Abstract
This report evaluates the protective effect of caffeoylquinic acid (CA) injury to oligodendrocyte precursor cells (OPCs) by promoting the formation of oligodendrocytes. Neonatal rat brain was used to isolate primary OPCs and non-lethal CoCl2 was used to induce hypoxic stress to inhibit the differentiation of OPCs. Differentiation of OPCs was estimated by survival assay and the expressions of myelin-basic-protein (MBP). Moreover, the effect of CA on the Akt signanling pathway was also estimated in the presence and absence of LY294002 (PI3K/Akt inhibitor) and adrenomedullin (AM) receptor antagonist (AM22-52) by using western blot assay. It was observed that CA enhances the differentiation OPCs in CoCl2 induced hypoxic stress condition. However treatment with CA in presence of LY294002 and AM22-52 was not able to enhance the differentiation of OPCs. Moreover treatment with CA significantly enhances the phosphorylation of Akt and presence of LY294002 and AM22-52 inhibits it. This report concludes that CA effectively attenuates the injury of white matter (OPCs) by enhancing the differentiation of OPCs. It enhances the formation of oligodendrocytes by activating AM receptor and thereby accelerates the regeneration of neuron in pathological condition.
Introduction
White matter of the central nervous system is made up of oligodendrocytes and these cells are responsible for the formation of myelin around axons [1]. It is very well documented that for neuronal homeostasis and effective conduction of nerve impulses, myelin sheath is required [2]. Oligodendrocytes mature during late embryonic development as oligodendrocyte precursor cells migrate and proliferate into oligodendrocytes [3, 4]. Moreover studies suggest that approximately 8% of adult brain cells as OPCs remain immature. These OPCs are responsible for the repair of neuronal injury in white matter by promoting myelinated neurons [5]. Literature reveals that degeneration of neurons and loss of myelin sheath from the neurons occurs in demyelinating diseases that causes permanent disability [6]. In the early stage of demyelinating disease proliferation of OPCs occur due to endogenous repair mechanism, but if disease progresses to an advanced stage, this neuron repair gets altered [7]. Therefore, for the treatment of demyelinating disease, promotion of proliferation of OPCs is a good target for the development of drug.
However, recently natural compounds have gained attention in the management of neuronal injury. Caffeoylquinic acid was isolated from the Chinese herb Aster tataricus, which belongs to the family Asteraceae [8]. It has been reported that caffeoylquinic acid is an ester of quinic and caffeic acid isolated from natural sources [9]. It has been reported to have anti-oxidant, anti-inflammatory, and anti-cancer activities [10-12]. 3,5-O-caffeoylquinic acid possesses strong anti-inflammatory activity, reducing the synthesis of cytokines, such as TNFα and IL-1β, and anti-oxidant properties [13]. Moreover, it reduces the generation of reactive oxygen species and, thus, reduces oxidative stress. This report estimates the effect of CA against demyelinating diseases.
Material and methods
Animals
Female Wistar rats were kept individually under observation for delivery in the cage. All the rats were housed under a controlled condition specified as per guidelines. All the experiments used in the given study are approved by animal ethical committee of the Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Cell Culture
A previously suggested method by Wang et al., 2011 was used for the proliferation of oligodendrocyte precursor cells. Cells were isolated from the cerebral cortex of pups by placing them in streptomycin (50 μg/ml) and penicillin (50 μg/ml) containing ice-cold DMEM/F12 medium. Cell suspension was prepared and filtered with cell strainer (70 μm). Later cell were centrifuged for the period of 10 min at 10000 rpm and collected cells were re suspended in DMEM/F12 medium and incubated it at 37 °C under controlled humid atmosphere for the duration of eight days. After every alternate day old medium was replaced with fresh medium. Later samples were kept on an orbital shaker to isolate the OPCs at 37 °C for the period of 60 min and the medium was replaced with fresh medium to remove the macrophages and microglial cells. The flask was again spun at 220 rpm for a period of 18 h by adding fresh DMEM/F12 medium (15 ml). Collected samples of cell suspension were kept in a Petri dish and incubated at 37 °C for 30 min. The cell suspension was then transferred to a 50 ml tube by passing it from the sieve of 15 μm size and later centrifuged it for 10 min at 1000 rpm to separate the cells. Medium of DMEM/F12 that contains FGFb (20 ng/ml), PDGF-BB (20 ng/ml) and 2 % B27 was used to re suspend the cells and thereafter placed it into 25 cm2 flasks at a quantity of 10,000 cells/cm2. Collected purified OPCs were differentiated.
CoCl2 treatment
Cobalt chloride (CoCl2) was incubated with cells at a non-lethal concentration for the production of hypoxia. Differential media in the presence and absence of CoCl2 (1 μM) was used to culture the cells for 7 days. On 0, 3rd and 5th day OPCs culture media was replaced with fresh CoCl2 containing media. Western blot assay, immunohistochemistry and survival assay was performed after the start of differentiation in OPCs i.e. on 7th day. Cells were separated into four groups such as control which do not receive the CoCl2, CoCl2 group which receives CoCl2 (1μm) for the duration of 7 days, CoCl2 +CA which receives both CoCl2 (1μm) and CA (10 nm) for the duration of 7 days.
Survival assay
WST reduction assay was used for the estimation of proliferation of cell using Cell Counting Kit- 8. All the cells were incubated at 37 °C for the duration of 60 min with WST solution (10 %). microplate reader was used for the estimation of absorbance of culture media at 630 nm for reference and 450 nm test wavelength.
Immunocytochemistry
Ice cold PBS solution was used for the washing of cells and thereafter with paraformaldehyde (4%) for the duration of 15 min. Triton X-100 (1%) contained PBS was used to wash the cells thrice and then incubated for the duration of 60 min. primary antibodies against MBP was incubated with cells for the overnight at 4 °C. Afterwards cells with secondary antibodies were incubated at room temperature for the duration of 60 min after washing with PBS solution. All the cells were stained with DAPI.
Western blot assay
Cell lyses buffer was used to collect the cell after washing them with PBS buffer. PBS was added in the sample in an equal amount so that concentration of estimated protein remains the same. SDS (91%) and 2-mercaptoethanol (9%) containing buffer was added in an equal amount of sample and mix it. Later 4–20% Tris–glycine gels was used to load the samples after heating them for the duration of 5 min at 95 °C. Tris buffered saline solution used to block the nitrocellulose membranes to which samples were transferred. Later Akt antibody phosphorylated Akt (Ser473) antibody and myelin basic protein (MBP) antibody was incubated with membrane at 4 °C for the duration of overnight. Thereafter visualize it under the chemiluminescence.
Statistical analysis
Values of the given data represented as mean ± SD (n=6) and the data of this study statistically analyzed by one-way ANOVA (Dunnett post hoc test). Level of significance was considered as p < 0.05.
Result
Effect of Caffeoylquinic acid on the proliferation and survival of OPCs
Effect of CA on the differentiation and proliferation of OPCs in CoCl2 induced hypoxia caused injury in neonatal rats was shown in Fig.1. Expressions of MBP are related to differentiation of OPCs. It was observed that CoCl2 group reduces the expressions of MBP and thereby decreases the differentiation of OPCs. However treatment with CA enhances the expressions of MBP as shown in Fig. 1 A. Fig 1B-C shows the effect of CA on the maturation of oligodendrocyte in hypoxia induced stress condition by western blot study. Result suggested that maturation of oligodendrocyte was promoted in the CA treated group than CoCl2 group. Moreover result of WST assay shows that both CoCl2 and CA didn’t reduces the count of OPCs in the culture medium.
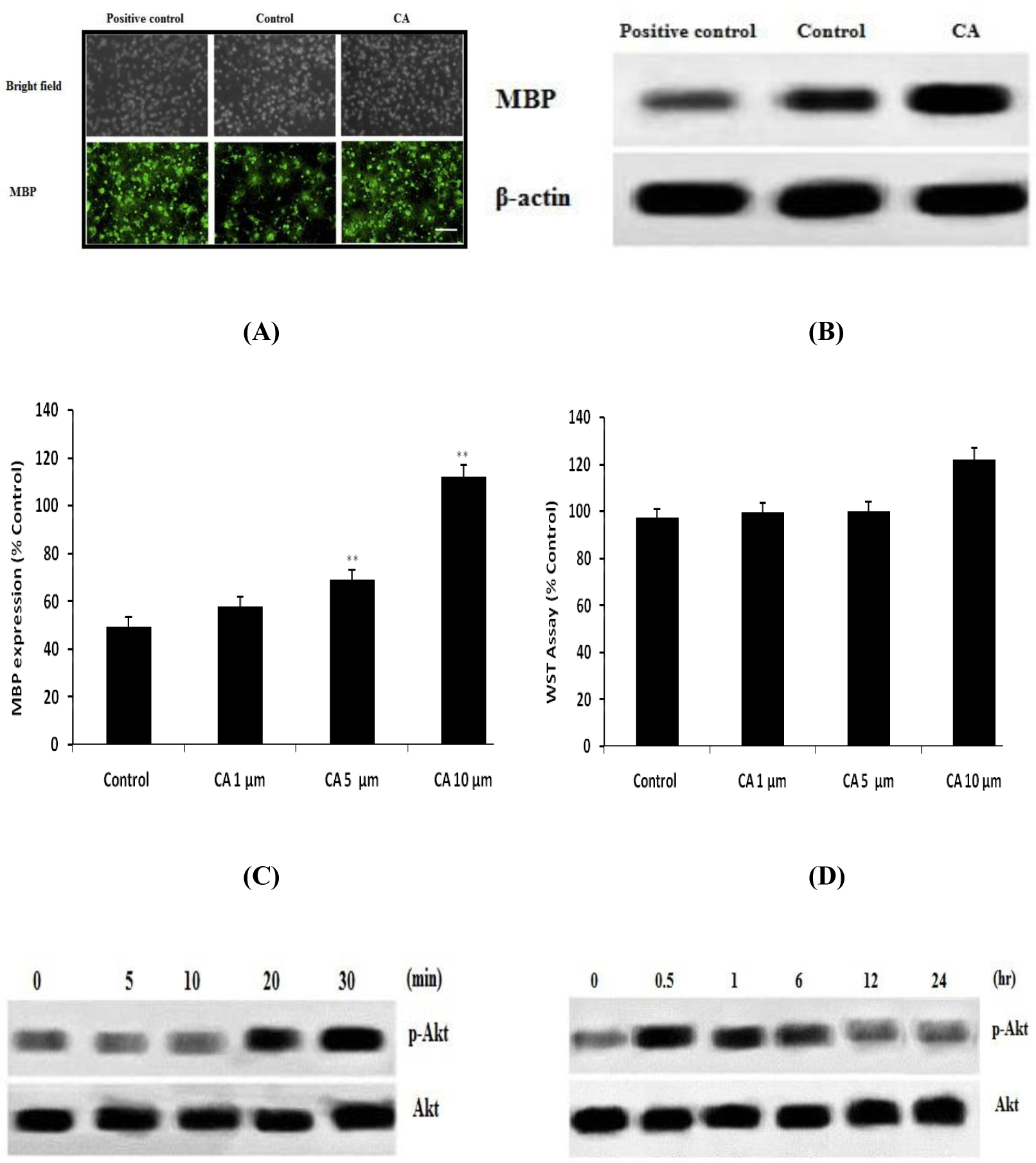
Effect of Caffeoylquinic acid on the proliferation and survival of OPCs in CoCl2 induced hypoxia. A. DAPI staining (Immunocytochemestry); B-C. Western blot for the expressions of MBP; D. WST assay for the assessment of cell death Values are expressed as Mean±SD (n=6) **p<0.01 Vs Control
Effect of Caffeoylquinic acid on signaling of Akt pathway
Effect of Caffeoylquinic acid on Akt signalling pathway in OPCs was shown in Fig. 2. It was observed that treatment with CA significantly enhances the level of phosphorylation of Akt in first 30 min and after 24 h it backs to normal in OPCs as shown in Fig. 2A-B. Moreover result suggested that treatment with AM 22-52 and LY294002 inhibit the level of phosphorylation of Akt that was induced by CA as shown in Fig. 2C-D.

Effect of Caffeoylquinic acid on Akt signalling pathway in OPCs. A-B, Effect of CA on the level of phosphorylation of Akt; C, Effect of CA on the level of phosphorylation of Akt in presence of LY294002 (PI3K inhibitor); D, Effect of CA on the level of phosphorylation of Akt in presence of AM receptor antagonist. Values are expressed as Mean±SD (n=6)
Effect of Caffeoylquinic acid on differentiation and survival of OPCs in presence AM 22-52 and LY294002
Effect of Caffeoylquinic acid on differentiation and survival of OPCs in presence AM 22-52 and LY294002 was shown in Fig. 3. It was observed that treatment with CA not able to enhance the expressions of MBP in presence of AM 22-52 and LY294002 than CA alone. It means in presence of AM 22-52 and LY294002, CA is not able to differentiate the OPCs in hypoxic condition.
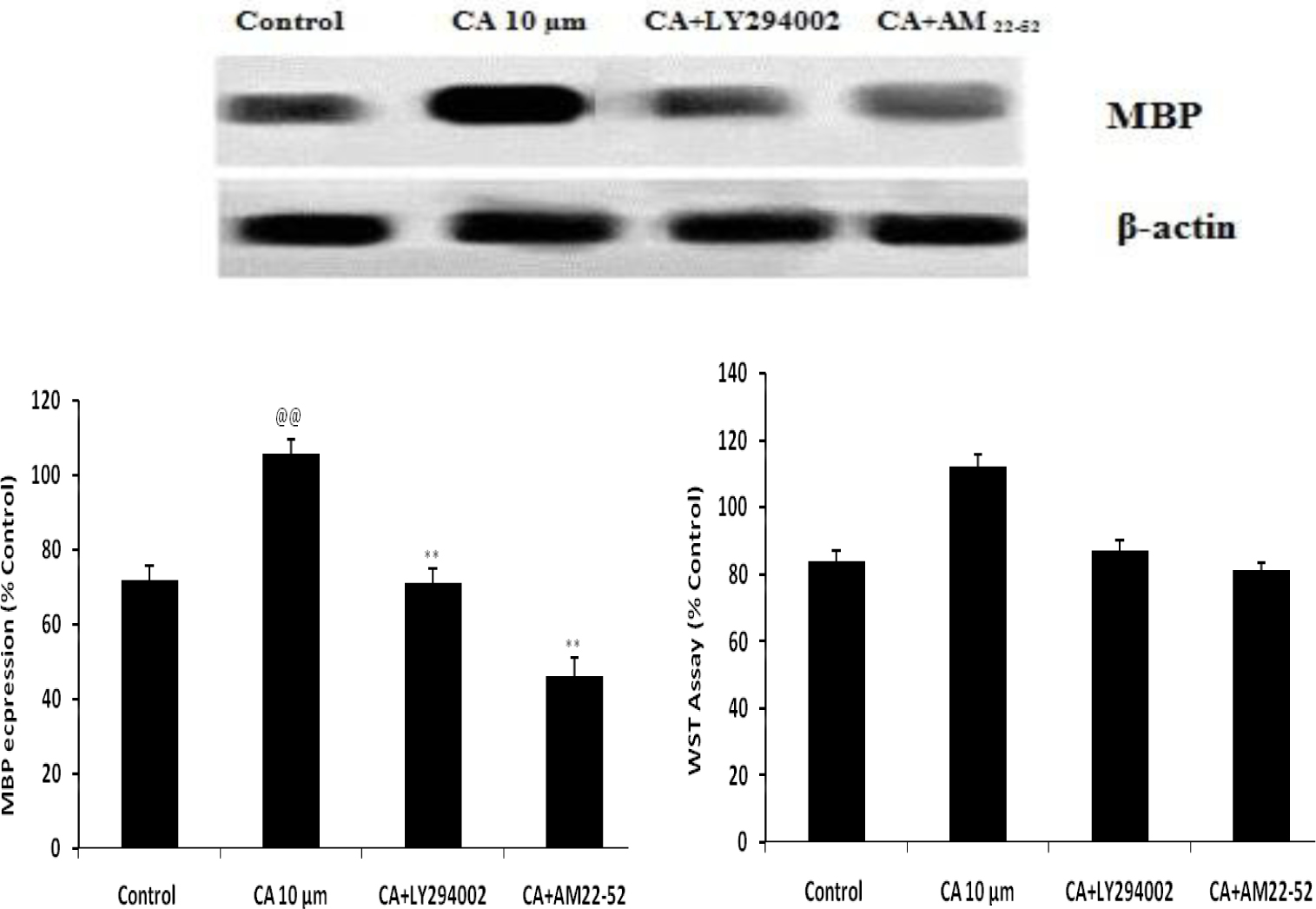
Effect of Caffeoylquinic acid on differentiation and survival of OPCs in presence AM 22-52 and LY294002 Values are expressed as Mean±SD (n=6) @@p<0.01 Vs Control; **p<0.01 Vs CA 10μm
Discussion
This study evaluates the protective effect of CA against CoCl2 induced hypoxia causing injury in OPCs. The effect of CA was evaluated by WST assay and MBP expressions in OPCs. Moreover a possible mechanism was postulated by evaluating its effect on Akt signaling.
Adrenomedullin receptors are widely distributed in the brain and AM is reported to act as a neurohormone and neurotransmitter [14]. Literature has also suggested that neuronal injury can be protected by activating the AM receptor, and thereby effectively aiding in the management of certain CNS disorders [15, 16]. In addition, several reports reveal that neurogenesis can be achieved by activating the AM receptor and it also helps in maturation of OPCs [17]. CA is a natural product, reported to possess anti-oxidant, anti-inflammatory, and anti-cancer activities by reducing the generation of reactive oxygen species, TNF-α and IL [10-12]. Injury caused by hypoxia results in the activation of these factors.
The results of our study suggest that CA enhances the expression of MBP and survival of OPCs. Expression of MBP enhancement is related to the differentiation of OPCs in to oligodendrocytes [18]. Results suggest that the expressions of MBP in hypoxia or in an injury condition become reduced, and several drugs protect the white matter injury by promoting the expressions of MBP [19]. It was also observed that CA enhances the phosphorylation of Akt. However it will not become enhanced in the presence of AM receptor antagonist and PI3K inhibitor. Furthermore, similar results were observed in the expressions of MBP and the survival of OPCs in presence of them.
Conclusion
This report concludes that CA effectively attenuates the injury of white matter (OPCs) by enhancing the differentiation of OPCs. It enhances the formation of oligodendrocytes by activating AM receptor and thereby accelerates the regeneration of neurons in pathological conditions.
References
[1] Baumann, N., Pham-Dinh, D., Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001, 81, 871–927.10.1152/physrev.2001.81.2.871Search in Google Scholar
[2] Levine, J.M., Reynolds, R., Fawcett, J.W., The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001, 24, 39–47.10.1016/S0166-2236(00)01691-XSearch in Google Scholar
[3] Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol 1993, 3, 191–197.10.1016/0962-8924(93)90213-KSearch in Google Scholar
[4] Back S.A., Han B.H., Luo N.L., Chricton C.A., Xanthoudakis S. et al., Selective vulnerability of late oligodendrocyte progenitors to hypoxia–ischemia. J Neurosci, 2002, 22, 455–463.10.1523/JNEUROSCI.22-02-00455.2002Search in Google Scholar
[5] Young, K.M., Psachoulia, K., Tripathi, R.B., Dunn, S.J., Cossell, L., Attwell, D., Tohyama, K., Richardson, W.D., Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 2013, 77, 873–885.10.1016/j.neuron.2013.01.006Search in Google Scholar
[6] Franklin, R.J., Ffrench-Constant, C., Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 2008, 9, 839–855.10.1038/nrn2480Search in Google Scholar
[7] Emery, B., Regulation of oligodendrocyte differentiation and myelination. Science 2010, 330, 779–782.10.1126/science.1190927Search in Google Scholar
[8] Dongliang C., Yu S., Terpenoid glycosides from the roots of Aster tataricus. Phytochemistry, 1993, 35(1), 173-176.10.1016/S0031-9422(00)90528-4Search in Google Scholar
[9] Wong SK, Lim YY, Ling SK, and Chan EW, Caffeoylquinic acids in leaves of selected Apocynaceae species: Their isolation and content. Pharmacognosy Res. 2014, 6(1), 67–72.10.4103/0974-8490.122921Search in Google Scholar PubMed PubMed Central
[10] Ma C, Dastmalchi K, Whitaker BD, Kennelly EJ., 2011. Two new antioxidant malonated caffeoylquinic acid isomers in fruits of wild eggplant relatives. J Agric Food Chem. 59(17), 9645-51.10.1021/jf202028ySearch in Google Scholar PubMed
[11] Duke. J. A. and Ayensu. E. S. Medicinal Plants of China Reference Publications, Inc. 1985 ISBN 0-917256-20-4.Search in Google Scholar
[12] Peluso G, De Feo V, De Simone F, Bresciano E, Vuotto ML., Studies on the inhibitory effects of caffeoylquinic acids on monocyte migration and superoxide ion production. J Nat Prod. 1995, 58(5), 639-46.10.1021/np50119a001Search in Google Scholar PubMed
[13] Abdel Motaal A, Ezzat SM, Tadros MG, El-Askary HI, In vivo anti-inflammatory activity of caffeoylquinic acid derivatives from Solidago virgaurea in rats. Pharm Biol. 2016, 54(12), 2864-2870.10.1080/13880209.2016.1190381Search in Google Scholar PubMed
[14] Martinez-Herrero, S., Larrayoz, I.M., Ochoa-Callejero, L., Garcia-Sanmartin, J., Martinez, A., Adrenomedullin as a growth and cell fate regulatory factor for adult neural stem cells. Stem Cells Int. 2012, 2012, 804717.10.1155/2012/804717Search in Google Scholar PubMed PubMed Central
[15] Miyamoto, N., Pham, L.D., Hayakawa, K., Matsuzaki, T., Seo, J.H., Magnain, C., Ayata, C., Kim, K.W., Boas, D., Lo, E.H., Arai, K., Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke 2013, 44, 2573–2578.10.1161/STROKEAHA.113.001530Search in Google Scholar PubMed PubMed Central
[16] Xia, C.F., Yin, H., Borlongan, C.V., Chao, J., Chao, L., Adrenomedullin gene delivery protects against cerebral ischemic injury by promoting astrocyte migration and survival. Hum. Gene Ther. 2004, 15, 1243–1254.10.1089/hum.2004.15.1243Search in Google Scholar PubMed
[17] Maki T., Takahashi Y., Miyamoto N., Liang A.C., Ihara M., Lo E.H., Arai K., Adrenomedullin promotes differentiation of oligodendrocyte precursor cells into myelin-basic-protein expressing oligodendrocytes under pathological conditions in vitro. Stem Cell Research 2015, 15, 68–74.10.1016/j.scr.2015.05.001Search in Google Scholar PubMed PubMed Central
[18] Mitome-Mishima, Y., Miyamoto, N., Tanaka, R., Shimosawa, T., Oishi, H., Arai, H., Hattori, N., Urabe, T., Adrenomedullin deficiency and aging exacerbate ischemic white matter injury after prolonged cerebral hypoperfusion in mice. BioMed Res. Int. 2014, 2014, 861632.10.1155/2014/861632Search in Google Scholar PubMed PubMed Central
[19] Nagaya, N., Mori, H., Murakami, S., Kangawa, K., Kitamura, S., Adrenomedullin: angiogenesis and gene therapy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1432–R1437.10.1152/ajpregu.00662.2004Search in Google Scholar PubMed
© 2017 Ying Yanqin et al.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Regular Articles
- ISS-N1 makes the first FDA-approved drug for spinal muscular atrophy
- Regular Articles
- Fragile X syndrome: Lessons learned from the most translated neurodevelopmental disorder in clinical trials
- Regular Articles
- Thymoquinone attenuates brain injury via an antioxidative pathway in a status epilepticus rat model
- Regular Articles
- Personality traits and perception of Müller-Lyer illusion in male Chinese military soldiers and university students
- Regular Articles
- Effects of mTOR on neurological deficits after transient global ischemia
- Regular Articles
- The concomitant association of thyroid disorders and Myasthenia gravis
- Regular Articles
- Dynamic expression of CX36 protein in kainic acid kindling induced epilepsy
- Regular Articles
- Autophagy, endoplasmic reticulum stress and the unfolded protein response in intracerebral hemorrhage
- Regular Articles
- White matter sexual dimorphism of the adult human brain
- Regular Articles
- Toward understanding non-coding RNA roles in intracranial aneurysms and subarachnoid hemorrhage
- Regular Articles
- COLQ-mutant congenital myasthenic syndrome with microcephaly: A unique case with literature review
- Regular Articles
- HILIC-MS rat brain analysis, a new approach for the study of ischemic attack
- Regular Articles
- SSTR4, childhood adversity, self-efficacy and suicide risk in alcoholics
- Regular Articles
- The essentials of a global index for cognitive function
- Regular Articles
- Solitaire stent in the treatment of acute ischemic stroke with large cerebral artery occlusion
- Regular Articles
- Grifolin attenuates white matter lesion in oxygen/glucose deprivation
- Regular Articles
- Caffeoylquinic acid enhances proliferation of oligodendrocyte precursor cells
- Regular Articles
- The arcuate fasciculus network and verbal deficits in psychosis
- Regular Articles
- Childhood adversities are not a predictors of SSTR4met in alcoholics
- Regular Articles
- A Rasch analysis between schizophrenic patients and the general population
- Regular Articles
- Assessment of R18, COG1410, and APP96-110 in excitotoxicity and traumatic brain injury
- Regular Articles
- Postural control and emotion in children with autism spectrum disorders
- Regular Articles
- One-shot synesthesia
- Regular Articles
- Chlorogenic acid prevents alcohol-induced brain damage in neonatal rat
- Regular Articles
- Task performance changes the amplitude and timing of the BOLD signal
- Regular Articles
- Effects of calcium on drinking fluorosis-induced hippocampal synaptic plasticity impairment in the offspring of rats
- Regular Articles
- Depression is associated with CRP SNPs in patients with family history
- Regular Articles
- Keyhole surgery of pineal area tumors - personal experience in 22 patients
- Regular Articles
- Models for preterm cortical development using non invasive clinical EEG
Articles in the same Issue
- Regular Articles
- ISS-N1 makes the first FDA-approved drug for spinal muscular atrophy
- Regular Articles
- Fragile X syndrome: Lessons learned from the most translated neurodevelopmental disorder in clinical trials
- Regular Articles
- Thymoquinone attenuates brain injury via an antioxidative pathway in a status epilepticus rat model
- Regular Articles
- Personality traits and perception of Müller-Lyer illusion in male Chinese military soldiers and university students
- Regular Articles
- Effects of mTOR on neurological deficits after transient global ischemia
- Regular Articles
- The concomitant association of thyroid disorders and Myasthenia gravis
- Regular Articles
- Dynamic expression of CX36 protein in kainic acid kindling induced epilepsy
- Regular Articles
- Autophagy, endoplasmic reticulum stress and the unfolded protein response in intracerebral hemorrhage
- Regular Articles
- White matter sexual dimorphism of the adult human brain
- Regular Articles
- Toward understanding non-coding RNA roles in intracranial aneurysms and subarachnoid hemorrhage
- Regular Articles
- COLQ-mutant congenital myasthenic syndrome with microcephaly: A unique case with literature review
- Regular Articles
- HILIC-MS rat brain analysis, a new approach for the study of ischemic attack
- Regular Articles
- SSTR4, childhood adversity, self-efficacy and suicide risk in alcoholics
- Regular Articles
- The essentials of a global index for cognitive function
- Regular Articles
- Solitaire stent in the treatment of acute ischemic stroke with large cerebral artery occlusion
- Regular Articles
- Grifolin attenuates white matter lesion in oxygen/glucose deprivation
- Regular Articles
- Caffeoylquinic acid enhances proliferation of oligodendrocyte precursor cells
- Regular Articles
- The arcuate fasciculus network and verbal deficits in psychosis
- Regular Articles
- Childhood adversities are not a predictors of SSTR4met in alcoholics
- Regular Articles
- A Rasch analysis between schizophrenic patients and the general population
- Regular Articles
- Assessment of R18, COG1410, and APP96-110 in excitotoxicity and traumatic brain injury
- Regular Articles
- Postural control and emotion in children with autism spectrum disorders
- Regular Articles
- One-shot synesthesia
- Regular Articles
- Chlorogenic acid prevents alcohol-induced brain damage in neonatal rat
- Regular Articles
- Task performance changes the amplitude and timing of the BOLD signal
- Regular Articles
- Effects of calcium on drinking fluorosis-induced hippocampal synaptic plasticity impairment in the offspring of rats
- Regular Articles
- Depression is associated with CRP SNPs in patients with family history
- Regular Articles
- Keyhole surgery of pineal area tumors - personal experience in 22 patients
- Regular Articles
- Models for preterm cortical development using non invasive clinical EEG

