Abstract
Cyclizations of Cbz-protected α,β-didehydro-β-arylalanine esters 1 with excess hydrazine hydrate afforded mixtures of the expected 3-pyrazolidinones 2 and the unexpected 1-amino-5-benzylidenehydantoins 6 and N-Cbz-β-arylalanine hydrazides 7. Presumably, the pyrazolidinones 2 and hydantoins 6 are formed as primary products via competitive 1,2- and 1,4-addition of hydrazine hydrate followed by cyclization, whereas β-arylalanine hydrazides 7 are formed as secondary products via reductive cleavage of the C(5)–N(1) bond in pyrazolidinones 2. The overall selectivity depends on the reaction time and on the β-substituent in the starting dehydroalanine ester 1.
1 Introduction
α,β-Didehydro-α-amino acid derivatives are important and useful intermediates and building blocks in organic synthesis. For example, they are employed in the synthesis of α-amino acids, peptides, and related compounds, as well as for the preparation of various heterocyclic compounds including natural products and their analogues [1–4].
3-Pyrazolidinones are cyclic (internal) hydrazides of 3-hydrazinopropanoic acid, which are commonly available by treatment of an α,β-unsaturated carboxylic acid derivative with hydrazine hydrate. The importance of 3-pyrazolidinones has risen significantly, owing to their applicability in industrial processes and biological activity. For example, pyrazolidinone derivatives have been employed as dyes and photographic developers, and their bioactivities range from antibacterial, analgesic, antipyretic, and anti-inflammatory to inhibition of cyclooxygenase and lipoxygenase [5–8].
In the last two decades, a substantial part of our research interest has also been devoted to the chemistry of 3-pyrazolidinones [8, 9]. Within this context, we recently reported the preparation of 5-substituted (4R*,5R*)-2-benzyloxycarbonylamino-3-pyrazolidinones 2 [7–11] from easily available methyl N-Cbz-3-aryl-α,β-didehydroalaninates 1 [12, 13]. Pyrazolidinones 2 were used as key intermediates in the synthesis of pyrazolo[1,2–a]pyrazole-based peptide mimetics [7] and aza-deoxa analogues of d-cycloserine [11]. Aiming at the preparation of novel fused 3-pyrazolidinones 3via intramolecular cyclization between the aryl group at position 5 and the amino function at position 4, we recently tried to prepare some 3-pyrazolidinones 2 with an ortho-substituted aryl group attached at position 5 (Fig. 1).
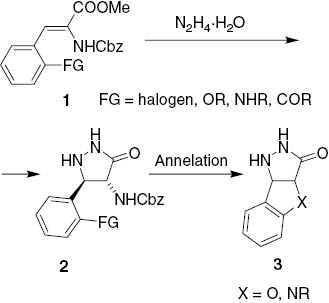
Planned synthesis of tricyclic pyrazolidinones 3via annelation of ortho-substituted 5-aryl group.
We were convinced that intermediates 2 should be easily available from the corresponding dehydroalaninates 1 following standard synthetic protocol [7–11]. However, the planned synthesis of target tricyclic pyrazolidinones 3 (cf. Fig. 1) could not be developed, as the key intermediates 2 could not be prepared, neither selectively nor effectively. It turned out that reactions of N-benzyloxycarbonyl-3-aryl-2,3-dehydroalaninates 1 with hydrazine hydrate did not give only the corresponding 3-pyrazolidinones 2 as believed previously, but rather mixtures of 3-pyrazolidinones 2, 1-aminohydantoins 6, and 3-arylalanine hydrazides 7. Further experiments were performed to obtain a closer insight into selectivity and the mechanism of these ring transformations. Herein, we report the results of this study.
2 Results and discussion
Starting acrylates 1a–n were prepared from methyl 2-benzyloxycarbonylamino-2-(dimethoxyphosphoryl)acetate (4) [13] and aldehydes 5a–n following the literature procedure (Scheme 1) [12]. First, we tried to prepare 3-pyrazolidinones 2a–e as the representative central intermediates. It is noteworthy that our previous reactions of acrylates 1 with hydrazine hydrate were carried out only for a few hours and furnished only the corresponding pyrazolidinones 2 upon workup by filtration [7–11]. Besides, chromatographic workup was not suitable for isolation of quite polar 1,2-unsubstituted 4-acylamino-3-pyrazolidinones 2 [5–11]. Following our previous examples [7–11], the acrylates 1a–e bearing an ortho-substituent at the 5-aryl group were treated with 3 equiv. of hydrazine hydrate in ethanol at r.t. Somewhat expectedly, reactions of 1a–e were slower than known reactions of their ortho-unsubstituted analogues [7–11]. As no precipitation occurred upon 24 h, the reaction mixtures were simply stirred for another 48 h, whereupon the precipitates were formed and collected by filtration. Only in the reaction of 2′-methoxycinnamate 1b, the precipitate was not formed. In the cyclizations of ortho-hydroxy- (1a) and ortho-benzyloxy-substituted cinnamate 1c, the expected 3-pyrazolidinones 2a and 2c were obtained in 32% and 18% yield, respectively. Meanwhile, cyclization of methyl 2′-chlorocinnamate 1d afforded a 4:1 mixture of the pyrazolidinone 2d and the 1-aminohydantoin derivative 6d, whereas reaction of 2′-nitrocinnamate 1e furnished only the corresponding hydantoin 6e in 48% yield. These unexpected results prompted us to treat also the acrylates 1f–n in the same way. Reactions of 1f, g, k gave mixtures of pyrazolidinones 2f, g, k and hydantoins 6f, g, k, whereas 3-(pyridyl)acrylates 1h–j furnished the hydantoins 6h–j in 3–50% yields. Formation of hydantoins 6f and 6g as by-products was not detected previously upon significantly shorter reaction times [11]. Acrylates 1m and 1n produced only the corresponding 3-pyrazolidinones 2m and 2n, which was in agreement with the literature data [7, 11]. No precipitate was formed in the reaction of dehydrotryptophan ester 1l (Scheme 1, Table 1).
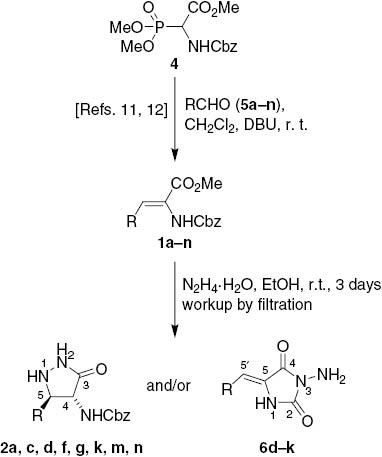
Isolation of 3-pyrazolidinones 2 and 1-aminohydantoins 6 upon treatment of 1 with hydrazine hydrate followed by filtration workup.
Experimental data for compounds 2 and/or 6 isolated upon workup by filtration.
| Entry | Compound | R | Yield (%) | 2:6 | Ref. |
|---|---|---|---|---|---|
| 1 | 1a, 2a, 5a, 6a | 2-Hydroxyphenyl | 32a | 100:0a | [11] |
| 2 | 1b, 2b, 5b, 6b | 2-Methoxyphenyl | b | b | c |
| 3 | 1c, 2c, 5c, 6c | 2-Benzyloxyphenyl | 18 | 100:0 | c |
| 4 | 1d, 2d, 5d, 6d | 2-Chlorophenyl | 65 | 81:19 | c |
| 5 | 1e, 2e, 5e, 6e | 2-Nitrophenyl | 48 | 0:100 | c |
| 6 | 1f, 2f, 5f, 6f | 3-Nitrophenyl | 17 | 88:12 | [11] |
| 7 | 1g, 2g, 5g, 6g | 4-Nitrophenyl | 57 | 93:7 | [11] |
| 8 | 1h, 2h, 5h, 6h | 2-Pyridyl | 50 | 0:100 | c |
| 9 | 1i, 2i, 5i, 6i | 3-Pyridyl | 5 | 0:100 | c |
| 10 | 1j, 2j, 5j, 6j | 4-Pyridyl | 3 | 0:100 | c |
| 11 | 1k, 2k, 5k, 6k | 2-Furyl | 79 | 71:29 | c |
| 12 | 1l, 2l, 5l, 6l | N-Boc-1H-indol-3-yl | b | b | c |
| 13 | 1m, 2m, 5m, 6m | Phenyl | 80a | 100:0a | [10] |
| 14 | 1n, 2n, 5n, 6n | Isopropyl | 80a | 100:0a | [7] |
aIn agreement with the literature data. bNo precipitate was formed. cThis paper.
Intrigued by these findings, we became interested in selectivity of the above transformations. Acrylates 1a–n were treated with 3 equiv of hydrazine hydrate in ethanol at r.t. for 3 days followed by evaporative workup. 1H NMR spectra of the crude reaction mixtures were taken in [D6]DMSO to determine the ratio of formed products. Surprisingly, the unexpected N-Cbz-3-arylalanine hydrazides 7 were also formed in addition to the expected pyrazolidinones 2 and hydantoins 6. In most cases, the hydantoins were the minor components, whereas pyrazolidinones 2 and hydrazides 7 were the major components of the product mixtures (Scheme 2, Table 2). In summary, three reactions were completely selective (cf. Table 2): (a) treatment of dehydroleucine ester 1n afforded only the 3-pyrazolidinone 2n (Entry 14) [7]; (b) reaction of 2-pyridyl substituted acrylate 1h gave the hydantoin 6h as the only product (Entry 8); and (c) its 4-pyridyl analogue 1j gave only the corresponding hydrazide 7j (Entry 10). Although the selectivity pattern could not be clearly discerned, preferential formation of hydrazides 7 was observed in the reactions of acrylates 1d–g, i, j bearing electron-withdrawing substituents at the 5-aryl group (Table 2, Entries 4–7, 9, 10), whereas reactions of 2′-hydroxy- and 2′-alkoxycinnamates 1a–c gave comparable amounts of 2 and 7 (Table 2, Entries 1–3). In the reactions of acrylates 1k–n with ortho-unsubstituted electron-rich groups the pyrazolidinones 2k–n were formed predominantly (Table 2, Entries 11–14).
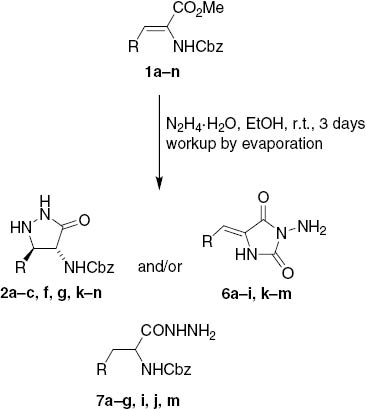
Formation of 3-pyrazolidinones 2, 1-aminohydantoins 6, and hydrazines 7 upon prolonged treatment of 1 with hydrazine hydrate followed by evaporative workup.
Ratio of products 2, 6, and 7 upon prolonged treatment of 1 with hydrazine hydrate.
| Entry | Compound | R | 2:6:7a |
|---|---|---|---|
| 1 | 2a, 6a, 7a | 2-Hydroxyphenyl | 45:15:40b |
| 2 | 2b, 6b, 7b | 2-Methoxyphenyl | 40:15:45b |
| 3 | 2c, 6c, 7c | 2-Benzyloxyphenyl | 35:10:55b |
| 4 | 2d, 6d, 7d | 2-Chlorophenyl | 0:21:79 |
| 5 | 2e, 6e, 7e | 2-Nitrophenyl | 0:35:65 |
| 6 | 2f, 6f, 7f | 3-Nitrophenyl | 34:8:58 |
| 7 | 2g, 6g, 7g | 4-Nitrophenyl | 30:4:66 |
| 8 | 2h, 6h, 7h | 2-Pyridyl | 0:100:0 |
| 9 | 2i, 6i, 7i | 3-Pyridyl | 0:3:97 |
| 10 | 2j, 6j, 7j | 4-Pyridyl | 0:0:100 |
| 11 | 2k, 6k, 7k | 2-Furyl | 55:45:0b |
| 12 | 2l, 6l, 7l | N-Boc-1H-indol-3-yl | 67:33:0b |
| 13 | 2m, 6m, 7m | Phenyl | 65:18:17b |
| 14 | 2n, 6n, 7n | Isopropyl | 100:0:0 |
aDetermined from the 1H NMR spectra of the crude reaction mixtures. bBecause of overlapping signals, the ratio of products could not be clearly discerned.
The next important issue was the tentative reaction pathway (mechanism) of these transformations. Ring transformations of 3-pyrazolidinones and their azomethine imine derivatives into N-aminohydantoins have been reported previously [10]. To get a deeper insight into the reaction pathway, further experiments were performed. First, treatment of a 2:1 mixture of 5-(2-chlorophenyl)-3-pyrazolidinone 2d and hydantoin 6d with 3 equiv of hydrazine hydrate in ethanol at r.t. for 7 days furnished a 2:1 mixture of the hydrazide 7d and hydantoin 6d. (Hydantoin 6d was obtained upon crystallization of a 4:1 mixture of 2d and 6d from ethanol.) Similarly, the 5-phenyl analogue 2m gave the 3-phenylalanine hydrazide 7m as the only product, whereas the 5-isopropyl analogue 2n did not react with hydrazine hydrate (Scheme 3).
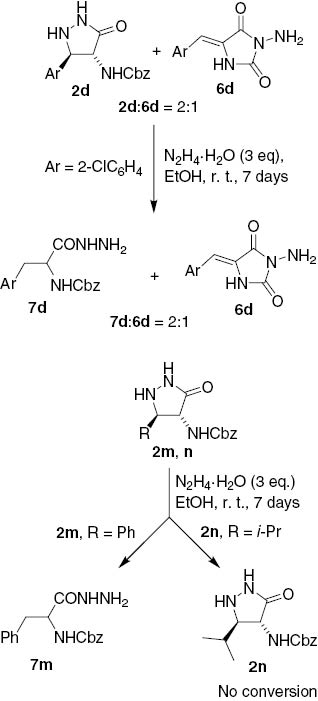
Control experiments performed to establish the reaction pathway.
The results of the above experiments were in agreement with formation of the pyrazolidinones 2 and hydantoins 6 as the two primary products in the reaction of acrylates 1 with excess hydrazine hydrate. Thus, hydantoins 6 were presumably formed directly from 1 and not via ring transformation of the pyrazolidinone intermediate 2. This is supported by the unchanged ratio of 7d and 6d compared to the ratio of starting compounds 2d and 6d (cf. Scheme 3). Similarly, the above unchanged ratio of 7d and 6d was in agreement with formation of 3-arylalanine hydrazides 7 through reductive cleavage of the benzylic C–N bond in the pyrazolidinones 2. This is additionally supported by the non-reactivity of the 5-alkyl substituted pyrazolidinone 2n devoid of benzylic C–N bond. The whole reaction sequence can thus be rationalized by initial competitive 1,4- and 1,2-addition of hydrazine hydrate to the unsaturated ester 1 to give the acrylohydrazide intermediate 8 (Scheme 4, Path A) and the β-hydrazino alaninate 9 (Scheme 4, Path B). The hydrazide 8 cyclizes by condensation to the carbamate group to give the hydantoin 6 [10], whereas cyclization of the hydrazine 9 to the ester group leads to the pyrazolidinone 2 [8]. Upon prolonged treatment with hydrazine hydrate, the relatively weak benzylic C–N bond in 5-aryl-3-pyrazolidinones 2 undergoes reductive ring opening to give the N-Cbz-3-arylalanine hydrazide 7 (Scheme 4).
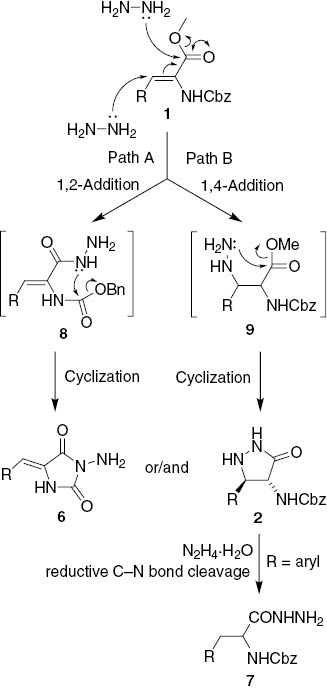
Plausible reaction pathway for formation of 2, 6, and 7.
In light of this explanation, the generally observed preferential formation of 2 and 7 is explainable by predominant 1,4-addition of hydrazine hydrate to acrylate 1 in the beginning of the reaction. This is in agreement with selective formation of 7i and 7j from 3-pyridylacrylates 1i and 1j, due to activation of the β-position by the electron-withdrawing 3- and 4-pyridyl groups. Meanwhile, selective transformation of their 2-pyridyl analogue 1h into hydantoin 6h does not fit into such an explanation because the β-position in 1h is activated similarly as in 1i and 1j. Therefore, there has to be another explanation for this exceptional selectivity. A plausible rationale is based on specificity of acrylate 1h with basic ring nitrogen atom at the ortho-position of the pyridyl residue. Thus, cyclization of the 1,2-adduct 8h should be much faster than cyclization of the Michael adduct 9h, which is hindered by intramolecular C=N···H–N hydrogen bond between the hydrazino group and the pyridine nitrogen atom, keeping the hydrazino group away from the ester function. Besides, the same ring nitrogen could also promote β-elimination from 9h leading to starting compound 1h or, alternatively, ring opening of the pyrazolidinone 2h to give the hydrazide 8h, which cyclizes into 6h (Scheme 5).
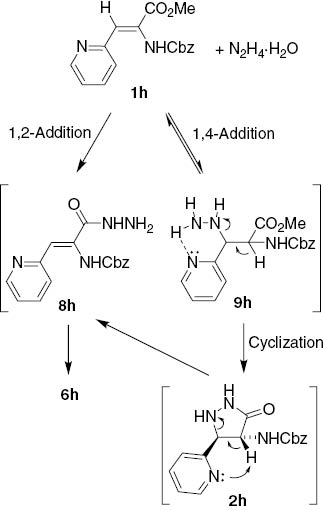
Proposed rationale for chemoselective formation of hydantoin 6h.
The structures of all novel compounds 1c, 1j, 1l, 2c, 2d, 2f, 2g, 2k, and 6c–k were determined by spectroscopic methods (IR, 1H and 13C NMR, HRMS). These novel compounds were not obtained in analytically pure form. Their identities were confirmed by 13C NMR and HRMS. Compounds 1c, 1j, 1l, 2c, 6e, and 6h–j were obtained and characterized as single components, whereas compounds 2d/6d, 2f/6f, 2g/6g, 2k/6k were obtained as inseparable mixtures of compounds and were characterized as such. The (Z)-configuration around the exocyclic C=C double bond of hydantoins 6 was determined on the basis of the characteristic chemical shift for the 5′-H, δ = 6.45–6.65 ppm, which was in agreement with the literature data for closely related (Z)-5-benzylidenehydantoins [14, 15].
3 Conclusion
Treatment of easily available N-Cbz-3-aryl-2,3-dehydroalanine esters 1 with excess hydrazine hydrate leads to three types of products, 3-pyrazolidinones 2, 1-amino-5-benzylidenehydantoins 6, and/or N-Cbz-3-arylalanine hydrazides 7. 3-Pyrazolidinones 2 and 1-aminohydantoins 6 are the primary cyclization products, which are presumably formed from 1via competitive 1,4- and 1,2-addition of hydrazine hydrate followed by cyclization of the respective adducts 8 and 9. Meanwhile, 3-arylalanine hydrazides 7 are formed from the corresponding 5-aryl-3-pyrazolidinones 2 by reductive cleavage of the benzylic C(5)–N(1) bond. The selectivity is dependent on the reaction time and on the 3-aryl substituent in starting acrylate 1. Upon short reaction times and workup by filtration, sparingly soluble pyrazolidinones 2 and/or hydantoins 6 are obtained. Prolonged reaction time followed by evaporative workup lead to mixtures of 2, 6, and 7. High overall selectivity was observed only in cyclizations of β-pyridylacrylates 1h–j, which furnished the respective hydantoin 6h and hydrazides 7i and 7j as the only products. In summary, the present study shows that the course of reactions of dehydroalanine esters 1 with hydrazine hydrate depends on the β-substituent of the starting acrylate 1. These reactions do not lead only to the corresponding 3-pyrazolidinones 2 as believed previously, but rather to mixtures of products 2, 6, and 7.
4 Experimental section
Melting points were determined on a Stanford Research Systems MPA100 OptiMelt automated melting point system. The NMR spectra were obtained on a Bruker Avance III UltraShield 500 plus at 500 MHz for 1H and at 126 MHz for 13C nucleus, respectively, using CDCl3 and [D6]DMSO as solvents with TMS as the internal standard. Mass spectra were recorded on an Agilent 6224 Accurate Mass TOF LC/MS spectrometer and IR spectra on a Bruker FTIR Alpha Platinum ATR spectrophotometer. Microanalyses were performed on a Perkin-Elmer CHN Analyzer 2400 II. Flash column chromatography (FC), was performed on silica gel (Fluka, Silica gel 60, particle size 35–70 μm).
Aldehydes 5a, b, d–k, m, n, DBU, and hydrazine hydrate are commercially available. Methyl 2-{[(benzyloxy)carbonyl]amino}-2-(dimethoxyphosphoryl)acetate (4) [13], 2-benzyloxybenzaldehyde (2c) [16], 1-tert-butoxycarbonyl-1H-indole-3-carbaldehyde (5l) [17], 3-substituted methyl 2-(benzyloxycarbonylamino)acrylates 1a, b, d–i, k, m, n [9–12], and 3-pyrazolidinones 2m [10] and 2n [7] were prepared according to the literature procedures.
4.1 General procedure for the synthesis of 3-substituted methyl 2-(benzyloxycarbonylamino)-acrylates 1c, j, l
Compounds 1c, j, l were prepared following a slightly modified literature procedures [11, 12]. A mixture of 4 (16.6 g, 50 mmol), CH2Cl2 (200 mL), DBU (7.83 mL, 52.5 mmol), and 5 (50 mmol) was stirred at 0°C or r.t. for 0.5–24 h.
Workup A: Volatile components were evaporated in vacuo, and the residue was diluted with AcOEt (150 mL) and washed with 1 M aqueous NaHSO4 (2 × 70 mL). The combined organic phase was dried over anhydrous Na2SO4 and filtered, and the filtrate was evaporated in vacuo to give 1.
Workup B: The reaction mixture was poured directly onto a stabilized column and the product was isolated by FC (silica gel, 3 × 15 cm, EtOAc). Fractions containing the product were combined and evaporated in vacuo to give 1.
The following compounds were prepared in this manner:
4.1.1 Methyl 2-benzyloxycarbonylamino-3-(2-benzyloxyphenyl)acrylate (1c)
Prepared from 4 (0.993 g, 3 mmol) and 2-benzyloxybenzaldehyde 5c (636 μL, 3 mmol), r.t., 12 h, workup A. Yield: 1.23 g (98%) of a light orange oil. – IR (ATR): v = 3314, 3063, 3032, 2950, 1708 (C=O), 1640 (C=O), 1597, 1577, 1484, 1450, 1435, 1380, 1303, 1219, 1162, 1139, 1112, 1048, 1025, 910, 847, 478, 733, 695, 623 cm−1. – 1H NMR (500 MHz, CDCl3): δ = 3.83 (s, 3H, OCH3), 5.11 and 5.15 (2s, 1:1, 4H, 2 × OCH2Ph), 6.95–7.01 (m, 2H, 1H of Ar, NH), 7.28–7.45 (m, 12H, 12 H of Ar), 7.52 (m, 2H, 1H of Ar, 3-H) ppm. – 13C NMR (126 MHz, CDCl3): δ = 52.6, 67.3, 70.9, 113.2, 121.2, 123.3, 125.4, 125.5, 127.3, 127.3, 128.1, 128.2, 128.3, 128.5, 128.6, 128.7, 130.0, 130.5, 136.4, 156.3, 165.8 ppm. – HRMS ((+)-ESI): m/z = 418.1647 (calcd. 418.1649 for C25H24NO5, [M+H]+).
4.1.2 Methyl 2-benzyloxycarbonylamino-3-(4-pyridyl)acrylate (1j)
Prepared from 4 (0.993 g, 3 mmol) and pyridine-4-carbaldehyde 5j (600 μL, 3 mmol), 0°C, 0.5 h, workup B. Yield: 0.936 g (100%) of a yellow oil. – IR (ATR): v = 3306, 3150, 3064, 3032, 2951, 2892, 2846, 1714 (C=O), 1646 (C=O), 1598, 1547, 1497, 1454, 1435, 1412, 1385, 1331, 1307, 1266, 1216, 1140, 1040, 990, 885, 850, 814, 769, 736, 696, 621 cm−1. – 1H NMR (500 MHz, CDCl3): δ 3.82 (s, 3H, OCH3), 5.11 (s, 2H, OCH2), 7.15 (s, 1H, 3-H), 7.29–7.39 (m, 7H, Ph, 2′-H, 6′-H), 8.11 (s, 1H, NH), 8.45–8.61 (m, 2H, 3′-H, 5′-H) ppm. – 13C NMR (126 MHz, CDCl3): δ = 52.6, 67.2, 123.2, 127.3, 128.0, 128.1, 128.1, 128.3, 135.7, 141.3, 149.7, 153.7, 165.0 ppm. – HRMS ((+)-ESI): m/z = 313.1181 (calcd. 313.1183 for C17H17N2O4, [M+H]+).
4.1.3 Methyl 2-benzyloxycarbonylamino-3-(1-tert-butoxycarbonyl-1H-indol-3-yl)acrylate (1l)
Prepared from 4 (0.662 g, 2 mmol) and 1-tert-butoxycarbonyl-1H-indole-3-carbaldehyde 5l (0.490 g, 2 mmol), r.t., 24 h, workup B. Yield: 0.930 g (50%) of a yellow oil. – IR (ATR): v = 3110, 3001, 2951, 2902, 1684 (C=O), 1597, 1558, 1533, 1498, 1464, 1439, 1404, 1387, 1364, 1326, 1302, 1282, 1249, 1221, 1200, 1187, 1167, 1134, 1090, 1077, 1009, 957, 919, 894, 857, 827, 813, 800, 791, 777, 759, 713, 670, 657, 628 cm−1. – 1H NMR (500 MHz, CDCl3): δ = 1.65 (s, 9H, tBu), 3.83 (s, 3H, OCH3), 5.15 (s, 2H, OCH2Ph), 6.37 (br s, 1H, NHCO), 7.23–7.40 (m, 7H, Ph, 5′-H, 6′-H), 7.66 (s, 1H, 3-H), 7.70 (d, J = 7.8 Hz, 1H, 4′-H), 7.96 (s, 1H, 3′-H), 8.14 (d, J = 8.1 Hz, 1H, 7′-H) ppm. – 13C NMR (126 MHz, CDCl3): δ = 28.2, 52.7, 65.5, 67.7, 84.7, 114.2, 115.5, 119.1, 123.4, 125.3, 127.1, 127.7, 127.7, 128.3, 128.4, 128.6, 128.7, 129.5, 136.0, 149.3, 165.7 ppm. – HRMS ((+)-ESI): m/z = 451.1860 (calcd. 451.1864 for C25H27N2O6, [M+H]+).
4.2 Synthesis of 5-substituted (4R*,5R*)-4-benzyloxycarbonylamino-3-pyrazolidinones 2a, c–k, m, n and (Z)-3-amino-5-(benzylidene)imidazolidine-2,4-diones 6d, f, g, k
Hydrazine hydrate (98%, 150 μL, 3 mmol) was added to a stirred solution of 1 (1 mmol) in EtOH (5 mL) and the mixture was stirred at r.t. for 3 days. The precipitate was collected by filtration and washed with EtOH (2 × 3 mL) to give 2 or/and 6. The following compounds were prepared in this manner:
4.2.1 (4R*,5R*)-4-benzyloxycarbonylamino-5-(2-hydroxyphenyl)-3-pyrazolidinone (2a)
Prepared from 1a (0.327 g, 1 mmol). Yield: 0.105 g (32%) of white crystals. Physical and spectral data of 2a were in agreement with the literature data [11].
4.2.2 (4R*,5R*)-4-benzyloxycarbonylamino-5-(2-benzyloxyphenyl)-3-pyrazolidinone (2c)
Prepared from 1c (417 g, 1 mmol). Yield: 75 mg (18%) of white crystals. M.p. 135°C–138°C. – IR (ATR): v = 3360, 3284, 3237, 3183, 3065, 3033, 1714 (C=O), 1695 (C=O), 1602, 1589, 1528, 1500, 1465, 1446, 1409, 1383 1350, 1294, 1241, 1202, 1176, 1152, 1105, 1044, 1028, 1004, 957, 940, 903, 857, 804, 771, 752, 735, 693, 640 cm–1. – 1H NMR (500 MHz, CDCl3): δ = 4.62 (t, J = 10.1 Hz, 1H, 5-H), 4.74 (t, J = 10.5 Hz, 1H, 4-H), 4.89–4.99 (m, 2H, OCH2Ph), 5.16 (s, 2H, OCH2Ph), 5.20 (d, J = 11.5 Hz, 1H, 1-H), 6.97 (t, J = 7.5 Hz, 1H, 1H of Ar), 7.09 (d, J = 8.3 Hz, 1H, 1H of Ar), 7.25–7.39 (m, 10H, 10H of Ar), 7.49 (d, J = 8.2 Hz, 2H, 2H of Ar), 7.67 (d, J = 9.2 Hz, 1H, NHCbz), 9.45 (br s, 1H, 2-H) ppm. – 13C NMR (126 MHz, [D6]DMSO): δ = 56.5, 61.5, 65.5, 69.3, 112.7, 120.6, 124.6, 127.2, 127.6, 127.8, 127.8, 128.3, 128.3, 128.7, 129.4, 136.8, 137.0, 156.0, 156.8, 173.0 ppm. – HRMS ((+)-ESI): m/z = 418.1759 (calcd. 418.1761 for C24H24N3O4, [M+H]+).
4.2.3 (4R*,5R*)-4-benzyloxycarbonylamino-5-(2-chlorophenyl)-3-pyrazolidinone (2d) and (Z)-3-amino-5-(2-chlorobenzylidene)imidazolidine-2,4-dione (6d)
Prepared from 1d (345 mg, 1 mmol). Yield: 224 mg (69%) of a white solid, 2d:6d = 81:19. – IR (ATR): v = 3246, 3064, 3032, 2999, 2949, 2949, 2894, 1727 (C=O), 1690 (C=O), 1642 (C=O), 1589, 1504, 1456, 1431, 1368, 1297, 1262, 1231, 1146, 1086, 1062, 996, 936, 915, 873, 843, 823, 779, 748, 719, 698, 665 cm−1.
NMR and MS data for compound 2d: 1H NMR (500 MHz, [D6]DMSO): δ = 4.47 (t, J = 9.7 Hz, 1H, 5-H), 4.81 (t, J = 10.5 Hz, 1H, 4-H), 4.98 and 5.02 (2d, 1:1, J = 12.4 Hz, 2H, OCH2Ph), 5.34 (d, J = 10.5 Hz, 1H, 1-H), 7.29–7.41 (m, 6H, 6H of Ar), 7.48 (dd, J = 7.7, 1.6 Hz, 1H, 1H of Ar), 7.71 (dd, J = 7.5, 2.0 Hz, 1H, 1H of Ar), 7.75 (br d, J = 9.3 Hz, 2H, NHCbz, 1H of Ar), 9.52 (s, 1H, 2-H) ppm. – 13C NMR (126 MHz, [D6]DMSO): δ = 57.2, 62.6, 65.6, 127.4, 127.8, 127.9, 128.4, 128.7, 129.7, 129.9, 134.1, 134.2, 136.8, 156.0, 172.2 ppm. – HRMS ((+)-ESI): m/z = 348.1102 (calcd. 348.1109 for C17H19ClN3O3, [M+H]+).
NMR and MS data for compound 6d: 1H NMR (500 MHz, [D6]DMSO): δ = 4.93 (s, 2H, NH2), 6.65 (s, 1H, CH), 7.35–7.43 (m, 3H, 3H of Ar), 7.55 (dd, J = 7.6, 1.6 Hz, 1H, 1H of Ar), 10.80 (s, 1H, 1-H) ppm. – 13C NMR (126 MHz, [D6]DMSO): δ = 104.2, 127.6, 127.8, 129.7, 130.0, 130.3, 130.7, 133.2, 154.7, 162.8 ppm. – HRMS ((+)-ESI): m/z = 238.0377 (calcd. 238.0383 for C10H9ClN3O2, [M+H]+).
4.2.4 (Z)-3-amino-5-(2-nitrobenzylidene)imidazolidine-2,4-dione (6e)
Prepared from 1e (89 g, 0.25 mmol). Yield: 30 mg (48%) of yellow crystals. M.p. 180°C (decomp). – IR (ATR): δ = 3331, 3252, 3171, 2970, 1773 (C=O), 1714 (C=O), 1658 (C=O), 1619, 1600, 1588, 1572, 1517, 1439, 1340, 1308, 1248, 1211, 1177, 1131, 1077, 1045, 949, 882, 859, 785, 747, 721, 694, 673, 660, 642, 606 cm−1. – 1H NMR (500 MHz, [D6]DMSO): δ = 4.92 (s, 2H, NH2), 6.61 (s, 1H, CH), 7.31 (ddd, J = 7.5, 4.8, 0.9 Hz, 1H, 1H of Ar), 7.63 (d, J = 7.8 Hz, 1H, 1H of Ar), 7.84 (td, J = 7.7, 1.8 Hz, 1H, 1H of Ar), 8.66 (d, J = 4.0 Hz, 1H, 1H of Ar), 10.52 (s, 1H, 1-H) ppm. – 13C NMR (126 MHz, [D6]DMSO): δ = 106.1, 122.5, 125.6, 129.3, 129.3, 137.2, 149.5, 153.7, 153.7, 162.7 ppm. – HRMS ((+)-ESI): m/z = 249.0619 (calcd. 249.0618 for C10H9N4O4, [M+H]+).
4.2.5 (4R*,5R*)-4-benzyloxycarbonylamino-5-(3-nitrophenyl)-3-pyrazolidinone (2f) and (Z)-3-amino-5-(3-nitrobenzylidene)imidazolidine-2,4-dione (6f)
Prepared from 1f (178 mg, 0.5 mmol). Yield: 30 mg (17%) of a yellow solid, 2f:6f = 88:12. – IR (ATR): v = 3368, 3285, 3231, 3186, 3095, 2945, 1775 (C=O), 1694 (C=O), 1661 (C=O), 1585, 1523, 1467, 1453, 1413, 1350, 1290, 1232, 1213, 1186, 1147, 1100, 1079, 1062, 1030, 964, 918, 897, 833, 816, 771, 755, 736, 686, 643, 609 cm−1.
NMR and MS data for compound 2f [11]: 1H NMR (500 MHz, [D6]DMSO): δ = 4.43 (t, J = 9.8 Hz, 1H, 4-H), 4.49 (t, J = 10.6 Hz, 1H, 5-H), 5.02 (s, J = 6.4 Hz, 2H, OCH2), 5.67 (d, J = 10.6 Hz, 1H, 1-H), 7.29–7.39 (m, 5H, Ph), 7.70 (t, J = 8.0 Hz, 1H, 1H of Ar), 7.83 (d, J = 8.8 Hz, 1H, NHCbz), 7.90 (d, J = 7.8 Hz, 1H, 1H of Ar), 8.21 (dd, J = 7.9, 2.2 Hz, 1H, 1H of Ar), 8.35 (t, J = 2.0 Hz, 1H, 1H of Ar), 9.65 (s, 1H, 2-H) ppm. – 13C NMR (126 MHz, [D6]DMSO): δ = 57.9, 65.0, 65.7, 121.9, 123.1, 127.7, 127.9, 128.4, 130.2, 134.1, 136.8, 139.4, 147.9, 156.2, 172.0 ppm. – HRMS ((+)-ESI): m/z = 357.1189 (calcd. 357.1193 for C17H17N4O5, [M+H]+).
NMR data for compound 6f: 1H NMR (500 MHz, [D6]DMSO): δ = 4.91 (s, 2H, NH2), 6.64 (s, 1H, CH), 7.77 (t, J = 8.0 Hz, 1H, 1H of Ar), 8.04 (d, J = 7.8 Hz, 1H, 1H of Ar), 8.13 (dd, J = 8.2, 1.7 Hz, 1H, 1H of Ar), 8.45 (t, J = 2.0 Hz, 1H, 1H of Ar), 9.36 (s, 1H, 1-H) ppm. – 13C NMR (126 MHz, [D6]DMSO): δ = 106.3, 123.7, 127.7, 127.9, 130.1, 135.4, 147.9, 148.2, 163.4, 166.7 ppm.
4.2.6 (4R*,5R*)-4-benzyloxycarbonylamino-5-(4-nitrophenyl)-3-pyrazolidinone (2g) and (Z)-3-amino-5-(4-nitrobenzylidene)imidazolidine-2,4-dione (6g)
Prepared from 1g 178 mg, 0.5 mmol). Yield: 100 mg (57%) of a yellow solid, 2g:6g = 93:7. – IR (ATR): v = 3337, 3224, 3176, 3082, 1715 (C=O), 1693 (C=O), 1604, 1518, 1454, 1419, 1374, 1345, 1286, 1238, 1208, 1179, 1147, 1107, 1083, 1049, 1013, 976, 948, 917, 889, 867, 846, 836, 776, 747, 723, 696, 685, 630 cm−1.
NMR and MS data for compound 2g [11]: 1H NMR (500 MHz, [D6]DMSO): δ = 4.40 (t, J = 9.8 Hz, 1H, 5-H), 4.49 (t, J = 10.5 Hz, 1H, 4-H), 5.01 and 5.03 (2d, 1:1, J = 12.6 Hz, 2H, OCH2), 5.68 (d, J = 10.6 Hz, 1H, 1-H), 7.29–7.40 (m, 5H, Ph), 7.72 (d, J = 8.7 Hz, 2H, 2H of Ar), 7.84 (d, J = 9.0 Hz, 1H, NHCbz), 8.25 (d, J = 8.7 Hz, 2H, 2H of Ar), 9.66 (s, 1H, 2-H) ppm. – 13C NMR (126 MHz, [D6]DMSO): δ = 58.0, 65.1, 65.7, 123.7, 127.8, 127.9, 128.4, 128.5, 136.8, 144.9, 147.3, 156.2, 172.0 ppm. – HRMS ((+)-ESI): m/z = 357.1190 (calcd. 357.1193 for C17H17N4O5, [M+H]+).
NMR data for compound 6g: 1H NMR (500 MHz, [D6]DMSO): δ = 4.94 (s, 2H, NH2), 6.62 (s, 1H, CH), 7.89 (d, J = 8.8 Hz, 2H, 2H of Ar), 8.22 (d, J = 8.9 Hz, 2H, 2H of Ar), 9.37 (br s, 1H, NH), ppm. – 13C NMR (126 MHz, [D6]DMSO): δ = 106.2, 123.0, 123.8, 127.3, 130.3, 146.2, 155.1, 163.0 ppm.
4.2.7 (Z)-3-amino-5-(2-pyridylmethylidene)imidazolidine-2,4-dione (6h)
Prepared from 1h (599 mg, 1,9 mmol). Yield: 194 mg (50%) of white crystals. M.p. 200°C (decomp). – IR (ATR): v = 3318, 3207, 3034, 1760 (C=O), 1706 (C=O), 1661 (C=O), 1615, 1575, 1556, 1470, 1432, 1394, 1356, 1261, 1238, 1182, 1151, 1136, 1090, 935, 917, 883, 789, 749, 715, 669, 622, 604 cm−1. – 1H NMR (500 MHz, [D6]DMSO): δ = 4.93 (s, 2H, NH2), 6.62 (s, 1H, CH), 7.31 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H, 5′-H), 7.63 (dt, J = 7.9, 1.1 Hz, 1H, 3′-H), 8.66 (ddd, J = 4.9, 1.9, 0.9 Hz, 1H, 6′-H), 8.84 (td, J = 7.7, 1.9 Hz, 1H, 4′-H), 10.51 (br s, 1H, NH) ppm. – 13C NMR (126 MHz, [D6]DMSO): δ = 106.1, 122.5, 125.6, 129.3, 137.2, 149.5, 153.7, 153.7, 162.7 ppm. – HRMS ((+)-ESI): m/z = 205.0719 (calcd. 205.072 for C9H9N4O2, [M+H]+). – C9H8N4O2 (204.2): calcd. C 52.94, H 3.95, N 27.44; found: C 52.90, H 3.48, N 26.80.
4.2.8 (Z)-3-amino-5-(3-pyridylmethylidene)imidazolidine-2,4-dione (6i)
Prepared from 1i (178 mg, 0.5 mmol). Yield: 5 mg (5%) of orange crystals. M.p. 180°C (decomp). – IR (ATR): v = 3359, 3285, 3197, 3052, 2764, 2165, 2096, 1966, 1771 (C=O), 1735 (C=O), 1718 (C=O), 1703 (C=O), 1658 (C=O), 1593, 1536, 1514, 1496, 1440, 1377, 1334, 1280, 1267, 1238, 1215, 1174, 1130, 1032, 961, 915, 879, 854, 843, 812, 778, 750, 708, 691, 652, 634, 607 cm−1. – 1H NMR (500 MHz, [D6]DMSO): δ = 4.93 (s, 2H, NH2), 6.56 (s, 1H, CH), 7.43 (dd, J = 8.0, 4.8 Hz, 1H, 5′-H), 8.06 (dt, J = 8.1, 2.0 Hz, 1H, 6′-H), 8.50 (dd, J = 4.8, 1.5 Hz, 1H, 4′-H), 8.80 (d, J = 2.3 Hz, 1H, 2′-H), 9.00 (s, 1H, 1-H) ppm. – 13C NMR (126 MHz, [D6]DMSO): δ = 105.7, 123.7, 129.0, 135.8, 148.8, 150.4, 154.7, 162.7, 169.2 ppm. – HRMS ((+)-ESI): m/z = 205.0720 (calcd. 205.0720 for C9H9N4O2, [M+H]+).
4.2.9 (Z)-3-amino-5-(4-pyridylmethylidene)imidazolidine-2,4-dione (6j)
Prepared from 1j (178 mg, 0.5 mmol). Yield: 3 mg (3%) of yellow crystals. M.p. 200°C (decomp). – IR (ATR): v = 3273, 3195, 3031, 2168, 1788 (C=O), 1730 (C=O), 1703 (C=O), 1659 (C=O), 1599, 1550, 1535, 1514, 1498, 1453, 1438, 1366, 1333, 1267, 1238, 1205, 1182, 1137, 1073, 1025, 998, 959, 935, 903, 845, 813, 778, 746, 724, 691, 669, 642, 610 cm−1. – 1H NMR (500 MHz, [D6]DMSO): δ = 4.93 (s, 2H, NH2), 6.47 (s, 1H, CH), 7.56–7.62 (m, 2H, 2′-H, 6′-H), 8.51–8.65 (m, 2H, 3′-H, 5′-H), 10.39 (br s, 1H, 1-H) ppm. – 13C NMR (126 MHz, [D6]DMSO): δ = 105.8, 123.3, 136.8, 140.1, 150.0, 154.9, 162.8 ppm. – HRMS ((+)-ESI): m/z = 205.0723 (calcd. 205.0720 for C9H9N4O2 [M+H]+).
4.2.10 (4R*,5R*)-4-benzyloxycarbonylamino-5-(2-furyl)-3-pyrazolidinone (2k) and (Z)-3-amino-5-(furan-2-ylmethylene)imidazolidine-2,4-dione (6k)
Prepared from 1k (150 mg, 0.5 mmol). Yield: 106 mg (79%) of a beige solid, 2k:6k = 71:29. – IR (ATR): v = 3338, 3291, 3208, 3175, 2942, 1718 (C=O), 1695 (C=O), 1661 (C=O), 1534, 1499, 1466, 1453, 141, 1403, 1356, 1285, 1238, 1196, 1160, 1134, 1060, 1027, 1016, 964, 936, 903, 884, 827, 804, 773, 739, 694, 675, 646, 612 cm−1.
NMR and MS data for compound 2k: 1H NMR (500 MHz, [D6]DMSO): δ = 4.38 (t, J = 11.2 Hz, 1H, 5-H), 4.52 (t, J = 11.2, 1H, 4-H), 5.03 (s, 2H, OCH2Ph), 5.39 (d, J = 11.2 Hz, 1H, 1-H), 6.46 (dd, J = 3.3, 1.8 Hz, 1H, 4′-H), 6.49 (d, J = 3.3 Hz, 1H, 3′-H), 7.29–7.38 (m, 5H, Ph), 7.67 (d, J = 1.3 Hz, 1H, 5′-H), 7.69 (d, J = 9.3 Hz, 1H, NHCbz), 9.51 (s, 1H, 2-H) ppm. – 13C NMR (126 MHz, [D6]DMSO): δ = 56.1, 59.7, 65.7, 108.5, 110.6, 112.7, 113.5, 127.8, 128.3, 136.8, 143.3, 156.2, 172.4 ppm. – HRMS ((+)-ESI): m/z = 302.1121 (calcd. 302.1135 for C15H16N3O4, [M+H]+).
NMR data for compound 6k: 1H NMR (500 MHz, [D6]DMSO): δ = 4.89 (s, 2H, NH2), 6.45 (s, 1H, CH), 6.64 (dd, J = 3.2, 1.6 Hz, 1H, 4′-H), 6.95 (d, J = 3.4 Hz, 1H, 3′-H), 7.78 (d, J = 1.3 Hz, 1H, 5′-H), 9.04 (s, 1H, 1-H) ppm. – 13C NMR (126 MHz, [D6]DMSO): δ = 97.7, 112.5, 115.9, 120.2, 127.6, 148.9, 154.2, 162.6 ppm.
4.2.11 (4R*,5R*)-4-benzyloxycarbonylamino-5-phenyl-3-pyrazolidinone (2m)
Prepared from 1m (0.327 g, 1 mmol). Yield: 0.147 g (47%) of white crystals. Physical and spectral data of 2m were in agreement with the literature data [10].
4.2.12 (4R*,5R*)-4-benzyloxycarbonylamino-5-isopropyl-3-pyrazolidinone (2n)
Prepared from 1n (0.275 g, 1 mmol). Yield: 0.200 g (72%) of white crystals. Physical and spectral data of 2n were in agreement with the literature data [7].
4.3 Synthesis of (RS)-benzyl (1-hydrazinyl-1-oxo-3-phenylpropan-2-yl)carbamate (7m)
Hydrazine hydrate (98%, 75 μL, 1.5 mmol) was added to a stirred suspension of 2m (0.156 g, 0.5 mmol) in EtOH (5 mL) and the mixture was stirred at r.t. for 7 days. Volatile components were evaporated in vacuo to afford 7m. Yield: 157 mg (100%) of a colorless resin. – IR (ATR): v = 3310, 3034, 2946, 1690 (C=O), 1646 (C=O), 1529, 1384, 1372, 1311, 1257, 1128, 1040, 910, 752, 698, 655 cm−1. – 1H NMR (500 MHz, [D6]DMSO): δ = 2.76 (dd, J = 13.7, 10.4 Hz, 1H, 3-Ha), 2.90 (dd, J = 13.7, 4.6 Hz, 1H, 3-Hb), 4.18 (ddd, J = 10.4, 8.7, 4.5 Hz, 1H, 2-H), 4.27 (s, 2H, NH2), 4.92 (s, 2H, OCH2Ph), 7.16–7.37 (m, 10H, 2 × Ph), 7.54 (d, J = 8.7 Hz, 1H, NHCH), 9.23 (s, 1H, NHNH2) ppm. – 13C NMR (126 MHz, [D6]DMSO): δ = 37.8, 55.0, 65.2, 126.3, 127.5, 127.7, 128.1, 128.3, 128.3, 129.2, 137.0, 138.1, 155.7, 170.8 ppm. – HRMS ((+)-ESI): m/z = 314.1498 (calcd. 314.1499 for C17H20N3O3, [M+H]+).
Acknowledgments:
We are grateful to the Slovenian Research Agency (Program Code P1-0179) for the financial support.
References
[1] U. Kazmaier in Amino Acids, Peptides and Proteins in Organic Chemistry: Modified Amino Acids, Organocatalysis and Enzyme, Vol. 2 (Ed.: A. B. Hughes), Wiley-VCH, Weinheim, 2009, chapter 1, p. 1.10.1002/9783527631780.ch1Search in Google Scholar
[2] B. Stanovnik, J. Svete, Chem. Rev.2004, 104, 2433.10.1021/cr020093ySearch in Google Scholar PubMed
[3] J. M. Humphrey, A. R. Chamberlin, Chem. Rev.1997, 97, 2243.10.1021/cr950005sSearch in Google Scholar PubMed
[4] U. Schmidt, A. Lieberknecht, J. Wild, Synthesis1988, 20, 159.10.1055/s-1988-27503Search in Google Scholar
[5] H. Dorn, Chem. Heterocycl. Compd. USSR1981, 17, 1.10.1007/BF00507082Search in Google Scholar
[6] R. M. Claramunt, J. Elguero, Org. Prep. Proced. Int. 1991, 23, 273.10.1080/00304949109458208Search in Google Scholar
[7] A. Novak, A. Testen, J. Bezenšek, U. Grošelj, M. Hrast, M. Kasunič, S. Gobec, B. Stanovnik, J. Svete, Tetrahedron2013, 69, 6648.10.1016/j.tet.2013.05.122Search in Google Scholar
[8] J. Svete in Stereochemistry Research Trends (Eds.: M. A. Horvat, J. H. Golob), Nova Science Publishers, New York, 2008, chapter 5, p. 129. (open access item).Search in Google Scholar
[9] U. Grošelj, J. Svete, ARKIVOC2015, Part (vi), 175.10.3998/ark.5550190.p009.129Search in Google Scholar
[10] A. Novak, J. Bezenšek, U. Grošelj, A. Golobič, B. Stanovnik, J. Svete, ARKIVOC2011, Part (vi), 18.10.3998/ark.5550190.0012.603Search in Google Scholar
[11] A. Novak, M. štefanič, U. Grošelj, M. Hrast, M. Kasunič, S. Gobec, B. Stanovnik, J. Svete, Helv. Chim. Acta2014, 97, 245.10.1002/hlca.201300169Search in Google Scholar
[12] U. Schmidt, H. Griesser, H. Lietenberger, A. Lieberknecht, R. Mangold, R. Meyer, B. Riedl, Synthesis1992, 24, 487.10.1055/s-1992-26143Search in Google Scholar
[13] U. Schmidt, A. Lieberknecht, J. Wild, Synthesis1984, 16, 53.10.1055/s-1984-30730Search in Google Scholar
[14] T. Sau-Fun, A. Kok-Peng, F. Yoke-Fan, J. Chem. Soc., Perkin Trans. 21986, 1941.Search in Google Scholar
[15] N. A. Meanwell, H. R. Roth, E. C. R. Smith, D. L. Wedding, J. J. K. Wright, J. Org. Chem.1991, 56, 6897.10.1021/jo00024a036Search in Google Scholar
[16] O. Ersoy, R. Fleck, M.-J. Blanco, S. Masamune, Bioorg. Med. Chem.1999, 7, 279.10.1016/S0968-0896(98)00203-XSearch in Google Scholar
[17] R. Guillon, C. Logé, F. Paginez, V. Ferchaud-Roucher, M. Duflos, C. Picot, P. Le Pape, J. Enzyme Inhib. Med. Chem.2011, 26, 261.10.3109/14756366.2010.503607Search in Google Scholar PubMed
©2016 by De Gruyter
Articles in the same Issue
- Frontmatter
- In this Issue
- Preface
- Chemistry of the iminium and imine functional groups
- Transformations of β-aryl-N-Cbz-α,β-didehydro-α-amino esters with hydrazine hydrate
- A short synthesis of pyridines from deprotonated α-aminonitriles by an alkylation/RCM sequence
- Palladium complexes of anionic N-heterocyclic carbenes derived from sydnones in catalysis
- Synthesis and characterisation of long wavelength-absorbing donor/acceptor-substituted methine dyes
- Selective di- and monochlorination of pyridazine-annelated bis(imidazolium) salts
- Synthesis and investigation of new cyclic haloamidinium salts
- A simple synthesis of dimethyl 2-[(Z)-3-amino-1-oxo-1-(substituted)but-2-en-2-yl]fumarates: potential intermediates in the synthesis of polysubstituted five- and six-membered heterocycles
- 2-(1,2,3-Triazol-4-yl)-imidazoline, -oxazoline, -thiazoline and -tetrahydropyrimidine as ligands in copper(II) and nickel(II) complexes
- Derivatives of the triaminoguanidinium ion, 4. O-Sulfonylation of N,N′,N″-tris(hydroxybenzylidenamino)guanidinium ions
- Consecutive three- and four-component coupling-Bagley-Bohlmann-Rahtz syntheses of tri- and tetrasubstituted pyridines
- Orthoamide und Iminiumsalze, XCI. N,N′,N″-Peralkylierte Guanidiniumsalze – Ionische Flüssigkeiten als Hilfsmittel in der Elektronenmikroskopie
Articles in the same Issue
- Frontmatter
- In this Issue
- Preface
- Chemistry of the iminium and imine functional groups
- Transformations of β-aryl-N-Cbz-α,β-didehydro-α-amino esters with hydrazine hydrate
- A short synthesis of pyridines from deprotonated α-aminonitriles by an alkylation/RCM sequence
- Palladium complexes of anionic N-heterocyclic carbenes derived from sydnones in catalysis
- Synthesis and characterisation of long wavelength-absorbing donor/acceptor-substituted methine dyes
- Selective di- and monochlorination of pyridazine-annelated bis(imidazolium) salts
- Synthesis and investigation of new cyclic haloamidinium salts
- A simple synthesis of dimethyl 2-[(Z)-3-amino-1-oxo-1-(substituted)but-2-en-2-yl]fumarates: potential intermediates in the synthesis of polysubstituted five- and six-membered heterocycles
- 2-(1,2,3-Triazol-4-yl)-imidazoline, -oxazoline, -thiazoline and -tetrahydropyrimidine as ligands in copper(II) and nickel(II) complexes
- Derivatives of the triaminoguanidinium ion, 4. O-Sulfonylation of N,N′,N″-tris(hydroxybenzylidenamino)guanidinium ions
- Consecutive three- and four-component coupling-Bagley-Bohlmann-Rahtz syntheses of tri- and tetrasubstituted pyridines
- Orthoamide und Iminiumsalze, XCI. N,N′,N″-Peralkylierte Guanidiniumsalze – Ionische Flüssigkeiten als Hilfsmittel in der Elektronenmikroskopie

