Enzymatic comparison and expression pattern of pig B4GALNT2 and B4GALNT2-like proteins
-
Anjing Zhang
, Zhining Zhong
, Dengke Pan
, Peidong Yang
, Shuqi Yang
, Jideng Ma
, Tingting Luo
, Li Chen
, Jinwei Zhang
, Jing Sun
, Jiaxiang Du
, Keren Long
, Mingzhou Li
and Lu Lu
Abstract
Objectives
The final step in the production of the human Sd(a) antigen is catalyzed by beta-1,4-N-acetyl-galactosamine transferase 2 (B4GALNT2). This is done by adding a N-acetylgalactosamine residue via a beta-1,4 linkage to a subterminal galactose residue that has been substituted with an alpha-2,3-linked sialic acid. The final stage of the production of the Cad antigen is also catalyzed by B4GALNT2. Knocking out pig B4GALNT2 gene decreased human serum antibodies binding to pig cells, which greatly reduces the immunological rejection in clinical xenotransplantation trials. Interestingly, gene region LOC110255214 (hereafter named B4GALNT2-like) showed high similarity with the B4GALNT2 gene in the pig genome in our previous work, but whether B4GALNT2-like shares similar biological properties like B4GALNT2 remains to be elucidated, whether B4GALNT2-like is a potential immune gene in xenotransplantation remains to be determined.
Methods
In this study, we compared the tissue expression pattern of B4GALNT2-like and B4GALNT2 in Bama pigs.
Results
We found the expression of B4GALNT2-like was significantly higher in the duodenum, but lower in the heart, spleen, lung, kidney, comparing to B4GALNT2. Applied the Escherichia coli recombinant expression, we obtained 768 and 1,300 μg protein for B4GALNT2 and B4GALNT2-like from 1 L culture, respectively. Using the expressed recombinant proteins, the enzymatic activity of the two proteins was determined and compared.
Conclusions
The enzymatic assay showed that B4GALNT2-like has comparable catalytic activity with B4GALNT2 (58.7 % of B4GALNT2), addressing an important question whether B4GALNT2-like is a new immunological rejection gene.
Introduction
Xenotransplantation refers to the transfer of vital organs (such as kidneys, hearts or livers) from one species to another, in which organs from non-human species are transplanted into human body to prolong human life, and is considered to be one of the most effective ways to solve the shortage of human organs [1]. The physiological tissues of pig heart, kidney, lung, islet, cornea and other organs are similar to humans, and pigs are currently the only xenograft donor animals that allow clinical research [2]. The main obstacle of xenogeneic organ transplantation in pigs is immune rejection. Human immune system can effectively resist the invasion of external pathogens, also attack the foreign organs and cause serious immune rejection during the transplantation [3]. At present, the use of gene editing technology to knock out immune rejection genes in pigs, integrate humanized antibody genes and knock out endogenous virus genes can effectively reduce the immune rejection reaction of pig xenotransplantation, improve the survival time of the donor in the human body, and reduce the potential risk of cross-species virus transmission [4].
The first method used to discover the pig 1,4-N-acetylgalactosaminotransferase 2 (B4GALNT2, also known as β4GalNT2) gene involved searching a library of swine EC cDNA for non-Gal antigens produced in human HEK293 cells [5]. Analysis of non-galactose antibodies induced by porcine cardiac xenografting in baboons confirmed that the glycan produced by porcine B4GALNT2 was an immunogenic xenograft antigen [5]. The pig B4GALNT2 gene has a conserved genome structure, and the open reading frame it encodes has 76 and 70 % amino acid homology with human and mouse B4GALNT2 genes, respectively, and can synthesize human Sda blood group antigens [6]. In swine endothelial cells, the B4GALNT2 gene has a widespread pattern of expression. Expression of porcine B4GALNT2 in human HEK cells (HEK-B4T) results in increased binding of antibody to the B4GALNT2 enzyme, and increased reactivity with anti-Sd(a) and DBA [7].Pigs and mice have been modified to have the B4GALNT2 gene mutation. B4GALNT2 knockout (B4GALNT2-KO) animals seemed normal in both species and no longer displayed Sda antigen expression. Reduced antibody binding was observed in B4GALNT2-mutated porcine tissue when combined with 1,3-galactotransferase (GGTA1-KO, also known as GalT) and cytidine monophosphate-acetyl neuraminic acid hydroxylase (CMAH-KO) deficiency, demonstrating the presence of B4GALNT2-dependent antibodies in humans and non-human primates [8]. B4GALNT2 in contrast to Gal and Neu5Gc, which are not expressed in humans, is involved in the manufacture of Sda antigen by transferring beta 1,4-linked GalNAc to an alpha 2,3 sialic acid-modified N-acetyl lactosamine acceptor oligosaccharide. It is also expressed in human and pig [9, 10]. In human, B4GALNT2 is predominantly expressed in gastrointestinal epithelium of the colon and with lower expression levels in the kidney, stomach, and ileum [11]. Consistently, pig B4GALNT2 gene was not only widely expressed in the gastrointestinal system but also in major vascularized organs [6].
Sda antigens are involved in a variety of physiological and pathological processes. Like other carbohydrates with a specific structure, Sda antigens can act as specific ligands or receptors for different cells or microorganisms, thus functioning in different situations [12]. Previous research on the B4GALNT2 gene’s functions has revealed that (1) Sda is essential for mouse embryo attachment [13, 14]; (2) Sda has been linked to the prevention of muscular dystrophy in a murine model [15]; (3) activating the expression of B4GALNT2 in the pig PK15 cell line can increase cell resistance to PRV or avian influenza A (SH13, H9N2) viral infection [16]; (4) B4GALNT2 deletion in mice changed the gut microbiota, and Sda antigen may act as a bridge between the host and the microbiota [17]. The further deletion of B4GALNT2 to the GGTA1 and CMAH-double KO background significantly lowers the level of human non-Gal IgM and IgG binding to pig PBMCs in triple knockout pigs (GGTA1, CMAH, and B4GALNT2), confirming the presence of human antibody that binds to the porcine glycan (s) produced by the B4GALNT2 gene [18, 19]. In our previous gene knockout experiment, a DNA region similar to the B4GALNT2 gene was found in the pig genome, indicating the complexity of porcine Sda antigen synthesis. In the most recent pig genome, Sus Scrofa11.1, this region is annotated as a novel gene LOC110255214 (hereafter named B4GALNT2-like) that has high nucleotide sequence similarity to B4GALNT2. However, whether this gene expresses a valid transcript or shares a similar function as the B4GALNT2 gene is of great value to reveal.
Thus, we investigated the expression profile of B4GALNT2 and B4GALNT2-like in Bama pigs and compared their expression in the main tissues that are used for xenotransplantation. We also expressed and purified the two recombinant proteins, and found B4GALNT2-like also maintains the enzymatic activity as B4GALNT2.
Materials and methods
Animals and tissue collection
In the present study, three six-months-old male Bama pigs were used and acquired from Chengdu Clonorgan Biotechnology Co., Ltd. After the animals were humanely executed by electric shock after being fasted for the previous night, blood was drawn from the jugular vein for sample. In a nutshell, the tissue samples were collected right away and kept in a freezer set to −80 °C until needed. The Institutional Animal Care and Use Committee of Sichuan Agricultural University granted approval for the animal experiment under permit number 20110235.
B4GALNT2 and B4GALNT2-like gene cloning
B4GALNT2 and B4GALNT2-like genes were obtained using the TA clone method. Total RNA was extracted from the kidney sample using the Animal Total RNA Isolation Kit (Foregene, Chengdu, China) according to the manufacturer’s instructions. mRNA reverse transcription was carried out. HisScript III 1st Strand cDNA (Vazyme, Chengdu, China) was used. PCR products were created gel-purified and cloned into Beijing TsingKe Biotech’s pClone007 basic vector for Sanger sequencing validation (Sangon Biotech, Shanghai, China). The universal primer pairs M13-47/M13-48 were used to sequence the products of each gene. After sequencing, the sequences of Bama pig B4GALNT2 and B4GALNT2-like were cloned into prokaryotic expression vector pET-28a(+) (Novagen, Madison, WI), within the multiple cloning sites BamHI and XhoI, using T4 DNA Ligase (NEB, Massachusetts, England). The sequence of specific primers was shown in Supplementary Table 1. Then the plasmid DNA is transformed into Escherichia coli T7E and Rosetta (DE3) for subsequent expression optimization [20].
Recombinant protein expression and purification
B4GLNT2 and B4GALNT2-like recombinant proteins were produced as previously reported [21]. Briefly, E. coli BL21 (DE3)/pET-28a(+) cells were induced by 1 mM Isopropyl-β-d-1-thiogalactopyranoside. We compared the induction conditions setting 16 °C for 16 h and 37 °C for 4 h. The supernatant and precipitation from cell lysates were collected and visualized in SDS-PAGE. Ni-TED 1 mL Sefinose (TM) Column (Sangon Biotech) was used to purify the target protein from the cell lysate according to the manufacturers instructions.
Enzymatic assay
We adopt a pH-sensitive assay as previously reported to measure the two galactosyltransferases, B4GALNT2 and B4GALNT2-like [22]. Briefly, the quantitative linear relationship between proton concentration and absorbance (OD577) was established by recording the absorbance and the corresponding serial proton dilution. Followingly, the enzymatic activity was measured in 0.01 mM Phenol red, 0.1 mM MnCl2 and 10 mM GlcNac, and 10 nM B4GALNT2 or B4GALNT2-like in total volume of 1 mL of phosphate buffer (pH8). The reaction was started once UDP-galactose (2 mM at final concentration) was added, and the absorbance at 577 nm was recorded for each sample at 1 min intervals for a total of 4 min. All the measurements were carried out at 25 °C degrees. The activities of enzymes were calculated from the calibration curve. The enzyme activity was defines as the amount of enzyme required to produce 1 μmol proton per minute.
qRCR
qRCR was performed to detect the B4GALNT2 and B4GALNT2-like gene expression in triplicates. Tissue RNA was collected following the method described above. cDNA was synthesized by PrimeScriptTM RT reagent Kit with gDNA Eraser (RR047A, Takara, USA) and qRT-PCR was performed using the TB Green Premix Ex Taq α (RR820A, Takara, Japan) on a CFX96 instrument (Bio-Rad, USA). To ensure the accuracy of expression detection, one of the primers used to detect the expression of B4GALNT2 and B4GALNT2-like must be in the different regions located in the second exon of the two genes. The sequence of specific primers was shown in Supplementary Table 1, all quantitative reactions were repeated three times. Relative expression levels of mRNA were calculated using the 2−ΔΔCt method [11]. ACTB was used as housekeeping genes for normalizing mRNA.
Statistical analysis
The values are shown as the mean ± SD Statistical analyses were performed by the Wilcoxon text using GraphPad Prism 8 software. Differences were considered statistically significant at p-value<0.05. The structures of the AlphaFold prediction were downloaded from the UniProt database (https://www.uniprot.org/), performing structure alignment using PyMOL 2.4 software.
Results
B4GALNT2 and B4GALNT2-like sequence identification in Bama pig
The nucleotide sequence of the pig B4GALNT2 cDNA was used to perform BLAST analysis of the pig HTG and EST divisions of the GenBank/EML database at the National Center for Biotechnology Information. The pig B4GALNT2 (ENSSSCG00000040942) and B4GALNT2-like (ENSSSCG00000030269) have sufficient homology with human B4GALNT2 cDNA. The two genes are in close proximity to chromosome 12, validated by sanger sequencing, the full length of B4GALNT2 and B4GALNT2-like transcripts were 1,509 and 1,521 bp, respectively (Supplementary Figure 1). As shown in Figure 1, B4GALNT2 and B4GALNT2-like showed the greatest difference in nucleotides at the end of exon-2. Besides, there were several nucleotide differences between both kinds of transcripts in exon −3, −5, −9, −10, and −11. Thus, we concluded here B4GALNT2-like, which was very similar to B4GALNT2, but a different gene.
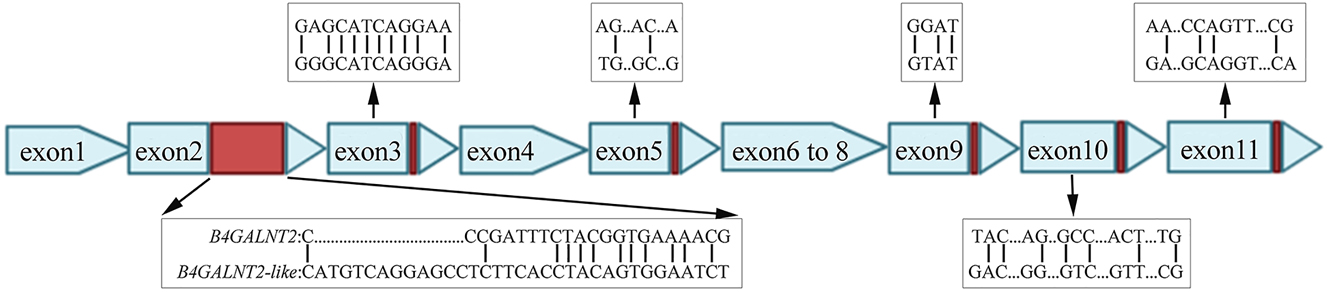
Schematic of gene structure difference between B4GALNT2 and B4GALNT2-like cDNA in Bama pig. The red boxes indicate the difference in exon −2, −3, −5, −9, −10 and −11.
Tissue expression of B4GALNT2 and B4GALNT2-like in Bama pig
Bama pig is the ideal model for xenotransplantation because their organ size, physiological metabolism and immune system are similar to those of human beings [23, 24]. We further performed qPCR to quantify the gene expression of B4GALNT2 and B4GALNT2-like in main pig organs and tissues, including heart, liver, spleen, lung, kidney, stomach, duodenum, jejunum and ileum tissues of Bama pigs. Like B4GALNT2, B4GALNT2-like was widely expressed in the gastrointestinal system and major vascularized organs (Figure 2A). However, the relative expression of B4GALNT2-like was significantly higher than B4GALNT2 in duodenum (p=0.014) and lower in spleen (p=0.005), lung (p=0.001), kidney (p=0.014), and heart (p=0.002).

Tissue expression of B4GALNT2 and B4GALNT2-like in Bama pig. The relative gene expression of B4GALNT2 and B4GALNT2-like in different tissues of Bama pig was detected by qPCR (n=6). ACTB was used as the internal reference for mRNA quantification. The data are presented as means ± SD. *p<0.05; **p<0.01.
Recombinant expression of B4GALNT2 and B4GALNT2-like
The CDS sequences of the Bama pig B4GALNT2 (1,509 bp) and B4GALNT2-like (1,521 bp) genes were cloned into pET-28a(+) plasmid using prokaryotic expression system in E. coli. Both B4GALNT2 and B4GALNT2-like are predominantly expressed in inclusion body. Optimizing the strain and induction conditions, we obtained yielding 0.768 mg B4GALNT2 and 1.3 mg B4GALNT2-like from 1 L culture, respectively (Figure 3). We predicted the molecular weights of B4GALNT2 and B4GALNT2-like to be 57.22 and 56.74 kDa respectively by using the protein molecular weight calculator (http://www.detaibio.com/sms2/protein_mw.html), which was consistent with our experimental results (Figure 3).

Recombinant expression of B4GALNT2 and B4GALNT2-like. (A) Preparation of B4GALNT2 and B4GALNT2-like recombinant protein. The B4GALNT2 and B4GALNT2-like recombinant protein bands are the clearest in T7E, induced 4 h at 37 °C. MW, molecular weight marker; NPE, the soluble supernatant fraction; DPE, the supernatant of denatured protein; Ø, non-induced bacteria culture (negative control); 16 and 37, incubation temperature (°C) during induction with IPTG (induction with IPTG) 1 mM during 16 h at 16 °C, or during 4 h at 37 °C. (B) We perform SDS-PAGE with Coomassie blue staining identification. Left: the Western blot with anti His-Tag antibody for B4GALNT2. Right: the Western blot with anti His-Tag antibody for B4GALNT2-like.
Enzymatic comparison of B4GALNT2 and B4GALNT2-like
Using the two recombinant expressed proteins, the enzymatic activity assay was performed to compare B4GALNT2 and B4GALNT2-like as previously reported [22]. We initially established a connection between proton concentration and phenolindicator absorbance (Figure 4A). The absorbance at 557 nm is linearly proportional to proton concentrations ranging from 0 to 0.4 mM, which covers the range of proton concentrations used in all tests in this study. The calibration curve was created using essentially the same enzyme mixture as used in the actual experiment, with the exception of the donor substrate. The proton concentration change corresponding to the absorbance change was converted and shown as a function of time using the calibration curve (Figure 4B and C). The slope of a linear regression for B4GALNT2 was calculated to be 0.0432 and 0.0234 for B4GALNT2-like. The enzyme activity was defined as the amount of enzyme required to produce 1 μmol proton per minute (1 U=1 μmol/min). We calculated B4GALNT2 and B4GALNT2-like have activity of about the enzyme activity of B4GALNT2=0.0432 mmol/(L/min)×1×1,000−3 μL/10−2 nmol mg=4,323 U/μmol and B4GALNT2-like is 2,340 U/μmol.

Enzyme activity determination of B4GALNT2 and B4GALNT2-like recombinant protein. (A) the calibration curve represents the relationship between proton concentration and absorbance at 557 nm. OD557 was measured after different concentrations of hydrochloric acid (0–0.4 mM). The measurement was performed three times and the results were averaged, the average results were used for plotting. (B) time-dependent release of protons from protein-catalyzed processes. The enzyme was tested in a total volume of 1 mL of phosphate buffer (pH=8) containing 0.01 mM phenol red, 0.1 mM MnCl2, 10 mM GlcNac, and 10 nM B4GALNT2 or B4GALNT2-like. The action was initiated by adding 2 mM UDP-galactose, and measurements were made at 557 nm for 5 min at 1 min intervals. The measurement was performed three times and the results were averaged, the average results were used for plotting. (C) absorbance at 557 nM changes with time for B4GALNT2 and B4GALNT2-like. The reaction was started by adding 2 mM UDP-galactose, and measurements were taken at 557 nM for 3 min with a time interval of 1 min. The curves show the mean and standard deviation. In blank, no UDP-galactose was introduced.
Discussion
Xenotransplantation is a potential solution to the continuing and critical shortage of deceased human organs for clinical transplantation. It is urgent to search for new immune rejection genes. Three major heterologous antigen genes have been discovered: GGTA1, B4GALNT2 and CMAH. Moreover, 9 anthropogenic genes that inhibit complement activation (hCD46, hCD55, hCD59), regulate coagulation disorders (hTHBD, hTFPI, hCD39), and anti-inflammation and anti-phagocytosis (hB2M, hHLA-E, hCD47) were introduced, which is an important means to reduce the immune response of primate receptors and reduce graft damage. For example, GGTA1-KO/hCD46/hTBM three-gene modified donor pigs for cardiac ectopic transplantation from pigs to baboons, with a minimum survival of 77 days and a maximum of 380 days [25], [26], [27]. On this basis, the team knocked out four genes (CD55/CD39/CD47/EPCR) and transplanted ectopic hearts to baboons, but the survival time of the graft was shorter than that of GGTA1T-KO/hCD46/hTBM triple gene modification [28]. Rhesus macaques survived for up to 499 days after receiving GGTAT-KO/hCD55 gene-edited pig kidneys transplantation [29]. After heart, liver and kidney transplants from the “13 gene-edited pigs”, three rhesus macaque respectively survived 7, 26 and 1 days [29]. Although donors of different gene editing types and gene combinations have been evaluated, but with limited effects [30, 31], intriguing us to ask if there any immune rejection genes that have not been identified?
More than 95 % of human anti-pig antibodies are directed against 3 pig carbohydrates: Gal, Neu5Gc and Sda [32, 33]. The swine B4GALNT2 enzyme is identical to the human enzyme that produces the human Sda blood group antigen [8]. In human HEK293 cells, the expression of swine B4GALNT2 increases anti-Sda antibody binding as well as Dolichos biflorus agglutinin (DBA), a lectin often employed to detect Sda [6]. In mice and pigs with the B4GALNT2 gene knocked out, the B4GALNT2-KO animals appeared to be normal and no longer showed evidence of Sda antigen expression. In GGTA1/CMAH double gene modified donor pigs, knockout of the B4GALNT2 gene resulted in a 49.1 and 43.2 % reduction respectively in IgG and IgM binding [8]. Furthermore, simultaneous silencing of the porcine GGTA1/CMAH/B4GALNT2 genes significantly reduced pig resistance to humans compared to CMAH/GGTA1 double-KO [28]. Despite the success in creating gene-edited pigs and further clinical xenotransplantation trials, even for heart [34], but the rejection still exist.
In the present study, we successfully expressed and purified pig B4GALNT2 and B4GALNT2-like and we found that B4GALNT2-like has comparable beta-1,4-N-acetyl-galactosaminyl transferase activity with B4GALNT2. As shown in Supplementary Figure 2, the two proteins shares conserved domains related to the enzymatic activities, specifically, the region ranged from 272 to 365th amino acid sequence represents a conserved GT-A fold and 325–339th amino acid sequence represents a conserved divalent cation binding domain [11, 35]. Consistent with this, in the UniProt database, we found that proteins A0A287BMC6 and I3L7N4 shared 98 % of the same site sequences with B4GALNT2 and B4GALNT2-like proteins, and further structural comparison using PyMOL also confirmed the conserved sites associated with enzyme activity (Figure 5). B4GALNT2-like is similar like to B4GALNT2, tracing its origin to possible gene replication [36]. Gene duplication is thought to be a central process in evolution to gain new functions [37]. Therefore, it is necessary to further study the function of B4GALNT2-like. However, the two amino acids in GT-A fold were different comparing B4GALNT2 and B4GALNT-like, which may ultimately result in the different enzymatic activities of B4GALNT2 and B4GALNT2-like recombinant proteins. High levels of B4GALNT2 enzymatic activity have been reported in porcine intestinal epithelial cells [38], but the distribution of expression levels of B4GALNT2-like in pigs is incomplete [6]. We found that B4GALNT2 and B4GALNT2-like were expressed in the heart, liver, spleen, lung, kidney, stomach, duodenum, jejunum, and ileal tissues of Bama pigs (Figure 2).

Structural alignment of B4GALNT2 and B4GALNT2-like proteins. The protein structure was aligned using PyMOL software. The green represents B4GALNT2, and the protein structure is from A0A287BMC6 in the UniProt database. The blue represents B4GALNT2-like, and the protein structure is from I3L7N4 in the UniProt database. The red conserved GT-A folding region is the 272–365 amino acid sequence on B4GALNT2-like, while the yellow conserved divalent cation binding domain is the 325–339 amino acid sequence on B4GALNT2-like.
In conclusion, B4GALNT2-like has a comparable enzymatic activity with B4GALNT2. The results cause our great concerns that whether B4GALNT2-like contributes immune rejection, but further works are needed to validate the role of B4GALNT2-like in xenotransplantation both ex vivo and in vivo as the present study only shed the light of the enzymatic role.
Funding source: National Key R & D Program of China
Funding source: National Key R & D Program of China
Funding source: National Natural Science Foundation of China
Funding source: National Natural Science Foundation of China
Funding source: Science Foundation of the Sichuan Province
Funding source: Science Foundation of the Sichuan Province
Funding source: Science Foundation of the Sichuan Province
-
Research ethics: The local Institutional Review Board deemed the study exempt from review.
-
Informed consent: Not applicable.
-
Author contributions: Performing experiments: Anjing Zhang, Zhining Zhong, Jideng Ma, Peidong Yang, Shuqi Yang, Xueming Li, Tingting Luo, Li Chen, Jinwei Zhang, Jing Sun, Jiaxiang Du; Data analysis and manuscript writing: Anjing Zhang, Zhining Zhong, Lu Lu, Tingting Luo, Keren Long, Mingzhou Li, Dengke Pan, Jiaxiang Du, Jideng Ma Manuscript editing and review: Anjing Zhang, Zhining Zhong, Peidong Yang, Li Chen, Jinwei Zhang, Jing Sun, Lu Lu, Mingzhou Li. Dengke Pan.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: Experimental work was supported by the National Key R & D Program of China (2021YFD1300800 to L.L. and 2020YFA0509500 to M.L.), the National Natural Science Foundation of China (32225046 to M.L. and 32202630 to F.K.), the Science Foundation of the Sichuan Province (2021YFS0008 and 2022YFQ0022 to L.L., 2021ZDZX0008 to K.L., and 2021YFYZ0009 to M.L.).
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Sim, K, Marinov, A, Levy, G. Xenotransplantation: a potential solution to the critical organ donor shortage. Can J Gastroenterol 1999;13:311–8, https://doi.org/10.1155/1999/231798.Search in Google Scholar PubMed
2. Zhang, X, Wang, Q, Zhao, J, Li, X, Peng, W, Yang, Z, et al.. The resurgent landscape of xenotransplantation of pig organs in nonhuman primates. Sci China Life Sci 2021;64:697–708, https://doi.org/10.1007/s11427-019-1806-2.Search in Google Scholar PubMed
3. Lei, T, Chen, L, Wang, K, Du, S, Gonelle-Gispert, C, Wang, Y, et al.. Genetic engineering of pigs for xenotransplantation to overcome immune rejection and physiological incompatibilities: the first clinical steps. Front Immunol 2022;13:1031185, https://doi.org/10.3389/fimmu.2022.1031185.Search in Google Scholar PubMed PubMed Central
4. Deng, J, Yang, L, Wang, Z, Ouyang, H, Yu, H, Yuan, H, et al.. Advance of genetically modified pigs in xeno-transplantation. Front Cell Dev Biol 2022;10:1033197, https://doi.org/10.3389/fcell.2022.1033197.Search in Google Scholar PubMed PubMed Central
5. Byrne, G, Stalboerger, P, Du, Z, Davis, T, McGregor, C. Identification of new carbohydrate and membrane protein antigens in cardiac. Xenotransplantation 2011;91:287–92, https://doi.org/10.1097/tp.0b013e318203c27d.Search in Google Scholar
6. Byrne, GW, Du, Z, Stalboerger, P, Kogelberg, H, McGregor, CG. Cloning and expression of porcine β1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation 2014;21:543–54, https://doi.org/10.1111/xen.12124(1399-3089).Search in Google Scholar
7. Ekser, B, Ezzelarab, M, Hara, H, van der Windt, D, Wijkstrom, M, Bottino, R, et al.. Clinical xenotransplantation: the next medical revolution? The Lancet 2012;379:672–83, https://doi.org/10.1016/s0140-6736(11)61091-x.Search in Google Scholar PubMed
8. Byrne, G, Ahmad-Villiers, S, Du, Z, McGregor, C. B4GALNT2 and xenotransplantation: a newly appreciated xenogeneic antigen. Xenotransplantation 2018;25:e12394, https://doi.org/10.1111/xen.12394.Search in Google Scholar PubMed PubMed Central
9. Galili, U, Rachmilewitz, E, Peleg, A, Flechner, I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med 1984;160:1519–31, https://doi.org/10.1084/jem.160.5.1519.Search in Google Scholar PubMed PubMed Central
10. Varki, A. Loss of N-glycolylneuraminic acid in humans: mechanisms, consequences, and implications for hominid evolution. Am J Phys Anthropol 2001;116:54–69, https://doi.org/10.1002/ajpa.10018.Search in Google Scholar
11. Montiel, M, Krzewinski-Recchi, M, Delannoy, P, Harduin-Lepers, A. Molecular cloning, gene organization and expression of the human UDP-GalNAc: Neu5Acalpha2-3Galbeta-R beta1, 4-N-acetylgalactosaminyltransferase responsible for the biosynthesis of the blood group Sda/Cad antigen: evidence for an unusual extended cytoplasmic domain. Biochem J 2003;373:369–79, https://doi.org/10.1042/bj20021892.Search in Google Scholar
12. Zhao, C, Cooper, D, Dai, Y, Hara, H, Cai, Z, Mou, L. The Sda and Cad glycan antigens and their glycosyltransferase, β1,4GalNAcT-II, in xenotransplantation. Xenotransplantation 2018;25:e12386, https://doi.org/10.1111/xen.12386.Search in Google Scholar PubMed
13. Li, P, Liao, C, Yu, L, Wu, W, Chu, S. Localization of B4GALNT2 and its role in mouse embryo attachment. Fertil Steril 2012;97:1206–12.e3, https://doi.org/10.1016/j.fertnstert.2012.02.019.Search in Google Scholar PubMed
14. Klisch, K, Contreras, D, Sun, X, Brehm, R, Bergmann, M, Alberio, R. The Sda/GM2-glycan is a carbohydrate marker of porcine primordial germ cells and of a subpopulation of spermatogonia in cattle, pigs, horses and llama. Reproduction 2011;142:667–74, https://doi.org/10.1530/rep-11-0007.Search in Google Scholar PubMed
15. Xu, R, DeVries, S, Camboni, M, Martin, P. Overexpression of Galgt2 reduces dystrophic pathology in the skeletal muscles of alpha sarcoglycan-deficient mice. Am J Pathol 2009;175:235–47, https://doi.org/10.2353/ajpath.2009.080967.Search in Google Scholar PubMed PubMed Central
16. Jiang, J, Sun, Y, Xiao, R, Wai, K, Ahmad, M, Khan, F, et al.. Porcine antiviral activity is increased by CRISPRa-SAM system. Biosci Rep 2019;39:BSR20191496, https://doi.org/10.1042/bsr20191496.Search in Google Scholar
17. Staubach, F, Künzel, S, Baines, A, Yee, A, McGee, B, Bäckhed, F, et al.. Expression of the blood-group-related glycosyltransferase B4galnt2 influences the intestinal microbiota in mice. ISME J 2012;6:1345–55, https://doi.org/10.1038/ismej.2011.204.Search in Google Scholar PubMed PubMed Central
18. Wang, Z, Li, P, Butler, J, Blankenship, R, Downey, S, Montgomery, J, et al.. Immunogenicity of renal microvascular endothelial cells from genetically modified pigs. Transplantation 2016;100:533–7, https://doi.org/10.1097/tp.0000000000001070.Search in Google Scholar
19. Wang, Z, Martens, G, Blankenship, R, Sidner, R, Li, P, Estrada, J, et al.. Eliminating xenoantigen expression on swine RBC. Transplantation 2017;101:517–23, https://doi.org/10.1097/tp.0000000000001302.Search in Google Scholar
20. Hayat, S, Farahani, N, Golichenari, B, Sahebkar, A. Recombinant protein expression in Escherichia coli (E.coli): what we need to know. Curr Pharm Des 2018;24:718–25, https://doi.org/10.2174/1381612824666180131121940.Search in Google Scholar PubMed
21. Lu, L, Wei, R, Prats-Ejarque, G, Goetz, M, Wang, G, Torrent, M, et al.. Human RNase3 immune modulation by catalytic-dependent and independent modes in a macrophage-cell line infection model. Cell Mol Life Sci 2021;78:2963–85, https://doi.org/10.1007/s00018-020-03695-5.Search in Google Scholar PubMed PubMed Central
22. Deng, C, Chen, R. A pH-sensitive assay for galactosyltransferase. Anal Biochem 2004;330:219–26, https://doi.org/10.1016/s0003-2697(04)00246-5.Search in Google Scholar
23. Cooper, D, Ekser, B, Ramsoondar, J, Phelps, C, Ayares, D. The role of genetically engineered pigs in xenotransplantation research. J Pathol 2016;238:288–99, https://doi.org/10.1002/path.4635.Search in Google Scholar PubMed PubMed Central
24. Xi, J, Zheng, W, Chen, M, Zou, Q, Tang, C, Zhou, X. Genetically engineered pigs for xenotransplantation: hopes and challenges. Front Cell Dev Biol 2023;10:1093534, https://doi.org/10.3389/fcell.2022.1093534.Search in Google Scholar PubMed PubMed Central
25. Mohiuddin, M, Singh, A, Corcoran, P, Hoyt, R, Thomas, ML, Lewis, B, et al.. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant 2014;14:488–9. https://doi.org/10.1111/ajt.12562.Search in Google Scholar PubMed PubMed Central
26. Burlak, C, Taylor, R, Wang, Z, Tector, A. Human anti-α-fucose antibodies are xenoreactive toward GGTA1/CMAH knockout pigs. Xenotransplantation 2020;27:e12629, https://doi.org/10.1111/xen.12629.Search in Google Scholar PubMed
27. Wang, Z, Burlak, C, Estrada, J, Li, P, Tector, M, Tector, AJ. Erythrocytes from GGTA1/CMAH knockout pigs: implications for xenotransfusion and testing in non-human primates. Xenotransplantation 2014;21:376–84, https://doi.org/10.1111/xen.12106.Search in Google Scholar PubMed PubMed Central
28. Mohiuddin, M, Singh, A, Corcoran, P, Thomas, MIII, Clark, T, Lewis, B, et al.. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun 2016;7:11138, https://doi.org/10.1038/ncomms11138.Search in Google Scholar PubMed PubMed Central
29. Kim, S, Mathews, D, Breeden, C, Higginbotham, L, Ladowski, J, Martens, G, et al.. Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion. Am J Transplant 2019;19:2174–85, https://doi.org/10.1111/ajt.15329.Search in Google Scholar PubMed PubMed Central
30. Morticelli, L, Rossdam, C, Cajic, S, Böthig, D, Magdei, M, Tuladhar, S, et al.. Genetic knockout of porcine GGTA1 or CMAH/GGTA1 is associated with the emergence of neo-glycans. Xenotransplantation 2023;30:e12804, https://doi.org/10.1111/xen.12804.Search in Google Scholar PubMed
31. Yoon, S, Lee, S, Park, C, Choi, H, Yoo, M, Lee, SC, et al.. An efficacious transgenic strategy for triple knockout of xeno-reactive antigen genes GGTA1, CMAH, and B4GALNT2 from Jeju native pigs. Vaccines 2022;10:1503, https://doi.org/10.3390/vaccines10091503.Search in Google Scholar PubMed PubMed Central
32. Martens, G, Reyes, L, Li, P, Butler, J, Ladowski, J, Estrada, J, et al.. Humoral reactivity of renal transplant-waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA class I knockout pigs. Transplantation 2017;101:e86–92, https://doi.org/10.1097/tp.0000000000001646.Search in Google Scholar PubMed PubMed Central
33. Pierson, R, Fishman, J, Lewis, G, D’Alessandro, D, Connolly, M, Burdorf, L, et al.. Progress toward cardiac xenotransplantation. Circulation 2020;142:1389–98, https://doi.org/10.1161/circulationaha.120.048186.Search in Google Scholar PubMed PubMed Central
34. Boulet, J, Cunningham, JW, Mehra, MR. Cardiac xenotransplantation: challenges, evolution, and advances. JACC (J Am Coll Cardiol) 2022;7:716–29, https://doi.org/10.1016/j.jacbts.2022.05.003.Search in Google Scholar PubMed PubMed Central
35. Marchler-Bauer, A, Panchenko, A, Shoemaker, B, Thiessen, P, Geer, L, Bryant, S. CDD: a database of conserved domain alignments with links to domain three-dimensional structure. Nucleic Acids Res 2002;30:281–3, https://doi.org/10.1093/nar/30.1.281.Search in Google Scholar PubMed PubMed Central
36. Assis, R, Bachtrog, D. Rapid divergence and diversification of mammalian duplicate gene functions. BMC Evol Biol 2015;15:138, https://doi.org/10.1186/s12862-015-0426-x.Search in Google Scholar PubMed PubMed Central
37. Fraimovitch, E, Hagai, T. Promoter evolution of mammalian gene duplicates. BMC Biol 2023;21:80. https://doi.org/10.1186/s12915-023-01590-6.Search in Google Scholar PubMed PubMed Central
38. Mansley, M, Watt, G, Francis, S, Walker, D, Land, S, Bailey, M, et al.. Dexamethasone and insulin activate serum and glucocorticoid-inducible kinase 1 (SGK1) via different molecular mechanisms in cortical collecting duct cells. Physiol Rep 2016;4:e12792, https://doi.org/10.14814/phy2.12792.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/tjb-2023-0148).
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review
- Quercetin induces cytotoxicity and apoptosis, reduces metastasis and drug resistance in oral cancer cells
- Research Articles
- Comparison of results of two hematological analyzer systems: Dirui BF-7200 and Sysmex XN-1000
- User verification of Abbott Alinity HQ hematology analyzer
- Erythrocyte labile iron pool indicating concealed iron overload in non-transfusion-dependent β-thalassemia
- Flow cytometric analysis of lymphocyte subsets, monocytes, and HLA-DR expressions on these cells in patients with COVID-19
- Comparative vascular effects of levetiracetam and valproate with hyperhomocysteinemia in rat models
- Evaluation of systemic inflammatory and fibrosis indices in Saprochaete capitata infections: a retrospective case-control study
- Class IA PI3K isoforms lead to differential signalling downstream of PKB/Akt
- Enzymatic comparison and expression pattern of pig B4GALNT2 and B4GALNT2-like proteins
- Does COVID-19 affect thyroid more than non-COVID-19 infections? A retrospective study
- Desmin’s conformational modulation by hydrophobicity
- Assessment of myogenic potency in patient-derived fibroblasts with c.1289-2A>G Desmin mutation
- GSH-related enzyme activity and tumor relation: glutathione peroxidase and glutathione reductase status under hypoxia in HepG2 cells
- Can triglyceride related indices be reliable markers in the assessment of polycystic ovarian syndrome?
- Vascular endothelial growth factor (VEGF)-C and its receptors, soluble VEGFR-2 and VEGFR-3, in polycystic ovary syndrome
- Platelet activating factor acetylhydrolase is associated with cardiac valvular calcification in dialysis patients
- Reticulated platelets and coronary slow flow: a study in stable coronary artery disease
- Identification of the role of TG2 on the expression of TGF-β, TIMP-1 and TIMP-2 in aged skin
- Unveiling the link: Helicobacter pylori infection and impact on ischemia modified albumin, thiol, and disulfide levels
Articles in the same Issue
- Frontmatter
- Review
- Quercetin induces cytotoxicity and apoptosis, reduces metastasis and drug resistance in oral cancer cells
- Research Articles
- Comparison of results of two hematological analyzer systems: Dirui BF-7200 and Sysmex XN-1000
- User verification of Abbott Alinity HQ hematology analyzer
- Erythrocyte labile iron pool indicating concealed iron overload in non-transfusion-dependent β-thalassemia
- Flow cytometric analysis of lymphocyte subsets, monocytes, and HLA-DR expressions on these cells in patients with COVID-19
- Comparative vascular effects of levetiracetam and valproate with hyperhomocysteinemia in rat models
- Evaluation of systemic inflammatory and fibrosis indices in Saprochaete capitata infections: a retrospective case-control study
- Class IA PI3K isoforms lead to differential signalling downstream of PKB/Akt
- Enzymatic comparison and expression pattern of pig B4GALNT2 and B4GALNT2-like proteins
- Does COVID-19 affect thyroid more than non-COVID-19 infections? A retrospective study
- Desmin’s conformational modulation by hydrophobicity
- Assessment of myogenic potency in patient-derived fibroblasts with c.1289-2A>G Desmin mutation
- GSH-related enzyme activity and tumor relation: glutathione peroxidase and glutathione reductase status under hypoxia in HepG2 cells
- Can triglyceride related indices be reliable markers in the assessment of polycystic ovarian syndrome?
- Vascular endothelial growth factor (VEGF)-C and its receptors, soluble VEGFR-2 and VEGFR-3, in polycystic ovary syndrome
- Platelet activating factor acetylhydrolase is associated with cardiac valvular calcification in dialysis patients
- Reticulated platelets and coronary slow flow: a study in stable coronary artery disease
- Identification of the role of TG2 on the expression of TGF-β, TIMP-1 and TIMP-2 in aged skin
- Unveiling the link: Helicobacter pylori infection and impact on ischemia modified albumin, thiol, and disulfide levels

