Abstract
Objectives
The purpose of this narrative review is to offer an updated perspective on the current research on the glycoprotein Osteoprotegerin (OPG), including its potential therapeutic impact and mechanisms of action, and interaction with bone and muscle tissues.
Content
As health and social care advances people are living longer, with projections suggesting that in 2050 there will be 2 billion people who are aged over 60 years. Yet musculoskeletal health still declines into older age and as a result there is an increase in the proportion of older populations that spend more time with persistent disabilities. Although physical exercise is repeatedly demonstrated to minimise detrimental effects of ageing, it is not always a feasible intervention, and other directions must be considered.
Summary and outlook
OPG, a glycoprotein decoy receptor for the receptor activator of nuclear factor kappa-β ligand (RANKL) is a key regulator of bone formation yet emerging evidence has presented its potential to offer positive outcomes in regard to the preservation of skeletal muscle mass and function. Animal models have shown that OPG levels increase during exercise, and independently acts to restore losses of muscle strength and reduce bone resorption. Interventions to increase circulating OPG alongside exercise may act as a therapeutic target to combat the decline in quality of life in older age in humans. Further research is needed on the mechanisms of its action and interaction in humans in combination with exercise.
Introduction
In 2006 it was estimated that globally there were 688 million people aged 60 years and over [1]. By 2050 this number is projected to grow to two billion, with approximately 20 % of those being over 80 years old [1]. The increasing proportions of older people living for longer is a positive reflection of health and social care advances, but there are also unintended negative consequences as health and physical function continue to decline with increasing age [2]. Consequently, people are living longer with musculoskeletal health conditions that impair the quality of life. Persistent physical disability as a result of declining musculoskeletal health increases with age, with current estimates for the UK suggesting a mean of 26.4 years will be spent in poorer health after the age of ∼52 years [3]. Understanding and combating the poorer musculoskeletal health in older age is public health priority, aiming to improve the quality of later life and ensure people remain independent for as long as possible.
Ageing is the chronic accumulation of cell and molecular damage imparting declines in both mental and physical function, ultimately leading to a heightened risk of disease [1]. Though not conclusive [4], 5], there are nine widely accepted hallmarks of ageing: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication [6]. There are many factors that can influence the physiological ageing process, such as dietary supplements, exercise, mental health and genetics. Sclerostin, Dickkopf-1 and Osteocalcin are widely studied circulatory markers that have potential to positively influence the ageing process by promoting neurogenesis [7], maintaining skeletal muscle mass [8] and by inhibiting the onset of age-related bone fragility [9]. Similarly, another molecule that has a growing body of evidence to suggest positive physiological effects within the ageing process is Osteoprotegerin (OPG).
OPG, identified by Simonet et al. [10] is a glycoprotein belonging to the TNFRα (Tumour necrosis factor receptor alpha) family and plays a large role in the bone remodelling cycle through both osteoclastogenesis and inhibition of the RANK/RANK Ligand (RANKL; Receptor Activator of Nuclear Factor Kappa β Ligand) system. The synthesis of OPG arises from the osteoblast as a propeptide from which a signal peptide of 21 amino acids then separates. This generates a 380 amino acid peptide in its mature form [11]. Its regulation can be impacted by some cytokines such as TNF-α, IL-1 and IL-6, hormones such as vitamin D and oestrogen, other mesenchymal transcription factors, and Wnt/β-catenin [12]. Structurally, OPG consists of 7 domains [13], 14]; domains 1–4 are cysteine-rick N-terminal domains, domains 5 and 6 are death domain regions, and the final domain is a C-terminal heparin binding domain. These first 4 domains are structurally similar to the TNF receptor (TNFR) family, whilst the primary physiological functions of domains 5 and 6 remain unestablished [15]. The 7th domain is a binding site for heparin, similar structures for which have been identified in fibroblast growth factors [16], 17]. This final domain is key for the antiresorptive role of OPG in bone remodelling. Heparin inhibits the binding of OPG to RANKL, enhancing osteoclastic bone resorption, thereby inhibiting OPG activity [18]. Within the general circulation OPG levels are found to be around 1–2 pmol.L−1, but can fluctuate acutely in response to exercise (detailed below) [12] and also over time with age [19], 20].
OPG lacks any direct signalling capacity of its own and is secreted from both osteoblasts and stromal cells to act as a decoy receptor, primarily functioning through the impairment of the RANK/RANKL complex, formed by binding to RANKL in place of the RANK receptor [11], 21]. By binding to the RANKL, the cognate receptor of RANK, the osteoclast receptor is prevented from activation [22]. There is also evidence to suggest OPG is secreted in response to mechanical stretching within differentiated C2C12 precursor cells, however the translation of these effects, via whole body exercise, on the stimulation of OPG secretion in mature skeletal muscle has not been defined [23]. There is also evidence from animal models to indicate that OPG possesses the capacity to offer protective effects against atrophy in dystrophic muscle and to restore function in fast-twitch extensor digitorum longus (EDL) [24]. In rodent models of dystrophic muscle, daily injections of 0.3 and 1.0 mg kg−1 OPG per day via OPG-fc (an Osteoprotegerin fragment complex), a dose-dependent increase in the contractile properties of muscle was observed, specifically in the Soleus (SOL) and EDL muscles [24]. Improvements in muscle specific force production reaching 114 and 233 % above baseline have been identified in the SOL and EDL, respectively [24]. However, these increases were observed only in dystrophic muscles and were absent in healthy (control) groups, suggesting a pathological process must first be active in order for OPG to exert any influence over the skeletal muscle. This research [24] highlights a potential therapeutic role for OPG in mitigating muscle wasting in dystrophic conditions and other similar physiological processes that encompass a loss of muscle mass and function, including muscle health in older age, particularly when combined with physical exercise.
Objectives
This narrative review will focus upon the role of Osteoprotegerin (OPG), a glycoprotein, as a potential molecule to support the alleviation of deterioration within the musculoskeletal system associated with ageing. The role of OPG within bone metabolism, with and without exercise, will be reviewed along with the current understanding of OPGs interactions with skeletal muscle. It is our aim to highlight, through the current body of evidence that demonstrates the beneficial effects of OPG within specified dystrophic muscular conditions, that OPG could act as a potential therapeutic target for future interventions to support and enhance musculoskeletal health in older age. The summary of this article is presented in Figure 1.
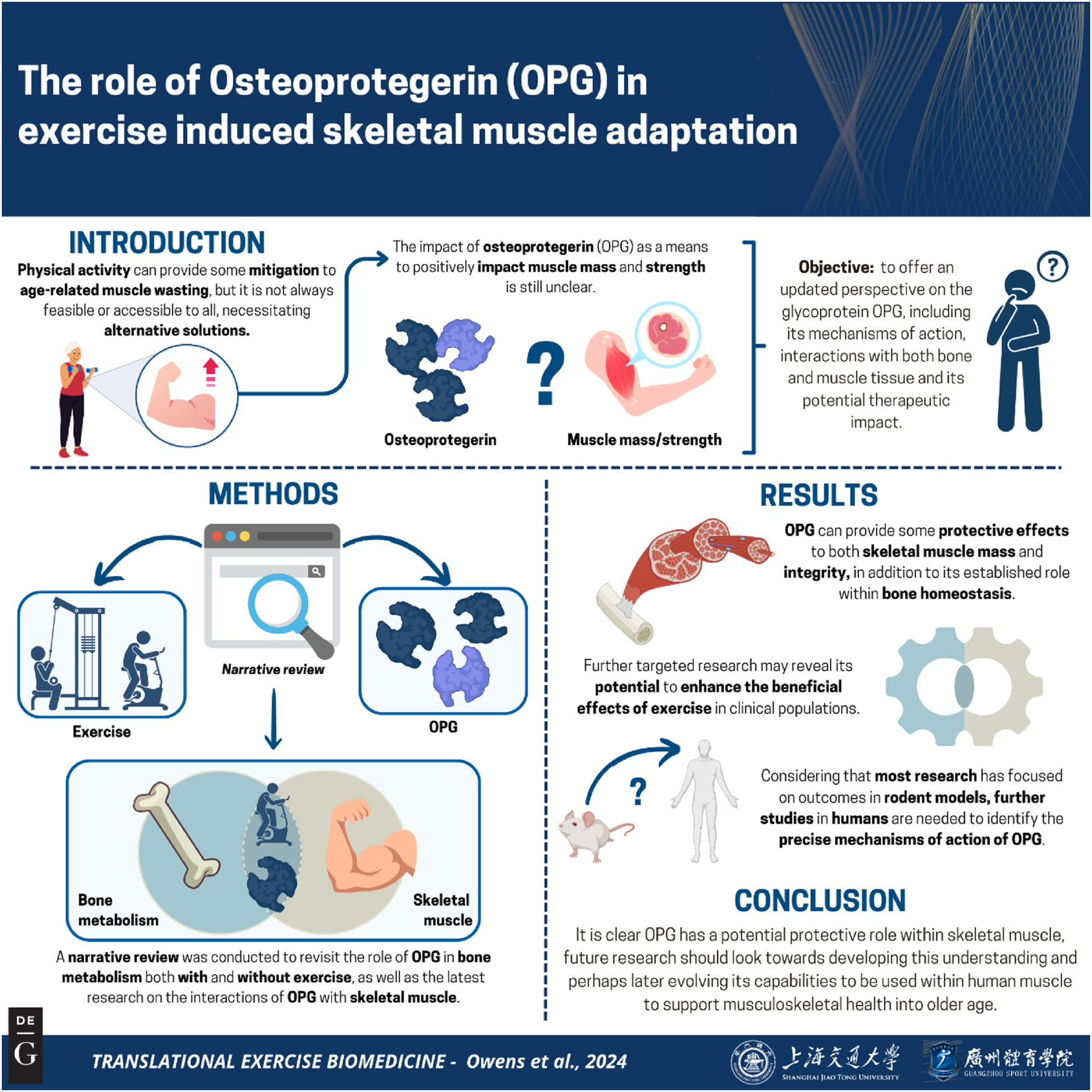
Graphical representation of this study. Key points: (1) Osteoprotegerin (OPG) is well known for its role in bone metabolism. (2) New evidence is emerging to suggest OPG can interact and preserve some muscle losses. (3) Given the relationship between muscle and bone, OPG could become a relevant glycoprotein to help mitigate some of the losses in bone and muscle through ageing. Figure created with biorender.
Content
Musculoskeletal ageing
Ageing of the musculoskeletal system is characterised by the gradual decrease in skeletal muscle mass (SkMM) and function [25], and bone mineral density (BMD) accompanied by an increased occurrence of intramuscular adipose tissue infiltration [26]. These factors do not decrease at the same rate, with skeletal muscle function showing decreases at a greater rate than that of mass [25] and therefore posing a greater risk in the loss of longevity and functional capacity with ageing [27]. Current estimates suggest that individuals from the age of 75 years lose SkMM at a rate of 0.64–0.7 % per year in females and 0.8–0.98 % per year in males [27], with force production loses at a rate of 3–4 % per year in males and 2.5–3 % per year in females [27]. In healthy individuals aged 77.4 ± 2.8 years, BMD declined ∼ 10 % over the preceding 25 year period [28].
Mechanical stimuli are imparted by the muscle in a number of forms, for example isometric, plyometric, static contractions etc. This interaction within the musculoskeletal system depends upon the conversion of mechanical forces exerted by the musculature into intracellular signals for cellular communication [29], 30]. In essence, the forces from muscles employed for movement and physical activity subsequently activate cellular mechanisms that, in turn, regulate the bone remodelling system [31]. Conversely, when little or no forces are provided by the muscles, such as during extended periods of bed rest following injury/illness, surgery or space flight in the absence of gravity, both skeletal muscle mass, bone mass and/or density are significantly reduced [32], [33], [34]. This mechanism is known as the mechanostat theory and states that bone adapts to increased mechanical loading (i.e. by impact/resistance exercise) by increasing in size and strength [35], [36], [37]. This is also evidenced in highly athletic populations [38], 39] whom typically have greater bone mass and strength [36].
The role of bone and muscle extends beyond structural features, with both acting as endocrine organs, secreting substances into the circulation to exert effects on other organs. Osteocytes primarily function as a regulator of bone break down, yet like myostatin and its primary association with muscle, osteocytes can also act as an endocrine organ influencing downstream circulating factors that, in turn, interact with other physiological systems [40]. Osteocalcin, an example of such circulating factors, is released by bone into the bloodstream where it can interact with substances from the liver and adipose tissue, supporting metabolism. Osteocalcin also inhibits bone formation by impairing osteoblast formation [41], 42]. OPG, is another example of the aforementioned factors and it functions to inhibit the production of osteoclasts, the primary cells of bone resorption. OPG primarily acts as a decoy receptor for the activator of nuclear factor kappa-B ligand (RANKL), consequently inhibiting the pathway by which osteocytes are differentiated into osteoclasts through stimulation by RANKL [43]. By reducing the activity of osteoclasts, OPG is able to limit bone resorption, creating a favourable environment for bone forming osteoblasts in which new bone matrix can be deposited. OPG also acts upon osteoblasts directly, stimulating osteoblast proliferation, differentiation and mineralisation, leading to further increased bone formation [44].
OPG is also able to bind and interact with TRIAL (TNF-related apoptosis inducing ligand). TRIAL is a member of the TNF superfamily and is normally produced by immune cells within the tumour environment. Both TRAIL and RANKL bind to OPG similarly, inhibiting TRAIL induced apoptosis in various cancer cells. To further contribute to muscle-bone cross talk, OPG has been highlighted to play a role within vascular physiology, secreted by endothelial cells of smooth muscle. Studies have shown recombinant OPG to promote survival of mature vascular endothelial cells [45], [46], [47]. Exogenous OPG administration is able to prevent vascular calcification [48] but conversely other studies have correlated high serum OPG levels with a greater severity of artery disease and heart failure [49], [50], [51]. The mechanisms of OPG within vascular pathologies remains unclear but there are suggestions that OPG blocks the apoptosis through binding with TRAIL and thereby enables stimulation of new endothelial cells and ultimately newer blood vessels [52], [53], [54]. Given the interaction of OPG with TRAIL within smooth muscle amongst pathologies such as heart disease and atherosclerosis, and the increased incidence of such pathologies within ageing, it is not implausible to suggest OPG to be able to interact with TRAIL of skeletal muscle in a similar ageing processes.
The resultant products of the endocrine functions of bone and muscle reaffirm the possibility of identifying therapeutic targets, within the bone/muscle interaction for the prevention and/or treatment of continued poor musculoskeletal health and ultimately the loss of independence and quality of later life.
Exercise and osteoprotegerin
With exercise the relationship between muscle and bone is enhanced via increased mechanical and biochemical signalling. The long-term musculoskeletal benefits are clearly demonstrated in competitive masters athletes [38], 55].
The majority of research investigating OPG and its relationship with exercise have been centred around bone health. Ziegler et al. [50] have reported elevated OPG levels immediately after long distance running in middle age men and women. Within cellular models, various levels of loading and stress have been noted to cause increases in levels of OPG [56], [57], [58], [59].
In premenopausal females OPG levels are found to be 26 % higher in those who exercise regularly compared to those who are sedentary, and OPG has been highlighted as a means to mitigate bone loss in females with an absence of menstrual cycles and subsequent oestrogen deficiency [60]. With a number of studies showing ultra endurance type activities to have ill effects on bone health, OPG has been suggested to become elevated as a compensatory mechanism to alleviate the increased resorption, but with comparative RANK-L measures, this is hard to confirm [61]. OPG levels have also improved in postmenopausal females following 1 year of light aerobic exercise, but whilst not affecting the RANKL levels [62].
Despite the majority of research noting positive changes in OPG levels and OPG/RANK ratios with exercise there are still equivocal findings, 8 weeks of either aerobic endurance or resistance exercise did not yield any significant changes in older women despite significant improvements in measures of bone mineral density [63]. In female gymnasts of a peripubertal age, undergoing very high impact type activity demonstrate similar levels of RANKL and OPG to those age matched swimmers (being a non impact activity) [64]. Yet a single stimulus of plyometric exercise can acutely increase levels of circulating OPG, without consideration of age and/or sex [65].
More generally, aerobic and resistance training have been shown to slow the age-related decline of muscle mass and strength [25], 66]. Similarly, improvements in BMD in older age have been established with weight bearing and resistance-based exercises, reducing the risk of falls and fractures [67], 68]. Despite well-established protocols of exercise declines in musculoskeletal health remains the most prominent cause of poor quality of life and a lack of independence [69], highlighting that the lack of adherence to exercise programs continues to remain an issue beyond the research intervention. Therefore, there is still a need to provide more viable and sustainable strategies, alongside exercise, that will support the maintenance of a higher quality of later life allowing older adults to remain in good health for a longer duration.
Future interventions that target both bone and muscle simultaneously, and can support the advantages of exercise, may hold potential in the successful alleviation of declines in musculoskeletal health and ultimately the onset of both sarcopenia and osteoporosis. Osteoprotegrin may provide a possible solution for beneficial effects at both the skeletal and muscle level.
Physiological relevance of osteoprotegerin
OPG functions to maintain skeletal density through the inhibition of bone resorption via the RANK/RANKL complex [70]. OPG is key in protecting the skeleton from excessive bone resorption, and the ratio of RANKL to OPG is an important measure of bone mass and skeletal integrity [22]. Preliminary research has identified in transgenic mice shows the overexpression of OPG is able to induce extreme osteopetrosis – a condition in which bones grow to an abnormal level of density [71]. Conversely, in mouse models with the gene responsible for OPG expression, TNFRSF11B is downregulated, severe osteoporosis is observed. The relative levels of OPG production in an organism is directly influential, dictating the extent to which bone resorption occurs. A recombinant viral vector containing genes expressing OPG has been utilised in mouse models via intramuscular injection to assess the effectiveness of reducing the breakdown of wear debris particulates, in orthopaedic implants, commonly associated with the impairment of osteoblastic bone formation and, therefore, bone formation within the bone resorption cycle [72]. Wear debris, which are artificial particles created by the abrasion of surgical implants against bone [72], can accumulate over an extended period of time and begin to cause osteolysis, which is a cause for concern in relation to surgical interventions. Increased osteolysis, caused by an upregulation of osteoclastic bone resorbing activity within bone tissue is a key factor in the onset of osteoporosis [73]. By introducing viral vectors containing OPG expressing genes, it was observed that the recombinant OPG blocked or inhibited the RANK/RANKL complex that would ordinarily be responsible for the stimulation of osteoclasts, resulting in downregulation of bone resorption [73]. These findings support the OPG’s impact and its use as a therapeutic target for the reduction of osteolysis in bone implants.
As well as its role within the skeleton, OPG has been implicated in the maintenance of human skeletal muscle mass and function, yet mechanisms remain undescribed [24]. There are preliminary findings that show positive results; exogenous OPG administration demonstrates inhibition of bone resorption and preservation of muscle mass within rodent models, leading to the development of osteopetrosis, when recombinant OPG (OPG-Fc) is administered to normal, healthy rodents [10]. As previously mentioned, when OPG-fc is administered to dystrophic mice, skeletal muscle integrity and function is increased by up to 233 %, identified in the EDL muscle, but provides no effects on healthy, normal muscle, suggesting a pathologic process must first be active to allow OPG to function [24]. OPG (−/−) mice exhibit greater reductions in bone health with prominent cases of osteoporosis, in addition these mice demonstrate selective weakness of type 2B skeletal muscle fibres only [74].
It is proposed that OPG could mechanistically work in a similar manner in skeletal muscle as it does in bone, through the interference of the binding of RANKL to surface RANK on skeletal muscles [22], 70]. As such, its effects may also be enhanced when combined with exercise. OPG-Fc and anti-RANKL antibodies appear to protect against muscle wasting in dystrophic mice, shown by the retention of 21 % more muscle mass in EDL muscle treated with OPG-Fc, compared with dystrophic mice injected with the same volume of Phosphate Buffered Saline (PBS), both over a 28-day period. Similarly, in the Diaphragm (DIA) during the same 28-day period a 12 % increase in muscle mass retention was identified in OPG-Fc injected dystrophic mice compared with dystrophic mice injected with PBS [24], 75], 76]. The notion of RANK/RANKL involvement has been further explored through the deletion of muscular RANK. Mouse models with such RANK deletion prevent the loss of maximum specific forces (primarily associated with type 2 fibres), but surprisingly decreased the overall size and fatigability of EDL muscle, which is primarily comprised of type 2A, 2X and 2B fibres [77]. This eludes to a preferential loss of type 2 fibres through RANK deletion, similarities of which are presented through muscular ageing and inactivity with specific fibre type loss, primarily type 2X, and a resulting fibre type grouping of type 1 fibres [66], 78], 79]. Thus, it is plausible the effects of OPG are fibre type specific further highlighting its potential to alleviate musculoskeletal deterioration in older humans. Albeit dystrophin models present different muscles to that of healthy aged, responsiveness to stimuli such as exercise show similar trajectories, thus the combined responses of muscular force and specific fibre type influences offer strong links to infer aged muscle will respond similarly to that of dystrophic muscle. Furthermore it has been highlighted that the poor differentiation capacity and premature senescence of satellite cells represents those of aged skeletal muscles, similar arguments can be presented for the onset of mitochondrial dysfunction and autophagy capacity, and thus provides a sound model for understanding mechanisms of action of specified molecules [80].
It is possible that if OPG does inhibit RANK on the surface of skeletal muscle then the regulation of Ca2+ influx is interrupted, through interference with the activity of sarcoplasmic reticulum Ca2+ ATPase, (SERCA) via activation of TRAF-6 (tumour necrosis factor receptor-associated factor 6), a process normally stabilised through the RANK/RANKL pathway and primarily responsible for intracellular calcium concentrations within the muscle [81]. Moreover, TRAF-6 has been shown to have influence across bone resorption as well as TRAF-6 deletion demonstrating preservation of muscle atrophy within a muscle wasting model, exhibiting roles across both bone and muscle pathophysiology [81]. If OPG is unable to inhibit RANK at the surface of the muscle then TRAF-6 is unable to regulate the SERCA activity with resulting rises of Ca2+ within the muscle, in turn activating calcineurin which dephosphorylates NAFTc1 and promotes slower muscle twitch phenotypes [81]. The NFATc1/calcineurin axis has been highlighted to initiate specific fibre type growth within other models, particularly in evolving slower type fibres in regeneration of muscle after injury [82]. The role of OPG, inhibiting RANK at skeletal muscle surface and subsequent TRAF-6 activation and increase of Ca2+ within the sarcoplasmic reticulum, could be a possible target to help to preserve muscle loss and restoration of the faster twitch muscle fibres, which could be further enhanced with incorporation of resistance based or anaerobic exercise activities. Comparatively, muscle specific RANK knockout mice exhibit reduced SERCA activity, favouring a fast muscle phenotype and protecting against sciatic denervation induced muscle dysfunction [81].
There are specific actions to SERCA; SERCA-1a is specific to the regulation of fast twitch muscle fibres whereas SERCA-2a is predominantly responsible for slow-twitch fibres. It is plausible that there may be a specified action of OPG on the SERCA pathways given the majority of influence is fibre type specific [81]. It is also possible that STIM-1 could control the influx of Ca2+ into skeletal muscle from the extracellular environment via the formation of the STIM-1/Orai-1 complex, opening the ORAI-1 Ca2+ ion channel. In the event of reduced SERCA activity, STIM-1 becomes activated through detection of lowered Ca2+ stores, and acts to promote the replenishment of Ca2+ to improve muscle function in denervated and atrophied EDL muscle [81]. Through OPG inhibition of the RANK/RANKL complex activity, it is possible that this same mechanism of SERCA activity reduction may occur, leading to STIM-1/Orai-1 complex mediated Ca2+ store replenishment and improvements in atrophied and dystrophic fast twitch muscle. This pathway could provide a novel approach to combat skeletal muscle dysfunction in ageing via SERCA modulation using OPG or similar acting agents. However, due to the lack of conclusive evidence to support this possible role of OPG within calcium homeostasis within skeletal muscle, further investigation into determining a proven role for OPG related to calcium homeostasis in skeletal muscle must be conducted in order to understand possible adverse effects to calcium homeostasis as a result of OPG treatments. Despite a role for OPG in calcium homeostasis not being proven in skeletal muscle to date, and the complete role RANK plays within skeletal remains partially understood, potential mechanisms for the action of OPG within skeletal muscle have been suggested (Figure 2). Furthermore, given RANKL deletions are able preserve the loss of muscle specific force but not the loss of muscle mass following denervation, it is likely that inhibition of SERCA allows a prolonged Ca2+ release supporting maintenance of muscle specific forces. However the exact mechanisms between a RANK deletion and reduced SERCA activity need to be fully identified before OPG can be utilised for the manipulation of SERCA and Calcium re uptake [81].
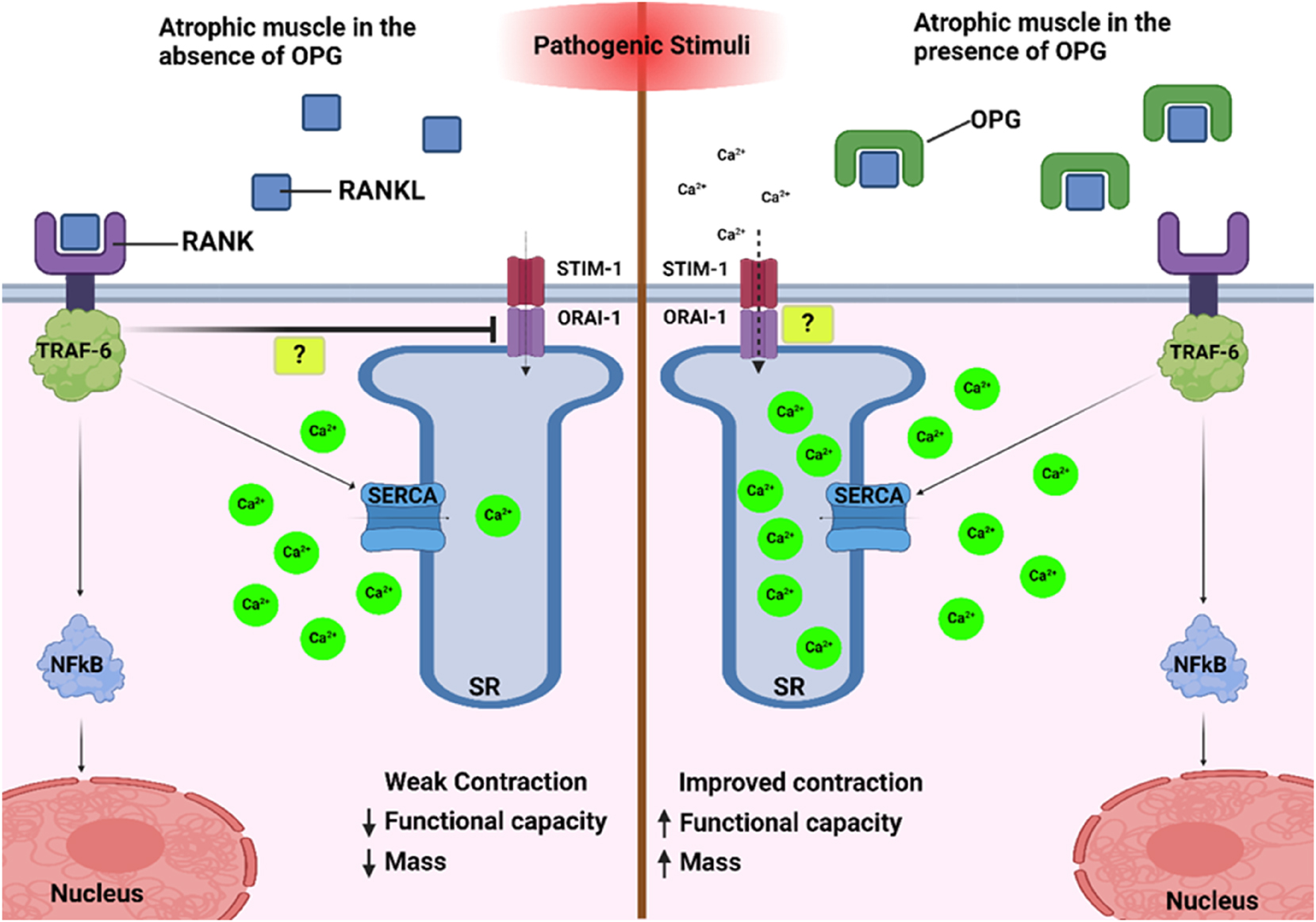
Within atrophic muscle, the absence of OPG allows the RANK/RANKL complex to form, leading to the stimulation of nuclear factor kappa beta (NFkB), in turn activating the ubiquitin-proteosome system and increasing atrophy. In addition, SERCA is weakly upregulated, leading to a reduced ability to transport Ca2+ ions into the sarcoplasmic reticulum (SR) to facilitate contraction. Within this pathway, it is suggested that the STIM-1/ORAI-1 complex method of Ca2+ replenishment is also inhibited, by a yet unknown mechanism. Conversely, when atrophic muscle is exposed to OPG, the RANK/RANKL complex is formed at a much-reduced rate, meaning SERCA activity is inhibited. In response to this inhibition, it is possible the STIM-1/ORAI-1 complex is upregulated, allowing the replenishment of Ca2+ ions into the SR and therefore increasing the capacity of the muscle to contract.
Another potential mechanism in which OPG may interact with skeletal muscle is via the stimulation or reduction of NF-kB. NF-kB activation causes profound muscle wasting by increasing activation of the ubiquitin proteasome system and has been linked to the loss of skeletal muscle mass in a number of physiological and pathophysiological conditions such as sarcopenia and Duchenne muscular dystrophy (DMD) [83]. RANK/RANKL interaction is the upstream activator of NF-kB; when NF-kB activates RANKL it causes stimulation and expression of TNF-α, IL1α, IL-1B [84], 85]. Treatment with anti-RANK molecules inhibits the NF-kB pathway in dystrophic mice, reducing the number of type 2 fibres [76]. OPG has also been shown to secrete from type 2 fast fibres, again highlighting specific fibre type action, and in cell culture models, can provide an anti-inflammatory effect protecting pancreatic beta-cells against TNFα induced insulin resistance [86]. However, more detailed research is required to evolve these mechanistic links to animal and human models. In clinical settings, Denosumab is an anti-RANKL drug that acts to inhibit the RANK/RANKL complex by acting as a decoy receptor for RANK, in much the same way as OPG, and is frequently used treatment for osteoporosis in post-menopausal females. Conversely, with over expression of RANKL, mice demonstrate muscle atrophy, fat infiltration, inflammation, necrosis and lower force production [87]. The clinical use of Denosumab has demonstrated improvements in lean mass and hang grip strength, as well as reductions in further bone fracture [88], 89]. The positive clinical outcomes for the use of the anti-RANK drug provide further rationale for the therapeutic target of OPG. Such interventions, in line with the general healthy ageing guidance regarding physical activity, nutrition and lifestyle would certainly provide a firm basis for the improvements in musculoskeletal health of older adults.
Other notable works investigating the role of OPG has involved the deletion of OPG in differing ages of mice [74]. The absence of OPG in those 1 and 3 months old did not seem to interfere with muscle function, but those of 5 months demonstrated atrophy of type 2B fibres, with an increased over expression of NF-kB, atrogin-1 and MuRF-1. Yet, when treating the 3-month OPG deficient mice with an anti-RANKL treatment for 2 months, muscle function improved both in vivo and ex vivo and biomechanical properties of bone were rescued. In vitro stimulation of RANKL within C2C12 myotubes increased the expression of transcription factors NF-kB, atrogin-1 and MuRF-1. Thus, it is clear that the absence of OPG, and resulting increase in RANKL within the surrounding circulation, results in increased bone resorption and muscle weakness of specific fibres, again primarily type 2, but these losses are somewhat rescued by anti-RANKL treatment [74]. Collectively this evidence further indicates the role that the OPG/RANK/RANKL pathway has within both bone and muscle structure and function [76] and its specificity for fibre type influence.
The inhibition of the activation of RANK by OPG still remains the leading explanation as to how OPG may be able to preserve skeletal muscle mass and integrity, whilst also mitigating the loss of muscle force within sarcopenic and dystrophic muscle [24]. In theory, the action of OPG to inhibit the formation of the RANK/RANKL complex within skeletal muscle leads to decreased Ca2+ concentrations, and uninhibited calcium flux. In dystrophic muscle, where the RANK/RANKL complex is uninhibited by OPG, increased Ca2+ concentration is observed, upregulating dystrophic muscular pathways and leading to decreased skeletal muscle mass and integrity [24], 77].
Summary and outlook
OPG can provide protective effects to both skeletal muscle mass and integrity, in addition to its established role within bone homeostasis [24], 90], and further targeted research may reveal its potential to enhance the beneficial effects of exercise in clinical populations. To understand how OPG could be used within patients it is imperative to understand its precise mechanisms of action and origination within both human skeletal muscle and bone, with current research having focussed outcomes within rodent models, within a number of varying models of physiological environments [24], 91]. As there is a lack of consistency in the molecular forms of OPG used within the literature, no conclusive consensus on the efficacy of it as a therapeutic target can be drawn. However, by understanding the mechanistic capacity of OPG within muscle, it will be possible to understand how OPG acts to preserve muscle mass and determine the effectiveness of OPG to mitigate some of the muscle and bone loss caused through ageing by disuse, disease, and illness. Future work is required to elucidate the molecular mechanisms, effectiveness and efficacy, behind the ability of OPG to retain skeletal muscle mass and integrity, with future research working towards producing a therapeutic model in which OPG’s protective effects could be investigated for use within clinical settings.
Further investigation into the apparent fibre specificity exhibited by both ageing and dystrophic musculature to preferentially decrease both type 2 fibre size and number over type 1 should be conducted to reveal the molecular mechanisms that drive this aligning animal models to those of relevant human population (i.e. aged). In addition to the relatively young mice used within these studies, much of the findings of studies utilising mouse models often fail to translate to humans due to a multiple factors, notably the often overlooked differences in muscle function between mice and humans [92]. Specifically, given the positive outcomes established thus far by the use of denosumab on skeletal muscle [89] and other pathologies such as cancers, often a heightened likelihood with ageing [93], further research should be targeted towards a better understanding of the anti-RANK agents interactions with OPG and muscle. There are many potential side effects with the use of anti-RANK therapies, such as denosumab and bisphosphonates, including hypocalcemia and osteonecrosis [94], 95]. By prioritising the therapeutic exploration of OPG, alternative routes minimising side effects and risk to patients may be uncovered allowing alternative treatment options for skeletal muscle atrophy due to ageing and dystrophies. Finally, it should not be omitted the positive influence that exercise has on OPG, with increases identified in both acute and chronic exercise regimes [12], given the premise of the review herein and the known benefits of exercise for musculoskeletal health in older age [96], 97]. OPG, or other anti-RANK agents used in conjunction with exercise throughout ageing could provide a much more sustainable/achievable approach to managing the reduced quality of life in older age as a result of deteriorations in musculoskeletal health.
Acknowledgments
This Review did not receive any specific funding. Aaron Owens is supported by the Biotechnology and Biological Sciences Research Council (BBSRC) Doctoral Training Partnership. Correspondence for this article should be addressed to Jessica Piasecki, PhD; School of Science & Technology, Nottingham Trent University, New Hall Building Room 168. E-mail: jessica.piasecki@ntu.ac.uk.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Aaron Owens: conceptualisation of the review; selection of studies; writing; figure design; review and editing of manuscript. Lívia Santos: review and editing of manuscript. John Hunt: review and proof reading of manuscript. Matthew Brook: proof reading of manuscript. Mathew Piasecki: neuromuscular physiology expertise; review and editing of manuscript Jessica Piasecki: skeletal muscle physiology expertise; selection of studies; review; editing and proof reading of manuscript.
-
Use of Large Language Models, AI and Machine Learning Tools: None decalred.
-
Conflict of interest: No conflicts of interest.
-
Research funding: This Review did not receive any specific funding. Aaron Owens is supported by the Biotechnology and Biological Sciences Research Council (BBSRC) Doctoral Training Partnership. Correspondence for this article should be addressed to Jessica Piasecki, PhD; School of Science & Technology, Nottingham Trent University, New Hall Building Room 168. E-mail: jessica.piasecki@ntu.ac.uk.
-
Data availability: Not applicable.
References
1. Ageing and health. 2022 [Internet] [cited 2023 Mar 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.Search in Google Scholar
2. de Villiers, TJ, Goldstein, SR. Bone health 2022: an update. Climacteric 2022;25:1–3. https://doi.org/10.1080/13697137.2021.1965408.Search in Google Scholar PubMed
3. Chief medical officer’s annual report 2023 – health in an ageing society: executive summary and recommendations. Available from: https://www.gov.uk/government/publications/chief-medical-officers-annual-report-2023-health-in-an-ageing-society.Search in Google Scholar
4. Keshavarz, M, Xie, K, Schaaf, K, Bano, D, Ehninger, D. Targeting the ‘hallmarks of aging’ to slow aging and treat age-related disease: fact or fiction? Mol Psychiatr 2023;28:242–55. https://doi.org/10.1038/s41380-022-01680-x.Search in Google Scholar PubMed PubMed Central
5. de Magalhães, JP. Distinguishing between driver and passenger mechanisms of aging. Nat Genet 2024;56:204–11. https://doi.org/10.1038/s41588-023-01627-0.Search in Google Scholar PubMed
6. López-Otín, C, Blasco, MA, Partridge, L, Serrano, M, Kroemer, G. The hallmarks of aging. Cell 2013;153:1194–217. https://doi.org/10.1016/j.cell.2013.05.039.Search in Google Scholar PubMed PubMed Central
7. Lu, B, Zhang, T, Yang, F. ‘Bone’ in the brain? osteocalcin-expressing neurons in adult hippocampus promote neurogenesis and suppress anxiety. Biol Psychiatr 2021;89:539–40. https://doi.org/10.1016/j.biopsych.2021.01.001.Search in Google Scholar PubMed
8. Mera, P, Laue, K, Wei, J, Berger, JM, Karsenty, G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab 2016;5:1042–7. https://doi.org/10.1016/j.molmet.2016.07.002.Search in Google Scholar PubMed PubMed Central
9. Marini, F, Giusti, F, Palmini, G, Brandi, ML. Role of Wnt signaling and sclerostin in bone and as therapeutic targets in skeletal disorders. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 2023;34:213–38. https://doi.org/10.1007/s00198-022-06523-7.Search in Google Scholar PubMed
10. Simonet, WS, Lacey, DL, Dunstan, CR, Kelley, M, Chang, MS, Lüthy, R, et al.. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997;89:309–19. https://doi.org/10.1016/s0092-8674(00)80209-3.Search in Google Scholar PubMed
11. Jayash, SN, Al-Namnam, NM, Shaghayegh, G. Osteoprotegerin (OPG) pathways in bone diseases and its application in therapeutic perspectives. Biointerf Res Appl Chem 2020;10:5193–200.10.33263/BRIAC102.193200Search in Google Scholar
12. Tobeiha, M, Moghadasian, MH, Amin, N, Jafarnejad, S. RANKL/RANK/OPG pathway: a mechanism involved in exercise-induced bone remodeling. BioMed Res Int 2020;2020. https://doi.org/10.1155/2020/6910312.Search in Google Scholar PubMed PubMed Central
13. Théoleyre, S, Kwan, TS, Vusio, P, Blanchard, F, Gallagher, J, Ricard-Blum, S, et al.. Characterization of osteoprotegerin binding to glycosaminoglycans by surface plasmon resonance: role in the interactions with receptor activator of nuclear factor kappaB ligand (RANKL) and RANK. Biochem Biophys Res Commun 2006;347:460–7. https://doi.org/10.1016/j.bbrc.2006.06.120.Search in Google Scholar PubMed
14. Baud’huin, M, Lamoureux, F, Duplomb, L, Rédini, F, Heymann, D. RANKL, RANK, osteoprotegerin: key partners of osteoimmunology and vascular diseases. Cell Mol Life Sci 2007;64:2334–50. https://doi.org/10.1007/s00018-007-7104-0.Search in Google Scholar PubMed PubMed Central
15. Yamaguchi, K, Kinosaki, M, Goto, M, Kobayashi, F, Tsuda, E, Morinaga, T, et al.. Characterization of structural domains of human osteoclastogenesis inhibitory factor. J Biol Chem 1998;273:5117–23. https://doi.org/10.1074/jbc.273.9.5117.Search in Google Scholar PubMed
16. Eswarakumar, VP, Lax, I, Schlessinger, J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 2005;16:139–49. https://doi.org/10.1016/j.cytogfr.2005.01.001.Search in Google Scholar PubMed
17. Guerrini, M, Hricovíni, M, Torri, G. Interaction of heparins with fibroblast growth factors: conformational aspects. Curr Pharmaceut Des 2007;13:2045–56. https://doi.org/10.2174/138161207781039733.Search in Google Scholar PubMed
18. Irie, A, Takami, M, Kubo, H, Sekino-Suzuki, N, Kasahara, K, Sanai, Y. Heparin enhances osteoclastic bone resorption by inhibiting osteoprotegerin activity. Bone 2007;41:165–74. https://doi.org/10.1016/j.bone.2007.04.190.Search in Google Scholar PubMed
19. Nagy, EE, Varga-Fekete, T, Puskas, A, Kelemen, P, Brassai, Z, Szekeres-Csiki, K, et al.. High circulating osteoprotegerin levels are associated with non-zero blood groups. BMC Cardiovasc Disord 2016;16:106. https://doi.org/10.1186/s12872-016-0287-2.Search in Google Scholar PubMed PubMed Central
20. Coulson, J, Bagley, L, Barnouin, Y, Bradburn, S, Butler-Browne, G, Gapeyeva, H, et al.. Circulating levels of dickkopf-1, osteoprotegerin and sclerostin are higher in old compared with young men and women and positively associated with whole-body bone mineral density in older adults. Osteoporos Int 2017;28:2683–9. https://doi.org/10.1007/s00198-017-4104-2.Search in Google Scholar PubMed
21. Cawley, KM, Bustamante-Gomez, NC, Guha, AG, MacLeod, RS, Xiong, J, Gubrij, I, et al.. Local production of osteoprotegerin by osteoblasts suppresses bone resorption. Cell Rep 2020;32. https://doi.org/10.1016/j.celrep.2020.108052.Search in Google Scholar PubMed PubMed Central
22. Wright, HL, McCarthy, HS, Middleton, J, Marshall, MJ. RANK, RANKL and osteoprotegerin in bone biology and disease. Curr Rev Musculoskelet Med 2009;2:56–64. https://doi.org/10.1007/s12178-009-9046-7.Search in Google Scholar PubMed PubMed Central
23. Yu, HC, Wu, TC, Chen, MR, Liu, SW, Chen, JH, Lin, KMC. Mechanical stretching induces osteoprotegerin in differentiating C2C12 precursor cells through noncanonical Wnt Pathways. J Bone Miner Res 2010;25:1128–37. https://doi.org/10.1002/jbmr.9.Search in Google Scholar PubMed
24. Dufresne, SS, Dumont, NA, Bouchard, P, Lavergne, É, Penninger, JM, Frenette, J. Osteoprotegerin protects against muscular dystrophy. Am J Pathol 2015;185:920–6. https://doi.org/10.1016/j.ajpath.2015.01.006.Search in Google Scholar PubMed
25. Wilkinson, DJ, Piasecki, M, Atherton, PJ. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev 2018;47:123–32. https://doi.org/10.1016/j.arr.2018.07.005.Search in Google Scholar PubMed PubMed Central
26. Engelke, K, Ghasemikaram, M, Chaudry, O, Uder, M, Nagel, AM, Jakob, F, et al.. The effect of ageing on fat infiltration of thigh and paraspinal muscles in men. Aging Clin Exp Res 2022;34:2089–98. https://doi.org/10.1007/s40520-022-02149-1.Search in Google Scholar PubMed PubMed Central
27. Mitchell, WK, Williams, J, Atherton, P, Larvin, M, Lund, J, Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 2012;3:260. https://doi.org/10.3389/fphys.2012.00260.Search in Google Scholar PubMed PubMed Central
28. Moilanen, A, Kopra, J, Kröger, H, Sund, R, Rikkonen, T, Sirola, J. Characteristics of long-term femoral neck bone loss in postmenopausal women: a 25-year follow-up. J Bone Miner Res 2022;37:173–8. https://doi.org/10.1002/jbmr.4444.Search in Google Scholar PubMed PubMed Central
29. Brotto, M, Bonewald, L. Bone and muscle: interactions beyond mechanical. Bone 2015;80. https://doi.org/10.1016/j.bone.2015.02.010.Search in Google Scholar PubMed PubMed Central
30. Gordon, E, Schimmel, L, Frye, M. The importance of mechanical forces for in vitro endothelial cell biology. Front Physiol 2020. https://doi.org/10.3389/fphys.2020.00684. [Internet] [cited 2023 Mar 1];11. Available from: https://www.frontiersin.org/articles/10.3389/fphys.2020.00684.Search in Google Scholar PubMed PubMed Central
31. Rosa, N, Simoes, R, Magalhães, FD, Marques, AT. From mechanical stimulus to bone formation: a review. Med Eng Phys 2015;37. https://doi.org/10.1016/j.medengphy.2015.05.015.Search in Google Scholar PubMed
32. Parry, SM, Puthucheary, ZA. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extreme Physiol Med 2015;4:16. https://doi.org/10.1186/s13728-015-0036-7.Search in Google Scholar PubMed PubMed Central
33. Genah, S, Monici, M, Morbidelli, L. The effect of space travel on bone metabolism: considerations on today’s major challenges and advances in pharmacology. Int J Mol Sci 2021;22:4585. https://doi.org/10.3390/ijms22094585.Search in Google Scholar PubMed PubMed Central
34. Juhl, OJ, Buettmann, EG, Friedman, MA, DeNapoli, RC, Hoppock, GA, Donahue, HJ. Update on the effects of microgravity on the musculoskeletal system. Npj Microgravity 2021;7:1–15. https://doi.org/10.1038/s41526-021-00158-4.Search in Google Scholar PubMed PubMed Central
35. Frost, HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec 1987;219:1–9. https://doi.org/10.1002/ar.1092190104.Search in Google Scholar PubMed
36. Bass, SL, Eser, P, Daly, R. The effect of exercise and nutrition on the mechanostat. J Musculoskelet Neuronal Interact 2005;5:239–54.Search in Google Scholar
37. Nichols, DL, Sanborn, CF, Essery, EV. Bone density and young athletic women. An update. Sports Med Auckl NZ 2007;37:1001–14. https://doi.org/10.2165/00007256-200737110-00006.Search in Google Scholar PubMed
38. Korhonen, MT, Heinonen, A, Siekkinen, J, Isolehto, J, Alén, M, Kiviranta, I, et al.. Bone density, structure and strength, and their determinants in aging sprint athletes. Med Sci Sports Exerc 2012;44:2340–9. https://doi.org/10.1249/mss.0b013e318267c954.Search in Google Scholar PubMed
39. Ireland, A, Degens, H, Ganse, B, Maden-Wilkinson, TM, Wilks, DC, Rittweger, J. Greater tibial bone strength in male tennis players than controls in the absence of greater muscle output. J Orthop Transl 2015;3:142–51. https://doi.org/10.1016/j.jot.2015.04.001.Search in Google Scholar PubMed PubMed Central
40. Winkler, DG, Sutherland, MK, Geoghegan, JC, Yu, C, Hayes, T, Skonier, JE, et al.. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 2003;22:6267–76. https://doi.org/10.1093/emboj/cdg599.Search in Google Scholar PubMed PubMed Central
41. Isaacson, J, Brotto, M. Physiology of mechanotransduction: how do muscle and bone “talk” to one another? Clin Rev Bone Miner Metab. 2014;12:77–85. https://doi.org/10.1007/s12018-013-9152-3.Search in Google Scholar PubMed PubMed Central
42. Komori, T. Functions of osteocalcin in bone, pancreas, testis, and muscle. Int J Mol Sci 2020;21. https://doi.org/10.3390/ijms21207513.Search in Google Scholar PubMed PubMed Central
43. Ikeda, K, Takeshita, S. The role of osteoclast differentiation and function in skeletal homeostasis. J Biochem (Tokyo) 2016;159:1–8. https://doi.org/10.1093/jb/mvv112.Search in Google Scholar PubMed PubMed Central
44. Hofbauer, LC, Khosla, S, Dunstan, CR, Lacey, DL, Spelsberg, TC, Riggs, BL. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology 1999;140:4367–70. https://doi.org/10.1210/endo.140.9.7131.Search in Google Scholar PubMed
45. Malyankar, UM, Scatena, M, Suchland, KL, Yun, TJ, Clark, EA, Giachelli, CM. Osteoprotegerin is an αvβ3-induced, NF-κB-dependent survival factor for endothelial cells. J Biol Chem 2000;275:20959–62. https://doi.org/10.1074/jbc.c000290200.Search in Google Scholar
46. Cross, SS, Yang, Z, Brown, NJ, Balasubramanian, SP, Evans, CA, Woodward, JK, et al.. Osteoprotegerin (OPG)–a potential new role in the regulation of endothelialcell phenotype and tumour angiogenesis? Int J Cancer 2006;118:1901–8. https://doi.org/10.1002/ijc.21606.Search in Google Scholar PubMed
47. Kobayashi-Sakamoto, M, Hirose, K, Nishikata, M, Isogai, E, Chiba, I. Osteoprotegerin protects endothelial cells against apoptotic cell death induced by Porphyromonas gingivalis cysteine proteinases. FEMS Microbiol Lett 2006;264:238–45. https://doi.org/10.1111/j.1574-6968.2006.00458.x.Search in Google Scholar PubMed
48. Price, PA, June, HH, Buckley, JR, Williamson, MK. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol 2001;21:1610–6. https://doi.org/10.1161/hq1001.097102.Search in Google Scholar PubMed
49. Ueland, T, Yndestad, A, Øie, E, Florholmen, G, Halvorsen, B, Frøland, SS, et al.. Dysregulated osteoprotegerin/RANK ligand/RANK Axis in clinical and experimental heart failure. Circulation 2005;111:2461–8. https://doi.org/10.1161/01.cir.0000165119.62099.14.Search in Google Scholar
50. Ziegler, S, Kudlacek, S, Luger, A, Minar, E. Osteoprotegerin plasma concentrations correlate with severity of peripheral artery disease. Atherosclerosis 2005;182:175–80. https://doi.org/10.1016/j.atherosclerosis.2005.01.042.Search in Google Scholar PubMed
51. Helske, S, Kovanen, PT, Lindstedt, KA, Salmela, K, Lommi, J, Turto, H, et al.. Increased circulating concentrations and augmented myocardial extraction of osteoprotegerin in heart failure due to left ventricular pressure overload. Eur J Heart Fail 2007;9:357–63. https://doi.org/10.1016/j.ejheart.2006.10.015.Search in Google Scholar PubMed
52. Pritzker, LB, Scatena, M, Giachelli, CM. The role of osteoprotegerin and tumor necrosis factor-related apoptosis-inducing ligand in human microvascular endothelial cell survival. Mol Biol Cell 2004;15:2834–41. https://doi.org/10.1091/mbc.e04-01-0059.Search in Google Scholar PubMed PubMed Central
53. McGonigle, JS, Giachelli, CM, Scatena, M. Osteoprotegerin and RANKL differentially regulate angiogenesis and endothelial cell function. Angiogenesis 2009;12:35–46. https://doi.org/10.1007/s10456-008-9127-z.Search in Google Scholar PubMed
54. Benslimane-ahmim, Z, Heymann, D, Dizier, B, Lokajczyk, A, Brion, R, Laurendeau, I, et al.. Osteoprotegerin, a new actor in vasculogenesis, stimulates endothelial colony-forming cells properties. J Thromb Haemost 2011;9:834–43. https://doi.org/10.1111/j.1538-7836.2011.04207.x.Search in Google Scholar PubMed
55. Nordström, A, Karlsson, C, Nyquist, F, Olsson, T, Nordström, P, Karlsson, M. Bone loss and fracture risk after reduced physical activity. J Bone Miner Res 2005;20:202–7. https://doi.org/10.1359/jbmr.041012.Search in Google Scholar
56. Kusumi, A, Sakaki, H, Kusumi, T, Oda, M, Narita, K, Nakagawa, H, et al.. Regulation of synthesis of osteoprotegerin and soluble receptor activator of nuclear factor-kappaB ligand in normal human osteoblasts via the p38 mitogen-activated protein kinase pathway by the application of cyclic tensile strain. J Bone Miner Metab 2005;23:373–81. https://doi.org/10.1007/s00774-005-0615-6.Search in Google Scholar PubMed
57. Kim, CH, You, L, Yellowley, CE, Jacobs, CR. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone 2006;39:1043–7. https://doi.org/10.1016/j.bone.2006.05.017.Search in Google Scholar PubMed
58. Saunders, MM, Taylor, AF, Du, C, Zhou, Z, Pellegrini, VD, Donahue, HJ. Mechanical stimulation effects on functional end effectors in osteoblastic MG-63 cells. J Biomech 2006;39:1419–27. https://doi.org/10.1016/j.jbiomech.2005.04.011.Search in Google Scholar PubMed
59. Tang, L, Lin, Z, Li, Y. Effects of different magnitudes of mechanical strain on Osteoblasts in vitro. Biochem Biophys Res Commun 2006;344:122–8. https://doi.org/10.1016/j.bbrc.2006.03.123.Search in Google Scholar PubMed
60. West, SL, Scheid, JL, De Souza, MJ. The effect of exercise and estrogen on osteoprotegerin in premenopausal women. Bone 2009;44:137–44. https://doi.org/10.1016/j.bone.2008.09.008.Search in Google Scholar PubMed
61. Scott, JPR, Sale, C, Greeves, JP, Casey, A, Dutton, J, Fraser, WD. The effect of training status on the metabolic response of bone to an acute bout of exhaustive treadmill running. J Clin Endocrinol Metab 2010;95:3918–25. https://doi.org/10.1210/jc.2009-2516.Search in Google Scholar PubMed
62. Bergström, I, Parini, P, Gustafsson, SA, Andersson, G, Brinck, J. Physical training increases osteoprotegerin in postmenopausal women. J Bone Miner Metab 2012;30:202–7. https://doi.org/10.1007/s00774-011-0304-6.Search in Google Scholar PubMed
63. Marques, EA, Wanderley, F, Machado, L, Sousa, F, Viana, JL, Moreira-Gonçalves, D, et al.. Effects of resistance and aerobic exercise on physical function, bone mineral density, OPG and RANKL in older women. Exp Gerontol 2011;46:524–32. https://doi.org/10.1016/j.exger.2011.02.005.Search in Google Scholar PubMed
64. Maïmoun, L, Coste, O, Philibert, P, Briot, K, Mura, T, Galtier, F, et al.. Peripubertal female athletes in high-impact sports show improved bone mass acquisition and bone geometry. Metab – Clin Exp. 2013;62:1088–98. https://doi.org/10.1016/j.metabol.2012.11.010.Search in Google Scholar PubMed
65. Kish, K, Mezil, Y, Ward, WE, Klentrou, P, Falk, B. Effects of plyometric exercise session on markers of bone turnover in boys and young men. Eur J Appl Physiol 2015;115:2115–24. https://doi.org/10.1007/s00421-015-3191-z.Search in Google Scholar PubMed
66. McPhee, JS, French, DP, Jackson, D, Nazroo, J, Pendleton, N, Degens, H. Physical activity in older age: perspectives for healthy ageing and frailty. Biogerontology 2016;17:567–80. https://doi.org/10.1007/s10522-016-9641-0.Search in Google Scholar PubMed PubMed Central
67. Santilli, V, Bernetti, A, Mangone, M, Paoloni, M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab Off J Ital Soc Osteoporos Miner Metab Skelet Dis. 2014;11:177–80.10.11138/ccmbm/2014.11.3.177Search in Google Scholar
68. de Villiers, TJ, Goldstein, SR. Bone health 2022: an update. Climacteric J Int Menopause Soc. 2022;25:1–3. https://doi.org/10.1080/13697137.2021.1965408.Search in Google Scholar PubMed
69. Verbrugge, LM, Latham, K, Clarke, PJ. Aging with disability for midlife and older adults. Res Aging 2017;39:741–77. https://doi.org/10.1177/0164027516681051.Search in Google Scholar PubMed
70. Kearns, AE, Khosla, S, Kostenuik, P. RANKL and OPG regulation of bone remodeling in health and disease. Endocr Rev 2007;29:155–92. https://doi.org/10.1210/er.2007-0014.Search in Google Scholar PubMed PubMed Central
71. Chandra, A, Rajawat, J. Skeletal aging and osteoporosis: mechanisms and therapeutics. Int J Mol Sci 2021;22:3553. https://doi.org/10.3390/ijms22073553.Search in Google Scholar PubMed PubMed Central
72. Longhofer, LK, Chong, A, Strong, NM, Wooley, PH, Yang, SY. Specific material effects of wear-particle-induced inflammation and osteolysis at the bone–implant interface: a rat model. J Orthop Transl 2017;8:5–11. https://doi.org/10.1016/j.jot.2016.06.026.Search in Google Scholar PubMed PubMed Central
73. Ulrich-Vinther, M, Carmody, EE, Goater, JJ, Soøballe, K, O’Keefe, RJ, Schwarz, EM. Recombinant adeno-associated virus-mediated osteoprotegerin gene therapy inhibits wear debris-induced osteolysis. J Bone Jt Surg – Ser A 2002;84:1405–12. https://doi.org/10.2106/00004623-200208000-00017.Search in Google Scholar PubMed
74. Bucay, N, Sarosi, I, Dunstan, CR, Morony, S, Tarpley, J, Capparelli, C, et al.. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 1998;12:1260–8. https://doi.org/10.1101/gad.12.9.1260.Search in Google Scholar PubMed PubMed Central
75. Dufresne, SS, Boulanger-Piette, A, Bossé, S, Argaw, A, Hamoudi, D, Marcadet, L, et al.. Genetic deletion of muscle RANK or selective inhibition of RANKL is not as effective as full-length OPG-fc in mitigating muscular dystrophy. Acta Neuropathol Commun 2018;6:1–10. https://doi.org/10.1186/s40478-018-0533-1.Search in Google Scholar PubMed PubMed Central
76. Hamoudi, D, Marcadet, L, Piette Boulanger, A, Yagita, H, Bouredji, Z, Argaw, A, et al.. An anti-RANKL treatment reduces muscle inflammation and dysfunction and strengthens bone in dystrophic mice. Hum Mol Genet 2019;28:3101–12. https://doi.org/10.1093/hmg/ddz124.Search in Google Scholar PubMed
77. Dufresne, S, Boulanger-Piette, A, Bossé, S, Frenette, J. Physiological role of receptor activator nuclear factor-kB (RANK) in denervation-induced muscle atrophy and dysfunction. Recept Clin Investig 2016;30:1323–1. https://doi.org/10.14800/rci.1323.Search in Google Scholar PubMed PubMed Central
78. Ciciliot, S, Rossi, AC, Dyar, KA, Blaauw, B, Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol 2013;45:2191–9. https://doi.org/10.1016/j.biocel.2013.05.016.Search in Google Scholar PubMed
79. Venturelli, M, Morgan, GR, Tarperi, C, Zhao, J, Naro, F, Reggiani, C, et al.. Physiological determinants of mechanical efficiency during advanced ageing and disuse. J Physiol 2024;602:355–72. https://doi.org/10.1113/jp285639.Search in Google Scholar
80. Mateos-Aierdi, AJ, Goicoechea, M, Aiastui, A, Fernández-Torrón, R, Garcia-Puga, M, Matheu, A, et al.. Muscle wasting in myotonic dystrophies: a model of premature aging. Front Aging Neurosci 2015;7:125. https://doi.org/10.3389/fnagi.2015.00125. [Internet] [cited 2024 Sep 16] Available from: https://www.frontiersin.org/journals/aging-neuroscience/articles/10.3389/fnagi.2015.00125/full.Search in Google Scholar PubMed PubMed Central
81. Dufresne, SS, Dumont, NA, Boulanger-Piette, A, Fajardo, VA, Gamu, D, Kake-Guena, SA, et al.. Muscle RANK is a key regulator of Ca2+ storage, SERCA activity, and function of fast-twitch skeletal muscles. Am J Physiol-Cell Physiol 2016;310:C663–72. https://doi.org/10.1152/ajpcell.00285.2015.Search in Google Scholar PubMed PubMed Central
82. Shin, J, Nunomiya, A, Gonda, K, Nagatomi, R. Specification of skeletal muscle fiber-type is determined by the calcineurin/NFATc1 signaling pathway during muscle regeneration. Biochem Biophys Res Commun 2023;659:20–8. https://doi.org/10.1016/j.bbrc.2023.03.032.Search in Google Scholar PubMed
83. Cai, D, Lee, KKH, Li, M, Tang, MK, Chan, KM. Ubiquitin expression is up-regulated in human and rat skeletal muscles during aging. Arch Biochem Biophys 2004;425:42–50. https://doi.org/10.1016/j.abb.2004.02.027.Search in Google Scholar PubMed
84. Ock, S, Ahn, J, Lee, SH, Park, H, Son, JW, Oh, JG, et al.. Receptor activator of nuclear factor-κB ligand is a novel inducer of myocardial inflammation. Cardiovasc Res 2012;94:105–14. https://doi.org/10.1093/cvr/cvs078.Search in Google Scholar PubMed
85. Zhao, Z, Hou, X, Yin, X, Li, Y, Duan, R, Boyce, BF, et al.. TNF induction of NF-κB RelB enhances RANKL-induced osteoclastogenesis by promoting inflammatory macrophage differentiation but also limits it through suppression of NFATc1 expression. PLoS One 2015;10:e0135728. https://doi.org/10.1371/journal.pone.0135728.Search in Google Scholar PubMed PubMed Central
86. Rutti, S, Dusaulcy, R, Hansen, JS, Howald, C, Dermitzakis, ET, Pedersen, BK, et al.. Angiogenin and Osteoprotegerin are type II muscle specific myokines protecting pancreatic beta-cells against proinflammatory cytokines. Sci Rep 2018;8:10072. https://doi.org/10.1038/s41598-018-28117-2.Search in Google Scholar PubMed PubMed Central
87. Bonnet, N, Bourgoin, L, Biver, E, Douni, E, Ferrari, S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest 2019;129:3214–23. https://doi.org/10.1172/jci125915.Search in Google Scholar PubMed PubMed Central
88. Miedany, YE, Gaafary, ME, Toth, M, Hegazi, MO, Aroussy, NE, Hassan, W, et al.. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol 2021;40:4225–32. https://doi.org/10.1007/s10067-021-05757-w.Search in Google Scholar PubMed
89. Rupp, T, von Vopelius, E, Strahl, A, Oheim, R, Barvencik, F, Amling, M, et al.. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int 2022;33:2177–84. https://doi.org/10.1007/s00198-022-06470-3.Search in Google Scholar PubMed PubMed Central
90. Min, H, Morony, S, Sarosi, I, Dunstan, CR, Capparelli, C, Scully, S, et al.. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med 2000;192:463–74. https://doi.org/10.1084/jem.192.4.463.Search in Google Scholar PubMed PubMed Central
91. Wise, GE, Yao, S, Liu, D. Injections of osteoprotegerin and PMA delay tooth eruption. Clin Anat 2006;19:19–24. https://doi.org/10.1002/ca.20144.Search in Google Scholar PubMed
92. Hu, X, Charles, JP, Akay, T, Hutchinson, JR, Blemker, SS. Are mice good models for human neuromuscular disease? Comparing muscle excursions in walking between mice and humans. Skelet Muscle 2017;7:26. https://doi.org/10.1186/s13395-017-0143-9.Search in Google Scholar PubMed PubMed Central
93. De Leon-Oliva, D, Barrena-Blázquez, S, Jiménez-Álvarez, L, Fraile-Martinez, O, García-Montero, C, López-González, L, et al.. The RANK–RANKL–OPG system: a multifaceted regulator of homeostasis, immunity, and cancer. Medicina (Mex). 2023;59:1752. https://doi.org/10.3390/medicina59101752.Search in Google Scholar PubMed PubMed Central
94. Tofé, VI, Bagán, L, Bagán, JV. Osteonecrosis of the jaws associated with denosumab: study of clinical and radiographic characteristics in a series of clinical cases. J Clin Exp Dent 2020;12:e676–81. https://doi.org/10.4317/jced.57019.Search in Google Scholar PubMed PubMed Central
95. Kalayanamitra, R, Yaghnam, I, Patel, R, Groff, A, Jain, R. The calcium culprit: a case of denosumab-induced hypocalcemia. Cureus;11:e4768. https://doi.org/10.7759/cureus.4768.Search in Google Scholar PubMed PubMed Central
96. Peterson, MD, Sen, A, Gordon, PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc 2011;43:249–58. https://doi.org/10.1249/mss.0b013e3181eb6265.Search in Google Scholar
97. El-Khoury, F, Cassou, B, Charles, MA, Dargent-Molina, P. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: systematic review and meta-analysis of randomised controlled trials. The BMJ 2013;347:f6234. https://doi.org/10.1136/bmj.f6234.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter on behalf of Shangai Jiao Tong University and Guangzhou Sport University
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- Validation of marathon performance model based on physiological factors in world-class East African runners: a case series
- Mitochondrial and cardiovascular responses to aerobic exercise training in supine and upright positions in healthy young adults: a randomized parallel arm trial
- Exercise-induced neurogenesis through BDNF-TrkB pathway: implications for neurodegenerative disorders
- The role of osteoprotegerin (OPG) in exercise-induced skeletal muscle adaptation
- Section: Personalized and advanced exercise prescription for health and chronic diseases
- Impact of early and late morning supervised blood flow restriction training on body composition and skeletal muscle performance in older inactive adults
- Section: Interaction of exercise with diet, nutrition and/or medication
- Anti-obesity drugs alone or combined with exercise training in the management of obesity: a systematic review with meta-analysis
Articles in the same Issue
- Frontmatter
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- Validation of marathon performance model based on physiological factors in world-class East African runners: a case series
- Mitochondrial and cardiovascular responses to aerobic exercise training in supine and upright positions in healthy young adults: a randomized parallel arm trial
- Exercise-induced neurogenesis through BDNF-TrkB pathway: implications for neurodegenerative disorders
- The role of osteoprotegerin (OPG) in exercise-induced skeletal muscle adaptation
- Section: Personalized and advanced exercise prescription for health and chronic diseases
- Impact of early and late morning supervised blood flow restriction training on body composition and skeletal muscle performance in older inactive adults
- Section: Interaction of exercise with diet, nutrition and/or medication
- Anti-obesity drugs alone or combined with exercise training in the management of obesity: a systematic review with meta-analysis


