Abstract
Objectives
The aims of the study were to assess the levels of serum TMAO, S-equol, and indoxyl sulfate in subjects with ocular active Behçet’s disease (OABD) and ocular inactive Behçet’s disease (OIBD).
Methods
The study involved 22 patients with OABD, 22 patients with OIBD, and thwentythree control participants. 5 mL venous blood was taken from the participants. The TMAO, S-equol, and indoxyl sulfate in the serum were measured using the ELISA method.
Results
When compared to the TMAO levels of the control group, the TMAO levels of the participants with OABD and OIBD were considerably greater (p<0.05). Similarly, when compared to the S-equol levels of the control group, the S-equol levels of the participants with OABD and OIBD were significantly higher (p<0.05). Additionally, when compared to the indoxyl sulfate of the control group, the indoxyl sulfate amounts of the participants OABD and OIBD were significantly higher (p<0.05).
Conclusions
It was first time shown that microbiota molecules could have an impact on Behçet’s disease (BD) pathogenesis. Additionally, measuring these molecules in addition to the BD Ocular Attack Score 24 (BOS24) might offer advice to medical professionals regarding the diagnosis and treatment of the illness.
Introduction
Behçet’s illness (BD) is a recurrent, chronic illness with multisystemic inflammatory characteristics, such as cutaneous, genital, vascular, and neurological symptoms, and ophthalmological involvement [1]. The Turkish physician (Hulusi Behçet) was the first to describe the illness in 1930 [2]. The etiopathology of the disease is not fully understood yet. Hypopyon iridocyclitis, neovascularization, tears, macular ischemia, retinal vasculitis, vitreous hemorrhage, and macular edema are catastrophic pathological conditions that can lead to blindness [3]. This disease is frequently encountered in Türkiye and Iran. Some cases have also been reported from Northern Europe, the United States, and Australia [4]. Both genders are equally affected, and the average onset age of the disease is around 20–35 years [5]. Variations in the ocular surface microbiota have been linked to a number of illnesses, including ocular surface dryness [6].
Recent research has suggested a potential genetic predisposition helping to advance BD’s development. Furthermore, many studies have suggested that cytokines such interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1, IL-6, IL-8, IL-17, IL-18, and IL-23 may have a role in the etiology of BD [7], [8], [9]. In addition, disulfide/total thiol (TT) and disulfide/native thiol (NT) ratios are higher in ocular active Behçet’s disease (OABD) and ocular inactive Behçet’s disease (OIBD) patients than in the general population, according to recent research [3], 10]. Moreover, Celik et al. found that individuals with ocular BD had significantly higher amount of neutrophil gelatinase-associated lipocalin (NGAL), sCD40, PF4V1, and IL-18 [11].
The effects of the ocular microbiota have recently been the subject of research on ocular health [12]. The structure of the ocular surface is constituted by the cornea and its adjacent covering tissue, the conjunctiva. In microbiological discourse, the term “ocular surface microbiota” is specifically applied to describe the stable communities of microorganisms residing on the cornea and conjunctiva, while deliberately not considering microbial inhabitants of the eyelids [13]. Eye illnesses can arise and worsen as a result of dysbiosis, or abnormalities in the ocular microbiota [12], 14], 15]. The microbiological makeup of the ocular surface can change according to a number of reasons, such as host physiology, exposure to the environment, and underlying medical problems. These changes can compromise the innate immunity of the corneal and conjunctival layers, permitting microbial elements to initiate inflammatory responses in the eye. Variations in ocular surface microbiota have been implicated in various conditions such as ocular surface dryness, antibiotic use, and infectious diseases [16].
A metabolite of the gut microbiota, trimethylamine N-oxide (TMAO) is created by bacteria from substances like betaine, γ-butyrobetaine, L-carnitine, and choline [17]. Trimethylamine (TMA) lyases, which have the ability to break the C-N link in these nutrients, release TMA, which the gut microbiota then uses as a carbon source. TMAO is a key metabolite of intestinal flora [16] and represents the degree of intestinal dysbiosis [17]. Becuase elevated TMAO levels can cause inflammation, disruption of gut microbial metabolism (increased TMAO) and decreased gut-blood barrier permeability has been linked to the development of metabolic disorders [18]. Among people with Type 2 diabetes mellitus, elevated amounts of plasma TMAO were linked to a higher risk and more severe form of diabetic retinopathy [19]. Atherosclerosis is commonly understood to be a long-term inflammatory condition that is instigated by inflammation in the vascular endothelium. Recent scientific investigations employing metabolomic techniques have implicated TMAO as an indicator for the onset of atherosclerosis. Moreover, evidence from in vitro and in vivo studies has confirmed that TMAO plays a part in endothelial cell inflammatory damage [20], 21].
S-equol [7-hydroxy-3-(4-hydroxyphenyl) chroman]-yielded by microorganisms in the human and animal gut from the soy isoflavone daidzein has a protective effect on the vascular system and has been shown to decrease the proliferation of human aortic smooth muscle cells (HASMCs), collagen and total protein synthesis, migration, and mitogen-activated protein kinase activity in a dose-dependent manner. A study that looked into S-equol’s possible inhibitory effects on NO production induced by lipopolysaccharide (LPS) was done. Astrocytes cultured in vitro were exposed to 1 μg/mL of LPS in the presence of different S-equol concentrations for a period of 24 h. Subsequent measurements revealed that LPS-induced nitric oxide (NO) generation was dose-dependently attenuated in the presence of S-equol [22], 23]. A novel investigation has recently uncovered that S-equol mitigates the generation of NO in astrocytes when triggered by LPS. Given that excessive NO is known to aggravate neuronal deterioration in diseases of neurodegeneration, these findings underscore the potential utility of S-equol in modulating inflammatory processes within the Central Nervous System [24]. This microbial metabolite with anti-inflammatory and anti-apoptotic effects may play a role in ocular Behçet’s disease.
Tryptophan, another amino acid, is metabolized by gut bacteria, such as Escherichia coli, through the tryptophanase enzyme. After entering the bloodstream through the digestive system, indole is then converted by the liver into indoxyl sulfate and eliminated by the urine. Levels of indoxyl sulfate increase when there is microbial dysbiosis [25]. Under such conditions, the cellular uptake of indoxyl sulfate is attributed to organic anion transporters (OATs), which also play a role in the impairment of smooth muscle and endothelial activities [26]. According to certain research, at the highest doses examined, indoxyl sulfate dramatically raises the production of two enzymes, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), which are mainly involved in inflammatory responses at the gut level. Indoxyl sulfate was also shown to significantly elevate serum levels of proinflammatory cytokines, including TNF-α, IL-6, and IL-1β, according to a study that examined these levels. At the systemic level, indoxyl sulfate therapy has a noteworthy time-dependent influence on these proinflammatory cytokines [27]. Indoxyl sulfate, through OATs, induces inflammation in biological systems by inducing several substances, including TNF [28]. Therefore, it may have a role in ocular BD.
Early diagnosis is important for reducing the risk of long-term vision loss. The diagnosis of ocular BD cannot be made with a pathognomonic laboratory test. As a result, the diagnosis is only supported by clinical observations. There are numerous ways to score ocular BD in order to determine its severity and level of activity [29]. The Tanaka et al.-developed BD Ocular Attack Score 24 (BOS24) is the most often utilized scoring system among these scoring systems [30]. Based on the aforementioned data, the purpose of this investigation was to ascertain the serum amount of TMAO, S-equol, and indoxyl sulfate in patients with OIBD and ocular active conditions, as well as to explore the potential correlation between these levels and disease activity.
Materials and methods
The Fırat University Faculty of Medicine Ethics Committee approved this study (approval number. 2023/04–36, dated 09.03.2023), and all subjects gave their informed consent. The study was conducted in accordance with the Helsinki Declaration’s principles. A total of 22 OABD patients and 22 OIBD patients, diagnosed by a rheumatology specialist and followed or recently diagnosed at the Universal Eye Diseases Hospital, were included in the study. They were matched with 23 healthy control volunteers in terms of gender and age. The healthy control group consisted of individuals without any known health problems, selected from routine annual check-up appointments. Using the updated criteria of the International BD Study Group, patients with a score of four or higher were classified as having BD. The BD Current Activity Form (BDCAF), which was applied to all study groups, was used to assess the systemic activity of BD. All ophthalmic examinations were conducted as previously described [3]. Participants with renal failure, alcohol consumption, diabetes mellitus, gastrointestinal diseases, ischemic heart failure, hypertension, body mass index greater than 30 kg/m2, recent use of immunosuppressive medications or systemic steroids during the previous four weeks, and those with severe cataracts preventing fundus examination were excluded from the study. The BOS24 was considered, and the investigation included eyes with high BOS24 value. Peripheral fundus lesions can receive up to eight points on the BOS24 scoring system, while anterior chamber cells can receive up to four points, posterior pole lesions can receive up to four points, vitreous opacity can receive up to four points, and six indices of ocular inflammation symptoms can total 24 points. Additionally, lesions of the fovea (maximum two points) and the optic disk (maximum two points) were graded [30], 31]. Following an overnight fasting period, 5 mL of venous blood was obtained from each participant in plain biochemistry tubes (Lot: 406, Hema & Lab Health Products, Ankara). After being centrifuged for 5 min at 4,000 rpm (1792 × g), the bloods were kept in Eppendorf tubes at −80 °C until they were examined further.
Biochemical analysis
The TMAO, S-equol, and indoxyl sulfate were assessed using the ELISA method as per the manufacturer’s guidelines (Sunred Biological Technology Co., Ltd., Shanghai, China). The detection limit for TMAO was 0.043 ng/mL, with a measurement scope of 0.05–10 ng/mL. The detection limit for S-equol was 0.247 ng/mL, with a measurement range of 0.25–70 ng/mL. The detection limit for indoxyl sulfate was 1.854 μg/mL, with a measurement range of 2–600 μg/mL. Plate washing was done using an automated Bio-Tek ELX50 washer (BioTek Instruments, USA), and using a ChroMate Microplate Reader P4300 (Awareness Technology Instruments, USA), absorbance measurements at 450 nm were obtained. The intra assay coefficients (CVs) for the ELISA kits employed in the study were below 10 %, while the inter-assay CVs were under 12 %.
Statistical analysis
The software program SPSS 22, which is produced by Chicago, Illinois-based SPSS Inc., was used to perform statistical analyses. Data are presented as median ± (minimum – maximum) for each data set. The Shapiro-Wilk (W) test was performed to determine whether the samples normally distributed (p<0.05). Mann–Whitney U test was used in pairwise comparisons. A statistically significant result was defined as a p-value of less than 0.05.
Results
There were no appreciable variations in the distribution of genders between the groups (Table 1, p=0.962). Both OABD and OIBD patients had significantly higher C-reactive protein (CRP) and neutrophil values compared to the control values (Table 1, p<0.005). The BOS24 score for OABD participants was 14.36 ± (8.53–21.18), while the BDCAF score was 3.96 ± (2.84–4.78). The BOS24 score for OIBD patients was 0.48 ± (0.38–0.57), while the BDCAF was 2.22 ± (1.76–2.74).
Comparison of demographic characteristics of patients with active and inactive ocular Behçet disease and controls.
| Parameters | Control (n=23) | OABD (n=22) | OIBD (n=22) | p-Value |
|---|---|---|---|---|
| Age, years (± SD) | 38.00 ± (30.00–40.00)a | 34.50 ± (31.00–40.00)a | 36.00 ± (31.00–42.00)a | 0.962 |
| Sex (female/male) | 11/12a | 9/13a | 12/10a | 0.86 |
| CRP, mg/dL | 2.34 ± (1.76–3.14)a | 6.19 ± (5.75–6.78)b | 1.98 ± (1.46–2.37) | 0.001 |
| WBC count, mm3 | 8.02 ± (7.56–9.56)a | 8.00 ± (7.45–8.54)b | 7.22 ± (6.38–8.16)a | 0.52 |
| Lymphocyte, 103/µL | 3.84 ± (2.69–4.34)a | 7.06 ± (6.56–7.45)b | 5.50 ± (4.86–6.27)a | 0.07 |
| Neutrophil, 103/µL | 1.90 ± (1.45–2.36)a | 1.65 ± (1.28–1.98)b | 1.82 ± (1.43–2.19)a | 0.002 |
| BOS24 score | – | 14.36 ± (8.53–21.18)b | 0.48 ± (0.38–0.57) | 0.001 |
| BDCAF scores | – | 3.96 ± (2.84–4.78)b | 2.22 ± (1.76–2.74) | 0.001 |
-
CRP, C-reactive protein; OABD, ocular active Behçet disease; OIBD, ocular inactive Behçet disease; WBC, white blood cell. BD ocular attack score 24 (BOS24), BD current activity form (BDCAF), acontrol vs. OABD vs. OIBD or bOABD vs. OIBD.
Patients with OABD showed a substantial increase in TMAO when compared to both OIBD and control values (p<0.05; Figure 1). Patients with OABD had greater TMAO levels than those with OIDB, although this difference was not statistically significant (p>0.05; Figure 1). Also, patients with OIBD had considerably greater amounts of TMAO compared to control values (p<0.05; Figure 1, Table 2).

Comparison of TMAO levels between patients with active and inactive ocular Behçet disease and controls. TMAO, trimethylamine N-oxide; OABD, ocular active Behçet disease; OIBD, ocular inactive Behçet disease; acontrol vs. OABD.
TMAO, S-equol and indoxyl sulfate levels of control, active and inactive ocular Behçet’s disease groups.
| Parameters | Control (n=23) | OABD (n=22) | OIBD (n=22) |
|---|---|---|---|
| TMAO, ng/mL | 4.70 ± (3.48–6.48) | 7.44 ± (4.96–8.86)a | 6.08 ± (4.76–8.34) |
| S-equol, ng/mL | 17.96 ± (14.56–23.86) | 33.25 ± (26.76–36.68)a | 26.53 ± (17.38–34.45)b |
| Indoxyl sulfate, mg/mL | 166.40 ± (149.10–200.30) | 328.00 ± (268.00–385.00)a | 178.00 ± (148.00–234.00) |
-
OABD, ocular active Behçet disease; OIBD, ocular inactive Behçet disease; TMAO, trimethylamine N-oxide; S-equol, 7-hydroxy-3-(4-hydroxyphenyl) chroman; acontrol vs. OABD, bcontrol vs. OIBD.
In patients with OABD, there was a notable increase in S-equol levels compared to those with OIBD and control values (p<0.05; Figure 2). Higher S-equol amounts were found in active patients with OABD compared to OIBD, however this difference did not achieve statistical significance (p>0.05, Figure 2, Table 2).
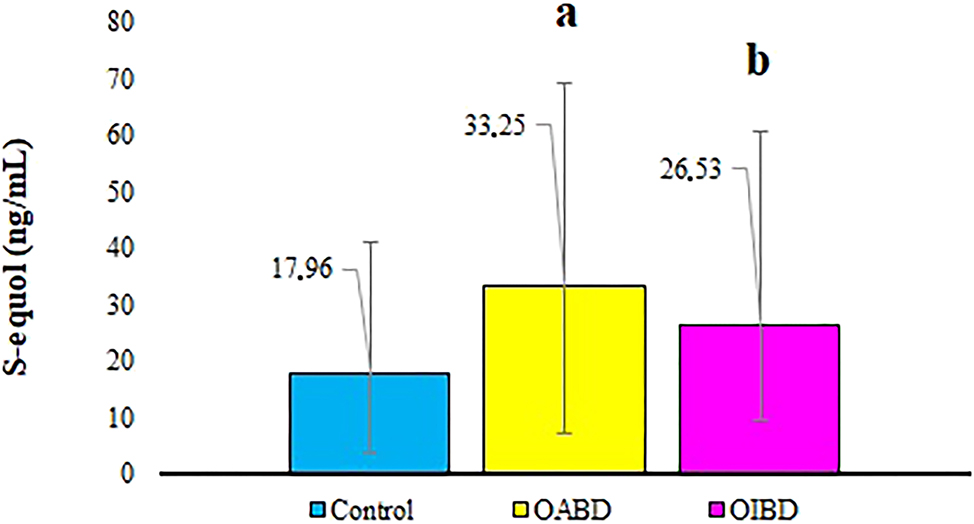
Comparison of S-equol levels between patients with active and inactive ocular Behçet disease and controls. OABD, ocular active Behçet disease; OIBD, ocular inactive Behçet disease; S-equol, 7-hydroxy-3-(4-hydroxyphenyl) chroman; acontrol vs. OABD, bcontrol vs. OIBD.
Moreover, individuals with OABD showed significantly higher amounts of indoxyl sulfate than did OIBD patients and control values (p<0.05; Figure 3). Despite the fact that individuals with OABD had higher levels of indoxyl sulfate than those with OIBD, this difference was not statistically significant (p>0.05; Figure 3, Table 2).

Comparison of indoxyl sulfate levels between patients with active and inactive ocular Behçet disease and controls. OABD, ocular active Behçet disease; OIBD, ocular inactive Behçet disease; acontrol vs. OABD.
Discussion
This current study is the first to reveal a connection between active and OIBD individuals and levels of TMAO, S-equol, and indoxyl sulfate. It was discovered in our study that OABD patients had higher TMAO amounts, but OIBD patients had lower TMAO amounts. The elevated TMAO in ocular BD may be a cause of the inflammation observed in this disease [21]. Recent findings suggest that diets high in TMAO-containing foods or high in choline trigger inflammation [32]. According to a TMAO study, endothelial dysfunction, vascular oxidative stress and inflammation, and cardiovascular disease are all facilitated by increased levels of circulating TMAO brought on by chronic kidney disease [33], 34]. Consequently, it’s thought that the rise in TMAO levels makes OABD worse. Perhaps the increase in TMAO contributes to parameters such as macular ischemia, retinal vasculitis, vitreous inflammation hemorrhage, macular edema, and blindness seen in ocular BD [29]. The increase in TMAO may be both the cause and result of oxidative stress and inflammation in ocular BD. Moreover, a connection between dysbiosis of the gut microbiota and elevated levels of TMAO in the blood has been noted in a number of pathophysiological diseases, including chronic kidney failure [35]. Thus, preventing and treating ocular BD may involve decreasing TMAO synthesis by the use of 3,3-dimethyl-1-butanol as a TMAO inhibitor [34].
In research conducted by Subedi and colleagues, the team employed microglial and astrocytic cell models to examine the neuroprotective and anti-neuroinflammatory properties of S-equol. Additionally, S-equol has been found to safeguard neurons against neuroinflammatory damage by decreasing neuronal apoptosis [27]. In a mice model of depression triggered by LPS, S-equol markedly reduced hippocampal concentrations of key inflammatory cytokines such as TNF-alpha [36]. In a cellular context, S-equol has been shown to enhance the dendritic branching of Purkinje neurons triggered by triiodothyronine, as well as promote neurite outgrowth in Neuro-2A cells during differentiation. These actions appear to be orchestrated via both Estrogen Receptor-ER and G Protein-Coupled Receptor 30 (GPR30). Furthermore, in astrocytic populations, S-equol stimulates both cellular multiplication and motility, activities principally mediated by GPR30 [37]. In ocular BD, the increase in S-equol levels may be due to its potential to prevent neuronal damage, macular ischemia, retinal vasculitis, and vitreous inflammation (compensatory mechanism). As a result, dietary isoflavones might play a significant role in ensuring that S-equol’s protective qualities are fulfilled.
Additionally, this study also identified an increase in indoxyl sulfate levels in ocular Behçet’s disease (Figure 3). Indoxyl sulfate is a metabolite formed during the breakdown of tryptophan by intestinal bacteria like E. coli [38]. Upon absorption in the intestines, it is converted to indoxyl sulfate in the liver. Indoxyl sulfate contributes to systemic inflammation, immune system alterations, and oxidative stress in different tissues of the body [39]. This substance is a uremic waste product that is mainly attached to bloodstream proteins as a result of the metabolic breakdown of dietary tryptophan. According to recent studies, indoxyl sulfate (IS) causes vascular problems in addition to acting as a uremic toxin.
Studies indicate that IS influences endothelial cell physiology, specifically by disrupting the equilibrium between oxidant and antioxidant systems, facilitating the discharge of endothelial-derived microparticles, and negatively affecting the regenerative capacity of endothelial tissue [40]. As previously mentioned, the indoxyl sulfate molecule may contribute to parameters such as macular ischemia, retinal vasculitis, and vitreous inflammation observed in ocular Behçet’s disease [29]. Therefore, increased circulating indoxyl sulfate has the potential to cause damage to the eye. The most significant data in favor of the hypothesised mechanism originates from Yu et al. [41], who claim that indoxyl sulfate contributes significantly to vascular inflammation by raising oxidative stress in the endothelium.
Our sample size is low due to the rarity of the disease in the population. Another limitation of the study is the influence of diet on all three molecules. For instance, intestinal microbes metabolize choline and l-carnitine, which are present in red meat, fish, dairy products, eggs, and poultry to create TMA and its oxidized version, TMAO, in the liver [42]. In the intestines of humans and animals, bacteria convert the soy isoflavone daidzein into S-equol [43]. Similar to this, the metabolism of dietary tryptophan results in the formation of indoxyl sulfate. Circulating levels of TMAO, S-equol, and indoxyl sulfate are determined by various factors such as dietary habits, gut microbiota, and enzyme flavin monooxygenase 3 (FMO3) activity [28]. Although efforts were made to ensure a similar diet in this study, participant compliance was challenging. Self-reporting from patients was relied upon.
Conclusions
The increase in TMAO, S-equol, and indoxyl sulfate molecules in ocular BD suggests their potential association with the etiopathology of this disease. The elevated levels of TMAO, S-equol, and indoxyl sulfate in ocular BD may also serve as helpful parameters for diagnosis and monitoring by ophthalmologists. Put another way, given the available evidence, we believe that future research on gut-derived TMAO, S-equol, and indoxyl sulfate will result in novel strategies for the diagnosis, management, and prevention of ocular BD.
Acknowledgments
We are grateful to Prof. Dr. Süleyman Serdar Koca for his help in BD diagnosis.
-
Research ethics: The Fırat University Faculty of Medicine Ethics Committee approved this study (approval number. 2023/04–36, dated 09.03.2023). The study was conducted in accordance with the Helsinki Declaration’s principles (as revised in 2013).
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
1. Nair, JR, Moots, RJ. Behcet’s disease. Clin Med 2017;17:71–7. https://doi.org/10.7861/clinmedicine.17-1-71.Search in Google Scholar PubMed PubMed Central
2. Tan, SY, Poole, PS. Hulusi Behçet (1889–1948): passion for dermatology. Singap Med J 2016;57:408–9. https://doi.org/10.11622/smedj.2016123.Search in Google Scholar PubMed PubMed Central
3. Balbaba, M, Ulaş, F, Postacı, SA, Öz, B, Aydın, S. Serum cortistatin levels in patients with ocular active and ocular inactive Behçet disease. Ocul Immunol Inflamm 2020;28:601–5. https://doi.org/10.1080/09273948.2019.1610461.Search in Google Scholar PubMed
4. Leonardo, NM, McNeil, J. Behçet’s disease: is there geographical variation? A review far from the Silk Road. Internet J Rheumatol 2015;2015:945262. https://doi.org/10.1155/2015/945262.Search in Google Scholar PubMed PubMed Central
5. Üsküdar Cansu, D, Kaşifoğlu, T, Korkmaz, C. Do clinical findings of Behcet’s disease vary by gender? A single-center experience from 329 patients. Eur J Rheumatol 2016;3:157–60. https://doi.org/10.5152/eurjrheum.2016.038.Search in Google Scholar PubMed PubMed Central
6. Kaya, TI. Genetics of Behçet’s disease. Pathol Res Int 2012;2012:912589. https://doi.org/10.1155/2012/912589.Search in Google Scholar PubMed PubMed Central
7. Cicioglu, AB, Yildirim, M, Baysal, V, Inaloz, HS, Baz, K, Kaya, S. Serum levels of IL‐4, IL‐10, IL‐12, IL‐13 and IFN‐gamma in Behçet’s disease. J Dermatol 2003;30:602–7. https://doi.org/10.1111/j.1346-8138.2003.tb00442.x.Search in Google Scholar PubMed
8. Talaat, RM, Sibaii, H, Bassyouni, IH, El-Wakkad, A. IL-17, IL-10, IL-6, and IFN-γ in Egyptian Behçet’s disease: correlation with clinical manifestations. Eur Cytokine Netw 2019;30:15–22. https://doi.org/10.1684/ecn.2019.0421.Search in Google Scholar PubMed
9. Chi, W, Zhu, X, Yang, P, Liu, X, Lin, X, Zhou, H, et al.. Upregulated IL-23 and IL-17 in Behçet patients with active uveitis. Invest Ophthalmol Vis Sci 2008;49:3058–64. https://doi.org/10.1167/iovs.07-1390.Search in Google Scholar PubMed
10. Balbaba, M, Ulaş, F, Yıldırım, H, Soydan, A, Dal, A, Aydın, S. Thiol/disulfide homeostasis in patients with ocular-active and ocular-inactive Behçet disease. Int Ophthalmol 2020;40:2643–50. https://doi.org/10.1007/s10792-020-01445-x.Search in Google Scholar PubMed
11. Celik, F, Coteli, E, Gul, FC, Ozsoy, E, Gungor Kobat, S, Karaca Karagoz, Z, et al.. Interleukin 18, soluble cluster of differentiation 40, platelet factor 4 variant 1, and neutrophil gelatinase-associated lipocalin can be used as biomarkers to aid activity and diagnosis in ocular Behçet’s disease. Int Ophthalmol 2022;42:3321–31. https://doi.org/10.1007/s10792-022-02331-4.Search in Google Scholar PubMed
12. Xue, W, Li, JJ, Zou, Y, Zou, B, Wei, L. Microbiota and ocular diseases. Front Cell Infect Microbiol 2021;11:759333. https://doi.org/10.3389/fcimb.2021.759333.Search in Google Scholar PubMed PubMed Central
13. Ueta, M, Kinoshita, S. Innate immunity of the ocular surface. Brain Res Bull 2010;81:219–28. https://doi.org/10.1016/j.brainresbull.2009.10.001.Search in Google Scholar PubMed
14. Miller, D, Iovieno, A. The role of microbial flora on the ocular surface. Curr Opin Allergy Clin Immunol 2009;9:466–70. https://doi.org/10.1097/aci.0b013e3283303e1b.Search in Google Scholar
15. Liu, Y, Dai, M. Trimethylamine N-oxide generated by the gut microbiota is associated with vascular inflammation: new insights into atherosclerosis. Mediat Inflamm 2020;2020:4634172. https://doi.org/10.1155/2020/4634172.Search in Google Scholar PubMed PubMed Central
16. Zhou, Y, Zhang, Y, Jin, S, Lv, J, Li, M, Feng, N. The gut microbiota derived metabolite trimethylamine N-oxide: its important role in cancer and other diseases. Biomed Pharmacother 2024;177:117031. https://doi.org/10.1016/j.biopha.2024.117031.Search in Google Scholar PubMed
17. Yin, J, Liao, SX, He, Y, Wang, S, Xia, GH, Liu, FT, et al.. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc 2015;4:e002699. https://doi.org/10.1161/jaha.115.002699.Search in Google Scholar
18. Zhu, Y, Li, Q, Jiang, H. Gut microbiota in atherosclerosis: focus on trimethylamine N‐oxide. APMIS 2020;128:353–66. https://doi.org/10.1111/apm.13038.Search in Google Scholar PubMed PubMed Central
19. Wilkinson, CP, Ferris, 3rd FL, Klein, RE, Lee, PP, Agardh, CD, Davis, M, et al.. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–82. https://doi.org/10.1016/s0161-6420(03)00475-5.Search in Google Scholar PubMed
20. Seldin, MM, Meng, Y, Qi, H, Zhu, W, Wang, Z, Hazen, SL, et al.. TrimethylamineN‐Oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear Factor‐Κb. J Am Heart Assoc 2016;5:e002767. https://doi.org/10.1161/jaha.115.002767.Search in Google Scholar
21. Sun, X, Jiao, X, Ma, Y, Liu, Y, Zhang, L, He, Y, et al.. TrimethylamineN-oxideinduces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun 2016;481:63–70. https://doi.org/10.1016/j.bbrc.2016.11.017.Search in Google Scholar PubMed
22. Setchell, KDR, Clerici, C. Equol: pharmacokinetics and biological actions. J Nutr 2010;140:1363S–8S. https://doi.org/10.3945/jn.109.119784.Search in Google Scholar PubMed PubMed Central
23. Gong, Y, Lv, J, Pang, X, Zhang, S, Zhang, G, Liu, L, et al.. Advances in the metabolic mechanism and functional characteristics of equol. Foods 2023;12:2334. https://doi.org/10.3390/foods12122334.Search in Google Scholar PubMed PubMed Central
24. Chinta, SJ, Ganesan, A, Reis-Rodrigues, P, Lithgow, GJ, Andersen, JK. Anti-inflammatory role of the isoflavone diadzein in lipopolysaccharide-stimulated microglia: implica- tions for Parkinson’s disease. Neurotox Res 2013;23:145–53. https://doi.org/10.1007/s12640-012-9328-5.Search in Google Scholar PubMed PubMed Central
25. Gao, K, Mu, CL, Farzi, A, Zhu, WY. Tryptophan metabolism: a link between the gut microbiota and brain. Adv Nutr 2020;11:709–23. https://doi.org/10.1093/advances/nmz127.Search in Google Scholar PubMed PubMed Central
26. Wu, W, Bush, KT, Nigam, SK. Key role for the organic anion transporters, OAT1 and OAT3, in the in vivo handling of uremic toxins and solutes. Sci Rep 2017;7:4939. https://doi.org/10.1038/s41598-017-04949-2.Search in Google Scholar PubMed PubMed Central
27. Subedi, L, Ji, E, Shin, D, Jin, J, Yeo, JH, Kim, SY. Equol, a dietary daidzein gut metabolite attenuates microglial activation and potentiates neuroprotection in vitro. Nutrients 2017;9:207. https://doi.org/10.3390/nu9030207.Search in Google Scholar PubMed PubMed Central
28. Matsumoto, T, Kojima, M, Takayanagi, K, Taguchi, K, Kobayashi, T. Role of S-equol, indoxyl sulfate, and trimethylamine N-oxide on vascular function. Am J Hypertens 2020;33:793–803. https://doi.org/10.1093/ajh/hpaa053.Search in Google Scholar PubMed PubMed Central
29. Zając, H, Turno-Kręcicka, A. Ocular manifestations of Behçet’s disease: an update on diagnostic challenges and disease management. J Clin Med 2021;10:5174. https://doi.org/10.3390/jcm10215174.Search in Google Scholar PubMed PubMed Central
30. Tanaka, R, Murata, H, Takamoto, M, Ohtomo, K, Okinaga, K, Yoshida, A, et al.. Behçet’s disease ocular attack score 24 and visual outcome in patients with Behçet’s disease. Br J Ophthalmol 2016;100:990–4. https://doi.org/10.1136/bjophthalmol-2015-307362.Search in Google Scholar PubMed
31. Kaburaki, T, Namba, K, Sonoda, K, Kezuka, T, Keino, H, Fukuhara, T, et al.. Ocular Behçet Disease Research Group of Japan. Behçet’s disease ocular attack score 24: evaluation of ocular disease activity before and after initiation of infliximab. Jpn J Ophthalmol 2014;58:120–30. https://doi.org/10.1007/s10384-013-0294-0.Search in Google Scholar PubMed
32. DeGruttola, AK, Low, D, Mizoguchi, A, Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis 2016;22:1137–50. https://doi.org/10.1097/mib.0000000000000750.Search in Google Scholar PubMed PubMed Central
33. Li, T, Gua, C, Wu, B, Chen, Y. Increased circulating trimethylamine N-oxide contributes to endothelial dysfunction in a rat model of chronic kidney disease. Biochem Biophys Res Commun 2018;495:2071–7. https://doi.org/10.1016/j.bbrc.2017.12.069.Search in Google Scholar PubMed
34. Wang, G, Kong, B, Shuai, W, Fu, H, Jiang, X, Huang, H. 3,3-Dimethyl-1-butanol attenuates cardiac remodeling in pressure-overload-induced heart failure mice. J Nutr Biochem 2020;78:108341. https://doi.org/10.1016/j.jnutbio.2020.108341.Search in Google Scholar PubMed
35. Wang, J, Gu, X, Yang, J, Wei, Y, Zhao, Y. Gut microbiota dysbiosis and increased plasma LPS and TMAO levels in patients with preeclampsia. Front Cell Infect Microbiol 2019;9:409. https://doi.org/10.3389/fcimb.2019.00409.Search in Google Scholar PubMed PubMed Central
36. Yasukawa, K, Tokuda, H, Tun, X, Utsumi, H, Yamada, K. The detrimental effect of nitric oxide on tissue is associated with inflammatory events in the vascular endothelium and neutrophils in mice with dextran sodium sulfate-induced colitis. Free Radic Res 2012;46:1427–36. https://doi.org/10.3109/10715762.2012.732698.Search in Google Scholar PubMed
37. Johnson, SL, Park, HY, Vattem, DA, Grammas, P, Ma, H, Seeram, NP. Equol, a blood-brain barrier permeable gut microbial metabolite of dietary isoflavone daidzein, exhibits neuroprotective effects against neurotoxins induced toxicity in human neuroblastoma SH-SY5Y cells and Caenorhabditis elegans. Plant Foods Hum Nutr 2020;75:512–7. https://doi.org/10.1007/s11130-020-00840-0.Search in Google Scholar PubMed
38. Leong, SC, Sirich, TL. Indoxyl sulfate—review of toxicity and therapeutic strategies. Toxins 2016;8:358. https://doi.org/10.3390/toxins8120358.Search in Google Scholar PubMed PubMed Central
39. Lai, YR, Cheng, BC, Lin, CN, Chiu, WC, Lin, TY, Chiang, HC, et al.. The effects of indoxyl sulfate and oxidative stress on the severity of peripheral nerve dysfunction in patients with chronic kidney diseases. Antioxidants 2022;11:2350. https://doi.org/10.3390/antiox11122350.Search in Google Scholar PubMed PubMed Central
40. Faure, V, Dou, L, Sabatier, F, Cerini, C, Sampol, J, Berland, Y, et al.. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost 2006;4:566–73. https://doi.org/10.1111/j.1538-7836.2005.01780.x.Search in Google Scholar PubMed
41. Yu, M, Kim, YJ, Kang, DH. Indoxyl sulfate–induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol 2011;6:30–9. https://doi.org/10.2215/cjn.05340610.Search in Google Scholar
42. Zhen, J, Zhou, Z, He, M, Han, HX, Lv, EH, Wen, PB, et al.. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front Endocrinol 2023;14:1085041. https://doi.org/10.3389/fendo.2023.1085041.Search in Google Scholar PubMed PubMed Central
43. Mayo, B, Vázquez, L, Flórez, AB. Equol: a bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients 2019;11:2231. https://doi.org/10.3390/nu11092231.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review
- Targeting oxidative stress, iron overload and ferroptosis in bone-degenerative conditions
- Research Articles
- Assessing medical biochemistry professionals’ knowledge, attitudes, and behaviors regarding green and sustainable medical laboratory practices in Türkiye
- The efficacy of high pressure liquid chromatography (HPLC) in detecting congenital glycosylation disorders (CDG)
- Atypical cells parameter in sysmex UN automated urine analyzer: a single center study
- The frequency of single specific immunoglobulin E and allergen mixes with a MAST (multiple-antigen simultaneous test) technique
- Differences in second trimester risk estimates for trisomy 21 between Maglumi X3/Preaccu and Immulite/Prisca systems
- Comparison of classical and flowcytometric osmotic fragility and flowcytometric eosin-5-maleimide binding tests in diagnosis of hereditary spherocytosis
- Casticin inhibits the hedgehog signaling and leads to apoptosis in AML stem-like KG1a and mature KG1 cells
- Trimethylamine N-oxide, S-equol, and indoxyl sulfate inflammatory microbiota players in ocular Behçet’s disease
- Genomic profiling of interferon signaling pathway gene mutations in type 2 diabetic individuals with COVID-19
- CDR1as/miR-7-5p/IGF1R axis contributes to the suppression of cell viability in prostate cancer
- Role of interferon regulatory factors in predicting the prognosis of Crimean-Congo hemorrhagic fever
- The significance of taurine for patients with Crimean-Congo hemorrhagic fever and COVID-19 diseases: a cross-sectional study
- Gene mining, recombinant expression and enzymatic characterization of N-acetylglucosamine deacetylase
- Ethanol inhibited growth hormone receptor-mediated endocytosis in primary mouse hepatocytes
- Gypsophila eriocalyx roots inhibit proliferation, migration, and TGF-β signaling in melanoma cells
- The role of kynurenine and kynurenine metabolites in psoriasis
- Tobacco induces abnormal metabolism of tryptophan via the kynurenine pathway
- Effect of vitamin D and omega-3 on the expression of endoplasmic reticulum-associated protein degradation and autophagic proteins in rat brain
Articles in the same Issue
- Frontmatter
- Review
- Targeting oxidative stress, iron overload and ferroptosis in bone-degenerative conditions
- Research Articles
- Assessing medical biochemistry professionals’ knowledge, attitudes, and behaviors regarding green and sustainable medical laboratory practices in Türkiye
- The efficacy of high pressure liquid chromatography (HPLC) in detecting congenital glycosylation disorders (CDG)
- Atypical cells parameter in sysmex UN automated urine analyzer: a single center study
- The frequency of single specific immunoglobulin E and allergen mixes with a MAST (multiple-antigen simultaneous test) technique
- Differences in second trimester risk estimates for trisomy 21 between Maglumi X3/Preaccu and Immulite/Prisca systems
- Comparison of classical and flowcytometric osmotic fragility and flowcytometric eosin-5-maleimide binding tests in diagnosis of hereditary spherocytosis
- Casticin inhibits the hedgehog signaling and leads to apoptosis in AML stem-like KG1a and mature KG1 cells
- Trimethylamine N-oxide, S-equol, and indoxyl sulfate inflammatory microbiota players in ocular Behçet’s disease
- Genomic profiling of interferon signaling pathway gene mutations in type 2 diabetic individuals with COVID-19
- CDR1as/miR-7-5p/IGF1R axis contributes to the suppression of cell viability in prostate cancer
- Role of interferon regulatory factors in predicting the prognosis of Crimean-Congo hemorrhagic fever
- The significance of taurine for patients with Crimean-Congo hemorrhagic fever and COVID-19 diseases: a cross-sectional study
- Gene mining, recombinant expression and enzymatic characterization of N-acetylglucosamine deacetylase
- Ethanol inhibited growth hormone receptor-mediated endocytosis in primary mouse hepatocytes
- Gypsophila eriocalyx roots inhibit proliferation, migration, and TGF-β signaling in melanoma cells
- The role of kynurenine and kynurenine metabolites in psoriasis
- Tobacco induces abnormal metabolism of tryptophan via the kynurenine pathway
- Effect of vitamin D and omega-3 on the expression of endoplasmic reticulum-associated protein degradation and autophagic proteins in rat brain

