Abstract
Introduction
In order to product (S)-2-pentanol which have been used as a key chiral intermediate required in the synthesis of several potential anti-Alzhemeir drugs, the effects of enzyme, acyl donor, substrate concentration and acyl donor/racemic-2-pentanol mole ratio were investigated on the kinetic resolution of racemic-2-pentanol.
Methods
Reactions were performed in a bioreactor of 50 mL capacity with a working volume of 30 mL on an orbital shaker at 150 rpm and at 30°C. Production parameters were investigated with different type of enzyme and acyl donor.
Results
The optimum conditions were obtained with Novozyme 435 and vinyl butyrate with the 50% conversion, 99% of enantiomeric excess for the substrate at 30 min. Optimum conditions are 1500 mM substrate and 4 mg/mL enzyme concentrations and 24.88 mM/min maximum initial reaction rate. It was obtained that Ping-Pong bi-bi mechanism was the appropriate reaction kinetic. Kinetic parameters were determined with Polymath 6.1 software as 4.16 mmol/min/g enzyme maximum reaction rates, 103.73 mM Km for (R)-2-pentanol and 51.18 mM Km for vinyl butyrate.
Conclusion
(S)-2-pentanol was obtained with 99% of enantiomeric excess. These data will be clear up to product (S)-2-pentanol at larger industrial scales in future.
Özet
Giriş ve Amaç
Alzheimer ilaçlarının üretiminde kiral ara bileşen olarak kullanılan (S)-2-pentanolün enantiyomerik saflıkta elde edilebilmesi için rasemik-2-pentanolün transesterleşmesi ile kinetik rezolüzosyununa enzim ve açil verici türünün, substratderişiminin, açil verici/rasemik-2-pentanol mol oranının etkisi ve tepkime kinetik modelinin belirlenmesi amaçlanmıştır.
Yöntem ve Gereçler
Çalışmalar ağzı kapaklı, 50 ml’lik biyoreaktörlerde, 30 mL çalışma hacminde, orbitalçarkalayıcıda, 150 rpm karıştırma hızında ve 30°C sıcaklıkta gerçekleştirilmiştir. Üretim parametrelerinin belirlenmesi için farklı enzim ve açil vericiler kullanılmıştır. Substrat ve enzim derişimi Cevap Yüzey Yöntemi (RSM) kullanılarak optimize edilmiştir.
Bulgular
Enzim olarak Novozyme 435, açil verici olarak vinilbütirat kullanımı ile 30 dakikada S-2-pentanol için %99 enantiyomerik aşırılığa ve %50 dönüşüme ulaşılmıştır. RSM ile başlangıç tepkime hızını maksimum yapan koşullar; 1500 mM substrat, 4 mg/mL enzim derişimi ile 24.88 mM/dk başlangıç tepkime hızı olarak elde edilmiştir. Tepkime kinetiğinin PingPongbibi modeline uyduğu belirlenmiştir. Polymath6.1 paket programı kullanılarak kinetik sabitler olarak maksimum hız, 4.16 mmol/dk/g enzim, alkol için KmA 103.73 mM, vinilbütirat için KmB 51.18 mM bulunmuştur.
Tartişma ve Sonuç
Bu çalışma ile %99 enantiyomerik aşırılıkta (S)-2-pentanol elde edilmiştir. Veriler ışığında literatürde eksikliği olan gelecekteki (S)-2-pentanolün büyük ölçekte ve sürekli sistemde üretim parametrelerine zemin oluşturarak katkı sağlanmıştır.
Introduction
Enantiomeric compounds can cause different biological effects, thus it is so important to use pure enantiomer in the drugs. Chiral secondary alcohols are biologically active naturally occurring compounds and are important intermediates for amines, ester, etc. They are also chiral auxiliaries in the syntheses of some drugs and insecticides [1], [2]. Asymmetric synthesis and kinetic resolution are the most used methods for producing optically active pure enantiomer from racemate. Kinetic resolution is more economical among these methods because of the high concentration of substrate can be employed [3].
Lipases (EC 3.1.1.3) are the most used class of enzymes in biotechnology in organic synthesis and kinetic resolution of racemic compounds, because of their major properties: industrial availability, unique class of enzymes in carrying out reactions in heterogeneous media and their substrate specificities to catalyze hydrolysis, esterification, interesterification and transesterification in low water media [4], [5]. Unlike most of the lipases, Candida antarctica lipase does not have a conserved pentapeptide sequence Gly-X-Ser-X-Gly, around the active site. The active site of lipases is formed by serine, histidine and aspartic acid/glutamic acid. In general lipases have a polypeptide chain called lid or flap at their active center, which protect the active center from the reaction medium. This lid may be simple or has a complex structure. It is known that lipase B from Candida antarctica has a very small and simple lid. In addition to these, lipases may show insignificant or strong stereoselectivity and the stereoselectivity of the enzyme may vary according to the structure of the substrate. For example, Pseudomonas fluorescens lipase have no stereospecificity vis-a-vis any of the primary positions of the triacylglycerol or alklydiacylglycerol [4].
Vinyl esters are widely used acyl donors for the transesterification reactions. Using vinyl esters as acyl donor prevent the reaction to reach equilibrium. In the literature vinyl esters have been commonly used for lipase catalyzed kinetic resolution, providing the optimum yield and effective transesterification. In addition to this, vinyl esters are used to evaluate the lipase activity in non-aqueous media [6]. It is known that first of all acyl-enzyme complex is formed, secondly it reacts with 2-pentanol [3], [7].
(S)-2-pentanol is a key chiral intermediate required in the synthesis of several potential anti-Alzheimer drugs that inhibit β-amyloid peptide release or synthesis [2], [6], [7], [8], [9], [10]. For this reason, resolution of the racemate is important to get enantiomerically pure (S)-2-pentanol. R-enantiomer of the (R,S)-2-pentanol is faster enantiomer and transesterification occurs with (R)-2-pentanol rapidly. In other word (R)-2-pentanol is the substrate for lipase catalyzed acylation reaction and the desired (S)-2-pentanol is the unreacted substrate and remains in the reaction medium [8]. There are several studies in the literature about the kinetic resolution of (R,S)-2-pentanol [6], [7], [8], [9], [10], [11]. Most of them are based on determination of the reaction conditions such as enzyme source, solvent and acyl donor types, water activity and temperature. Studies were commonly started with the investigation of the enzyme source. Wang et al. [10] investigated the solvent effect on the enantioselectivity of kinetic resolution with the thermophilic lipase QLM (Alcaligenes sp.). They used seven different classical solvents including dichloromethane, hexane, acetone etc. and concluded that the improvement of the enzyme enantioselectivity could achieve with choosing a small molecular sized solvent. For their reactions the highest enantiomeric ratio (E) were obtained with dichloromethane. In other respects some of the researchers studied the ionic liquids for the reaction. Noel et al. [7] reported that with ionic liquid, [Bmim][NT12], synthetic efficiency was obtained 2.5 times greater than hexane. In the investigation of Hernandez et al. [9], [Bmim+][BF4−] was the most appropriate ionic liquid for their conditions. On the other hand, according to the literature, high enantioselectivities were obtained with the classical solvents like hexane, heptane, and diethylether [3], [8], [11]. Some of the researchers evaluated the water activity for the transesterification and found that enantioselectivity was not affected with water activity [3], [7], [9].
As can be seen in the literature, only process parameters have been investigated for enantioselective production of (S)-2-pentanol. For industrial scale production of (S)-2-pentanol, there is need to investigate mass transfer limitations with chemical engineering principles. It is important to achieve the high enantioselective and productive production and to investigate the reactor hydrodynamics. For this aim, firstly, we investigated the effects of enzyme source, type of acyl donor, amount of alcohol, amount of enzyme, and acyl donor/alcohol mole ratio, high substrate concentration on the enantiomeric excess of the substrate (eeS) and initial reaction rate. Secondly, internal and external mass transfer limitations were determined and estimation of the kinetic modeling was performed in the batch heterogen bioreactor system.
Materials and methods
Materials
All the enzymes used in this study were obtained commercially. Novozyme 435 (Nov.435, lipase B from Candida antarctica immobilized on acrylic resin), Lipozyme RM IM (Rhizomucormeihei lipase) was obtained from Novozymes. Amona lipase AK (lipase from Pseudomonas fluorescens), vinyl acetate and (R,S)-2-pentanol were purchased from Aldrich (Germany). Vinyl laureate, vinyl benzoate, vinyl butyrate were obtained from Fluka (Japan) and n-hexane, ethyl propionate were purchased from Merck (Germany). All chemicals were analytical grade and were used without any pre-treatment.
Transesterification reaction
Batch reactions for transesterification (Figure 1) were performed in a bioreactor of 50 mL capacity with a working volume of 30 mL. (R,S)-2-pentanol, acyl donor and n-hexane were added into the bioreactor. After taking the t=0 initial concentration sample, the biocatalyst was added to initiate reaction. Bioreactor, placed in an orbital shaker, was incubated at 30°C at desired agitation speed (rpm). At the 50% of the conversion, reaction was ended. Samples from reaction medium were taken at regular time intervals, diluted with n-hexane and cooled in ice bath. All experiments were carried out in duplicate. 200 μL 30 mM internal standard (ethyl propionate) was added to 800 μL reaction medium solution to prepare 1 mL solvent for gas chromatography (GC) analyses and the samples were mixed by vortex.
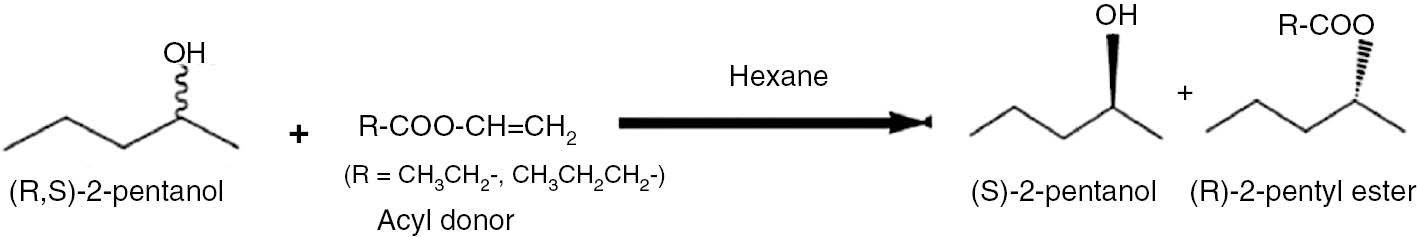
Transesterification reaction of (R,S)-2-pentanol with vinyl ester in hexane medium with lipase.
Analytical method
The reaction mixtures were analyzed using GC-2010 Shimadzu gas chromatography with flame-ionization detector (FID) and a β-DEX-120 capillary column with helium as carrier gas. The injector and detector temperatures were kept at 250°C and oven temperature was held at 50°C for 10 min, then increased at a rate of 5°C min−1 to 75°C and kept at this temperature for 2 min; then increased at a rate of 5°C min−1 to final temperature of 100°C and kept at that temperature for an additional 10 min.
Experimental design
The Design Expert (version 6.01, Stat. Ease, Inc., Minneapolis, MN, USA) was used for regression and graphical analyses of the data obtained.
Determination of enantiomeric values
eeS, the conversion (c) and enantiomeric ratio (E) were calculated from Eq. (1) to Eq. (3), respectively, where CS, CR, CS0 and CR0 denotes concentrations of (S)-2-pentanol and (R)-2-pentanol, and initial concentrations of (S)-2-pentanol and (R)-2-pentanol, respectively.
Results
Screening of different biocatalysts
To find the most effective enzyme for the kinetic resolution of (R,S)-2-pentanol, screening of three commercial lipases (Nov.435, Lipozyme RM IM and lipase AK) were incubated in 30 mL reaction medium containing 150 mM (R,S)-2-pentanol, 150 mM vinyl butyrate and 150 mg of enzyme at 30°C and 150 rpm on the orbital shaker.
Enantiomeric excess and conversion reached 99% and 50% in a short time (30 min) with immobilized Candida antarctica lipase B (Nov.435) (Figure 2). Lipozyme RM-IM and Lipase-AK had lower enantiomeric excess and conversion for a long time. Lipozyme RM-IM had higher activity than Lipase-AK. Nov.435 was found as the best biocatalyst for the reaction. In the literature, it was reported that some of the lipases like lipase AK, was an inactive biocatalyst for the reaction [8] and crude enzymes had lower enantioselectivities as compared to the immobilized lipases [6]. Jacobsen et al. [12] investigated the enzyme immobilization on the kinetic resolution of secondary alcohols. They used pure protein formulation of lipase B from Candida antarctica (Novozyme 525 F) and immobilized lipase B (Nov.435). They reported that enantiomeric ratio and conversion were the same for the both enzymes and the enantioselectivity was not effect by the enzyme immobilization. Patel et al. [8] reached 98% ee at 6 h for preparative scale enzymatic acetylation of (R,S)-2-pentanol using lipase B from Candida antarctica. In studies of Wang et al. [11] Cal B, Pseudomonas fluorescens lipase (PFL), Pseudomonas cepacia lipase (PCL) were used for kinetic resolution of (R,S)-2-pentanol and 75% eeS, 67% conversion were obtained for Cal B at 48 h. As can be seen in the literature, the higher enantioselectivity was obtained in a short reaction time in our study. The significant higher activity of Nov.435 probably comes from the characteristic property of Candida Antarctica lipase B, which has a very small and simple lid at the active center that does not isolate the active center of the enzyme in the closed form [4]. Therefore Nov.435 was selected for all experiments.

Effect of different catalysts on conversion by time. One hundred and fifty millimolar (R,S)-2-pentanol, 150 mM vinyl butyrate, 150 mg enzyme, 30°C, 150 rpm.
Screening of acyl donor
Screening of various acyl donors [vinyl laurate (C14H26O2,  ), vinyl acetate (C4H6O2,
), vinyl acetate (C4H6O2,  ), vinyl benzoate (C9H8O2,
), vinyl benzoate (C9H8O2,  ) and vinyl butyrate (C6H10O2,
) and vinyl butyrate (C6H10O2,  )] for the kinetic resolution of (R,S)-2-pentanol was carried out in 30 mL reaction medium containing 150 mM (R,S)-2-pentanol, 150 mM acyl donor and 150 mg Nov.435 at 30°C and 150 rpm on the orbital shaker.
)] for the kinetic resolution of (R,S)-2-pentanol was carried out in 30 mL reaction medium containing 150 mM (R,S)-2-pentanol, 150 mM acyl donor and 150 mg Nov.435 at 30°C and 150 rpm on the orbital shaker.
Results show that (Figure 3) Nov.435 had an effective transesterification for all tested vinyl esters except vinyl benzoate. Among these acyl donors, 99% eeS were obtained in 20 min with vinyl butyrate. Other acyl donors except vinyl benzoate, high eeS were obtained at 30 min. Studies in the literature showed that using vinyl esters as acyl donors give high activity for transesterification of (R,S)-2-pentanol. Unlike other acyl donors, vinyl benzoate has an aromatic chain. It is concluded that the differences in enantioselectivity come from the aromatic chain in its structure. Similarly, Bakker et al. [2] studied the effects of acyl donor type with a wide range of vinyl carboxylates on the reaction rate and the enantioselectivity for the transesterification of 1-phenylethanol. They obtained maximum reaction rate with vinyl butyrate and a low reaction rate with vinyl benzoate. They concluded that this situation occurred because of the steric hindrance of vinyl benzoate.
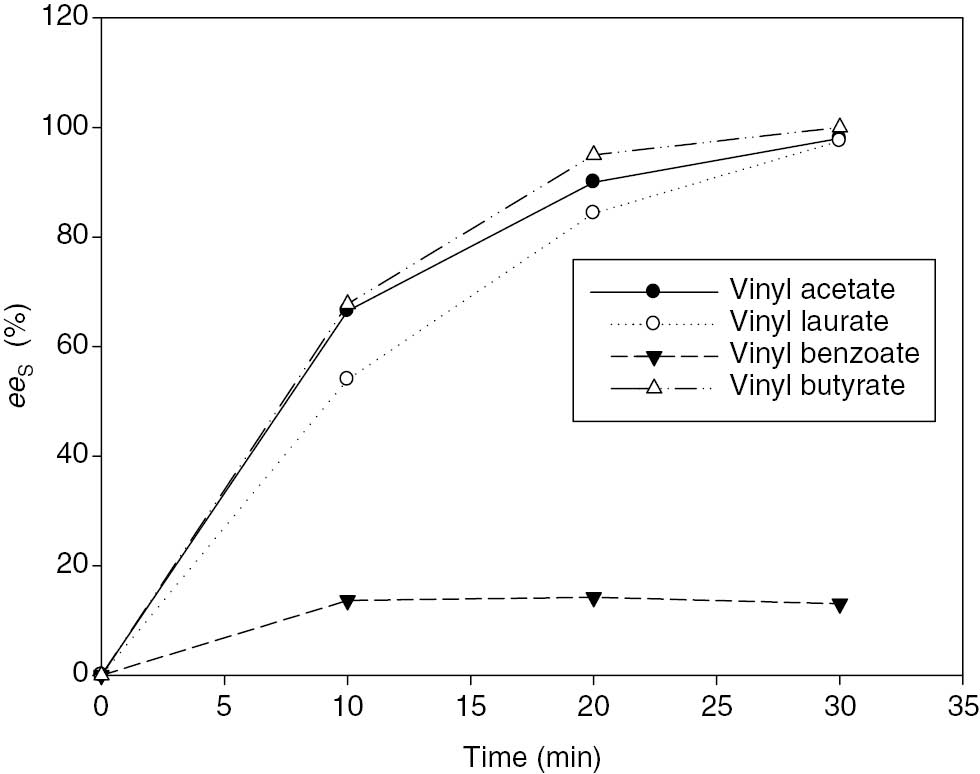
Effect of different acyldonor on eeS by time. One hundred and fifty millimolar (R,S)-2-pentanol, 150 mM acyl donor, 150 mg Nov.435, 30°C, 150 rpm.
Because of obtained slightly higher values in a short time with vinyl butyrate, it was selected as the acyl donor in this study.
Effect of substrate concentration
The effect of substrate concentration on the reaction rate was investigated by varying the initial concentrations of (R,S)-2-pentanol and vinyl butyrate from 800 mM via 2000 mM in equimolar vinyl butyrate and (R,S)-2-pentanol concentrations. The reactions were performed at 30°C, 150 rpm with the 150 mg of Nov.435. Results were shown in Figures 4 and 5. As can be seen from the Figures, with the increase in substrate concentration, no decrease has been seen for eeS and initial reaction rate. Therefore, it was concluded that there was no substrate inhibition; high substrate concentration can be employed in kinetic resolution by transesterification of (R,S)-2-pentanol.

Effect of substrate concentration on eeS. Acyl donor vinyl butyrate, 150 mg Nov.435, 30°C, 150 rpm.

Effect of substrate concentration on initial reaction rate. Acyl donor vinyl butyrate, 150 mg Nov.435, 30°C, 150 rpm.
The effect of acyl donor/(R,S)-2-pentanol mole ratio on transesterification
The effect of substrate acyl donor/(R,S)-2-pentanol mole ratio (n) on transesterification was investigated between n=0.3 and n=2.0. For n=0.6 and higher, eeS and c rapidly reaches 100% and 50%, respectively (Figure 6). This shows that acyl donor was the limiting reactant for the reaction. This situation may be explained by the rapid formation of enzyme and acyl donor complex (acylation step), following reaction with 2-pentanol. During the transesterification reaction, lipase is initially acylated by an acyl donor, and the acylated enzyme reacts with an alcohol enantioselectivitely [13]. In order to give an acyl-enzyme intermediate, the serine of the lipase reacts with acyl donor, and then reacts with the chiral alcohol [14].

Effect of acyl donor/(R,S)-2-pentanol mole ratio on eeS. Acyl donor vinyl butyrate 150 mg Nov.435, 30°C, 150 rpm.
Therefore, amount of acyl donor is important. An increase in molar ratio of acyl donor/alcohol may prevent the depletion of acyl donor on the active site of the enzyme. Moreover, an increase in alcohol concentration may prevent the acyl donor reaching the active site. Therefore, high acyl donor is preferred [1].
The optimization of substrate and enzyme concentration with response surface methodology (RSM)
After determining the source of enzyme, type of acyl donor, the other reaction conditions were investigated by RSM. It was aimed to obtain a high reaction rate with low enzyme concentrations and high substrate concentration. According to the initial experiments, enzyme and substrate concentrations are important parameters for this reaction. Thus RSM was used to better understand the relationship between substrate and enzyme concentration on initial reaction rate. The range and levels of the variables, which were chosen based on previous results in this study, are given in Table 1. Two factor five level design consisted of 13 experiments and the results are listed in Table 2.
Experimental range and levels of the independent variables.
| Independent variables | Range and levels | ||||
|---|---|---|---|---|---|
| −1.41 | −1 | 0 | 1 | 1.41 | |
| A: Substrate concentartion (mM) | 293 | 500 | 750 | 1500 | 1707 |
| B: Enzyme concentration (mg/mL) | 0.03 | 1.50 | 2.75 | 4.00 | 4.50 |
Design matrix of two variables.
| No | Independent variables | Response, r0 (mM/min) | ||||
|---|---|---|---|---|---|---|
| Coded value | Natural value | Model | Experimental | |||
| A | B | CS0 (mM) | CE (mg/mL) | |||
| 1 | −1 | −1 | 500 | 1.5 | 7.12 | 5.76 |
| 2 | 1 | −1 | 1500 | 1.5 | 6.81 | 7.30 |
| 3 | −1 | 1 | 500 | 4 | 12.94 | 14.68 |
| 4 | 1 | 1 | 1500 | 4 | 24.88 | 28.48 |
| 5 | −1.41421 | 0 | 292.89 | 2.75 | 8.83 | 7.56 |
| 6 | 1.414214 | 0 | 1707.11 | 2.75 | 17.05 | 13.16 |
| 7 | 0 | −1.41421 | 1000 | 0.982233 | 4.49 | 5.84 |
| 8 | 0 | 1.414214 | 1000 | 4.517767 | 21.38 | 18.34 |
| 9 | 0 | 0 | 1000 | 2.75 | 12.94 | 13.08 |
| 10 | 0 | 0 | 1000 | 2.75 | 12.94 | 13.42 |
| 11 | 0 | 0 | 1000 | 2.75 | 12.94 | 13.41 |
| 12 | 0 | 0 | 1000 | 2.75 | 12.94 | 13.81 |
| 13 | 0 | 0 | 1000 | 2.75 | 12.94 | 13.40 |
The results of 2FI response surface model fitting in the form of analysis of variance (ANOVA) are given in Table 3. Values of “Prob>F” <0.0500 indicate that model terms are significant. In this case A, B and A*B were found to be significant model terms. Enzyme concentration is the most significant parameter (Prob>F<0.0001).
ANOVA for response surface 2FI model.
| Source | Sum of squares | DF | Mean square | F-value | Prob>F |
|---|---|---|---|---|---|
| Model | 390.5412 | 3 | 130.1804 | 24.77176 | 0.0001 |
| A | 67.6261 | 1 | 67.6261 | 12.86843 | 0.0059 |
| B | 285.3382 | 1 | 285.3382 | 54.29641 | <0.0001 |
| AB | 37.5769 | 1 | 37.5769 | 7.150429 | 0.0255 |
| Residual | 47.29676 | 9 | 5.255195 | ||
| Pure error | 0.26112 | 4 | 0.06528 | ||
| Cor total | 437.838 | 12 |
Final equation in terms of coded factors:
Final equation in terms of actual factors:
The initial reaction rate drew a parallel increase with the increase of the substrate concentration and amount of the enzyme (Figure 7). According to the RSM, maximum initial rate obtained was 24.88 mM min−1 with 1500 mM substrate concentration and 120 mg of enzyme. For this conditions r0, was experimentally obtained as 28.48 mM min−1. The close rate values show that the equation is suitable for the reaction. At the optimum conditions, 99%≪eeS, 48% conversion and 70 enantiomeric ratio were obtained.

The effect of substrate and enzyme concentration on the initial reaction rate.
External and internal mass-transfer limitations
The transesterification of (R,S)-2-pentanol was catalyzed by an immobilized lipase in this study. Thus, internal and external mass transfer limitations must be considered for the reaction kinetics and modeling of the reactor.
For determining the external mass-transfer limitation, kinetic resolutions of (R,S)-2-pentanol (150 mM) catalyzed by Nov.435 (150 mg) with vinyl butyrate (150 mM) as acyl donor were studied at different agitation speeds (100, 150 and 175 rpm) at 30°C. According to Figure 8, it was obvious that there was a significant difference for 100 rpm and 150 rpm. Fifty percent conversion was not reached for a long time for 100 rpm. Similarly, eeS value for 150 and 175 rpm was achieved 100% in a short time, but 50% of eeS value could not be obtained for 100 rpm. Reaction rate increased quite a few with increasing agitation speeds from 100 to 150 rpm (Figure 9). According to the results for low agitation speeds, external mass transfer limitations existed and external mass transfer limitation decreased with increasing the agitation speed. In addition, as can be seen from Figure 9, there is a slight increase of initial reaction rate from 150 rpm to 175 rpm. Also taking into account the shear stress on enzyme, 150 rpm was chosen as the optimum agitation speed.

Effect of agitation speed on eeS by time. One hundred and fifty millimolar (R,S)-2-pentanol, 150 mM acyl donor, 150 mg Nov.435, 30°C.

Effect of agitation speed on initial reaction rate. One hundred and fifty millimolar (R,S)-2-pentanol, 150 mM acyl donor, 150 mg Nov.435, 30°C.
For determining the internal mass-transfer limitations, Nov.435 was fractionated based on particle size on 300, 425, 600 mesh sieves (0.050–0.023 mm). Four different particle sizes were obtained for Nov.435. Kinetic resolutions of (R,S)-2-pentanol (150 mM) with vinyl butyrate (150 mM) as acyl donor were studied with these different particle sizes of Nov.435 with an amount of 150 mg at 30°C temperature and 150 rpm agitation speed.
For the internal mass transfer limitation (Figure 10), similar enantiomeric excesses were obtained for all particle sizes. In addition to this, there was no significant difference on initial reaction rate for different particle sizes of the enzyme (Figure 11). Zhang and Liu [15] investigated the influences of internal and external diffusion limitations on the kinetic resolution of (R,S)-ketoprofen ethyl ester. They used immobilized lipase with different particle diameter range from 0.2 to 1.7 mm. They found that conversion unchanged when the diameters increased from 0.2 to 0.5 mm and decreased with the increase of diameter from 0.5 to 1.7 mm. They concluded that for the 0.2–0.5 mm particle diameter there was no considerable effect of the internal diffusion on the kinetics and internal mass transfer limitation could be neglected. For this study it is concluded that there were no internal mass transfer limitations. Although the diffusion path increases with increasing particle size, a significant difference of initial rate was not observed. Besides the particle size of the used enzyme (Nov.435) is not big.

Effect of biocatalyst particle size on eeS. One hundred and fifty millimolar (R,S)-2-pentanol, 150 mM acyl donor, 150 mg Nov.435, 30°C, 150 rpm.

Effect of biocatalyst particle size on initial reaction rate. One hundred and fifty millimolar (R,S)-2-pentanol, 150 mM acyl donor, 150 mg Nov.435, 30°C, 150 rpm.
Kinetic constant determination
The initial reaction rate obtained at various vinyl butyrate (50–2000 mM) and (R,S)-2-pentanol concentrations (50–2000 mM) were fitted to Michaelis-Menten kinetics with Ping-Pong bi-bi mechanism by a nonlinear regression method (software Polymath 6.1). The concentrations of (R)-2-pentanol and vinyl butyrate versus time were plotted for each experiment. A linear trend was observed for 0–5 min and its slope gave the initial rate for each experiment. Initial reaction rates were plotted versus the concentration of the substrates ((R)-2-pentanol and vinyl butyrate).
We first investigated Ping-Pong bi-bi mechanism with substrate inhibition. We found that there was no substrate inhibition according to the unrealistic values obtained for kinetic constants. Thus the kinetic model was chosen as Ping-Pong bi-bi mechanism without substrate inhibition. Maximum reaction rate (rM), KM for (R)-2-pentanol (KMA) and KM for vinyl butyrate (KMB) were obtained as 20.82 mM min−1 (4.16 mmol genzyme−1 min−1), 103.73 mM and 51.18 mM, respectively (Eq. 6).
Discussion
The kinetic resolution of (R,S)-2-pentanol by transesterification was investigated systematically. According to the obtained results the internal and external mass transfer limitations and the kinetic model for the enantioselective transesterification was investigated. Consequently, the overall parameters of kinetic model have obtained. In literature, for industrial usage there was lack in optimization of the parameters using chemical engineering principles. Here, we filled this gap for future studies for continuous processes. Parameters found in this study should be examined in larger industrial scales to optimize the production of (S)-2-pentanol in future.
Acknowledgments
The authors gratefully acknowledge the financial support given to this work by Ankara University, BAP (Project No. 14L0443003).
Conflict of interest: There are no conflict of interest among the authors.
References
1. Soyer A, Bayraktar E, Mehmetoglu Ü. Optimization of lipase-catalyzed enantioselective production of 1-phenyl-1-propanol using response surface methodology. Prep Biochem Biotechnol 2010;40:389–404.10.1080/10826068.2010.525433Search in Google Scholar
2. Bakker M, Sprujit AS, Rantwijk F, Sheldon RA. Highly enantioselective aminoacylase-catalyzed transesterification of secondary alcohols. Tetrahedron Assymetr 2000;11:1801–8.10.1016/S0957-4166(00)00118-XSearch in Google Scholar
3. de los Rios AP, Hernandez-Fernandez FI, Tomas-Alonso F, Gomez D. Biocatalytic kinetic resolution of rac-1-phenylethanol and ras-2-pentanol in hexane medium: acyl donor and water content effects. Can J Chem Eng 2010;88:442–6.10.1002/cjce.20285Search in Google Scholar
4. Kapoor M, Gupta MN. Lipase promiscuity and its biochemical applications. Process Biochem 2012;47:555–69.10.1016/j.procbio.2012.01.011Search in Google Scholar
5. Rodrigues RC, Fernandez-Lafuente RJ. Lipase from Rhizomucor miehei as a biocatalyst in fats and oils modification. Mol Catal B Enzym 2010;66:15–32.10.1016/j.molcatb.2010.03.008Search in Google Scholar
6. Goujard L, Villeneuve P, Barea B, Lecomte J, Pina M, Claude S, et al. A spectrophotometric transesterification-based assay for lipases in organic solvent. Anal Biochem 2009;385:161–7.10.1016/j.ab.2008.10.025Search in Google Scholar PubMed
7. Noel M, Lozano P, Vaultier M, Iborra J. Kinetic resolution of rac-2-pentanol catalyzed by Candida antarctica lipase B in the ionic liquid, 1-butyl-3-methylimidazolium bis[(trifluoromethyl) sulfonyl] amide. Biotechnol Lett 2004;26:301–6.10.1023/B:BILE.0000015429.28402.50Search in Google Scholar
8. Patel RN, Banerjee A, Nanduri V, Goswami A, Comezoglu FT. Enzymatic resolution of racemic secondary alcohols by lipase B from Candida antarctica. J Am Oil Chem Soc 2000;77:1015–9.10.1007/s11746-000-0161-ySearch in Google Scholar
9. Hernandez-Fernandez FJ, de los Rios AP, Tomas-Alonsa F, Gomez D, Villora G. On the development of an integrated membrane process with ionic liquids for the kinetic resolution of rac-2-pentanol. J Memb Sci 2008;314:238–46.10.1016/j.memsci.2008.01.043Search in Google Scholar
10. Wang Y, Wang R, Li Q, Zhang Z, Maa J, Feng Y. Solvent effects on the enantioselectivity of the thermophilic lipase QLM in the resolution of (R,S)-2-octanol and (R,S)-2-pentanol. J Mol Catal B Enzym 2009;56:146–50.10.1016/j.molcatb.2008.01.010Search in Google Scholar
11. Wang Y, Wang R, Li Q, Zhang Z, Feng Y. Kinetic resolution of rac-alkyl alcohols via lipase-catalyzed enantioselective acylation using succinic anhydride as acylating agent. J Mol Catal B Enzym 2009;56:142–5.10.1016/j.molcatb.2008.02.002Search in Google Scholar
12. Jacobsen EE, Andresen LS, Anthonsen T. Immobilization does not influence the enantioselectivity of CAL-B catalyzed kinetic resolution of secondary alcohols. Tetrahedron Assymetr 2005;16:847–50.10.1016/j.tetasy.2004.11.081Search in Google Scholar
13. Mehmetoğlu Ü, Bayraktar E, Babaarslan Ç. Production of enantiomerically pure pharmaceutical compounds using biocatalysts. In: Bhattacharya S, editor. Enzyme mixture and complex biosynthesis. Texas: Landes Bioscience, 2007: 65–78.Search in Google Scholar
14. Reetz MT. Lipases as practical biocatalysts. Curr Opin Chem Biol 2002;6:145–50.10.1016/S1367-5931(02)00297-1Search in Google Scholar
15. Zhang Y, Liu J. Kinetic study of enantioselective hydrolysis of (R,S)-ketoprofen ethyl ester usingimmobilized T. laibacchii lipase. Biochem Eng J 2011;54:40–6.10.1016/j.bej.2011.01.005Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Opinion Paper
- How can I protect my telomeres and slow aging?
- Research Articles
- Biochemical changes in the leaves of mungbean (Vigna radiata) plants infected by phytoplasma
- Optimization of process parameters and kinetic modeling for the enantioselective kinetic resolution of (R,S)-2-pentanol
- Determination of petroleum biodegradation by bacteria isolated from drilling fluid, waste mud pit and crude oil
- Variations in the fatty acid compositions of the liver and gonad tissue of spiny eel (Mastacembelus mastacembelus) from Atatürk Dam Lake
- Purification and characterization of two isoforms of native α amylase from Ok-Rong mango (Mangifera indica Linn. cv. Ok-Rong)
- Purification, immobilization and characterization of thermostable α-amylase from a thermophilic bacterium Geobacillus sp. TF14
- Development and characterization of a formulation based covalent conjugation with polyacrylic acid and recombinant major surface antigen (SAG1) of Toxoplasma gondii
- Reasearch Article
- Effect of elevation and phenological stages on essential oil composition of Stachys
- Letter to the Editor
- Some errors in the measurement of neutrophil-to-lymphocyte ratio
- Indices
- Reviewers 2017
- Yazar Dizini/Author Index
Articles in the same Issue
- Frontmatter
- Opinion Paper
- How can I protect my telomeres and slow aging?
- Research Articles
- Biochemical changes in the leaves of mungbean (Vigna radiata) plants infected by phytoplasma
- Optimization of process parameters and kinetic modeling for the enantioselective kinetic resolution of (R,S)-2-pentanol
- Determination of petroleum biodegradation by bacteria isolated from drilling fluid, waste mud pit and crude oil
- Variations in the fatty acid compositions of the liver and gonad tissue of spiny eel (Mastacembelus mastacembelus) from Atatürk Dam Lake
- Purification and characterization of two isoforms of native α amylase from Ok-Rong mango (Mangifera indica Linn. cv. Ok-Rong)
- Purification, immobilization and characterization of thermostable α-amylase from a thermophilic bacterium Geobacillus sp. TF14
- Development and characterization of a formulation based covalent conjugation with polyacrylic acid and recombinant major surface antigen (SAG1) of Toxoplasma gondii
- Reasearch Article
- Effect of elevation and phenological stages on essential oil composition of Stachys
- Letter to the Editor
- Some errors in the measurement of neutrophil-to-lymphocyte ratio
- Indices
- Reviewers 2017
- Yazar Dizini/Author Index

