Abstract
Two new cationic four-coordinate Cu(I) complexes supported by different chelating N-heterocyclic carbene ligands and the diphosphine ligand bis[2-(diphenylphosphino)phenyl]ether (POP) have been synthesized. The chemical structures of both complexes have been characterized by 1H NMR, 13C NMR, 31P NMR, and mass spectroscopy, and the crystal structure of one complex has been determined by single-crystal X-ray diffraction. Results of theoretical calculations indicate that the lowest energy electronic transitions of these complexes are mainly the metal-to-ligand charge transfer and ligand-to-ligand charge transfer transitions. The complexes in solid state show intense emissions with high photoluminescence quantum yields. The photophysical behavior at 298 and 77K shows that emissions of these complexes at room temperature are thermally activated delayed fluorescence mixed with phosphorescence.
1 Introduction
Luminescent transition metal complexes have attracted much attention for their applications in electroluminescence (EL) devices, such as organic light-emitting diodes (OLEDs) and light-emitting electrochemical cells (LECs) [1]. Particularly, the phosphorescent Ir(III) and Pt(II) complexes are widely employed as emitters in EL devices, because both the singlet and triplet excitons can be harvested by these emitters via strong spin-orbital coupling between singlet and triplet states [2], [3], [4], [5], [6], [7], [8], [9]. The OLEDs/LECs based on phosphorescent Ir(III) and Pt(II) complexes have far higher performances compared to those based on conventional fluorescent emitters, but the metals iridium and platinum are very scarce and highly expensive, which limits the large-scale applications of these complexes [10], [11], [12]. In recent years, researchers devoted much efforts into developing low-cost luminescent Cu(I) complexes as emitters of EL devices. It has been proven that many Cu(I) complexes emit efficient thermally activated delayed fluorescence (TADF) at ambient temperature due to their small energy gaps (ΔEST) between the lowest singlet state (S1) and the lowest triplet states (T1) [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]. Thus, the EL devices based on Cu(I) complexes can also harness both singlet and triplet excitons via reverse intersystem crossing (RISC) resulting in high efficiency [24], [25], [26], [27], [28], [29].
N-heterocyclic carbenes (NHCs) have emerged as excellent ligands for luminescent Cu(I) complexes, and a large number of Cu(I)-NHC complexes with efficient TADF were reported in the past decade [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], including also the highly efficient EL devices based on Cu(I)-NHC complexes [40], [41], [42], [43]. For example, the Cu(I)–NHC complex (MAC*)Cu(Cz) reported by the Thompson group not only shows an outstanding TADF (Φ = 90% and τ = 1.4 μs in polystyrene film), but also the OLED based on (MAC*)Cu(Cz) achieves a high external quantum efficiency (EQE) of 19.4% with small roll off [41]. Most recently, the Gong and Wu groups reported a Cu(I)-NHC complex MAC*-Cu-DPAC with efficient red TADF. The EQEmax of the OLED based on MAC*-Cu-DPAC is as high as 21.1%, and the EQE is still 20.1% at 1000 cd m−2 [43]. In view of these findings and our continuous interest in luminescent four-coordinate Cu(I)–NHC complexes [44], [45], [46], we synthesized two new cationic four-coordinate Cu(I)–NHC complexes [Cu(4-Me-Pyim)(POP)](PF6) and [Cu(4-MeO-Pyim)(POP)](PF6) which consist of chelating NHC ligands and the diphosphine ligand POP (Scheme 1). Both Cu(I)–NHC complexes show intense emissions with high PL efficiencies in the solid state. The emission data acquired at T = 298 and 77K indicate that the emissions of these complexes at room temperature are TADF mixed with phosphorescence.
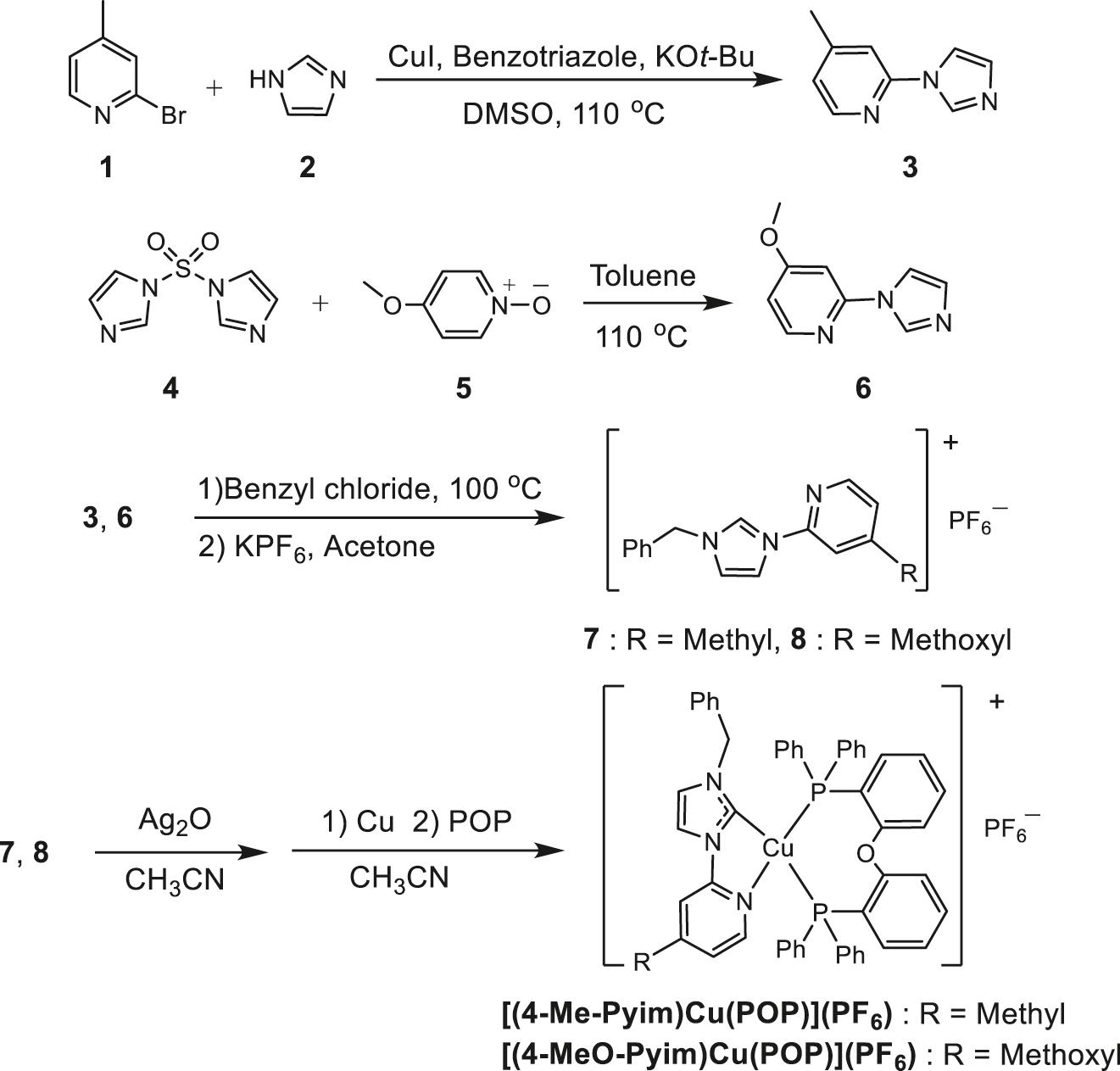
Synthesis routes to the Cu(I)–NHC complexes.
2 Experimental
All starting materials were purchased from commercial suppliers and used as received, and all solvents were analytical grade and used without further purification unless otherwise stated. 1H NMR, 13C NMR, and 31P NMR spectra were recorded on the Bruker Avance 400 spectrometer. Mass spectra were obtained on a Bruker APEX II FT-ICR instrument. The thermogravimetric analysis (TGA) measurement was performed on a TG/DTA 6300 instrument with a heating rate of 10 K min−1 under N2 atmosphere. Absorption spectra were recorded on a Hitachi U-3010 UV/Vis spectrophotometer. Photoluminescence (PL) spectra, emission lifetimes, and absolute emission quantum yields were recorded on an Edinburgh FLS980 spectrometer equipped with an integrating sphere.
2.1 Synthesis
2.1.1 Synthesis of 2-(1H-imidazol-1-yl)-4-methylpyridine (3)
CuI (38.0 mg, 0.2 mmol) and benzotriazole (47.6 mg, 0.4 mmol) were dissolved in 4 mL dimethyl sulfoxide (DMSO) by stirring. Then 2-bromo-4-methylpyridine 1 (684 mg, 4 mmol), imidazole 2 (272 mg, 4 mmol), and KOt-Bu (628 mg, 5.6 mmol) were added in sequence. Under N2 atmosphere, the mixture was stirred for 8 h at 110 °C. After cooling, 10 mL of ethyl acetate was added. The resulting organic solution was washed with water three times, dried over anhydrous MgSO4, and evaporated with a rotary evaporator. The residue was purified by column chromatography to give the desired product as a white powder, the eluent being ethyl acetate-dichloromethane (2:1 v/v). Yield: 93% (591 mg). – 1H NMR (400 MHz, CDCl3): δ (ppm) = 8.33 (1H, s), 8.29–8.28 (1H, d, J = 6.30 Hz), 7.62 (1H, s), 7.17–7.15 (2 H, d, J = 9.95 Hz), 7.03–7.02 (1H, d, J = 6.20 Hz), 2.40 (3H, s). – ESI-MS: m/z = 159.1 [M]+.
2.1.2 Synthesis of 2-(1H-imidazol-1-yl)-4-methoxypyridine (6)
1,1′-Sulfonyldiimidazole 4 (991.0 mg, 5 mmol) and 4-methoxypyridine N-oxide (625.6 mg, 5 mmol) were added to 25 mL of toluene, and then the mixture was stirred for 8 h at 110 °C. After cooling, the resulting organic solution was evaporated with a rotary evaporator. The residue was purified by column chromatography to give the desired product as a white powder, the eluent being ethyl acetate-dichloromethane (2:1 v/v). Yield: 76% (665 mg). – 1H NMR (400 MHz, CDCl3): δ (ppm) = 8.35 (1H, s), 8.32–8.31 (1H, d, J = 6.0 Hz), 7.63 (1H, s), 7.21 (1H, s), 6.85 (1H, s), 6.80–6.78 (1H, d, J = 8.0 Hz), 3.94 (3H, s). – ESI-MS: m/z = 175.3 [M]+.
2.1.3 Synthesis of compound 7
Compound 3 (477 mg, 3 mmol) was added to 1.7 mL of benzyl chloride (1.90 g, 15 mmol), then the mixture was refluxed at 100 °C for 2 h. After the excess benzyl chloride was evaporated under vacuum, the residue was put in 15 mL of acetone, and KPF6 (1.10 g, 6 mmol) was added and the mixture stirred for 3 h. The precipitate was filtered and the organic solvent was evaporated with a rotary evaporator to give the desired product as a white powder. Yield: 86% (1.02 g). – 1H NMR (400 MHz, acetone-d6): δ (ppm) = 9.31 (1H, s), 8.41–8.38 (1H, m), 8.04 (1H, s), 7.58 (1H, s), 7.54 (1H, s), 7.47–7.45 (5H, m), 7.39–7.37 (1H, m), 5.43 (2 H, s), 2.46 (3H, s). – ESI-MS: m/z = 250.3 [M–PF6−]+.
2.1.4 Synthesis of compound 8
Compound 6 (525 mg, 3 mmol) was added to 1.7 mL of benzyl chloride (1.90 g, 15 mmol), then the mixture was refluxed at 100 °C for 2 h. After the excess benzyl chloride was evaporated under vacuum, the residue was put in 15 mL of acetone, and then KPF6 (1.10 g, 6 mmol) was added and the mixture stirred for 3 h. The precipitate was filtered and the organic solvent was evaporated with a rotary evaporator to give the desired product as a white powder. Yield: 85% (1.05 g). – 1H NMR (400 MHz, acetone-d6): δ (ppm) = 9.33 (1H, s), 8.38–8.35 (1H, m), 8.07 (1H, s), 7.53 (1H, s), 7.47–7.45 (5H, m), 7.23 (1H, s), 7.08–7.05 (1H, m), 5.43 (2H, s), 3.95 (3H, s). – ESI-MS: m/z = 266.2 [M–PF6−]+.
2.1.5 Synthesis of [Cu(4-Me-Pyim)(POP)](PF6)
Compound 7 (395 mg, 1 mmol) and Ag2O (115 mg, 0.5 mmol) were added to 10 mL of CH3CN, and the mixture was reacted overnight at 50 °C. After the solvent was evaporated with a rotary evaporator, the residue was washed with ethanol three times and the Ag(I) complex was obtained as a white powder. At room temperature, the Ag(I) complex (250 mg, 0.25 mmol) was reacted with copper powder (33 mg, 0.5 mmol) for 3 h in degassed anhydrous CH3CN under N2 atmosphere, then the ligand POP (269 mg, 0.5 mmol) was added and the mixture reacted for 3 h. After the precipitate was filtrated, the solvent was evaporated with a rotary evaporator. The residue was dissolved in dichloromethane-ethanol solvent, and the product was obtained as a pale green powder by slowly evaporating the solvent. Yield: 58% (577 mg). – 1H NMR (400 MHz, CD3CN): δ (ppm) = 7.96 (1H, s), 7.81 (1H, s), 7.54–7.52 (1H, d, J = 8.0 Hz), 7.37–7.32 (4H, m), 7.28–7.11 (15H, m), 7.06–6.93 (12H, m), 6.82–6.75 (2H, m), 6.64–6.62 (2H, d, J = 7.6 Hz), 5.18 (2H, s), 1.87 (3H, s). – 13C NMR (100 MHz, CD3CN): δ (ppm) = 160.04, 158.80, 150.90, 140.38, 136.85, 134.90, 133.13, 133.00, 130.98, 129.77, 129.72, 129.68, 128.84, 128.01, 125.89, 123.73, 120.96, 118.30, 110.20, 55.60, 25.09. – 31P NMR (162 MHz, CD3CN): δ (ppm) = −8.19 (s), −144.2 (quint). – ESI-MS: m/z = 851.5 [M–PF6−]+.
2.1.6 Synthesis of [Cu(4-MeO-Pyim)(POP)](PF6)
Compound 8 (411 mg, 1 mmol) and Ag2O (115 mg, 0.5 mmol) were added to 10 mL of CH3CN, and the mixture was reacted overnight at 50 °C. After the solvent was evaporated with a rotary evaporator, the residue was washed with ethanol three times, and the Ag(I) complex was obtained as a white powder. At room temperature, the Ag(I) complex (259 mg, 0.25 mmol) was reacted with copper powder (33 mg, 0.5 mmol) for 3 h in degassed anhydrous CH3CN under N2 atmosphere, then the ligand POP (269 mg, 0.5 mmol) was added and the mixture reacted for 3 h. After the precipitate was filtrated, the solvent was evaporated with a rotary evaporator. The residue was dissolved in dichloromethane-ethanol solvent, and the product was obtained as pale green crystals by slowly evaporating the solvent. Yield: 62% (627 mg). – 1H NMR (400 MHz, CD3CN): δ (ppm) = 7.86 (1H, t, J = 8.2 Hz), 7.73 (1H, s), 7.37–7.28 (6H, m), 7.26–7.17 (9H, m), 7.16–7.05 (7H, m), 7.04–6.89 (8H, m), 6.72–6.59 (4H, m), 6.52 (1H, s), 5.03 (2H, s), 3.87 (3H, s). – 13C NMR (100 MHz, CD3CN): δ (ppm) = 168.75, 159.29, 152.93, 150.43, 136.98, 135.17, 134.56, 134.37, 133.89, 133.02, 131.23, 130.98, 129.71, 128.85, 128.27, 125.95, 123.68, 121.49, 110.03, 99.19, 57.05, 54.86. – 31P NMR (162 MHz, CD3CN): δ (ppm) = −8.79 (s), −144.2 (quint). – ESI-MS: m/z = 867.5 [M–PF6−]+.
2.2 Single-crystal structure determination
Diffraction data was collected on a Rigaku Saturn 724 CCD diffractometer with graphite-monochromatized MoKα radiation (λ = 0.71073 Å) at room temperature. An empirical absorption correction was applied with the program Sadabs [47]. The structure was solved by Direct Methods with Shelxs-97 [48] and refined by the full-matrix least-squares method on F2 with anisotropic displacement parameters for all non-H atoms (Shelxs-97 [49]). The hydrogen atoms were assigned with common isotropic displacement factors and included in the final refinement by use of geometrical restraints.
CCDC 2151403 ([Cu(4-MeO-Pyim)(POP)](PF6)) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
3 Results and discussion
3.1 Synthesis and structures
Scheme 1 shows the synthesis routes of these Cu(I)–NHC complexes. The N-arylated imidazoles 3 and 6 were synthesized through the methods reported by Verma and Keith, respectively [50, 51]. The compounds 3/6 were reacted with excess benzyl chloride and the product anion-exchanged with KPF6 to give the imidazolium salts 7/8. Finally, the Cu(I)–NHC complexes were prepared using the imidazolium salts 7/8, Ag2O, Cu powder and the ligand POP by the reported method [44]. Both these Cu(I)–NHC complexes in solid state are stable in air. Under N2 atmosphere, the thermal decomposition temperatures (3% weight loss) were determined to be 340 °C for [Cu(4-Me-Pyim)(POP)](PF6) and 336 °C for [Cu(4-MeO-Pyim)(POP)](PF6).
Single crystals of [Cu(4-MeO-Pyim)(POP)](PF6) were obtained through slowly evaporating the solvent from the ethanol/dichloromethane solution of the complex, the crystallographic data is listed in Table 1, the important bond lengths and bond angles are listed in Table 2. The complex [Cu(4-MeO-Pyim)(POP)](PF6) adopts the typical distorted tetrahedral configuration as shown in Figure 1. The bond lengths of Cu–C, Cu–N and Cu–P are similar to those of reported four-coordinate Cu(I)–NHC complexes [44], [45], [46]. Additionally, the imidazolylidene and pyridine rings of the NHC ligand are nearly coplanar in this complex with the dihedral angle of 4.8°.
Crystallographic data and experimental details for [Cu(4-MeO-Pyim)(POP)](PF6).
| Empirical formula | C52H43CuF6N3O2P3 |
| Formula weight | 1012.34 |
| Temperature, K | 287.89(10) |
| Crystal system | Triclinic |
| Space group |
P
|
| a, Å | 10.5629(5) |
| b, Å | 16.4099(7) |
| c, Å | 16.7040(8) |
| α, deg | 109.280(4) |
| β, deg | 90.171(4) |
| γ, deg | 100.803(4) |
| Volume, Å3 | 2678.0(2) |
| Z | 2 |
| Dcalc, gcm−3 | 1.26 |
| µ, mm−1 | 0.6 |
| F (000), e | 1040.0 |
| 2θ range data collection, deg | 6.588–50.998 |
| Refl. collected | 28,524 |
| Refl. unique/Rint/Rσ | 9829/0.0714/0.1085 |
| Data/parameters | 9829/605 |
| Goodness-of-fit on F2 | 1.064 |
| Final R1/wR2 [I > 2σ(I)] | 0.1011/0.2836 |
| Final R1/wR2 (all data) | 0.1575/0.3207 |
| Δρfin (max/min), e Å−3 | 1.31/−0.55 |
Selected bond lengths (Å) and bond angles (deg) of [Cu(4-MeO-Pyim)(POP)](PF6).
| Cu01–C1 | 1.995(7) | Cu01–P1 | 2.274(2) |
| Cu01–N3 | 2.171(6) | Cu01–P2 | 2.2401(19) |
| C1–Cu01–N3 | 78.8(3) | C1–Cu01–P1 | 116.1(2) |
| C1–Cu01–P2 | 126.2(2) | P1–Cu01–P2 | 112.31(7) |
| N3–Cu01–P1 | 104.68(17) | N3–Cu01–P2 | 110.24(16) |
. Hydrogen atoms and the hexafluorophosphate anion are omitted for clarity.](/document/doi/10.1515/znb-2022-0092/asset/graphic/j_znb-2022-0092_fig_001.jpg)
Crystal structure of [Cu(4-MeO-Pyim)(POP)](PF6). Hydrogen atoms and the hexafluorophosphate anion are omitted for clarity.
3.2 Computational studies
To understand the electronic structures and the nature of the transitions of the Cu(I)–NHC complexes, density functional theory (DFT) and time-dependent DFT calculations were performed at the B3LYP/6-31G(d) level using the Gaussian 09 program [52]. As shown in Figure 2, both complexes have spatially separated highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs). The LUMOs are mostly located on the imidazolylidene and pyridine rings of the NHC ligands, while the central Cu(I) ion and the ligand POP have little contributions to the LUMOs. Contrarily, the HOMOs are mainly distributed on the Cu(I) ion and the ligand POP, but the imidazolylidene unit also makes a contribution to the HOMOs. It is well known that spatially separated HOMOs and LUMOs determine the molecular orbital character of TADF materials [10, 53, 54]. The lowest energy electronic transitions of these complexes are summarized in Table 3. It can be found that the excited states S1, T1, and T2 of these complexes are formed from the HOMO→LUMO and HOMO-1→LUMO electronic transitions. The results indicate that the lowest energy electronic transitions of the Cu(I)–NHC complexes are mainly metal-to-ligand charge transfer (MLCT) and ligand-to-ligand charge transfer (LLCT) transitions.

Calculated molecular orbitals for the Cu(I)–NHC complexes.
Calculated lowest energy transitions for the Cu(I)–NHC complexes.
| Complex | State | f | Major contribution |
|---|---|---|---|
| [Cu(4-Me-Pyim)(POP)](PF6) | S1 | 0.0513 | HOMO→LUMO (72%) |
| HOMO-1→LUMO (25%) | |||
| T2 | 0.0000 | HOMO→LUMO (43%) | |
| HOMO-1→LUMO (53%) | |||
| T1 | 0.0000 | HOMO→LUMO (55%) | |
| HOMO-1→LUMO (38%) | |||
| [Cu(4-MeO-Pyim)(POP)](PF6) | S1 | 0.0521 | HOMO→LUMO (62%) |
| HOMO-1→LUMO (35%) | |||
| T2 | 0.0000 | HOMO→LUMO (56%) | |
| HOMO-1→LUMO (42%) | |||
| T1 | 0.0000 | HOMO→LUMO (39%) | |
| HOMO-1→LUMO (54%) |
3.3 Photophysical properties
The UV/Visible absorption spectra of the complexes in dilute CH2Cl2 solution are shown in Figure 3. The intense absorption bands between 210 and 330 nm with large molar absorption coefficients (ε) can be assigned to the ligand-centered π–π* transitions. The weaker absorption bands above 330 nm should be the charge transfer (CT) transitions, which include MLCT and LLCT transitions according to the theoretical calculations. Both complexes do not show luminescence in CH2Cl2 solution at room temperature, which can be attributed to Jahn–Teller distortion and solvent-induced exciplex formation [55].

Absorption spectra of the Cu(I)–NHC complexes in CH2Cl2 solution at room temperature.
Both complexes in solid state show intense emissions at room temperature (Figure 4). The emission peaks of [Cu(4-Me-Pyim)(POP)](PF6) and [Cu(4-MeO-Pyim)(POP)](PF6) are located at 526 and 505 nm, respectively, and the PL quantum yields were measured to be 58% for [Cu(4-Me-Pyim)(POP)](PF6) and 53% for [Cu(4-MeO-Pyim)(POP)](PF6). The complex [Cu(4-MeO-Pyim)(POP)](PF6) shows a shorter emission wavelength compared to [Cu(4-Me-Pyim)(POP)](PF6), the reason being that the electron-donating ability of methoxy group is stronger than that of methyl group. The results of theoretical calculations indicate that a considerable amount of LUMO of these complexes is distributed over the pyridine ring of the NHC ligands, and thus the methoxy group can raise the LUMO level more than the methyl group, which causes [Cu(4-MeO-Pyim)(POP)](PF6) to have a larger band gap. As expected, [Cu(4-MeO-Pyim)(POP)](PF6) shows a blue-shifted emission in comparison with the reported complex [Cu(Pyim)(POP)](PF6) [44]. However, the emission wavelength of [Cu(4-Me-Pyim)(POP)](PF6) is slightly longer than that of [Cu(Pyim)(POP)](PF6), which may be caused by intermolecular interactions in the crystal.

Emission spectra of the Cu(I)–NHC complexes in the solid state at T = 298 and 77K.
As shown in Figure 5, both complexes in the solid state show microsecond-scale emission lifetimes at room temperature, 87.4 μs for [Cu(4-Me-Pyim)(POP)](PF6) and 65.6 μs for [Cu(4-MeO-Pyim)(POP)](PF6), which implies that the excited triplet states participate in the photophysical processes. To understand the emissive states of these complexes, emission properties at 77K were measured. It can be clearly observed that the emission spectra are red-shifted and the emission lifetimes increase when the measurement temperature was lowered from 298 to 77K (Figures 4 and 5), which matches with the TADF character. The emission data acquired at 298 and 77K are summarized in Table 4. The emissions of the Cu(I) complexes with TADF contain more or less phosphorescence components according to previous reports [27, 31, 56]. These Cu(I)–NHC complexes show relatively long emission lifetimes and relatively small increases in emission lifetime upon lowering the measurement temperature, which should be caused by a significant phosphorescence component.

Emission decay behavior of the Cu(I)–NHC complexes at T = 298 and 77K.
Emission data of the Cu(I)–NHC complexes as polycrystalline powders at T = 298 and 77 K.
| Complexes | Emission (T = 298K) | Emission (T = 77 K) | |||
|---|---|---|---|---|---|
| λem (nm) | τa (μs) | Φb (%) | λem (nm) | τa (μs) | |
| [Cu(4-Me-Pyim)(POP)](PF6) | 526 | 87.4 | 58 | 551 | 200.6 |
| [Cu(4-MeO-Pyim)(POP)](PF6) | 505 | 65.6 | 53 | 533 | 146.2 |
-
aAverage lifetime calculated by the equation τave = ∑Aiτi/∑Ai with Ai as the pre-exponential factor. bAbsolute photoluminescence quantum yield with relative error = ±10%.
4 Conclusions
In summary, two new cationic four-coordinate Cu(I) complexes supported by NHC and the POP diphosphine ligands have been synthesized. These complexes in solid state are very stable to air and have high thermal stability. Results of theoretical calculations indicate that these complexes have spatially separated HOMOs and LUMOs, the lowest energy electronic transitions being mainly MLCT and LLCT transitions. At room temperature, the complexes in solid state show efficient emission with long lifetimes. The photophysical data measured at 298 and 77K indicate that the emissions of the Cu(I)–NHC complexes at room temperature are of TADF character mixed with phosphorescence.
Funding source: Science and Technology Department of Henan Province (Natural Science Foundation of Henan Province)
Award Identifier / Grant number: No. 182300410230
Acknowledgments
We thank Jiange Wang for the single-crystal structure determination.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was funded by Science and Technology Department of Henan Province (Natural Science Foundation of Henan Province, No. 182300410230).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Pashaei, B., Karimi, S., Shahroosvand, H., Abbasi, P., Pilkington, M., Bartolotta, A., Fresta, E., Fernandez-Cestau, J., Costa, R. D., Bonaccorso, F. Chem. Soc. Rev. 2019, 48, 5033–5139. https://doi.org/10.1039/c8cs00075a.Search in Google Scholar PubMed
2. Meng, X., Wang, P., Bai, R., He, L. J. Mater. Chem. C 2020, 8, 6236–6244. https://doi.org/10.1039/d0tc01054e.Search in Google Scholar
3. Shafikov, M. Z., Daniels, R., Kozhevnikov, V. N. J. Phys. Chem. Lett. 2019, 10, 7015–7024. https://doi.org/10.1021/acs.jpclett.9b03002.Search in Google Scholar PubMed
4. Ma, H., Shen, K., Wu, Y., Xia, F., Yu, F., Sun, Z., Qian, C., Peng, Q., Zhang, H.-H., You, C., Xie, G., Hang, X.-C., Huang, W. Mater. Chem. Front. 2019, 3, 2448–2454. https://doi.org/10.1039/c9qm00347a.Search in Google Scholar
5. Li, W., Miao, T., Wang, B., Liu, J., Lü, X., Fu, G., Feng, W., Wong, W.-Y. J. Mater. Chem. C 2021, 9, 8337–8344. https://doi.org/10.1039/d1tc01977e.Search in Google Scholar
6. Sun, Y., Chen, C., Liu, B., Guo, Y., Feng, Z., Zhou, G., Chen, Z., Yang, X. J. Mater. Chem. C 2021, 9, 5373–5378. https://doi.org/10.1039/d0tc05965j.Search in Google Scholar
7. Zhang, M., Zhang, S.-W., Wu, C., Li, W., Wu, Y., Yang, C., Meng, Z., Xu, W., Tang, M.-C., Xie, R., Meng, H., Wei, G. ACS Appl. Mater. Interfaces 2022, 14, 1546–1556. https://doi.org/10.1021/acsami.1c19127.Search in Google Scholar PubMed
8. Chen, Y., Qian, C., Qin, K., Li, H., Shi, X., Lu, Z., Ma, H., Qin, T., Hang, X.-C., Huang, W. ACS Appl. Mater. Interfaces 2021, 13, 52833–52839. https://doi.org/10.1021/acsami.1c13843.Search in Google Scholar PubMed
9. Li, G., Zhao, X., Fleetham, T., Chen, Q., Zhan, F., Zheng, J., Yang, Y.-F., Lou, W., Yang, Y., Fang, K., Shao, Z., Zhang, Q., She, Y. Chem. Mater. 2020, 32, 537–548. https://doi.org/10.1021/acs.chemmater.9b04263.Search in Google Scholar
10. Yang, Z., Mao, Z., Xie, Z., Zhang, Y., Liu, S., Zhao, J., Xu, J., Chi, Z., Aldred, M. P. Chem. Soc. Rev. 2017, 46, 915–1016. https://doi.org/10.1039/c6cs00368k.Search in Google Scholar PubMed
11. Wang, Z.-Q., Xu, C., Wang, W.-Z., Duan, L.-M., Li, Z., Zhao, B.-T., Ji, B.-M. New J. Chem. 2012, 36, 662–667. https://doi.org/10.1039/c2nj20809a.Search in Google Scholar
12. Wang, Z., Zheng, C., Fu, W., Xu, C., Wu, J., Ji, B. New J. Chem. 2017, 41, 14152–14160. https://doi.org/10.1039/c7nj02806g.Search in Google Scholar
13. Mohankumar, M., Holler, M., Meichsner, E., Nierengarten, J.-F., Niess, F., Sauvage, J.-P., Delavaux-Nicot, B., Leoni, E., Monti, F., Malicka, J. M., Cocchi, M., Bandini, E., Armaroli, N. J. Am. Chem. Soc. 2018, 140, 2336–2347.https://doi.org/10.1021/jacs.7b12671.Search in Google Scholar PubMed
14. Schinabeck, A., Leitl, M. J., Yersin, H. J. Phys. Chem. Lett. 2018, 9, 2848–2856. https://doi.org/10.1021/acs.jpclett.8b00957.Search in Google Scholar PubMed
15. Olaru, M., Rychagova, E., Ketkov, S., Hynkarenko, Y., Yakunin, S., Kovalenko, M. V., Yablonskiy, A., Andreev, B., Kleemiss, F., Beckmann, J., Vogt, M. J. Am. Chem. Soc. 2020, 142, 373–381. https://doi.org/10.1021/jacs.9b10829.Search in Google Scholar PubMed
16. Xu, K., Chen, B.-L., Yang, F., Liu, L., Zhong, X.-X., Wang, L., Zhu, X.-J., Li, F.-B., Wong, W.-Y., Qin, H.-M. Inorg. Chem. 2021, 60, 4841–4851. https://doi.org/10.1021/acs.inorgchem.0c03755.Search in Google Scholar PubMed
17. Yamazaki, Y., Tsukuda, T., Furukawa, S., Dairiki, A., Sawamura, S., Tsubomura, T. Inorg. Chem. 2020, 59, 12375–12384. https://doi.org/10.1021/acs.inorgchem.0c01445.Search in Google Scholar PubMed
18. He, T.-F., Ren, A.-M., Chen, Y.-N., Hao, X.-L., Shen, L., Zhang, B.-H., Wu, T.-S., Zhang, H.-X., Zou, L.-Y. Inorg. Chem. 2020, 59, 12039–12053. https://doi.org/10.1021/acs.inorgchem.0c00980.Search in Google Scholar PubMed
19. Yu, P., Peng, D., He, L.-H., Chen, J.-L., Wang, J.-Y., Liu, S.-J., Wen, H.-R. Inorg. Chem. 2022, 61, 254–264. https://doi.org/10.1021/acs.inorgchem.1c02807.Search in Google Scholar PubMed
20. Lee, K., Lai, P.-N., Parveen, R., Donahue, C. M., Wymore, M. M., Massman, B. A., Vlaisavljevich, B., Teets, T. S., Daly, S. R. Chem. Commun. 2020, 56, 9110–9113. https://doi.org/10.1039/d0cc03427d.Search in Google Scholar PubMed
21. Huang, C.-H., Yang, M., Chen, X.-L., Lu, C.-Z. Dalton Trans. 2021, 50, 5171–5176. https://doi.org/10.1039/d0dt04424e.Search in Google Scholar PubMed
22. Meyer, M., Mardegan, L., Tordera, D., Prescimone, A., Sessolo, M., Bolink, H. J., Constable, E. C., Housecroft, C. E. Dalton Trans. 2021, 50, 17920–17934. https://doi.org/10.1039/d1dt03239a.Search in Google Scholar PubMed PubMed Central
23. Farias, G., Salla, C. A. M., Heying, R. S., Bortoluzzi, A. J., Curcio, S. F., Cazati, T., dos Santos, P. L., Monkman, A. P., de Souza, B., Bechtold, I. H. J. Mater. Chem. C 2020, 8, 14595–14604. https://doi.org/10.1039/d0tc03660a.Search in Google Scholar
24. Li, C., Mackenzie, C. F. R., Said, S. A., Pal, A. K., Haghighatbin, M. A., Babaei, A., Sessolo, M., Cordes, D. B., Slawin, A. M. Z., Kamer, P. C. J., Bolink, H. J., Hogan, C. F., Zysman-Colman, E. Inorg. Chem. 2021, 60, 10323–10339. https://doi.org/10.1021/acs.inorgchem.1c00804.Search in Google Scholar PubMed
25. Li, X., Zhang, J., Zhao, Z., Yu, X., Li, P., Yao, Y., Liu, Z., Jin, Q., Bian, Z., Lu, Z., Huang, C. ACS Appl. Mater. Interfaces 2019, 11, 3262–3270. https://doi.org/10.1021/acsami.8b15897.Search in Google Scholar PubMed
26. Housecroft, C. E., Constable, E. C. J. Mater. Chem. C 2022, 10, 4456–4482. https://doi.org/10.1039/d1tc04028f.Search in Google Scholar PubMed PubMed Central
27. Lin, L., Chen, D.-H., Yu, R., Chen, X.-L., Zhu, W.-J., Liang, D., Chang, J.-F., Zhang, Q., Lu, C.-Z. J. Mater. Chem. C 2017, 5, 4495–4504. https://doi.org/10.1039/c7tc00443e.Search in Google Scholar
28. Zhang, F., Guan, Y., Chen, X., Wang, S., Liang, D., Feng, Y., Chen, S., Li, S., Li, Z., Zhang, F., Lu, C., Cao, G., Zhai, B. Inorg. Chem. 2017, 56, 3742–3753. https://doi.org/10.1021/acs.inorgchem.6b01847.Search in Google Scholar PubMed
29. Klein, M., Rau, N., Wende, M., Sundermeyer, J., Cheng, G., Che, C.-M., Schinabeck, A., Yersin, H. Chem. Mater. 2020, 32, 10365–10382. https://doi.org/10.1021/acs.chemmater.0c02683.Search in Google Scholar
30. Krylova, V. A., Djurovich, P. I., Aronson, J. W., Haiges, R., Whited, M. T., Thompson, M. E. Organometallics 2012, 31, 7983–7993. https://doi.org/10.1021/om300656v.Search in Google Scholar
31. Leitl, M. J., Krylova, V. A., Djurovich, P. I., Thompson, M. E., Yersin, H. J. Am. Chem. Soc. 2014, 136, 16032–16038. https://doi.org/10.1021/ja508155x.Search in Google Scholar PubMed
32. Gernert, M., Balles-Wolf, L., Kerner, F., Müller, U., Schmiedel, A., Holzapfel, M., Marian, C. M., Pflaum, J., Lambert, C., Steffen, A. J. Am. Chem. Soc. 2020, 142, 8897–8909. https://doi.org/10.1021/jacs.0c02234.Search in Google Scholar PubMed
33. Chotard, F., Sivchik, V., Linnolahti, M., Bochmann, M., Romanov, A. S. Chem. Mater. 2020, 32, 6114–6122. https://doi.org/10.1021/acs.chemmater.0c01769.Search in Google Scholar
34. Föller, J., Ganter, C., Steffen, A., Marian, C. M. Inorg. Chem. 2019, 58, 5446–5456.10.1021/acs.inorgchem.9b00334Search in Google Scholar PubMed
35. Jazzar, R., Soleilhavoup, M., Bertrand, G. Chem. Rev. 2020, 120, 4141–4168. https://doi.org/10.1021/acs.chemrev.0c00043.Search in Google Scholar PubMed
36. Romanov, A. S., Chotard, F., Rashid, J., Bochmann, M. Dalton Trans. 2019, 48, 15445–15454. https://doi.org/10.1039/c9dt02036e.Search in Google Scholar PubMed
37. Lin, S., Peng, Q., Ou, Q., Shuai, Z. Inorg. Chem. 2019, 58, 14403–14409. https://doi.org/10.1021/acs.inorgchem.9b01705.Search in Google Scholar PubMed
38. Ruduss, A., Turovska, B., Belyakov, S., Stucere, K. A., Vembris, A., Traskovskis, K. Inorg. Chem. 2022, 61, 2174–2185. https://doi.org/10.1021/acs.inorgchem.1c03371.Search in Google Scholar PubMed
39. Hamze, R., Shi, S., Kapper, S. C., Ravinson, D. S. M., Estergreen, L., Jung, M.-C., Tadle, A. C., Haiges, R., Djurovich, P. I., Peltier, J. L., Jazzar, R., Bertrand, G., Bradforth, S. E., Thompson, M. E. J. Am. Chem. Soc. 2019, 141, 8616–8626. https://doi.org/10.1021/jacs.9b03657.Search in Google Scholar PubMed
40. Elie, M., Sguerra, F., Meo, F. D., Weber, M. D., Marion, R., Grimault, A., Lohier, J.-F., Stallivieri, A., Brosseau, A., Pansu, R. B., Renaud, J.-L., Linares, M., Hamel, M., Costa, R. D., Gaillard, S. ACS Appl. Mater. Interfaces 2016, 8, 14678–14691.10.1021/acsami.6b04647Search in Google Scholar PubMed
41. Shi, S., Jung, M. C., Coburn, C., Tadle, A., Sylvinson, M. R. D., Djurovich, P. I., Forrest, S. R., Thompson, M. E. J. Am. Chem. Soc. 2019, 141, 3576–3588. https://doi.org/10.1021/jacs.8b12397.Search in Google Scholar PubMed
42. Romanov, A. S., Yang, L., Jones, S. T. E., Di, D., Morley, O. J., Drummond, B. H., Reponen, A. P. M., Linnolahti, M., Credgington, D., Bochmann, M. Chem. Mater. 2020, 31, 3613–3623.10.1021/acs.chemmater.8b05112Search in Google Scholar
43. Ying, A., Huang, Y.-H., Lu, C.-H., Chen, Z., Lee, W.-K., Zeng, X., Chen, T., Cao, X., Wu, C.-C., Gong, S., Yang, C. ACS Appl. Mater. Interfaces 2021, 13, 13478–13486. https://doi.org/10.1021/acsami.0c22109.Search in Google Scholar PubMed
44. Wang, Z., Zheng, C., Wang, W., Xu, C., Ji, B., Zhang, X. Inorg. Chem. 2016, 55, 2157–2164. https://doi.org/10.1021/acs.inorgchem.5b02546.Search in Google Scholar PubMed
45. Wang, Z., Sun, X., Fu, W., Xu, C., Ji, B. J. Lumin. 2018, 204, 618–625. https://doi.org/10.1016/j.jlumin.2018.08.064.Search in Google Scholar
46. Wang, Z., Sun, X., Xu, C., Ji, B. Front. Chem. 2019, 7, 422. https://doi.org/10.3389/fchem.2019.00422.Search in Google Scholar PubMed PubMed Central
47. Sheldrick, G. M. Sadabs, Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, 1994.Search in Google Scholar
48. Sheldrick, G. M. Shelxs-97, Program for the Solution of Crystal Structures; University of Göttingen: Göttingen, 1997.Search in Google Scholar
49. Sheldrick, G. M. Shelxl-97, Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, 1997.Search in Google Scholar
50. Verma, A. K., Singh, J., Sankar, V. K., Chaudhary, R., Chandra, R. Tetrahedron Lett. 2007, 48, 4207–4210. https://doi.org/10.1016/j.tetlet.2007.04.061.Search in Google Scholar
51. Keith, J. M. J. Org. Chem. 2008, 73, 327–330. https://doi.org/10.1021/jo702038g.Search in Google Scholar PubMed
52. Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J. A.Jr., Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, J. M., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, Ö., Foresman, J. B., Ortiz, J. V., Cioslowski, J., Fox, D. J. Gaussian 09 (Revision E.01); Gaussian, Inc.: Wallingford, CT, 2009.Search in Google Scholar
53. Wang, Z., Cai, J., Zhang, M., Zheng, C., Ji, B. Acta Chim. Sin. 2019, 77, 263–268. https://doi.org/10.6023/a18100437.Search in Google Scholar
54. Wang, Z., Bai, M., Zhang, M., Zhang, Z., Feng, X., Zheng, C. Acta Chim. Sin. 2020, 78, 140–146. https://doi.org/10.6023/a19100372.Search in Google Scholar
55. Armaroli, N. Chem. Soc. Rev. 2001, 30, 113–124. https://doi.org/10.1039/b000703j.Search in Google Scholar
56. Hofbeck, T., Monkowius, U., Yersin, H. J. Am. Chem. Soc. 2015, 137, 399–404. https://doi.org/10.1021/ja5109672.Search in Google Scholar PubMed
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this issue
- Research Articles
- Crystal structures of sildenafil compounds with nitrate and di(citrato)zinc counterions
- Synthesis, crystal structure, and properties of three lead(II) complexes based on the 1,10-phenanthroline ligand
- A highly selective and sensitive fluorescent sensor based on a 1,8-naphthalimide with a Schiff base function for Hg2+ in aqueous media
- Co-crystallization of dimethyl N-cyanodithioiminocarbonate and bis[(aqua)-µ2-hydroxy-n-butyldichlorotin(IV)]
- Synthesis of ring-A serjanic acid derivatives and their cytotoxic evaluation through the brine shrimp lethality assay (BSLA)
- Electron density of the 1:2 complex of valinomycin with calcium triflate observed in crystals of the composition (valinomycin)Ca2(OTf)4(THF)5(H2O)4
- Two zinc and cadmium coordination polymers constructed with bis(4-(1H-imidazol-1-yl)phenyl)methanone and naphthalene-1,4-dicarboxylate ligands: synthesis and structural characterization
- Characterization of hydrophilic carbon nanohorns prepared by the arc-in-water method
- Crystal structure determination and characterization of Sm3SiO5F3
- A four-fold three-dimensional zinc(II) coordination polymer based on 4,4′-bis(2-methyl-imidazolyl)biphenyl and 5-sulfoisophthalate ligands: synthesis, structure and properties
- Synthesis, structures, and photophysical properties of two Cu(I) complexes supported by N-heterocyclic carbene and phosphine ligands
- Synthesis of chiral binaphthol-based bishydroxylamines
Articles in the same Issue
- Frontmatter
- In this issue
- Research Articles
- Crystal structures of sildenafil compounds with nitrate and di(citrato)zinc counterions
- Synthesis, crystal structure, and properties of three lead(II) complexes based on the 1,10-phenanthroline ligand
- A highly selective and sensitive fluorescent sensor based on a 1,8-naphthalimide with a Schiff base function for Hg2+ in aqueous media
- Co-crystallization of dimethyl N-cyanodithioiminocarbonate and bis[(aqua)-µ2-hydroxy-n-butyldichlorotin(IV)]
- Synthesis of ring-A serjanic acid derivatives and their cytotoxic evaluation through the brine shrimp lethality assay (BSLA)
- Electron density of the 1:2 complex of valinomycin with calcium triflate observed in crystals of the composition (valinomycin)Ca2(OTf)4(THF)5(H2O)4
- Two zinc and cadmium coordination polymers constructed with bis(4-(1H-imidazol-1-yl)phenyl)methanone and naphthalene-1,4-dicarboxylate ligands: synthesis and structural characterization
- Characterization of hydrophilic carbon nanohorns prepared by the arc-in-water method
- Crystal structure determination and characterization of Sm3SiO5F3
- A four-fold three-dimensional zinc(II) coordination polymer based on 4,4′-bis(2-methyl-imidazolyl)biphenyl and 5-sulfoisophthalate ligands: synthesis, structure and properties
- Synthesis, structures, and photophysical properties of two Cu(I) complexes supported by N-heterocyclic carbene and phosphine ligands
- Synthesis of chiral binaphthol-based bishydroxylamines

