Abstract
Background and aims
The choice of testing site for quantitative sensory testing (QST) of pain sensitivity is important and previous studies have demonstrated patterns in pain sensitivity within discrete areas in different body regions. Some areas are characterized by a relatively high degree of spatial pain discrimination and recognizable patterns of pain referral, whilst others are not. The lumbar region is likely to have relatively low pain acuity and overlapping of pain referral. The current study was conducted to determine whether patterns of pain sensitivity (detection thresholds) could be identified in the lower back, whether differences in such patterns exist between different groups and whether such patterns could help identify a clinical source of pain and localized increased pain sensitivity.
Methods
Twenty-one patients with non-specific chronic low back pain and 21 healthy controls were tested for pressure and heat pain thresholds on 30 pre-defined locations over the mid and lower back. Topographical maps of mean pain thresholds and variability were produced, inspected visually and analyzed statistically. Between group differences in pain threshold were analyzed statistically as an indicator of widespread increased pain sensitivity. Evidence of segmental increased pain sensitivity was examined by group statistical comparison of mid-line lower range.
Results
A clear pattern of higher pain thresholds in the mid-line was evident in both groups and for both pain modalities. No discernible patterns were evident for variability within groups, but marked differences were seen between groups: variability for pressure pain thresholds appeared similar between groups, however for heat pain threshold, variability was uniformly low in the control group and uniformly high in the patient group. A highly significant (p<0.0001) difference in pain thresholds for pressure and heat was found with patients exhibiting lower thresholds than controls. No between group difference was found for mid-line lower range for either modality (p>0.05).
Conclusions
The current study supports previous findings of widespread, increased pain sensitivity in chronic non-specific low-back pain patients. It also indicates that there are discernible and similar topographical patterns of pain sensitivity in the dorsal area in both groups, but that this pattern is related to the lateral position of the test site and not the segmental level. Specific segments with increased pain sensitivity could not be identified in the patient group, which casts doubt on the utility of pressure and heat pain thresholds as indicators of the clinical source of spinal pain – at least in a population of chronic non-specific low-back pain.
Implications
In a cohort of chronic non-specific low-back pain patients and with the chosen methodology, topographical QST mapping in the lumbar region does not appear useful for identifying the spinal segment responsible for clinical pain, but it does demonstrate widespread group differences in pain sensitivity.
1 Introduction
Several studies using quantitative sensory testing (QST) have reported widespread hyperalgesia and lowered pain thresholds in chronic low back pain [1], [2], [3], [4], [5], [6], [7], [8], [9]. It is not clear how best to assess hyperalgesia in a clinical setting [10], [11], but such perturbed pain sensitivity may be important for both prognosis and clinical management [3], [4], [7], [9], [12], [13], [14].
The test site is one of the variables that need careful consideration when assessing pain sensitivity and a number of studies have examined the spatial distributions of pain sensitivity in the neck/shoulder region [15], [16], [17], [18], [19], the head and scalp [20], [21], [22], the upper extremities [23], [24], the lower extremities [25], [26], [27], and lower back [17]. Most studies have used mechanical pressure pain thresholds and some thermal stimuli [23], [24]. Many reported distinct patterns of pain sensitivity in healthy volunteers and musculoskeletal pain patients, but understanding of the clinical importance is still lacking. See Alburquerque-Sendin et al. for a review [28].
The ability to spatially discriminate superficial nociception appears to be relatively poor in general [29], and deteriorates during acute pain [30]. Furthermore, Mancini et al. [31] described the whole-body spatial resolution of superficial pain as becoming progressively poorer further inferiorly in the torso and distally in the extremities (the feet and hands being notable exceptions) probably due to different nociceptor density.
Spatial discrimination of deep somatic pain is generally poorer and more diffuse. Whilst deep pain from individual cervical segments produce recognizable distributions of referred pain [32], considerable overlap exists between perceived pain area from different lumbar and thoracic segments [33], [34]. Pain from lumbar segments may overlap considerably with pain from the sacroiliac joints [35] and even the hip joints [36].
The development of centrally mediated hyperalgesia may muddy the picture even further: animal studies demonstrate that the receptive fields of dorsal horn neurons increase when they become sensitized [37], [38] and in a clinical context, referred pain and secondary hyperalgesia is believed to reflect similar adaptations at the spinal level [39], [40]. As the nociceptive drive is non-proportionally amplified it may become evident as spreading pain and hyperalgesia, and widespread painful comorbidity is a common clinical finding in chronic low back pain [41], [42] and widespread hyperalgesia has been demonstrated experimentally in several studies [3], [4], [5], [8], [9]. In knee osteoarthrosis, the temporal development of expanding and increasingly diffuse pain has been demonstrated in step with persistent pain from the underlying disease [43] and in LBP pressure pain threshold and temporal summation of pain have been shown to decrease and increase, respectively, as pain persists [44].
The aims of this study were to (a) develop topographical maps of pressure and heat pain threshold in healthy volunteers and chronic non-specific LBP patients and (b) examine within- and between-group differences of such topographical mapping.
The hypotheses were that (a) recognizable patterns of pain sensitivity could be discerned within and between groups, (b) patients would in general be more pain sensitive than healthy controls as a reflection of generalized increased pain sensitivity, and (c) mid-line within individual lower range would be greater in the LBP group as a reflection of localized increased pain sensitivity in relation to the underlying cause of LBP.
2 Materials and methods
2.1 Participants
Participants were recruited from two different populations; (a) healthy controls and (b) patients with chronic non-specific low back pain. The local Ethics Committee approved the study (approval no: S-20150117), all participants gave informed consent and the study was conducted in accordance with the Helsinki Declaration [45]. Informed consent for participation was secured prior to the project.
2.1.1 Healthy controls
Inclusion criteria
Age between 18 and 65
No back pain or trouble prompting treatment within the last year
No other chronic pain condition requiring regular or recurring treatment
No use of pain medication within the last week
No psychiatric diagnoses
No non-specific pain syndromes
No malignant or infections conditions
Not pregnant
2.1.2 Non-specific low-back pain patients
Inclusion criteria
Age between 18 and 65
LBP for more than 3 months
LBP currently and on average scored as more than 3 (0–10 visual analog pain scale)
No radicular pain below knee level
No psychiatric diagnosis, non-specific pain syndromes, malignant or infectious diseases
No inflammatory spinal disorders, osteoporosis or other specific LBP diagnoses, apart from degenerative changes
No clinically significant disk herniation/protrusion on magnetic resonance imaging (MRI)
No moderate-to-severe degenerative changes on MRI
No significant narrowing of the central spinal canal or nerve root canals (lateral foraminae) on MRI
2.2 Clinical data
Prior to the QST examination, data was collected on: sex (male/female), age (years), height (cm), weight (kg), duration of low-back pain (years/months), average low-back pain intensity (0–10 NRS), smoking status (yes/no and amount and duration), previous spinal surgery (yes/no and type).
2.3 QST procedure
QST procedures were performed by four research assistants (two for each group), following a detailed, scripted procedure which included the exact wording of verbal instructions as well as the QST procedures themselves.
Time was allocated before data collection for the assistants to practice the procedures, both supervised (by SON) and independently.
2.3.1 Test pattern
With a black felt-tip pen, 30 test sites were marked on the back of the study participants in five columns (A-to-E from left-to-right) (Fig. 1).

Test pattern. The 30 test sites were tested in random order.
Initially, the height of the iliac crests were palpated as an indicator of the L4-5 level and the inter-spinous space of this segment was marked in the mid-line. In instances where the L4-5 inter-spinous space was difficult to identify by palpation, an ultrasound scanner was used to locate it (Sonosite Titan, Bothwell, WA, USA 98021. L38 linear probe).
The inter-spinous spaces below and above this level was marked for the L5/S1, L3/4, L2/3, L1/2, T11/12, T9/10, T7/8, T5/6 and T3/4 segments. This mid-line column of test sites was denoted “C”.
A further four columns were marked – “A” and “B” on the left hand side of the mid-line and “D” and “E” on the right. These four columns were only marked at the five lumbar levels (L1/2 to L5/S1).
Columns “A” and “E” were placed lateral to the mid-line, at a distance of half the width of the iliac crest. Columns “B” and “D” were placed midway between columns “A” and “E”, and the mid-line “C”, respectively.
A-priori, a computer-generated randomized test order was produced for each test subject which included all 30 test sites. For each subject and each QST procedure, five test sites were randomly chosen and tested twice to allow for test/re-test analysis. These five extra tests were deliberately performed as the first five tests.
The entire procedure lasted approximately 45 min.
2.3.2 Pressure pain threshold
Using a pressure algometer (Somedic model 2, 1 cm2 probe, Hørby, Sweden), the pressure pain thresholds was measured by applying a gradually increasing pressure of approximately 50 kPa/s until the participant indicated the pressure was becoming painful (by pressing an indicator button connected to the algometer).
In the mid-line (column “C”) the probe of the algometer was placed in the inter-spinous space and stabilized by the examiners non-dominant hand to prevent it slipping.
2.3.3 Heat pain threshold
Heat pain threshold was tested using a peltier thermode (Medoc TSA II, 30×30 mm2, baseline temp=32°C, rate of increase=1°C/s). The test subject indicated pain threshold with an indicator button after which the temperature returned to baseline within 1–2 s.
2.3.4 Procedure for both QST
Pressure pain threshold was tested first for all test sites, followed by heat pain threshold:
Initially, a single trial pain threshold test was performed on the lower leg (dominant side) to familiarize the participant with the stimulus.
Before the trial test and before the first pain threshold test, participants were instructed (in Danish):
“I will now apply gradually increasing [stimulus] here (test site indicated by light touch). When you find the [stimulus] becomes painful, press this button and I will stop. It’s important that you indicate when the [stimulus] starts to become painful – it’s not a question of how much you can tolerate, but where the threshold is, between something which is [stimulus] that doesn’t hurt and where it starts to hurt. Do you understand? Are you ready?”
For each of the QST, the “[stimulus]” was replaced with “pressure” or “heat” as appropriate.
On the subsequent 34 tests, participants were instructed (in Danish):
“I will now repeat the same test, but at this point (test site indicated by light touch). Are you ready?”
A minimum 10 s rest interval was observed between tests. Each test site on the list was tested only once, except for the five randomly chosen test/re-test sites. If no pressure pain had been elicited by 1,000 kPa, this was recorded as the PPT. If no heat pain had been elicited by 50°C, the system would interrupt the stimulus, return the thermode to baseline temperature and a heat pain threshold of 50 would be recorded before testing proceeded to the next test site.
2.4 Statistical analysis
2.4.1 Summary and descriptive statistics
Descriptive statistics are presented as mean values with standard deviation. Summary statistics of QST are presented as both non-parametric and parametric for completeness. Analysis was performed using R (v3.4.4) for Linux (R Core Team, Vienna, Austria, 2018), including packages “tidyverse” (v1.2.1), “stringr” (v1.3.0), “ggthemes” (v3.4.2), “pander” (v0.6.1), “BlandAltmanLeh” (v0.3.1), “car” (ed2, 2011), “cccrm” (v1.2.1), “gridExtra” (v2.3), “gstat”, “sp” and “colorRamps” (v2.3).
2.4.2 Topographical mapping
The pain sensitivity heat maps over the lumbar region were generated using R packages “gstat” [46] and “ggplot2” [47] based on inverse distance weighted interpolation. For ease of comparison, mean values of pain sensitivity was mapped onto the same color gradient scales (“matlab.like2”) as used by Binderup et al. and Ribeiro et al. [17], [19]. Variation in pain threshold was mapped to a blue-to-yellow color scale.
2.4.3 Group differences in mean pain threshold
Levenes test indicated a significant difference in variance (heteroscedasticity) between groups for heat pain threshold only, but group size was roughly equal and the difference in variance was considerably less than a factor 4 [variance ratio (heat)=1.64], and thus ANOVA was considered appropriate for use [48]. ANOVA is reported with test site lateral-position (x), test site axial-position (y) and group as independent variables for each QST procedure.
Post-hoc between-group comparison af pain thresholds is reported as unpaired Wilcoxon test and distributions are presented as smoothed density plots.
2.4.4 Group differences in mid-line lower range
Pain sensitivity in the mid-line over the lumbar and thoracic regions is represented as bi-plots. The within-individual lower range (difference between median and minimum pain thresholds) was calculated for mid-line pain thresholds and group comparison thereof was performed as un-paired Wilcoxon test. Also the interaction of group and axial y-position was included in the ANOVA described above.
2.4.5 Test/re-test reliability
Test/re-test reliability was assessed using Intra-class Correlation Coefficients ICC3.2 for single fixed raters (similar to Pearsons R) as a measure of correlation and Concordance Correlation Coefficient as a measure of agreement, as recommended by Carrasco [49]. Graphical illustrations of test/re-test reliability are provided as customized Bland-Altman plots, as recommended by Berchtold [50].
2.4.6 Analysis and annotation
Where p-values are presented as star notation, the following applies: “ns”>0.05, *≤ 0.05, **<0.01, ***<0.001 and ****<0.0001.
Analysis was performed using R for Linux [51] version 3.4.4, extended with relevant add-on packages.
3 Results
3.1 Descriptive
Twenty-one healthy volunteers (10 females) were recruited from a population of 5th year university students. The average age was 27.9 years (SD=5.2), average height was 175.7 cm (SD=9.3) and average weight was 76.5 kg (SD=20). None were tobacco smokers.
Twenty-one consecutive patients with non-specific LBP (13 females) were recruited from the patient population at the Spinecenter of Southern Denmark. The average age was 45.3 years (SD=15), average height was 170.2 cm (SD=9.5) (one missing value), average weight was 82.6 kg (SD=17.7). The mean LBP intensity (0–10 NRS) was 4.3 (SD=1.2) and duration ranged from 6 months to “many years” (median=29 months). Two patients had had back surgery: one cosmetic and could not specify. Seven were tobacco smokers.
3.2 Summary
For the LBP group, no correlations were found between mean pain thresholds (30 test sites) and clinical pain duration (ρ=0.21, p=0.37 for pressure and ρ=0.26, p=0.26 for heat pain threshold). Similarly, no correlations were found between mean pain threshold and clinical pain intensity (ρ=−0.37, p=0.1 for pressure and ρ=−0.35, p=0.12 for heat pain threshold). A highly significant correlation was found for heat and pressure pain thresholds (both groups) (ρ=0.58, p<0.0001).
A highly statistically significant (p<0.0001) difference (17.5 years) in mean age was found between groups. No significant difference was found in height or weight.
Summary statistics of QST are presented in Table 1.
Summary statistics by group and QST.
| QST | Group | Median | q25 | q75 | n | Mean | SD | p-Value |
|---|---|---|---|---|---|---|---|---|
| Heat | Control | 40 | 38 | 42 | 630 | 40 | 2.8 | p<0.0001 |
| Heat | nsLBP | 38 | 36 | 41 | 630 | 39 | 3.5 | |
| Pressure | Control | 398 | 306 | 565 | 630 | 443 | 194 | p<0.0001 |
| Pressure | nsLBP | 322 | 227 | 469 | 630 | 372 | 196 |
-
Summary statistics (median, 25% and 75% quartiles, n, mean and standard deviation) of pain thresholds. p-Values represent unpaired Wilcoxon group comparison (control vs. LBP) for each QST. “nsLBP”=“non-specific low-back pain”.
3.3 Group differences
Levenes test indicated significantly different variabilities (heteroscedasticity) between groups for heat (p<0.0001), but not for mechanical pressure.
ANOVA (Table 2) showed that pain threshold was dependent upon group (and lateral but not vertical position of the test-site), for both heat and pressure.
Anova.
| Var | Df | Sum.Sq | Mean.Sq | F | p-Value |
|---|---|---|---|---|---|
| Heat | |||||
| x | 4 | 480 | 120 | 12 | p<0.0001 |
| y | 9 | 35 | 3.9 | 0.4 | p≥0.005 |
| Group | 1 | 599 | 599 | 61 | p<0.0001 |
| y:group | 9 | 30 | 3.4 | 0.34 | p≥0.05 |
| Residuals | 1,236 | 12,206 | 9.9 | ||
| Pressure | |||||
| x | 4 | 1,103,870 | 2,75,968 | 7.4 | p<0.0001 |
| y | 9 | 4,10,778 | 45,642 | 1.2 | p≥0.05 |
| Group | 1 | 1,609,930 | 1,609,930 | 43 | p<0.0001 |
| y:group | 9 | 62,710 | 6,968 | 0.19 | p≥0.05 |
| Residuals | 1,236 | 4.6e+07 | 37,417 | ||
-
Anova table of pain thresholds.
Highly significant between-group differences in pain thresholds were found (Table 1), with LBP patients having a lower pain threshold than healthy controls for both pressure and heat (Fig. 2).

Distribution of pain threshold. A density plot (smoothed histogram) of pain thresholds for each stimulus type, by group. Between-group differences were highly significant (p<0.001, Wilcoxon test) for both stimulus types. Units: Celsius (heat) and kPa (pressure). “nsLBP”=non-specific low-back pain.
3.4 Topographical mapping
Topographical mapping of means and variance were assessed by visual inspection of the lumbar region (Fig. 3 and 4) and the midline separately (Fig. 5).

Heat maps of mean pain threshold – lumbar. Spatial distribution of mean pain threshold by group. Y-axis (L5–L1) refers to lumbar segment. X-axis (A–E) refers to sagittal position, with C in the midline and A and E being midway to the Iliac crest. Units: Celsius (heat) and kPa (pressure). “nsLBP”=non-specific low-back pain.
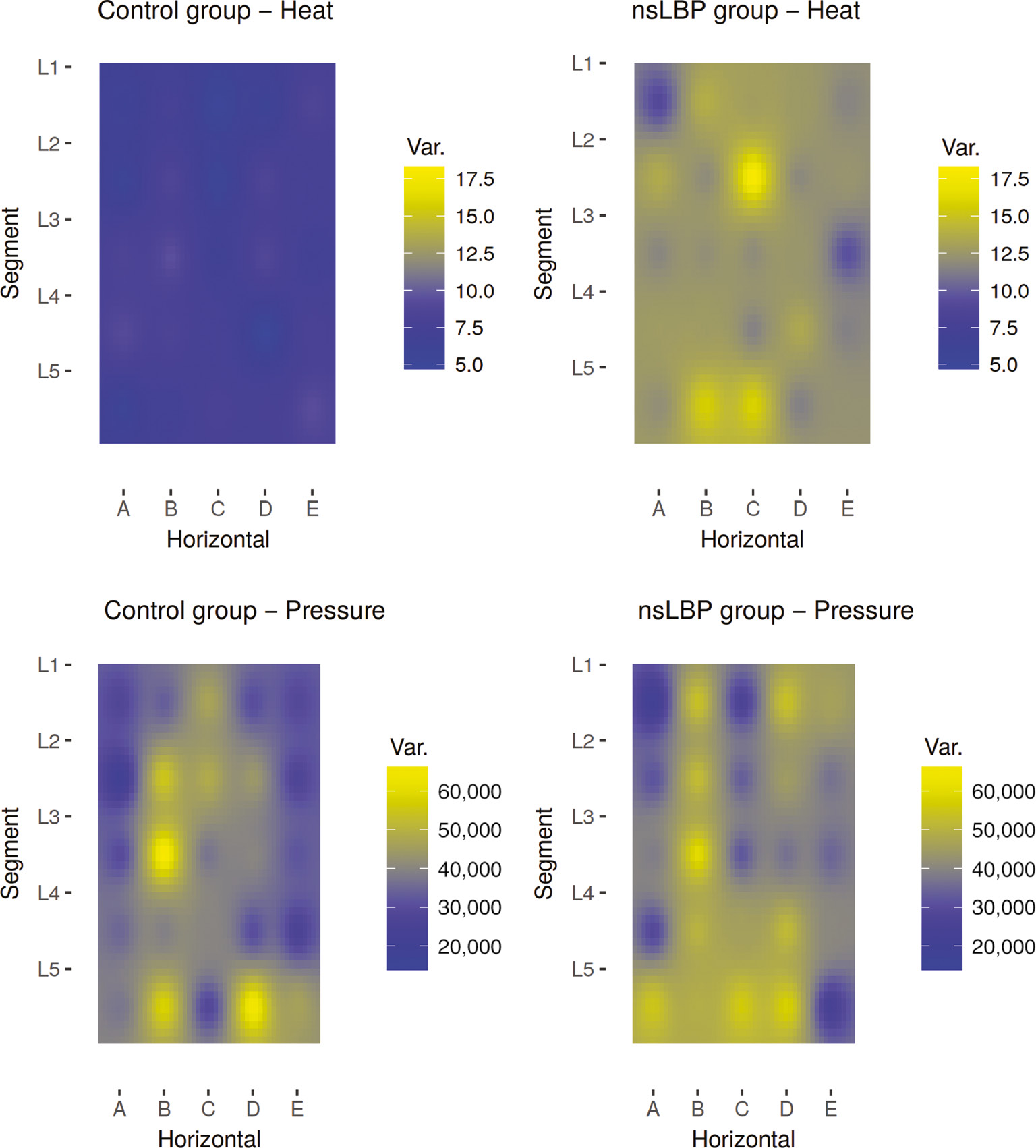
Heat maps of variation in pain threshold – lumbar. Spatial distribution of pain threshold variation by group. Y-axis (L5–L1) refers to lumbar segment. X-axis (A–E) refers to sagittal position, with C in the midline and A and E being midway to the Iliac crest. Units: Celsius2 (heat) and kPa2 (pressure). “nsLBP”=non-specific low-back pain.
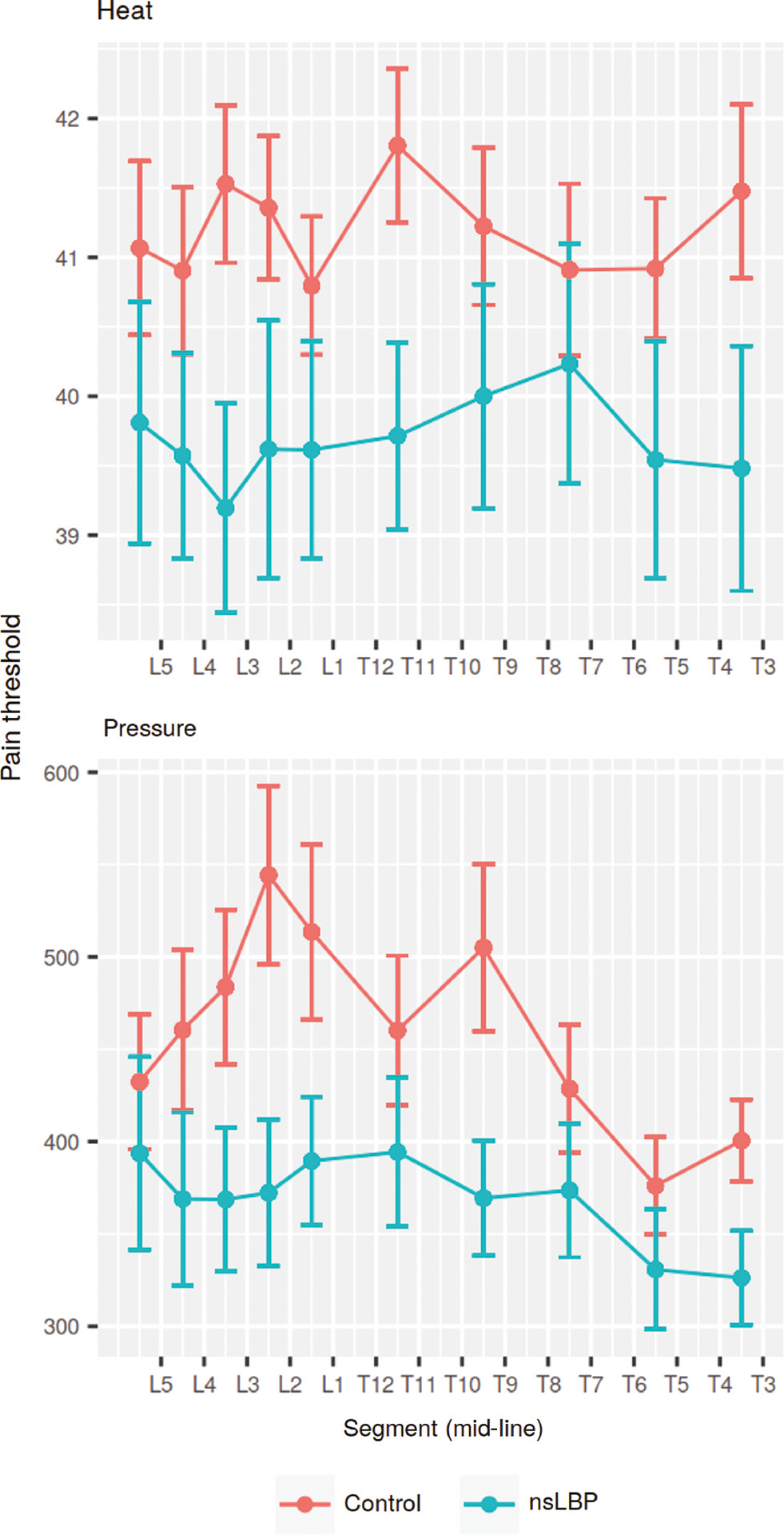
Mid-line pain threshold. Mean values and standard error of measurement of pain sensitivity in the mid-line. Units: Celsius (heat) and kPa (pressure). “nsLBP”=non-specific low-back pain.
Statistical analysis (ANOVA) indicated that pain threshold was dependent on lateral position of the test-site in the lumbar region, but not segmental level. Figure 3 suggested that the main difference was between the mid-line (column C) and paraspinal test sites. Post-hoc testing confirmed a significant difference between pain thresholds in the mid-line versus lateral positions of the lumbar region [unpaired Wilcoxon test p<0.0001 (heat), p<0.05 (pressure)].
3.5 Lower range
For the individual lower range of pain thresholds in the mid-line (Table 3 and Fig. 6), no significant difference between groups was found (unpaired Wilcoxon test p≥0.05 for heat, p≥0.05 for pressure).
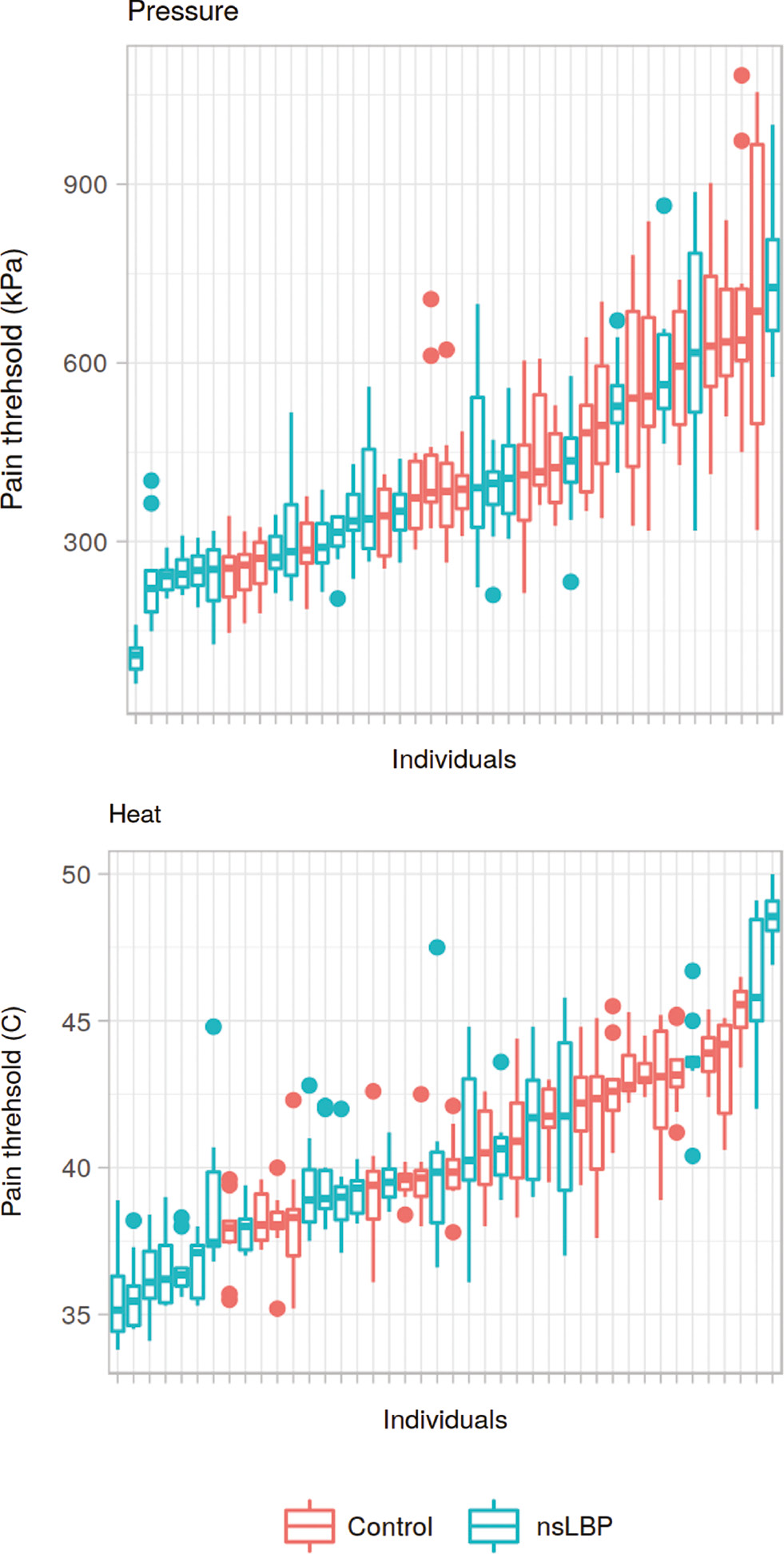
Mid-line pain threshold per individual. Box-plot of pain sensitivity (all tested mid-line segments) for each individual, sorted by median value. Box plots illustrate median value and inter-quartile range. Whiskers represents nearest value to 1.5 * IQR. The lowest value is thus represented as either the end of the lower whisker or as an outlier (dot). Units: Celsius (heat) and kPa (pressure). “nsLBP”=non-specific low-back pain.
Lower range (mid-line).
| QST | Group | Mean | SD | p-Value |
|---|---|---|---|---|
| Heat | Control | 2.3 | 1.1 | p≥0.05 |
| Heat | nsLBP | 2 | 1.2 | |
| Pressure | Control | 142 | 74 | p≥0.05 |
| Pressure | nsLBP | 109 | 64 |
-
Mean and standard deviation of individual lower range (median–minimum difference) of pain thresholds in the mid-line. p-Value represents un-paired Wilcoxon test. “nsLBP”=non-specific low-back pain.
No significant interaction of group and axial y-position was found on analysis of variance (Table 2).
3.6 Test-retest reliability
The test-retest reliabilities (correlation and agreement) were found to be acceptable, with coefficients between 0.82 and 0.86. The ICC3.2 and CCC are listed in Table 4 and shown in Fig. 7.
Test-retest correlation.
| QST | ICC | p-Value | CCC |
|---|---|---|---|
| Heat | 0.86 [0.82; 0.89] | p<0.0001 | 0.83 [0.8; 0.87] |
| Pressure | 0.82 [0.77; 0.86] | p<0.0001 | 0.82 [0.78; 0.85] |
-
Intra-class correlation coefficient (ICC3.2) [95% CI], statistical significance levels of ICC, and concordance correlation coefficient [95% CI].

Test-retest Bland-Altman plot. Bland-Altman plot of test-retest. Units: Celsius (heat) and kPa (pressure).
When comparing the results of the first and second QST value for each test/re-test site, a significant difference was found for heat (paired Wilcoxon test p<0.001) with lower mean threshold (39.3°C) for the first tests compared to the re-tests (40°C). No significant difference was observed for pressure pain thresholds (426.49 kPa vs. 420.11 kPa, p≥0.05).
4 Discussion
A clear pattern emerged from topographic mapping: pain thresholds were higher in the mid-line compared to para-spinal tissues, in both groups and for both pain stimuli. The data also supports significant group difference in pain sensitivity with lower thresholds in the LBP group. No significant differences in within-individual lower ranges were observed between the two groups.
4.1 Patterns in topographical mapping of mean pain thresholds
In the control group, pain thresholds for pressure were higher para-spinally at the L5-S1 segment, but otherwise pain thresholds were uniformly higher in the mid-line compared to lateral test sites in both groups. These findings are in line with the study on pressure pain thresholds of Binderup et al. [17].
The difference in pain thresholds over the superficial bony structures in the mid-line, compared to the soft tissues para-spinally was observed for both pressure and heat. Whilst, the difference in pressure pain threshold could be due to differences in mechanical properties of underlying structures, this does not explain the differences observed for heat pain. In fact, the difference was more pronounced for heat than for pressure, which suggests that the primary cause of the difference is unlikely to be mechanical in nature.
A possible explanation for this finding, could be differences in sensory innervation and acuity for the two modalities, including spatial discriminatory ability between the mid-line and lateral test sites. Mørch et al. [52] reported generally poorer acuity for nociceptive graphesthesia (laser stimulation) compared to tactile graphesthesia and using two-point discrimination, Mancini et al. [31] demonstrated that important differences in spatial pain acuity exist between proximal and distal areas, and that these differences were not simply a reflection of differences in tactile acuity. Our finding may be interpreted to reflect such differences in spatial nociceptive acuity.
4.2 Patterns in topographical mapping of variability of pain thresholds
There seems to be no obvious difference in variability of pressure pain thresholds between groups, but an (albeit imperfect) pattern within groups is evident: greater variability in test columns B and D, corresponding to the bulk of the erector spinae muscles.
The greater variability in pressure pain threshold para-spinally is likely a reflection of common muscle tenderness, present to varying degrees in the erector spinae muscles. Such muscle tenderness is seen in low back pain patients and pain free individuals alike, and albeit trigger-points in lower back muscles are more prevalent in LBP patients, they are also quite common in healthy controls [53], [54]. Whilst such commonplace muscle soreness is insufficient to conceal a group difference in pain thresholds, it does explain the similarity in patterns of variation between groups.
By contrast, there appears to be no discernible pattern in variability for heat pain thresholds within groups – variability is uniformly low in the control group and uniformly high in the LBP group. i.e. A very striking difference is evident between groups but no pattern discernible within groups for heat pain. This observation can be explained by widespread increased heat pain sensitivity in some, but not all LBP patients and no such increase in the control group.
Some studies have reported changes in pain sensitivity in areas of referred pain, whilst others have not [55], [56], [57], [58], [59] and a study by Bajaj et al. suggests that such changes may be modality-dependent [60]. As clinical low back pain is typically of deep somatic origin it is likely to cause such referred pain and could thus account for greater variability within the test area of the present study.
4.3 Group differences and widespread increased pain sensitivity
The wide-spread lower pressure and heat pain thresholds in the LBP group is in concordance with previous findings [4], [5], [6], [8], [61] and may be interpreted as indicative of altered central pain processing, more specifically sensitization of central pain pathways and/or perturbations in descending inhibitory control.
A significant difference in age was observed at baseline, which means groups were not directly comparable. However, a recent systematic review and meta-analysis of the influence of age on pain sensitivity [62] based on 31 published articles, reported higher pain thresholds in older individuals compared to younger individuals. In the current study, the LBP patients had lower pain thresholds, despite being on average 18 years older than the healthy controls. In other words, the group difference in age does not account for the observed difference in pain sensitivity in the current study.
4.4 Localized increased pain sensitivity – within-individual differences in mid-line pain thresholds
The rationale for assessing localized increased pain sensitivity by examining the individual lower range of pain thresholds was, that a spinal segment with such increased sensitivity would likely be the segment with the lowest pain thresholds for that individual. No group difference was found for either pain modality, however.
Care should be taken when interpreting this finding, but it could indicate that any effect of localized increased pain sensitivity at the site of clinical pain is obfuscated by the effects of widespread increased pain sensitivity, in this group of chronic LBP patients. As pain thresholds were generally lower in the LBP group, any further localized increased pain sensitivity would be less obvious due to a flooring effect.
Also, the patients recruited in the present study were included on the particular criterion that they had non-specific low back pain. Patients with moderate or severe degenerative changes on MRI, central or foraminal stenosis etc. were excluded. It is therefore perhaps unreasonable to expect an anatomically or segmentally well-localized source of LBP in the current patient group.
4.5 Test/re-test reliability
Of the five pairs of repeat-tests used for test/re-test analysis, the first tests were performed as the very first five tests for each participant for each QST. This might have biased the test/re-test analysis towards lower coefficients of reliability and agreement as it is often the case that the first pain threshold measurements are lower than the following. In any case, the test/re-test reliability and agreement coefficients in the present study were found to be high and comparable to those of previous studies [63], [64], [65].
Arguably, the shear volume of measurements could be a separate problem. It is conceivable that repeated measurements could lead to altered sensitivity, fatigue and loss of concentration. In the present study 70 pain threshold measurements were performed for each participant, but a post-hoc test (not presented here) did not indicate any significant correlation between test-order and pain threshold.
5 Conclusions
Recognizable patterns of pain sensitivity were observed as hypothesized and specifically high thresholds (lower pain sensitivity) in the mid-line was apparent in both groups.
The current data supported the hypothesis that LBP patients were more pain sensitive than healthy controls in a large dorsal area, as a reflection of spreading increased pain sensitivity. This was evident despite a baseline age difference which would tend to mask such a difference.
No discernible pattern between segments was observed in the mid-line, in either group and the hypothesis that within-individual differences (lower range) between segments would be greater in LBP patients as an indication of localized increased pain sensitivity, was not supported.
-
Authors’ statements
-
Research funding: The project was entirely funded through research tenure positions – no external funding was sought or received.
-
Conflict of interest: We, the authors state no conflicts of interest.
-
Informed consent: All participants in the study provided written informed consent.
-
Ethical approval: The study was approved by the local science Ethics Committee – Region of Southern Denmark, approval number S-20150117.
References
[1] Schmidt AJ, Brands AM. Persistence behavior of chronic low back pain patients in an acute pain situation. J Psychosom Res 1986;30:339–46.10.1016/0022-3999(86)90011-5Search in Google Scholar PubMed
[2] Brands AM, Schmidt AJ. Learning processes in the persistence behavior of chronic low back pain patients with repeated acute pain stimulation. Pain 1987;30:329–37.10.1016/0304-3959(87)90021-2Search in Google Scholar PubMed
[3] Clauw D, Williams D, Lauerman W, Dahlman M, Aslami A, Nachemson A, Kobrine A, Wiesel S. Pain sensitivity as a correlate of clinical status in individuals with chronic low back pain. Spine 1999;24:2035–41.10.1097/00007632-199910010-00013Search in Google Scholar PubMed
[4] Giesecke T, Gracely R, Grant M, Nachemson A, Petzke F, Williams D, Clauw D. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum 2004; 50:613–23.10.1002/art.20063Search in Google Scholar PubMed
[5] O’Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L. Generalized deep-tissue hyperalgesia in patients with chronic low-back pain. Eur J Pain 2007;11:415–20.10.1016/j.ejpain.2006.05.009Search in Google Scholar PubMed
[6] Blumenstiel K, Gerhardt A, Rolke R, Bieber C, Tesarz J, Friederich H-C, Eich W, Treede R-D. Quantitative sensory testing profiles in chronic back pain are distinct from those in fibromyalgia. Clin J Pain 2011;27:682–90.10.1097/AJP.0b013e3182177654Search in Google Scholar PubMed
[7] O’Neill S, Kjær P, Graven-Nielsen T, Manniche C, Arendt-Nielsen L. Low pressure pain thresholds are associated with, but does not predispose for, low back pain. Eur Spine J 2011;20:2120–5.10.1007/s00586-011-1796-4Search in Google Scholar PubMed PubMed Central
[8] Puta C, Schulz B, Schoeler S, Magerl W, Gabriel B, Gabriel HHW, Miltner WHR, Weiss T. Enhanced sensitivity to punctate painful stimuli in female patients with chronic low back pain. BMC Neurol 2012;12:98.10.1186/1471-2377-12-98Search in Google Scholar PubMed PubMed Central
[9] O’Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L. Association between a composite score of pain sensitivity and clinical parameters in low-back pain. Clin J Pain 2014;30:831–8.10.1097/AJP.0000000000000042Search in Google Scholar PubMed
[10] Courtney CA, Kavchak AE, Lowry CD, O’Hearn MA. Interpreting joint pain: quantitative sensory testing in musculoskeletal management. J Orthop Sports Phys Ther 2010;40:818–25.10.2519/jospt.2010.3314Search in Google Scholar PubMed
[11] Starkweather AR, Heineman A, Storey S, Rubia G, Lyon DE, Greenspan J, Dorsey SG. Methods to measure peripheral and central sensitization using quantitative sensory testing: a focus on individuals with low back pain. Appl Nurs Res 2016;29:237–41.10.1016/j.apnr.2015.03.013Search in Google Scholar PubMed
[12] Wilder-Smith OHG, Tassonyi E, Arendt-Nielsen L. Preoperative back pain is associated with diverse manifestations of central neuroplasticity. Pain 2002;97:189–94.10.1016/S0304-3959(01)00430-4Search in Google Scholar PubMed
[13] Schiff E, Eisenberg E. Can quantitative sensory testing predict the outcome of epidural steroid injections in sciatica? A preliminary study. Anesth Analg 2003;97:828–32.10.1213/01.ANE.0000078583.47735.69Search in Google Scholar PubMed
[14] Wilder-Smith OHG, Tassonyi E, Crul BJP, Arendt-Nielsen L. Quantitative sensory testing and human surgery: effects of analgesic management on postoperative neuroplasticity. Anesthesiology 2003;98:1214–22.10.1097/00000542-200305000-00025Search in Google Scholar PubMed
[15] Nie H, Kawczynski A, Madeleine P, Arendt-Nielsen L. Delayed onset muscle soreness in neck/shoulder muscles. Eur J Pain 2005;9:653–3.10.1016/j.ejpain.2004.12.009Search in Google Scholar PubMed
[16] Ge H-Y, Fernández-de-las-Peñas C, Madeleine P, Arendt-Nielsen L. Topographical mapping and mechanical pain sensitivity of myofascial trigger points in the infraspinatus muscle. Eur J Pain 2008;12:859–65.10.1016/j.ejpain.2007.12.005Search in Google Scholar PubMed
[17] Binderup AT, Arendt-Nielsen L, Madeleine P. Pressure pain sensitivity maps of the neck-shoulder and the low back regions in men and women. BMC Musculoskelet Disord 2010;11:234.10.1186/1471-2474-11-234Search in Google Scholar PubMed PubMed Central
[18] Fernández-de-las-Peñas C, Madeleine P, Caminero AB, Cuadrado ML, Arendt-Nielsen L, Pareja JA. Generalized neck-shoulder hyperalgesia in chronic tension-type headache and unilateral migraine assessed by pressure pain sensitivity topographical maps of the trapezius muscle. Cephalalgia 2010;30:77–86.10.1111/j.1468-2982.2009.01901.xSearch in Google Scholar PubMed
[19] Ribeiro IL, Camargo PR, Alburquerque-Sendín F, Madeleine P, Fernández-de-las-Peñas C, Salvini TF. Topographical pressure pain sensitivity maps of the shoulder region in individuals with subacromial pain syndrome. Man Ther 2016;21:134–43.10.1016/j.math.2015.07.002Search in Google Scholar PubMed
[20] Fernndez-de-las-Peas C, Ge H-Y, Cuadrado ML, Madeleine P, Pareja JA, Arendt-Nielsen L. Bilateral pressure pain sensitivity mapping of the temporalis muscle in chronic tension-type headache. Headache 2008;48:1067–75.10.1111/j.1526-4610.2007.01005.xSearch in Google Scholar PubMed
[21] Binderup AT, Arendt-Nielsen L, Madeleine P. Cluster analysis of pressure pain threshold maps from the trapezius muscle. Comput Methods Biomech Biomed Eng 2010;13:677–83.10.1080/10255840903446979Search in Google Scholar PubMed
[22] Cuadrado ML, Valle B, Fernández-de-las-Peñas C, Madeleine P, Barriga FJ, Arias JA, Arendt-Nielsen L, Pareja JA. Pressure pain sensitivity of the scalp in patients with nummular headache: a cartographic study. Cephalalgia 2010;30:200–6.10.1111/j.1468-2982.2009.01895.xSearch in Google Scholar PubMed
[23] Li X, Petrini L, Defrin R, Madeleine P, Arendt-Nielsen L. High resolution topographical mapping of warm and cold sensitivities. Clin Neurophysiol 2008;119:2641–6.10.1016/j.clinph.2008.08.018Search in Google Scholar PubMed
[24] Ruiz-Ruiz B, Fernández-de-las-Peñas C, Ortega-Santiago R, Arendt-Nielsen L, Madeleine P. Topographical pressure and thermal pain sensitivity mapping in patients with unilateral lateral epicondylalgia. J Pain 2011;12:1040–8.10.1016/j.jpain.2011.04.001Search in Google Scholar PubMed
[25] Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. Pain 2010;149:573–81.10.1016/j.pain.2010.04.003Search in Google Scholar PubMed
[26] Arendt-Nielsen L, Egsgaard LL, Petersen KK, Eskehave TN, Graven-Nielsen T, Hoeck HC, Simonsen O. A mechanism-based pain sensitivity index to characterize knee osteoarthritis patients with different disease stages and pain levels. Eur J Pain 2015;19:1406–17.10.1002/ejp.651Search in Google Scholar PubMed
[27] Tornero-Caballero MC, Salom-Moreno J, Cigarán-Méndez M, Morales-Cabezas M, Madeleine P, Fernández-de-las-Peñas C. Muscle trigger points and pressure pain sensitivity maps of the feet in women with fibromyalgia syndrome. Pain Med 2016;17:1923–32.10.1093/pm/pnw090Search in Google Scholar PubMed
[28] Alburquerque-Sendín F, Madeleine P, Fernández-de-las-Peñas C, Camargo PR, Salvini TF. Spotlight on topographical pressure pain sensitivity maps: a review. J Pain Res 2018;11:215–25.10.2147/JPR.S135769Search in Google Scholar PubMed PubMed Central
[29] Frahm KS, Mørch CD, Andersen OK. Tempo-spatial discrimination is lower for noxious stimuli than for innocuous stimuli. Pain 2018;159:393.10.1097/j.pain.0000000000001095Search in Google Scholar PubMed
[30] Adamczyk WM, Saulicz O, Saulicz E, Luedtke K. Tactile acuity (dys)Function in acute nociceptive low back pain: a double-blind experiment. Pain 2018;159:427.10.1097/j.pain.0000000000001110Search in Google Scholar PubMed
[31] Mancini F, Bauleo A, Cole J, Lui F, Porro CA, Haggard P, Iannetti GD. Whole-body mapping of spatial acuity for pain and touch. Ann Neurol 2014;75:917–24.10.1002/ana.24179Search in Google Scholar PubMed PubMed Central
[32] Fukui S, Ohseto K, Shiotani M, Ohno K, Karasawa H, Naganuma Y, Yuda Y. Referred pain distribution of the cervical zygapophyseal joints and cervical dorsal rami. Pain 1996;68:79–83.10.1016/S0304-3959(96)03173-9Search in Google Scholar PubMed
[33] Fukui S, Ohseto K, Shiotani M. Patterns of pain induced by distending the thoracic zygapophyseal joints. Reg Anesth 1997;22:332–6.10.1016/S1098-7339(97)80007-7Search in Google Scholar
[34] Fukui S, Ohseto K, Shiotani M, Ohno K, Karasawa H, Naganuma Y. Distribution of referred pain from the lumbar zygapophyseal joints and dorsal rami. Clin J Pain 1997;13:303–7.10.1097/00002508-199712000-00007Search in Google Scholar PubMed
[35] Fukui S, Nosaka S. Pain patterns originating from the sacroiliac joints. J Anesth 2002;16:245–7.10.1007/s005400200033Search in Google Scholar PubMed
[36] Kurosawa D, Murakami E, Aizawa T. Referred pain location depends on the affected section of the sacroiliac joint. Eur Spine J 2015;24:521–7.10.1007/s00586-014-3604-4Search in Google Scholar PubMed
[37] Hoheisel U, Mense S, Simons DG, Yu XM. Appearance of new receptive fields in rat dorsal horn neurons following noxious stimulation of skeletal muscle: a model for referral of muscle pain? Neurosci Lett 1993;153:9–12.10.1016/0304-3940(93)90064-RSearch in Google Scholar PubMed
[38] Hoheisel U, Koch K, Mense S. Functional reorganization in the rat dorsal horn during an experimental myositis. Pain 1994;59:111–8.10.1016/0304-3959(94)90054-XSearch in Google Scholar PubMed
[39] Mense S. Referral of muscle pain: new aspects. APS J 1994; 3:1–9.10.1016/S1058-9139(05)80227-XSearch in Google Scholar
[40] Graven-Nielsen T. Fundamentals of muscle pain, referred pain, and deep tissue hyperalgesia. Scand J Rheumatol 2006;35:1–43.10.1080/03009740600865980Search in Google Scholar PubMed
[41] Hestbaek L, Leboeuf-Yde C, Manniche C. Is low back pain part of a general health pattern or is it a separate and distinctive entity? A critical literature review of comorbidity with low back pain. J Manipulative Physiol Ther 2003;26:243–52.10.1016/S0161-4754(03)00003-4Search in Google Scholar
[42] Hestbaek L, Leboeuf-Yde C, Kyvik KO, Vach W, Russell MB, Skadhauge L, Svendsen A, Manniche C. Comorbidity with low back pain: a cross-sectional population-based survey of 12- to 22-year-olds. Spine (Phila Pa 1976) 2004;29:1483–91.10.1097/01.BRS.0000129230.52977.86Search in Google Scholar PubMed
[43] Thompson L, Boudreau R, Newman A, Hannon M, Chu C, Nevitt M, Kent Kwoh C. The association of osteoarthritis risk factors with localized, regional and diffuse knee pain. Osteoarthr Cartilage 2010;18:1244–9.10.1016/j.joca.2010.05.014Search in Google Scholar PubMed PubMed Central
[44] Marcuzzi A, Wrigley PJ, Dean CM, Graham PL, Hush JM. From acute to persistent low back pain: a longitudinal investigation of somatosensory changes using quantitative sensory testingan exploratory study. Pain Rep 2018;3:e641.10.1097/PR9.0000000000000641Search in Google Scholar PubMed PubMed Central
[45] Charlton E. Ethical guidelines for pain research in humans. Committee on Ethical Issues of the International Association for the Study of Pain. Pain 1995;63:277–8.Search in Google Scholar
[46] Gräler B, Pebesma E, Heuvelink G. Spatio-temporal interpolation using gstat. R J 2016;8:204–18.10.32614/RJ-2016-014Search in Google Scholar
[47] Wickham H. Ggplot2: elegant graphics for data analysis, 1st ed. 2009. Corr. 3rd printing 2010 edition. New York: Springer, 2010.10.1007/978-0-387-98141-3_1Search in Google Scholar
[48] Dean A, Voss D, Draguljić D. Design and analysis of experiments, 2nd ed. 2017 edition. New York, NY: Springer, 2017.10.1007/978-3-319-52250-0Search in Google Scholar
[49] Carrasco JL, Phillips BR, Puig-Martinez J, King TS, Chinchilli VM. Estimation of the concordance correlation coefficient for repeated measures using SAS and R. Comp Methods Programs Biomed 2013;109:293–304.10.1016/j.cmpb.2012.09.002Search in Google Scholar PubMed
[50] Berchtold A. TestRetest: agreement or reliability? Method Innov 2016;9:1–7.10.1177/2059799116672875Search in Google Scholar
[51] R Core Team. R: a Language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2016.Search in Google Scholar
[52] Mørch CD, Andersen OK, Quevedo AS, Arendt-Nielsen L, Coghill RC. Exteroceptive aspects of nociception: insights from graphesthesia and two-point discrimination. Pain 2010;151:45–52.10.1016/j.pain.2010.05.016Search in Google Scholar PubMed PubMed Central
[53] Iglesias-González JJ, Muñoz-García MT, Rodrigues-de-Souza DP, Alburquerque-Sendín F, Fernández-de-las-Peñas C. Myofascial trigger points, pain, disability, and sleep quality in patients with chronic nonspecific low back pain. Pain Med 2013;14:1964–70.10.1111/pme.12224Search in Google Scholar PubMed
[54] Hua NK, Van der Does E. The occurrence and inter-rater reliability of myofascial trigger points in the quadratus lumborum and gluteus medius: a prospective study in non-specific low back pain patients and controls in general practice. Pain 1994;58:317–23.10.1016/0304-3959(94)90125-2Search in Google Scholar PubMed
[55] Kellgren J. Observations on referred pain arising from muscles. Clin Sci 1938;3:175–90.Search in Google Scholar
[56] Steinbrocker O, Isenberg SA, Silver M, Neustadt D, Kuhn P, Schittone M. Observations on pain produced by injection of hypertonic saline into muscles and other supportive tissues. J Clin Invest 1953;32:1045–51.10.1172/JCI102815Search in Google Scholar PubMed PubMed Central
[57] Feinstein B, Langton JN, Jameson RM, Schiller F. Experiments on pain referred from deep somatic tissues. J Bone Joint Surg Am 1954;36-A:981–97.10.2106/00004623-195436050-00007Search in Google Scholar
[58] Graven-Nielsen T, Arendt-Nielsen L, Svensson P, Staehelin Jensen T. Stimulus response functions in areas with experimentally induced referred muscle pain a psychophysical study. Brain Res 1997;744:121–8.10.1016/S0006-8993(96)01077-3Search in Google Scholar
[59] Graven-Nielsen T, Babenko V, Svensson P, Arendt-Nielsen L. Experimentally induced muscle pain induces hypoalgesia in heterotopic deep tissues, but not in homotopic deep tissues. Brain Res 1998;787:203–10.10.1016/S0006-8993(97)01480-7Search in Google Scholar PubMed
[60] Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L. A comparison of modality-specific somatosensory changes during menstruation in dysmenorrheic and nondysmenorrheic women. Clin J Pain 2002;18:180–90.10.1097/00002508-200205000-00007Search in Google Scholar PubMed
[61] Hüppe A, Brockow T, Raspe H. Chronic widespread pain and tender points in low back pain: a population-based study. Z Rheumatol 2004;63:76–83.10.1007/s00393-004-0531-5Search in Google Scholar PubMed
[62] Lautenbacher S, Peters JH, Heesen M, Scheel J, Kunz M. Age changes in pain perception: a systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci Biobehav Rev 2017;75:104–13.10.1016/j.neubiorev.2017.01.039Search in Google Scholar PubMed
[63] Potter L, McCarthy C, Oldham J. Algometer reliability in measuring pain pressure threshold over normal spinal muscles to allow quantification of anti-nociceptive treatment effects. Int J Osteopath Med 2006;9:113–9.10.1016/j.ijosm.2006.11.002Search in Google Scholar
[64] Paungmali A, Sitilertpisan P, Taneyhill K, Pirunsan U, Uthaikhup S. Intrarater reliability of pain intensity, tissue blood flow, thermal pain threshold, pressure pain threshold and lumbo-pelvic stability tests in subjects with low back pain. Asian J Sports Med 2012;3:8–14.10.5812/asjsm.34718Search in Google Scholar PubMed PubMed Central
[65] O’Neill S, O’Neill L. Improving QST reliability more raters, tests, or occasions? A multivariate generalizability Study. J Pain 2015;16:454–62.10.1016/j.jpain.2015.01.476Search in Google Scholar PubMed
©2018 Scandinavian Association for the Study of Pain. Published by Walter de Gruyter GmbH, Berlin/Boston. All rights reserved
Articles in the same Issue
- Frontmatter
- Editorial comment
- The Fear Avoidance Beliefs Questionnaire – the FABQ – for the benefit of another 70 million potential pain patients
- The Yaksh-model of intrathecal opioid-studies: still exciting four decades later
- Pain is common in chronic fatigue syndrome – current knowledge and future perspectives
- Systematic review
- Use of multidomain management strategies by community dwelling adults with chronic pain: evidence from a systematic review
- Clinical pain research
- Topographic mapping of pain sensitivity of the lower back – a comparison of healthy controls and patients with chronic non-specific low back pain
- A prospective study of patients’ pain intensity after cardiac surgery and a qualitative review: effects of examiners’ gender on patient reporting
- Correlations between the active straight leg raise, sleep and somatosensory sensitivity during pregnancy with post-partum lumbopelvic pain: an initial exploration
- Pain is associated with reduced quality of life and functional status in patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
- Does validation and alliance during the multimodal investigation affect patients’ acceptance of chronic pain? An experimental single case study
- Translation, cross-cultural adaptation, and psychometric properties of the Hausa version of the Fear-Avoidance Beliefs Questionnaire in patients with low back pain
- Observational study
- Cause-specific mortality of patients with severe chronic pain referred to a multidisciplinary pain clinic: a cohort register-linkage study
- Pain self-efficacy moderates the association between pain and somatization in a community sample
- Pediatric chronic pain and caregiver burden in a national survey
- Psychometric evaluation of the Danish version of a modified Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R-D) for patients hospitalized with acute abdominal pain
- Musculoskeletal pain in multiple body sites and work ability in the general working population: cross-sectional study among 10,000 wage earners
- Prediction of running-induced Achilles tendinopathy with pain sensitivity – a 1-year prospective study
- Original experimental
- Body image is more negative in patients with chronic low back pain than in patients with subacute low back pain and healthy controls
- Identifying pain in children with CHARGE syndrome
- Patients’ perspective of the effectiveness and acceptability of pharmacological and non-pharmacological treatments of fibromyalgia
- Exercise-induce hyperalgesia, complement system and elastase activation in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome – a secondary analysis of experimental comparative studies
- Characterization of the antinociceptive effects of intrathecal DALDA peptides following bolus intrathecal delivery
- The effects of auditory background noise and virtual reality technology on video game distraction analgesia
- Book review
- Atlas of Common Pain Syndromes, 4th Edition
- Atlas of Ultrasound-Guided Regional Anesthesia, 3rd Edition
- Anaesthesia, Intensive Care and Perioperative Medicine A-Z, 6th Edition
Articles in the same Issue
- Frontmatter
- Editorial comment
- The Fear Avoidance Beliefs Questionnaire – the FABQ – for the benefit of another 70 million potential pain patients
- The Yaksh-model of intrathecal opioid-studies: still exciting four decades later
- Pain is common in chronic fatigue syndrome – current knowledge and future perspectives
- Systematic review
- Use of multidomain management strategies by community dwelling adults with chronic pain: evidence from a systematic review
- Clinical pain research
- Topographic mapping of pain sensitivity of the lower back – a comparison of healthy controls and patients with chronic non-specific low back pain
- A prospective study of patients’ pain intensity after cardiac surgery and a qualitative review: effects of examiners’ gender on patient reporting
- Correlations between the active straight leg raise, sleep and somatosensory sensitivity during pregnancy with post-partum lumbopelvic pain: an initial exploration
- Pain is associated with reduced quality of life and functional status in patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
- Does validation and alliance during the multimodal investigation affect patients’ acceptance of chronic pain? An experimental single case study
- Translation, cross-cultural adaptation, and psychometric properties of the Hausa version of the Fear-Avoidance Beliefs Questionnaire in patients with low back pain
- Observational study
- Cause-specific mortality of patients with severe chronic pain referred to a multidisciplinary pain clinic: a cohort register-linkage study
- Pain self-efficacy moderates the association between pain and somatization in a community sample
- Pediatric chronic pain and caregiver burden in a national survey
- Psychometric evaluation of the Danish version of a modified Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R-D) for patients hospitalized with acute abdominal pain
- Musculoskeletal pain in multiple body sites and work ability in the general working population: cross-sectional study among 10,000 wage earners
- Prediction of running-induced Achilles tendinopathy with pain sensitivity – a 1-year prospective study
- Original experimental
- Body image is more negative in patients with chronic low back pain than in patients with subacute low back pain and healthy controls
- Identifying pain in children with CHARGE syndrome
- Patients’ perspective of the effectiveness and acceptability of pharmacological and non-pharmacological treatments of fibromyalgia
- Exercise-induce hyperalgesia, complement system and elastase activation in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome – a secondary analysis of experimental comparative studies
- Characterization of the antinociceptive effects of intrathecal DALDA peptides following bolus intrathecal delivery
- The effects of auditory background noise and virtual reality technology on video game distraction analgesia
- Book review
- Atlas of Common Pain Syndromes, 4th Edition
- Atlas of Ultrasound-Guided Regional Anesthesia, 3rd Edition
- Anaesthesia, Intensive Care and Perioperative Medicine A-Z, 6th Edition

