Abstract
Gaseous diffusion of n-hexane into the reaction mixture of sodium dicyanamide NaN(CN)2 with 15-crown-5 in tetrahydrofuran leads to a sodium dicyanamide compound [Na(15-crown-5)]N(CN)2 (1). Compound 1 exhibits a centrosymmetric dumbbell-like structure constructed by dicyanamide ligands double-bridging [Na(15-crown-5)]+ moieties with a μ-1,5 coordination mode. Solid-state photoluminescence experiments have shown that compound 1 emits violet luminescence, and its possible emission mechanism was investigated in detail based on theoretical calculations.
1 Introduction
Great interest is continuously being focused on the metal dicyanamide (dca) compounds due to the variety of observed topologies and the diversity of their magnetic properties [1], [2]. Within these compounds, the topologies of the structures are correlated with the coordination behavior of the dicyanamide ligand N(CN)2−. The compounds prefer to show three-dimensional [3], [4] or two-dimensional frameworks [5], [6] where the dca ligands adopt a μ3-1,3,5 coordination mode, whereas layers [7], [8] or chain structures [9], [10] appear when the dca ligands adopt a μ-1,5 coordination fashion. Isolated structures built from dca ligands are very limited in the literature [11], [12] because the dca ligand commonly has a good multidentate coordination ability leading to extended molecular structures. Low-dimensional molecules can be directly used as the structural models for theoretical calculations, and these treatments usually lead to more accurate theoretical results, which are very useful to explain the experimental data. In the present paper, we report the synthesis, crystal structure, photoluminescence, and possible emission mechanism of a dumbbell-like dinuclear sodium compound, {[Na(15-crown-5)]N(CN)2}2 (1). The calculations on the excited-state electron density distributions of corresponding frontier orbitals and density of states (DOS) for 1 show good agreement with the photoluminescence data.
Compound 1 displays a centrosymmetric dumbbell-like structure in which each Na+ cation is coordinated by five O atoms from a molecule 15-crown-5 to form [Na(15-crown-5)]+ moieties. Two of these cations are linked by two bridging dca ligands with a μ-1,5 coordination mode (Fig. 1). This double-bridging leads to a rare dinuclear sodium compound. The entire dimer {[Na(15-crown-5)]N(CN)2}2 has crystallographic inversion symmetry.
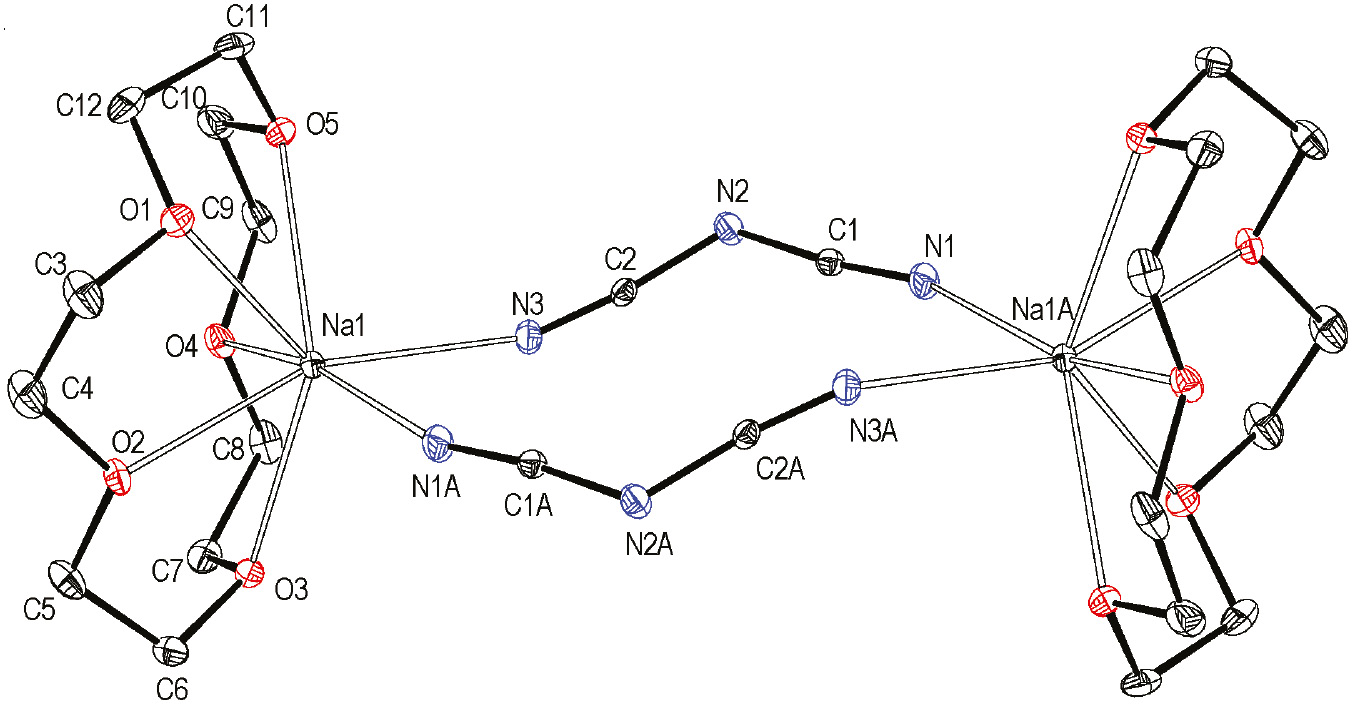
ORTEP plot of 1 with displacement ellipsoids at the 30% probability level and atom labeling adopted. Selected bond lengths (Å) and angles (°): Na1–N1A 2.460(2), Na1–N3 2.515(2), Na1–O1 2.568(2), Na1–O2 2.513(2), Na1–O3 2.594(2), Na1–O4 2.594(2), Na1–O5 2.590(2), N1–C1 1.120(3), N2–C2 1.300(3), N2–C1 1.300(3), N3–C2 1.118(3), C2–N2–C1 123.2(2), N1–C1–N2 173.0(2), N3–C2–N2 171.9(2). All hydrogen atoms are omitted for clarity. Symmetry code: A: 1−x, 1−y, 1−z.
In the [Na(15-crown-5)]+ moiety, the Na–O bond lengths vary from 2.513(2) to 2.594(2) Å, which are in agreement with the reported Na–O bond lengths in other [Na(15-crown-5)]+ complex cations [13], [14]. The nonadjacent O···O distances range between 3.779(2) and 4.679(2) Å, which are shorter than twice a Na–O bond length, causing the Na+ cation to be located outside the distorted plane (the least squares plane contains the five oxygen atoms of 15-crown-5, and its calculated function is −8.6733x−6.3977y+9.9784z=−2.4240) of the 15-crown-5 molecule with a deviation distance of 1.147(2) Å. The N(CN)2− linkers adopt μ-1,5 coordination mode with the mean terminal C–N bond length of 1.119(3) Å and the mean middle C–N bond length of 1.300(3) Å, revealing that the terminal C–N bonds have triple bond character whereas the middle C-N bonds possess delocalized bond character.
As is shown in Fig. 2, the isolated [Na(15-crown-5)]N(CN)2 molecules are stacked along the b direction, while being completely superimposed over each other along the a and c directions. It should be noted that there are no obvious supramolecular interactions other than the van der Waals forces between these sodium dimers.
![Fig. 2: The {[Na(15-crown-5)]N(CN)2}2 dimers are stacked together complementarily to form a three-dimensional structure. The complex cation [Na(15-crown-5)]+ is simplified as a pentagonal pyramid.](/document/doi/10.1515/znb-2016-0192/asset/graphic/j_znb-2016-0192_fig_002.jpg)
The {[Na(15-crown-5)]N(CN)2}2 dimers are stacked together complementarily to form a three-dimensional structure. The complex cation [Na(15-crown-5)]+ is simplified as a pentagonal pyramid.
Solid-state luminescence spectra show that complex 1 exhibits a relatively strong violet emission band around 436 nm upon photoexcitation at 366 nm (Fig. 3). Its lifetime was measured to be 2.3 ns, suggesting that compound 1 is a good potential candidate for violet luminescent material. Time-dependent DFT calculations [15], [16], [17] on one repeating unit of 1 (Fig. 4 and Table S1; Supporting information available online) indicate that the lowest singlet excitation for 1 is dominated by the degenerate combination of HOMO→LUMO, HOMO–1→LUMO, and HOMO→LUMO+1 transitions, in which the HOMO and HOMO−1 are mostly composed of occupied π* orbitals of the dca groups, whereas both the LUMO and the LUMO+1 mainly consist of σ* orbital of the crown ethers and a small portion of the unoccupied π orbital of the dca groups. DOS calculation [18], [19], [20], [21] of 1 (Figs. 5 and S1) indicates that the top of the valence bands (VBs) appears to be relatively flat and the bottom of the conduction bands (CBs) have small dispersion. The top of VBs (−1.7 to 0.0 eV) mainly originates from Ocrown-2p states (37.3 electrons per electron volt) mixing with small amounts of (CN)dca-2p states (14.7 electrons per electron volt), whereas the bottom of the CBs (4.5–7.9 eV) have contributions from both the Ccrown-2p states (9.4 electrons per electron volt) and the (CN)dca-2p states (15.0 electrons per electron volt). Together with the structural features of complex 1, the origin of the emission band at 436 nm may be mainly ascribed to the coupling of two types of intraligand charge transfer (ILCT, n→σ* or π*→π*) where electrons are transferred from the Ocrown-2p (Ocrown-2p states, VBs) to the unoccupied σ* orbitals of the crowns or transferred from the occupied π* orbitals of dca ligands ((CN)dca-2p states, VBs) to the unoccupied π* orbital of the dca ligands ((CN)dca-2p states, CBs).

Solid-state electronic emission and excitation spectra of 1 at room temperature.

The electron-density distributions of the corresponding frontier orbitals calculated for 1.

Density of states (total and partial) of 1. The Fermi level is set at 0 eV.
In summary, a luminescent dumbbell-like dinuclear sodium dicyanamide compound was synthesized, and its possible emission mechanism was investigated through theoretical calculations. The results are significant not only for synthesizing a luminescent material but also for elucidating the relationship of the luminescent properties and the crystal structure of the compound, which is important for the design and synthesis of more efficient luminescent materials.
2 Experimental
2.1 Synthesis of 1
NaN(CN)2 (32 mg, 0.4 mmol) and 15-crown-5 (88 mg, 0.4 mmol) were added to 10 mL of dry and distilled THF solvent, and this reaction mixture was stirred at 60°C for 8 h under an atmosphere of N2. After filtration, the filtrate was reduced to 5 mL in a small tube, which was loaded into a large vial containing 5 mL of n-hexane. The large vial was sealed and left undisturbed at room temperature. Colorless crystals of 1 formed in 10 days. Yield: 76%. – C12H20N3NaO5: calcd. C 46.60, H 6.53, N 13.59; found C 46.53, H 6.48, N 13.12%. – FT-IR (KBr, 4000–400 cm−1): 3515(br, m), 2916(m), 2878(w), 2245(s), 2202(m), 2148(vs), 1623(w), 1473(w), 1353(m), 1324(m), 1289(w), 1245(w), 1117(s), 1089(s), 1038(m), 946(s), 901(w), 862(m), 834(w), 524(m). ν (C≡N) vibrations of dicyanamide anions [22]: 2245 (νas+νs), 2202 (νas), 2148 (νs).
2.2 Crystallographic data for 1
C12H20N3NaO5, Mr=309.30, 0.30×0.10×0.10 mm3, monoclinic space group, P21/c, a=11.279(6), b=10.922(5), c=15.523(8) Å, β=124.066(4)°, V=1584(1) Å3, T=293(2) K, Z=4, Dcalcd.=1.30 g cm−3, μ=0.1 mm−1, F(000)=656 e, λ(MoKα)=0.71073 Å, 10239 reflections measured (3.17<θ<25.02°), 2795 unique (Rint=0.0364), 1310 of which were considered observed with I>2 σ(I). Refinement of 190 parameters gave R1=0.0634, wR2=0.1636 (I>2 σ(I)), and S=1.001. Further details about the crystal structure determination are listed in the Supporting Information.
CCDC 922765 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
3 Supporting information
Details on materials and physical measurements, structure determination, time-dependent DFT calculations with Gaussian2009, DOS calculations with the program CASTEP, and descriptions of other computational details are given as Supporting Information available online (DOI: 10.1515/znb-2016-0192).
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 21071156
Funding source: Natural Science Foundation of Chongqing
Award Identifier / Grant number: CSTC2015JCYJA50007
Award Identifier / Grant number: CSTC2014GJHZ0030
Funding statement: The authors gratefully acknowledge financial support of the National Natural Science Foundation of China (grant no. 21071156) and the Natural Science Foundation of Chongqing (grant nos. CSTC2015JCYJA50007 and CSTC2014GJHZ0030).
Acknowledgments
The authors gratefully acknowledge financial support of the National Natural Science Foundation of China (grant no. 21071156) and the Natural Science Foundation of Chongqing (grant nos. CSTC2015JCYJA50007 and CSTC2014GJHZ0030).
References
[1] M. Ohba, H. Ōkawa, Coord. Chem. Rev.2000, 198, 313, and references therein.10.1016/S0010-8545(00)00233-2Search in Google Scholar
[2] S. R. Batten, K. S. Murray, Coord. Chem. Rev.2003, 246, 103, and references therein.10.1016/S0010-8545(03)00119-XSearch in Google Scholar
[3] P. Jensen, D. J. Price, S. R. Batten, B. Moubaraki, K. S. Murray, Chem. Eur. J.2000, 6, 3186.10.1002/1521-3765(20000901)6:17<3186::AID-CHEM3186>3.0.CO;2-VSearch in Google Scholar
[4] S. R. Batten, P. Jensen, B. Moubaraki, K. S. Murray, R. Robson, Chem. Commun.1998, 439.10.1039/a707264cSearch in Google Scholar
[5] M. Wriedt, C. Näther, Dalton Trans.2011, 40, 886.10.1039/C0DT00864HSearch in Google Scholar
[6] H.-L. Sun, S. Gao, B.-Q. Ma, G. Su, Inorg. Chem.2003, 42, 5399.10.1021/ic034202iSearch in Google Scholar
[7] Y. M. Chow, Inorg. Chem.1971, 10, 1938.10.1021/ic50103a022Search in Google Scholar
[8] D. Armentano, G. De Munno, F. Guerra, M. Julve, F. Lloret, Inorg. Chem.2006, 45, 4626.10.1021/ic060044uSearch in Google Scholar
[9] D. Ghoshal, A. K. Ghosh, J. Ribas, E. Zangrando, G. Mostafa, T. K. Maji, N. R. Chaudhuri, Cryst. Growth Des. 2005, 5, 941.10.1021/cg049669ySearch in Google Scholar
[10] S. R. Batten, P. Jensen, C. J. Kepert, M. Kurmoo, B. Moubaraki, K. S. Murray, D. J. Price, J. Chem. Soc. Dalton Trans.1999, 2987.10.1039/a903487kSearch in Google Scholar
[11] K. E. Bessler, L. L. Romualdo, V. M. Deflon, A. Hagenbach, Z. Anorg. Allg. Chem.2000, 626, 1942.10.1002/1521-3749(200009)626:9<1942::AID-ZAAC1942>3.0.CO;2-QSearch in Google Scholar
[12] W.-Z. Shen, X.-Y. Chen, P. Cheng, D.-Z. Liao, S.-P. Yan, Z.-H. Jiang, Z. Anorg. Allg. Chem.2003, 629, 2591.10.1002/zaac.200300319Search in Google Scholar
[13] R. D. Moulton, D. J. Chandler, A. M. Arif, R. A. Jones, A. J. Bard, J. Am. Chem. Soc.1988, 110, 5714.10.1021/ja00225a023Search in Google Scholar
[14] J. A. Bandy, A. Berry, M. L. H. Green, K. Prout, Chem. Commun.1985, 1462–1463.10.1039/C39850001462Search in Google Scholar
[15] X. Liu, G.-C. Guo, M.-L. Fu, W.-T. Chen, M.-S. Wang, J.-S. Huang, Inorg. Chem.2006, 45, 3679.10.1021/ic0601539Search in Google Scholar
[16] X. Liu, G.-C. Guo, M.-L. Fu, W.-T. Chen, J.-Z. Zhang, J.-S. Huang, Dalton Trans.2006, 884.10.1039/B515652CSearch in Google Scholar
[17] X. Liu, G.-C. Guo, Aust. J. Chem.2008, 61, 481.10.1071/CH08025Search in Google Scholar
[18] X. Liu, K.-L. Huang, Inorg. Chem.2009, 48, 8653.10.1021/ic900611uSearch in Google Scholar
[19] X. Liu, K.-L. Huang, G.-M. Liang, M.-S. Wang, G.-C. Guo, CrystEngComm2009, 11, 1615.10.1039/b902121cSearch in Google Scholar
[20] X. Liu, L. Li, Y.-Z. Yang, K.-L. Huang, Dalton Trans.2014, 43, 4086.10.1039/C3DT53219DSearch in Google Scholar
[21] L. Li, C.-H. Wang, X.-L. Zhang, X. Liu, Eur. J. Inorg. Chem.2015, 859.10.1002/ejic.201403037Search in Google Scholar
[22] J. Carranza, J. Sletten, F. Lloret, M. Julve, Inorg. Chim. Acta2004, 357, 3304.10.1016/j.ica.2004.01.044Search in Google Scholar
Supplemental Material:
The online version of this article (DOI: 10.1515/znb-2016-0192) offers supplementary material, available to authorized users.
©2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this Issue
- Crystal and molecular structure of 1-picryl-2-phenyl-2-(4-picrylamidophenyl)-diazenium betaine: analogy between a picramido group and an oxygen atom
- Synthesis and crystal structure of the rare earth borogermanate EuGeBO5
- Alkylation of tetrathiotungstate anions: crystal structures of the alkylthiolatotrithiotungstate complexes [PPh4]2[WS3(Sn Pr)][WS3(Sn Bu)]·½C6H6 and [PPh4][WS3(SCH2C6H4CH2Cl-4)]
- Two isostructural fluorinated metal-organic frameworks with rare rod-packing architecture: syntheses, structures and luminescent properties
- Synthesis and characterization of a macrocyclic copper complex containing the 14-membered 1,3,5,8,10,12-hexaazacyclotetradecane unit
- Synthesis of lignin model compound containing a β-O-4 linkage
- Crystal structures and third-order optical properties of three manganese(II) complexes constructed from N-heterocyclic and polycarboxylate ligands
- Trigonal dodecahedral sodium coordination in a trinuclear copper(II)-sodium complex incorporating a salen-type compartmental Schiff base
- X-ray and NQR studies of bromoindate(III) complexes: [C2H5NH3]4InBr7, [C(NH2)3]3InBr6, and [H3NCH2C(CH3)2CH2NH3]InBr5
- Synthesis and structural characterization of Li3Y(BO3)2
- About the air- and water-stable copper(I) dicyanamide: synthesis, crystal structure, vibrational spectra and DSC/TG analysis of Cu[N(CN)2]
- Note
- Synthesis, crystal structure, and photoluminescence of a dumbbell-like sodium dicyanamide compound with 15-crown-5
Articles in the same Issue
- Frontmatter
- In this Issue
- Crystal and molecular structure of 1-picryl-2-phenyl-2-(4-picrylamidophenyl)-diazenium betaine: analogy between a picramido group and an oxygen atom
- Synthesis and crystal structure of the rare earth borogermanate EuGeBO5
- Alkylation of tetrathiotungstate anions: crystal structures of the alkylthiolatotrithiotungstate complexes [PPh4]2[WS3(Sn Pr)][WS3(Sn Bu)]·½C6H6 and [PPh4][WS3(SCH2C6H4CH2Cl-4)]
- Two isostructural fluorinated metal-organic frameworks with rare rod-packing architecture: syntheses, structures and luminescent properties
- Synthesis and characterization of a macrocyclic copper complex containing the 14-membered 1,3,5,8,10,12-hexaazacyclotetradecane unit
- Synthesis of lignin model compound containing a β-O-4 linkage
- Crystal structures and third-order optical properties of three manganese(II) complexes constructed from N-heterocyclic and polycarboxylate ligands
- Trigonal dodecahedral sodium coordination in a trinuclear copper(II)-sodium complex incorporating a salen-type compartmental Schiff base
- X-ray and NQR studies of bromoindate(III) complexes: [C2H5NH3]4InBr7, [C(NH2)3]3InBr6, and [H3NCH2C(CH3)2CH2NH3]InBr5
- Synthesis and structural characterization of Li3Y(BO3)2
- About the air- and water-stable copper(I) dicyanamide: synthesis, crystal structure, vibrational spectra and DSC/TG analysis of Cu[N(CN)2]
- Note
- Synthesis, crystal structure, and photoluminescence of a dumbbell-like sodium dicyanamide compound with 15-crown-5

