Abstract
The drastic growth of the population on our planet requires the efficient and sustainable use of our natural resources. Enzymes are indispensable tools for a wide range of industries producing food, pharmaceuticals, pesticides, or biofuels. Because insects constitute one of the most species-rich classes of organisms colonizing almost every ecological niche on earth, they have developed extraordinary metabolic abilities to survive in various and sometimes extreme habitats. Despite this metabolic diversity, insect enzymes have only recently generated interest in industrial applications because only a few metabolic pathways have been sufficiently characterized. Here, we address the biosynthetic route to iridoids (cyclic monoterpenes), a group of secondary metabolites used by some members of the leaf beetle subtribe Chrysomelina as defensive compounds against their enemies. The ability to produce iridoids de novo has also convergently evolved in plants. From plant sources, numerous pharmacologically relevant structures have already been described. In addition, in plants, iridoids serve as building blocks for monoterpenoid indole alkaloids with broad therapeutic applications. As the commercial synthesis of iridoid-based drugs often relies on a semisynthetic approach involving biocatalysts, the discovery of enzymes from the insect iridoid route can account for a valuable resource and economic alternative to the previously used enzymes from the metabolism of plants. Hence, this review illustrates the recent discoveries made on the steps of the iridoid pathway in Chrysomelina leaf beetles. The findings are also placed in the context of the studied counterparts in plants and are further discussed regarding their use in technological approaches.
1 Introduction
Iridoids comprise a large family of biologically active molecules that have thus far been found in plants and insects. Structurally, they are known as cyclopentan-[c]-pyran with a hydroxyl (iridoid aglucones) or glucosyl group (iridoid glucosides) at the C-1 position of the pyran ring. In particular, plants produce manifold structures subgrouped according to, for example, substituents, linkage to other molecules, or modifications of the ring structures (summarized, e.g. in Refs. [1], [2], [3], [4]). Among the 2500–3000 identified plant iridoids/secoiridoids are the secologanins, which serve as key building blocks in the synthesis of thousands of monoterpenoid indole alkaloids, including vinblastine/vincristine or camptothecin that are widely used as anticancer agents [5], [6]. Besides anticancer activity, iridoids have additional pharmaceutical potentials that provide valuable resources for the development of novel drugs and therapeutic strategies against diverse diseases [7], [8], [9]. In the natural environment, iridoids benefit plants by preventing microbial invasions [10], [11], [12], [13], [14], and by repelling herbivorous vertebrates and invertebrates [10], [15], [16].
The protective effect of iridoids can be based on their bitter taste, making these phytochemicals in particular distasteful to mammals [17], and on their physiological toxicity, which also affects invertebrates and pathogens in a dose-dependent manner [2]. To date, it has been shown that iridoid toxicity can be attributed to the highly reactive aglycones that are released from the corresponding nontoxic iridoid glucosides that are often safely stored in plant organelles [18], [19]. Glycoside hydrolysis can be achieved nonenzymatically or enzymatically by β-glucosidases (hydrolases, EC 3.2.1.21) produced by the plants themselves [20], [21], [22] or by their enemies [12], [13], [23], [24]. If the resulting compound is a reactive aldehyde, it has the ability to link irreversibly and nonselectively essential cellular components including proteins. This may affect the physiological processes of invasive organisms directly and/or decrease the nutritive value of dietary proteins considerably [25], [26], [27], [28]. Although a universal target of iridoids has not been defined, these rather nonspecific effects contribute to an increased mortality of, for example, nonadapted herbivores feeding on iridoid glycoside-containing diets [24].
The biosynthesis of iridoids in plants proceeds through C5-isopentenyl diphosphate (C5-IDP) and C5-dimethylallyl diphosphate (C5-DMADP) – the universal building blocks for all terpenoids. C5-IDP and C5-DMADP are biosynthesized in two pathways existing side by side in higher plants – namely, the cytosolic mevalonic acid (MVA) pathway and the plastid 2-methyl-d-erythritol 4-phosphate pathway. The 2-methyl-d-erythritol 4-phosphate route was found to be the main route for the synthesis of iridoid precursors in plants [7]. The iridoid pathway starts with its key intermediate, geraniol diphosphate, and comprises a number of oxidation, reduction, glycosylation, and methylation reactions [5], [29]. The biosynthesis of secologanin through the intermediates iridodial and iridotrial in the Madagascar periwinkle, Catharanthus roseus, has been best understood [30], [31], [32], [33], [34], [35]. The pathway in C. roseus is organized in a complex manner, with the enzymes localized in different cell types and subcellular compartments [36], [37].
Although iridoids are typically encountered in the plant kingdom, these secondary metabolites can also be identified in insects. In fact, the name iridoid is a generic term derived from iridomyrmecin, a component of defensive secretions identified from species of the ant genus Iridomyrmex [38] (Figure 1). Insects use iridoids frequently as chemical stimuli for communication or defense [41], [42], [43], [44], [45], [46], [47]. Although many insects profit from the sequestration of iridoid glucosides from their host plants [16], [48], [49], [50], others, such as stick insects [51], rove beetles [40], or leaf beetles [52], [53], [54], [55], [56] are able to produce iridoids de novo.
The biosynthetic steps are thought to proceed in a similar way to the known pathway in plants. However, as in plants, in insects, the iridoid pathway has also been characterized in only a few species and, even in these pioneering examples, the pathway is not yet fully resolved. The best investigated insect species regarding iridoid synthesis thus far belong to the family of leaf beetles (Chrysomelidae, subtribe Chrysomelina) (Table 1). In particular, the juveniles of Chrysomelina beetles evolved specialized pair-wise exocrine glands (composed of a reservoir with adhered glandular cells) on their dorsal segments to release iridoids in droplets as defensive secretions. Considering the evolutionary aspects of Chrysomelina beetles, it has been shown that iridoid de novo synthesis precedes the sequestration of plant-derived secondary metabolites for the production of defensive compounds [57]. To achieve the exploitation of phytochemicals, the larvae use transport and metabolic mechanisms already present in the iridoid de novo-producing species. These mechanisms were adapted according to host plant affiliations during the evolutionary sequence of Chrysomelina species.
Iridoid compounds in defensive secretions from the larval stages of selected Chrysomelina (Chrysomelidae) species specialized to different host plants.
| Species | Compounds | Configuration | de (%) | Host plant |
|---|---|---|---|---|
| Phaedon cochleariae | Chrysomelidial | (5R,8R) | 94 | Brassica rapa subsp. chinensis |
| Hydrothassa marginella | Chrysomelidial, plagiolactone | (5S,8S) | 95 | Ranunculus acris |
| Phratora vulgatissima | Chrysomelidial, plagiodial | (5S,8S) | 93 | Salix caprea |
| Gastrophysa viridula | Chrysomelidial | (5R,8R) | 96 | Rumex obtusifolius |
| Gastrophysa polygoni | Chrysomelidial | (5R,8R) | 92 | Polygonum aviculare |
| Gastrophysa cyanea | Chrysomelidial, gastrolactone | (5R,8R) | 97 | Rumex obtusifolia |
| Gastrophysa atrocyanea | Chrysomelidial | (5R,8R) | 91 | Rumex obtusifolia |
| Plagiodera versicolora | Plagiodial, plagiolactone | Salix fragilis | ||
| Linea aenea | Plagiodial, plagiolactone | Alnus glutinosa | ||
| Prasocuris phellandrii | Plagiodial | Caltha palustris | ||
| Phratora laticollis | Plagiodial | Populus canadensis |
The absolute configuration of chrysomelidial has been measured by gas chromatography–mass spectrometry (GC-MS) on a chiral column (adapted from Kunert et al. [39]). de, diastereomeric excess.
Based on the fact that iridoid/secoiridoid-derived compounds have tremendous pharmacological potential, the plant enzymes of this pathway are the focus of the current research as biocatalysts in commercial drug production [58], [59]. Additionally, because iridoids are also used as sex pheromones by aphids, which are agriculturally relevant pest species, they are exploited for the development of innovative and integrated pest management strategies [60], [61]. Hence, a molecular understanding of the iridoid metabolism, not only in plants but also in insects, has the potential to expand the repertoire of enzymatic workhorses available for different industrial approaches in the field of human health or nutrition. Due to the ecological and socioeconomic relevance of the iridoid pathway, we highlight in this review the most recent developments in our understanding of the iridoid biosynthesis and its enzymatic machinery in Chrysomelina beetles.
2 Iridoid de novo synthesis: early steps
The iridoid de novo biosynthesis in the larvae of Chrysomelina species starts with the formation of the isoprene units C5-DMADP and C5-IDP, which are derived from the MVA pathway [62]. To date, two enzymes have been studied in this early part of the pathway: the 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR, EC 1.1.1.34) and the short-chain isoprenyl diphosphate synthase (scIDS) whose properties are described below in more detail.
HMGR catalyzes the rate-limiting step in the MVA pathway [63]. The enzyme utilizes two molecules of NADPH to mediate the four-electron reduction of 3-hydroxy-3-methyl-glutaryl-coenzyme A to the carboxylic acid mevalonate. Because HMGR is one of the most regulated enzymes known [64], its regulatory features may also be important for the biosynthesis of iridoids in chrysomelids. Consistently, analyses of different larval tissues from the iridoid synthesizing species Phaedon cochleariae and Gastrophysa viridula revealed high HMGR mRNA levels, high HMGR activity, and accumulation of the iridoid intermediate, 8-hydroxygeraniol-8-O-β-d-glucoside, in the fat body tissue of the iridoid de novo producers [65]. Hence, the fat body – the most prominent tissue in the larvae performing myriad metabolic functions throughout the insects’ development [66] – is implicated in de novo production of the glucosidically bound iridoid precursor. It is further reasonable to assume that iridoid biosynthesis is spatially distributed and the glucosidically bound intermediate is released from fat body tissue into the hemolymph followed by transport into the defensive glands for further conversion.
HMGR is regulated on very different levels, pre- and posttranslationally [64]. In insects, for example, it is known that HMGR transcription is affected by juvenile hormones [67], [68]. From Chrysomelina beetles, we reported that HMGR is negatively regulated by 8-hydroxygeraniol, another intermediate of iridoid biosynthesis [69]. Purification of the catalytic HMGR domain revealed that inhibition by 8-hydroxygeraniol is subject to the catalytic domain, which was corroborated by docking analyses on the modeled HMGR catalytic portion. De novo producing larvae possess the potential to sequester glucosidically bound 8-hydroxygeraniol if present in the diet [70], [71], [72], [73]. After cleavage of the sugar moiety, the aglucon may interfere with HMGR and, consequently, the enzyme may represent a key regulator to maintain homeostasis of endo- and exogenous metabolites of the iridoid synthesis. Inhibition was also observed for other insect HMGRs including Drosophila melanogaster.
The second characterized enzyme of the early steps in the iridoid pathway is a member of the scIDS. Generally, catalysis by scIDSs follows a sequential mechanism called “head-to-tail alkylation”. During chain elongation, the allylic cosubstrate, e.g. C5-DMADP or C10-geranyl diphosphate (C10-GDP), undergoes coupling with homoallylic C5-IDP through electrophilic alkylation at its carbon-carbon double bond. For the scIDS in the iridoid pathway, it is expected that the enzyme produces C10-GDP, the ubiquitous C10-building block of many monoterpenes [74], [75], [76], [77]. The reaction mechanism depends on the activation on a trinuclear metal cluster usually containing Mg2+ or Mn2+ [78].
Compared with plants, only a few GDPs have thus far been characterized in insects [75]. Strikingly, most of them have the ability to form multiple products. For example, an enzyme studied from the bark beetle, Ips pini, displayed prenyltransferase and terpene synthase activity [79], [80], [81], resulting in the formation of precursors for the de novo synthesis of monoterpenoid aggregation pheromones such as ipsdienol, which coordinates the colonization of coniferous trees [82]. Another scIDS from Dendroctonus spp. bark beetles produced C10-GDP and C15-farnesyl diphosphate (C15-FDP) depending on the C5-IDP/C5-DMADP substrate ratio [79], [80], [81]. Bifunctionality was also observed from the scIDSs characterized from different aphid species [83], [84], [85], [86], [87]. Here, the recombinant proteins generated both GDP and FDP in parallel, and hence may be involved in the biosynthesis of either aphid sex pheromones or the sesquiterpene (E)-β-farnesene, the most common component of alarm pheromones.
Based on earlier studies describing the role of metal cofactors for scIDS catalysis, the product composition of a scIDS discovered from juvenile P. cochleariae has been tested in the presence of different metal ions [88]. Surprisingly, we found the enzyme isoprenyl diphosphate synthase 1 (PcIDS1) from P. cochleariae possessing an unusual product regulation mechanism not previously described for scIDSs. It alters the chain length of its products depending on the cofactor: the recombinant PcIDS1 yielded 96% C10-GDP and only 4% C15-FDP in the presence of Co2+ or Mn2+ as a cofactor, whereas it yielded only 18% C10-GDP but 82% C15-FDP in the presence of Mg2+. Kinetic studies further reinforced their assertion that PcIDS1 has an energetic preference for Co2+ with C5-DMADP as an allylic cosubstrate for C10-GDP production but showed that C15-FPP production was favored when Mg2+ was the cofactor. Cation quantification studies in P. cochleariae larval tissues strengthened the physiological plausibility that the flux of carbon into separate metabolic pathways (C10- vs. C15-isoprenoids) could be accomplished by these ions in vivo.
Inspired by our work, the functional characterization of a farnesyl diphosphate synthase from the yellow fever mosquito, Aedes aegyptii, for example, revealed a similar dependency from the divalent cation of product condensation as observed for PcIDS1 [89]. Given that plants possess a number of genes encoding IDS’s (e.g. at least 10 in Arabidopsis thaliana), whereas insects possess only a few (e.g. 3 in Bombyx mori), insects may compensate for this disparity by generating different chain-length products in other ways. Instead of “inventing” a new IDS, insects seem to use different cofactors to add products to an enzyme’s repertoire, thereby lowering metabolic costs. This type of “adjustable” enzyme may afford insects an efficient mechanism for the generation of chemical diversity that is critical for adaptation to ever-changing ecological contexts. Compared with plants, the functions of the many predicted isoprenyl diphosphate synthases in insects are much less understood. For example, putative trans-isoprenyl diphosphate synthases recently characterized from the flea beetle, Phyllotreta striolata, displayed terpene synthase activity [90]. Hence, the few functionally characterized isoprenyl diphosphate synthases from insects have already shown the potential of these enzymes or of chimeric insect-plant/microbe proteins [91] for use in a biotechnological context, such as in the optimization of carbon fluxes during the production processes of pharmaceuticals.
Later in the iridoid pathway, geranyl diphosphate is converted into 8-hydroxygeraniol through geraniol, thereby removing the diphosphate moiety (Figure 2). The enzymes responsible for these reactions still remain to be elucidated in iridoid de novo-producing leaf beetles. In the plant C. roseus, the conversion of GDP into geraniol is catalyzed by a terpene synthase (geraniol synthase), but in beetles, a homologous sequence has not been identified. It is conceivable that a phosphatase is involved in the PPi group cleavage followed by a cytochrome P450 mediated ω-hydroxylation to obtain 8-hydroxy geraniol [92], [93]. An alternative would be the implication of a so-called “moonlighting P450” enzyme that possesses two catalytic centers exerting monooxygenase and terpene synthase activity [94]. Such an enzyme could cleave the diphosphate and oxidize geraniol to form 8-hydroxygerniol. In C. roseus, it is known that the P450 enzyme CYP76B6 (G8O) oxidizes geraniol to 8-hydroxygeraniol [95]. Following oxidation, a glucose unit is transferred onto 8-hydroxygeraniol to enable the translocation of the precursor from the hemolymph into the defensive glands. The responsible glycosyltransferase, however, remains elusive.
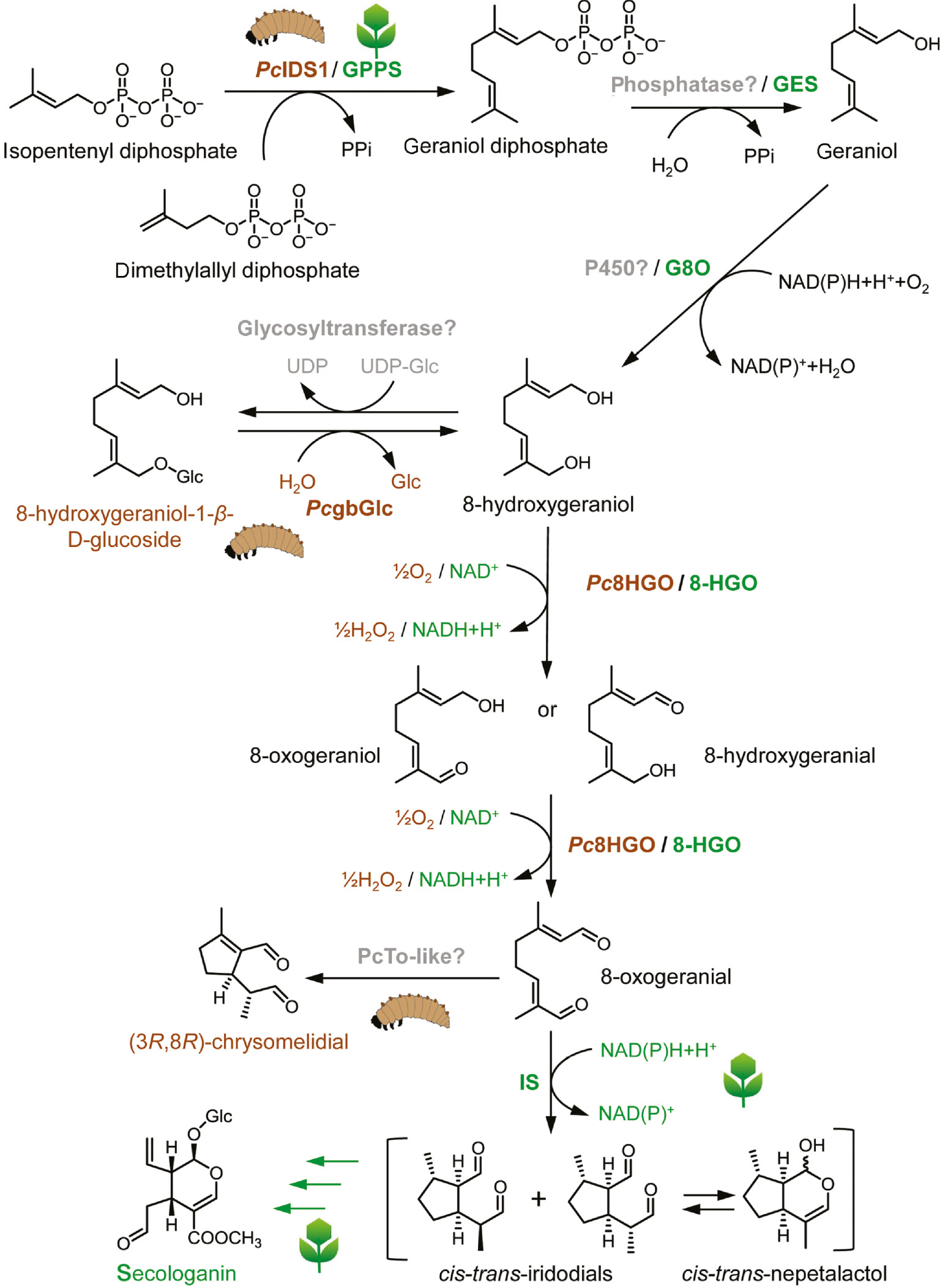
Comparative illustration of key steps in the iridoid/secoidoid biosynthesis from mustard leaf beetle, P. cochleariae (Pc), and Madagascar periwinkle, C. roseus. GPPS, geranyl diphosphate synthase; GES, geraniol synthase; G8O, geraniol 8-oxidase; 8-HGO, 8-hydroxygeraniol oxidoreductase; IS, iridoid synthase; PcIDS1, isoprenyl diphosphate synthase; PcgbGlc, glandular β-glucosidase; Pc8HGO, 8-hydroxy geraniol oxidoreductase (GMC superfamily); PcTo-like, takeout-like protein.
3 Iridoid de novo synthesis: late steps
In P. cochleariae, the iridoid biosynthesis proceeds not entirely in the glands but it is most likely compartmented within the larval body [69]. After translocation of 8-hydroxygeraniol-O-β-d-glucoside into the defensive glands, its final transformation into iridoids occurs in the secretions. This transformation involves the hydrolysis of the glucoside and oxidation of the two primary hydroxy groups to produce the dialdehyde 8-oxogeranial, which is followed by a cyclization [52], [54], [39].
In general, hydrolysis of glycosides occurs in the metabolism of all organisms. Enzymes that catalyze such reactions often belong to the glycosyl hydrolase family 1 (GH1) according to CAZy [96], [97]. Whereas in plants, GH1s play an important role in the activation of glucosides for defense purpose [98], [99], [100], [101], insects use those enzymes mainly for digestion, either in the gut or in the salivary glands [24], [102], [103]. In addition to this, GH1s may also be involved in the production of chemical defenses widely distributed in insects [104]. However, because only a few metabolic pathways have been characterized to date, it is not surprising that only a few insect β-glucosidases are known to be utilized for the synthesis of deterrents. Examples include the β-glucosidase linamarase from the caterpillars of the six-spot burnet moth, Zygaena filipendulae [105], [106], the ascorbate-dependent β-thioglucosidases (called myrosinases) from the cabbage aphid, Brevicoryne brassicae [107], [108], [109], and from the flea beetle, P. striolata [110].
To assess their importance in deterrent biosynthesis, we identified and functionally characterized GH1 glucosidases from different Chrysomelina species [111]. Determination of the kinetic parameters of heterologously expressed enzyme from P. cochleariae revealed hydrolase activity in the presence of physiological precursors from different deterrent pathways found in Chrysomelina beetles, i.e. activity was determined in the presence of 8-hydroxygeraniol-O-β-d-glucoside (intermediate in the iridoid metabolism of P. cochleariae), salicin (intermediate in the salicyl aldehyde synthesis of Chrysomela populi), and 2-phenylethyl-β-d-glucoside (intermediate in the ester production found in Chrysomela lapponica). Evidently, the intrinsic broad substrate selectivity of the enzyme does not require changes in the catalytic center to allow the conversion of different plant-derived compounds.
β-glucosidases play an important role in complex biomass hydrolysis from renewable sources for the production of biofuels, food, or food additives [112], [113]. However, product inhibition impairing yields, thermal inactivation of enzymes, and the high cost of enzyme production are still the main obstacles to commercial hydrolysis processes. Hence, the demand for alternatives to the currently available enzyme preparations has prompted researchers to further characterize enzymes from the most diverse organisms. The hydrolysis reactions from insects represent one of these sources that have not yet been sufficiently exploited. For example, the glucosidases from Chrysomelina leaf beetles exhibited an optimal hydrolytic activity at particularly low pH (optimal range 4.5–6), a property that might be of interest for specific technological processes [111].
The following reaction, flavin adenine dinucleotide (FAD)-dependent oxidation, is known to be catalyzed from members of the multigene family of glucose-methanol-choline (GMC) oxidoreductases [114]. Interestingly, plants use a completely different enzyme family for the oxidation of 8-hydroxygeraniol. In C. roseus, an NAD(P)-binding Rossmann fold domain-type oxidoreductase (8-hydroxygeraniol oxidoreductase, 8-HGO) contributes to the formation of 8-oxogeranial by the catalysis of two successive and reversible oxidation steps [30].
The GMC protein from the larvae of P. cochleariae (Pc8HGO) has been identified in defensive secretions by proteomic analyses [115]. The importance of this enzyme for the formation of chrysomelidial has been further verified by RNAi in vivo. Functional characterization, including the substrate specificity of this enzyme after heterologous expression, revealed the selective oxidation of 8-hydroxygeraniol to 8-oxogeranial. In addition, the substrate specificity of Pc8HGO was tested by incubating the oxidase with salicyl alcohol, the substrate of Chrysomela spp. salicyl alcohol oxidase (SAO). No enzyme-based conversion to salicyl aldehyde could be detected, indicating that this particular enzyme does not react with salicyl alcohol. Also, the recently tested SAO is selective only for the genuine substrate of the salicyl aldehyde pathway [116], [117], [118]. Thus, unlike the glandular β-glucosidases, the GMC oxidases have a narrow substrate spectrum.
In comparison to other oxidoreductases, the members of the GMC oxidoreductase family share a conserved sequence motif, the β-α-β dinucleotide binding motif (GxGxxG(x)18E) responsible for the binding of cofactor FAD. Despite the diversity of substrates that can be converted by the members of this protein family, the majority of GMC oxidoreductases seem to share a catalysis mechanism involving a hydride transfer from the substrate to FAD that is promoted by a conserved histidine residue. Subsequently, molecular oxygen is utilized as the acceptor for the hydride and is further reduced to hydrogen peroxide. In insects, the GMC oxidoreductase multigene family has undergone a massive expansion. For comparison, in vertebrates, only one to two GMC genes are known, whereas, in insects, 15–43 genes are known. It is believed that these GMC genes are involved in developmental, immune, or defensive processes in insects. It seems that the substrate diversity in redox reactions potentially supplied by this multigene family equips insects with a toolbox that allows them to adjust to the particular biotic and abiotic conditions that may result, for example, when shifting host plants [119], [120]. Although the exact functions of many of these GMC genes have not been elucidated, it can already be suggested that GMC members have the potential to catalyze reactions that are valuable from a biotechnological perspective.
Whereas deglucosylation and oxidation reactions are also found in sequestering species, the final cyclization of acyclic dialdehydes to generate iridoids proceeds exclusively in iridoid de novo producing species. Interestingly, with the same precursor, isotopic tracing studies have shown that there are two mechanistically different cyclization modes in different leaf beetle groups [39]. When deuterium atom labeled [2H5]Ger-8-OH was used for the feeding experiments, the precursor lost a single deuterium atom from C(4) in P. cochleariae, Hydrothassa marginella, and Phratora vulgatissima. In contrast, in Gastrophysa cyanea, Gastrophysa polygoni, Gastrophysa atrocyanea, and G. viridula, [2H5]Ger-8-OH was observed to exchange all three deuterium atoms from the methyl group at C(3) (Figure 3). Based on these isotopic labeling studies, two different cyclization mechanisms have been proposed proceeding through either a “transoid” orientation of the dienamine intermediate (Phaedon-type cyclization) or a “cisoid” orientation of the dienamine (Gastrophysa-type cyclization).
![Figure 3: Metabolism of [2H5]-8-hydroxygeraniol toward chrysomelidial in different leaf beetle larvae. The proposed mechanism for iridoid cyclization includes the formation of a dienamine. The dienamine intermediate is “transoid” for Phaedon and “cisoid” for Gastrophysa (adapted from Kunert et al. [39]).](/document/doi/10.1515/znc-2017-0015/asset/graphic/j_znc-2017-0015_fig_003.jpg)
Metabolism of [2H5]-8-hydroxygeraniol toward chrysomelidial in different leaf beetle larvae. The proposed mechanism for iridoid cyclization includes the formation of a dienamine. The dienamine intermediate is “transoid” for Phaedon and “cisoid” for Gastrophysa (adapted from Kunert et al. [39]).
Moreover, the absolute configuration and optical purity of chrysomelidial secreted by different families was determined by GC-MS. Curiously, except for those in H. marginella and P. vulgatissima, which are (5S,8S)-chrysomelidial, secretions in P. cochleariae and all investigated members of the genus Gastrophysa contain (5R,8R)-chrysomelidial. To date, however, the only enzyme capable of performing the reductive cyclization step of 8-oxogeranial has been identified in C. roseus. The iridoid synthase is a member of the Rossmann-fold NAD(P)+-binding protein superfamily. It cyclizes 8-oxogeranial to cis-trans-iridodials and cis-trans-nepetalactol under the consumption of NAD(P)H [32]. In a proteomic analysis, however, no protein similar to the iridoid synthase from C. roseus could be detected in the secretions of iridoid-producing Chrysomelina larvae. Chemical analysis of the cyclization reaction predicted a NAD(P)H-independent reaction leading to the assumption that a different class of enzymes is catalyzing the reaction in insects compared with plants.
The most promising candidate to be involved in cyclization is a takeout-like protein, a member of the juvenile hormone-binding protein superfamily, which comprises ligand-binding proteins for juvenile hormones or similar hydrophobic terpenoids [121]. The detailed biochemical characterization of this protein in vitro, however, is still in progress. As the enzymes from Chrysomelina leaf beetles are known to differ in their stereoselectivity [39], a variety of cyclic products can be produced that could serve as building blocks for new metabolites of pharmacological relevance. Hence, deciphering the reaction mechanism would make the enzyme prospectively a candidate for application in drug development.
4 Conclusion and future aspects
Important gaps in our understanding of insect metabolism have been filled in recent years. However, we are still only scratching the surface of the metabolic complexity present in the approximately one million insect species on our planet [122]. Every newly discovered and functional studied protein contributes to the growing set of biocatalysts that can render chemical synthesis more efficient and sustainable. Iridoid biosynthesis in leaf beetles definitely offers special features that could be attractive for industrial applications. Because the iridoid pathway in beetles diverges from that in plants, its catalytic proteins can come into consideration as valuable tools for alternative synthesis strategies for iridoid-derived drugs. Our mechanistic studies on the insect iridoid route hence provide a framework for further enzyme engineering.
The intrinsic catalytic activity itself as well as the physicochemical properties of the enzymes can be exploited for industrial applications. For example, the secretory proteins localized in the defensive secretions of Chrysomelina larvae have to function in an extracellular milieu not comparable with the normal cellular interior. Defensive secretions possess nonphysiologic pH values and contain the enzymes together with the end products. Often, these metabolic end products are lipophilic and form separated phases or a kind of oil-water emulsion in the secretions [123]. Furthermore, the deterrents can have damaging effects on the proteins, e.g. iridoids can cause a nonspecific cross-linking of proteins. Hence, the enzymes in emulsions or on the interface between hydrophilic and hydrophobic phases have to meet special requirements to fulfill their function. Often, special decorations of proteins or interactions with chaperone-like proteins improve stability in nonphysiological environments.
In particular, protein modifications by N- or O-glycanes modulate the physicochemical properties of proteins including thermodynamics, kinetics, chemical stability, or 3D architecture [124]. Because protein-based pharmaceuticals such as antibodies or enzymes require long-term stability, much emphasis has been placed on optimal glycosylation parameters by researchers in the field of glycoprotein engineering [125], [126]. An understanding of the sugar composition of secretory proteins in defensive secretions could thus contribute alternative glycosylation patterns that might benefit protein design in modern medicine.
Because secretory enzymes have to ensure deterrent production in a closed reservoir “outside” the insect body, they are exposed to a fluctuating surrounding temperature and should be able to operate in a varying range of temperatures. As technological processes often have to proceed under nonphysiological conditions simply to be profitable, an understanding of the secretory proteins from a thermodynamic perspective might promote these processes. Our current understanding of the secretory proteins in insect iridoid producers provides a platform to build on future research on defensive secretions from insects with regard to biochemical as well as technological aspects.
References
1. Jensen SR. Plant iridoids their biosynthesis and distribution in angiosperms. In: Harborne JB, Tomas-Barberan FA, editors. Ecological chemistry and biochemistry of plant terpenoids. Oxford: Clarendon Press, 1991:133–58.Search in Google Scholar
2. Dinda B, Chowdhury DR, Mohanta BC. Naturally occurring iridoids, secoiridoids and their bioactivity. An updated review, Part 3. Chem Pharm Bull 2009;57:765–96.10.1248/cpb.57.765Search in Google Scholar
3. Dinda B, Debnath S, Banik R. Naturally occurring iridoids and secoiridoids. An updated review, Part 4. Chem Pharm Bull 2011;59:803–33.10.1248/cpb.59.803Search in Google Scholar
4. Elnaggar LJ, Beal JL. Iridoids – a review. J Nat Prod 1980;43: 649–707.10.1021/np50012a001Search in Google Scholar
5. De Luca V, Salim V, Thamm A, Masada SA, Yu F. Making iridoids/secoiridoids and monoterpenoid indole alkaloids: progress on pathway elucidation. Curr Opin Plant Biol 2014;19:35–42.10.1016/j.pbi.2014.03.006Search in Google Scholar
6. Almagro L, Fernandez-Perez F, Pedreno MA. Indole alkaloids from Catharanthus roseus: bioproduction and their effect on human health. Molecules 2015;20:2973–3000.10.3390/molecules20022973Search in Google Scholar
7. Dinda B, Debnath S. Monoterpenes: iridoids. In: Ramawat KG, Mérillon J-M, editors. Natural products: phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes. Berlin, Heidelberg: Springer, 2013:3009–67.10.1007/978-3-642-22144-6_132Search in Google Scholar
8. Tundis R, Loizzo MR, Menichini F, Statti GA, Menichini F. Biological and pharmacological activities of iridoids: recent developments. Mini Rev Med Chem 2008;8:399–420.10.2174/138955708783955926Search in Google Scholar
9. Ghisalberti EL. Biological and pharmacological activity of naturally occurring iridoids and secoiridoids. Phytomedicine 1998;5:147–63.10.1016/S0944-7113(98)80012-3Search in Google Scholar
10. Biere A, Marak HB, van Damme JM. Plant chemical defense against herbivores and pathogens: generalized defense or trade-offs? Oecologia 2004;140:430–41.10.1007/s00442-004-1603-6Search in Google Scholar
11. Davini E, Javarone C, Trogolo C, Aureli P, Pasolini B. The quantitative isolation and antimicrobial activity of the aglycone of aucubin. Phytochemistry 1986;25:2420–2.10.1016/S0031-9422(00)81711-2Search in Google Scholar
12. Marak HB, Biere A, van Damme JM. Two herbivore-deterrent iridoid glycosides reduce the in vitro growth of a specialist but not of a generalist pathogenic fungus of Plantago lanceolata L. Chemoecology 2002;12:185–92.10.1007/PL00012667Search in Google Scholar
13. Baden CU, Dobler S. Potential benefits of iridoid glycoside sequestration in Longitarsus melanocephalus (Coleoptera, Chrysomelidae). Basic Appl Ecol 2009;10:27–33.10.1016/j.baae.2007.12.003Search in Google Scholar
14. Whitehead SR, Tiramani J, Bowers MD. Iridoid glycosides from fruits reduce the growth of fungi associated with fruit rot. J Plant Ecol 2015;9:357–66.10.1093/jpe/rtv063Search in Google Scholar
15. Bowers MD. Iridoid glycosides. In: Rosenthal GA, Berenbaum MR, editors. Herbivores: their interactions with secondary plant metabolites, Vol. 1: the chemical participants. San Diego, CA: Academic Press, 1991:297–325.10.1016/B978-0-12-597183-6.50013-9Search in Google Scholar
16. Dobler S, Petschenka G, Pankoke H. Coping with toxic plant compounds – the insect’s perspective on iridoid glycosides and cardenolides. Phytochemistry 2011;72:1593–604.10.1016/j.phytochem.2011.04.015Search in Google Scholar
17. Rodriguez S, Marston A, Wolfender JL, Hostettmann K. Iridoids and secoiridoids in the Gentianaceae. Curr Org Chem 1998;2:627–48.10.2174/1385272802666220130082729Search in Google Scholar
18. Kim D-H, Kim B-R, Kim J-Y, Jeong Y-C. Mechanism of covalent adduct formation of aucubin to proteins. Toxicol Lett 2000;114:181–8.10.1016/S0378-4274(99)00295-7Search in Google Scholar
19. Konno K, Hirayama C, Yasui H, Nakamura M. Enzymatic activation of oleuropein: a protein crosslinker used as a chemical defense in the privet tree. Proc Natl Acad Sci USA 1999;96: 9159–64.10.1073/pnas.96.16.9159Search in Google Scholar PubMed PubMed Central
20. Pankoke H, Buschmann T, Muller C. Role of plant beta-glucosidases in the dual defense system of iridoid glycosides and their hydrolyzing enzymes in Plantago lanceolata and Plantago major. Phytochemistry 2013;94:99–107.10.1016/j.phytochem.2013.04.016Search in Google Scholar PubMed
21. Pankoke H, Gehring R, Muller C. Impact of the dual defence system of Plantago lanceolata (Plantaginaceae) on performance, nutrient utilisation and feeding choice behaviour of Amata mogadorensis larvae (Lepidoptera, Erebidae). J Insect Physiol 2015;82:99–108.10.1016/j.jinsphys.2015.08.006Search in Google Scholar PubMed
22. Koudounas K, Banilas G, Michaelidis C, Demoliou C, Rigas S, Hatzopoulos P. A defence-related Olea europaea β-glucosidase hydrolyses and activates oleuropein into a potent protein cross-linking agent. J Exp Bot 2015;66:2093–106.10.1093/jxb/erv002Search in Google Scholar
23. Pankoke H, Dobler S. Low rates of iridoid glycoside hydrolysis in two Longitarsus leaf beetles with different feeding specialization confer tolerance to iridoid glycoside containing host plants. Physiol Entomol 2015;40:18–29.10.1111/phen.12085Search in Google Scholar
24. Pankoke H, Bowers MD, Dobler S. The interplay between toxin-releasing β-glucosidase and plant iridoid glycosides impairs larval development in a generalist caterpillar, Grammia incorrupta (Arctiidae). Insect Biochem Mol Biol 2012;42:426–34.10.1016/j.ibmb.2012.02.004Search in Google Scholar
25. Konno K, Hirayama C, Yasui H, Okada S, Sugimura M, Yukuhiro F, et al. GABA, β-alanine and glycine in the digestive juice of privet-specialist insects: convergent adaptive traits against plant iridoids. J Chem Ecol 2010;36:983–91.10.1007/s10886-010-9842-ySearch in Google Scholar
26. Bartholomaeus A, Ahokas J. Inhibition of P-450 by aucubin: is the biological activity of aucubin due to its glutaraldehyde-like aglycone? Toxicol Lett 1995;80:75–83.10.1016/0378-4274(95)03339-MSearch in Google Scholar
27. Ling SK, Tanaka T, Kouno I. Effects of iridoids on lipoxygenase and hyaluronidase activities and their activation by beta-glucosidase in the presence of amino acids. Biol Pharm Bull 2003;26:352–6.10.1248/bpb.26.352Search in Google Scholar PubMed
28. Pungitore CR, Ayub MJ, García M, Borkowski EJ, Sosa ME, Ciuffo G, et al. Iridoids as allelochemicals and DNA polymerase inhibitors. J Nat Prod 2004;67:357–61.10.1021/np030238bSearch in Google Scholar PubMed
29. Huang YX, Tan HX, Guo ZY, Wu XX, Zhang QL, Zhang L, et al. The biosynthesis and genetic engineering of bioactive indole alkaloids in plants. J Plant Biol 2016;59:203–14.10.1007/s12374-016-0032-5Search in Google Scholar
30. Miettinen K, Dong L, Navrot N, Schneider T, Burlat V, Pollier J, et al. The seco-iridoid pathway from Catharanthus roseus. Nat Commun 2014;5:3606.10.1038/ncomms4606Search in Google Scholar PubMed PubMed Central
31. Loyola-Vargas VM, Galaz-Ávalos RM, Kú-Cauich R. Catharanthus biosynthetic enzymes: the road ahead. Phytochem Rev 2007;6:307–39.10.1007/s11101-007-9064-2Search in Google Scholar
32. Geu-Flores F, Sherden NH, Courdavault V, Burlat V, Glenn WS, Wu C, et al. An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis. Nature 2012;492:138–42.10.1038/nature11692Search in Google Scholar
33. Salim V, De Luca V. Chapter one– towards complete elucidation of monoterpene indole alkaloid biosynthesis pathway: Catharanthus roseus as a pioneer system. Adv Bot Res 2013;68:1–37.10.1016/B978-0-12-408061-4.00001-8Search in Google Scholar
34. Thamm AM, Qu Y, De Luca V. Discovery and metabolic engineering of iridoid/secoiridoid and monoterpenoid indole alkaloid biosynthesis. Phytochem Rev 2016;15:339–61.10.1007/s11101-016-9468-ySearch in Google Scholar
35. Kries H, Caputi L, Stevenson CE, Kamileen MO, Sherden NH, Geu-Flores F, et al. Structural determinants of reductive terpene cyclization in iridoid biosynthesis. Nat Chem Biol 2016;12:6–8.10.1038/nchembio.1955Search in Google Scholar
36. Verma P, Mathur AK, Srivastava A, Mathur A. Emerging trends in research on spatial and temporal organization of terpenoid indole alkaloid pathway in Catharanthus roseus: a literature update. Protoplasma 2012;249:255–68.10.1007/s00709-011-0291-4Search in Google Scholar
37. Mahroug S, Burlat V, St-Pierre B. Cellular and sub-cellular organisation of the monoterpenoid indole alkaloid pathway in Catharanthus roseus. Phytochem Rev 2007;6:363–81.10.1007/s11101-006-9017-1Search in Google Scholar
38. Cavill GW, Ford DL, Locksley HD. The chemistry of ants. 1. Terpenoid constituents of some Australian Iridomyrmex species. Aust J Chem 1956;9:288–93.10.1071/CH9560288Search in Google Scholar
39. Kunert M, Rahfeld P, Shaker KH, Schneider B, David A, Dettner K, et al. Beetles do it differently: two stereodivergent cyclisation modes in iridoid-producing leaf-beetle larvae. ChemBioChem 2013;14:353–60.10.1002/cbic.201200689Search in Google Scholar
40. Weibel DB, Oldham NJ, Feld B, Glombitza G, Dettner K, Boland W. Iridoid biosynthesis in staphylinid rove beetles (Coleoptera: Staphylinidae, Philonthinae). Insect Biochem Mol Biol 2001;31:583–91.10.1016/S0965-1748(00)00163-6Search in Google Scholar
41. Pasteels JM, Braekman JC, Daloze D, Ottinger R. Chemical defence in chrysomelid larvae and adults. Tetrahedron 1982;38:1891–7.10.1016/0040-4020(82)80038-0Search in Google Scholar
42. Dawson GW, Griffiths DC, Pickett JA, Wadhams LJ, Woodcock CM. Plant-derived synergists of alarm pheromone from turnip aphid, Lipaphis (Hyadaphis) erysimi (Homoptera, Aphididae). J Chem Ecol 1987;13:1663–71.10.1007/BF00980207Search in Google Scholar
43. Tschuch G, Lindemann P, Moritz G. An unexpected mixture of substances in the defensive secretions of the tubuliferan thrips, Callococcithrips fuscipennis (Moulton). J Chem Ecol 2008;34:742–7.10.1007/s10886-008-9494-3Search in Google Scholar
44. Meinwald J, Chadha MS, Hurst JJ, Eisner T. Defense mechanisms of arthropods. DC. Anisomorphal, the secretion of a phasmid insect. Tetrahedron Lett 1962;1:29–33.10.1016/S0040-4039(00)62038-5Search in Google Scholar
45. Sugawara F, Matsuda K, Kobayashi A, Yamashita K. Defensive secretion of chrysomelid beetles: defensive secretion of chrysomelid larvae Gastrophysa atrocyanea Motschulsky and Phaedon brassicae Baly. J Chem Ecol 1979;5:635–41.10.1007/BF00986548Search in Google Scholar
46. Cavill GW, Robertson PL, Brophy JJ, Duke RK, McDonald J, Plant WD. Chemical ecology of the meat ant, Iridomyrmex purpureus sens. strict. Insect Biochem 1984;14:505–13.10.1016/0020-1790(84)90004-0Search in Google Scholar
47. Pickett JA, Allemann RK, Birkett MA. The semiochemistry of aphids. Nat Prod Rep 2013;30:1277–83.10.1039/c3np70036dSearch in Google Scholar PubMed
48. Nishida R. Sequestration of defensive substances from plants by Lepidoptera. Annu Rev Entomol 2002;47:57–92.10.1146/annurev.ento.47.091201.145121Search in Google Scholar PubMed
49. Opitz SE, Mueller C. Plant chemistry and insect sequestration. Chemoecology 2009;19:117–54.10.1007/s00049-009-0018-6Search in Google Scholar
50. Petschenka G, Agrawal AA. How herbivores coopt plant defenses: natural selection, specialization, and sequestration. Curr Opin Insect Sci 2016;14:17–24.10.1016/j.cois.2015.12.004Search in Google Scholar PubMed
51. Meinwald J, Happ GM, Labows J, Eisner T. Cyclopentanoid terpene biosynthesis in a phasmid insect and in catmint. Science 1966;151:79–80.10.1126/science.151.3706.79Search in Google Scholar PubMed
52. Oldham NJ, Veith M, Boland W, Dettner K. Iridoid monoterpene biosynthesis in insects: evidence for a de novo pathway occurring in the defensive glands of Phaedon armoraciae (Chrysomelidae) leaf beetle larvae. Naturwissenschaften 1996;83:470–3.10.1007/s001140050318Search in Google Scholar
53. Veith M, Dettner K, Boland W. Stereochemistry of an alcohol oxidase from the defensive secretion of larvae of the leaf beetle Phaedon armoraciae (Coleoptera, Chrysomelidae). Tetrahedron 1996;52:6601–12.10.1016/0040-4020(96)00298-0Search in Google Scholar
54. Veith M, Lorenz M, Boland W, Simon H, Dettner K. Biosynthesis of iridoid monoterpenes in insects: defensive secretions from larvae of leaf beetles (Coleoptera:Chrysomelidae). Tetrahedron 1994;50:6859–74.10.1016/S0040-4020(01)81338-7Search in Google Scholar
55. Lorenz M, Boland W, Dettner K. Biosynthesis of iridodials in the defense glands of beetle larvae (Chrysomelinae). Angew Chem Int Ed Engl 1993;32:912–4.10.1002/anie.199309121Search in Google Scholar
56. Laurent P, Braekman JC, Daloze D, Pasteels J. Biosynthesis of defensive compounds from beetles and ants. Eur J Org Chem 2003;2733–43.10.1002/ejoc.200300008Search in Google Scholar
57. Termonia A, Hsiao TH, Pasteels JM, Milinkovitch MC, Feeding specialization and host-derived chemical defense in Chrysomeline leaf beetles did not lead to an evolutionary dead end. Proc Natl Acad Sci USA 2001;98:3909–14.10.1073/pnas.061034598Search in Google Scholar
58. Kries H, O’Connor SE. Biocatalysts from alkaloid producing plants. Curr Opin Chem Biol 2016;31:22–30.10.1016/j.cbpa.2015.12.006Search in Google Scholar
59. O’Connor SE. Engineering of secondary metabolism. Annu Rev Genet 2015;49:71–94.10.1146/annurev-genet-120213-092053Search in Google Scholar
60. Birkett MA, Pickett JA. Aphid sex pheromones: from discovery to commercial production. Phytochemistry 2003;62:651–6.10.1016/S0031-9422(02)00568-XSearch in Google Scholar
61. Birkett MA, Hassanali A, Hoglund S, Pettersson J, Pickett JA. Repellent activity of catmint, Nepeta cataria, and iridoid nepetalactone isomers against Afro-tropical mosquitoes. Ixodid ticks and red poultry mites. Phytochemistry 2011;72:109–14.10.1016/j.phytochem.2010.09.016Search in Google Scholar PubMed
62. Morgan ED. Biosynthesis in insects: advanced edition. Cambridge: Royal Society Chemistry, 2010.10.1039/9781839169281Search in Google Scholar
63. Friesen J, Rodwell V. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol 2004;5:248.1–248.7.10.1186/gb-2004-5-11-248Search in Google Scholar PubMed PubMed Central
64. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 1990;343:425–30.10.1038/343425a0Search in Google Scholar PubMed
65. Burse A, Schmidt A, Frick S, Kuhn J, Gershenzon J, Boland W. Iridoid biosynthesis in Chrysomelina larvae: fat body produces early terpenoid precursors. Insect Biochem Mol Biol 2007;37:255–65.10.1016/j.ibmb.2006.11.011Search in Google Scholar PubMed
66. Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol 2010;55:207–25.10.1146/annurev-ento-112408-085356Search in Google Scholar PubMed PubMed Central
67. Belles X, Martin D, Piulachs MD. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu Rev Entomol 2005;50:181–99.10.1146/annurev.ento.50.071803.130356Search in Google Scholar PubMed
68. Keeling CI, Bearfield JC, Young S, Blomquist GJ, Tittiger C. Effects of juvenile hormone on gene expression in the pheromone-producing midgut of the pine engraver beetle, Ips pini. Insect Mol Biol 2006;15:207–16.10.1111/j.1365-2583.2006.00629.xSearch in Google Scholar PubMed
69. Burse A, Frick S, Schmidt A, Buechler R, Kunert M, Gershenzon J, et al. Implication of HMGR in homeostasis of sequestered and de novo produced precursors of the iridoid biosynthesis in leaf beetle larvae. Insect Biochem Mol Biol 2008;38:76–88.10.1016/j.ibmb.2007.09.006Search in Google Scholar PubMed
70. Feld BK, Pasteels JM, Boland W. Phaedon cochleariae and Gastrophysa viridula (Coleoptera:Chrysomelidae) produce defensive iridoid monoterpenes de novo and are able to sequester glycosidically bound terpenoid precursors. Chemoecology 2001;11:191–8.10.1007/PL00001851Search in Google Scholar
71. Kunert M, Soe A, Bartram S, Discher S, Tolzin-Banasch K, Nie L, et al. De novo biosynthesis versus sequestration: a network of transport systems supports in iridoid producing leaf beetle larvae both modes of defense. Insect Biochem Mol Biol 2008;38:895–904.10.1016/j.ibmb.2008.06.005Search in Google Scholar PubMed
72. Kuhn J, Pettersson EM, Feld BK, Burse A, Termonia A, Pasteels JM, et al. Selective transport systems mediate sequestration of plant glucosides in leaf beetles: a molecular basis for adaptation and evolution. Proc Natl Acad Sci USA 2004;101:13808–13.10.1073/pnas.0402576101Search in Google Scholar PubMed PubMed Central
73. Soe AR, Bartram S, Gatto N, Boland W. Are iridoids in leaf beetle larvae synthesized de novo or derived from plant precursors? A methodological approach. Isotopes Environ Health Stud 2004;40:175–80.10.1080/10256010410001674994Search in Google Scholar
74. Liang PH, Ko TP, Wang AH. Structure, mechanism and function of prenyltransferases. Eur J Biochem 2002;269:3339–54.10.1046/j.1432-1033.2002.03014.xSearch in Google Scholar
75. Vandermoten S, Haubruge E, Cusson M. New insights into short-chain prenyltransferases: structural features, evolutionary history and potential for selective inhibition. Cell Mol Life Sci 2009;66;3685–95.10.1007/s00018-009-0100-9Search in Google Scholar
76. Wang KC, Ohnuma S. Isoprenyl diphosphate synthases. Biochim Biophys Acta 2000;1529:33–48.10.1016/S1388-1981(00)00136-0Search in Google Scholar
77. Gao Y, Honzatko RB, Peters RJ. Terpenoid synthase structures: a so far incomplete view of complex catalysis. Nat Prod Rep 2012;29:1153–75.10.1039/c2np20059gSearch in Google Scholar PubMed PubMed Central
78. Brandt W, Braeuer L, Guennewich N, Kufka J, Rausch F, Schulze D, et al. Molecular and structural basis of metabolic diversity mediated by prenyldiphosphate converting enzymes. Phytochemistry 2009;70:1758–75.10.1016/j.phytochem.2009.09.001Search in Google Scholar PubMed
79. Gilg AB, Bearfield JC, Tittiger C, Welch WH, Blomquist GJ. Isolation and functional expression of an animal geranyl diphosphate synthase and its role in bark beetle pheromone biosynthesis. Proc Natl Acad Sci USA 2005;102:9760–5.10.1073/pnas.0503277102Search in Google Scholar PubMed PubMed Central
80. Gilg AB, Tittiger C, Blomquist GJ. Unique animal prenyltransferase with monoterpene synthase activity. Naturwissenschaften 2009;96:731–5.10.1007/s00114-009-0521-1Search in Google Scholar PubMed
81. Keeling CI, Chiu CC, Aw T, Li M, Henderson H, Tittiger C, et al. Frontalin pheromone biosynthesis in the mountain pine beetle, Dendroctonus ponderosae, and the role of isoprenyl diphosphate synthases. Proc Natl Acad Sci USA 2013;110:18838–43.10.1073/pnas.1316498110Search in Google Scholar PubMed PubMed Central
82. Blomquist GJ, Figueroa-Teran R, Aw M, Song M, Gorzalski A, Abbott NL, et al. Pheromone production in bark beetles. Insect Biochem Mol Biol 2010;40:699–712.10.1016/j.ibmb.2010.07.013Search in Google Scholar PubMed
83. Lewis MJ, Prosser IM, Mohib A, Field LM. Cloning and characterisation of a prenyltransferase from the aphid Myzus persicae with potential involvement in alarm pheromone biosynthesis. Insect Mol Biol 2008;17:437–43.10.1111/j.1365-2583.2008.00815.xSearch in Google Scholar PubMed
84. Ma G-Y, Sun X-F, Zhang Y-L, Li Z-X, Shen Z-R. Molecular cloning and characterization of a prenyltransferase from the cotton aphid, Aphis gossypii. Insect Biochem Mol Biol 2010;40: 552–61.10.1016/j.ibmb.2010.05.003Search in Google Scholar PubMed
85. Vandermoten S, Charloteaux B, Santini S, Sen SE, Beliveau C, Vandenbol M, et al. Characterization of a novel aphid prenyltransferase displaying dual geranyl/farnesyl diphosphate synthase activity. FEBS Lett 2008;582:1928–34.10.1016/j.febslet.2008.04.043Search in Google Scholar PubMed
86. Vandermoten S, Santini S, Haubruge E, Heuze F, Francis F, Brasseur R, et al. Structural features conferring dual geranyl/farnesyl diphosphate synthase activity to an aphid prenyltransferase. Insect Biochem Mol Biol 2009;39:707–16.10.1016/j.ibmb.2009.08.007Search in Google Scholar PubMed
87. Zhang Y-L, Li Z-X. Functional analysis and molecular docking identify two active short-chain prenyltransferases in the green peach aphid, Myzus persicae. Arch Insect Biochem Physiol 2012;81:63–76.10.1002/arch.21032Search in Google Scholar PubMed
88. Frick S, Nagel R, Schmidt A, Bodemann RR, Rahfeld P, Pauls G, et al. Metal ions control product specificity of isoprenyl diphosphate synthases in the insect terpenoid pathway. Proc Natl Acad Sci USA 2013;110:4194–9.10.1073/pnas.1221489110Search in Google Scholar PubMed PubMed Central
89. Rivera-Perez C, Nyati P, Noriega FG. A corpora allata farnesyl diphosphate synthase in mosquitoes displaying a metal ion dependent substrate specificity. Insect Biochem Mol Biol 2015;64:44–50.10.1016/j.ibmb.2015.07.010Search in Google Scholar PubMed PubMed Central
90. Beran F, Rahfeld P, Luck K, Nagel R, Vogel H, Wielsch N, et al. Novel family of terpene synthases evolved from trans-isoprenyl diphosphate synthases in a flea beetle. Proc Natl Acad Sci USA 2016;113:2922–7.10.1073/pnas.1523468113Search in Google Scholar PubMed PubMed Central
91. Xi J, Rossi L, Lin XL, Xie DY. Overexpression of a synthetic insect-plant geranyl pyrophosphate synthase gene in Camelina sativa alters plant growth and terpene biosynthesis. Planta 2016;244:215–30.10.1007/s00425-016-2504-8Search in Google Scholar PubMed
92. Daloze D, Pasteels J. Isolation of 8-hydroxygeraniol-8-O-β-d-glucoside, a probable intermediate in biosynthesis of iridoid monoterpenes, from defensive secretions of Plagiodera versicolora and Gastrophysa viridula (Coleoptera:Chrysomelidae). J Chem Ecol 1994;20:2089–97.10.1007/BF02066245Search in Google Scholar PubMed
93. Veith M, Oldham NJ, Dettner K, Pasteels JM, Boland W. Biosynthesis of defensive allomones in leaf beetle larvae: stereochemistry of salicylalcohol oxidation in Phratora vitellinae and comparison of enzyme substrate and stereospecificity with alcohol oxidases from several iridoid producing leaf beetles. J Chem Ecol 1997;23:429–43.10.1023/B:JOEC.0000006369.26490.c6Search in Google Scholar
94. Zhao B, Waterman MR. Moonlighting cytochrome P450 monooxygenases. IUBMB Life 2011;63:473–7.10.1002/iub.501Search in Google Scholar
95. Collu G, Unver N, Peltenburg-Looman AM, van der Heijden R, Verpoorte R, Memelink J. Geraniol 10-hydroxylase, a cytochrome P450 enzyme involved in terpenoid indole alkaloid biosynthesis. FEBS Lett 2001;508:215–20.10.1016/S0014-5793(01)03045-9Search in Google Scholar
96. Henrissat B, Callebaut I, Fabrega S, Lehn P, Mornon JP, Davies G. Conserved catalytic machinery and the prediction of a common fold for several families of glycosyl hydrolases. Proc Natl Acad Sci USA 1995;92:7090–4.10.1073/pnas.92.15.7090Search in Google Scholar
97. Henrissat B, Davies G. Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol 1997;7:637–44.10.1016/S0959-440X(97)80072-3Search in Google Scholar
98. Cairns JR, Esen A. Beta-glucosidases. Cell Mol Life Sci 2010;67:3389–405.10.1007/s00018-010-0399-2Search in Google Scholar PubMed
99. Morant AV, Jorgensen K, Jorgensen C, Paquette SM, Sanchez-Perez R, Moller BL, et al. Beta-glucosidases as detonators of plant chemical defense. Phytochemistry 2008;69:1795–813.10.1016/j.phytochem.2008.03.006Search in Google Scholar PubMed
100. Pentzold S, Zagrobelny M, Rook F, Bak S. How insects overcome two-component plant chemical defence: plant beta-glucosidases as the main target for herbivore adaptation. Biol Rev 2014;89:531–51.10.1111/brv.12066Search in Google Scholar PubMed
101. Berenbaum MR. The chemistry of defense – theory and practice. Proc Natl Acad Sci USA 1995;92:2–8.10.1073/pnas.92.1.2Search in Google Scholar PubMed PubMed Central
102. Terra WR, Ferreira C. Biochemistry and molecular biology of digestion. Insect Biochem Mol Biol 2012;365–418.10.1016/B978-0-12-384747-8.10011-XSearch in Google Scholar
103. Tokuda G, Saito H, Watanabe H. A digestive beta-glucosidase from the salivary glands of the termite, Neotermes koshunensis (Shiraki): distribution, characterization and isolation of its precursor cDNA by 5′- and 3′-RACE amplifications with degenerate primers. Insect Biochem Mol Biol 2002;32:1681–9.10.1016/S0965-1748(02)00108-XSearch in Google Scholar
104. Dobler S. Evolutionary aspects of defense by recycled plant compounds in herbivorous insects. Basic Appl Ecol 2001;2:15–26.10.1078/1439-1791-00032Search in Google Scholar
105. Zagrobelny M, Moller BL. Cyanogenic glucosides in the biological warfare between plants and insects: the Burnet moth-Birdsfoot trefoil model system. Phytochemistry 2011;72:1585–92.10.1016/j.phytochem.2011.02.023Search in Google Scholar
106. Franzl S, Ackermann I, Nahrstedt A. Purification and characterization of a beta-glucosidase (linamarase) from the hemolymph of Zygaena trifolii Esper, 1783 (Insecta, Lepidoptera). Experientia 1989;45:712–8.10.1007/BF01974565Search in Google Scholar
107. Jones AM, Bridges M, Bones AM, Cole R, Rossiter JT. Purification and characterisation of a non-plant myrosinase from the cabbage aphid Brevicoryne brassicae (L.). Insect Biochem Mol Biol 2001;31:1–5.10.1016/S0965-1748(00)00157-0Search in Google Scholar
108. Jones AM, Winge P, Bones AM, Cole R, Rossiter JT. Characterization and evolution of a myrosinase from the cabbage aphid Brevicoryne brassicae. Insect Biochem Mol Biol 2002;32:275–84.10.1016/S0965-1748(01)00088-1Search in Google Scholar
109. Kazana E, Pope TW, Tibbles L, Bridges M, Pickett JA, Bones AM, et al. The cabbage aphid: a walking mustard oil bomb. Proc R Soc Lond B Biol Sci 2007;274:2271–7.10.1098/rspb.2007.0237Search in Google Scholar PubMed PubMed Central
110. Beran F, Pauchet Y, Kunert G, Reichelt M, Wielsch N, Vogel H, et al. Phyllotreta striolata flea beetles use host plant defense compounds to create their own glucosinolate-myrosinase system. Proc Natl Acad Sci USA 2014;111:7349–54.10.1073/pnas.1321781111Search in Google Scholar PubMed PubMed Central
111. Rahfeld P, Haeger W, Kirsch R, Pauls G, Becker T, Schulze E, et al. Glandular beta-glucosidases in juvenile Chrysomelina leaf beetles support the evolution of a host-plant-dependent chemical defense. Insect Biochem Mol Biol 2015;58:28–38.10.1016/j.ibmb.2015.01.003Search in Google Scholar PubMed
112. Sørensen A, Lübeck M, Lübeck PS, Ahring BK. Fungal beta-glucosidases: a bottleneck in industrial use of lignocellulosic materials. Biomolecules 2013;3:612–31.10.3390/biom3030612Search in Google Scholar PubMed PubMed Central
113. Mika N, Zorn H, Ruhl M. Insect-derived enzymes: a treasure for industrial biotechnology and food biotechnology. Adv Biochem Eng Biotechnol 2013;136:1–17.10.1007/10_2013_204Search in Google Scholar PubMed
114. Sampson NS. Dissection of a flavoenzyme active site: the reaction catalyzed by cholesterol oxidase. Antioxid Redox Signal 2001;3:839–46.10.1089/15230860152665019Search in Google Scholar PubMed
115. Rahfeld P, Kirsch R, Kugel S, Wielsch N, Stock M, Groth M, et al. Independently recruited oxidases from the glucose-methanol-choline oxidoreductase family enabled chemical defences in leaf beetle larvae (subtribe Chrysomelina) to evolve. Proc R Soc Lond B Biol Sci 2014;281:20140842.10.1098/rspb.2014.0842Search in Google Scholar PubMed PubMed Central
116. Kirsch R, Vogel H, Muck A, Reichwald K, Pasteels JM, Boland W. Host plant shifts affect a major defense enzyme in Chrysomela lapponica. Proc Natl Acad Sci USA 2011;108:4897–901.10.1073/pnas.1013846108Search in Google Scholar PubMed PubMed Central
117. Kirsch R, Vogel H, Muck A, Vilcinskas A, Pasteels JM, Boland W. To be or not to be convergent in salicin-based defence in chrysomeline leaf beetle larvae: evidence from Phratora vitellinae salicyl alcohol oxidase. Proc R Soc Lond B Biol Sci 2011;278:3225–32.10.1098/rspb.2011.0175Search in Google Scholar PubMed PubMed Central
118. Michalski C, Mohagheghi H, Nimtz M, Pasteels JM, Ober D. Salicyl alcohol oxidase of the chemical defense secretion of two chrysomelid leaf beetles – molecular and functional characterization of two new members of the glucose-methanol-choline oxidoreductase gene family. J Biol Chem 2008;283:19219–28.10.1074/jbc.M802236200Search in Google Scholar PubMed
119. Sun W, Shen YH, Yang WJ, Cao YF, Xiang ZH, Zhang Z. Expansion of the silkworm GMC oxidoreductase genes is associated with immunity. Insect Biochem Mol Biol 2012;42:935–45.10.1016/j.ibmb.2012.09.006Search in Google Scholar PubMed
120. Iida K, Cox-Foster DL, Yang XL, Ko WY, Cavener DR. Expansion and evolution of insect GMC oxidoreductases. BMC Evol Biol 2007;7:75.10.1186/1471-2148-7-75Search in Google Scholar PubMed PubMed Central
121. Bodemann RR, Rahfeld P, Stock M, Kunert M, Wielsch N, Groth M, et al. Precise RNAi-mediated silencing of metabolically active proteins in the defence secretions of juvenile leaf beetles. Proc R Soc Lond B Biol Sci 2012;279:4126–34.10.1098/rspb.2012.1342Search in Google Scholar PubMed PubMed Central
122. May RM. How many species are there on earth? Science 1988;241:1441–9.10.1126/science.241.4872.1441Search in Google Scholar PubMed
123. Brueckmann M, Termonia A, Pasteels JM, Hartmann T. Characterization of an extracellular salicyl alcohol oxidase from larval defensive secretions of Chrysomela populi and Phratora vitellinae (Chrysomelina). Insect Biochem Mol Biol 2002;32:1517–23.10.1016/S0965-1748(02)00072-3Search in Google Scholar
124. Walsh CT, Garneau-Tsodikova S, Gatto GJ. Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem Int Ed Engl 2005;44:7342–72.10.1002/anie.200501023Search in Google Scholar PubMed
125. Sola RJ, Griebenow K. Effects of glycosylation on the stability of protein pharmaceuticals. J Pharm Sci 2009;98;1223–45.10.1002/jps.21504Search in Google Scholar PubMed PubMed Central
126. Al-Rubeai M. Cell engineering glycosylation. Dordrecht, Netherlands: Springer, 2002.10.1007/0-306-47525-1Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Insect biotechnology – a major challenge in the 21st century
- Research Articles
- Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed
- The black soldier fly, Hermetia illucens – a promising source for sustainable production of proteins, lipids and bioactive substances
- Applicability of biotechnologically produced insect silks
- Biotechnological potential of insect fatty acid-modifying enzymes
- Strategies for the construction of insect P450 fusion enzymes
- Deciphering the route to cyclic monoterpenes in Chrysomelina leaf beetles: source of new biocatalysts for industrial application?
- The components of shear stress affecting insect cells used with the baculovirus expression vector system
- Abstracts
- 1st Gießen Symposium of Insect Biotechnology, October 9-10, 2017 (published online)
Articles in the same Issue
- Frontmatter
- Editorial
- Insect biotechnology – a major challenge in the 21st century
- Research Articles
- Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed
- The black soldier fly, Hermetia illucens – a promising source for sustainable production of proteins, lipids and bioactive substances
- Applicability of biotechnologically produced insect silks
- Biotechnological potential of insect fatty acid-modifying enzymes
- Strategies for the construction of insect P450 fusion enzymes
- Deciphering the route to cyclic monoterpenes in Chrysomelina leaf beetles: source of new biocatalysts for industrial application?
- The components of shear stress affecting insect cells used with the baculovirus expression vector system
- Abstracts
- 1st Gießen Symposium of Insect Biotechnology, October 9-10, 2017 (published online)


![Figure 1: Iridoid defense molecules identified from insects (according to Kunert et al. [39] and Weibel et al. [40]).](/document/doi/10.1515/znc-2017-0015/asset/graphic/j_znc-2017-0015_fig_001.jpg)
