleming FJ, Aquina CT, Dolan JG, Monson JR, et al. Treatments for stage IV colon cancer and overall survival. J Surg Res 2019;242:47-54.10.1016/j.jss.2019.04.034Suche in Google Scholar PubMed
4 Osterman E, Glimelius B. Recurrence risk after up-to-date colon cancer staging, surgery, and pathology: analysis of the entire swedish population. Dis Colon Rectum 2018;61:1016-25.10.1097/DCR.0000000000001158Suche in Google Scholar PubMed
5 Seok H, Ham J, Jang ES, Chi SW. MicroRNA target recognition: insights from transcriptome-wide non-canonical interactions. Mol Cells 2016;39:375-81.10.14348/molcells.2016.0013Suche in Google Scholar PubMed PubMed Central
6 Chaudhuri K, Chatterjee R. MicroRNA detection and target prediction: integration of computational and experimental approaches. DNA Cell Biol 2007;26:321-37.10.1089/dna.2006.0549Suche in Google Scholar PubMed
7 Leclercq M, Diallo AB, Blanchette M. Prediction of human miRNA target genes using computationally reconstructed ancestral mammalian sequences. Nucleic Acids Res 2017;45:556-66.10.1093/nar/gkw1085Suche in Google Scholar PubMed PubMed Central
8 Gu Y, Ma L, Song L, Li X, Chen D, Bai X. miR-155 inhibits mouse osteoblast differentiation by suppressing SMAD5 expression. Biomed Res Int 2017;2017:1893520.10.1155/2017/1893520Suche in Google Scholar PubMed PubMed Central
9 Xiao L, Jiang L, Hu Q, Li Y. MicroRNA-133b Ameliorates Allergic Inflammation and Symptom in Murine Model of Allergic Rhinitis by Targeting Nlrp3. Cell Physiol Biochem 2017;42:901-12.10.1159/000478645Suche in Google Scholar PubMed
10 Song L, Li D, Li X, Ma L, Bai X, Wen Z, et al. Exposure to PM2.5 induces aberrant activation of NF-kappaB in human airway epithelial cells by downregulating miR-331 expression. Environ Toxicol Pharmacol 2017;50:192-9.10.1016/j.etap.2017.02.011Suche in Google Scholar PubMed
11 Mohamed AA, Omar AAA, El-Awady RR, Hassan SMA, Eitah WMS, Ahmed R, et al. MiR-155 and MiR-665 role as potential non-invasive biomarkers for hepatocellular carcinoma in egyptian patients with chronic hepatitis c virus infection. J Transl Int Med 2020;8:32-40.10.2478/jtim-2020-0006Suche in Google Scholar PubMed PubMed Central
12 Yan Z, Hong S, Song Y, Bi M. microR-4449 Promotes Colorectal Cancer Cell Proliferation via Regulation of SOCS3 and Activation of STAT3 Signaling. Cancer Manag Res 2021;13:3029-39.10.2147/CMAR.S266153Suche in Google Scholar PubMed PubMed Central
13 Xu RS, Wu XD, Zhang SQ, Li CF, Yang L, Li DD, et al. The tumor suppressor gene RhoBTB1 is a novel target of miR-31 in human colon cancer. Int J Oncol 2013;42:676-82.10.3892/ijo.2012.1746Suche in Google Scholar PubMed
14 Liu Y, Chen X, Chen X, Liu J, Gu H, Fan R, et al. Long non-coding RNA HOTAIR knockdown enhances radiosensitivity through regulating microRNA-93/ATG12 axis in colorectal cancer. Cell Death Dis 2020;11:175.10.1038/s41419-020-2268-8Suche in Google Scholar PubMed PubMed Central
15 Shi H, Li K, Feng J, Liu G, Feng Y, Zhang X. LncRNA-DANCR interferes with miR-125b-5p/HK2 axis to desensitize colon cancer cells to cisplatin vis activating anaerobic glycolysis. Front Oncol 2020;10:1034.10.3389/fonc.2020.01034Suche in Google Scholar PubMed PubMed Central
16 Fu Z, Yu W, Wang H, Chen X. Overexpression of RNF187 induces cell EMT and apoptosis resistance in NSCLC. J Cell Physiol 2019;234:14161-9.10.1002/jcp.28111Suche in Google Scholar PubMed
17 Zhang L, Chen J, Yong J, Qiao L, Xu L, Liu C. An essential role of RNF187 in Notch1 mediated metastasis of hepatocellular carcinoma. J Exp Clin Cancer Res 2019;38:384.10.1186/s13046-019-1382-xSuche in Google Scholar PubMed PubMed Central
18 Wan WB, Wu K, Peng K, Qiu ZQ, Duan ZB, Chen X, et al. High level of RNF187 contributes to the progression and drug resistance of osteosarcoma. J Cancer 2020;11:1351-8.10.7150/jca.33488Suche in Google Scholar PubMed PubMed Central
19 Wang Z, Kong Q, Su P, Duan M, Xue M, Li X, et al. Regulation of Hippo signaling and triple negative breast cancer progression by an ubiquitin ligase RNF187. Oncogenesis 2020;9:36.10.1038/s41389-020-0220-5Suche in Google Scholar PubMed PubMed Central
20 Davies CC, Chakraborty A, Diefenbacher ME, Skehel M, Behrens A. Arginine methylation of the c-Jun coactivator RACO-1 is required for c-Jun/AP-1 activation. EMBO J 2013;32:1556-67.10.1038/emboj.2013.98Suche in Google Scholar PubMed PubMed Central
21 Chakraborty A, Diefenbacher ME, Mylona A, Kassel O, Behrens A. The E3 ubiquitin ligase Trim7 mediates c-Jun/AP-1 activation by Ras signalling. Nat Commun 2015;6:6782.10.1038/ncomms7782Suche in Google Scholar PubMed PubMed Central
22 Pang D, Wang W, Zhou X, Lu K, Zhang J, Chen Z, et al. RACO-1 modulates Hippo signalling in oesophageal squamous cell carcinoma. J Cell Mol Med 2020;24:11912-21.10.1111/jcmm.15811Suche in Google Scholar PubMed PubMed Central
23 Gao J, Song L, Xia H, Peng L, Wen Z. 6’-O-galloylpaeoniflorin regulates proliferation and metastasis of non-small cell lung cancer through AMPK/miR-299-5p/ATF2 axis. Respir Res 2020;21:39.10.1186/s12931-020-1277-6Suche in Google Scholar PubMed PubMed Central
24 Song L, Li D, Gu Y, Wen ZM, Jie J, Zhao D, et al. MicroRNA-126 Targeting PIK3R2 inhibits NSCLC A549 cell proliferation, migration, and invasion by regulation of PTEN/PI3K/AKT pathway. Clin Lung Cancer 2016;17:e65-75.10.1016/j.cllc.2016.03.012Suche in Google Scholar PubMed
25 Zhu Y, Wang C, Luo J, Hua S, Li D, Peng L, et al. The protective role of Zingerone in a murine asthma model via activation of the AMPK/Nrf2/ HO-1 pathway. Food Funct 2021;12:3120-31.10.1039/D0FO01583KSuche in Google Scholar
26 Wan WB, Wu K, Peng K, Qiu ZQ, Duan ZB, Chen X, et al. High level of RNF187 contributes to the progression and drug resistance of osteosarcoma. J Cancer 2020;11:1351-8.10.7150/jca.33488Suche in Google Scholar PubMed PubMed Central
27 Chen J, Chen K, Zhou Z, Huang L, Cai Y, Tu H, et al. RING finger protein 187 as a novel potential biomarker for predicting the prognosis of ovarian carcinoma in 2 cancer centers. Curr Probl Cancer 2020;44:100555.10.1016/j.currproblcancer.2020.100555Suche in Google Scholar PubMed
28 Davies CC, Chakraborty A, Cipriani F, Haigh K, Haigh JJ, Behrens A. Identification of a co-activator that links growth factor signalling to c-Jun/AP-1 activation. Nat Cell Biol 2010;12:963-72.10.1038/ncb2098Suche in Google Scholar PubMed
29 Satoh J, Kino Y, Niida S. MicroRNA-Seq Data analysis pipeline to identify blood biomarkers for alzheimer’s disease from public data. Biomark Insights 2015;10:21-31.10.4137/BMI.S25132Suche in Google Scholar PubMed PubMed Central
30 Wang X, Sundquist K, Hedelius A, Palmer K, Memon AA, Sundquist J. Circulating microRNA-144-5p is associated with depressive disorders. Clin Epigenetics 2015;7:69.10.1186/s13148-015-0099-8Suche in Google Scholar PubMed PubMed Central
31 Stokowy T, Eszlinger M, Swierniak M, Fujarewicz K, Jarzab B, Paschke R, et al. Analysis options for high-throughput sequencing in miRNA expression profiling. BMC Res Notes. 2014;7:144.10.1186/1756-0500-7-144Suche in Google Scholar PubMed PubMed Central
32 Gao Z, Liu R, Liao J, Yang M, Pan E, Yin L, et al. Possible tumor suppressive role of the miR-144/451 cluster in esophageal carcinoma as determined by principal component regression analysis. Mol Med Rep 2016;14:3805-13.10.3892/mmr.2016.5691Suche in Google Scholar PubMed
33 Chang CW, Wu HC, Terry MB, Santella RM. microRNA expression in prospectively collected blood as a potential biomarker of breast cancer risk in the BCFR. Anticancer Res 2015;35:3969-77.Suche in Google Scholar
34 Li CY, Liang GY, Yao WZ, Sui J, Shen X, Zhang YQ, et al. Identification and functional characterization of microRNAs reveal a potential role in gastric cancer progression. Clin Transl Oncol 2017;19:162-72.10.1007/s12094-016-1516-ySuche in Google Scholar PubMed
35 Mah SM, Buske C, Humphries RK, Kuchenbauer F. miRNA*: a passenger stranded in RNA-induced silencing complex? Crit Rev Eukaryot Gene Expr 2010;20:141-8.10.1615/CritRevEukarGeneExpr.v20.i2.40Suche in Google Scholar PubMed
36 Meijer HA, Smith EM, Bushell M. Regulation of miRNA strand selection: follow the leader? Biochem Soc Trans 2014;42:1135-40.10.1042/BST20140142Suche in Google Scholar PubMed
37 Qiu Z, Tu L, Hu X, Zhou Z, Lin Y, Ye L, et al. A Preliminary Study of mir-144 inhibiting the stemness of colon cancer stem cells by targeting kruppel-like factor 4. J Biomed Nanotechnol 2020;16:1102-9.10.1166/jbn.2020.2952Suche in Google Scholar PubMed
38 Kapral M, Wawszczyk J, Weglarz L. Regulation of MicroRNA-155 and Its Related Genes Expression by Inositol Hexaphosphate in Colon Cancer Cells. Molecules 2019;24:4153.10.3390/molecules24224153Suche in Google Scholar PubMed PubMed Central
39 Sheng S, Xie L, Wu Y, Ding M, Zhang T, Wang X. MiR-144 inhibits growth and metastasis in colon cancer by down-regulating SMAD4. Biosci Rep 2019;39: BSR20181895.10.1042/BSR20181895Suche in Google Scholar PubMed PubMed Central
40 Jiang Y, Cai Y, Shao W, Li F, Guan Z, Zhou Y, et al. MicroRNA144 suppresses aggressive phenotypes of tumor cells by targeting ANO1 in colorectal cancer. Oncol Rep 2019;41:2361-70.10.3892/or.2019.7025Suche in Google Scholar
41 Cai SD, Chen JS, Xi ZW, Zhang LJ, Niu ML, Gao ZY. MicroRNA144 inhibits migration and proliferation in rectal cancer by downregulating ROCK1. Mol Med Rep 2015;12:7396-402.10.3892/mmr.2015.4391Suche in Google Scholar PubMed PubMed Central
42 Matsushita R, Seki N, Chiyomaru T, Inoguchi S, Ishihara T, Goto Y, et al. Tumour-suppressive microRNA-144-5p directly targets CCNE1/2 as potential prognostic markers in bladder cancer. Br J Cancer 2015;113:282-9.10.1038/bjc.2015.195Suche in Google Scholar PubMed PubMed Central
43 Song L, Peng L, Hua S, Li X, Ma L, Jie J, et al. miR-144-5p Enhances the radiosensitivity of non-small-cell lung cancer cells via targeting ATF2. Biomed Res Int 2018;2018:5109497.10.1155/2018/5109497Suche in Google Scholar PubMed PubMed Central
44 Fu W, Yu G, Liang J, Fan P, Dong K, Zhang B, et al. miR-144-5p and miR-451a inhibit the growth of cholangiocarcinoma cells through decreasing the expression of ST8SIA4. Front Oncol 2020;10:563486.10.3389/fonc.2020.563486Suche in Google Scholar PubMed PubMed Central
© 2022 Zhuo Gao, Junnan Jiang, Lijian Hou, Bin Zhang, published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Artikel in diesem Heft
- Editorial
- Single-nucleotide polymorphisms in medical nutritional weight loss: Challenges and future directions
- Perspective
- Current development of a nonpharmacological intervention approach for mild cognitive impairment patients and a clinical trial in China
- Immune checkpoint inhibitor-related endocrinopathies
- Commentary
- 10.2478/jtim-2022-0008
- Review Article
- Basic pancreatic lesions: Radiologic-pathologic correlation
- Advances in the application of regenerative medicine in prevention of post-endoscopic submucosal dissection for esophageal stenosis
- Inflammatory factors driving atherosclerotic plaque progression new insights
- Original Article
- Prevalence of left atrial septal pouch among patients with embolic stroke of undetermined source or stroke of known etiology: A retrospective study
- Predicting survival for hepatic arterial infusion chemotherapy of unresectable colorectal liver metastases: Radiomics analysis of pretreatment computed tomography
- Dysregulation of miR-144-5p/RNF187 axis contributes to the progression of colorectal cancer
- Letter to Editor
- The first patient with sporadic Huntington’s disease due to a de novo (CAG)n expansion in China
- Hyperchloremic metabolic acidosis post hysteroscopy: A place for balanced solutions?
Artikel in diesem Heft
- Editorial
- Single-nucleotide polymorphisms in medical nutritional weight loss: Challenges and future directions
- Perspective
- Current development of a nonpharmacological intervention approach for mild cognitive impairment patients and a clinical trial in China
- Immune checkpoint inhibitor-related endocrinopathies
- Commentary
- 10.2478/jtim-2022-0008
- Review Article
- Basic pancreatic lesions: Radiologic-pathologic correlation
- Advances in the application of regenerative medicine in prevention of post-endoscopic submucosal dissection for esophageal stenosis
- Inflammatory factors driving atherosclerotic plaque progression new insights
- Original Article
- Prevalence of left atrial septal pouch among patients with embolic stroke of undetermined source or stroke of known etiology: A retrospective study
- Predicting survival for hepatic arterial infusion chemotherapy of unresectable colorectal liver metastases: Radiomics analysis of pretreatment computed tomography
- Dysregulation of miR-144-5p/RNF187 axis contributes to the progression of colorectal cancer
- Letter to Editor
- The first patient with sporadic Huntington’s disease due to a de novo (CAG)n expansion in China
- Hyperchloremic metabolic acidosis post hysteroscopy: A place for balanced solutions?

