Abstract
Objective
The pathogenesis of depression is not fully understood, but studies have suggested that higher circulating levels of C reactive protein (CRP) might relate to depression occurrence. However, due to the highly variability of individual patients’ conditions, the results to date are inconsistent. Considering Single nucleotide polymorphisms (SNPs) of CRP gene have also been suggested to predict plasma CRP levels. In the present study, we hypothesize that inherited CRP allelic variations may co-vary with depressive symptomatology.
Methods
We recruited patients with a diagnosis of depression, with or without family depression history. We then detected serum CRP levels, as well as genome CRP SNPs from participants of this project.
Results
We found a significantly higher circulating CRP levels in patients with a positive family history. Furthermore, we also identified certain inherited CRP SNPs (A allele in rs1417938 and C allele in rs1205) which could up-regulate serum CRP levels and thus be associated with depression occurrence.
Conclusion
Our findings raise new evidence for the relationship between circulating CRP level and depression occurrence.
1 Introduction
C-reactive protein (CRP) is a conserved protein which has existed for more than 400 million years and is found among vertebrate and invertebrate animals. CRP can strongly react with C polysaccharide of pneumococcus and thus is how its name is derived [1]. CRP was initially indentified in the serum of patients with an acute bacterial infection [2]. At the acute inflammation phase, CRP is produced mostly by the liver and its concentration might rise 2000-fold during the first 24-48 hours of infection. Because serum CRP level is barely detectable under healthy conditions and is usually higher than 10 mg/L in acute inflammation conditions; people have long thought a relatively low level of serum CRP (3-10mg/L) doesn’t represent any physiological or pathological situations. However, recent studies have found under some chronic inflammation conditions, such as cardiovascular disease, metabolic syndrome or cancer, patients will usually have a serum CRP level higher than 3mg/L [3]. Furthermore, studies also indicate an increase in serum CRP may be related to some physical activities, such as smoking [4].
It has been widely acknowledged that chronic inflammation is largely involved in depression [5]. Considering elevated CRP is a primary marker for chronic inflammation, two hypotheses in the field have been raised regarding to inflammation and depression [7]: The first hypothesis is depression related neurobiological conditions may lead to enhanced inflammation and CRP levels. In contrast, the second hypothesis states that elevated CRP or inflammation conditions, in turn, may increase the risk of depression. Plenty of investigations had been made to find out more precise mechanism under CRP and depression, however, the results to date were inconsistent and full of controversy.
Interestingly, both CRP polymorphism and depression are about 40% inheritable [8]. Furthermore, identical twin studies have suggested a potential genetic pathway which may link depression and elevated CRP levels [9]. In addition, there are several CRP genetic polymorphisms which have been found to regulate serum CRP levels. However, although some studies have implicated CRP in depression occurrence; whether and how CRP involved in it still remains controversial. Considering there is evidence suggesting CRP gene polymorphisms and serum CRP level were susceptibility factors for depression in elder people [10], in the present study, we have investigated family inherited depression and calculated its potential relations with certain inherited CRP SNPs. The aim here is to more accurately describe the role of the CRP gene polymorphisms and serum CRP levels in depression, and also to discuss whether circulating CRP levels may contribute to depression occurrence.
2 Materials and Methods
2.1 Participants and grouping
This investigation recruited 200 adults (100 positive and 100 control, age from 18-65) who participated in 2th affiliated hospital of Xinjiang Medical University research program (2013/7-2016/7). Collected data included social-demographic measurements; character of personality, temperament, and psychopathology; general social and cognitive functioning; health-impairing attributes of habit and lifestyle; biological measurements of serum makers; and DNA for the study of genetic variation and phenotypes. All included participants fulfilled ICD-10 diagnosis standard.
Exclusions from our participation including: history of chronic kidney or liver disease, atherosclerotic cardiovascular disease, prior years cancer treatment, and major neurological disorders, schizophrenia or other chronic inflammation related illness such as smoking. Other exclusions included pregnancy and medicine history, such as: insulin, glucocorticoid, antiarrhythmic, psychotropic, or prescription weight-loss medications.
Data collections under approved protocols and guidelines of the Ethics Committee of 2th affiliated hospital of Xinjiang Medical University. 100 depression patients with at least one family members also have depression symptom were assigned as family-DEP group (fDEP), in contrast, 100 depression patients without any clear depression family members were assigned as non-fDEP group (non-fEDP).
2.2 Procedure
Prior to the laboratory visit, participants were asked to get through 8 hours fast and also asked to avoid exercise for 12 hours. Participants were also asked to abstain from alcohol for 24 hours. Laboratory samples were all collected in the morning. Upon subjects’ arrival, they were asked to complete a brief medical history and medication use interview with the help of nurse. After this interview, we recorded and calculated subjects BMI (kg/m2) as well as drew 40 ml blood sample for later use. This 40 ml blood sample was separated into 2 portion of use. The first portion of blood was used for CRP level detection and the second portion was used for DNA isolation and PCR exam later.
2.3 Variable measures
2.3.1 Circulating CRP
Plasma CRP was assayed by using the Abbott Architect c16000 chemistry analyzer (Diamond Diagnostics, MA, USA) as previously described [23]. In this procedure, polystyrene beads are coated with monoclonal antibodies against CRP, which can aggregate CRP antigen and thereby increase the intensity of detecting light. The light intensity is related to the amount of CRP concentrated on the beads. The assay has a detection range of 0.175–1100 mg/L. Logarithmic transformation was used to normalize CRP values distribution.
2.3.2 Depressive symptoms
Depressive symptoms were measured by using Hamilton Rating Scale for Depression (HAMD-17). The patient is rated by a clinician among 17 dimensions with a score on a 3 or 5 point scale. A score of 0-7 is considered to be normal. Scores of 18 or higher indicate moderate or severe depression.
2.3.3 DNA isolation and SNPs analysis
DNA was isolated from frozen whole blood samples as previously described [22, 24]. The CRP gene is located on chromosome 1 (1q21– 23), which has two exons and encodes a protein with 204 amino acid length. In the present study, we have employed five common CRP gene polymorphisms: rs3093059, rs1417938, rs1800947, rs1130864 and rs1205 (Figure 1). We selected these markers by using Tagger algorithm and the HapMap genotype data in China [6]. Subjects were genotyped by using high resolution melting method (LightCycler 480) followed by software analyze (LightCycler 480 software release 1.5.1.62 SP1).
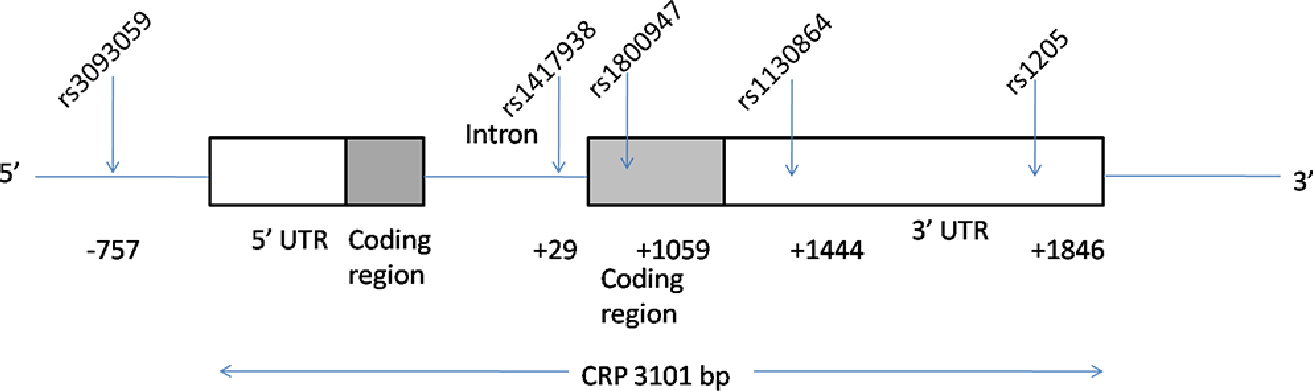
The C-reactive protein (CRP) gene region (chromosome 1q21–q23) and approximate locations of the tag single-nucleotide polymorphism (SNP) locations (dbSNP) relative to the transcription start site.
2.4 Statistical analyses
All data statistical analyses were performed by using SPSS v15.0 (SPSS, Inc., Chicago, IL). X2-Tests was used to compare CRP genotypes distribution within those predicted SNPs, Bonferroni correction was used in post-hoc test to correct for multiple testing (one genotype vs two other genotypes), with a new level of significance as 0.017[27]. The association between CRP SNPs and circulating CRP concentration was investigated and evaluated by using one-way ANOVA with tukey post hoc. The Data were expressed as Mean±SEM.
3 Results
3.1 Population characteristics
Table 1 shows demographic, biochemical and inflammatory values in fDEP and non-fDEP groups. No statistically significant differences (p<0.05) was found in all the parameters we have collected here.
Demographic features, biochemical and inflammatory variables of the study groups
| Variable | non-fDEP groups (n=100) | fDEP groups (n=100) | p value |
|---|---|---|---|
| Gender (male/female) | 59/41 | 56/44 | 0,571 |
| Age (years) | 47.85± 7.23 | 45.09± 8.12 | 0,801 |
| BMI (kg/m2) | 26.64± 4.22 | 25.31± 5.01 | 0,839 |
| Chol (mmol/L) | 5.31±0.55 | 5.18±0.89 | 0,901 |
| Tg (mmol/L) | 1.71±0.79 | 1.85±0.98 | 0,912 |
| LDL-C (mmol/L) | 3.01±0.98 | 2.98±0.85 | 0,981 |
| HDL-C (mmol/L) | 1.21±0.76 | 1.18±0.89 | 0,977 |
| Gly (mmol/L) | 4.71±1.22 | 4.36±0.87 | 0,785 |
| Cortisol (nmol/L) | 454.4± 26.76 | 461.32±41.21 | 0,888 |
| Uric acid (umol/L) | 276.64± 17.99 | 257.68± 17.98 | 0,458 |
| Homocysteine (umol/L) | 14.53± 1.36 | 18.12±2.11 | 0,155 |
| Ceruloplasmin (mg/L) | 298.4 ± 31.22 | 269.84±19.68 | 0,441 |
| HAMD-17 (score) | 24.46± 1.77 | 24.06 ± 1.46 | 0,862 |
abbreviation: Chol:cholesterol; Tg: triglyceride; LDL-C: Low-density lipoprotein-Cholesterol; HDL-C: High-density lipoproteins-Cholesterol; Gly-fasting glycose
3.2 Circulating CRP levels and depression
In our research, we have found fDEP patients have a significant higher circulating CRP level than non-fDEP patients. In non-fDEP group, the circulating CRP level was 1.144±1.452 mg/L, while in fDEP patients, this number was increased to 1.744±1.635 mg/L (p=0.035) (Figure 2).

Circulating CRP level in f-DEP and non-DEP groups. Mean ±SEM, * p<0.05.
3.3 CRP polymorphisms and fDEP
Here we involved 5 well established CRP SNPs (rs3093059, rs1417938, rs 1800947, rs1130864 and rs1205, Figure 1) polymorphism and found A allele in rs1417938 and C allele in rs1205 SNP were overrepresented in fDEP patients. On the other hand, rs3093059, rs1800947 and rs1130864 did not differ between fDEP and non-fDEP patients (table 2).
Frequency of SNPs in different groups
| SN Pand genotype | non-fDEP (n=100) | fDEP (n=100) | P | post-hoc (genotype vs all others) | |
|---|---|---|---|---|---|
| % | % | ||||
| rs3093059 | TT | 88 | 86 | 0,902 | |
| TC | 10 | 12 | |||
| CC | 2 | 2 | |||
| rs1417938 | TT | 56 | 37 | 0,005 | |
| TA | 43 | 48 | |||
| AA | 3 | 13 | 0,016 | ||
| rs1800947 | GG | 84 | 89 | 0,32 | |
| GC | 4 | 5 | |||
| CC | 12 | 6 | |||
| rs1130864 | CC | 45 | 50 | 0,172 | |
| CT | 45 | 45 | |||
| TT | 10 | 5 | |||
| rs1205 | TT | 48 | 39 | 0,013 | |
| CT | 46 | 41 | |||
| CC | 6 | 20 | 0,003 | ||
3.4 CRP polymorphism and circulation CRP level
The serum CRP level among genotypes of rs3093059 and rs1130864 polymorphisms was comparable. However, we found compare with its major allele, both CC genotype in rs1205 and AA genotype in rs1417938 SNP showed a significant higher serum CRP level (table 3).
Associations between CRP SNPs and serum CRP levels
| SNP and genotype | N | Mean serum CRP | SE | P | |
|---|---|---|---|---|---|
| (mg/L) | |||||
| rs3093059 | TT | 174 | 1,39 | 0,31 | 0,976 |
| TC | 22 | 1,58 | 0,52 | ||
| CC | 4 | 1,51 | 0,89 | ||
| rs1417938 | TT | 93 | 1,01 | 0,13 | 0,031 |
| TA | 91 | 1,36 | 0,18 | ||
| AA | 16 | 1,98 | 0,19 | <0.05 compare with TT | |
| rs1800947 | GG | 173 | 1,45 | 0,35 | 0,61 |
| GC | 9 | 0,89 | 0,17 | ||
| CC | 18 | 0,48 | 0,28 | ||
| rs1130864 | CC | 95 | 1,13 | 0,31 | 0,753 |
| CT | 90 | 1,44 | 0,29 | ||
| TT | 15 | 1,28 | 0,18 | ||
| rs1205 | TT | 87 | 0,49 | 0,26 | 0,024 |
| CT | 87 | 0,97 | 0,21 | ||
| CC | 26 | 1,87 | 0,54 | <0.05 compare with TT |
4 Discussion
In our study, we examined multiple demographic, biochemical and inflammatory values between fDEP patients and non-fDEP controls, and as shown in Table 1.Most of those factors were not significantly changed between these two groups. However, as shown in Fig 2, we have found an increase in serum CRP level in fDEP patients. Furthermore, we found that two of five variants of the CRP gene were modest but significantly associated with depression patients who had a positive depression family history. These associations were not influenced by other potential mediating factors, such as age, gender and physical or mental health. Furthermore, we reported the circulation CRP level in depression patients with family history was significantly higher than those patients without family history.
To date, inconsistent results have been reported from studies for CRP variants and depression. Two studies have found no associations between depressive and affective symptoms [11, 12]. In contrast, Luciano et al [13] examined the relationship between depressive mood state and CRP SNPs in two elderly cohorts while adjusting for age and gender. They reported a modest significant association between rs1800947 and depression in one of the cohorts. Although they found no association with depression and rs1205, Almeida et al [14] reported an association between rs1205 and depression in 3700 men aged 70 years and over. Another study also indicated certain CRP gene haplotype was positively related with depression and high serum CRP level [15].
Considering the difference between reports may come from huge individual patients variations, the current viewpoint in literatures is certain CRP SNPs could regulate serum CRP level [16, 17, 18, 19, 25]. For example, CC genotype in rs1205 could up regulate serum CRP level [20], while T allele of rs1417938 could down regulate serum CRP level [21]. In the present study, we also found CC genotype in rs1205 and AA genotype in rs1417938 showed a significant higher serum CRP level. The detailed mechanisms for those regulations are still unknown and might also include epigenetic modifications of the gene, which have not been investigated in this study.
Because both depression and CRP polymorphism are around 40% inheritable, we thus analyzed our data and tried to find out whether CRP polymorphism might be related to depression in an inherited manner. Our results here have showed that A allele in rs1417938 and C allele in rs1205 SNP were potentially related with fDEP patients. Those data indicated certain CRP polymorphisms were positive with depression family history. Considering inherited factors would usually show dominant effects than other sporadic factors in diseases, our data further support CRP polymorphism may relate with depression occurrence.
As many previously reports have indicated CRP gene polymorphisms may regulate serum CRP level, we thus also investigated serum CRP level. An interesting result in comparing the major alleles, is that both A allele in rs1417938 and C allele in rs1205 could up regulate serum CRP level, which is consistent with previous reports [16, 17, 18, 19]. Finally, we also found serum CRP level in fDEP patients was significantly higher than non-fDEP, which fits the above data regard to high-CRP expression related polymorphisms distribution between non-fDEP and fDEP groups. Although we have to acknowledge that CRP polymorphisms may have functions other than regulating serum CRP, our data still strongly suggests that higher level of serum CRP level might be one reason for depression occurrence.
Previous literature have found serum CRP level might be gender determined or related. Furthermore, a large population scale survey has found serum CRP level were significantly higher in female than male [26]. To investigate whether gender was involved in our study as a correlated variation, we analyzed our data carefully but failed to find a gender dependent CRP difference. This inconsistency with previous studies may come from: 1. our project recruited fewer patients than previously reported, which might be insufficient to show the gender influence on serum CRP; 2. participants in previous studies included both depressed and non-depressed people; however in our study, all participants were patients with depression.
According to our hypothesis, participants in our project may all maintain a relative higher CRP level than healthy controls, which might overcome gender influence. All above reasons might lead to failure to show a gender dependent serum CRP changes.
In summary, by researching inherited CRP SNPs and depression, our data have showed certain high-CRP expression SNPs appeared in fDEP patients with higher frequency, which strongly proved those CRP polymorphisms were positively related with depression. Furthermore, our results also showed fDEP patients had higher serum CRP level than non-fDEP, which indicated higher serum CRP level was positively related with depression. Our results could supply novel information for depression occurrence and treatment.
References
[1] Devaraj S, Valleggi S, Siegel D, Jialal I, Role of C-reactive protein in contributing to increased cardiovascular risk in metabolic syndrome. Curr Atheroscler Rep. 2010, 12:110-118.10.1007/s11883-010-0098-3Search in Google Scholar PubMed PubMed Central
[2] The polarity protein Par3 regulates APP trafficking and processing through the endocytic adaptor protein Numb M Sun, SZ Asghar, H Zhang. Neurobiology of disease. 2016. 93:1-1110.1016/j.nbd.2016.03.022Search in Google Scholar PubMed PubMed Central
[3] McDade TW, Borja JB, Kuzawa CW, Perez TL, Adair LS. C-reactive protein response to influenza vaccination as a model of mild inflammatory stimulation in the Philippines. Vaccine. 2015, 33 :2004-200810.1016/j.vaccine.2015.03.019Search in Google Scholar PubMed PubMed Central
[4] Siedlinski M, Klanderman B, Sandhaus RA, Barker AF, Brantly ML, Eden E, McElvaney NG, Rennard SI, Stocks JM, Stoller JK, Strange C, Turino GM, Campbell EJ, Demeo DL. Association of cigarette smoking and CRP levels with DNA methylation in α-1 antitrypsin deficiency. Epigenetics. 2012, 7: 720-72810.4161/epi.20319Search in Google Scholar PubMed PubMed Central
[5] Lucassen PJ, Oomen CA, Naninck EF, Fitzsimons CP, van Dam AM, Czeh B, Korosi A. Regulation of Adult Neurogenesis and Plasticity by (Early) Stress, Glucocorticoids, andInflammation. Cold Spring Harb Perspect Biol. 2015, 7:a02130310.1101/cshperspect.a021303Search in Google Scholar PubMed PubMed Central
[6] Sun, M., Bernard, L. P., DiBona, V. L., Wu, Q., Zhang, H. Calcium Phosphate Transfection of Primary Hippocampal Neurons. J. Vis. Exp. (81), e50808, 10.3791/50808 (2013).Search in Google Scholar PubMed PubMed Central
[7] Pankow, J.S., Folsom, A.R., Cushman, M., Borecki, I.B., Hopkins, P.N., Eckfeldt, J.H., Tracy, R.P. Familial and genetic determinants of systemic markers of inflammation: the NHLBI family heart study. Atherosclerosis, 2001, 154: 681–689.10.1016/S0021-9150(00)00586-4Search in Google Scholar PubMed
[8] Yang, Kun, Chuanfeng Zhang, Lei Sun, Dong Li, and Xin Hong. Cigarette smoke condensate could promote human bronchial epithelial BEAS-2B cell migration through shifting neprilysin trafficking. Journal of Cancer Research and Therapeutics. 2016.10.4103/0973-1482.183182Search in Google Scholar PubMed
[9] Su S, Miller AH, Snieder H, Bremner JD, Ritchie J, Maisano C Jones L, Murrah NV, Goldberg J, Vaccarino V. Common genetic contributions to depressive symptoms and inflammatory markers in middle-aged men: the Twins Heart Study. Psychosom Med 2009, 71: 152–15810.1097/PSY.0b013e31819082efSearch in Google Scholar PubMed PubMed Central
[10] Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol 2014, 35: 320–33010.1016/j.yfrne.2014.05.004Search in Google Scholar PubMed PubMed Central
[11] Bremmer, M.A., Beekman, A.T., Deeg, D.J., Penninx, B.W., Dik, M.G., Hack, C.E., Hoogendijk, W.J., 2008. Inflammatory markers in late-life depression: results from a population-based study. J. Affect. Disord. 106, 249–255.10.1016/j.jad.2007.07.002Search in Google Scholar PubMed
[12] Gaysina D, Pierce M, Richards M, Hotopf M, Kuh D, Hardy R. Association between adolescent emotional problems and metabolic syndrome: the modifying effect of C-reactive protein gene (CRP) polymorphisms. Brain Behav Immun, 2011, 25: 750–75810.1016/j.bbi.2011.01.019Search in Google Scholar PubMed PubMed Central
[13] Luciano M, Houlihan LM, Harris SE, Gow AJ, Hayward C, Starr JM, Deary IJ. Association of existing and new candidate genes for anxiety, depression and personality traits in older people. Behav Genet 2010, 40: 518–53210.1007/s10519-009-9326-4Search in Google Scholar PubMed
[14] Almeida OP, Norman PE, Allcock R, van Bockxmeer F, Hankey GJ, Jamrozik K, Flicker L. Polymorphisms of the CRP gene inhibit inflammatory response and increase susceptibility to depression: the Health in Men Study. Int J Epidemiol. 2009, 38: 1049–105910.1093/ije/dyp199Search in Google Scholar PubMed PubMed Central
[15] Halder I, Marsland AL, Cheong J, Muldoon MF, Ferrell RE, Manuck SB. Polymorphisms in the CRP gene moderate an association between depressive symptoms andcirculating levels of C-reactive protein. Brain Behav Immun. 2010, 24:160-167.10.1016/j.bbi.2009.09.014Search in Google Scholar PubMed PubMed Central
[16] Fan AZ, Yesupriya A, Chang MH, House M, Fang J, Ned R, Hayes D, Dowling NF, Mokdad AH. Gene polymorphisms in association with emerging cardiovascular risk markers in adult women. BMC Med Genet. 2010, 11: 610.1186/1471-2350-11-6Search in Google Scholar PubMed PubMed Central
[17] M Sun, T Zhou, L Zhou, Q Chen, Y Yu, H Yang, K Zhong, X Zhang, F Xu. Formononetin protects neurons against hypoxia-induced cytotoxicity through upregulation of ADAM10 and sAβPPα, Journal of Alzheimer’s Disease 28 (4), 795-80810.3233/JAD-2011-110506Search in Google Scholar PubMed
[18] Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013, 150:736-744.10.1016/j.jad.2013.06.004Search in Google Scholar PubMed
[19] H Wang, M Sun, H Yang, X Tian, Y Tong, T Zhou, T Zhang, Y Fu, X Guo. Hypoxia-inducible factor-1 mediates up-regulation of neprilysin by histone deacetylase-1 under hypoxia condition in neuroblastoma cells, Journal of neurochemistry 131 (1), 4-1110.1111/jnc.12795Search in Google Scholar PubMed
[20] Ancelin ML, Farré A, Carrière I, Ritchie K, Chaudieu I, Ryan J. C-reactive protein gene variants: independent association with late-life depression andcirculating protein levels. Transl Psychiatry. 2015, 5:e499.10.1038/tp.2014.145Search in Google Scholar PubMed PubMed Central
[21] Davey Smith G, Ebrahim S, Lewis S, Hansell AL, Palmer LJ, Burton PR. Genetic epidemiology and public health: hope, hype, and future prospects. Lancet. 2005, 366: 1484–149810.1016/j.ajo.2005.11.030Search in Google Scholar
[22] Y Yu, L Zhou, M Sun, T Zhou, K Zhong, H Wang, Y Liu, X Liu, Xylocoside G reduces amyloid-β induced neurotoxicity by inhibiting NF-κB signaling pathway in neuronal cells. Journal of Alzheimer’s Disease 30 (2), 263-27510.3233/JAD-2012-110779Search in Google Scholar PubMed
[23] Komurcu-Bayrak E, Erginel-Unaltuna N, Onat A, Ozsait B, Eklund C, Hurme M, Mononen N, Laaksonen R, Hergenc G, Lehtimäki T. Association of C-reactive protein (CRP) gene allelic variants with serum CRP levels and hypertension in Turkish adults. Atherosclerosis. 2009, 206: 474–47910.1016/j.atherosclerosis.2009.03.030Search in Google Scholar PubMed
[24] J Yu, M Sun, Z Chen, J Lu, Y Liu, L Zhou, X Xu, D Fan, D Chui, Magnesium modulates amyloid-β protein precursor trafficking and processing. Journal of Alzheimer’s Disease 20 (4), 1091-110610.3233/JAD-2010-091444Search in Google Scholar PubMed
[25] Lacoma A, Bas A, Tudela P, Giménez M, Mòdol JM, Pérez M, Ausina V, Dominguez J, Prat-Aymerich C. Correlation of inflammatory and cardiovascular biomarkers with pneumonia severity scores. Enferm Infecc Microbiol Clin. 2014, 32 :140-14610.1016/j.eimc.2013.07.006Search in Google Scholar PubMed
[26] Liu Y, Al-Sayegh H, Jabrah R, et al. Association between C-reactive protein and depression: modulated by gender and mediated by body weight. Psychiatry Res (Ireland), 2014, 219: 103-10810.1016/j.psychres.2014.05.025Search in Google Scholar PubMed
[27] Latta, S.C., C.A. Howell, M.D. Dettling, and R.L. Cormier. Use of data on avian demographics and site persistence during overwintering to assess quality of restored riparian habitat. Conservation Biology 2012, 26: 482-49210.1111/j.1523-1739.2012.01828.xSearch in Google Scholar PubMed
©2017 Hasiyeti-Yibulaiyin et al.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Regular Articles
- ISS-N1 makes the first FDA-approved drug for spinal muscular atrophy
- Regular Articles
- Fragile X syndrome: Lessons learned from the most translated neurodevelopmental disorder in clinical trials
- Regular Articles
- Thymoquinone attenuates brain injury via an antioxidative pathway in a status epilepticus rat model
- Regular Articles
- Personality traits and perception of Müller-Lyer illusion in male Chinese military soldiers and university students
- Regular Articles
- Effects of mTOR on neurological deficits after transient global ischemia
- Regular Articles
- The concomitant association of thyroid disorders and Myasthenia gravis
- Regular Articles
- Dynamic expression of CX36 protein in kainic acid kindling induced epilepsy
- Regular Articles
- Autophagy, endoplasmic reticulum stress and the unfolded protein response in intracerebral hemorrhage
- Regular Articles
- White matter sexual dimorphism of the adult human brain
- Regular Articles
- Toward understanding non-coding RNA roles in intracranial aneurysms and subarachnoid hemorrhage
- Regular Articles
- COLQ-mutant congenital myasthenic syndrome with microcephaly: A unique case with literature review
- Regular Articles
- HILIC-MS rat brain analysis, a new approach for the study of ischemic attack
- Regular Articles
- SSTR4, childhood adversity, self-efficacy and suicide risk in alcoholics
- Regular Articles
- The essentials of a global index for cognitive function
- Regular Articles
- Solitaire stent in the treatment of acute ischemic stroke with large cerebral artery occlusion
- Regular Articles
- Grifolin attenuates white matter lesion in oxygen/glucose deprivation
- Regular Articles
- Caffeoylquinic acid enhances proliferation of oligodendrocyte precursor cells
- Regular Articles
- The arcuate fasciculus network and verbal deficits in psychosis
- Regular Articles
- Childhood adversities are not a predictors of SSTR4met in alcoholics
- Regular Articles
- A Rasch analysis between schizophrenic patients and the general population
- Regular Articles
- Assessment of R18, COG1410, and APP96-110 in excitotoxicity and traumatic brain injury
- Regular Articles
- Postural control and emotion in children with autism spectrum disorders
- Regular Articles
- One-shot synesthesia
- Regular Articles
- Chlorogenic acid prevents alcohol-induced brain damage in neonatal rat
- Regular Articles
- Task performance changes the amplitude and timing of the BOLD signal
- Regular Articles
- Effects of calcium on drinking fluorosis-induced hippocampal synaptic plasticity impairment in the offspring of rats
- Regular Articles
- Depression is associated with CRP SNPs in patients with family history
- Regular Articles
- Keyhole surgery of pineal area tumors - personal experience in 22 patients
- Regular Articles
- Models for preterm cortical development using non invasive clinical EEG
Articles in the same Issue
- Regular Articles
- ISS-N1 makes the first FDA-approved drug for spinal muscular atrophy
- Regular Articles
- Fragile X syndrome: Lessons learned from the most translated neurodevelopmental disorder in clinical trials
- Regular Articles
- Thymoquinone attenuates brain injury via an antioxidative pathway in a status epilepticus rat model
- Regular Articles
- Personality traits and perception of Müller-Lyer illusion in male Chinese military soldiers and university students
- Regular Articles
- Effects of mTOR on neurological deficits after transient global ischemia
- Regular Articles
- The concomitant association of thyroid disorders and Myasthenia gravis
- Regular Articles
- Dynamic expression of CX36 protein in kainic acid kindling induced epilepsy
- Regular Articles
- Autophagy, endoplasmic reticulum stress and the unfolded protein response in intracerebral hemorrhage
- Regular Articles
- White matter sexual dimorphism of the adult human brain
- Regular Articles
- Toward understanding non-coding RNA roles in intracranial aneurysms and subarachnoid hemorrhage
- Regular Articles
- COLQ-mutant congenital myasthenic syndrome with microcephaly: A unique case with literature review
- Regular Articles
- HILIC-MS rat brain analysis, a new approach for the study of ischemic attack
- Regular Articles
- SSTR4, childhood adversity, self-efficacy and suicide risk in alcoholics
- Regular Articles
- The essentials of a global index for cognitive function
- Regular Articles
- Solitaire stent in the treatment of acute ischemic stroke with large cerebral artery occlusion
- Regular Articles
- Grifolin attenuates white matter lesion in oxygen/glucose deprivation
- Regular Articles
- Caffeoylquinic acid enhances proliferation of oligodendrocyte precursor cells
- Regular Articles
- The arcuate fasciculus network and verbal deficits in psychosis
- Regular Articles
- Childhood adversities are not a predictors of SSTR4met in alcoholics
- Regular Articles
- A Rasch analysis between schizophrenic patients and the general population
- Regular Articles
- Assessment of R18, COG1410, and APP96-110 in excitotoxicity and traumatic brain injury
- Regular Articles
- Postural control and emotion in children with autism spectrum disorders
- Regular Articles
- One-shot synesthesia
- Regular Articles
- Chlorogenic acid prevents alcohol-induced brain damage in neonatal rat
- Regular Articles
- Task performance changes the amplitude and timing of the BOLD signal
- Regular Articles
- Effects of calcium on drinking fluorosis-induced hippocampal synaptic plasticity impairment in the offspring of rats
- Regular Articles
- Depression is associated with CRP SNPs in patients with family history
- Regular Articles
- Keyhole surgery of pineal area tumors - personal experience in 22 patients
- Regular Articles
- Models for preterm cortical development using non invasive clinical EEG

