Characterization of the antinociceptive effects of intrathecal DALDA peptides following bolus intrathecal delivery
-
Shinichi Kokubu
Abstract
Background and aims
We systematically characterized the potency and side effect profile of a series of small opioid peptides with high affinity for the mu opioid receptor.
Methods
Male Sprague Dawley rats were prepared with intrathecal (IT) catheters, assessed with hind paw thermal escape and evaluated for side effects including Straub tail, truncal rigidity, and pinnae and corneal reflexes. In these studies, DMT-DALDA (dDAL) (H-Dmt-D-Arg-Phe-Lys-NH2 MW=981), dDALc (H-Dmt-Cit-Phe-Lys-NH2 MW=868), dDALcn (H-Dmt-D-Cit-Phe-Nle-NH2 MW=739), TAPP (H-Tyr-D-Ala-Phe-Phe-NH2 MW=659), dDAL-TICP ([Dmt1]DALDA-(CH2)2-NH-TICP[psi]; MW=1519), and dDAL-TIPP (H-Dmt-D-Arg-Phe-Lys(Nε-TIPP)-NH2 were examined. In separate studies, the effects of approximately equiactive doses of IT DMT DALDA (10 pmol), morphine (30 nmol) and fentanyl (1 nmol) were examined on formalin-induced flinching at different pretreatment intervals (15 min – 24 h).
Results
(1) All agents resulted in a dose-dependent reversible effect upon motor function (Straub Tail>Truncal rigidity). (2) The ordering of analgesic activity (%MPE) at the highest dose lacking reliable motor signs after bolus delivery was: DMT-DALDA (80%±6/3 pmol); dDALc (75%±8/1 pmol); dDALcn (84%±10/300 pmol); TAPP (56%±12/10 nmol); dDAL-TICP (52%±27/300 pmol). (3) All analgesic effects were reversed by systemic (IP) naloxone (1 mg/kg). Naltrindole (3 mg/kg, IP) had no significant effect upon the maximum usable peptide dose. (4) Tolerance and cross-tolerance development after 5 daily boluses of DMT-DALDA (3 pmol) and morphine (30 nmol) revealed that both agents displayed a progressive decline over 5 days. (5) Cross-tolerance assessed at day 5 revealed a reduction in response to morphine in DMT-DALDA treated animal but not DMT-DALDA in the morphine treated animal, indicating an asymmetric cross-tolerance. (6) IT DMT-DALDA, morphine and fentanyl resulted in significant reductions in phase 1 and phase 2 flinching. With a 15 min pretreatment all drugs resulted in comparable reductions in flinching. However, at 6 h, the reduction in flinching after DMT-DALDA and morphine were comparably reduced while fentanyl was not different from vehicle. All effects on flinching were lost by 24 h.
Conclusions
These results emphasize the potent mu agonist properties of the DALDA peptidic structure series, their persistence similar to morphine and their propensity to produce tolerance. The asymmetric cross-tolerance between equiactive doses may reflect the relative intrinsic activity of morphine and DMT-DALDA.
Implications
These results suggest that the DALDA peptides with their potency and duration of action after intrathecal delivery suggest their potential utility for their further development as a spinal therapeutic to manage pain.
1 Introduction
Mu receptor-preferring opioids given intrathecally yield a potent suppression of nociceptive signaling through μ-opioid receptors (MORs) located presynaptically on small nociceptive afferents and postsynaptically upon second order projection neurons [1]. In the present study we sought to characterize the spinal actions of a family of dermorphin-derived mu opioid peptide agonists. Previous work has reported that several of these agents had potent analgesic effects. For example, one of the analogues, DMT-DALDA (H-Dmt-D-Arg-Phe-Lys-NH2; Dmt-2′,6′dimethyltyrosine), showed high μ-opioid receptor selectivity (Kiδ/Kiμ>10,000 and Kiκ/Kiμ>100) with a high binding affinity at the MOR (Kiμ=0.143 nM) as well as high metabolic stability [2], [3]. This peptide showed significant potency in blocking a spinal reflex (the tail-flick) after intrathecal (IT) administration [4]. Earlier work with dermorphin and dermorphin-based peptides have indicated their analgesic actions after IT delivery on both spinal reflex [5] and complex behavioral tasks [6], [7], [8]. Of interest, several of these peptides carry a net positive charge at physiological pH and are thus very hydrophilic and polar [4], which suggests that, like morphine, they should display an extended duration of action, following IT delivery. In developing drugs for IT delivery, the aim is to define highly potent, water-soluble MOR agonists, permitting the use of low delivery concentrations. These considerations prompted us to address in chronically spinal catheterized rats the profile of selected members of this family of peptides on pain behavior (hind paw thermal escape latencies and formalin-evoked flinching) and to assess the contribution of possible delta receptor interactions of these compounds. Of note, while both MOR and ∂-opioid receptor (DOR) agonists given IT can alter nociceptive processing, several groups have indicated that DOR antagonists can enhance MOR-mediated analgesia. This has led to the design of molecules with mixed MOR agonist/DOR antagonist properties. Two such compounds, dDAL-TICP ([Dmt1]DALDA-(CH2)2-NH←TICPΨ) and dDAL-TIPP (H-Dmt-D-Arg-Phe-Lys(Nε-TIPP)-NH2 were also examined in the present studies [9]. Such studies as outlined here provide the enabling data for potentially developing such selective peptides as IT therapeutics. Accordingly, in the present studies, we undertook an assessment of the analgesic activity of these peptides after bolus intrathecal delivery (thermal escape), the reversibility by a non-selective (naloxone) and delta opioid receptor specific (naltrindole) antagonist, confirmation of the antinociceptive activity of DMT-DALDA on formalin flinching and duration of action by increasing pretreatment intervals and, finally, assessment of loss of effect with repeated delivery and cross-tolerance between morphine and DMT-DALDA.
2 Methods
2.1 Animals
Male Sprague-Dawley rats (225–300 g; Harlan, Indianapolis, IN, USA) were individually housed in standard cages and maintained on a 12-h light/dark cycle (lights on at 7 am). Testing occurred during the light cycle. Food and water were available ad libitum. Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 85-23, Bethesda, MD, USA) and as approved by the institutional Animal Care and Use Committee of the University of California, San Diego, La Jolla, CA, USA.
2.2 Surgical preparation
Each rat was implanted with a single IT catheter for drug delivery, as described previously [10]. In brief, rats were anesthetized by induction with 4% isoflurane in a room air/oxygen mixture (1:1), the anesthesia was maintained with 2% isoflurane delivery by mask, and the animal placed in a head holder. The cisternal membrane was exposed through a midline incision and a polyethylene (outer diameter 0.36 mm) catheter was passed into the IT space to the level of the L2–L3 spinal segments (8.5 cm). The catheter was externalized on the top of the head. Rats were given carprofen (5 mg/kg in lactated Ringer’s solution) subcutaneously and allowed to recover under a heat lamp. Rats exhibiting motor weakness or signs of paresis were sacrificed. Animals were allowed to recover for 5–7 days before the experiment.
2.3 Study paradigm
In the present work, opioid peptide agonists and morphine sulfate were delivered exclusively via an implanted IT catheter. Antagonists (naloxone, naltrindole) were given intraperitoneally (IP).
2.3.1 Establishing the maximum tolerable dose (MTD)
To efficiently establish the MTD we employed the following paradigm. The first rat received a given dose; if the drug resulted in any adverse effects upon locomotion or evidence of neurological change (placing and stepping, pinnae/blink), the next rat received a half log lower dose and so on until no adverse signs were evident. Once the effect was submaximal, the next rat received the immediately preceding dose. If the starting dose had no evident effect upon function, the next animal received a half log higher dose and so on. As a result, for some drugs at some doses only a single animal was presented for a given dose; however, for the dose response and the MTD, a minimum of 5 animals were employed. This sequence was repeated at least once and the maximum dose that produced a minimum change in motor function was defined.
2.3.2 Dose response curve
After establishing the MTD, each compound was then assessed over a range of doses for effects upon thermal escape.
2.3.3 Effects of antagonists on MTD
After generating a dose response curve, each compound displaying analgesic activity at the MTD was tested in the presence of an antagonist. To assess the antagonist effect, each animal was given an intraperitoneal pretreatment at 10-min of naloxone 1 mg/kg or a 15-min pretreatment of naltrindole 3 mg/kg prior to the IT injection.
2.4 Behavioral testing
Before initiation of drug delivery, baseline behavioral and testing data were taken. At selected times after infusion of the test or control article, these data were again collected. All assessments were made with the observer blinded as to the drug being delivered.
2.4.1 General functional evaluation
These studies were not targeted at defining the side effect profile of the IT drugs except in so far as it would impact upon the ability to display the appropriate behavioral response in nociceptive assessment. Accordingly, end points included the following specific observations. (i) Absence of spontaneous activity in the test environment (otherwise evoked by a hand clap) [11]. (ii) Straub (stiff) tail defined as a tail that displays a lack of curvature when moved to one side or the other and the tail has sufficient rigidity that it does not make contact with floor of the cage during the observation interval. (iii) Truncal rigidity defined as the animal displaying a posture in which the chest wall and back are maintained in a rigid, unbending posture. (iv) Severe hind limb dysfunction, defined by loss of hind paw placing and stepping reflex, loss of weight bearing and/or failure of symmetrical ambulatory patterns. For analysis, the presence of these adverse events was limited to the first 3–4 h after IT delivery.
2.4.2 Acute thermal escape thresholds
A Hargreaves-type hind paw thermal stimulator system was employed [12] (Torrey Pines Instruments, San Diego, CA, USA). This system allows the direction of a focused light beam on the plantar surface of the paw through a glass plate upon which the rat stands. Surface temperature was maintained at 30°C. Rats were placed in individual plexiglass chambers on the thermal escape surface and allowed to acclimate for 30 min before testing. A brisk withdrawal of the paw after the initiation of the thermal stimulus was taken as the response. Lack of a response within 20 s was cause to terminate the test and assign the score of “20 s”. A latency measurement was taken for the right and left hind paws and averaged. Latency assessments were then made at various time points after drug administration. Each time point signifies the time that the hind paws were tested. Unless otherwise stated, animals were assessed for thermal escape latencies at baseline and again at 15, 30, 60, 90, 120, 180 and 240 min after bolus injection after initiation of infusion.
2.4.3 Formalin-induced flinching
Animals were allowed to acclimate in individual plexiglass chambers for 30 min before experimental manipulation. Rats were administered IT vehicle 10 min before the formalin injection. Flinching was evoked by a subcutaneous injection of formalin (2.5%, 50 μL) into the dorsal side of left hind paw. A metal band was placed around the hind paw that was being injected, and flinching and shaking of the injected paw was quantified by an automatic device (Torrey Pines Instruments, San Diego, USA) [13]. Flinches were counted in 1-min intervals for 60 min.
2.5 Drugs and chemicals
The following drugs were synthesized as previously described [2], [14]: dDALDA (H-Dmt-D-Arg-Phe-Lys-NH2; Dmt=2′,6′-dimethyltyrosine) (MW 981). (DMT-DALDA is abbreviated as dDAL in Figs. 2 and 4–6).
dDALc (H-Dmt-D-Cit-Phe-Lys-NH2; Cit=citrulline) (MW 868).
dDALcn (H-Dmt-D-Cit-Phe-Nle-NH2; Nle=norleucine) (MW 739).
TAPP (H-Tyr-D-Ala-Phe-Phe-NH2) (MW 659).
dDAL-TICP (H-Dmt-D-Arg-Phe-Lys-NH-(CH2)2-NH←TICP[Ψ]; TICP[Ψ]=H-Tyr-TicΨ[CH2-NH]Cha-Phe-OH [Tic=1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid; Cha=cyclohexylalanine]) (MW 1519).
dDAL-TIPP (H-Dmt-D-Arg-Phe-Lys(Nε←TIPP)-NH2; TIPP=H-Tyr-Tic-Phe-Phe-OH (MW 1604).
Morphine SO4 (MS) (MW: 759) (MOR) was obtained from Merck Pharmaceuticals (Rahway, NJ, USA). Naloxone hydrochloride (Nx) and Naltrindole (Ntd) were obtained from Sigma Chemical (St. Louis, MO, USA). Each drug was dissolved in saline such that the total dose was delivered in 10 μL. Isoflurane was obtained from VETone (Meridian, ID, USA).
2.6 Sample size
For the thermal escape studies group size was based on a power analysis of the area under the curve (%MPE×h) observed with the appropriate control group, e.g. animals receiving intrathecal saline. For the IT saline control group, the mean±SD group thermal escape response latency with each animal assessed out to 4 h was 10.5±1.3 s (vs. 10.1±1.5 s in uninjected rats; p>0.10), which corresponded to a mean±SD AUC of 569±427 %MPE×h. An increase of approximately 50% over baseline (6 s) for 0.5 h was considered to be behaviorally meaningful and this corresponded to an AUC of 1350 %MPE×h. Assuming β=0.8 and designating p<0.05 as statistically significant, a power analysis indicated the need for 5 animals. For the formalin model, the effects on phase 2 flinching of the IT saline control, was 1458±321 flinches/phase 2 (vs. 1521±220 flinches/phase 2 in uninjected rats; p>0.10). A decrease in phase 2 flinching of approximately 50% below IT saline baseline (729 flinches/phase 2) was considered to be behaviorally meaningful. Assuming β=0.8 and designating p=0.05 as statistical significance, a power analysis indicated the need for 4 animals. Analyses were based on assumptions of normality https://www.stat.ubc.ca/∼rollin/stats/ssize/n2.html).
2.7 Statistical analysis
The data were compiled in Excel (v.14.4.9, Microsoft Corporation, USA), and statistical analyses were performed using Prism (v.6.0, GraphPad Software, Inc., La Jolla, CA, USA).
The response latencies were normalized by converting them to percentage of the maximum possible effect [(Post–Pre)/20 s – Pre]×100 and the area under the percentage max possible effect curve calculated (AUC). Treatments were compared using one-way or two-way analysis of variance as indicated, and post hoc comparisons across treatment groups accomplished with Sidak post hoc comparison. Differences reaching the p<0.05 level of rejection were considered to be statistically significant.
3 Results
3.1 IT bolus delivery and thermal escape
3.1.1 Morphine
IT morphine sulfate (MS) resulted in a dose-dependent (3–30 nmol) increase in acute thermal escape latencies starting at 30 min and lasting in excess of 90 min (Fig. 1A). Pretreatment with naloxone (intraperitoneal administration; IP 1 mg/kg) but not naltrindole (IP 3 mg/kg) prevented this increase (Fig. 1B and C). Straub tail displayed a dose-dependent incidence across the range of doses which was unaltered by naloxone or naltrindole. Doses of >40 nmol resulted in severe truncal rigidity (Fig. 1D).
![Fig. 1:
(A) Dose-response curve of thermal latencies (seconds) vs. time post IT administration of morphine sulfate or saline vehicle. (B) Scattergraph of area under the curve of MTD (1, 1/2 log, 1 log), MTD with pretreatment of antagonists, and saline vehicle. One-way ANOVA analysis yielded p<0.0001. Sidak post hoc comparison showed **p<0.01, ***p<0.001, ****p<0.0001 compared to MTD (30 nmol) and ##p<0.01, ####p<0.0001 compared to saline vehicle. (C) Time effect graph of IT morphine with pretreatment (15 min) of systemic antagonists (naloxone [Nx] and naltrindole [Ntd]). (D) Scattergraph of the observance of Straub tail and motor dysfunction at any time during the time course.](/document/doi/10.1515/sjpain-2018-0120/asset/graphic/j_sjpain-2018-0120_fig_008.jpg)
(A) Dose-response curve of thermal latencies (seconds) vs. time post IT administration of morphine sulfate or saline vehicle. (B) Scattergraph of area under the curve of MTD (1, 1/2 log, 1 log), MTD with pretreatment of antagonists, and saline vehicle. One-way ANOVA analysis yielded p<0.0001. Sidak post hoc comparison showed **p<0.01, ***p<0.001, ****p<0.0001 compared to MTD (30 nmol) and ##p<0.01, ####p<0.0001 compared to saline vehicle. (C) Time effect graph of IT morphine with pretreatment (15 min) of systemic antagonists (naloxone [Nx] and naltrindole [Ntd]). (D) Scattergraph of the observance of Straub tail and motor dysfunction at any time during the time course.
3.1.2 DMT-DALDA
IT DMT-DALDA resulted in a dose-dependent (1–10 pmol) increase in acute thermal escape latencies starting by 15 min and lasting 2–4 h (Fig. 2A). Pretreatment with naloxone (IP1 mg/kg) but not naltrindole (IP 3 mg/kg) prevented this increase. IT DMT-DALDA resulted in a dose-dependent incidence of Straub tail during the first 30 min after injection at doses up to 10 pmol, which was not prevented by either naloxone or naltrindole (Fig. 2A). Strong, reversible, truncal rigidity was observed at doses of 30 pmol.
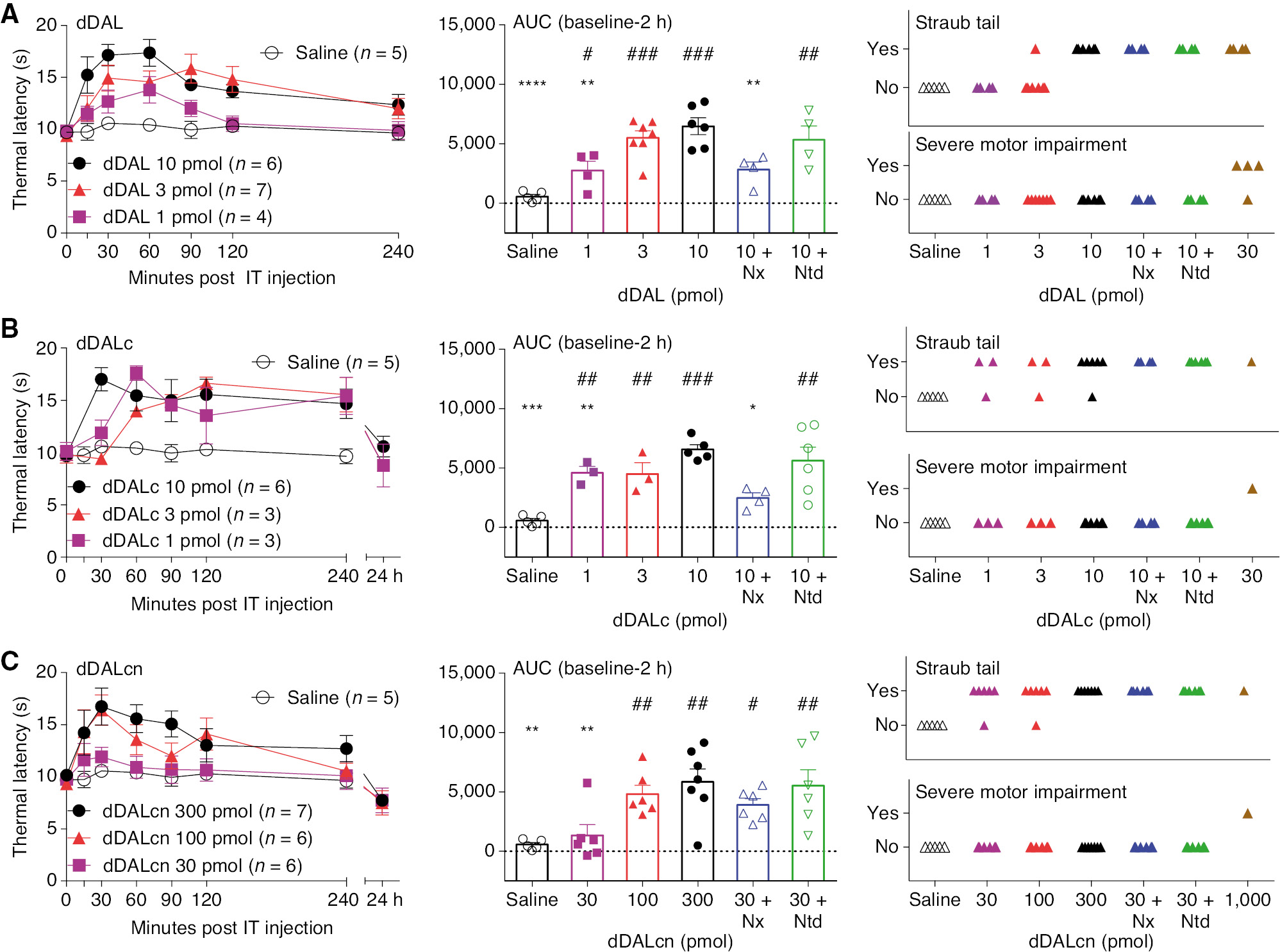
(A) Dose-response curve of thermal latencies (seconds) vs. time post IT administration of dDALDA or saline vehicle, scattergraph of area under the curve and observance of Straub tail or motor dysfunction of MTD (1, 1/2 log, 1 log), MTD with pretreatment of antagonists or saline vehicle at any time during the time course. One-way ANOVA analysis showed statistical significance of p<0.0001 with Sidak post hoc comparisons of **p<0.01, ****p<0.0001 compared to MTD (10 pmol) and #p<0.05, ##p<0.01, ###p<0.001 compared to saline vehicle. (B) Dose-response curve of thermal latencies (seconds) vs. time post IT administration of cDALc or saline vehicle and scattergraph of area under the curve and observance of Straub tail or motor dysfunction of MTD (1, 1/2 log, 1 log), MTD with pretreatment of antagonists or saline vehicle at any time during the time course. One-way ANOVA analysis showed statistical significance of p=0.0002 with Sidak post hoc comparisons of *p<0.05, **p<0.01, ***p<0.001 compared to MTD (10 pmol) and ##p<0.01, ###p<0.001 compared to saline vehicle. (C) Dose-response curve of thermal latencies (seconds) vs. time post IT administration of cDALcn or saline vehicle and scattergraph of area under the curve and observance of Straub tail or motor dysfunction of MTD (1, 1/2 log, 1 log), MTD with pretreatment of antagonists or saline vehicle at any time during the time course. One-way ANOVA analysis showed statistical significance of p=0.0014 with Sidak post hoc comparisons of **p<0.01 compared to MTD (300 pmol) and #p<0.05, ##p<0.01 compared to saline vehicle.
3.1.3 dDALc
IT dDALc resulted in a dose-dependent (1–10 pmol) increase in acute thermal escape latencies starting at 15 min and lasting in excess of 4 h post-dosing (Fig. 2B). Pretreatment with naloxone (IP 1 mg/kg) but not naltrindole (IP 3 mg/kg) prevented this increase (Fig. 1C). Brief intervals of Straub tail were observed in animals dosed with dDALc at doses up to 10 pmol, with a long-lasting but reversible paralysis observable at higher doses.
3.1.4 dDALcn
IT dDALcn resulted in a dose-dependent (30–300 pmol) increase in thermal escape latencies (Fig. 2A). Pretreatment with systemic naloxone or naltrindole had no statistically significant effect upon these increases. Straub tail was noted at doses as low as 30 pmol and were not altered by naloxone or naltrindole.
3.1.5 TAPP
IT TAPP was with minimal effect on thermal escape latencies at 10 nmol. Higher doses were not examined due to truncal paralysis at 46 nmol (Fig. 3A).
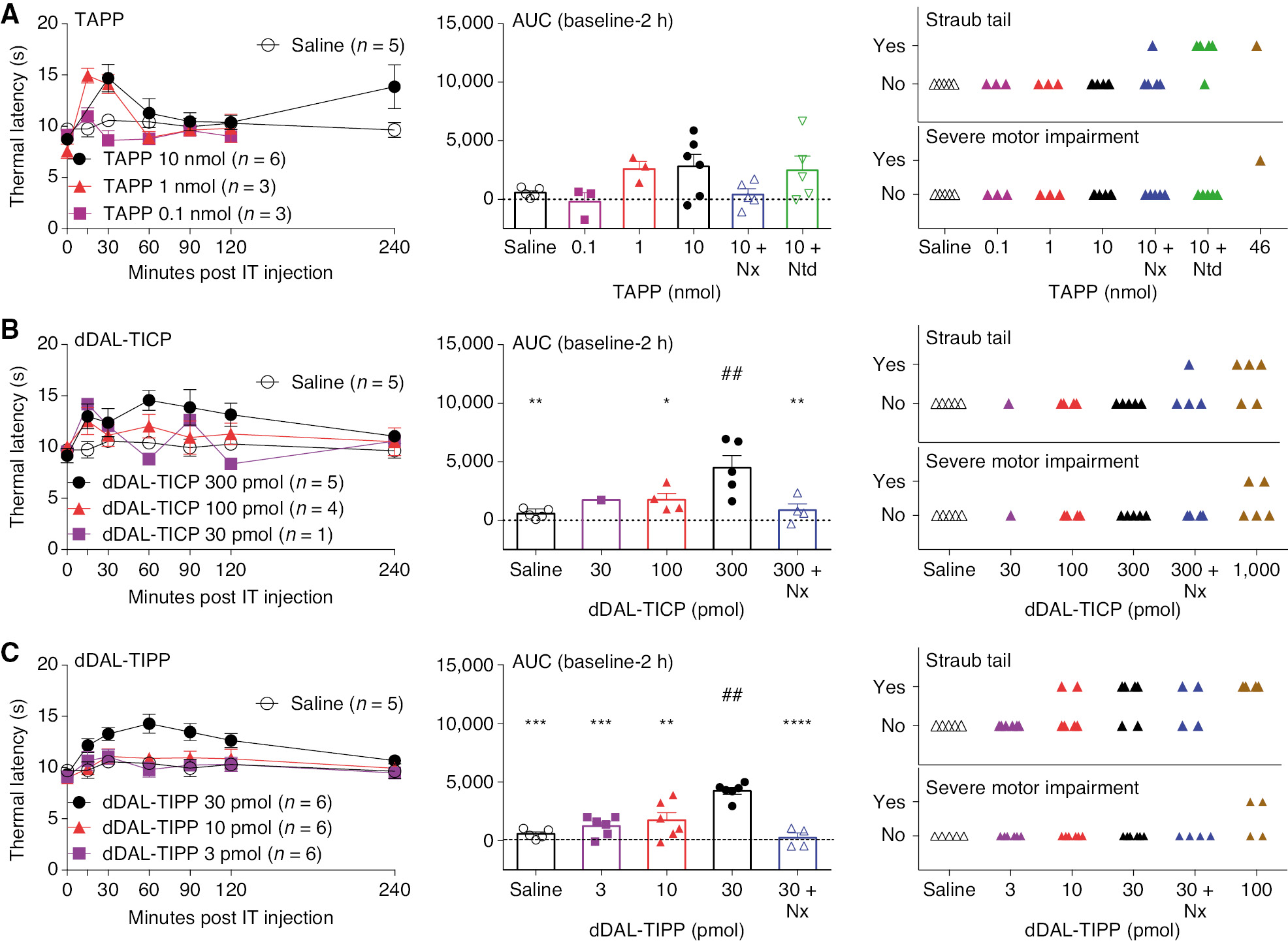
(A) Dose-response curve of thermal latencies (seconds) vs. time post IT administration of TAPP or saline vehicle, scatter of area under the curve and observance of Straub tail or motor dysfunction of MTD (1, 1/2 log, 1 log), MTD with pretreatment of antagonists or saline vehicle at any time during the time course. One-way ANOVA analysis shows p=0.0906 and post hoc analysis were no significant difference compared to MTD (10 nmol). (B) Dose-response curve of thermal latencies (seconds) vs. time post IT administration of dDAL-TICP or saline vehicle and scattergraph of area under the curve and observance of straub tail or motor dysfunction of MTD (1, 1/2 log, 1 log), MTD with pretreatment of antagonists or saline vehicle at any time during the time course. One-way ANOVA analysis showed statistical significance of p=0.0003 with Sidak post hoc comparisons of *p<0.05, **p<0.01 compared to MTD (300 pmol) and ##p<0.01 compared to saline vehicle. (C) Dose-response curve of thermal latencies (seconds) vs. time post IT administration of dDAL-TIPP or saline vehicle and scattergraph of area under the curve and observance of Straub tail or motor dysfunction of MTD (1, 1/2 log, 1 log), MTD with pretreatment of antagonists or saline vehicle at any time during the time course. One-way ANOVA analysis showed statistical significance of p=0.0014 with Sidak post hoc comparisons of **p<0.01, ***p<0.001, ****p<0.0001 compared to MTD (30 pmol) and ##p<0.01 compared to saline vehicle.
3.1.6 dDAL-TICP
IT dDAL-TICP was with minimum effect on thermal escape latencies at 300 pmol. Higher doses were not examined as higher doses led to truncal rigidity (Fig. 3B).
3.1.7 dDAL-TIPP
IT dDAL-TIPP had modest effects upon thermal escape latency at doses up to 30 pmol. Higher doses were not examined as strong truncal rigidity was observed at 100 pmol (Fig. 3C).
3.1.8 Vehicle
IT NaCl 0.9% was without effect on thermal escape latencies. Incidence of Straub tail or motor dysfunction was not observed. No other untoward effects were observed (Figs. 1 and 2).
3.2 IT bolus DMT-DALDA: formalin flinching
Intraplantar delivery of formalin resulted in biphasic flinching of the injected hind paw over the 60 min after formalin injection. IT delivery of DMT-DALDA (10 pmol) resulted in a significant reduction in phases 1 and 2 flinching (Fig. 4A–D) as compared to IT vehicle control when administered as soon at 5–6 h in advance of formalin, but not when given 24 h before formalin, emphasizing the long duration of action (Fig. 4E and F).
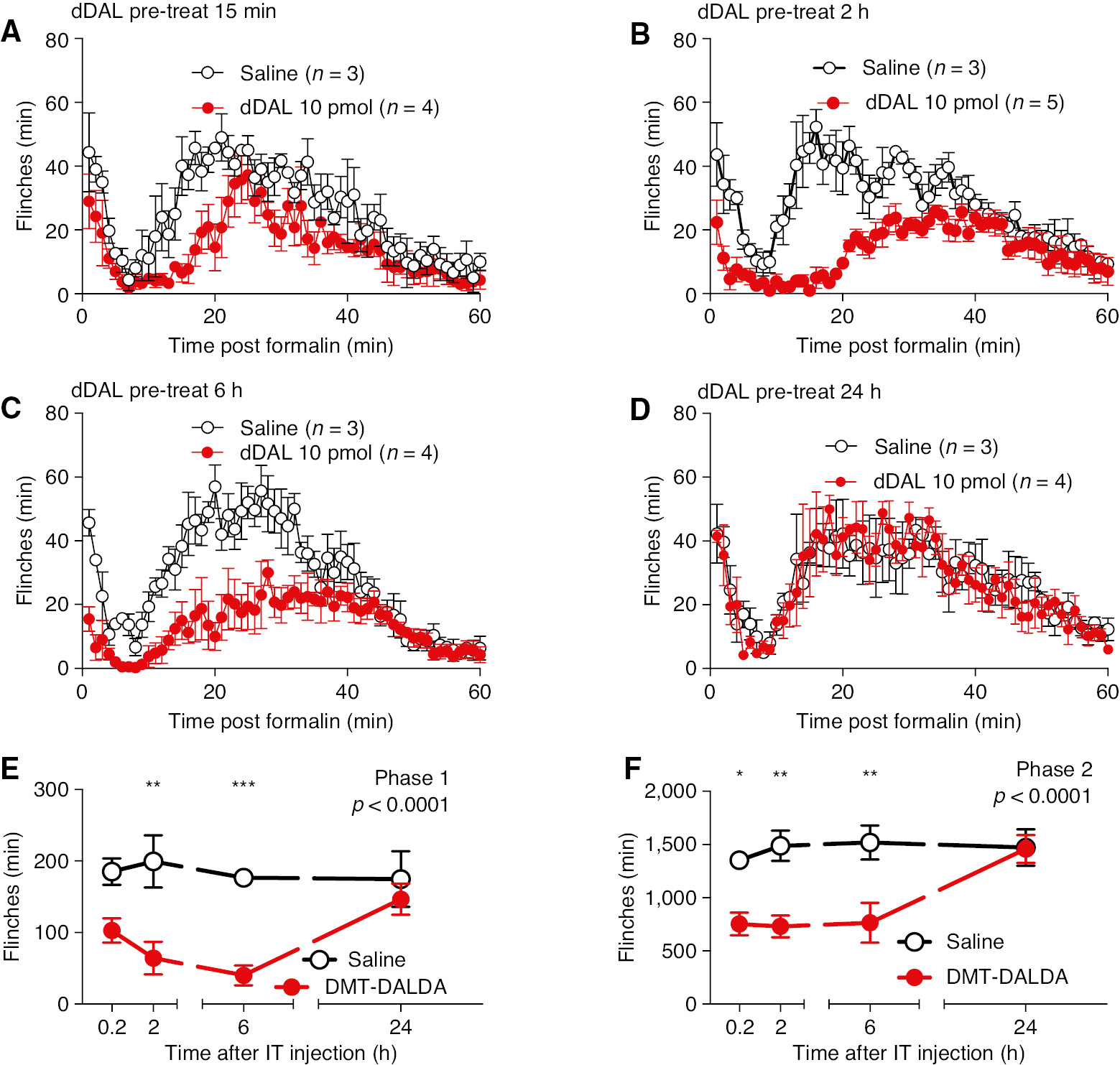
(A, B, C, D). Minute by minute plot of formalin-induced flinching at 0.2, 2, 6 and 24 h after pretreatment with IT DMT-DALDA (10 pmol/10 L) or IT saline vehicle (10 μL). (E/F) Total flinch count at 15 min, 2, 6 and 24 h after pretreatment with IT DMT-DALDA or IT vehicle for phase 1 (E) and phase 2 (F). Comparisons were made with two-way ANOVA (Treatment main effect p-value shown in each graph). Post hoc comparisons were carried out between each drug treatment and vehicle using Sidak post hoc test (*p<0.05, **p<0.01, ***p<0.01).
3.3 IT bolus morphine and fentanyl: formalin flinching
For comparison with standard opiate analgesic molecules, pretreatment studies were carried out with morphine (30 nmol/10 μL) and fentanyl (1 nmol/10 μL). As indicated in Fig. 5, Morphine produced significant reductions of phase 1 and phase 2 through 6 h, but not 24 h. In contrast, fentanyl (1 nmol/10 μL) resulted in a significant elevation at 15 min pretreatment, but not at 2 h reflecting its rapid spinal clearance and short duration of action (Fig. 5).
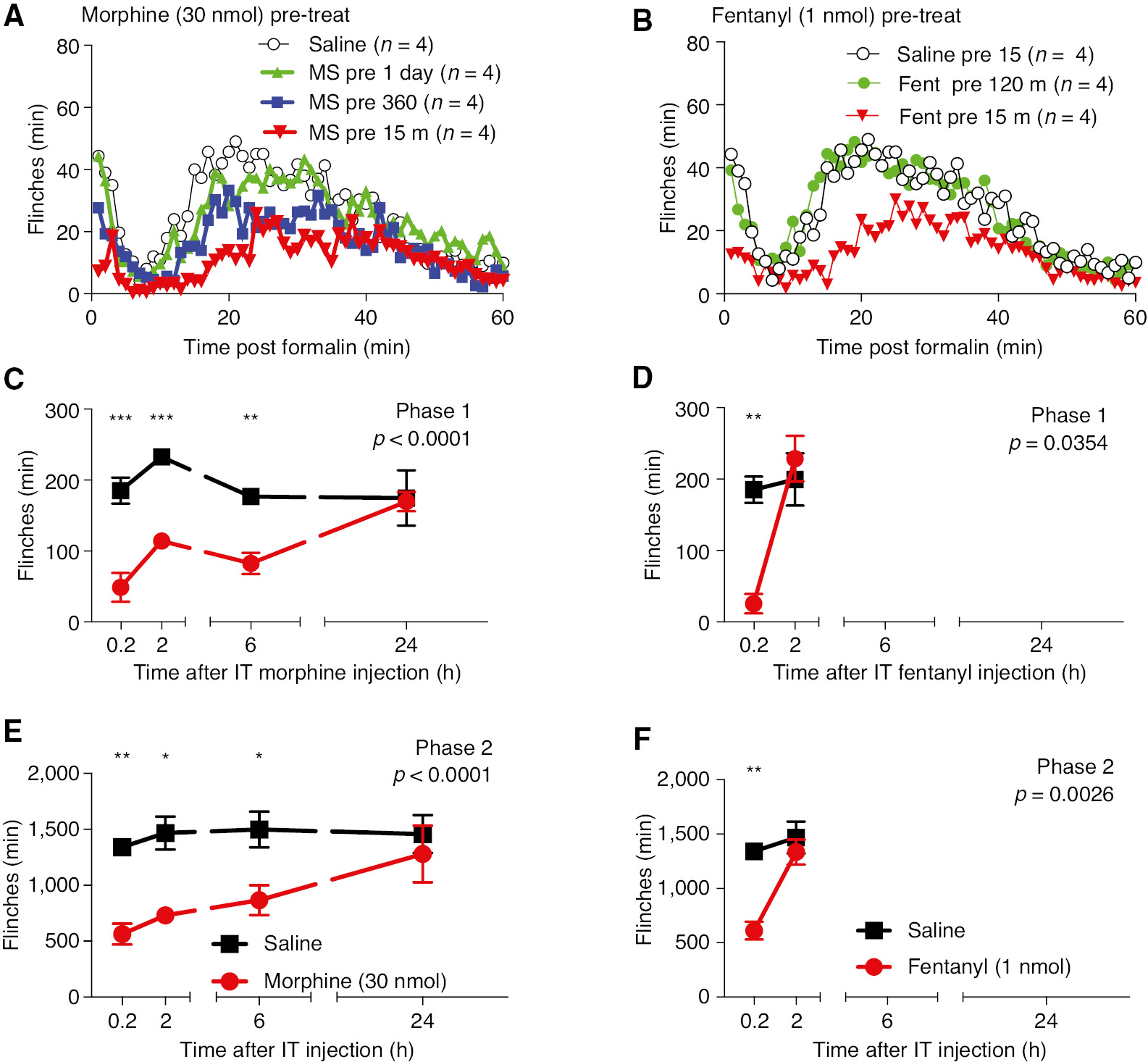
(A) Minute by minute plot of formalin-induced flinching at 15 min and 2, 6 and 24 h after pretreatment with IT morphine (30 nmol/10 μL) or the IT vehicle (10 μL). (B) Minute by minute plot of formalin induced flinching at 15 min (0.2 h) and 2 h after pretreatment of IT Fentanyl (1 nmol/10 μL) or the IT vehicle (10 μL). (C) Total flinch count during phase 1 (0–10 min) and phase 2 (11–60 min) at (C) 15 min, 2, 6 and 24 h after pretreatment with Morphine or (D) 15 min or 2 h for IT Fentanyl. Comparisons were made with two-way ANOVA (Treatment main effect p-value shown in each graph). Post hoc comparisons were carried out between each drug treatment and vehicle using Sidak post hoc test. (*p<0.05, **p<0.01, ***p<0.01).
3.4 IT bolus DMT-DALDA: tolerance and cross-tolerance on thermal escape
3.4.1 Tolerance after repeated bolus delivery of DMT-DALDA
DMT-DALDA (10 pmol) or saline were administered IT for 3 days (Fig. 6). On day 4, both groups received DMT-DALDA. As shown, on day 4 DMT-DALDA in animals which had received DMT-DALDA on three preceding days showed significantly less analgesia than did animals receiving three preceding days of saline, indicating a loss of DMT-DALDA activity (e.g. tolerance) (Fig. 6B). On day 5, all animals received IT morphine (30 nmol). As shown in Fig. 6B, animals that received 4 days of saline and 1 day of DMT-DALDA showed a greater response to morphine than did animals with 4 preceding days of DMT-DALDA (e.g. evidence of a cross-tolerance between morphine and DMT-DALDA).
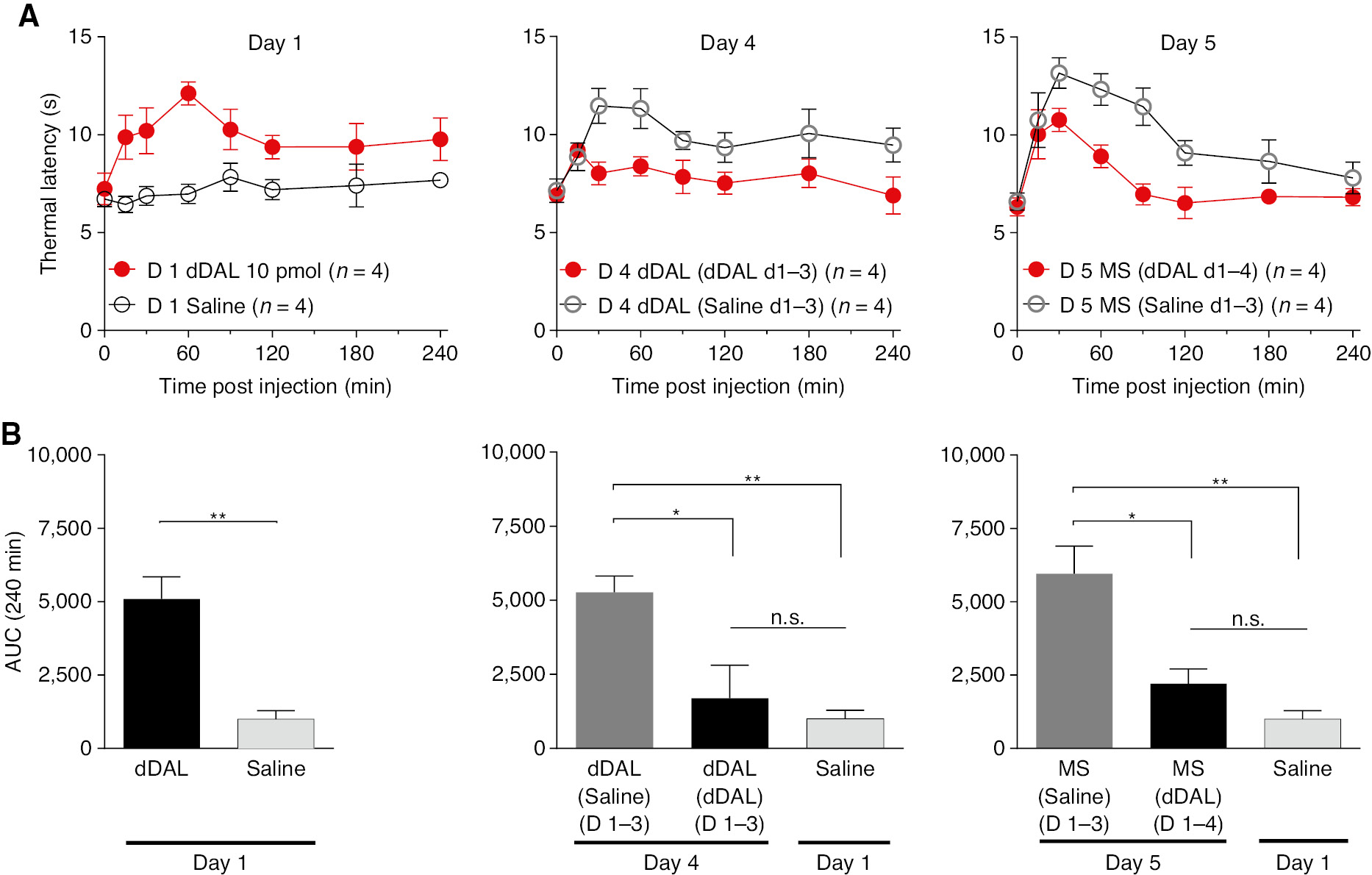
(A) Time effect graph of thermal latencies of daily bolus IT DMT-DALDA for 4 days group vs. one-time bolus IT DMT-DALDA on day 4 group. On Day 5 both groups were given morphine. (B) Area under the curve (AUC) of each group on Days 1, 4, 5 and saline vehicle given on Day 1. One-way ANOVA analysis yielded p<0.0001 with Sidak post hoc comparisons of *p<0.05, **p<0.01 compared to each group.
3.5 Tolerance after repeated bolus delivery of morphine
The same experimental paradigm was used to examine the properties of IT morphine and its interaction with DMT-DALDA. In Fig. 7, IT morphine or saline was given for 3 days. On day 4, morphine was given to both groups. As indicated (Fig. 7B) morphine given to animals receiving morphine for the three preceding days showed a significantly lesser response as compared to animals that had received saline on days 1–3, e.g. tolerance (Fig. 7B). On day 5, DMT-DALDA showed an equal degree of analgesia whether given in a rat that had 3 days of saline and 1 day of morphine as compared to 4 days of morphine (Fig. 7C).
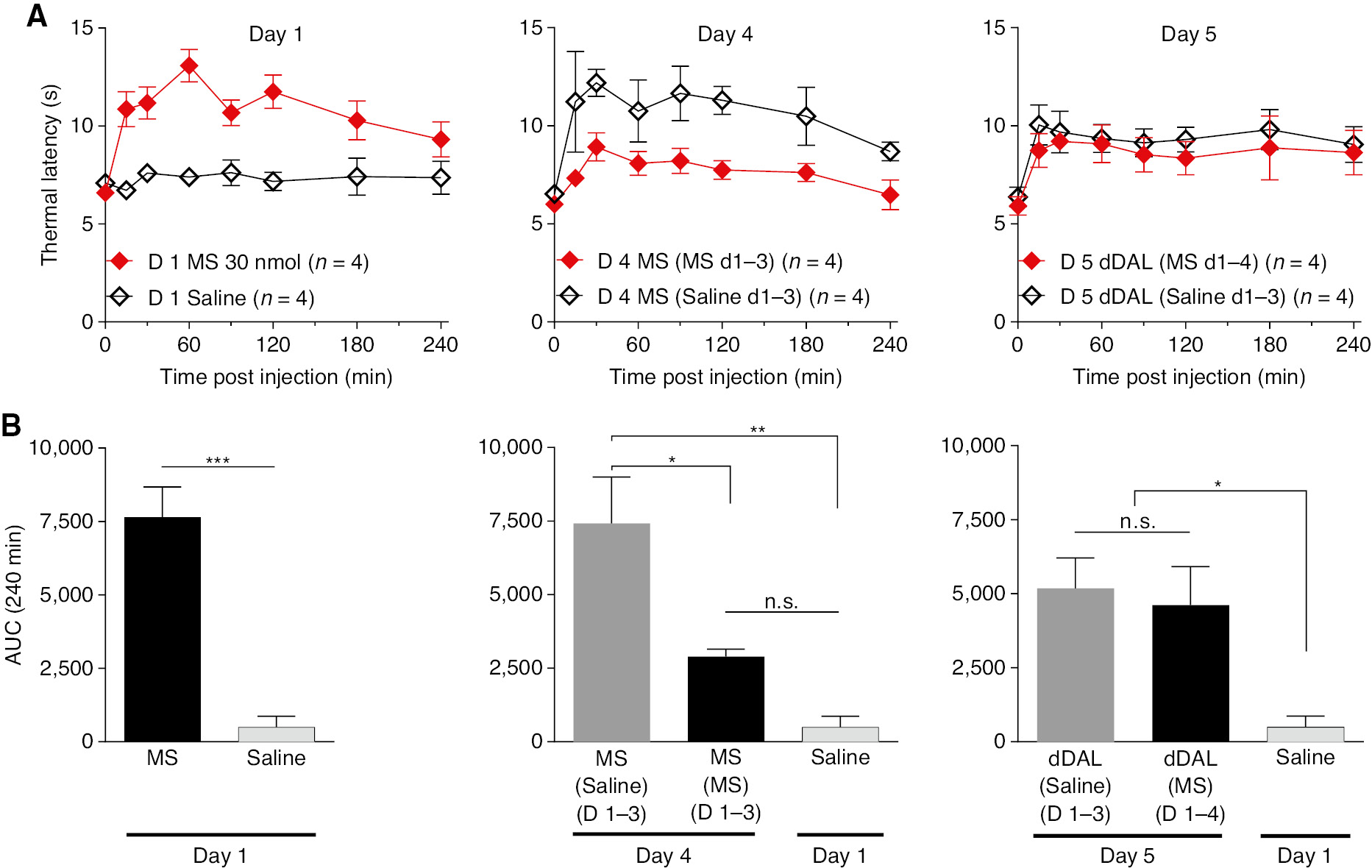
(A) Time effect graph of thermal latencies of daily bolus IT Morphine for 4 days group vs. one-time bolus IT morphine on Day 4 group. On Day 5 both group were given DMT-DALDA. (B) Area under the curve (AUC) of each group on Days 1, 4, 5, and saline vehicle given on Day 1. One-way ANOVA analysis yielded p<0.0001 with Sidak post hoc comparisons of *p<0.05, **p<0.01, ***p<0.001 compared to each group.
4 Discussion
The rationale underlying the present series of studies was to consider the potential utility of mu opioid peptides for development as IT analgesics. Such considerations hinge upon pharmacology, potency, duration of action and side effect profiles.
4.1 Efficacy and potency
In the present work, these agents displayed a dose dependent effect on acute thermal escape. Further, with DMT-DALDA, at doses effective in blocking acute thermal escape, a significant suppression was noted in phase 1 and phase 2 formalin flinching. Phase 1 is considered to be an endpoint reflective of acute nociception, while phase 2 reflects the role of a facilitated state of spinal nociceptive processing [13].
4.2 Duration of action
The duration of action of spinal drugs depends largely upon the rate at which they are cleared into the systemic circulation after delivery and to a lesser degree by their metabolism in the CNS [1]. Early work with peptides such has β endorphin and DADLE ((D-Ala2, D-Leu5)-enkephalin) [15], [16], [17] revealed long durations of actions after IT delivery as compared to a small polar molecule such as morphine and in particular when compared to small lipophilic molecules (such as remifentanil, fentanyl or sufentanil) [18], [19]. In this work we systematically compared the bolus effects of a number of dermorphin-derived peptides and analogues that ranged in IT potency from pmol to nmol doses. Previous work had shown that the DALDA analogues were resistant to metabolism in ex vivo blood incubation studies [20]. Importantly, this duration of activity was noted in not just an acute thermal threshold model but was confirmed by the potent effects upon the two-phased flinching observed in the formalin model. The use of increased pretreatment intervals also emphasized the extended duration of action of the potent DMT-DALDA molecule, which compared most favorably to that associated with morphine. The fentanyl-formalin data in contrast emphasizes the brevity of the behavioral action of mu opioids that are rapidly cleared.
4.3 Pharmacology
The antinociception observed after IT agents were uniformly reversed by naloxone and not by naltrindole. The doses employed were previously shown to be respectively shown to be effective in reversing the analgesia produced by the IT delivery of mu and delta-preferring ligands [21]. An interesting variant to the possible mechanisms involved in DMT-DALDA is that this tetrapeptide amide (H-Dmt-D-Arg-Phe-Lys-NH2) is not only a potent and selective μ opioid receptor (MOR) agonist but is reported to display antioxidant activity due to its N-terminal Dmt (2′,6′-dimethyltyrosine) residue [22] and to block norepinephrine reuptake in synaptosomes [23]. Given the efficacy of naloxone in reversing these effects, the role of the antioxidant and uptake blocking properties of these molecules in the present model of acute thermal nociception and formalin evoked flinching appear minimal, though if there is a robust synergy between mu receptor activity and the other two mechanisms, then loss of mu function would result in a total collapse of effect. This possibility was not studied in the present work.
While both MOR and DOR-preferring agonists can block substance P release from C-fibers to yield analgesia after IT delivery [21], [24] and interact synergistically [25], it has been reported that the severity of tolerance development in morphine-treated animals is reduced in DOR knockout mice [26] or when using DOR antagonists [27], [28], [29]. This phenomenon has been suggested to reflect a modulatory role of DOR on MOR function [30]. In the present study IT delivery of dDAL-TICP and dDAL-TIPP (agents with significant MOR agonist and DOR antagonist activity) displayed a surprisingly minimal effect on pain behavior. The reason for the contrast with previous reports may reflect spinal vs. supraspinal systems. The interpretation of the spinal reflex as a marker of nociceptive processing may be rendered moot where there is an effect of the drug on motor function, which, in this case, results in bilateral increases in motor out flow to the tail musculature.
4.4 Motor effects
The principal motor effect first observed with escalating doses of IT morphine and peptides was the appearance of the classic Straub tail [31]. This is a marker of increased motor tone in the sacrococcygeus dorsalis muscle, mediated by increased opiate receptor-initiated activation of lumbosacral outflow. The lack of effect of spinal section on the Straub tail phenomena argues that it can be mediated at the spinal level [32]. Importantly, the appearance of Straub tail renders a spinal response such as the tail flick difficult to interpret as indices of nociception. In the present studies, IT saline had no effect upon tail appearance, whereas the agents employed resulted in an increased incidence of at least brief signs of Straub (stiffened) tail. Several studies have reported that the motor effects after systemic [33], [34], [35] or IT opiates [36] are at least partially diminished by naloxone. In the present work, naloxone at a dose completely reversing the effect on thermal escape latency had no effect upon the incidence of the Straub tail endpoint. A gradation assessment of the magnitude of the Straub tail response was not made, and we recognize that changes in the degree of motor effects may have been missed. Further, we note our studies were undertaken with a single antagonist dose (albeit one that completely blocked the intrathecal drug effects upon thermal escape) in the face of a single just maximally usable agonist dose. At this moment we speculate that these data support the possibility that a nonopiate receptor mechanism may be at play in this spinal effect in the rat, including a possible role involving toll receptors [37] or Mas-related G-protein–coupled receptors (MRGPRs) [38].
4.5 Tolerance
In the present studies repeated bolus delivery of equianalgesic doses of morphine and DMT-DALDA resulted in evidence of tolerance over a 5-day interval. Of note, animals tolerant to morphine continued to respond to DMT-DALDA, whereas animals tolerant to DMT-DALDA did not respond to morphine. Such asymmetric cross-tolerance has been previously described for IT infusions of mu agonists (morphine and sufentanil) [39], [40], [41]. The mechanisms of such asymmetry are not clear. Previous work has suggested that it may reflect the relative intrinsic activity of the two agonists, reflecting different degrees of receptor occupancy required by different agonists to yield a criterion effect, e.g. a measure of intrinsic activity. With continued drug exposure, the pharmacodynamics resemble the condition associated with a loss of functional receptors over time, [42] with the changes having a greater impact upon the agent with lower intrinsic activity [39], reflecting the magnitude of a receptor reserve [43]. In addition, as noted above, DMT-DALDA, aside from being a MOR agonist, also has other pro-analgesic actions that might account for the asymmetry in tolerance.
4.6 Mu/delta interactions
Several studies have suggested that μ-receptor activity could be modulated by δ-ligands. Several disparate conclusions have been noted. First, it has been reported that IT delivery of μ and ∂ ligands can result in a positive synergy [25], [44], [45]. Second, other observations have suggested that antagonists for the δ-receptor can enhance the analgesic potency and efficacy of μ-agonists [9]. The present examination of two such compounds, dDAL-TICP and dDAL-TIPP [9], were without evident activity in thermal escape at maximal doses without effects upon motor function. These results with these agents at these doses employed do not support a positive synergy between spinal μ agonism and δ antagonism.
5 Conclusions
The present work characterized the profile of several structural analogues of dermorphin delivered IT as a bolus. These several DALDA analogues resulted in potent, long-lasting and naloxone reversible but not naltrindole reversible analgesia. These initial studies showing tolerability confirms the extreme potency of these molecules as μ receptor agonists, as compared to many of the opiates, such as morphine and even fentanyl. While considerable work remains to be done, we note that IT morphine is beset by several properties, including mast cell degranulation [46]. This ability to degranulate mast cells has been argued to account for its propensity to produce IT masses [47], [48]. While agents such as fentanyl and alfentanil do not produce masses [47], [49], their lipid solubility leads them to display rapid clearances rendering them less suitable for IT use than agents that are cleared more slowly. The high potency of the dermorphin analogues raises the possibility that they may have the ability to produce analgesia at lower concentrations than those required to degranulate mast cells. Further work addressing this potential are required. While IT boluses of DMT-DALDA displayed significant tolerance over 5 days, it failed to show loss of effect in a morphine tolerant rat. Future work for development of these peptides requires definition of the intrathecal pharmacokinetics and safety after Intrathecal infusion.
6 Implications
These results showing tolerability, duration of action and potency point to the potential utility of this family of μ peptide as a spinal therapeutic with a possibly improved safety profile. Studies on spinal histopathology are required before further considerations into spinal utilization can be made.
-
Authors’ statements
-
Research funding: This work was supported completely by a NIH grant DA15353 (TY).
-
Conflict of interest: Peter Schiller has a patent position on the structures of the DALDA analogues.
-
Informed consent: not applicable.
-
Ethical approval: Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 85-23, Bethesda, MD, USA) and as approved by the institutional Animal Care and Use Committee of the University of California, San Diego, CA, USA.
References
[1] Yaksh TL, Fisher CJ, Hockman TM, Wiese AJ. Current and future issues in the development of spinal agents for the management of pain. Curr Neuropharmacol 2017;15:232–59.10.2174/1570159X14666160307145542Search in Google Scholar
[2] Schiller PW, Nguyen TM, Berezowska I, Dupuis S, Weltrowska G, Chung NN, Lemieux C. Synthesis and in vitro opioid activity profiles of DALDA analogues. Eur J Med Chem 2000;35:895–901.10.1016/S0223-5234(00)01171-5Search in Google Scholar
[3] Zhao GM, Qian X, Schiller PW, Szeto HH. Comparison of [Dmt1]DALDA and DAMGO in binding and G protein activation at mu, delta, and kappa opioid receptors. J Pharmacol Exp Ther 2003;307:947–54.10.1124/jpet.103.054775Search in Google Scholar PubMed
[4] Novoa A, Van Dorpe S, Wynendaele E, Spetea M, Bracke N, Stalmans S, Betti C, Chung NN, Lemieux C, Zuegg J, Cooper MA, Tourwé D, De Spiegeleer B, Schiller PW, Ballet S. Variation of the net charge, lipophilicity, and side chain flexibility in Dmt(1)-DALDA: effect on opioid activity and biodistribution. J Med Chem 2012;55:9549–61.10.1021/jm3008079Search in Google Scholar PubMed PubMed Central
[5] Shimoyama M, Szeto HH, Schiller PW, Tagaito Y, Tokairin H, Eun C, Shimoyama, N. Differential analgesic effects of a mu-opioid peptide, [Dmt(1)]DALDA, and morphine. Pharmacology 2009;83:33–7.10.1159/000167878Search in Google Scholar PubMed PubMed Central
[6] Stevens CW, Yaksh TL. Spinal action of dermorphin, an extremely potent opioid peptide from frog skin. Brain Res 1986;385:300–4.10.1016/0006-8993(86)91076-0Search in Google Scholar PubMed
[7] Sullivan AF, Dickenson AH. Electrophysiological studies on the spinal effects of dermorphin, an endogenous mu-opioid agonist. Brain Res 1988;461:182–5.10.1016/0006-8993(88)90738-XSearch in Google Scholar PubMed
[8] Ro S, Zhu Q, Lee CW, Goodman M, Darlak K, Spatola AF, Chung, NN, Schiller, PW, Malmbergm, AB, Yaksh, TL. Highly potent side chain-main chain cyclized dermorphin-deltorphin analogues: an integrated approach including synthesis, bioassays, NMR spectroscopy and molecular modelling. J Pept Sci 1995;1:157–74.10.1002/psc.310010303Search in Google Scholar PubMed
[9] Schiller PW. Opioid peptide-derived analgesics. AAPS J 2005;7:E560–5.10.1208/aapsj070356Search in Google Scholar PubMed PubMed Central
[10] Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav 1976;17:1031–6.10.1016/0031-9384(76)90029-9Search in Google Scholar PubMed
[11] Malmberg AB, Yaksh TL. Voltage-sensitive calcium channels in spinal nociceptive processing: blockade of N- and P-type channels inhibits formalin-induced nociception. J Neurosci 1994;14:4882–90.10.1523/JNEUROSCI.14-08-04882.1994Search in Google Scholar PubMed PubMed Central
[12] Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. J Neurosci Methods 1997;76:183–91.10.1016/S0165-0270(97)00097-6Search in Google Scholar PubMed
[13] Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, Yaksh MC. An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol 2001;90:2386–402.10.1152/jappl.2001.90.6.2386Search in Google Scholar PubMed
[14] Ding J, Lemieux C, Chung NN, Schiller PW. Bifunctional µ/δ opioid peptides: variation of the type and length of the linker connecting the two components. Chem Biochem Drug Des 2012;79:186–93.10.1111/j.1747-0285.2011.01268.xSearch in Google Scholar PubMed PubMed Central
[15] Yaksh TL, Henry JL. Antinociceptive effects of intrathecally administered human beta-endorphin in the rat and cat. Can J Physiol Pharmacol 1978;56:754–9.10.1139/y78-120Search in Google Scholar PubMed
[16] Tung AS, Yaksh TL. In vivo evidence for multiple opiate receptors mediating analgesia in the rat spinal cord. Brain Res 1982;247:75–83.10.1016/0006-8993(82)91029-0Search in Google Scholar PubMed
[17] Schmauss C, Shimohigashi Y, Jensen TS, Rodbard D, Yaksh TL. Studies on spinal opiate receptor pharmacology. 3. Analgetic effects of enkephalin dimers as measured by cutaneous-thermal and visceral-chemical evoked-responses. Brain Res 1985;337:209–15.10.1016/0006-8993(85)90056-3Search in Google Scholar PubMed
[18] Yaksh TL, Noueihed RY, Durant PA. Studies of the pharmacology and pathology of intrathecally administered 4-anilinopiperidine analogues and morphine in the rat and cat. Anesthesiol 1986;64:54–66.10.1097/00000542-198601000-00009Search in Google Scholar PubMed
[19] Buerkle H, Yaksh TL. Continuous intrathecal administration of shortlasting mu opioids remifentanil and alfentanil in the rat. Anesthesiol 1996;84:926–35.10.1097/00000542-199604000-00021Search in Google Scholar PubMed
[20] Szeto HH, Lovelace JL, Fridland G, Soong Y, Fasolo J, Wu D, Desiderio DM, Schiller PW. In vivo pharmacokinetics of selective mu-opioid peptide agonists. J Pharmacol Exp Ther 2001;298:57–61.Search in Google Scholar
[21] Kouchek M, Takasusuki T, Terashima T, Yaksh TL, Xu Q. Effects of intrathecal SNC80, a delta receptor ligand, on nociceptive threshold and dorsal horn substance p release. J Pharmacol Exp Ther 2013;347:258–64.10.1124/jpet.113.206573Search in Google Scholar PubMed PubMed Central
[22] Schiller PW, Nguyen TM, Saray A, Poon AW, Laferrière A, Coderre TJ. The bifunctional μ opioid agonist/antioxidant [Dmt(1)]DALDA is a superior analgesic in an animal model of complex regional pain syndrome-type i. ACS Chem Neurosci 2015;6:1789–93.10.1021/acschemneuro.5b00228Search in Google Scholar PubMed PubMed Central
[23] Shimoyama M, Shimoyama N, Zhao GM, Schiller PW, Szeto HH. Antinociceptive and respiratory effects of intrathecal H-Tyr-D-Arg-Phe-Lys-NH2 (DALDA) and [Dmt1] DALDA. J Pharmacol Exp Ther 2001;297:364–71.Search in Google Scholar
[24] Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh, TL. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci 2005;25:3651–60.10.1523/JNEUROSCI.0252-05.2005Search in Google Scholar PubMed PubMed Central
[25] Malmberg AB, Yaksh TL. Isobolographic and dose-response analyses of the interaction between intrathecal mu-agonists and delta-agonists – effects of naltrindole and its benzofuran analog (Ntb). J Pharmacol Exp Ther 1992;263:264–75.Search in Google Scholar
[26] Kest B, Lee CE, McLemore GL, Inturrisi CE. An antisense oligodeoxynucleotide to the delta opioid receptor (DOR-1) inhibits morphine tolerance and acute dependence in mice. Brain Res Bull 1996;39:185–8.10.1016/0361-9230(95)02092-6Search in Google Scholar PubMed
[27] Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther 1991;258:299–303.Search in Google Scholar
[28] Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. P Natl Acad Sci USA 2005;102:19208–13.10.1073/pnas.0506627102Search in Google Scholar PubMed PubMed Central
[29] Fundytus ME, Schiller PW, Shapiro M, Weltrowska G, Coderre TJ. Attenuation of morphine tolerance and dependence with the highly selective delta-opioid receptor antagonist TIPP[psi]. Eur J Pharmacol 1995;286:105–8.10.1016/0014-2999(95)00554-XSearch in Google Scholar
[30] Rozenfeld R, Abul-Husn NS, Gomez I, Devi LA. An emerging role for the delta opioid receptor in the regulation of mu opioid receptor function. ScientificWorldJournal 2007;7:64–73.10.1100/tsw.2007.219Search in Google Scholar PubMed PubMed Central
[31] Straub W. Eine Empfindliche Biologische Reaktion auf Morphin. Dtsch Med Wochenschr 1911;37:1462–8.10.1055/s-0028-1130858Search in Google Scholar
[32] Bilbey DL, Salem H, Grossman MH. The anatomical basis of the straub phenomenon. Br J Pharmacol Chemother 1960;15:540–3.10.1111/j.1476-5381.1960.tb00277.xSearch in Google Scholar PubMed PubMed Central
[33] Aceto MD, McKean DB, Pearl J. Effects of opiates and opiate antagonists on the Straub tail reaction in mice. Br J Pharmacol 1969;36:225–39.10.1111/j.1476-5381.1969.tb09500.xSearch in Google Scholar PubMed PubMed Central
[34] Nath C, Gupta MB, Patnaik GK, Dhawan KN. Morphine-induced straub tail response: mediated by central mu2-opioid receptor. Eur J Pharmacol 1994;263:203–5.10.1016/0014-2999(94)90543-6Search in Google Scholar PubMed
[35] Zarrindast MR, Alaei-Nia K, Shafizadeh M. On the mechanism of tolerance to morphine-induced Straub tail reaction in mice. Pharmacol Biochem Behav 2001;69:419–24.10.1016/S0091-3057(01)00519-6Search in Google Scholar
[36] Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol 1980;67:313–6.10.1016/0014-2999(80)90515-4Search in Google Scholar PubMed
[37] Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal 2007;7:98–111.10.1100/tsw.2007.230Search in Google Scholar PubMed PubMed Central
[38] Lansu K, Karpiak J, Liu J, Huang XP, McCorvy JD, Kroeze WK, Che T, Nagase H, Carroll FI, Jin J, Shoichet BK, Roth BL. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol 2017;13:529–36.10.1038/nchembio.2334Search in Google Scholar PubMed PubMed Central
[39] Sosnowski M, Yaksh TL. Differential cross-tolerance between intrathecal morphine and sufentanil in the rat. Anesthesiol 1990;73:1141–7.10.1097/00000542-199012000-00012Search in Google Scholar PubMed
[40] Laurido C, Hernandez A, Perez H. Cross-tolerance to acute administration of Mu and kappa opioid agonists at the spinal cord level in the rat. Int J Neurosci 1996;87:191–9.10.3109/00207459609070837Search in Google Scholar PubMed
[41] Nielsen CK, Ross FB, Smith MT. Incomplete, asymmetric, and route-dependent cross-tolerance between oxycodone and morphine in the Dark Agouti rat. J Pharmacol Exp Ther 2000;295:91–9.Search in Google Scholar
[42] Mjanger E, Yaksh TL. Characteristics of dose-dependent antagonism by beta-funaltrexamine of the antinociceptive effects of intrathecal mu agonists. J Pharmacol Exp Ther 1991;258:544–50.Search in Google Scholar
[43] Chavkin C, Goldstein A. Reduction in opiate receptor reserve in morphine tolerant guinea pig ilea. Life Sci 1982;31:1687–90.10.1016/0024-3205(82)90186-2Search in Google Scholar PubMed
[44] Negus SS, Bear AE, Folk JE, Rice KC. Role of delta opioid efficacy as a determinant of mu/delta opioid interactions in rhesus monkeys. Eur J Pharmacol 2009;602:92–100.10.1016/j.ejphar.2008.11.004Search in Google Scholar PubMed PubMed Central
[45] Sutters KA, Miaskowski C, Taiwo YO, Levine JD. Analgesic synergy and improved motor function produced by combinations of mu-delta- and mu-kappa-opioids. Brain Res 1990;530:290–4.10.1016/0006-8993(90)91297-TSearch in Google Scholar PubMed
[46] Schmidt-Rondon E, Wang Z, Malkmus SA, Di Nardo A, Hildebrand K, Page L, Yaksh TL. Effects of opioid and nonopioid analgesics on canine wheal formation and cultured human mast cell degranulation. Toxicol Appl Pharmacol 2018;338:54–64.10.1016/j.taap.2017.10.017Search in Google Scholar PubMed PubMed Central
[47] Yaksh TL, Steinauer JJ, Veesart SL, Malkmus SA. Alfentanil: correlations between absence of effect upon subcutaneous mast cells and absence of granuloma formation after intrathecal infusion in the dog. Neuromodulation 2013;16:459–66.10.1111/j.1525-1403.2012.00534.xSearch in Google Scholar PubMed PubMed Central
[48] Yaksh TL, Allen JW, Veesart SL, Horais KA, Malkmus SA, Scadeng M, Steinauer JJ, Rossi SS. Role of meningeal mast cells in intrathecal morphine-evoked granuloma formation. Anesthesiol 2013;118:664–78.10.1097/ALN.0b013e31828351aaSearch in Google Scholar PubMed PubMed Central
[49] Allen JW, Horais KA, Tozier NA, Yaksh TL. Opiate pharmacology of intrathecal granulomas. Anesthesiol 2006;105:590–8.10.1097/00000542-200609000-00025Search in Google Scholar PubMed
©2018 Scandinavian Association for the Study of Pain. Published by Walter de Gruyter GmbH, Berlin/Boston. All rights reserved.
Articles in the same Issue
- Frontmatter
- Editorial comment
- The Fear Avoidance Beliefs Questionnaire – the FABQ – for the benefit of another 70 million potential pain patients
- The Yaksh-model of intrathecal opioid-studies: still exciting four decades later
- Pain is common in chronic fatigue syndrome – current knowledge and future perspectives
- Systematic review
- Use of multidomain management strategies by community dwelling adults with chronic pain: evidence from a systematic review
- Clinical pain research
- Topographic mapping of pain sensitivity of the lower back – a comparison of healthy controls and patients with chronic non-specific low back pain
- A prospective study of patients’ pain intensity after cardiac surgery and a qualitative review: effects of examiners’ gender on patient reporting
- Correlations between the active straight leg raise, sleep and somatosensory sensitivity during pregnancy with post-partum lumbopelvic pain: an initial exploration
- Pain is associated with reduced quality of life and functional status in patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
- Does validation and alliance during the multimodal investigation affect patients’ acceptance of chronic pain? An experimental single case study
- Translation, cross-cultural adaptation, and psychometric properties of the Hausa version of the Fear-Avoidance Beliefs Questionnaire in patients with low back pain
- Observational study
- Cause-specific mortality of patients with severe chronic pain referred to a multidisciplinary pain clinic: a cohort register-linkage study
- Pain self-efficacy moderates the association between pain and somatization in a community sample
- Pediatric chronic pain and caregiver burden in a national survey
- Psychometric evaluation of the Danish version of a modified Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R-D) for patients hospitalized with acute abdominal pain
- Musculoskeletal pain in multiple body sites and work ability in the general working population: cross-sectional study among 10,000 wage earners
- Prediction of running-induced Achilles tendinopathy with pain sensitivity – a 1-year prospective study
- Original experimental
- Body image is more negative in patients with chronic low back pain than in patients with subacute low back pain and healthy controls
- Identifying pain in children with CHARGE syndrome
- Patients’ perspective of the effectiveness and acceptability of pharmacological and non-pharmacological treatments of fibromyalgia
- Exercise-induce hyperalgesia, complement system and elastase activation in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome – a secondary analysis of experimental comparative studies
- Characterization of the antinociceptive effects of intrathecal DALDA peptides following bolus intrathecal delivery
- The effects of auditory background noise and virtual reality technology on video game distraction analgesia
- Book review
- Atlas of Common Pain Syndromes, 4th Edition
- Atlas of Ultrasound-Guided Regional Anesthesia, 3rd Edition
- Anaesthesia, Intensive Care and Perioperative Medicine A-Z, 6th Edition
Articles in the same Issue
- Frontmatter
- Editorial comment
- The Fear Avoidance Beliefs Questionnaire – the FABQ – for the benefit of another 70 million potential pain patients
- The Yaksh-model of intrathecal opioid-studies: still exciting four decades later
- Pain is common in chronic fatigue syndrome – current knowledge and future perspectives
- Systematic review
- Use of multidomain management strategies by community dwelling adults with chronic pain: evidence from a systematic review
- Clinical pain research
- Topographic mapping of pain sensitivity of the lower back – a comparison of healthy controls and patients with chronic non-specific low back pain
- A prospective study of patients’ pain intensity after cardiac surgery and a qualitative review: effects of examiners’ gender on patient reporting
- Correlations between the active straight leg raise, sleep and somatosensory sensitivity during pregnancy with post-partum lumbopelvic pain: an initial exploration
- Pain is associated with reduced quality of life and functional status in patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
- Does validation and alliance during the multimodal investigation affect patients’ acceptance of chronic pain? An experimental single case study
- Translation, cross-cultural adaptation, and psychometric properties of the Hausa version of the Fear-Avoidance Beliefs Questionnaire in patients with low back pain
- Observational study
- Cause-specific mortality of patients with severe chronic pain referred to a multidisciplinary pain clinic: a cohort register-linkage study
- Pain self-efficacy moderates the association between pain and somatization in a community sample
- Pediatric chronic pain and caregiver burden in a national survey
- Psychometric evaluation of the Danish version of a modified Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R-D) for patients hospitalized with acute abdominal pain
- Musculoskeletal pain in multiple body sites and work ability in the general working population: cross-sectional study among 10,000 wage earners
- Prediction of running-induced Achilles tendinopathy with pain sensitivity – a 1-year prospective study
- Original experimental
- Body image is more negative in patients with chronic low back pain than in patients with subacute low back pain and healthy controls
- Identifying pain in children with CHARGE syndrome
- Patients’ perspective of the effectiveness and acceptability of pharmacological and non-pharmacological treatments of fibromyalgia
- Exercise-induce hyperalgesia, complement system and elastase activation in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome – a secondary analysis of experimental comparative studies
- Characterization of the antinociceptive effects of intrathecal DALDA peptides following bolus intrathecal delivery
- The effects of auditory background noise and virtual reality technology on video game distraction analgesia
- Book review
- Atlas of Common Pain Syndromes, 4th Edition
- Atlas of Ultrasound-Guided Regional Anesthesia, 3rd Edition
- Anaesthesia, Intensive Care and Perioperative Medicine A-Z, 6th Edition

