The substitution Fe3+-Al and the isosymmetric displacive phase transition in synthetic zoisite: A powder X-ray and infrared spectroscopy study
Abstract
The Fe3+-Al substitution in synthetic zoisite was studied in the system CFASH at 2.0 GPa and 750°C (compositional range: 0.0-0.14 Xps). The samples were characterized by powder X-ray diffraction, FTIR, and electron microprobe. Discontinuities in refined lattice parameters at ~0.05 Xps are attributed to two distinct and hitherto unknown modifications, zoisite I (<0.05 Xps) and zoisite II (>0.05 Xps). The following lattice parameters were derived
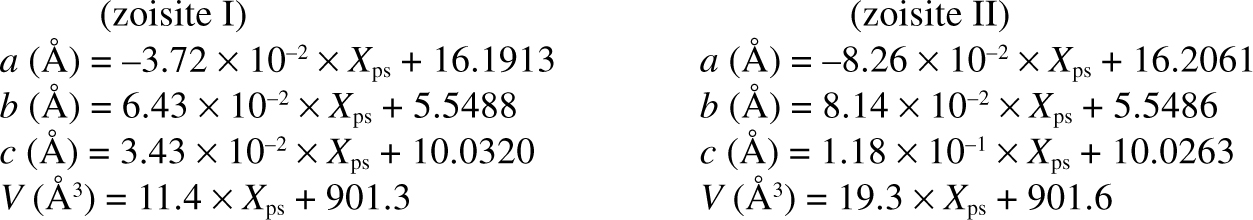
In both modifications, substitution of Fe3+ expands the M3 octahedron, resulting in opposed rotations of the corner-linked T1 and T2 tetrahedra of the Si2O7 group. The extent of rotation is limited and controls the maximum Fe3+ content in zoisite I and II. With increasing Fe3+ content, zoisite I transforms to zoisite II and zoisite II to clinozoisite. The transformation from zoisite I to II can be classified as a substitutionally induced isosymmetric displacive phase transition.
Four significant IR bands were observed at ~3250, ~3195, ~3155, and ~2170 cm-1. The first three bands are attributed to the configurations Al2[M1,2]-O10-H···O2-Al2[M1,2](Al,Fe3+[M3], Al2[M1,2]-O10- H···O4-Al2[M1,2]Fe3+[M3], and Al2[M1,2]-O10-H···O4-Al2[M1,2]Al[M3]. O10-H···O2 is bifurcated between the two symmetrically arranged O2 and O2' atoms. The band at ~2170 cm-1 is interpreted as the first overtone of the bending vibration of O10-H···O2. In analogy with the results from powder XRD the IR bands show discontinuities at ~0.05 Xps, confirming the two modifications of zoisite
© 2015 by Walter de Gruyter Berlin/Boston
Artikel in diesem Heft
- Thermal equations of state for B1 and B2 KCl
- Determination of molar absorptivities for infrared absorption bands of H2O in andesitic glasses
- H2O activity in H2O-N2 fluids at high pressure and temperature measured by the brucitepericlase equilibrium
- Kinetics of iron oxidation-reduction in hydrous silicic melts
- Kinetics of cation ordering in synthetic MgAl2O4 spinel
- Structural properties and heat-induced oxidation-dehydrogenation of manganoan ilvaite from Perda Niedda mine, Sardinia, Italy
- Ultrahigh-pressure metamorphism in western Tianshan, China: Part I. Evidence from inclusions of coesite pseudomorphs in garnet and from quartz exsolution lamellae in omphacite in eclogites
- Ultra-high pressure metamorphism in western Tianshan, China: Part II. Evidence from magnesite in eclogite
- Discovery of clinoenstatite in garnet pyroxenites from the Dabie-Sulu ultrahigh-pressure terrane, east-central China
- Ultrahigh-pressure (UHP) low-Al titanites from carbonate-bearing rocks in Dabieshan- Sulu UHP terrane, eastern China
- Metamictization and recrystallization of titanite: An infrared spectroscopic study
- Fine structure of infrared OH-stretching bands in natural and heat-treated amphiboles of the tremolite-ferro-actinolite series
- Correlation between OH concentration and oxygen isotope diffusion rate in diopsides from the Adirondack Mountains, New York
- The substitution Fe3+-Al and the isosymmetric displacive phase transition in synthetic zoisite: A powder X-ray and infrared spectroscopy study
- In situ X-ray observation of the reaction dolomite = aragonite + magnesite at 900–1300 K
- High-pressure single-crystal X-ray and powder neutron study of F,OH/OD-chondrodite: Compressibility, structure, and hydrogen bonding
- Quantitative characterization of biotic iron oxides by analytical electron microscopy
- Synthesis and NMR characterization (1H and 31P MAS) of the fluorine-free hydroxylapatite–britholite-(Y) series
- Quantum mechanical calculations of dioctahedral 2:1 phyllosilicates: Effect of octahedral cation distributions in pyrophyllite, illite, and smectite
- The nature of disorder in montmorillonite by simulation of X-ray powder patterns
- Ferripedrizite, a new monoclinic BLi amphibole end-member from the Eastern Pedriza Massif, Sierra de Guadarrama, Spain, and a restatement of the nomenclature of Mg-Fe-Mn-Li amphiboles
- Description and crystal structure of vajdakite, [(Mo6+O2)2(H2O)2 As3+ 2O5]·H2O—A new mineral from Jáchymov, Czech Republic
- Some remarks on fission-track observational biases and crystallographic orientation effects
- Surface reconstruction and As-polymerization at fractured loellingite (FeAs2) surfaces
- The strength of moissanite
- Diamond formation through carbonate-silicate interaction
- Anisotropic Fe-Mg diffusion in biotite
- Characterization of a high-pressure phase of silica from the Martian meteorite Shergotty
Artikel in diesem Heft
- Thermal equations of state for B1 and B2 KCl
- Determination of molar absorptivities for infrared absorption bands of H2O in andesitic glasses
- H2O activity in H2O-N2 fluids at high pressure and temperature measured by the brucitepericlase equilibrium
- Kinetics of iron oxidation-reduction in hydrous silicic melts
- Kinetics of cation ordering in synthetic MgAl2O4 spinel
- Structural properties and heat-induced oxidation-dehydrogenation of manganoan ilvaite from Perda Niedda mine, Sardinia, Italy
- Ultrahigh-pressure metamorphism in western Tianshan, China: Part I. Evidence from inclusions of coesite pseudomorphs in garnet and from quartz exsolution lamellae in omphacite in eclogites
- Ultra-high pressure metamorphism in western Tianshan, China: Part II. Evidence from magnesite in eclogite
- Discovery of clinoenstatite in garnet pyroxenites from the Dabie-Sulu ultrahigh-pressure terrane, east-central China
- Ultrahigh-pressure (UHP) low-Al titanites from carbonate-bearing rocks in Dabieshan- Sulu UHP terrane, eastern China
- Metamictization and recrystallization of titanite: An infrared spectroscopic study
- Fine structure of infrared OH-stretching bands in natural and heat-treated amphiboles of the tremolite-ferro-actinolite series
- Correlation between OH concentration and oxygen isotope diffusion rate in diopsides from the Adirondack Mountains, New York
- The substitution Fe3+-Al and the isosymmetric displacive phase transition in synthetic zoisite: A powder X-ray and infrared spectroscopy study
- In situ X-ray observation of the reaction dolomite = aragonite + magnesite at 900–1300 K
- High-pressure single-crystal X-ray and powder neutron study of F,OH/OD-chondrodite: Compressibility, structure, and hydrogen bonding
- Quantitative characterization of biotic iron oxides by analytical electron microscopy
- Synthesis and NMR characterization (1H and 31P MAS) of the fluorine-free hydroxylapatite–britholite-(Y) series
- Quantum mechanical calculations of dioctahedral 2:1 phyllosilicates: Effect of octahedral cation distributions in pyrophyllite, illite, and smectite
- The nature of disorder in montmorillonite by simulation of X-ray powder patterns
- Ferripedrizite, a new monoclinic BLi amphibole end-member from the Eastern Pedriza Massif, Sierra de Guadarrama, Spain, and a restatement of the nomenclature of Mg-Fe-Mn-Li amphiboles
- Description and crystal structure of vajdakite, [(Mo6+O2)2(H2O)2 As3+ 2O5]·H2O—A new mineral from Jáchymov, Czech Republic
- Some remarks on fission-track observational biases and crystallographic orientation effects
- Surface reconstruction and As-polymerization at fractured loellingite (FeAs2) surfaces
- The strength of moissanite
- Diamond formation through carbonate-silicate interaction
- Anisotropic Fe-Mg diffusion in biotite
- Characterization of a high-pressure phase of silica from the Martian meteorite Shergotty

