Abstract
Objectives
Growth hormone (GH) exhibits various essential physiological functions, which are exerted by its binding to growth hormone receptor (GHR). Ethanol has been demonstrated to have an impact on GH’s biological activity. Nevertheless, mechanism underlying the regulation of the biological activity of GH by ethanol have yet to be fully elucidated.
Methods
This study utilized an indirect immunofluorescence assay to identify GHR expression in mouse hepatocytes. Western blot was used to determine the impact of ethanol on GH-induced intracellular signalling. Indirect immunofluorescence and colocalization experiments were used to determine the effect of ethanol on GH-GHR’s nuclear localization and endocytosis.
Results
GHR was primarily localized in the cell membrane and cytoplasm. The phosphorylation levels of JAK2 and STAT1/3/5 were markedly lowered after treatment with ethanol. On this basis, we further explored the mechanism underlying the regulation of GH biological activity by ethanol from the perspective of cell internalization. We found that the nuclear translocation of GH-GHR was inhibited when treated with ethanol. In addition, the results of colocalization analyses revealed that ethanol inhibited GHR-mediated nuclear translocation may mainly by inhibiting caveolin-dependent endocytosis.
Conclusions
Our study showed that ethanol inhibits GH signaling ability in a time-dependent manner. Ethanol could inhibit the nuclear localization of GH-GHR, which may be linked to the inhibition of the interaction between GHR and caveolin. The combined effect of these factors downregulated the GH-GHR signal. This study laid a foundation for further exploring the mechanism that the effects of ethanol on GH biological activity.
Introduction
Approximately 2 billion people worldwide drink alcoholic beverages. Moderate wine consumption is reported to reduce the risk of developing Alzheimer’s disease and has hepatoprotective function [1], [2], [3]. However, long-term or heavy drinking clearly leads to liver damage [4]. The mechanism underlying this phenomenon is still not comprehensively understood. Several studies have reported that alcohol have harmful effects on body via inducing significant changes in cell metabolism and signal transduction [5]. As early as in the 1980s, it was discovered that alcohol can impair receptor-mediated endocytosis (RME) [6]. Subsequently, multiple pathways by which alcohol affects RME were discovered. For example, alcohol can affect the internalization of epidermal growth factor (EGF) by diminishing the number of cell surface receptors [7]. It can affect the internalization of envelope protein gp120 of human immunodeficiency virus 1 in hepatocytes though the lysosome/ubiquitin proteolytic pathways [8]. Moreover, it can change the fluidity of plasma membrane through the cholesterol in the plasma membrane and manage the intracellular distribution of Rab protein to affect the clathrin-independent endocytosis [9]. Alcohol increases the level of Arf6 in the nervous system, which controls the assembly of AP-2/clathrin coat in clathrin-mediated endocytosis [10].
Growth hormone (GH) is a 191‐amino acid polypeptide hormone. GH can serve various physiological functions including regulate the metabolism of proteins, lipids, and carbohydrates [11]. GH exerts its biological effects via binding to the growth hormone receptor (GHR) [12]. GHR is a class-I cytokine receptor containing 638 amino acids. It is widely expressed as a dimer on the body’s cell membrane and greater levels in the liver. GH can activate the extracellular domain (EDC) of GHR, which in turn activates Janus kinase2 (JAK2). After this, a number of down-stream intracellular signaling molecules, including signal transducers and activators of transcription (STAT; STATs 1/3/5), are phosphorylated to enable GH to perform related biological functions [13], [14], [15], [16].
Internalized GH and GHR exist in the form of GH-GHR dimers. It has been reported that ethanol suppresses the GHR signaling rather than ligand binding [17], 18]. The nuclear localization of GHR-dependent GH is crucial for initiating various GH-induced signal transduction pathways [19], [20], [21]. Endocytosis is a process necessary for many important physiological functions of cells. The intracellular endocytosis occurs via multiple pathways. For instance, caveolin‐dependent, clathrin‐dependent, and caveolin‐ and clathrin‐independent. Ethanol could inhibit caveolin-dependent or clathrin- and caveolin-independent endocytosis by impairing asialoglycoprotein receptor function, disturbing protein trafficking [22], 23]. Moreover, ethanol impairs receptor-mediated endocytosis of various proteins, including EGFR, via some endocytosis defect or receptor recycling [7], [24], [25], [26]. However, whether ethanol affects GHR endocytosis has yet to be elucidated.
In the present study, we investigated the molecular mechanism that ethanol inhibits GH biological activity from the perspective of cell internalization. We initially explored the impact of ethanol on GH-induced intracellular signalling by Western blot analysis. Furthermore, we determined the effect of ethanol on GH-GHR’s nuclear localization and endocytosis by indirect immunofluorescence and colocalization experiments. We postulated that ethanol may inhibit the endocytosis mediated by GHR. This study reported a new mechanism via which ethanol inhibits the biological activity of GH.
Materials and methods
Antibodies, reagents, and cell lines
Total JAK2 (Cat#3230) and phospho-JAK2 (Cat#3771); Total STAT5 (Cat#94205) and phospho-STAT5 (Cat#9359); Total STAT3 (Cat#9139) and phospho-STAT3 (Cat#9145); Total STAT1 (Cat#14994) and phospho-STAT1 (Cat#14995), β-actin (Cat#3700), Caveolin-1 antibody (Cat#3238), were obtained from Cell Signaling Technology (Boston USA). Human growth hormone (Cat#CYT-202) was obtained from ProSpec (Ness-Ziona Israel). Anti‐GHR antibody (Cat#ab65304), Alexa Fluor 488 (Cat#ab150077) were obtained from Abcam (Cambridge, UK). Gibco (Grand Island) supplied the fetal bovine serum (FBS) and cell culture medium. Beyotime (Shanghai, China) supplied the bicinchoninic acid (BCA) kits. Pierce (Rockford, USA) provided enhanced chemiluminescence (ECL) and bovine serum albumin (BSA). Millipore (Bedford, MA, USA) provided polyvinylidene fluoride (PVDF) membranes. Sigma-Aldrich (USA) was the source of all additional reagents, unless otherwise specified.
All experiments involving animals were performed with the approval of Binzhou Medical University’s animal ethics committee (No. 2024-096). In this investigation, adult C57/BL6 mice were employed, which were accommodated in a controlled environment free from specific pathogens. The mice were allowed unrestricted accessibility to sterilized water and granular food, and they were subjected to alternating 12 h light/dark cycles. The ambient humidity was sustained between 40 and 60 %, while the temperature was preserved at a range of 22–24 °C. Based on our previously methods [27], primary mouse hepatocytes endogenously abundantly expressing GHR were isolated utilizing the two-step collagenase perfusion technique by Seglen [28], with minor changes.
Indirect immunofluorescence assay (IFA)
The mouse primary hepatocytes were starved for 1 h by culturing in the medium without serum. After washing with phosphate buffered saline (PBS), the hepatocytes were stimulated with 1 % v/v ethanol for 30 min, then treated with Alexa Fluor 488-GH (20 μg/mL) for 30 min. Further, the cells were fixed with 4 % paraformaldehyde (PFA) for 10 min, then washed three times with PBS. 4′,6-diamidino-2-phenylindole (DAPI) was employed to stain the cell nuclei. The cells were observed utilizing confocal laser scanning microscopy (CLSM, Zeiss, Oberkochen, Germany). Image analysis was carried out utilizing Zeiss ZEN Blue software and ImageJ software.
To dynamically detect the localization of GH, mouse primary hepatocytes were cultured in a laser confocal petri dish. The cells were treated with ethanol (1 % v/v) for 30min, then stimulated with Alexa Fluor 488-GH (20 μg/mL) for various durations (5, 15, 30, 45, 60, 90 min). After washing three times, the cells were observed using CLSM.
For the colocalization analysis, the hepatocytes were treated with GH or Ethanol (EtOH)+GH. Then fixed with 4 % PFA, blocked with 3 % BSA. After washing 3 times with PBS, anti‐cavoelin and anti‐GHR antibodies were incubated. After that, the cells were washed and probed with fluorescence-labeled secondary antibody. After washing three times, the cells were observed using CLSM.
Western blotting
The mouse primary hepatocytes were exposed to ethanol for 30 min, then treated with GH for 0, 15, 30, 45, 60, 90 min. Using BCA kits, the protein content in these cells was determined. Afterward, a 7–12 % sodium dodecyl sulfate-polyacrylamide gel was utilized to electrophoresis 30 μg of protein per lane which was subsequently loaded onto polyvinylidene fluoride membranes. The membranes were subjected to a blocking step using 5 % BSA. After washing thrice in Tris-buffered saline, primary antibodies were added to incubate the membranes, then incubating the membranes with secondary antibodies. Finally, an enhanced chemiluminescence detection technique was utilized to facilitate protein visualization.
Statistical analysis
The mean values ± standard error are employed to present the data. The statistical analysis was executed employing one-way ANOVA or Tukey’s multiple comparisons test, utilizing the Statistical Analysis System (SAS) software (SAS version 9.0; Institute Inc., Cary, NC, USA). A p-value of less than 0.05 was deemed to be statistically significant.
Results
GHR expression on mouse hepatocytes
First, we examined the GHR expression on hepatocytes. As depicted in Figure 1, GHR was primarily found in the cell membrane and cytoplasm. The nucleus was stained blue by DAPI. The control antibody did not demonstrate any positive staining (data not presented).

CLSM analysis to evaluate the expression of GHR on hepatocytes. After rinsing, the cells were, fixed, then blocked with BSA. After washing with PBS, the cells were incubated with anti-GHR antibodies at 4 °C overnight. Then the cells were incubated with secondary. The cell nuclei were stained with DAPI. The cell samples were visualized by CLSM. Bar=10 μm. The figures shown represent at least three separate experiments.
GHR-mediated intracellular signaling is suppressed by ethanol
The endogenous expression of GHR in the hepatocytes were assessed via western blotting. The results showed that GHR expression decreased after GH treatment in a time‐dependent manner (Figure 2A). Furthermore, the impact of ethanol on GH-induced intracellular signaling was assessed. When the cells were treated with GH or EtOH+GH, the phosphorylation levels of intracellular signaling proteins (JAK2, and STAT1/3/5) first increased and then decreased. Ethanol strongly reduced the phosphorylation levels of these signaling proteins (Figure 2B–E). These findings indicated that ethanol inhibited GHR-mediated intracellular signaling in a time-dependent manner.

The impact of EtOH on GHR-mediated intracellular signaling proteins. (A) Serum-starved mouse hepatocytes were treated with GH (20 μg/mL) for various time periods (0, 15, 30, 60, and 90 min). Then, the expression levels of GHR protein were established via western blot. (B–E) effect of ethanol on GH induced intracellular signal transduction. The expression of phosphorylated and total JAK2 (B), STAT1 (C), STAT3 (D), and STAT5 (E) was detected by western blot. The figures shown represent at least three separate experiments. *p<0.05, **p<0.01 compared with the GH group.
Internalization and nuclear localization of GH-GHR with or without ethanol stimulation
To observe the internalization of GH after ethanol stimulation, cells were treated with GH or EtOH+GH (Figure 3A). The IFA experiments indicated that amounts of GH (green) were observed in the nucleus in the control group. And the nuclear localization of GH was significantly reduced after ethanol stimulation compared with GH alone. Following ethanol treatment, we also examined GHR expression in the cell membrane and nucleus using Western blot analysis. Figure 3B illustrates that upon GH administration, GHR was present in both the nuclear extract and the cytoplasm. However, after exposure to ethanol, GHR was primarily detected in the cytoplasm, with minimal expression observed in the nucleus. These results illustrated that ethanol may inhibit the nuclear translocation of GH.
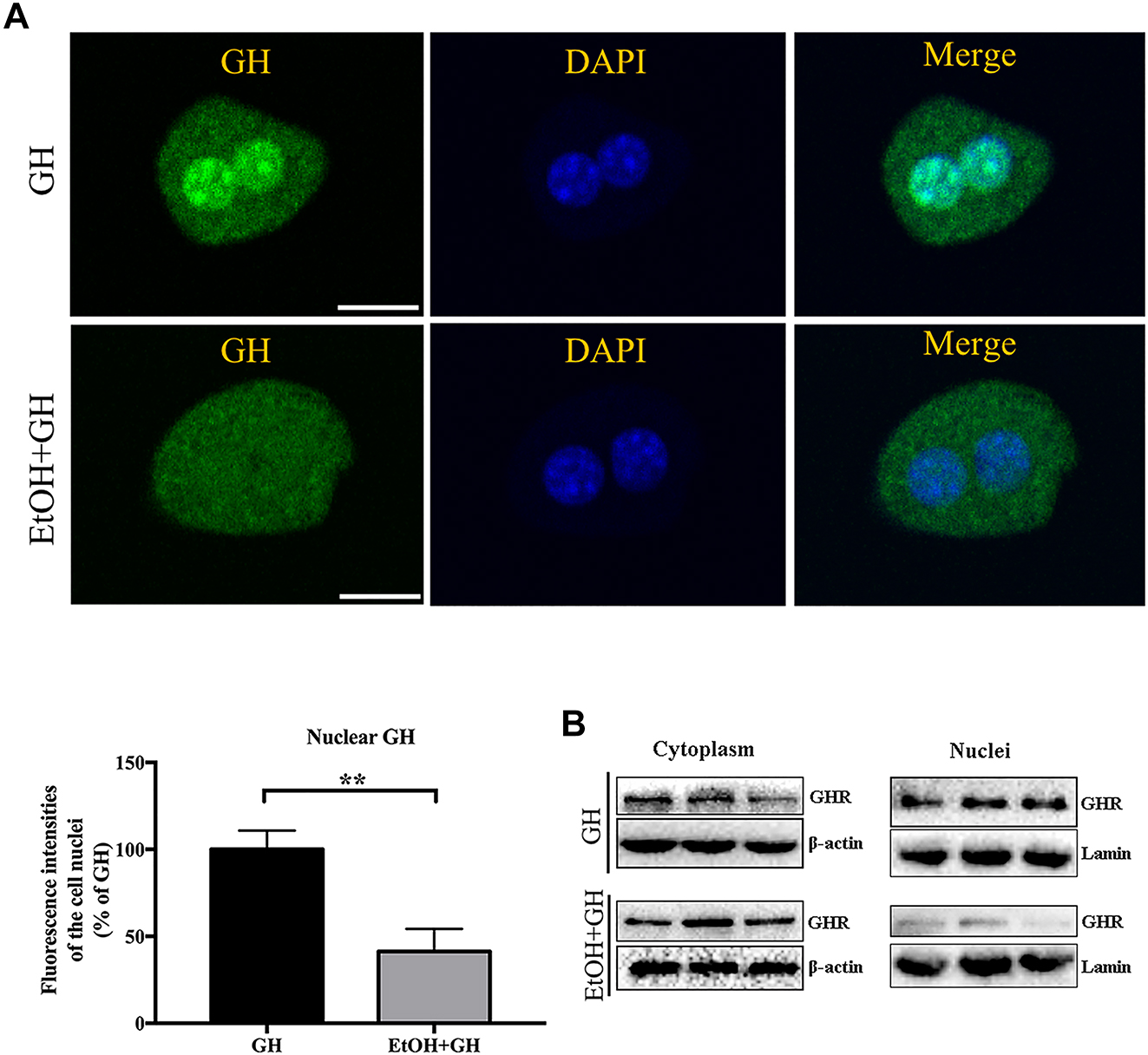
The impact of EtOH on the nuclear localization of GH in mouse hepatocytes. (A) The cells were exposed to alexa fluor 488-GH (20 μg/mL) for 30min, or exposed to alexa fluor 488-GH (20 μg/mL) after EtOH (1 % v/v) treated for 30min. After washing, the cells were fixed and stained with DAPI (nucleus). The cells were subjected to CLSM. Bar=10 μm **p<0.01 compared with the GH group. (B) The expression of GHR in the membrane and nuclei of mouse hepatocytes. The cells were exposed to GH (20 μg/mL) for 30 min, or exposed to GH (20 μg/mL) after EtOH (1 % v/v) treated for 30min, the expression levels of GHR protein in the membrane and nuclei were detected by western blot. The figures shown represent at least three separate experiments.
Ethanol inhibited the nuclear localization of GH in a time‐dependent manner
CLSM was employed to investigate the impact of ethanol on the internalization of GH in the hepatocytes at various times (5–90 min). Without ethanol treatment, a substantial quantity of GH (green) was located on the plasma membrane and in the cytoplasm and nucleus in 30–60 min (Figure 4). However, subsequent to ethanol treatment, GH was found to be mostly present on the plasma membrane with minimal presence within the cell nucleus. The findings indicated that ethanol suppresses the nuclear localization of GH in a time-dependent manner.

The dynamics of GH internalization after stimulation with GH or EtOH+GH. The cells were exposed to alexa fluor 488-GH (20 μg/mL), or exposed to alexa fluor 488-GH (20 μg/mL) after EtOH (1 % v/v) treated for different durations. The cells were fixed with 4 % paraformaldehyde. After washing with PBS, the cells were visualized using CLSM. Bar=10 μm. The figures shown represent at least three separate experiments. Distinct letters indicate a statistically significant difference (p<0.05).
GH‐GHR endocytosis is affected by ethanol
The endocytosis pathway mediated by clathrin and cavoelin is reported to have a vital function in the internalization of GHR. To identify whether ethanol affects GH-GHR internalization, colocalization experiments were conducted using CLSM. The presence of cavoelin and GHR colocalization signals was identified when the cells were exposed alone to GH (Figure 5). However, after treating both ethanol and GH, the colocalization signals of GHR and cavoelin markedly decreased in the nucleus. The results suggesting that ethanol can not only inhibit GH-mediated intracellular signaling at the plasma membrane but can also affect GH‐GHR endocytosis (summarized mechanisms are depicted in Figure 6).

The endocytosis of GH or EtOH+GH. The cells were exposed to GH (20 μg/mL) for 30 min, or exposed to GH (20 μg/mL) after EtOH (1 % v/v) treated for 30min, after which the cells were washed three times. Then cells were further fixed with 4 % paraformaldehyde, then blocked with 3 % BSA, and immunostained utilizing anti‐cavoelin (green), and anti‐GHR antibodies (red). Following the last round of washing, the cell samples were visualized using CLSM. Bar=10 μm. The figures shown represent at least three separate experiments.

Possible mechanisms by which ethanol inhibits the biological activity of GH. GH binding with GHR can not only activate GHR-mediated intracellular signaling but also lead to GHR transport into nucleus. In traditional research, ethanol inhibits the biological activity of GH by inhibiting GHR-mediated signal transduction. The present study found a novel possible molecular mechanism: ethanol could inhibit the biological activity of GH via inhibiting GHR-mediated endocytosis, which may be related to the inhibition of the interaction between GHR and caveolin. This study laid a foundation for further exploring the mechanism underlying the effects of ethanol on GH biological activity.
Discussion
It is well known that GH can modulate various metabolic processes, and the suppression of GH-mediated intracellular signaling by ethanol may be a contributing factor to the onset of numerous ethanol-related diseases. Previous studies have reported that ethanol can inhibit spontaneous pulsatile GH secretion [17]. Ethanol can inhibit GH-induced protein synthesis without altering the number or binding characteristics of GHR. Traditional views suggest that ethanol inhibits the biological activity of GH by inhibiting GH-induced signal transduction [18]. However, the precise mechanism responsible for this inhibition is still not completely comprehended. In this investigation, to our knowledge, we present the first exploration of the possible molecular mechanism that ethanol could inhibit biological activity from the perspective of cell internalization.
Our experimental results indicate that GHR was abundantly expressed in the cytoplasm and on the cell membrane. According to the previous studies, JAK2 and GHR are tyrosine-phosphorylated subsequently, and down-stream signaling is subsequently activated after GH interacts with GHR on the cell membrane [13], [14], [15], [16]. In the current study, we found that ethanol inhibited GHR-mediated phosphorylation levels of JAK2 and STAT1/3/5 proteins in a time-dependent manner. However, it has been reported that GH not only displays physiological roles at the cell membrane but also exhibits physiological functions in the cytoplasm and cell nuclei [20], 29].
Internalization is the starting step of nuclear transport of the GH-GHR complex. After binding with GHR, intracellular signaling molecules are activated, and the internalization process of the GH-GHR complex is also initiated. Nuclear localization of GH-GHR can still exhibit important biological activities [20]. Thus, we investigated how ethanol influences the activity of GH-induced intracellular signaling molecules by focusing on the process of cell internalization. It was observed that ethanol suppressed the nuclear localization of GH-GHR. Furthermore, it was noted that following GH stimulation, GH-GHR underwent rapid nuclear translocation in a time-dependent manner, and ethanol blocked the nuclear localization of GH-GHR. This suggested that ethanol may inhibit GH signaling ability through inhibiting GHR-mediated nuclear translocation.
Lipid rafts/vesicles are used as signal transduction platforms by many membrane proteins, mediating important physiological processes [30]. Alcohol can damage the lipid rafts/vesicles of membrane proteins. High concentrations of alcohol can induce complete translocation of these membrane proteins from lipid rafts/vesicles, leading to various pathological changes in various tissues [31]. Strous and Lobie et al. reported that the GH-GHR complex mainly enters the cytoplasm through internalization mediated by clathrin and caveolin [32]. Dalke et al. reported that after long-term alcohol intake, EGFR-mediated endocytosis in rat liver cells was inhibited, affecting multiple parts of the body including surface receptor expression, internalization, intracellular transport to lysosomes, and receptor recycling [7]. Long-term consumption of alcohol can damage protein transport and selectively damage receptor-mediated endocytosis [33]. In our study, GH-GHR could enter into the cytoplasm via caveolin-mediated endocytosis. However, the colocalization signals of GHR-cavoelin were significantly reduced in the nucleus when the cells were exposed to with ethanol and GH. These findings indicate that ethanol may inhibit GHR-mediated nuclear translocation through inhibiting caveolin-dependent endocytosis. However, in addition to caveolin-mediated endocytosis, endocytosis is inhibited in cells via many ways. Thus, we are unable to exclude additional unidentified factors.
In conclusion, we explored the mechanisms underlying the inhibition of GHR-mediated signaling by ethanol in primary mouse hepatocytes. Ethanol could inhibit the nuclear localization of GH-GHR, which may be linked to the inhibition of the interaction between GHR and caveolin. The combined effect of these factors downregulated the GH-GHR signal. Thus, we postulated that ethanol inhibits the endocytosis mediated by GHR. The groundwork for further investigation into the mechanism behind ethanol’s impact on GH biological activity was established by this study. Nevertheless, further research should be done to identify the precise mechanism by which ethanol impacts the biological action of GH.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 32102629
-
Research ethics: All animal studies were approved by the animal ethics committee of Binzhou Medical University (No. 2024-096).
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission. Ruonan Li and Li Xian conceived and designed the experiments. Zihan Ge, Xingjie Liu, Yu Yang, Hainan Lan, Yawen Zhang and Wei Zhang performed all experiments and analyzed the data. Ruonan Li, Zihan Ge and Xingjie Liu wrote the paper.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interests: The authors state no conflict of interest.
-
Research funding: This work was supported by the National Natural Science Foundation of China-Young Scientists Fund (Grant number 32102629). Scientific Research Launch Fund of Binzhou Medical College (Grant Nos.BY2020KYQD19).
-
Data availability: Not applicable.
References
1. Deng, J, Zhou, DH, Li, J, Wang, YJ, Gao, C, Chen, M. A 2-year follow-up study of alcohol consumption and risk of dementia. Clin Neurol Neurosur 2006;108:378–83. https://doi.org/10.1016/j.clineuro.2005.06.005.Search in Google Scholar PubMed
2. Gao, HY, Li, GY, Huang, J, Han, Y, Sun, FZ, Du, XW, et al.. Protective effects of Zhuyeqing liquor on the immune function of normal and immunosuppressed mice in vivo. Bmc Complem Altern M 2013;13:1–7. https://doi.org/10.1186/1472-6882-13-252.Search in Google Scholar PubMed PubMed Central
3. Gao, HY, Huang, J, Wang, HY, Du, XW, Cheng, SM, Han, Y, et al.. Protective effect of Zhuyeqing liquor, a Chinese traditional health liquor, on acute alcohol-induced liver injury in mice. J Inflamm 2013;10:1–9. https://doi.org/10.1186/1476-9255-10-30.Search in Google Scholar PubMed PubMed Central
4. Asrani, SK, Devarbhavi, H, Eaton, J, Kamath, PS. Burden of liver diseases in the world. J Hepatol 2019;70:151–71. https://doi.org/10.1016/j.jhep.2018.09.014.Search in Google Scholar PubMed
5. Nowak, AJ, Relja, B. The impact of acute or chronic alcohol intake on the NF-κB signaling pathway in alcohol-related liver disease. Int J Mol Sci 2020;21:9407. https://doi.org/10.3390/ijms21249407.Search in Google Scholar PubMed PubMed Central
6. Casey, C, Kragskow, S, Sorrell, M, Tuma, D. Chronic ethanol administration impairs the binding and endocytosis of asialo-orosomucoid in isolated hepatocytes. J Biol Chem 1987;262:2704–10. https://doi.org/10.1016/s0021-9258(18)61564-9.Search in Google Scholar
7. Dalke, DD, Sorrell, MF, Casey, CA, Tuma, DJ. Chronic ethanol administration impairs receptor-mediated endocytosis of epidermal growth factor by rat hepatocytes. Hepatology 1990;12:1085–91. https://doi.org/10.1002/hep.1840120502.Search in Google Scholar PubMed
8. Singh, AK, Jiang, Y, Gupta, S. Effects of chronic alcohol drinking on receptor-binding, internalization, and degradation of human immunodeficiency virus 1 envelope protein gp120 in hepatocytes. Alcohol 2007;41:591–606. https://doi.org/10.1016/j.alcohol.2007.08.003.Search in Google Scholar PubMed
9. Parmahamsa, M, Reddy, KR, Varadacharyulu, N. Changes in composition and properties of erythrocyte membrane in chronic alcoholics. Alcohol Alcohol 2004;39:110–2. https://doi.org/10.1093/alcalc/agh034.Search in Google Scholar PubMed
10. Jaworski, J. ARF6 in the nervous system. Eur J Cell Biol 2007;86:513–24. https://doi.org/10.1016/j.ejcb.2007.04.007.Search in Google Scholar PubMed
11. Vijayakumar, A, Novosyadlyy, R, Wu, Y, Yakar, S, LeRoith, D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm Igf Res 2010;20:1–7. https://doi.org/10.1016/j.ghir.2009.09.002.Search in Google Scholar PubMed PubMed Central
12. Dehkhoda, F, Lee, CM, Medina, J, Brooks, AJ. The growth hormone receptor: mechanism of receptor activation, cell signaling, and physiological aspects. Front Endocrinol 2018;9:35. https://doi.org/10.3389/fendo.2018.00035.Search in Google Scholar PubMed PubMed Central
13. Cunningham, BC, Ultsch, M, de Vos, AM, Mulkerrin, MG, Clauser, KR, Wells, JA. Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science 1991;254:821–5. https://doi.org/10.1126/science.1948064.Search in Google Scholar PubMed
14. De Vos, AM, Ultsch, M, Kossiakoff, AA. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 1992;255:306–12. https://doi.org/10.1126/science.1549776.Search in Google Scholar PubMed
15. Wells, JA. Binding in the growth hormone receptor complex. P Natl A Sci 1996;93:1–6. https://doi.org/10.1073/pnas.93.1.1.Search in Google Scholar PubMed PubMed Central
16. Waters, MJ, Brooks, AJ. JAK2 activation by growth hormone and other cytokines. Biochem J 2015;466:1–11. https://doi.org/10.1042/bj20141293.Search in Google Scholar
17. Lang, CH, Liu, X, Nystrom, G, Wu, D, Cooney, RN, Frost, RA. Acute effects of growth hormone in alcohol-fed rats. Alcohol Alcohol 2000;35:148–58. https://doi.org/10.1093/alcalc/35.2.148.Search in Google Scholar PubMed
18. Xu, X, Ingram, RL, Sonntag, WE. Ethanol suppresses growth hormone‐mediated cellular responses in liver slices. Alcohol Clin Exp Res 1995;19:1246–51. https://doi.org/10.1111/j.1530-0277.1995.tb01607.x.Search in Google Scholar PubMed
19. Brooks, AJ, Waters, MJ. The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol 2010;6:515–25. https://doi.org/10.1038/nrendo.2010.123.Search in Google Scholar PubMed
20. Conway-Campbell, BL, Wooh, JW, Brooks, AJ, Gordon, D, Brown, RJ, Lichanska, AM, et al.. Nuclear targeting of the growth hormone receptor results in dysregulation of cell proliferation and tumorigenesis. P Natl A Sci 2007;104:13331–6. https://doi.org/10.1073/pnas.0600181104.Search in Google Scholar PubMed PubMed Central
21. Lanning, NJ, Carter-Su, C. Recent advances in growth hormone signaling. Rev Endocr Metab Dis 2006;7:225–35. https://doi.org/10.1007/s11154-007-9025-5.Search in Google Scholar PubMed
22. Casey, CA, Lee, SM, Aziz-Seible, R, McVicker, BL. Impaired receptor-mediated endocytosis: its role in alcohol-induced apoptosis. J Gastroen Hepatol 2008;23:S46–9. https://doi.org/10.1111/j.1440-1746.2007.05275.x.Search in Google Scholar PubMed
23. Marin, MP, Esteban-Pretel, G, Ponsoda, X, Romero, AM, Ballestin, R, López, C, et al.. Endocytosis in cultured neurons is altered by chronic alcohol exposure. Toxicol Sci 2010;115:202–13. https://doi.org/10.1093/toxsci/kfq040.Search in Google Scholar PubMed
24. Dunn, WA, Hubbard, AL. Receptor-mediated endocytosis of epidermal growth factor by hepatocytes in the perfused rat liver: ligand and receptor dynamics. J Cell Biol 1984;98:2148–59. https://doi.org/10.1083/jcb.98.6.2148.Search in Google Scholar PubMed PubMed Central
25. Gladhaug, I, Christoffersen, T. Rapid constitutive internalization and externalization of epidermal growth factor receptors in isolated rat hepatocytes. Monensin inhibits receptor externalization and reduces the capacity for continued endocytosis of epidermal growth factor. J Biol Chem 1988;263:12199–203. https://doi.org/10.1016/s0021-9258(18)37739-1.Search in Google Scholar
26. Sharma, RJ, Grant, DA. A differential effect between the acute and chronic administration of ethanol on the endocytotic rate konstant, ke, for internalisation of asialoglycoproteins by hepatocytes. BBA-Biomembranes 1986;862:199–204. https://doi.org/10.1016/0005-2736(86)90483-9.Search in Google Scholar PubMed
27. Lan, H, Li, W, Fu, Z, Yang, Y, Wu, T, Liu, Y, et al.. Differential intracellular signalling properties of the growth hormone receptor induced by the activation of an anti-GHR antibody. Mol Cell Endocrinol 2014;390:54–64. https://doi.org/10.1016/j.mce.2014.04.004.Search in Google Scholar PubMed
28. Seglen, PO. Preparation of isolated rat liver cells. Method Cell Biol 1976;13:29–83. https://doi.org/10.1016/s0091-679x(08)61797-5.Search in Google Scholar PubMed
29. van Kerkhof, P, Govers, R, dos Santos, CM, Strous, GJ. Endocytosis and degradation of the growth hormone receptor are proteasome-dependent. J Biol Chem 2000;275:1575–80. https://doi.org/10.1074/jbc.275.3.1575.Search in Google Scholar PubMed
30. Cuddy, LK, Winick‐Ng, W, Rylett, RJ. Regulation of the high-affinity choline transporter activity and trafficking by its association with cholesterol-rich lipid rafts. J Neurochem 2014;128:725–40. https://doi.org/10.1111/jnc.12490.Search in Google Scholar PubMed
31. González-Reimers, E, Santolaria-Fernández, F, Martín-González, MC, Fernández-Rodríguez, CM, Quintero-Platt, G. Alcoholism: a systemic proinflammatory condition. World J Gastroentero 2014;20. https://doi.org/10.3748/wjg.v20.i40.14660.Search in Google Scholar PubMed PubMed Central
32. Slotman, JA, da Silva Almeida, AC, Hassink, GC, van de Ven, RH, van Kerkhof, P, Kuiken, HJ, et al.. Ubc13 and COOH terminus of Hsp70-interacting protein (CHIP) are required for growth hormone receptor endocytosis. J Biol Chem 2012;287:15533–43. https://doi.org/10.1074/jbc.m111.302521.Search in Google Scholar
33. McVicker, BL, Casey, CA. Effects of ethanol on receptor-mediated endocytosis in the liver. Alcohol 1999;19:255–60. https://doi.org/10.1016/s0741-8329(99)00043-9.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review
- Targeting oxidative stress, iron overload and ferroptosis in bone-degenerative conditions
- Research Articles
- Assessing medical biochemistry professionals’ knowledge, attitudes, and behaviors regarding green and sustainable medical laboratory practices in Türkiye
- The efficacy of high pressure liquid chromatography (HPLC) in detecting congenital glycosylation disorders (CDG)
- Atypical cells parameter in sysmex UN automated urine analyzer: a single center study
- The frequency of single specific immunoglobulin E and allergen mixes with a MAST (multiple-antigen simultaneous test) technique
- Differences in second trimester risk estimates for trisomy 21 between Maglumi X3/Preaccu and Immulite/Prisca systems
- Comparison of classical and flowcytometric osmotic fragility and flowcytometric eosin-5-maleimide binding tests in diagnosis of hereditary spherocytosis
- Casticin inhibits the hedgehog signaling and leads to apoptosis in AML stem-like KG1a and mature KG1 cells
- Trimethylamine N-oxide, S-equol, and indoxyl sulfate inflammatory microbiota players in ocular Behçet’s disease
- Genomic profiling of interferon signaling pathway gene mutations in type 2 diabetic individuals with COVID-19
- CDR1as/miR-7-5p/IGF1R axis contributes to the suppression of cell viability in prostate cancer
- Role of interferon regulatory factors in predicting the prognosis of Crimean-Congo hemorrhagic fever
- The significance of taurine for patients with Crimean-Congo hemorrhagic fever and COVID-19 diseases: a cross-sectional study
- Gene mining, recombinant expression and enzymatic characterization of N-acetylglucosamine deacetylase
- Ethanol inhibited growth hormone receptor-mediated endocytosis in primary mouse hepatocytes
- Gypsophila eriocalyx roots inhibit proliferation, migration, and TGF-β signaling in melanoma cells
- The role of kynurenine and kynurenine metabolites in psoriasis
- Tobacco induces abnormal metabolism of tryptophan via the kynurenine pathway
- Effect of vitamin D and omega-3 on the expression of endoplasmic reticulum-associated protein degradation and autophagic proteins in rat brain
Articles in the same Issue
- Frontmatter
- Review
- Targeting oxidative stress, iron overload and ferroptosis in bone-degenerative conditions
- Research Articles
- Assessing medical biochemistry professionals’ knowledge, attitudes, and behaviors regarding green and sustainable medical laboratory practices in Türkiye
- The efficacy of high pressure liquid chromatography (HPLC) in detecting congenital glycosylation disorders (CDG)
- Atypical cells parameter in sysmex UN automated urine analyzer: a single center study
- The frequency of single specific immunoglobulin E and allergen mixes with a MAST (multiple-antigen simultaneous test) technique
- Differences in second trimester risk estimates for trisomy 21 between Maglumi X3/Preaccu and Immulite/Prisca systems
- Comparison of classical and flowcytometric osmotic fragility and flowcytometric eosin-5-maleimide binding tests in diagnosis of hereditary spherocytosis
- Casticin inhibits the hedgehog signaling and leads to apoptosis in AML stem-like KG1a and mature KG1 cells
- Trimethylamine N-oxide, S-equol, and indoxyl sulfate inflammatory microbiota players in ocular Behçet’s disease
- Genomic profiling of interferon signaling pathway gene mutations in type 2 diabetic individuals with COVID-19
- CDR1as/miR-7-5p/IGF1R axis contributes to the suppression of cell viability in prostate cancer
- Role of interferon regulatory factors in predicting the prognosis of Crimean-Congo hemorrhagic fever
- The significance of taurine for patients with Crimean-Congo hemorrhagic fever and COVID-19 diseases: a cross-sectional study
- Gene mining, recombinant expression and enzymatic characterization of N-acetylglucosamine deacetylase
- Ethanol inhibited growth hormone receptor-mediated endocytosis in primary mouse hepatocytes
- Gypsophila eriocalyx roots inhibit proliferation, migration, and TGF-β signaling in melanoma cells
- The role of kynurenine and kynurenine metabolites in psoriasis
- Tobacco induces abnormal metabolism of tryptophan via the kynurenine pathway
- Effect of vitamin D and omega-3 on the expression of endoplasmic reticulum-associated protein degradation and autophagic proteins in rat brain

