Rituximab in the treatment of anti-HMGCR immune-mediated necrotizing myopathy: Two cases successfully treated
-
Giulia Micheli
, Lorenzo Salvati
Dear Editor,
Anti-HMGCR-positive myositis is an immune-mediated necrotizing myopathy (IMNM) characterized by the presence of antibodies directed against hydroxymethylglutaryl-CoA reductase (HMGCR), a key enzyme involved in cholesterol metabolism. Clinically, it presents with symmetric proximal muscle weakness, markedly elevated serum creatine kinase (CK) levels, abnormal findings on electromyography (EMG) and histopathological evidence of muscle fibre necrosis with macrophage infiltrate. A history of statins exposure is often reported, although the disease can also develop in statin-naïve individuals.[1,2] Myositis persists even after drug discontinuation and requires immunosuppressive/immunomodulatory therapies, mainly represented by glucocorticoids, methotrexate (MTX) and high-dose intravenous immunoglobulins (IVIG).[3] Although the response to treatment is generally good, some patients do not respond to initial therapy or relapse after steroid discontinuation. Rituximab (RTX) has been successfully used as second-line therapy, due to the putative pathogenic role of anti-HMGCR antibodies.[4,5] Herein, we report two patients with anti-HMGCR-positive IMNM successfully treated with rituximab, in addition to standard therapy.
A 57-year-old man (case 1) and a 70-year-old woman (case 2) presented with progressive asthenia and limb muscle weakness. Both showed significantly elevated serum CK levels (12,946 and 11,344 U/L, respectively; reference range 39–308 U/L). Only case 2 had a history of statin use, but both had taken red yeast rice supplements prior to symptom onset. EMG revealed myositic and myopathic changes in both patients. In case 1, magnetic resonance image (MRI) was showed hyperintensities on FAT-suppressed images (Figure 1A). Anti-HMGCR antibodies were strongly positive on an enzyme-linked immunosorbent assay (ELISA) in both cases (315 and 402 U, respectively; reference range < 20 U). Initial treatment included high-dose intravenous steroids and IVIG followed by oral high-dose corticosteroids and MTX, with a rapid CK reduction and progressive improvement of muscle strength. However, CK and anti-HMGCR antibody levels remained elevated, and symptoms persisted, especially in case 1. In this patient a muscle biopsy was performed and discrete inhomogeneity in fibres calibre, nuclear internalizations, isolated nicotinamide adenine dinucleotide (NADH)-positive hypotrophic and diverse regenerating fibres, diffuse modest CD68 expression and increased expression of membrane attack complex (MAC) and major histocompatibility complex (MHC) class I on cellular surfaces in a picture of myopathic damage were found. For this reason, RTX therapy was proposed (1000 mg two weeks apart and then every 6 months for 3 times). Following RTX therapy, case 1 achieved full recovery, with normalization of CK, complete negativization of anti-HMGCR antibodies, resolution of MRI findings, and discontinuation of IVIG (Figure 1B, 2A-B). No adverse events occurred during and after treatment. Case 2 improved clinically, discontinued MTX and IVIG, and achieved reduction of anti-HMGCR antibody levels (Figure 2C-D). In this patient, a mild IgG1 deficiency occurred after RTX, without increased infections.
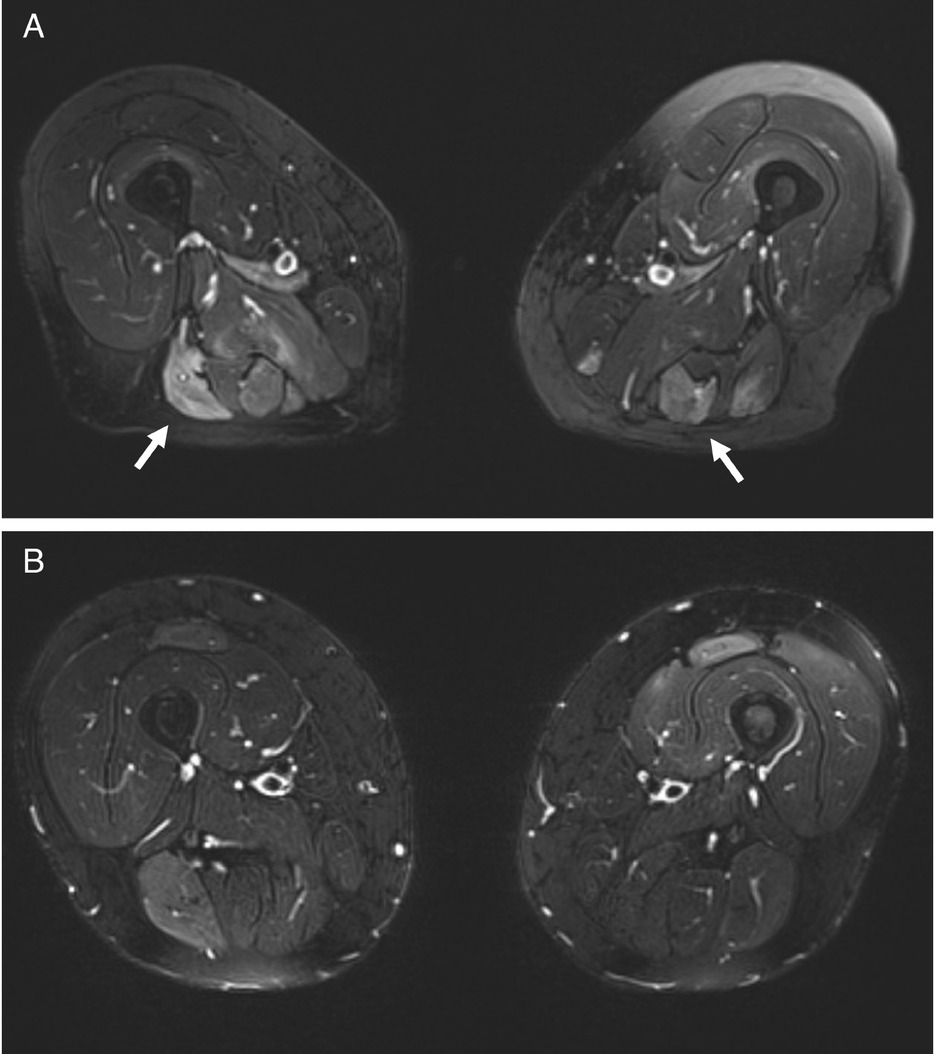
MRI scans of the thighs before and after rituximab therapy in Case 1. (A) Axial T2-weighted images of the upper thigh (December 2020) showing extensive areas of hyperintensity with muscle edema (white arrows). (B) Follow-up axial T2-weighted images (May 2022) of the same region demonstrate marked resolution of the previously observed edema.
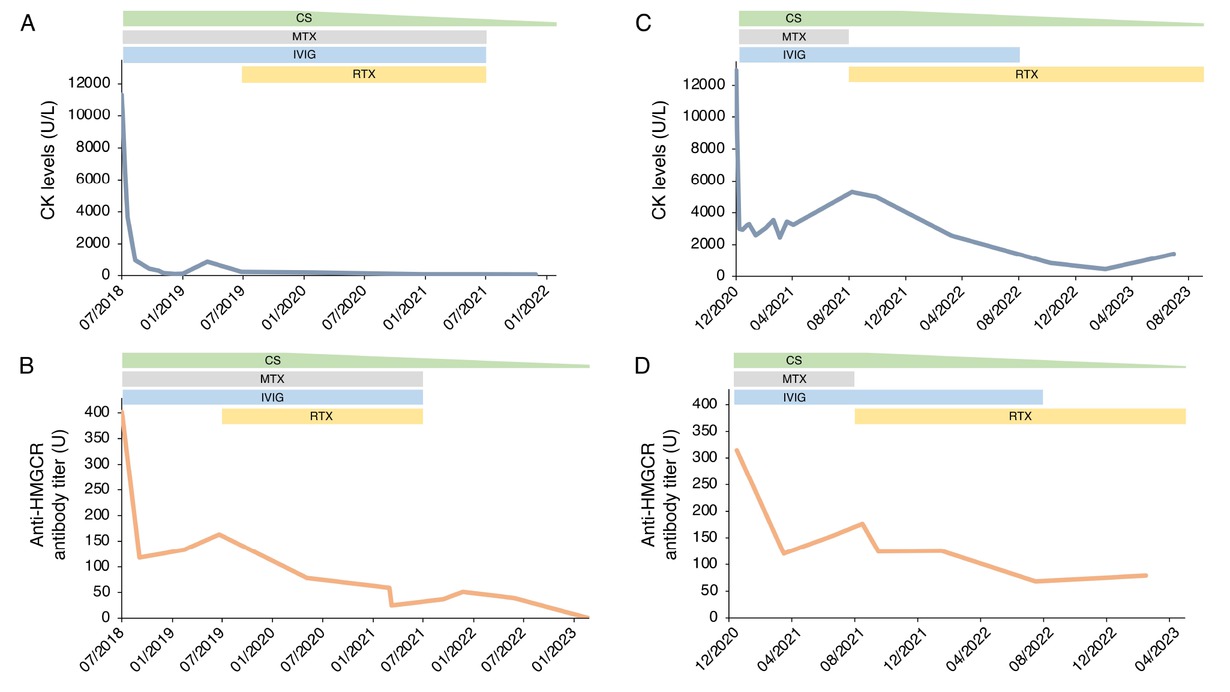
Disease progression in Case 1 and Case 2: longitudinal evaluation of CK levels (A-C) and anti-HMGCR antibody titers (B-D) in relation to treatment. CK levels (U/L) and anti-HMGCR antibody titers (U) are shown over time for Case 1 (panel A-B) and Case 2 (panel C-D). Reference ranges CK: 39-308 U/L; anti-HMGCR antibody titer: <20 U. CK, creatine kinase; CS, corticosteroid; MTX, methotrexate; IVIG, intravenous immunoglobulin; RTX, rituximab.
Anti-HMGCR myopathy is a rare subtype of IMNM.[6,7] To date, prospective studies and randomized clinical trials specifically addressing the optimal therapeutic approach for this entity are limited. Anti-HMGCR antibody titres have been reported to correlate with disease severity, supporting a potential pathogenic role.[8,9] Accordingly, B-cell depletion with RTX has emerged as a promising effective therapeutic strategy. However, published data on RTX efficacy in this subset of myopathies remain limited with currently no consensus regarding the optimal RTX protocol.[10,11] In conclusion, the few retrospective studies published to date show substantial heterogeneity in the patient characteristics, treatment regimens, timing of RTX initiation, and biomarkers used to monitor response. Our findings contribute to the growing evidence supporting the use of RTX in anti-HMGCR-positive IMNM and emphasize the urgent need for prospective randomized clinical trials to evaluate its efficacy and safety in this context.
Acknowledgments
We thank the patients and all those who care for them.
-
Author contributions
Conceptualization: G.M., L.S., P.P. Writing – Original Draft Preparation: G.M., L.S., P.P. Investigation and Laboratory Evaluation: G.M., L.S., B.P., Al.M., N.V. Clinical Management and Patient Care: G.M., L.S., E.V., A.V., An.M., L.C., D. C., P.P. Writing – Review and Editing: All authors.
-
Source of funding
The study did not receive any source of funding.
-
Ethical approval
Not applicable.
-
Informed consent
The participants of the study have consented to the submission and publication to the present journal. The authors confirm that both participants provided written informed consent for publication of the case report and any accompanying images.
-
Conflict of interest
Authors state no conflict of interest.
-
Use of large language models, AI and machine learning tools
This study did not used large language models, AI and machine learning tools.
-
Data availability statement
Additional data are available upon reasonable request.
References
[1] Mohassel P, Mammen AL. Anti-HMGCR Myopathy. J Neuromuscul Dis. 2018;5:11–20.10.3233/JND-170282Suche in Google Scholar PubMed PubMed Central
[2] Giudizi MG, Cammelli D, Vivarelli E, et al. Anti-HMGCR antibody-associated necrotizing myopathy: diagnosis and treatment illustrated using a case report. Scand J Rheumatol. 2016;45:427–429.10.3109/03009742.2015.1132761Suche in Google Scholar PubMed
[3] Allenbach Y, Mammen AL, Benveniste O, et al. 224th ENMC International Workshop:: Clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul Disord. 2018;28:87–99.10.1016/j.nmd.2017.09.016Suche in Google Scholar PubMed
[4] Landon-Cardinal O, Allenbach Y, Soulages A, et al. Rituximab in the Treatment of Refractory Anti-HMGCR Immune-mediated Necrotizing Myopathy. J Rheumatol. 2019;46:623–627.10.3899/jrheum.171495Suche in Google Scholar PubMed
[5] Silva SP, Eugénio G, Pinto M, et al. Clinical and persistent remission in anti-HMGCR immune-mediated necrotizing myopathy to a single cycle of rituximab - a case-based review.” “Clinical and persistent remission in anti-HMGCR immune-mediated necrotizing myopathy to a single cycle of rituximab – a case-based review. ARP Rheumatol. 2024;3:231–236.10.63032/ZVNO7794Suche in Google Scholar PubMed
[6] Selva-O’Callaghan A, Alvarado-Cardenas M, Pinal-Fernández I, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol. 2018;14:215–224.10.1080/1744666X.2018.1440206Suche in Google Scholar PubMed PubMed Central
[7] Khoo T, Chinoy H. Anti-HMGCR immune-mediated necrotising myopathy: Addressing the remaining issues. Autoimmun Rev. 2023;22:103468.10.1016/j.autrev.2023.103468Suche in Google Scholar PubMed
[8] Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum. 2012;64:4087–4093.10.1002/art.34673Suche in Google Scholar PubMed PubMed Central
[9] Martinez-Lopez D, Corrales Selaya C, Prieto-Peña D, et al. Anti-HMGCR Autoantibody Levels in the Follow-up of Statin-induced Immune-mediated Necrotizing Myopathy: Multicentric Study of 24 Patients [abstract]. Arthritis Rheumatol. 2023;75 (suppl 9). https://acrabstracts.org/abstract/anti-hmgcr-autoantibody-levels-in-the-follow-up-of-statin-induced-immune-mediated-necrotizing-myopathy-multicentric-study-of-24-patients/. Accessed June 5, 2025.10.1136/annrheumdis-2023-eular.2742Suche in Google Scholar
[10] Zhang W, Prince HM, Reardon K. Statin-induced anti-HMGCR antibody-related immune-mediated necrotising myositis achieving complete remission with rituximab. BMJ Case Rep. 2019;12:e232406.10.1136/bcr-2019-232406Suche in Google Scholar PubMed PubMed Central
[11] Gupta S, Rakhra A, Thallapally V, et al. Rituximab use for refractory anti-HMGCR immune-mediated necrotizing myopathy: A case report. Intractable Rare Dis Res. 2021;10:122–125.10.5582/irdr.2020.03144Suche in Google Scholar PubMed PubMed Central
© 2025 Giulia Micheli, Lorenzo Salvati, Boaz Palterer, Emanuele Vivarelli, Alessio Mazzoni, Nila Volpi, Alessandra Vultaggio, Andrea Matucci, Lorenzo Cosmi, Daniele Cammelli, Paola Parronchi, published by De Gruyter on behalf of NCRC-DID
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Editorial
- Anti-CD20 therapy in lupus nephritis: A revisit
- Original Article
- Predicting response to infliximab and interferon-α in Behçet’s syndrome: An exploratory analysis from the BIO-BEHÇET’S randomized controlled trial
- Sirolimus versus mycophenolate mofetil for the treatment of lupus nephritis: Results from a real-world CSTAR cohort study
- Tadalafil plus endothelin receptor antagonists in connective tissue disease-associated pulmonary arterial hypertension: A multicenter study on exercise capacity and cardiac outcomes
- Prevalence of rheumatoid arthritis in China: Variations and trends from the global burden of disease study 2021
- Letter to the Editor
- Rituximab in the treatment of anti-HMGCR immune-mediated necrotizing myopathy: Two cases successfully treated
- Images
- Medusas petrifying gaze: Severe, diffused and refractory calcinosis from a patient with ACA-negative CREST syndrome
Artikel in diesem Heft
- Editorial
- Anti-CD20 therapy in lupus nephritis: A revisit
- Original Article
- Predicting response to infliximab and interferon-α in Behçet’s syndrome: An exploratory analysis from the BIO-BEHÇET’S randomized controlled trial
- Sirolimus versus mycophenolate mofetil for the treatment of lupus nephritis: Results from a real-world CSTAR cohort study
- Tadalafil plus endothelin receptor antagonists in connective tissue disease-associated pulmonary arterial hypertension: A multicenter study on exercise capacity and cardiac outcomes
- Prevalence of rheumatoid arthritis in China: Variations and trends from the global burden of disease study 2021
- Letter to the Editor
- Rituximab in the treatment of anti-HMGCR immune-mediated necrotizing myopathy: Two cases successfully treated
- Images
- Medusas petrifying gaze: Severe, diffused and refractory calcinosis from a patient with ACA-negative CREST syndrome

