Abstract
The equiatomic plumbides SrPdPb and SrPtPb were synthesized by induction-melting of the elements in sealed tantalum ampoules followed by annealing in muffle furnaces. Both crystal structures were refined from single crystal X-ray diffractometer data: TiNiSi type, Pnma, a = 764.58(4), b = 478.23(7), c = 832.20(7) pm, wR2 = 0.0432, 643 F2 values, 20 variables for SrPdPb and a = 765.05(2), b = 476.31(3), c = 825.25(4), wR2 = 0.0691, 642 F2 values, 20 variables for SrPtPb. The palladium (platinum) and lead atoms built orthorhombically distorted and strongly puckered Pd3Pb3 (283–290 pm Pd–Pb) respectively Pt3Pb3 (281–291 pm Pt–Pb) hexagons that coordinate the strontium atoms.
1 Introduction
The ternary systems RE-T-Pb (RE = rare earth element, T = electron-rich transition metal) have intensively been studied in the past 30 years with respect to phase formation and the rare earth magnetism. 1 , 2 So far, more than 280 RE x T y Pb z plumbides 3 have been characterized. These plumbides have two pitfalls: (i) Due to the comparatively low boiling temperature of lead (2013 K), the samples need to be synthesized in sealed high-melting metal ampoules. Synthesis by arc-melting would result in too large lead evaporation. (ii) The high heavy metal toxicity of lead hampers any application. Nevertheless, these RE x T y Pb z plumbides are an important family of intermetallic compounds for basic research in order to study structure property relationships. An advantage is the possibility to grow single crystals via a lead self-flux technique. Two striking examples are GdPtPb and Yb2Pt2Pb. GdPtPb orders antiferromagnetically at TN = 15.5 K with a planar, collinear magnetic structure and a pronounced metamagnetic step at a critical field of 20 kOe. 4 Ytterbium is trivalent in Yb2Pt2Pb and orders antiferromagnetically below TN = 2.07 K. 5 , 6 The Shastry-Sutherland lattice geometry leads to high magnetic anisotropy what is also evident from the multistep metamagnetic behavior in the low-temperature magnetization isotherms. 7
Since the europium-based plumbides EuPdPb, 8 EuRhPb2 and EuPdPb2 9 all exhibit stable divalent ground states, at least related strontium-based alkaline earth (AE) plumbides should exist. A look at the Pearson database readily revealed that only few AE x T y Pb z plumbides have been synthesized. Figure 1 gives a short overview on the ternary alkaline earth plumbides that have been structurally characterized. It is remarkable that no phases have yet been reported for the Mg-T-Pb systems. 3
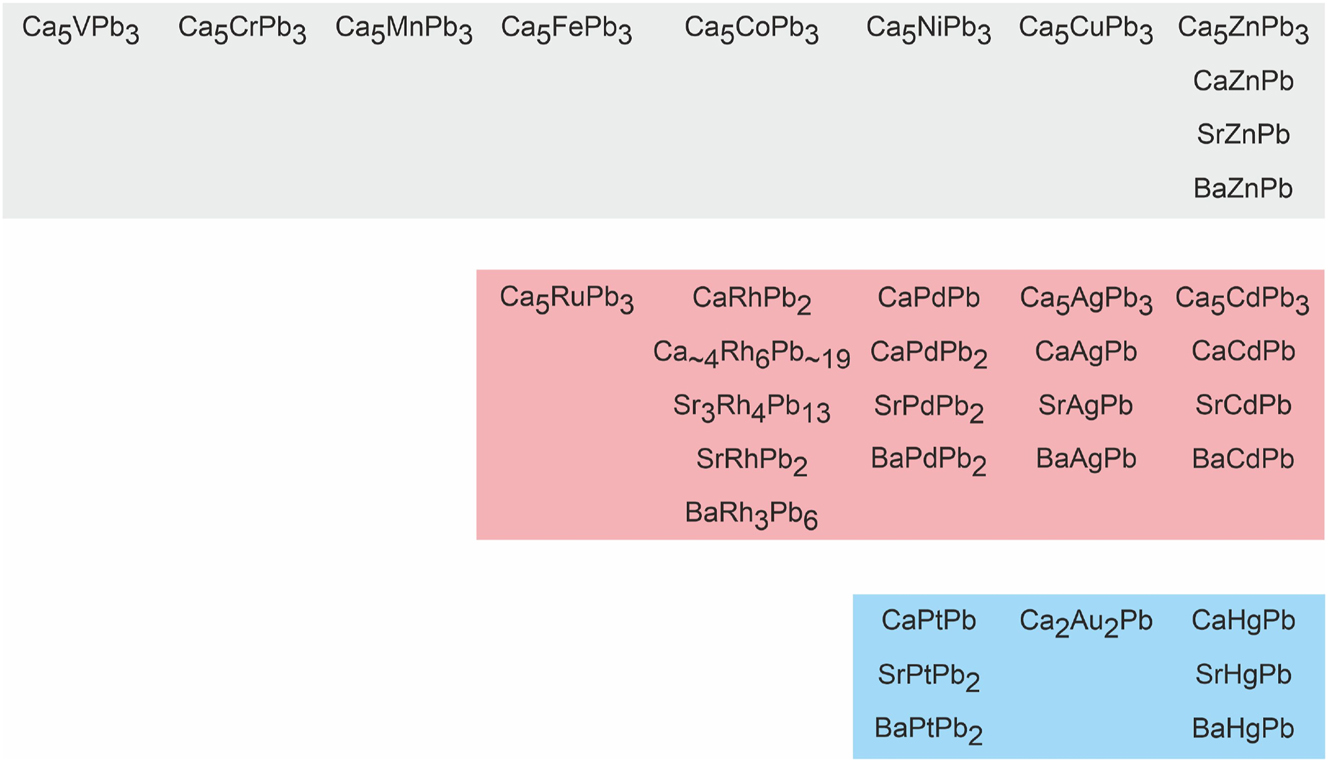
Structurally characterized alkaline earth plumbides.
The largest series of compounds comprises the Ca5TPb3 (T = V, Cr, Mn, Fe, Co. Ni, Cu, Zn, Ru, Ag, Cd) 10 phases which crystallize with a stuffed Mn5Si3 variant. Most of the equiatomic plumbides AETPb (AE = Ca, Sr, Ba, T = Zn, Pd, Ag, Cd, Pt, Hg) 11 , 12 , 13 , 14 , 15 , 16 crystallize with superstructure variants of the aristotype AlB2. 17 CaCdPb 12 adopts the hexagonal ZrNiAl type. Ca2Au2Pb 18 is a singular compound which crystallizes with the Mo2B2Fe-type structure. With higher lead content, the AETPb2 (AE = Ca, Sr, Ba; T = Rh, Pd, Pt) 9 phases crystallize with the MgCuAl2-type structure with a lonsdaleite-related lead substructure. Further lead-rich compounds are BaRh3Pb6 (own type) 19 and the Remeika phases Ca∼4Rh6Pb∼19 and Sr3Rh4Pb13. 20 , 21
Further phase analytical studies in the AE-T-Pb systems now led to the synthesis and structural characterization of the plumbides SrPdPb and SrPtPb. So far, SrPdPb was structurally characterized only on the basis of a Rietveld refinement. 22 Herein we report on single crystal X-ray diffraction data on SrPdPb and SrPtPb with higher resolution.
2 Experimental
2.1 Synthesis
Starting materials for the synthesis of SrPdPb and SrPtPb were strontium pieces (Onyxmet, 99.95 %), palladium and platinum sheet (Agosi, 99.9 %) and lead granules (ABCR, 99.9 %). The moisture sensitive strontium pieces were kept in Schlenk tubes under dry argon (Westfalen, 99.998 %, purified by using titanium sponge (T = 873 K), silica gel and molecular sieves). Prior to use they were mechanically cleaned from surface impurities under dry paraffin oil (sodium wire) and washed with dry cyclohexane (sodium wire). The elements were then weighed in the ideal atomic ratio of Sr:(Pd/Pt):Pb = 1:1:1 (around 400 mg total sample mass) and arc-welded 23 in tantalum ampoules under an argon pressure of ca. 800 mbar. The latter were placed in a water-cooled sample chamber of an induction furnace (Hüttinger Elektronik, Freiburg, Typ TIG 1.5/300), 24 rapidly heated to 1370 K and kept at that temperature for 15 min. The temperature was then gradually lowered to room temperature over a period of 30 min. The temperature was controlled through a radiation pyrometer (Metis MS09, Sensortherm) with an accuracy of ± 50 K.
For homogenization and crystal growth, the reaction ampoules were subsequently sealed in evacuated silica tubes (oxidation protection) and heated to 1173 K within 60 min. The temperature was kept for 1 h and then reduced to room temperature at a rate of 5 K h−1. The samples could easily be separated from the crucibles by careful mechanical deformation of the tubes. The light grey polycrystalline samples are moisture sensitive and were kept in Schlenk tubes under argon.
2.2 X-ray diffraction
The polycrystalline SrPdPb and SrPtSb samples were characterized by powder X-ray diffraction using the Guinier technique: Enraf-Nonius FR552 camera, CuKα1 radiation, imaging plate detector, Fujifilm BAS–1800 readout system. α-Quartz (a = 491.30 and c = 540.46 pm) was used as an internal standard. The orthorhombic lattice parameters (Table 1) were obtained from least-squares fits of the experimental 2θ values. The correct indexing of the patterns was ensured with the help of intensity calculations (Lazy Pulverix routine 27 ).
Refined lattice parameters (Guinier powder data) of equiatomic ternary alkaline earth tetrelides with TiNiSi-type structure, space group Pnma. Also data of EuPdPb is listed for comparison. Standard deviations are given in parentheses.
| Compound | a/pm | b/pm | c/pm | V/nm³ | Reference |
|---|---|---|---|---|---|
| CaPdPb | 730.6(5) | 465.8(5) | 822.6(5) | 0.2799 | 13 , 22 |
| SrPdSn | 758.3(3) | 470.6(3) | 816.7(2) | 0.2914 | 22 |
| SrPdSn | 758.71(9) | 471.20(5) | 816.63(9) | 0.2919 | 25 |
| SrPdPb | 765.6(1) | 479.0(1) | 833.4(2) | 0.3056 | this work |
| SrPdPb | 765.7(1) | 479.3(1) | 835.9(1) | 0.3068 | 22 |
| EuPdPb | 752.4(2) | 476.0(2) | 826.8(2) | 0.2961 | 8 |
| CaPtPb | 729.7(5) | 464.3(5) | 816.9(8) | 0.2768 | 13 |
| SrPtSn | 764.16(7) | 470.74(4) | 799.64(7) | 0.2877 | 26 |
| SrPtPb | 766.4(2) | 477.0(1) | 826.7(2) | 0.3022 | this work |
Pieces of the annealed samples were carefully crushed and small single crystalline splinters were selected under an optical microscope. They were glued to thin glass fibers using beeswax and coated with Parabar 10312. Due to their moisture sensitivity, the mounted crystals were directly transferred to the goniometer of a Stoe StadiVari diffractometer (Mo-Kα micro focus source and a Pilatus detection system). The Gaussian-shaped profile of the micro focus X-ray source required scaling along with a numerical absorption correction. Details about the data collections and the structure refinements are summarized in Table 2.
Single crystal data and refinement parameters of SrPdPb and SrPtPb, Z = 4, space group Pnma and Pearson code oP12. The data sets were collected at room temperature.
| Formula | SrPdPb | SrPtPb |
| Molar mass/g mol−1 | 401.2 | 489.9 |
| Cell parameters/pm | a = 764.58(4) | a = 765.05(2) |
| b = 478.23(7) | b = 476.31(3) | |
| c = 832.20(7) | c = 825.25(4) | |
| Cell volume/nm3 | V = 0.3043 | V = 0.3007 |
| Calc. density/g cm−3 | 8.76 | 10.82 |
| Diffractometer | Stoe StadiVari | Stoe StadiVari |
| Radiation | Mo-Kα | Mo-Kα |
| Crystal size/µm3 | 20 × 25 × 40 | 15 × 25 × 70 |
| Absorpt. corr. | numerical | numerical |
| Absorpt. coeff./mm−1 | 78.2 | 119.6 |
| Detector distance/mm | 40 | 40 |
| Irradiation time/s | 72 | 54 |
| ω range, increment/◦ | 0-180; 1.0 | 0-180; 1.0 |
| Integr. param. (A; B; EMS) | 7.0; −2.0; 0.030 | 7.0; −6.0; 0.030 |
| F(000)/e | 664 | 792 |
| θ range/◦ | 3.6–33.3 | 3.6–33.5 |
| hkl range | ±11; ±7; ±12 | ±11; ±7; ±12 |
| No. Refl. | 4273 | 9783 |
| Indep. Refl./Rint | 643/0.0370 | 642/0.0737 |
| Refl. with I ≥ 3σ(I)/R σ | 559/0.0123 | 560/0.0162 |
| Data/parameters | 643/20 | 642/20 |
| Goodness-of-fit | 1.38 | 1.92 |
| R1/wR2 (I ≥ 3σ(I)) | 0.0190/0.0421 | 0.0284/0.0683 |
| R1/wR2 (all data) | 0.0237/0.0432 | 0.0334/0.0691 |
| Extinct. coeff. | 169(10) | 30(7) |
| Largest diff. peak, hole/e Å−3 | 1.68/−1.66 | 4.23/−2.64 |
2.3 EDX analysis
The SrPdPb and SrPtPb single crystals were semiquantitatively analyzed by EDX after the data collections using a Zeiss EVO® MA10 scanning electron microscope which was operated in variable pressure mode (60 Pa N2). The microscope was equipped with a LaB6 cathode and an Oxford Instruments INCA® x-act detector. SrF2, Pd, Pt and PbF2 were used as standards. The analyses on the Parabar 10312 coated irregular crystal surfaces resulted in the compositions 34 ± 2 at.% Sr : 33 ± 2 at.% Pd : 33 ± 2 at.% Pb and 34 ± 2 at.% Sr : 34 ± 2 at.% Pt : 32 ± 2 at.% Pb, in excellent agreement with the ideal composition (33.3 : 33.3 : 33.3). No impurity elements (especially tantalum as the ampoule material) were detected.
3 Structure refinements
Both data sets showed primitive orthorhombic lattices and the systematic extinctions were in agreement with space group Pnma. The starting atomic parameters were then deduced with the charge-flipping algorithm 28 implemented in Superflip 29 and the structures were refined on F 2 with the Jana2020 software package, 30 , 31 with anisotropic displacement parameters for all sites. Separate refinements of the occupancy parameters gave no hints for deviations from the ideal compositions. The final difference Fourier analysis were contourless. Further details on the refinements, the atomic coordinates, the displacement parameters and the interatomic distances are listed in Tables 2–4.
Atomic coordinates and anisotropic displacement parameters (pm2) for SrPdPb and SrPtPb (space group Pnma). The anisotropic displacement factor exponent takes the form: –2π2[(ha*)2U11 + … + 2hka*b*U12]. Ueq is defined as one third of the trace of the orthogonalized U ij tensor. All atoms lie on Wyckoff positions 4c (x,1/4, z). U12 = U23 = 0.
| Atom | x | z | U 11 | U 22 | U 33 | U 13 | U eq |
|---|---|---|---|---|---|---|---|
| SrPdPb | |||||||
| Sr | 0.02117(9) | 0.68842(8) | 206(3) | 206(3) | 226(3) | −7(2) | 213(2) |
| Pd | 0.28877(8) | 0.39502(7) | 227(3) | 203(2) | 237(2) | 13(2) | 223(1) |
| Pb | 0.16615(4) | 0.07384(3) | 218(1) | 191(1) | 211(1) | 10(1) | 207(1) |
| SrPtPb | |||||||
| Sr | 0.01711(18) | 0.68960(18) | 225(5) | 207(5) | 241(6) | −13(4) | 225(3) |
| Pt | 0.28927(7) | 0.39857(7) | 236(2) | 192(2) | 248(3) | 13(2) | 225(1) |
| Pb | 0.16871(7) | 0.07642(7) | 234(2) | 190(2) | 237(2) | 8(2) | 220(1) |
Interatomic distances (pm) for SrPdPb and SrPtPb. All distances of the first coordination spheres are listed. Standard deviations are equal or smaller than 0.2 pm.
| SrPdPb | SrPtPb | ||||||
| Sr: | 1 | Pd | 318.6 | Sr: | 1 | Pt | 317.9 |
| 2 | Pd | 328.4 | 2 | Pt | 329.2 | ||
| 1 | Pb | 339.4 | 1 | Pb | 338.0 | ||
| 2 | Pb | 341.8 | 2 | Pb | 339.6 | ||
| 2 | Pd | 343.7 | 2 | Pt | 342.0 | ||
| 1 | Pb | 348.3 | 1 | Pb | 345.3 | ||
| 2 | Pb | 351.3 | 2 | Pb | 351.0 | ||
| 1 | Pd | 389.6 | 1 | Pt | 382.0 | ||
| 2 | Sr | 395.7 | 2 | Sr | 394.1 | ||
| 2 | Sr | 395.8 | 2 | Sr | 395.3 | ||
| Pd: | 1 | Pb | 283.3 | Pt: | 1 | Pb | 281.4 |
| 2 | Pb | 283.7 | 2 | Pb | 281.6 | ||
| 1 | Pb | 289.7 | 1 | Pb | 291.0 | ||
| 1 | Sr | 318.6 | 1 | Sr | 317.9 | ||
| 2 | Sr | 328.4 | 2 | Sr | 329.2 | ||
| 2 | Sr | 343.7 | 2 | Sr | 342.0 | ||
| 1 | Sr | 389.6 | 1 | Sr | 382.0 | ||
| Pb: | 1 | Pd | 283.3 | Pb: | 1 | Pt | 281.4 |
| 2 | Pd | 283.7 | 2 | Pt | 281.6 | ||
| 1 | Pd | 289.7 | 1 | Pt | 291.0 | ||
| 1 | Sr | 339.4 | 2 | Sr | 338.0 | ||
| 2 | Sr | 341.8 | 1 | Sr | 339.6 | ||
| 1 | Sr | 348.3 | 1 | Sr | 345.3 | ||
| 2 | Sr | 351.3 | 2 | Sr | 351.0 | ||
| 2 | Pb | 369.9 | 2 | Pb | 372.2 | ||
CCDC–2478225 (SrPdPb) and CCDC–2478227 (SrPtPb) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
4 Crystal chemistry
The plumbides SrPdPb and SrPtPb crystallize with the orthorhombic TiNiSi-type 32 structure, space group Pnma. They are ternary ordered variants of the aristotype AlB2. This crystal chemical relation is well documented via group-subgroup relations. 17 The near-neighbor coordination of the strontium atoms in SrPdPb and SrPtPb is presented in Figure 2 along with some closely related tetrelides. Each strontium atom is coordinated by two tilted and orthorhombically distorted Pd3Pb3 respectively Pt3Pb3 hexagons. The strong tilts lead to inter-layer bonding and this directly affects the strontium atoms. Instead of the regular 6 + 2 coordination in the aristotype AlB2, the tilting pushes four of the six strontium neighbors out of the first coordination sphere and the strontium atoms get a 2 + 2 strontium coordination.
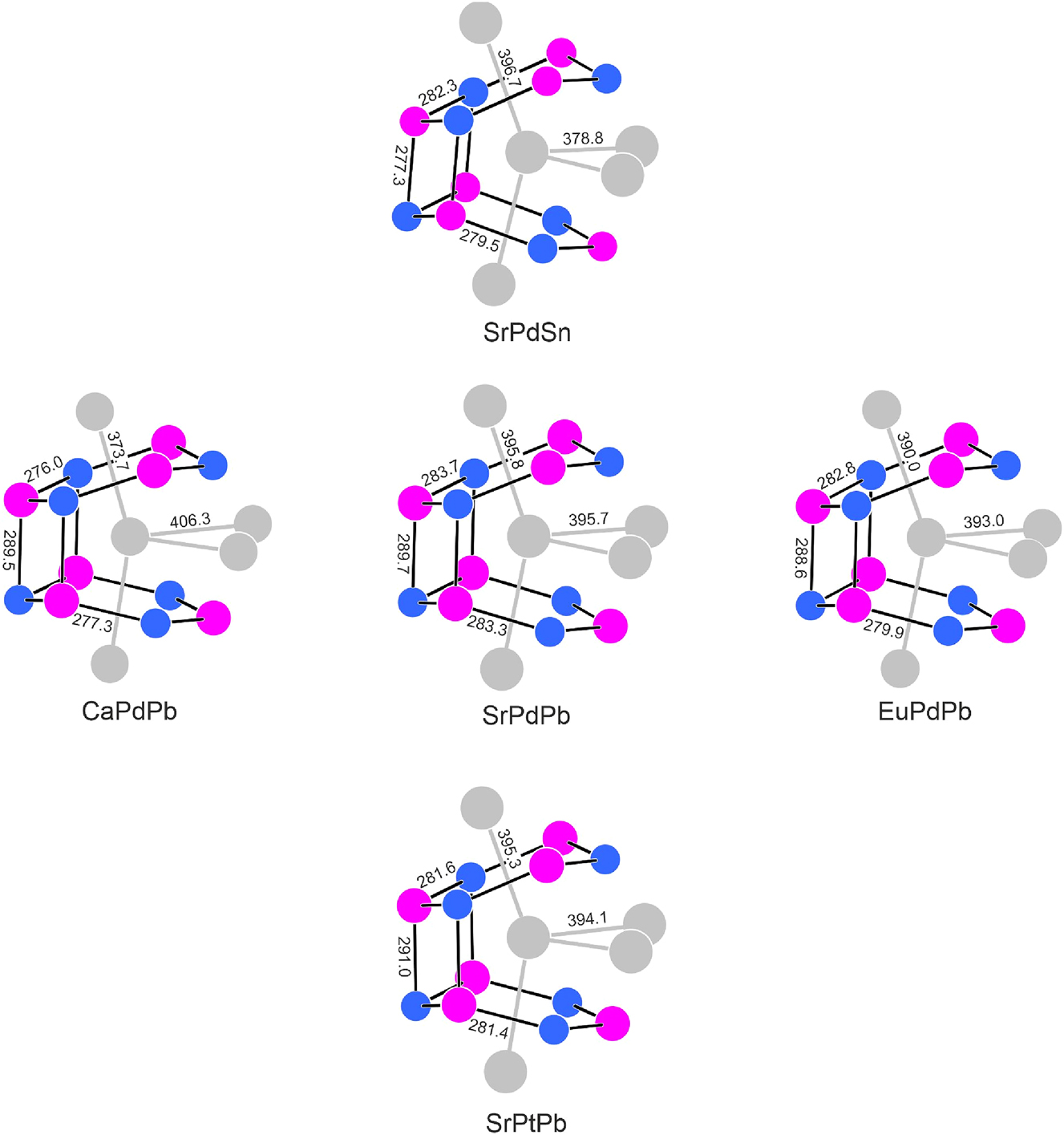
Coordination of the calcium, strontium and europium atoms in the structures of SrPdSn, 25 CaPdPb, 22 SrPdPb, EuPdPb 8 and SrPtSb. Calcium (strontium, europium), palladium (platinum) and tin (lead) atoms are drawn as medium grey, blue and magenta circles, respectively. Relevant interatomic distances (pm) are indicated.
Figure 2 shows the flexibility of the structure type. In the four near-neighbor coordinations drawn around SrPdPb, always one of the atoms is substituted, keeping the same valence electron count. The structure can adjust changes in the atom size and the electronegativity by small changes in the interatomic distances and by the puckering. The largest changes are observed for the substitutions Ca→Sr and Sn→Pb, accounting for the larger differences in size. In agreement with the course of the radii, the cell volumes increase in the sequence CaPdPb→EuPdPb→SrPdPb. This is similar to the AETPb2 plumbides. 9 SrPtPb has a slightly smaller cell volume than SrPdPb, a consequence of the relativistic contraction of platinum. Again, this trend was also observed for the AEPdPb2 and AEPtPb2 plumbides. 9
Exemplarily we discuss the interatomic distances in SrPdPb. The palladium atoms have a strongly distorted tetrahedral lead coordination with Pd–Pb distances in the comparatively small range from 283–290 pm, comparable to the sum of the covalent radii 33 of 282 pm for Pd + Pb. The palladium atoms in MgCuAl2-type EuPdPb2 have coordination number 6 with slightly longer Pd–Pb distances of 286–291 pm. 9 Within the [Pd2Pb2] rhombs that form between the tilted hexagons we observe weak Pb–Pb interactions. The Pb–Pb distances of 370 pm are only slightly longer than in fcc lead (12 × 350 pm). 34
Within the Sr@Pd6Pb6Sr4 coordination, the strontium atom has three shorter Sr–Pd (319–328 pm) and three Sr–Pb (339–342 pm) distances. Both are comparable to the sums of the covalent radii (320 pm for Sr + Pd and 346 pm for Sr + Pb), 33 indicating substantial Sr–Pd and Sr–Pb bonding. This is in line with the Pauling electronegativities 33 of 0.95 (Sr), 2.20 (Pd) and 2.33 (Pb).
The four strontium neighbors of each strontium atom have Sr–Sr distances 396 pm, somewhat smaller than the Sr–Sr distances in fcc strontium (12 × 430 pm), 34 but comparable to twice the covalent radius of 384 pm. 33 This expresses the partial ionization of strontium in SrPdPb, comparable to EuPdPb where 151Eu Mössbauer spectroscopic data revealed a stable divalent ground state. 8
For further general crystal chemical trends in the large family of TiNiSi related intermetallic phases we refer to relevant review articles. 17 , 35 , 36 , 37 , 38 , 39 , 40
Acknowledgements
We thank Dr. R.-D. Hoffmann and Dipl.-Ing. U. Ch. Rodewald for the intensity data collections.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contribution: All authors have accepted responsibility for the entire content of this submitted manuscript and approved the submission.
-
Use of Large Language Models, AI and Machine Learning Tools: Not relevant. Our group is able to think and act independently.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: This research was funded by Universität Münster.
-
Data availability: Data is available from the corresponding author on well-founded request.
References
1. Pöttgen, R.; Rodewald, U.Ch. Rare Earth–Transition Metal–Plumbides. In Handbook on the Physics and Chemistry of Rare Earths; Gschneider, Jr., K. A.; Pecharsky, V. K.; Bünzli, J.-C., Eds.; North-Holland/Elsevier: Amsterdam, Vol. 38, 2008; pp. 55–103. Chapter 237; https://doi.org/10.1016/S0168-1273-07-38002-1.Suche in Google Scholar
2. Klenner, S.; Pöttgen, R. Rare Earth Transition Metal Plumbides – an Update. In Handbook on the Physics and Chemistry of Rare Earths; Pecharsky, V. K.; Bünzli, J.-C., Eds.; North-Holland/Elsevier: Amsterdam, Vol. 57, 2020; pp. 1–44. Chapter 312; https://doi.org/10.1016/bs.hpcre.2020.06.001.Suche in Google Scholar
3. Villars, P.; Cenzual, K., Eds. Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds (Release 2024/25); ASM International®: Materials Park, Ohio (USA), 2024.Suche in Google Scholar
4. Manni, S.; Bud’ko, S. L.; Canfield, P. C. Phys. Rev. B 2017, 96, 054435 (7 pages); https://doi.org/10.1103/physrevb.96.054435.Suche in Google Scholar
5. Pöttgen, R.; Arpe, P. E.; Felser, C.; Kußmann, D.; Müllmann, R.; Mosel, B. D.; Künnen, B.; Kotzyba, G. J. Solid State Chem. 1999, 145, 668–677.Suche in Google Scholar
6. Kim, M. S.; Bennett, M. C.; Aronson, M. C. Phys. Rev. B 2008, 77, 144425 (7 pages); https://doi.org/10.1103/physrevb.77.144425.Suche in Google Scholar
7. Shimura, Y.; Sakakibara, T.; Iwakawa, K.; Ōnuki, Y.; Sugiyama, K. J. Korean Phys. Soc. 2013, 63, 551–554; https://doi.org/10.3938/jkps.63.551.Suche in Google Scholar
8. Heletta, L.; Klenner, S.; Block, T.; Pöttgen, R. Z. Naturforsch. 2017, 72b, 989–994.Suche in Google Scholar
9. Klenner, S.; Bönnighausen, J.; Pöttgen, R. Z. Naturforsch. 2020, 75b, 903–911.Suche in Google Scholar
10. Gulyo, A. M.; Mudring, A.-V.; Corbett, J. D. Inorg. Chem. 2003, 42, 6673–6681; https://doi.org/10.1021/ic0301728.Suche in Google Scholar
11. Iandelli, A. Rev. Chim. Miner. 1987, 24, 28–32.Suche in Google Scholar
12. Merlo, F.; Pani, M.; Fornasini, M. L. J. Less-Common Met. 1991, 171, 329–336; https://doi.org/10.1016/0022-5088-91-90155-w.Suche in Google Scholar
13. Evers, J.; Oehlinger, G.; Polborn, K.; Sendlinger, B. J. Solid State Chem. 1993, 103, 45–56; https://doi.org/10.1006/jssc.1993.1077.Suche in Google Scholar
14. Merlo, F.; Pani, M.; Fornasini, M. L. J. Alloys Compd. 1993, 196, 145–148; https://doi.org/10.1016/0925-8388-93-90585-b.Suche in Google Scholar
15. Iandelli, A. J. Alloys Compd. 1994, 203, 137–138; https://doi.org/10.1016/0925-8388-94-90724-2.Suche in Google Scholar
16. Merlo, F.; Pani, M.; Fornasini, M. L. J. Alloys Compd. 1996, 232, 289–295; https://doi.org/10.1016/0925-8388-95-01952-9.Suche in Google Scholar
17. Hoffmann, R.-D.; Pöttgen, R. Z. Kristallogr. 2001, 216, 127–145.Suche in Google Scholar
18. Fornasini, M. L.; Merlo, F.; Pani, M. Z. Kristallogr. NCS 2001, 216, 23; https://doi.org/10.1524/ncrs.2001.216.14.23.Suche in Google Scholar
19. Venturini, G.; Kamta, M.; Malaman, B.; Marêché, J. F.; Roques, B. Mater. Res. Bull. 1987, 22, 359–364; https://doi.org/10.1016/0025-5408-87-90053-5.Suche in Google Scholar
20. Venturini, G.; Kamta, M.; Mc Rae, E.; Marêché, J. F.; Malaman, B.; Roques, B. Mater. Res. Bull. 1986, 21, 1203–1208; https://doi.org/10.1016/0025-5408-86-90048-6.Suche in Google Scholar
21. Malaman, B.; Venturini, G. J. Less-Common Met. 1989, 155, L1–L4; https://doi.org/10.1016/0022-5088-89-90458-x.Suche in Google Scholar
22. Sendlinger, B. Hochdruck-Untersuchungen an den ambivalenten Verbindungen MTX (M = Yb, Ca, Eu, Sr, Ba; T = Pd, Pt; X = Si, Ge, Sn, Pb). PhD Thesis: Universität München, München, 1993.Suche in Google Scholar
23. Pöttgen, R.; Gulden, Th.; Simon, A. GIT Labor-Fachzeitschrift 1999, 43, 133–136.Suche in Google Scholar
24. Pöttgen, R.; Lang, A.; Hoffmann, R.-D.; Künnen, B.; Kotzyba, G.; Müllmann, R.; Mosel, B. D.; Rosenhahn, C. Z. Kristallogr. 1999, 214, 143–150.Suche in Google Scholar
25. Bönnighausen, J.; Block, T.; Pöttgen, R. Z. Naturforsch. 2024, 79b, 513–519.Suche in Google Scholar
26. Hoffmann, R.-D.; Pöttgen, R.; Kußmann, D.; Niepmann, D.; Trill, H.; Mosel, B. D. Solid State Sci. 2002, 4, 481–487.Suche in Google Scholar
27. Yvon, K.; Jeitschko, W.; Parthé, E. J. Appl. Crystallogr. 1977, 10, 73–74.Suche in Google Scholar
28. Palatinus, L. Acta Crystallogr. 2013, B69, 1–16.Suche in Google Scholar
29. Palatinus, L.; Chapuis, G. J. Appl. Crystallogr. 2007, 40, 786–790; https://doi.org/10.1107/s0021889807029238.Suche in Google Scholar
30. Petříček, V.; Dušek, M.; Palatinus, L. Z. Kristallogr. 2014, 229, 345–352.Suche in Google Scholar
31. Petříček, V.; Palatinus, L.; Plášil, J.; Dušek, M. Z. Kristallogr. 2023, 238, 271–282.Suche in Google Scholar
32. Shoemaker, C. B.; Shoemaker, D. P. Acta Crystallogr. 1965, 18, 900–905; https://doi.org/10.1107/s0365110x65002189.Suche in Google Scholar
33. Emsley, J. The Elements; Oxford University Press: Oxford, 1999.Suche in Google Scholar
34. Donohue, J. The Structures of the Elements; Wiley: New York, 1974.Suche in Google Scholar
35. Parthé, E.; Gelato, L.; Chabot, B.; Penzo, M.; Cenzual, K.; Gladyshevskii, R. TYPIX-Standardized Data and Crystal Chemical Characterization of Inorganic Structure Types. In Gmelin Handbook of Inorganic and Organometallic Chemistry, 8th edition; Springer: Berlin, 1993.Suche in Google Scholar
36. Nuspl, G.; Polborn, K.; Evers, J.; Landrum, G. A.; Hoffmann, R. Inorg. Chem. 1996, 35, 6922–6932; https://doi.org/10.1021/ic9602557.Suche in Google Scholar
37. Landrum, G. A.; Hoffmann, R.; Evers, J.; Boysen, H. Inorg. Chem. 1998, 37, 5754–5763; https://doi.org/10.1021/ic980223e.Suche in Google Scholar
38. Bojin, M. D.; Hoffmann, R. Helv. Chim. Acta 2003, 86, 1653–1682; https://doi.org/10.1002/hlca.200390140.Suche in Google Scholar
39. Bojin, M. D.; Hoffmann, R. Helv. Chim. Acta 2003, 86, 1683–1708; https://doi.org/10.1002/hlca.200390141.Suche in Google Scholar
40. Janka, O.; Niehaus, O.; Pöttgen, R.; Chevalier, B. Z. Naturforsch. 2016, 71b, 737–764.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

