Abstract
Objectives
Lung cancer remains a predominant cancer type with high incidence and low survival rates. Key challenges in its treatment include impaired cellular mechanisms, notably resistance to apoptosis and altered immune responses. A critical aspect in this context is the heightened TLR4-mediated signaling, known to promote cell survival, metastasis, and resistance to cell death, particularly impacting immune microenvironment regulation. This study focuses on evaluating the impact of TLR4 signaling activation on potential therapeutic strategies.
Methods
Our research utilizes cannabidiol (CBD), a compound already employed in mitigating chemotherapy side effects in lung adenocarcinoma, recognized for its antitumor properties including antiproliferative, antimetastatic, and apoptosis-inducing effects. However, the effectiveness of CBD in lung cancer cells with elevated TLR4 signaling remains uncertain.
Results
Our findings reveal that the combination of CBD and TLR4 agonist affects cell viability, proliferation, cell cycle progression, apoptosis, and gene expression related to immune response and extracellular matrix regulation. In lung adenocarcinoma cells with activated TLR4, CBD shows an increased IC50 value, reflecting reduced antiproliferative capacity. Furthermore, its efficacy in arresting the cell cycle and inducing apoptosis is also compromised. The influence on immune response and extracellular matrix regulation is also altered in TLR4-activated cells.
Conclusions
These results indicate that TLR4 activation significantly diminishes the antitumor efficacy of CBD. This highlights the importance of considering TLR4 signaling activation in future research on therapeutic agents like CBD for cancer treatment.
Introduction
Lung cancer ranks as the most frequently diagnosed cancer among men globally. The prognosis and clinical outcomes in lung cancer cases are closely associated with the cancer’s stage at diagnosis [1]. Systemic treatment options for lung cancer include chemotherapy, targeted therapy, and immunotherapy. The search for new potential targets to achieve desired survival rates continues [2, 3]. Non-small cell lung cancer (NSCLC) accounts for approximately 85 % of all newly diagnosed lung cancers, while small cell lung cancer makes up about 15 % of cases [4]. NSCLC has a complex and heterogeneous structure signaling and genetic alterations. Numerous proto-oncogenes and tumor suppressor genes play important roles in lung carcinogenesis [5]. In addition to, tumor-inducing changes occur in genes and receptors related to the immune system. Toll-like receptors (TLRs), which determine the major responses of the innate immune system. TLR4 has been shown to be highly expressed in lung cancer tissue at both mRNA and protein levels compared to tumor-free lung tissue [6]. TLR4 signaling has been shown to promote apoptosis resistance and the escape of immune cells [7]. It also regulates the antitumor responses of cells in the tumor microenvironment. Thus, cross-talk between tumor and immune cells is critical for tumor development [8]. TLR4 overexpression in patients with NLSLC has been shown to indicate poor prognosis [9]. We should understand that the level of activation of TLR4 signaling directly affects the prognosis of lung cancer due to its effects on both the tumor cell and the microenvironment.
Cannabidiol (CBD) is currently used in palliative care to reduce the side effects of painkillers and chemotrapathics in patients with terminal stage lung cancer [10]. In a case study, CBD oil intake caused tumor regression in a patient with lung adenocarcinoma without further intervention [11]. The literature review indicates that CBD has direct antineoplastic effects on lung cancer cells either through cannabinoid receptors or independently [12]. CBD exerts selective cytotoxicity and stops the proliferation of many types of cancer. In in vitro experiments, CBD has been established as a potent anti-metastatic agent in the treatment of breast carcinoma, in cervical, alveolar and lung cancer cells [13]. CBD has been shown to exert its effects such as proliferation, invasion, tumor size, apoptosis, metastasis, and autophagy [14].
On the other hand, it is known to have an effect on CBD and TLR signaling. Cannabinoid receptors 1 and 2 are the main receptors mediating the biological effects of phytocannabinoids [15]. The endocannabinoid system consists not only of cannabinoid receptors but also of endogenous cannabinoids and their biosynthetic and metabolizing enzymes. Both exogenous and endogenous cannabinoids inhibit proinflammatory cytokine production by macrophages stimulated through TLRs [16].
Considering the literature, it is observed that CBD and its products have a high potential as a therapeutic candidate in lung cancer. However, it is not known how the activation level of TLR4, which is an essential regulator in lung cancer, alters the effects of CBD. In this study, we aimed to evaluate the effects of TLR4 activation and CBD on anti-tumorigenic properties in lung cancer cells, both individually and in combination. We conducted analyses on cell viability, proliferation, cell cycle, apoptosis, gene expression related to the immune response and extracellular matrix (ECM) related genes in lung adenocarcinoma cells. We observed that the high TLR4 activation, when treated along with candidate and potentially approved medical agents like CBD, could reduce the effectiveness of therapeutics. In future studies, we believe that the administration of candidate therapeutics in combination with TLR4 inhibitors could significantly enhance the anti-tumorigenic effects in lung cancer cells.
Materials and methods
Materials
The TLR4 agonist (T4Ag), which was Ultrapure LPS from E. coli 0111: B4 (Cat no: tlrl-3pelps), was purchased from InvivoGen (San Diego, CA, USA). CBD was purchased in as a pure crystal form CBDepot (Teplice, Czech Republic). All cell culture chemicals were purchased from Capricorn Scientific (Capricorn, Germany). MTT salt was purchased from (Biotium, California, USA Cat No: 10059). Calcein-acetoxymethyl ester dye obtained from Santa Cruz Biotechnology (Dallas, TX, USA). The Cell Cycle Analysis Kit (THOR-CCK-100, Thorvacs Biotechnology, Turkey) was used following the manufacturer’s protocol. The FITC Annexin V Apoptosis Detection Kit with 7-AAD [Tonbo Bioscience, Cat. No. 35–6410 (San Diego, United States)] was utilized following the protocol provided with the kit. A.B.T.™ 2X qPCR SYBR-Green Master Mix (ATLAS Biotechnology, Turkey) for used qRT-PCR.
CBD treatment
CBD was used for experiments without further purification. CBD was dissolved in a 70 % ethanol solution. The 1 mM stock solution was diluted with complete medium and used in the experiments.
Cell culture
A549 cell line (CCL-185™), adenocarcinomic human alveolar basal epithelial cells, were purchased from the American Type Culture Collection (ATCC, Wesel, Germany). The cells were cultured in DMEM supplemented with 10 % fetal bovine serum, 2 mM l-glutamine and 1 % antibiotic solution. The culture was maintained at 37 °C, 5 % CO2, and 100 % humidity.
Cell viability assay
The viability effects of CBD and TLR4 signaling on A549 cells using the MTT assay. For each experimental group, cannabis cells were seeded per well in 96-well plates. T4Ag group was cultured with complete medium containing 100 ng/mL at 24 h. After 24 h of incubation, 10 μL of MTT solution with a final concentration of 0.5 mg/mL was added to each well at both 0 and 24 h. Following 4 h incubation period, the formazan crystals that had formed were dissolved in each well using 100 μL of Dimethyl sulfoxide (DMSO) [17]. The absorbance rate measured at a wavelength of 570 nm (BioTek Synergy h1, BioTek Instruments, Winooski, VT, USA). We calculated half maximal inhibitory concentration (IC50) values by linear regression analysis based on viability rates.
Cell proliferation analysis
This analysis evaluating the effects of CBD, T4Ag and both was performed on A549 cells. T4Ag group was treated with 100 ng/mL T4Ag for 24 h simultaneously. Cells were incubated with 2 μM calcein-acetoxymethyl ester. Live cells were visualized and photographed using a fluorescence inverted microscope (Leica, Wetzlar, Germany). For each experimental group, A549 cells in the image area were counted using Image J software (NIH, Bethesda, Maryland, USA). The number of cells and mean values were determined for each group [18].
Cell cycle and apoptosis analysis
Cell cycle analysis aims to rate the phase of cells in the cycle based on the DNA content of the cells [19]. TLR4 signaling was analyzed in activated and unstimulated A549 cells at 24 and 72 h after CBD was applied at an IC50 dose. After preparing the cells according to the protocol, Propidium Iodide was added and incubated at 37 °C. After reading with the NovoCyte Flow Cytometer, histograms were generated using NovoExpress Software (1.6.1). For apoptosis analysis, we aimed to determine the proportions of viable, early and late apoptotic cells. TLR4 was used to evaluate the effect of CBD in stimulated and unstimulated A549 cells [20]. In this study, FITC Annexin V Apoptosis Detection Kit with 7-AAD was performed at 24 and 72 h. Graphs were generated using NovoExpress Software.
Gene expression analysis
Gene expression analysis was performed to evaluate the effects of CBD on the immune response and metastasis of TLR4 signaling activated A549 cells. Cells were treated with IC50 CBD, 100 ng/mL T4Ag and both for 24 h. Real Time PCR reaction was performed on a Lightcycler® 96 instrument (Roche Diagnostic Systems, Indianapolis, IN) using the A.B.T.™ 2X qPCR SYBR-Green Master Mix kit. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), TIMP-1 metallopeptidase Inhibitor 1 (TIMP1), matrix metallopeptidase-2 (MMP-2), plasminogen activator urokinase (PLAU), interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α) and interferon-gamma (IFN-γ) primers were used [21]. Primer sequences are given in Table 1. The GAPDH gene threshold cycle value was used to normalize the data.
Primers used for qRT-PCR expression analysis.
| Gene name | Forward sequence | Reverse sequence |
|---|---|---|
| GAPDH | 5′‐GTCTCCTCTGACTTCAACAGCG‐3′ | 5′‐ACCACCCTGTTGCTGTAGCCAA ‐3′ |
| IFN-γ | 5′-GCTGTTACTGCCAGGACCC-3′ | 5′-TTTTCTGTCACTCTCCTCTTTCC-3′ |
| TNF-α | 5′-GCCCATGTTGTAGCAAACCCTC-3′ | 5′-GGTTATCTCTCAGCTCCACGCC-3′ |
| MMP-2 | 5′‐TTCATTTGGCGGACTGTGAC ‐3′ | 5′‐GTGCTGGCTGAGTAGATCCA ‐3′ |
| PLAU | 5′-CCAAAATGCTGTGTGCTGCT-3′ | 5′-CCCTTAAGCTGGCTGGAACA-3′ |
| TIMP-1 | F: 5′-ACCCCTGGAGCACGGCT-3′ | R: 5′-CCCACCTTCCAAGTTAGTGACA-3′ |
| IL-6 | F: 5′-CTCCACAAGCGCCTTCGGT-3′ | R: 5′-GAATCTTCTCCTGGGGGTACTGG-3′ |
Statistical analysis
The data is presented as mean±SD GraphPad Prism 8.1 (GraphPad Software, San Diego, CA, USA) was utilized to create the graphs. Statistical analysis was carried out using one-way or two-way ANOVA GraphPad Prism 8.1 followed by Bonferroni, Sidak’s or Tukey post hoc testing to compare differences among groups.
Results
TLR4 signaling affects CBD-induced cell death and antiproliferative effects on A549 cells
We evaluated the effects of CBD on the viability and proliferation of A549 lung cancer cells. Additionally, we comparatively assessed its effects on TLR4-activated A549 cells. CBD was applied at various concentrations (0–25 μM), and after a 24-h application, cell viability was measured using the MTT test, and proliferation was assessed using the calcein staining method. CBD dose-dependently reduced viability in both A549 cell lines (0–25 μM). The IC50 value for untreated A549 cells was calculated as 20.48 μM, while for TLR4-activated A549 cells, it was calculated as 25.28 μM (Figure 1A). In this case, we observed that in cells with activated TLR4 signaling, CBD was less effective at a concentration close to 5 μM. When evaluating proliferation under the same conditions, T4Ag (100 ng/mL) applied at 0 h was found to be higher compared to unstimulated A549 cells at 0 h. Subsequently, significant differences were found between the two groups at four different CBD concentrations (Figure 1B). In CBD applications close to IC50 values (20 and 25 μM), images indicate that TLR4 signaling reduces CBD’s antiproliferative effects (Figure 1C). It appears that CBD cannot equally reduce proliferation and viability against TLR4 signaling at the same ratios. TLR4 signaling largely protects against CBD’s reduction of viability and proliferation.
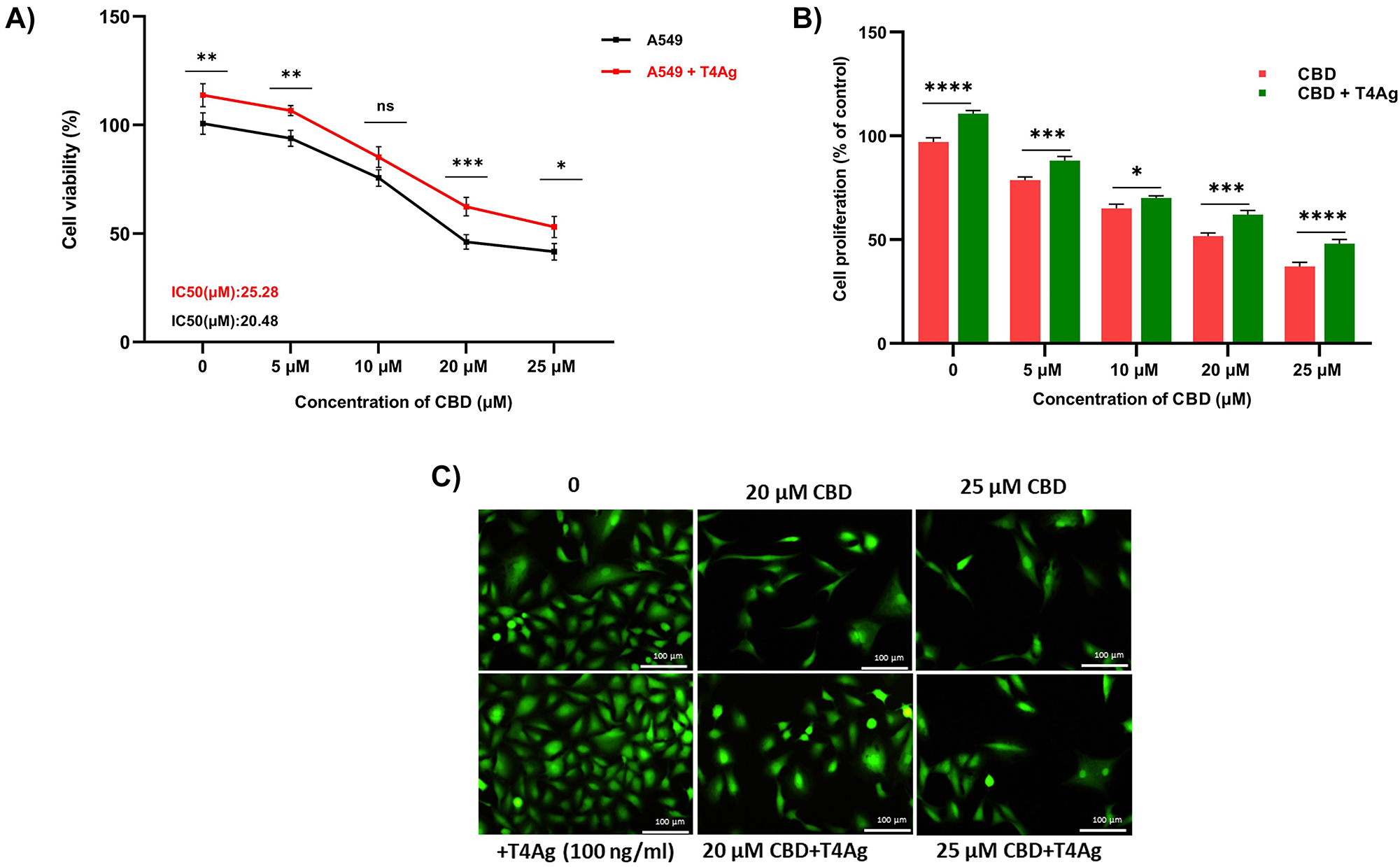
Effects of T4Ag treatment along with CBD on the viability and proliferation in A549 cells. A549 cells were exposed to various concentrations (0, 5, 10, 20, 25 μM) of CBD and T4Ag (100 ng/mL) and CBD for 24 h to determine the IC50 (A). The results are presented as the mean of relative viability from three independent experiments (mean±SD, n=3). (B) presents the quantified cell proliferation rate compare the control groups. (C) shows representative images of cell proliferation assessed using calcein AM staining. Statistical significance is represented by stars: * for p<0.05, ** for p<0.01, *** for p<0.001, and **** for p<0.0001, indicating a significant difference from the control group. The data were analyzed by two-way ANOVA followed by Sidak’s post hoc testing.
Modulation of CBD-induced cell cycle arrest in A549 lung cancer cells by TLR4 activation
In this comprehensive flow cytometry study, we scrutinized the modulatory effects of CBD and TLR4 activation on the cell cycle distribution of A549 lung cancer cells. Application of the IC50 concentration of CBD alone for 72 h markedly augmented the Sub-G1 cell population, indicative of CBD-mediated apoptosis and potential cell cycle arrest. This effect was notably less pronounced at the 24-h interval, suggesting a time-dependent induction of cell cycle arrest by CBD (Figure 2A, B, G). Concurrently, co-stimulation with a T4Ag appeared to transiently counteract the CBD-induced S phase accumulation at 24 h, as evidenced by a reduced S phase fraction compared to CBD treatment alone, implying an initial mitigation of cell cycle arrest by TLR4 signaling. However, this interaction between CBD and TLR4 signaling did not persist over time, with no significant difference in the S phase fraction observed at the 72-h time point between CBD alone and the combination treatment, relative to an elevated level compared to the control (Figure 2C, D, G). Moreover, TLR4 activation with T4Ag independently led to a substantial enrichment of cells in the G2/M phase at 72 h, signifying a distinct cell cycle arrest at this later stage, independent of CBD’s influence. Histogram analyses from NovoExpress Software supported these observations, revealing a reduced S phase peak in the combination treatment at 24 h, yet a reversion to levels similar to the control group at 72 h (Figure 2E, F, G).
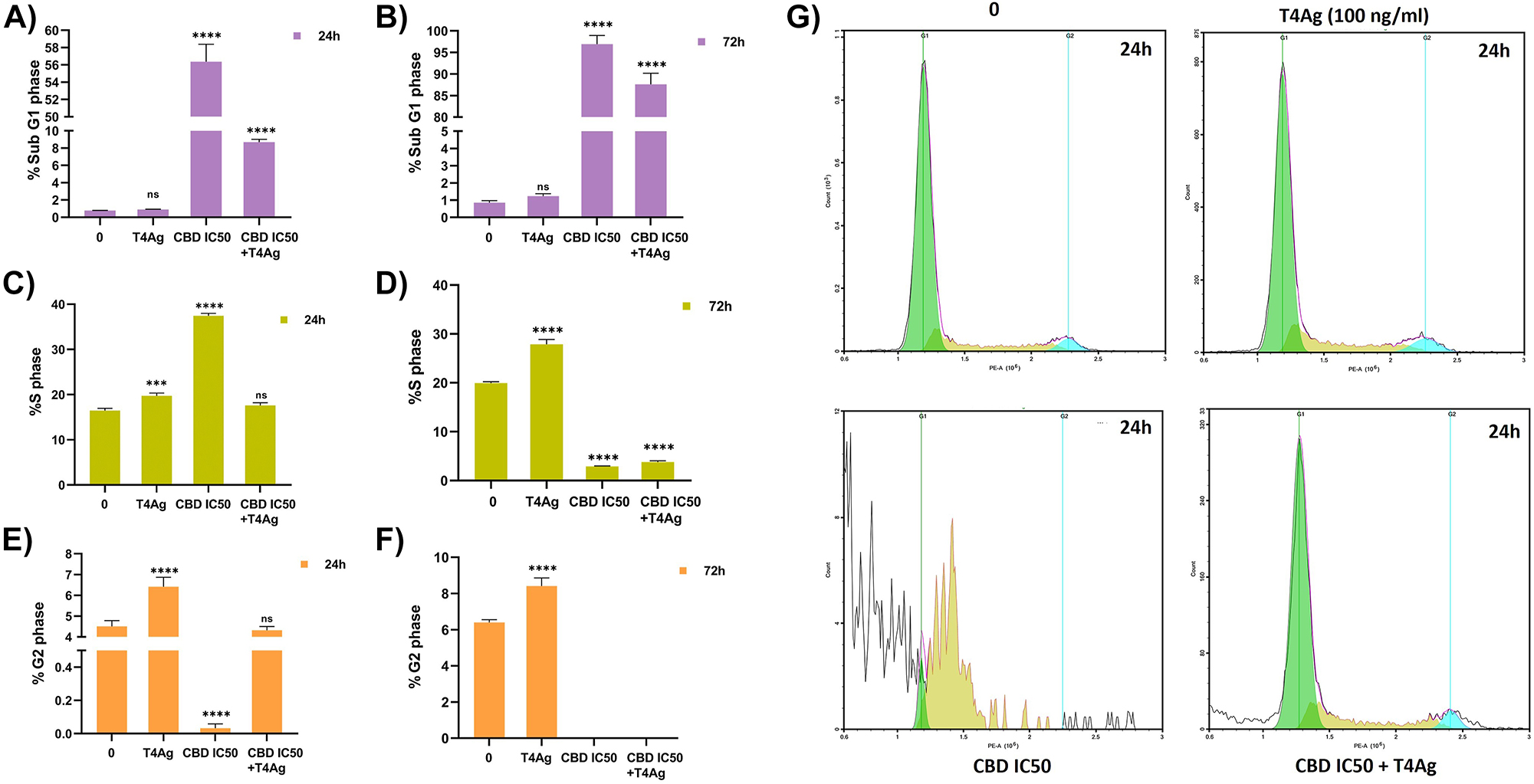
Effect of CBD and T4Ag cell cycle flows in A549 cells. Cell cycle histograms of the experimental groups (g) 24 h and the number of cells belonging to the SubG1 (A, B), S (C, D) and G2 (E, F) phases are given graphically 24 and 72 h. The untreated cells served as the control. The statistical significance level is denoted by asterisks as follows: * for p<0.05, ** for p<0.01, *** for p<0.001, and **** for p<0.0001, indicating the mean statistical difference. The data were analyzed by one-way ANOVA followed by Sidak’s post hoc testing. The values are represented as mean±SD, with n=3.
Our data elucidate a complex temporal interplay between the pro-apoptotic and cell cycle arrest effects of CBD and the modulatory role of TLR4 signaling. While TLR4 activation can attenuate the cell cycle inhibitory effects of CBD in the short term, our findings suggest that this modulation is not sustained, with both treatments converging to a similar cell cycle profile after 72 h. These results highlight the potential of CBD as an antitumorigenic agent, whose efficacy may be subject to temporal modulation by TLR4 signaling but remains effective over an extended period.
Impact of TLR4 activation on CBD-ınduced apoptosis in A549 cells
In assessing the efficacy of CBD and T4Ag on A549 lung cancer cells, distinct patterns of cell viability and apoptosis emerged. Treatment with CBD for 24 h resulted in a pronounced increase in late apoptotic cells, underpinning its potent apoptotic induction capabilities. This finding aligns with the recognized pro-apoptotic properties of CBD, thereby validating its potential as an anti-cancer agent. This pronounced rise in early apoptotic rates, alongside the escalation in late apoptosis, underscores CBD’s robust capability to induce apoptosis at various stages. When TLR4 was co-activated with CBD (CBD+T4Ag), we observed a mitigation in the proportion of late apoptotic cells at the 24-h mark compared to CBD treatment alone, which indicates a potential interference by TLR4 signaling in the apoptotic pathway activated by CBD (Figure 3A, B, E). The presence of T4Ag seems to delay the onset of late apoptosis, as evidenced by the higher proportion of cells in the early apoptotic stage. This could be interpreted as a protective mechanism activated by TLR4 signaling against CBD-induced apoptosis. Moreover, the proportion of live cells in the CBD + TLR4 group remained higher than in the CBD alone group after 24 h, providing further evidence of the modulatory role of TLR4 in tempering the apoptotic effect of CBD. However, this protective effect was not evident at the 72-h time point, where the rates of live and apoptotic cells in treatments involving CBD converged to similar levels regardless of TLR4 signaling (Figure 3C, D). This suggests that the influence of TLR4 is transient, with the anti-cancer effects of CBD prevailing over time. Overall, our data suggest that while TLR4 activation can initially modulate the apoptotic effects of CBD, its impact is not durable, with CBD demonstrating a sustained ability to diminish cell viability and drive late-stage apoptosis in A549 lung cancer cells.
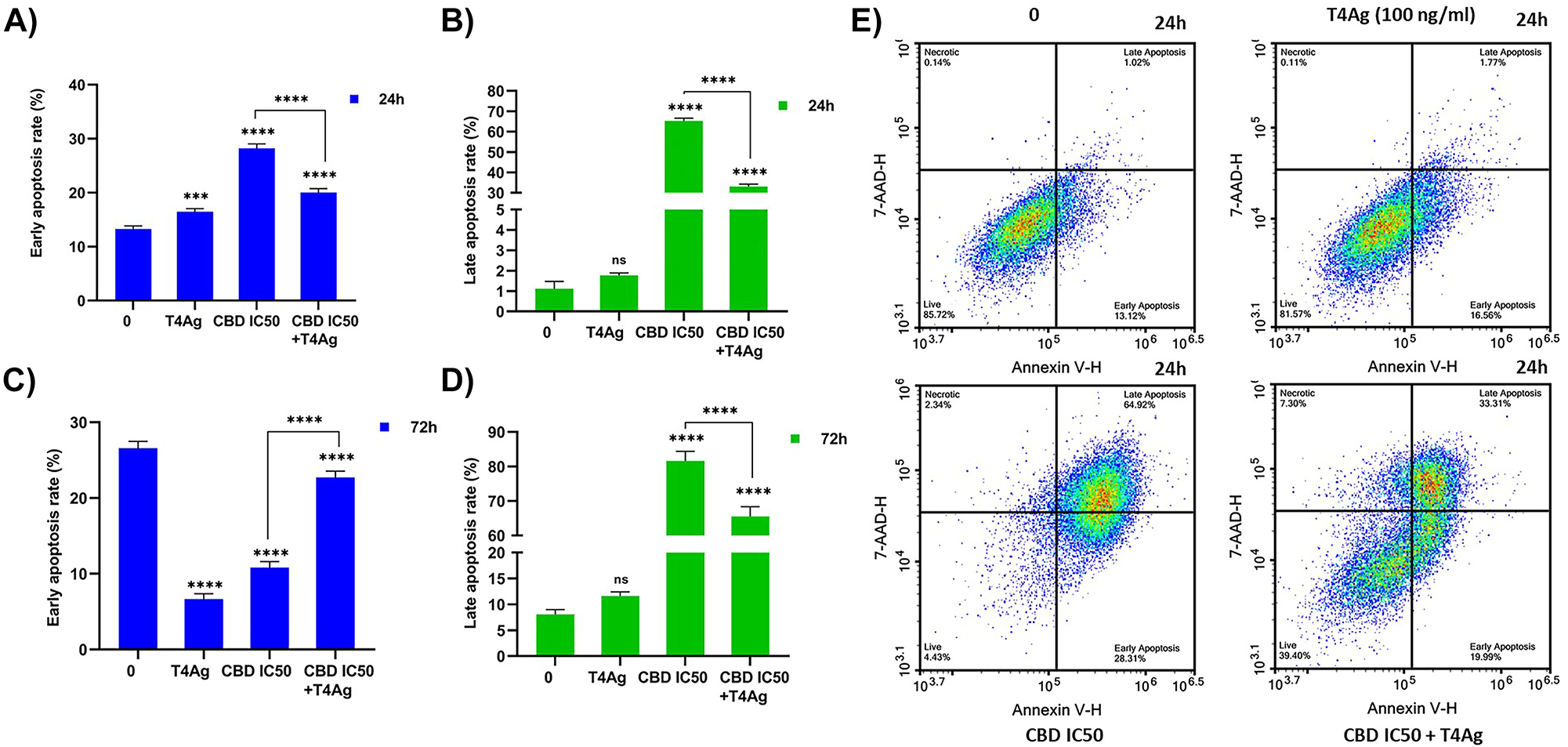
Effect of T4Ag, CBD and T4Ag+CBD on apoptosis rate in A549 cells. The cell ratios in early and late apoptosis for all experimental groups were presented graphically at 24 h (A, B) and 72 h (C, D). The plot graphs for the 24 h time point was also provided (E). The values are represented as mean±SD, with n=3. Statistical significance is represented by stars: * for p<0.05, ** for p<0.01, *** for p<0.001, and **** for p<0.0001, indicating a significant difference from the control group. The data were analyzed by one-way ANOVA followed by Tukey post hoc testing.
Differential cytokine and extracellular matrix-associated gene expression in A549 lung cancer cells: effects of CBD and TLR4 agonist treatment.
In A549 lung cancer cells, cytokine gene expression was measured after treatments with CBD, a T4Ag, and their combination. The relative fold change in expression levels, normalized to GAPDH, showed a significant increase in TNF-α, IFN-γ, and IL-6 upon treatment with T4Ag alone and in combination with the IC50 concentration of CBD. The combination treatment compared to single therapy TNF-α resulted in upregulation of IFN-γ cytokines and downregulation of IL-6 cytokine (Figure 4A, B, C). These findings illustrate that the interaction between CBD and T4Ag leads to a heightened inflammatory cytokine response in A549 lung cancer cells.
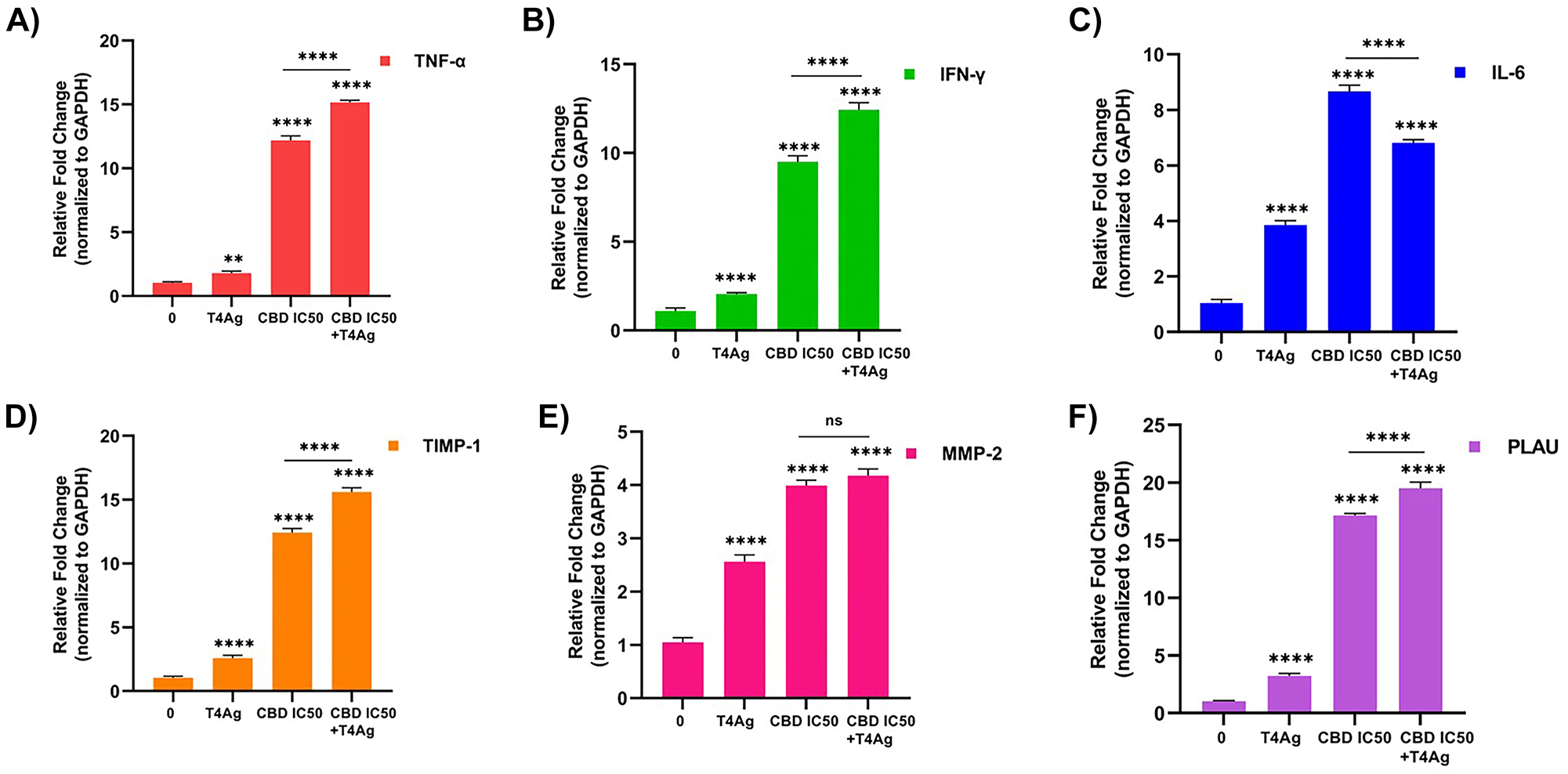
The 24 h effect of CBD and T4Ag on cytokines and ECM regulated gene expression of A549 cells. Fold change graphs of TNF-α (A) IFN-γ (B), and IL-6 (C) gene expression of genes related to immune response are given. Gene expression fold change graphs of ECM associated genes belonging to the experimental groups TIMP-1 (D), MMP-2 (E), and PLAU (F) were given. The results were normalized to the expression of GAPDH. Statistical significance is represented by stars: * for p<0.05, ** for p<0.01, *** for p<0.001, and **** for p<0.0001, indicating a significant difference from the control group. The data were analyzed by two-way ANOVA followed by Tukey’s post hoc testing.
In A549 lung cancer cells, the impact of CBD and T4Ag treatment on genes associated with the ECM was evaluated. The expression levels of TIMP-1, MMP-2, and PLAU were quantified (Figure 4D, E, F). Relative to the control, treatment with T4Ag alone resulted in an upregulation of TIMP-1, which was further enhanced by the addition of CBD. This suggests a potential inhibitory effect on ECM degradation, potentially reducing cell invasiveness. In contrast, both MMP-2 and PLAU expressions were significantly elevated upon treatment with T4Ag, and this elevation persisted when CBD was combined with T4Ag, indicating an active ECM remodeling process. The differential regulation of these ECM-related genes suggests a complex response of the ECM to the treatments, with implications for the invasiveness and metastatic potential of lung cancer cells.
Discussion
In the comprehensive analysis of A549 lung cancer cell response to CBD and T4Ag, our study elucidates a multi-faceted interaction influencing cell cycle progression, apoptosis, cytokine expression, and extracellular matrix (ECM) dynamics. The dichotomous nature of these responses underscores the intricate balance of antitumorigenic and pro-survival pathways in the modulation of cancer cell behavior.
In a study evaluating the cytotoxic activity of CBD on two lung cancer cell lines over a 72-h application, IC50 values were found to be A549: 16.4 µM and H1299: 23 µM [13]. In the presence of serum, a concentration of 24 µM CBD applied for 24 h has killed approximately 50 % of A549 cells [22]. Since our application was for 24 h, observing a higher IC50 value appears consistent. CBD has been shown to inhibit the proliferation and migration of human breast cancer cells through differential modulation of the MAPK/ERK pathway pathways in breast cancer cells [23]. It is thought to be regulated through the same signaling pathways in lung cancer. Cannabis sativa extract, at concentrations ranging from 50 to 900 ng/mL, dose-dependently reduced viability in A549 cells by inducing early apoptosis [24].
The 24-h stimulation with different concentrations of T4Ag increased the viability and proliferation of A549 cells in a concentration-dependent manner. At a concentration of 0.1 μg/ml, it elevated by approximately 10 % compared to control cells, at 1 μg/ml concentration by approximately 20 %, and at 10 μg/ml concentration by approximately 27 %. In the in vitro part of the same study, mice stimulated with T4Ag showed an approximately 18 % increase in Ki-67 expression in lung cancer tissue [25]. Similarly, our stimulation with 100 ng/mL T4Ag resulted in a 10 % increase in proliferation. We believe the variations in these percentages stem from the differences in T4Ag type and dosage. Nevertheless, it is evident that TLR4 activation induces proliferation signals and chemoresistance in lung cancer cells [26]. It is known that there is interaction in the regulation of immune responses between TLR-mediated activation and the endocannabinoid system [27]. However, there are no studies in the literature examining the outcome of this interaction on proliferation signals in cancer cells.
It is known that cannabinoids stimulate apoptosis through classical cannabinoid receptors and halt the cell cycle at the G1 phase by activating cyclin kinase inhibitors. Additionally, cannabinoids interact with receptors that control proliferation and signal activations [28]. In the A549 cancer cell line, approximately 15 % of cells exhibited apoptosis when treated with CBD at an IC50 of 16.4 µM, while at IC75, the induction of apoptosis was approximately 31 % [13]. In the study conducted by Ramer et al., an application of CBD (3 μmol/L) for 18 h resulted in an observed apoptosis rate of over 50 % in A549 cells [29]. In our applied IC50 value, the apoptosis rate is 90 %. Apoptosis has been induced in both cases. CBD has shown apoptotic effect in human lung adenocarcinoma tumors via reactive oxygen species (ROS) and transient receptor potential vanilloid-2 (TRPV2) pathway [30]. Further studies are needed to explore the mechanisms underlying inducing apoptosis.
CBD, has been shown to halt the cell cycle at the G1/S transition in breast cancer cell lines through the cannabinoid type 2 receptor [31]. The treatment of CBD (12.5 μM) in multiple myeloma cell lines has significantly increased the cell population in the sub-G1 phase 24 h later [32]. CBD’s ability to induce cell cycle arrest and apoptosis is well-documented and our findings corroborate this antitumorigenic potential, with a clear indication of a time-dependent augmentation of sub-G1 populations, indicative of apoptosis. The early mitigation of these effects by TLR4 agonist co-treatment suggests a temporary activation of pro-survival pathways, which may serve as an initial defense mechanism of the cancer cells. However, the reversion to a cell cycle and apoptosis profile similar to that of CBD treatment alone at the 72-h mark highlights the transient nature of TLR4’s impact.
It is argued that TLR signals also alter cannabinoid receptor expression in innate immune cells, influencing their sensitivity to cannabinoids. However, the relationship between the endocannabinoid system and the innate immune system may not be unidirectional [27]. Cytokine profiling in response to the treatments revealed an upregulation of TNF-α, IFN-γ, and IL-6. Typically, TNF-α and IFN-γ are associated with antitumorigenic immune responses, yet their role in cancer progression is nuanced, potentially contributing to a pro-tumorigenic environment under certain conditions. Similarly, the role of IL-6 in cancer is contentious, with evidence of both tumorigenic and antitumorigenic effects [33]. CBD is generally known for its anti-inflammatory and immune-suppressive properties. However, it has also been demonstrated to induce the production of proinflammatory cytokines in non-immune cells [34]. It has been shown that the CBD analog Cannabidivarin acts as a TLR4 antagonist, inhibiting TLR4 signaling and LPS-induced proinflammatory cytokine expression [35]. Clear conclusions can only be drawn as the receptors involved in CBD’s effects are elucidated, and its interactions with other signaling mediators are better understood.
The release of proinflammatory cytokines from cells can lead to a favorable condition for activating immune cells in the tumor microenvironment. Supporting both the inhibition of various mechanisms in cancer cells and the activation of the immune system, cannabinoid receptor ligands such as CBD may have promising potential for cancer treatment [36]. The upregulation of these cytokines suggests that CBD, in conjunction with T4Ag, may promote an inflammatory response that could have dual effects on tumor behavior. Therefore, we do not make a definitive claim regarding their impact on inflammatory responses.
CBD has been shown to reduce invasion by upregulating intercellular adhesion molecule-1 (ICAM-1) and TIMP-1 genes in three different lung cancer cell lines (A549, H358, and H460) and in an in vivo model [37]. The application of CBD has been shown to suppress endothelial angiogenesis in A549 cells by increasing the secretion of TIMP-1 [38]. The examination of ECM-related gene expression further adds to the complexity of the interplay between CBD and TLR4 signaling. The upregulation of TIMP-1 in response to combined treatment implies a reduction in ECM degradation, potentially inhibiting tumor invasiveness. Yet, the simultaneous increase in MMP-2 and PLAU expression, known facilitators of ECM breakdown and cancer cell invasion, indicates an active remodeling of the ECM, which could either suppress or promote tumor progression. At a concentration of 1 µM, CBD resulted in a 25 % reduction in urokinase-type plasminogen activator (uPA, PLAU) expression up to 12 h, followed by a subsequent increase to nearly control levels at 24 h and a further 25 % increase at 48 h. However, CBD did not exhibit a significant modulation in the secretion of uPA [39].
It has been reported that the expression and activity of endocannabinoid system receptors vary significantly among different cancer types due to changes occurring during carcinogenesis. This variation may explain the contradictory results of both inhibiting and inducing cancer cells [23]. In lung cancer treatment, although CBD appears to be a highly promising agent, mechanistic studies are necessary to determine its full range of effects and identify the complete mechanisms of action to assess its candidate potential [40]. The contrasting effects observed raise critical questions about the net impact of CBD and T4Ag on tumor progression. The dual regulatory roles on key factors of ECM remodeling and cytokine expression may reflect a cellular adaptive response to maintain homeostasis in the face of external stimuli.
We emphasized that CBD affects numerous molecular mechanisms in TLR4-activated A549 cells, including cell viability, proliferation, apoptosis, cell cycle flow, ECM regulation and immune response-related gene expression effects. In conclusion, the effects of CBD may vary depending on TLR4 activation state. Our study is a first in the literature in demonstrating the TLR4 activation-dependent anti-carcinogenic effects of CBD. In the light of our results, we think that it will provide a perspective for its use as a therapeutic approach in lung cancer. However, further in vitro and in vivo studies are needed to elucidate the relevant molecular mechanisms in detail. We believe that particularly elucidating the interaction with the major regulator in lung cancer, the TLR4 signal, through mechanistic studies, can enhance the efficiency of therapeutic approaches.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission. Concept – D.K., M.P.K. and S.Y.; supervision – S.Y.; materials – D.K. and M.P.K.; data collection and/or processing – D.K.; analysis and/or Interpretation – D.K. and M.P.K.; writing –D.K. and S.Y.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Thandra, KC, Barsouk, A, Saginala, K, Aluru, JS, Barsouk, A. Epidemiology of lung cancer. Wspólczesna Onkol 2021;25:45–52. https://doi.org/10.5114/wo.2021.103829.Search in Google Scholar PubMed PubMed Central
2. Mithoowani, H, Febbraro, M. Non-small-cell lung cancer in 2022: a review for general practitioners in oncology. Curr Oncol 2022;29:1828–39. https://doi.org/10.3390/curroncol29030150.Search in Google Scholar PubMed PubMed Central
3. Derman, BA, Mileham, KF, Bonomi, PD, Batus, M, Fidler, MJ. Treatment of advanced squamous cell carcinoma of the lung: a review. Transl Lung Cancer Res 2015;4:524–32. https://doi.org/10.3978/j.issn.2218-6751.2015.06.07.Search in Google Scholar PubMed PubMed Central
4. Liao, RG, Watanabe, H, Meyerson, M, Hammerman, PS. Targeted therapy for squamous cell lung cancer. Lung Cancer Manag 2012;1:293–300. https://doi.org/10.2217/lmt.12.40.Search in Google Scholar PubMed PubMed Central
5. Singh, CR, Kathiresan, K. Molecular understanding of lung cancers – A review. Asian Pac J Trop Biomed 2014;4:S35–41. https://doi.org/10.12980/APJTB.4.2014C597.Search in Google Scholar PubMed PubMed Central
6. Zhang, YB, He, FL, Fang, M, Hua, TF, Hu, BD, Zhang, ZH, et al.. Increased expression of Toll-like receptors 4 and 9 in human lung cancer. Mol Biol Rep 2009;36:1475–81. https://doi.org/10.1007/s11033-008-9338-9.Search in Google Scholar PubMed
7. Yang, LS, Wu, WS, Zhang, F, Jiang, Y, Fan, Y, Fang, HX, et al.. Role of toll-like receptors in lung cancer. J Recept Signal Transduction 2014;34:342–4. https://doi.org/10.3109/10799893.2014.903418.Search in Google Scholar PubMed
8. Hoden, B, DeRubeis, D, Martinez-Moczygemba, M, Ramos, KS, Zhang, D. Understanding the role of toll-like receptors in lung cancer immunity and immunotherapy. Front Immunol 2022;13:1033483. https://doi.org/10.3389/fimmu.2022.1033483.Search in Google Scholar PubMed PubMed Central
9. Wang, K, Wang, J, Wei, F, Zhao, N, Yang, F, Ren, X. Expression of TLR4 in non-small cell lung cancer is associated with PD-L1 and poor prognosis in patients receiving pulmonectomy. Front Immunol 2017;8:456. https://doi.org/10.3389/fimmu.2017.00456.Search in Google Scholar PubMed PubMed Central
10. Kyriakou, I, Yarandi, N, Polycarpou, E. Efficacy of cannabinoids against glioblastoma multiforme: a systematic review. Phytomedicine 2021;88:153533. https://doi.org/10.1016/J.PHYMED.2021.153533.Search in Google Scholar PubMed
11. Sulé-Suso, J, Watson, NA, van Pittius, DG, Jegannathen, A. Striking lung cancer response to self-administration of cannabidiol: a case report and literature review. SAGE Open Med Case Rep; 2019;7:2050313X1983216. https://doi.org/10.1177/2050313x19832160.Search in Google Scholar
12. Vasileios, I, Milas, GP, Zareifopoulos, N. Antitumorigenic effect of cannabidiol in lung cancer: what do we know so far? A mini review. Iran Biomed J 2022;26:406–13. https://doi.org/10.52547/ibj.3732.Search in Google Scholar PubMed PubMed Central
13. Todorova, J, Lazarov, LI, Petrova, M, Tzintzarov, A, Ugrinova, I. The antitumor activity of cannabidiol on lung cancer cell lines A549 and H1299: the role of apoptosis. Biotechnol Biotechnol Equip 2021;35:873–9. https://doi.org/10.1080/13102818.2021.1915870.Search in Google Scholar
14. Seltzer, ES, Watters, AK, Mackenzie, D, Granat, LM, Zhang, D. Cannabidiol (Cbd) as a promising anti-cancer drug. Cancers 2020;12:1–26. https://doi.org/10.3390/cancers12113203.Search in Google Scholar PubMed PubMed Central
15. Wang, B, Li, D, Fiselier, A, Kovalchuk, I, Kovalchuk, O. High-CBD cannabis extracts inhibit the expression of proinflammatory factors via miRNA-mediated silencing in human small intestinal epithelial cells. Heliyon 2023;9:18817. https://doi.org/10.1016/j.heliyon.2023.e18817.Search in Google Scholar PubMed PubMed Central
16. McCoy, KL. Interaction between cannabinoid system and toll-like receptors controls inflammation. Mediators Inflamm 2016;2016:5831315. https://doi.org/10.1155/2016/5831315.Search in Google Scholar PubMed PubMed Central
17. Kouokam, JC, Huskens, D, Schols, D, Johannemann, A, Riedell, SK, Walter, W, et al.. Investigation of griffithsin’s interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS One 2011;6:e22635. https://doi.org/10.1371/journal.pone.0022635.Search in Google Scholar PubMed PubMed Central
18. Li, N, Du, H, Mao, L, Xu, G, Zhang, M, Fan, Y, et al.. Reciprocal regulation of NRF2 by autophagy and ubiquitin-proteasome modulates vascular endothelial injury induced by copper oxide nanoparticles. J Nanobiotechnology 2022;20:270. https://doi.org/10.1186/s12951-022-01486-7.Search in Google Scholar PubMed PubMed Central
19. Feitelson, MA, Arzumanyan, A, Kulathinal, RJ, Blain, SW, Holcombe, RF, Mahajna, J et al.. Sustained proliferation in cancer: mechanisms and novel therapeutic targets. Semin Cancer Biol. 2015;35 (Suppl):S25–54. https://doi.org/10.1016/j.semcancer.2015.02.006.Search in Google Scholar PubMed PubMed Central
20. Modi, S, Kir, D, Banerjee, S, Saluja, A. Control of apoptosis in treatment and biology of pancreatic cancer. J Cell Biochem 2016;117:279–88. https://doi.org/10.1002/jcb.25284.Search in Google Scholar PubMed PubMed Central
21. Kaçaroğlu, D, Kalaycıoğlu, GD, Özden, AK. Carthamus Tınctorius L. (Safflower) extracts inhibit expression of metastatic genes of MDA-MB-231 breast cancer cells. Cell Mol Biol 2023;69:19–25. https://doi.org/10.14715/cmb/2023.69.12.4.Search in Google Scholar PubMed
22. Hamad, H, Olsen, BB. Cannabidiol induces cell death in human lung cancer cells and cancer stem cells. Pharmaceuticals 2021;14:1169. https://doi.org/10.3390/ph14111169.Search in Google Scholar PubMed PubMed Central
23. Śledziński, P, Nowak-Terpiłowska, A, Zeyland, J. Cannabinoids in medicine: cancer, immunity, and microbial diseases. Int J Mol Sci 2021;22:1–22. https://doi.org/10.3390/ijms22010263.Search in Google Scholar PubMed PubMed Central
24. Hosami, F, Manayi, A, Salimi, V, Khodakhah, F, Nourbakhsh, M, Nakstad, B, et al.. The pro-apoptosis effects of Echinacea purpurea and Cannabis sativa extracts in human lung cancer cells through caspase-dependent pathway. BMC Complement Med Ther 2021;21:37. https://doi.org/10.1186/s12906-021-03204-6.Search in Google Scholar PubMed PubMed Central
25. Hattar, K, Savai, R, Subtil, FSB, Wilhelm, J, Schmall, A, Lang, DS, et al.. Endotoxin induces proliferation of NSCLC in vitro and in vivo: role of COX-2 and EGFR activation. Cancer Immunol Immunother 2013;62:309–20. https://doi.org/10.1007/s00262-012-1341-2.Search in Google Scholar PubMed PubMed Central
26. Shcheblyakov, DV, Logunov, DY, Tukhvatulin, AI, Shmarov, MM, Naroditsky, BS, Gintsburg, AL. Toll-like receptors (TLRs): the role in tumor progression. Acta Naturae 2010;2:21–9. https://doi.org/10.32607/20758251-2010-2-3-21-29.Search in Google Scholar
27. Mccoy, KL. Interaction between cannabinoid system and toll-like receptors controls inflammation. Mediators Inflamm 2016;2016:5831315. https://doi.org/10.1155/2016/5831315.Search in Google Scholar PubMed PubMed Central
28. Afrin, F, Chi, M, Eamens, AL, Duchatel, RJ, Douglas, AM, Schneider, J, et al.. Can hemp help? Low-THC cannabis and non-THC cannabinoids for the treatment of cancer. Cancers 2020;12:1033. https://doi.org/10.3390/cancers12041033.Search in Google Scholar PubMed PubMed Central
29. Ramer, R, Heinemann, K, Merkord, J, Rohde, H, Salamon, A, Linnebacher, M, et al.. COX-2 and PPAR-γ confer cannabidiol-induced apoptosis of human lung cancer cells. Mol Cancer Ther 2013;12:69–82. https://doi.org/10.1158/1535-7163.MCT-12-0335.Search in Google Scholar PubMed
30. Misri, S, Kaul, K, Mishra, S, Charan, M, Verma, AK, Barr, MP, et al.. Cannabidiol inhibits tumorigenesis in cisplatin-resistant non-small cell lung cancer via TRPV2. Cancers 2022;14:1181. https://doi.org/10.3390/cancers14051181.Search in Google Scholar PubMed PubMed Central
31. Kisková, T, Mungenast, F, Suváková, M, Jäger, W, Thalhammer, T. Future aspects for cannabinoids in breast cancer therapy. Int J Mol Sci 2019;20:1673. https://doi.org/10.3390/ijms20071673.Search in Google Scholar PubMed PubMed Central
32. Nabissi, M, Morelli, MB, Offidani, M, Amantini, C, Gentili, S, Soriani, A, et al.. Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget 2016;7:7754357. https://doi.org/10.18632/oncotarget.12721.Search in Google Scholar PubMed PubMed Central
33. Essogmo, FE, Zhilenkova, AV, Tchawe, YSN, Owoicho, AM, Rusanov, AS, Boroda, A, et al.. Cytokine profile in lung cancer patients: anti-tumor and oncogenic cytokines. Cancers 2023;15:5383. https://doi.org/10.3390/cancers15225383.Search in Google Scholar PubMed PubMed Central
34. Nichols, JM, Kaplan, BLF. Immune responses regulated by cannabidiol. Cannabis Cannabinoid Res 2020;5:12–31. https://doi.org/10.1089/can.2018.0073.Search in Google Scholar PubMed PubMed Central
35. Wang, X, Lin, C, Wu, S, Zhang, T, Wang, Y, Jiang, Y, et al.. Cannabidivarin alleviates neuroinflammation by targeting TLR4 co-receptor MD2 and improves morphine-mediated analgesia. Front Immunol 2022;13:929222. https://doi.org/10.3389/fimmu.2022.929222.Search in Google Scholar PubMed PubMed Central
36. Braile, M, Marcella, S, Marone, G, Galdiero, MR, Varricchi, G, Loffredo, S. The interplay between the immune and the endocannabinoid systems in cancer. Cells 2021;10:1282. https://doi.org/10.3390/cells10061282.Search in Google Scholar PubMed PubMed Central
37. Ramer, R, Bublitz, K, Freimuth, N, Merkord, J, Rohde, H, Haustein, M, et al.. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule‐1. Faseb J 2012;26:1535–48. https://doi.org/10.1096/fj.11-198184.Search in Google Scholar PubMed
38. Ramer, R, Fischer, S, Haustein, M, Manda, K, Hinz, B. Cannabinoids inhibit angiogenic capacities of endothelial cells via release of tissue inhibitor of matrix metalloproteinases-1 from lung cancer cells. Biochem Pharmacol 2014;91:202–16. https://doi.org/10.1016/j.bcp.2014.06.017.Search in Google Scholar PubMed
39. Ramer, R, Rohde, A, Merkord, J, Rohde, H, Hinz, B. Decrease of plasminogen activator inhibitor-1 may contribute to the anti-invasive action of cannabidiol on human lung cancer cells. Pharm Res 2010;27:2162–74. https://doi.org/10.1007/s11095-010-0219-2.Search in Google Scholar PubMed
40. Drozd, M, Marzęda, P, Czarnota, J, Dobrzyński, M, Skubel, T, Dudek, I, et al.. The potential of cannabinoids in the treatment of lung cancer. J Educ Health Sport 2022;12:1100–10. https://doi.org/10.12775/jehs.2022.12.08.094.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- A brief history of hematology analyzers and recent advancements: the available testing wealth
- Research Articles
- Correlation between serum 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D in response to analytical procedures; a systematic review and meta-analysis
- Investigation of CDH1 germline mutations in Turkish patients with Kaposi’s sarcoma
- Frequency and pattern of test utilization rate in clinical biochemistry laboratory: two different large hospital examples
- High serum angiopoietin-like protein-4 levels are associated with gestational hypertension and preeclampsia: a case-control study
- Comparison of efficacy and reliability of six commercial COVID-19 diagnostic PCR kits
- Does COVID-19 infection alter serum biochemical and hematological biomarkers in deceased dementia patients?
- Activity of protein C, protein S and antithrombin 3 in COVID-19 patients treated with different modalities of oxygen supplementation
- Effectiveness after immunization with BNT162b2 and Gam-COVID-Vac for SARS-CoV-2 and neutralizing antibody titers in health care workers
- Relationship between methylation pattern of the SYN2 gene and schizophrenia
- Revealing distinct DNA methylation patterns in hepatic carcinoma through high-throughput sequencing
- 4-h mean lactate clearance as a good predictor of adverse outcome in acute cardiogenic pulmonary edema: a pilot study
- Elucidating the role of ZRF1 in monocyte-to-macrophage differentiation, cell proliferation and cell cycle in THP-1 cells
- Inflammatory factors secreted from endothelial cells induced by high glucose impair human retinal pigment epithelial cells
- Influence of TLR4 signaling on cannabidiol’s antitumor effectiveness in lung adenocarcinoma cells
- Renoprotective effect of diacerein in rats with partial unilateral ureteral obstruction model
- Cytotoxic and apoptotic effectiveness of Cypriot honeybee (Apis mellifera cypria) venom on various cancer cells
- Resveratrol modulates signalling to inhibit vascular smooth muscle cell proliferation induced by angiotensin II and high glucose
- Development and production of antibodies against gamma inactivated pathogenic bacterial spores
Articles in the same Issue
- Frontmatter
- Editorial
- A brief history of hematology analyzers and recent advancements: the available testing wealth
- Research Articles
- Correlation between serum 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D in response to analytical procedures; a systematic review and meta-analysis
- Investigation of CDH1 germline mutations in Turkish patients with Kaposi’s sarcoma
- Frequency and pattern of test utilization rate in clinical biochemistry laboratory: two different large hospital examples
- High serum angiopoietin-like protein-4 levels are associated with gestational hypertension and preeclampsia: a case-control study
- Comparison of efficacy and reliability of six commercial COVID-19 diagnostic PCR kits
- Does COVID-19 infection alter serum biochemical and hematological biomarkers in deceased dementia patients?
- Activity of protein C, protein S and antithrombin 3 in COVID-19 patients treated with different modalities of oxygen supplementation
- Effectiveness after immunization with BNT162b2 and Gam-COVID-Vac for SARS-CoV-2 and neutralizing antibody titers in health care workers
- Relationship between methylation pattern of the SYN2 gene and schizophrenia
- Revealing distinct DNA methylation patterns in hepatic carcinoma through high-throughput sequencing
- 4-h mean lactate clearance as a good predictor of adverse outcome in acute cardiogenic pulmonary edema: a pilot study
- Elucidating the role of ZRF1 in monocyte-to-macrophage differentiation, cell proliferation and cell cycle in THP-1 cells
- Inflammatory factors secreted from endothelial cells induced by high glucose impair human retinal pigment epithelial cells
- Influence of TLR4 signaling on cannabidiol’s antitumor effectiveness in lung adenocarcinoma cells
- Renoprotective effect of diacerein in rats with partial unilateral ureteral obstruction model
- Cytotoxic and apoptotic effectiveness of Cypriot honeybee (Apis mellifera cypria) venom on various cancer cells
- Resveratrol modulates signalling to inhibit vascular smooth muscle cell proliferation induced by angiotensin II and high glucose
- Development and production of antibodies against gamma inactivated pathogenic bacterial spores

