Abstract
Objectives
Whether low-load resistance training (RT) without muscle failure, with or without blood flow restriction (BFR), is sufficient to increase strength and muscle growth of calf muscles in trained individuals is still unclear. This study aimed to compare the effects of low-intensity BFR RT vs. traditional low-intensity RT (noBFR) with moderate training volume on strength and circumference.
Methods
We designed a parallel, randomized controlled trial including 36 RT-trained participants (BFR: 7 females, 32.9 ± 8.8 years, 11 males, 28.4 ± 3.6 years; noBFR; 8 females, 29.6 ± 3.4 years; 10 males, 28.6 ± 4.9 years) who underwent eight weeks of twice-weekly low-load RT with a total of 16 RT sets (30 % of one-repetition maximum [1RM]). RT consisted of bilateral calf raises and seated unilateral calf raises, each conducted with 4 sets (30, 15, 15, 15 repetitions not to failure) of either BFR or noBFR. Outcome measures included calf circumference (CC), leg stiffness (LS), and various strength tests (seated and standing calf raise 1RM, isokinetic strength of plantar- and dorsiflexion).
Results
There were no significant interactions or group effects for most measures. Both groups showed significant improvements in seated calf raise strength (p=0.046, η 2 p=0.17). Pairwise comparisons indicated moderate to large effect sizes for strength improvements (standardized mean differences: 0.35–1.11), but no changes in calf circumference were observed in either group.
Conclusions
Low-load RT with and without BFR are useful to increase strength without necessarily affecting hypertrophy. Low-intensity BFR training did not confer additional benefits over traditional low-intensity RT for calf muscle strength or circumference, questioning its general advantage under such conditions.
Introduction
The maintenance of muscle mass and strength is crucial for supporting health, well-being, and physical performance throughout the lifespan [1], [2], [3], [4]. Resistance training (RT) has emerged as the most effective method to increase strength and muscle mass in nearly all age groups [5] and has been recommended for everyone by the World Health Organization (WHO) as lifelong training strategy [6]. RT exerts a mechanical stimulation on skeletal muscle, inducing increases in muscular strength and protein synthesis, which, in the long term, accumulates myofibrillar proteins in skeletal muscle, leading to hypertrophy [7].
However, reduced physical activity due to aging [8] or disease [9] significantly reduces strength and muscle mass, particularly affecting the lower limb muscles [10]. Maximal strength capacity reaches its peak between the ages of 20 and 30, and begins a gradual decline by the time individuals reach their 40s [11]. Muscle mass, on the other hand, has been shown to decrease by 3–8 % per decade in both men and women after the age of 30 [12].
The calves (trizeps surae), consisting of the soleus muscle as well as the medial and lateral gastrocnemius, are particularly prone to strength and muscle loss upon immobilization [10, 13, 14]. Even astronauts who train extensively onboard the International Space Station (ISS) lose up to 15 % of their soleus muscle mass during six months of spaceflight [15]. Functionally, the calf muscles are vital for stabilizing the body during gait and preventing falls emphasizing the importance to maintain their functional capacity during aging [16].
In contrast, calf muscles are sometimes considered more resistant to growth compared to other muscle groups [17, 18] and a study has shown that the anabolic response to acute bouts of RT is significantly lower in the soleus muscle compared to the knee extensors [19]. Nevertheless, studies have shown that the calf muscles can adapt to RT in terms of hypertrophy and strength to a certain extent [20, 21]. For example, after an 8 week RT intervention (3 sessions/week, 4 sets of 9–13 RM each), Weiss et al. [17] found an increase in strength of 13 % in both men and women. A recent study by Kassiano et al. [20] demonstrated that after six weeks of RT three times per week, depending on the RT volume (6–12 sets/week with 15–20 repetitions), there were significant improvements in calf muscle thickness measured via ultrasound in untrained women. A study by Kinoshita and colleagues [21] compared the effects of standing vs. seated calf raise RT (12 weeks, twice weekly, 5 sets of 10 repetitions) on triceps surea muscle hypertrophy and found that both exercises led to significant increases in muscle volume measured using magnetic resonance imaging.
In contrast to traditional RT, another strength training method known as blood flow restriction (BFR) training has been established in recent decades. This technique involves using low training intensities (typically <40 % 1RM) and reducing arterial inflow to the working muscles while preventing venous return flow using elastic bands or specialized cuffs applied proximally on the extremities [22, 23]. The effectiveness of BFR calf training has been studied in previous research. For example, Gavanda et al. [24] compared the effects of a six-week low-intensity calf muscle RT (twice a week, 4 sets at 30 % of one-repetition maximum [RM] to concentric muscle failure) with and without blood flow restriction (BFR) on muscle thickness and performance. They found significant increases in gastrocnemius muscle thickness measured by ultrasound, as well as strength increases of 25 % in the BFR group compared to 21 % in the intervention group without BFR. Two other BFR training studies by Centner et al. [25, 26] demonstrated significant but comparable increases in plantar flexion strength and the cross-sectional area of the gastrocnemius medialis muscle after 14 weeks of thrice-weekly sitting and standing calf raises. Participants in the BFR groups used low loads (20–35 % 1RM, first set 30 reps, then three sets of 15 repetitions) or a conventional high-load RT protocol using 70–85 % 1RM (3 sets, 6–12 reps).
From the available data, it can be concluded that calf training with BFR appears to be promising in terms of hypertrophy and strength gains. Especially for patients who are likely unable to regularly lift heavier weights [27], [28], [29]. However, the search for a highly effective training regimen to consistently induce calf muscle growth and strength is still ongoing, and there is a lack of additional data to make recommendations for effective calf training. For example, the total number of participants in the study by Gavanda et al. [24] was quite small (BFR group n=12; no BFR group n=9), and the intervention period of six weeks was relatively short. Furthermore, the weekly volume of eight RT sets was relatively low for trained participants (8 sets per week). On the other hand, the interventions by Centner et al. [25, 26] were considerably longer (14 weeks), and the number of participants was slightly higher (high-load RT groups n=14 and n=15; BFR groups n=11 and n=14). However, these studies, together with a study by Kataoka et al. [18], lack the comparison of identical intensities, for example, comparing 30 % BFR with 30 % traditional RT.
Whether BFR can further and reliably enhance the adaptation of calf muscles with moderate volume and without muscle failure is still controversial. Therefore, the aim of this study was to investigate the effects of a traditional low-intensity RT compared to a standard BFR RT protocol, both not to failure, on strength and calf circumference in trained participants with an appropriate training volume (16 weekly sets) [24, 30]. We assume that a traditional low-intensity RT, when combined with BFR, will produce significant improvements in both strength and calf circumference. The potential impact of our findings could provide valuable insights into developing more effective RT regimens for maintaining muscle mass and strength. This could contribute to improved physical function and quality of life in various populations. The summary of this article is presented in Figure 1.

Graphical representation of this article. Figure created with BioRender.
Methods
Study design
A parallel, two-group, randomized study design was used to assess the effects of an 8-week, twice-weekly low-intensity BFR or low-intensity conventional calf muscle RT (noBFR) on functional and structural adaptations. Participants were assigned to groups using blocked randomization via studyrandomizer.com to ensure equal distribution. The independent variables were the two training modalities (BFR and noBFR). The dependent variables were calf circumference (CC), leg stiffness (LS), maximum plantar flexion strength tests, and isokinetic ankle strength tests. Prior to the investigation, ethical clearance was obtained from the IST University of Applied Sciences ethics committee (Ethic Decision No. 022023IST233) according to the Declaration of Helsinki [31].
Participants
Before the recruitment of volunteers, an a priori power analysis for repeated measures analyses of variance (2 groups, 4 measurements) was conducted using G*Power software (Version 3.1.9.7, Universität Düsseldorf, Düsseldorf, Germany) to determine the number of participants needed. The analysis was based on detecting a medium effect size (f=0.25) as seen in a previous study [24], with a significance level of α=0.05 and a desired power of 1-β=0.95 [32]. According to this power analysis, a total of 36 subjects would have been sufficient. However, to account for possible dropouts, a total of 43 healthy men and women between the ages of 18 and 45 were initially verbally recruited in a rehabilitation center. Participants had to be healthy with no absolute or relative contraindications to BFR training [33] and no lower extremity injury in the past 6 months prior to this study. Additionally, volunteers were required to have at least two years of RT experience and not to be taking any legal (i.e., nutritional supplements) or illegal performance-enhancing substances (i.e., anabolic steroids). Informed consent was obtained from all individuals included in this study. One subject was eliminated because it was technically not possible to determine arterial occlusion pressure (AOP). Six participants did not reach the minimum of 12 out of 16 training sessions (75 % adherence) and were therefore also eliminated from the study. Data from 36 subjects were included for final analysis (BFR: 7 females, 32.9 ± 8.8 years, 171 ± 6 cm, 62.6 ± 8.5 kg; 11 males, 28.4 ± 3.6 years, 181 ± 8 cm, 82.2 ± 13.0 kg; noBFR; 8 females, 29.6 ± 3.4 years, 166 ± 5 cm, 65.6 ± 6.6 kg; 10 males, 28.6 ± 4.9 years, 181 ± 7 cm, 84.5 ± 12.9 kg).
Testing
All tests were conducted in a rehabilitation center by the same researchers, using the same equipment, and at a similar time of the day. All participants were familiar with the tests employed in this study, having undergone these assessments previously. Participants were instructed to refrain from any rigorous exercise 48 h prior to the start of the study.
Anthropometry
First, participants’ body weight (BW) was measured using a digital scale (ES-26M-W, Renpho, Eastvale, California, USA) and height was asked for descriptive purposes. Subsequently, CC of both shanks was measured by a well-trained, experienced member of the research team 10 cm below the patella using a tape measure. Such measurements have been previously used and reported to demonstrate excellent reliability [34]. The mean of both sides of a participant was used for further analysis.
Leg stiffness
Following a low intensity, 10-min warm-up on a stationary bike (Cybex 770C Upright Fitness Bike, Cybex International, Inc., Owatonna, Minnesota, USA), LS was assessed using the Optojump photocell system (Microgate Srl, Bolzano, Italy) [24]. Participants first completed 20, 10 and then 7 hops with their hands on hips, with increasing intensity and 30, 60, and 90 s of rest between sets, respectively. They then completed two trials of a seven-maximal hopping test with 90 s of rest between trials. Volunteers were instructed to jump as high as possible with minimal ground contact time. Mean flight and ground contact times from the seven jumps of a single trial, along with participants’ BW, were used to calculate LS using the formula proposed by Ruggiero et al. [35]. The best LS value from the two trials was used for the analysis.
One-repetition maximum
Thereafter, 1RM testing was performed for the bilateral calf raise with extended knees using a seated leg press (Quadrus Funktionsstemme, Kanzlsperger GmbH, Berngau, Germany). Participants first completed three warm-up sets (10, 5, and 2 repetitions) with increasing intensity (80, 100, 120 % of their BW), and 60, 90, and 120 s of rest between sets. The 1RM was then assessed by increasing the resistance for a single repetition until a valid attempt could no longer be performed [36, 37]. A repetition was considered valid if the weight could be lifted through the full range of motion (ROM), verified using a digital goniometer (EasyAngle, Meloq AB, Stockholm, Sweden). The 1RM was identified in all participants within a maximum of three trials.
Next, the 1RM in the unilateral seated calf raise with 90° flexed knees (NA9, Kaphingst GmbH, Lahntal, Germany) was estimated using the Epley formula [38]. The reason we estimated rather than actually testing the 1RM was because the seated calf raise machine did not offer enough resistance for our trained participants. In a previous study, the Epley formula was found to have a low average error [38] and was deemed accurate for machine exercise testing of plantar flexion [39]. The warm-up consisted of 10 repetitions at 70 % BW for each leg. Following a 60 s rest period, participants completed as many valid repetitions as possible at 130 % of their BW per leg. The criteria for a valid repetition correspond to the requirements described in the previous section. Subsequently, the 1RM was calculated from the repetitions completed and the resistance used. The mean of both sides of a participant was used for further analysis.
Isokinetic strength
Afterwards, isokinetic strength testing of plantar- and dorsiflexion was performed using the CON-TREX® MJ dynamometer. Isokinetic testing has been reported to be reliable and valid for assessing ankle strength parameters [40, 41]. For this, an ankle adapter adjusted at 4 cm height (PHYSIOMED ELEKTROMEDIZIN AG, Schnaittach, Germany), the trunk, the pelvis at 20° hip flexion, and the tested limb was fixed with constraining and Velcro straps. In addition, a hard roll of foam with a diameter of 10 cm was placed under the knees for support. The arms were crossed over the chest and the leg not being tested was placed in a rest position. The participants then performed five submaximal exercise cycles (repetitions) at 60°/s as a specific warm-up. Each cycle included a movement from full plantar flexion to full dorsiflexion (with −3° in each position for safety reasons) and back. Subsequently, to determine mean peak torque in Newton-meter two tests with five repetitions at 60°/s and one test with 25 repetitions at 210°/s and maximal contraction per leg were carried out with 2 min of rest in-between.
Post-testing occurred three to five days after the last training session [24]. All tests were carried out identically to the pre-tests.
Cuff pressure
In the participants allocated to the BFR group, the individual AOP was determined prior to the first training session following the protocol proposed by Vehrs et al. [42]. For this, a 7 cm wide pneumatic BFR cuff (Occlusion Cuff Elite®, The Occlusion Cuff®, Belfast, United Kingdom) was applied to participants’ right leg as proximally as possible. Volunteers were then placed half-sitting on a therapy bench to prevent the cuff from being compressed by the benches surface. Subsequently, pulse sounds were monitored dorsomedially at the level of the ankle joint on the anterior tibial artery using a hand-held Doppler device (The Occlusion Cuff Vascular Doppler, The Occlusion Cuff®, Belfast, United Kingdom). The cuff was then gradually inflated every 10 s by 10 mmHg starting from 50 mmHg until pulse sounds were no longer detectable. This pressure was defined as 100 % AOP. During the intervention, 60 % AOP was used in the BFR group, as described earlier [43]. The mean AOP was 209 ± 54 mmHg, with a range from 130 to 330 mmHg.
Intervention
The intervention lasted eight weeks and consisted of two RT sessions per week. All participants agreed to refrain from any additional lower-body RT for the duration of the study. Additionally, they were instructed to maintain their normal dietary habits to minimize dietary bias. Training sessions were carried out on non-consecutive days with a minimum of 48 and maximum of 96 h between. All training sessions were supervised by a member of the research team or a qualified therapist with a trainer to participant ratio of 1:1–4. Prior to each training session, a standardized warm-up was completed, consisting of 10 minutes of low intensity cycling on a stationary bike (Cybex 770C Upright Fitness Bike, Cybex International, Inc., Owatonna, Minnesota, USA). Thereafter, two calf exercises were performed in randomized order. The bilateral calf raises with knees extended and unilateral seated calf raises, as described in the testing section. For both exercises, one set of 30 and three sets of 15 repetitions using 30 % of their pre-intervention 1RM with 30 s of inter-set rest were completed as described by Patterson and colleagues [43]. Cadence of every repetition was set as 2 s eccentric and 1 s concentric, with no isometric hold at the top or the bottom. Each repetition was performed with full ROM, either with or without BFR, according to group assignment. In the BFR group, the cuffs were placed and inflated to 60 % AOP before the first set of the first exercise, as described previously, and not loosened or taken off during the inter-set rest periods until the last set of the second exercise was completed.
Statistics
The summary statistics are presented as mean ± standard deviation. Relevant data were initially tested and verified for normal distribution via Shapiro-Wilk tests and variance homogeneity. To examine baseline group differences (BFR vs. noBFR) for age, height, and BW, several 2 (group: BFR vs. noBFR) × 2 (sex: male vs. female) repeated measures analyses of variance (rANOVA) were conducted. Furthermore, separate 2 (group: BFR vs. noBFR) × 2 (time: pre vs. post) × 2 (sex: male vs. female) repeated measures analyses of variance with covariate (rANCOVA) were conducted for each outcome measure: CC, LS, leg press calf raise 1RM, seated calf raise 1RM, plantar flexion 5RM, dorsal extension 5RM, plantar flexion 25RM, and dorsal extension 25RM. Pre-intervention individual values were used as covariates. Effect sizes for rANOVA are provided as partial eta squared (η 2 p), with ≥0.01, ≥0.06, ≥0.14 indicating small, moderate, and large effects, respectively [33]. In case of significant interaction, group, time, or sex effects, relevant Bonferroni post-hoc tests were subsequently computed. Additionally, to examine group differences in total training sessions, an independent t-test was calculated. For pairwise effect size comparison, standardized mean differences (SMD) were calculated as the differences between means divided by the pooled standard deviations (trivial: |SMD|<0.2, small: 0.2≤|SMD|<0.5, moderate: 0.5≤|SMD|<0.8, large: |SMD|≥0.8) [33]. Statistical analyses were performed using R in its integrated development environment RStudio (version 4.1.1; The R Foundation for Statistical Computing). For all calculations, an α-level of 0.05 was used as threshold for statistical significance.
Results
Output parameters
The rANCOVA revealed no statistically significant interaction (0.056≤p≤0.941; 0.01≤η 2 p≤0.06) or group (0.162≤p≤0.996; 0.01≤η 2 p≤0.02) effects for all output parameters (Table 1). While seated calf raise 1RM revealed statistically significant time effects (p=0.046; η 2 p=0.17;), all other output parameters showed no relevant time effects (0.275≤p≤0.900; 0.01≤η 2 p≤0.06) regarding the rANCOVA results. However, leg press calf raise 1RM (Figure 3), seated calf raise 1RM (Figure 3), plantar flexion 5RM (Figure 4), dorsal extension 5RM (Figure 4) and plantar flexion 25RM (Figure 5) revealed moderate to large pairwise pre to post comparison effect sizes (0.35≤SMD≤1.11) for both BFR and noBFR. Except for CC (Figure 2), dorsal extension 25RM (Figure 5) and LS (0.077≤p≤0.120; 0.19≤η 2 p≤0.57; Figure 2), all other output parameters revealed statistically significant sex effects (0.005≤p≤0.024; 0.20≤η 2 p≤0.68). Sub sequent performed post hoc tests revealed statical significant higher CC (Figure 2), LS (Figure 2), leg press calf raise 1RM (Figure 3), seated calf raise 1RM (Figure 3), plantar flexion 5RM (Figure 4), dorsal extensions 5RM (Figure 4), plantar flexion 25RM (Figure 5), and dorsal extensions 25RM (Figure 5) values (p≤0.001; SMD≥0.77) for men compared to female participants.
Output parameters for pre and post testing of both intervention groups: blood flow restriction (BFR) and conventional resistance training without BFR (noBFR). rANCOVA interaction, group, time, and sex significances (p) and partial eta squared (η 2 p) are provided. In addition, post hoc significances (p), mean differences (MD), and standardized mean differences (SMD) for pre to post comparison of each group are given.
| Parameter | BFR pre | BFR post | Post hoc | noBFR pre | noBFR post | Post hoc | rANCOVA |
|---|---|---|---|---|---|---|---|
| Calf circumference, cm | 37.8 ± 2.9 | 37.9 ± 3.0 | p=0.982; MD=0.0 ± 0.8; SMD=0.03; |
37.7 ± 2.7 | 38.0 ± 2.7 | p=0.714; MD=0.3 ± 0.5; SMD=0.61; |
Interaction: p=0.394, η
2
p=0.01; group: p=0.996, η 2 p=0.00; time: p=0.596, η 2 p=0.00; sex: p=0.111; η 2 p=0.20; |
| Seated calf raise 1RM, kg | 172.2 ± 47.2 | 207.6 ± 51.2 | p=0.039; MD=35.3 ± 31.8; SMD=1.11; |
180.7 ± 40.4 | 214.9 ± 52.4 | p=0.036; MD=34.2 ± 32.2; SMD=1.06; |
Interaction: p=0.941, η
2
p=0.00; group: p=0.616, η 2 p=0.01; time: p=0.046, η 2 p=0.17; sex: p=0.024; η 2 p=0.20; |
| Leg press calf raise 1RM, kg | 143.8 ± 45.5 | 157.5 ± 44.4 | p=0.366; MD=13.7 ± 15.6; SMD=0.88; |
154.4 ± 43.3 | 163.2 ± 40.2 | p=0.533; MD=8.8 ± 17.2; SMD=0.51; |
Interaction: p=0.241, η
2
p=0.02; group: p=0.91, η 2 p=0.02; time: p=0.9, η 2 p=0.04; sex: p=0.011; η 2 p=0.43; |
| Plantar flexion 5RM, Nm | 106.4 ± 29.6 | 116.4 ± 32.4 | p=0.340; MD=10.0 ± 11.9; SMD=0.84; |
104.1 ± 28.4 | 111.8 ± 23.9 | p=0.384; MD=7.7 ± 12.5; SMD=0.62; |
Interaction: p=0.859, η
2
p=0.00; group: p=0.569, η 2 p=0.01; time: p=0.388, η 2 p=0.04; sex: p=0.005; η 2 p=0.42; |
| Dorsal extension 5RM, Nm | 24.8 ± 6.4 | 26.5 ± 7.7 | p=0.467; MD=1.7 ± 2.7; SMD=0.65; |
24.5 ± 7.4 | 26.7 ± 8.4 | p=0.402; MD=2.2 ± 3.2; SMD=0.71; |
Interaction: p=0.062, η
2
p=0.05; group: p=0.162, η 2 p=0.00; time: p=0.728, η 2 p=0.06; sex: p=0.006; η 2 p=0.68; |
| Plantar flexion 25RM, Nm | 74.1 ± 19 | 81.6 ± 22.9 | p=0.292; MD=7.5 ± 9.9; SMD=0.76; |
77.5 ± 23.4 | 82.5 ± 18.4 | p=0.482; MD=5.0 ± 14.3; SMD=0.35; |
Interaction: p=0.626, η
2
p=0.00; group: p=0.88, η 2 p=0.01; time: p=0.275, η 2 p=0.04; sex: p=0.016; η 2 p=0.40; |
| Dorsal extension 25RM, Nm | 15.1 ± 4.1 | 15.3 ± 4.6 | p=0.899; MD=0.2 ± 3.3; SMD=0.06; |
14.7 ± 5.4 | 15.2 ± 4.6 | p=0.777; MD=0.5 ± 3.0; SMD=0.16; |
Interaction: p=0.056, η
2
p=0.06; group: p=0.257, η 2 p=0.00; time: p=0.332, η 2 p=0.00; sex: p=0.077; η 2 p=0.57; |
| Leg stiffness, kN/m | 22.5 ± 7.6 | 22.9 ± 6.6 | p=0.838; MD=0.5 ± 2.9; SMD=0.17; |
22.1 ± 6.0 | 22.1 ± 6.7 | p=0.995; MD=0.0 ± 4.0; SMD=0.00; |
Interaction: p=0.195, η
2
p=0.03; group: p=0.308, η 2 p=0.00; time: p=0.398, η 2 p=0.00; sex: p=0.12; η 2 p=0.19; |
-
1RM, one-repetition maximum; 5RM, five-repetition maximum; 25, twenty-five-repetition maximum.

Pre (circles) to post (triangles) data of both intervention groups: blood flow restriction (BFR) and conventional resistance training without BFR (noBFR). Calf circumference (left) and leg stiffness (right) are displayed. Individual male (blue) and female (orange) data are presented. Mean with standard derivation is given in black.
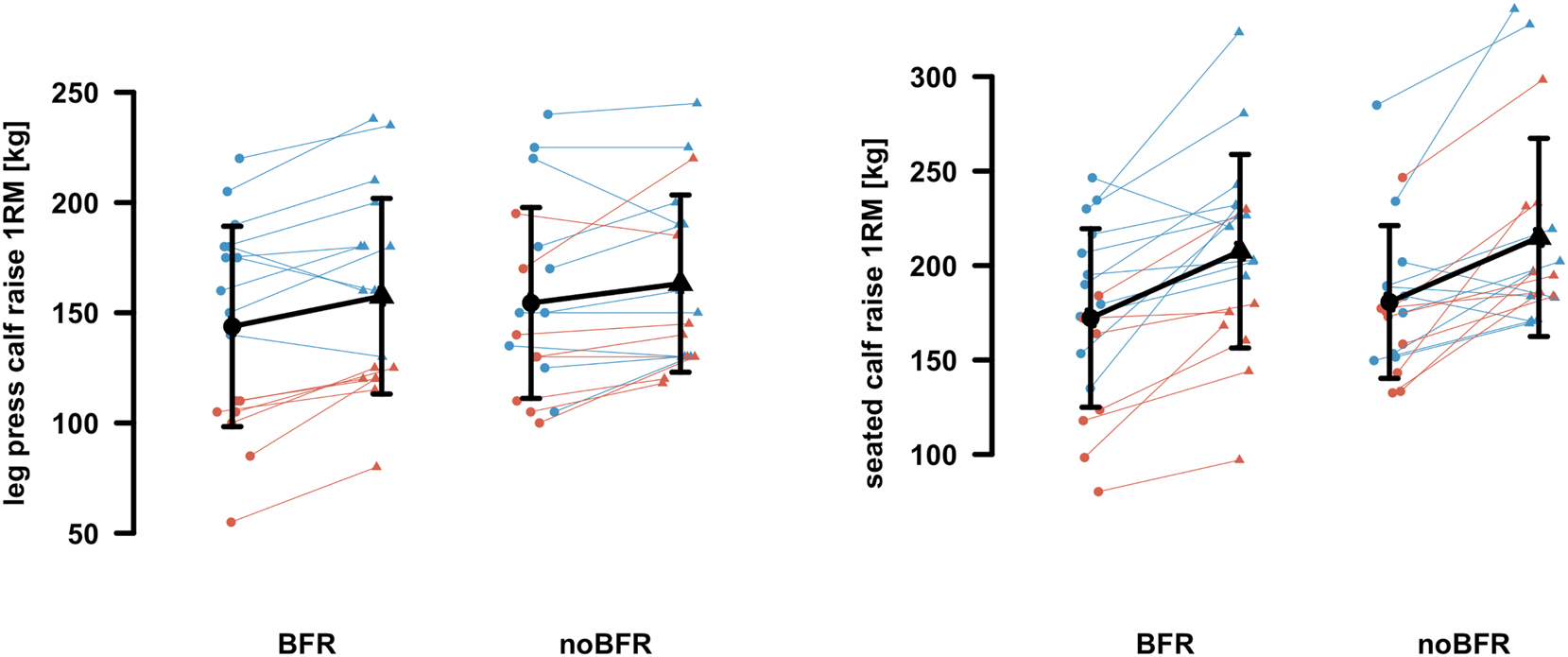
Pre (circles) to post (triangles) data of both intervention groups: blood flow restriction (BFR) and conventional resistance training without BFR (noBFR). Leg press calf raise one-repetition maximum (1RM) (left) and seated calf raise 1RM (right) are displayed. Individual male (blue) and female (orange) data are presented. Mean with standard derivation is given in black.

Pre (circles) to post (triangles) data of both intervention groups: blood flow restriction (BFR) and conventional resistance training without BFR (noBFR). Plantar flexion five-repetition maximum (5RM) (left) and dorsal extension 5RM (right) are displayed. Individual male (blue) and female (orange) data are presented. Mean with standard derivation is given in black.

Pre (circles) to post (triangles) data of both intervention groups: blood flow restriction (BFR) and conventional resistance training without BFR (noBFR). Plantar flexion twenty-five-repetition maximum (25RM) (left) and dorsal extension 25RM (right) are displayed. Individual male (blue) and female (orange) data are presented. Mean with standard derivation is given in black.
Participants data
Regarding the participant data, the 2 × 2 rANOVA revealed no statistical significant interaction (0.256≤p≤0.935; 0.01≤η 2 p≤0.04) and group (0.633≤p≤0.919; η 2 p≤0.01) effects for age, height and body mass. In contrast, age, height and body mass showed statistical relevant sex effects (0.001≤p≤0.006; 0.22≤η 2 p≤0.29). Subsequent performed post hoc tests revealed statical significant higher height and body mass values (0.001≤p≤0.006; 0.22≤η 2 p≤0.29) for male compared to female participants. In addition, age revealed no statistical relevant sex effects (p=0.088; η 2 p=0.09). Furthermore, post hoc testing revealed no significant group differences (0.56≤p≤0.754; 0.11≤SMD≤0.28) for age, height and body mass between BFR and noBFR. Apart from this, total number of sessions were not statistical different (p=0.99; MD=0.1 ± 1.4; SMD=0.07) between BFR (14.7 ± 1.6) and noBFR (14.8 ± 1.3).
Discussion
In the current study, 36 RT trained participants performed eight weeks of either BFR or regular RT for the calf muscles, twice per week, with 150 contractions at 30 % of 1RM without momentary muscle failure. We observed significant increases in seated calf raise 1RM in both groups, while all other strength measures, as well as calf muscle circumference, did not change in any group. It should to be noted that, despite the important role of muscle mass in maintaining health during aging, maintaining strength capacities effectively counteracts age-related dynapenia [44], which reduces the functional capacities of calf muscles for gait and stair walking [45].
Hence, in this study, low-load BFR training did not induce superior strength-enhancing and hypertrophy effects compared to regular low-intensity RT. This is in contrast to previous meta-analysis showing low-load BFR to be effective and superior to conventional low-load RT in various populations [46–49]. Additionally, low-load BFR has been found to be more effective than high-load conventional RT in trained individuals, both for strength and hypertrophy in a variety of muscle groups [50].
The absence of substantial gains in strength and hypertrophy in our study may have several reasons. For example, Gavanda et al. [24] found that even just 6 weeks of twice-weekly low-load BFR and NoBFR RT (both 30 % 1RM), using less accumulated volume (BFR group 614 ± 121 reps; NoNFR group 814 ± 196 reps vs. 2,400 repetitions in our groups), were effective for increasing strength and muscle thickness in trained males. This can be explained by two factors. First, their RT protocol consisted of 4 sets until failure, whereas ours used 30, 15, 15, 15 repetitions not to failure. However, it seems that training to failure is necessary to elicit positive outcomes, since low-intensity RT not done to muscle failure does not lead to substantial increases in hypertrophy or strength [47, 51]. Second, since all sets in the Gavanda et al. study [24] were performed until failure, RT volume progressed over the course of the study, while our protocol had no progression of any type.
Regarding calf muscle BFR RT in untrained individuals, two studies by Centner et al. [25, 26] found similar strength and hypertrophy increases in both the BFR and the NoBFR groups following 14 weeks of thrice-weekly RT using the same repetition scheme and similar training intensity in their BFR group as in our study. However, their NoBFR group used higher intensities (70–85 % 1RM) compared to our NoBFR group (30 % 1RM). The presence of significant improvements in strength and hypertrophy in the two studies by Centner et al. [25, 26] leaves room for interpretation. On one hand, it can be speculated that low-load BFR might only be effective for untrained individuals. On the other hand, it may be necessary to train the calves at higher intensities during traditional RT to achieve strength and hypertrophy gains, as higher RT intensities have been shown to be superior for strength increases [11, 52, 53].
To summarize, while the duration of the intervention and the training volume (total repetitions and sets per week) likely were chosen adequately based on results of previous studies [20, 24, 30, 54, 55], the lack of progression (volume or intensity) [24], [25], [26], the absence of muscular failure, and the participants’ training level may have been decisive. Specifically, regarding RT experience, it was shown that trained participants adapt less well to BFR RT than untrained ones [50].
Future studies with trained subjects are needed to determine whether failure training is necessary for optimal results in low-load calf BFR RT. Additionally, studies comparing different RT intensities in both trained and untrained individuals are needed. This would help to determine which training intensity is most effective in traditional calf RT for strength and hypertrophy gains. Besides, in future studies, it would be beneficial to consider more sensitive measurement techniques for hypertrophy, such as ultrasound or magnetic resonance imaging (MRI).
Some molecular aspects may help explain the lower hypertrophic response of calf muscles compared to leg extensors, which are more often analyzed in RT studies. First, calf muscles, specifically the soleus muscle, show a lower response in RT-stimulated muscle protein synthesis compared to the leg extensors [19]. Over time, this may lead to a significantly lower myofibrillar protein accumulation within the calf muscles, which may only become significant with a more extended training duration (e.g., exceeding 8 weeks). A second factor that determines the accumulation of myofibrillar proteins within muscle is the rate of protein degradation [56]. Proteoasomal degradation of myofibrillar proteins is rapidly activated after acute RT [57] and is increased when myofibrillar damage is more extensive, such as at the beginning of a RT period [58, 59]. Lastly, calf muscles have a higher atrophy potential compared to other muscles of the lower limb, and it has been argued that calf muscles have a higher proteasomal capacity [13]. However, what consequences these aspects have for training practice still need to be investigated in further studies. Our data also show high interindividual variability in the hypertrophic response, affecting men and women to a similar extent, with some participants showing a slightly decrease and others a remarkable increase in CC. Low responders to RT can be statistically found in all randomly arranged study cohorts. For example, Hubal and colleagues [60] impressively demonstrated the high variability in strength and hypertrophy responsiveness of the arm flexors in response to 10 weeks of RT within a cohort of several hundred male and female participants.
Lastly, Hopping tests are commonly used to characterize LS [61]. This depends on various factors, including ankle stiffness [62] and properties of the Achilles tendon [63]. Previous studies on the effects of low-load BFR RT on morphological and mechanical Achilles tendon adaptations found increases in tendon stiffness and tendon cross-sectional area after 14 weeks of training [25, 26]. In contrast, we found that LS was unaffected by low-load RT with and without BFR. This is in line with previous research showing that low-load BFR training was not sufficient to elicit changes in tendon stiffness [64] nor LS [24]. It remains unclear whether the absence of changes in the LS in the present study is due to the shorter intervention period compared to the studies by Centner et al. [25, 26] or whether the hopping test is not suitable for detecting tendon adaptations. Future studies may use both indirect (hopping tests) and laboratory methods (ultrasound, MRI) to determine this.
Strengths and limitations
We also have to acknowledge some methodological limitations of our approach. Firstly, there was no time matched non-exercise control group. Moreover, the number of women and men in this study was too small to adequately investigate any differences in adaptations between the sexes. This should be investigated in future studies with larger numbers of participants from both sexes. Secondly, we determined CC using measuring tape. At the area of measurement, there is an overlap of gastrocnemius and soleus muscles, making it impossible to separately determine muscle growth between the two muscles. Additionally, we have no information on whether type I or type II muscle fibers were affected by any adaptive changes in response to our RT intervention, as we were technically unable to collect muscle biopsies. It has been shown that the molecular signature of low-intensity BFR within skeletal muscle differs from high-intensity RT without BFR. Due to technical limitations concerning weight loading on the seated calf raise machine, the Epley formula used to estimate the 1RM might have introduced a deviation from the actual 1RM. However, because the distribution of such an error was equal in both groups, we assume that it has not influenced our results, specifically not for the between-groups comparison. Finally, it should be noted that our total training volume, considered one primary driver for hypertrophy [65], was likely adequate [30], although it was distributed across two exercises. However, given the specificity of strength adaptations [66], the question remains whether significant increases in strength would occur with the same volume concentrated in a single exercise. This is a topic that could be explored in future studies.
On the other hand, this study is strengthened by its individual determination of AOP following a gold standard protocol. Additionally, the adequate sample size enabled robust statistical analysis, ensuring strong statistical power. Besides, a variety of different strength tests (1RMs, isokinetic testing) were conducted, allowing for valid conclusions from the study. Therefore, the presented data contribute to identifying a highly effective training regimen for inducing calf muscle growth and strength.
Conclusion and practical application
In conclusion, our findings demonstrate that low-load BFR does not offer superior benefits in terms of muscle hypertrophy and strength adaptations in young healthy adults compared to low-load RT when both are conducted at 30 % of 1 RM without muscle failure. Both training modalities resulted in significant strength improvements in seated calf raise, but neither produced notable increases in muscle circumference. This suggests that for individuals seeking strength enhancements, traditional low-load RT is a viable option sufficient to increase strength with a moderate volume of 150 contractions per week, without the need for additional BFR techniques. From a practical standpoint, these findings suggest, that low-load RT provides a safe and effective method to improve strength without the risks associated with higher-intensity training or the complexities of BFR. This may be especially important for older adults who need to maintain functional capacity and independence, as regular strength training can counteract age-related muscle loss and improve mobility. However, this should be investigated in future studies. Additionally, fitness professionals can incorporate low-load RT into training programs to provide clients with a method that balances efficacy and safety, promoting long-term muscle health and overall well-being. Thus, incorporating low-load RT into regular exercise regimens can support muscle maintenance and strength improvement across diverse populations, ensuring broad applicability and accessibility.
Acknowledgments
We are deeply grateful for each participant involved in this project and for the efforts and hard work they have put into it.
-
Research ethics: Prior to the investigation, ethical clearance was obtained from the IST University of Applied Sciences ethics committee (Ethic Decision No. 022023IST233) according to the Declaration of Helsinki.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission. SiGa: conceptualization, methodology, writing – original draft, writing – review & editing, supervision, project administration. ME: conceptualization, methodology, investigation, data curation. SH: formal analysis, data curation, writing – original draft, visualization. StGe: resources, writing – review & editing. SeGe: writing – original draft.
-
Competing interests: All other authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Izquierdo, M, Merchant, RA, Morley, JE, Anker, SD, Aprahamian, I, Arai, H, et al.. International exercise recommendations in older adults (ICFSR): expert consensus guidelines. J Nutr Health Aging 2021;25:824–53. https://doi.org/10.1007/s12603-021-1665-8.Search in Google Scholar PubMed
2. Srikanthan, P, Karlamangla, AS. Muscle mass index as a predictor of longevity in older adults. Am J Med 2014;127:547–53. https://doi.org/10.1016/j.amjmed.2014.02.007.Search in Google Scholar PubMed PubMed Central
3. Tieland, M, Trouwborst, I, Clark, BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle 2018;9:3–19. https://doi.org/10.1002/jcsm.12238.Search in Google Scholar PubMed PubMed Central
4. Westcott, WL. Resistance training is medicine: effects of strength training on health. Curr Sports Med Rep 2012;11:209–16. https://doi.org/10.1249/jsr.0b013e31825dabb8.Search in Google Scholar PubMed
5. Bersiner, K, Park, S-Y, Schaaf, K, Yang, W-H, Theis, C, Jacko, D, et al.. Resistance exercise: a mighty tool that adapts, destroys, rebuilds and modulates the molecular and structural environment of skeletal muscle. Phys Act Nutr 2023;27:78–95. https://doi.org/10.20463/pan.2023.0021.Search in Google Scholar PubMed PubMed Central
6. Bull, FC, Al-Ansari, SS, Biddle, S, Borodulin, K, Buman, MP, Cardon, G, et al.. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–62. https://doi.org/10.1136/bjsports-2020-102955.Search in Google Scholar PubMed PubMed Central
7. Figueiredo, VC. Revisiting the roles of protein synthesis during skeletal muscle hypertrophy induced by exercise. Am J Physiol Regul Integr Comp Physiol 2019;317:R709–18. https://doi.org/10.1152/ajpregu.00162.2019.Search in Google Scholar PubMed
8. Breen, L, Stokes, KA, Churchward-Venne, TA, Moore, DR, Baker, SK, Smith, K, et al.. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab 2013;98:2604–12. https://doi.org/10.1210/jc.2013-1502.Search in Google Scholar PubMed
9. Powers, SK, Lynch, GS, Murphy, KT, Reid, MB, Zijdewind, I. Disease-Induced skeletal muscle atrophy and fatigue. Med Sci Sports Exerc 2016;48:2307–19. https://doi.org/10.1249/mss.0000000000000975.Search in Google Scholar
10. Kilroe, SP, Fulford, J, Jackman, SR, van Loon, LJC, Wall, BT. Temporal muscle-specific disuse atrophy during one week of leg immobilization. Med Sci Sports Exerc 2020;52:944–54. https://doi.org/10.1249/mss.0000000000002200.Search in Google Scholar PubMed
11. Peterson, MD, Rhea, MR, Sen, A, Gordon, PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev 2010;9:226–37. https://doi.org/10.1016/j.arr.2010.03.004.Search in Google Scholar PubMed PubMed Central
12. Volpi, E, Nazemi, R, Fujita, S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care 2004;7:405–10. https://doi.org/10.1097/01.mco.0000134362.76653.b2.Search in Google Scholar PubMed PubMed Central
13. Bodine, SC. Disuse-induced muscle wasting. Int J Biochem Cell Biol 2013;45:2200–8. https://doi.org/10.1016/j.biocel.2013.06.011.Search in Google Scholar PubMed PubMed Central
14. Nunes, EA, Stokes, T, McKendry, J, Currier, BS, Phillips, SM. Disuse-induced skeletal muscle atrophy in disease and nondisease states in humans: mechanisms, prevention, and recovery strategies. Am J Physiol Cell Physiol 2022;322:C1068–84. https://doi.org/10.1152/ajpcell.00425.2021.Search in Google Scholar PubMed
15. Trappe, S, Costill, D, Gallagher, P, Creer, A, Peters, JR, Evans, H, et al.. Exercise in space: human skeletal muscle after 6 months aboard the International Space Station. J Appl Physiol 1985) 2009;106:1159–68. https://doi.org/10.1152/japplphysiol.91578.2008.Search in Google Scholar PubMed
16. do Rosario, JT, Da Fonseca Martins, NS, Peixinho, CC, Oliveira, LF. Effects of functional training and calf stretching on risk of falls in older people: a pilot study. J Aging Phys Activ 2017;25:228–33. https://doi.org/10.1123/japa.2015-0316.Search in Google Scholar PubMed
17. Weiss, LW, Clark, FC, Howard, DG. Effects of heavy-resistance triceps surae muscle training on strength and muscularity of men and women. Phys Ther 1988;68:208–13. https://doi.org/10.1093/ptj/68.2.208.Search in Google Scholar PubMed
18. Kataoka, R, Vasenina, E, Hammert, WB, Ibrahim, AH, Dankel, SJ, Buckner, SL. Muscle growth adaptations to high-load training and low-load training with blood flow restriction in calf muscles. Eur J Appl Physiol 2022;122:623–34. https://doi.org/10.1007/s00421-021-04862-7.Search in Google Scholar PubMed
19. Trappe, TA, Raue, U, Tesch, PA. Human soleus muscle protein synthesis following resistance exercise. Acta Physiol Scand 2004;182:189–96. https://doi.org/10.1111/j.1365-201x.2004.01348.x.Search in Google Scholar PubMed
20. Kassiano, W, Costa, BDV, Kunevaliki, G, Lisboa, F, Tricoli, I, Francsuel, J, et al.. Bigger calves from doing higher resistance training volume? Int J Sports Med 2024. https://doi.org/10.1055/a-2316-7885.Search in Google Scholar PubMed
21. Kinoshita, M, Maeo, S, Kobayashi, Y, Eihara, Y, Ono, M, Sato, M, et al.. Triceps surae muscle hypertrophy is greater after standing versus seated calf-raise training. Front Physiol 2023;14:1272106. https://doi.org/10.3389/fphys.2023.1272106.Search in Google Scholar PubMed PubMed Central
22. Loenneke, JP, Fahs, CA, Rossow, LM, Thiebaud, RS, Mattocks, KT, Abe, T, et al.. Blood flow restriction pressure recommendations: a tale of two cuffs. Front Physiol 2013;4:249. https://doi.org/10.3389/fphys.2013.00249.Search in Google Scholar PubMed PubMed Central
23. Wilson, JM, Lowery, RP, Joy, JM, Loenneke, JP, Naimo, MA. Practical blood flow restriction training increases acute determinants of hypertrophy without increasing indices of muscle damage. J Strength Condit Res 2013;27:3068–75. https://doi.org/10.1519/jsc.0b013e31828a1ffa.Search in Google Scholar
24. Gavanda, S, Isenmann, E, Schlöder, Y, Roth, R, Freiwald, J, Schiffer, T, et al.. Low-intensity blood flow restriction calf muscle training leads to similar functional and structural adaptations than conventional low-load strength training: a randomized controlled trial. PLoS One 2020;15:e0235377. https://doi.org/10.1371/journal.pone.0235377.Search in Google Scholar PubMed PubMed Central
25. Centner, C, Lauber, B, Seynnes, OR, Jerger, S, Sohnius, T, Gollhofer, A, et al.. Low-load blood flow restriction training induces similar morphological and mechanical Achilles tendon adaptations compared with high-load resistance training. J Appl Physiol 1985) 2019;127:1660–7. https://doi.org/10.1152/japplphysiol.00602.2019.Search in Google Scholar PubMed
26. Centner, C, Jerger, S, Lauber, B, Seynnes, O, Friedrich, T, Lolli, D, et al.. Similar patterns of tendon regional hypertrophy after low-load blood flow restriction and high-load resistance training. Scand J Med Sci Sports 2023;33:848–56. https://doi.org/10.1111/sms.14321.Search in Google Scholar PubMed
27. Bentzen, A, Jørgensen, SL, Birch, S, Mortensen, L, Toft, M, Lindvig, MG, et al.. Feasibility of blood flow restriction exercise in adults with a non-surgically treated Achilles tendon rupture; a case series. Int J Exerc Sci 2024;17:140–53.Search in Google Scholar
28. Merry, K, MacPherson, M, Vis-Dunbar, M, Whittaker, JL, Grävare Silbernagel, K, Scott, A. Identifying characteristics of resistance-based therapeutic exercise interventions for Achilles tendinopathy: a scoping review. Phys Ther Sport 2023;63:73–94. https://doi.org/10.1016/j.ptsp.2023.06.002.Search in Google Scholar PubMed
29. Mortensen, L, Mechlenburg, I, Langgård Jørgensen, S. Low-load blood-flow-restricted exercise to prevent muscle atrophy and decline in functional performance in a patient recovering from a malleolus fracture. A case report. Clin J Sport Med 2023;33:97–100. https://doi.org/10.1097/jsm.0000000000001072.Search in Google Scholar
30. Schoenfeld, BJ, Ogborn, D, Krieger, JW. Dose-response relationship between weekly resistance training volume and increases in muscle mass: a systematic review and meta-analysis. J Sports Sci 2017;35:1073–82. https://doi.org/10.1080/02640414.2016.1210197.Search in Google Scholar PubMed
31. World Medical Association. WMA declaration of Helsinki: ethical principles for medical research involving human subjects; 2013. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ [Accessed 16 Feb 2024].Search in Google Scholar
32. Faul, F, Erdfelder, E, Lang, A-G, Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. https://doi.org/10.3758/bf03193146.Search in Google Scholar PubMed
33. Kelly, MR, Cipriano, KJ, Bane, EM, Murtaugh, BT. Blood flow restriction training in athletes. Curr Phys Med Rehabil Rep 2020;8:329–41. https://doi.org/10.1007/s40141-020-00291-3.Search in Google Scholar
34. Carmont, MR, Silbernagel, KG, Mathy, A, Mulji, Y, Karlsson, J, Maffulli, N. Reliability of Achilles tendon resting angle and calf circumference measurement techniques. Foot Ankle Surg 2013;19:245–9. https://doi.org/10.1016/j.fas.2013.06.007.Search in Google Scholar PubMed
35. Ruggiero, L, Dewhurst, S, Bampouras, TM. Validity and reliability of two field-based leg stiffness devices: implications for practical use. J Appl Biomech 2016;32:415–9. https://doi.org/10.1123/jab.2015-0297.Search in Google Scholar PubMed
36. Haff, G, Triplett, NT, editors. Essentials of strength training and conditioning, 4th ed. Champaign, IL, Windsor, ON, Leeds: Human Kinetics; 2016.Search in Google Scholar
37. Grgic, J, Lazinica, B, Schoenfeld, BJ, Pedisic, Z. Test-retest reliability of the one-repetition maximum (1RM) strength assessment: a systematic review. Sports Med Open 2020;6:31. https://doi.org/10.1186/s40798-020-00260-z.Search in Google Scholar PubMed PubMed Central
38. Wood, TM, Maddalozzo, GF, Harter, RA. Accuracy of seven equations for predicting 1-RM performance of apparently healthy, sedentary older adults. Meas Phys Educ Exerc Sci 2002;6:67–94. https://doi.org/10.1207/s15327841mpee0602_1.Search in Google Scholar
39. Knutzen, KM, Brilla, LR, Caine, D. Validity of 1RM prediction equations for older adults. J Strength Condit Res 1999;13:242–6. https://doi.org/10.1519/00124278-199908000-00011.Search in Google Scholar
40. Gaines, JM, Talbot, LA. Isokinetic strength testing in research and practice. Biol Res Nurs 1999;1:57–64. https://doi.org/10.1177/109980049900100108.Search in Google Scholar PubMed
41. Karnofel, H, Wilkinson, K, Lentell, G. Reliability of isokinetic muscle testing at the ankle. J Orthop Sports Phys Ther 1989;11:150–4. https://doi.org/10.2519/jospt.1989.11.4.150.Search in Google Scholar PubMed
42. Vehrs, PR, Richards, S, Blazzard, C, Hart, H, Kasper, N, Lacey, R, et al.. Use of a handheld Doppler to measure brachial and femoral artery occlusion pressure. Front Physiol 2023;14:1239582. https://doi.org/10.3389/fphys.2023.1239582.Search in Google Scholar PubMed PubMed Central
43. Patterson, SD, Brandner, CR. The role of blood flow restriction training for applied practitioners: a questionnaire-based survey. J Sports Sci 2018;36:123–30. https://doi.org/10.1080/02640414.2017.1284341.Search in Google Scholar PubMed
44. Manini, TM, Clark, BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci 2012;67:28–40. https://doi.org/10.1093/gerona/glr010.Search in Google Scholar PubMed PubMed Central
45. Lenhart, RL, Francis, CA, Lenz, AL, Thelen, DG. Empirical evaluation of gastrocnemius and soleus function during walking. J Biomech 2014;47:2969–74. https://doi.org/10.1016/j.jbiomech.2014.07.007.Search in Google Scholar PubMed PubMed Central
46. Chang, H, Yao, M, Chen, B, Qi, Y, Zhang, J. Effects of blood flow restriction combined with low-intensity resistance training on lower-limb muscle strength and mass in post-middle-aged adults: a systematic review and meta-analysis. Int J Environ Res Publ Health 2022;19. https://doi.org/10.3390/ijerph192315691.Search in Google Scholar PubMed PubMed Central
47. Loenneke, JP, Wilson, JM, Marín, PJ, Zourdos, MC, Bemben, MG. Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol 2012;112:1849–59. https://doi.org/10.1007/s00421-011-2167-x.Search in Google Scholar PubMed
48. Slysz, J, Stultz, J, Burr, JF. The efficacy of blood flow restricted exercise: a systematic review & meta-analysis. J Sci Med Sport 2016;19:669–75. https://doi.org/10.1016/j.jsams.2015.09.005.Search in Google Scholar PubMed
49. Zhang, T, Wang, X, Wang, J. Effect of blood flow restriction combined with low-intensity training on the lower limbs muscle strength and function in older adults: a meta-analysis. Exp Gerontol 2022;164:111827. https://doi.org/10.1016/j.exger.2022.111827.Search in Google Scholar PubMed
50. Geng, Y, Wu, X, Zhang, Y, Zhang, M. Potential moderators of the effects of blood flow restriction training on muscle strength and hypertrophy: a meta-analysis based on a comparison with high-load resistance training. Sports Med Open 2024;10:58. https://doi.org/10.1186/s40798-024-00719-3.Search in Google Scholar PubMed PubMed Central
51. Lasevicius, T, Schoenfeld, BJ, Silva-Batista, C, Barros, TS, Aihara, AY, Brendon, H, et al.. Muscle failure promotes greater muscle hypertrophy in low-load but not in high-load resistance training. J Strength Condit Res 2022;36:346–51. https://doi.org/10.1519/jsc.0000000000003454.Search in Google Scholar
52. Lixandrão, ME, Ugrinowitsch, C, Berton, R, Vechin, FC, Conceição, MS, Damas, F, et al.. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med 2018;48:361–78. https://doi.org/10.1007/s40279-017-0795-y.Search in Google Scholar PubMed
53. Schoenfeld, BJ, Grgic, J, Ogborn, D, Krieger, JW. Strength and hypertrophy adaptations between low- vs. High-load resistance training: a systematic review and meta-analysis. J Strength Condit Res 2017;31:3508–23. https://doi.org/10.1519/jsc.0000000000002200.Search in Google Scholar
54. Ralston, GW, Kilgore, L, Wyatt, FB, Baker, JS. The effect of weekly set volume on strength gain: a meta-analysis. Sports Med 2017;47:2585–601. https://doi.org/10.1007/s40279-017-0762-7.Search in Google Scholar PubMed PubMed Central
55. Libardi, CA, Godwin, JS, Reece, TM, Ugrinowitsch, C, Herda, TJ, Roberts, MD. Effects of low-load resistance training with blood flow restriction on muscle fiber myofibrillar and extracellular area. Front Physiol 2024;15:1368646. https://doi.org/10.3389/fphys.2024.1368646.Search in Google Scholar PubMed PubMed Central
56. Chinkes, DL. Methods for measuring tissue protein breakdown rate in vivo. Curr Opin Clin Nutr Metab Care 2005;8:534–7. https://doi.org/10.1097/01.mco.0000170754.25372.37.Search in Google Scholar PubMed
57. Phillips, SM, Tipton, KD, Aarsland, A, Wolf, SE, Wolfe, RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 1997;273:E99–107. https://doi.org/10.1152/ajpendo.1997.273.1.e99.Search in Google Scholar
58. Ulbricht, A, Gehlert, S, Leciejewski, B, Schiffer, T, Bloch, W, Höhfeld, J. Induction and adaptation of chaperone-assisted selective autophagy CASA in response to resistance exercise in human skeletal muscle. Autophagy 2015;11:538–46. https://doi.org/10.1080/15548627.2015.1017186.Search in Google Scholar PubMed PubMed Central
59. Damas, F, Phillips, SM, Libardi, CA, Vechin, FC, Lixandrão, ME, Jannig, PR, et al.. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol 2016;594:5209–22. https://doi.org/10.1113/jp272472.Search in Google Scholar PubMed PubMed Central
60. Hubal, MJ, Gordish-Dressman, H, Thompson, PD, Price, TB, Hoffman, EP, Angelopoulos, TJ, et al.. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc 2005;37:964–72.Search in Google Scholar
61. De Ste, Croix MBA, Hughes, JD, Lloyd, RS, Oliver, JL, Read, PJ. Leg stiffness in female soccer players: intersession reliability and the fatiguing effects of soccer-specific exercise. J Strength Condit Res 2017;31:3052–8. https://doi.org/10.1519/jsc.0000000000001715.Search in Google Scholar
62. Farley, CT, Morgenroth, DC. Leg stiffness primarily depends on ankle stiffness during human hopping. J Biomech 1999;32:267–73. https://doi.org/10.1016/s0021-9290(98)00170-5.Search in Google Scholar PubMed
63. Wikstrom, EA, Tillman, MD, Chmielewski, TL, Borsa, PA. Measurement and evaluation of dynamic joint stability of the knee and ankle after injury. Sports Med 2006;36:393–410. https://doi.org/10.2165/00007256-200636050-00003.Search in Google Scholar PubMed
64. Kubo, K, Komuro, T, Ishiguro, N, Tsunoda, N, Sato, Y, Ishii, N, et al.. Effects of low-load resistance training with vascular occlusion on the mechanical properties of muscle and tendon. J Appl Biomech 2006;22:112–9. https://doi.org/10.1123/jab.22.2.112.Search in Google Scholar PubMed
65. Krzysztofik, M, Wilk, M, Wojdała, G, Gołaś, A. Maximizing muscle hypertrophy: a systematic review of advanced resistance training techniques and methods. Int J Environ Res Publ Health 2019;16. https://doi.org/10.3390/ijerph16244897.Search in Google Scholar PubMed PubMed Central
66. Folland, JP, Williams, AG. The adaptations to strength training : morphological and neurological contributions to increased strength. Sports Med 2007;37:145–68. https://doi.org/10.2165/00007256-200737020-00004.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter on behalf of Shangai Jiao Tong University and Guangzhou Sport University
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Beyond the Olympic Games
- Beyond the Olympic and Paralympic Games
- Practical steps to develop a transcriptomic test for blood doping
- A unique pseudo-eligibility analysis of longitudinal laboratory performance data from a transgender female competitive cyclist
- Why the dominance of East Africans in distance running? A narrative review
- Gender equality policy of the Olympic Movement in Chinese sport governing bodies: the case of elite volleyball
- Movement Science
- Associations of strength indices and cycling economy in young adults
- Are calves trainable? Low-intensity calf muscle training with or without blood flow restriction: a randomized controlled trial
- Exercise Biology
- Caveolin-3 regulates slow oxidative myofiber formation in female mice
- Effect of aerobic intermittent exercise on the decreased cognitive ability induced by PM2.5 exposure in rats
Articles in the same Issue
- Frontmatter
- Beyond the Olympic Games
- Beyond the Olympic and Paralympic Games
- Practical steps to develop a transcriptomic test for blood doping
- A unique pseudo-eligibility analysis of longitudinal laboratory performance data from a transgender female competitive cyclist
- Why the dominance of East Africans in distance running? A narrative review
- Gender equality policy of the Olympic Movement in Chinese sport governing bodies: the case of elite volleyball
- Movement Science
- Associations of strength indices and cycling economy in young adults
- Are calves trainable? Low-intensity calf muscle training with or without blood flow restriction: a randomized controlled trial
- Exercise Biology
- Caveolin-3 regulates slow oxidative myofiber formation in female mice
- Effect of aerobic intermittent exercise on the decreased cognitive ability induced by PM2.5 exposure in rats

