Abstract
Squaraines, two-fold condensation products in 1,3-position of squaric acid, represent dyes or pigments of high actuality. After their first boom in electrophotography diverse applications are presently studied in a wide area of research, which reaches from electrooptical materials to biosensors and compounds used in photodynamic therapy. Absorption and/or emission ranges in the NIR are mandatory for many of these techniques. The present article deals with stilbenoid squaraines, which feature an extended conjugation in their biradicaloid D-π-A-π-D structure. Due to the charge-transfer character of the excitation, boundaries are set for the optimal length of the conjugation. The absorption maxima of the stilbenoid squaraines and their aggregates are lying in chloroform as a solvent between 600 and 1000 nm. In the solid state panchromatic absorptions can be observed, which reach far into the NIR region. The facile preparation of squaraines bearing stilbene building blocks in one or two of their arms and moreover the easy access to dyes with multiple squaraine units fixed to stilbenoid scaffolds promise a wide palette of further applications in materials science.
1 Introduction
Soon after their first syntheses [1], [2] squaraines (1) reached a great significance as bright violet, blue or blue-green dyes or pigments [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]. Their primary boom in electrophotography (xerography) [7] was soon ensued by many diverse applications. The following, partly overlapping topics shall be outlined here:
Figure 1 shows the canonical representations of 1,3-diarylsquaraines (1). The name squaraine for such derivatives of squaric acid was first suggested by A. H. Schmidt [56]. These inner salts (betaines) can be described by resonance structures, which contain a positive charge in the four-membered ring and a negative charge on one of the exocyclic oxygen atoms. Within this article the core is drawn by a cross-conjugated cyclobutenediylium diolate structure bearing a +2 charge in the aromatic four-membered ring and a –1 charge on both exocyclic oxygen atoms. However, the delocalization of the positive charge into the lateral aryl substituents in 1,3-position is important for the stability of the squaraines. 1,3-Diphenylsquaraine for example is not sufficiently stable. At least one phenyl substituent R must have an electron-donating character.
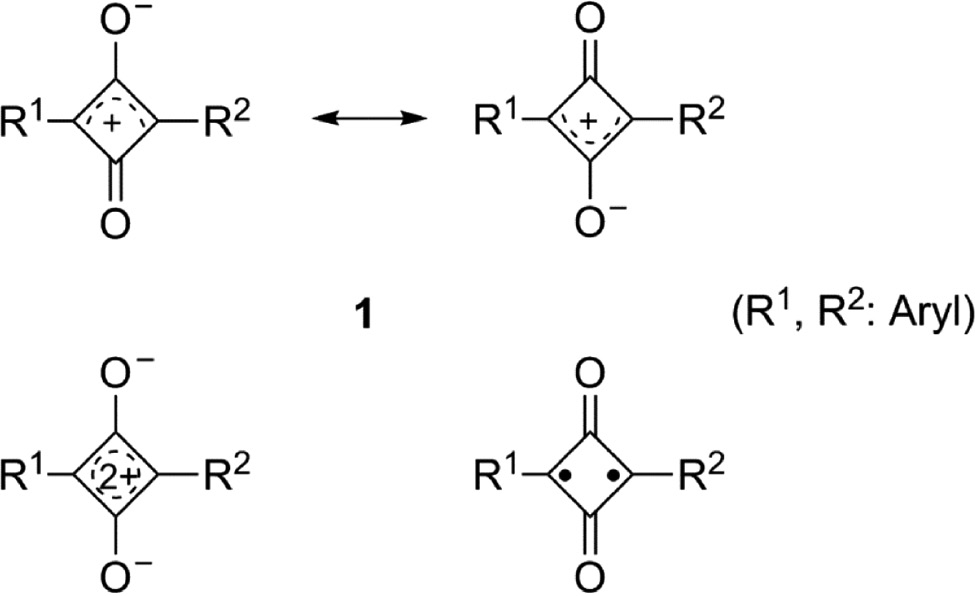
Canonical structures of 1,3-diarylsquaraines 1.
The singlet biradical structure of 1 (Fig. 1) was underestimated for a long time in favor of the quadrupolar donor-acceptor-donor (D-π-A-π-D) structure. The results of more recent calculations indicate a coupling between the mesoionic and the biradical form and assign a biradicaloid character to the 1,3-diarylsquaraines (1) [57], [58], [59], [60], [61].
1,3-Diarylsquaraines (1) exhibit an intense absorption of visible light. 1,3-Bis[4-(dimethylamino)phenyl]squaraine (1a) [2] represents a typical example.
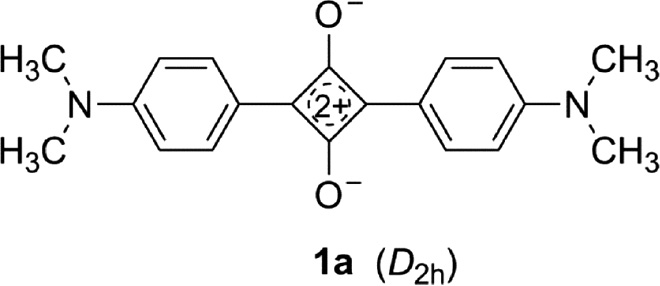
The strong, narrow long-wavelength absorption band (λmax=624 nm, εmax=105.43 L mol−1 cm−1 in CHCl3) [62] corresponds to a π→π* transition (HOMO→LUMO) which arises predominantly from the core (Fig. 2) [63], [64], [65]. The charge-transfer character of the band is small (qCT=0.25 e) as calculated by the SAC-CI method, which gives in this case better results than the TD-DFT method [57]. The first excited singlet state gives rise to a strong fluorescence (λmax=642 nm, quantum yield ϕF=0.70). The fluorescence lifetime amounts to 1.27 ns in CHCl3 at room temperature [64]. Embedded in PPMA, the absorption of the molecule becomes panchromatic with ϕF=0.65 [66].
The quadrupole character of 1a is responsible for the formation of aggregates [68], [69]. In many UV/Vis/NIR spectra of squaraines, absorptions of monomers, dimers and higher aggregates up to hexamers are superimposed.
Extension of the conjugation in these D-π-A-π-D systems should shift the absorption and fluorescence far into the NIR region. This argument is also valid for the biradical structure. An intense absorption and/or fluorescence in the NIR is mandatory for many applications of squaraines.
Vinylene or 1,4-phenylenevinylene segments in the lateral substituents (arms) seem to be suitable for this purpose. Thus, the structures 2–5 in Fig. 3 were conceived as target structures for dyes which absorb and fluoresce in the NIR. Stilbene units, which are present in the structures of type 2 and 3, are important building blocks in many compounds used in materials science [70], [71].
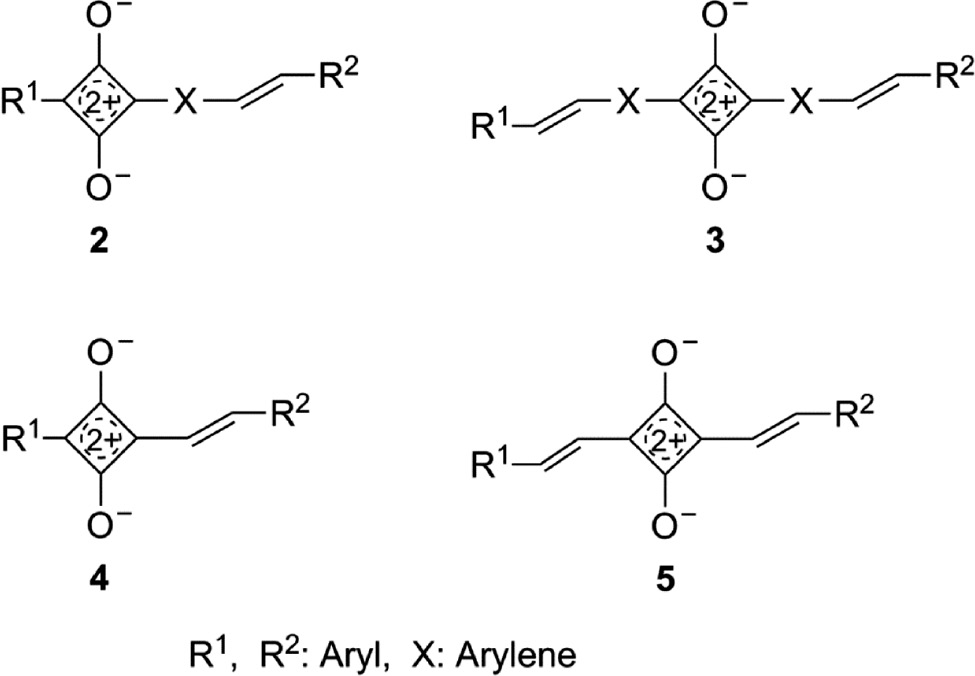
Possible extension of conjugation in 1,3-diarylsquaraines (1) to 1-aryl-3-stilbenylsquaraines (2), 1,3-distilbenylsquaraines (3) or 1-aryl-3-styrylsquaraines (4), and 1,3-distyrylsquaraines (5).
Whereas a large number of squaraines 2 and 3 were synthesized, only a single copper complex 4a [72] of a 1-aryl-3-styrylsquaraine (4) has been described and 1,3-distyrylsquaraines (5) were only included in theoretical studies [73].
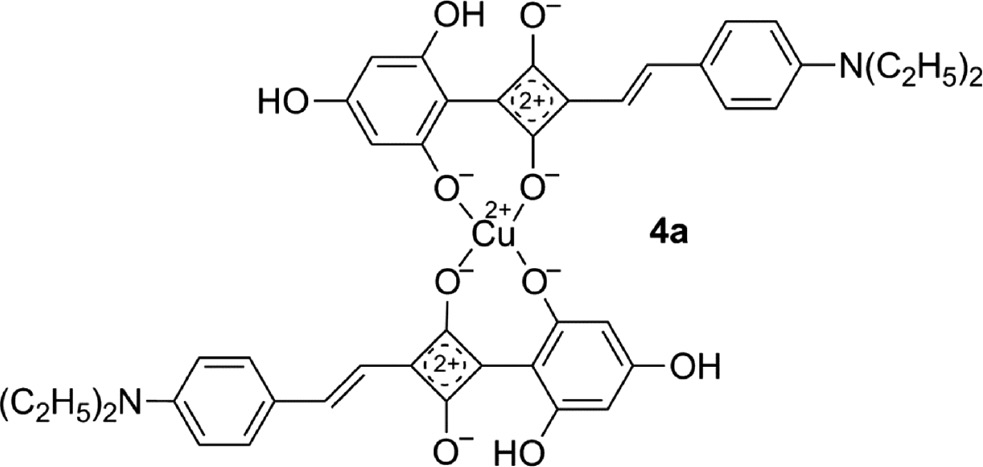
Nevertheless, many squaraines 4′ and 5′ were synthesized which bear one or two heterocyclic substituents, with R1 or R2 or both featuring thiophene, thiadiazole, thiatriazole, pyridine or condensed heterocyclic ring systems [9], [15], [74].
2 1-Aryl-3-stilbenylsquaraines
The preparation of 1-aryl-3-stilbenylsquaraines (2) is shown in Schemes 1 and 2 .
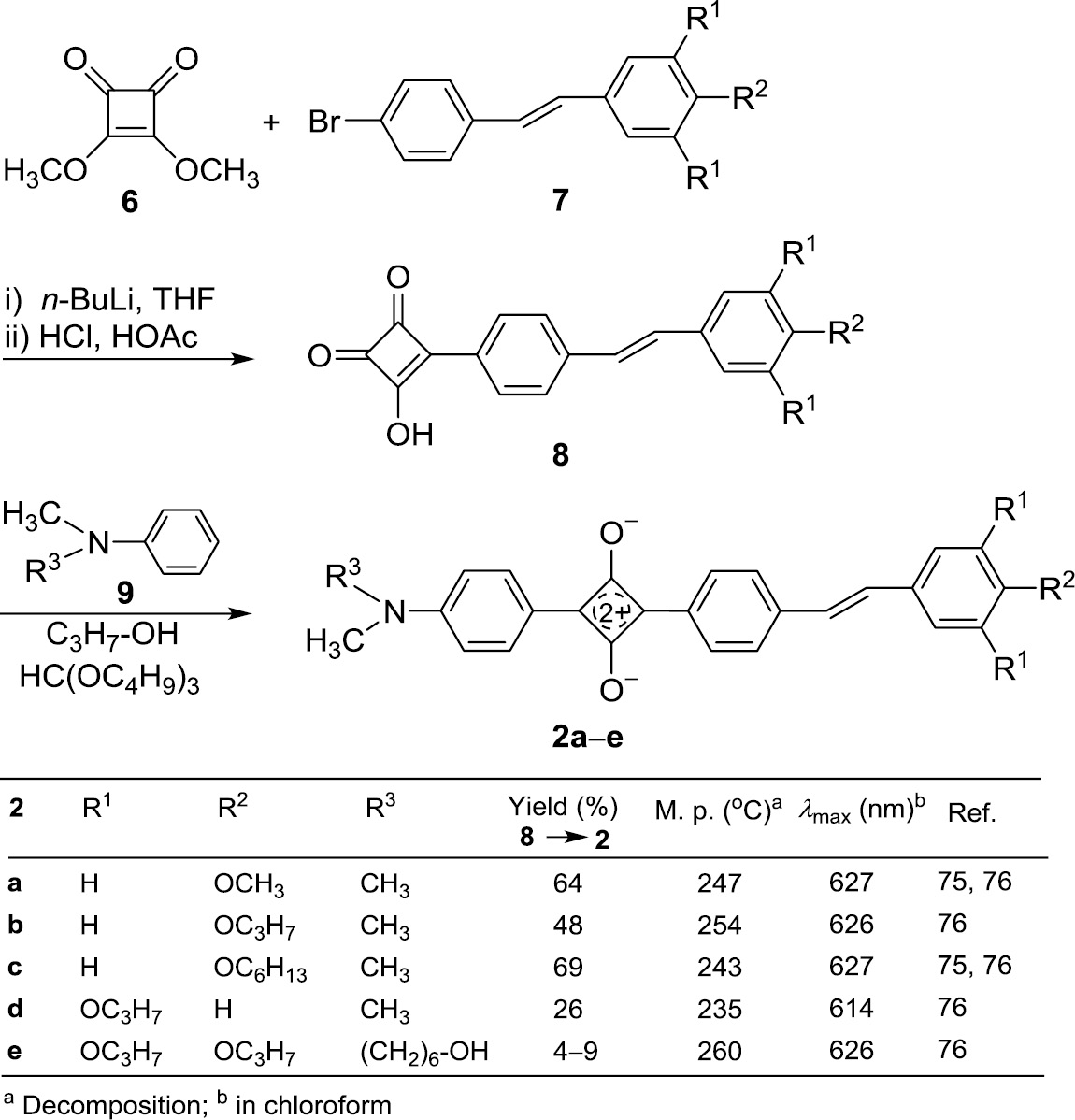
Preparation of the 1-alkyl-3-stilbenylsquaraines 2a–e.
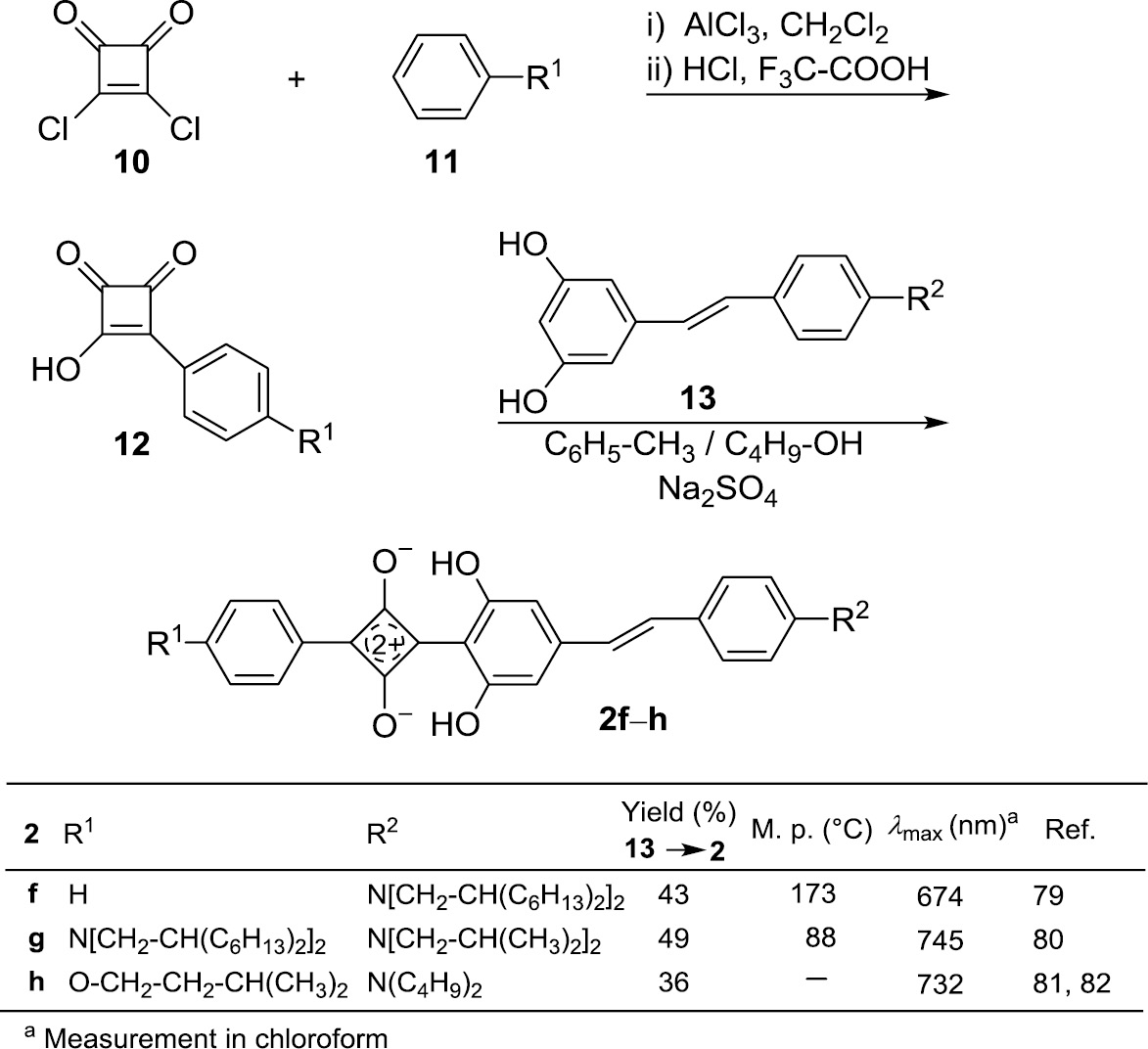
Preparation of the 1-aryl-3-stilbenylsquaraines 2f–h.
Squaric acid dimethyl ester (6) reacts with the trans-stilbene derivatives 7 to yield the semisquaric acids 8 (Scheme 1). In the first step bromine is exchanged by lithium and then a 1,4-addition of the organometallic compound to the enone 6 takes place. A spontaneous elimination of methanol and subsequent hydrolysis gives the semisquaric acid 8, which reacts as an electrophile in the aromatic substitution of the anilines 9. Due to electronic and steric effects the C-3 position of 8 proved to have the highest reactivity, so that the squaraines 2a–e are generated as the major products [75], [76]. The trans-configuration of 7 is preserved in the whole process.
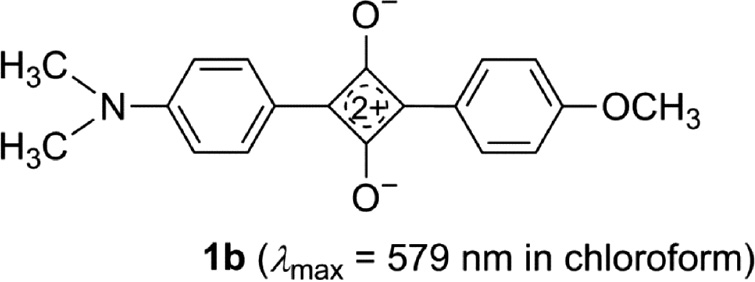
The squaraines 2a–e are high-melting compounds which are poorly soluble in organic solvents. Their absorption maxima in chloroform lie between 614 and 627 nm in the orange region of the visible light so that their solutions are green-blue. The comparison of 2a to the 1,3-diarylsquaraine 1b [77], [78], which both bear a dimethylamino and a methoxy group as electron-donating groups, reveals a bathochromic shift of 48 nm for the absorption maximum. This proves, that the effect of extension of conjugation is as predicted – even when the corresponding electronic transition is predominantly localized in the core [63], [64], [65].
An alternative synthetic route to 1-aryl-3-stilbenylsquaraines 2 is shown in Scheme 2. The Friedel-Crafts reaction of squaric acid dichloride (10) and the benzene derivatives 11 yields after hydrolysis the semisquaric acids 12, which can be coupled directly to the resorcinol systems 13. The two hydroxy groups of 13 enhance the nucleophilicity and establish hydrogen bonds in the resulting squaraines 2f–h [79], [80], [81], [82]. Stilbenes without or with just one hydroxy group do not react in this kind of condensation reactions. The mixed solvent 1-butanol/toluene forms an azeotrope with water, which allows to remove continuously the generated water, when the refluxing solvent was previously dried with Na2SO4 [77]. The trans-configuration of 13 is maintained in the products 2f–h. The squaraine 2h, prepared by Marder and coworkers [81], was the very first stilbenoid squaraine.
Due to the long and branched alkyl chains the melting points of 2f–h are much lower than those of 2a–e and their solubility in organic solvents is much better. The absorption maximum of 2f, which has an electron-releasing end group only on one side, lies at 674 nm, whereas 2g and 2h (with electron-donating substituents on both sides) exhibit absorption maxima at 745 and 732 nm, respectively.
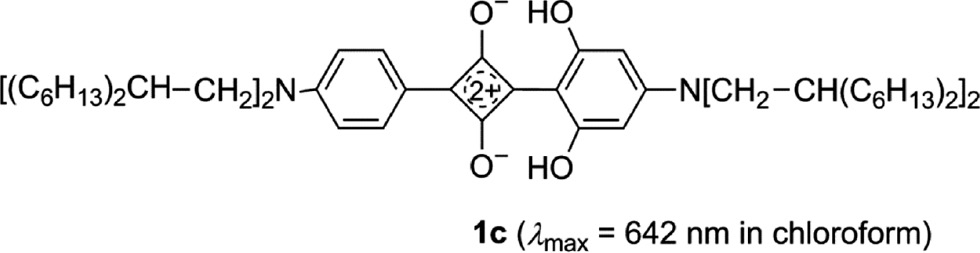
The comparison of the absorption of 2g to that of the related 1,3-diarylsquaraine 1c [80] reveals a strong red-shift of 103 nm to the long-wavelength end of the visible region.
In principle, the resorcinol structure of 2f–h enables a tautomeric equilibrium. However, a significant participation of a tautomeric form 2g′ could be ruled out for 2g (Scheme 3) on the basis of an NMR study of the 1H,13C spin couplings [67].
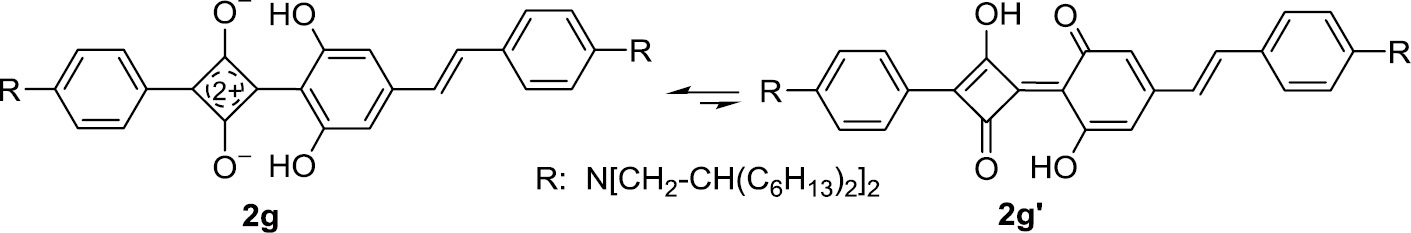
Resorcinol-derived squaraines such as 2g do not exist to a perceptible amount in the tautomeric form 2g′.
3 1,3-Distilbenylsquaraines
The direct coupling of semisquaric acids 12 and dihydroxystilbene 13, shown in Scheme 2 can be applied twice in the condensation reaction of squaric acid (14) and two molecules of 15. The yields of the reaction are low to moderate; however, the process is a very facile one-step reaction (Scheme 4), which is a great advantage in materials science [75], [80], [83], [84], [85], [86], [87], [88], [89], [90].
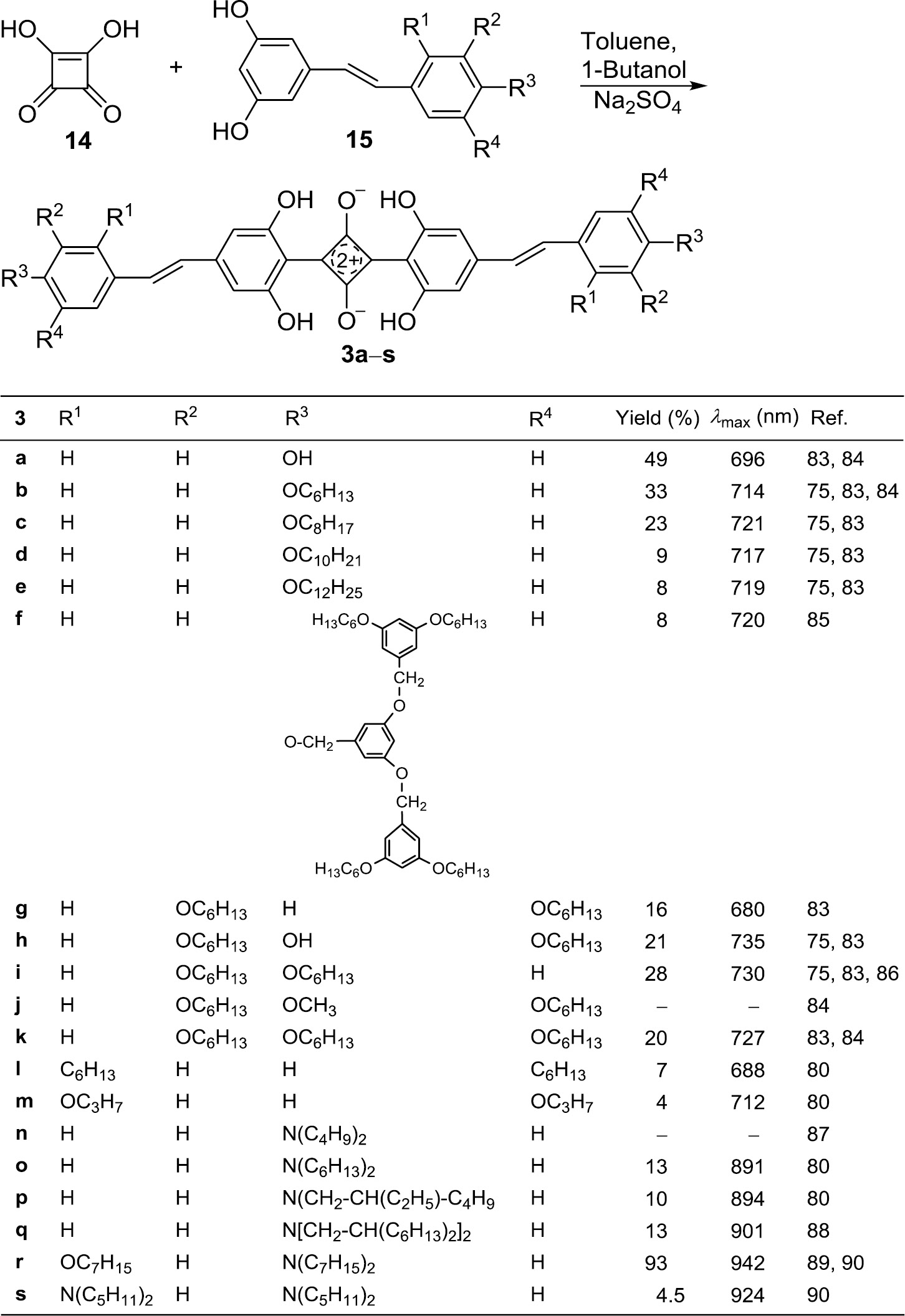
The 1,3-distilbenylsquaraines 3a–s form dark blue, blue-green or blue-violet crystals. Dissolved in chloroform they show an intense absorption with λmax between 680 and 950 nm. The higher the electron releasing tendency of the terminal substituents is, the more is the band shifted into the NIR region. The extension of the conjugation on both sides of the core has a dramatic effect. The comparison of for example 3q and 1d, which have identical donor groups, reveals a red-shift of 244 nm (in chloroform) [88].
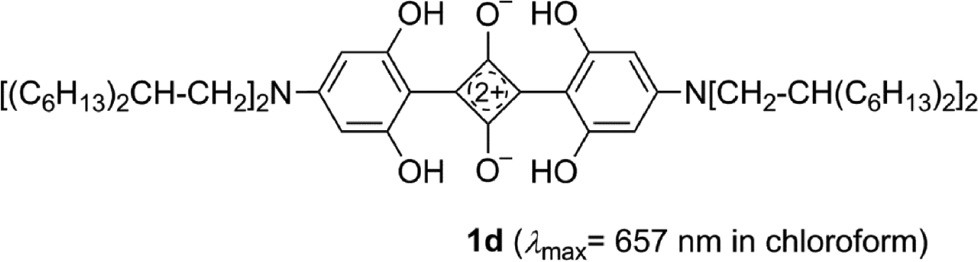
Preparation of the symmetrical 1,3-distilbenylsquaraines 3a–s.
The unsymmetrical 1,3-distilbenylsquaraines 3t–w can be obtained by the condensation reaction of dihydroxystilbenes 16 and stilbenylsemisquaric acids 17 (Scheme 5) [67], [75], [76]. The process resembles the synthesis of 2f–h (Scheme 2).
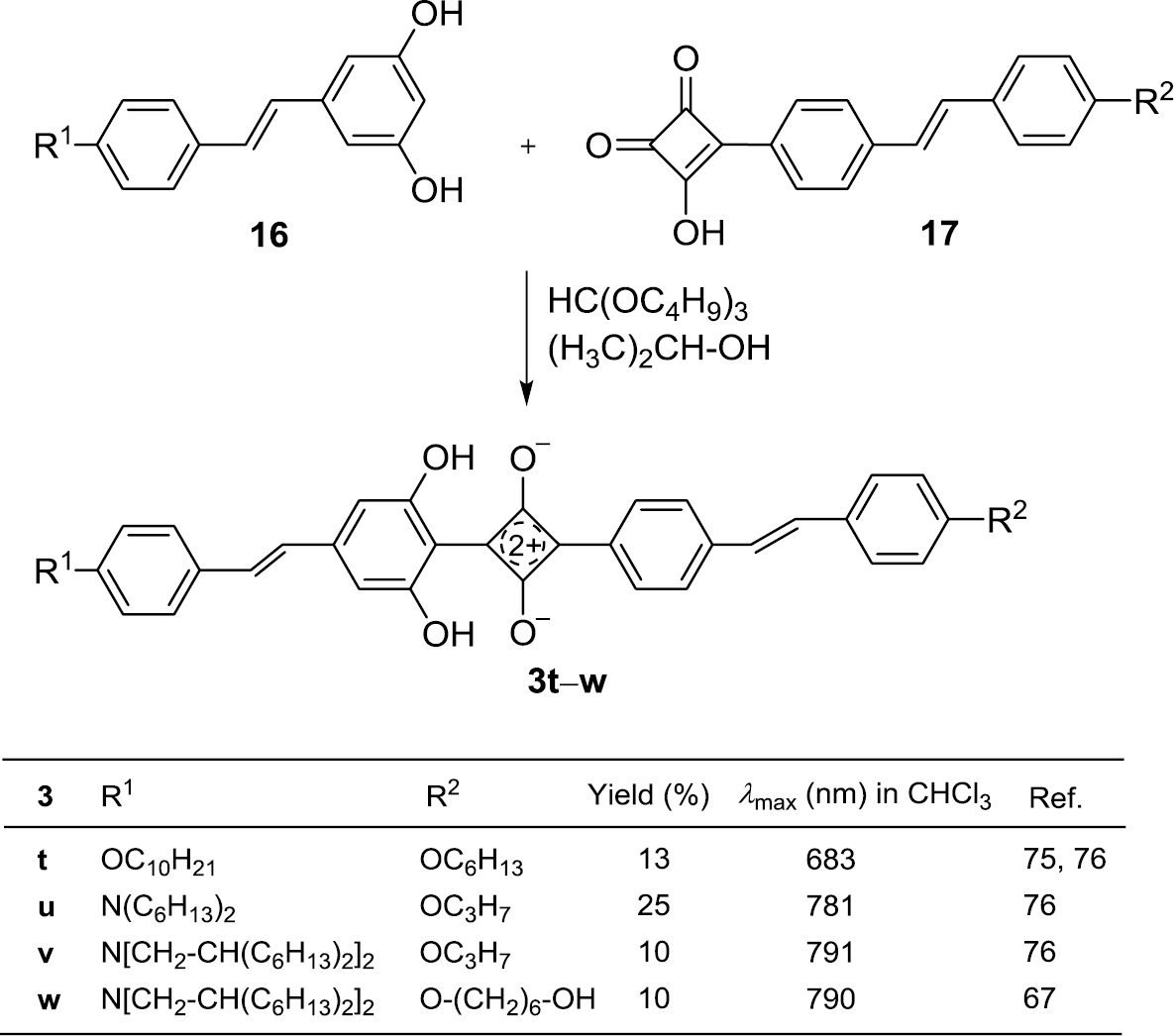
Preparation of the unsymmetrical 1,3-distilbenyl-squaraines 3t–w.
4 Multiple squaraine chromophores attached to stilbenoid scaffolds
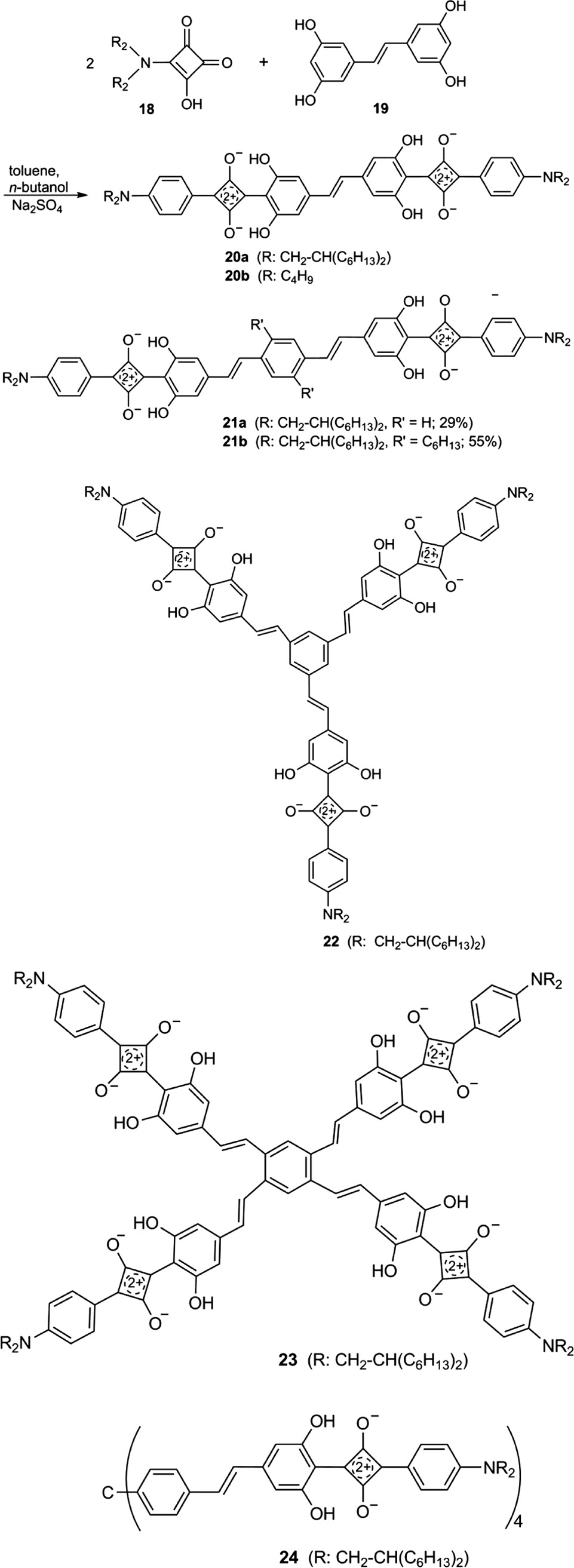
Multiple squaraine dyes can be obtained by two-, three- or fourfold coupling reactions of semisquaric acid 18 with stilbenoid resorcinol derivatives [79]. The reaction conditions are identical to those in Schemes 2 and 4. Bis(2-hexyloctyl)amino groups were used as electron donating and solubilizing substituents. Scheme 6 depicts the reaction of 18 with tetrahydroxystilbene 19 to yield 20a (22%) [79]. Analogous reactions gave 20b [81] and 21a (29%) [79], 21b (55%) [79], 22 (49%) [79], 23 (20%) [79] and 24 (35%) [79]. Dye 20b was studied for an application in display front filters [72], [91].
Table 1 summarizes absorption and emission data of the multiple squaraines 20–24 in comparison to the monosquaraine 2f.
Preparation of multiple squaraine dyes fixed to stilbenoid scaffolds.
Vis/NIR spectroscopic data of the multiple squaraines 20–24 and of the model compound 2f (measurements in chloroform).
| Dye | Number of squaraine units | Absorption λmax (nm) | 10−5 εmax (L mol−1 cm−1) | Fluorescence λmax (nm) |
|---|---|---|---|---|
| 2f | 1 | 674 | 2.70 | 702 |
| 20a | 2 | 778 | 2.58 | 798 |
| 21a | 2 | 724 | 4.56 | 750 |
| 21b | 2 | 720 | 3.34 | 761 |
| 22 | 3 | 693 | 8.11 | 714 |
| 23 | 4 | 707 | 3.93 | 753 |
| 24 | 4 | 687 | 7.23 | 702 |
The major goal of the investigation of the multiple squaraines was the enhancement of the absorbance. The absorption bands of 20–24 have very different εmax values (Table 1) and very different widths at half height between 552 and 1334 cm−1 [79]. Together with the different aggregation tendency, a quantitative comparison with 2f is difficult. However, the statement that the absorption intensities per squaraine unit of 21a, 21b, 22, 23 and 24 are much higher than that of the model compound 2f is certainly correct [79]. Conjugation, cross-conjugation and even homoconjugation of squaraine building blocks have obviously an effect on the absorption intensity per squaraine unit.
5 Oligophenylenevinylene (OPV) chains for the extension of conjugation in squaraine dyes
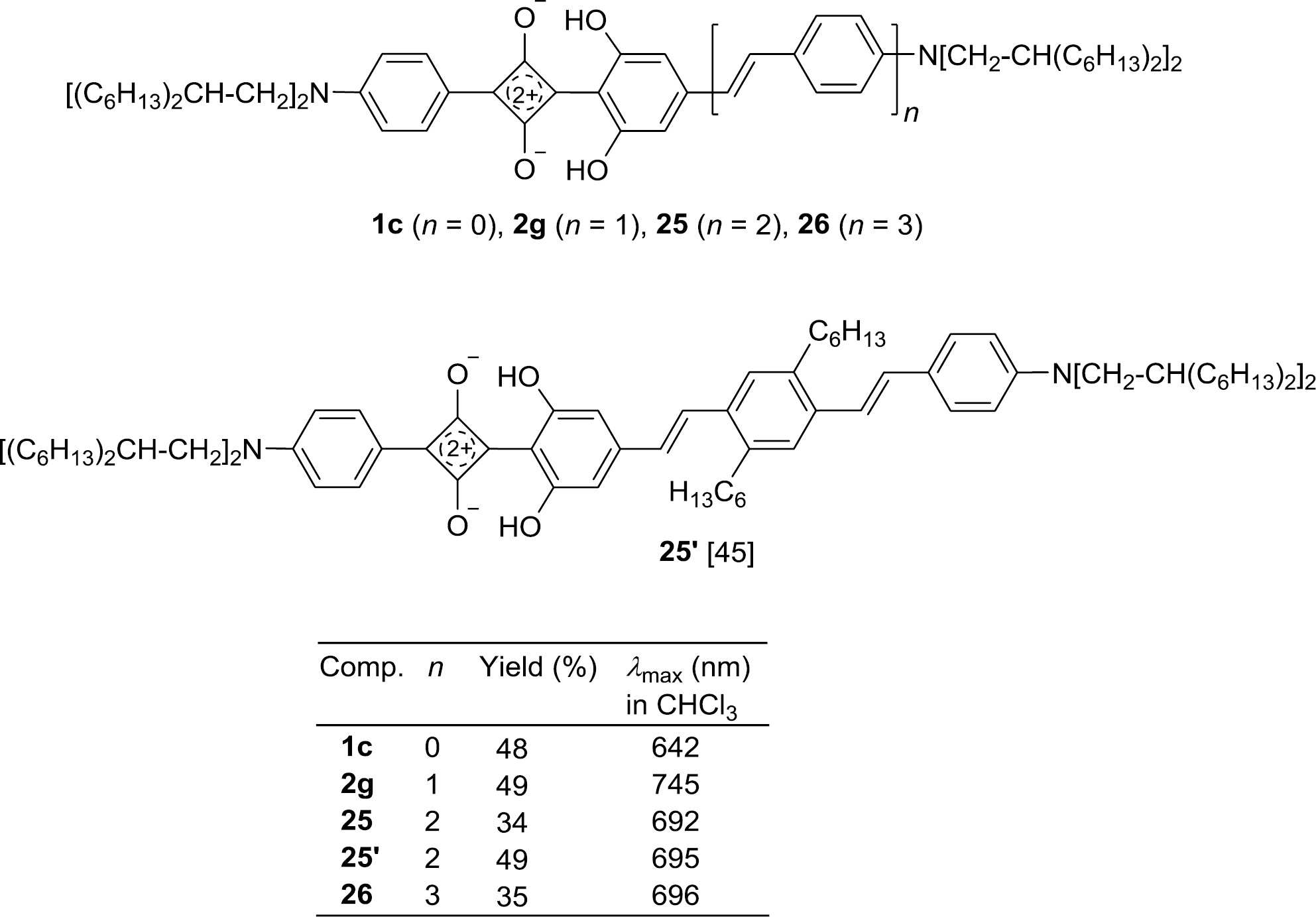
The bathochromic shift observed for the absorptions of 1,3-diarylsquaraines, whose conjugation was extended by styryl building blocks, stimulated activities to study the incorporation of a larger number of 1,4-phenylenevinylene segments. According to the procedure discussed in Scheme 2 the series 1c, 2g, 25, and 26 (Fig. 4) was synthesized by condensation reactions of the corresponding semisquaric acid and a resorcinol of the OPV type [88]. The solubility of the resulting squaraines could be improved by amino groups which bear 2-hexyloctyl substituents [88].
1,3-Diarylsquaraines with OPV chains on one side, and the model compound 1c.
Series of squaraines bearing OPV chains on both sides, and the model compounds 1d and 1e.
The absorption maximum is bathochromically shifted on going from n=0 to n=1 (Δλ=103 nm), but then a saturation tendency can be observed for higher n values; λmax reaches a limiting value of 694±2 nm (n=2, 3).
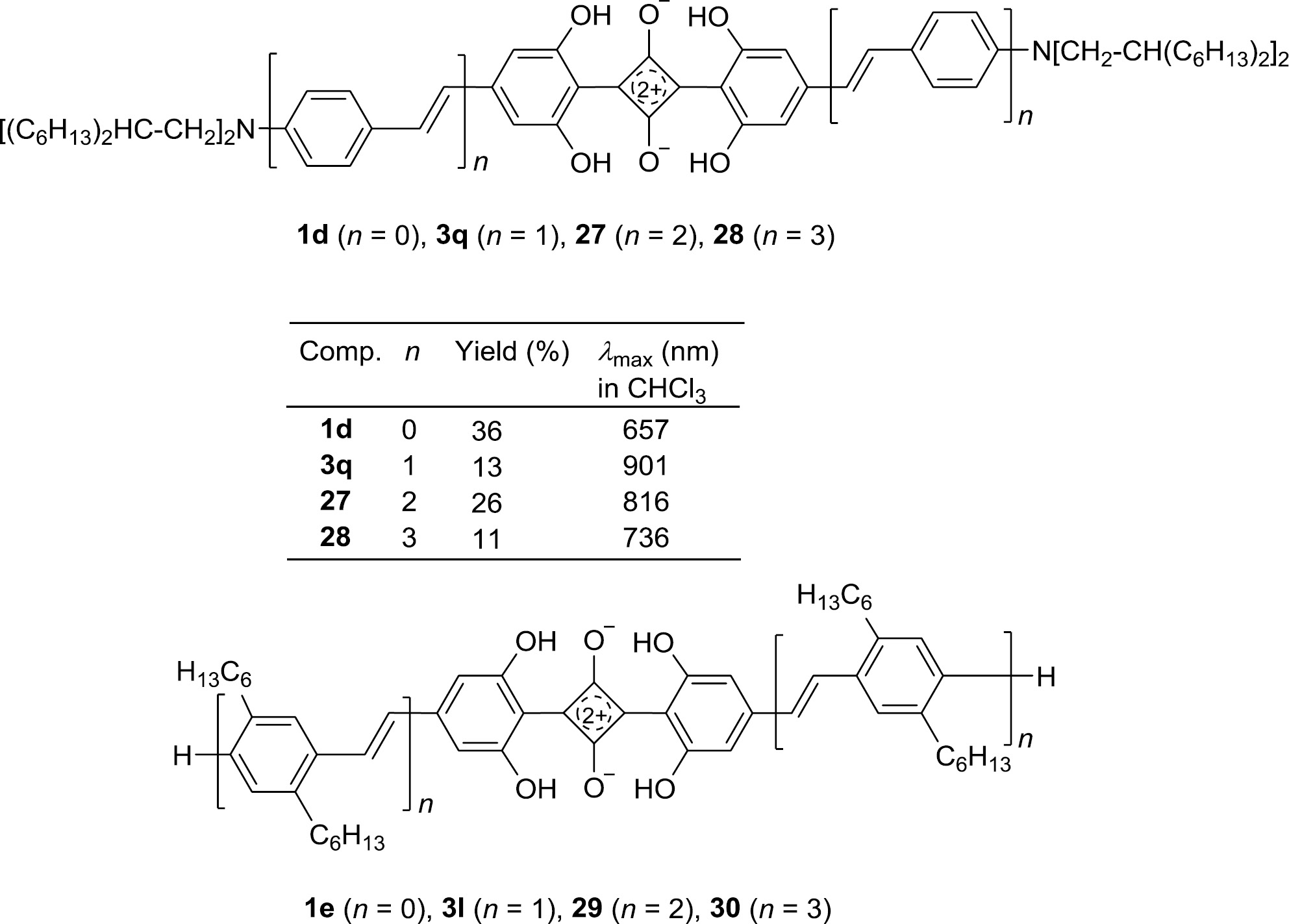
The symmetrical series 1d, 3q, 27 and 28 (n=0–3) with OPV building blocks on both sides was prepared according to Scheme 4 from squaric acid and the corresponding resorcinol derivatives [88]. An analogous process led to the related series 3l, 29 and 30 (n=1–3) without amino substituents as terminal donor groups [80]. The first member 1e (n=0) of this series is still unknown. The different absorption behavior of the two series is interpreted in Section 6.
6 Spectroscopic characterization of stilbenoid squaraines
The distribution of the electron density of the squaraines is characteristically reflected in the 13C chemical shifts of their core units. Whereas the CO groups (C-2 and C-4) of the four-membered rings have fairly constant δ values of 183±2 ppm, the chemical shifts of C-1 and C-3 depend strongly on the nature of the substituents Ri in these positions and vary between 160 and 190 ppm. The higher the partial positive charge in C-1 or C-3 is, the higher is the corresponding δ (13C) value. The different types of squaraines discussed in this article and their 13C chemical shifts are listed in Table 2.
13C Chemical shifts (δ values in ppm) of C-1 and C-3 in the squaraine rings bearing the substituents Ri (i=1–3); measurements in CDCl3).
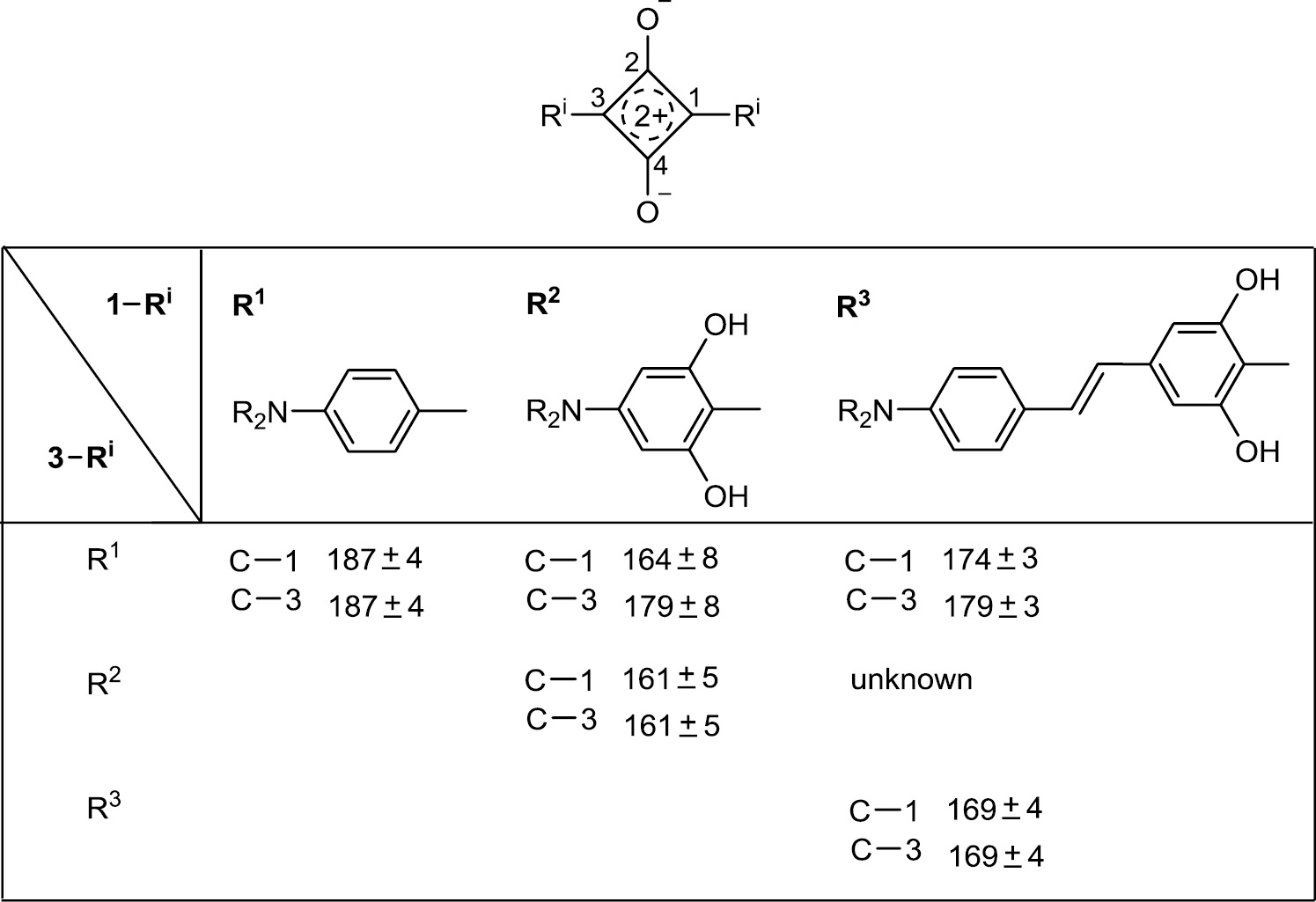 |
The substituents R2 take over more of the positive charge of the squaraine core than R3, and much more than R1.
The distribution of the electron density along the OPV chain can be traced by the 1H and 13C chemical shifts [92]. Figure 6 shows the comparison of the 13C NMR data of squaraine 3q and its precursor 15q for the positions of partial positive charges in 3q. Down-field shifts can be observed for all these positions. The most pronounced effect is found for the phenylene carbon atom in p-position to the squaraine ring (Δδ=154.1–141.2=12.9 ppm) and for the olefinic β carbon atom (Δδ=137.9–129.8=8.1 ppm).
![Fig. 6: Comparison of the 13C chemical shifts of the 1,3-distil-benylsquaraine 3q and the corresponding resorcinol 15q for the positions bearing a partial positive charge in 3q (measurements in CDCl3 at room temperature) [88].](/document/doi/10.1515/znb-2018-0260/asset/graphic/j_znb-2018-0260_fig_006.jpg)
Comparison of the 13C chemical shifts of the 1,3-distil-benylsquaraine 3q and the corresponding resorcinol 15q for the positions bearing a partial positive charge in 3q (measurements in CDCl3 at room temperature) [88].
Orientated at the various potential applications of the squaraine compounds, their most important spectroscopic property concerns the UV/Vis/NIR absorption and emission. Figure 7a shows as an example the spectrum of 1,3-distilbenylsquaraine 3i in CHCl3 [83]. The long-wavelength band of 3i is relatively broad and has its maximum at 730 nm. The band arises from the superposition of monomer and aggregate absorptions. The monomer 3i gives a fluorescence band with a maximum at 787 nm and an excitation spectrum with a maximum at 753 nm. The difference between absorption and excitation spectrum results from H-aggregates of 3i which do not fluoresce.
![Fig. 7: (a) UV/Vis/NIR spectrum of 1,3-bis(4-{2-[3,4-bis(hexyloxy)-phenyl]ethenyl}-2,6-dihydroxyphenyl)squaraine (3i) in CHCl3. The insert exhibits the fluorescence and the excitation spectrum in CHCl3; (b) solid-state reflection measurement of a dispersion of 3i in a not specified silane matrix.](/document/doi/10.1515/znb-2018-0260/asset/graphic/j_znb-2018-0260_fig_007.jpg)
(a) UV/Vis/NIR spectrum of 1,3-bis(4-{2-[3,4-bis(hexyloxy)-phenyl]ethenyl}-2,6-dihydroxyphenyl)squaraine (3i) in CHCl3. The insert exhibits the fluorescence and the excitation spectrum in CHCl3; (b) solid-state reflection measurement of a dispersion of 3i in a not specified silane matrix.
The intermolecular interactions of 3i are even more obvious in the solid state spectrum (Fig. 7b), which shows a panchromatic absorption from the UV far into the NIR region [83].
The long-wavelength bands of squaraines are in many cases a superposition of monomer and aggregate absorptions because squaraines have a high tendency to form H- and J-aggregates [68], [69], [92]. The individual squaraine structure, the solvent, the concentration and the temperature determine the type of aggregate and its stability. Figure 8 shows the absorption of 1,3-distilbenylsquaraine 3o [85] dissolved in n-heptane at a total concentration of about 10−5 mol L−1. At least four absorbing species can be recognized. Addition of dichloromethane leads to a strong band, whose maximum is red-shifted by 140 nm. It can be assigned to the monomer, because highly diluted solutions in n-heptane (c≤10−6 mol L−1) show only this band.
![Fig. 8: UV/Vis/NIR spectrum of 1,3-bis{4-[2-(4-dihexylaminophenyl) ethenyl]-2,6-dihydroxyphenyl}squaraine (3o) in an apolar and a partly polar solvent.](/document/doi/10.1515/znb-2018-0260/asset/graphic/j_znb-2018-0260_fig_008.jpg)
UV/Vis/NIR spectrum of 1,3-bis{4-[2-(4-dihexylaminophenyl) ethenyl]-2,6-dihydroxyphenyl}squaraine (3o) in an apolar and a partly polar solvent.
The conclusion has been supported by a temperature-dependant measurement in acetonitrile (Fig. 9). Raising the temperature from 35 to 60°C leads to the decrease of absorptions of H- and J-aggregates and to a strong increase of the absorption of the monomer at λmax to 815 nm [85].
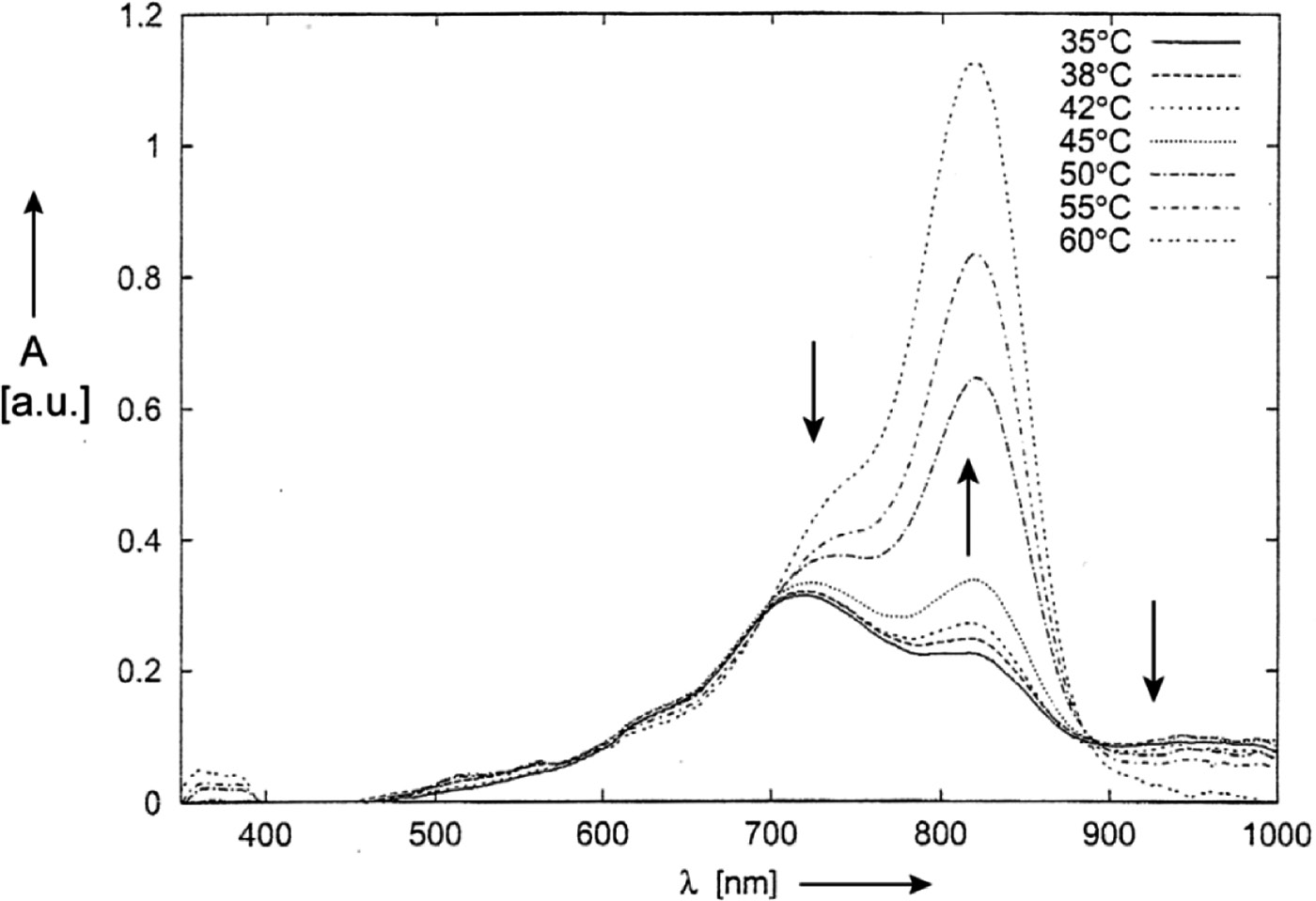
UV/Vis/NIR absorption spectra of 3o in acetonitrile at different temperatures.
The solvent dependence of the absorption of 1,3-distilbenylsquaraine 3q [85] at room temperature is presented in Table 3: λmax varies between 835 and 909 nm. The correlation of λmax with the solvent parameters ET (30) [93] is very crude (Fig. 10) [74], [85]. Different aggregates in equilibria with the monomers exist in solvents with different polarity. Obviously, chlorine containing solvents (numbers 5, 7 and 8 of the list) give rise to absorption maxima at particularly low wavenumbers (large wavelengths). Solvents with a high nucleophilicity can attack on the squaraine core and destroy the dye.
![Fig. 10: Correlation of the absorption maximum of 1,3-bis[4-(2-{4-[bis(2-hexyloctyl)amino]phenyl}ethenyl)-2,6-dihydroxyphenyl]squaraine (3q) in nine different solvents (see Table 3) with the ET(30) parameters.](/document/doi/10.1515/znb-2018-0260/asset/graphic/j_znb-2018-0260_fig_010.jpg)
Correlation of the absorption maximum of 1,3-bis[4-(2-{4-[bis(2-hexyloctyl)amino]phenyl}ethenyl)-2,6-dihydroxyphenyl]squaraine (3q) in nine different solvents (see Table 3) with the ET(30) parameters.
Solvent dependence of the UV/Vis/NIR absorption of 1,3-distilbenyl-squaraine 3q.
| Solvent | ET (30) (kcal mol−1) | Absorption λmax (nm) | max (cm−1) |
|---|---|---|---|
| 1 Cyclohexane | 30.9 | 843 | 11,862 |
| 2 n-Heptane | 31.3 | 835 | 11,976 |
| 3 Toluene | 33.9 | 846 | 11,820 |
| 4 1,4-Dioxane | 36.0 | 848 | 11,792 |
| 5 1,1,1-Trichloroethane | 36.2 | 894 | 11,186 |
| 6 Tetrahydrofuran | 37.4 | 879 | 11,377 |
| 7 Chloroform | 39.1 | 901 | 11,099 |
| 8 Dichloromethane | 40.7 | 909 | 11,001 |
| 9 Acetone | 42.2 | 890 | 11,236 |
The UV/Vis/NIR absorption of squaraines bearing OVP chains deserves special attention. The wavelengths of the absorption maxima exhibit first a bathochromic shift for the extension of conjugation (n: 0→1) in the unsymmetrical series 1c, 2g, 25, 26 (Fig. 4) as well as in the symmetrical series 1d, 3q, 27, 28 (Fig. 5). However, further extension of the conjugation (n: 1→2, 3) leads then to a hypsochromic shift in both series. This effect represents a typical behavior of series of conjugated oligomers bearing very strong terminal donor and acceptor groups [70]. The amount of intramolecular charge transfer (ICT) decreases when the distance between D and A becomes larger and the increasing length of the conjugated arm A-π-D cannot compensate that. Increasing conjugation and decreasing ICT have an opposite effect on λmax in organic solvents (Fig. 11). When the terminal amino groups are protonated, they lose their donor character. The original structure D-π-A-π-D adopts an A-π-A-π-A character, and a continuously bathochromic shift to a limiting value for high numbers n can be observed in this series [70].
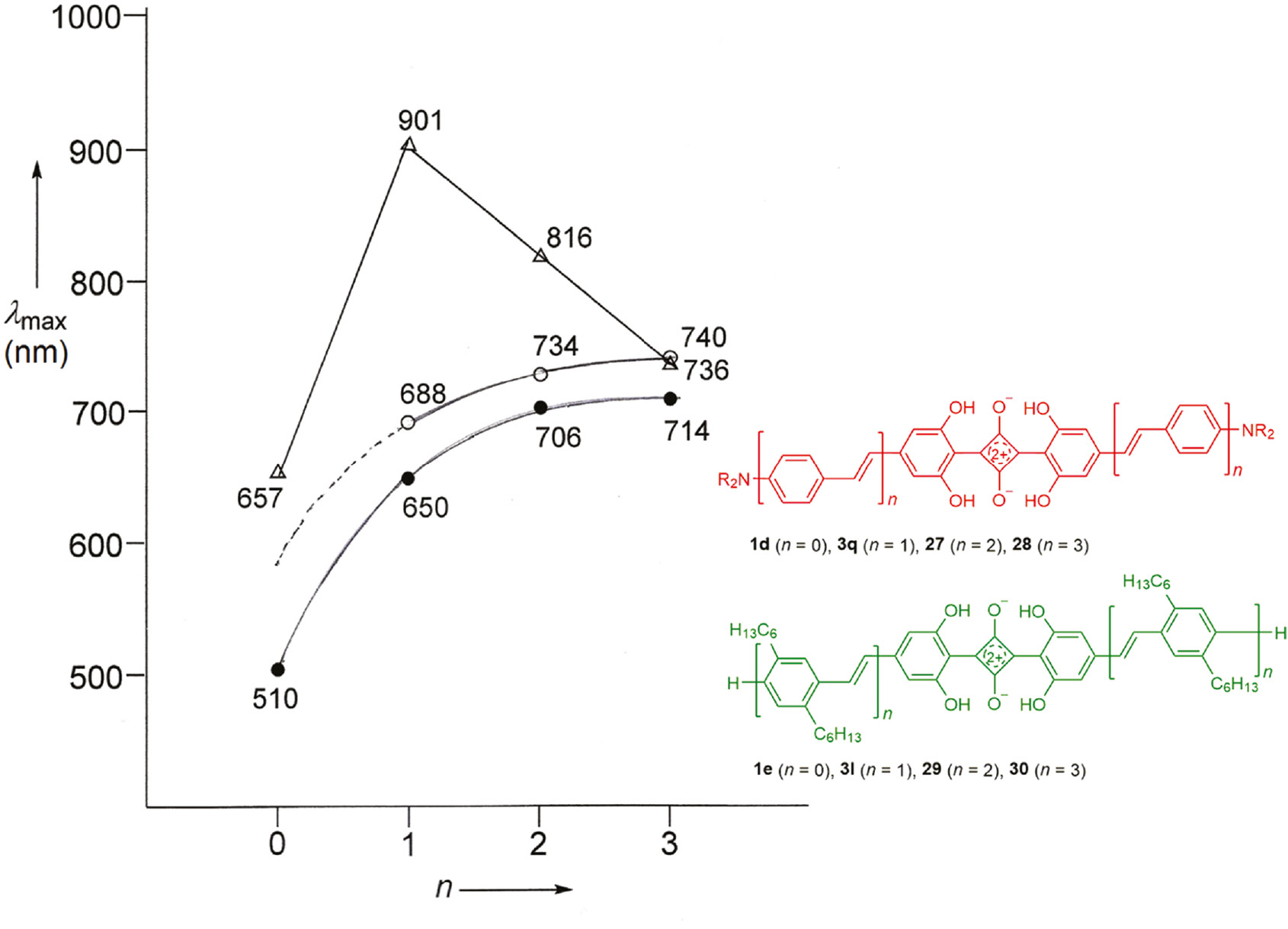
Absorption maxima of different squaraine series: Δ: 1d, 3q, 27, 28 (n=0–3) in CHCl3; : 1d·H+, 3q·H+, 27·H+, 28·H+ (n=0–3) in CHCl3-CF3COOH; ο: 3l, 29, 30 (n=1–3) in CHCl3.
The squaraine series 1e, 3l, 29, 30 has OPV chains without terminal donor groups and behaves as observed for many series of conjugated oligomers, and also for the protonated series introduced above: an increasing number n gives rise to a bathochromic shift increasing to a limiting value λ∞. The first member of the series, 1,3-bis(2,6-dihydroxyphenyl)squaraine (1e) is an unknown compound. On the basis of the data for the isomeric compound 1,3-bis(2,4-dihydroxyphenyl)squaraine (λmax=567 nm) and for 1,3-bis(2,4,6-trihydroxyphenyl)squaraine (λmax=563 nm) a λmax value of 565±5 nm can be estimated for 1e and its aggregates [69], [94].
7 Conclusion
Squaraines have the character of biradicaloid donor-acceptor-donor (D-π-A-π-D) compounds, whose absorption and emission can be bathochromically shifted into the NIR region, when the π-conjugation in one or both branches A-π-D is extended from phenyl to stilbenyl groups. Further extension to oligo(1,4-phenylenevinylene) chains (OPV) has the contrary effect for systems bearing strong terminal donor groups, because the intramolecular charge-transfer (ICT) between donor D and acceptor A is impaired by the increasing distance of A and D.
For optimum performance, trans-stilbene building blocks, present e. g. in 2,6-dihydroxy-4-[2-(4-dialkylaminophenyl)-ethenyl]phen-1-yl groups, are best suited, because the hydroxyl groups guarantee a more perfect planarity by intramolecular hydrogen bonds and because they carry additional electron donor groups. Amino groups bearing long branched alkyl chains such as 2-hexyloctyl ensure a fairly good solubility in di- or trichloromethane.
The absorption intensity of such squaraine dyes and their often present H- and/or J-aggregates is high and can still be enhanced, when two to four arylsquaraine units are attached to give conjugated, cross-conjugated or homo-conjugated stilbenoid scaffolds.
All the squaraines presented in this article can be readily synthesized (Schemes 1, 2, 4–6). The series of dihydroxyphenyl compounds for example can be obtained by the direct condensation reaction of a dihydroxystilbene derivative and squaric acid. The easy access together with the strong absorption and fluorescence in the Vis/NIR region renders the stilbenoid squaraine dyes promising candidates for many applications in materials science.
References
[1] A. Treibs, K. Jacob, Angew. Chem., Int. Ed. Engl.1965, 4, 694.10.1002/anie.196506941Search in Google Scholar
[2] W. Ziegenbein, H. E. Sprenger, Angew. Chem., Int. Ed. Engl. 1966, 5, 893.10.1002/anie.196608932Search in Google Scholar
[3] A. H. Schmidt in Oxocarbons, (Ed.: R. West), Academic Press, New York, 1980, p. 185.10.1016/B978-0-12-744580-9.50014-2Search in Google Scholar
[4] S. Das, K. G. Thomas, M. V. George, Mol. Supramol. Photochem.1997, 1, 467.10.1201/b16837-11Search in Google Scholar
[5] K.-Y. Law, Mol. Supramol. Photochem. 1997, 1, 519.10.1201/b16837-13Search in Google Scholar
[6] A. Ajayaghosh, Acc. Chem. Res. 2005, 38, 449.10.1021/ar0401000Search in Google Scholar PubMed
[7] B. Halton, Chem. N. Z.2008, 72, 57.10.3917/lig.721.0057Search in Google Scholar
[8] Z. Chen, A. Lohr, C. R. Saha-Möller, F. Würthner, Chem. Soc. Rev. 2009, 38, 564.10.1039/B809359HSearch in Google Scholar PubMed
[9] L. Beverina, P. Salice, Eur. J. Org. Chem. 2010, 2010, 1207.10.1002/ejoc.200901297Search in Google Scholar
[10] L. D. Patsenker, A. L. Tatarets, O. P. Klochko, E. A. Terpetschnig, Springer Ser. Fluoresc. 2010, 9, 159.10.1007/978-3-642-04701-5_5Search in Google Scholar
[11] L. D. Patsenker, A. L. Tatarets, E. A. Terpetschnig, Springer Ser. Fluoresc. 2010, 8, 65.10.1007/978-3-642-04702-2_3Search in Google Scholar
[12] R. R. Avirah, D. T. Jayaram, N. Adarsh, D. Ramalah, Org. Biomol. Chem. 2012, 10, 911.10.1039/C1OB06588BSearch in Google Scholar PubMed
[13] L. Yuan, W. Lin, K. Zheng, L. He, W. Huang, Chem. Soc, Rev.2013, 42, 622.10.1039/C2CS35313JSearch in Google Scholar
[14] J. Roncali, P. Leriche, P. Blanchard, Adv. Mater.2014, 26, 3821.10.1002/adma.201305999Search in Google Scholar PubMed
[15] L. Beverina, M. Sassi, Synlett2014, 25, 477.10.1055/s-0033-1340482Search in Google Scholar
[16] M. Gsänger, D. Bialas, L. Huang, M. Stolte, F. Würthner, Adv. Mater.2016, 28, 3615.10.1002/adma.201505440Search in Google Scholar PubMed
[17] A. Broggi, H. Kim, J. Jung, M. P. Bracciale, M. L. Santarelli, C. Kim, A. Marrocchi, Macromol. Chem. Phys. 2017, 218, 1600487.10.1002/macp.201600487Search in Google Scholar
[18] H.-W. Lin, Y.-C. Shiau, U.S. 9871217 B1 20180116, 2018.Search in Google Scholar
[19] V. Maltese, S. Cospito, A. Beneduci, B. C. De Simone, N. Russo, G. Chidichimo, R. A. J. Janssen, Chem. Eur. J.2016, 22, 10179.10.1002/chem.201601281Search in Google Scholar PubMed
[20] Q. Xiao, S. Yang, R. Wang, Y. Zhang, H. Zhang, H. Zhou, Z. Li, Dyes Pigm.2018, 154, 137.10.1016/j.dyepig.2018.03.003Search in Google Scholar
[21] Q.-J. Sun, G.-F. Dong, H.-Y. Zheng, H.-Y. Zhao, J. Qiao, L. Duan, L.-D. Wang, F.-S. Zhang, Y. Qiu, Wuli Huaxue Xuebao2011, 27, 1893.Search in Google Scholar
[22] U. Mayerhöffer, K. Deing, K. Gruss, H. Braunschweig, K. Meerholz, F. Würthner, Angew. Chem. Int. Ed. Engl. 2009, 48, 8776.10.1002/anie.200903125Search in Google Scholar PubMed
[23] R. Bisht, V. Sudhakar, K. Mele, F. Munavvar, N. Jarjule, J. Nithyanandhan, ACS Appl. Mater. Interfaces2018, 10, 26335.10.1021/acsami.8b09866Search in Google Scholar PubMed
[24] Q. Xiao, F. Wu, M. Han, Z. Li, L. Zhu, Z. Li, J. Mat. Chem. A, 2018, 6, 13644.10.1039/C8TA05026KSearch in Google Scholar
[25] H.-C. Zhu, C.-F. Li, Z.-H. Fu, S.-S. Wei, X.-F. Zhu, J. Zhang, Appl. Surf. Sci. 2018, 455, 1095.10.1016/j.apsusc.2018.06.081Search in Google Scholar
[26] V. Punitharasu, K. Mele, F. Munavvar, J. Nithyanandhan, ACS Appl. Mater. Interfaces2018, 10, 16541.10.1021/acsami.8b03106Search in Google Scholar
[27] Y. Haishima, Y. Kubota, K. Manseki, J. Jin, Y. Sawada, T. Inuzuka, K. Funabiki, M. Matsui, J. Org. Chem. 2018, 83, 4389.10.1021/acs.joc.8b00070Search in Google Scholar
[28] G. M. Paterno, N. Barbero, S. Galliano, C. Barolo, G. Lanzani, F. Scotognella, R. Borrelli, J. Mat. Chem. C2018, 6, 2778.10.1039/C7TC05078JSearch in Google Scholar
[29] J. Wu, C. Si, Y. Chen, L. Yang, B. Hu, G. Chen, Z. Lu, Y. Huang, Chem. Eur. J. 2018, 24, 3234.10.1002/chem.201705140Search in Google Scholar
[30] Y. Chen, G. Wang, L. Yang, J. Wu, F. S. Melkonyan, Y. Huang, Z. Lu, T. J. Marks, A. Facchetti, J. Mat. Chem. C2018, 6, 847.10.1039/C7TC04639ASearch in Google Scholar
[31] Z. Li, S. Xu, Y. Du, Appl. Mech. Mater. 2011, 80–81, 360.10.4028/www.scientific.net/AMM.80-81.360Search in Google Scholar
[32] G. J. Ashwell, J. Ewington, K. Moczko, J. Mat. Chem. 2005, 15, 1154.10.1039/b413480jSearch in Google Scholar
[33] M. Yang, S. Li, J. Ma, Y. Jiang, Chem. Phys. Lett. 2002, 354, 316.10.1016/S0009-2614(02)00127-6Search in Google Scholar
[34] L. Beverina, M. Crippa, P. Salice, R. Ruffo, C. Ferrante, I. Fortunati, R. Signorini, C. M. Mari, R. Bozio, A. Facchetti, G. A. Pagani, Chem. Mater. 2008, 20, 3242.10.1021/cm800714kSearch in Google Scholar
[35] H. Abrahamse, M. R. Hamblin, Biochem. J. 2016, 473, 347.10.1042/BJ20150942Search in Google Scholar PubMed PubMed Central
[36] T. Liu, X. Liu, Y. Zhang, M. V. Bondar, Y. Fang, K. D. Belfield, Eur. J. Org. Chem.2018, 2018, 4095.10.1002/ejoc.201800584Search in Google Scholar
[37] B. Wu, Y. Lin, B. Li, C. Zhan, F. Zeng, S. Wu, Analyt. Chem. 2018, ahead of print.Search in Google Scholar
[38] S. Khopkar, S. Deshpande, G. Shankarling, ACS Sustainable Chem. Eng. 2018, 6, 10798.10.1021/acssuschemeng.8b02095Search in Google Scholar
[39] K. Strassel, A. Kaiser, S. Jenatsch, A. C. Veron, S. B. Anantharaman, E. Hack, M. Diethelm, F. Nüsch, R. Aderne, C. Legnani, S. Yakunin, M. Cremona, R. Hany, ACS Appl. Mater. Interfaces2018, 10, 11063.10.1021/acsami.8b00047Search in Google Scholar PubMed
[40] T. S. Jarvis, F. M. Roland, K. M. Dubiak, P. W. Huber, B. D. Smith, J. Mat. Chem. B2018, 30, 4963.10.1039/C8TB01495GSearch in Google Scholar PubMed PubMed Central
[41] G. Wang, W. Xu, H. Yang, N. Fu, Dyes Pigm.2018, 157, 369.10.1016/j.dyepig.2018.05.016Search in Google Scholar
[42] S. K. Shaw, C. L. Schreiber, F. M. Roland, P. M. Battles, S. P. Brennan, S. J. Padanilam, B. D. Smith, Bioorg. Med. Chem.2018, 26, 2085.10.1016/j.bmc.2018.03.007Search in Google Scholar PubMed PubMed Central
[43] J. Gayton, L. McNamara, A. Huckaba, T. Rill, E. Sharpe, S. Autry, N. Hammer, J. Delcamp, Abstracts of Papers, 255th ACS National Meeting & Exposition, New Orleans, LA (USA) March 18–22, 2018, ORGN-292.Search in Google Scholar
[44] S. Dong, J. D. W. Teo, L. Y. Chan, C.-L. K. Lee, K. Sou, ACS Appl. Nano Mater.2018, 1, 1009.10.1021/acsanm.8b00084Search in Google Scholar
[45] W. Liu, A. Johnson, B. D. Smith, J. Am. Chem. Soc. 2018, 140, 3361.10.1021/jacs.7b12991Search in Google Scholar PubMed
[46] M. Saikiran, D. Sato, S. S. Pandey, S. Hayase, T. Kato, Bioorg. Med. Chem. Lett.2017, 27, 4024.10.1016/j.bmcl.2017.07.057Search in Google Scholar PubMed
[47] G. Saranya, P. Anees, M. M. Joseph, K. K. Maiti, A. Ajayaghosh, Chem. Eur. J. 2017, 23, 7191.10.1002/chem.201700839Search in Google Scholar PubMed
[48] M. Shimi, V. Sankar, M. K. A. Rahim, P. R. Nitha, S. Das, K. V. Radhakrishnan, K. G. Raghu, Chem. Commun. 2017, 53, 5433.10.1039/C6CC10282DSearch in Google Scholar PubMed
[49] G. Wang, W. Xu, Y. Guo, N. Fu, Sens. Actuators B2017, 245, 932.10.1016/j.snb.2017.01.172Search in Google Scholar
[50] M. Ptaszek, Progr. Mol. Biol. Transl. Sci.2013, 143, 59.10.1016/B978-0-12-386932-6.00003-XSearch in Google Scholar PubMed
[51] I. C. Wu, J. Yu, F. Ye, Y. Rong, M. E. Gallina, B. S. Fujimoto, Y. Zhang, Y.-H. Chan, W. Sun, X.-H. Zhou, C. Wu, D. T. Chiu, J. Am. Chem. Soc. 2015, 137, 173.10.1021/ja5123045Search in Google Scholar PubMed PubMed Central
[52] D. Shangguan, Y. Wei, X. Liu, L. Shen, X. Zhang, T. Bing, Faming Zhuanli Shenquing CN 108070275, 2018.Search in Google Scholar
[53] I. Miletto, A. Fraccarollo, N. Barbero, C. Barolo, M. Cossi, L. Marchese, E. Gianotti, J. Chem. Soc., Dalton Trans. 2018, 47, 3038.10.1039/C7DT03735JSearch in Google Scholar
[54] M. S. Soumya, D. Gayathri Devi, K. M. Shafeekh, S. Das, A. Abraham, Photodiagn. Photodyn. Ther.2017, 18, 302.10.1016/j.pdpdt.2017.03.009Search in Google Scholar PubMed
[55] P. S. S. Babu, P. M. Manu, T. J. Dhanya, P. Tapas, R. N. Meera, A. Surendran, K. A. Aneesh, S. J. Vadakkancheril, D. Ramaiah, S. A. Nair, M. R. Pillai, Sci. Rep.2017, 7, 42126.10.1038/srep42126Search in Google Scholar PubMed PubMed Central
[56] A. H. Schmidt, Synthesis1980, 961.10.1055/s-1980-29291Search in Google Scholar
[57] C. G. Krishna, L. P. Avinash, K. Bhanuprakash, RSC Adv.2015, 5, 18813.10.1039/C4RA10649KSearch in Google Scholar
[58] A. L. Puyad, C. Prabhakar, K. Yesudas, K. Bhanuprakash, V. J. Rao, J. Mol. Struct. Theochem. 2009, 904, 1.10.1016/j.theochem.2009.02.038Search in Google Scholar
[59] K. Srinivas, C. Prabhakar, C. L. Devi, K. Yesudas, K. Bhanuprakash, V. J. Rao, J. Phys. Chem. A2007, 111, 3378.10.1021/jp067410fSearch in Google Scholar
[60] A. L. Puyad, G. K. Chaitanya, C. Prabhakar, K. Bhanuprakash, J. Mol. Model.2013, 19, 275.10.1007/s00894-012-1543-8Search in Google Scholar
[61] K. Yesudas, G. K. Chaitanya, K. Bhanuprakash, V. J. Rao, J. Phys. Chem. A2006, 110, 11717.10.1021/jp064074uSearch in Google Scholar
[62] K.-Y. Law, F. C. Bailey, Canad. J. Chem. 1993, 71, 494.10.1139/v93-070Search in Google Scholar
[63] H. Ozawa, K. Yoshiro, S. Yabushita, Chem. Phys. Lett. 2015, 625, 78.10.1016/j.cplett.2015.02.025Search in Google Scholar
[64] C. Cornelissen-Gude, W. Rettig, R. Lapouyade, J. Phys. Chem. A1997, 101, 9673.10.1021/jp971577eSearch in Google Scholar
[65] R. W. Bigelow, H.-J. Freund, Chem. Phys.1986, 107, 159.10.1016/0301-0104(86)85001-7Search in Google Scholar
[66] I. B. Burgess, P. Rochon, N. Cunningham, Opt. Mater. 2008, 30, 1478.10.1016/j.optmat.2007.09.004Search in Google Scholar
[67] H. Meier, 2019, unpublished work.Search in Google Scholar
[68] A. Kaczmarek-Kedziera, D. Kedziera, Theor. Chem. Acc. 2016, 135:214 and references therein.10.1007/s00214-016-1971-0Search in Google Scholar
[69] S. Das, T. L. Thanulingam, K. G. Thomas, P. V. Kamet, M. V. George, J. Phys. Chem.1993, 97, 13620.10.1021/j100153a032Search in Google Scholar
[70] H. Meier, Angew. Chem. Int. Ed.2005, 44, 2482.10.1002/anie.200461146Search in Google Scholar
[71] H. Meier, M. Lehmann in Encycl. Nanosci. Nanotechnol., (Ed.: H. S. Nalwa), Vol. 10, American Scientific Publishers, Stevensons Ranch 2004, p. 95.Search in Google Scholar
[72] N. Sekine, K. Ookuba, PCT Int. Appl. WO 2008035554 A1 20080327, 2008.Search in Google Scholar
[73] S. Ohira, I. Rudra, K. Schmidt, S. Barlow, S.-J. Chung, Q. Zhang, J. Matichak, S. R. Marder, J.-L. Bredas, Chem Eur. J. 2008, 14, 11082.10.1002/chem.200801055Search in Google Scholar
[74] See however: D. Keil, H. Hartmann, Dyes Pigm.2001, 49, 161.10.1016/S0143-7208(01)00016-XSearch in Google Scholar
[75] H. Meier, U. Dullweber, Tetrahedron Lett. 1996, 37, 1191.10.1016/0040-4039(95)02414-XSearch in Google Scholar
[76] H. Meier, R. Petermann, U. Dullweber, J. Prakt. Chem./Chem. Ztg. 1998, 340, 744.10.1002/prac.19983400807Search in Google Scholar
[77] K.-J. Law, F. C. Bailey, J. Org. Chem. 1992, 57, 3278.10.1021/jo00038a010Search in Google Scholar
[78] F. Meyers, C.-T. Chen, S. R. Marder, J.-L. Bredas, Chem. Eur. J.1997, 3, 530.10.1002/chem.19970030408Search in Google Scholar
[79] J. Gerold, U. Holzenkamp, H. Meier, Eur. J. Org. Chem. 2001, 2001, 2757.10.1002/1099-0690(200107)2001:14<2757::AID-EJOC2757>3.0.CO;2-2Search in Google Scholar
[80] J. Gerold, Doctoral Thesis, University of Mainz, Mainz, 2001.Search in Google Scholar
[81] C. T. Chen, S. R. Marder, L. T. Cheng, J. Am. Chem. Soc. 1994, 116, 3117.10.1021/ja00086a049Search in Google Scholar
[82] S. R. Marder, C. T. Chen, L. T. Cheng, US 5500156A 19960319, 1996.Search in Google Scholar
[83] H. Meier, U. Dullweber, J. Org. Chem. 1997, 62, 4821.10.1021/jo970284eSearch in Google Scholar
[84] J. H. Banning, J. W. Thomas, Jr. B. Wu, S. V. Drappel, Ger. Offen. DE 102014216684 A1 20150226, 2015.Search in Google Scholar
[85] R. Petermann, Doctoral Thesis, University of Mainz, Mainz, 2001.Search in Google Scholar
[86] N. Takiguchi, PCT Int. Appl. WO 2017110511 A1 20170629, 2017.Search in Google Scholar
[87] Y. Hirai, D. Sasaki, Y. Jimbo, PCT Int. Appl. WO 2017043175 A1 20170316, 2017.Search in Google Scholar
[88] H. Meier, R. Petermann, J. Gerold, Chem. Commun.1999, 1999, 977.10.1039/a900888hSearch in Google Scholar
[89] R. Petermann, M. Tian, S. Shinura, M. Furuki, Jpn. Kokai Tokkyo Koho JP 2004067892 A 20040304, 2004.Search in Google Scholar
[90] R. Petermann, M. Tian, S. Tatsuura, M. Furuki, Dyes Pigm.2003, 57, 43.10.1016/S0143-7208(03)00003-2Search in Google Scholar
[91] N. Sekine, K. Ookubo, Kokai Tokkyo Koho 2009 JP 2009040860 A 20090226, 2009.Search in Google Scholar
[92] Y. Xu, Z. Li, A. Malkovsky, S. Sun, Y. Pang, J. Phys. Chem. B2010, 114, 8574.10.1021/jp1029536Search in Google Scholar PubMed
[93] C. Reichardt, Solvents and Solvent Effects in Organic Chemistry, VCH, Weinheim, 1988, p. 365.Search in Google Scholar
[94] S. Rosselli, D. Danner, Z. A. Bamedi, G. Nelles, T. Miteva, G. Fuhrmann, PCT Int. Appl. WO 2016120166 A1 20160804, 2016.Search in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this Issue
- Review
- Extended conjugation in stilbenoid squaraines
- Research Articles
- Synthesis, crystal structure and magnetic properties of an octanuclear cerium(III) complex of pyridine-2,6-dicarboxylate
- Synthesis and structural characterization of two coordination polymers constructed by bis(4-(1H-imidazol-1-yl)phenyl)methanone and 5-(tert-butyl)isophthalate ligands
- Syntheses and properties of cyclometalated ruthenium(II) complexes with 1,10-phenanthroline and phenylphthalazine ligands
- Synthesis of novel 1,2,3-triazole-substituted tomentosins
- New antifungal prenylated flavanones from Colutea armata
- Crystal structure of Sc3Co1.64In4 and Sc10Co9In20 from single-crystal data
- Ternary transition metal gallides with TiNiSi, ZrBeSi and MgZn2-type structure
Articles in the same Issue
- Frontmatter
- In this Issue
- Review
- Extended conjugation in stilbenoid squaraines
- Research Articles
- Synthesis, crystal structure and magnetic properties of an octanuclear cerium(III) complex of pyridine-2,6-dicarboxylate
- Synthesis and structural characterization of two coordination polymers constructed by bis(4-(1H-imidazol-1-yl)phenyl)methanone and 5-(tert-butyl)isophthalate ligands
- Syntheses and properties of cyclometalated ruthenium(II) complexes with 1,10-phenanthroline and phenylphthalazine ligands
- Synthesis of novel 1,2,3-triazole-substituted tomentosins
- New antifungal prenylated flavanones from Colutea armata
- Crystal structure of Sc3Co1.64In4 and Sc10Co9In20 from single-crystal data
- Ternary transition metal gallides with TiNiSi, ZrBeSi and MgZn2-type structure

![Fig. 2: Frontier orbitals of 1,3-bis(4-dimethylaminophenyl)squaraine (1a) and long-wavelength electron transition (absorption and emission in CH2Cl2) [63], [64], [67].](/document/doi/10.1515/znb-2018-0260/asset/graphic/j_znb-2018-0260_fig_002.jpg)
