Abstract
Background
Paracetamol is one of the widely used antipyretic and analgesic drug around the world. Many researchers showed that paracetamol caused to hepatotoxicity or nephrotoxicity.
Objective
In the present study, we aimed to determine whether betaine has protective effects on hepatotoxicity and nephrotoxicity in neonate rats, following to long term maternal paracetamol exposure.
Materials and methods
Randomly chosen neonates, from the neonate pools, were divided into three groups; Control (n=13), APAP (n=13), and APAP+Betaine (n=13). Physiological saline, paracetamol (30 mg/kg/day), and paracetamol (30 mg/kg/day)+betaine (800 mg/kg/day) were orally administered to the relevant groups during the pregnancy period (approximately 21 day). Following to the birth, neonates were decapitated under anaesthesia and tissue samples were taken for biochemical and histological analyses.
Results
The statistical analysis showed that, malondialdehyde and nitric oxide levels increase significantly in APAP group, while paraoxonase, arylesterase activity and glutathione levels decrease. After the betaine administration, glutathione levels, paraoxonase and arylesterase activities increased while malondialdehyde and nitric oxide levels decreased in APAP+betaine group. These biochemical findings also were supported by histological results.
Conclusion
In this study, our biochemical and histological findings indicate that betaine can protect the tissue injury caused by paracetamol.
Öz
Amaç
Parasetamol, dünyada yaygın olarak kullanılan antipiretik ve analjezik ilaçlardan biridir. Birçok araştırmacı parasetamolün hepatotoksisiteye veya nefrotoksisiteye neden olduğunu göstermiştir. Bu çalışmada, uzun süreli maternal parasetamol maruziyetini takiben, betainin yenidoğan sıçanlarda hepatotoksisite ve nefrotoksisite üzerinde koruyucu etkileri olup olmadığını belirlemeyi amaçladık.
Materyal ve Metot
Yenidoğan havuzlarından rastgele seçilen yenidoğanlar üç gruba ayrıldı; Kontrol (n=13), APAP (n=13) ve APAP+Betain (n=13). Fizyolojik salin, parasetamol (30 mg/kg/gün) ve parasetamol (30 mg/kg/gün)+betain (800 mg/kg/gün) hamilelik döneminde (yaklaşık 21 gün) ilgili gruplara oral yoldan uygulandı. Doğumdan sonra yenidoğanlar anestezi altında kesildi ve biyokimyasal ve histolojik analizler için doku örnekleri alındı.
Bulgular
İstatistiksel analiz APAP grubunda malondialdehit ve nitrik oksit seviyelerinin önemli ölçüde arttığını, paraoksonaz, arilesteraz aktivitesi ve glutatyon seviyelerinin azaldığını göstermiştir. Betain uygulamasından sonra APAP+betain grubunda glutatyon seviyeleri, paraoksonaz ve arilesteraz aktiviteleri artarken, malondialdehit ve nitrik oksit seviyeleri azaldı. Bu biyokimyasal bulgular histolojik sonuçlarla da desteklenmiştir.
Sonuç
Bu çalışmada biyokimyasal ve histolojik bulgularımız betainin parasetamolün neden olduğu doku hasarını koruyabildiğini göstermektedir.
Introduction
Paracetamol (APAP; acetaminophen) is one of the non-opiate analgesics and the main antipyretic or analgesic taken by the pregnant women for mild-to-moderate pain and fever [1]. It is known to cross the placenta freely, and thus its long-term use during pregnancy might cause neonatal side effects which results in liver or kidney injuries, because its large amounts of harmful metabolites [2], [3].
Paracetamol is transformed into its metabolites such as n-acetyl-p-benzoquinoneimine (NAPQI) by the CYP450 enzyme system in the liver [4], [5] and excreted by the urinary system. NAPQI is a highly electrophilic molecule and particularly attacks to the sulfhydryl-containing structures, and therefore it binds to cellular reduced glutathione (GSH) immediately and is detoxified by the clearance pathways [6]. As a result of its binding feature, cellular GSH levels start to decline and its tendency to bind sulfhydryl-containing proteins increases. Free NAPQI molecules bind to nitric oxide (NO) to produce peroxynitrite radicals (ONOO−) in the mitochondrial matrix. Peroxynitrite radicals increase in the mitochondria and disrupt its membrane permeability [7]. NAPQI also cause to cell damage by the formation of reactive oxygen species such as superoxide, hydrogen peroxide and hydroxyl radicals which react with cellular lipids and lead to the lipid peroxidation (LPO) [8].
Betaine, has electrophilic methyl groups and attends to many trans-methylation reactions as a methyl donor [9]. The reaction catalysed by betaine homocysteine methyl transferase (BHMT) has a central role on the homocysteine-methionine cycle; because the antioxidant GSH molecule is produced depend on this pathway [10].
PON (paraoxonase) is a calcium-dependent serum esterase and a member of a multigene family with at least three types such as PON1, PON2 and PON3 [11]. Amino acid sequences of these three PON isoenzymes show high level of similarity among the mammalian species. It is showed that PON2 and PON3 have antioxidant activity similarly like PON1 [12]. Although these isoenzymes of PON are expressed in all tissues; PON1 and PON3 are mainly expressed in the liver, while PON2 is primarily expressed in the brain, liver kidney, and testis. As a single enzyme, PON1 shows three different activity: paraoxonase, arylesterase and dyazoxonas activities. PON family are mainly responsible for hydrolyses of organophosphates such as, paraoxon, aromatic esters and phenyl acetate [13].
In the light of this information, we aimed to investigate the potential protective effect of betaine against prenatal exposure of paracetamol in the neonatal rat.
Materials and methods
Animals
Twelve female Sprague-Dawley rats weighing 200–250 g were obtained from TICAM (Medical and Surgical Experimental Research Center, Eskişehir). Rats were acclimatized to the laboratory conditions for 1-week prior to the experiments. All of the experiments involved in the study were carried out according to the Animal Experiments Local Ethics Committee of Eskişehir Osmangazi University (Approval Date: 02/09/2014; Protocol Number: 410). Female rats were divided randomly into the three groups and caged with male rats (four females and one male per cage).
Chemicals
The chemicals which were used in this study are as follows: Betaine Hydrochloride (cat. no. 164552500) was purchased from Acros Organics (USA). Cadmium (cat. no. 20890) was purchased from Fluka (Germany). Coomassie Brilliant Blue G-250 (cat. no. 42673616) was obtained from Molekula (USA). Acetic acid (CH3COOH, cat. no. 27225), Sodium dihydrogen phosphate (NaH2PO4, cat. no. 04269), Sulphuric acid (H2SO4, cat. no. 07208) were purchased from Riedel-de Haën (Germany). Potassium chloride (KCl, cat. no. 104935), Sodium hydroxide (NaOH, cat. no. 106462), Metaphosphoric acid (cat. no. 100546), Sodium chloride (NaCl, cat. no. 106400), Disodium hydrogen phosphate (Na2HPO4, 106574), Sodium citrate (cat. no. A228431), Zinc sulphate (cat. no. 2450472), Copper sulphate (cat. no. A278687), N-(1-naphtyl)-ethylendiamine (cat. no. 106237), Sodium nitrite (NaNO2, cat. no.106549) were obtained from Merck (Germany). All other chemicals were purchased from Sigma-Aldrich (USA).
Determination of pregnancy and study groups
Vaginal smear was used as a positive indication of pregnancy and was taken daily between 8:00 and 09:00 a.m. via lavage using physiological saline (0.9% NaCl). The day after sperm detection in the vaginal smear was designed as day one of pregnancy.
Pregnant rats were divided into three groups: control group (n=4), APAP group (n=4) and APAP+betaine group (n=4). Two millilitre saline solution, 30 mg/kg paracetamol [14] and 30 mg/kg paracetamol+800 mg/kg betaine [15] were orally administered to the related groups each morning (from first day until the last day of pregnancy) as a single dose during the pregnancy, respectively. Paracetamol and betaine were prepared daily by dissolving in 2 mL saline solution. Following delivery (approximately on 21st day of the pregnancy for all the pregnant rats), neonates from the three pregnant rat groups were used to constitute of three neonate-pools.
Randomly chosen rats from each neonate-pools were divided into three neonate study-groups: control group (n=13), APAP group (n=13) and APAP+betaine group (n=13). In the 24 h after birth, all neonates were decapitated under the anaesthesia. Their kidney and liver tissues were collected immediately in order to realize the biochemical (n=8, neonates) and the histological (n=5, neonates) analysis.
Measurement of malondialdehyde, nitric oxide and glutathione levels in tissue samples
As an important indicator of lipid peroxidation, malondialdehyde (MDA) levels of kidney and liver tissues were measured by the thiobarbituric acid (TBA) method as previously described [16]. According to the method, the tissue samples were homogenized in ice-cold 1.15% wt/vol KCl (9 mL KCl per 1 g of wet tissue) with an Ultra-Turrax T25 (Janke&Kunkel, Staufen, Germany), and the homogenates were centrifuged at 1000×g (JOUAN MR 22, rotor AM 10.17, Thermo Fisher, Waltham, MA, USA) for 10 min at 4°C. To 0.4 mL of supernatants were added 0.2 mL of 8.1% SDS, 1.5 mL of 20% acetate buffer pH 3.5 adjusted with NaOH, 1.5 mL of 0.8% solution of freshly prepared TBA solution and distilled water to adjust the volume to 4 mL. The mixtures were incubated in boiling water for 60 min and centrifuged at 1000 g for 10 min. The absorbance of the purple reaction-product in supernatants was measured photometrically at 532 nm. Results were expressed as nmol/mg protein by using the standard curve generated with 1,1,3,3-Tetraethoxypropane.
The measurement of GSH content in tissue samples was based on the 5,5′-Dithiobis (2-nitrobenzoic acid)-thiol (DTNB-thiol) reaction as described by Beutler et al. [17]. Tissue samples were homogenized in ice-cold 0.1 M phosphate buffer at pH 7.4, in a ratio of 1 g wet tissue to 9 mL buffer. The homogenates were centrifuged at 4000×g for 10 min at 4°C. After the centrifugation, to the supernatants (0.5 mL) were added 0.75 mL of precipitate solution (1.67 g of glacial meta-phosphoric acid, 0.2 g of EDTA and 30 g of NaCl in 100 mL of distilled water), and then, the mixtures were centrifuged at 4000×g for 15 min at 4°C to precipitate of proteins. To the protein-free supernatants (0.5 mL) were added 2 mL of 0.3 M Na2HPO4 solution and 1.5 mL of 6 mM DTNB solution (dissolved in 100 mL of 1% wt/vol sodium citrate) and incubated for 5 min at room temperature in the dark. Absorbance was measured by UV/vis spectrophotometer (Shimadzu, Model UV-1601, Tokyo, Japan) at 412 nm. The tissue GSH levels were calculated by the GSH standard curve and expressed as μmol/mg protein.
Nitric oxide assay was performed according to a previously described method [18], which is based on the cadmium-dependent reduction of nitrate (NO3−) to nitrite (NO2−). Briefly, the tissue samples were first homogenized in the cold PBS (9 mL PBS per 1 g wet tissue). The homogenates were centrifuged at 1370 g for 10 min and to the supernatants (0.125 mL) were added 0.5 mL of 5 mM ZnSO4 and 0.625 mL of 55 mM NaOH solutions for deproteinization of proteins. Ten minutes after, the mixtures were centrifuged at 1370 g for 10 min. After the Cd activation according to the assay method, to the supernatants (0.25 mL) were added 0.25 mL of 0.2 M glycine, 0.5 mL distilled water and the activated cadmium. After an incubation period for 90 min at room temperature, 0.5 mL this mixture was added 0.125 mL distilled water, 0.25 mL of 58 mM sulphanilamide and 0.25 mL of 0.77 M N-(1-naphtyl)-ethylendiamine solution. After 45 min of the incubation, absorbance was measured spectrophotometrically at 545 nm. The tissue nitrite levels were calculated by using NaNO2 standard curve and expressed as μmol/mg protein.
Paraoxonase 1 and arylesterase activities in tissue samples
Paraoxon is a synthetic substrate hydrolysed by PON1 to p-nitrophenol. PON1 activity measurement is based on the absorbance change depend on p-nitrophenol consumption during the reaction [19]. Tissue samples were homogenized in ice-cold 50 mM Tris-HCl buffer at pH 8.0 (including final 2 mM CaCl2 and 1 M NaCl), in a ratio of 1 g wet tissue to 9 mL buffer. The homogenates were centrifuged at 10,000 g for 15 min at 4°C. After the centrifugation, to the supernatants (50 μL for liver tissue and 150 μL for kidney tissue) were added 950 μL of 100 mM Tris-HCl buffer and 235 μL of 10 μM paraoxon. Absorbance change in the mixture was followed at 405 nm for 5 min spectrophotometrically. Results were expressed as U/mg protein.
Measurement of arylesterase (ARE) activity is based on the phenyl acetate hydrolysation which catalysed by arylesterase [19]. Tissue samples were homogenized in ice-cold 50 mM Tris-HCl buffer at pH 8.0 (including final 2 mM CaCl2), in a ratio of 1 g wet tissue to 9 mL buffer. After the centrifugation at 10,000 g for 15 min at 4°C, 1 mL of 5 mM phenyl acetate was added to the supernatants (50 μL for liver tissue and 150 μL for kidney tissue) for reaction mixture. Changes in absorbance were followed at 270 nm for 3 min spectrophotometrically and results were expressed as U/mg protein.
Determination of protein concentration
Protein concentrations of the kidney and liver tissue samples were determined according to the Bradford method using bovine serum albumin as a standard [20].
Histological analyses
Kidney and liver tissues from neonates were processed in 10% formalin solution, exposed to graded ethyl-alcohols, and embedded in paraffin. The paraffin blocks were sliced with 5 μm thickness, and stained with haematoxylin and eosin (H&E). The histological assessments were performed using a light microscope (NIKON, Japan).
Statistical analysis
Data were analysed using SPSS 22.0 version for Windows (IBM Corp., USA) and presented as mean±standard deviation (SD). The Shapiro-Wilk test was used to decide to normal distribution, and One-Way ANOVA test was used to reveal the statistical significance among the groups. Significance level was p<0.05.
Results
Results of biochemical analyses
The results of PON1 and ARE activity, MDA, GSH and NO levels in neonate liver were summarized in Table 1. While MDA and NO levels increased, PON1, ARE activities and GSH levels decreased in paracetamol group compared to control (p<0.05). PON1 activity of APAP+betaine group was higher than paracetamol group (p<0.05). On the other hand, GSH levels and ARE activities declined in APAP group compared to control. Results of MDA, GSH, NO levels and PON1 and ARE activities indicated that there was no difference between control and APAP+betaine groups.
MDA, GSH, NO levels and PON1, ARE activities in neonate liver (SD, standard deviation).
| Groups | Liver MDA levels (nmol/mg protein±SD) | Liver GSH levels (μmol/mg protein±SD) | Liver NO levels (μmol/mg protein±SD) | Liver PON1 activity (U/mg protein±SD) | Liver ARE activity (U/mg protein±SD) |
|---|---|---|---|---|---|
| Control | 49.78±19.37 | 0.78±0.11 | 1.62±0.30 | 26.29±2.48 | 10.79±3.36 |
| APAP | 88.52±27.51a | 0.67±0.13 | 1.99±0.23a | 18.93±2.93a | 8.36±3.53 |
| APAP+betaine | 61.11±21.42 | 0.73±0.09 | 1.60±0.25 | 23.62±4.35b | 12.60±3.12 |
APAP, Paracetamol; GSH, reduced glutathione; NO, nitric oxide; PON1, paraoxonase 1; ARE, arylesterase. aSignificantly decrease or increase compared to control group (p<0.05). bSignificantly decrease or increase compared to paracetamol group (p<0.05).
Table 2 shows PON1 and ARE activity, MDA, GSH and NO levels in kidney tissues. MDA levels in APAP and APAP+betaine group were higher than control group (p<0.05). NO levels increased in APAP+betaine group compared to APAP group, whereas there were no differences between control and APAP+betaine group (p<0.05). PON1 activity declined in APAP group compared to control group, betaine increased PON1 activity close to control group (p<0.05). Similar to the results of liver tissues, GSH levels and ARE activities declined compared to control group.
MDA, GSH, NO levels and PON1, ARE activities in neonate kidneys (SD, standard deviation).
| Groups | Kidney MDA levels (nmol/mg protein±SD) | Kidney GSH levels (μmol/mg protein±SD) | Kidney NO levels (μmol/mg protein±SD) | Kidney PON1 activity (U/mg protein±SD) | Kidney ARE activity (U/mg protein±SD) |
|---|---|---|---|---|---|
| Control | 27.76±5.14 | 0.30±0.10 | 1.91±0.04 | 9.93±2.29 | 35.36±5.63 |
| APAP | 40.81±9.79a | 0.23±0.10 | 2.06±0.22 | 6.48±2.60a | 30.98±4.91 |
| APAP+betaine | 38.65±8.01a | 0.28±0.05 | 1.84±0.07b | 9.12±0.98b | 35.90±6.78 |
APAP, Paracetamol; GSH, reduced glutathione; NO, nitric oxide; PON1, paraoxonase 1; ARE, arylesterase. aSignificantly decrease or increase compared to control group (p<0.05). bSignificantly decrease or increase compared to paracetamol group (p<0.05).
Results of histological analyses
Figure 1A shows normal cell structure of hepatocytes and sinusoids in neonate’s liver. There were intensive cellular degenerations, congestions in central vein and infiltrations at parenchymal tissue of paracetamol group, in liver histology (Figure 1B and C). After the betaine administration, cellular degeneration and infiltration decreased and the liver structure was seen as normal (Figure 1D).
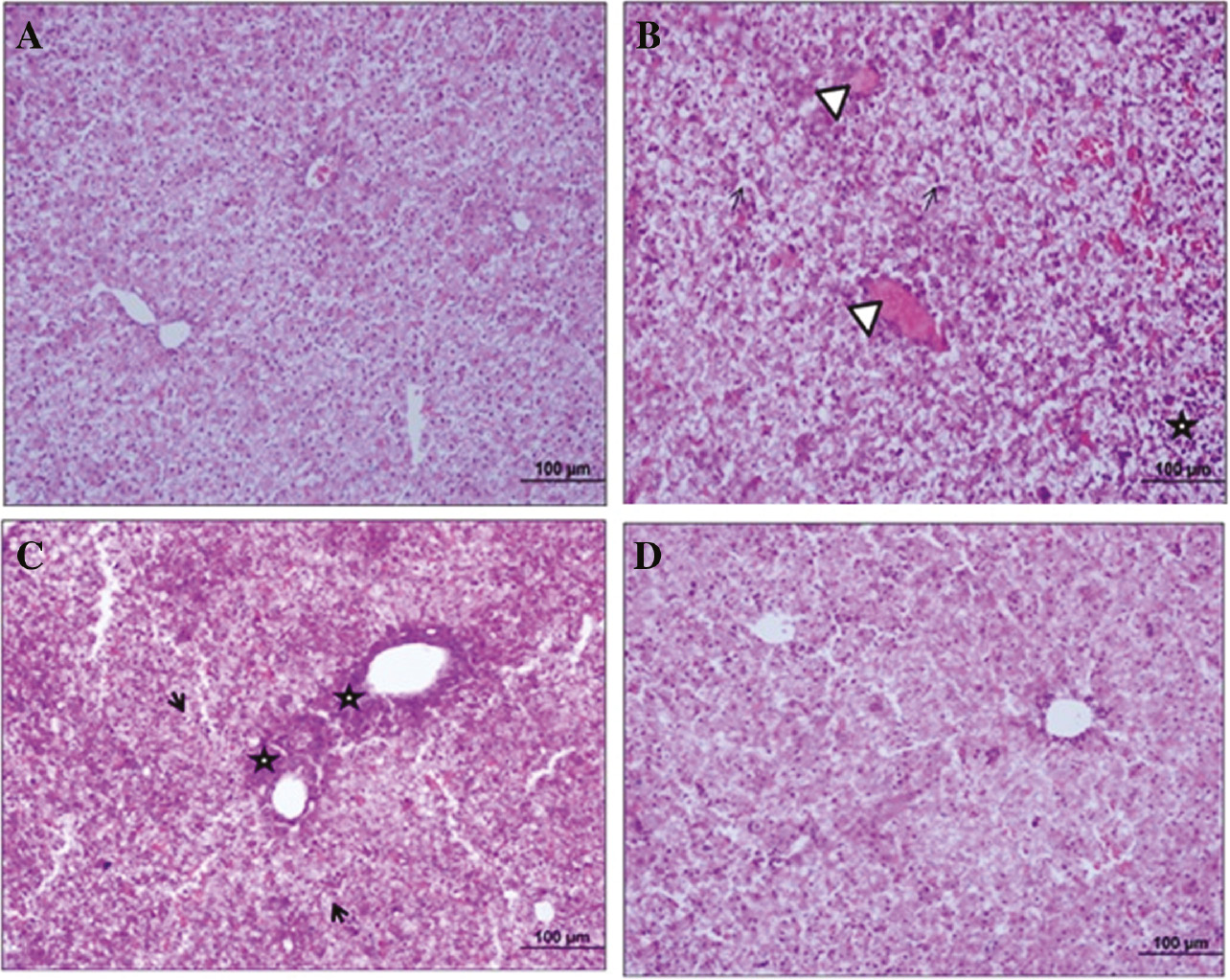
Images of histological analysis of neonate rat’s liver tissue.
This sections shows control (A), APAP (B and C) and APAP+betaine (D) groups histology of neonate’s liver (H&E, scale bar: 100 μm, ×20). →, Shows intensive degeneration areas; Δ, congestion in central vein; *, cellular infiltration in perivascular and parenchymal areas.
Figure 2A shows normal glomerular structure of Malpighian corpuscle and renal tubules (Figure 2A). Necrotic tubular structure, contraction in Bowman capsule and interstitial fibrosis are seen in kidney of APAP group (Figure 2B–D). Figure 2E and F shows close to normal appearance of Malpighian corpuscle and tubular damage decreased in APAP+betaine group.
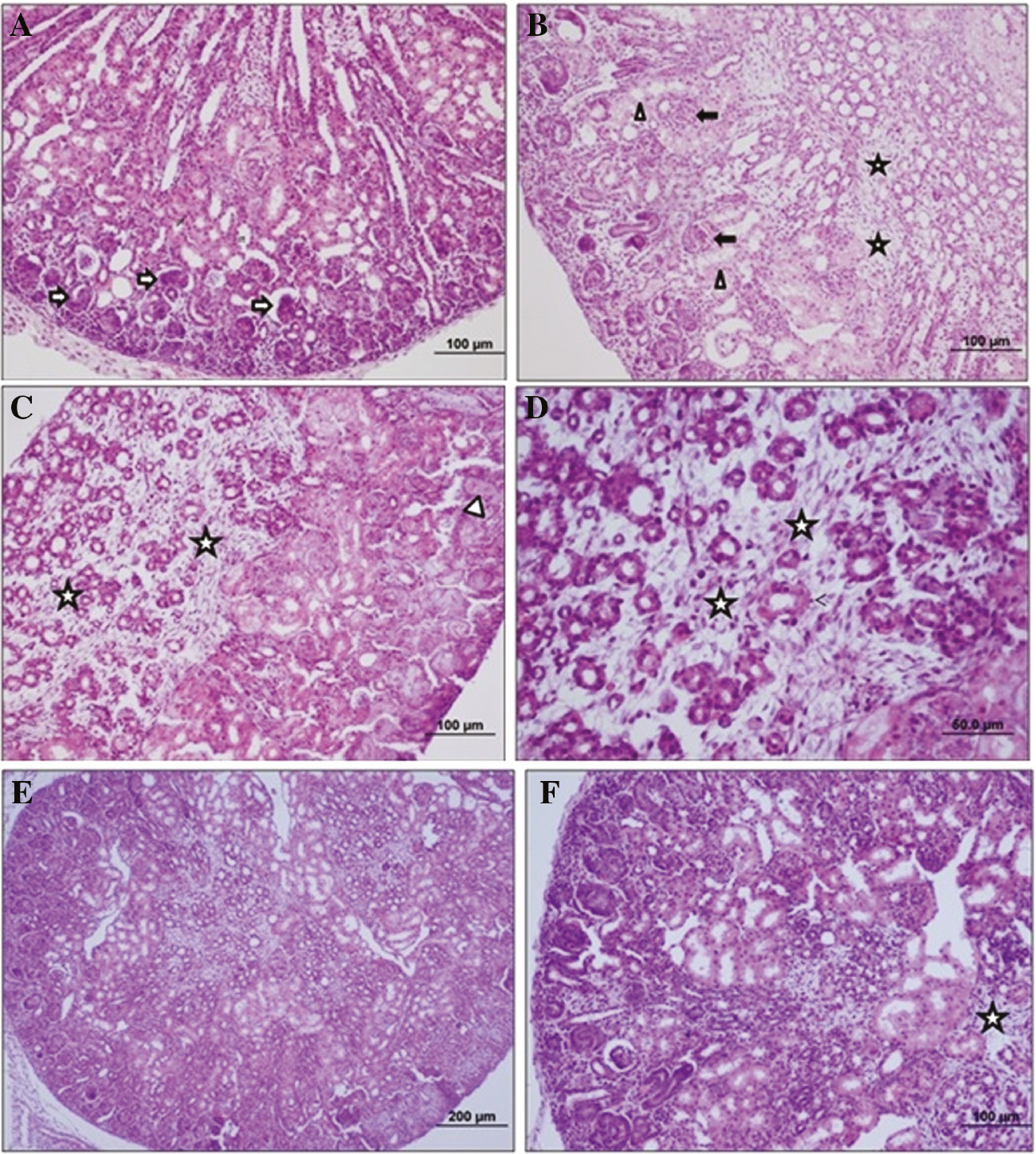
Images of histological analysis of neonate rat’s kidney tissue.
This kidney tissue sections shows control (A), APAP (B–D) and APAP+betaine (E and F) groups histology of neonates (H&E, scale bar: 60, 100, 200 μm, ×20). →, Shows narrowing in the range of Bowman; Δ, necrotic tubular structure; *, interstitial fibrosis.
Discussion
Paracetamol is widely used and recommended drug for mild pains in pregnancy. The blood clearance of paracetamol is lower in neonates compared to children or adults. Paracetamol is conjugated with sulphate or glucuronic acid in the liver and discarded via the urinary system [14], and a small amount of paracetamol transforms to its toxic metabolite, NAPQI [4].
In a study [21], which performed to investigate the paracetamol-caused oxidative kidney damage, 750 mg/kg paracetamol was orally administered to rats for seven consecutive days. According to the results of the study, MDA levels increased and GSH levels decreased in the paracetamol group compared to the control group. Another study showed that a single dose of paracetamol (2 g/kg) caused to a rise on lipid peroxidation and decreased by 66% of GSH stores in the liver cells [22]. To examine the effects of paracetamol on hepatotoxicity and nephrotoxicity, Naguib et al. [23] administered 500 mg/kg dose of paracetamol to mice intraperitoneally. According to the study, there was a significant increase in MDA and decrease in GSH levels in the liver and kidney tissues, in addition to inhibition of the antioxidant enzymes such as glutathione peroxidase and superoxide dismutase [23].
Zaher et al. [24] examined the toxic effects of paracetamol in the liver and kidney tissues of rats. In the same study, the researchers revealed that paracetamol caused to damage of centrilobular areas in the liver which is resulted in induction of the NO synthesis due to increase iNOS expression [24]. Similarly, another study showed that a single dose of 250 mg/kg paracetamol administration resulted with rise on NO level of liver tissues due to the increased iNOS expression in rabbits [25].
There have not been found any study on paracetamol-induced hepatotoxicity or nephrotoxicity, related with PON1 and ARE activity in rats. However, in a human study on paracetamol intoxication, it was detected a decrease serum PON and ARE activities because of their antioxidant activity [26]. In a hepatotoxicity rat model induced by carbon tetrachloride (CCl4), it was demonstrated that diminish of PON1 activity in CCl4 group as a result of the ROS reaction with sulfhydryl groups of the PON [27].
Betaine-homocysteine methyltransferase (BHMT), which is expressed especially in liver and kidney, remethylated homocysteine to methionine by using betaine as a methyl donor [28]. GSH is another important molecule that is produced depends on this pathway, and is a significant endogenous antioxidant molecule [10]. GSH not only react with ROS but also conjugate with different drugs or their metabolites in hepatocytes. Betaine is a lipotropic molecule that can pass freely through the cell membrane and protects the cells against necrotic damage [29].
Protective effect of betaine was examined on alcohol induced hepatotoxicity by Kanbak et al. [30]. In the study, researchers showed that MDA levels dropped following to betaine administration roughly to the control group levels [30]. In another study on diethyl nitrosamine-induced hepatocyte injury, researchers revealed that MDA levels increased in diethyl nitrosamine-treated rats while decreased in betaine-treated. According to the researchers, betaine had no effect on antioxidant enzyme activities but caused to increase of s-adenosyl methionine (SAM) levels which reduces the membrane damage and the lipid peroxidation in hepatocytes [31].
In a cisplatin-induced nephrotoxicity study to investigate the protective effects of betaine in rats, betaine applied by gavage for 21 days [32]. Thiobarbituric acid reactive substances (TBARs), GSH and NO levels were measured in the kidney tissue samples, and the results showed that betaine administration improved the NO levels which were increased by cisplatin. In the same study, researchers emphasized that betaine protects the cells via its antioxidant properties and roles in the GSH synthesis, to maintain the stability of antioxidant/oxidant balance positively.
In sodium selenite-induced hepatotoxicity in rats, it was found that PON1 activity was lower in sodium selenite-treated rats than betaine-treated [33]. The view of author was that selenium interacts with thiol groups of the PON1 and decreases the enzyme activity, and betaine administration as a precursor of GSH synthesis protected the cells from oxidative stress by its antioxidant activity.
In our study, we found that MDA levels of the liver tissue increased significantly and decreased following to the betaine administration in the APAP group (p<0.05). Although there was a decline in GSH levels in the APAP group (14% in liver and 23% in kidney), the decline was not statistically significant. We have several views related with our findings of GSH levels: (a) this result could be depended on the high standard deviations. (b) In contrast to the many studies used toxic and single dose of paracetamol, we examined long term effect paracetamol (30 mg/kg) in neonates. Our selected dose of paracetamol may have no effect on the GSH levels. (c) It has been shown that the majority of hepatic GSH levels were recovered in first 24 h in paracetamol administered groups [34]. Another important reason also can be the regeneration of hepatic GSH levels, since we performed paracetamol every 24 h during pregnancy.
We showed that the NO levels had a significant increase in APAP groups, and the betaine administration caused to decrease in the liver or kidney tissues of neonates. In our study, NO levels were high in paracetamol treated rats, similarly to the study which was reported by Ghosh et al. [35]. In our study, the major opinion about the high MDA levels and cellular injuries are related with the increased NO levels.
In our study, both the PON1 and the ARE activities dropped in the APAP group whereas they increased in betaine administered group. Currently, PON1 and ARE are presenting as antioxidant enzymes. Thus, in this study PON1 and ARE contributed to protect the cells by their antioxidant properties against to oxidative stress formed by NAPQI, which attacks to the thiol groups of PON1 and ARE, and caused to the reduction on the enzyme activities, as described above. High NO level is another factor which cause to decrease of PON1 and ARE activities and the antioxidant capacity.
Betaine provided improvement in MDA levels, NO levels, PON1 and ARE activities. Betaine has electrophilic methyl groups and antioxidant feature in addition to attend to the GSH synthesis via trans-methylation and trans-sulphuration reactions. Amiraslani et al. showed that betaine inhibits the NO production in microglia cells due to the inhibition of iNOS [36]. In our study we revealed that NO level increase in the APAP groups. In our study the dominant view is that the high NO level causes to the negative effects on MDA levels, PON1 and ARE activities. At this point, NO has been a significant molecule to illuminate all related pathways about the injury of kidney and liver tissues. In the light of this information, we understand that betaine protects to the cell damage by scavenging the free radicals via its electrophilic property, and by the inhibiting the NO synthesis.
Our histological results supported our biochemical parameters. In the liver parenchyma tissue of APAP group showed intense cellular degeneration, congestion in the central vein and intensive cellular infiltration in the perivascular areas and parenchymal tissue compared to control group. On the other hand, APAP+betaine group had decreased cellular damage, decreased infiltration and near normal liver structure compared to APAP group. In the kidney; In the APAP group Bowman range narrowing, necrotic tubular structures and interstitial fibrosis were observed compared to the control group; In the APAP+betaine group, near-normal Malpigian bodies, decreased tubular damage and decreased interstitial fibrosis areas were observed. These results show that betaine might have both hepatoprotective and nephroprotective effects which also supporting by biochemical analyses.
In conclusion, we showed that paracetamol cause to hepatotoxicity and nephrotoxicity on fetuses; when it is used during the whole pregnancy period without giving any break in a rat dose of 30 mg/kg. The results were supported by biochemical and histological analyses. In addition, we suggest that betaine might be protective agent against to APAP hepato- and nephrotoxicity of fetuses. There are not adequate studies in literature about the effects of long term maternal paracetamol administration in neonates. It is obvious that much more in vitro and in vivo researches are necessary to illuminate the molecular mechanisms related with pregnancy or neonates.
Acknowledgements
I am very thankful to my supervisor, Prof. Dr. Güngör Kanbak, as he shared his pearls of wisdom during my thesis study. I would also like to show my gratitude to Betül Can for her all support on me in all the process. I am also immensely grateful to Hadi Karimkhani and Dilek Burukoğlu Dönmez for their support during animal and laboratory studies. This study, which was also my master thesis, was presented in 41. The FEBS Congress as a poster.
References
1. Bertolini A, Ferrari A, Ottani A, Guerzoni S, Tacchi R, LeoneS. Paracetamol: new vistas of an old drug. CNS Drug Rev 2006;12:250–75.10.1111/j.1527-3458.2006.00250.xSearch in Google Scholar PubMed PubMed Central
2. Blecharz-Klin K, Joniec-Maciejak I, Jawna K, PyrzanowskaJ, Piechal A, Wawer A, et al. Effect of prenatal and early life paracetamol exposure on the level of neurotransmitters in rats – focus on the spinal cord. Int J Dev Neurosci 2015;47:133–9.10.1016/j.ijdevneu.2015.09.002Search in Google Scholar PubMed
3. Dean A, Driesche SV, Wang Y, McKinnell C, Macpherson S, EddieSL, et al. Analgesic exposure in pregnant rats affects fetal germ cell development with inter-generational reproductive consequences. Sci Rep 2016;6:1–12.10.1038/srep19789Search in Google Scholar PubMed PubMed Central
4. Pacifici GM, Allegaert K. Clinical pharmacology of paracetamol in neonates: a review. Curr Ther Res 2015;77:24–30.10.1016/j.curtheres.2014.12.001Search in Google Scholar PubMed PubMed Central
5. Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol 1980;10:291–8.10.1111/j.1365-2125.1980.tb01812.xSearch in Google Scholar PubMed PubMed Central
6. Graham GG, Scott KF, Day RO. Tolerability of paracetamol. Drug Safety 2005;28:227–40.10.2165/00002018-200528030-00004Search in Google Scholar PubMed
7. Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity – a clinically relevant model to test the efficacy of natural products. Life Sci 2011;88:737–45.10.1016/j.lfs.2011.01.025Search in Google Scholar PubMed PubMed Central
8. James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Am Soc Pharmacol Exp Ther 2003;31:499–1506.10.1124/dmd.31.12.1499Search in Google Scholar PubMed
9. McCue KF, Hanson AD. Salt-inducible betaine aldehyde dehydrogenase from sugar beet: cDNA cloning and expression. Plant Mol Biol 1991;18:1–11.10.1007/BF00018451Search in Google Scholar PubMed
10. Liu HH, Lu P, Guo Y, Farrel E, Zhang X, Zheng M, et al. An integrative genomic analysis identifies Bhmt2 as a diet-dependent genetic factor protecting against acetaminophen-induced liver toxicity. Genome Res 2010;20:28–35.10.1101/gr.097212.109Search in Google Scholar PubMed PubMed Central
11. Labrecque B, Beaudry D, Mayhue M, Halle C, Bordignon V, Murphy BD, et al. Molecular characterization and expression analysis of the porcine. Gene 2009;443:110–20.10.1016/j.gene.2009.04.026Search in Google Scholar
12. Rodrigo L, Gil F, Hernandez AF, Lopez O, Pla A. Identification of paraoxonase 3 in rat liver microsomes: purification and biochemical properties. Biochem J 2003;376:261–8.10.1042/bj20030732Search in Google Scholar
13. Kartkaya K, Oğlakçı A, Şentürk H, Bayramoğlu G, Canbek M, Kanbak G. Investigation of possible protective role of gallic acid on paraoxanase and arylesterase activities in levers of rats with acute alcohol intoxication. Cell Biochem Funct 2012;31:208–13.10.1002/cbf.2874Search in Google Scholar
14. Scialli AR, Ang R, Breitmeyer J, Royal MA. A review of the literature on the effects of acetaminophen on pregnancy outcome. Reprod Toxicol 2010;30:495–507.10.1016/j.reprotox.2010.07.007Search in Google Scholar
15. Kitamura H, Yamauchi A, Sugiura T, Matsuoka Y, Horio M, Tohyama M, et al. Inhibition of myo-inositol transport causes acute renal failure with selective medullary injuryin the rat. Kidney Int 1998;53:146–53.10.1046/j.1523-1755.1998.00747.xSearch in Google Scholar
16. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351–8.10.1016/0003-2697(79)90738-3Search in Google Scholar
17. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:883–90.Search in Google Scholar
18. Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem 1990;36:1440–3.10.1093/clinchem/36.8.1440Search in Google Scholar
19. Haagen L, Brok A. A new automated method for phenotyping arylesterase (EC 3.1.1.2) based upon inhibition of enzymatic hydrolysis of 4-nitrophenyl acetate by phenyl acetate. Eur J Clin Chem Clin Biochem 1992;30:391–5.10.1515/cclm.1992.30.7.391Search in Google Scholar
20. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54.10.1016/0003-2697(76)90527-3Search in Google Scholar
21. Hamid ZA, Budin SB, Jie NW, Hamid A, Husain K, MohamedJ. Nephroprotective effects of Zingiber zerumbet Smith ethyl acetate extract against paracetamol-induced nephrotoxicity and oxidative stress in rats. J Zhejiang Univ Sci B 2012;13:176–85.10.1631/jzus.B1100133Search in Google Scholar PubMed PubMed Central
22. Galal RM, Zaki HF, El-Nasr MM, Agha AM. Potential protective effect of honey against paracetamol-induced hepatotoxicity. Arch Iran Med 2012;15:674–80.Search in Google Scholar
23. Naguib YM, Azmy RM, Samaka RM, Salem MF. Pleurotus ostreatus opposes mitochondrial dysfunction and oxidative stress in acetaminophen-induced hepato-renal injury. BMC Complement Altern Med 2014;14:494.10.1186/1472-6882-14-494Search in Google Scholar
24. Zaher AO, Rahman MM, Hafez MM, Omran FM. Role of nitric oxide and reduced glutathione in the protective effects of aminoguanidine, gadolinium chloride and oleanolic acid against acetaminophen-induced hepatic and renal damage. Toxicology 2007;234:124–34.10.1016/j.tox.2007.02.014Search in Google Scholar
25. Cigremis Y, Turel H, Adiguzel K, Akgoz M, Kart A, Karaman M, et al. The effects of acute acetaminophen toxicity on hepatic mRNA expression of SOD, CAT, GSH-Px, and levels of peroxynitrite, nitric oxide, reduced glutathione, and malondialdehyde in rabbit. Mol Cell Biochem 2009;323:31–8.10.1007/s11010-008-9961-8Search in Google Scholar
26. Karadas S, Aslan M, Gonullu H, Kati C, Duran L, Olmez S, et al. Acetaminophen intoxication is associated with decreased serum paraoxonase and arylesterase activities and increased lipid hydroperoxide levels. Hum Exp Toxicol 2014;33:1134–40.10.1177/0960327113511477Search in Google Scholar
27. Hafez MM, Al-Shabanah OA, Al-Harbi NO, Al-Harbi MM, Al-Rejaie SS, Alsurayea SM, et al. Association between paraoxonases gene expression and oxidative stress in hepatotoxicity induced by CCl4. Oxid Med Cell Longev 2014;2014:893212.10.1155/2014/893212Search in Google Scholar
28. Schwahn BC, Laryea MD, Chen Z, Melnyk S, Pogribny I, Garrow T, et al. Betaine rescue of an animal model with methylenetetrahydrofolate reductase deficiency. Biochem J 2004;382:831–40.10.1042/BJ20030822Search in Google Scholar
29. Kim SK, Kim YC. Effects of singly administered betaine on hepatotoxicity of chloroform in mice. Food Chem Toxicol 1998;36:655–61.10.1016/S0278-6915(98)00024-6Search in Google Scholar
30. Kanbak G, İnal M, Bayçu C. Ethanol-induced hepatoxicity and protective effect of betaine. Cell Biochem Funct 2001;19:281–5.10.1002/cbf.926Search in Google Scholar PubMed
31. Küçükgergin CB, Bingül İ, Tekkeşin MS, Olgaç V, Doğru-Abbasoğlu S, Uysal M. Effects of carnosine, taurine, and betaine pretreatments on diethylnitrosamine-induced oxidative stress and tissue injury in rat liver. Toxicol Ind Health 2016;32:1405–13.10.1177/0748233714563432Search in Google Scholar PubMed
32. Hagar H, El Medany A, Salam R, El Medany G, Nayal OA. Betaine supplementation mitigates cisplatin-induced nephrotoxicity by abrogation of oxidative/nitrosative stress and suppression of inflammation and apoptosis in rats. Exp Toxicol Pathol 2015;67:133–41.10.1016/j.etp.2014.11.001Search in Google Scholar PubMed
33. Harisa GI. Oxidative stress and paraoxonase activity in experimental selenosis: effects of betaine administration. Biol Trace Elem Res 2013;152:258–66.10.1007/s12011-013-9618-7Search in Google Scholar PubMed
34. Ben-Shachar R, Chen Y, Luo S, Hartman C, Reed M, Nijhout HF. The Biochemistry of acetaminophen hepatotoxicty and rescue: a mathematical model. Theor Biol Med Model 2012;9:1–22.10.1186/1742-4682-9-55Search in Google Scholar PubMed PubMed Central
35. Ghosh J, Das J, Manna P, Sil PC. Acetaminophen induced renal injury via oxidative stress and TNF-production: therapeutic potential of arjunolic acid. Toxicology 2010;268:8–18.10.1016/j.tox.2009.11.011Search in Google Scholar PubMed
36. Amiraslani B, Sabouni F, Abbasi S, Nazem H, Sabet M. Recognition of betaine as an inhibitor of lipopolysaccharide-induced nitric oxide production in activated microglial cells. Iran Biomed J 2012;16:84–9.Search in Google Scholar
©2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Review Article
- Establishing and using reference intervals
- Research Articles
- Evaluation of the clinical chemistry tests analytical performance by using different models and specifications
- Impact of preventive actions on rejection rates in the preanalytical period
- Comparison of some biochemical tests in different blood collection tubes in hemodialysis patients
- Evaluation of percentage recovery together with modified reference range in hyperprolactinemia
- Fractalkine (CX3CL1) and its receptor (CX3CR1) in children with hypertrophic adenoid and chronic otitis media with effusion
- Neutrophil gelatinase-associated lipocalin as a potential biomarker for pulmonary thromboembolism
- Diagnostic values of neutrophil/lymphocyte ratio, platelet/lymphocyte ratio and procalcitonin in early diagnosis of bacteremia
- The distribution indices of erythrocytes: which one for acute ischemic stroke?
- Evaluating serum elastin levels in striae gravidarum
- Spectrum of BRCA1/BRCA2 variants in 1419 Turkish breast and ovarian cancer patients: a single center study
- Investigation of effect of vitamin D receptor, calcium-sensing receptor and β-catenin on cutaneous squamous cell carcinoma
- Hepatotoxicity and nephrotoxicity following long-term prenatal exposure of paracetamol in the neonatal rat: is betaine protective?
Articles in the same Issue
- Frontmatter
- Review Article
- Establishing and using reference intervals
- Research Articles
- Evaluation of the clinical chemistry tests analytical performance by using different models and specifications
- Impact of preventive actions on rejection rates in the preanalytical period
- Comparison of some biochemical tests in different blood collection tubes in hemodialysis patients
- Evaluation of percentage recovery together with modified reference range in hyperprolactinemia
- Fractalkine (CX3CL1) and its receptor (CX3CR1) in children with hypertrophic adenoid and chronic otitis media with effusion
- Neutrophil gelatinase-associated lipocalin as a potential biomarker for pulmonary thromboembolism
- Diagnostic values of neutrophil/lymphocyte ratio, platelet/lymphocyte ratio and procalcitonin in early diagnosis of bacteremia
- The distribution indices of erythrocytes: which one for acute ischemic stroke?
- Evaluating serum elastin levels in striae gravidarum
- Spectrum of BRCA1/BRCA2 variants in 1419 Turkish breast and ovarian cancer patients: a single center study
- Investigation of effect of vitamin D receptor, calcium-sensing receptor and β-catenin on cutaneous squamous cell carcinoma
- Hepatotoxicity and nephrotoxicity following long-term prenatal exposure of paracetamol in the neonatal rat: is betaine protective?

