Hyperoxaluria: An early diagnosis will allow a timely combined renal and liver transplantations to avoid irreversible damages to all other organs
-
Elisa Kottos
ABSTRACT
A 4-month-old patient was admitted to the emergency room for vomiting, weight gain, food refusal and hypertension. Blood gases showed a metabolic acidosis with increased anion gap. Laboratory finding revealed severe renal failure (creatinine 8 mg/dL). Renal ultrasound showed an important hyperechogenicity of the parenchyma with loss of cortico-medullar differentiation suggesting a nephronophytosis. Genetic testing was negative. Urine oxalate levels were increased to 140 μmol/L. New genetic tests were positive for type I hyperoxaluria. The authors discuss the management of hyperoxaluria.
INTRODUCTION
Acute renal failure is a rare entity in infants presenting with non-specific symptoms such as nausea or delayed weight gain. Some severe forms of renal failure escape antenatal diagnosis and are discovered in advanced forms after several months.
CASE REPORT
A 4-month-old child was admitted to the emergency department for vomiting, diarrhea, loss of appetite, abdominal cramps and oliguria. In his history, there was an uncomplicated urinary tract infection induced by a Klebsiella oxytoca three weeks earlier. In this context, a renal ultrasound revealed a suspicion of polycystic kidney disease. A follow-up in pediatric nephrology was organized. His parents were of Turkish origin, consanguineous in the 5th degree and the patient had two brothers of 8 and 10 years, both in good health. His maternal grandfather died at the age of 69 years from a severe kidney disease of unknown origin that required hemodialysis. In the emergency department, parents reported a 500 g weight gain in a few days. Blood pressure was 177/104 mm hg with a mean of 128 mmHg. Clinical examination was normal and there was no evidence of dehydration. No fever was found. Ear, nose and throat examinations, ophthalmologic assessments and abdominal and cardiac ultrasounds were normal.
Given his urinary history, a urinary test strip was performed and revealed proteinuria. Capillary pH showed a metabolic acidosis with an increased anion gap (Table 1). Laboratory findings revealed a severe renal failure, a normochromatic, normocytic anemia, a hyponatremia and an increased parathormone.
Laboratory value of the patient
| Blood gas | Blood sample |
|---|---|
| pH: 7.21 | Hb 9.4 g/dL |
| PCO2: 27 mmHg | Plt 537000/μL |
| pO2: 48 mm | WBC 18260/μL |
| HCO3: 11 mmol/L | CRP 1.5 mg/L |
| BE: 15.3 mmol/L | Na 114 mmol/L |
| Lactate: 3.86 mmol/L | K 4.6 mmol/L |
| Glycemia: 89 mg/dL | Cl 77 mmol/L |
| Na: 113 | HCO3 10 mmol/L |
| K: 7.74 | Proteins 65.2 g/L |
| Cl: 82 | Urea 149 mg/dL |
| AnGap 24.9 | Creatinine 8.03mg/dL |
| HHg: testosterone < 300 mg/dL | Uric acid 10 mg/dL PTH 445 pg/mL |
Extended blood tests (Infectious, Immunoglobulins, Complement, Autoantibodies, Immune complexes, Thyroid) were normal. Renal ultrasound revealed an important hyperechogenicity of the parenchyma with loss of cortico-medullar differentiation suggesting a nephronophtisis. Peritoneal dialysis was started through a temporary catheter. The patient was fed by nasogastric tube following dietary difficulties with food stagnation and had psychomotor retardation (brain MRI was normal, Figure 1).

Normal brain magnetic resonance imaging
A search for NPHP2 gene mutation (nephronophtysis) gave negative results after 6 months but a control ultrasound suggested a total calcification of the kidneys. Plasma oxalic acid was then assayed and found to be abnormally high at 140 μmol/L. Ophthalmological examination was repeated, and this time, the fundus revealed a brownish-black scar with white punctiform deposits all over the retina (Figure 2). New genetic tests finally came positive for type I hyperoxaluria. The bone radiography performed afterwards actually showed osteopenia of the metacarpals and phalanges as well as oxalate deposits (Figure 3) and the abdominal x-ray confirmed coralliform calcifications of both kidneys (Figure 4).
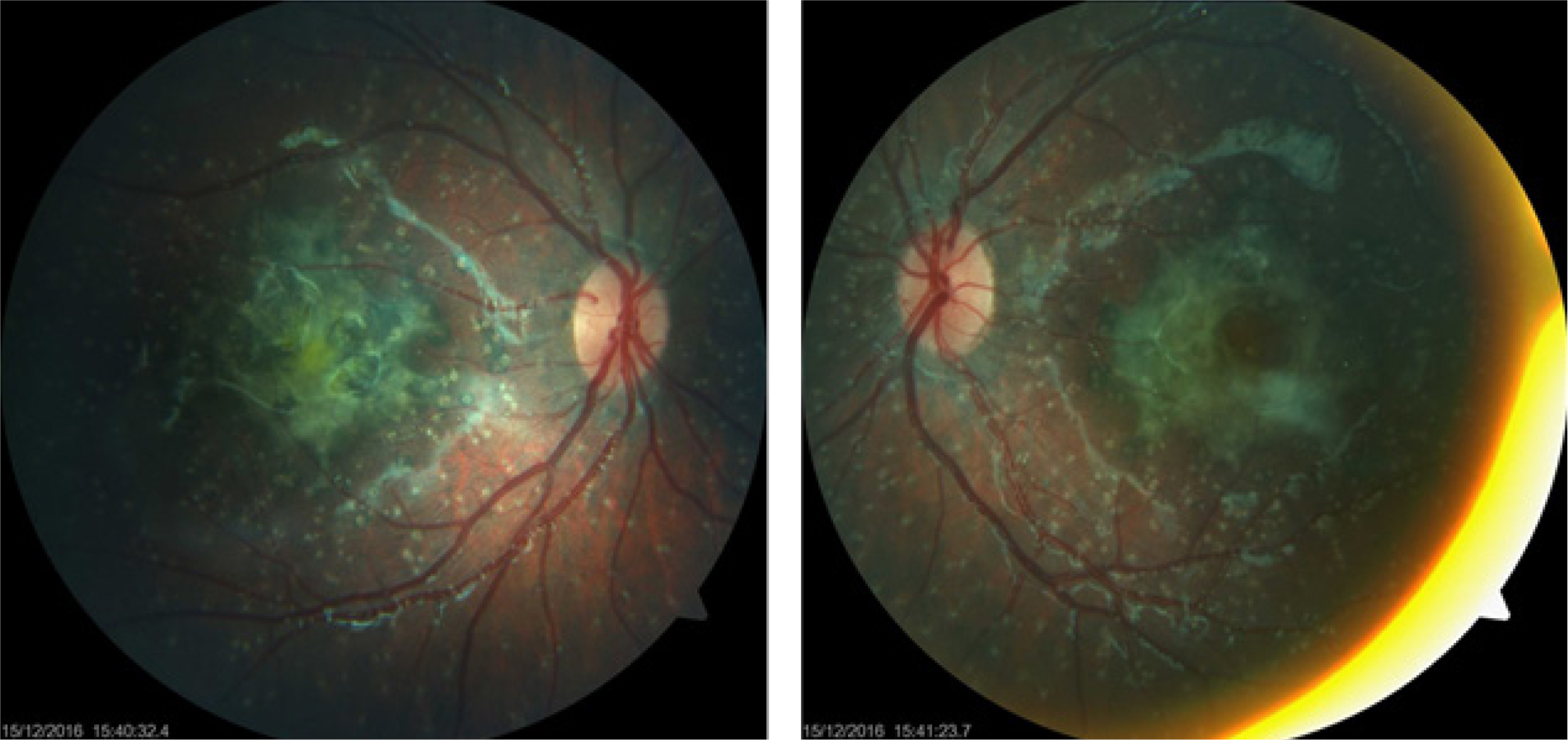
Bottom of eye : black-brown scar with white punctiform deposits all over the retina

Bone age that corresponds to the actual age with osteopenia of metacarpus and phalanges, and oxalate deposits

X-ray of abdomen with coralliform calcifications of both kidneys
The patient was put on an intensive dialysis program (6 days a week). At the age of 22 months, he had an orthotopic liver transplant. He received a segment II and III from a deceased adult donor. He was treated with tacrolimus, basiliximab and prednisone. The median value of his oxalate levels dropped from 144 µmol/L [95–200] to 97 µmol/L [68–190] (P = 0.0125). The patient continued dialysis until he received an ectopic iliac kidney transplant (from a deceased adult donor) at the age of 2 years and 8 months. The immunosuppressive treatment included tacrolimus, mycophenolate and prednisone. Mycophenolate was changed to tacrolimus due to the patient’s digestive intolerance. The median value of the oxalate levels measured post-transplant was 52 µmol/L [38–89] (P = 0.0096 as compared to the values of the period between liver and kidney transplant) (Figure 5).

Evolution of oxalate levels as a function of the period preceding the liver transplant (LT), the period between the hepatic transplant and the kidney transplant (KT) and the period after the KT (P < 0.05 between the three periods)
Oxalate deposits in the retina persisted with the appearance of new vessels. The patient was treated with an intraocular injection of ranibizumab. His visual acuity during the last follow up was 3/10 with the right eye and 2/10 with the left eye.
DISCUSSION
Primary hyperoxaluria type I (OMIM259900) is an innate abnormality of glyoxalate metabolism caused by a deficiency of the liver enzyme L-alanine-glyoxylate aminotransferase[1] and resulting in an overproduction of oxalate, which forms crystals with calcium in the kidneys.[2] When the glomerular filtration rate (GFR) reaches 30–45 mL/min/1.73 m2, oxalate excretion becomes insufficient and the latter accumulates in the other tissues (Figure 6).[3] This autosomal recessive gene disorder (AGXT gene) affects 1 to 3/1,000,000 births in Europe and is responsible for 1–2% of terminal deficiencies in children.[2]

Deficiency L-alanine-glyoxylate aminotransferase (AGT) that leads to hyperoxaluria
The clinical presentation of type I hyperoxaluria is highly variable and correlates with the type of mutation in the AGXT gene. The disease is most often reported by urinary lithiasis in adulthood. It almost always progresses to an end-stage renal failure. In less than 10% of cases, onset is abrupt with severe hyperoxaluria and end-stage renal failure appearing in the early childhood.[4] In about 10% of cases, the diagnosis is made after recurrence in the post-renal transplant period.[3] The most common sites of crystal deposition are the kidneys, the blood vessels and bones with frequent fractures. Oxalosis can also affect the joints, the retina, the skin, the bone marrow, the heart and the central nervous system. Retinal damage is all the more important as hyperoxaluria is early and irreversible, even after transplantation.[5] The median age of onset of dialysis is 1.5 years and the 5-year survival rate after the start of dialysis is 76%, compared to 96% for end-stage renal failure of other origin. The prognosis depends on the speed of diagnosis and the timing of management, but also on the affected tissues.[3] The diagnosis is based on the presence of oxalate in the urine and genetic analysis. There are three types of primary hyperoxaluria, depending on the mutated gene. Type I represents 80% of the cases. Genetic analysis must therefore focus on the AGXT gene in the first place.[3] The only effective treatment is a combined or sequential hepato-renal transplantation.[6–7] In the early stages of the disease, management is based on increased hydration, vitamin B6 supplementation (a cofactor of the defective enzyme), citrate intake (decreases the precipitation of oxalate in the urine), and elimination of lithiasis when they appear. When end-stage renal disease develops, dialysis should be started, which may be necessary up to 7 times a week, sometimes with a combination of hemodialysis and peritoneal dialysis, pending transplantation.[7] The persistence of high oxalate levels long after the liver transplant reflect the large amount of oxalate stored in the body. This significant reduction justified the chosen strategy to separate liver transplant and kidney transplantations to ensure the kidney graft survival.
CONCLUSION
This clinical case illustrates that the diagnosis of hyperoxaluria is difficult and often delayed. However, it is important to make the diagnosis as quickly as possible to avoid sequelae such as retinitis. One can also remember that the first symptoms of kidney failure can be very insidious and that it is important to listen to the parents.
Declaration of Patient Consent
-
The authors certify that they have obtained all appropriate consent forms from the guardians.
Conflict of Interests
-
Patrick M. Honore Co-Editor-in-Chief of the journal. The article was subject to the journal’s standard procedures, with peer review handled independently of the editor and his research groups.
REFERENCES
1. Cochat P, Koch Nogueira PC, Mahmoud MA, Jamieson NV, Scheinman JI, Rolland MO. Primary hyperoxaluria in infants: Medical, ethical and economic issues. J Pediatr 1999;135:746-50.10.1016/S0022-3476(99)70095-8Search in Google Scholar
2. Harambat J, van Stralen KJ, Espinosa L, Groothoff JW, Hulton SA, Cerkauskiene R, et al. Characteristics and Outcomes of Children with Primary Oxalosis Requiring Renal Replacement Therapy. Clin J Am Soc Nephrol 2012;7:458-65.10.2215/CJN.07430711Search in Google Scholar PubMed PubMed Central
3. Rumsby G, Cochat P. Primary Hyperoxaluria. N Engl J Med 2013;369:2163.10.1056/NEJMc1311606Search in Google Scholar
4. Mandrile G, van Woerden CS, Berchialla P, Beck BB, Acquaviva Bourdain C, Hulton SA, et al. Data from a large European study indicate that the outcome of primary hyperoxaluria type 1 correlates with the AGXT mutation type. Kidney Int 2014;86:1197-204.10.1038/ki.2014.222Search in Google Scholar PubMed
5. Atiskova Y, Dulz S, Schmäschke K, Oh J, Grabhorn E, Kemper MJ, et al. Oxalate retinopathy is irreversible despite early combined liver-kidney transplantation in primary hyperoxaluria type 1. Am J Transplant 2019;19:3328-34.10.1111/ajt.15484Search in Google Scholar PubMed
6. Ozer A, Aktas H, Bulum B, Emiroglu R. The experience of combined and sequential liver and kidney transplantation from a single living donor in patients with primary hyperoxaluria type 1. Pediatr Transplant 2019;23:e13406.10.1111/petr.13406Search in Google Scholar PubMed
7. Weigert A, Martin-Higueras C, Hoppe B. Novel therapeutic approaches in primary hyperoxaluria. Expert OpinEmerg Drugs 2018;23:349-57.10.1080/14728214.2018.1552940Search in Google Scholar PubMed
How to cite this article: Kottos E, Adams B, Biarent D, Beretta-Piccoli X, Ismaili K, De Bels D, et al. Hyperoxaluria: an early diagnosis will allow a timely combined renal and liver transplantations to avoid irreversible damages to all other organs. J Transl Intern Med 2021; 9: 318-22.
© 2021 Elisa Kottos et al., published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Editorial
- Translational research in anti-pancreatic fibrosis drug discovery and development
- Dawning precision treatment for gastric cancer: The latest biomarkers
- Commentary
- Can gamma entrainment of the brain rhythms prevent or alleviate Alzheimer’s disease?
- Review
- The challenges and optimization of cell-based therapy for cardiovascular disease
- New insights of epigenetics in vascular and cellular senescence
- Original Article
- Lung adenocarcinoma-specific three-integrin signature contributes to poor outcomes by metastasis and immune escape pathways
- Relationship between homocysteine levels and post-stroke cognitive impairment in female and male population: from a prospective multicenter study
- Assessment of 17 clinically available renal biomarkers to predict acute kidney injury in critically ill patients
- Elevated resting heart rates are a risk factor for mortality among patients with coronavirus disease 2019 in Wuhan, China
- Lipid-related protein NECTIN2 is an important marker in the progression of carotid atherosclerosis: An intersection of clinical and basic studies
- Effect of local anti-vascular endothelial growth factor therapy to prevent the formation of stenosis in outflow vein in arteriovenous fistula
- Case Report
- Hyperoxaluria: An early diagnosis will allow a timely combined renal and liver transplantations to avoid irreversible damages to all other organs
Articles in the same Issue
- Editorial
- Translational research in anti-pancreatic fibrosis drug discovery and development
- Dawning precision treatment for gastric cancer: The latest biomarkers
- Commentary
- Can gamma entrainment of the brain rhythms prevent or alleviate Alzheimer’s disease?
- Review
- The challenges and optimization of cell-based therapy for cardiovascular disease
- New insights of epigenetics in vascular and cellular senescence
- Original Article
- Lung adenocarcinoma-specific three-integrin signature contributes to poor outcomes by metastasis and immune escape pathways
- Relationship between homocysteine levels and post-stroke cognitive impairment in female and male population: from a prospective multicenter study
- Assessment of 17 clinically available renal biomarkers to predict acute kidney injury in critically ill patients
- Elevated resting heart rates are a risk factor for mortality among patients with coronavirus disease 2019 in Wuhan, China
- Lipid-related protein NECTIN2 is an important marker in the progression of carotid atherosclerosis: An intersection of clinical and basic studies
- Effect of local anti-vascular endothelial growth factor therapy to prevent the formation of stenosis in outflow vein in arteriovenous fistula
- Case Report
- Hyperoxaluria: An early diagnosis will allow a timely combined renal and liver transplantations to avoid irreversible damages to all other organs


