Santalum Genus: phytochemical constituents, biological activities and health promoting-effects
-
Javad Sharifi-Rad
, Cristina Quispe
, Krishnendu Acharya
and Daniela Calina
Abstract
Santalum genus belongs to the family of Santalaceae, widespread in India, Australia, Hawaii, Sri Lanka, and Indonesia, and valued as traditional medicine, rituals and modern bioactivities. Sandalwood is reported to possess a plethora of bioactive compounds such as essential oil and its components (α-santalol and β-santalol), phenolic compounds and fatty acids. These bioactives play important role in contributing towards biological activities and health-promoting effects in humans. Pre-clinical and clinical studies have shown the role of sandalwood extract as antioxidant, anti-inflammatory, antibacterial, antifungal, antiviral, neuroleptic, antihyperglycemic, antihyperlipidemic, and anticancer activities. Safety studies on sandalwood essential oil (EO) and its extracts have proven them as a safe ingredient to be utilized in health promotion. Phytoconstituents, bioactivities and traditional uses established sandalwood as one of the innovative materials for application in the pharma, food, and biomedical industry.
1 Introduction
The genus Santalum is a woody flowering plant that belongs to the family Santalaceae commonly known as sandalwood. The members of the genus are generally trees or shrubs. The plant is obligate hemiparasite attaching itself by haustoria to establish contact with the host and extracts xylem sap for nutrients and water [1]. The family Santalaceae comprises 29 genera with around 400 species out of which 18 well-recognized species are under the genus Santalum [1], [2], [3], [4], [5], [6], [7] (Table 1).
Section, recognized species, according to the International Union for Conservation of Nature (IUCN) category, common name and geographical distribution of Santalum species.
| Taxonomic group/Section | Species and IUCN Red List Category | Common name | Geographical Occurrence | Reference |
|---|---|---|---|---|
| Section Santalum skottsb. | S. album L. | Indian sandalwood | Australia, Belgium, Cambodia, China, Germany, Great britain, Holand, India, Indonesia, Japan, Madagaskar, Malaysia, Norway, Spain, Srilanka, Switzerland, and the United States. | [3, 8, 9] |
| Section Solenantha Tuyama | S. fernandezianum Phil. | Freycinet sandalwood, or iliahi | Hawaiian islands (O‘ahu, Moloka‘i) | [10, 11] |
| S. haleakalae Hillebr. | Haleakala sandalwood or iliahi | Hawaiian islands (Maui) | [11, 12] | |
| S. pyrularium A. Gray | Hawaiian sandalwood or iliahi | Hawaiian islands (Kaua‘i) | [3, 5, 13, 14] | |
| Section Hawaiiensia skottsb. | S. ellipticum Gaudich. | Coastal sandalwood or ʻIliahialoʻe | Hawaiian islands | [3, 11, 12] |
| S. paniculatum Hook. & Arn. | Hawai‘i | Hawaiian islands | [3, 11] | |
| Section Polynesica skottsb. | S. fernandezianum F.Phil. | Chile sandalwood | Juan fernandez islands | [3] |
| Genus Eucarya T.Mitch. | S. acuminatum (R.Br.) A.DC. | Desert Quandong, native Peach | Australia | [15], [16], [17] |
Sandalwood is generally popular for its fragrant heartwood oil used by cosmetic industries for the production of perfume [13, 18], [19], [20], [21]. The high demand for sandalwood oil and timber has resulted in drastic over-harvesting; as a result, many taxa are now considered as rare, threatened or listed as endangered [22]. Moreover, one species Santalum fernandezianum Phil. from the Juan Fernandez Islands (South Pacific Ocean), has been reported extinct due to over-exploitation by human beings [23]. About 25 species belong to the genus Santalum, they are evergreen trees or shrubs characterized by a semi-parasitic lifestyle. They conduct photosynthesis; however, they take in water and inorganic nutrients by parasitizing on the roots of other plant species.
Plants of the genus Santalum are characterized by the production of EOs with many biological properties due to the high content of bioactive substances such as lignans, glycosides, triterpenoids, and sesquiterpenoids (α/β-santalol - the compound found in the largest amount). These bioactive compounds include antioxidant, anti-inflammatory, antibacterial, antifungal, antiviral, neuroleptic, antihyperglycemic, antihyperlipidemic, and anticancer activities.
Santalum genus has been known to possess many health benefits proved based on traditional uses and modern biological approaches through preclinical studies. Traditionally, Santalum genus has been used as an antipyretic, immune booster, antidiarrhea, and for treating cold and cough. Modern uses have shown their effect as antioxidant, anti-inflammatory, antibacterial, antifungal, antiviral, neuroleptic, antihyperglycemic, antihyperlipidemic, and anticancer activities. One of the most important parameters considered for the application of plant extracts in the biomedical field is its safety. The safety aspects of EO components have been studied by various researchers and it is concluded that extracts from Santalum genus are fairly safe to be used as health-promoting effects. The current review is the first of its kind that gives a snapshot of the Santalum genus concerning its traditional uses, bioactive components, bioactivities (in vitro, in vivo and clinical trials), and its safety aspects while using it as a health-promoting agent in humans.
2 Review methodology
Available information on the genus Santalum, its biological properties, and its potential mechanisms of action was collected by searching the following databases: PubMed/Medline, Web of Science and ScienceDirect. The following MeSH terms were used: “Santalum/growth & development”, “Santalum/chemistry”, “Plant Oils/isolation & purification”, “Animals”, “Apoptosis/drug effects”, “Carcinogenesis/drug effects”, “Cell Cycle Checkpoints/drug effects”, “Humans”, “Mice”, “Plant Extracts/chemistry”, “Plant Extracts/therapeutic use”, “Plant Oils/therapeutic use”, “Antioxidants/metabolism”.
The study included research articles and reviews published in extenso, written in English language in scientific journals, book chapters and books with information about Santalum genum and sandalwood. Editorials/letters to publishers, case reports, conference abstracts, studies that included homeopathic preparations were excluded. The PlantList database was used to verify the taxonomy and provide information on the classification and distribution of Santalum subspecies [24, 25].
3 Botany
Santalum is widely distributed to semi-arid areas from Indonesia in the West to Juan Fernandez Islands (Chile) in the East and from Hawaiian Archipelago in the North to New Zealand in the South [8] (Figure 1). The major production places of the plant are shown in Table 1.

Geographical distribution of Santalum genus.
The well-recognized species are broadly grouped into four categories viz. Indian sandalwood (Santalum album L.), Australian sandalwood (Santalum acuminatum (R. Br.) A. DC.), Hawaiian sandalwood (Santalum ellipticum Gaudich., Santalum freycinetianum F. Phil., Santalum haleakalae Hillebr., Santalum paniculatum Hook. & Arn., and Santalum pyrularium A. Gray), and Pacific Islands sandalwood (S. fernandezianum Phil.).
A taxonomic grouping in Santalum is purely based on morphological characters. It has been reported that Section Santalum is described as reddish corollas that are longer than wide and partly superior ovaries [12, 26, 27]. Based on smaller ovaries, longer perianth tubes and absence of hairs to the filament [28] separated the two Hawaiian members (S. freycinetianum and S. haleakalae) from the section Santalum into the endemic section Solenantha. The characteristic features of the section Hawaiiensia are explained as having white, brown, orange or green corollas that are as wide as long and inferior ovaries [12, 26, 27].
Section Polynesica is similar in appearance to the section Hawaiiensia but with partly superior ovaries [26]. A molecular phylogenetic study reveals that the sectional classification of Santalum needs revision [3]. Skottsberg [26, 29] suggested that sections Hawaiiensia and Polynesica were closely related based on morphological characters and section Polynesica was treated as a synonym of section Hawaiiensia by [30]. But molecular phylogenetic analysis indicates that sections Hawaiiensia and Polynesica are not close to one another rather related to other taxa of section Santalum and should not be united taxonomically [3]. Revisionary studies based on molecular data considered six species of Santalum in Hawaii, whereas previously there were only four recognized species [5]. Hawaiian species are considered to be a result of two colonization processes which comprises four species within red-flowered section Solenantha (i.e., S. freycinetianum, S. haleakalae, and S. pyrularium); and two species within white-flowered section Hawaiiensia (i.e., S. ellipticum, and S. paniculatum) [14].
General features of the genus Santalum are evergreen trees or shrubs; leaves opposite rarely alternate, sometimes in whorls, glabrous or sometimes glaucous, ovate, obovate or lanceolate, coriaceous [31]. Flowers cymose panicle, axillary or in the terminal, tetra or pentamerous, hermaphrodite; bracts small. Perianth-tube campanulate to conical or ovoid, adnate to the base of the ovary; stamens 4-5, dorsifixed, filament slender, short, anthers ovate; 4-lobed; style long, stigma 2-4 lobed; ovary inferior or partly inferior; ovules 2-3. Flowers produce sweet to a week or no fragrance. Fruit globose to sub-globose drupe, annulate on the top by the deciduous perianth; seed subglobose; albumen copious. Morphological differences of some important species are summarized in Table 2.
Contrasting morphological characters of important Santalum species.
| Category | Species | Size | Leaves | Flower | Fruit | Reference |
|---|---|---|---|---|---|---|
| Indian sandalwood | S. album L. | Small to the medium-size tree with slender drooping branches | Opposite, lanceolate to ovate; acute to obtuse at base, entire; apex acute to acuminate; pale green to lush green | Initially, straw yellow coloured and gradually turn to deep purplish or brown | Green to purplish–black; succulent | [8, 9, 31, 32] |
| Australian sandalwood | S. acuminatum (R. Br.) A. DC. | A shrubby small tree | Opposite, more or less lanceolate; pale green to olive-green; acute apex | Small, creamy white or greenish-white | Globose, green turning to orange–red to bright, glossy red; persistent tepal scar | [15, 16, 33] |
| Hawaiian sandalwood | S. ellipticum Gaudich. | Shrub to small tree | Elliptic to orbicular, ovate, or obovate; leathery to succulent; glaucous; dull, greyish green | Greenish in bud but tinged with brown, orange, or salmon after opening; produce a sweet fragrance; flower as long as wide | Purple to black drupes, with a distinctive apical receptacular ring | [11, 34] |
| S. paniculatum Hook. & Arn. | Shrub or tree | Ovate, obovate or elliptic; upper surfaces glossy and lower surface dull; yellowish orange to bluish or olive green. | Greenish in bud but tinged with brown, orange, or salmon after opening; produce a sweet fragrance; flowers as long as wide | Purple to black with a distinctive apical receptacular ring. | [11, 34] | |
| S. freycinetianum F. Phil. | Shrub to tree | Narrowly elliptic, oblong, to narrowly ovate; acute to rounded apex; bit glaucous; green | Light pink turning deep pink with maturity (rarely with white interiors); produce a weak fragrance; flowers longer than wide | Reddish–purple to almost black with a distinctive sub-apical receptacular ring | [5, 10, 11] | |
| S. haleakalae Hillebr. | Small tree | Ovate, obovate, or orbicular; stiff to coriaceous surfaces; olive green | Deep pink to red throughout, or with white to pink interiors; produce a weak fragrance; flowers longer than wide | Black or purplish–black with a distinctive sub-apical receptacular ring. | [5, 11] | |
| S. pyrularium A. Gray | Small tree or shrubby tree | Opposite; elliptic, ovate, to oblong; glaucous abaxially not much paler on abaxial surface; acute to obtuse apices; medium to dark green | Cream to purple throughout, greenish with the purple interior, or greenish-white turning red with age | Red, elliptic, with subapical ring | [5] |
4 Traditional uses
The close-grained heartwood of Santalum is used for ornamental and carving work. Santalum fruits are edible and the seeds contain fatty oil which is suitable for the manufacture of paint. Incense sticks are made of powdered heartwood and are used in houses and temples. In addition, powered heartwood is ground into a paste and used as a cosmetic [35]. Santalum genus is mentioned in Indian mythology, folklore, scripture, and the oldest literature (for example, Vinaya Pitaka (400–300 BC) and Milinda Pahna (200 BC)) and also in the epic Ramayana and Mahabharata. The ancient Egyptians used Santalum plants oil for embalming the dead and in the ritual burning to venerate the gods. In certain communities among the Hindus it is traditional to put a piece of sandalwood in the funeral pyre. A beige-coloured paste obtained from sandalwood is put in on the forehead and other body parts, especially by devotees of God Krishna (Vaishnavites) and for ritual bathing of Hindu gods [36]. In Zoroastrian temples, Santalum burns in sacred lights to soothe the problems of all mankind. It is used by Jews, Buddhists, Hindus, as well as almost all other belief systems for its huge variety in attributes [35].
5 Bioactive composition
5.1 Essential oil, terpenes, and derivatives
After 30 years of growth with a natural condition, oil is collected from the heartwood of sandalwood. The yield of the oil depends on the age of the tree; an old mature tree gives an oil yield between 2.5–6%; the colour of the heartwood, individual tree understudy, location within the tree, and the environment of growth of the tree. Sandalwood oil consists of main terpenoids: mono- and sesquiterpenes and their oxygenated derivatives (mostly the alcohols, ketones, and aldehydes) and also some fatty acids, and phenylpropanoids chemical compounds [37], [38], [39] (Figure 2).
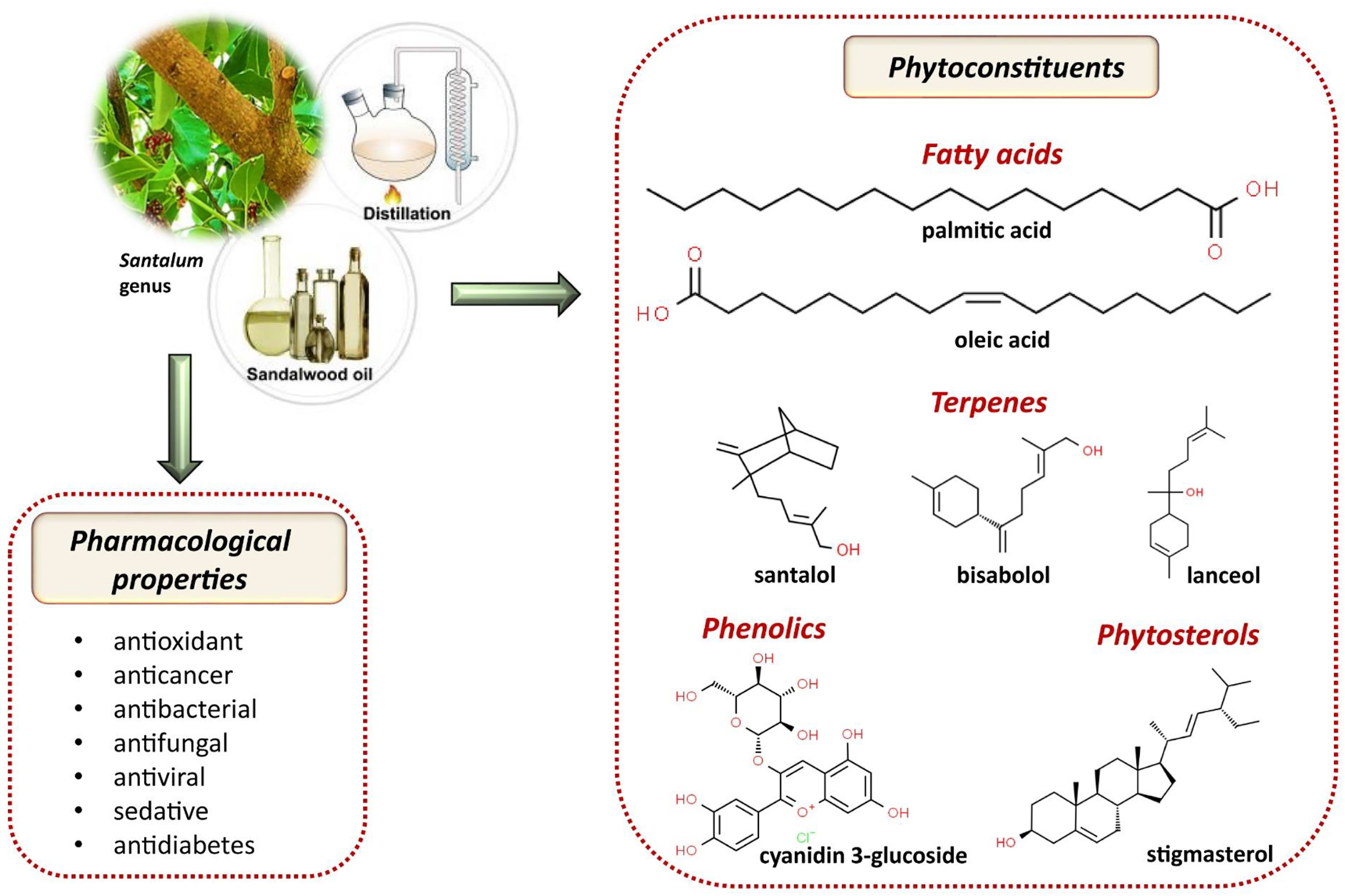
Illustrative scheme with the most important bioactive constituents of Santalum essential oil and their pharmacological properties.
The bark extract of S. album contains mainly santalol (90%) [40, 41], exo-norbicycloekasantalal, β-santalic, teresantalic, nortricycloekasantalic, bicycloekasantalic, di-hydro-β-santalic acids, urs-12-en-3b-il-palmitate, β -sitosterol, (+)epi- β-santalol, (-) β-santalol, (-)trans-β-santalol, α-santalol (52%), β-santalol (23%), epi-β-santalene, cis-lanceol, cis-nuciferol, β-, epi-β-teresantalic acid, β-, epi-β-norekasantalic acid, β-, epi-β-ekasantalic acid, α-santalic acid, 11-keto-dihydro- α -santalic acid, bisabolenols A, B, C, D and E, tricycloekasan-talol, α- and β-santalenes, trans- α -bergamotene, α-curcumone, nuciferol. The bark extract of S. album includes l-allohydroxiproline, betulinic acid, β-sitosterol, and fatty acids. The bark extract of S. album contains betulinic acid (0.05%), β -sitosterol, glucose, fructose, and sucrose [38, 40]. Although including a low amount of trans-β-santalol, cis-lanceol hydrocarbons, α-santalene, β-santalene, α-bergamotene, epi-β-santalene, as α-curcumene, β-curcumene, γ-curcumene, β-bisabolene and α-bisabolol; cis-α-santalol (53%), cis-β-santalol (23%), α-trans-bergamotol, epi-cis-β-santalol sesquiterpene alcohols are the major components of the sandalwood oil [42], [43], [44], [45], [46].
5.1.1 Extraction of the Santalum Oil
EO is one of the important components of an important component of sandalwood and its isolation from sandalwood depends on the methods of extraction. The EO of the sandalwood is widely used in the fragrance industry due to having a strong aroma and has various biological activities such as: anticancer, antiviral, antidiarrheal, cytotoxic activities, among others [47]. The different extraction methods can be applied to the Santalum during oil extraction. Therefore, the composition and amount of the fragrance and volatile compounds found in oil may vary dependent on the extraction methods. The conventional steam distillation and hydrodistillation methods are performed under high temperatures (around 100 °C) which can often result in loss of volatile compounds and changes in the odour [48], [49], [50]. Maceration or Soxhlet type solvent extraction are other techniques that have some the drawbacks such as large volume of solvent usage, exposure to hazardous and flammable liquid organic solvents, and environmental issues [51]. Therefore, the use of some solvent-free “green methods” during the extraction of EO has gained prominence in recent years. Microwave-assisted extraction, subcritical CO2 (SC–CO2) extraction and some other combined novel technologies, such as microwave-assisted hydrodistillation method, are preferred due to having higher selectivity and extraction yield, need for less time for analysis and not posing environmental and safety concerns [52, 53].
Kusuma and Mahfud [52] objected to looking into the effects of the newly employed microwave air-hydrodistillation method for extraction of EOs and comparison with classical microwave hydrodistillation method. Results of this research showed that additional airflow to the microwave hydrodistillation can help obtain the sandalwood oil in higher yield directly proportional with air flow rate. The compound composition of microwave air-hydro distilled sandalwood oil is larger than another method concerning identification 43 compounds whereas 37 compounds are recorded in microwave hydrodistillation. Microwave air-hydrodistillation provides better aroma/fragrance quality than microwave hydrodistillation extracts [52].
Nautiyal [54] mentioned that extraction yield and quality affect the trade of sandalwood oil. It was also highlighted that heartwood preparation and the extraction method have an influence on α- and β-santalol levels in the obtained oil. In the study, eight different extraction methods which are SC–CO2, ethyl alcohol, benzene, diethyl ether, toluene, steam distillation, hydrodistillation, and alkaline-hydro distillation are examined. The highest yield is obtained from SC–CO2 extraction, 3.83 grams per liter (g/L). In the analysis of extracted sandalwood oil for α- and β-santalol levels were examined through gas chromatography (GC). The most efficient extraction methods are SC–CO2, ethyl alcohol, and steam distillation; they include nearly 84% total α- and β- santalol. Hydrodistillation is the least efficient in terms of having α:β- santalol ratio, 3:1, whereas SC–CO2, ethyl alcohol, and steam distillation had 1.9:1. Furthermore, Nautiyal [54] stated that organoleptic characteristics are affected by the levels of α- and β-santalol, besides other compounds. Pleasant sandalwood oil extracts are found via SC–CO2 extraction, hydro, alkaline-hydro, and steam distillation. Furthermore, Nautiyal [55] extracted sandalwood (S. album) oil via SC–CO2 at 200 bar and 28 °C under two conditions, and the fractionation of the extract was analyzed continuously. Extractions by steam distillation, hydro distillation, Soxhlet extraction were conducted for comparison. The results showed that SC–CO2 extraction is much more effective in terms of the physical properties of oil than commercial sandalwood oil [55].
Over the last 25 years, about 65,000 chemical structures of the terpenoids and over 7,000 sesquiterpenes (C15) have been reported in previous studies [56]. The EO of the S. album tree is composed of the mixture of sesquiterpenes i.e., α-santalol, β-santalol, epi-β-santalol, α-trans-bergamotol, α-bisabolol, lanceol, sesquisabinene hydrate, and farnesol [57]. According to the literature research, α-santalol and β-santalol (Figure 3) which are the main sesquiterpene alcohol compounds found in sandalwood oil are known to indicate biological activities against the skin and prostate cancer and malaria [58]. In the same context, GC analysis of S. album oil shows that Z-α-santalol and Z- β-santalol are found with proportions 41–55% and 16–24%, respectively according to the standard [52]; identified some of the sesquiterpenes and monoterpenols, such as α-santalol, β-santalol, α-bergamotol, and cis-lanceol.

Structure of α-santalol and β-santalol.
According to this chromatographic analysis, santalol levels below these specifications can be related to extraction from undeveloped heartwood, adulteration with synthetic or semi-synthetic substitutes, or substitution with EOs from other species [42].
Mohankumar et al. [59] conducted a study on the heartwood of S. album EO concerning antioxidant and stress modulatory efficacy. The traditional steam distillation method is preferred for S. album oil extraction and the oil chemical profile identified by the GC–MS technique. Santalum album oil has at least 19 main components, accounting for 96.81% of the total content. The main compounds of S. album oil followed the order as α-santalol with 41.77% > β-santalol with 18.02% > (Z)-α-trans-bergamotol (8.50%) > (Z)-lanceol (6.57%) > epi-β-santalol (5.78%), cis-nuciferol (3.21%) > docosahexaenoic acid (2.54%) > β-trans-santalol (2.24%) > β-costol (1.41%) > β-santalene (1.24%) > (Z)-β-curcumen-12-ol (1.02%). Besides all components, the pleasant odour of S. album oil was contributed by α- and β-santalol.
Subasinghe et al. [60] investigate the Indian sandalwood (S. album) EO content and composition in Sri Lanka. Two naturally grown trees heartwoods are studied for comparing the oil properties. The maceration method is applied overnight with deionized water. One of the three the oil yield was measured at 15 cm below ground and found with the highest yield of EO whereas other trees showed a yield varying from 1.46 to 3.35 % w/w.
Another study examines the phytochemical analysis and antibacterial efficiency of extracts of S. album in preclinical studies. In vitro extracts contain callus, somatic embryo, and seedlings; non-oil-yielding young and oil-yielding matured trees are included in vivo part. Combined dichloromethane and methanol are used for the 18 h maceration method. Seedlings have the highest amount of sesquiterpenoids with 51.4 mg/g, and the old tree has the least (8.07 mg/g). Monoterpenoids compound content range changes between 3.1 and 4.5 mg/g, except young tree leave extract that has the highest content with 9.5 mg/L [61].
The volatile oil from S. album wood and of Boswellia sacra Flueck, (syn. Boswellia carteri Birdw.) the resin obtained by SC–CO2 extraction and the effects of extraction conditions on the composition is analyzed in the study of [37]. In general, oxygenated sesquiterpenes dominate the composition of the oil with a 90% ratio and hydrocarbon sesquiterpenes follow these compounds around 5%. According to the results, the best operative conditions is obtained working at 120 bar and 45 °C with the 0.658 g/mL density of CO2 in the extraction vessels for both matrices.
Another research was conducted on EO composition from roots of S. album [62]. Samples were kept in ethyl ether at 48 h for extracting the oil from the root bark. Santalum album root heartwood had 10.3% in fresh weight oil yield. Fifty-three different chemical compounds are detected by GC-MS; moreover, β-santalol and α- santalol were included in the ethanolic extract at the highest level with 19.6 and 16%.
In the study of Jones et al. [63]; the yield of the oil from 22 S. album trees was evaluated with the use of core sampling at two different heights (30 cm and 100 cm ground level). The results showed that the total concentration of sesquiterpene hydrocarbons is found in a slightly higher proportion in samples. On the other hand, the ratio of α-santalol and β-santalol is lower generally at 100 cm above ground level.
In recent years, procurement of sandalwood resources and their biologically active compounds such as EO or terpenoids have been decreased due to the devastating of the natural stocks and habitats. Therefore, some of the strategies like the heterologous expression, plant cell cultures and plant cell bioreactors have gained prominence to promote the synthesis of sandalwood terpenoids [47, 64].
5.2 Fatty acids
The identification of fatty acid in the oil is generally performed using gas chromatography/mass spectrometry (GC-MS) [65], [66], [67]. Zhang et al. [68] examined the 60 compounds from the pericarp-derived volatile oil of S. album with different extraction methods. Colourless EOs are obtained in 2.6 and 5% yield by hydrolyzation and n-hexane extraction and analyzed by GC and GC-MS. Fatty acids, especially palmitic and oleic acids, dominated the total extracted oil with 40–70% depending on the extraction method. Santalum album berries proximate analysis, and in vitro activities of these compounds have been done by Sri Harsha et al. [69]. Soxhlet method with hexane is used for taking off the oil. The oil that contains a higher amount of oleic acid (45.4%) and palmitic acid (32.5%) is measured 1.5 g/100 g fresh weight. Berries have a very low amount of α-tocopherol when compared to other berry tocopherol content.
5.3 Phenolics and saponins
Santalum album berries phenolic content is found 310 mg gallic acid equivalents (GAE)/100 g fresh weight in methanolic extract of berries. Acidified methanolic extract of S. album berries anthocyanin level is measured 0.21% in fresh weight, and the anthocyanin is confirmed as cyanidin 3-glucoside [69]. Various extract of S. album has antioxidant activity and a significant role in fighting against free radicals. Kaur et al. [70] reported that the methanolic extract of this strain indicates higher phenolic fractions than other extracts. Besides, cyanidin-3-glucoside is one of the anthocyanin pigments that show antioxidant properties and nutritional potential in S. album.
Like S. album callus, somatic embryo, and seedlings (in vitro); non-oil-yielding young and oil-yielding matured trees (in vivo) phenolic results, a similar result is shown in saponin content, in vivo extracts with 31.6 and 43.6 mg/g show higher saponin content than in vitro extract (9.4 and 17.1 mg/g) [61]. Phytoconstituents and antioxidant activity were analyzed in vitro grown callus cultures of S. album. The yield of the extract for a dichloromethane–methanol (1:1) solvent mixture was found as 4.3%. The results uncover the abundance of phenolic extracts (18.2 µg). Other major phytoconstituents are found in the extract as terpenoids (16.4 µg/mg), saponins (9.4 µg/mg) and flavan-3-ols (7.0 µg/mg) [71].
Chintamani and Dikshit [72] investigated the antioxidant potential and secondary metabolite of the fruit pulp and the kernels of the S. album. As a result of the GC-MS analysis, phenols that have been found in the free form are detected in the acetonitrile extract of fruit kernel and sterol derivatives such as cholest-4-en-3 one compound is recorded mostly in the dichloromethane extract of fruit pulp and. Besides, pyrazine amide and acetamide-2-cyano were obtained as a major constituent of the kernel with the extraction in the methanol and acetonitrile, respectively.
5.4 Phytosterols
β-Sitosterol is found in the S. album combined hexane and isopropanol solvent extract (85.35 mg/100 g oil) and S. album supercritical CO₂ extract (88.9 mg/100 g oil) at the highest level. Stigmasterol and δ-5-avenasterol amounts are quite higher than other types of chemical compounds [73].
6 Pharmacological activities
6.1 Anticancer activity
Cancer is a population of cell cells with uncontrolled growth and multiplication [74], [75], [76], [77]. Natural bioactive compounds help us with anti-inflammatory qualities to fight infections like bacterial, fungal and viral, but also with antioxidant properties with cancer [78], [79], [80], [81]. Cytotoxicity is one of the biological activities that characterize sandalwood oil. Mishra et al. [82] in their study showed that new cyclic octapeptide cyclosaplin was cytotoxic against MDA-MB-231 that are human breast cancer cells. Its anticancer activity is based on inducing apoptosis in cells but also on suppression of viability of the cells (Figure 4). Different scientists from the world found that compounds from sandalwood have anticancer activities in many types of skin cancer and leukaemia cells [82], [83], [84], [85], [86]. Matsuo and Mimaki [83] found new neolignan and known lignans in sandalwood and this study, they showed that new neolignan was cytotoxic towards HL-60 cells, which are human promyelocytic leukaemia cells. In different work, Matsuo et al. [84] showed that cis-β-santalol and -β-santaldiol were cytotoxic against HL-60 human promyelocytic leukaemia cells by inducing apoptosis in them. According to Santha and Dwivedi [85]; α-santalol from sandalwood oil from Santalum album have anticancer properties, because it can induce apoptosis, have an anti-angiogenic effect and also antioxidant activity on various types of cancer cells.
6.2 Antibacterial, antifungal and antiviral activities
Antibacterial activities are another one that was found among compounds of sandalwood oil due to the content of α/β-santalol and were active against Salmonella typhimurium and Staphylococcus aureus which are bacteria that cause well known and still threatening diseases throughout the whole world [39, 61, 87]. Epi-β-santalene was found to effective against S. typhimurium [88] (Figure 4).
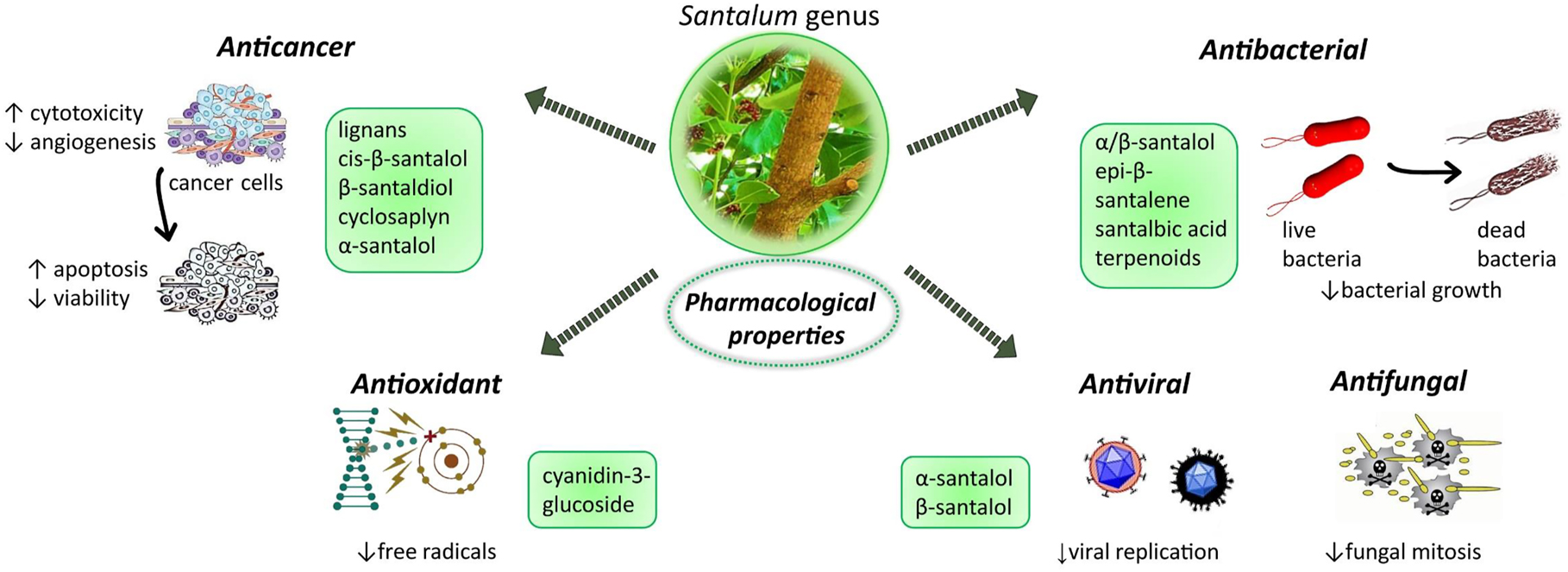
Illustrative scheme with the most representative pharmacological properties of Santalum genus and the correlation with bioactive compounds. Symbols: ↑ increase, ↓ decrease.
In seeds of Santalum album there is a compound known as santalbic acid, which has antibacterial properties against gram-positive bacteria and antifungal effect on many types of pathogenic fungi [89]. Ochi et al. [90] found that crude organic fractions and sesquiterpenoids from sandalwood oil have antimicrobial activity against Helicobacter pylori which caused peptic ulcers and also can be the cause of gastric cancer. Vadnere et al. [91] purposed to conduct phytochemical analysis and antimicrobial screening of S. album seeds petroleum ether and ethanol extracts. In vitro antimicrobial activity of both extracts was analyzed using a disk diffusion method for Bacillus subtilis, S. aureus, Pseudomonas aeruginosa, Escherichia coli and C. albicans. The outcome of the investigations highlights the potential high efficacy of petroleum ether extract related to santalbic acid, which can function as an antimicrobial agent [91].
Different research showed that derivatives from S. album possess significant antifungal properties against species as Microsporum canis, Trichophyton mentagrophytes, and Trichophyton rubrum which is due to the inhibitory effect on mitosis [39, 87, 92]. Powers et al. [93] in their studies have shown that the most active, of the 60 EOs obtained from commercial sources against Aspergillus niger, Candida albicans, and Cryptococcus neoformans, both in terms of antifungal and cytotoxic activity, were the sandalwood species (S. album, S. paniculatum), rich in santalols.
Gupta and Chaphalkar [94] found that aqueous root extract of S. album has anti-inflammatory and antiviral activities because it inhibits proliferation, production of monocytes marker – CD14 and also inhibition of nitric oxide in their study on immunopharmalogical activities of it against hepatitis B virus surface antigen – HbsAg, and New Castle disease virus. Antiviral properties were also reported by other scientists in the study of Herpes simplex viruses type 1 and 2 [95]. The authors showed that antiviral activity is dose-dependent and didn’t exist due to virucidal activity but rather because of the effect on the replication. Sandalwood oil has been also shown to be used against warts, skin blemishes, and other viral-induced tumours on the skin [96].
6.3 Antioxidant
Antioxidants are a group of compounds that protect the body from the chemical process called oxidation [97], [98], [99]. This process produces free radicals that attack cell membranes, and for this reason natural antioxidants are important for human health [100, 101]. Antioxidant efficacy is also a known property of sandalwood oil and methanolic extracts from the heartwood of S. album [102] but Misra and Dey [71] found it in vitro in callus extract of Sandalwood tree and their study showed that it is comparable. Also, antioxidative properties were found in anthocyanins pigment cyanidin-3-glucoside [69].
6.4 Other pharmacological activities
Diabetes is a disease that is widespread along with all the world and sandalwood oil is also found effective in managing the complications of this disease [103]. Kulkarni et al. [104] found that it has antihyperglycemic and antihyperlipidemic activities in their studies with diabetic rats, because of the antihyperlipidemic properties it can also help with protecting the liver and also with cardiovascular diseases. Sandalwood extract was reported to inhibit the cardiac tissue damage via reduction of lipid peroxidation damage on the doxorubicin induced cardiotoxicity rat model and significant protective effect against induced myocardial infarction in albino rats in a dose-dependent manner [105].
Sedative activities are known as properties of derivatives from sandalwood [106], [107], [108]. Sandalwood oil is reported to produce a relaxing effect on the nerves and is used for headaches, insomnia and nervous tensions. Studies carried out by [109] observed that inhalation of sandalwood oil decreased the motility of mice to an extent of 40–78%. Also showed in their studies that a mild sedative effect occurred in female Swiss albino mice after inhaling sandalwood oil.
Okugawa et al. [107] showed an antipsychotic effect in vitro and in vivo on mice. In addition, α-santalol is a strong inhibitor of both tyrosinase and cholinesterase in vivo, and hence there is a great potential of the EO for use in the treatment of Alzheimer’s disease [39].
The potential pharmacological property of S. album oil in infective skin conditions have been examined during a few clinical against a wide range of skin conditions. The therapeutic potential of S. album oil in dermatology is attributed to its antioxidant, anti-inflammatory and antimicrobial properties. Furthermore, S. album oil inhibits the hyper-proliferation of keratinocytes, which is problematic in eczema and psoriasis [110]. Dulal et al. [111] reported that sandalwood oil restores and rejuvenates ageing and wrinkled skin. Sandalwood oil has anti-inflammatory activity as well as emollient used in skincare.
All these pharmacological activities show the value of genus Santalum (Figure 4). Sandalwood or sandalwood oil can be used in medicine, cosmetology, and aromatherapy. These innovative materials can solve major issues or diseases such as diabetes, cardiovascular problems, infections of different types, cancer, and also help assist to maintain healthy and beautiful skin and a calm mind.
7 Health-promoting effects: clinical studies
Among all the Santalum species, the results of several preclinical studies on S. album revealed the vast variety of pharmaceutical properties of this valuable medicinal plant [61, 91, 94, 112]. Although there are promising in vitro and in vivo research results on S. album oil that shows the high potential capacity of S. album oil to treat skin cancer, to date there are limited human studies. Although the available information on sandalwood oil toxicity is limited, it is considered safe due its long history of oral use without any reported adverse effects.
Regarding skin safety, S. album oil has a good safety profile in terms of patch testing for contact dermatitis in both irritation and allergy. According to Burdock and Carabin [43]; undiluted S. album oil and 10% S. album oil are non-irritant. In five dermatology reports, some allergic reactions have been reported. Number of 12 out of 3,542 patients (0.34%) were sensitive to a 2% dilution of S. album oil, and in three reports, 69 of 5,595 patients (1.2%) exhibited sensitivity to a 10% dilution [113]. In a subsequent multicenter European study, 3 fragrance markers (FMs) (fragrance mix I, fragrance mix II, and Myroxylon pereirae) have been tested on consecutive patients to determine the frequency of positive patch-test reactions to EOs tested in the baseline. The result revealed that 656 of 48,956 dermatitis patients (1.38%) revealed positive reactions to 10% S. album oil [114].
Skin inflammation and irritation, known as radiodermatitis, are common side effects in radiation therapy for cancer patients [115]. Radio dermatitis is associated with oxidative stress and an increase in cytokines, including interleukin (IL)-1β, IL-6, and IL-8 [116]. In a study conducted by [117]; the effectiveness of a turmeric and sandalwood oil containing proprietary cream [Vicco® turmeric cream (VTC); Vicco Laboratories, Parel, India] on radiodermatitis in patients with head and neck cancer undergoing radiotherapy have been assessed. In this nine-week open-label clinical study, the degree of radiodermatitis of 46 cancer patients experiencing radiotherapy, significantly inhibited (24 patients) compared to baby oil (22 patients) applying Vicco® cream containing 16% turmeric extract and 0.5% S. album oil [117]. In a similar study, the same product (Vicco®) exhibited significantly delayed and moderated on 40 breast cancer patients (20 in each group) radiodermatitis in the sandalwood/turmeric group compared to the control group [118].
Based on four clinical trial projects on photoallergy testing, nine of 621 patients (1.45%) tested demonstrated positive effect to S. album oil at 2% [119], [120], [121], [122]. It should be noted that photoallergy to EOs is very rare, and its clinical application was generally not established.
The potential clinical anti-inflammatory action of sandalwood oil was tested in a clinical trial performed in 50 patients with mild to moderate facial acne for 8 weeks. This pilot study of a topical regimen treatment (foaming cleanser, serum, spot treatment, and mask) containing 0.5% salicylic acid and up to 2% S. album oil was conducted in teenage and adult subjects with mild to moderate facial acne [123]. For the eight-week treatment period, treatment was well tolerated by nearly all patients (42 of 47 participants (89.4%). Patients experienced an improvement when compared with baseline with notable reductions in lesion counts in patients with more severe or inflamed lesions, using the Global Aesthetic Improvement Scale (GAIS). There is no report of limitation of use of this regimen due to no adverse events [123].
According to the literature, it is assumed that S. album oil might have therapeutic benefits to psoriasis patients due to its anti-inflammatory, antiproliferative properties via inducing autophagy and cell death in proliferating keratinocytes [124], [125], [126]. A Phase 2 clinical trial results in patients with mild to moderate psoriasis illustrated that the topically applied 10% S. album oil serum administered twice a day for 28 days was well tolerated and alleviates mild to moderate psoriasis symptoms [127].
In a pilot study, undiluted S. album oil to common warts twice daily for 12 weeks has been applied to ten candidates with the age range from six to adult. The results showed that 10 of the 12 (80%) participants had complete resolution of all treated warts over their hands, feet, legs, or face, with the other two subjects experiencing moderate improvement. There was no report of skin irritation, redness, pain or other adverse symptoms [128].
8 Safety, adverse effects and therapeutic limitations
Due to the chemical composition of the sandalwood, its EO is most popularly used in folk medicine, cosmetics, pharmacy, as well as the food industry. The list of internal and external health problems, in which the oils of Santalum plants are used, contains inter alia general weakness, headache and stomach ache, common colds, bronchitis, skin diseases such as infectious sores, ulcers, acne, and rashes, heart ailments, fever, infection of the urinary tract, and inflammation of the mouth [129, 130].
Just as the positive effect of EOs depend on their chemical composition, their safety and side effects result from the main phytochemicals and as well as the synergistic action of compounds that are present in lower concentrations. The main components present in the sandalwood EOs, that should be taken into the consideration regarding the safety are α-santalol, β-santalol, β-santalene, Z-α-trans-bergamotol [43, 131].
In the food industry, natural flavouring substances may be safely used in the products, meeting some criteria: must be used in the appropriate forms, in the minimum quantity required to produce their intended physical or technical effect, and following all the principles of the good manufacturing practice. Following this, the Food and Drug Administration (FDA) recommendation, a wide range of EO (clove, oregano, thyme, nutmeg, basil, mustard, and cinnamon) and components (linalool, thymol, eugenol, carvone, cinnamaldehyde, vanillin, carvacrol, citral) are classified as generally recognized as safe (GRAS) and have been accepted in the application in food products [132].
According to the FDA, S. album is an accepted natural flavouring substance and can be used in the food industry in any kind of product, without restrictions.
Although some limitations with the dosage of the S. album EOs are recommended. According to the data of Flavor and Extract Manufacturers Association (FEMA) published in the article of Burdock and Carabin [43] the maximum doses of sandalwood oil in alcoholic beverages should not exceed 0.77 ppm; in non-alcoholic beverages 1.96 ppm, hard candy 89.98 ppm, while in the case of baked products the maximum level is 9.72 ppm. Even though it is difficult to determine how much sandalwood EO is consumed with food by humans, National Academy of Sciences (NAS) data are estimated to 0.0074 mg/day or 0.000123 mg/kg/day sandalwood oil for 60 kg individual.
On the other hand, FEMA reported that these values are 0.0058 mg/day and 0.0001 mg/kg/day, while the mean consumption of foods containing the usual amount- PADI (Possible Average Daily Intake) - is estimated to 0.97 mg/person/day or 0.016 mg/kg/day of sandalwood oil [43]. In general, the information on the safety and adverse effects of the genus Santalum is extremely limited.
Even though the EOs are generally considered as safe, toxicological studies showed that some of them may be harmful to human health. Studies have been shown that different chemicals of EOs (menthol, carvone, limonene, citral, cinnamaldehyde, benzaldehyde, as well as methyl anthranilate, geranyl acetate, furfural, and eugneol) taken at high levels showed no carcinogenic effects [133]. Although low concentrations of EOs are usually devoid of mutagenicity and carcinogenicity, some single components or crude EOs may act as carcinogens. For example, estrogen-dependent malignancy can be induced by Salvia sclarea L. EOs, while estragole from Artemisia dracunculus L. shows carcinogenic potential in rodents. Following, psoralen (bergamia EOs) is photosensitive compound that may induce DNA adduct formation and skin cancer, while methyleugenol (Laurus nobilis L.) and D-limonene (citrus EOs) is being known as carcinogenic in rodents [134].
The lethal dose (LD50) of the sandalwood EOs was evaluated for rats (5.58 g/kg body weight) and rabbits (>5 g/kg BW, body weight). The LD50 was also estimated for the major constituent of the EOs, α-santalol and the values for rats were 3.8 g/kg BW, and for rabbits >5 g/kg BW. 3 mL/kg of α-bisabolol showed a reduction in fetal numbers in rats and rabbits, while 1 mL/kg showed no teratogenic effects [113].
Interestingly, studies on the effects of the inhaled sandalwood oil have shown that female Swiss exposed to the oil for 1 h shown 40% decreased motility, and in the blood of these animals, α- and β-santalols were present [43, 135]. Some studies with animal models suggest that sandalwood EO can irritate rabbit skin, but it seems that this oil has no such effect on human skin [113]. The results of studies showed that sandalwood EO is characterized by low sensitization potential. In the work of Paulsen and Andersen [136] of 318 patients responded that 10% of sandalwood EOs gave a positive effect.
At the same time, 2% concentration did not cause any negative effects on any of the respondents [136]. A total of 1.4% of all tested dentists and dental nurses responded to sandalwood oil in the case of the paper published by Kiec-swierczyńska and Krecisz [137]. A total of 0.9% of patients (total of 1606 patients) responded to 10% sandalwood oil, at the same time 0.4% of patients responded to 2% concentration [138].
In the study with 641 patients with eczema, sandalwood oil had no response by any of the tested patients [139], while in the case of 422 patients with suspected contact allergy, 2.4% gave the positive response to sandalwood, and 3.1% to cinnamic alcohol [140]. Similar percentage results were obtained in the studies on the photoallergies’ caused by sandalwood oil. 2.2% (3 of 138 patients) were positive to sandalwood oil reaction in the study conducted by Fotiades et al. [119] while 2 of 1050 probable photodermatitis patients (0.19%) in the study of Pigatto et al. [141].
In general sandalwood, EOs are recognized as nontoxic in the matter of phototoxicity, but the suggested maximum dose of the S. album EO is 2% [113]. The results of the study with 4266 Japanese people with cosmetic dermatitis showed that 57 (1.34%) was positive to 2% α-santalol. This suggests that the concentration of the sandalwood EOs should be lower for people of Japanese origin [113]. In general, the use of sandalwood oil in eczema, psoriasis, radiation dermatitis, and antifungal is reported in the literature, and the EOs is well tolerated with acceptable safety [142]. What is more, the major constituent of S. album EOs, α-santalol is being recognized as a chemopreventive effect with nontoxic side effects against normal cells [143].
9 Conclusions and future perspectives
The review highlighted the bioactive compounds present in the sandalwood and bioactivities of its extract proven by the in vivo, in vitro and clinical trials. The EO components such as α-santalol and β-santalol are considered important for evaluating the commercial value of the sandalwood. These components are responsible for most of the biological activities along with the soothing aroma of Santalum species. Traditional uses of the EO from sandalwood have been proven to be beneficial in treating somatic and other disorders such as common cold, fever, lung infection, and many types of inflammations. Antioxidant, anti-inflammatory, antibacterial, antifungal, antiviral, neuroleptic, antihyperglycemic, antihyperlipidemic, and anticancer activities of Santalum extracts have been recently proved through clinical trials. Recent scientific studies have not shown any adverse effect of consumption of sandalwood EO in in vivo trials. Hence, extracts from sandalwood are presently used in cosmetic products and as a flavouring agent in food items. More detailed studies are needed to decipher the exact molecular mechanism of the sandalwood extracts in improving human health. These molecular studies will also assist in delineating the more precise use of sandalwood extracts for human consumption. Exhaustive clinical studies are also needed to further promote the use of sandalwood ingredients in food and pharma application. Pharmaceutical formulation of the sandalwood extracts is another area that needs the attention of the scientific community to further improve the use of Santalum species in health promotion.
Acknowledgments
MM wants to thank ANID CENTROS BASALES ACE210012.
-
Author contributions: All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas that is, revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and confirming to be accountable for all aspects of the work.
-
Research funding: None declared.
-
Competing interest: The author declares no conflict of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
-
Data availability: All supporting data for this manuscript are included in the figures and available from the corresponding authors on reasonable request.
References
1. Subasinghe, U. Sandalwood research: a global perspective. J Trop For Environ 2013;3:1–8. https://doi.org/10.31357/jtfe.v3i1.1117.Search in Google Scholar
2. Fox, J. Sandalwood: the royal tree. Biologist 2000;47:31. https://doi.org/10.7748/ns.14.52.31.s51.Search in Google Scholar
3. Harbaugh, DT, Baldwin, BG. Phylogeny and biogeography of the sandalwoods (Santalum, Santalaceae): repeated dispersals throughout the Pacific. Am J Bot 2007;94:1028–40. https://doi.org/10.3732/ajb.94.6.1028.Search in Google Scholar PubMed
4. Harbaugh, D. A taxonomic revision of Australian northern sandalwood (Santalum lanceolatum, Santalaceae). Aust Syst Bot 2007;20:409–16. https://doi.org/10.1071/sb07009.Search in Google Scholar
5. Harbaugh, DT, Oppenheimer, HL, Wood, KR, Wagner, WL. Taxonomic revision of the endangered Hawaiian red-flowered sandalwoods (Santalum) and discovery of an ancient hybrid species. Syst Bot 2010;35:827–38. https://doi.org/10.1600/036364410x539899.Search in Google Scholar
6. Nageswara rao, M, Soneji, J, Sudarshana, P. Santalum. In: Kole, C, editor. Wild crop relatives: genomic and breeding resources. Berlin: Springer; 2011.Search in Google Scholar
7. Teixeira da silva, JA, Kher, MM, Soner, D, Page, T, Zhang, X, Nataraj, M, et al.. Sandalwood: basic biology, tissue culture, and genetic transformation. Planta 2016;243:847–87. https://doi.org/10.1007/s00425-015-2452-8.Search in Google Scholar PubMed
8. Srinivasan, V, Sivaramakrishnan, V, Rangaswamy, C, Ananthapadmanabha, H, Shanakaranarayana, K. Sandal. Dehra Dun, India: ICFRE; 1992.Search in Google Scholar
9. Purohit, P. Reviving the royal tree Santalum album linn.: Santalaceae. J Med Plants Stud 2018;6:273–6.Search in Google Scholar
10. James, A. Santalum freycinetianum Gaudich. In: Vozzo, J, editor. Tropical tree seed manual: part II, species descriptions. Washington, DC: U.S: Department of Agriculture; 2002.Search in Google Scholar
11. Mark, D, Thomson, L, Elevitch, C. Santalum ellipticum, S. freycinetianum, S. haleakalae, and S. paniculatum (Hawaiian sandalwood). In: Elevitch, C, editor. Species profiles for Pacific Island agroforestry. ver 4.1. Hawaii: Professional Development Program Grant; 2006.Search in Google Scholar
12. Wagner, W, Herbst, D, Sohmer, S. Santalum manual of the flowering plants of Hawaii. Honolulu: University of Hawaii Press; 1999.Search in Google Scholar
13. Kepler, A. Hawaiian heritage plants. Honolulu, Hawaii, USA: University of Hawaii Press; 1998.10.1515/9780824843922Search in Google Scholar
14. Wood, K. Notes on Santalum involutum (Santalaceae) Kaua‘i, Hawai‘i. Technical Report. Hawai‘i: National Tropical Botanical Garden; 2015.Search in Google Scholar
15. George, A. Santalaceae. In: Jessop, J, editor. Flora of central Australia. Sydney: Reed Books; 1981.Search in Google Scholar
16. George, A. Santalaceae. In: George, A, editor. Flora of Australia 22 Australian. Canberra: Government Publishing Service; 1984.Search in Google Scholar
17. Nge, FJ, Ranathunge, K, Kotula, L, Cawthray, GR, Lambers, H. Strong host specificity of a root hemi-parasite (Santalum acuminatum) limits its local distribution: beggars can be choosers. Plant Soil 2019;437:159–77. https://doi.org/10.1007/s11104-019-03966-6.Search in Google Scholar
18. Barrett, D, Fox, J. Geographical distribution of santalaceae and botanical characteristics of species in the genus. In: Cjerum, L, Fox, J, Ehrhart, Y, editors. Sandalwood seed nursery and plantation technology. Proceedings of a regional workshop for Pacific Island countries, 1994, noume’a, new caledonia, RAS field document 8. Suva, Fiji: South Pacific Forestry Development Programme; 1995.Search in Google Scholar
19. Cherrier, J. Sandalwood in French polynesia. In: Mckinnell, F, editor. Sandalwood in the Pacific Region: proceedings of a symposium at the XVII Pacific science congress. Honolulu, Hawaii: Australian Center for International Agriculture Research Proceedings; 1991:24–5 pp.Search in Google Scholar
20. Whistler, A. Polynesian herbal medicine. Kauai, Hawaii, USA: National Tropical Botanical Garden Press; 1992.Search in Google Scholar
21. Payne, K. Native plant guide: karlkurla bushland park and the goldfields of Western Australia. Kalgoorlie, Western Australia, Australia: Kalgoorlie-Boulder Urban Landcare Group; 2003.Search in Google Scholar
22. Herbst, D. Endangered and threatened wildlife and plants; determination of endangered status for Santalum freycinetianum var. lanaiense (Lanai sandalwood or ‘iliahi). Fed Regist 1986;51:3182–5.Search in Google Scholar
23. Stuessy, TF, Marticorena, C, Roberto.rodri guez, R, Crawford, DJ, Mariosilva, O. Endemism in the vascular flora of the Juan Fernandez islands. Aliso 1992;13:297–307. https://doi.org/10.5642/aliso.19921302.03.Search in Google Scholar
24. PlantList T. Available from: http://www.theplantlist.org/ [Accessed 15 Jun 2022].Search in Google Scholar
25. Heinrich, M, Appendino, G, Efferth, T, Fürst, R, Izzo, AA, Kayser, O, et al.. Best practice in research – overcoming common challenges in phytopharmacological research. J Ethnopharmacol 2020;246:112230. https://doi.org/10.1016/j.jep.2019.112230.Search in Google Scholar PubMed
26. Skottsberg, C. The geographical distribution of the sandalwoods and its ignificance. In: Proceedings of the fourth Pacific science congress, java, Indonesia; 1930;vol 3:435–40 pp.Search in Google Scholar
27. Stemmermann, L. Observations on the genus Santalum (Santalaceae) in hawai’i. Pac Sci 1980;34:41–54.Search in Google Scholar
28. Tuyama, T. On Santalum boninense, and the distribution of the species of Santalum. J Jpn Bot 1939;15:697–712.Search in Google Scholar
29. Skottsberg, C. Further notes on Pacific sandalwoods. Meddelanden från Göteborgs Botaniska Trädgard 1930;5:135–45.Search in Google Scholar
30. Fosberg, F, Sachet, M. Santalum in eastern polynesia. Candollea 1985;40:459–70.Search in Google Scholar
31. Kulkarni, H. Sandal (Santalum album L.) descriptor. In: Srimathi, R, Kulkarni, H, KR, V, editors. Recent advances in research and management of sandal (Santalum album L.) in India. New Delhi: Associated Publishing Company; 1995.Search in Google Scholar
32. Srimathi, R, Kulkarni, H, Venkatesan, K. Phenotypes of sandal. J Bombay Nat Hist Soc 1983;80:245–6.Search in Google Scholar
33. Lim, T. Santalum acuminatum. In: Edible medicinal and non-medicinal plants. Berlin, Germany: Springer Nature; 2013:785–9 pp.10.1007/978-94-007-5653-3_43Search in Google Scholar
34. Batabyal, S. Growth variability of Santalum album L in different edaphic conditions and formulation of protocols for its propagation [Ph.D. thesis]. Bardhaman, India: University of Burdwan; 2015.Search in Google Scholar
35. Chintamani, K, Dikshit, M. Extraction, antioxidant potential and identification of scondary metabolites of whole fruits of Santalum album Linn by GC-MS. Int J Curr Pharmaceut Res 2015;7:40–2.Search in Google Scholar
36. Kumar, ANA, Joshi, G, Ram, HYM. Sandalwood: history, uses, present status and the future. Curr Sci 2012;103:1408–16.Search in Google Scholar
37. Marongiu, B, Piras, A, Porcedda, S, Tuveri, E. Extraction of Santalum album and Boswellia carterii Birdw. volatile oil by supercritical carbon dioxide: influence of some process parameters. Flavour Fragrance J 2006;21:718–24. https://doi.org/10.1002/ffj.1718.Search in Google Scholar
38. Sindhu, R, Arora, S. Sandalwood oil: phytochemical and pharmacological updates. In: Govil, J, editor. Recent progress in medicinal plants. Studium Press LLC; 2013.Search in Google Scholar
39. Misra, BB, Dey, S. Biological activities of East Indian sandalwood tree, Santalum album. PeerJ PrePrints 2013;1:e96v1.10.7287/peerj.preprints.96v1Search in Google Scholar
40. Scartezzini, P, Speroni, E. Review on some plants of Indian traditional medicine with antioxidant activity. J Ethnopharmacol 2000;71:23–43. https://doi.org/10.1016/s0378-8741(00)00213-0.Search in Google Scholar PubMed
41. Banerjee, S, Ecavade, A, Rao, AR. Modulatory influence of sandalwood oil on mouse hepatic glutathione S-transferase activity and acid soluble sulphydryl level. Cancer Lett 1993;68:105–9. https://doi.org/10.1016/0304-3835(93)90135-v.Search in Google Scholar PubMed
42. Howes, MJ, Simmonds, MS, Kite, GC. Evaluation of the quality of sandalwood essential oils by gas chromatography-mass spectrometry. J Chromatogr A 2004;1028:307–12. https://doi.org/10.1016/j.chroma.2003.11.093.Search in Google Scholar PubMed
43. Burdock, GA, Carabin, IG. Safety assessment of sandalwood oil (Santalum album L.). Food Chem Toxicol 2008;46:421–32. https://doi.org/10.1016/j.fct.2007.09.092.Search in Google Scholar PubMed
44. Jones, CG, Ghisalberti, EL, Plummer, JA, Barbour, EL. Quantitative co-occurrence of sesquiterpenes; a tool for elucidating their biosynthesis in Indian sandalwood, Santalum album. Phytochemistry 2006;67:2463–8. https://doi.org/10.1016/j.phytochem.2006.09.013.Search in Google Scholar PubMed
45. Braun, NA, Meier, M, Pickenhagen, W. Isolation and chiral GC analysis of β-bisabolols—trace constituents from the essential Oil of Santalum album L. (Santalaceae). J Essent Oil Res 2003;15:63–5. https://doi.org/10.1080/10412905.2003.9712064.Search in Google Scholar
46. Verghese, J, Sunny, T, Balakrishnan, K. (+)-α-santalol and (-)-β-santalol(Z) concentration, a new quality determinant of East Indian sandalwood oil. Flavour Fragrance J 1990;5:223–6. https://doi.org/10.1002/ffj.2730050407.Search in Google Scholar
47. Zhang, Y, Yan, H, Niu, M, Cheng, Q, Zhang, X, Teixeira da silva, JA, et al.. Multiple strategies for increasing yields of essential oil and obtaining sandalwood terpenoids by biotechnological methods in sandalwood. Trees 2018;32:17–28. https://doi.org/10.1007/s00468-017-1558-y.Search in Google Scholar
48. Sharifi-Rad, J, Quispe, C, Herrera-Bravo, J, Akram, M, Abbaass, W, Semwal, P, et al.. Phytochemical constituents, biological activities, and health-promoting effects of the Melissa officinalis. Oxid Med Cell Longev 2021;2021:6584693. https://doi.org/10.1155/2021/6584693.Search in Google Scholar
49. Taheri, Y, Quispe, C, Herrera-Bravo, J, Sharifi-Rad, J, Ezzat, SM, Merghany, RM, et al.. Urtica dioica-derived phytochemicals for pharmacological and therapeutic applications. Evid Based Complement Alternat Med 2022;2022:4024331. https://doi.org/10.1155/2022/4024331.Search in Google Scholar
50. Salehi, B, Rescigno, A, Dettori, T, Calina, D, Docea, AO, Singh, L, et al.. Avocado–soybean unsaponifiables: a panoply of potentialities to Be exploited. Biomolecules 2020;10:130. https://doi.org/10.3390/biom10010130.Search in Google Scholar
51. Azwanida, N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromatic Plants 2015;4:1–6.Search in Google Scholar
52. Kusuma, HS, Mahfud, M. Kinetic studies on extraction of essential oil from sandalwood (Santalum album) by microwave air-hydrodistillation method. Alex Eng J 2018;57:1163–72. https://doi.org/10.1016/j.aej.2017.02.007.Search in Google Scholar
53. Tyśkiewicz, K, Gieysztor, R, Konkol, M, Szałas, J, Rój, E. Essential oils from humulus lupulus scCO- extract by hydrodistillation and microwave-assisted hydrodistillation. Molecules 2018;23:2866.10.3390/molecules23112866Search in Google Scholar
54. Nautiyal, O. Subcritical carbon dioxide and conventional extraction techniques of Sandalwood oil: an industry project. Sandalwood Research Newsletter 2010;25:5–8.Search in Google Scholar
55. Nautiyal, OH. Process optimization of sandalwood (Santalum album) oil extraction by subcritical carbon dioxide and conventional techniques. Indian J Chem Technol 2014;21:290–7.Search in Google Scholar
56. Rusdi, NA, Goh, HH, Sabri, S, Ramzi, AB, Mohd noor, N, Baharum, SN. Functional characterisation of new sesquiterpene synthase from the Malaysian herbal plant, polygonum minus. Molecules 2018;23. https://doi.org/10.3390/molecules23061370.Search in Google Scholar
57. Srivastava, PL, Daramwar, PP, Krithika, R, Pandreka, A, Shankar, SS, Thulasiram, HV. Functional characterization of novel sesquiterpene synthases from Indian sandalwood, Santalum album. Sci Rep 2015;5:10095. https://doi.org/10.1038/srep10095.Search in Google Scholar
58. Bommareddy, A, Rule, B, Vanwert, AL, Santha, S, Dwivedi, C. α-Santalol, a derivative of sandalwood oil, induces apoptosis in human prostate cancer cells by causing caspase-3 activation. Phytomedicine 2012;19:804–11. https://doi.org/10.1016/j.phymed.2012.04.003.Search in Google Scholar
59. Mohankumar, A, Kalaiselvi, D, Levenson, C, Shanmugam, G, Thiruppathi, G, Nivitha, S, et al.. Antioxidant and stress modulatory efficacy of essential oil extracted from plantation-grown Santalum album L. Ind Crop Prod 2019;140:111623. https://doi.org/10.1016/j.indcrop.2019.111623.Search in Google Scholar
60. Subasinghe, U, Gamage, M, Hettiarachchi, DS. Essential oil content and composition of Indian sandalwood (Santalum album) in Sri Lanka. J For Res 2013;24:127–30. https://doi.org/10.1007/s11676-013-0331-3.Search in Google Scholar
61. Misra, BB, Dey, S. Comparative phytochemical analysis and antibacterial efficacy of in vitro and in vivo extracts from East Indian sandalwood tree (Santalum album L.). Lett Appl Microbiol 2012;55:476–86. https://doi.org/10.1111/lam.12005.Search in Google Scholar PubMed
62. Xin-Hua, Z, Silva, JATD, Yong-Xia, J, Jian, Y, Guo-Hua, M. Essential oils composition from roots of Santalum album L. J Essen Oil Bear Plant 2012;15:1–6. https://doi.org/10.1080/0972060x.2012.10644011.Search in Google Scholar
63. Jones, CG, Plummer, JA, Barbour, EL. Non-destructive sampling of Indian sandalwood (Santalum album L.) for oil content and composition. J Essent Oil Res 2007;19:157–64. https://doi.org/10.1080/10412905.2007.9699250.Search in Google Scholar
64. Rani, A, Meghana, R, Kush, A. Squalene production in the cell suspension cultures of Indian sandalwood (Santalum album L.) in shake flasks and air lift bioreactor. Plant Cell Tissue Organ Cult 2018;135:155–67. https://doi.org/10.1007/s11240-018-1452-3.Search in Google Scholar
65. Semwal, P, Painuli, S, Abu-Izneid, T, Rauf, A, Sharma, A, Daştan, SD, et al.. Diosgenin: an updated pharmacological review and therapeutic perspectives. Oxid Med Cell Longev 2022;2022:1035441. https://doi.org/10.1155/2022/1035441.Search in Google Scholar PubMed PubMed Central
66. Quetglas-Llabrés, MM, Quispe, C, Herrera-Bravo, J, Catarino, MD, Pereira, OR, Cardoso, SM, et al.. Pharmacological properties of bergapten: mechanistic and therapeutic aspects. Oxid Med Cell Longev 2022;2022:8615242. https://doi.org/10.1155/2022/8615242.Search in Google Scholar PubMed PubMed Central
67. Sharifi-Rad, J, Quispe, C, Bouyahya, A, El-Menyiy, N, El Omari, N, Shahinozzaman, M, et al.. Ethnobotany, phytochemistry, biological activities, and health-promoting effects of the genus Bulbophyllum. Evid Based Complement Alternat Med 2022;2022:6727609. https://doi.org/10.1155/2022/6727609.Search in Google Scholar PubMed PubMed Central
68. Zhang, XH, Da silva, JA, Jia, YX, Zhao, JT, Ma, GH. Chemical composition of volatile oils from the pericarps of Indian sandalwood (Santalum album) by different extraction methods. Nat Prod Commun 2012;7:93–6. https://doi.org/10.1177/1934578x1200700132.Search in Google Scholar
69. Sri Harsha, PSC, Khan, MI, Prabhakar, P, Giridhar, P. Cyanidin-3-glucoside, nutritionally important constituents and in vitro antioxidant activities of Santalum album L. berries. Food Res Int 2013;50:275–81. https://doi.org/10.1016/j.foodres.2012.10.024.Search in Google Scholar
70. Kaur, N, Sarkar, B, Gill, I, Kaur, S, Mittal, S, Dhiman, M, et al.. Indian herbs and their therapeutic potential against alzheimer’s disease and other neurological disorders. In: Farooqui, T, Farooqui, A, editors. Neuroprotective effects of phytochemicals in neurological disorders. New Jersey, United States: John Wiley & Sons, Ltd; 2017.10.1002/9781119155195.ch4Search in Google Scholar
71. Misra, B, Dey, S. Phytochemical analyses and evaluation of antioxidant efficacy of in vitro callus extract of East Indian sandalwood tree (Santalum album L.). J Pharmacogn Phytochem 2012;1:8–18.10.7287/peerj.preprints.96v1Search in Google Scholar
72. Chintamani, K, Dikshit, M. Extraction, identification and antioxidant potential of secondary metabolites of whole fruits of Santalum album Linn by GC-MS. Int J Curr Pharmaceut Res 2015;7:40–2.Search in Google Scholar
73. Hettiarachchi, DS, Liu, YD, Boddy, MR, Fox, JED, Sunderland, VB. Contents of fatty acids, selected lipids and physicochemical properties of western Australian sandalwood seed oil. J Am Oil Chem Soc 2013;90:285–90. https://doi.org/10.1007/s11746-012-2162-3.Search in Google Scholar
74. Shahinozzaman, M, Islam, M, Basak, B, Sultana, A, Emran, R, Ashrafizadeh, M, et al.. A review on chemistry, source and therapeutic potential of lambertianic acid. Z Naturforsch C Biosci 2021;76:347–56. https://doi.org/10.1515/znc-2020-0267.Search in Google Scholar PubMed
75. Ianoși, SL, Batani, A, Ilie, MA, Tampa, M, Georgescu, SR, Zurac, S, et al.. Non-invasive imaging techniques for the in vivo diagnosis of Bowen’s disease: three case reports. Oncol Lett 2019;17:4094–101. https://doi.org/10.3892/ol.2019.10079.Search in Google Scholar PubMed PubMed Central
76. Mitrut, P, Docea, AO, Kamal, AM, Mitrut, R, Calina, D, Gofita, E, et al.. Colorectal cancer and inflammatory bowel disease. London, United Kingdom: IntechOpen; 2016.10.5772/63408Search in Google Scholar
77. Zlatian, OM, Comanescu, MV, Rosu, AF, Rosu, L, Cruce, M, Gaman, AE, et al.. Histochemical and immunohistochemical evidence of tumor heterogeneity in colorectal cancer. Rom J Morphol Embryol 2015;56:175–81.Search in Google Scholar
78. Salehi, B, Sharifi-Rad, J, Cappellini, F, Reiner, A, Zorzan, D, Imran, M, et al.. The therapeutic potential of anthocyanins: current approaches based on their molecular mechanism of action. Front Pharmacol 2020b;11:20. https://doi.org/10.3389/fphar.2020.01300.Search in Google Scholar PubMed PubMed Central
79. Sharifi-Rad, J, Bahukhandi, A, Dhyani, P, Sati, P, Capanoglu, E, Docea, AO, et al.. Therapeutic potential of neoechinulins and their derivatives: an overview of the molecular mechanisms behind pharmacological activities. Front Nutr 2021;8:664197. https://doi.org/10.3389/fnut.2021.664197.Search in Google Scholar PubMed PubMed Central
80. Dhyani, P, Quispe, C, Sharma, E, Bahukhandi, A, Sati, P, Attri, DC, et al.. Anticancer potential of alkaloids: a key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int 2022;22:206. https://doi.org/10.1186/s12935-022-02624-9.Search in Google Scholar PubMed PubMed Central
81. Sharifi-Rad, J, Quispe, C, Patra, JK, Singh, YD, Panda, MK, Das, G, et al.. Paclitaxel: application in modern oncology and nanomedicine-based cancer therapy. Oxid Med Cell Longev 2021;2021:3687700. https://doi.org/10.1155/2021/3687700.Search in Google Scholar PubMed PubMed Central
82. Mishra, A, Gauri, SS, Mukhopadhyay, SK, Chatterjee, S, Das, SS, Mandal, SM, et al.. Identification and structural characterization of a new pro-apoptotic cyclic octapeptide cyclosaplin from somatic seedlings of Santalum album L. Peptides 2014;54:148–58. https://doi.org/10.1016/j.peptides.2014.01.023.Search in Google Scholar PubMed
83. Matsuo, Y, Mimaki, Y. Lignans from Santalum album and their cytotoxic activities. Chem Pharm Bull (Tokyo) 2010;58:587–90. https://doi.org/10.1248/cpb.58.587.Search in Google Scholar PubMed
84. Matsuo, Y, Sakagami, H, Mimaki, Y. A rare type of sesquiterpene and β-santalol derivatives from Santalum album and their cytotoxic activities. Chem Pharm Bull (Tokyo) 2014;62:1192–9. https://doi.org/10.1248/cpb.c14-00457.Search in Google Scholar PubMed
85. Santha, S, Dwivedi, C. Anticancer effects of sandalwood (Santalum album). Anticancer Res 2015;35:3137–45.Search in Google Scholar
86. Kim, TH, Ito, H, Hayashi, K, Hasegawa, T, Machiguchi, T, Yoshida, T. Aromatic constituents from the heartwood of Santalum album L. Chem Pharm Bull 2005;53:641–4. https://doi.org/10.1248/cpb.53.641.Search in Google Scholar PubMed
87. Butaud, JF, Gaydou, V, Bianchini, JP, Faure, R, Raharivelomanana, P. Dihydroxysesquiterpenoids from Santalum insulare of French polynesia. Nat Prod Commun 2007;2:239–42. https://doi.org/10.1177/1934578x0700200303.Search in Google Scholar
88. Simanjuntak, P. Antibacterial assay of sandalwood (Santalum album L.) extract. Majalah Farm 2003;14:326–32.Search in Google Scholar
89. Jones, GP, Rao, KS, Tucker, DJ, Richardson, B, Barnes, A, Rivett, DE. Antimicrobial activity of santalbic acid from the oil of Santalum acuminatum (quandong). Int J Pharmacogn 1995;33:120–3. https://doi.org/10.3109/13880209509055210.Search in Google Scholar
90. Ochi, T, Shibata, H, Higuti, T, Kodama, KH, Kusumi, T, Takaishi, Y. Anti-Helicobacter pylori compounds from Santalum album. J Nat Prod 2005;68:819–24. https://doi.org/10.1021/np040188q.Search in Google Scholar PubMed
91. Vadnere, GP, Usman, MR, Lodhi, S, Patil, V. Phytochemical investigation and in vitro antimicrobial screening of Santalum album seeds extracts. Int J Pharm Pharmaceut Sci 2017;9:117–24. https://doi.org/10.22159/ijpps.2017v9i11.21216.Search in Google Scholar
92. Kim, TH, Hatano, T, Okamoto, K, Yoshida, T, Kanzaki, H, Arita, M, Ito, H. Antifungal and Ichthyotoxic sesquiterpenoids from Santalum album heartwood. Molecules 22; 2017. https://doi.org/10.3390/molecules22071139.Search in Google Scholar PubMed PubMed Central
93. Powers, CN, Osier, JL, Mcfeeters, RL, Brazell, CB, Olsen, EL, Moriarity, DM, et al.. Antifungal and cytotoxic activities of sixty commercially-available essential oils. Molecules 2018;23:1549–62. https://doi.org/10.3390/molecules23071549.Search in Google Scholar PubMed PubMed Central
94. Gupta, A, Chaphalkar, S. Immunopharmacological screening of aqueous root extract of Santalum album. J HerbMed Pharma 2015;5:7–11.Search in Google Scholar
95. Benencia, F, Courrèges, M. Antiviral activity of sandalwood oil against Herpes simplex viruses-1 and -2. Phytomedicine 1999;6:119–23. https://doi.org/10.1016/s0944-7113(99)80046-4.Search in Google Scholar
96. Haque, M, Haque, A. Use of α- and β-santalols, major constituents of sandalwood oil, in the treatment of warts, skin blemishes and other viral- induced tumors. US Patent, 2002:6406706.Search in Google Scholar
97. Salehi, B, Prakash Mishra, A, Nigam, M, Karazhan, N, Shukla, I, Kiełtyka-Dadasiewicz, A, et al.. Ficus plants: state of the art from a phytochemical, pharmacological, and toxicological perspective. Phytother Res 2021;35:1187–217. https://doi.org/10.1002/ptr.6884.Search in Google Scholar PubMed
98. Sharifi-Rad, J, Quispe, C, Durazzo, A, Lucarini, M, Souto, EB, Santini, A, et al.. Resveratrol’ biotechnological applications: enlightening its antimicrobial and antioxidant properties. J Herb Med 2022b;32:100550. https://doi.org/10.1016/j.hermed.2022.100550.Search in Google Scholar
99. Salehi, B, Sharifi-Rad, J, Capanoglu, E, Adrar, N, Catalkaya, G, Shaheen, S, et al.. Cucurbita plants: from farm to industry. Appl Sci-Basel 2019;9:21. https://doi.org/10.3390/app9163387.Search in Google Scholar
100. Scheau, C, Caruntu, C, Badarau, IA, Scheau, AE, Docea, AO, Calina, D, et al.. Cannabinoids and inflammations of the gut-lung-skin barrier. J Personalized Med 2021;11. https://doi.org/10.3390/jpm11060494.Search in Google Scholar PubMed PubMed Central
101. Salleh, WMNHW. A systematic review of botany, phytochemicals and pharmacological properties of “Hoja santa” (Piper auritum Kunth). Z Naturforsch C Biosci 2021;76:93–102. https://doi.org/10.1515/znc-2020-0116.Search in Google Scholar PubMed
102. Saneja, A, Kaushik, P, Kaushik, D, Kumar, S, Kumar, D. Antioxidant, analgesic and anti-inflammatory activities of Santalum album linn. Planta Med 2009;75:452. https://doi.org/10.1055/s-2009-1216540.Search in Google Scholar
103. Quispe, C, Herrera-Bravo, J, Javed, Z, Khan, K, Raza, S, Gulsunoglu-Konuskan, Z, et al.. Therapeutic applications of curcumin in diabetes: a review and perspective. BioMed Res Int 2022;2022:1375892. https://doi.org/10.1155/2022/1375892.Search in Google Scholar PubMed PubMed Central
104. Kulkarni, CR, Joglekar, MM, Patil, SB, Arvindekar, AU. Antihyperglycemic and antihyperlipidemic effect of Santalum album in streptozotocin induced diabetic rats. Pharm Biol 2012;50:360–5. https://doi.org/10.3109/13880209.2011.604677.Search in Google Scholar PubMed
105. Khan, M, Singh, M, Khan, M, Ahmad, S. Protective effect of Santalum album on doxorubicin induced cardiotoxicity in rats. World J Pharmaceut Res 2014;3:2760–71.Search in Google Scholar
106. Kumar, R, Anjum, N, Tripathi, YC. Phytochemistry and pharmacology of Santalum album L.: a review. World J Pharmaceut Res 2015;4:1842–76.Search in Google Scholar
107. Okugawa, H, Ueda, R, Matsumoto, K, Kawanishi, K, Kato, A. Effect of α-santalol and β-santalol from sandalwood on the central nervous system in mice. Phytomedicine 1995;2:119–26. https://doi.org/10.1016/s0944-7113(11)80056-5.Search in Google Scholar
108. Okugawa, H, Ueda, R, Matsumoto, K, Kawanishi, K, Kato, K. Effects of sesquiterpenoids from “Oriental incenses” on acetic acid-induced writhing and D2 and 5-HT2A receptors in rat brain. Phytomedicine 2000;7:417–22. https://doi.org/10.1016/s0944-7113(00)80063-x.Search in Google Scholar PubMed
109. Jackson, D, Shiju, L, Jebasingh, D, Huxley, V. Memory enhancement potential of Santalum album extracts on albino mice. J Theor Biol 2009;5:151.Search in Google Scholar
110. Inouye, S, Takahashi, M, Abe, S. Composition, antifungal and radical scavenging activities of 15 rare essential oils. Inter J Essential Oil Therap 2010;4:1–10.Search in Google Scholar
111. Dulal, S, Taher, M, Sheikh, H. Sandalwood oil can be miraculous tackle on skin aging, skin appearance and wrinkle skin – a review. World J Pharm Res 2019;5:51–5.Search in Google Scholar
112. Ahmed, N, Ali khan, M, Mat jais, A, Mohtarrudin, N, Ranjbar, M, Amjad, M, et al.. Anti-ulcer activity of sandalwood (Santalum album L.) stem hydroalcoholic extract in three gastric-ulceration models of wistar rats. Bol Latinoam Caribe Plantas Med Aromat 2013;12:81–91.Search in Google Scholar
113. Tisserand, R, Young, R. Essential oil safety: a guide for health care professionals, 2nd ed. St. Louis: Churchill Livingstone; 2014.10.1016/B978-0-443-06241-4.00013-8Search in Google Scholar
114. Warshaw, EM, Zug, KA, Belsito, DV, Fowler, JFJR., Dekoven, JG, Sasseville, D, et al.. Positive patch-test reactions to essential oils in consecutive patients from North America and central europe. Derm 2017;28:246–52. https://doi.org/10.1097/der.0000000000000293.Search in Google Scholar
115. Sharifi-Rad, J, Kamiloglu, S, Yeskaliyeva, B, Beyatli, A, Alfred, MA, Salehi, B, et al.. Pharmacological activities of psoralidin: a comprehensive review of the molecular mechanisms of action. Front Pharmacol 2020;11:11. https://doi.org/10.3389/fphar.2020.571459.Search in Google Scholar PubMed PubMed Central
116. de sanctis, V, Agolli, L, Visco, V, Monaco, F, Muni, R, Spagnoli, A, et al.. Cytokines, fatigue, and cutaneous erythema in early stage breast cancer patients receiving adjuvant radiation therapy. BioMed Res Int 2014:523568. https://doi.org/10.1155/2014/523568.Search in Google Scholar PubMed PubMed Central
117. Palatty, PL, Azmidah, A, Rao, S, Jayachander, D, Thilakchand, KR, Rai, MP, et al.. Topical application of a sandal wood oil and turmeric based cream prevents radiodermatitis in head and neck cancer patients undergoing external beam radiotherapy: a pilot study. Br J Radiol 2014;87:20130490. https://doi.org/10.1259/bjr.20130490.Search in Google Scholar PubMed PubMed Central
118. Rao, S, Hegde, SK, Baliga-Rao, MP, Lobo, J, Palatty, PL, George, T, Baliga, MS. Sandalwood oil and turmeric-based cream prevents ionizing radiation-induced dermatitis in breast cancer patients. Clin Study Med 2017;4:43–51. https://doi.org/10.3390/medicines4030043.Search in Google Scholar PubMed PubMed Central
119. Fotiades, J, Soter, NA, Lim, HW. Results of evaluation of 203 patients for photosensitivity in a 7.3-year period. J Am Acad Dermatol 1995;33:597–602. https://doi.org/10.1016/0190-9622(95)91277-0.Search in Google Scholar PubMed
120. Scalf, LA, Davis, MD, Rohlinger, AL, Connolly, SM. Photopatch testing of 182 patients: a 6-year experience at the Mayo clinic. Dermatitis 2009;20:44–52. https://doi.org/10.2310/6620.2008.08049.Search in Google Scholar
121. Victor, FC, Cohen, DE, Soter, NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol 2010;62:605–10. https://doi.org/10.1016/j.jaad.2009.06.084.Search in Google Scholar PubMed
122. Greenspoon, J, Ahluwalia, R, Juma, N, Rosen, CF. Allergic and photoallergic contact dermatitis: a 10-year experience. Dermatitis 2013;24:29–32. https://doi.org/10.1097/der.0b013e31827edc8b.Search in Google Scholar PubMed
123. Moy, RL, Levenson, C, So, JJ, Rock, JA. Single-center, open-label study of a proprietary topical 0.5% salicylic acid-based treatment regimen containing sandalwood oil in adolescents and adults with mild to moderate acne. J Drugs Dermatol JDD 2012;11:1403–8.Search in Google Scholar
124. Itoi-Ochi, S, Matsumura, S, Terao, M, Murota, H, Katayama, I. Sandalwood oil downregulates skin inflammation through 11β-HSD1 activation in keratinocytes. J Dermatol Sci 2016;84:e134. https://doi.org/10.1016/j.jdermsci.2016.08.401.Search in Google Scholar
125. Dickinson, SE, Olson, ER, Levenson, C, Janda, J, Rusche, JJ, Alberts, DS, et al.. A novel chemopreventive mechanism for a traditional medicine: East Indian sandalwood oil induces autophagy and cell death in proliferating keratinocytes. Arch Biochem Biophys 2014;558:143–52. https://doi.org/10.1016/j.abb.2014.06.021.Search in Google Scholar PubMed PubMed Central
126. Sharma, M, Levenson, C, Bell, RH, Anderson, SA, Hudson, JB, Collins, CC, et al.. Suppression of lipopolysaccharide-stimulated cytokine/chemokine production in skin cells by sandalwood oils and purified α-santalol and β-santalol. Phytother Res 2014;28:925–32. https://doi.org/10.1002/ptr.5080.Search in Google Scholar PubMed
127. Sharma, M, Levenson, C, Clements, I, Castella, P, Gebauer, K, Cox, ME. East Indian sandalwood oil (EISO) alleviates inflammatory and proliferative pathologies of psoriasis. Front Pharmacol 2017;8:125. https://doi.org/10.3389/fphar.2017.00125.Search in Google Scholar PubMed PubMed Central
128. Haque, M, Coury, DL. Topical sandalwood oil for common warts. Clin Pediatr (Phila) 2018;57:93–5. https://doi.org/10.1177/0009922817691536.Search in Google Scholar PubMed
129. Preedy, VR. Essential oils in food preservation, flavor and safety. San Diego: Academic Press; 2016.Search in Google Scholar
130. Goswami, NB, Jagatpati, T. White sandal (Santalum album L.), a precious medicinal and timber yielding plant: a short review. Plant Archives 2018;18:1048–56.Search in Google Scholar
131. Misra, BB, Dey, S. Evaluation of in vivo anti-hyperglycemic and antioxidant potentials of α-santalol and sandalwood oil. Phytomedicine 2013;20:409–16. https://doi.org/10.1016/j.phymed.2012.12.017.Search in Google Scholar PubMed
132. Es, I, Khaneghah, AM, Akbariirad, H. Global regulation of essential oils. In: Essential oils in food processing. New Jersey, United States: John Wiley & Sons, Ltd; 2017.10.1002/9781119149392.ch11Search in Google Scholar
133. Smith, RL, Cohen, SM, Doull, J, Feron, VJ, Goodman, JI, Marnett, LJ, et al.. A procedure for the safety evaluation of natural flavor complexes used as ingredients in food: essential oils. Food Chem Toxicol 2005;43:345–63. https://doi.org/10.1016/j.fct.2004.11.007.Search in Google Scholar PubMed
134. Kuttan, R, Liju, VB. Safety evaluation of essential oils. New Jersey, United States: John Wiley & Sons, Ltd; 2017.10.1002/9781119149392.ch12Search in Google Scholar
135. Buchbauer, G, Jirovetz, L, Jäger, W, Plank, C, Dietrich, H. Fragrance compounds and essential oils with sedative effects upon inhalation. J Pharm Sci 1993;82:660–4. https://doi.org/10.1002/jps.2600820623.Search in Google Scholar PubMed
136. Paulsen, E, Andersen, KE. Colophonium and Compositae mix as markers of fragrance allergy: cross-reactivity between fragrance terpenes, colophonium and compositae plant extracts. Contact Derm 2005;53:285–91. https://doi.org/10.1111/j.0105-1873.2005.00704.x.Search in Google Scholar PubMed
137. Kiec-Swierczyńska, M, Krecisz, B. Allergic contact dermatitis in dentists and dental nurses. Exog Dermatol 2002;1:27–31.10.1159/000047988Search in Google Scholar
138. Frosch, PJ, Johansen, JD, Menné, T, Pirker, C, Rastogi, SC, Andersen, KE, et al.. Further important sensitizers in patients sensitive to fragrances. Contact Dermatitis 2002;47:279–87. https://doi.org/10.1034/j.1600-0536.2002.4704171.x.Search in Google Scholar PubMed
139. Trattner, A, David, M. Patch testing with fine fragrances: comparison with fragrance mix, balsam of Peru and a fragrance series. Contact Derm 2003;49:287–9. https://doi.org/10.1111/j.0105-1873.2003.0264.x.Search in Google Scholar PubMed
140. An, S, Lee, AY, Lee, CH, Kim, DW, Hahm, JH, Kim, KJ, et al.. Fragrance contact dermatitis in Korea: a joint study. Contact Dermatitis 2005;53:320–3. https://doi.org/10.1111/j.0105-1873.2005.00720.x.Search in Google Scholar PubMed
141. Pigatto, PD, Legori, A, Bigardi, AS, Guarrera, M, Tosti, A, Santucci, B, et al.. Gruppo Italiano Ricerca Dermatiti da Contatto ed Ambientali Italian Multicenter Study of Allergic Contact Photodermatitis: epidemiological aspects. Am J Contact Derm 1996;7:158–63. https://doi.org/10.1097/01206501-199609000-00006.Search in Google Scholar
142. Jain, R, Nair, S. Sandalwood oil for the chemoprevention of skin cancer: mechanistic insights, anti-inflammatory, and in vivo anticancer potential. Current Pharmacology Reports 2019;5:345–58. https://doi.org/10.1007/s40495-019-00195-4.Search in Google Scholar
143. Bommareddy, A, Brozena, S, Steigerwalt, J, Landis, T, Hughes, S, Mabry, E, et al.. Medicinal properties of alpha-santalol, a naturally occurring constituent of sandalwood oil: review. Nat Prod Res 2019;33:527–43. https://doi.org/10.1080/14786419.2017.1399387.Search in Google Scholar PubMed
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Articles
- Covid-19 vaccines and neurological complications: a systematic review
- Santalum Genus: phytochemical constituents, biological activities and health promoting-effects
- Sakuranetin and its therapeutic potentials – a comprehensive review
- Novel sialidase from non-pathogenic bacterium Oerskovia paurometabola strain O129
- Do Colletotrichum gloeosporioides and Rhizopus stolonifer induce alkaloidal and antifungal responses in Annona muricata seedlings?
- Sesquiterpenoids from Tithonia diversifolia (Hemsl.) A. Gray induce apoptosis and inhibit the cell cycle progression of acute myeloid leukemia cells
- Isolation and purification of 12 flavonoid glycosides from Ginkgo biloba extract using sephadex LH-20 and preparative high-performance liquid chromatography
- Insecticidal activities of the essential oil of Rhynchanthus beesianus rhizomes and its constituents against two species of grain storage insects
Articles in the same Issue
- Frontmatter
- Review Articles
- Covid-19 vaccines and neurological complications: a systematic review
- Santalum Genus: phytochemical constituents, biological activities and health promoting-effects
- Sakuranetin and its therapeutic potentials – a comprehensive review
- Novel sialidase from non-pathogenic bacterium Oerskovia paurometabola strain O129
- Do Colletotrichum gloeosporioides and Rhizopus stolonifer induce alkaloidal and antifungal responses in Annona muricata seedlings?
- Sesquiterpenoids from Tithonia diversifolia (Hemsl.) A. Gray induce apoptosis and inhibit the cell cycle progression of acute myeloid leukemia cells
- Isolation and purification of 12 flavonoid glycosides from Ginkgo biloba extract using sephadex LH-20 and preparative high-performance liquid chromatography
- Insecticidal activities of the essential oil of Rhynchanthus beesianus rhizomes and its constituents against two species of grain storage insects

