Abstract
Objectives
Previous research on stress-induced pain modulation suggests that moderate psychological stress usually leads to hyperalgesia while more severe threat results in hypoalgesia. However, existing studies often lack suitable control conditions imperative to identify mere stress effects. Similarly, research mainly focused on pure anticipation of a social threat, not taking into consideration actual experiences of social evaluation. Therefore, we set out to investigate actual social up- and downgrading combined with a standardized stress paradigm to evaluate short-term and prolonged changes in pain perception and their potential association with neuroendocrine and subjective stress parameters.
Methods
We allocated 177 healthy women to four experimental conditions, either the standard version of the Trier Social Stress Test (TSST) followed by positive, negative or no performance feedback, or a well-matched but less demanding placebo version of the TSST. Stress responses were assessed with ratings, salivary alpha-amylase, and salivary cortisol. To capture putative effects of stress on pain, heat pain threshold, ratings of phasic heat pain stimuli, and conditioned pain modulation were measured.
Results
Despite a largely successful stress induction, results do not support a reliable influence of experimentally induced social stress–with or without subsequent performance feedback–on pain in women. Further, we found no clear association of pain modulation and changes in neuroendocrine or subjective stress responses.
Conclusions
Our results contrast previous studies, which repeatedly demonstrated stress-induced hypo- or hyperalgesia. This might be due to methodological reasons as former research was often characterized by high heterogeneity regarding the applied stressors, low sample sizes, and lacking or inconclusive control conditions. Thus, our results raise the question whether pain modulation in women by experimental psychosocial stress might have been overestimated in the past. Future research is necessary, which should employ parametric stress induction methods including well-matched control tasks, taking into consideration the participants’ gender/sex and the time course of the stress response relative to pain assessment.
The study is registered as DRKS00026946 at ‘Deutsches Register Klinischer Studien’ (DRKS) and can be also found at the World Health Organization’s search portal.
Introduction
Pain is prone to be modulated by psychological factors such as affect [1], [2], [3] and experimentally induced stress [4], [5], [6], [7], [8], [9], [10]. In view of quite inconsistent findings regarding stress effects on pain in humans, it is assumed that certain characteristics of stressors play a major role in determining the magnitude and direction of stress-induced pain modulation. Thus, some authors hypothesise that severe stress causes analgesia (SIA) while moderate stressors result in hyperalgesia [4, 5, 10, 11]. However, findings are rather mixed, probably due to methodological inconsistencies across studies, such as using distinct stress induction paradigms varying in type and severity, low sample sizes, and–probably most crucial–lacking or inappropriate control conditions [4, 12].
Main indicators of successful stress induction are cortisol and catecholamine levels [13]. Nevertheless, it is still unclear to what extent their release is essential for stress-related pain modulation [8, 14]. While some animal studies demonstrated their involvement in stress-induced pain modulation [15], comparable endocrinological evidence in humans is limited [8]. While cortisol can be measured accurately via saliva, alpha-amylase is recommended as a substitute for catecholamine measurements since saliva levels of epinephrine and norepinephrine do not highly correlate with blood plasma levels [16, 17].
Stress paradigms used for investigating pain modulation are manifold [4, 12]. Here, we chose the Trier Social Stress Test (TSST) [18], which comprises a brief public speech and the execution of a demanding cognitive task in front of an allegedly specially trained committee. The TSST reliably elicits substantial cortisol releases [12, 18] and elevated levels of stress and anxiety [19], [20], [21]. We also included a validated control condition: the placebo version of the TSST, which was designed to generate similar physical and cognitive load without provoking social stress [22], while controlling for potential time effects.
The TSST, like other paradigms designed to induce psychosocial stress, mainly focuses on the perception of anticipated social threat, while actual interpersonal harm such as social up- or downgrading were only rarely investigated [12, 20, 23]. Therefore, we decided to innovatively complement the TSST with one critical component, namely randomized positive vs. negative feedback regarding participants’ task performance. Thereby, our aim, on the one hand, was to further increase stress responses triggered by the TSST. Additive effects in this respect are expected since negative feedback represents an actual social rejection already associated with higher stress responsiveness [23]. We expected to show, on the other hand, stress reducing effects in the positive feedback condition as such social upgrading can be considered as an affirmatory social interaction known to exert protective effects on stress (and pain) outcomes [24], [25], [26], [27], [28], [29]. As certain distinguishable stress profiles among our experimental conditions seem reasonable, stress-induced pain modulation should–according to theories assuming a dose-dependent continuum of the stress-induced pain response [11, 30] – be highest in the negative feedback and lowest in the positive feedback group, with the mere TSST performance, supposedly, lying somewhere in between.
To this end, we recruited 177 females to compare four experimental groups, that is the TSST combined with no feedback, positive feedback or negative feedback and the so-called placebo TSST as a control condition. A rather young, all-female sample was chosen as we decided to focus on only one gender and age-range in this first approach to study additive feedback effects and because of well-known gender-specific stress responses after the TSST [31, 32].
Subjective (stress ratings and anxiety scores) and physiological (cortisol and alpha-amylase) stress markers were determined for checking if the manipulation succeeded. However, we did not hypothesize that changes of cortisol and alpha-amylase have to be equally pronounced, since the link between these parameters is not correlated one-to-one [16]. Furthermore, we set out to analyse correlations between change scores of stress and pain.
Stress effects on pain were explored by means of phasic heat pain ratings, heat pain threshold, and conditioned pain modulation (CPM). In line with recent reports [5, 9, 33], we hypothesized stress-induced hyperalgesia and a more deficient CPM in the TSST compared to the placebo TSST group directly after stress induction. Furthermore, we expected–in a more exploratory way–additional feedback effects on stress-induced pain modulation. Being to our knowledge the first investigation not only focusing on simple pre-post comparisons, we implemented a third pain measurement to assess delayed stress effects on pain.
Methods and materials
Participants
186 females were recruited via SONA Systems (Sona Systems Ltd., Tallinn, Estonia), a web-based software for recruiting participants, postings at the Department of Psychology, University of Würzburg, Germany, and advertisements in local online newspaper.
Exclusion criteria were male gender, mental disorders, acute or chronic pain, regular smoking, and psychopharmacological or analgesic medication (self-report). Participants had to be between the age of 18 and 35 years and speak German fluently. Due to saliva collection, they should not eat or drink anything except water half an hour before the experiment. For compensation, participants received either 20 Euro or course credit.
Five participants were excluded from analyses due to high discomfort during the experiment or failure to meet all inclusion criteria. Another two participants from the placebo group were excluded due to pronounced cortisol increases of more than 1.5 nmol/L, as defined by Miller, Plessow [34]. Thus, the final sample size consisted of 177 females (Age M=23.83, SD=3.45; Table 1).
Sample Characteristics.
| Stress paradigm type of feedback | TSST positive | TSST negative | TSST no FB | Placebo no FB | ||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| Age, years | 24.16 | 3.34 | 23.63 | 3.70 | 24.02 | 3.29 | 23.51 | 3.51 |
| Education, years | 12.91 | 0.61 | 12.96 | 0.21 | 12.77 | 0.77 | 12.86 | 0.52 |
| Disstress1 | 3.20 | 1.90 | 3.13 | 1.61 | 2.89 | 1.28 | 3.23 | 1.72 |
| STAI SKD1 | 8.00 | 2.27 | 7.62 | 2.11 | 8.07 | 2.28 | 8.35 | 1.91 |
| Cortisol, nmol/L | 7.76 | 5.98 | 7.84 | 7.07 | 7.34 | 5.99 | 9.29 | 6.82 |
| Alpha-amylase, U/mL | 88.78 | 93.95 | 86.64 | 94.93 | 96.90 | 96.27 | 115.24 | 111.85 |
| STAI-trait | 37.88 | 10.27 | 36.74 | 7.96 | 38.78 | 9.95 | 39.29 | 10.85 |
| PCS | 13.49 | 8.64 | 13.70 | 8.34 | 17.87 | 8.01 | 16.23 | 8.70 |
| BDI-II | 7.12 | 5.29 | 7.09 | 5.70 | 7.51 | 6.22 | 7.60 | 7.04 |
| RS-25 | 136.07 | 20.93 | 136.50 | 17.04 | 133.73 | 20.86 | 133.40 | 15.52 |
| SPAI | 1.94 | 1.09 | 2.25 | 0.97 | 2.30 | 0.94 | 2.32 | 1.01 |
| LOT-R | 7.16 | 2.60 | 7.50 | 2.62 | 6.73 | 2.71 | 4.67 | 2.34 |
| PSQ | 3.84 | 1.32 | 4.15 | 1.47 | 4.56 | 1.40 | 4.46 | 1.66 |
| SVF (pos.) | 22.60 | 3.76 | 22.74 | 3.00 | 22.91 | 3.61 | 22.98 | 3.21 |
| SVF (neg.) | 15.05 | 2.84 | 14.72 | 2.14 | 15.56 | 2.41 | 14.88 | 2.70 |
| ASP (RO) | 31.34 | 27.37 | 27.17 | 19.82 | 30.68 | 23.68 | 32.28 | 24.45 |
| ASP (SI) | 60.17 | 26.25 | 58.88 | 23.82 | 60.08 | 24.72 | 55.67 | 24.99 |
| ASP (CI) | 78.72 | 14.40 | 76.85 | 16.68 | 74.56 | 11.62 | 75.00 | 11.04 |
| ASP (TC) | 51.60 | 26.48 | 48.78 | 24.78 | 49.72 | 23.76 | 48.66 | 24.29 |
| Sample size | 43 | 46 | 45 | 43 | ||||
| of which cortisol responders2 | 16 (37.21%) | 15 (32.61%) | 17 (37.78%) | 0 | ||||
-
M, mean; SD, standard deviation; TSST, trier social stress test; FB, feedback; pos., positive strategies; neg., negative strategies; 1Baseline measurement, 2Defined by cortisol changes of more than 1.5 nmol/L between baseline and (approximate) peak; STAI, State-Trait-anxiety-inventory; STAI-SKD, German short version of the STAI-State subscale; PCS, pain catastrophizing scale; BDI-II, beck-depression-inventory; RS-25, resilience scale; SPAI, social phobia and expressions inventory; LOT-R, life orientation test; PSQ, pain catastrophizing scale; SVF, Stressverarbeitungsfragebogen (questionnaire about coping in stressful environments); ASP, Ausdrucksformen der Spiritualität (expressions of spirituality) with the following subscales; ASP (RO), religious orientation; ASP (SI), search for insight; ASP (CI), conscious interaction; ASP (TC), transcendence conviction. Please see Appendix S3 for references.
Participants were pseudo-randomly (following a predetermined order) allocated to either perform the TSST with positive feedback (pos. FB, n=43), negative feedback (neg. FB, n=46) or no FB (no FB, n=45). Furthermore, n=43 females participated in the control condition. As contraceptives or certain menstrual cycle phases were no exclusion criteria, we checked that the intake of contraceptive hormones did not differ significantly across groups (p=0.072). Furthermore, we assured that in those not taking medication (no FB: n=25, pos. FB: n=32, neg FB: n=25, placebo: n=20), days since the last menses were not influenced by group (p=0.757).
A priori sample size calculation was performed using G*Power (Version 3.1.9.2, University of Kiel, Germany). Performance of a mixed model analysis of variance (ANOVA) including one within-subjects (WS) factor (3 factor levels) and one between-subjects (BS) factor (4 factor levels) revealed an optimal sample size of n=184 (assuming an effect size of f=0.25, power of 0.80, and a significance level of alpha=0.05).
Thermal pain stimulation
Phasic heat pain stimuli were delivered via a thermal stimulator with an active thermode area of 25 × 50 mm (Somedic SenseLab Inc., Sösdala, Sweden). For tonic heat pain stimulation, the water bath circulator WiseCircu WCB-11 (Witeg Labortechnik Inc., Wertheim, Germany) was used. The thermode was attached on the left medial or lateral proximal volar forearm (balanced across participants). The pain testing sequence always started with assessment of heat pain tolerance followed by measurement of temporal summation, heat pain threshold, ratings of phasic heat pain stimuli, and conditioned pain modulation of heat pain threshold and the rating of phasic heat pain stimuli. Facing rather complex and diverse measurements, analyses of heat pain tolerance and temporal summation are beyond the scope of this paper and will be reported elsewhere.
Throughout each pain test, participants were asked to indicate heat pain threshold/tolerance and to verbally rate their pain experience. A computer flat screen (EIZO Flatscreen EV2416W, Eizo Inc., Hakusan, Japan) in front of them was used to visualize and thus facilitate comprehension of the test-specific response requirements.
Heat pain threshold
Heat pain threshold was assessed using a method of limits protocol based on the approach of Lautenbacher, Roscher [34]. Participants were asked to indicate with a manual stop signal when the pain sensation turned from hot to just noticeable painful. Starting temperature was set to 32 °C and heated up with a rate of 1 °C/s. After the participant indicated a pain sensation or the temperature reached the maximum of 50 °C, it declined immediately at a rate of 5 °C/s back to baseline (32 °C). A series of four threshold determinations were performed. Although beyond the scope of this article, it should be noted that heat pain tolerance was assessed analogously except that participants were asked to indicate unbearable pain. Thus, we were able to calculate lacking differentiation between heat pain threshold and heat pain tolerance (i.e., a negative difference between the mean of pain tolerance subtracted from the mean of pain threshold). Due to such non-differentiation, one participant from the positive feedback group, three participants from the negative feedback group, four participants from the no feedback group, and five participants from the placebo group had to be excluded from analyses of heat pain threshold and tolerance levels at all pain measurements. Nevertheless, participants’ data was included in the analyses of the remaining pain variables.
Ratings of phasic heat pain stimuli
Eight phasic heat pain stimuli with a temperature of 48 °C and an inter-stimulus-interval of 15 s (peak to peak) were applied. Temperature was set to 35 °C (baseline) and increased/decreased at a rate of 5 °C/s. Temperature declined back to baseline immediately after target temperature was reached. Shortly thereafter, participants were asked by the experimenter (whilst cooling of the thermode proceeded) to rate verbally the just given stimulus on an 11-point numeric rating scale (NRS) ranging from 0 (‘no pain at all’) to 10 (‘extremely painful’).
Conditioned pain modulation
Conditioned pain modulation was assessed by two different paradigms: first, by measuring differences of heat pain threshold and second, by determining change values of phasic heat pain ratings–both pain tests serving as test stimuli (TS) frequently used in the context of CPM assessments [35]. Differences were made calculable by a simultaneous immersion of the right hand into the hot water bath–representing a frequently used conditioning stimulus (CS) [35]. Constant water circulation guaranteed the maintenance of an equal temperature distribution. The temperature of the CS was set to 46 °C, as already done in previous research [36, 37]. Maximum temperature variance was ±0.1 °C. During hand immersion, both types of TS (separated by a break of 5 s) were applied and compared with pain levels in absence of the CS.
Before the test stimuli were applied, a sensory stabilization phase [36, 37] of 20 s took place at the beginning of participants’ hand immersion into the hot water bath. Subsequently, measurements of heat pain threshold and the rating of phasic heat pain took place in the manner described in the respective pain test section above. The total immersion time into the water bath was about 180 s.
Experimental stress induction
TSST without feedback
The exact procedure used in this study was adapted from a revised TSST protocol, for further details see Kudielka, Hellhammer [21] and Appendix S1. It can be described as a reliable, highly standardized psychobiological instrument, which examines the responsiveness of the organism to psychosocial stress by means of different stress-generating tasks, which include elements of public speaking, mental arithmetic, and anticipation [18].
TSST with feedback
In the two groups receiving feedback, the TSST was performed analogously to the descriptions in Appendix S1, except that in the end, participants were told that a detailed analysis and evaluation of their performances had to be carried out at a later point in time, but that first impressions would be discussed immediately to promptly provide individual feedback. Accordingly, the committee left the room for 2 min to allegedly discuss the participants’ performance and provided negative or positive verbal feedback–depending on the experimental condition. In case of negative feedback, the committee said that the speech was not convincing, and that authenticity, facial expressions, and gesturing did not fit task demands. Additionally, feedback was given regarding the arithmetic task by stating that in comparison to standard values of age-matched controls, participants achieved sub-standard results. In the positive feedback condition, the same aspects were addressed, but in orthogonal manner. In both groups, feedback lasted about 2 min.
Placebo TSST
The exact procedure of the placebo TSST was adapted from Het, Rohleder [22] and is described in detail in Appendix S2. The tasks applied had the same time course and a comparable cognitive and physical load to that of the TSST but without being stressful [22].
Questionnaires and physiological recording
State anxiety was captured using the STAI-SKD [38], a short version of the state anxiety scale of the German State-Trait Anxiety Inventory [39]. Stress was rated on a 10-point NRS ranging from 1 (‘not stressed at all’) to 10 (‘very stressed’). Additionally, participants answered sociodemographic questions and completed further questionnaires on affective states, pain perception/pain experiences, and spirituality as these concepts were considered to potentially modulate pain [2, 40], [41], [42]. A listing of all questionnaires including post-experimental questions can be found in Appendix S3.
Furthermore, physiological data was recorded, which will not be reported due to bad recording quality whilst performing the TSST. For measuring electrocardiography, three Ag/AgCl electrodes were attached on the torso of the participants (right collarbone, left lower costal arch; ground electrode: left lower side of the torso). Skin conductance was recorded using two 8 mm Ag/AgCl surface electrodes (electrode gel: 0.5% NaCl) attached to the thenar and hypothenar eminence of participants’ left hand.
Saliva collection and analyses
All saliva samples for the measurement of cortisol and alpha-amylase were collected with salivettes (Sarstedt Inc., Nürmbrecht, Germany) and analysed by the Department of Biological Psychology, University of Dresden, Germany. Further details regarding the biochemical analyses are provided in Appendix S4.
Study procedure (see Figure 1)
Experimental testing took place between 8:00 a.m. and 8:00 p.m. The ratio of testing time was balanced across groups.

Timeline and measurements conducted during the experimental procedure, CG = control group (placebo TSST); FB = feedback; T0 – T2 = #run of experimental pain assessment; R−45 – R+55 = run of completion of STAI-SKD or STAI, respectively, and distress rating, referring to (placebo-) TSST offset (in min); S−45 – S+55 = run of saliva collection referring to (placebo-) TSST offset (in min). The following pain tests were conducted at T0, T1, and T2 in this order: assessment of temporal summation, heat pain tolerance, heat pain threshold, ratings pf phasic heat pain stimuli, CPM effect of heat pain threshold and the rating of phasic heat pain stimuli.
Upon arrival, participants received information about study procedures as well as data handling and signed informed consent. Afterwards, electrodes for psychophysiological recording were attached. A saliva sample was taken 45 min before the TSST was over (S−45, please note that saliva samples and ratings/scores are named in the following always with reference to time in minutes until/since TSST offset). Participants completed the sociodemographic questionnaire as well as the first STAI-SKD and stress rating (R−45). Subsequently, participants received detailed written procedural information regarding pain tests and rating procedures, followed by a short training phase of some of the pain tests. Thereafter, the thermode was moved from the right to the left arm and the first experimental pain assessment (T0) took place, after which the second STAI-SKD and stress rating were filled out (R−15).
Subsequently, participants received instructions detailing the next experimental exercises to carry out the TSST or placebo TSST, respectively. After offset of the tasks, participants rated state anxiety and stress levels for the third time (R±0). Since the feedback procedure (fictive discussion and provision of feedback) lasted approximately 4 min, participants in conditions without feedback had a concordant waiting episode to keep time courses identical across groups. After feedback or waiting time, respectively, the STAI-SKD plus stress rating was completed for the fourth time (R+5) and a second saliva sample (S+5) was collected. Subsequently, another pain assessment (T1) took place, after which the third saliva sample (S+25) was collected. Next, participants were asked to fill out questionnaires for the upcoming 15 min. At the beginning of this time window, participants completed the fifth self-report regarding stress and anxiety levels (R+25). After time was up, the third pain assessment (T2) took place–even in the common case that participants did not finish all questionnaires. T2 was followed by the last STAI-SKD plus stress rating (R+55) and saliva collection (S+55). Finally, participants completed the post-experimental questionnaire and had unlimited time to finish the remaining questionnaires provided between T1 and T2. After removing the electrodes, all females were debriefed intensively according to the recommendations from McFarland, Cheam [43]. Please see Figure 1 for a timeline of the experimental procedure.
The study was conducted in accordance with the Declaration of Helsinki and was approved in all aspects by the local ethics Committee of the Department of Psychology of the University of Würzburg, Germany (#GZ 2018-07). Informed consent was obtained for every participant.
Data analysis
All analyses were performed using IBM SPSS statistics software version 25 (IBM Corp., Armonk, USA). Effect sizes are reported in partial eta squared ηp2 or Cohen’s d [44], respectively. Alpha≤0.05 was considered significant (two-tailed). Greenhouse Geisser (GG)-correction was applied in case the assumption of sphericity was violated (Mauchly test). Post hoc tests were performed using least significant differences (LSD) [45]. If homoscedasticity was violated (Levene test), independent samples t-tests with un-pooled variances and a correction of the degrees of freedom were utilized for test statistics [46].
Effects of experimental stress on pain measurements
Mean heat pain thresholds and mean phasic heat pain ratings were used for all subsequent analyses, even though we observed a systematic intrasession habituation of pain in all groups, as reported in previous research [47, 48].
The magnitude of CPM-effects was calculated by subtracting heat pain threshold in absence of the CS from the threshold in presence of the CS. Analogously, ratings of phasic heat pain stimuli in presence of the CS were subtracted from phasic heat pain ratings without CS. Thus, negative change scores indicated a successful endogenous pain inhibition, thereby following recommendations from Yarnitsky [49]. Mixed model ANOVAs were calculated for every pain variable with the BS-factor group (pos. FB, neg. FB, no FB, placebo) and the WS-factor time (T0, T1, T2).
Correlation analyses
TSST reactivity scores, i.e., the increase between each (logarithmized) stress-related baseline measurement and stress ratings/anxiety scores at R±0, logarithmized alpha-amylase concentrations at S+5 (after feedback or waiting time, respectively), and–due to typical response latency [18]– logarithmized salivary cortisol concentrations at S+25, respectively, were correlated with changes in pain perception (heat pain thresholds at T0 subtracted from thresholds at T1, phasic heat pain ratings at T1 subtracted from pain intensities at T0, and CPM effects at T0 subtracted from CPM effects at T1, respectively) using Pearson’s correlation coefficient r.
Correlative analyses of pain outcomes with all questionnaires were also planned, however, due to the lack of variance in our results and the big amount of data we refrained from reporting each single comparison.
Manipulation check
TSST reactivity scores were used to compare changes across groups via single one-way ANOVAs for each stress parameter. To obtain appropriate classifications of cortisol responders vs. non-responders, the response criterion by Miller, Plessow [50] of 1.5 nmol/L, which is based on a physiologically plausible response (an autoregressive latent trajectory mixture model, fitted to response levels of the TSST protocol vs. its placebo version), was additionally reported referring to non-logarithmized cortisol changes between baseline and R+25.
The area under the curve with respect to increase (AUCI) was calculated for each subject to approximate the integral for the cortisol change in response to the TSST [8, 51]. Its magnitude between groups was compared by a one-way ANOVA.
Feedback manipulation check was performed using two-sample t-tests comparing pos. FB and neg. FB regarding the feedback compatibility and unpleasantness ratings from the post-experimental survey. Additionally, one-way ANOVAs with feedback reactivity scores, defined as changes between baseline measurements and post-feedback/waiting time values of stress and anxiety at R+5 were calculated to compare change scores across groups.
To explore whether CPM in general was successfully induced by the experimental procedure applied here, mean change scores (TS without vs. with CS) between phasic heat pain ratings or thresholds at T0 were compared using dependent t-tests across all participants.
Results
TSST effects on stress (manipulation check)
The one-way ANOVA with the TSST reactivity score of ln cortisol was significant across groups, F(3, 142)=12.04, p<0.001, ηp2=0.20, with significantly more positive cortisol responses in pos. FB (M=0.23, SD=0.78), neg. FB (M=0.22, SD=0.47), and no FB (M=0.27, SD=0.47) vs. placebo (M=−0.64, SD=0.38), all ps<0.001. Other comparisons were not significant (all ps>0.202).
The analysis of the TSST reactivity score of ln alpha-amylase similarly was significant across groups, F(3, 135)=4.78, p=0.003, ηp2=0.10. Post hoc comparisons showed higher increases for pos. FB (M=0.43, SD=0.79) and neg. FB (M=0.55, SD=0.73) compared to placebo (M=0.01, SD=0.45), both ps<0.006. Remaining comparisons were not significant (all ps>0.429).
The analysis of the TSST reactivity score of stress ratings was also significant across groups, F(3, 172)=21.68, p<0.001, ηp2=0.27, driven by higher ratings in pos. FB (M=2.44, SD=2.35), neg. FB (M=3.04, SD=1.89), and no FB (M=2.64, SD=2.24) compared to placebo (M=−0.24, SD=1.90), all ps<0.001. In the neg. FB group (M=4.74, SD=1.84), a slower recovery was observable, reflected by higher stress ratings at R+5 compared to the pos. FB group (M=3.28, SD=1.64), the no FB group (M=3.84, SD=1.89), and obviously the placebo condition (M=2.51, SD=1.58), all ps<0.016. The remaining comparisons were not significant (all ps>0.140).
The analysis of the TSST reactivity score of anxiety ratings similarly was significant across groups in the one-way ANOVA, F(3, 172)=14.22, p<0.001, ηp2=0.20. LSD post hoc tests showed higher changes in anxiety levels in pos. FB (M=7.07, SD=2.92), neg. FB (M=3.33, SD=3.58), and no FB (M=2.64, SD=3.45) compared to placebo (M=−1.07, SD=2.56), all ps<0.001. Also, recovery from anxiety at R+5 was worst in neg. FB (M=9.00, SD=2.98) compared to pos. FB (M=7.07, SD=2.06), no FB (M=7.82, SD=2.37), and obviously placebo (M=6.65, SD=2.40), all ps<0.025. Other comparisons were not significant (all ps>162). All time courses of manipulation check parameters separated for each group are shown in Figure 2. Please note that both cortisol and alpha-amylase are presented in (non-logarithmized) raw values for easier interpretation.

Time courses of salivary cortisol (raw values) (Panel A), salivary alpha-amylase (raw values) (Panel B), stress ratings (Panel C), and anxiety scores (Panel D). Depicted are means ± standard errors. pos. FB = positive feedback condition, neg. FB = negative feedback condition, no FB = no feedback condition. Collection of saliva samples took place as following: Measurement 1 (S−45) = after informed consent (baseline), measurement 2 (S+5) = after feedback/waiting time, measurement 3 (S+25) = after 2nd pain assessment (T1), measurement 4 (S+55) = after 3rd pain assessment (T2). Measurements of stress ratings and anxiety scores took place as following: Measurement 1 (R−45) = after informed consent (baseline), measurement 2 (R−15) = after 1st pain assessment (T0), measurement 3 (R±0) = after TSST/Placebo TSST, measurement 4 (R+5) = after feedback/waiting time, measurement 5 (R+25) = after 2nd pain assessment (T1), measurement 6 (R+55) = after 3rd pain assessment (T2). Please note that for the sake of clarity, significant comparisons can be taken from the text.
Besides these comparisons, we applied the criterion of 1.5 nmol/L to effectively distinguish between cortisol responders and non-responders according to Miller, Plessow [50]. We used change scores of non-logarithmized cortisol levels between baseline and R+25 resulting in n=16 (37.21%) responders in positive feedback, n=15 (32.61%) responders in negative feedback, n=17 (37.78%) responders in no feedback and n=0 responders in the placebo condition. Please note that each ratio of cortisol responders per group reported formerly was calculated comprising missing values. Thus, some participants could neither be classified as responders nor as non-responders (due to unsuccessful laboratory analyses of one or more saliva sample(s) taken) but were still kept in the sample (pos. FB: n=7 corresponding to 16.73% of group’s sample size; neg. FB: n=8 corresponding to 17.39% of group’s sample size; no FB: n=8 corresponding to 17.78% of groups sample size).
Differences regarding the AUCI between groups turned out to be significant in ln cortisol, F(3, 138)=10.77, p<0.001, ηp2=0.19. LSD-based post hoc comparisons revealed that this effect was driven by a higher AUCI in pos. FB (M=10.61, SD=54.68), neg. FB (M=2.49, SD=49.73), and no FB (M=9.24, SD=55.22), each compared to the control condition (M=−46.59, SD=21.58), all ps<0.001. All remaining comparisons were not significant (all ps>0.489).
Also, differences in AUCI regarding ln alpha-amylase using a one-way ANOVA were significant between groups, F(3, 128)=3.18, p=0.026, ηp2=0.07. LSD-based post hoc comparisons revealed that this effect was driven by significantly higher AUCI in pos. FB (M=20.16, SD=46.40) and neg. FB (M=26.77, SD=48.83) compared to placebo (M=−2.12, SD=33.79), all ps<0.038. Besides, the AUCI of neg. FB (M=26.77, SD=48.83) was significantly higher than the AUCI of no FB (M=4.53, SD=41.35), p=0.035. All remaining comparisons were not significant (all ps>0.135).
Overall, physiological and subjective stress markers showed mostly successful stress inductions, however, feedback-specific variance across groups was sparse. Additionally, compatibility of the received feedback with participants’ expectation as well as feelings of discomfort regarding the given feedback (both captured post-experimentally) did not differ across groups. More detailed results regarding feedback manipulation checks can be found in Appendix S5.
Effects of stress on pain measurements
Heat pain threshold and ratings of phasic heat pain stimuli
Heat pain threshold: Analysis of heat pain threshold revealed no differences between groups (F(3, 159)=0.53, p=0.662, ηp2=0.01), but a significant main effect of time (GG-F(1.85, 294.80)=38.93, p<0.001, ηp2=0.20). The interaction of group x time was not significant, GG-F(5.56, 294.80)=0.33, p=0.909, ηp2=0.01. LSD post hoc tests showed that pain thresholds generally increased from T0 (M=43.81, SD=2.26) to T1 (M=44.76, SD=2.03) and T2 (M=44.79, SD=1.93), both ps<0.001, while the difference between T1 and T2 was not significant (p=0.795).
Ratings of phasic heat pain stimuli: Analysis of phasic heat pain stimuli revealed a significant main effect of group (F(3, 173)=36.63, p=0.047, ηp2=0.05) and of time (GG-F(1.93, 333.74)=30.14, p<0.001, ηp2=0.15), but no significant interaction of group x time, GG-F(5.79, 333.74)=0.19, p=0.977, ηp2= >0.01. LSD post hoc tests showed that the main effect of time resulted from significant differences between T0 (M=5.98, SD=2.11) and T1 (M=5.40, SD=2.33) as well as T2 (M=5.27, SD=2.39), both ps<0.001, but not between T1 and T2 (p=0.157). The main effect of group was driven by lower values in pos. FB (M=5.09, SD=2.55) and neg. FB (M=5.16, SD=2.30) compared to no FB (M=6.20, SD=2.12), both ps<0.020. Remaining comparisons were not significant (all ps>0.161).
Time courses of both pain tests separated by groups are shown in Figure 3.
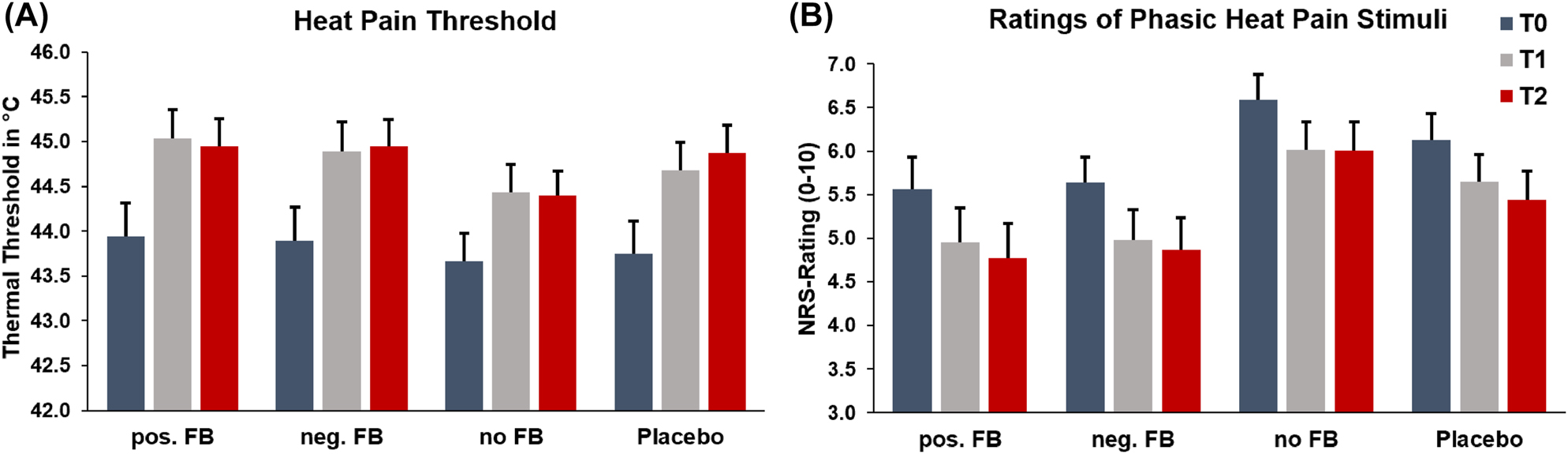
Heat pain threshold (Panel A) and ratings of phasic heat pain stimuli (Panel B). Depicted are means and standard errors, split by time points and each experimental condition. pos. FB = positive feedback condition, neg. FB = negative feedback condition, no FB = no feedback condition, T0 = 1st pain assessment, T1 = 2nd pain assessment, T2 = 3rd pain assessment. Please note that the lack of interaction effects does not permit significance testing on a single group level.
Conditioned pain modulation of heat pain threshold and ratings of phasic heat pain stimuli
CPM effect of heat pain threshold: A mixed-model ANOVA revealed no significant differences between group (F(3, 160)=0.24 p=0.870, ηp2=0.01), but a significant main effect of time (GG-F(1.91, 305.19)=9.06, p<0.001, ηp2=0.05). No significant interaction of group x time was found, GG-F(5.72, 305.19)=9.06 p=0.521, ηp2=0.02. LSD post hoc tests revealed that the main effect of time was driven by significant differences between T0 (M=−0.74, SD=1.98) compared to T1 (M=−0.14, SD=1.54) and T2 (M=−0.21, SD=1.39), both ps<0.001. The comparison between T1 and T2 was not significant (p=0.612).
CPM effect of the rating of phasic heat pain stimuli: A mixed-model ANOVA revealed no significant main effect of group (F(3, 173)=1.37, p=0.255, ηp2=0.02) or time (F(2, 346)=1.82, p=0.164, ηp2=0.01) and no significant interaction of group x time, F(2, 346)=0.60, p=0.732, ηp2=0.01. Both CPM effects are shown in Figure 4. Please note that CPM in general was successfully induced by both protocols applied in this study, for further details see Appendix S5.

CPM effect of heat pain threshold (Panel A) and the rating of phasic heat pain stimuli (Panel B). Depicted are means and standard errors, split by time points and each experimental condition. pos. FB = positive feedback condition, neg. FB = negative feedback condition, no FB = no feedback condition, T0 = 1st pain assessment, T1 = 2nd pain assessment, T2 = 3rd pain assessment. Please note that the lack of interaction effects does not permit significance testing on a single group level.
Since all pain variables did not show any interaction effects, analyses were performed again using pooled stress groups (no FB, neg. FB, pos. FB). However, there were still no interaction effects (all ps>0.130) despite the increase of statistical power.
Correlation of stress parameters and pain variables
Correlations between changes in stress parameters and changes in pain perception/modulation were generally very sparse and do not support a clear influence of stress on pain outcomes. Overall, we conclude, that cortisol effects on changes in pain perception/modulation can barely be clearly interpreted based on the observed distribution of our data set. Results are reported in Appendix S6.
Discussion
Stress-induced changes in pain perception
The results of our study suggest an overall decrease in pain perception and modulation in all three TSST (+FB) conditions instead of an increase as was expected a priori. However, a similar response pattern was also evident in the placebo condition, thus questioning stress-induced modulation of pain reported in previous studies [7], [8], [9]. One reason for this inconsistency to former research likely stems from certain–in our opinion crucial–methodological considerations [4, 12]. Whereas previous studies on stress-induced hypo-/hyperalgesia often neglected the necessity of a control condition [4, 5, 10], we addressed this issue by incorporating a highly standardized placebo group [22, 52]. Furthermore, in contrast to previous studies reporting TSST driven hyperalgesia [9], we complemented a rather large sample with several conceptual manipulation checks and various indicators of pain processing and modulation.
With respect to ‘dynamic’ pain parameters, which likely represent traits of central pain processing and capture inhibitory (CPM) aspects of endogenous pain modulation [49], our study is more in line with research showing hampered CPM effects following stress induction by different stress paradigms [6, 7, 32]. However, as indicated by the data from the control group, our results do not point towards stress or stress plus feedback specific effects impacting CPM across time. Since at T0, both CPM paradigms (threshold and the rating of phasic heat pain stimuli) showed significant inhibitory capabilities, the lack of group specific changes is probably not driven by a general absence of CPM (at T0), albeit effects were smaller compared to previous studies [53]. Generally, CPM responses were found to be lower in female samples [11, 12] and the use of fixed instead of individually tailored test stimuli bears greater risk for floor or ceiling effects [13], which might explain why CPM effects in the present study were rather small. That being said, the all-female sample, the applied stressor (resulting in a rather low cortisol response), and our specific CPM paradigms (even though common testing protocols were used [14], [15], [16], [17]), might to some degree explain the contrasting finding compared to previous experiments [18], [19], [20]. Still, the quoted studies applied a crossover-design or had no dedicated control conditions. Such experimental approaches may lead to an overestimation and misallocation of effects, underlining the importance of placebo-controlled experimental plans [54].
Additionally, in the present study we recruited mainly young female participants who were investigated by young female experimenters. This constellation already demonstrated to result in less intense stress responses following TSST [55]. Thus, despite the observed subjective and–to a lesser degree–physiological stress induction, responses might not have been sufficient to actually alter pain.
Furthermore, even though the placebo TSST can be considered a very well-designed reference condition providing a maximally unbiased baseline [22], one may argue that during the free speech task participants might have remembered predominantly pleasant events similar to psychological tasks intended to induce positive affective states [56, 57], which are known to decrease pain [4, 11, 29]. A similar argument might apply to the observed pain decrease from T0 to T2 in all experimental groups. Supposedly, pain testing became less aversive during the time course of the experiment, since perceived controllability and predictability regarding the testing procedure likely increased across time. The decline of pain perception over time might be interpreted in the light of habituation as a predominantly behavioural phenomenon [58], which is at least in part mediated by increased central anti-nociceptive activity [59]. Additionally, adaption-level processes might have had an distinguishable impact on pain decrement as they are–in contrast to habituation–largely based on sensory and motor fatigue (e.g. resulting in less nociceptor action potentials) [60, 61].
Lastly, the fact that pain assessment always started with heat pain tolerance might have led to anchor effects and hampered differentiation between self-reported levels of heat pain tolerance and threshold observed in some participants. Using a fixed testing order, however, controls for the risk of prolonged CPM effects, which may confound subsequent intrasession pain assessments [62]. Additionally, a limitation of our study might be the fact that we only used heat pain stimuli. However, this pain modality is advantageous according to Churyukanov, Plaghki [2] as the two most important transmission systems, i.e., Aδ- and C-fiber-mediated signaling, can be activated together.
Manipulation check of TSST and feedback
Overall, manipulation checks suggest a successful stress induction by TSST performance that was especially evident for subjective, but less clear for physiological parameters. One reason for the rather sparse cortisol response might be our prolonged window of experimental testing (8:00 a.m. – 8:00 p.m.) regardless of endocrine circadian rhythmics [63]. Even though net increases of cortisol should be rather independent of testing time [21], one meta-analysis reported an influence of daytime on cortisol reactivity and suggests prolonged acclimatization of participants in the lab [55]. However, with respect to the general decrease of cortisol across our whole experiment in the placebo condition–AUCI trapezoids were significantly larger in TSST groups compared to the placebo condition–the performance of the stress paradigm appears to compensate and even to turn around this downward trend, as observed previously by von Dawans, Ditzen [64].
This downward trend of corstisol might be explained to some degree by the characteristics of the control task, which might be perceived as somewhat positive instead of solely neutral (see 4.1 for further discussion) and the common chronobiological decline of cortisol following the early ‘awakening response’ [50, 63]. That being said, it should be recognized, however, that hormone increases were rather low when considering the response patterns of each single TSST group using a fixed criterion (as defined by Miller, Stalder [65], showing a responder rate of about only one third of all TSST participants). Even so, one should contrast this with the fact that only two persons from the placebo group were classified as significant cortisol responders and that other studies using the TSST also showed only moderate cortisol releases in all-female samples, which are less pronounced than comparable hormone increases in men [52, 66]. However, given that we set out to distinguish between stress responders and non-responders based on a valid criterion to describe the overall effectiveness of stress induction [50], the finding questions clarity and success of our cortisol manipulation check.
Anxiety and stress reports showed very pronounced increases in all TSST conditions, however, both ratings rapidly decreased after the feedback or the waiting period, respectively. Given that the end of the TSST was very evident for the participants, subjective stress measurements might have taken place as social stress was already declining and replaced by feelings of relief [67]. Nonetheless, recovery from stress induction was different across experimental groups: especially, in the negative feedback group the return towards baseline levels was delayed, in line with theories postulating increased discomfort following negative evaluation [68, 69].
Both feedback groups rated their individual performance feedback similarly unpleasant and plausible, i.e., compatible to their own impression of performance. This suggests that feedback was experienced in general as neither overly aversive nor diagnostic and thus might explain why TSST plus feedback demonstrated only small additive stress effects. Importantly, heat pain stimulation itself in the sense of a physical stressor did not seem to affect stress responses as reflected by a lack of subjective changes (at R−15) in stress parameters across groups.
Conclusion and outlook
Despite a successful induction of psychosocial stress (especially as evident in the subjective data) we did not replicate previously reported stress-associated changes in pain perception or modulation.
The general decrease in pain over time reported here is most likely neither a specific consequence of the TSST nor of the performance feedback given afterwards but probably the result of the participants’ habituation/adaptation to pain assessment. This implies that negative feedback, even though capable to delay recovery from stress, is not generally additive with regards to the stress or pain response. Furthermore, humoral and subjective stress responses were not systematically associated with changes in pain measurements, partly contradicting earlier findings [10, 70].
As previous research often lacked a suitable reference conditions or sufficiently large samples [4, 5], and might not have induced enough psychological stress [4, 12], our findings raise the question whether former studies might have overestimated social stress–and especially TSST−associated changes in pain perception.
Besides the crucial methodological considerations as listed above, future research is necessary to explore sex or gender specific effects in TSST driven pain modulation. Furthermore, future studies might consider the investigation of participants at risk for pain chronification or even employ pain patients to gain broader understandings of the interaction of pain and stress. Given that experimentally induced stress responses seemed to be relatively short-lived here, task instructions, which determine when and how long to anticipate a psychosocial threat, should be considered carefully in order to perform pain measurements actually peri instead of post stress experiences. Despite all inconsistencies reported here, holding on this line of research is particularly important in the light of some insight promoting results since the social domain of the biopsychosocial model of (chronic) pain has been widely understudied, although its understanding holds large promise for an improved treatment of pain.
Funding source: Faculty of Humanities and the Gender Equality
Funding source: Women Empowerment Commissioner of the University of Würzburg, Germany
Acknowledgments
This study is part of the dissertation project of SKS supported by a stipend of the Evang. Studienwerk Villigst, Germany, within the project DoloRES (resilience in pain processing). We would like to thank Michael Jost for technical assistance, Mareike Koolman, Elisabeth Schwarz, Christina Bielek and Lena Pflückebaum for their support in data collection and preparation as well as Corinna Rothenbücher and Magdalena Fröhling for their work on layout revision and text formatting.
-
Research funding: Data collection, participants’ reimbursement, and saliva analyses were funded by the Faculty of Humanities and the Gender Equality and Women Empowerment Commissioner of the University of Würzburg, Germany.
-
Author contributions: All authors contributed to study’s design and conception. SKS acquired the data, which were analyzed by SKS and PR. All authors discussed the results. SKS drafted the manuscript. All authors commented and revised it critically for important intellectual content. All authors approved the final version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
-
Competing interests: The authors have no conflicts of interest to declare.
-
Informed consent: Informed consent has been obtained from all individuals included in this study.
-
Ethical approval: We hereby declare our compliance with the ethical guidelines published by the SJP. Our research complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as amended in 2013). It has been approved by our local ethics committee (Department of Psychology, University of Würzburg, Germany) in all aspects (#GZ 2018-07).
References
1. Reicherts, P, Gerdes, AB, Pauli, P, Wieser, MJ. On the mutual effects of pain and emotion: facial pain expressions enhance pain perception and vice versa are perceived as more arousing when feeling pain. Pain 2013;154:793–800. https://doi.org/10.1016/j.pain.2013.02.012.Search in Google Scholar PubMed
2. Dickens, C, McGowan, L, Dale, S. Impact of depression on experimental pain perception: a systematic review of the literature with meta-analysis. Psychosom Med 2003;65:369–75. https://doi.org/10.1097/01.PSY.0000041622.69462.06.Search in Google Scholar PubMed
3. Reicherts, P, Wiemer, J, Gerdes, AB, Schulz, SM, Pauli, P, Wieser, MJ. Anxious anticipation and pain: the influence of instructed vs conditioned threat on pain. Soc Cognit Affect Neurosci 2017;12:544–54. https://doi.org/10.1093/scan/nsw181.Search in Google Scholar PubMed PubMed Central
4. Ahmad, AH, Zakaria, R. Pain in times of stress. Malays J Med Sci 2015;22:52–61.Search in Google Scholar
5. Olango, WM, Finn, DP. Neurobiology of stress-induced hyperalgesia. Curr Top Behav Neurosci 2014;20:251–80. https://doi.org/10.1007/7854_2014_302.Search in Google Scholar PubMed
6. Geva, N, Defrin, R. Opposite effects of stress on pain modulation depend on the magnitude of individual stress response. J Pain 2018;19:360–71. https://doi.org/10.1016/j.jpain.2017.11.011.Search in Google Scholar PubMed
7. Geva, N, Pruessner, JC, Defrin, R. Acute psychosocial stress reduces pain modulation capabilities in healthy men. Pain 2014;155:2418–25. https://doi.org/10.1016/j.pain.2014.09.023.Search in Google Scholar PubMed
8. Gaab, J, Jimenez, J, Voneschen, L, Oschwald, D, Meyer, AH, Nater, UM, et al.. Psychosocial stress-induced analgesia: an examination of effects on heat pain threshold and tolerance and of neuroendocrine mediation. Neuropsychobiology 2016;74:87–95. https://doi.org/10.1159/000454986.Search in Google Scholar PubMed
9. Crettaz, B, Marziniak, M, Willeke, P, Young, P, Hellhammer, DH, Stumpf, A, et al.. Stress-induced allodynia – evidence of increased pain sensitivity in healthy humans and patients with chronic pain after experimentally induced psychosocial stress. PLoS One 2013;8:e69460. https://doi.org/10.1371/journal.pone.0069460.Search in Google Scholar PubMed PubMed Central
10. Butler, RK, Finn, DP. Stress-induced analgesia. Prog Neurobiol 2009;88:184–202. https://doi.org/10.1016/j.pneurobio.2009.04.003.Search in Google Scholar PubMed
11. Rhudy, JL, Williams, AE. Gender differences in pain: do emotions play a role? Gend Med 2005;2:208–26. https://doi.org/10.1016/S1550-8579(05)80051-8.Search in Google Scholar PubMed
12. Dickerson, SS, Kemeny, ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 2004;130:355–91. https://doi.org/10.1037/0033-2909.130.3.355.Search in Google Scholar PubMed
13. Herman, J, Cullinan, W. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 1997;20:78–84. https://doi.org/10.1016/s0166-2236(96)10069-2.Search in Google Scholar PubMed
14. Al’Absi, M, Petersen, KL. Blood pressure but not cortisol mediates stress effects on subsequent pain perception in healthy men and women. Pain 2003;106:285–95. https://doi.org/10.1016/S0304-3959(03)00300-2.Search in Google Scholar PubMed
15. Jennings, EM, Okine, BN, Roche, M, Finn, DP. Stress-induced hyperalgesia. Prog Neurobiol 2014;121:1–18. https://doi.org/10.1016/j.pneurobio.2014.06.003.Search in Google Scholar PubMed
16. Engert, V, Vogel, S, Efanov, SI, Duchesne, A, Corbo, V, Ali, N, et al.. Investigation into the cross-correlation of salivary cortisol and alpha-amylase responses to psychological stress. Psychoneuroendocrinology 2011;36:1294–302. https://doi.org/10.1016/j.psyneuen.2011.02.018.Search in Google Scholar PubMed
17. Nater, UM, Skoluda, N, Strahler, J. Biomarkers of stress in behavioural medicine. Curr Opin Psychiatr 2013;26:440–5. https://doi.org/10.1097/YCO.0b013e328363b4ed.Search in Google Scholar PubMed
18. Kirschbaum, C, Pirke, K, Hellhammer, DH. The ‘Trier Social Stress Test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993;28:76–81. https://doi.org/10.1159/000119004.Search in Google Scholar PubMed
19. Maruyama, Y, Kawano, A, Okamoto, S, Ando, T, Ishitobi, Y, Tanaka, Y, et al.. Differences in salivary alpha-amylase and cortisol responsiveness following exposure to electrical stimulation versus the Trier Social Stress Tests. PLoS One 2012;7:e39375. https://doi.org/10.1371/journal.pone.0039375.Search in Google Scholar PubMed PubMed Central
20. Allen, AP, Kennedy, PJ, Dockray, S, Cryan, JF, Dinan, TG, Clarke, G. The trier social stress test: principles and practice. Neurobiol Stress 2017;6:113–26. https://doi.org/10.1016/j.ynstr.2016.11.001.Search in Google Scholar PubMed PubMed Central
21. Kudielka, BM, Hellhammer, DH, Kirschbaum, C, Harmon-Jones, E, Winkielman, P. Ten years of research with the trier social stress test – revisited. In: Harmon-Jones, E, Winkielman, P, editors. Social neuroscience: integrating biological and psychological explanations of social behavior 56–83. New York: Guilford Press; 2007:56–83 pp.Search in Google Scholar
22. Het, S, Rohleder, N, Schoofs, D, Kirschbaum, C, Wolf, OT. Neuroendocrine and psychometric evaluation of a placebo version of the ‘trier social stress test. Psychoneuroendocrinology 2009;34:1075–86. https://doi.org/10.1016/j.psyneuen.2009.02.008.Search in Google Scholar PubMed
23. Phan, JM, Schneider, E, Peres, J, Miocevic, O, Meyer, V, Shirtcliff, EA. Social evaluative threat with verbal performance feedback alters neuroendocrine response to stress. Horm Behav 2017;96:104–15. https://doi.org/10.1016/j.yhbeh.2017.09.007.Search in Google Scholar PubMed PubMed Central
24. Brown, JL, Sheffield, D, Leary, MR, Robinson, ME. Social support and experimental pain. Psychosom Med 2003;65:276–83. https://doi.org/10.1097/01.psy.0000030388.62434.46.Search in Google Scholar PubMed
25. Eisenberger, NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci 2012;13:421–34. https://doi.org/10.1038/nrn3231.Search in Google Scholar PubMed
26. Geva, N, Uzefovsky, F, Levy-Tzedek, S. Touching the social robot PARO reduces pain perception and salivary oxytocin levels. Sci Rep 2020;10:1–15. https://doi.org/10.1038/s41598-020-66982-y.Search in Google Scholar PubMed PubMed Central
27. Masten, CL, Eisenberger, NI, Borofsky, LA, McNealy, K, Pfeifer, JH, Dapretto, M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents’ risk for depression. Dev Psychopathol 2011;23:283–92. https://doi.org/10.1017/S0954579410000799.Search in Google Scholar PubMed PubMed Central
28. Montoya, P, Larbig, W, Braun, C, Preissl, H, Birbaumer, N. Influence of social support and emotional context on pain processing and magnetic brain responses in fibromyalgia. Arthritis Rheum 2004;50:4035–44. https://doi.org/10.1002/art.20660.Search in Google Scholar PubMed
29. Hanssen, MM, Peters, ML, Boselie, JJ, Meulders, A. Can positive affect attenuate (persistent) pain? State of the art and clinical implications. Curr Rheumatol Rep 2017;19:80. https://doi.org/10.1007/s11926-017-0703-3.Search in Google Scholar PubMed PubMed Central
30. Williams, AE, Rhudy, JL. The influence of conditioned fear on human pain thresholds: does preparedness play a role? J Pain 2007;8:598–606. https://doi.org/10.1016/j.jpain.2007.03.004.Search in Google Scholar PubMed
31. Lopez-Duran, NL, Mayer, SE, Abelson, JL. Modeling neuroendocrine stress reactivity in salivary cortisol: adjusting for peak latency variability. Stress 2014;17:285–95. https://doi.org/10.3109/10253890.2014.915517.Search in Google Scholar PubMed
32. Nilsen, KB, Christiansen, SE, Holmen, LB, Sand, T. The effect of a mental stressor on conditioned pain modulation in healthy subjects. Scand J Pain 2012;3:142–8. https://doi.org/10.1016/j.sjpain.2012.04.005.Search in Google Scholar PubMed
33. Geva, N, Pruessner, JC, Defrin, R. Triathletes lose their advantageous pain modulation under acute psychosocial stress. Med Sci Sports Exerc 2017;49:333–41. https://doi.org/10.1249/mss.0000000000001110.Search in Google Scholar
34. Lautenbacher, S, Roscher, S, Strian, D, Fassbender, K, Krumrey, K, Krieg, JC. Pain perception in depression: relationships to symptomatology and naloxone-sensitive mechanisms. Psychosom Med 1994;56:345–52. https://doi.org/10.1097/00006842-199407000-00010.Search in Google Scholar PubMed
35. Nir, RR, Yarnitsky, D. Conditioned pain modulation. Curr Opin Support Palliat Care 2015;9:131–7. https://doi.org/10.1097/SPC.0000000000000126.Search in Google Scholar PubMed
36. Horn-Hofmann, C, Kunz, M, Madden, M, Schnabel, EL, Lautenbacher, S. Interactive effects of conditioned pain modulation and temporal summation of pain—the role of stimulus modality. Pain 2018;159:2641–8. https://doi.org/10.1097/j.pain.0000000000001376.Search in Google Scholar PubMed
37. Honigman, L, Yarnitsky, D, Sprecher, E, Weissman-Fogel, I. Psychophysical testing of spatial and temporal dimensions of endogenous analgesia: conditioned pain modulation and offset analgesia. Exp Brain Res 2013;228:493–501. https://doi.org/10.1007/s00221-013-3580-7.Search in Google Scholar PubMed
38. Englert, C, Bertrams, A, Dickhäuser, O. Entwicklung der fünf-item-Kurzskala STAI-SKD zur Messung von Zustandsangst. Z für Gesundheitspsychol 2011;19:173–80. https://doi.org/10.1026/0943-8149/a000049.Search in Google Scholar
39. Laux, L, Glanzmann, P, Schaffner, P, Spielberger, CD. State-Trait-Angstinventar. Göttingen: Beltz; 1981.Search in Google Scholar
40. Tang, J, Gibson, SJ. A psychophysical evaluation of the relationship between trait anxiety, pain perception, and induced state anxiety. J Pain 2005;6:612–9. https://doi.org/10.1016/j.jpain.2005.03.009.Search in Google Scholar PubMed
41. Riva, P, Williams, KD, Gallucci, M. The relationship between fear of social and physical threat and its effect on social distress and physical pain perception. Pain 2014;155:485–93. https://doi.org/10.1016/j.pain.2013.11.006.Search in Google Scholar PubMed
42. Hemington, KS, Cheng, JC, Bosma, RL, Rogachov, A, Kim, JA, Davis, KD. Beyond negative pain-related psychological factors: resilience is related to lower pain affect in healthy adults. J Pain 2017;18:1117–28. https://doi.org/10.1016/j.jpain.2017.04.009.Search in Google Scholar PubMed
43. McFarland, C, Cheam, A, Buehler, R. The perseverance effect in the debriefing paradigm: replication and extension. J Exp Soc Psychol 2007;43:233–40. https://doi.org/10.1016/j.jesp.2006.01.010.Search in Google Scholar
44. Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 2013;4:863. https://doi.org/10.3389/fpsyg.2013.00863.Search in Google Scholar PubMed PubMed Central
45. Fisher, RA. Design of experiments. Br Med J 1936;1:554. https://doi.org/10.1136/bmj.1.3923.554-a.Search in Google Scholar
46. Kent State University Libraries. SPSS tutorials: independent samples t-test; 2021. Available from: https://libguides.library.kent.edu/spss/independentttest.Search in Google Scholar
47. Pontén, M, Fust, J, D’Onofrio, P, Dorp, RV, Sunnergård, L, Ingre, M, et al.. The pain alarm response – an example of how conscious awareness shapes pain perception. Sci Rep 2019;9:12478. https://doi.org/10.1038/s41598-019-48903-w.Search in Google Scholar PubMed PubMed Central
48. Condes-Lara, M, Calvo, JM, Fernandez-Guardiola, A. Habituation to bearable experimental pain elicited by tooth pulp electrical stimulation. Pain 1981;11:185–200. https://doi.org/10.1016/0304-3959(81)90004-x.Search in Google Scholar
49. Yarnitsky, D. Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain 2015;156:24–31. https://doi.org/10.1097/01.j.pain.0000460343.46847.58.Search in Google Scholar PubMed
50. Miller, R, Plessow, F, Kirschbaum, C, Stalder, T. Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: evaluation of salivary cortisol pulse detection in panel designs. Psychosom Med 2013;75:832–40. https://doi.org/10.1097/PSY.0000000000000002.Search in Google Scholar PubMed
51. Pruessner, JC, Kirschbaum, C, Meinlschmid, G, Hellhammer, DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003;28:916–31. https://doi.org/10.1016/s0306-4530(02)00108-7.Search in Google Scholar PubMed
52. Reschke-Hernández, AE, Okerstrom, KL, Bowles Edwards, A, Tranel, D. Sex and stress: men and women show different cortisol responses to psychological stress induced by the trier social stress test and the Iowa singing social stress test. J Neurosci Res 2017;95:106–14. https://doi.org/10.1002/jnr.23851.Search in Google Scholar PubMed PubMed Central
53. Pud, D, Granovsky, Y, Yarnitsky, D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain 2009;144:16–29. https://doi.org/10.1016/j.pain.2009.02.015.Search in Google Scholar PubMed
54. Schnell, R, Hill, PB, Esser, E. Methoden der empirischen Sozialforschung. Munich: Oldenbourg; 1992.Search in Google Scholar
55. Goodman, WK, Janson, J, Wolf, JM. Meta-analytical assessment of the effects of protocol variations on cortisol responses to the trier social stress test. Psychoneuroendocrinology 2017;80:26–35. https://doi.org/10.1016/j.psyneuen.2017.02.030.Search in Google Scholar PubMed
56. Snyder, CR, Shane, J. The oxford handbook of positive psychology. Oxford: Oxford University Press; 2021.Search in Google Scholar
57. Fredrickson, BL. Cultivating positive emotions to optimize health and well-being. Prev Treat 2000;3:1a. https://doi.org/10.1037/1522-3736.3.1.31a.Search in Google Scholar
58. Habituation, RT. International encyclopedia of the social & behavioral sciences. In: Smelser, NJ, Baltes, PB, editors. Oxford: Pergamon; 2001:6458–62 pp.Search in Google Scholar
59. Bingel, U, Schoell, E, Herken, W, Büchel, C, May, A. Habituation to painful stimulation involves the antinociceptive system. Pain 2007;131:21–30. https://doi.org/10.1016/j.pain.2006.12.005.Search in Google Scholar PubMed
60. Rollman, GB. Signal detection theory pain measures: empirical validation studies and adaptation-level effects. Pain 1979;6:9–21. https://doi.org/10.1016/0304-3959(79)90136-2.Search in Google Scholar PubMed
61. Bowling, N. Adaptation-level theory. In: Michalos, AC, editor. Encyclopedia of quality of life and well-being research. Dordrecht: Springer; 2014:28–9 pp.10.1007/978-94-007-0753-5_25Search in Google Scholar
62. Lewis, GN, Rice, DA, McNair, PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain 2012;13:936–44. https://doi.org/10.1016/j.jpain.2012.07.005.Search in Google Scholar PubMed
63. Chan, S, Debono, M. Replication of cortisol circadian rhythm: new advances in hydrocortisone replacement therapy. Ther Adv Endocrinol Metab 2010;1:129–38. https://doi.org/10.1177/2042018810380214.Search in Google Scholar PubMed PubMed Central
64. von Dawans, B, Ditzen, B, Trueg, A, Fischbacher, U, Heinrichs, M. Effects of acute stress on social behavior in women. Psychoneuroendocrinology 2019;99:137–44. https://doi.org/10.1016/j.psyneuen.2018.08.031.Search in Google Scholar PubMed
65. Miller, R, Stalder, T, Jarczok, MN, Almeida, DM, Badrick, E, Bartels, M, et al.. The CIRCORT database: reference ranges and seasonal changes in diurnal salivary cortisol derived from a meta-dataset comprised of 15 field studies. Psychoneuroendocrinology 2016;73:16–23. https://doi.org/10.1016/j.psyneuen.2016.07.201.Search in Google Scholar PubMed PubMed Central
66. Lopez-Duran, NL, Mayer, SE, Abelson, JL. Modeling neuroendocrine stress reactivity in salivary cortisol: adjusting for peak latency variability. Stress 2014;17:285–95.10.3109/10253890.2014.915517Search in Google Scholar PubMed
67. Vors, O, Marqueste, T, Mascret, N. The Trier social stress test and the Trier social stress test for groups: qualitative investigations. PLoS One 2018;13:e0195722. https://doi.org/10.1371/journal.pone.0195722.Search in Google Scholar PubMed PubMed Central
68. Shrauger, JS, Lund, AK. Self-evaluation and reactions to evaluations from others. J Pers 1975;43:94–108. https://doi.org/10.1111/j.1467-6494.1975.tb00574.x.Search in Google Scholar PubMed
69. Moreland, RL, Sweeney, PD. Self-expectancies and reactions to evaluations of personal performance. J Pers 1984;52:156–76. https://doi.org/10.1111/j.1467-6494.1984.tb00350.x.Search in Google Scholar
70. Timmers, I, Kaas, AL, Quaedflieg, C, Biggs, EE, Smeets, T, de Jong, JR. Fear of pain and cortisol reactivity predict the strength of stress-induced hypoalgesia. Eur J Pain 2018;22:1291–303. https://doi.org/10.1002/ejp.1217.Search in Google Scholar PubMed PubMed Central
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/sjpain-2021-0204).
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial Comment
- Chronic pain and health inequalities: why we need to act
- Systematic Reviews
- Resilience as a protective factor in face of pain symptomatology, disability and psychological outcomes in adult chronic pain populations: a scoping review
- Is intravenous magnesium sulphate a suitable adjuvant in postoperative pain management? – A critical and systematic review of methodology in randomized controlled trials
- Topical Review
- Pain assessment 3 × 3: a clinical reasoning framework for healthcare professionals
- Clinical Pain Researches
- The treatment lottery of chronic back pain? A case series at a multidisciplinary pain centre
- Parameters of anger as related to sensory-affective components of pain
- Loneliness in patients with somatic symptom disorder
- The development and measurement properties of the Dutch version of the fear-avoidance components scale (FACS-D) in persons with chronic musculoskeletal pain
- Observational Studies
- Can interoceptive sensitivity provide information on the difference in the perceptual mechanisms of recurrent and chronic pain? Part I. A retrospective clinical study related to multidimensional pain assessment
- Distress intolerance and pain catastrophizing as mediating variables in PTSD and chronic noncancer pain comorbidity
- Stress-induced headache in the general working population is moderated by the NRCAM rs2300043 genotype
- Does poor sleep quality lead to increased low back pain the following day?
- “I had already tried that before going to the doctor” – exploring adolescents’ with knee pain perspectives on ‘wait and see’ as a management strategy in primary care; a study with brief semi-structured qualitative interviews
- Problematic opioid use among osteoarthritis patients with chronic post-operative pain after joint replacement: analyses from the BISCUITS study
- Worst pain intensity and opioid intake during the early postoperative period were not associated with moderate-severe pain 12 months after total knee arthroplasty – a longitudinal study
- Original Experimentals
- How gender affects the decoding of facial expressions of pain
- A simple, bed-side tool to assess evoked pressure pain intensity
- Effects of psychosocial stress and performance feedback on pain processing and its correlation with subjective and neuroendocrine parameters
- Participatory research: a Priority Setting Partnership for chronic musculoskeletal pain in Denmark
- Educational Case Report
- Hypophosphatasia as a plausible cause of vitamin B6 associated mouth pain: a case-report
- Short Communications
- Pain “chronification”: what is the problem with this model?
- Korsakoff syndrome and altered pain perception: a search of underlying neural mechanisms
Articles in the same Issue
- Frontmatter
- Editorial Comment
- Chronic pain and health inequalities: why we need to act
- Systematic Reviews
- Resilience as a protective factor in face of pain symptomatology, disability and psychological outcomes in adult chronic pain populations: a scoping review
- Is intravenous magnesium sulphate a suitable adjuvant in postoperative pain management? – A critical and systematic review of methodology in randomized controlled trials
- Topical Review
- Pain assessment 3 × 3: a clinical reasoning framework for healthcare professionals
- Clinical Pain Researches
- The treatment lottery of chronic back pain? A case series at a multidisciplinary pain centre
- Parameters of anger as related to sensory-affective components of pain
- Loneliness in patients with somatic symptom disorder
- The development and measurement properties of the Dutch version of the fear-avoidance components scale (FACS-D) in persons with chronic musculoskeletal pain
- Observational Studies
- Can interoceptive sensitivity provide information on the difference in the perceptual mechanisms of recurrent and chronic pain? Part I. A retrospective clinical study related to multidimensional pain assessment
- Distress intolerance and pain catastrophizing as mediating variables in PTSD and chronic noncancer pain comorbidity
- Stress-induced headache in the general working population is moderated by the NRCAM rs2300043 genotype
- Does poor sleep quality lead to increased low back pain the following day?
- “I had already tried that before going to the doctor” – exploring adolescents’ with knee pain perspectives on ‘wait and see’ as a management strategy in primary care; a study with brief semi-structured qualitative interviews
- Problematic opioid use among osteoarthritis patients with chronic post-operative pain after joint replacement: analyses from the BISCUITS study
- Worst pain intensity and opioid intake during the early postoperative period were not associated with moderate-severe pain 12 months after total knee arthroplasty – a longitudinal study
- Original Experimentals
- How gender affects the decoding of facial expressions of pain
- A simple, bed-side tool to assess evoked pressure pain intensity
- Effects of psychosocial stress and performance feedback on pain processing and its correlation with subjective and neuroendocrine parameters
- Participatory research: a Priority Setting Partnership for chronic musculoskeletal pain in Denmark
- Educational Case Report
- Hypophosphatasia as a plausible cause of vitamin B6 associated mouth pain: a case-report
- Short Communications
- Pain “chronification”: what is the problem with this model?
- Korsakoff syndrome and altered pain perception: a search of underlying neural mechanisms

