Abstract
In this work, a new macrocyclic copper complex, [Cu(ACE)(SCN)2]; ACE: 1,3,6,10,12,15-hexaazatricyclo[13.3.1.16,10]eicosane, was prepared and characterized by elemental analysis, FT-IR, Raman spectroscopy and single-crystal X-ray diffraction. X-ray analysis of [Cu(ACE)(SCN)2] reveals an elongated octahedral geometry around the copper atom in a centrosymmetric CuN6 environment.
1 Introduction
Macrocyclic complexes have attracted much attention for the unique properties offered by the macrocyclic environment, such as the ability to access unusual oxidation states of the metal center [1], metal ion-selective reagents [2], models for metalloenzyme active sites [3], applications in biology, medicine and also as catalysts [4]. There are various reactions for the synthesis of the macrocyclic compounds including the formation of the C–C, C–O, C–S, C–N, C=N and B–O bonds [5].
Among the macrocyclic rings, the 14-membered 1,3,5,8,10,12-hexaazacyclotetradecane (ACD, Scheme 1) unit is interesting owing to its presence in the structure of a variety of compounds including aromatic polycarboxylate ligands [6], enantiopure complexes [7], coordination polymers [8], [9], [10] and supramolecular coordination polymers [11]. Asymmetric derivatives of this unit are chiral [5]; thus stereochemistry for these ligands is important and has been studied [8], [12]. This unit also shows pH-dependent coordination behavior [13].
![Scheme 1: The structure of 1,3,6,10,12,15-hexaazatricyclo[13.3.1.16,10]eicosane (ACE) and 1,3,5,8,10,12-hexaazacyclotetradecane (ACD).](/document/doi/10.1515/znb-2016-0199/asset/graphic/j_znb-2016-0199_scheme_001.jpg)
The structure of 1,3,6,10,12,15-hexaazatricyclo[13.3.1.16,10]eicosane (ACE) and 1,3,5,8,10,12-hexaazacyclotetradecane (ACD).
Copper complexes of this unit have been used as molecular precursors to construct magnetic materials [14], [15], [16], photoluminescent materials [8], [17], [18], DNA-binding compounds [19], [20], heterometallic trinuclear complexes [21], bimetallic cyanido-bridged complexes [14], [22], [23], [24], oxo-anion recognition complexes [25] and also complexes for the electrocatalytic reduction of CO2 [26]. Coordination polymers containing copper complexes of this unit form different cavities including honeycombs [9], [14], [15] or square grids [8].
In this work, the structural and spectral (FT-IR and Raman) properties of a new example, [Cu(ACE)(NCS)2] (1); ACE: 1,3,6,10,12,15-hexaazatricyclo[13.3.1.16,10]eicosane, in which ACE is a derivative of the ACD ligand containing two fused 1,3-diazacyclohexane rings (Scheme 1), are presented and discussed.
2 Results and discussion
Template condensation involving amine and formaldehyde followed by ion exchange was employed for the synthesis of 1.
In the IR spectrum of 1, there is a band at 2047 cm−1 which can be attributed to the stretching vibrations of the thiocyanate ion. This band also guides us to determine the coordination mode of the thiocyanate ion. According to the literature [27], the thiocyanate ion can coordinate to metal centers as N-donor, S-donor or bridging NS-donor. The CN stretching frequencies are generally lower in N-bonded complexes (near and above 2050 cm−1) than in S-bonded (near 2100 cm−1) and bridging ones (above 2100 cm−1) [27]. On the basis of these rules, we can decide that the thiocyanate ion is coordinated to the copper atom as N-donor ligand. The Raman spectrum also confirms this donation type.
Information about the low metal–ligand vibration frequency can be obtained by Raman spectroscopy [28]. In the Raman spectrum of 1, stretching vibrations of the metal–ligand bonds are present in the region of 400–550 cm−1 [29].
In crystals of 1 the complex has crystallographically induced centrosymmetry (Ci) with the copper atom residing on the center of inversion (Fig. 1). The copper atom is in an elongated octahedral coordination environment, in which four nitrogen atoms of ACE are located in the equatorial positions with the Cu–N bond lengths averaging to 2.047 Å, which is comparable to the Cambridge structural database (CSD) average (2.0126 Å, Scheme 2). The axial sites are occupied by the two nitrogen atoms of the two thiocyanate ions. The axial bond length (2.596 Å) is much longer than those of the equatorial ones which may also be due to the Jahn–Teller effect. The coordinated atoms N1/N3/N1i/N3i of ACE and the copper atom are strictly located in one plane.

The Ortep diagram of the molecular structure of 1. The ellipsoids for 1 are drawn at the 30% probability level.

The bond length and angle averages for different geometries in complexes of ACE/Cu.
The macrocyclic ligand is tetradentate forming two five- and two six-membered chelate rings which alternate on the equatorial plane. Similar to the CSD results (Scheme 2), the average of the N–Cu–N angles in the six-membered chelate rings (93.07°) is larger than that in the five-membered chelate rings (86.93°). The 1,3-diazacyclohexane subunits are fused to each of the six-membered chelate rings and have a chair conformation and an anticonfiguration with respect to the equatorial plane. The ligand itself has no chiral center and four new ones including N1, N1i, N3 and N3i are formed after coordination. The two nitrogen atoms of the five- and six-membered chelate rings have identical and different configurations, respectively. Due to the centrosymmetric space group, the crystals contain a racemic mixture of R,S,R,S and S,R,S,R isomers.
In the network of 1 (Fig. 2), there are intermolecular C–H···C, C–H···N and N–H···S hydrogen bonds. The sulfur atoms act as proton acceptors, whereas the carbon and nitrogen atoms participate in hydrogen bonding as proton donors and acceptors at the same time.
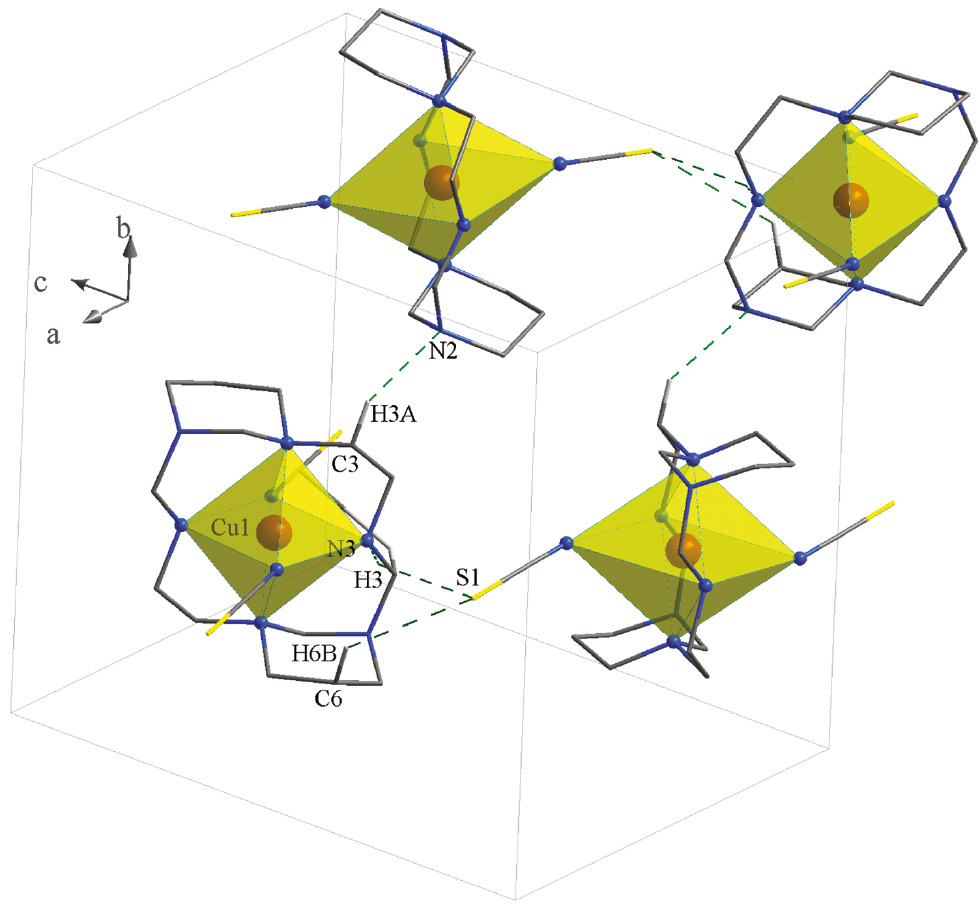
Packing of 1 showing the hydrogen bonds. Only the hydrogen atoms involved in hydrogen bonding are shown.
3 Conclusions
In this work, a new complex of 1,3,6,10,12,15-hexaazatricyclo[13.3.1.16,10]eicosane (ACE) [Cu(ACE)(SCN)2] (1) was synthesized and its spectral (IR, Raman) and structural properties were investigated. The ACE ligand acts as N4-donor by forming two five- and two six-membered chelate rings with the Cu center. X-ray analysis of 1 revealed a copper atom with an elongated octahedral geometry (CuN6).
4 Experimental section
4.1 Materials and instrumentation
All starting chemicals and solvents were reagent or analytical grade and used as received. The microwave-assisted synthesis was carried out using a Microwave Laboratory Systems MicroSYNTH, Milestone s.r.l. The infrared spectrum of a KBr pellet was recorded in the range 400–4000 cm−1 using an FT-IR 8400-Shimadzu spectrometer. The Raman spectrum was obtained using a Nicolet Model 910 Fourier-transform spectrometer. The carbon, hydrogen and nitrogen contents were determined in a Thermo Finnigan Flash Elemental Analyzer 1112 EA. Melting points were determined using a Barnsted Electrothermal 9200 electrically heated apparatus.
4.2 [Cu(ACE)(NCS)2] (1)
The [Cu(ACE)(NO3)2] complex was synthesized according to the literature [8] by the reaction of N-(2-aminoethyl)-1,3-diaminopropane with Cu(NO3)2·3H2O, and then the addition of aqueous formaldehyde in a microwave oven under the reflux condition. A solution of potassium thiocyanate (KSCN) (2 mmol, 0.19 g), dissolved in H2O (10 mL), was added to the solution of [Cu(ACE)(NO3)2] (1 mmol, 0.47 g) in H2O (20 cm3). The reaction mixture was stirred at 60°C for 5 h. Purple crystals of the product suitable for X-ray diffraction studies were obtained by slow evaporation of the solution. The crystals were then collected by filtration. Yield: 0.37 g, 80%; m.p.: 229°C. – C16H30CuN8S2 (462.14): calcd. C 41.58, H 6.54, N 24.25; found C 40.68, H 6.14, N 23.82. – IR (KBr disk): ν=3202 (NH), 2963 (CH2), 2047 (CNSCN), δas=1435 (CH2), δs=1381 (CH2), ν=1150 m (CN), 536 (CuN) cm−1. – Raman: ν=2962 (CH2), δs=1357 (CH2), δas=1415 (CH2), ν=1132 (CN), 527 and 409 (CuN) cm−1.
4.3 X-ray structure determination
Suitable crystals of 1 were placed on Oxford diffraction Gemini Ultra and kept at 100 K during data collection. Using Olex2 [30], the structure was solved with the Shelxs [31] structure solution program using Direct Methods and refined with the Shelxl [31] refinement package using least-squares minimization. Diagrams of the molecular structure and unit cell were created using Ortep-III [32], [33] and Diamond [34], [35]. Crystallographic data and details of the data collection and structure refinement are listed in Table 1, selected bond lengths and angles in Table 2 and hydrogen bond geometries in Table 3.
Crystal data and structure refinement for complex 1.
| Empirical formula | C16H30CuN8S2 |
| Formula weight, g mol−1 | 462.14 |
| Temperature, K | 100 |
| Crystal size, mm3 | 0.14×0.13×0.10 |
| Crystal system | Orthorhombic |
| Space group | Pbca |
| Unit cell dimensions | |
| a, Å | 10.7587(12) |
| b, Å | 13.0929(7) |
| c, Å | 14.0178(9) |
| Volume, Å3 | 1974.6(3) |
| Z | 4 |
| Calculated density, mg m−3 | 1.56 |
| Absorption coefficient, mm−1 | 1.3 |
| F(000), | 972 |
| θ range for data collection, deg | 2.9–33.1 |
| hkl ranges | −16:16, −20:20, −21:20 |
| Reflections collected | 39563 |
| Independent reflections | 3768 |
| Rint | 0.050 |
| Data/restraints/parameters | 3768/0/124 |
| Goodness-of-fit (F2) | 1.052 |
| Final R1/wR2 [I>2σ(I)] | 0.042/0.127 |
| Final R1/wR2 (all data) | 0.068/0.111 |
| Largest diff. peak/hole, e Å−3 | 1.36/−0.82 |
Selected bond lengths (Å) and angles (deg) of complex 1 with estimated standard deviations in parentheses.a
| Bond lengths (Å) | Angles (deg) | ||
|---|---|---|---|
| Cu(1)–N(1) | 2.0854(15) | N(1)–Cu(1)–N(1)i | 180 |
| Cu(1)–N(4) | 2.0089(15) | N(4)–Cu(1)–N(1)i | 86.93(5) |
| Cu(1)–N(11) | 2.596(2) | N(4)–Cu(1)–N(1) | 93.07(5) |
| C(12)–N(11) | 1.154(3) | N(11)–C(12)–S(2) | 178.9(2) |
| C(12)–S(2) | 1.653(2) | C(12)–N(11)–Cu(1) | 143.5(2) |
aAtoms marked with an i are related to the respective unmarked ones by a center of inversion.
Hydrogen bond dimensions (Å and deg) in complex 1.
| D–H···A | d(D–H) | d(H···A) | ∠(DHA) | d(D···A) | Symmetry code of A |
|---|---|---|---|---|---|
| C(5)–H(5A)···C(12) | 0.970 | 2.875 | 141.7 | 3.686(3) | 1.5 − x, −y, −0.5 + z |
| C(3)–H(3A)···N(2) | 0.970 | 2.710 | 131.1 | 3.427(2) | 0.5+x, 0.5 −y, 1−z |
| C(10)–H(10A)···C(3) | 0.970 | 2.898 | 132.7 | 3.626(2) | 1 −x, −0.5 + y, 0.5 −z |
| N(4)–H(4)···S(2) | 0.980 | 2.632 | 142.83 | 3.464(2) | 0.5−x, −0.5+y, z |
CCDC 1473426 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
[1] G. Melson (Ed.), Coordination Chemistry of Macrocyclic Compounds, Plenum Press, New York, 1979.10.1007/978-1-4613-2928-2Search in Google Scholar
[2] M. K. Beklemishev, S. Elshani, C. M. Wai, Anal. Chem.1994, 66, 3521.10.1021/ac00092a036Search in Google Scholar
[3] D. E. Fenton, H. Okawa, J. Chem. Soc., Dalton Trans.1993, 1349.10.1039/DT9930001349Search in Google Scholar
[4] J. Costamagna, G. Ferraudi, B. Matsuhiro, M. Campos-Vallette, J. Canales, M. Villagrán, J. Vargas, M. J. Aguirre, Coord. Chem. Rev.2000, 196, 125.10.1016/S0010-8545(99)00165-4Search in Google Scholar
[5] M. Hakimi, K. Moeini, Z. Mardani, M. A. Fernandes, F. Mohr, E. Schuh, J. Coord. Chem.2012, 65, 1232.10.1080/00958972.2012.669834Search in Google Scholar
[6] J. Cho, A. J. Lough, J. C. Kim, Inorg. Chim. Acta2003, 342, 305.10.1016/S0020-1693(02)01149-0Search in Google Scholar
[7] M. J. Kobyłka, T. Bereta, J. Janczak, J. Lisowski, Polyhedron2012, 42, 1.10.1016/j.poly.2012.04.016Search in Google Scholar
[8] M. Hakimi, K. Moeini, Z. Mardani, F. Mohr, Polyhedron2014, 70, 92.10.1016/j.poly.2013.12.033Search in Google Scholar
[9] J. W. Ko, K. S. Min, M. P. Suh, Inorg. Chem.2002, 41, 2151.10.1021/ic011281uSearch in Google Scholar PubMed
[10] J. W. Shin, S. M. Yeo, K. S. Min, Inorg. Chem. Commun.2012, 22, 162.10.1016/j.inoche.2012.05.051Search in Google Scholar
[11] X. Jiang, B. Tao, H. Xia, G.-Y. Liao, CrystEngComm2012, 14, 3271.10.1039/c2ce06494dSearch in Google Scholar
[12] C.-H. Kwak, H. Nam, H.-I. Kim, M.-J. Park, J.-E. Jee, J. Kim, Inorg. Chim. Acta2004, 357, 1325.10.1016/j.ica.2003.12.007Search in Google Scholar
[13] G.-C. Ou, C.-Y. Su, J.-H. Yao, T.-B. Lu, Inorg. Chem. Commun.2005, 8, 421.10.1016/j.inoche.2005.01.030Search in Google Scholar
[14] Y. S. You, J. H. Yoon, J. H. Lim, H. C. Kim, C. S. Hong, Inorg. Chem.2005, 44, 7063.10.1021/ic050634cSearch in Google Scholar PubMed
[15] X. Shen, H. Zhou, Q. Zhang, Y. Xu, H. Zhou, Eur. J. Inorg. Chem.2012, 2012, 5050.10.1002/ejic.201200480Search in Google Scholar
[16] A.-H. Yuan, W.-Y. Liu, H. Zhou, Y.-Y. Chen, X.-P. Shen, J. Mol. Struct.2009, 919, 356.10.1016/j.molstruc.2008.10.012Search in Google Scholar
[17] B. Tao, F. Cheng, X. Jiang, H. Xia, J. Mol. Struct.2012, 1028, 176.10.1016/j.molstruc.2012.06.043Search in Google Scholar
[18] J. H. Park, A. R. Jeong, D. K. A. K. Hastuti, M. J. Jeong, K. S. Min, J. Incl. Phenom. Macrocycl. Chem.2015, 82, 153.10.1007/s10847-015-0489-8Search in Google Scholar
[19] J. Liu, H. Zhang, C. Chen, H. Deng, T. Lu, L. Ji, J. Chem. Soc., Dalton Trans.2003, 114.10.1039/b206079pSearch in Google Scholar
[20] S. Sujatha, S. Balasubramanian, B. Varghese, M. Jayaprakashvel, N. Mathivanan, Inorg. Chim. Acta2012, 386, 109.10.1016/j.ica.2012.02.011Search in Google Scholar
[21] B. Zhang, Z.-H. Ni, A.-L. Cui, H.-Z. Kou, New J. Chem.2006, 30, 1327.10.1039/b605521dSearch in Google Scholar
[22] Z. Trávníček, R. Herchel, J. Mikulík, R. Zbořil, J. Solid State Chem.2010, 183, 1046.10.1016/j.jssc.2010.03.001Search in Google Scholar
[23] S.-L. Ma, S. Ren, Y. Ma, S.-P. Yan, D.-Z. Liao, J. Chem. Crystallogr.2010, 40, 1011.10.1007/s10870-010-9786-7Search in Google Scholar
[24] X.-P. Shen, S. Gao, G. Yin, K.-B. Yu, Z. Xu, New J. Chem.2004, 28, 996.10.1039/B401557FSearch in Google Scholar
[25] M. Boiocchi, M. Licchelli, M. Milani, A. Poggi, D. Sacchi, Inorg. Chem.2015, 54, 47.10.1021/ic501527kSearch in Google Scholar
[26] M. Isaacs, J. C. Canales, M. J. Aguirre, G. Estiú, F. Caruso, G. Ferraudi, J. Costamagna, Inorg. Chim. Acta2002, 339, 224.10.1016/S0020-1693(02)00942-8Search in Google Scholar
[27] K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, John Wiley & Sons, Inc., Hoboken, 2009, p. 283.10.1002/9780470405888Search in Google Scholar
[28] M. Hakimi, Z. Mardani, K. Moeini, F. Mohr, M. A. Fernandes, Polyhedron2014, 67, 27.10.1016/j.poly.2013.08.065Search in Google Scholar
[29] M. Hakimi, Z. Mardani, K. Moeini, F. Mohr, Polyhedron2015, 102, 569.10.1016/j.poly.2015.10.038Search in Google Scholar
[30] O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Puschmann, J. Appl. Crystallogr.2009, 42, 339.10.1107/S0021889808042726Search in Google Scholar
[31] G. Sheldrick, Acta Crystallogr.2008, A64, 112.10.1107/S0108767307043930Search in Google Scholar PubMed
[32] C. K. Johnson, M. N. Burnett, Ortep-III (version 1.0.2), Oak Ridge Thermal Ellipsoid Plot Program for Crystal Structure Illustrations, Rep. ORNL-6895, Oak Ridge National Laboratory, Oak Ridge, TN (USA) 1996.10.2172/369685Search in Google Scholar
[33] L. J. Farrugia, J. Appl. Crystallogr.2012, 45, 849.10.1107/S0021889812029111Search in Google Scholar
[34] K. Brandenburg, Diamond, Crystal and Molecular Structure Visualization, Crystal Impact—H. Putz and K. Brandenburg GbR, Bonn (Germany) 2012. See also: http://www.crystalimpact.com/diamond/.Search in Google Scholar
[35] G. Bergerhof, M. Berndt, K. Brandenburg, J. Res. Natl. Stand. Technol.1996, 101, 221.10.6028/jres.101.023Search in Google Scholar PubMed PubMed Central
©2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this Issue
- Crystal and molecular structure of 1-picryl-2-phenyl-2-(4-picrylamidophenyl)-diazenium betaine: analogy between a picramido group and an oxygen atom
- Synthesis and crystal structure of the rare earth borogermanate EuGeBO5
- Alkylation of tetrathiotungstate anions: crystal structures of the alkylthiolatotrithiotungstate complexes [PPh4]2[WS3(Sn Pr)][WS3(Sn Bu)]·½C6H6 and [PPh4][WS3(SCH2C6H4CH2Cl-4)]
- Two isostructural fluorinated metal-organic frameworks with rare rod-packing architecture: syntheses, structures and luminescent properties
- Synthesis and characterization of a macrocyclic copper complex containing the 14-membered 1,3,5,8,10,12-hexaazacyclotetradecane unit
- Synthesis of lignin model compound containing a β-O-4 linkage
- Crystal structures and third-order optical properties of three manganese(II) complexes constructed from N-heterocyclic and polycarboxylate ligands
- Trigonal dodecahedral sodium coordination in a trinuclear copper(II)-sodium complex incorporating a salen-type compartmental Schiff base
- X-ray and NQR studies of bromoindate(III) complexes: [C2H5NH3]4InBr7, [C(NH2)3]3InBr6, and [H3NCH2C(CH3)2CH2NH3]InBr5
- Synthesis and structural characterization of Li3Y(BO3)2
- About the air- and water-stable copper(I) dicyanamide: synthesis, crystal structure, vibrational spectra and DSC/TG analysis of Cu[N(CN)2]
- Note
- Synthesis, crystal structure, and photoluminescence of a dumbbell-like sodium dicyanamide compound with 15-crown-5
Articles in the same Issue
- Frontmatter
- In this Issue
- Crystal and molecular structure of 1-picryl-2-phenyl-2-(4-picrylamidophenyl)-diazenium betaine: analogy between a picramido group and an oxygen atom
- Synthesis and crystal structure of the rare earth borogermanate EuGeBO5
- Alkylation of tetrathiotungstate anions: crystal structures of the alkylthiolatotrithiotungstate complexes [PPh4]2[WS3(Sn Pr)][WS3(Sn Bu)]·½C6H6 and [PPh4][WS3(SCH2C6H4CH2Cl-4)]
- Two isostructural fluorinated metal-organic frameworks with rare rod-packing architecture: syntheses, structures and luminescent properties
- Synthesis and characterization of a macrocyclic copper complex containing the 14-membered 1,3,5,8,10,12-hexaazacyclotetradecane unit
- Synthesis of lignin model compound containing a β-O-4 linkage
- Crystal structures and third-order optical properties of three manganese(II) complexes constructed from N-heterocyclic and polycarboxylate ligands
- Trigonal dodecahedral sodium coordination in a trinuclear copper(II)-sodium complex incorporating a salen-type compartmental Schiff base
- X-ray and NQR studies of bromoindate(III) complexes: [C2H5NH3]4InBr7, [C(NH2)3]3InBr6, and [H3NCH2C(CH3)2CH2NH3]InBr5
- Synthesis and structural characterization of Li3Y(BO3)2
- About the air- and water-stable copper(I) dicyanamide: synthesis, crystal structure, vibrational spectra and DSC/TG analysis of Cu[N(CN)2]
- Note
- Synthesis, crystal structure, and photoluminescence of a dumbbell-like sodium dicyanamide compound with 15-crown-5

