Abstract
Chitin and keratin are naturally abundant biopolymers. They hold significant potential for sustainable applications due to their chemical structure, (nano)structural properties, biodegradability and nontoxicity. Chitin, a polysaccharide contained in exoskeletons of arthropods and the cell walls of fungi, forms strong hydrogen bonds that confer mechanical stability, which is ideal for use in protective structures and lightweight composites. Keratin, a fibrous protein found in vertebrate epithelial tissues such as wool, feathers and hair, is characterized by its high sulfur content and the formation of disulfide bonds, which provide both mechanical strength and flexibility. Utilizing chitin and keratin waste materials from the food industry, such as shrimp shells, chicken feathers and sheep wool, offers an eco-friendly alternative to synthetic materials and leverages their inherent biocompatibility. Additionally to the common macroscale reuse of chitin and keratin waste as fertilizer or livestock feed, using chitin and keratin as functional materials adds further uses for these versatile materials. The waste is increasingly being utilized specifically for its superior structural properties resulting from nanoscale functionalities. Chitin and keratin exhibit excellent thermal insulation properties, making them suitable for energy-efficient building materials. Their structural colours (e.g., in butterflies and birds), arising from micro- and nanoscale arrangements, offer non-fading colouration for textiles and coatings without the need for potentially harmful dyes. Additionally, these biopolymers provide lightweight yet strong materials ideal for packaging, consumer products, and – when smartly structured – even passive radiative cooling applications. Biomimetic designs based on chitin and keratin promise advancements across multiple fields by harnessing their natural properties and converting waste into high-value products, thereby addressing recycling issues and promoting sustainability.
1 Introduction
Chitin and keratin are ubiquitous materials in organisms. Chitin is a structural polysaccharide constructed from chains of modified glucose. It is found in single-celled organisms such as certain diatoms 1 and yeasts 2 and in fungal cell walls, 3 insects, 4 spiders 5 and crustaceans 6 as well as in molluscs 4 and (less commonly) some other invertebrates such as annelids, nematodes, bryozoans and cnidarians. 7 Keratin is a fibrous protein produced in the epithelial tissues of various vertebrates. Keratin is contained in wool, hair, feathers, hooves, horns, claws, beaks and skin. 8 , 9
The functionality of keratin and chitin in biological systems arises from the synergy between their unique chemical compositions and nanoscale to macroscale hierarchical structuring. The biomimetic translation of this structuring enhances material properties such as strength, flexibility, and durability. Additionally, the localized availability of resources for the biosynthesis of keratin and chitin underscores their sustainability and recyclability, highlighting their potential as eco-friendly materials for innovative applications in material science. 10 , 11 , 12
Chemically, keratin is distinguished by its high sulfur content due to the abundance of cysteine amino acids, which form disulfide bonds. 13 These bonds are crucial for mechanical stability and flexibility. 14 , 15 Chitin can form strong hydrogen bonds between adjacent chains. 16 These hydrogen bonds enhance its structural stability, which is crucial, for example, the protective exoskeletons of insects and crustaceans and the cell walls of fungi. 17 , 18
The structural arrangement of chitin and keratin on the micro- and nanometer length scales contributes to more than just mechanical strength: the hierarchical structuring of chitin in butterfly wing scales and of keratin in the feathers of birds such as hummingbirds, pigeons and peacocks, for example, interacts with light through complex photonic structures, resulting in brilliant colours (without the use of pigments). 19
Organism-inspired structural functionalities are primarily translated into technology through the use of highly engineered materials, 20 , 21 such as structural colours inspired by butterfly wings, achieved using metal oxides and volatile solvent-based systems. 22 , 23 Alternatively, structure-based functionalities can be implemented using chitin and keratin waste such as shrimp shells or sheep wool. 24 This alternative offers clear advantages: it utilises renewable, non-toxic, biodegradable, and recyclable materials. 25 The functional properties intrinsic to chitin and keratin are directly transferred to biomimetic applications, preserving the natural base materials without modification.
Incorporating biomimetic principles into material design requires synergising chemistry-based functionalities and structure-based characteristics on various length scales, reflecting the multi-scale strategies observed in natural systems (integration instead of additive manufacturing 26 ). This combined approach enhances material performance and sustainability, demonstrating that chemistry and structural design are most effective when applied together rather than in isolation.
2 Chitin and keratin in organisms and related biomimetics
Keratin is widespread across various classes of animals, including mammals, birds, and reptiles. It is a significant component of humans’ hair, skin, nails, and cornea. Human hair is composed of approximately 80 % hard keratins, 27 though its concentration varies based on location and function within the body. In other mammals, keratin forms the structure of horns, hooves, and claws, while in birds, it is present in feathers and beaks. 28 Reptiles have keratin in their scales and claws. 29
Chitin is a crucial structural component in the exoskeletons of various arthropods, including insects, spiders, and crustaceans such as crabs and lobsters. 30 , 31 It is also present in the cell walls of certain fungi 32 and contributes to the radula – a feeding structure – in some molluscs. Chitin content varies significantly across organisms: squid, cuttlefish, and octopuses contain between 6 % and 40 % chitin, 11 , 33 , 34 , 35 while the exoskeleton of insects can incorporate up to 40 %. 36 Specific insect chitin content ranges from 18.4 % in cockroaches (Blattella sp.) to 64 % in butterflies (Pieris sp.). 37 In crustaceans, chitin typically constitutes 20–30 % of the exoskeleton, 38 ranging from 17.8 % in shrimp (Crangon crangon) to 75 % in lobster (Homarus sp.). 39 Fungal species such as Aspergillus niger (black mould) can contain chitin levels between 2.3 % and 42 %. 39 The determination of chitin content across different organisms varies depending on the species and the specific composition under different conditions. 37
Keratin and chitin are structural biopolymers known for their durability and resistance to environmental degradation. 40 , 41 Keratin protects against mechanical wear and tear, forming intermediate filaments within cells that reinforce tissues such as skin, hair and nails, shielding tissues from physical and chemical damage. 42 , 43 Chitin resists microbial degradation, forming long chains that enhance the strength and rigidity of exoskeletons and cell walls, fortifying exoskeletons and cell walls and offering structural defence for organisms. 44 , 45
3 Waste management and sustainable utilization of keratin
Much keratin-rich waste originates from the food industry, particularly slaughterhouses, meat markets, and the wool industry. 28 This waste comprises keratin-containing animal by-products, such as wool, hair, hooves, and feathers. Only a few microorganisms, such as keratinophilic microflora (bacteria and fungi), can break down keratin proteins. 46 , 47 Biological degradation of keratin waste is considered more efficient than physical and chemical methods. 48 Organic and biodegradable keratin waste can be repurposed as a sustainable resource via chemical and mechanical processing, transforming it into valuable biomaterials. 49 , 50 Keratin derived from biomass offers an eco-friendly alternative that is naturally free from synthetic chemicals and thus reduces environmental impact. 51
3.1 Keratin-enriched waste
Poultry slaughterhouses generate substantial feather waste, as only 60–70 % of poultry parts are deemed suitable for human consumption. 52 Keratinous by-products comprise roughly 15–20 % of slaughterhouse waste, with feathers accounting for 7–9 %. 53 Feather waste is challenging for livestock to digest due to its keratin content, necessitating various pre-treatment methods – physical, chemical, or biological – for effective utilization. 54 While some feather waste is processed into feather fodder for animal feed or fertilizer, the majority is discarded via landfill disposal or incineration, contributing to pollution concerns. The environmental impact of these disposal methods underscores the need to increase feather recycling. Feathers comprise approximately 91 % β-keratin, a highly sought-after resource. 55 , 56 , 57 Keratin extracted from organic waste is a sustainable toxin-free resource. 51
Wool is a historically significant natural fibre which has long played a crucial role in global trade and agricultural economies. 58 , 59 Wool’s fibrous structure is composed of approximately 95 % keratin by weight, with a cysteine content between 11 % and 17 %, and highly resistant to degradation in common solvents. This resilience is attributed to the tightly packed α-helix and β-sheet in keratin. 58 , 60 Wool’s market share faces increasing competition from synthetic fibres as consumer preference shifts toward lighter-weight products. 61 Environmental concerns, such as the water and resource intensity of wool production and pollution associated with processing, have also contributed to declining demand, confining wool use primarily to luxury goods. 62
Market statistics typically account only for wool that enters the manufacturing chain and is processed into various products, overlooking raw wool produced on farms that remains unused, often due to low quality unsuitable for textiles. 63 In recent years, sheep shearing has sometimes been performed solely for animal welfare, offering no profit to farmers, with the resultant wool shifting from a valuable product to a waste management challenge. 64 The accumulation of wool waste has become a significant issue in waste management, underscoring the need for sustainable options. 58 , 65
In addition to wool, other keratin-rich waste by-products include human hair, animal hair, and hooves from various sources, such as cattle, horses, oxen and pigs (260 million pigs were slaughtered in the EU in 2016 66 ). 55
Keratin extraction employs various methods, including oxidation, 67 reduction, 68 sulfitolysis 69 and superheated hydrolysis. 70 , 71 Each technique targets the cleavage of cystine disulfide bonds within the keratin structure, yielding low molecular weight proteins and peptides. 72 The resulting keratin extracts are typically characterized through molecular weight analysis and amino acid profiling to evaluate their composition and functionality. The choice of extraction method is highly dependent on the starting material, such as hooves, feathers, or wool, each possessing distinct structural and chemical properties. These differences necessitate tailored adjustments to optimize the extracted keratin’s yield and quality. 55
4 Waste management and sustainable utilization of chitin
With population growth, 73 waste generation has risen significantly, 12 particularly from food production, 74 where valuable by-products are discarded. 75 For instance, seafood waste is often incinerated, landfilled, discarded at sea, or left to decompose, which poses risks to human health and biodiversity and causes environmental problems. 76 Chitin and chitosan (derived from chitin) possess high economic value due to their versatile biological properties. 77
The seafood processing industry generates substantial waste, including shells and exoskeletons that are slow to biodegrade due to their high structural components. 78 Disposing seafood waste in marine environments contributes to coastal pollution by affecting water quality and marine ecosystems. 79 Accumulated organic waste can lead to nutrient overload, fostering algal blooms and depleting oxygen levels, which harm local biodiversity. 80
Seafood by-products (waste) can be utilized as valuable raw materials for chitosan and chitin production. 81 Chitosan’s excellent adsorption capabilities make it suitable for removing heavy metals and dyes from wastewater. 82 , 83 Edible chitosan-based films extend the shelf life of perishable products as a barrier against moisture and microbial contamination. 84 , 85 Crustacean exoskeletons are the primary source of chitin for commercial use due to their high chitin content and wide availability. 86
Chitin nanomaterials can be extracted in the form of chitin nanocrystals or chitin nanofibers, each exhibiting unique structural and functional properties. 87
The cyclic use and reuse of chitin and chitosan can be effectively achieved through large-scale fermentation processes, leveraging their biodegradability and renewability. This approach enables the sustainable production of value-added products, minimizes waste, and supports circular bioeconomy frameworks by integrating microbial fermentation for efficient biopolymer recovery and transformation. 88
4.1 Chitin-enriched waste
The shrimp industry is a substantial source of chitin waste worldwide, driven by high global shrimp consumption. 89 In 2023, shrimp production reached 5.6 million tons globally, 90 with projections indicating an increase to 7.28 million tons by 2025, reflecting a compound annual growth rate (CAGR) of 6.1 % from 2020 to 2025. 91 Asian countries collectively contributed over 80 % of global shrimp production in 2023. 92
The shrimp processing stage, particularly peeling and deveining, generates considerable chitin waste, primarily from the shells, with 45–48 % of raw shrimp material by weight discarded as waste. 93
Shrimp waste is a valuable source of animal protein and essential biomaterials, including chitin, lipids (e.g., glycerides, phospholipids, carotenoids), and calcium carbonate. Studies indicate that dried shrimp waste contains approximately 18 % chitin, 43 % protein, 29 % ash, and 10 % fat. 94 In addition, shrimp waste harbours bioactive compounds such as unsaturated fatty acids, carotenoid pigments, free amino acids, and trace elements. 95 Efficient recovery of these components presents substantial industrial, environmental and research opportunities. 96
Beyond shrimp shell waste, insect-derived chitin is an emerging source of this versatile biopolymer, with insects containing 5 %–10 % chitin (dry weight). 97 , 98 Chitin is readily separable from the protein-rich parts of insects, such as their bodies, larvae, or exoskeletons. During various life stages (e.g., larvae, pupae, prepupae, adults), insects shed exoskeletons, providing a chitin-rich substrate for extraction. 36 , 99 Although chitin from shrimp, crabs, squid pens, and mushrooms has been widely studied, the production and comparative analysis of chitin nanofibers (ChNFs) from different insect sources is an emerging research field. 100
While insect and crustacean chitin share similar chemical structures, crystalline arrangements, and thermal properties, they vary in morphology and fibre accessibility, which affects extraction efficiency. 36 , 101 The extraction processes must be tailored to the source; crustacean shells contain predominantly calcium carbonate, whereas insect exoskeletons have a higher protein and lipid content, requiring adjusted demineralization and deproteinization steps. 102 Fungal chitin sources have glucan residues on their fibre surface, which is challenging to purify. 103
Chemical chitosan extraction is a multi-step process comprising demineralization, deproteinization, and deacetylation. 104 , 105 , 106 Demineralization typically employs a dilute hydrochloric acid (HCl) solution to dissolve calcium carbonate and calcium chloride, the predominant inorganic components in the exoskeletons of crustaceans. 107 Deproteinization follows, where an alkaline treatment is applied to eliminate proteins, resulting in purified chitin. The final step, deacetylation, transforms chitin into chitosan by removing acetyl groups. Apart from the chemical extraction of chitin and chitosan, there is a biological alternative method. 36 , 108 Biological methods offer an environmentally friendly alternative by utilizing lactic acid for demineralization and proteases secreted into the fermentation medium for deproteinization. 33 , 37 , 107
5 Chemical properties of chitin and keratin
The structural polymers chitin and keratin fulfil distinct functions due to their unique chemical compositions and structural configurations. 109 , 110 Despite differences in their molecular makeup, both serve essential structural roles, exhibit fibrous properties, and provide protective functions. Chitin is a polysaccharide consisting of long chains of N-acetylglucosamine, a glucose derivative, which lends rigidity to the exoskeletons and cell walls in various organisms. 11 , 111 In contrast, keratin is a sulfur-containing protein rich in the amino acid cysteine. It forms disulfide bonds that enhance its strength and resilience, making it ideal for protective tissues like hair, nails, and feathers. 13 , 72
5.1 Chemistry of keratin
The amino acid sequence forms alpha-helices and beta-sheets, which assemble into intermediate filaments within cells, contributing to the tissue’s mechanical stability and resilience. 42 Keratins are particularly abundant in the outer layers of the skin and protective structures. 112 Their high cysteine content allows for disulfide bonds, giving keratin durability and resistance to environmental stresses. 113
Unlike specific biological tissues, the keratin-based matrix lacks the capacity for self-repair, as it primarily comprises non-living tissue formed by keratinocytes (keratin-producing cells) that die and accumulate in the outermost layers. This structural framework provides resilience and protection but remains incapable of regenerating in the same manner as bone or other living tissues. 42 , 114 , 115
5.1.1 Primary structure
At the molecular level, keratin exists in two main types – alpha-keratin and β-keratin – which are distinguished by their amino acid composition and structural organization. α-keratin is primarily found in mammals, with some exceptions like the pangolin, which has α- and β-keratin. In contrast, β-keratin is characteristic of birds and reptiles. 116 , 117 , 118 , 119 Each type has distinct atomic-scale and sub-nanoscale structures (Figure 1). 8 , 120

The hierarchical structure of α-keratin and β-keratin spans multiple scales, from the amino acid sequence forming alpha-helices and beta-sheets to protofilaments and intermediate filaments. These intermediate filaments further organize into larger macroscopic structures, demonstrating higher-order arrangement and functionality. This multi-scale architecture underpins the remarkable mechanical and biological properties of keratin-based materials. Source: 8 licensed under CC BY.
In α-keratin, the structure is divided into three regions: crystalline fibrils (helices), the matrix, and the terminal domains of the filaments. 116 Its α-helical shape arises from internal hydrogen bonds, which coil the chain. 121 The α-helical chains are stabilized by sulfur-based cross-links, forming dimers that further assemble into protofilaments. 117 , 122 These protofilaments feature N- and C-terminal ends rich in cysteine, essential for bonding with other intermediate filament (IF) molecules, thus creating a robust and organized network. 123 α-keratin itself is divided into type I (acidic) and type II (basic) categories, further classified into hard keratin (5 % sulfur content) and soft keratin (1 % sulfur content) based on structural properties. 124 , 125 Hard keratin – found in hair, feathers, horns, claws, hooves, and exoskeletons – is sub-classified into Ia (acidic-hard) and IIa (basic-hard), while soft keratin – found in cellular tissue like calluses – is categorized as Ib (acidic-soft) and IIb (basic-soft). 117 , 126 The molecular mass of α-keratin ranges from approximately 40 kDa–68 kDa, 127 with a notable cysteine content (7–20 %), essential for forming disulfide linkages that provide mechanical strength and durability. 128
In contrast, β-keratin has a different molecular structure, featuring a central domain with β-sheet residues flanked by N- and C-terminal regions. 129 In β-keratin, the β-strands align antiparallelly, stabilized by hydrogen bonds that create a stable, planar, and pleated sheet structure. 130 The beta sheets form a rigid, planar surface due to this arrangement. Both filament and matrix regions integrate within a single protein structure, resulting in a comparatively smaller molecular mass, ranging between 10 kDa and 22 kDa. 127
These structural distinctions between α- and β-keratin – particularly in cysteine content, molecular mass, and bonding types – underlie their differing mechanical properties and functional roles across species.
5.1.2 Secondary structure
The two main regular secondary protein structures, α-helices and β-sheets, are crucial internal support frameworks within extended polypeptide chains and serve as critical classifications for keratins. Both α- and β-keratins exhibit a distinctive filament-matrix architecture at the nanoscale (Figure 1). 116 , 131
α-Keratin proteins organize into a coiled-coil structure, where α-helices wind around each other to form superhelical bundles. 117 , 132 These bundles typically consist of two or three helices in parallel or antiparallel orientation, although bundles with up to seven helices have been observed. 133 The right-handed helices are stabilized by interchain hydrogen bonds, resulting in a twisted helical structure. In α-keratins, the term ‘filament’ refers to intermediate filaments (IFs) visible under transmission electron microscopy, with a diameter of approximately 7–10 nm. 134 The spacing between the α-helical axes within these structures measures 0.98 nm. 117 , 135
In β-keratin, the ‘filament’ – often termed the ‘β-keratin filament’ – is about 3–4 nm’ in diameter and is organized into a pleated-sheet structure. 136 This structure is stabilized by hydrogen bonds between β-strands, as well as the planarity of the peptide bond, resulting in a robust, planar sheet. 137 The sheet is formed as a single polypeptide chain that folds into four parallel and antiparallel strands, maintained by intermolecular hydrogen bonds, with an inter-sheet spacing of approximately 0.97 nm. 130
5.1.3 Hierarchical structures
Hair and wool fibres serve as exemplary models to illustrate keratin’s hierarchical structure. As shown in Figure 2, this structure comprises several distinct layers. 138 The outermost layer, the cuticle, which constitutes approximately 10 % of the fibre’s volume, consists of overlapping cells that secure the hair within the follicle by firmly attaching it to the root shaft. 115 Each scale cell in the cuticle comprises three sub-layers: the epicuticle, the exocuticle, and the endocuticle. The arrangement and morphology of these cuticle cells influence the frictional properties of hair. In human hair (Figure 3), the cuticle typically has five to ten layers of scales, while wool fibres usually consist of only one to two layers. 139 Generally, fibres with greater diameters have multiple layers of cuticle cells, and the thickness of individual cuticle cells varies, measuring around 0.3 µm in Merino wool, 0.5–0.66 µm in Lincoln wool, and approximately 0.33 µm in human hair. 140 , 141

The hierarchical structure of human hair spans scales from nanometers to micrometres, beginning with alpha-helices and intermediate filaments (as detailed in Figure 1) and extending to microfibrils, cortical cells, and the entire hair fibre. This layered organization, ranging from ∼2 nm to over 100 μm, highlights the complexity of keratin’s architecture and its role in imparting strength, flexibility, and functionality to hair. Source: 142 permission granted.

The chemical structures of chitin and chitosan. Chitosan is obtained through partial deacetylation of chitin in the solid state under alkaline conditions or via enzymatic hydrolysis catalyzed by chitin deacetylase. This structural difference imparts distinct solubility and functionality, enabling diverse biological and industrial applications. 150 Source: 174 licensed under CC BY.
The cortex layer, constituting roughly 90 % of the fibre’s mass, 115 comprises three distinct zones: the orthocortex, paracortex, and mesocortex, each characterized by unique structural arrangements that influence the fibre’s curliness. 143 The paracortex’s Intermediate Filaments (IFs) display a more uniform hexagonal arrangement. In contrast, the IFs in the orthocortex form discrete bundles and are surrounded by a smaller matrix volume than in the paracortex. 42 , 144 The paracortex also has a smaller diameter than the orthocortex. The lipid-rich cell membrane complex provides cohesion among cortical cells, with microfibrils (formed from IFs) and matrix proteins visible within. 145
At the nanoscale, alpha-helical chains assemble to form IFs embedded in a sulfur-rich matrix containing proteins, nuclear remnants, cell membrane complexes, and intercellular cement. 116 , 146 , 147 The medulla, a central porous layer comprising foamy keratin, shows notable variation among fibre types. Fine fibres, such as Merino wool and polar bear fur, typically lack a medulla (a hollow core), enhancing their thermal insulation properties. 8 , 115 , 148
5.2 Chemistry of chitin and chitosan
Chitin, or poly (β-(1 → 4)-N-acetyl-D-glucosamine), is the second most abundant natural polysaccharide after cellulose and is integral to various organisms. 149 , 150 , 151 Chitin provides structural integrity in single-celled organisms, such as diatoms, protists (eukaryotic microorganisms), and fungal cell walls. In multicellular organisms, it is found in sponges, corals, molluscs, worms, and notably in the exoskeletons of arthropods. 152 The crystalline structure of chitin varies by source, resulting in three distinct polymorphic forms: α-chitin, β-chitin and γ-chitin. These forms differ primarily in the stacking arrangement of chitin chains. In α-chitin, the chains are arranged in an alternating antiparallel orientation, contributing to its rigidity, while in β-chitin, all chains align in a parallel arrangement, which offers greater flexibility. 153 The third form, γ-chitin, is a structural variant of the α-family, exhibiting a combination of parallel and antiparallel stack configurations. 154
5.2.1 α-chitin
α-chitin is the predominant polysaccharide in krill, lobster and crab shells, yeast cell walls, shrimp shells, and insect cuticles. Its crystalline structure is highly organized with a space group symmetry designated as P212121, an orthorhombic crystal system. 155 Here, “P” signifies a primitive cell with a single lattice point per unit cell, while 21 represents a two-fold rotational axis, of which three are in this arrangement. 156 This orthorhombic structure is characterized by a symmetry group of 222 and demonstrates high crystallinity, observed in X-ray diffraction (XRD) patterns. XRD reveals a hallmark diffraction ring for α-chitin at approximately 0.338 nm alongside an inner ring at 0.943 nm, both stable even in hydrated conditions, reflecting chitin chains’ dense, ordered packing.
Electron diffraction further elucidates α-chitin’s structural parameters, with cell dimensions nearly twice those of β-chitin in one axis. Detailed crystallographic analysis shows that α-chitin’s unit cell accommodates two antiparallel chitin chains per unit, aligned in sheets and bonded by intra-sheet solid hydrogen interactions, such as C=O⋯NH. These interactions form a cohesive network that significantly enhances the material’s mechanical stability and rigidity. 150 , 155
At the molecular level, α-chitin consists of long chains of N-acetylglucosamine units linked via β-(1 → 4) glycosidic bonds. 149 The N-acetyl glycosyl moiety is the independent crystallographic unit in α- and β-chitin, serving as a recurring structural element. 157 Regarding its interaction with solvents, α-chitin exhibits selective swelling behaviour; water and alcohol cannot penetrate its crystalline lattice. However, aliphatic diamines, acting as potent swelling agents, can intercalate within the lattice, creating highly crystalline complexes. These agents fit between the chitin sheets, causing directional expansion. Interestingly, this inter-sheet swelling correlates with the number of carbon atoms in the diamine, while the chitin structure’s original crystallinity is restored upon acid removal. 150 , 158 , 159 This selective swelling behaviour highlights the material’s unique capacity for guest-molecule intercalation, with implications for various applications.
5.2.2 β-chitin
β-chitin is relatively rare, found in specific marine and deep-sea biological structures such as squid pens, diatom spines, and the tubes of certain deep-sea worms, including pogonophores and vestimentiferans, typically at depths of 1,000–10,000 m. 160 , 161 Although β-chitin also occurs in a few other organisms, it is significantly less prevalent than α-chitin. 162 Structurally, β-chitin differs subtly but distinctly from α-chitin, particularly in its crystallographic and hydration properties. 163 , 164 The characteristic diffraction ring of β-chitin appears at 0.324 nm, similar to that of α-chitin. However, upon hydration, this ring expands to approximately 1.16 nm, indicating a substantial increase of 0.242 nm compared to α-chitin, highlighting its unique response to moisture. 150
β-chitin’s crystalline structure consists of a single chitin molecule per unit cell, arranged in parallel chains within stacked sheets. These sheets are stabilized primarily by intra-sheet hydrogen bonds, which confer structural cohesion but lack significant inter-sheet hydrogen bonding along one crystallographic axis. Consequently, β-chitin exhibits pronounced intercrystalline swelling when exposed to water, alcohol, or amines, with these molecules able to penetrate its crystal lattice without disrupting overall crystallinity. 165 , 166 This intra-crystalline swelling is reversible; however, exposure to solid acids induces an irreversible conversion of β-chitin to α-chitin. 167 This transformation involves breaking intra- and inter-sheet hydrogen bonds, resulting in a loss of crystallinity and significant shrinkage. This irreversible β-to-α transition suggests the superior thermodynamic stability of α-chitin, which recrystallizes into a more energetically favoured arrangement. 155
5.2.3 Chitosan
Chitosan is a non-toxic, linear polysaccharide and the primary derivative of chitin, comprising randomly distributed N-acetylglucosamine and glucosamine units. 168 It is obtained through partial deacetylation of chitin in the solid state under alkaline conditions or via enzymatic hydrolysis catalyzed by chitin deacetylase. 169 Chitosan is predominantly sourced from crustaceans, especially squid pens. 170 In its solid form, it has a semi-crystalline structure. Electron diffraction analysis shows an orthorhombic unit cell with space group (P212121), containing two antiparallel chitosan chains without intercalated water molecules. 171 The molecular weight of chitosan ranges from 10,000 to 1 million Daltons, 172 and its structure includes an amino group and two hydroxyl groups, which form a network of intermolecular hydrogen bonds that contribute significantly to its stability. 173
6 Biomimetics using chitin and chitosan
Biomimetics, bio-inspiration, and bio-utilization are distinct yet interconnected concepts, as systematically defined by Drack and Gebeshuber. 175 This work proposes an integrative approach that merges these methodologies to optimize biopolymers such as chitin, keratin, and chitosan, effectively bridging biomimetic principles with bio-inspired innovations. This approach becomes particularly compelling when these materials are sourced through recycling. Recycling facilitates sustainable material recovery while maintaining their intrinsic functional properties.
Chitin is a highly versatile biopolymer found in marine organisms and insects, offering significant potential across various applications, from biomedicine to materials science. Both chitin and its derivative, chitosan, exhibit notable biological activities, including antitumor, antimicrobial, and antioxidant properties, primarily influenced by their unique chemical compositions and structural configurations. 150 , 174 , 176 A detailed examination of chitin’s hierarchical structure provides critical insights into its mechanical properties and informs the development of advanced materials. Understanding these properties supports innovations in protective armour and structural materials inspired by chitin’s role in providing strength and resilience to various biological organisms. 177
The list of properties presented is intentionally selective and not comprehensive, focusing on the most notable characteristics of chitin and chitosan that are particularly pertinent for biomimetic applications without claiming to provide a conclusive inventory of their potential attributes.
6.1 Mechanical properties
Chitin’s unique chemistry and hierarchical structure confer remarkable mechanical properties, which can be further enhanced through polymorphic forms, specifically α- and β-chitin crystals, that provide additional structural strength. 178 These polymorphs introduce distinct molecular arrangements and bonding patterns contributing to increased rigidity and resilience. 177
6.1.1 Tensile strength
Uniaxial tension simulations were conducted to investigate α- and β-chitin crystal’s tensile properties along the [001] direction, chosen due to the more muscular backbone strength of carbohydrate chains compared to the weaker non-bonded interchain interactions. The results indicated that both chitin polymorphs exhibited linear elasticity at minor strains, with α-chitin showing an elastic modulus nearly twice that of β-chitin. 179 , 177 Under increasing strain, both forms displayed nonlinear elasticity preceding failure, with α-chitin uniquely featuring a yielding plateau absent in β-chitin. Throughout loading, α-chitin sustained higher stress up to yielding. 180 However, post-yield, the stress-strain curves of both polymorphs converged, ultimately showing equivalent tensile strength and critical fracture strain at failure. 181 , 182 The work of fracture and effective modulus of α-chitin exceeded those of β-chitin, underscoring its superior resistance to deformation. At the atomic scale, failure in both polymorphs originated from the β-(1 → 4) linkages within chitin backbone chains. However, α-chitin underwent a structural transformation under substantial tension, leading to yielding, whereas β-chitin maintained its crystalline structure until the backbone ruptured. 177 , 183 , 184 Despite their comparable ultimate tensile strength and fracture strain, these observations clarify α- and β-chitin’s differing failure modes and deformation mechanisms under tensile load. 177
Chitosan, derived from shrimp shells, is an environmentally friendly, biodegradable, and antibacterial polysaccharide with a chemical structure similar to cellulose, allowing for easy integration with cellulose-based surfaces, such as hand sheets. Researchers examined the influence of chitosan on mechanical properties by incorporating shrimp-derived chitosan into cellulose hand sheets at 0.25 %–0.50 % of the oven-dry pulp weight. 185 The tensile index, indicating sheet resistance to breaking under tension, increased significantly with chitosan addition, suggesting enhanced tear resistance. 186 , 187 The modulus of elasticity, reflecting the sheet’s capacity to recover its shape post-deformation, also improved markedly, implying increased elasticity and deformation tolerance. The enhancement of tensile strength and elasticity demonstrates chitosan’s potential to improve cellulose-based sheet performance for various applications, contributing to durability and flexibility. 188 , 189 , 190
6.1.2 Wear resistance
The anti-wear performance of chitin crystals is crucial for the protective armour in crustaceans and beetles, which safeguards softer tissues from environmental abrasions. These biological structures must effectively counteract surface scratches and material degradation over time, which may otherwise compromise their protective function. 191 , 192 When subjected to forces at steep attack angles, these surfaces consistently exhibit a characteristic wear pattern known as “cutting mode,” marked by scratch grooves and material debris accumulation. 191
To assess the anti-scratch performance of chitin, a minimum applied force, denoted Fcut, is required to initiate surface scratching. Higher Fcut values correlate with improved wear resistance. 192 Factors influencing Fcut include shear strength, attack angle, penetration depth, and surface energy, particularly on the [010] chitin surface. Surface energy represents the energy required to create a new surface area, which can be determined by evaluating changes in total potential energy when a chitin crystal is split. 192 Comparative analyses of Fcut for α- and β-chitin crystals reveal that α-chitin demonstrates superior wear resistance across various conditions. This superior performance aligns with the frequent occurrence of α-chitin in natural protective structures, suggesting a potential relationship between wear resistance and crystal polymorphism. 177
6.2 Bactericidal properties
The antibacterial chemical properties of chitin, chitosan, and their derivatives have garnered significant attention in research. The antibacterial activity against various pathogens makes them potential candidates for various antimicrobial applications. 193 , 194 Chitosan is recognized for its broad-spectrum antimicrobial properties, which offer significant advantages over conventional disinfectants, including a faster action rate, enhanced effectiveness, and lower mammalian cell toxicity. 195 While the precise mechanisms behind chitosan’s antimicrobial properties are still under investigation, it is widely believed that chitosan’s positively charged polysaccharide interacts with the negatively charged bacterial cell membrane, resulting in increased cell permeability. 196 , 197 , 198
The antibacterial effects of chitosan are proposed to involve several mechanisms. Low molecular weight (MW) chitosan can penetrate bacterial cell walls and inhibit essential processes, such as mRNA and DNA synthesis, especially in gram-positive bacteria. In contrast, high-MW chitosan is thought to act primarily at the cell surface, altering its permeability or forming a barrier that restricts the influx of essential solutes. 196 , 199 Chitosan’s antibacterial efficacy is also affected by environmental pH, with acidic conditions enhancing its adsorption onto bacterial cells due to increased positive ionic charge. 174 , 198 , 200 At pH 6, chitosan’s higher solubility further enhances its antibacterial action compared to chitin. 193
Other factors impacting chitosan’s antimicrobial activity include its degree of acetylation (DA) and molecular weight. 198 , 201 Lower DA and MW, combined with acidic pH, have been shown to enhance antibacterial effectiveness Salah et al., 202 Yun, Kim, and Lee, 203 Liu et al. 204 Chitosan also exhibits differential effects on gram-positive versus gram-negative bacteria, 174 , 205 increasing activity against gram-positive bacteria with MW. In contrast, the opposite trend is observed with gram-negative bacteria. It remains unclear, however, whether chitosan’s action is bacteriostatic (inhibiting growth) or bactericidal (inducing cell death). 193
In addition to antibacterial effects, chitosan has antifungal activity, effectively inhibiting fungal species by forming a permeable film at the cell interface and activating plant defence mechanisms. 206 Its antifungal efficiency varies with properties such as MW and DA. 207 Numerous modifications to chitosan have been developed to enhance solubility and antimicrobial effectiveness, introducing functional groups along polymeric chains. 208 Common modifications include N-modification, O-modification, and combined N–O-modification, with approaches such as quaternization, carboxymethylation, and sulfonation, significantly improving solubility and antimicrobial potential. 193
Heedy et al. explored the synergistic antimicrobial effects of chitosan nanostructures, 209 revealing that a chitosan hydrogel with nanopillars showed enhanced antimicrobial properties, markedly inhibiting the growth of the gram-negative bacterium Pseudomonas aeruginosa and the fungus Fusarium oxysporum. Chitosan based nanostructures avoid unwanted toxic chemicals and harsh fabrication processes. 210 Surfaces with nanopillar topographies inspired by cicada wings, 194 butterfly wings, 211 and shark skin 212 exhibit antimicrobial properties. These materials act antimicrobially due to the nanopillars deforming bacterial membranes, causing oxidative stress and rupturing. 213
Natural polymer hydrogels have gained increasing attention compared to synthetic polymer hydrogels due to their low toxicity, excellent biocompatibility, ease of degradation, abundant availability, and lower cost. 214 Hydrogels made from chitin and chitosan can be tailored through various modifications to serve as scaffolds for ideal wound dressings or as “intelligent” hydrogels for controlled and targeted drug release. 215 , 216 , 217 These hydrogels have been found to exhibit self-healing properties. 218 Keratin-based hydrogels exhibit valuable properties for wound healing, drug release, and neural regeneration. 219 , 220
6.3 Structural colour
Chitin exhibits various structural conformations shaped by natural processes such as biopolymerization, crystallization, and non-equilibrium self-assembly. 178 These processes produce unique physical effects, including intricate light scattering, polarization, and mechanical properties, driven by the balance between highly structured chain conformations within nanofibrils and disordered regions. 178 Colours play essential roles in Nature for communication, defence, signalling, and environmental adaptation, including camouflage. 221 , 222 , 223 , 224 Unlike pigments that selectively absorb light due to electron delocalization within specific molecules, structural colouration arises from scattering, interference, and diffraction at the nanoscale, with structures on the order of the wavelength of visible light. 19
Chitin serves as the primary structural component in the exoskeletons of various species, providing both robustness and an adaptable colour palette. 120 , 178 , 225 , 226 Recent studies have focused on understanding how butterfly wing-scale nanostructures influence light reflection at different angles. For instance, butterflies with structural colours, such as Papilio memnon and Parides sesostris, utilize grating structures with ridges and cross-ribs, producing strong orientation-dependent reflection when ridges are perpendicular to incident light. 227 This orientation-based reflectance produces varied appearances based on viewing angle, which can be replicated in synthetic materials for technological applications. 228
A notable example of chitin’s optical properties is the blue colour of the Morpho butterfly (Morpho peleides see Figure 4), which displays a vibrant metallic-blue hue due to coherent scattering from layered nano- and microscale structures. Unlike conventional multilayers, Morpho butterflies have tilted, Christmas tree-like multilayered surfaces that generate a metallic blue with minimal angle dependence. 231 This unique colour is produced by periodic surface nanostructures that cause diffraction from additional grooves, with organized chitin fibres in the cuticle contributing to birefringence due to their polymer orientation. Refractive indices between 1.54 and 1.62 and air-filled nanostructures enhance Morpho’s brilliant, angle-independent colour. 232 , 233

Nanostructures are responsible for structural colouration in various insects. These features demonstrate how nanoscale architecture, rather than pigments, produces vibrant colours through interference, diffraction, and selective light reflection. (a) (left) Morpho peleides butterfly image. (right) Transverse section of wing scales showing christmas-tree like structure and ridges (SEM image). (b) (left) Female of a Japanese jewel beetle. Source, 229 permission granted. (right) Transverse TEM sections of cuticle showing multi-layered structures. (c) (left) Glorious scarab image. (right) SEM image of the cuticle with its multi-layered structure with helicoidal cholesteric twist. (d) (left) Snot weevil image. Source, 230 permission granted. (middle) Bright-field light microscopy image. (right) Single green-coloured scale (SEM image). Source: 178 licensed under CC BY.
Chitin also enables beetles to achieve diverse optical effects such as luminescence, iridescence, photonic structures, and polarized reflectance. Beetles like the scarab feature helicoidal nano- and microstructures in their chitin layers, selectively reflecting left-circularly polarized light. 234 , 235 Chrysomelid beetles, for instance, utilize quasi-ordered structures that produce diffuse, non-iridescent colours through light scattering. 236 At the same time, interaction with water can shift colours in both beetles and butterflies by altering refractive index and reflectance. 237 Dense chitin fibril networks in species like Cyphochilus beetles and Lepidiota stigma achieve bright whiteness through multiple scattering, whereas transparent species like the glasswing butterfly (Greta oto) use chitin nanopillars for transparency by reducing scattering along one axis. 238 , 239
Weevils demonstrate complex three-dimensional photonic crystals, such as gyroid and diamond networks, contributing to iridescent, rainbow-like appearances. 240 The rainbow weevil (Pachyrrhynchus congestus) achieves this through an inverse opal-like chitin structure with diamond symmetry. 230 In crustaceans, chitin-based Bouligand layers – periodically arranged uniaxial fibre layers in a helicoidal pattern – enhance resistance to mechanical damage. 241 , 242 Crustaceans typically do not display iridescence, unlike beetles, but species like green and blue crabs may use pigments combined with Bouligand layers as waveguides, creating mixed-colour effects. 178 , 243
Chitin’s nanostructures enable a broad spectrum of optical and mechanical functions across species. Whether enhancing structural colour, modulating transparency, or optimizing mechanical strength, chitin nanofibrils play a central role in biological adaptations, often combined with materials like CaCO3, proteins, and pigments in exoskeletons. These complex structural designs underscore the interplay between optical functionality and mechanical resilience in natural materials, guiding biomimetic advances in material science. 18 , 244
6.4 Pharmaceutical and biomedical properties
The remarkable attributes of chitin and chitosan, including biocompatibility, 245 renewable sourcing, 246 nontoxicity, 172 non-allergenicity, 247 and biodegradability, 248 enable their use across diverse biomedical and industrial applications. These biopolymers demonstrate a wide range of biological functions, such as antifungal, 249 antibacterial, 250 antitumor, 251 immunoadjuvant, 252 anti-thrombogenic, 253 and anti-cholesteremic properties, 254 enhancing their value for applications in drug delivery, tissue engineering, wound care, and film production.
Chitin and chitosan can be fabricated into various forms, including powders, films, fibres, solutions, sponges, gels, beads, and capsules, enabling their use in oral, nasal, and ocular drug delivery systems, as well as in wound dressings and vaccine delivery platforms. 255 Due to its cationic nature, chitosan can interact with cell surfaces, modulating ion transport and enhancing absorption and hydration. Its mucoadhesive properties make it particularly useful as an excipient and drug carrier, improving bioavailability and therapeutic efficacy. 256
While its insolubility and processing challenges somewhat constrain chitin’s applications, its combination with chitosan significantly expands its functional utility. Chitosan’s solubility in acidic environments, coupled with its tunable chemical and physical properties that can be further enhanced through modifications, significantly increases its versatility. 174 , 257
6.4.1 Tissue engineering
Tissue engineering aims to develop materials capable of replacing damaged tissue or stimulating advanced regenerative processes within the body. Tissue engineering involves manipulating living cells by modulating their environment or genetic engineering to create biological materials suitable for implantation. Implantable materials must meet stringent criteria, including biocompatibility, functional efficiency, mechanical strength, and long-term stability. 258
Designing polymer scaffolds for tissue engineering requires meeting several critical requirements. These include high porosity with an optimized pore size distribution and a large surface area to facilitate cell infiltration and nutrient exchange. 245 Biodegradability is equally vital, as the scaffold’s degradation rate must align with the pace of new tissue formation. 259 Structural integrity is necessary to prevent pore collapse during tissue growth, demanding robust mechanical properties. Furthermore, the scaffold must be nontoxic and biocompatible, promoting cellular adhesion, proliferation, migration, and differentiation to support effective tissue regeneration. 110 , 260
Chitin has emerged as a promising material for scaffolds in tissue engineering due to its advantageous properties. 261 It can be moulded into various forms, including hydrogels, porous sponges, and fibrous scaffolds, each tailored to specific tissue engineering applications. 262 Physical or biochemical modifications often enhance chitin utility. 176 These modifications include blending chitin with other polymers or chemically functionalizing its surface with sugars, peptides, or proteins to improve cell adhesion and bioactivity. For example, hybrid chitin nanofibers combined with silk fibroin or poly(glycolic acid) have been developed to support cell attachment and proliferation. 263 , 264
Chitin scaffolds find particular application in cartilage, bone, and tendon tissue engineering, where their mechanical and biological properties are critical for mimicking the natural extracellular matrix. 265
6.4.2 Wound healing
Research has highlighted the significant potential of chitin and chitosan in accelerating wound healing, particularly for patients with impaired healing, such as those with diabetes. 266 , 267 Chitin-based products, including powders, fabrics, beads, and films, have been extensively investigated for their medical applications due to their biocompatibility, safety, and versatility. 268
Studies demonstrate the effectiveness of chitosan scaffolds and membranes in treating deep burns and chronic wounds. 269 , 270 Innovative approaches, such as employing fungal mycelia to produce bioactive wound-healing membranes, further expand the applicability of chitosan-based materials. 271 Composite scaffolds, such as α-chitin/nanosilver and β-chitin/nanosilver, have exhibited superior antibacterial activity and enhanced hemostatic properties, making them promising candidates for wound care applications. 215 , 272
Clinical evaluations of chitosan-based dressings on skin graft donor sites have shown accelerated wound healing, improved tissue quality, and reduced healing times compared to conventional dressings. These advancements underscore the therapeutic potential of chitin and chitosan in wound healing. 110
6.4.3 Drug delivery systems
Drugs typically consist of one or more pharmacologically active agents combined with a suitable carrier to facilitate administration Smolen. 273 Effective drug delivery systems must meet several criteria: the active ingredient must be delivered selectively to the target site within the body, in precise amounts, and at a controlled rate over a specified period Cirillo et al., 274 Coelho et al., 275 , Baharlouei and Rahman. 276 Various polymers, including hydrogels, capsules, and tablets, are employed as carriers to achieve this. These carriers must exhibit biodegradability, biocompatibility, and non-toxicity, and their three-dimensional structure enables precise control over the timing and rate of drug release. 168 , 277
Polymers play a pivotal role in modulating the pharmacokinetics of active ingredients, enhancing therapeutic efficacy while minimizing side effects. 168 Natural polymers, derived from plants, animals, fungi, and bacteria, offer distinct advantages over synthetic counterparts, such as inherent biodegradability, biocompatibility, and accessibility. 278 Widely used natural polymers in pharmaceutical applications include cellulose, chitosan, and alginate. 279
Chitosan, in particular, stands out for its versatility. Its properties, including solubility, bioactivity, and functionality, are influenced by its degree of deacetylation and molecular weight, allowing for tailored applications. 280 Chitosan can be formulated into diverse dosage forms, such as tablets, capsules, hydrogels, films, membranes, and nanocomposites. 281 This adaptability enables its use in various drug delivery systems, making it an essential material for advancing precision medicine and patient-specific therapies. 282
6.4.4 Cancer treatment
Chitosan and its derivatives demonstrate considerable potential in cancer therapy, particularly for targeted drug delivery and cancer cell imaging. Advances in nanoparticle engineering have enabled the development of chitosan-based systems that facilitate precise drug delivery, controlled release, and the visualization of cancerous tissues (see Section 6.4.3). Chitosan’s biocompatibility, biodegradability, and ability to sustain the release of therapeutic agents also make it an attractive candidate for gene therapy applications Chuan et al., 283 Baharlouei and Rahman. 276
Chitosan-coated nanoparticles have shown efficacy in targeting specific cancer markers, inhibiting tumour growth, and inducing apoptosis in cancer cells. 110 , 284 , 285 The antitumor effectiveness of chitosan is influenced by its structural properties, such as degree of deacetylation, molecular weight, and the type of cancer being targeted. 286 Research indicates that chitosan oligosaccharides with specific molecular weights exhibit significant tumour-inhibiting capabilities in animal models. 287 , 288 Lower molecular weight chitosan often demonstrates enhanced antimetastatic effects and immune-stimulatory properties, critical for slowing cancer progression. 287
However, conflicting data exist regarding the influence of molecular weight on chitosan’s cytotoxicity towards cancer cells. Some studies suggest that lower molecular weight enhances antitumor activity, 287 , 288 , 289 while others report no significant differences in toxicity between varying molecular weights. 290 , 291 Despite these discrepancies, chitosan shows promise not only as an anticancer agent but also as a carrier for encapsulating and delivering anticancer drugs.
Further preclinical studies are required to unravel the precise mechanisms of chitosan’s anticancer activity, optimize its formulation, and enhance its therapeutic efficacy. These efforts are crucial to advancing chitosan-based systems into viable clinical applications and next-generation cancer treatments. 174 , 292
6.4.5 Bone regeneration
Studies highlight the structural similarity between chitin- and chitosan-based nanofibrous scaffolds and the extracellular matrix of bone, positioning these scaffolds as promising candidates for bone regeneration applications (see Section 6.4.1). These scaffolds exhibit essential features, including a high surface area, small pore size, and substantial porosity, collectively mimicking the natural bone matrix. 293 This biomimetic architecture supports cell adhesion, proliferation, and differentiation, facilitating osteogenesis. 294
Incorporating carbon nanotubes into chitosan composites has significantly enhanced their mechanical strength, improving their suitability for load-bearing applications. 295 , 296 Additionally, chitosan composites integrated with calcium phosphates, such as beta-tricalcium phosphate and hydroxyapatite, have demonstrated favourable outcomes in bone repair and regeneration, as evidenced by various animal studies. 297 , 298 These combinations capitalise on the osteoconductive properties of calcium phosphates and the biocompatibility of chitosan to promote bone healing and integration.
Researchers have employed strategies such as reinforcing the composite matrix and designing multilayered nanocomposites to optimise chitosan-based materials for bone tissue engineering further. 299 These approaches aim to improve mechanical properties, such as tensile strength and elasticity while maintaining biocompatibility and bioactivity. 245 Future research directions include fine-tuning scaffold fabrication techniques, exploring novel reinforcement materials, and conducting long-term in vivo studies to assess the efficacy and safety of these composites in clinical settings. 300 , 301 These efforts could pave the way for advanced bone tissue engineering and regenerative medicine solutions. 110 , 302
6.5 Biomimetic passive radiative cooling
Every physical object with a temperature above absolute zero (0 K or −273.15 °C) emits electromagnetic radiation, a phenomenon described by Planck’s law. Within the Earth’s atmosphere is a spectral range between 7.5 µm and 13 µm, referred to as the atmospheric window, in which atmospheric gases exhibit minimal absorption and are nearly transparent to infrared radiation. This transparency allows Earth’s surfaces to emit thermal radiation within this range directly into outer space. 303
When a surface combines high solar reflectivity with thermal solid emissivity in the atmospheric window, it can achieve cooling below ambient temperatures without requiring external energy input. This natural phenomenon, passive daytime radiative cooling (PDRC), has garnered significant interest due to its potential applications in energy-efficient cooling systems. 304
Certain species of butterflies 305 and ants 306 have evolved nanostructures on their wing scales or bodies that exploit PDRC to regulate body temperature. The Saharan Silver Ant (Cataglyphis bombycina) possesses specialized chitinous hairs with a triangular cross-section and surface indentations. 307 These structures efficiently reflect sunlight while enhancing infrared emission via Mie scattering and total internal reflection, enabling effective cooling in extreme desert environments. The hairs act as an antireflection layer in the mid-infrared, increasing heat emission, while the bare underside reflects heat from the hot desert ground more effectively than if it were covered with hairs. 308
Within the triangular hairs, Mie scattering captures light, which is then emitted in multiple directions. The hairs increase reflection at certain wavelengths due to fundamental and higher-order Mie resonance. Variations in the cross-sectional sizes of the hairs smooth out these resonance peaks, resulting in a coating with enhanced broadband reflectivity. 309 , 310
Inspired by such biological strategies, researchers investigate chitosan films derived from shrimp shells for their potential to mimic these cooling properties. Engineering these biopolymer-based materials to exhibit high solar reflectivity and tailored emissivity within the atmospheric window holds promise for sustainable cooling applications, offering a biomimetic approach to passive thermal management. 311
7 Biomimetics using keratin structures
Keratin is a structural protein with diverse morphologies tailored to its functions, from providing waterproof barriers to forming impact-resistant materials. 312 Unlike collagen, another prominent structural protein, keratin assembles through the twisting of polypeptide chains into a coiled-coil configuration. 313 Its structure consists of crystalline and amorphous regions, resulting in a combination of high toughness and a substantial Young’s modulus, enabling it to resist deformation under stress.
A defining feature of keratin is its abundance of cysteine residues, which form robust disulfide bonds. 314 These bonds cross-link the protein chains and matrix molecules, enhancing mechanical strength and stability. 315 The hierarchical organization of keratin, from its molecular configuration to its macroscopic structure, contributes to its remarkable versatility and mechanical properties, solidifying its role as an essential biomaterial in Nature. 115
7.1 Hydrophobic and bactericidal properties
Hydrophobicity is a crucial surface property often observed in natural systems. Known as the “lotus effect”, micro- and nanoscale surface roughness minimizes the contact area of water droplets, promoting self-cleaning by enabling droplets to roll off at small tilt angles, carrying dirt and debris. 316 Despite keratin’s inherent hydrophilic tendencies, this mechanism keeps surfaces dry and clean in organisms such as lotus plants and geckos.
An exemplary application of this property is the anti-icing ability of penguin feathers. 317 Their micro- and nanostructured surfaces trap air within crevices, preventing water adhesion and freezing. This air entrapment is a thermal barrier, reducing ice formation and heat transfer. Penguin feathers’ barbules and hamuli (hook-like structures) feature grooves approximately 100 nm deep, which contribute to the roughness essential for air pocket formation. 8 , 317 Hamuli are also found in the hindwings of Hymenoptera (bees, wasps, ants, and sawflies), where they connect forewings and hindwings, enhancing aerodynamic stability and efficiency during flight. 318
Various natural surfaces exhibit bactericidal properties, such as dragonfly wings, 319 cicadas wings(chitin-based), 194 and geckos (keratin-based). 320 These surfaces disrupt bacterial membranes, with some demonstrating both anti-biofouling and bactericidal effects. 321 , 322 In lizards and geckos, the outer layer of the skin, the “Oberhäutchen” (upper membrane), is composed of β-keratin. This layer often contains small spinules, which confer superhydrophobic properties, repel water, and hinder bacterial colonization. Gecko skin has been found to exhibit antibacterial properties, achieving an 88 % efficacy against gram-negative bacteria and a 66 % efficacy against gram-positive bacteria. 323
Gecko skin exhibits a static water contact angle of 151°–155°, qualifying it as superhydrophobic (contact angles >150° 324 ). 325 Unlike surfaces such as insect hairs or penguin feathers, where intricate roughness patterns dominate, gecko skin’s superhydrophobicity arises primarily from spinules’ density. 325 These structures prevent water accumulation and enable self-cleaning by facilitating droplet coalescence and runoff with minimal disturbance, effectively removing bacteria and debris. 326
These findings highlight the multi-functionality of gecko skin in resisting microbial growth and maintaining a clean, water-repellent surface, as well as the remarkable adhesive system geckos possess, which is discussed in detail in Section 7.6, offering significant inspiration for biomimetic material design.
7.2 Structural color
It is crucial to distinguish between chemical and structural colours arising from fundamentally different processes. Chemical colours result from the selective absorption and reflection of light by pigments, where specific wavelengths are absorbed by conjugated electron systems within the pigments. The absorbed energy excites electrons to higher states and is later dissipated as heat, emitted as fluorescence, or transferred to other molecules. 327 , 328
Structural colours, in contrast, arise when incident light is reflected, scattered, or deflected by structures on the scale of light’s wavelengths (380–700 nm), with minimal energy exchange between the light and the material. This type of colouration can produce vibrant, iridescent hues. Five fundamental physical phenomena contribute to structural colouration: multilayer interference, scattering, diffraction, thin film interference, and photonic crystals. 19 Many of the green, blue, and violet colours observed in Nature and all iridescent colours result from structural colouration. Such colours are typical in birds, insects, plants, algae, and viruses. The morphologies of such nanostructures include multilayer structures (such as in the iridescent throat patch of hummingbirds), two-dimensional photonic crystals (such as in the peacock and in mallard feathers) or spinodal-like channel structures (such as in the Eurasian Jay Garrulus glandarius). 8 , 329 , 330 , 331
In avian species, non-iridescent structural colours are often due to light scattering within nanostructures composed of β-keratin and air within the medullary cells of feather barb rami (branches of the feather’s central shaft). 332 These nanostructures can be categorized into two main morphological types: ‘channel’ nanostructures, which feature elongated, tortuous air channels surrounded by β-keratin bars, and ’spherical’ nanostructures, which consist of spherical air cavities encased in thin β-keratin bars, sometimes interconnected by small passages. 333 These structures form through self-assembly, creating multi-layered assemblies of β-keratin alongside pigment-based proteins such as melanin or carotenoids. The vivid colours observed in bird feathers result from combining nanostructure colouring and colour pigment mixing. 334
Despite its relatively low refractive index (∼1.5), β-keratin significantly produces high reflectance and vibrant colouration when incorporated into multi-layered structures. 8 , 334 Studies by Jeon et al. and colleagues explored how the keratin cortex surrounding multi-layered melanosomes in common feather barbules influences feather colour. 329 They discovered that altering the thickness of the keratin cortex could substantially affect the colouration, particularly the hue, while leaving other optical properties, such as saturation and brightness, largely unaffected. Both optical simulations and empirical observations underscored the importance of the keratin cortex in regulating feather colour, offering a detailed understanding of how structural elements influence colour perception.
7.3 Specific mechanical properties
Keratin-based materials, widely found in Nature, are integral to the mechanical performance of organisms, offering load-bearing capacity, impact resistance, and protective functions. One of keratin’s distinctive features is its hydration-dependent mechanical properties. When dry, keratin exhibits a high stiffness and load-bearing capacity with Young’s modulus of approximately 10 GPa. 335 , 336 , 337 In contrast, it becomes ductile when fully hydrated and exhibits a significantly reduced Young’s modulus (∼0.1 GPa), highlighting its remarkable adaptability to varying environmental conditions. 336 , 338
Keratin materials display higher-order assemblies with geometric complexity across multiple length scales, contributing to their mechanical excellence. 339 For example, the hoof walls of horses and bovines illustrate how keratin’s higher-order architecture supports energy dissipation and structural resilience. Horse hooves, subject to impact speeds of around eight m/s and forces approximating 16.1 N/kg, are primarily composed of dead keratinocytes, providing resilience without self-repair. 337 , 340 The hoof wall features a mesoscale architecture of hollow tubules (∼40 µm in diameter) encased in rigid elliptical regions (200 µm × 100 µm) embedded within a lamellar matrix of keratinocytes (Figure 5). This arrangement enhances fracture toughness by controlling crack propagation and redirection. The intertubular matrix, tubule orientation, and the volume fraction of intermediate filaments (IFs) vary through the hoof wall’s thickness, contributing to fracture resistance. Cracks initiated along tubules often divert outward by intertubular materials, sparing inner living tissues. 336 , 337 , 341 , 342 Fracture surfaces reveal energy dissipation mechanisms within IFs, keratin matrices, cell boundaries, and tubular components, ensuring efficient crack deflection and damage resistance. Hydration aids in restoring the initial shape of compressed keratin samples, demonstrating the material’s reversible deformation capabilities. 343

Hierarchical structure of the horse hoof wall, from nanoscale intermediate filaments to macroscale features. The diagram traces the formation of intermediate filaments into microfibrils, their organization within keratinocytes, hollow tubular alignment, and their integration into the hoof wall, showcasing the multiscale design contributing to its mechanical strength. Source: 8 licensed under CC BY.
Fingernails and claws, composed of keratin, further illustrate its structural diversity. These structures, critical for providing rigid support and protecting soft pads, consist of a sandwich-like tri-laminar architecture: a dorsal layer, a transverse-fibre-dominated intermediate layer and a thin ventral layer. 115 The fibre orientation influences fracture behaviour, with cracks typically propagating parallel to the nail’s free edge, avoiding damage to underlying tissues. Lamellar epithelial cells within these layers, combined with surface sutures, enhance bonding and structural integrity. 344
Environmental humidity significantly affects keratin’s mechanical properties, with increased moisture leading to reduced tensile strength. 345 This variability underscores keratin’s adaptability and its pivotal role in structural biology. From hooves to claws and nails, keratin’s multi-scale architecture and hydration-dependent properties exemplify its biological significance and potential for biomimetic applications.
7.4 Thermal insulation
Keratinous systems are highly effective thermal insulators due to their hierarchical structures and porous properties, enabling efficient air trapping. 346 These natural materials help regulate body temperatures in animals such as polar bears and penguins, enabling survival in extreme environments. 347
Penguin feathers include contour (body) and down (plume) feathers, each with distinct structures, roles and localisation. 348 Down feathers primarily comprise thermal insulation, characterised by a branched hierarchical arrangement. 349 Their structure features a central shaft (calamus) from which barbs and barbules extend, interlocked by hamuli for enhanced stability. The barbs contain a cellular core with progressively increasing porosity toward the centre, creating a foam-like structure that maximises surface area for heat retention and provides exceptional insulation. 350 , 351 This structural adaptation parallels the design of porcupine quills, which also exploit porosity and hierarchy for lightweight yet effective insulation. 352 This arrangement is similar to porcupine quills (see Section 7.5, Figure 6).
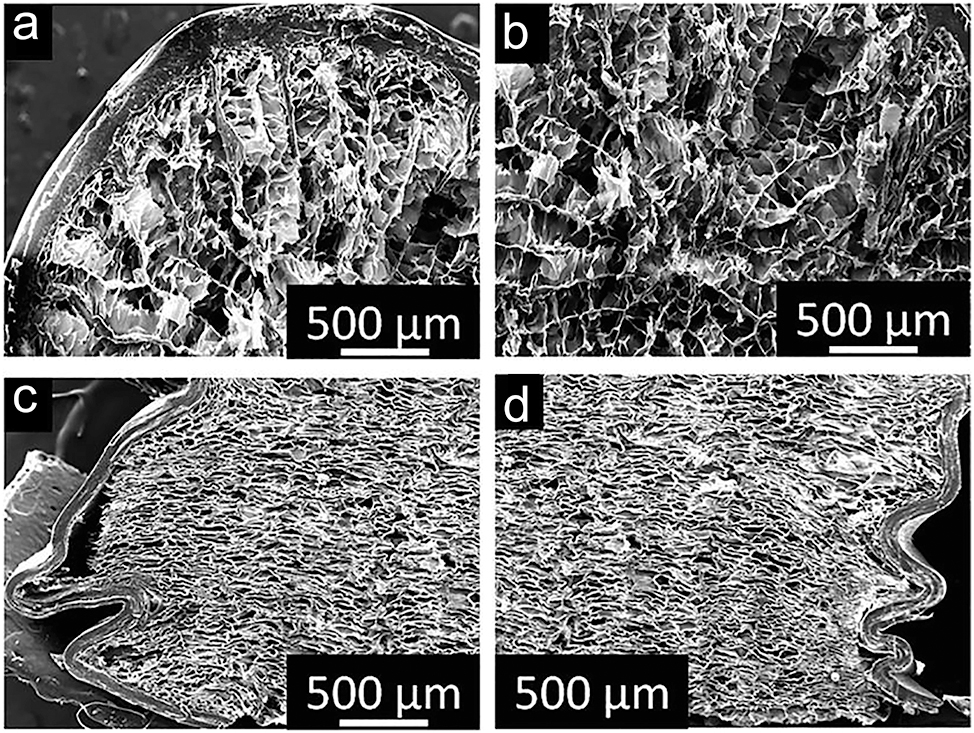
Cross-sectional micrographs of a porcupine quill: (a, b) SEM images showing the horizontal cross-section of the quill prior to compression, highlighting its structural integrity. (c, d) SEM images of the vertical cross-section post-compression, revealing deformation patterns and mechanical response. Source: 360 licensed under CC BY.
Polar bear fur, by contrast, achieves insulation through its unique dual-layered hair system. 353 The hollow guard hairs absorb ultraviolet light and channel it to the bear’s black skin, where it generates heat. 354 These hollow hairs and their air-filled medulla minimise heat loss while providing mechanical resilience. The overlapping keratinocyte-derived scales comprise the rough surface of the hair. 355 , 356 Pores in these scales connect to the cortex, tightly packed with keratin fibre bundles. These fibres, composed of coiled-coil α-keratin, are aligned along the hair shaft. Between the fibres, tubular pores prevent crack propagation, ensuring flexibility, mechanical strength, and durability. 357
The medulla’s architecture distinguishes between penguin feathers and polar bear hair. Penguin feathers exhibit foam-like porosity, whereas polar bear guard hairs contain hollow vacuoles. 356 Despite these differences, both systems demonstrate that hierarchical structures and porosity are fundamental to thermal insulation. Penguin feathers consist of β-keratin sheets, while polar bear hair comprises α-keratin. Both materials feature wrinkled outer surfaces, porosity within and on the main shafts, aligned keratin bundles in the cortex, and tubular porosity between these bundles. 356 , 358 , 359
7.5 Lightweight structures
Sandwich structures are widely utilized in engineering applications due to their unique combination of ultra-lightweight composition, efficient energy absorption, and mechanical strength comparable to bulk materials. 361 These structures achieve tailored performance by modifying the material properties and geometry of their face layers (outer cortex) and core (foam-like interior). 362 , 363 Typically, sandwich structures incorporate a high Young’s modulus material in the face layers for stiffness and a low Young’s modulus core for weight reduction, resulting in a lightweight and structurally robust material. 364 , 365 Such structures often exhibit rectangular or cylindrical cross-sections optimized for specific mechanical needs. 361
Keratin-based systems such as beaks, quills, feathers, spines, and baleen are biological analogues of engineered sandwich structures. 366 , 367 , 368 These natural designs often utilize the same material in the face and core but in different physical forms: a dense, compact face layer and a porous, foam-like core. For instance, porcupine quills, primarily composed of alpha-keratin, exemplify this arrangement 367 (Figure 6). These quills possess a thin-walled cylindrical cortex surrounding a closed-cell foam core, providing resilience against compressive and flexural loads. 367 Experiments reveal that the foam core stabilizes the structure under load, mitigating severe failures like buckling in hollow or foam-free quills. 360 The outer shell buckles into a wavy pattern upon compression, but the foam core maintains integrity and energy absorption. 366
Similarly, hedgehog spines exhibit comparable sandwich structures reinforced with longitudinal stringers and transverse plates. 369 While structurally similar to porcupine quills, hedgehog spines differ in function, serving primarily as shock absorbers during falls. In contrast, porcupine quills act as a defensive mechanism, detaching from the body and inflicting injury upon predators. These natural designs demonstrate the potential of lightweight, resilient materials in bioinspired engineering.
The combination of dense outer layers and porous cores in keratin-based systems serves as a model for designing advanced materials. By integrating foam within a rigid outer layer, these natural structures achieve remarkable mechanical properties such as impact resistance and energy dissipation, all while maintaining a low mass. 8 , 109 This bioinspired approach has significant implications for developing efficient, lightweight materials for modern engineering applications.
7.6 Reversible adhesion
Flight feathers have an additional feature. Their barbules overlap those of neighboring filaments and hook them onto one another so that they are united into a continuous vane. There are several hundred such hooks on a single barbule, a million or so in a single feather, and a bird the size of a swan has about twenty-five thousand feathers.
(David Attenborough, 370 )
Reversible adhesion refers to the ability to adhere and detach from surfaces repeatedly without causing damage to the substrate or the adhesive organ itself. 372 This capability, widely observed in Nature, can be categorized into various mechanisms, including dry adhesion (e.g., van der Waals forces), wet adhesion (e.g., capillary forces), mechanical interlocking friction, chemical bonding, and suction resulting from pressure differences. 373 , 374 The specific mechanism employed by an organism often depends on its environmental conditions and the functional requirements of its adhesive organ. Various natural systems leverage hierarchical and nanostructured surfaces, such as those seen in keratin-based materials, to optimize adhesion properties. 8 , 375 , 376
Feather vanes provide an illustrative example of mechanical interlocking friction. Bird feathers exhibit interlocking mechanisms comprising tiny hooks (hamuli) on the barbules, which latch onto adjacent barbs to form a cohesive vane. 350 , 377 This structure is essential for maintaining the feather’s aerodynamic and mechanical integrity during flight. 350 , 378 , 379 The directional permeability of feather vanes facilitates efficient air capture, enhancing lift. The branching barbs and attached barbule network, supported by the rachis, form a lattice structure. 379 The hamuli ensure a secure connection, while these components’ geometric arrangement and rigidity contribute to efficient energy transfer and flexibility. 380 , 381 This interlocking system allows birds to “zip” and “unzip” their feathers during preening, restoring functionality and ensuring durability under mechanical stress. 382
The gecko’s adhesive system exemplifies dry reversible adhesion. Gecko feet are equipped with adhesive pads composed of microscopic and nanoscale setae arrays, which branch hierarchically into spatula-shaped tips. 383 , 384 This configuration maximizes the contact area with the surface, enabling strong adhesion. 376 The hierarchical system consists of lamellae (soft ridges), densely packed setae (composed of beta-keratin), and spatulae at the nanoscale (Figure 7). The spatulae conform to surface irregularities, allowing geckos to generate adhesion forces primarily through van der Waals interactions. 385 These weak intermolecular forces provide a non-destructive yet strong attachment, which can detach instantly via a peeling motion. 386 Geckos achieve detachment by altering the angle of setae relative to the surface, allowing rapid movement without residue or wear. 384 , 387
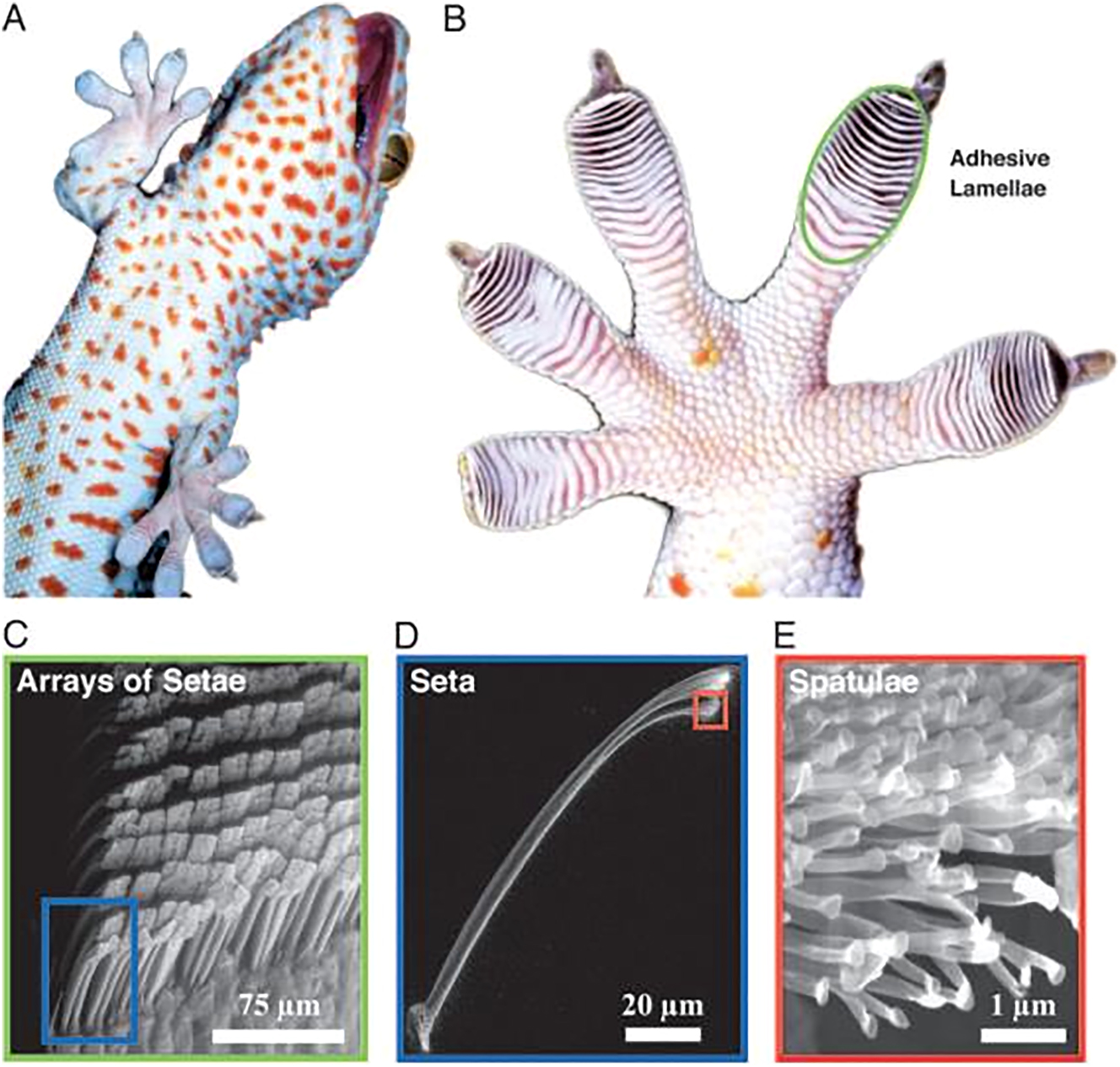
Hierarchical structure of the gecko adhesive system. (A) Ventral view of the tokay gecko climbing vertical glass. (B) Ventral view of the foot, with adhesive lamellae visible as overlapping pads. (C) Single lamella, with arrays of setae visible. (D) Single seta with branched structure. (E) Hundreds of spatulae tips reaching out of the seta. 371 Source: 371 permission granted. Copyright (2005) National Academy of Sciences, U.S.A.
Van der Waals forces (Eq. (1)) arise due to interactions between molecular dipoles and are influenced by the separation distance (D) and the Hamaker constant (A). 388 Despite being weak, these forces operate universally across all materials, ranging from approximately 0.3 to 50 nm. 388 , 389 They are central in gecko adhesion, mainly when dry conditions dominate surface interactions. 376
The capillary adhesion model suggests liquid-mediated contact could enhance gecko adhesion under certain conditions. 390 , 391 Capillary forces result from liquid condensation in the contact area, forming menisci that bridge the surfaces. 392 These forces arise from Laplace pressure (difference across the curved liquid interface) and surface tension Orr, Scriven, and Rivas, 393 Bhushan. 394 Relative humidity influences the adhesion strength, as increased humidity promotes capillary condensation. 386 Experimental studies and computational models have shown that adhesion decreases with increasing contact angles, supporting the hypothesis that capillary forces complement van der Waals interactions in humid environments. 386 , 395
Nature’s reversible adhesive systems inspire the development of advanced materials. The hierarchical architecture, interlocking mechanisms, and the interplay of multiple adhesion forces highlight the efficiency of natural designs.
8 Summary, conclusions and outlook
This work showcases current research while guiding and inspiring the chemical research community to explore the combined functional potential of biomimetic hierarchical (nano)structures in chitin, chitosan, and keratin. Emphasizing these structured biopolymers’ strength, durability, and sustainability possibilities highlights their relevance for high-value applications across diverse technological domains.
By harnessing the natural design principles of these biopolymers, research can create innovative, sustainable materials that address modern engineering challenges while ensuring environmental compatibility. This strategy avoids the toxicity, biodegradability, and waste issues often associated with synthetic materials, offering significant advantages in industries such as medicine, (bio)chemistry, energy-efficient construction, advanced textiles, pharmaceuticals, packaging, cooling technologies (e.g., facades, computer parts, clothing), and colourants.
Valorizing waste materials rich in chitin and keratin – such as shrimp shells and poultry feathers – addresses critical sustainability challenges by employing waste into high-value, biodegradable alternatives to synthetic polymers. These bioinspired materials reduce environmental impact and provide solutions in areas like passive cooling technologies, self-cleaning surfaces, and advanced drug delivery systems.
Future research should focus on elucidating hierarchical design principles to engineer advanced biomimetic materials with customized properties, fully leveraging the potential of chitin and keratin. Developing cost-effective, scalable, and sustainable strategies for recycling keratin- and chitin-rich waste is critical for ensuring these applications’ economic feasibility and environmental compatibility. Advancements in biotechnology can further augment the functional versatility of these biopolymers, enabling broader utilization across diverse sectors. Promoting interdisciplinary collaboration among chemistry, biomimetics, materials science, nanotechnology, and bioengineering will catalyze innovation and unlock new opportunities for these versatile, sustainable materials.
While challenges such as processing scalability and competition with established synthetic materials remain, integrating chitin- and keratin-based materials into industrial applications aligns with global sustainability frameworks. It supports the development of a circular economy. Continued investment in research and innovation will solidify their role as eco-friendly, high-performance materials, exemplifying the convergence of biology and technology to address pressing global challenges.
Funding source: FFG
Award Identifier / Grant number: 53090840
Funding source: TU Wien Bibliothek
Award Identifier / Grant number: Open Access Funding Programme
Acknowledgment
The authors thank generations of their students who have performed specialist studies on cicadas, butterflies, and Silver Ants regarding their nanostructure-based functionalities. Thank you, dear Alexander Bürger, Viola Kaser, Florian Zischka, Markus Moser and Markus Zimmerl for your fabulous work!
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: AI tools were used to improve English sentence clarity, grammar, and formulation.
-
Conflict of interest: The author states no conflict of interest.
-
Research funding: The authors acknowledge TU Wien Bibliothek for financial support through its Open Access Funding Programme. FFG Grant No. 53090840 to I.C. Gebeshuber.
-
Data availability: Not applicable.
References
1. Durkin, C. A.; Mock, T.; Armbrust, E. V. Chitin in Diatoms and its Association with the Cell Wall. Eukaryotic Cell 2009, 8 (7), 1038–1050. https://doi.org/10.1128/ec.00079-09.Search in Google Scholar PubMed PubMed Central
2. Roelofsen, P. A.; Hoette, I. Chitin in the Cell Wall of Yeasts. Antonie Leeuwenhoek 1951, 17 (1), 297–313. https://doi.org/10.1007/bf02062277.Search in Google Scholar
3. Brown, H. E.; Esher, S. K.; Alspaugh, J. A. Chitin: A “Hidden Figure” in the “Fungal Cell Wall”. In The Fungal Cell Wall; Springer International Publishing: Cham, Switzerland, 2019; pp 83–111.10.1007/82_2019_184Search in Google Scholar PubMed
4. Moussian, B. Chitin: Structure, Chemistry and Biology. In Targeting Chitin-Containing Organisms; Springer: Singapore, 2019; pp. 5–18.10.1007/978-981-13-7318-3_2Search in Google Scholar PubMed
5. Machałowski, T.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Bechmann, N.; Binnewerg, B.; Schubert, M.; Guan, K.; Bornstein, S. R.; Czaczyk, K.; Pokrovsky, O.; Kraft, M.; Bertau, M.; Schimpf, C.; Rafaja, D.; Tsurkan, M.; Galli, R.; Meissner, H.; Petrenko, I.; Fursov, A.; Voronkina, A.; Figlerowicz, M.; Joseph, Y.; Jesionowski, T.; Ehrlich, H. Spider Chitin. The Biomimetic Potential and Applications of Caribena Versicolor Tubular Chitin. Carbohydr. Polym. 2019, 226, 115301. https://doi.org/10.1016/j.carbpol.2019.115301.Search in Google Scholar PubMed
6. Kurita, K. Chitin and Chitosan: Functional Biopolymers from Marine Crustaceans. Mar. Biotechnol. 2006, 8 (3), 203–226. https://doi.org/10.1007/s10126-005-0097-5.Search in Google Scholar PubMed
7. Zakrzewski, A.-C.; Weigert, A.; Helm, C.; Adamski, M.; Adamska, M.; Bleidorn, C.; Raible, F.; Hausen, H. Early Divergence, Broad Distribution, and High Diversity of Animal Chitin Synthases. Genome Biol. Evol. 2014, 6 (2), 316–325. https://doi.org/10.1093/gbe/evu011.Search in Google Scholar PubMed PubMed Central
8. Lazarus, B. S.; Chadha, C.; Velasco-Hogan, A.; Barbosa, J. D.; Jasiuk, I.; Meyers, M. A. Engineering with Keratin: A Functional Material and a Source of Bioinspiration. iScience 2021, 24 (8), 102798. https://doi.org/10.1016/j.isci.2021.102798.Search in Google Scholar PubMed PubMed Central
9. Bragulla, H. H.; Homberger, D. G. Structure and Functions of Keratin Proteins in Simple, Stratified, Keratinized and Cornified Epithelia. J. Anat. 2009, 214 (4), 516–559. https://doi.org/10.1111/j.1469-7580.2009.01066.x.Search in Google Scholar PubMed PubMed Central
10. Ward, W. H.; Lundgren, H. P. The Formation, Composition, and Properties of the Keratins. Adv. Protein Chem. 1954, 243–297. https://doi.org/10.1016/s0065-3233(08)60208-9.Search in Google Scholar PubMed
11. Muzzarelli, R. A. A. Chitin Nanostructures in Living Organisms. In Chitin; Springer: Netherlands, 2010; pp. 1–34.Search in Google Scholar
12. van Nieuwenhoven, R. W.; Drack, M.; Gebeshuber, I. C. Engineered Materials: Bioinspired “Good Enough” versus Maximized Performance. Adv. Funct. Mater. 2023, 34 (35). https://doi.org/10.1002/adfm.202307127.Search in Google Scholar
13. Gillespie, J. M. The Proteins of Hair and Other Hard α-Keratins. In Cellular and Molecular Biology of Intermediate Filaments; Springer US: New York, USA, 1990; pp 95–128.10.1007/978-1-4757-9604-9_4Search in Google Scholar
14. Parbhu, A. N.; Bryson, W. G.; Lal, R. Disulfide Bonds in the Outer Layer of Keratin Fibers Confer Higher Mechanical Rigidity: Correlative Nano-Indentation and Elasticity Measurement with an AFM. Biochemistry 1999, 38 (36), 11755–11761. https://doi.org/10.1021/bi990746d.Search in Google Scholar PubMed
15. Harland, D. P.; Popescu, C.; Richena, M.; Deb-Choudhury, S.; Wichlatz, C.; Lee, E.; Plowman, J. E. The Susceptibility of Disulfide Bonds to Modification in Keratin Fibers Undergoing Tensile Stress. Biophys. J. 2022, 121 (11), 2168–2179. https://doi.org/10.1016/j.bpj.2022.04.029.Search in Google Scholar PubMed PubMed Central
16. Dweltz, N. E. The Structure of Chitin. Biochim. Biophys. Acta 1960, 44, 416–435. https://doi.org/10.1016/0006-3002(60)91597-3.Search in Google Scholar PubMed
17. Grunenfelder, L. K.; Herrera, S.; Kisailus, D. Crustacean-Derived Biomimetic Components and Nanostructured Composites. Small 2014, 10 (16), 3207–3232. https://doi.org/10.1002/smll.201400559.Search in Google Scholar PubMed
18. Bai, L.; Liu, L.; Esquivel, M.; Tardy, B. L.; Huan, S.; Niu, X.; Liu, S.; Yang, G.; Fan, Y.; Rojas, O. J. Nanochitin: Chemistry, Structure, Assembly, and Applications. Chem. Rev. 2022, 122 (13), 11604–11674. https://doi.org/10.1021/acs.chemrev.2c00125.Search in Google Scholar PubMed PubMed Central
19. Kinoshita, S. Structural Colors in the Realm of Nature; World Scientific: Singapore, 2008.10.1142/9789812709752Search in Google Scholar
20. Kumar, N. S.; Padma Suvarna, R.; Chandra Babu Naidu, K.; Banerjee, P.; Ratnamala, A.; Manjunatha, H. A Review on Biological and Biomimetic Materials and Their Applications. Appl. Phys. A 2020, 126 (6). https://doi.org/10.1007/s00339-020-03633-z.Search in Google Scholar
21. Aththanayaka, S.; Thiripuranathar, G.; Ekanayake, S. Emerging Advances in Biomimetic Synthesis of Nanocomposites and Potential Applications. Mater. Today Sustain. 2022, 20, 100206. https://doi.org/10.1016/j.mtsust.2022.100206.Search in Google Scholar
22. Piszter, G.; Kertész, K.; Nagy, G.; Kovács, D.; Zámbó, D.; Baji, Z.; Pap, J. S.; Biró, L. P. Photocatalytic Bioheteronanostructures by Integrating Multicomponent Cu2O–Au Nanoparticles into ZnO-Coated Butterfly Wings Colored by Photonic Nanoarchitectures. J. Mater. Sci. 2024, 59 (38), 17877–17896. https://doi.org/10.1007/s10853-024-09764-5.Search in Google Scholar
23. Chatterjee, A. At the Intersection of Natural Structural Coloration and Bioengineering. Biomimetics 2022, 7 (2), 66. https://doi.org/10.3390/biomimetics7020066.Search in Google Scholar PubMed PubMed Central
24. Biswal, T. Future Perspectives of Biopolymeric Industry. Phys. Sci. Rev. 2023, 9 (9), 2965–2988. https://doi.org/10.1515/psr-2022-0192.Search in Google Scholar
25. Ur Rehman, N.; Ullah, K. S.; Sajid, M.; Ihsanullah, I.; Waheed, A. Preparation of Sustainable Composite Materials from Bio-Based Domestic and Industrial Waste: Progress, Problems, and Prospects-A Review. Adv. Sustain. Syst. 2024, 8 (8). https://doi.org/10.1002/adsu.202300587.Search in Google Scholar
26. Nachtigall, W. Zehn Grundprinzipien natürlicher Konstruktionen – “10 Gebote bionischen Designs”. In Vorbild Natur: Bionik-Design für funktionelles Gestalten; Springer Berlin Heidelberg: Berlin, Heidelberg, 1997; pp. 21–34.10.1007/978-3-642-60866-7_4Search in Google Scholar
27. Shubha, A.; Sharmita, G.; Anita, L. Production and Characterization of Human Hair Keratin Bioplastic Films with Novel Plasticizers. Sci. Rep. 2024, 14 (1). https://doi.org/10.1038/s41598-023-44905-x.Search in Google Scholar PubMed PubMed Central
28. Sharma, S.; Gupta, A.; Kumar, A. Keratin: An Introduction. In Keratin as a Protein Biopolymer; Springer International Publishing: Cham, Switzerland, 2018; pp 1–18.10.1007/978-3-030-02901-2_1Search in Google Scholar
29. Wyld, J. A.; Brush, A. H. Keratin Diversity in the Reptilian Epidermis. J. Exp. Zool. 1983, 225 (3), 387–396. https://doi.org/10.1002/jez.1402250306.Search in Google Scholar
30. Zhao, X.; Zhang, J.; Zhu, K. Y. Chito-Protein Matrices in Arthropod Exoskeletons and Peritrophic Matrices. In Extracellular Sugar-Based Biopolymers Matrices; Springer International Publishing: Cham, Switzerland, 2019; pp 3–56.10.1007/978-3-030-12919-4_1Search in Google Scholar
31. Iber, B. T.; Azman Kasan, N.; Torsabo, D.; Wese Omuwa, J. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew. Mater. 2022, 10 (4), 1097–1123. https://doi.org/10.32604/jrm.2022.018142.Search in Google Scholar
32. Gooday, G. W. Cell Walls. In The Growing Fungus; Springer: Netherlands, 1995; pp. 43–62.10.1007/978-0-585-27576-5_3Search in Google Scholar
33. Hamed, I.; Özogul, F.; Regenstein, J. M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. https://doi.org/10.1016/j.tifs.2015.11.007.Search in Google Scholar
34. Youn, D. K.; No, H. K.; Prinyawiwatkul, W. Preparation and Characteristics of Squid Pen β-chitin Prepared under Optimal Deproteinisation and Demineralisation Condition. Int. J. Food Sci. Technol. 2012, 48 (3), 571–577. https://doi.org/10.1111/ijfs.12001.Search in Google Scholar
35. Jung, H.-S.; Kim, M. H.; Park, W. H. Preparation and Structural Investigation of Novel β-Chitin Nanocrystals from Cuttlefish Bone. ACS Biomater. Sci. Eng. 2019, 5 (4), 1744–1752. https://doi.org/10.1021/acsbiomaterials.8b01652.Search in Google Scholar PubMed
36. Hahn, T.; Roth, A.; Ji, R.; Schmitt, E.; Zibek, S. Chitosan Production with Larval Exoskeletons Derived from the Insect Protein Production. J. Biotechnol. 2020, 310, 62–67. https://doi.org/10.1016/j.jbiotec.2019.12.015.Search in Google Scholar PubMed
37. Kaur, S.; Dhillon, G. S. Recent Trends in Biological Extraction of Chitin from Marine Shell Wastes: A Review. Crit. Rev. Biotechnol. 2013, 35 (1), 44–61. https://doi.org/10.3109/07388551.2013.798256.Search in Google Scholar PubMed
38. Ngasotter, S.; Sampath, L.; Xavier, K. A. M. Nanochitin: An Update Review on Advances in Preparation Methods and Food Applications. Carbohydr. Polym. 2022, 291, 119627. https://doi.org/10.1016/j.carbpol.2022.119627.Search in Google Scholar PubMed
39. Synowiecki, J.; Ali Al-Khateeb, N. Production, Properties, and Some New Applications of Chitin and its Derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43 (2), 145–171. https://doi.org/10.1080/10408690390826473.Search in Google Scholar PubMed
40. Kumawat, T. K.; Sharma, A.; Sharma, V.; Chandra, S. Keratin Waste: The Biodegradable Polymers. In Keratin; IntechOpen: London, UK, 2018.Search in Google Scholar
41. Zhou, Y.; He, Y.; Lin, X.; Feng, Y.; Liu, M. Sustainable, High-Performance, and Biodegradable Plastics Made from Chitin. ACS Appl. Mater. Interfaces 2022, 14 (41), 46980–46993. https://doi.org/10.1021/acsami.2c12764.Search in Google Scholar PubMed
42. Ling, S., Ed. Fibrous Proteins: Design, Synthesis, and Assembly; Humana Press: New York, USA, 2021.10.1007/978-1-0716-1574-4Search in Google Scholar
43. Jacob, J. T.; Coulombe, P. A.; Kwan, R.; Omary, M. B. Types I and II Keratin Intermediate Filaments. Cold Spring Harbor Perspect. Biol. 2018, 10 (4), a018275. https://doi.org/10.1101/cshperspect.a018275.Search in Google Scholar PubMed PubMed Central
44. Feroz, S.; Muhammad, N.; Ratnayake, J.; Dias, G. Keratin – Based Materials for Biomedical Applications. Bioact. Mater. 2020, 5 (3), 496–509. https://doi.org/10.1016/j.bioactmat.2020.04.007.Search in Google Scholar PubMed PubMed Central
45. Naleway, S. E.; Taylor, J. R.; Porter, M. M.; Meyers, M. A.; McKittrick, J. Structure and Mechanical Properties of Selected Protective Systems in Marine Organisms. Mater. Sci. Eng. C 2016, 59, 1143–1167. https://doi.org/10.1016/j.msec.2015.10.033.Search in Google Scholar PubMed
46. Kunert, J. Physiology of Keratinophilic Fungi. Rev. Iberoam. De. Micol. 2000, 1, 77–85.Search in Google Scholar
47. Sharma, R.; Rajak, R. C. Keratinophilic Fungi: Nature’s Keratin Degrading Machines!: Their Isolation, Identification and Ecological Role. Resonance 2003, 8 (9), 28–40. https://doi.org/10.1007/bf02837919.Search in Google Scholar
48. Brandelli, A. Bacterial Keratinases: Useful Enzymes for Bioprocessing Agroindustrial Wastes and beyond. Food Bioprocess Technol. 2007, 1 (2), 105–116. https://doi.org/10.1007/s11947-007-0025-y.Search in Google Scholar
49. Yan, R.-R.; Gong, J. S.; Su, C.; Liu, Y. L.; Qian, J. Y.; Xu, Z. H.; Shi, J. S. Preparation and Applications of Keratin Biomaterials from Natural Keratin Wastes. Appl. Microbiol. Biotechnol. 2022, 106 (7), 2349–2366. https://doi.org/10.1007/s00253-022-11882-6.Search in Google Scholar PubMed
50. Santos, M. M. F.; Grisi, C. V. B.; Lima, D. A. S.; Florentino, G. I. B.; Ferreira, V. C. D. S.; Madruga, M. S.; Silva, F. A. P. D. Biodegradable and Active Film Based on Keratin and Protein Hydrolysate of Free-Range Chicken Feathers: Structural and Antioxidant Properties. Food Biosci. 2024, 62, 105048. https://doi.org/10.1016/j.fbio.2024.105048.Search in Google Scholar
51. Sharma, S.; Gupta, A. Sustainable Management of Keratin Waste Biomass: Applications and Future Perspectives. Braz. Arch. Biol. Technol. 2016, 59. https://doi.org/10.1590/1678-4324-2016150684.Search in Google Scholar
52. Kasulla, S.; Malik, S. J. Enzymatic Pre-Treatment of Chicken Feather Waste to Generate Biogas. Int. J. Trend Sci. Res. Dev. 2021, 5 (5), 510–514; https://doi.org/10.5281/ZENODO.5094514.Search in Google Scholar
53. Patil, R.; Wagh, C.; Kavathekar, B. Microbial Degradation of Keratin Waste: A Review. In National Conference on Environment Pollution Control and Management (EPCM-2019); College of Engineering: Pune, 2019.Search in Google Scholar
54. Muduli, S.; Champatin, A.; Popalghat, H. K.; Patel, P.; Sneha, K. Poultry Waste Management: An Approach for Sustainable Development. Int. J. Adv. Sci. Res. 2019, 4 (1), 8–14.Search in Google Scholar
55. Senthilkumar, N.; Chowdhury, S.; Sanpui, P. Extraction of Keratin from Keratinous Wastes: Current Status and Future Directions. J. Mater. Cycles Waste Manag. 2022, 25 (1), 1–16. https://doi.org/10.1007/s10163-022-01492-9.Search in Google Scholar
56. Wang, Y.-X.; Cao, X.-J. Extracting Keratin from Chicken Feathers by Using a Hydrophobic Ionic Liquid. Process Biochem. 2012, 47 (5), 896–899. https://doi.org/10.1016/j.procbio.2012.02.013.Search in Google Scholar
57. Khumalo, M.; Sithole, B.; Tesfaye, T. Valorisation of Waste Chicken Feathers: Optimisation of Keratin Extraction from Waste Chicken Feathers by Sodium Bisulphite, Sodium Dodecyl Sulphate and Urea. J. Environ. Manage. 2020, 262, 110329. https://doi.org/10.1016/j.jenvman.2020.110329.Search in Google Scholar PubMed
58. Rajabinejad, H.; Buciscanu, I.-I.; Maier, S. S. Current Approaches for Raw Wool Waste Management and Unconventional Valorization: A Review. Environ. Eng. Manag. J. 2019, 18 (7), 1439–1456. https://doi.org/10.30638/eemj.2019.136.Search in Google Scholar
59. Allafi, F.; Hossain, M. S.; Lalung, J.; Shaah, M.; Salehabadi, A.; Ahmad, M. I.; Shadi, A. Advancements in Applications of Natural Wool Fiber: Review. J. Nat. Fibers 2020, 19 (2), 497–512. https://doi.org/10.1080/15440478.2020.1745128.Search in Google Scholar
60. Cardamone, J. M.; Nuñez, A.; Garcia, R. A.; Aldema-Ramos, M. Characterizing Wool Keratin. Adv. Mater. Sci. Eng. 2009, 2009 (1), 147175; https://doi.org/10.1155/2009/147175.Search in Google Scholar
61. Ozek, H. Z. Wool Production Steps and Global Trade with Recent Statistics. In The Wool Handbook; Woodhead Publishing: Cambridge, UK, 2024; pp 25–74.10.1016/B978-0-323-99598-6.00024-4Search in Google Scholar
62. Textile Exchange. Preferred Fiber & Materials Market Report 2021, 2021. https://textileexchange.org/ (accessed 2024-11-26).Search in Google Scholar
63. Pattinson, R.; Wilcox, C.; Williams, S.; Curtis, K. The Wool Supply Chain and Selling System in New South Wales (Supporting Paper: 3). NSW Wool Industries and Future Opportunities Report; NSW Department of Primary Industries, 2015.Search in Google Scholar
64. Henry, B. Understanding the Environmental Impacts of Wool: A Review of Life Cycle Assessment Studies; International Wool Textile Organisation: Brussels, 2012.Search in Google Scholar
65. Kulkarni, M. B.; Gavande, V.; Mahanwar, P. A.; Shah, A. R.; Shuib, R. K.; Khare, A.; Radhakrishnan, S. Review on Biomass Sheep Wool–Based Polymer Composites. Biomass Convers. Biorefin. 2023, 14, 30961–30982; https://doi.org/10.1007/s13399-023-04912-4.Search in Google Scholar
66. Augère-Granier, M.-L. The EU Pig Meat Sector, 2020. https://www.europarl.europa.eu/RegData/etudes/BRIE/2020/652044/EPRS_BRI(2020)652044_EN.pdf (accessed 2024-11-6).Search in Google Scholar
67. Brown, E. M.; Pandya, K.; Taylor, M. M.; Liu, C. K. Comparison of Methods for Extraction of Keratin from Waste Wool. Agric. Sci. 2016, 7 (10), 670–679. https://doi.org/10.4236/as.2016.710063.Search in Google Scholar
68. Alahyaribeik, S.; Ullah, A. Methods of Keratin Extraction from Poultry Feathers and Their Effects on Antioxidant Activity of Extracted Keratin. Int. J. Biol. Macromol. 2020, 148, 449–456. https://doi.org/10.1016/j.ijbiomac.2020.01.144.Search in Google Scholar PubMed
69. Hussain, F. S.; Memon, N. Recent Developments in Extraction of Keratin from Industrial Wastes. In Extraction of Natural Products from Agro-Industrial Wastes; Elsevier: Amsterdam, Netherlands, 2023; pp 281–302.10.1016/B978-0-12-823349-8.00010-1Search in Google Scholar
70. Staroń, P.; Banach, M.; Kowalski, Z.; Staroń, A. Hydrolysis of Keratin Materials Derived from Poultry Industry. Proc. ECOpole 2014, 8 (2), 443–448.Search in Google Scholar
71. Bhavsar, P. S.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Mossotti, R.; Giansetti, M.; Rovero, G.; Maier, S. S.; Muresan, A.; Tonin, C. Superheated Water Hydrolyzed Keratin: A New Application as a Foaming Agent in Foam Dyeing of Cotton and Wool Fabrics. ACS Sustain. Chem. Eng. 2017, 5 (10), 9150–9159. https://doi.org/10.1021/acssuschemeng.7b02064.Search in Google Scholar
72. Chilakamarry, C. R.; Mahmood, S.; Saffe, S. N. B. M.; Arifin, M. A. B.; Gupta, A.; Sikkandar, M. Y.; Begum, S. S.; Narasaiah, B. Extraction and Application of Keratin from Natural Resources: A Review. 3 Biotech 2021, 11 (5). https://doi.org/10.1007/s13205-021-02734-7.Search in Google Scholar PubMed PubMed Central
73. Singh, J.; Laurenti, R.; Sinha, R.; Frostell, B. Progress and Challenges to the Global Waste Management System. Waste Manag. Res. J. Sustain. Circular Econ. 2014, 32 (9), 800–812. https://doi.org/10.1177/0734242x14537868.Search in Google Scholar
74. Morone, P.; Koutinas, A.; Gathergood, N.; Arshadi, M.; Matharu, A. Food Waste: Challenges and Opportunities for Enhancing the Emerging Bio-Economy. J. Clean. Prod. 2019, 221, 10–16. https://doi.org/10.1016/j.jclepro.2019.02.258.Search in Google Scholar
75. Yan, N.; Chen, X. Sustainability: Don’t Waste Seafood Waste. Nature 2015, 524 (7564), 155–157. https://doi.org/10.1038/524155a.Search in Google Scholar PubMed
76. Chandrasekharan, M. Biotechnology for Utilization of Marine Byproducts. In Fish Processing Byproducts; Sachindra, N. M.; Studium Press: Houston, TX, 2015; pp. 43–62.Search in Google Scholar
77. Philibert, T.; Lee, B. H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2016, 181 (4), 1314–1337. https://doi.org/10.1007/s12010-016-2286-2.Search in Google Scholar PubMed
78. Lal, J.; Deb, S.; Singh, S. K.; Biswas, P.; Debbarma, R.; Yadav, N. K.; Debbarma, S.; Vaishnav, A.; Meena, D. K.; Waikhom, G.; Patel, A. B. Diverse Uses of Valuable Seafood Processing Industry Waste for Sustainability: A Review. Environ. Sci. Pollut. Res. 2023, 31 (53), 62249–62263; https://doi.org/10.1007/s11356-023-28890-2.Search in Google Scholar PubMed
79. Islam, M. S.; Khan, S.; Tanaka, M. Waste Loading in Shrimp and Fish Processing Effluents: Potential Source of Hazards to the Coastal and Nearshore Environments. Mar. Pollut. Bull. 2004, 49 (1–2), 103–110. https://doi.org/10.1016/j.marpolbul.2004.01.018.Search in Google Scholar PubMed
80. Hendrawan, D. I.; Rinanti, A.; Fachrul, M. F.; Tazkiaturrizki; Minarti, A.; Marendra, S. M. P.; Zahra, L. A. Addressing Algal Bloom and Other Ecological Issues Caused by Microalgae Biomass Conversion Technology. In Algae as a Natural Solution for Challenges in Water-Food-Energy Nexus; Springer Nature: Singapore, 2024; pp 373–431.10.1007/978-981-97-2371-3_15Search in Google Scholar
81. Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood Waste: A Source for Preparation of Commercially Employable Chitin/Chitosan Materials. Bioresour. Bioprocess. 2019, 6 (1). https://doi.org/10.1186/s40643-019-0243-y.Search in Google Scholar
82. Sheth, Y.; Dharaskar, S.; Khalid, M.; Sonawane, S. An Environment Friendly Approach for Heavy Metal Removal from Industrial Wastewater Using Chitosan Based Biosorbent: A Review. Sustain. Energy Technol. Assessments 2021, 43, 100951. https://doi.org/10.1016/j.seta.2020.100951.Search in Google Scholar
83. Wan Ngah, W. S.; Teong, L. C.; Hanafiah, M. A. K. M. Adsorption of Dyes and Heavy Metal Ions by Chitosan Composites: A Review. Carbohydr. Polym. 2011, 83 (4), 1446–1456. https://doi.org/10.1016/j.carbpol.2010.11.004.Search in Google Scholar
84. Elsabee, M. Z. Chitosan – Based Edible Films. In Polysaccharides; Macmillan: Houndmills, UK, 2014; pp 1–37.10.1007/978-3-319-03751-6_7-1Search in Google Scholar
85. Barik, M.; BhagyaRaj, G.; Dash, K. K.; Shams, R. A Thorough Evaluation of Chitosan-Based Packaging Film and Coating for Food Product Shelf-Life Extension. J. Agric. Food Res. 2024, 16, 101164. https://doi.org/10.1016/j.jafr.2024.101164.Search in Google Scholar
86. Hours, R. A.; Gortari, M. C. Biotechnological Processes for Chitin Recovery Out of Crustacean Waste: A Mini-Review. Electron. J. Biotechnol. 2013, 16 (3). https://doi.org/10.2225/vol16-issue3-fulltext-10.Search in Google Scholar
87. Ngasotter, S.; Xavier, K.; Meitei, M. M.; Waikhom, D.; Madhulika; Pathak, J.; Singh, S. K. Crustacean Shell Waste Derived Chitin and Chitin Nanomaterials for Application in Agriculture, Food, and Health – A Review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100349. https://doi.org/10.1016/j.carpta.2023.100349.Search in Google Scholar
88. Zhang, H.; Yun, S.; Song, L.; Zhang, Y.; Zhao, Y. The Preparation and Characterization of Chitin and Chitosan under Large-Scale Submerged Fermentation Level Using Shrimp By-Products as Substrate. Int. J. Biol. Macromol. 2017, 96, 334–339. https://doi.org/10.1016/j.ijbiomac.2016.12.017.Search in Google Scholar PubMed
89. Paul, T.; Halder, S. K.; Das, A.; Ghosh, K.; Mandal, A.; Payra, P.; Barman, P.; Das Mohapatra, P. K.; Pati, B. R.; Mondal, K. C. Production of Chitin and Bioactive Materials from Black Tiger Shrimp (Penaeus monodon) Shell Waste by the Treatment of Bacterial Protease Cocktail. 3 Biotech 2014, 5 (4), 483–493. https://doi.org/10.1007/s13205-014-0245-6.Search in Google Scholar PubMed PubMed Central
90. Jory, D. Annual Farmed Shrimp Production Survey: A Slight Decrease in Production Reduction in 2023 with Hopes for Renewed Growth in 2024; Global Seafood Alliance: Portsmouth, NH, USA, 2023.Search in Google Scholar
91. IMARC. Shrimp Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2022–2027. In Glob. Shrimp Mark. Res. Rep, 2022.Search in Google Scholar
92. Rossi, N.; Grosso, C.; Delerue-Matos, C. Shrimp Waste Upcycling: Unveiling the Potential of Polysaccharides, Proteins, Carotenoids, and Fatty Acids with Emphasis on Extraction Techniques and Bioactive Properties. Mar. Drugs 2024, 22 (4), 153. https://doi.org/10.3390/md22040153.Search in Google Scholar PubMed PubMed Central
93. Sachindra, N. M.; Mahendrakar, N. S. Process Optimization for Extraction of Carotenoids from Shrimp Waste with Vegetable Oils. Bioresour. Technol. 2005, 96 (10), 1195–1200. https://doi.org/10.1016/j.biortech.2004.09.018.Search in Google Scholar PubMed
94. Synowiecki, J.; Al-Khateeb, N. A. A. Q. The Recovery of Protein Hydrolysate during Enzymatic Isolation of Chitin from Shrimp Crangon crangon Processing Discards. Food Chem. 2000, 68 (2), 147–152. https://doi.org/10.1016/s0308-8146(99)00165-x.Search in Google Scholar
95. Venugopal, V. Valorization of Seafood Processing Discards: Bioconversion and Bio-Refinery Approaches. Front. Sustain. Food Syst. 2021, 5. https://doi.org/10.3389/fsufs.2021.611835.Search in Google Scholar
96. Mao, X.; Guo, N.; Sun, J.; Xue, C. Comprehensive Utilization of Shrimp Waste Based on Biotechnological Methods: A Review. J. Clean. Prod. 2017, 143, 814–823. https://doi.org/10.1016/j.jclepro.2016.12.042.Search in Google Scholar
97. Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional Composition of Black Soldier Fly (Hermetia illucens) Prepupae Reared on Different Organic Waste Substrates. J. Sci. Food Agric. 2016, 97 (8), 2594–2600. https://doi.org/10.1002/jsfa.8081.Search in Google Scholar PubMed
98. Hahn, T.; Roth, A.; Febel, E.; Fijalkowska, M.; Schmitt, E.; Arsiwalla, T.; Zibek, S. New Methods for High-Accuracy Insect Chitin Measurement. J. Sci. Food Agric. 2018, 98 (13), 5069–5073. https://doi.org/10.1002/jsfa.9044.Search in Google Scholar PubMed
99. Song, Y.-S.; Kim, M.; Moon, C.; Seo, D.; Han, Y. S.; Jo, Y. H.; Noh, M. Y.; Park, Y.; Kim, S.; Kim, Y. W.; Jung, W. Extraction of Chitin and Chitosan from Larval Exuvium and Whole Body of Edible Mealworm, <scp> Tenebrio molitor </scp>. Entomol. Res. 2018, 48 (3), 227–233. https://doi.org/10.1111/1748-5967.12304.Search in Google Scholar
100. Mushi, N. E. A Review on Native Well-Preserved Chitin Nanofibrils for Materials of High Mechanical Performance. Int. J. Biol. Macromol. 2021, 178, 591–606. https://doi.org/10.1016/j.ijbiomac.2021.02.149.Search in Google Scholar PubMed
101. Huet, G.; Hadad, C.; Husson, E.; Laclef, S.; Lambertyn, V.; Araya Farias, M.; Jamali, A.; Courty, M.; Alayoubi, R.; Gosselin, I.; Sarazin, C.; Van Nhien, A. N. Straightforward Extraction and Selective Bioconversion of High Purity Chitin from Bombyx Eri Larva: Toward an Integrated Insect Biorefinery. Carbohydr. Polym. 2020, 228, 115382. https://doi.org/10.1016/j.carbpol.2019.115382.Search in Google Scholar PubMed
102. Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current State of Chitin Purification and Chitosan Production from Insects. J. Chem. Technol. Biotechnol. 2020, 95 (11), 2775–2795. https://doi.org/10.1002/jctb.6533.Search in Google Scholar
103. Pasquier, E.; Beaumont, M.; Mattos, B. D.; Otoni, C. G.; Winter, A.; Rosenau, T.; Belgacem, M. N.; Rojas, O. J.; Bras, J. Upcycling Byproducts from Insect (Fly Larvae and Mealworm) Farming into Chitin Nanofibers and Films. ACS Sustain. Chem. Eng. 2021, 9 (40), 13618–13629. https://doi.org/10.1021/acssuschemeng.1c05035.Search in Google Scholar
104. Kumari, S.; Rath, P.; Sri Hari Kumar, A.; Tiwari, T. Extraction and Characterization of Chitin and Chitosan from Fishery Waste by Chemical Method. Environ. Technol. Innovation 2015, 3, 77–85. https://doi.org/10.1016/j.eti.2015.01.002.Search in Google Scholar
105. Abdou, E. S.; Nagy, K. S. A.; Elsabee, M. Z. Extraction and Characterization of Chitin and Chitosan from Local Sources. Bioresour. Technol. 2008, 99 (5), 1359–1367. https://doi.org/10.1016/j.biortech.2007.01.051.Search in Google Scholar PubMed
106. Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and Chitosan Preparation from Shrimp Shells Penaeus monodon and its Human Ovarian Cancer Cell Line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. https://doi.org/10.1016/j.ijbiomac.2017.09.035.Search in Google Scholar PubMed
107. El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, Chemical Modification and Characterization of Chitin and Chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. https://doi.org/10.1016/j.ijbiomac.2018.08.139.Search in Google Scholar PubMed
108. Benhabiles, M. S.; Abdi, N.; Drouiche, N.; Lounici, H.; Pauss, A.; Goosen, M.; Mameri, N. Protein Recovery by Ultrafiltration during Isolation of Chitin from Shrimp Shells Parapenaeus Longirostris. Food Hydrocolloids 2013, 32 (1), 28–34. https://doi.org/10.1016/j.foodhyd.2012.11.035.Search in Google Scholar
109. Donato, R. K.; Mija, A. Keratin Associations with Synthetic, Biosynthetic and Natural Polymers: An Extensive Review. Polymers 2019, 12 (1), 32. https://doi.org/10.3390/polym12010032.Search in Google Scholar PubMed PubMed Central
110. Islam, S.; Rahman Bhuiyan, M. A.; Islam, M. N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2016, 25 (3), 854–866. https://doi.org/10.1007/s10924-016-0865-5.Search in Google Scholar
111. Tharanathan, R. N.; Kittur, F. S. Chitin – the Undisputed Biomolecule of Great Potential. Crit. Rev. Food Sci. Nutr. 2003, 43 (1), 61–87. https://doi.org/10.1080/10408690390826455.Search in Google Scholar PubMed
112. Sarma, A. Biological Importance and Pharmaceutical Significance of Keratin: A Review. Int. J. Biol. Macromol. 2022, 219, 395–413. https://doi.org/10.1016/j.ijbiomac.2022.08.002.Search in Google Scholar PubMed
113. Salleh, K. M.; Abd Rashid, N. F. Keratin-Based Biomaterials for Biomedical Applications. In Polymer Composites Derived from Animal Sources; Woodhead Publishing: Cambridge, UK, 2024; pp 219–242.10.1016/B978-0-443-22414-0.00012-0Search in Google Scholar
114. Fuchs, E. Keratins and the Skin. Annu. Rev. Cell Dev. Biol. 1995, 11.1, 123–154. https://doi.org/10.1146/annurev.cb.11.110195.001011.Search in Google Scholar PubMed
115. McKittrick, J.; Chen, P. Y.; Bodde, S. G.; Yang, W.; Novitskaya, E. E.; Meyers, M. A. The Structure, Functions, and Mechanical Properties of Keratin. JOM 2012, 64 (4), 449–468. https://doi.org/10.1007/s11837-012-0302-8.Search in Google Scholar
116. Wang, B.; Yang, W.; McKittrick, J.; Meyers, M. A. Keratin: Structure, Mechanical Properties, Occurrence in Biological Organisms, and Efforts at Bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. https://doi.org/10.1016/j.pmatsci.2015.06.001.Search in Google Scholar
117. Fraser, R. D. B.; MacRae, T. P.; Suzuki, E. Structure of the α-Keratin Microfibril. J. Mol. Biol. 1976, 108 (2), 435–452. https://doi.org/10.1016/s0022-2836(76)80129-5.Search in Google Scholar PubMed
118. Sawyer, R. H.; Glenn, T.; French, J. O.; Mays, B.; Shames, R. B.; Barnes, G. L.Jr.; Rhodes, W.; Ishikawa, Y. The Expression of Beta (β) Keratins in the Epidermal Appendages of Reptiles and Birds. Am. Zool. 2000, 40 (4), 530–539. https://doi.org/10.1093/icb/40.4.530.Search in Google Scholar
119. Alexander, N. J. Comparison of α and β Keratin in Reptiles. Z. für Zellforsch. Mikrosk. Anat. 1970, 110 (2), 153–165. https://doi.org/10.1007/bf00335521.Search in Google Scholar PubMed
120. Zhang, W.; Fan, Y. Structure of Keratin. In Fibrous Proteins; Humana Press: New York, 2021; pp 41–53.10.1007/978-1-0716-1574-4_5Search in Google Scholar PubMed
121. Parry, D. A. D.; Bruce Fraser, R. D.; Squire, J. M. Fifty Years of Coiled-Coils and α-Helical Bundles: A Close Relationship Between Sequence and Structure. J. Struct. Biol. 2008, 163 (3), 258–269. https://doi.org/10.1016/j.jsb.2008.01.016.Search in Google Scholar PubMed
122. Pauling, L.; Corey, R. B.; Branson, H. R. The Structure of Proteins: Two Hydrogen-Bonded Helical Configurations of the Polypeptide Chain. Proc. Natl. Acad. Sci. 1951, 37 (4), 205–211. https://doi.org/10.1073/pnas.37.4.205.Search in Google Scholar PubMed PubMed Central
123. Parry, D. A. D.; Steinert, P. M. Intermediate Filaments: Molecular Architecture, Assembly, Dynamics and Polymorphism. Q. Rev. Biophys. 1999, 32 (2), 99–187. https://doi.org/10.1017/s0033583500003516.Search in Google Scholar PubMed
124. Yu, J.; Yu, D. W.; Checkla, D. M.; Freedberg, I. M.; Bertolino, A. P. Human Hair Keratins. J. Invest. Dermatol. 1993, 101 (s1), 56S–59S. https://doi.org/10.1111/1523-1747.ep12362635.Search in Google Scholar PubMed
125. Hatzfeld, M.; Burba, M. Function of Type I and Type II Keratin Head Domains: Their Role in Dimer, Tetramer and Filament Formation. J. Cell Sci. 1994, 107 (7), 1959–1972. https://doi.org/10.1242/jcs.107.7.1959.Search in Google Scholar PubMed
126. Coulombe, P. A.; Omary, M. B. ‘Hard’ and ‘Soft’ Principles Defining the Structure, Function and Regulation of Keratin Intermediate Filaments. Curr. Opin. Cell Biol. 2002, 14 (1), 110–122. https://doi.org/10.1016/s0955-0674(01)00301-5.Search in Google Scholar PubMed
127. Alibardi, L.; Dalla Valle, L.; Toffolo, V.; Toni, M. Scale Keratin in Lizard Epidermis Reveals Amino Acid Regions Homologous with Avian and Mammalian Epidermal Proteins. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2006, 288A (7), 734–752. https://doi.org/10.1002/ar.a.20342.Search in Google Scholar PubMed
128. Basit, A.; asghar, F.; Sadaf, S.; Akhtar, M. W. Health Improvement of Human Hair and Their Reshaping Using Recombinant Keratin K31. Biotechnol. Rep. 2018, 20, e00288. https://doi.org/10.1016/j.btre.2018.e00288.Search in Google Scholar PubMed PubMed Central
129. Holthaus, K. B.; Eckhart, L.; Dalla Valle, L.; Alibardi, L. Review: Evolution and Diversification of Corneous Beta-Proteins, the Characteristic Epidermal Proteins of Reptiles and Birds. J. Exp. Zool. B Mol. Dev. Evol. 2018, 330 (8), 438–453. https://doi.org/10.1002/jez.b.22840.Search in Google Scholar PubMed
130. Bear, R. S.; Rugo, H. J. The Results of X-ray Diffraction Studies on Keratin Fibers. Ann. N. Y. Acad. Sci. 1951, 53 (3), 627–648. https://doi.org/10.1111/j.1749-6632.1951.tb31964.x.Search in Google Scholar PubMed
131. Qiu, J.; Wilkens, C.; Barrett, K.; Meyer, A. S. Microbial Enzymes Catalyzing Keratin Degradation: Classification, Structure, Function. Biotechnol. Adv. 2020, 44, 107607. https://doi.org/10.1016/j.biotechadv.2020.107607.Search in Google Scholar PubMed PubMed Central
132. Feughelman, M. Mechanical Properties and Structure of Alpha-Keratin Fibres: Wool, Human Hair and Related Fibres; UNSW Press: Sydney, Australia, 1997.Search in Google Scholar
133. Lupas, A. N.; Bassler, J.; Dunin-Horkawicz, S. The Structure and Topology of α-Helical Coiled Coils. In Fibrous Proteins: Structures and Mechanisms; Humana Press: New York, 2017; pp 95–129.10.1007/978-3-319-49674-0_4Search in Google Scholar PubMed PubMed Central
134. Wegst, U. G. K.; Ashby, M. F. The Mechanical Efficiency of Natural Materials. Philos. Mag. 2004, 84 (21), 2167–2186. https://doi.org/10.1080/14786430410001680935.Search in Google Scholar
135. Yang, F.-C.; Zhang, Y.; Rheinstädter, M. C. The Structure of People’s Hair. PeerJ 2014, 2, e619. https://doi.org/10.7717/peerj.619.Search in Google Scholar PubMed PubMed Central
136. Alibardi, L. Cornification in Reptilian Epidermis Occurs through the Deposition of Keratin-Associated Beta-Proteins (Beta-Keratins) onto a Scaffold of Intermediate Filament Keratins. J. Morphol. 2012, 274 (2), 175–193. https://doi.org/10.1002/jmor.20086.Search in Google Scholar PubMed
137. Loughlin, W. A.; Tyndall, J. D. A.; Glenn, M. P.; Fairlie, D. P. Beta-Strand Mimetics. Chem. Rev. 2004, 104 (12), 6085–6118. https://doi.org/10.1021/cr040648k.Search in Google Scholar PubMed
138. Martínez-Hernández, A. L.; Velasco-Santos, C.; de Icaza, M.; Castaño, V. Hierarchical Microstructure in Keratin Biofibers. Microsc. Microanal. 2003, 9 (S02), 1282–1283. https://doi.org/10.1017/s1431927603446412.Search in Google Scholar
139. Swift, J. A.; Smith, J. R. Microscopical Investigations on the Epicuticle of Mammalian Keratin Fibres. J. Microsc. 2001, 204 (3), 203–211. https://doi.org/10.1046/j.1365-2818.2001.00957.x.Search in Google Scholar PubMed
140. Bradbury, J. H.; King, N. L. R. The Chemical Composition of Wool. IV. The Quantity of Each Histological Component. Aust. J. Chem. 1967, 20 (12), 2803. https://doi.org/10.1071/ch9672803.Search in Google Scholar
141. Appleyard, H. M.; Greville, C. M. The Cuticle of Mammalian Hair. Nature 1950, 166 (4233), 1031. https://doi.org/10.1038/1661031a0.Search in Google Scholar PubMed
142. Yu, Y.; Yang, W.; Wang, B.; Meyers, M. A. Structure and Mechanical Behavior of Human Hair. Mater. Sci. Eng. C 2017, 73, 152–163. https://doi.org/10.1016/j.msec.2016.12.008.Search in Google Scholar PubMed
143. Thibaut, S.; Barbarat, P.; Leroy, F.; Bernard, B. A. Human Hair Keratin Network and Curvature. Int. J. Dermatol. 2007, 46 (s1), 7–10. https://doi.org/10.1111/j.1365-4632.2007.03454.x.Search in Google Scholar PubMed
144. Dobb, M. G. Electron-Diffraction Studies of Keratin Cells. J. Text. Inst. 1970, 61 (5), 232–234. https://doi.org/10.1080/00405007008631783.Search in Google Scholar
145. Zhang, D. Physical, Morphological and Chemical Structure & Property Relationships for α-Keratins in Bleached Human Hair; The University of Manchester: United Kingdom, 2013.Search in Google Scholar
146. Chou, C.-C.; Lepore, E.; Antonaci, P.; Pugno, N.; Buehler, M. J. Mechanics of Trichocyte Alpha-Keratin Fibers: Experiment, Theory, and Simulation. J. Mater. Res. 2015, 30 (1), 26–35. https://doi.org/10.1557/jmr.2014.267.Search in Google Scholar
147. Rogers, G. E. Electron Microscopy of Wool. J. Ultrastruct. Res. 1959, 2 (3), 309–330. https://doi.org/10.1016/s0022-5320(59)80004-6.Search in Google Scholar PubMed
148. Cui, Y.; Gong, H.; Wang, Y.; Li, D.; Bai, H. A Thermally Insulating Textile Inspired by Polar Bear Hair. Adv. Mater. 2018, 30 (14). https://doi.org/10.1002/adma.201706807.Search in Google Scholar PubMed
149. Abidin, N. A. Z.; Kormin, F.; Mohamed Anuar, N. A. F.; Abu Bakar, M. F. The Potential of Insects as Alternative Sources of Chitin: An Overview on the Chemical Method of Extraction from Various Sources. Int. J. Mol. Sci. 2020, 21 (14), 4978. https://doi.org/10.3390/ijms21144978.Search in Google Scholar PubMed PubMed Central
150. Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31 (7), 603–632. https://doi.org/10.1016/j.progpolymsci.2006.06.001.Search in Google Scholar
151. Carlström, D. The Crystal Structure of α-Chitin (Poly-N-Acetyl-D-Glucosamine). J. Cell Biol. 1957, 3 (5), 669–683. https://doi.org/10.1083/jcb.3.5.669.Search in Google Scholar PubMed PubMed Central
152. Satitsri, S.; Muanprasat, C. Chitin and Chitosan Derivatives as Biomaterial Resources for Biological and Biomedical Applications. Molecules 2020, 25 (24), 5961. https://doi.org/10.3390/molecules25245961.Search in Google Scholar PubMed PubMed Central
153. Jang, M.-K.; Kong, B.; Jeong, Y.; Lee, C. H.; Nah, J. Physicochemical Characterization of α-Chitin, β-Chitin, and γ-Chitin Separated from Natural Resources. J. Polym. Sci., Part A: Polym. Chem. 2004, 42 (14), 3423–3432. https://doi.org/10.1002/pola.20176.Search in Google Scholar
154. Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A. M.; Baran, T.; Amemiya, C. T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On Chemistry of γ-Chitin. Carbohydr. Polym. 2017, 176, 177–186. https://doi.org/10.1016/j.carbpol.2017.08.076.Search in Google Scholar PubMed
155. Saito, Y.; Okano, T.; Gaill, F.; Chanzy, H.; Putaux, J. L. Structural Data on the Intra-Crystalline Swelling of β-Chitin. Int. J. Biol. Macromol. 2000, 28 (1), 81–88. https://doi.org/10.1016/s0141-8130(00)00147-1.Search in Google Scholar PubMed
156. Hahn, T.; Looijenga-Vos, A.; Aroyo, M. I.; Flack, H. D.; Momma, K.; Konstantinov, P. Guide to the Use of the Space-Group Tables. In International Tables for Crystallography; International Union of Crystallography: Chester, UK, 2016; pp 142–174.10.1107/97809553602060000926Search in Google Scholar
157. Minke, R.; Blackwell, J. The Structure of α-Chitin. J. Mol. Biol. 1978, 120 (2), 167–181. https://doi.org/10.1016/0022-2836(78)90063-3.Search in Google Scholar PubMed
158. Rössle, M.; Flot, D.; Engel, J.; Burghammer, M.; Riekel, C.; Chanzy, H. Fast Intracrystalline Hydration of β-Chitin Revealed by Combined Microdrop Generation and On-Line Synchrotron Radiation Microdiffraction. Biomacromolecules 2003, 4 (4), 981–986. https://doi.org/10.1021/bm0340218.Search in Google Scholar PubMed
159. Noishiki, Y.; Nishiyama, Y.; Wada, M.; Kuga, S. Complexation of α-Chitin with Aliphatic Amines. Biomacromolecules 2005, 6 (4), 2362–2364. https://doi.org/10.1021/bm0500446.Search in Google Scholar PubMed
160. Rudall, K. M. Chitin and its Association with Other Molecules. J. Polym. Sci., Part C: Polym. Symp. 1969, 28 (1), 83–102. https://doi.org/10.1002/polc.5070280110.Search in Google Scholar
161. Blackwell, J.; Parker, K. D.; Rudall, K. M. Chitin in Pogonophore Tubes. J. Mar. Biol. Assoc. U. K. 1965, 45 (3), 659–661. https://doi.org/10.1017/s0025315400016489.Search in Google Scholar
162. Rudall, K. M.; Kenchington, W. The Chitin System. Biol. Rev. 1973, 48 (4), 597–633. https://doi.org/10.1111/j.1469-185x.1973.tb01570.x.Search in Google Scholar
163. Mogilevskaya, E. L.; Akopova, T. A.; Zelenetskii, A. N.; Ozerin, A. N. The Crystal Structure of Chitin and Chitosan. Polym. Sci. Ser. A 2006, 48 (2), 116–123. https://doi.org/10.1134/s0965545x06020039.Search in Google Scholar
164. Clark, G. L.; Smith, A. F. X-ray Diffraction Studies of Chitin, Chitosan, and Derivatives. J. Phys. Chem. 1936, 40 (7), 863–879. https://doi.org/10.1021/j150376a001.Search in Google Scholar
165. Ogawa, Y.; Kimura, S.; Wada, M. Electron Diffraction and High-Resolution Imaging on Highly-Crystalline β-Chitin Microfibril. J. Struct. Biol. 2011, 176 (1), 83–90. https://doi.org/10.1016/j.jsb.2011.07.001.Search in Google Scholar PubMed
166. Bogdanova, O. I.; Polyakov, D. K.; Streltsov, D. R.; Bakirov, A. V.; Blackwell, J.; Chvalun, S. N. Structure of β-Chitin from Berryteuthis Magister and its Transformation during Whisker Preparation and Polymerization Filling. Carbohydr. Polym. 2016, 137, 678–684. https://doi.org/10.1016/j.carbpol.2015.11.027.Search in Google Scholar PubMed
167. Noishiki, Y.; Takami, H.; Nishiyama, Y.; Wada, M.; Okada, S.; Kuga, S. Alkali-Induced Conversion of β-Chitin to α-Chitin. Biomacromolecules 2003, 4 (4), 896–899. https://doi.org/10.1021/bm0257513.Search in Google Scholar PubMed
168. Riva, R.; Ragelle, H.; des Rieux, A.; Duhem, N. J.; Jérôme, C.; Preat, V. Chitosan and Chitosan Derivatives in Drug Delivery and Tissue Engineering. In Chitosan for Biomaterials II; Springer: Berlin Heidelberg, 2011; pp 19–44.10.1007/12_2011_137Search in Google Scholar
169. Sivashankari, P. R.; Prabaharan, M. Deacetylation Modification Techniques of Chitin and Chitosan. In Chitosan Based Biomaterials Volume 1; Elsevier: Amsterdam, Netherlands, 2017; pp 117–133.10.1016/B978-0-08-100230-8.00005-4Search in Google Scholar
170. Shepherd, R.; Reader, S.; Falshaw, A. Glycoconj. J. 1997, 14 (4), 535–542. https://doi.org/10.1023/a:1018524207224.10.1023/A:1018524207224Search in Google Scholar
171. Cartier, N.; Domard, A.; Chanzy, H. Single Crystals of Chitosan. Int. J. Biol. Macromol. 1990, 12 (5), 289–294. https://doi.org/10.1016/0141-8130(90)90015-3.Search in Google Scholar PubMed
172. Pillai, C. K. S.; Paul, W.; Sharma, C. P. Chitin and Chitosan Polymers: Chemistry, Solubility and Fiber Formation. Prog. Polym. Sci. 2009, 34 (7), 641–678. https://doi.org/10.1016/j.progpolymsci.2009.04.001.Search in Google Scholar
173. Sánchez-Machado, D. I.; Lopez-Cervantes, J.; Correa-Murrieta, M. A.; Sanchez-Duarte, R. G. G.; Flores, P. C.; de la Mora-López, G. S. Chitosan. In Nonvitamin and Nonmineral Nutritional Supplements; Academic Press: London, UK, 2019; pp 485–493.10.1016/B978-0-12-812491-8.00064-3Search in Google Scholar
174. Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13 (3), 1133–1174. https://doi.org/10.3390/md13031133.Search in Google Scholar PubMed PubMed Central
175. Drack, M.; Gebeshuber, I. C. Comment on “Innovation through Imitation: Biomimetic, Bioinspired and Biokleptic Research” by A. E. Rawlings, J. P. Bramble and S. S. Staniland, Soft Matter, 2012, 8, 6675. Soft Matter 2013, 9 (7), 2338. https://doi.org/10.1039/c2sm26722e.Search in Google Scholar
176. Prashanth, K. V. H.; Tharanathan, R. N. Chitin/Chitosan: Modifications and Their Unlimited Application Potential – An Overview. Trends Food Sci. Technol. 2007, 18 (3), 117–131. https://doi.org/10.1016/j.tifs.2006.10.022.Search in Google Scholar
177. Wei, A.; Fu, J.; Guo, F. Mechanical Properties of Chitin Polymorphs: A Computational Study. J. Mater. Sci. 2021, 56 (20), 12048–12058. https://doi.org/10.1007/s10853-021-06086-8.Search in Google Scholar
178. Hou, J.; Aydemir, B. E.; Dumanli, A. G. Understanding the Structural Diversity of Chitins as a Versatile Biomaterial. Phil. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2021, 379 (2206). https://doi.org/10.1098/rsta.2020.0331.Search in Google Scholar
179. Nishino, T.; Matsui, R.; Nakamae, K. Elastic Modulus of the Crystalline Regions of Chitin and Chitosan. J. Polym. Sci. B Polym. Phys. 1999, 37 (11), 1191–1196. https://doi.org/10.1002/(sici)1099-0488(19990601)37:11<1191::aid-polb13>3.0.co;2-h.10.1002/(SICI)1099-0488(19990601)37:11<1191::AID-POLB13>3.3.CO;2-8Search in Google Scholar
180. Ren, J.; Wang, Y.; Yao, Y.; Wang, Y.; Fei, X.; Qi, P.; Lin, S.; Kaplan, D. L.; Buehler, M. J.; Ling, S. Biological Material Interfaces as Inspiration for Mechanical and Optical Material Designs. Chem. Rev. 2019, 119 (24), 12279–12336. https://doi.org/10.1021/acs.chemrev.9b00416.Search in Google Scholar
181. Salavati, M. Mechanical Properties of α-Chitin and Chitosan Biocomposite: A Molecular Dynamic Study. J. Compos. Sci. 2023, 7 (11), 464. https://doi.org/10.3390/jcs7110464.Search in Google Scholar
182. Montroni, D.; Sparla, F.; Fermani, S.; Falini, G. Influence of Proteins on Mechanical Properties of a Natural Chitin-Protein Composite. Acta Biomater. 2021, 120, 81–90. https://doi.org/10.1016/j.actbio.2020.04.039.Search in Google Scholar
183. Hajiali, F.; Jin, T.; Yang, G.; Santos, M.; Lam, E.; Moores, A. Mechanochemical Transformations of Biomass into Functional Materials. ChemSusChem 2022, 15 (7). https://doi.org/10.1002/cssc.202102535.Search in Google Scholar
184. Gao, K.; Guo, Y.; Niu, Q.; Fang, H.; Zhang, L.; Zhang, Y.; Wang, L.; Zhou, L. Effects of Chitin Nanofibers on the Microstructure and Properties of Cellulose Nanofibers/Chitin Nanofibers Composite Aerogels. Cellulose 2018, 25 (8), 4591–4602. https://doi.org/10.1007/s10570-018-1899-8.Search in Google Scholar
185. Khantayanuwong, S.; Khemarom, C.; Salaemae, S. Effects of Shrimp Chitosan on the Physical Properties of Handsheets. Agric. Nat. Resour. 2017, 51 (1), 53–56. https://doi.org/10.1016/j.anres.2016.07.006.Search in Google Scholar
186. Lertsutthiwong, P.; Chandrkrachang, S.; Nazhad, M. M.; Stevens, W. F. Chitosan as a Dry Strength Agent for Paper. Appita J. 2002, 55 (3), 208–212.Search in Google Scholar
187. Ashori, A.; Harun, J.; Zin, W. M.; Yusoff, M. N. M. Enhancing Dry-Strength Properties of Kenaf (Hibiscus cannabinus) Paper through Chitosan. Polym.-Plast. Technol. Eng. 2006, 45 (1), 125–129. https://doi.org/10.1080/03602550500373709.Search in Google Scholar
188. Vikele, L.; Laka, M.; Sable, I.; Rozenberga, L.; Grinfelds, U.; Zoldners, J.; Passas, R.; Mauret, E. Effect of Chitosan on Properties of Paper for Packaging. Cellul. Chem. Technol. 2017, 51 (1–2), 67–73.Search in Google Scholar
189. Habibie, S.; Hamzah, M.; Anggaravidya, M.; Kalembang, E. The Effect of Chitosan on Physical and Mechanical Properties of Paper. J. Chem. Eng. Mater. Sci. 2016, 7 (1), 1–10. https://doi.org/10.5897/jcems2015.0235.Search in Google Scholar
190. Nada, A. M. A.; El-Sakhawy, M.; Kamel, S.; Eid, M.; Adel, A. M. Mechanical and Electrical Properties of Paper Sheets Treated with Chitosan and its Derivatives. Carbohydr. Polym. 2006, 63 (1), 113–121. https://doi.org/10.1016/j.carbpol.2005.08.028.Search in Google Scholar
191. Fu, J.; He, C.; Xia, B.; Li, Y.; Feng, Q.; Yin, Q.; Shi, X.; Feng, X.; Wang, H.; Yao, H. C-axis Preferential Orientation of Hydroxyapatite Accounts for the High Wear Resistance of the Teeth of Black Carp (Mylopharyngodon piceus). Sci. Rep. 2016, 6 (1). https://doi.org/10.1038/srep23509.Search in Google Scholar PubMed PubMed Central
192. Sedriks, A. J.; Mulhearn, T. O. Mechanics of Cutting and Rubbing in Simulated Abrasive Processes. Wear 1963, 6 (6), 457–466. https://doi.org/10.1016/0043-1648(63)90281-3.Search in Google Scholar
193. Khattak, S.; Wahid, F.; Liu, L. P.; Jia, S. R.; Chu, L. Q.; Xie, Y. Y.; Li, Z. X.; Zhong, C. Applications of Cellulose and Chitin/chitosan Derivatives and Composites as Antibacterial Materials: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2019, 103 (5), 1989–2006. https://doi.org/10.1007/s00253-018-09602-0.Search in Google Scholar PubMed
194. van Nieuwenhoven, R. W.; Bürger, A. M.; Mears, L. L. E.; Kienzl, P.; Reithofer, M.; Elbe-Bürger, A.; Gebeshuber, I. C. Verifying Antibacterial Properties of Nanopillars on Cicada Wings. Appl. Nanosci. 2024, 14 (3), 531–541. https://doi.org/10.1007/s13204-024-03030-5.Search in Google Scholar
195. Kong, M.; Chen, X. G.; Xing, K.; Park, H. J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144 (1), 51–63. https://doi.org/10.1016/j.ijfoodmicro.2010.09.012.Search in Google Scholar PubMed
196. Sudarshan, N. R.; Hoover, D. G.; Knorr, D. Antibacterial Action of Chitosan. Food Biotechnol. 1992, 6 (3), 257–272. https://doi.org/10.1080/08905439209549838.Search in Google Scholar
197. Papineau, A. M.; Hoover, D. G.; Knorr, D.; Farkas, D. F. Antimicrobial Effect of Water-Soluble Chitosans with High Hydrostatic Pressure. Food Biotechnol. 1991, 5 (1), 45–57. https://doi.org/10.1080/08905439109549790.Search in Google Scholar
198. Helander, I. M.; Nurmiaho-Lassila, E. L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan Disrupts the Barrier Properties of the Outer Membrane of Gram-Negative Bacteria. Int. J. Food Microbiol. 2001, 71 (2–3), 235–244. https://doi.org/10.1016/s0168-1605(01)00609-2.Search in Google Scholar PubMed
199. Leuba, J. L.; Stossel, P. Chitosan and Other Polyamines: Antifungal Activity and Interaction with Biological Membranes. In Chitin in Nature and Technology; Plenum Press: London, 1986; pp 215–222.10.1007/978-1-4613-2167-5_29Search in Google Scholar
200. Rhoades, J.; Roller, S. Antimicrobial Actions of Degraded and Native Chitosan against Spoilage Organisms in Laboratory Media and Foods. Appl. Environ. Microbiol. 2000, 66 (1), 80–86. https://doi.org/10.1128/aem.66.1.80-86.2000.Search in Google Scholar PubMed PubMed Central
201. No, H. Antibacterial Activity of Chitosans and Chitosan Oligomers with Different Molecular Weights. Int. J. Food Microbiol. 2002, 74 (1–2), 65–72. https://doi.org/10.1016/s0168-1605(01)00717-6.Search in Google Scholar PubMed
202. Salah, F.; Ghoul, Y. E.; Mahdhi, A.; Majdoub, H.; Jarroux, N.; Sakli, F. Effect of the Deacetylation Degree on the Antibacterial and Antibiofilm Activity of Acemannan from Aloe Vera. Ind. Crops Prod. 2017, 103, 13–18. https://doi.org/10.1016/j.indcrop.2017.03.031.Search in Google Scholar
203. Yun, Y. S.; Kim, K. S.; Lee, Y. N. Antibacterial and Antifungal Effect of Chitosan. J. Chitin Chitosan 1999, 4 (1), 8–14.Search in Google Scholar
204. Liu, N.; Chen, X. G.; Park, H. J.; Liu, C. G.; Liu, C. S.; Meng, X. H.; Yu, L. J. Effect of MW and Concentration of Chitosan on Antibacterial Activity of Escherichia coli. Carbohydr. Polym. 2006, 64 (1), 60–65. https://doi.org/10.1016/j.carbpol.2005.10.028.Search in Google Scholar
205. Jeon, Y. Antimicrobial Effect of Chitooligosaccharides Produced by Bioreactor. Carbohydr. Polym. 2001, 44 (1), 71–76. https://doi.org/10.1016/s0144-8617(00)00200-9.Search in Google Scholar
206. Bai, R.-K.; Huang, M.-Y.; Jiang, Y.-Y. Selective Permeabilities of Chitosan-Acetic Acid Complex Membrane and Chitosan-Polymer Complex Membranes for Oxygen and Carbon Dioxide. Polym. Bull. 1988, 20 (1). https://doi.org/10.1007/bf00262253.Search in Google Scholar
207. Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of Acetylation Degree and Molecular Weight of Homogeneous Chitosans on Antibacterial and Antifungal Activities. Int. J. Food Microbiol. 2014, 185, 57–63. https://doi.org/10.1016/j.ijfoodmicro.2014.04.029.Search in Google Scholar PubMed
208. Mourya, V. K.; Inamdar, N. N. Chitosan-Modifications and Applications: Opportunities Galore. React. Funct. Polym. 2008, 68 (6), 1013–1051. https://doi.org/10.1016/j.reactfunctpolym.2008.03.002.Search in Google Scholar
209. Heedy, S.; Marshall, M. E.; Pineda, J. J.; Pearlman, E.; Yee, A. F. Synergistic Antimicrobial Activity of a Nanopillar Surface on a Chitosan Hydrogel. ACS Appl. Bio Mater. 2020, 3 (11), 8040–8048. https://doi.org/10.1021/acsabm.0c01110.Search in Google Scholar PubMed
210. Frost, S. J.; Mawad, D.; Wuhrer, R.; Myers, S.; Lauto, A. Semitransparent Bandages Based on Chitosan and Extracellular Matrix for Photochemical Tissue Bonding. BioMed. Eng. Online 2018, 17 (1). https://doi.org/10.1186/s12938-018-0444-1.Search in Google Scholar PubMed PubMed Central
211. Narasimhan, V.; Siddique, R. H.; Lee, J. O.; Kumar, S.; Ndjamen, B.; Du, J.; Hong, N.; Sretavan, D.; Choo, H. Multifunctional Biophotonic Nanostructures Inspired by the Longtail Glasswing Butterfly for Medical Devices. Nat. Nanotechnol. 2018, 13 (6), 512–519. https://doi.org/10.1038/s41565-018-0111-5.Search in Google Scholar PubMed PubMed Central
212. Arisoy, F. D.; Kolewe, K. W.; Homyak, B.; Kurtz, I. S.; Schiffman, J. D.; Watkins, J. J. Bioinspired Photocatalytic Shark-Skin Surfaces with Antibacterial and Antifouling Activity via Nanoimprint Lithography. ACS Appl. Mater. Interfaces 2018, 10 (23), 20055–20063. https://doi.org/10.1021/acsami.8b05066.Search in Google Scholar PubMed PubMed Central
213. Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A. H.; Su, B. Antibacterial Effects of Nanopillar Surfaces Are Mediated by Cell Impedance, Penetration and Induction of Oxidative Stress. Nat. Commun. 2020, 11 (1). https://doi.org/10.1038/s41467-020-15471-x.Search in Google Scholar PubMed PubMed Central
214. Mi, F.-L.; Tan, Y. C.; Liang, H. F.; Sung, H. W. In Vivo Biocompatibility and Degradability of a Novel Injectable-Chitosan-Based Implant. Biomaterials 2002, 23 (1), 181–191. https://doi.org/10.1016/s0142-9612(01)00094-1.Search in Google Scholar PubMed
215. Madhumathi, K.; Sudheesh Kumar, P. T.; Abhilash, S.; Sreeja, V.; Tamura, H.; Manzoor, K.; Nair, S. V.; Jayakumar, R. Development of Novel Chitin/Nanosilver Composite Scaffolds for Wound Dressing Applications. J. Mater. Sci.: Mater. Med. 2009, 21 (2), 807–813. https://doi.org/10.1007/s10856-009-3877-z.Search in Google Scholar PubMed
216. Tan, H.; Chu, C. R.; Payne, K. A.; Marra, K. G. Injectable In Situ Forming Biodegradable Chitosan–Hyaluronic Acid Based Hydrogels for Cartilage Tissue Engineering. Biomaterials 2009, 30 (13), 2499–2506. https://doi.org/10.1016/j.biomaterials.2008.12.080.Search in Google Scholar PubMed PubMed Central
217. Chen, M.-C.; Ling, M. H.; Lai, K. Y.; Pramudityo, E. Chitosan Microneedle Patches for Sustained Transdermal Delivery of Macromolecules. Biomacromolecules 2012, 13 (12), 4022–4031. https://doi.org/10.1021/bm301293d.Search in Google Scholar PubMed
218. Wei, Z.; Yang, J. H.; Liu, Z. Q.; Xu, F.; Zhou, J. X.; Zrínyi, M.; Osada, Y.; Chen, Y. M. Novel Biocompatible Polysaccharide-Based Self-Healing Hydrogel. Adv. Funct. Mater. 2015, 25 (9), 1352–1359. https://doi.org/10.1002/adfm.201401502.Search in Google Scholar
219. Chen, L.; Meng, R.; Qing, R.; Li, W.; Wang, Z.; Hou, Y.; Deng, J.; Pu, W.; Gao, Z.; Wang, B.; Hao, S. Bioinspired Robust Keratin Hydrogels for Biomedical Applications. Nano Lett. 2022, 22 (22), 8835–8844. https://doi.org/10.1021/acs.nanolett.2c02530.Search in Google Scholar PubMed
220. Wang, J.; Hao, S.; Luo, T.; Cheng, Z.; Li, W.; Gao, F.; Guo, T.; Gong, Y.; Wang, B. Feather Keratin Hydrogel for Wound Repair: Preparation, Healing Effect and Biocompatibility Evaluation. Colloids Surf. B Biointerfaces 2017, 149, 341–350. https://doi.org/10.1016/j.colsurfb.2016.10.038.Search in Google Scholar PubMed
221. Stuart-Fox, D.; Moussalli, A. Camouflage, Communication and Thermoregulation: Lessons from Colour Changing Organisms. Philos. Trans. R. Soc. B Biol. Sci. 2008, 364 (1516), 463–470. https://doi.org/10.1098/rstb.2008.0254.Search in Google Scholar PubMed PubMed Central
222. Miller, R.; Owens, S. J.; Rørslett, B. Plants and Colour: Flowers and Pollination. Opt Laser. Technol. 2011, 43 (2), 282–294. https://doi.org/10.1016/j.optlastec.2008.12.018.Search in Google Scholar
223. Shawkey, M. D.; D’Alba, L. Interactions between Colour-Producing Mechanisms and Their Effects on the Integumentary Colour Palette. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372 (1724), 20160536. https://doi.org/10.1098/rstb.2016.0536.Search in Google Scholar PubMed PubMed Central
224. Cuthill, I. C.; Allen, W. L.; Arbuckle, K.; Caspers, B.; Chaplin, G.; Hauber, M. E.; Hill, G. E.; Jablonski, N. G.; Jiggins, C. D.; Kelber, A.; Mappes, J.; Marshall, J.; Merrill, R.; Osorio, D.; Prum, R.; Roberts, N. W.; Roulin, A.; Rowland, H. M.; Sherratt, T. N.; Skelhorn, J.; Speed, M. P.; Stevens, M.; Stoddard, M. C.; Stuart-Fox, D.; Talas, L.; Tibbetts, E.; Caro, T. The Biology of Color. Science 2017, 357 (6350). https://doi.org/10.1126/science.aan0221.Search in Google Scholar PubMed
225. Lizundia, E.; Nguyen, T. D.; Winnick, R. J.; MacLachlan, M. J. Biomimetic Photonic Materials Derived from Chitin and Chitosan. J. Mater. Chem. C 2021, 9 (3), 796–817. https://doi.org/10.1039/d0tc05381c.Search in Google Scholar
226. Nikolov, S.; Fabritius, H.; Petrov, M.; Friák, M.; Lymperakis, L.; Sachs, C.; Raabe, D.; Neugebauer, J. Robustness and Optimal Use of Design Principles of Arthropod Exoskeletons Studied by Ab Initio-Based Multiscale Simulations. J. Mech. Behav. Biomed. Mater. 2011, 4 (2), 129–145. https://doi.org/10.1016/j.jmbbm.2010.09.015.Search in Google Scholar PubMed
227. Zobl, S.; Wilts, B. D.; Salvenmoser, W.; Pölt, P.; Gebeshuber, I. C.; Schwerte, T. Orientation-Dependent Reflection of Structurally Coloured Butterflies. Biomimetics 2020, 5 (1), 5. https://doi.org/10.3390/biomimetics5010005.Search in Google Scholar PubMed PubMed Central
228. Zobl, S.; Salvenmoser, W.; Schwerte, T.; Gebeshuber, I. C.; Schreiner, M. Morpho Peleides Butterfly Wing Imprints as Structural Colour Stamp. Bioinspiration Biomimetics 2016, 11 (1), 016006. https://doi.org/10.1088/1748-3190/11/1/016006.Search in Google Scholar PubMed
229. Schenk, F.; Wilts, B. D.; Stavenga, D. G. The Japanese Jewel Beetle: A Painter’s Challenge. Bioinspiration Biomimetics 2013, 8 (4), 045002. https://doi.org/10.1088/1748-3182/8/4/045002.Search in Google Scholar PubMed
230. Wilts, B. D.; Saranathan, V. A Literal Elytral Rainbow: Tunable Structural Colors Using Single Diamond Biophotonic Crystals in Pachyrrhynchus Congestus Weevils. Small 2018, 14 (46). https://doi.org/10.1002/smll.201802328.Search in Google Scholar PubMed
231. Butt, H.; Yetisen, A. K.; Mistry, D.; Khan, S. A.; Hassan, M. U.; Yun, S. H. Morpho Butterfly-Inspired Nanostructures. Adv. Opt. Mater. 2016, 4 (4), 497–504. https://doi.org/10.1002/adom.201500658.Search in Google Scholar
232. Leertouwer, H. L.; Wilts, B. D.; Stavenga, D. G. Refractive Index and Dispersion of Butterfly Chitin and Bird Keratin Measured by Polarizing Interference Microscopy. Opt. Express 2011, 19 (24), 24061. https://doi.org/10.1364/oe.19.024061.Search in Google Scholar
233. Castle, E. S. The Double Refraction of Chitin. J. Gen. Physiol. 1936, 19 (5), 797–805. https://doi.org/10.1085/jgp.19.5.797.Search in Google Scholar PubMed PubMed Central
234. Seago, A. E.; Brady, P.; Vigneron, J. P.; Schultz, T. D. Gold Bugs and beyond: A Review of Iridescence and Structural Colour Mechanisms in Beetles (Coleoptera). J. R. Soc. Interface 2008, 6 (Suppl. 2). https://doi.org/10.1098/rsif.2008.0354.focus.Search in Google Scholar PubMed PubMed Central
235. Prum, R. O.; Cole, J. A.; Torres, R. H. Blue Integumentary Structural Colours in Dragonflies (Odonata) are Not Produced by Incoherent Tyndall Scattering. J. Exp. Biol. 2004, 207 (22), 3999–4009. https://doi.org/10.1242/jeb.01240.Search in Google Scholar PubMed
236. McDonald, L. T. Circularly Polarised Optics in Scarabaeidae; University of Exeter: United Kingdom, 2017.Search in Google Scholar
237. Mouchet, S. R.; Lobet, M.; Kolaric, B.; Kaczmarek, A.; Van Deun, R.; Vukusic, P.; Deparis, O.; Van Hooijdonk, E. Photonic Scales of Hoplia Coerulea Beetle: Any Colour You like. Mater. Today: Proc. 2017, 4 (4), 4979–4986. https://doi.org/10.1016/j.matpr.2017.04.104.Search in Google Scholar
238. Burresi, M.; Cortese, L.; Pattelli, L.; Kolle, M.; Vukusic, P.; Wiersma, D. S.; Steiner, U.; Vignolini, S. Bright-White Beetle Scales Optimise Multiple Scattering of Light. Sci. Rep. 2014, 4 (1). https://doi.org/10.1038/srep06075.Search in Google Scholar PubMed PubMed Central
239. Siddique, R. H.; Gomard, G.; Hölscher, H. The Role of Random Nanostructures for the Omnidirectional Anti-Reflection Properties of the Glasswing Butterfly. Nat. Commun. 2015, 6 (1). https://doi.org/10.1038/ncomms7909.Search in Google Scholar PubMed
240. Saranathan, V.; Seago, A. E.; Sandy, A.; Narayanan, S.; Mochrie, S. G. J.; Dufresne, E. R.; Cao, H.; Osuji, C. O.; Prum, R. O. Structural Diversity of Arthropod Biophotonic Nanostructures Spans Amphiphilic Phase-Space. Nano Lett. 2015, 15 (6), 3735–3742. https://doi.org/10.1021/acs.nanolett.5b00201.Search in Google Scholar PubMed
241. Suksangpanya, N.; Yaraghi, N. A.; Kisailus, D.; Zavattieri, P. Twisting Cracks in Bouligand Structures. J. Mech. Behav. Biomed. Mater. 2017, 76, 38–57. https://doi.org/10.1016/j.jmbbm.2017.06.010.Search in Google Scholar PubMed
242. Luo, Y.; Li, Y.; Liu, K.; Li, L.; Wen, W.; Ding, S.; Huang, Y.; Liu, M.; Zhou, C.; Luo, B. Modulating of Bouligand Structure and Chirality Constructed Bionically Based on the Self-Assembly of Chitin Whiskers. Biomacromolecules 2023, 24 (6), 2942–2954. https://doi.org/10.1021/acs.biomac.3c00419.Search in Google Scholar PubMed
243. Nekvapil, F.; Pinzaru, S. C.; Barbu-Tudoran, L.; Suciu, M.; Glamuzina, B.; Tamaș, T.; Chiș, V. Color-Specific Porosity in Double Pigmented Natural 3d-Nanoarchitectures of Blue Crab Shell. Sci. Rep. 2020, 10 (1). https://doi.org/10.1038/s41598-020-60031-4.Search in Google Scholar PubMed PubMed Central
244. Muzzarelli, R. A. A. Chitin Nanostructures in Living Organisms. In Chitin: Formation and Diagenesis; Springer: Dordrecht, 2011; pp 1–34.10.1007/978-90-481-9684-5_1Search in Google Scholar
245. Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.; Tamura, H. Biomedical Applications of Chitin and Chitosan Based Nanomaterials – A Short Review. Carbohydr. Polym. 2010, 82 (2), 227–232. https://doi.org/10.1016/j.carbpol.2010.04.074.Search in Google Scholar
246. Kostag, M.; El Seoud, O. A. Sustainable Biomaterials Based on Cellulose, Chitin and Chitosan Composites – A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100079. https://doi.org/10.1016/j.carpta.2021.100079.Search in Google Scholar
247. Gurau, G.; Rogers, R. D. Which Part of a Shrimp has More Economic Value, the Shell or the Meat? ECS Meet. Abstr. 2018, MA2018-02 (53), 1864. https://doi.org/10.1149/ma2018-02/53/1864.Search in Google Scholar
248. Zhu, K.; Tu, H.; Yang, P.; Qiu, C.; Zhang, D.; Lu, A.; Luo, L.; Chen, F.; Liu, X.; Chen, L.; Fu, Q.; Zhang, L. Mechanically Strong Chitin Fibers with Nanofibril Structure, Biocompatibility, and Biodegradability. Chem. Mater. 2019, 31 (6), 2078–2087. https://doi.org/10.1021/acs.chemmater.8b05183.Search in Google Scholar
249. Verlee, A.; Mincke, S.; Stevens, C. V. Recent Developments in Antibacterial and Antifungal Chitosan and its Derivatives. Carbohydr. Polym. 2017, 164, 268–283. https://doi.org/10.1016/j.carbpol.2017.02.001.Search in Google Scholar PubMed
250. Benhabiles, M. S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.; Mameri, N. Antibacterial Activity of Chitin, Chitosan and its Oligomers Prepared from Shrimp Shell Waste. Food Hydrocolloids 2012, 29 (1), 48–56. https://doi.org/10.1016/j.foodhyd.2012.02.013.Search in Google Scholar
251. Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides. J. Funct. Biomater. 2015, 6 (1), 33–49. https://doi.org/10.3390/jfb6010033.Search in Google Scholar PubMed PubMed Central
252. Muzzarelli, R. A. A. Chitins and Chitosans as Immunoadjuvants and Non-Allergenic Drug Carriers. Mar. Drugs 2010, 8 (2), 292–312. https://doi.org/10.3390/md8020292.Search in Google Scholar PubMed PubMed Central
253. Qiu, Y.; Zhang, N.; Kang, Q.; An, Y.; Wen, X. Fabrication of Permeable Tubular Constructs from Chemically Modified Chitosan with Enhanced Antithrombogenic Property. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2009, 90B (2), 668–678. https://doi.org/10.1002/jbm.b.31333.Search in Google Scholar PubMed
254. NorMazila Ramli, A.; Mahadi, N. M.; Rabu, A.; Murad, A. M.; Bakar, F. D.; Illias, R. M. Molecular Cloning, Expression and Biochemical Characterisation of a Cold-Adapted Novel Recombinant Chitinase from Glaciozyma Antarctica PI12. Microb. Cell Factories 2011, 10 (1). https://doi.org/10.1186/1475-2859-10-94.Search in Google Scholar PubMed PubMed Central
255. Singh, R.; Shitiz, K.; Singh, A. Chitin and Chitosan: Biopolymers for Wound Management. Int. Wound J. 2017, 14 (6), 1276–1289. https://doi.org/10.1111/iwj.12797.Search in Google Scholar PubMed PubMed Central
256. Kumar, A.; Vimal, A.; Kumar, A. Why Chitosan? From Properties to Perspective of Mucosal Drug Delivery. Int. J. Biol. Macromol. 2016, 91, 615–622. https://doi.org/10.1016/j.ijbiomac.2016.05.054.Search in Google Scholar PubMed
257. Jin, T.; Liu, T.; Lam, E.; Moores, A. Chitin and Chitosan on the Nanoscale. Nanoscale Horiz. 2021, 6 (7), 505–542. https://doi.org/10.1039/d0nh00696c.Search in Google Scholar PubMed
258. Dutta, P. K.; Dutta, J.; Tripathi, V. S. J. Sci. Ind. Res. 2004, 63, 20–31.10.5005/jp/books/10631_6Search in Google Scholar
259. Nagahama, H.; Nwe, N.; Jayakumar, R.; Koiwa, S.; Furuike, T.; Tamura, H. Novel Biodegradable Chitin Membranes for Tissue Engineering Applications. Carbohydr. Polym. 2008, 73 (2), 295–302. https://doi.org/10.1016/j.carbpol.2007.11.034.Search in Google Scholar
260. Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and Cells for Tissue Regeneration: Different Scaffold Pore Sizes–Different Cell Effects. Cytotechnology 2015, 68 (3), 355–369. https://doi.org/10.1007/s10616-015-9895-4.Search in Google Scholar PubMed PubMed Central
261. Ding, F.; Deng, H.; Du, Y.; Shi, X.; Wang, Q. Emerging Chitin and Chitosan Nanofibrous Materials for Biomedical Applications. Nanoscale 2014, 6 (16), 9477–9493. https://doi.org/10.1039/c4nr02814g.Search in Google Scholar PubMed
262. Shen, X.; Shamshina, J. L.; Berton, P.; Gurau, G.; Rogers, R. D. Hydrogels Based on Cellulose and Chitin: Fabrication, Properties, and Applications. Green Chem. 2016, 18 (1), 53–75. https://doi.org/10.1039/c5gc02396c.Search in Google Scholar
263. Wang, J.; Zhuang, S. Chitosan-Based Materials: Preparation, Modification and Application. J. Clean. Prod. 2022, 355, 131825. https://doi.org/10.1016/j.jclepro.2022.131825.Search in Google Scholar
264. Xing, X.; Han, Y.; Cheng, H. Biomedical Applications of Chitosan/Silk Fibroin Composites: A Review. Int. J. Biol. Macromol. 2023, 240, 124407. https://doi.org/10.1016/j.ijbiomac.2023.124407.Search in Google Scholar PubMed
265. Wan, A. C. A.; Tai, B. C. U. Chitin – A Promising Biomaterial for Tissue Engineering and Stem Cell Technologies. Biotechnol. Adv. 2013, 31 (8), 1776–1785. https://doi.org/10.1016/j.biotechadv.2013.09.007.Search in Google Scholar PubMed
266. Vazquez-Ayala, L.; Del Ángel-Olarte, C.; Escobar-García, D. M.; Rosales-Mendoza, S.; Solis-Andrade, I.; Pozos-Guillén, A.; Palestino, G. Chitosan Sponges Loaded with Metformin and Microalgae as Dressing for Wound Healing: A Study in Diabetic Bio-Models. Int. J. Biol. Macromol. 2024, 254, 127691. https://doi.org/10.1016/j.ijbiomac.2023.127691.Search in Google Scholar PubMed
267. Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a Starting Material for Wound Healing Applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. https://doi.org/10.1016/j.ejpb.2015.08.004.Search in Google Scholar PubMed
268. Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future – Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15 (4), 793. https://doi.org/10.3390/polym15040793.Search in Google Scholar PubMed PubMed Central
269. Dai, T.; Tanaka, M.; Huang, Y. Y.; Hamblin, M. R. Chitosan Preparations for Wounds and Burns: Antimicrobial and Wound-Healing Effects. Expert Rev. Anti-infect. Ther. 2011, 9 (7), 857–879. https://doi.org/10.1586/eri.11.59.Search in Google Scholar PubMed PubMed Central
270. Sánchez-Machado, D. I.; López-Cervantes, J.; Martínez-Ibarra, D. M.; Escárcega-Galaz, A. A.; Vega-Cázarez, C. A. The Use of Chitosan as a Skin-Regeneration Agent in Burns Injuries: A Review. E-Polymers 2022, 22 (1), 75–86. https://doi.org/10.1515/epoly-2022-0011.Search in Google Scholar
271. Muzzarelli, R.; Baldassarre, V.; Conti, F.; Ferrara, P.; Biagini, G.; Gazzanelli, G.; Vasi, V. Biological Activity of Chitosan: Ultrastructural Study. Biomaterials 1988, 9 (3), 247–252. https://doi.org/10.1016/0142-9612(88)90092-0.Search in Google Scholar PubMed
272. Kumar, P. T. S.; Abhilash, S.; Manzoor, K.; Nair, S.; Tamura, H.; Jayakumar, R. Preparation and Characterization of Novel β-Chitin/Nanosilver Composite Scaffolds for Wound Dressing Applications. Carbohydr. Polym. 2010, 80 (3), 761–767. https://doi.org/10.1016/j.carbpol.2009.12.024.Search in Google Scholar
273. Smolen, V. F. Bioavailability and Pharmacokinetic Analysis of Drug Responding Systems. Annu. Rev. Pharmacol. Toxicol. 1978, 18 (1), 495–522. https://doi.org/10.1146/annurev.pa.18.040178.002431.Search in Google Scholar PubMed
274. Cirillo, G.; Hampel, S.; Spizzirri, U. G.; Parisi, O. I.; Picci, N.; Iemma, F. Carbon Nanotubes Hybrid Hydrogels in Drug Delivery: A Perspective Review. BioMed Res. Int. 2014, 2014, 1–17. https://doi.org/10.1155/2014/825017.Search in Google Scholar PubMed PubMed Central
275. Coelho, J. F.; Ferreira, P. C.; Alves, P.; Cordeiro, R.; Fonseca, A. C.; Góis, J. R.; Gil, M. H. Drug Delivery Systems: Advanced Technologies Potentially Applicable in Personalized Treatments. EPMA J. 2010, 1 (1), 164–209. https://doi.org/10.1007/s13167-010-0001-x.Search in Google Scholar PubMed PubMed Central
276. Baharlouei, P.; Rahman, A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Mar. Drugs 2022, 20 (7), 460. https://doi.org/10.3390/md20070460.Search in Google Scholar PubMed PubMed Central
277. Gandhi, K. J.; Deshmane, S. V.; Biyani, K. R. Polymers in Pharmaceutical Drug Delivery System: A Review. Int. J. Pharm. Sci. Rev. Res. 2012, 14 (2), 57–66.Search in Google Scholar
278. Muhamad, I. I.; Selvakumaran, S.; Md Lazim, N. A. Designing Polymeric Nanoparticles for Targeted Drug Delivery System. Nanomed 2014, 287, 287.Search in Google Scholar
279. Ahmed, T. A.; Aljaeid, B. M. Preparation, Characterization, and Potential Application of Chitosan, Chitosan Derivatives, and Chitosan Metal Nanoparticles in Pharmaceutical Drug Delivery. Drug Des. Dev. Ther. 2016, 483–507.10.2147/DDDT.S99651Search in Google Scholar PubMed PubMed Central
280. Egorov, A. R.; Kirichuk, A. A.; Rubanik, V. V.; Rubanik, V. V.Jr.; Tskhovrebov, A. G.; Kritchenkov, A. S. Chitosan and its Derivatives: Preparation and Antibacterial Properties. Materials 2023, 16 (18), 6076. https://doi.org/10.3390/ma16186076.Search in Google Scholar PubMed PubMed Central
281. Ali, A.; Ahmed, S. A Review on Chitosan and its Nanocomposites in Drug Delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. https://doi.org/10.1016/j.ijbiomac.2017.12.078.Search in Google Scholar PubMed
282. Parhi, R. Drug Delivery Applications of Chitin and Chitosan: A Review. Environ. Chem. Lett. 2020, 18 (3), 577–594. https://doi.org/10.1007/s10311-020-00963-5.Search in Google Scholar
283. Chuan, D.; Jin, T.; Fan, R.; Zhou, L.; Guo, G. Chitosan for Gene Delivery: Methods for Improvement and Applications. Adv. Colloid Interface Sci. 2019, 268, 25–38. https://doi.org/10.1016/j.cis.2019.03.007.Search in Google Scholar PubMed
284. Pillé, J.-Y.; Li, H.; Blot, E.; Bertrand, J. R.; Pritchard, L. L.; Opolon, P.; Maksimenko, A.; Lu, H.; Vannier, J. P.; Soria, J.; Malvy, C.; Soria, C. Intravenous Delivery of Anti-RhoA Small Interfering RNA Loaded in Nanoparticles of Chitosan in Mice: Safety and Efficacy in Xenografted Aggressive Breast Cancer. Hum. Gene Ther. 2006, 17 (10), 1019–1026. https://doi.org/10.1089/hum.2006.17.1019.Search in Google Scholar PubMed
285. Murata, J.; Saiki, I.; Nishimura, S.; Nishi, N.; Tokura, S.; Azuma, I. Inhibitory Effect of Chitin Heparinoids on the Lung Metastasis of B16-BL6 Melanoma. Jpn. J. Cancer Res. 1989, 80 (9), 866–872. https://doi.org/10.1111/j.1349-7006.1989.tb01728.x.Search in Google Scholar PubMed PubMed Central
286. Park, J. K.; Chung, M. J.; Choi, H. N.; Park, Y. I. Effects of the Molecular Weight and the Degree of Deacetylation of Chitosan Oligosaccharides on Antitumor Activity. Int. J. Mol. Sci. 2011, 12 (1), 266–277. https://doi.org/10.3390/ijms12010266.Search in Google Scholar PubMed PubMed Central
287. Suzuki, K.; Mikami, T.; Okawa, Y.; Tokoro, A.; Suzuki, S.; Suzuki, M. Antitumor Effect of Hexa-N-Acetylchitohexaose and Chitohexaose. Carbohydr. Res. 1986, 151, 403–408. https://doi.org/10.1016/s0008-6215(00)90359-8.Search in Google Scholar PubMed
288. Jeon, Y.-J.; Kim, S.-K. Antitumor Activity of Chitosan Oligosaccharides Produced in Ultrafiltration Membrane Reactor System. J. Microbiol. Biotechnol. 2002, 12 (3), 503–507.Search in Google Scholar
289. Salah, R.; Michaud, P.; Mati, F.; Harrat, Z.; Lounici, H.; Abdi, N.; Drouiche, N.; Mameri, N. Anticancer Activity of Chemically Prepared Shrimp Low Molecular Weight Chitin Evaluation with the Human Monocyte Leukaemia Cell Line, THP-1. Int. J. Biol. Macromol. 2013, 52, 333–339. https://doi.org/10.1016/j.ijbiomac.2012.10.009.Search in Google Scholar PubMed
290. Huang, M.; Khor, E.; Lim, L.-Y. Uptake and Cytotoxicity of Chitosan Molecules and Nanoparticles: Effects of Molecular Weight and Degree of Deacetylation. Pharm. Res. 2004, 21 (2), 344–353. https://doi.org/10.1023/b:pham.0000016249.52831.a5.10.1023/B:PHAM.0000016249.52831.a5Search in Google Scholar PubMed
291. Younes, I.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Cytotoxicity of Chitosans with Different Acetylation Degrees and Molecular Weights on Bladder Carcinoma Cells. Int. J. Biol. Macromol. 2016, 84, 200–207. https://doi.org/10.1016/j.ijbiomac.2015.09.031.Search in Google Scholar PubMed
292. Karagozlu, M. Z.; Kim, S.-K. Anticancer Effects of Chitin and Chitosan Derivatives. In Marine Carbohydrates: Fundamentals and Applications, Part A; Academic Press: Waltham, USA, 2014; pp 215–225.10.1016/B978-0-12-800269-8.00012-9Search in Google Scholar PubMed
293. Przekora, A. The Summary of the Most Important Cell-Biomaterial Interactions that Need to be Considered during In Vitro Biocompatibility Testing of Bone Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C 2019, 97, 1036–1051. https://doi.org/10.1016/j.msec.2019.01.061.Search in Google Scholar PubMed
294. Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of Chitin and Chitosan Nanofibers in Bone Regenerative Engineering. Carbohydr. Polym. 2020, 230, 115658. https://doi.org/10.1016/j.carbpol.2019.115658.Search in Google Scholar PubMed
295. Dresselhaus, M. S.; Ajayan, P. M.; Zhou, O. Z. Applications of Carbon Nanotubes. In Carbon Nanotubes-Synthesis, Structure, Properties, and Applications; Springer: Berlin, Heidelberg, 2001; pp 391–425.Search in Google Scholar
296. Wang, S.-F.; Shen, L.; Zhang, W. D.; Tong, Y. J. Preparation and Mechanical Properties of Chitosan/Carbon Nanotubes Composites. Biomacromolecules 2005, 6 (6), 3067–3072. https://doi.org/10.1021/bm050378v.Search in Google Scholar PubMed
297. Kucharska, M.; Walenko, K.; Lewandowska-Szumieł, M.; Brynk, T.; Jaroszewicz, J.; Ciach, T. Chitosan and Composite Microsphere-Based Scaffold for Bone Tissue Engineering: Evaluation of Tricalcium Phosphate Content Influence on Physical and Biological Properties. J. Mater. Sci.: Mater. Med. 2015, 26 (3). https://doi.org/10.1007/s10856-015-5464-9.Search in Google Scholar PubMed
298. Zhou, D.; Qi, C.; Chen, Y. X.; Zhu, Y. J.; Sun, T. W.; Chen, F.; Zhang, C. Q. Comparative Study of Porous Hydroxyapatite/Chitosan and Whitlockite/Chitosan Scaffolds for Bone Regeneration in Calvarial Defects. Int. J. Nanomed. 2017, 12, 2673–2687. https://doi.org/10.2147/ijn.s131251.Search in Google Scholar PubMed PubMed Central
299. Hu, Q. Preparation and Characterization of Biodegradable Chitosan/Hydroxyapatite Nanocomposite Rods via In Situ Hybridization: A Potential Material as Internal Fixation of Bone Fracture. Biomaterials 2004, 25 (5), 779–785. https://doi.org/10.1016/s0142-9612(03)00582-9.Search in Google Scholar PubMed
300. Todd, E. A.; Mirsky, N. A.; Silva, B. L. G.; Shinde, A. R.; Arakelians, A. R. L.; Nayak, V. V.; Marcantonio, R. A. C.; Gupta, N.; Witek, L.; Coelho, P. G. Functional Scaffolds for Bone Tissue Regeneration: A Comprehensive Review of Materials, Methods, and Future Directions. J. Funct. Biomater. 2024, 15 (10), 280. https://doi.org/10.3390/jfb15100280.Search in Google Scholar PubMed PubMed Central
301. Reddy, M. S. B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K. K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13 (7), 1105. https://doi.org/10.3390/polym13071105.Search in Google Scholar PubMed PubMed Central
302. Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D. A.; Quiñones-Olvera, L. F. Chitosan and its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 1–15. https://doi.org/10.1155/2015/821279.Search in Google Scholar PubMed PubMed Central
303. Zhao, D.; Aili, A.; Zhai, Y.; Xu, S.; Tan, G.; Yin, X.; Yang, R. Radiative Sky Cooling: Fundamental Principles, Materials, and Applications. Appl. Phys. Rev. 2019, 6 (2). https://doi.org/10.1063/1.5087281.Search in Google Scholar
304. Chen, M.; Pang, D.; Chen, X.; Yan, H.; Yang, Y. Passive Daytime Radiative Cooling: Fundamentals, Material Designs, and Applications. EcoMat 2021, 4 (1). https://doi.org/10.1002/eom2.12153.Search in Google Scholar
305. Tsai, C.-C.; Childers, R. A.; Nan Shi, N.; Ren, C.; Pelaez, J. N.; Bernard, G. D.; Pierce, N. E.; Yu, N. Physical and Behavioral Adaptations to Prevent Overheating of the Living Wings of Butterflies. Nat. Commun. 2020, 11 (1). https://doi.org/10.1038/s41467-020-14408-8.Search in Google Scholar PubMed PubMed Central
306. Wu, W.; Lin, S.; Wei, M.; Huang, J.; Xu, H.; Lu, Y.; Song, W. Flexible Passive Radiative Cooling Inspired by Saharan Silver Ants. Sol. Energy Mater. Sol. Cells 2020, 210, 110512. https://doi.org/10.1016/j.solmat.2020.110512.Search in Google Scholar
307. Willot, Q.; Simonis, P.; Vigneron, J. P.; Aron, S. Total Internal Reflection Accounts for the Bright Color of the Saharan Silver Ant. PLoS One 2016, 11 (4), e0152325. https://doi.org/10.1371/journal.pone.0152325.Search in Google Scholar PubMed PubMed Central
308. Shi, N. N.; Tsai, C. C.; Camino, F.; Bernard, G. D.; Yu, N.; Wehner, R. Keeping Cool: Enhanced Optical Reflection and Radiative Heat Dissipation in Saharan Silver Ants. Science 2015, 349 (6245), 298–301. https://doi.org/10.1126/science.aab3564.Search in Google Scholar PubMed
309. Bohren, C. F.; Huffman, D. R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: Weinheim, Germany, 2008.Search in Google Scholar
310. Schuller, J. A.; Taubner, T.; Brongersma, M. L. Optical Antenna Thermal Emitters. Nat. Photonics 2009, 3 (11), 658–661. https://doi.org/10.1038/nphoton.2009.188.Search in Google Scholar
311. Zimmerl, M.; van Nieuwenhoven, R.; Whitmore, K.; Vetter, W.; Gebeshuber, I. Biomimetic Cooling: Functionalizing Biodegradable Chitosan Films with Saharan Silver Ant Microstructures. Biomimetics 2024, 9 (10), 630. https://doi.org/10.3390/biomimetics9100630.Search in Google Scholar PubMed PubMed Central
312. Huang, W. Impact Resistant and Energy Absorbent Natural Keratin Materials: Horns and Hooves; University of California: San Diego, 2018.Search in Google Scholar
313. Steinert, P. M. Structure of the Three-Chain Unit of the Bovine Epidermal Keratin Filament. J. Mol. Biol. 1978, 123 (1), 49–70. https://doi.org/10.1016/0022-2836(78)90376-5.Search in Google Scholar PubMed
314. Shavandi, A.; Silva, T. H.; Bekhit, A. A.; Bekhit, A. E. D. A. Keratin: Dissolution, Extraction and Biomedical Application. Biomater. Sci. 2017, 5 (9), 1699–1735. https://doi.org/10.1039/c7bm00411g.Search in Google Scholar PubMed
315. Suzuta, K. Studies on the Disulfide Cross-Linked Structure and Mechanical Properties of Keratin Fibers. Ph.D. Thesis, Shinshu University, Japan, 2015.Search in Google Scholar
316. Barthlott, W.; Neinhuis, C. Purity of the Sacred lotus, or Escape from Contamination in Biological Surfaces. Planta 1997, 202 (1), 1–8. https://doi.org/10.1007/s004250050096.Search in Google Scholar
317. Wang, S.; Yang, Z.; Gong, G.; Wang, J.; Wu, J.; Yang, S.; Jiang, L. Icephobicity of Penguins Spheniscus Humboldti and an Artificial Replica of Penguin Feather with Air-Infused Hierarchical Rough Structures. J. Phys. Chem. C 2016, 120 (29), 15923–15929. https://doi.org/10.1021/acs.jpcc.5b12298.Search in Google Scholar
318. Michels, J.; Appel, E.; Gorb, S. N. Coupling Wings with Movable Hooks – Resilin in the Wing-Interlocking Structures of Honeybees. Arthropod Struct. Dev. 2021, 60, 101008. https://doi.org/10.1016/j.asd.2020.101008.Search in Google Scholar PubMed
319. Mainwaring, D. E.; Nguyen, S. H.; Webb, H.; Jakubov, T.; Tobin, M.; Lamb, R. N.; Wu, A. H. F.; Marchant, R.; Crawford, R. J.; Ivanova, E. P. The Nature of Inherent Bactericidal Activity: Insights from the Nanotopology of Three Species of Dragonfly. Nanoscale 2016, 8 (12), 6527–6534. https://doi.org/10.1039/c5nr08542j.Search in Google Scholar PubMed
320. Green, D. W.; Lee, K. K. H.; Watson, J. A.; Kim, H. Y.; Yoon, K. S.; Kim, E. J.; Lee, J. M.; Watson, G. S.; Jung, H. S. High Quality Bioreplication of Intricate Nanostructures from a Fragile Gecko Skin Surface with Bactericidal Properties. Sci. Rep. 2017, 7 (1). https://doi.org/10.1038/srep41023.Search in Google Scholar PubMed PubMed Central
321. Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P. K. D. V. Bio-Mimicking Nano and Micro-Structured Surface Fabrication for Antibacterial Properties in Medical Implants. J. Nanobiotechnol. 2017, 15 (1). https://doi.org/10.1186/s12951-017-0306-1.Search in Google Scholar PubMed PubMed Central
322. Hasan, J.; Webb, H. K.; Truong, V. K.; Pogodin, S.; Baulin, V. A.; Watson, G. S.; Watson, J. A.; Crawford, R. J.; Ivanova, E. P. Selective Bactericidal Activity of Nanopatterned Superhydrophobic Cicada Psaltoda Claripennis Wing Surfaces. Appl. Microbiol. Biotechnol. 2012, 97 (20), 9257–9262. https://doi.org/10.1007/s00253-012-4628-5.Search in Google Scholar PubMed
323. Li, X.; Cheung, G. S.; Watson, G. S.; Watson, J. A.; Lin, S.; Schwarzkopf, L.; Green, D. W. The Nanotipped Hairs of Gecko Skin and Biotemplated Replicas Impair and/or Kill Pathogenic Bacteria with High Efficiency. Nanoscale 2016, 8 (45), 18860–18869. https://doi.org/10.1039/c6nr05046h.Search in Google Scholar PubMed
324. Ma, M.; Hill, R. M. Superhydrophobic Surfaces. Curr. Opin. Colloid Interface Sci. 2006, 11 (4), 193–202. https://doi.org/10.1016/j.cocis.2006.06.002.Search in Google Scholar
325. Watson, G. S.; Green, D. W.; Schwarzkopf, L.; Li, X.; Cribb, B. W.; Myhra, S.; Watson, J. A. A Gecko Skin Micro/Nano Structure – A Low Adhesion, Superhydrophobic, Anti-wetting, Self-Cleaning, Biocompatible, Antibacterial Surface. Acta Biomater. 2015, 21, 109–122. https://doi.org/10.1016/j.actbio.2015.03.007.Search in Google Scholar PubMed
326. Liu, K.; Jiang, L. Bio-Inspired Self-Cleaning Surfaces. Annu. Rev. Mater. Res. 2012, 42 (1), 231–263. https://doi.org/10.1146/annurev-matsci-070511-155046.Search in Google Scholar
327. Christie, R. Colour Chemistry; Royal Society of Chemistry: London, UK, 2014.10.1039/9781782626510Search in Google Scholar
328. Zollinger, H. Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments; John Wiley & Sons: Weinheim, Germany, 2003.Search in Google Scholar
329. Jeon, D.-J.; Ji, S.; Lee, E.; Kang, J.; Kim, J.; D’Alba, L.; Manceau, M.; Shawkey, M. D.; Yeo, J. S. How Keratin Cortex Thickness Affects Iridescent Feather Colours. R. Soc. Open Sci. 2023, 10 (1). https://doi.org/10.1098/rsos.220786.Search in Google Scholar PubMed PubMed Central
330. Yoda, M.; Minami, G.; Garden, J.-L.; Bourgeois, O.; Haque, A.; Kumar, A.; Deyhle, H.; Hieber, S.; Müller, B.; Cano, M.; Maspoch, D.; Sobolev, K.; Sanchez, F.; Jabbari, E.; Nevill, J.; Malleo, D.; Bøggild, P.; Chen, W.; Wang, C.; Bhushan, B.; Rao, A. Nano-FET. In Encyclopedia of Nanotechnology; Springer Netherlands: Dorthrecht, Netherlands, 2012; p 1543.10.1007/978-90-481-9751-4_100497Search in Google Scholar
331. Gruson, H.; Elias, M.; Andraud, C.; Djediat, C.; Berthier, S.; Doutrelant, C.; Gomez, D. Hummingbird Iridescence: An Unsuspected Structural Diversity Influences Colouration at Multiple Scales. 2019. https://doi.org/10.1101/699744.Search in Google Scholar
332. Saranathan, V.; Finet, C. Cellular and Developmental Basis of Avian Structural Coloration. Curr. Opin. Genet. Dev. 2021, 69, 56–64. https://doi.org/10.1016/j.gde.2021.02.004.Search in Google Scholar PubMed
333. Prum, R. O.; Dufresne, E. R.; Quinn, T.; Waters, K. Development of Colour-Producing β-Keratin Nanostructures in Avian Feather Barbs. J. R. Soc. Interface 2009, 6 (Suppl. 2). https://doi.org/10.1098/rsif.2008.0466.focus.Search in Google Scholar PubMed PubMed Central
334. Burg, S. L.; Parnell, A. J. Self-Assembling Structural Colour in Nature. J. Phys.: Condens. Matter 2018, 30 (41), 413001. https://doi.org/10.1088/1361-648x/aadc95.Search in Google Scholar
335. Lee, S.; Novitskaya, E. E.; Reynante, B.; Vasquez, J.; Urbaniak, R.; Takahashi, T.; Woolley, E.; Tombolato, L.; Chen, P. Y.; McKittrick, J. Impact Testing of Structural Biological Materials. Mater. Sci. Eng. C 2011, 31 (4), 730–739. https://doi.org/10.1016/j.msec.2010.10.017.Search in Google Scholar
336. Bertram, J. E. A.; Gosline, J. M. Functional Design of Horse Hoof Keratin: The Modulation of Mechanical Properties Through Hydration Effects. J. Exp. Biol. 1987, 130 (1), 121–136. https://doi.org/10.1242/jeb.130.1.121.Search in Google Scholar PubMed
337. Huang, W.; Yaraghi, N. A.; Yang, W.; Velazquez-Olivera, A.; Li, Z.; Ritchie, R. O.; Kisailus, D.; Stover, S. M.; McKittrick, J. A Natural Energy Absorbent Polymer Composite: The Equine Hoof Wall. Acta Biomater. 2019, 90, 267–277. https://doi.org/10.1016/j.actbio.2019.04.003.Search in Google Scholar PubMed
338. Collins, S. N.; Cope, B. C.; Hopegood, L.; Latham, R. J.; Linford, R. G.; Reilly, J. D. Stiffness as a Function of Moisture Content in Natural Materials: Characterisation of Hoof Horn Samples. J. Mater. Sci. 1998, 33 (21), 5185–5191. https://doi.org/10.1023/a:1004479803611.10.1023/A:1004479803611Search in Google Scholar
339. Lazarus, B. S.; Luu, R. K.; Ruiz-Pérez, S.; Bezerra, W. B. A.; Becerra-Santamaria, K.; Leung, V.; Durazo, V. H. L.; Jasiuk, I.; Barbosa, J. D.; Meyers, M. A. Equine Hoof Wall: Structure, Properties, and Bioinspired Designs. Acta Biomater. 2022, 151, 426–445. https://doi.org/10.1016/j.actbio.2022.08.028.Search in Google Scholar PubMed
340. Setterbo, J. J.; Garcia, T. C.; Campbell, I. P.; Reese, J. L.; Morgan, J. M.; Kim, S. Y.; Hubbard, M.; Stover, S. M. Hoof Accelerations and Ground Reaction Forces of Thoroughbred Racehorses Measured on Dirt, Synthetic, and Turf Track Surfaces. Am. J. Vet. Res. 2009, 70 (10), 1220–1229. https://doi.org/10.2460/ajvr.70.10.1220.Search in Google Scholar PubMed
341. Kasapi, M. A.; Gosline, J. M. Design Complexity and Fracture Control in the Equine Hoof Wall. J. Exp. Biol. 1997, 200 (11), 1639–1659. https://doi.org/10.1242/jeb.200.11.1639.Search in Google Scholar PubMed
342. Kasapi, M. A.; Gosline, J. M. Micromechanics of the Equine Hoof Wall: Optimizing Crack Control and Material Stiffness through Modulation of the Properties of Keratin. J. Exp. Biol. 1999, 202 (4), 377–391. https://doi.org/10.1242/jeb.202.4.377.Search in Google Scholar PubMed
343. Quan, H.; Kisailus, D.; Meyers, M. A. Hydration-Induced Reversible Deformation of Biological Materials. Nat. Rev. Mater. 2020, 6 (3), 264–283. https://doi.org/10.1038/s41578-020-00251-2.Search in Google Scholar
344. Farren, L.; Shayler, S.; Ennos, A. R. The Fracture Properties and Mechanical Design of Human Fingernails. J. Exp. Biol. 2004, 207 (5), 735–741. https://doi.org/10.1242/jeb.00814.Search in Google Scholar PubMed
345. Johnson, K. L.; Trim, M.; Francis, D.; Whittington, W.; Miller, J.; Bennett, C.; Horstemeyer, M. Moisture, Anisotropy, Stress State, and Strain Rate Effects on Bighorn Sheep Horn Keratin Mechanical Properties. Acta Biomater. 2017, 48, 300–308. https://doi.org/10.1016/j.actbio.2016.10.033.Search in Google Scholar PubMed
346. Ma, Y.-S.; Kuo, F. M.; Liu, T. H.; Lin, Y. T.; Yu, J.; Wei, Y. Exploring Keratin Composition Variability for Sustainable Thermal Insulator Design. Int. J. Biol. Macromol. 2024, 275, 133690. https://doi.org/10.1016/j.ijbiomac.2024.133690.Search in Google Scholar PubMed
347. McCafferty, D. J.; Pandraud, G.; Gilles, J.; Fabra-Puchol, M.; Henry, P. Y. Animal Thermoregulation: A Review of Insulation, Physiology and Behaviour Relevant to Temperature Control in Buildings. Bioinspiration Biomimetics 2017, 13 (1), 011001. https://doi.org/10.1088/1748-3190/aa9a12.Search in Google Scholar PubMed
348. Williams, C. L.; Hagelin, J. C.; Kooyman, G. L. Hidden Keys to Survival: The Type, Density, Pattern and Functional Role of Emperor Penguin Body Feathers. Proc. R. Soc. B: Biol. Sci. 2015, 282 (1817), 20152033. https://doi.org/10.1098/rspb.2015.2033.Search in Google Scholar PubMed PubMed Central
349. D’alba, L.; Carlsen, T. H.; Ásgeirsson, Á.; Shawkey, M. D.; Jónsson, J. E. Contributions of Feather Microstructure to Eider Down Insulation Properties. J. Avian Biol. 2017, 48 (8), 1150–1157. https://doi.org/10.1111/jav.01294.Search in Google Scholar
350. Sullivan, T. N.; Chon, M.; Ramachandramoorthy, R.; Roenbeck, M. R.; Hung, T.; Espinosa, H. D.; Meyers, M. A. Reversible Attachment with Tailored Permeability: The Feather Vane and Bioinspired Designs. Adv. Funct. Mater. 2017, 27 (39). https://doi.org/10.1002/adfm.201702954.Search in Google Scholar
351. Lu, Y.; Chen, Z.; Guo, E.; Kong, X.; Kang, H.; Xu, Y.; Li, R.; Fan, G.; Wang, T. Micro Hierarchical Structure and Mechanical Property of Sparrow Hawk (Accipiter nisus) Feather Shaf. Comput. Model. Eng. Sci. 2021, 127 (2), 705–720. https://doi.org/10.32604/cmes.2021.015426.Search in Google Scholar
352. Liu, L.; Wang, Y.; Zhao, J.; Cai, Z.; Guo, C. Multiscale Characterization and Biomimetic Design of Porcupine Quills for Enhanced Mechanical Performance. Materials 2024, 17 (9), 1949. https://doi.org/10.3390/ma17091949.Search in Google Scholar PubMed PubMed Central
353. Shao, Z.; Wang, Y.; Bai, H. A Superhydrophobic Textile Inspired by Polar Bear Hair for Both in Air and Underwater Thermal Insulation. Chem. Eng. J. 2020, 397, 125441. https://doi.org/10.1016/j.cej.2020.125441.Search in Google Scholar
354. Zhao, N.; Wang, Z.; Cai, C.; Shen, H.; Liang, F.; Wang, D.; Wang, C.; Zhu, T.; Guo, J.; Wang, Y.; Liu, X.; Duan, C.; Wang, H.; Mao, Y.; Jia, X.; Dong, H.; Zhang, X.; Xu, J. Bioinspired Materials: From Low to High Dimensional Structure. Adv. Mater. 2014, 26 (41), 6994–7017. https://doi.org/10.1002/adma.201401718.Search in Google Scholar PubMed
355. Bhushan, B. Biomimetics: Lessons from Nature – An Overview. Phil. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2009, 367 (1893), 1445–1486. https://doi.org/10.1098/rsta.2009.0011.Search in Google Scholar PubMed
356. Metwally, S.; Martínez Comesaña, S.; Zarzyka, M.; Szewczyk, P. K.; Karbowniczek, J. E.; Stachewicz, U. Thermal Insulation Design Bioinspired by Microstructure Study of Penguin Feather and Polar Bear Hair. Acta Biomater. 2019, 91, 270–283. https://doi.org/10.1016/j.actbio.2019.04.031.Search in Google Scholar PubMed
357. Zhuge, Y.; Liu, F. Recent Progress of Bionic Hierarchical Structure in the Field of Thermal Insulation Protection. J. Bionic Eng. 2023, 21 (1), 1–18. https://doi.org/10.1007/s42235-023-00425-y.Search in Google Scholar
358. Dresp, B.; Langley, K. Fine Structural Dependence of Ultraviolet Reflections in the King Penguin Beak Horn. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2006, 288A (3), 213–222. https://doi.org/10.1002/ar.a.20314.Search in Google Scholar PubMed
359. Greenwold, M. J.; Bao, W.; Jarvis, E. D.; Hu, H.; Li, C.; Gilbert, M. T. P.; Zhang, G.; Sawyer, R. H. Dynamic Evolution of the Alpha (α) and Beta (β) Keratins Has Accompanied Integument Diversification and the Adaptation of Birds into Novel Lifestyles. BMC Evol. Biol. 2014, 14 (1). https://doi.org/10.1186/s12862-014-0249-1.Search in Google Scholar PubMed PubMed Central
360. Tee, Y. L.; Maconachie, T.; Pille, P.; Leary, M.; Do, T.; Tran, P. From Nature to Additive Manufacturing: Biomimicry of Porcupine Quill. Mater. Des. 2021, 210, 110041. https://doi.org/10.1016/j.matdes.2021.110041.Search in Google Scholar
361. Seki, Y.; Kad, B.; Benson, D.; Meyers, M. A. The Toucan Beak: Structure and Mechanical Response. Mater. Sci. Eng. C 2006, 26 (8), 1412–1420. https://doi.org/10.1016/j.msec.2005.08.025.Search in Google Scholar
362. Lingham-Soliar, T. Feather Structure, Biomechanics and Biomimetics: The Incredible Lightness of Being. J. Ornithol. 2014, 155 (2), 323–336. https://doi.org/10.1007/s10336-013-1038-0.Search in Google Scholar
363. Achrai, B.; Bar-On, B.; Wagner, H. D. Bending Mechanics of the Red-Eared Slider Turtle Carapace. J. Mech. Behav. Biomed. Mater. 2014, 30, 223–233. https://doi.org/10.1016/j.jmbbm.2013.09.009.Search in Google Scholar PubMed
364. Sahu, S. K.; Sreekanth, P. S. R.; Reddy, S. V. K. A Brief Review on Advanced Sandwich Structures with Customized Design Core and Composite Face Sheet. Polymers 2022, 14 (20), 4267. https://doi.org/10.3390/polym14204267.Search in Google Scholar PubMed PubMed Central
365. Sullivan, T. N.; Pissarenko, A.; Herrera, S. A.; Kisailus, D.; Lubarda, V. A.; Meyers, M. A. A Lightweight, Biological Structure with Tailored Stiffness: The Feather Vane. Acta Biomater. 2016, 41, 27–39. https://doi.org/10.1016/j.actbio.2016.05.022.Search in Google Scholar PubMed
366. Liu, Z. Q.; Jiao, D.; Meyers, M.; Zhang, Z. Structure and Mechanical Properties of Naturally Occurring Lightweight Foam-Filled Cylinder – The Peacock’s Tail Coverts Shaft and its Components. Acta Biomater. 2015, 17, 137–151. https://doi.org/10.1016/j.actbio.2015.01.035.Search in Google Scholar PubMed
367. Yang, W.; Chao, C.; McKittrick, J. Axial Compression of a Hollow Cylinder Filled with Foam: A Study of Porcupine Quills. Acta Biomater. 2013, 9 (2), 5297–5304. https://doi.org/10.1016/j.actbio.2012.09.004.Search in Google Scholar PubMed
368. Wang, B.; Sullivan, T. N.; Pissarenko, A.; Zaheri, A.; Espinosa, H. D.; Meyers, M. A. Lessons from the Ocean: Whale Baleen Fracture Resistance. Adv. Mater. 2018, 31 (3). https://doi.org/10.1002/adma.201804574.Search in Google Scholar PubMed
369. Vincent, J. F. V.; Owers, P. Mechanical Design of Hedgehog Spines and Porcupine Quills. J. Zool. 1986, 210 (1), 55–75. https://doi.org/10.1111/j.1469-7998.1986.tb03620.x.Search in Google Scholar
370. Attenborough, D. Life on Earth: A Natural History; Little Brown & Company: Boston, United States, 1979.Search in Google Scholar
371. Hansen, W. R.; Autumn, K. Evidence for Self-Cleaning in Gecko Setae. Proc. Natl. Acad. Sci. 2005, 102 (2), 385–389. https://doi.org/10.1073/pnas.0408304102.Search in Google Scholar PubMed PubMed Central
372. Wu, J.; Kim, S.; Chen, W.; Carlson, A.; Hwang, K. C.; Huang, Y.; Rogers, J. A. Mechanics of Reversible Adhesion. Soft Matter 2011, 7 (18), 8657–8662. https://doi.org/10.1039/c1sm05915g.Search in Google Scholar
373. Arzt, E.; Gorb, S.; Ralph, S. From Micro to Nano Contacts in Biological Attachment Devices. Proc. Natl. Acad. Sci. 2003, 100 (19), 10603–10606. https://doi.org/10.1073/pnas.1534701100.Search in Google Scholar PubMed PubMed Central
374. Gorb, S. N. Biological Attachment Devices: Exploring Nature’s Diversity for Biomimetics. Phil. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2008, 366 (1870), 1557–1574. https://doi.org/10.1098/rsta.2007.2172.Search in Google Scholar PubMed
375. Palacio, M. L. B.; Bhushan, B.; Schricker, S. R. Gecko-Inspired Fibril Nanostructures for Reversible Adhesion in Biomedical Applications. Mater. Lett. 2013, 92, 409–412. https://doi.org/10.1016/j.matlet.2012.11.023.Search in Google Scholar
376. Autumn, K.; Sitti, M.; Liang, Y. A.; Peattie, A. M.; Hansen, W. R.; Sponberg, S.; Kenny, T. W.; Fearing, R.; Israelachvili, J. N.; Full, R. J. Evidence for van der Waals Adhesion in Gecko Setae. Proc. Natl. Acad. Sci. 2002, 99 (19), 12252–12256. https://doi.org/10.1073/pnas.192252799.Search in Google Scholar PubMed PubMed Central
377. Alibardi, L. Cell Organization of Barb Ridges in Regenerating Feathers of the Quail: Implications of the Elongation of Barb Ridges for the Evolution and Diversification of Feathers. Acta Zool. 2007, 88 (2), 101–117. https://doi.org/10.1111/j.1463-6395.2007.00257.x.Search in Google Scholar
378. Tang, D.; Liu, D.; Fan, Z. Study on the Interlocking Mechanism of Barbules of an Eagle Feather and the Corresponding Microstructures to Reconstitute Their Integrity. J. Test. Eval. 2020, 48 (3), 2494–2504. https://doi.org/10.1520/jte20190475.Search in Google Scholar
379. Chang, W.-L.; Wu, H.; Chiu, Y. K.; Wang, S.; Jiang, T. X.; Luo, Z. L.; Lin, Y. C.; Hsu, J. T.; Huang, H. L.; Gu, H. J.; Lin, T. Y.; Yang, S. M.; Lee, T. T.; Lai, Y. C.; Lei, M.; Shie, M. Y.; Yao, C. T.; Chen, Y. W.; Tsai, J.; Shieh, S. J.; Hwu, Y. K.; Cheng, H. C.; Tang, P. C.; Hung, S. C.; Chen, C. F.; Habib, M.; Widelitz, R. B.; Wu, P.; Juan, W. T.; Chuong, C. M. The Making of a Flight Feather: Bio-Architectural Principles and Adaptation. Cell 2019, 179 (6), 1409–1423.e17. https://doi.org/10.1016/j.cell.2019.11.008.Search in Google Scholar PubMed PubMed Central
380. Roland Ennos, A.; Hickson, J. R. E.; Roberts, A. Functional Morphology of the Vanes of the Flight Feathers of the Pigeon Columba Livia. J. Exp. Biol. 1995, 198 (5), 1219–1228. https://doi.org/10.1242/jeb.198.5.1219.Search in Google Scholar PubMed
381. Dyck, J. The Evolution of Feathers*. Zool. Scripta 1985, 14 (2), 137–154. https://doi.org/10.1111/j.1463-6409.1985.tb00184.x.Search in Google Scholar
382. Gebeshuber, I. C. Bird Biomimetics. In Newsletter of the Malaysian Nature Society, 2014; p. 4. https://www2.iap.tuwien.ac.at/∼gebeshuber/MNS_Newsletter_Dec_2014.pdf.Search in Google Scholar
383. Li, X.; Tao, D.; Lu, H.; Bai, P.; Liu, Z.; Ma, L.; Meng, Y.; Tian, Y. Recent Developments in Gecko-Inspired Dry Adhesive Surfaces from Fabrication to Application. Surf. Topogr. Metrol. Prop. 2019, 7 (2), 023001. https://doi.org/10.1088/2051-672x/ab1447.Search in Google Scholar
384. Russell, A. P.; Stark, A. Y.; Higham, T. E. The Integrative Biology of Gecko Adhesion: Historical Review, Current Understanding, and Grand Challenges. Integr. Comp. Biol. 2019, 59 (1), 101–116. https://doi.org/10.1093/icb/icz032.Search in Google Scholar PubMed
385. Boesel, L. F.; Greiner, C.; Arzt, E.; del Campo, A. Gecko-Inspired Surfaces: A Path to Strong and Reversible Dry Adhesives. Adv. Mater. 2010, 22 (19), 2125–2137. https://doi.org/10.1002/adma.200903200.Search in Google Scholar PubMed
386. Kwak, J.-S.; Kim, T.-W. A Review of Adhesion and Friction Models for Gecko Feet. Int. J. Precis. Eng. Manuf. 2010, 11 (1), 171–186. https://doi.org/10.1007/s12541-010-0020-5.Search in Google Scholar
387. Prowse, M. Environmental Effects on the Mechanical Properties and Adhesion of Gecko Setae. Ph.D. Thesis, University of Washington, 2012.Search in Google Scholar
388. Israelachvili, J. N.; Tabor, D. Van der Waals Forces: Theory and Experiment. In Progress in Surface and Membrane Science; Academic Press: New York, Vol. 7, 1973; pp 1–55.10.1016/B978-0-12-571807-3.50006-5Search in Google Scholar
389. Hamaker, H. C. The London – van der Waals Attraction Between Spherical Particles. Physica 1937, 4 (10), 1058–1072. https://doi.org/10.1016/s0031-8914(37)80203-7.Search in Google Scholar
390. Hiller, U. Untersuchungen zum Feinbau und zur Funktion der Haftborsten von Reptilien. Z. Morphol. Tiere 1968, 62 (4), 307–362. https://doi.org/10.1007/bf00401561.Search in Google Scholar
391. Stork, N. E. Experimental Analysis of Adhesion of Chrysolina Polita (Chrysomelidae: Coleoptera) on A Variety of Surfaces. J. Exp. Biol. 1980, 88 (1), 91–108. https://doi.org/10.1242/jeb.88.1.91.Search in Google Scholar
392. Fan, P. L.; O’Brien, W. J. Adhesion in Deformable Isolated Capillaries. Adhes. Sci. Technol. 1975, 635–646. https://doi.org/10.1007/978-1-4613-4331-8_14.Search in Google Scholar
393. Orr, F. M.; Scriven, L. E.; Rivas, A. P. Pendular Rings between Solids: Meniscus Properties and Capillary Force. J. Fluid Mech. 1975, 67 (4), 723–742. https://doi.org/10.1017/s0022112075000572.Search in Google Scholar
394. Bhushan, B. Principles and Applications to Tribology: Bhushan/Introduction; John Wiley & Sons, Ltd: New York, USA, 2013.10.1002/9781118403020Search in Google Scholar
395. Wan Kim, T.; Bhushan, B. The Adhesion Model Considering Capillarity for Gecko Attachment System. J. R. Soc. Interface 2007, 5 (20), 319–327. https://doi.org/10.1098/rsif.2007.1078.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Preface
- Preface
- Contribution to “From Nanostructure to Function”
- Temporal airy pulses efficiency in thin glass dicing
- Surface investigations of bronze and brass statuary monuments in open-air exposure
- Dual dynamic voltammetric study of the formation of ferrate ions during the electrochemical dissolution of white cast iron in the transpassive region
- Cetuximab-induced changes to tumor oral mucosa models probed by stimulated Raman spectromicroscopy
- From nanostructure to function: hierarchical functional structures in chitin and keratin
- The influence of glycine on β-lactoglobulin amyloid fibril formation – computer simulation study
Articles in the same Issue
- Frontmatter
- Preface
- Preface
- Contribution to “From Nanostructure to Function”
- Temporal airy pulses efficiency in thin glass dicing
- Surface investigations of bronze and brass statuary monuments in open-air exposure
- Dual dynamic voltammetric study of the formation of ferrate ions during the electrochemical dissolution of white cast iron in the transpassive region
- Cetuximab-induced changes to tumor oral mucosa models probed by stimulated Raman spectromicroscopy
- From nanostructure to function: hierarchical functional structures in chitin and keratin
- The influence of glycine on β-lactoglobulin amyloid fibril formation – computer simulation study


