New cation-disordered quaternary selenides Tl2Ga2TtSe6 (Tt=Ge, Sn)
-
Volodymyr Babizhetskyy
, Volodymyr Levytskyy
Abstract
Two new quaternary selenides of the α-TlSe structure type have been synthesized and characterized. Single crystal X-ray diffraction analysis has revealed that Tl2Ga2SnSe6 crystallizes with space group I4/mmc, a = 8.095(1), c = 6.402(1) Å, with a refined composition of Tl1−xGa1−ySnySe2 (x = y = 0.345(5)), Z = 4, R1 = 0.028; wR2 = 0.066. The crystal structure of the isostructural compound Tl2Ga2GeSe6 has been determined by means of powder X-ray diffraction: space group I4/mmc, Z = 4, a = 8.0770(4), c = 6.2572(5) Å, refined composition Tl1−xGa1−yGeySe2, x = 0.343(5), y = 0.35(2), (RB(I) = 0.084; RP = 0.041; RPw = 0.058). According to their optical absorption spectra all compounds are semiconductors with relatively narrow direct band gaps of 2.15(3) and 2.05(5) eV for the Ge and Sn phase, respectively.
1 Introduction
The family of binary TlX (X=S, Se, Te) compounds and their derivatives is well known as optical and semiconducting materials [1]. Ordered ternary representatives TlTtX2 (Tt=Al, Ga, In) also exist featuring two crystallographic sites with different coordination environments, but the existence of solid solutions for different chalcogenides cannot be excluded [2], [3], [4], [5], [6]. The light atoms in those structures usually exhibit the oxidation state +3, while the heavy ones rather adopt the oxidation state +1. This fact provoked further investigation towards formation of solid solutions or quaternary compounds in the series TlTtX2–Tt′X2 (Tt′=Si, Ge, Sn) exhibiting aliovalent metal substitutions.
It should be noted that a few representatives of the Tt″TtX2–Tt′X2 systems are known with Tt″=Li, Na, Cu, Ag having approximate stoichiometry Tt″2Tt2Tt′X6. For instance, lithium-containing systems with gallium include the Li2Ga2GeS6 compound that crystallizes in the non-centrosymmetric orthorhombic space group Fdd2 which is a promising non-linear optical material [7]. The substitution of In for Ga results in a series of compounds Li2In2Si(Ge)S6(Se6) that crystallize in a monoclinic structure, space group Cc [8], [9]. Isostructural sodium-containing compounds Na2In2Si(Ge)S6(Se6) and Na2CdGe2S6(Se6) have also been reported [10], [11], [12], [13]. It has been found that Na2Ga2GeS6 crystallizes in the orthorhombic structure (space group Fdd2), while Na2In2SiS6 and Na2In2GeS6 have monoclinic structures (space group Cc). Na2Ga2SnS6 is dimorphic and crystallizes in both of the above structures.
The formation of the 2-2-1-6 compounds is also common in silver-containing systems. They all crystallize in either of two structures. The monoclinic structure (space group Cc) is typical of indium-containing compounds (Ag2In2Si(Ge)S6 [14], Ag2In2SiSe6 [15], Ag2In2GeSe6 [16]), while compounds with Ga (Ag2Ga2SiS6 [17], Ag2Ga2SiSe6 [18]) crystallize in the tetragonal structure (space group I4̅2d). Finally, unique cases of this composition are known in a copper-containing system Cu2In2SiS6 (space group Cc) [19] and in a combination with thallium, Tl2In2SnSe6 (space group I4/mcm) [20].
For that reason, the TlGaSe2–SnSe2 system has been investigated in more detail, followed by iso-compositional representatives with Ge. At ambient conditions TlGaSe2 has a monoclinic crystal structure [10] (structure type KInS2 [21]), whereas a tetragonal structure (high-temperature inherent variant, α-TlSe type [6]) has been observed within the TlGaSe2–SnSe2 cross-section. The composition, defined by energy-dispersive X-ray spectroscopy (EDX), indicated the existence of a new quaternary compound. In this work we present the results of structural, spectroscopic and theoretical investigations of the nonstoichiometric Tl1−xGa1−xTtxSe2 series.
2 Experimental
2.1 Synthesis
The Tl2Ga2TtSe6 samples were prepared from high-purity elements (Tl, 99.99 wt.%; Ga, 99.999 wt.%; Si, 99.9999 wt.%; Ge, 99.9999 wt.%; Sn, 99.9999 wt.%; Se, 99.999 wt.%). Single crystals of Tl2Ga2TtSe6 were grown using the Bridgman-Stockbarger method [22]. Crystals were synthesized and grown in fused silica ampoules with a conical bottom. Calculated amounts of 37 mol% GeSe2/63 mol% TlGaSe2 and 40 mol% SnSe2/60 mol% TlGaSe2 with the total weights of 2 g were placed in a fused silica tube, evacuated to a residual pressure of 0.1 Pa, and then sealed. The samples were synthesized in a shaft-type furnace by heating to T=1170 K at the rate of 40 K·h−1. The melt was kept at this temperature for 6 h with periodic vibration to assure homogeneity, cooled to T=870 K at the rate of 20 K·h−1, and annealed for 10 days. The process ended in cooling the ampoule to room temperature at 8.4 K·h−1.
2.2 Microprobe analysis
Quantitative sample analysis was performed by the EDX technique with a scanning electron microscope REMMA-102-02. EDX analysis of pieces of mechanically crushed samples showed that the Tl2Ga2SnSe6 sample was homogeneous and its chemical composition corresponded to Tl18.7(2)Ga21.0(3)Sn8.8(2)Se51.5(3). The Tl2Ga2GeSe6 sample average is Tl19(1)Ga20(2)Ge10(2)Se51(2) in good agreement with the nominal sample composition, though the refined Se content using this method is slightly underestimated compared to the loaded one.
2.3 X-ray single-crystal diffraction
A shiny metallic black needle-shaped single crystal was isolated from the crushed sample. Its quality was evaluated by the Laue method using white X-ray Mo radiation. The single-crystal X-ray diffraction data were collected at room temperature on a STOE IPDS II image plate diffractometer with monochromatized MoKα radiation.
The unit cell parameters and extinction rules suggested I4/mcm as the most appropriate space group. The WinGX program package [23] was used for the hkl data treatment. Multi-scan absorption corrections were made with the use of the program Platon [24]. The basic model of the crystal structure was obtained by Direct Methods using the program Sir2014 [25]. Only three different atomic coordinates were obtained (in 4a, 4b and 8h positions) equivalent to those of β-TlGaSe2 [6]. First the Tl, Ga and Se atoms were appointed in 4a, 4b and 8h positions, according to the β-TlGaSe2 structure, respectively. Refinement of the crystal structure with the program Shelxl [26] in isotropic approximation of atomic displacement showed three residual electron density peaks in the difference Fourier synthesis close to the Tl atom (10.7 e Å−3; 0.59 Å) and a high reliability factor R1=0.163. Anisotropic refinement yielded a substantial decrease of R1 to 9.8% but still a considerable (9.1 e Å−3) residual electron density peak at the Ga position. Statistical occupation of the 4b and partial occupation of the 4a positions revealed significant improvement of the R values and a satisfactory chemical composition with respect to the EDX analysis (R1=0.028; wR2=0.066; S=1.154; Tl0.655Ga0.657Sn0.343Se2). Finally, taking into account the valences of the elements, the refinement was simplified for a more reasonable form Tl1−xGa1−ySnxSe2, x=y=0.345(5). The refined composition is close to the initial value Tl2Ga2SnSe6, indicating a narrow homogeneity range of the compound. The crystal data and information on data collection and evaluation are summarized in Table 1. The atomic coordinates, equivalent and anisotropic displacement parameters and interatomic distances are listed in Tables 2– 4, respectively. Crystal structure illustrations have been prepared with the program Diamond [27].
Crystal data and structure refinement details of Tl2Ga2SnSe6.
| Empirical formula | Tl2Ga2SnSe6 |
| Moiety formula | Tl1−xGa1−ySnySe2, x=y=0.345(5) |
| Equal to composition (in at.%) | Tl17.9Ga17.9Sn9.5Se54.7 |
| Composition by EDX (in at.%) | Tl18.7Ga21.0Sn8.8Se51.5 |
| Z | 4 |
| Structure type | α-TlSe |
| Crystal system | Tetragonal |
| Space group, Pearson symbol | I4/mcm (no. 140), tI16 |
| Unit cell parameters | |
| a, Å | 8.095(1) |
| c, Å | 6.402(1) |
| Unit cell volume V, Å3 | 419.5(2) |
| Number of formula units per cell Z | 4 |
| Calculated density, g cm−3 | 5.99 |
| Absorption coefficient μ, mm−1 | 48.6 |
| Radiation/wavelength, Å | MoKα/0.71073 |
| Diffractometer | STOE IPDS II |
| Absorption correction | Multi-scan (Platon [24]) |
| θ range, ° | 3.6–29.0 |
| hkl indices range | −10≤h≤10, −10≤k≤11, −8≤l≤7 |
| Collected reflections | 1793 |
| Independent reflections/Rint/Rσ | 167/0.060/0.131 |
| Reflections with I>2 σ(I) | 122 |
| Refinement | F2 |
| Refined parameters | 10 |
| Final R1/wR2 [I>2 σ(I)]a,b | 0.028/0.064 |
| Final R1/wR2 (all data)a,b | 0.043/0.066 |
| Goodness-of-fitc on F2 | 1.153 |
| Largest diff. peak/hole, e Å−3 | 0.93/−1.46 |
aR1=Σ||Fo|–|Fc||/Σ|Fo|; bwR2=[Σw(Fo2–Fc2)2/Σw(Fo2)2]1/2, w=[σ2(Fo2)+(0.0170P)2+1.1085P]−1, where P=(Max(Fo2, 0)+2Fc2)/3; cGoF=S=[Σw(Fo2–Fc2)2/(nobs–nparam)]1/2.
Atomic coordinates and equivalent displacement parameters (in Å2) for Tl2Ga2SnSe6.a
| Atom | Wyckoff position | G (site occupancy) | x | y | z | Ueq |
|---|---|---|---|---|---|---|
| Se | 8h | 1 | 0.16418(11) | 1/2+x | 0 | 0.0334(4) |
| M | 4b | 1 | 0 | ½ | 1/4 | 0.0309(4) |
| Tl | 4a | 0.655(5) | 0 | 0 | 1/4 | 0.0724(7) |
aM=0.655(5)Ga+0.345(5)Sn.
Anisotropic atomic displacement parameters (in Å2) for Tl2Ga2SnSe6.a,b
| Atom | U11=U22 | U33 | U12 |
|---|---|---|---|
| Se | 0.0329(4) | 0.0344(7) | −0.0043(6) |
| M | 0.0329(5) | 0.0270(9) | 0 |
| Tl | 0.0440(6) | 0.1291(17) | 0 |
aM=0.655(5)Ga+0.345(5)Sn. bU13=U23=0.
Interatomic distances (δ) and coordination numbers (CN) of atoms in the crystal structure of Tl2Ga2SnSe6.a
| Atoms | δ (Å) | CN | Atoms | δ (Å) | CN | Atoms | δ (Å) | CN |
|---|---|---|---|---|---|---|---|---|
| Tl–2 Tl | 3.2010(7) | 10 | M–4 Se | 2.469(1) | 4 | Se–2 M | 2.469(1) | 6 |
| 8 Se | 3.423(1) | 4 Tl | 3.423(1) |
aM=0.655(5)Ga+0.345(5)Sn.
2.4 X-ray powder diffraction
Experimental diffraction data for the isostructural compounds Tl2Ga2GeSe6 and Tl2Ga2GeSe6 were obtained on a DRON 4-13 diffractometer (45 kV and 30 mA, CuKα radiation, Bragg-Brentano geometry) and analyzed with the WinCSD program package [28].
Phase analysis showed that the XRD pattern of Tl2Ga2GeSe6 was similar to that of the tetragonal II-TlGaSe2 phase (high temperature-high pressure modification) [6] with slightly different unit cell parameters. In the first stage of the treatment of the crystal structure was refined as TlGaSe2 using the initial atomic coordinates of HT-TlGaSe2 [6]. After accounting for the predominant orientation factor (texture) along the 0 0 1 direction, refinement of isotropic displacement parameters for Tl, Ga and Se atoms showed significantly increased values of the latter, especially for the Tl and Ga sites. Taking into account the EDX results for the elemental composition, a statistical mixture M=0.65 Ga+0.35 Ge was restrained. The RB and Biso values for the M and Se positions were reduced, but for Tl Biso they increased. Refinement of the 4a site occupancy (G) by thallium atoms resulted in G=0.66. Finally both the 4a and 4b occupancies and, afterwards, the isotropic displacement parameters for all Tl, M, and Se atoms were refined. Experimental, calculated and difference powder XRD profiles of the Tl2Ga2GeSe6 sample are shown in Fig. 1. Details of the refinement are listed in Table 5, atomic coordinates and their displacement parameters in Table 6. High correlation of the refined composition with the elemental analysis was observed, despite comparatively higher Biso values for Tl, caused by structural features of this structure type, since Tl atoms are localized in Se tunnels along the 0 0 1 direction (see Fig. 3a). Unfortunately, anisotropic displacement parameters could not be refined reliably from powder XRD due to a poor data to parameter ratio.

Observed (dots) and calculated (line) profiles and their difference plot (bottom) of the XRD patterns of the Tl2Ga2GeSe6 sample. Peak positions are marked by short vertical bars.
Details of data collection and refinement of the crystal structure of Tl2Ga2GeSe6.
| Refined composition | Tl1−xGa1−yGeySe2, x=0.343(5), y=0.35(2) |
| Space group | I4/mcm (no. 140) |
| Structure type | α-TlSe |
| Pearson symbol and Z | tI16, 4 |
| Unit cell parameters | |
| a, Å | 8.0770(4) |
| c, Å | 6.2572(5) |
| V, Å3 | 408.20(7) |
| Calculated density, g·cm−3 | 5.91 |
| Diffractometer | DRON 4-13 |
| Radiation/λ, Å | CuKα/1.54185 |
| Mode of refinement | Full with fixed elements per cycle |
| 2θ range/step, ° | 10.0–100.0/0.02 |
| (sinθ/λ)max, Å−1 | 0.497 |
| Detector | NaI(Tl) scintillation counter |
| Scanning time (in s) per step of 0.02° in 2θ | 20 |
| No. of reflections | 69 |
| No. of parameters (all/free) | 24/5 |
| Scale factor | 0.0682(1) |
| Goodness-of-fit | 2.46 |
| RB(I)/RP/RPw, % | 8.4/4.1/5.8 |
Refined atomic coordinates and isotropic displacement parameters for Tl2Ga2GeSe6.a
| Atom | Wyckoff position | G (site occupancy) | x | y | z | Biso (Å2) |
|---|---|---|---|---|---|---|
| Se | 8h | 1 | 0.1577(2) | x+½ | 0 | 1.18(7) |
| M | 4b | 1 | 0 | ½ | ¼ | 2.87(12) |
| Tl | 4a | 0.657(5) | 0 | 0 | ¼ | 3.48(9) |
aM=0.65(2) Ga+0.35(2) Ge.
CCDC 1960737 (Tl2Ga2SnSe6) and 1960739 (Tl2Ga2GeSe6) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
2.5 Electronic structure calculations
Tight binding electronic structure calculations for a few idealized models of Tl1−xGa1−ySnySe2 assuming fully occupied sites were performed with the linear-muffin-tin-orbital (LMTO) method in the atomic sphere approximation (ASA) using the Stuttgart TB-LMTO-ASA code [29]. The radii of the Wigner-Seitz spheres were assigned automatically (1.84, 1.47 and 1.39 Å for Tl, Ga, and Se, respectively) to achieve the best possible approximations to the full potentials [30]. Two additional empty spheres were necessary with a 16% overlap restriction. Basis sets of Tl 6s,6p,(5d), Ga 4s,4p,(3d), Sn 5s,5p,(4d) and Se 4s,4p,(3d) (downfolded [31] orbitals in parentheses) were employed. Scalar relativistic corrections were included in the calculations. The band structure was sampled for 1063 k-points in the corresponding irreducible wedge of the Brillouin zone.
2.6 UV/Vis diffuse reflectance spectroscopy
UV/Vis diffuse reflectance spectra were recorded on powder samples using an Agilent Technologies Cary 5000 UV-Vis-NIR spectrophotometer equipped with a diffuse-reflectance accessory (Praying Mantis, Harrick). A Spectralon disk was used as the reference material.
3 Results and discussion
The crystal structure of Tl2Ga2SnSe6 is closely related to the structures of the stoichiometric 2-2-1-6 compounds and contains three main building units – MSe4 tetrahedra, SeM2Tl4 trigonal prisms and Tl@Tl2Se8 bi-capped tetragonal antiprisms (Fig. 2). Atomic coordination numbers and selected interatomic distances are summarized in Table 4. The simplest structural representation appears in the form of chains of edge-sharing [MSe4] tetrahedra along [001], connected in the ab plane with Tl+ cations occupying the channels (Fig. 3a). This structural arrangement is related to the one in the tetragonal quaternary selenide Ag2Ga2SiSe6 (Fig. 4f), that exhibits a three-dimensional framework composed of corner-sharing [(Ga/Si)Se4] and [AgSe4] tetrahedra with empty cavities [17]. In the disordered crystal structure of Ag2Ga2SiSe6 the Ga and Si atoms form a statistical mixture, while the Ag atoms partially occupy the 4b site. The crystal structure of the monoclinic quaternary selenide Na2In2GeSe6 (Fig. 3d), exhibits a three-dimensional framework composed of corner-sharing [InSe4] and [GeSe4] tetrahedra with Na+ cations occupying the voids [11]. The basic building unit of the Na2In2GeSe6 framework is a [In4Ge2Se16] block comprised of two smaller [In2GeSe9] units. The latter in turn consists of two [InSe4] tetrahedra and one [GeSe4] tetrahedron linked to each other via Se vertex-sharing. The [In4Ge2Se16] blocks are connected to each other via corner-sharing to generate the three-dimensional framework.
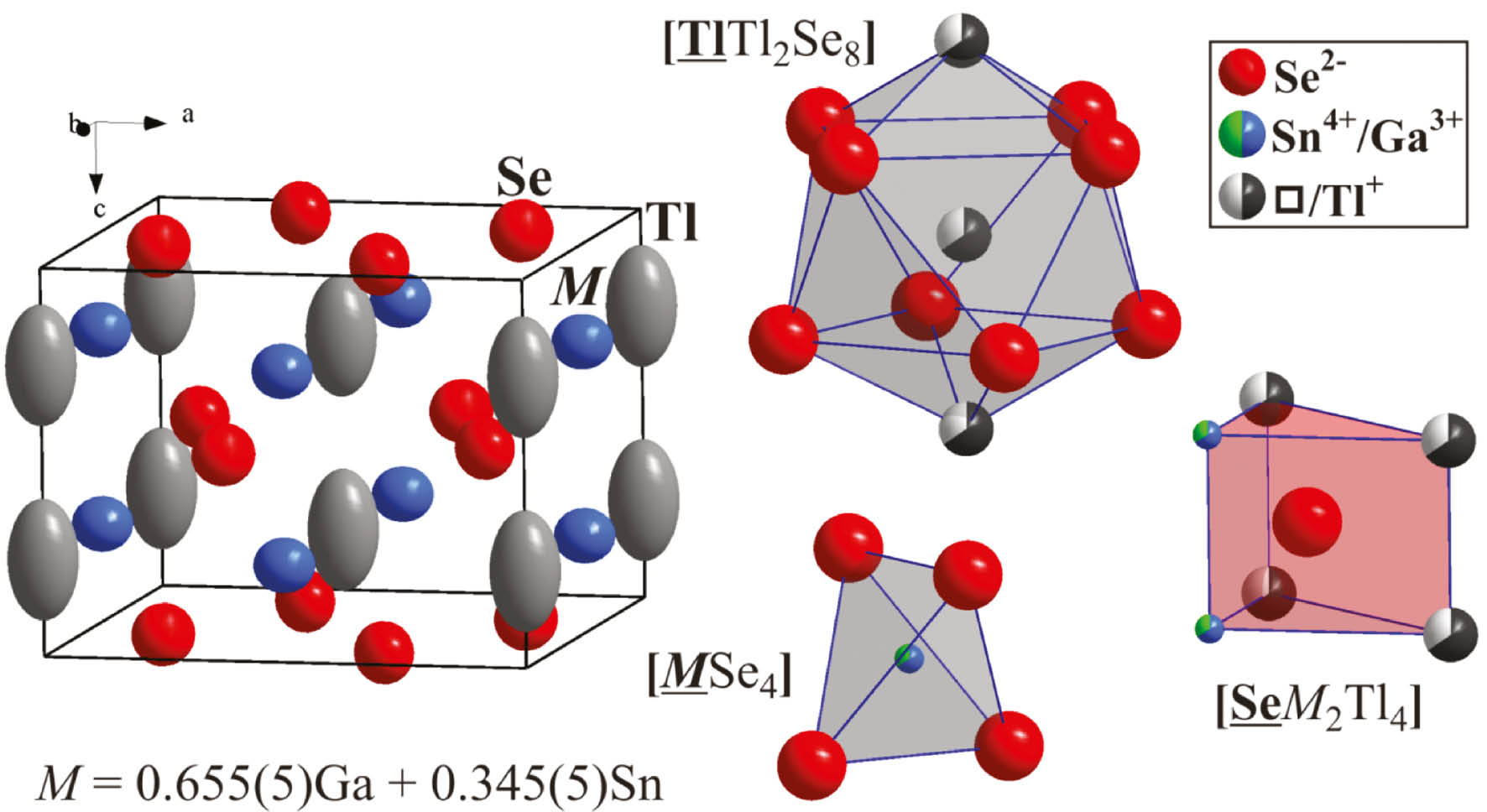
Filled unit cell (atoms are shown as their anisotropic ellipsoids with 95% probability) and atomic coordination polyhedra (ions are shown as circles with color distinguishing sectors, indicating partial or mixed site occupancy) of Tl2Ga2SnSe6.

Packing of tetrahedra of selenium and sulfur atoms in the crystal structures of Tl2Ga2SnSe6 (a), K2Sn2ZnSe6 (b) and Na2Ge2ZnSe6 (c), Na2In2GeSe6 (d), Li2Ga2GeS6 (e), and Ag2Ga2SiSe6 (f).

Band structure and projected densities of state (DOS) for an idealized model of Tl2In2SnSe6.
The lithium sulfide Li2Ga2GeS6 crystallizes in the non-centrosymmetric orthorhombic space group Fdd2 [7]. The crystal structure is built of [GeS4] tetrahedra linked by corners to other four identical units to create a three dimensional framework with tunnels along the c axis, in which the Li+ ions are located (Fig. 3e). The Li atoms in distorted tetrahedral coordination (Li@S4) form an infinite chain parallel to the c axis by vertex-sharing. K2Sn2ZnSe6 and Na2Ge2ZnSe6 ([11], Fig. 3b and c) crystallize in the space groups P4/ncc and I4/mcm, respectively. In these structures [SnSe4] or [GeSe4] and [ZnSe4] edge-sharing tetrahedra appear in similar chains. Two [Sn(Ge)Se4] tetrahedra are first connected to each other by sharing two Se atoms to form the [Sn(Ge)2Se6] block, which is further linked via edge-sharing two other Se atoms with one [ZnSe4] tetrahedron to build up a [Sn(Ge)2ZnSe6] infinite chain along [001]. The chains are separated from each other by K+ or Na+ cations occupying the voids. All K+ and Na+ cations are coordinated by eight Se atoms in tetragonal antiprismatic arrangement. The unit cell expansion is in good agreement with the atomic sizes of Na/Ge vs. K/Sn [32].
Observed interatomic distances indicate that in Tl2Ga2SnSe6 thallium is monovalent (ionic radius [33] r(Tl+)=1.49 Å), gallium trivalent (r(Ga3+)=0.62 Å) and selenium divalent (r(Se2−)=1.91 Å) showing a reasonable correlation of their coordination polyhedra (Fig. 2) and earlier evaluations for β-TlGaSe2 [6]. The shortest interatomic distances are those between the M and Se atoms indicating stronger chemical bonding between them. From the viewpoint of the charge balance each Sn atom introduced to the structure leads to simultaneous exclusion of both a Ga and a Tl atom according to
Electronic structure calculations were performed for a couple of slightly modified models of Tl2Ga2SnSe6 because of the difficulties in the treatment of partially occupied sites. TlGaSe2, TlInSe2 and TlSnSe2 have been examined in order to estimate changes in the electronic structure with regard to the atomic size, electronegativity and valence electron concentration. All three models revealed qualitatively identical results with reasonable variation of the monitored output parameters as a good approximation for the observed disordered structure. Expectedly, the fully occupied Sn position led to the shift of the Fermi level into the areas with non-zero densities of states. The proper adjustment to the corresponding valence electron count revealed a comparable gap in the electronic structure (Fig. 4). The electronic densities of state (DOS) exhibit broad valence s, p bands reaching 8 eV below the Fermi level including large contributions from mostly Se-4p bands located 1–2.5 eV below EF. In this region they exhibit the largest overlap with the Ga-4p and Tl-6s,6p bands. The contributions from the Ga and Tl bands are significantly smaller being comparable to each other in the range from –5 to 2 eV. This agrees well with the formally cationic roles of both metals in this compound. The Tl-6s bands dominate at –5 to –6.5 eV while the Ga-4s bands appear around –7 eV.
The maximum of the valence band is located at the Z point, while the pronounced minimum of the conduction band is found between the X and P points. Consequently, the model suggests an indirect band gap semiconductor with a calculated band gap size varied between the Ga and Sn model from 0.74 to 0.93 eV. These values are considerably lower than the expected range based on the samples’ color and optical appearance. Since the size of the band gap is usually underestimated by the DFT methods, UV/Vis diffuse reflectance measurements were performed to obtain more accurate values. The UV/Vis diffuse reflectance spectra, recorded at room temperature, show an intense broad band with an absorption edge at 728 nm for 1 and at 779 nm for 2 (Fig. 5). These spectral features correlate well with the red-brown color of the compounds. The UV/Vis data were then employed to determine the band gap of the compounds. The values of the energy band gap (Eg) were estimated by linear extrapolation of the Tauc plot [34] [(αhν)2 vs. hν] to (αhν)2=0. The values of Eg are 2.15(3) eV for 1 and 2.05(5) eV for 2, which is consistent with the transparency of the crystals and the color of the compounds. However, the band gap of 2 is smaller compared with that of 1. A similar observation can be made in the case of other quaternary selenides, like BaGa2GeSe6 (2.22 eV) [35] and BaGa2SnSe6 (1.95 eV) [36]. It can be concluded that the heavier the employed group 14 elements is, the narrower is the band gap observed in this series of compounds. This can be explained in terms of differences in electron binding capacities of the various ions. Nuclei of heavier tetravalent ions are larger and exhibit weaker electron binding capacities than those of lighter ones. Therefore, the valence orbitals of the heavier tetravalent ions contribute to the bottom of the conduction bands to a larger extent, resulting in smaller band gaps.

UV/Vis diffuse reflectance spectra and energy band gap of 1–2.
4 Conclusions
In summary, two new quaternary selenides
Dedicated to: Professor Arndt Simon on the occasion of his 80th birthday.
Acknowledgments
The authors thank W. Hölle (MPI FKF, Stuttgart) for X-ray intensity data collection. AVM and VS would like to thank Energimydigheten, the Swedish Energy Agency for support.
References
[1] R. S. Itoga, C. R. Kannewurf, J. Phys. Chem. Solids1971, 32, 1099.10.1016/S0022-3697(71)80168-3Suche in Google Scholar
[2] A. M. Ulubey, F. M. Hashimzade, D. A. Huseinova, M. A. Nizametdinova, G. S. Orudzhev, K. R. Allakhverdiev, N. T. Yıldız, Phys. Status Solidi B2011, 248, 181.10.1002/pssb.201046367Suche in Google Scholar
[3] C. R. Whitehouse, A. A. Balchin, J. Mater. Sci.1978, 13, 2394.10.1007/BF00808054Suche in Google Scholar
[4] T. Isaacs, Z. Kristallogr.1975, 141, 104.10.1524/zkri.1975.141.1-2.104Suche in Google Scholar
[5] D. Müller, F. E. Poltmann, H. Hahn, Z. Naturforsch.1974, 29b, 117.10.1515/znb-1974-1-237Suche in Google Scholar
[6] K.-J. Range, G. Mahlberg, S. Obenland, Z. Naturforsch.1977, 32b, 1354.10.1515/znb-1977-1129Suche in Google Scholar
[7] Y. Kim, I. Seo, S. W. Martin, J. Baek, P. S. Halasyamani, N. Arumugam, H. Steinfink, Chem. Mater.2008, 20, 6048.10.1021/cm8007304Suche in Google Scholar
[8] W. Yin, K. Feng, W. Hao, J. Yao, Y. Wu, Inorg. Chem.2012, 51, 5839.10.1021/ic300373zSuche in Google Scholar PubMed
[9] S.-F. Li, B.-W. Liu, M.-J. Zhang, Y.-H. Fan, H.-Y. Zeng, G.-C. Guo, Inorg. Chem.2016, 55, 1480.10.1021/acs.inorgchem.5b02211Suche in Google Scholar PubMed
[10] G. E. Delgado, A. J. Mora, F. V. Pérez, J. González, Cryst. Res. Technol.2007, 42, 663.10.1002/crat.200610885Suche in Google Scholar
[11] M. Zhou, C. Li, X. Li, J. Yao, Y. Wu, Dalton Trans.2016, 45, 7627.10.1039/C6DT00143BSuche in Google Scholar
[12] G. Li, Q. Liu, K. Wu, Z. Yang, S.-L. Pan, Dalton Trans.2017, 46, 2778.10.1039/C7DT00087ASuche in Google Scholar
[13] J. P. Yohannan, K. Vidyasagar, J. Solid State Chem.2016, 238, 147.10.1016/j.jssc.2016.03.026Suche in Google Scholar
[14] V. P. Sachanyuk, G. P. Gorgut, V. V. Atuchin, I. D. Olekseyuk, O. V. Parasyuk, J. Alloys Compd.2008, 452, 348.10.1016/j.jallcom.2006.11.043Suche in Google Scholar
[15] I. D. Olekseyuk, V. P. Sachanyuk, O. V. Parasyuk, J. Alloys Compd.2006, 414, 73.10.1016/j.jallcom.2005.07.025Suche in Google Scholar
[16] O. V. Krykhovets, L. V. Sysa, I. D. Olekseyuk, T. Glowyak, J. Alloys Compd.1999, 287, 181.10.1016/S0925-8388(99)00016-XSuche in Google Scholar
[17] M. Piasecki, G. L. Myronchuk, O. V. Parasyuk, O. Y. Khyzhun, A. O. Fedorchuk, V. V. Pavlyuk, V. R. Kozer, V. P. Sachanyuk, A. M. El-Naggar, A. A. Albassam, J. Jedryka, I. V. Kityk, J. Solid State Chem.2017, 246, 363.10.1016/j.jssc.2016.12.011Suche in Google Scholar
[18] O. V. Parasyuk, V. V. Pavlyuk, O. Y. Khyzhun, V. R. Kozer, G. L. Myronchuk, V. P. Sachanyuk, G. S. Dmytriv, A. Krymus, I. V. Kityk, A. M. El-Naggar, A. A. Albassam, M. Piasecki, RSC Adv.2016, 6, 90958.10.1039/C6RA19558JSuche in Google Scholar
[19] V. P. Sachanyuk, I. D. Olekseyuk, O. V. Parasyuk, J. Alloys Compd.2007, 443, 61.10.1016/j.jallcom.2006.09.131Suche in Google Scholar
[20] G. E. Davydyuk, O. Y. Khyzhun, A. H. Reshak, H. Kamarudin, G. L. Myronchuk, S. P. Danylchuk, A. O. Fedorchuk, L. V. Piskach, M. Y. Mozolyuk, O. V. Parasyuk, Phys. Chem. Chem. Phys.2013, 15, 6965.10.1039/c3cp50836fSuche in Google Scholar PubMed
[21] B. Eisenmann, A. Hofmann, Z. Kristallogr.1991, 195, 318.10.1524/zkri.1991.195.3-4.318Suche in Google Scholar
[22] P. Capper, P. Rudolph (Eds.), Crystal Growth Technology. Semiconductors and Dielectrics, Wiley-VCH, Weinheim, 2011.10.1002/9783527632879Suche in Google Scholar
[23] L. J. Farrugia, J. Appl. Crystallogr.1999, 32, 837.10.1107/S0021889899006020Suche in Google Scholar
[24] A. L. Spek, J. Appl. Crystallogr.2003, 36, 7.10.1107/S0021889802022112Suche in Google Scholar
[25] M. C. Burla, R. Caliandro, B. Carrozzini, G. L. Cascarano, C. Cuocci, C. Giacovazzo, M. Mallamo, A. Mazzone, G. Polidori, J. Appl. Crystallogr.2015, 48, 306.10.1107/S1600576715001132Suche in Google Scholar
[26] G. M. Sheldrick, Acta Crystallogr.2015, C71, 3.Suche in Google Scholar
[27] K. Brandenburg, Diamond (version 2.1c), Crystal and Molecular Structure Visualization, Crystal Impact – H. Putz & K. Brandenburg GbR, Bonn (Germany) 1999.Suche in Google Scholar
[28] L. Akselrud, Y. Grin, WinCSD (version 4), Software Package for Crystallographic Calculations, Max-Planck-Institut für Chemische Physik fester Stoffe, Dresden (Germany) 2014. See also: L. Akselrud, Y. Grin, J. Appl. Crystallogr.2014, 47, 803.10.1107/S1600576714001058Suche in Google Scholar
[29] O. Jepsen, O. K. Andersen, The Stuttgart TB-LMTO-ASA programm (version 4.7), Max-Plank-Institut für Festkörperforschung, Stuttgart (Germany) 2000.Suche in Google Scholar
[30] O. Jepsen, O. K. Andersen, Z. Phys. B1995, 97, 35.10.1007/BF01317585Suche in Google Scholar
[31] W. R. Lambrecht, O. K. Andersen, Phys. Rev. B1986, 34, 2439.10.1103/PhysRevB.34.2439Suche in Google Scholar
[32] B. Cordero, V. Gomez, A. E. Platero-Prats, M. Reves, J. Echeverria, E. Cremades, F. Barragan, S. Alvarez, Dalton Trans.2008, 21, 2832.10.1039/b801115jSuche in Google Scholar PubMed
[33] J. Emsley, The Elements, 3rd ed., Clarendon Press, Oxford, 1998.Suche in Google Scholar
[34] J. Tauc, R. Grigorovici, A. Vancu, Phys. Status Solidi B1966, 15, 627.10.1002/pssb.19660150224Suche in Google Scholar
[35] W. Yin, K. Feng, R. He, D. Mei, Z. Lin, J. Yao, Y. Wu, Dalton Trans.2012, 41, 5653.10.1039/c2dt12493aSuche in Google Scholar PubMed
[36] X. Li, C. Li, P. Gong, Z. Lin, J. Yao, Y. Wu, J. Mater. Chem. C2015, 3, 10998.10.1039/C5TC02337HSuche in Google Scholar
©2020 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Laudatio/Preface
- Arndt Simon zum 80. Geburtstag gewidmet
- Research Articles
- High-temperature superconductors: underlying physics and applications
- Chemical resistance and chemical capacitance
- Investigations of the optical and electronic effects of silicon and indium co-doping on ZnO thin films deposited by spray pyrolysis
- Electrochemical synthesis of transition metal oxide nitrides with ε-TaN, δ-NbN and γ′-Mo2N structure type in a molten salt system
- Preferred selenium incorporation and unexpected interlayer bonding in the layered structure of Sb2Te3−xSex
- BOX A-type monopyrrolic heterocycles modified via the Suzuki-Miyaura cross-coupling reaction
- Cooperative activation of azides by an Al/N-based active Lewis pair – unexpected insertion of nitrogen atoms into C–Si bonds and formation of AlCN3 heterocycles
- Squares of gold atoms and linear infinite chains of Cd atoms as building units in the intermetallic phases REAu4Cd2 (RE=La–Nd, Sm) with YbAl4Mo2-type structure
- Synthesis and characterization of K5Sn2OF11
- Li vs. Zn substitution in Li17Si4 – Li17–ε–δZnεSi4 connecting the structures of Li21Si5 and Li17Si4
- Cs2Zn(CN)4: a first example of a non-cyano spinel of composition A2M(CN)4 with A=alkali metal and M=group 12 metal
- Magnetic and electronic properties of CaFeO2Cl
- A spatially separated [KBr6]5− anion in the cyanido-bridged uranium(IV) compound [U2(CN)3(NH3)14]5+[KBr6]5−·NH3
- Crystal structures of the tetrachloridoaluminates(III) of rubidium(I), silver(I), and lead(II)
- A new theoretical model for hexagonal ice, Ih(d), from first principles investigations
- Structural characterization and Raman spectrum of Cs[OCN]
- New cation-disordered quaternary selenides Tl2Ga2TtSe6 (Tt=Ge, Sn)
- The UV-phosphor strontium fluorooxoborate Sr[B5O7F3]:Eu
- Extending the knowledge on the quaternary rare earth nickel aluminum germanides of the RENiAl4Ge2 series (RE=Y, Sm, Gd–Tm, Lu) – structural, magnetic and NMR-spectroscopic investigations
- Structural diversity in Cd(NCS)2-3-cyanopyridine coordination compounds: synthesis, crystal structures and thermal properties
- Cation-anion pairs of niobium clusters of the type [Nb6Cl12(RCN)6][Nb6Cl18] (R=Et, nPr, iPr) with nitrile ligands RCN forming stabilizing inter-ionic contacts
- “Flat/steep band model” for superconductors containing Bi square nets
- La- and Lu-agardite – preparation, crystal structure, vibrational and magnetic properties
- A series of new layered lithium europium(II) oxoniobates(V) and -tantalates(V)
- The untypical high-pressure Zintl phase SrGe6
- A new ternary silicide GdFe1−xSi2 (x=0.32): preparation, crystal and electronic structure
- Lanthanide orthothiophosphates revisited: single-crystal X-ray, Raman, and DFT studies of TmPS4 and YbPS4
- A hexaniobate expanded by six [Hg(cyclam)]2+ complexes via Hg–O bonds yields a positively charged polyoxoniobate cluster
- Preliminary communication
- A hexaniobate expanded by six [Hg(cyclam)]2+ complexes via Hg–O bonds yields a positively charged polyoxoniobate cluster
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Laudatio/Preface
- Arndt Simon zum 80. Geburtstag gewidmet
- Research Articles
- High-temperature superconductors: underlying physics and applications
- Chemical resistance and chemical capacitance
- Investigations of the optical and electronic effects of silicon and indium co-doping on ZnO thin films deposited by spray pyrolysis
- Electrochemical synthesis of transition metal oxide nitrides with ε-TaN, δ-NbN and γ′-Mo2N structure type in a molten salt system
- Preferred selenium incorporation and unexpected interlayer bonding in the layered structure of Sb2Te3−xSex
- BOX A-type monopyrrolic heterocycles modified via the Suzuki-Miyaura cross-coupling reaction
- Cooperative activation of azides by an Al/N-based active Lewis pair – unexpected insertion of nitrogen atoms into C–Si bonds and formation of AlCN3 heterocycles
- Squares of gold atoms and linear infinite chains of Cd atoms as building units in the intermetallic phases REAu4Cd2 (RE=La–Nd, Sm) with YbAl4Mo2-type structure
- Synthesis and characterization of K5Sn2OF11
- Li vs. Zn substitution in Li17Si4 – Li17–ε–δZnεSi4 connecting the structures of Li21Si5 and Li17Si4
- Cs2Zn(CN)4: a first example of a non-cyano spinel of composition A2M(CN)4 with A=alkali metal and M=group 12 metal
- Magnetic and electronic properties of CaFeO2Cl
- A spatially separated [KBr6]5− anion in the cyanido-bridged uranium(IV) compound [U2(CN)3(NH3)14]5+[KBr6]5−·NH3
- Crystal structures of the tetrachloridoaluminates(III) of rubidium(I), silver(I), and lead(II)
- A new theoretical model for hexagonal ice, Ih(d), from first principles investigations
- Structural characterization and Raman spectrum of Cs[OCN]
- New cation-disordered quaternary selenides Tl2Ga2TtSe6 (Tt=Ge, Sn)
- The UV-phosphor strontium fluorooxoborate Sr[B5O7F3]:Eu
- Extending the knowledge on the quaternary rare earth nickel aluminum germanides of the RENiAl4Ge2 series (RE=Y, Sm, Gd–Tm, Lu) – structural, magnetic and NMR-spectroscopic investigations
- Structural diversity in Cd(NCS)2-3-cyanopyridine coordination compounds: synthesis, crystal structures and thermal properties
- Cation-anion pairs of niobium clusters of the type [Nb6Cl12(RCN)6][Nb6Cl18] (R=Et, nPr, iPr) with nitrile ligands RCN forming stabilizing inter-ionic contacts
- “Flat/steep band model” for superconductors containing Bi square nets
- La- and Lu-agardite – preparation, crystal structure, vibrational and magnetic properties
- A series of new layered lithium europium(II) oxoniobates(V) and -tantalates(V)
- The untypical high-pressure Zintl phase SrGe6
- A new ternary silicide GdFe1−xSi2 (x=0.32): preparation, crystal and electronic structure
- Lanthanide orthothiophosphates revisited: single-crystal X-ray, Raman, and DFT studies of TmPS4 and YbPS4
- A hexaniobate expanded by six [Hg(cyclam)]2+ complexes via Hg–O bonds yields a positively charged polyoxoniobate cluster
- Preliminary communication
- A hexaniobate expanded by six [Hg(cyclam)]2+ complexes via Hg–O bonds yields a positively charged polyoxoniobate cluster

