Abstract
Objective
We aimed to investigate the frequency of delayed notifications and probable causes of delays for critical value notification in clinical laboratory of university hospital.
Materials and methods
All data was obtained from critical value reporting forms and laboratory information system. The frequency and location of critical and delayed results, latencies throughout a working day and the professional status who received the critical callbacks were shown as percentages.
Results
A total of 2018 (1.02%) critical values were reported and 13.1% of them were delayed notifications. Most of them were observed in laboratory tests ordered from patients of service and polyclinics compared to ICU and emergency department (26.7%, 26%, 6.2% and 4.9%, respectively, p<0.01). Delayed notifications were significantly higher for biochemical parameters (19.7%, p<0.001) and observed particularly in morning hours (06:00 a.m.–10:00 a.m.), lunch break time (12:00–14:00) and end of the working day (16:00–18:00). Latencies of mild-delayed reporting were 18.5±4.4 min for 62.8% and advanced-delayed reporting were 47.1±11.3 min for 37.2% of total delayed notifications. Most of the critical results were reported to the health care staff other than physician (55.6%).
Conclusion
Laboratory professionals should work in collaboration with responsible clinician and healthcare staff in critical value reporting process.
Özet
Amaç
Bu çalışmada üniversite hastanesi klinik laboratuvarından yapılan kritik değer bildirimlerinde yaşanan gecikmelerin sıklığını ve olası gecikme nedenlerini araştırmayı amaçladık.
Materyal ve Metotlar
Kritik değerlerin ve geciken bildirimlerin sıklığı, bildirim yeri, bildirim yapılan personel ve gün içi gecikme süreleri yüzdeler halinde gösterilmiştir.
Sonuçlar
Toplamda 2018 (%1.02) kritik değer bildirimi yapılmış olup bunların %13.1’i gecikmiş bildirim olarak gerçekleşmiştir. Geciken bildirimler servis ve poliklinik hastalarından yapılan istemlerde acil servis ve yoğun bakımlara göre daha yüksek oranda gözlenmiştir (sırasıyla, %26.7, %26, %6.2 ve %4.9 p<0.01). En fazla gecikme biyokimya parametrelerinde (%19.7, p<0.001) ortaya cıkmış olup en çok gecikmenin sırasıyla sabah saatlerinde (06:00–10:00), öğle arası (12:00–14:00) ve mesai bitiminde görev değişim saatlerinde (16:00–18:00) gerçekleştiği gözlenmiştir. Bildirimlerin %62.8’inde ortalama 18.5±4.4 dakika (hafif gecikme), %37.2’sinde ise ortalama olarak 47.1±11.3 dakika (ileri derecede gecikme) gecikme yaşanmıştır. Kritik bildirimlerin çoğu sorumlu hekim dışındaki sağlık çalışanlarına yapılmıştır (%55.6).
Sonuç
Laboratuvar uzmanları kritik değer bildirim süreçlerini sorumlu klinisyen ve sağlık çalışanları ile işbirliği içerisinde planlanmalıdır.
Introduction
Critical or panic values were first described by Lundberg as “potentially life-threatening test results unless necessary treatment is done promptly in clinical laboratories” [1], [2]. Reporting and documentation of critical values has been performed for patient safety in clinical laboratories and written procedures for reporting critical values are recommended as a quality requirement by national regulatory authorities. Many clinical laboratories have prepared and used their quality procedures for defining critical values, their limits, reporting time and documentation process [3], [4], [5]. Various reporting systems have gradually developed for effective notification including phone calls, online reporting and text messages, but all have some limitations [6], [7]. Delays in critical value reporting due to these limitations are considered as laboratory error and may lead to increases in mortality and morbidity. Lack of access to healthcare staff can be an important limitation and lead to delays in reporting critical values. It also can cause overloading of laboratory staffs because of recurrent phone calls.
Despite its importance there are few studies in literature evaluating delaying times and causes of delays in critical value notification [8], [9] and to our knowledge no study in Turkey.
In this study we aimed to investigate the frequency of delayed notifications and probable causes of delays in critical value notification respective to the time of the day and related clinical departments in our university hospital.
Materials and methods
All data were collected from reports generated by laboratory information system (LIS) and written documents during the 2 months period in biochemistry laboratory of Mustafa Kemal University, beginning in May 2015. Written consent was obtained from hospital administration.
Critical value reporting procedure
We used critical value limits determined according to the reports published earlier [7] and clinician recommendations in our hospital. The critical limits are equal for all clinical departments. Selected laboratory parameters in this study and cut-off values were as follows: Biochemical parameters; serum glucose (<2.2 or >25 mmol/L), BUN (>35.7 mmol/L), creatinin (>663 μmol/L), calcium (<1.5 or >3 mmol/L), sodium (<120 or >160 mmol/L), potassium (<2.8 or >6.2 mmol/L), total bilirubin (>257 μmol/L), ALT (>15 μkat/L), AST (>15 μkat/L), albumin (17 g/L), total protein (25 g/L) and troponin (>0.3 μg/L). Hematologial parameters; hemoglobin (<70 or >200 g/L), leucocyte count (<2 or >50×109/L) and PLT count (<20 or >1000×109/L). Blood gas parameters; pH (<7.1, >7.7 units), pCO2 (<25, >60 mmHg) and pO2 (<60 mmHg).
A laboratory information system is used in our laboratory that automatically alerts laboratory technician with both audible and visual warnings for test results requiring critical value notification. The relevant laboratory technician evaluates each critical value according to the written procedure for critical value reporting. After approving critical values, they are reported to the responsible clinician, intern doctor and nursing staff or service secretary by phone call and recorded in critical value notification forms.
During this observational study, we aimed to analyze delayed notifications. Therefore, all critical values were considered as separate events. Result generation time and reporting time for each critical value were recorded separately and delayed tests and delay times were evaluated daily. Time intervals exceeding 15 min between critical value alarming time and reporting time were considered as mild-delayed notification and exceeding 30 min were considered as advanced-delayed notification [8].
Results were analyzed in terms of test origin and frequency of critical value reporting and healthcare personnel to whom the result was notified by phone call. The rate of delayed notifications, delay times and working hours at which delaying notifications occurred were also evaluated. Regulatory-preventive action forms (RPAF) were prepared for delayed notifications in the end of the study as a requirement for routine quality procedure in our laboratory. Responsible service nurses and clinicians were questioned for the probable reasons of late answering.
Statistical analyses were performed using statistical software (SPSS for Windows, verison 21.0, Chicago, IL, USA). Critical value, delayed reporting frequencies and their percentages were calculated and statistical difference was determined by χ2-test. p<0.05 was accepted as statistically significant.
Results
A total of 197,654 test parameters were performed in our clinical laboratory (biochemistry: 112,615, hematology: 78,190, blood gases: 6849) and 2018 (1.02%) critical values were reported during the 2-month study. 86.9% of critical values were notified within 15 min. Delayed notifications occurred in 13.1% (n=265) of total critical values. Latencies of delayed notifications were 18.5±4.4 min (mild-delayed reporting) for 62.8% and 47.1±11.3 min (advanced-delayed reporting) for 37.2% of total delayed notifications. Most of them were observed in laboratory tests ordered from patients in service and polyclinics (26.7%, and 26%, respectively, p<0.01) (Table 1). Delayed notifications were higher for biochemical parameters (19.7%, p<0.001) (Figure 1). Most frequently delayed notifications were observed between 6:00 a.m and 8:00 a.m. and their distributions through working hours are shown in Figure 2. Delayed notifications were higher in biochemistry parameters (19.7%) compared to hematology (12.6%) and blood gas parameters (5.6%) (p<0.001). Nearly more than half of the critical values (55.6%) were reported to non-physician personnel (21.6% for nurses, 17.6% for intern doctors and 16.1 for service secretaries and 44.4% of total critical values were reported to the responsible physicians in our hospital (Figure 3).
Location and frequency of critical test values and delayed notifications.
| Locations | Total reportedcritical values, n (%) | Critical values no delay, n (%) | Critical values delayed, n (%) | X-square |
|---|---|---|---|---|
| Services | 725 (35.9) | 572 (73.3) | 153 (26.7) | a |
| ICU | 639 (31.7) | 609 (95) | 30 (4.9) | b |
| Polyclinics | 296 (14.7) | 235 (74) | 61 (26) | a |
| Emergency | 358 (17.7) | 337 (93.8) | 21 (6.2) | b |
| Total | 2018 (100) | 1753 (86.9) | 265 (13.1) | – |
ICU, Intensive care unit. a,b, The locations which has different letters are significantly different from each other, (p<0.01).
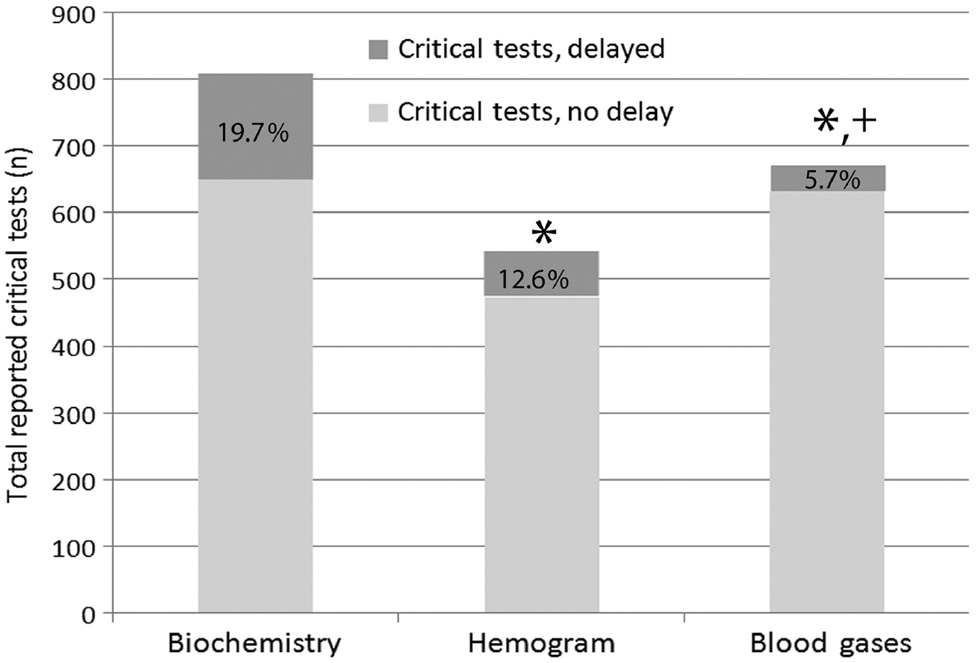
Percent distribution of delayed critical test notifications for 2 months in clinical laboratory.
“*” Denotes significant difference at p<0.001 in comparison with biochemistry parameters. “+” Denotes significant difference at p<0.001 in comparison with whole blood count parameters.

Distribution of delayed notifications throughout 24-h day in University Hospital.

Percentages of reported critical values according to the notified personnel.
Discussion
In this study, we evaluated the delaying frequencies and delay times for critical value reporting by phone call in the clinical biochemistry laboratory. Reducing preventable laboratory errors is an important goal for the clinical laboratories [10]. This evaluation provides a context for prevention of delayed notification and improvement of critical value reporting because effective reporting impacts on patient safety and efficiency of interaction between clinics and clinical laboratories.
A total of 2018 critical values were reported by phone call and documented in our laboratory during the 2-month study. As shown in Table 1, critical value frequency was approximately two times higher in tests ordered from service and ICU patients than those of polyclinics similar to previously reported by Dighe et al. [8]. They found 3.5 times higher critical callbacks for inpatient tests than outpatients. This is an expected result because patients in services especially in ICU’s have more life-threatening values than the outpatients. We also observed that the mean delaying times were 18.5±4.4 min for 62.5% and 47.1±11.3 min for 37.5% of total delayed tests. In literature there is no consensus about the exact time period for an appropriate reporting time. Some studies have recommended that critical values should be reported within 15–30 min after testing is completed [8], [11]. Some others reported higher durations (30–120 min) [12]. After consulting responsible clinicians, we consider that these threshold limits seem appropriate for our hospital standards. 13.1% of total critical value notifications by phone call were accepted as delayed notifications and most of them for biochemistry and hematology parameters because of late answering (also called as dropped call) by responsible healthcare staff. Delayed reports from blood gas analyses were less frequent than biochemical and hematology parameters. Delayed notifications were remarkably higher in services and polyclinics patients compared to those of emergency departments and intensive care units (Table 1). It is well known that critical value reporting is an important issue because of patient safety and test results (e.g. blood gas parameters) of critically ill patients in these departments (ED and ICU) are monitored closely by the nursing staff and clinicians. Therefore, these lower percentages can be resulted from the nursing staff working in these departments being more aware of the importance of critical values compared to services and polyclinics. In literature, different dropped call rates between 0.1 and 0.3% have been reported previously [13]. Our rates were higher than those of reported previously. To find out the reasons of dropped calls, we analyzed the distribution of delayed notifications throughout the 24-h day (Figure 2). Most of the delayed notifications were prominent from 06:00 a.m. until 10:00 a.m., at the beginning of working hours which are the busiest times of a work-day. Other delayed notifications were observed between 12:00 and 14:00, lunch break time, and between 16:00 and 18:00, shift change time for nursing and other healthcare staff.
The most three prominent reasons we observed from RPAF’s were preparation of patients for morning visits, beginning of phlebotomy process for test ordering and personnel task switching occurred during this period. We concluded that relatively increased workload at these hours and ineffective workflow planning during shift times can cause late answering for critical callbacks by healthcare staffs in our hospital.
Many laboratories have used different alerting (visual or audible) and reporting systems such as phone call, online reporting and text message for preventing delays in critical value reporting [7], [14]. Nevertheless, phone call is still the most common method used in clinical laboratories because of its ease of use for asking all recipients to read back the critical values [15], [16]. In a study Piva et al. recommended alternative reporting system ensuring read-back procedure. They used a software which generates automated notification by a specific text message sent to the cell phone of the responsible healthcare personnel. The alert message is appeared on the monitor until the responsible clinician acknowledges its receipt. If delay occurs within the timeliness, the notification is communicated by phone call [17]. Reporting of critical values through LIS to the smart phones of the responsible personnel of the clinics can be another alternative for critical value reporting. Because smart phones and its commercial applications (message groups or networks) have gradually increased. Additionally, some of them also include read-back applications. We have used phone call for critical value notification in our laboratory because lack of specific softwares mentioned above and “read back” is not possible by online reporting system used in our laboratory.
The other issue in critical value reporting relates to who should receive the phone call when laboratory technician reports out a critical value. In our hospital, critical values are reported to the intern doctors, nurses or service secretary by phone call if the responsible clinician is not available. In this study we found that 44.4% of critical values were reported to the responsible clinician. Remaining 55.6% of total notifications were made to the other healthcare staff. In a study, Howanitz et al. reported that nursing staff surveyed were not aware of critical values and did not think the calls for critical value were helpful. Whereas, majority of the clinicians surveyed were aware of the critical values and thought the calls were helpful [15]. However, the responsible clinician may not always be available because of increased workload or being in different locations (polyclinics, services or operating room) during working hours. Therefore critical value reporting to the non-physician nursing staff is inevitable in our hospital condition. We speculate that critical value education to increase awareness of health care staff about the importance of critical values in patient safety may be helpful in preventing delayed reports.
Critical value reporting is a medically important issue and its procedure varies between the laboratories and hospitals.
In conclusion, each laboratory should determine their critical value reporting policy according to their needs and facilities. However, there is a need for developing more effective technologies supporting phone call and decreasing workload of nursing staffs to maximize clinical benefits. Wireless technology usage (i.e. wireless phone) can be a useful choice because it is portable and can make clinicians always available. We also recommend that laboratory professionals should involve not only healthcare staffs but also administrative staffs who are responsible for personnel switching in developing critical value reporting policy to ensure patient safety.
Limitations, this study has focused on only delays between result generation time and reporting time for critical values. We did not evaluate the delays for overall testing process starting from sampling time. Further research is needed to evaluate this process more comprehensive.
Conflict of interest: Authors have no conflict of interest regarding this study.
References
1. Lundberg G. When to panic over an abnormal value. Med Lab Obs 1972;4:47–54.Search in Google Scholar
2. Lundberg GD. Critical (panic) value notification: an established laboratory practice policy (parameter). J Am Med Assoc 1990;263:709.10.1001/jama.1990.03440050103044Search in Google Scholar
3. Kost GJ. Critical limits for urgent clinician notification at US medical centers. J Am Med Assoc 1990;263:704–7.10.1001/jama.1990.03440050098042Search in Google Scholar
4. Kost GJ. Critical limits for emergency clinician notification at United States children’s hospitals. Pediatrics 1991;88:597–603.10.1542/peds.88.3.597Search in Google Scholar
5. Tillman J, Barth JH, ACB National Audit Group. A survey of laboratory ‘critical (alert) limits’ in the UK. Ann Clin Biochem 2003;40:181–4.10.1258/000456303763046148Search in Google Scholar PubMed
6. Kuperman GJ, Teich JM, Tanasijevic MJ, Ma’Luf N, Rittenberg E, Jha A, et al. Improving response to critical laboratory results with automation: results of a randomised controlled trial. J Am Med Inform Assoc 1999;6:512–22.10.1136/jamia.1999.0060512Search in Google Scholar PubMed PubMed Central
7. Piva E, Sciacovelli L, Zaninotto M, Laposata M, Plebani M. Evaluation of effectiveness of a computerized notification system for reporting critical values. Am J Clin Pathol 2009;131:432–41.10.1309/AJCPYS80BUCBXTUHSearch in Google Scholar PubMed
8. Dighe AS, Rao A, Coakley AB, Lewandrowski KB. Analysis Of laboratory critical value reporting at a large academic medical center. Am J Clin Pathol 2006;125:758–64.10.1309/R53XVC2U5CH6TNG8Search in Google Scholar
9. Onyenekwu CP, Hudson CL, Zemlin AE, Erasmus RT. The impact of repeat-testing of common chemistry analytes at critical concentrations. Clin Chem Lab Med 2014;52:1739–45.10.1515/cclm-2014-0331Search in Google Scholar PubMed
10. Sirota RL. The Institute of Medicine’s report on medical error: implications for pathology. Arch Pathol Lab Med 2000;124:1674–8.10.5858/2000-124-1674-TIOMSRSearch in Google Scholar PubMed
11. Agarwal R, Chhillar N, Tripathi CB. Study of variables affecting critical value notification in a laboratory catering to tertiary care hospital. Indian J Clin Biochem 2015;30:89–93.10.1007/s12291-013-0409-xSearch in Google Scholar PubMed PubMed Central
12. Valenstein PN, Wagar EA, Stankovic AK, Walsh MK, Schneider F. Notification of critical results: a College of American Pathologists Q-Probes study of 121 institutions. Arch Pathol Lab Med 2008;132:1862–7.10.5858/132.12.1862Search in Google Scholar PubMed
13. Wagar EA, Stankovic AK, Wilkinson DS, Walsh M, Souers RH. Assessment monitoring of laboratory critical values: a College of American Pathologists Q-Tracks study of 180 institutions. Arch Pathol Lab Med 2007;131:44–9.10.5858/2007-131-44-AMOLCVSearch in Google Scholar PubMed
14. Carroll AE, Saluja S, Tarczy-Hornoch P. Development of a personal digital assistant (PDA) based client/server NICU patient data and charting system. Proc Am Med Inform Assoc Symp 2001;100–4.Search in Google Scholar
15. Howanitz PJ, Steindel SJ, Heard NV. Laboratory critical values policies and procedures a college of american pathologists Q-probes study in 623 institutions. Arch Pathol Lab Med 2002;126:663–9.10.5858/2002-126-0663-LCVPAPSearch in Google Scholar PubMed
16. The Joint Commission’s Annual Report 2015. America’s Hospitals: Improving Quality and Safety. Available at: https://www.jointcommission.org/assets/1/18/TJC_Annual_Report_2015_EMBARGOED_11_9_15.pdf. (Last accessed: August 29 2015).Search in Google Scholar
17. Piva E, Pelloso M, Penello L, Plebani M. Laboratory critical values: Automated notifi cation supports effective clinical decision making. Clin Biochem 2014;47:1163–8.10.1016/j.clinbiochem.2014.05.056Search in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Review Article
- Automation in the clinical laboratory: integration of several analytical and intralaboratory pre- and post-analytical systems
- Research Articles
- Flow cytometric detection of endothelial progenitor cells (EPC) in acute coronary syndrome
- Evaluation of prolidase activity in uremic bone disease
- The independent relationship between hemoglobin A1c and homeostasis model assessment of insulin resistance in non-diabetic subjects
- Association of missense substitution of A49T and V89L in the SRD5A2 gene with prostate cancer in Turkish patients
- Delays in reporting critical values from clinical laboratories to responsible healthcare staff
- Re-determining the cut-off points of FIB-4 for patients monoinfected with chronic hepatitis B virus infection
- Approach to pre-analytical errors in a public health laboratory
- Serum proPSA as a marker for reducing repeated prostate biopsy numbers
- NT-proBNP levels in β-thalassemia major patients without cardiac hemosiderosis
- Comparison of high sensitive and conventional troponin assays in diagnosis of acute myocardial infarction
- Assessment of macroprolactinemia rate in a training and research hospital from Turkey
- Opinion Papers
- Evaluation of the first Turkish in vitro diagnostic symposium
- The report of the 1st Turkey in vitro diagnostic symposium results
- Venous blood gases: is it useful in COPD?
- Letter to the Editor
- How are ethical issues in the laboratory medicine held in Turkey? A perspective view through medical ethics and clinical laboratory science
Articles in the same Issue
- Frontmatter
- Review Article
- Automation in the clinical laboratory: integration of several analytical and intralaboratory pre- and post-analytical systems
- Research Articles
- Flow cytometric detection of endothelial progenitor cells (EPC) in acute coronary syndrome
- Evaluation of prolidase activity in uremic bone disease
- The independent relationship between hemoglobin A1c and homeostasis model assessment of insulin resistance in non-diabetic subjects
- Association of missense substitution of A49T and V89L in the SRD5A2 gene with prostate cancer in Turkish patients
- Delays in reporting critical values from clinical laboratories to responsible healthcare staff
- Re-determining the cut-off points of FIB-4 for patients monoinfected with chronic hepatitis B virus infection
- Approach to pre-analytical errors in a public health laboratory
- Serum proPSA as a marker for reducing repeated prostate biopsy numbers
- NT-proBNP levels in β-thalassemia major patients without cardiac hemosiderosis
- Comparison of high sensitive and conventional troponin assays in diagnosis of acute myocardial infarction
- Assessment of macroprolactinemia rate in a training and research hospital from Turkey
- Opinion Papers
- Evaluation of the first Turkish in vitro diagnostic symposium
- The report of the 1st Turkey in vitro diagnostic symposium results
- Venous blood gases: is it useful in COPD?
- Letter to the Editor
- How are ethical issues in the laboratory medicine held in Turkey? A perspective view through medical ethics and clinical laboratory science

