Abstract
Neurodegenerative diseases (NDDs) are age-related disorders characterized by progressive neurodegeneration and neuronal cell loss in the central nervous system. Neuropathological conditions such as the accumulation of misfolded proteins can cause neuroinflammation, apoptosis, and synaptic dysfunction in the brain, leading to the development of NDDs including Alzheimer’s disease (AD) and Parkinson’s disease (PD). MicroRNAs (miRNAs) are small noncoding RNA molecules that regulate gene expression post-transcriptionally via RNA interference. Recently, some studies have reported that some miRNAs play an important role in the development of NDDs by regulating target gene expression. MiRNA-485 (miR-485) is a highly conserved brain-enriched miRNA. Accumulating clinical reports suggest that dysregulated miR-485 may be involved in the pathogenesis of AD and PD. Emerging studies have also shown that miR-485 plays a novel role in the regulation of neuroinflammation, apoptosis, and synaptic function in the pathogenesis of NDDs. In this review, we introduce the biological characteristics of miR-485, provide clinical evidence of the dysregulated miR-485 in NDDs, novel roles of miR-485 in neuropathological events, and discuss the potential of targeting miR-485 as a diagnostic and therapeutic marker for NDDs.
Introduction
Neurodegenerative diseases (NDDs) are age-related disorders characterized by progressive neuronal degeneration and neuronal cell death in the central nervous system (CNS) (Grasso et al. 2015; Paul et al. 2020; Sheinerman et al. 2017). The most prevalent NDDs include Alzheimer’s disease (AD) and Parkinson’s disease (PD) (de Lau and Breteler 2006; Grasso et al. 2015; Paul et al. 2020; Querfurth and LaFerla 2010; Reitz and Mayeux 2014). Previous studies have indicated that the etiologies of NDDs include neuropathological conditions such as the accumulation of misfolded proteins, failure of axonal transport, mitochondrial damage, oxidative injury, and altered autophagy (Kim et al. 2015; Millecamps and Julien 2013; Nixon and Yang 2012; Sweeney et al. 2017; Wang et al. 2019b). These pathological conditions can cause neuroinflammation and neuronal cell loss in the CNS, leading to impaired brain function in NDDs (Gcwensa et al. 2021; Grasso et al. 2015; Yu et al. 2021).
MicroRNAs (miRNAs) are endogenous small noncoding single-stranded RNA molecules containing approximately 21–25 nucleotides (Sharma and Lu 2018; Zhang and Bian 2021). It is well-known that miRNAs are involved in modulating the expression of target genes at the post-mRNA transcription level by cutting off the RNA molecules of the target gene, inhibiting target gene translation (Aalaei-Andabili and Rezaei 2016; Winter et al. 2009). Previous studies have demonstrated that miRNAs play important roles in several biological metabolic processes, such as cell growth, proliferation, differentiation, and apoptosis, which are closely related to the development of various diseases, especially tumors (Aalaei-Andabili and Rezaei 2016; Dickson et al. 2013; Hwang and Mendell 2006; Jovanovic and Hengartner 2006; Otto et al. 2017).
Recently, some studies have reported that some miRNAs are widely distributed in the CNS and play an important role in neural development, differentiation, maturation, and synaptic plasticity (Aksoy-Aksel et al. 2014; Cho et al. 2019; Cohen et al. 2011; Sharma and Lu 2018). Significant dysregulation of miR-124 or miR-132 in the brain underlie physiological and pathological processes associated with AD, including dysfunction of synaptic plasticity, accumulation of aggregated proteins in the brain, and cognitive impairment (Fang et al. 2012; Paul et al. 2020; Zhang and Bian 2021). In addition, Yao and colleagues have demonstrated that the downregulated miR-124 expression in the brain induces the upregulation of inflammatory signals through autophagy and microglial activation in the PD brain (Yao et al. 2019). Moreover, many studies showed that the dysfunction of many different miRNAs (e.g., miR-34, miR-132, miR-153) is closely related to the development of pathogenesis in AD and PD (Goh et al. 2019; Wang et al. 2019a). Therefore, miRNAs play important roles in the occurrence and development of neurodegenerative diseases and may be applied as new biomarkers and therapeutic targets for brain diseases.
MiRNA-485 (miR-485) is a highly conserved brain-enriched miRNA (Faghihi et al. 2010; Pasquinelli et al. 2000; Ravanidis et al. 2020). Similar to the roles of other miRNAs, it has been reported that miR-485 is involved in several physiological functions such as metabolism, cell cycle, migration, and differentiation of various types of cancers (Gao et al. 2019; Gu et al. 2020; Lou et al. 2016; Wang et al. 2018). A few recent studies have reported that miR-485 is dysregulated in the brains of patients with AD and PD (Cogswell et al. 2008; Faghihi et al. 2010; Gui et al. 2015) (Koh et al. 2021), which causes neuroinflammation (Lin et al. 2022; Yu et al. 2021) (Koh et al. 2021) and dysfunction of synaptic plasticity (Cohen et al. 2011, 2014; Zolochevska and Taglialatela 2020) (Koh et al. 2021). Rani and colleagues have reported that the expression of circulating miR-485 is correlated with age (Rani et al. 2017) and associated with age-related cognitive decline (Rani et al. 2017). These findings suggest that age-related miR-485 dysregulation plays an important role in the development of neurodegenerative diseases, such as AD and PD. In this review, we summarized the findings of recent studies on the clinical evidence of dysregulated miR-485 in NDDs, the novel roles of miR-485 in the pathogenesis of neurodegenerative diseases, and discussed the potential of targeting miR-485 as a diagnostic and therapeutic marker for NDDs (Figure 1).
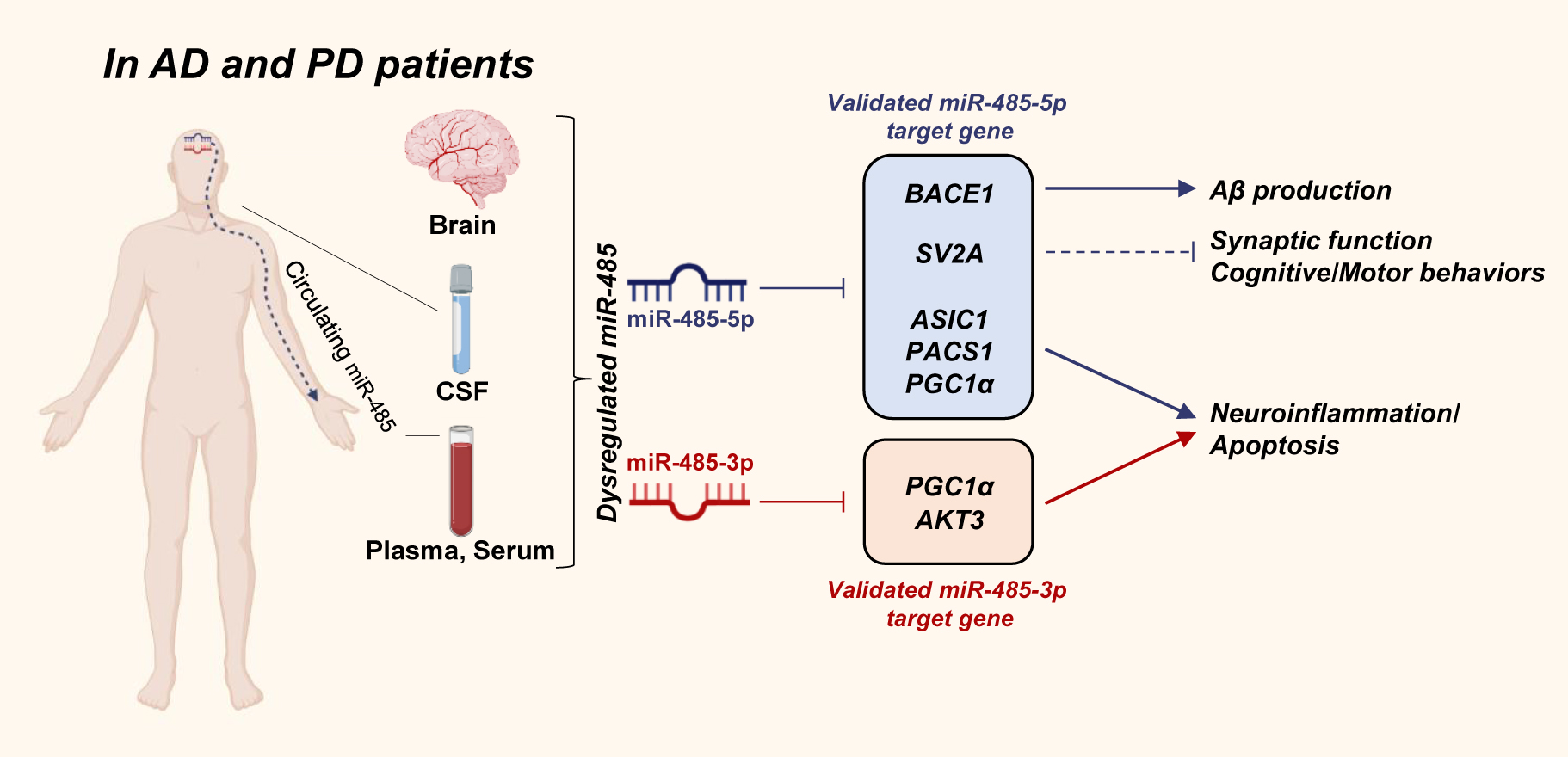
The novel roles of miR-485 in the pathogenesis of Alzheimer’s disease (AD) and Parkinson’s disease (PD).
The dysregulated miR-485 (miR-485-5p or miR-485-3p) is found in the brain, cerebrospinal fluid (CSF), plasma, and serum in the patients with AD and PD. MiR-485 plays role in the regulation of beta-amyloid (Aβ) production, synaptic function, cognitive, and motor behaviors, neuroinflammation, and apoptosis via regulating the validated target genes of miR-485-5p (represented in blue box) and miR-485-3p (represented in red box). BACE1, β-site amyloid precursor protein-cleaving enzyme 1; SV2A, synaptic vesicle glycoprotein 2A; ASIC1, acid-sensing ion channel 1; PACS1, phosphofurin acidic cluster sorting protein 1; PGC1α, peroxisome proliferator-activated receptor-gamma coactivator 1-alpha; AKT3, AKT serine/threonine kinase 3.
Biological characteristics of miR-485
Structure and conservation of miR-485
In humans, the miR-485 gene is located at the 101.055.419–101.055.491 (+) gene region on chromosome 14 at 14q32.31 (Gene ID: 574436) (Formosa et al. 2014; Krokker et al. 2021). Like other miRNAs (Sharma and Lu 2018; Winter et al. 2009), miR-485 is also a small single-stranded protein non-coding RNA molecule that regulates gene expression post-transcriptionally by RNA interference (Lou et al. 2016; Yu et al. 2021). In humans, it has been reported that mature miR-485 is composed of two homologous miRNAs, hsa-miR-485-5p and hsa-miR-485-3p, comprising 22 nucleotides and is processed from a 73-nucleotide sequence of the stem-loop precursor miR-485 (Lou et al. 2016) (miRbase.org).
In the general miRNA biogenesis pathway, mature miRNAs are generated from primary miRNAs/precursor miRNAs by sequential enzymatic cleavage processes (Winter et al. 2009). According to genomic research in mice, the miR-485 gene is encoded within a single transcript, Meg9, which is highly expressed in the mouse brain (Hagan et al. 2009; Lackinger et al. 2019). Hagan and colleagues reported that a truncated transcript, the precursor miR-485, is generated from the Meg9 transcript during alternative RNA splicing of Meg9 transcript to generate a truncated Mirg RNA (Hagan et al. 2009). However, the molecular mechanisms underlying the miR-485-specific biogenesis pathway in the generation of mature miR-485 remain unknown.
Most miRNAs are evolutionally conserved in related species, and some are conserved in invertebrates and vertebrates (Bentwich et al. 2005; Lagos-Quintana et al. 2001; Landgraf et al. 2007; Pasquinelli et al. 2000). Emerging evidence has demonstrated that mature miR-485, miR-485-5p, and miR-485-3p are well conserved and have similar sequences in humans, mice, rats, and other species (Liu et al. 2012). MiR-485-5p and miR-485-3p have well conserved seed sequences (known as GAGGCUGG in miR-485-5p; GUCAUACA in miR-485-3p), which are essential for complementary binding to their target mRNAs (Cohen et al. 2014; Lou et al. 2016). Table 1 shows the conserved miR-485-5p and miR-485-3p sequences in different species.
Conserved sequences of mature miR-485 in different species.
| Species | miR-485-5p sequence | miR-485-3p sequence |
|---|---|---|
| Homo sapiens | A GAGGCUGG CCGUGAUGAAUUC | G UCAUACA CGGCUCUCCUCUCU |
| Mus musculus | A GAGGCUGG CCGUGAUGAAUUC | AG UCAUACA CGGCUCUCCUCUC |
| Rattus norvegicus | A GAGGCUGG CCGUGAUGAAUUC | CAUACA CGGCUCUCCUCUCUUC |
| Macaca mulatta | A GAGGCUGG CCGUGAUGAAUUC | G UCAUACA CGGCUCUCCUCUCU |
| Canis familiaris | A GAGGCUGG CCGUGAUGAAUUCG | Not determined |
| Equus caballus | A GAGGCUGG CCGUGAUGAAUUC | G UCAUACA CGGCUCUCCUCUCU |
| Pongo pygmaeus | A GAGGCUGG CCGUGAUGAAUUC | G UCAUACA CGGCUCUCCUCUCU |
| Ovis aries | A GAGGCUGG CCGUGAUGAAUUCG | G UCAUACA CGGCUCUCCUCUCU |
| Carpa hircus | A GAGGCUGG CCGUGAUGAAUUC | AG UCAUACA CGGCUCUCCUCUCU |
-
Both mature miR-485, miR-485-5p, and miR-485-3p are well conserved and have similar sequences in humans, mice, rats, and other species. Underlined bold text indicates seed sequences of miR-485-5p and miR-485-3p.
Age-related expression of miR-485
Recent studies have demonstrated that miRNAs, such as miR-30d-5p and miR-505-5p, are differentially expressed with age in whole blood, serum, and peripheral blood mononuclear cells (Fehlmann et al. 2020; Freedman et al. 2016; Huan et al. 2018; Noren Hooten et al. 2010; Noren Hooten et al. 2013). Rani et al. reported increased plasma miR-485-3p expression with advancing age, which showed a highly positive correlation with age (Rani et al. 2017). Similarly, miR-485-3p expression in the skin was higher in aged people than in young people (Muther et al. 2017). Other studies have demonstrated the dysregulation of miR-485-3p and miR-485-5p expression in senescent cells (Maes et al. 2009; Suh 2018). Cellular senescence is a key biological process underlying aging (Childs et al. 2015), and it has been suggested that age-dependent differentially expressed miR-485 may accelerate the progression of several age-related diseases.
Brain-enriched miR-485
Brain-enriched miRNAs are defined as miRNAs with the highest expression in the brain. Several studies have demonstrated the high expression of miR-485 in the brain relative to other peripheral regions such as the liver, heart, spleen, kidney, and muscles in humans, mice, and felines (Faghihi et al. 2010; Lagana et al. 2017; Ravanidis et al. 2020). MiR-485-3p is enriched in the gray matter of the brain, which mostly comprises neurons and glial cells (Wang et al. 2011). In addition, Faghihi et al. found that miR-485-5p expression was 2-4-fold higher in the entorhinal cortex, hippocampus, and superior frontal gyrus than in the cerebellum (Faghihi et al. 2010). These findings indicate that miR-485 expression is differentially regulated in a tissue-specific manner, and it may function as region-specific gene regulation in the brain.
Dysregulation of miR-485 in neurodegenerative diseases
Alzheimer’s disease
AD is an irreversible progressive neurodegenerative disease and is one of the most prevalent neurodegenerative diseases globally. AD is considered the most common cause of senile dementia in adults older than 65 years (Querfurth and LaFerla 2010; Reitz and Mayeux 2014). The main neuropathological hallmarks of AD are the accumulation of beta-amyloid (Aβ) oligomers/plaques by amyloidogenic processing and neurofibrillary tangles by abnormally phosphorylated and aggregated forms of tau protein in neurons of the nervous system (Querfurth and LaFerla 2010; Wang et al. 2019a). Previous studies have demonstrated that these pathological changes in AD can lead to long-term memory loss and impairment of cognitive function in patients (Forstl and Kurz 1999; Hector and Brouillette 2020; Jahn 2013; Wang et al. 2019a). Clinical evidence for the dysregulated miR-485 expression in neurodegenerative diseases such as AD and PD is presented in Table 2.
Clinical evidence of dysregulated miR-485 in AD and PD.
| NDDs | miRNAs | Trend | Sample size | Sources | Target mRNAs (validated) | Target mRNAs (predicted) | Possible pathological implications | References |
|---|---|---|---|---|---|---|---|---|
| AD | miR-485-5p | Down | 15 AD, 12 controls | Hippocampus | – | – | – | Cogswell et al. (2008) |
| miR-485-5p | Down | 35 AD, 35 controls | Entorhinal cortex and hippocampus | BACE1 | – | Increased BACE1 mRNA level, increased Aβ production | Faghihi et al. (2010) | |
| miR-485-5p | Down | 42 AD, 7 controls | Prefrontal cortex | – | – | – | Lau et al. (2013) | |
| miR-485-5p | Up | 28 AD, 27 contorls | Exosomes isolated from CSF | – | 42 genes in the neurotrophin signaling pathway, 41 genes in the dopaminergic synapse, 40 genes in cholinergic synapse | – | Gui et al. (2015) | |
| miR-485-5p | Down | 208 AD, 205 controls | Serum | – | – | – | Tan et al. (2014) | |
| miR-485-5p | Up | 4 AD, 5 NDAN, 6 controls | Frontal cortex | – | App, Syn1, Ppp3ca, Mapt, Snap25, Snca, Dnm1 | Increased Aβ oligomer binding | Zolochevska and Taglialatela (2020) | |
| miR-485-3p | Up | 89 AD, 62 controls | Serum | AKT3 | – | Inhibit neuronal cell proliferation Increased neuroinflammation and apoptosis |
Yu et al. (2021) | |
| miR-485-3p | Up | 10 AD, 10 MCI, 10 controls |
Frontal cortex | – | – | – | Weinberg et al. (2015) | |
| miR-485-3p | Up | 1021 AD, 91 VaD, 169 DLB, 32 MCI, 288 controls |
Serum | – | – | – | Shigemizu et al. (2019) | |
| miR-485-3p | Down | 9 AD, 1 control | Gray matter | – | – | – | Wang et al. (2011) | |
| PD | miR-485-5p | Down | 8 PD, 4 controls | Subtantia nigra tissues | – | ADH1C, FUT1 | – | Cardo et al. (2014) |
| miR-485-5p | Up | 47 PD, 27controls | Exosomes isolated from CSF | – | 42 genes in the neurotrophin signaling pathway, 41 genes in the dopaminergic synapse, 40 genes in cholinergic synapse | – | Gui et al. (2015) | |
| miR-485-5p | Down | 65 PD, 70 controls | CSF | – | – | – | Burgos et al. (2014) | |
| miR-485-5p | Down | 32 PD, 32 controls | Plasma | – | – | – | Khoo et al. (2012) | |
| miR-485-5p | Down | 25 PD, 25 controls | Plasma | – | – | – | Chen et al. (2018) | |
| miR-485-5p | Down | 152 PD, 101 controls | Plasma | – | – | – | Ravanidis et al. (2020) | |
| miR-485-3p | Up | 12 PD, 12 controls | Putamen | – | LTA, SLC5A3 | – | Nair and Ge (2016) | |
| miR-485-3p | Up | 92 PD, 64 controls | Serum | – | FBXO45 | Increased neuroinflammation and apoptosis | Lin et al. (2022) |
-
Table shows the clinical evidence for the dysregulated miR-485 expression in various sample sources of the patients with neurodegenerative diseases (NDDs). MiR-485 regulates pathological conditions in NDDs via regulating their validated and predicted target mRNA expression. AD, Alzheimer’s disease; PD, Parkinson’s disease; CSF, cerebrospinal fluid; Down, downregulated; Up, upregulated; NDAN, non-demented individuals with AD pathology; MCI, mild cognitive impairment; VaD, vascular dementia; DLB, dementia with Lewy Bodies.
Cogswell et al. first reported aberrant miR-485 expression in patients with AD using qRT-PCR analysis (Cogswell et al. 2008). According to this clinical study, miR-485-5p expression was significantly decreased in the hippocampus of early stage AD patients relative to age-matched non-demented controls (Cogswell et al. 2008). Two different clinical studies have also demonstrated that miR-485-5p is downregulated in the prefrontal cortex, entorhinal cortex, and hippocampus of AD patients (Faghihi et al. 2010; Lau et al. 2013). Additionally, miR-485-5p expression is reduced in peripheral blood and cerebrospinal fluid (CSF) exosomes in patients with AD (Gui et al. 2015; Tan et al. 2014). These findings indicate that the dysregulation of miR-485-5p in the CSF and brain may be considered one of the novel pathologic features of AD patients, which may be related to the development and progression of AD. Interestingly, a recent study has demonstrated that the dysregulated miR-485-5p expression was observed in the postmortem frontal cortex of AD patients relative to healthy controls, but there was no change in miR-485-5p expression in non-demented individuals with AD pathology (NDAN) patients (Zolochevska and Taglialatela 2020). These findings suggest that the dysregulated miR-485-5p expression may be among the mechanisms responsible for the clinical manifestations and pathological signs and symptoms of patients with AD and NDAN.
Lau et al. studied 49 prefrontal cortex samples from patients with early- and late-stage clinically well-defined AD for the temporal analysis of miRNAs in patients with AD (Lau et al. 2013). They found that miR-485-5p expression was significantly downregulated in AD patients relative to healthy controls. This dynamic change in miR-485-5p expression in the prefrontal cortex showed a gradual downregulation from the early stage of AD to the late stage of AD (Lau et al. 2013). These findings indicate that miR-485-5p expression was gradually decreased with the progression of AD, and it has the potential to be used as a diagnostic biomarker for inferring the severity and stage of a patient through the degree of dysregulation of miR-485-5p.
Recent evidence indicates that Aβ oligomers are initiating factors of AD and play a major role in the induction of neuronal dysfunction in the early stage of AD (Busche et al. 2012; Busche et al. 2015; Hector and Brouillette 2020). During amyloidogenic processing, β−site amyloid precursor protein-cleaving enzyme 1 (BACE1) is a key cleaver of amyloid precursor protein (APP) that plays a pivotal role in Aβ production, leading to the pathogenesis of AD (Querfurth and LaFerla 2010). Emerging evidence has demonstrated that a large number of miRNAs (e.g., miR-29a, miR-107, and miR-124) can combine with the 3′-UTR of BACE1 mRNA and reduce BACE1 protein expression in mice, rats, and humans (Deng et al. 2014; Fang et al. 2012; Hebert et al. 2008; Wang et al. 2008). Using a luciferase reporter assay, Faghihi et al. found that miR-485-5p can strongly bind to the open-reading frame (exon 6) of BACE1 mRNA. The upregulation of miR-485-5p represses BACE1 mRNA translation by 30%, whereas the knockdown of miR-485-5p is associated with an increase in the concentrations of the BACE1 protein in vitro (Faghihi et al. 2010). In addition, treatment of anti-miR molecules for miR-485-5p blocked miRNA binding and reversed the miR-485-5p-mediated BACE1 protein reduction (Faghihi et al. 2010). In this study, the level of miR-485-5p expression was reduced while BACE1 mRNA expression was increased in the entorhinal cortex and hippocampus of AD patients relative to normal older adults (Faghihi et al. 2010). Taken together, these findings indicate that miR-485-5p plays important role in regulating the amyloidogenic pathway by the regulation of BACE1 mRNA expression in the pathogenesis of early stage AD.
In addition, phosphofurin acidic cluster sorting protein 1 (PACS1) is also targeted by miR-485-5p (He et al. 2021). PACS1, a connector protein, can interact directly with acidic clusters; it is involved in the trafficking of integral membrane proteins from the endosomal compartment to the trans-Golgi network (Crump et al. 2001, 2003). A previous study demonstrated that PACS1 participates in amyloidogenesis by regulating the trafficking and processing of the APP in the mouse brain (Burgert et al. 2013; Kottgen et al. 2005). The upregulation of miR-485-5p levels improved pericyte viability and suppressed Aβ-induced pericyte apoptosis of APP/PS1 mice; this ameliorating effect of miR-485-5p on Aβ-induced apoptosis was countervailed by PACS1 overexpression (He et al. 2021). Moreover, in APP/PS1 AD animal model, hippocampal overexpression of miR-485-5p significantly alleviated cognitive deficits and reduced Aβ oligomers in the brain (He et al. 2021). These findings suggest that miR-485-5p may be a crucial mediator for preventing pericytes from apoptosis in AD development via the regulation of PACS1-mediated amyloidogenesis.
It is well-reported that Aβ oligomers can bind to several cell surface proteins, such as EphA4, EphB2, and Sortilin (Dinamarca et al. 2012; Smith and Strittmatter 2017). As the accumulated Aβ oligomer binds to these putative Aβ oligomer receptors, in turn, it activates intracellular signaling cascades that mediate the synaptotoxic effects of Aβ oligomers (Smith and Strittmatter 2017; Spires-Jones and Hyman 2014). Zolochevska and Taglialatela reported that upregulated miR-485-5p significantly decreased the amount of Aβ oligomers associated with the SH-SY5Y cell surface (Zolochevska and Taglialatela 2020). Additionally, the intracerebroventricular injection of the miR-485-5p significantly reduced synaptosome membrane-bound Aβ oligomers in the frontal cortex of mice (Zolochevska and Taglialatela 2020). These findings suggest that miR-485-5p may play a pivotal role in synapse resilience in response to Aβ oligomers binding in the brains of AD patients.
Similar to the observation of dysregulated miR-485-5p expression in AD patients, the dysregulation of miR-485-3p expression in AD patients has been also reported (Koh et al. 2021; Yu et al. 2021). Previous studies demonstrate that miR-485-3p was upregulated in serum and CSF exosomes of AD patients than that of healthy controls (Koh et al. 2021; Shigemizu et al. 2019; Yu et al. 2021). The level of miR-485-3p was significantly upregulated in the postmortem frontal cortex, precentral gyrus of patients with AD, and in SH-SY5Y and BV2 in vitro cell models (Koh et al. 2021; Weinberg et al. 2015; Yu et al. 2021). Consistently, miR-485-3p expression was upregulated in mild cognitive impairment (MCI) patients with positive Aβ positron emission tomography (PET) compared to that in MCI patients with negative Aβ PET (Koh et al. 2021). These findings suggest that the upregulated miR-485-3p expression is closely related to AD pathology such as Aβ plagues accumulation. CD36 is a well-recognized integral microglial cell membrane protein known to mediate phagocytosis of damaged, apoptotic cells, aggregates like Aβ plagues in vitro and in vivo (Grajchen et al. 2020; Zhao et al. 2009). Pharmacological manipulation of CD36 improved microglial Aβ phagocytosis, resulting in Aβ clearance and cognitive improvement in AD mouse models (Dobri et al. 2021; Yamanaka et al. 2012). Consistently, pharmacological inhibition of miR-485-3p significantly increased CD36-mediated Aβ phagocytosis in microglia, leading to decrease Aβ plaques deposition and Aβ aggregation in the hippocampus and cortex of 5XFAD mice (Koh et al. 2021). Additionally, the cognitive decline such as impairment of spatial working memory was improved by miR-485-3p suppression in 5XFAD mice (Koh et al. 2021). These findings suggest that miR-485-3p plays important role in the development of AD pathological phenotypes such as Aβ plaques accumulation and cognitive impairment via regulation of CD36-mediated microglial Aβ phagocytosis.
The Mini-Mental State Examination (MMSE) score is a measure of the degree of dementia, and it has been the most widely used tool for detecting cognitive impairment and dementia severity in patients with AD (Folstein et al. 1975; Henneges et al. 2016). The severity of dementia can be classified as mild, moderate, or severe based on the MMSE score (Folstein et al. 1975; Henneges et al. 2016). Yu and colleagues found that the serum concentration of miR-485-3p was negatively correlated with the MMSE scores of patients with mild, moderate, and severe AD. Additionally, ROC curve analysis of serum miR-485-3p concentrations showed high diagnostic accuracy (cut-off value, 1.435; sensitivity, 84.3%; specificity, 96.8%) for distinguishing between AD patients and healthy controls (Yu et al. 2021). Moreover, the level of miR-485-3p expression in the frontal cortex, precentral gyrus, and CSF are also positively correlated with the level of Aβ plagues accumulation (Koh et al. 2021). These findings suggest that the degree of abnormal expression of circulating miR-485-3p may be used as a diagnostic biomarker for predicting the severity of AD.
Parkinson’s disease
PD is considered a debilitating neurodegenerative disorder in the aging population older than 60 years of age (de Lau and Breteler 2006). The main pathological hallmarks of PD are the formation of Lewy bodies and cytoplasmic inclusions of α-synuclein (SNCA)–ubiquitin complex, which cannot be degraded by the proteasome (Ibáñez et al. 2004). Abnormal deposition of SNCA within the substantia nigra of the brain causes degeneration of dopaminergic neurons (Hallett et al. 2019; Zheng et al. 2010), resulting in motor dysfunction such as bradykinesia, rigidity, resting tremor, and postural instability (Jankovic 2008). The etiology of PD is a complex combination of brain aging, environmental causes, and genetic causes, such as mutations in SNCA (Coppede 2012).
Numerous studies have demonstrated that the dysregulation of miRNAs such as miR-19, miR-29, and miR-30 in the brain, CSF, and blood contribute to PD pathogenesis (Briggs et al. 2015; Burgos et al. 2014; Goh et al. 2019; Gui et al. 2015; Ma et al. 2016; Tatura et al. 2016). Recently, the dysregulation of miR-485 expression was also reported in patients with PD (Burgos et al. 2014; Cardo et al. 2014; Chen et al. 2018; Gui et al. 2015; Khoo et al. 2012; Nair and Ge 2016; Ravanidis et al. 2020). Using a TaqMan low-density array, Cardo et al. reported that miR-485-5p was downregulated in the substantia nigra of the postmortem human brain of PD patients (Cardo et al. 2014). Similar to human brain tissue, miR-485-5p was found to be downregulated in the plasma samples of patients with PD (Chen et al. 2018; Khoo et al. 2012). Consistent with these findings, Ravanidis and colleagues have reported that the plasma concentration of miR-485-5p was downregulated in patients with different types of PD, including idiopathic PD (iPD), GBA-PD (mutations in glucocerebrosidase gene), and SNCAA53T-PD (point mutation in SNCA) (Ravanidis et al. 2020). Taken together, it could be suggested that the dysregulated miR-485-5p expression is one of the hallmarks of PD patients and it may be related to PD pathogenesis.
Ravanidis et al. profiled the demographic and clinical characteristics of iPD, GBA-PD, and SNCAA53T-PD patients and healthy controls. The age of disease onset and the average age at examination were significantly lower for SNCAA53T-PD patients than for iPD and GBA-PD patients (Ravanidis et al. 2020). However, there was no difference in the MMSE scores among the patients with PD. In the changes in miR-485-5p expression, the miR-485-5p expression in the plasma of SNCAA53T-PD patients was significantly decreased relative to that in iPD patients (Ravanidis et al. 2020). These findings suggest that the dysregulation of miR-485-5p may play a role in the pathophysiological processes underlying genetic PD linked to synucleinopathies.
However, a conflicting trend in miR-485-5p expression was observed in CSF samples. Burgos et al. have demonstrated the downregulation of miR-485-5p in the CSF of PD patients (Burgos et al. 2014), while Gui et al. showed that the concentration of miR-485-5p was upregulated in the exosomes isolated from the CSF of PD patients (Gui et al. 2015). These findings suggest that the dysregulation of miR-485-5p may be used as a diagnostic biomarker for PD; however, further studies are needed to clarify the conflicting trend of miR-485 expression.
In contrast, two different clinical studies have demonstrated increased miR-485-3p expression in patients with PD. Using the NanoString nCounter microRNA assay, Nair and Ge showed that miR-485-3p expression was upregulated in the putamen of patients with PD relative to healthy controls (Nair and Ge 2016). Consistently, the serum expression of miR-485-3p was significantly increased in different cohorts of patients with PD relative to healthy controls (Lin et al. 2022). ROC curve analysis of serum miR-485-3p concentrations showed high diagnostic accuracy (cut-off value, 1.055; sensitivity, 86.96%; specificity, 85.94%) in differentiating PD patients from healthy controls (Lin et al. 2022). These findings suggest that the dysregulated miR-485-3p may serve as a diagnostic biomarker for distinguishing PD patients from healthy controls.
Role of miR-485 in neuroinflammation and apoptosis in neurodegenerative diseases
As previously demonstrated, neuroinflammation and neuronal cell death are major hallmarks of neurodegenerative diseases, including AD and PD (Ekshyyan and Aw 2004; Kwon and Koh 2020; Mattson 2000). Under neuropathological conditions, microglia produce pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6 (Kwon and Koh 2020). Recent studies have demonstrated that the dysregulated miR-485 regulates neuroinflammatory responses and apoptotic pathways in neuronal diseases, including NDDs (He et al. 2021; Lin et al. 2022; Xu et al. 2020; Yu et al. 2021).
MiR-485-5p can target acid-sensing ion channel 1 (ASIC1), a voltage-insensitive proton-gated cation channel that is highly expressed in the CNS (Rhoades et al. 2019; Xu et al. 2020). It has been demonstrated that ASIC1 is extensively involved in neurological and neurodegenerative diseases, such as AD and PD (Gonzales and Sumien 2017; Joch et al. 2007; Komnig et al. 2016; Mango and Nistico 2018; Ortega-Ramirez et al. 2017; Ruan et al. 2021). In primary cultured microglia, the upregulated ASIC1 induced neuroinflammatory responses including releasing cytokines (Yu et al. 2015). Especially, the regulation of mitochondrial permeability transition pores by mitochondrial ASIC1 is closely related to oxidative neuronal cell death in PD model (Wang et al. 2013). In an animal model of inflammatory pain, the overexpression of miR-485-5p significantly decreased ASIC1 expression and alleviated inflammatory pain response (Xu et al. 2020). These findings indicate that miR-485-5p may serve as a regulator in ASIC1-mediated neuroinflammation and apoptosis. Some clinical studies have demonstrated that miR-485-5p is downregulated in AD and PD patients (Burgos et al. 2014; Cardo et al. 2014; Chen et al. 2018; Faghihi et al. 2010; Gui et al. 2015; Khoo et al. 2012; Lau et al. 2013; Nair and Ge 2016; Ravanidis et al. 2020; Tan et al. 2014). Taken together, it could be speculated that ASIC1-mediated inflammatory responses and apoptosis may be involved in the pathogenesis of AD and PD in patients whose miR-485-5p is downregulated.
Yu et al. demonstrated that the concentrations of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 were significantly increased in the serum of AD patients, which were positively correlated with serum miR-485-3p expression (Yu et al. 2021). In a cellular model of AD and PD, miR-485-3p overexpression enhanced LPS-induced pro-inflammatory cytokine release in microglial BV2 cells (Lin et al. 2022). The silencing of miR-485-3p significantly alleviated the Aβ-induced pro-inflammatory responses in microglial BV2 cells and apoptosis in SH-5YSY cells, respectively. A subsequent luciferase assay showed that AKT3 directly targets miR-485-3p (Yu et al. 2021). AKT3, one of serine/threonine protein kinases, is involved in a wide variety of biological processes including cell proliferation, neuroinflammatory responses, and apoptosis (DuBois et al. 2019; Wang et al. 2017). Taken together, these findings suggest that miR-485-3p may regulate neuroinflammatory responses and neuronal cell viability in the pathogenesis of AD and PD via the negative regulation of the AKT3 signaling pathway.
MiR-485-5p and miR-485-3p can target the two different seed sequences of peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC1α), respectively, and can suppress PGC1α expression (Lou et al. 2016). PGC1α is involved in various cellular processes associated with inflammatory signaling, oxidative stress, and mitochondrial dysfunction (Sweeney and Song 2016). In the brain, the impairment of PGC1α activity triggers mitochondrial dysfunction, leading to neuronal degeneration. In AD pathology, hippocampal PGC1α promotes non-amyloidogenic processing of APP protein, precluding the generation of amyloidogenic Aβ peptides (Qin et al. 2009). In PD pathology, PGC1α knockout mice showed increased SNCA accumulation and increased vulnerability to MPTP-induced degeneration of nigral dopamine neurons (Ciron et al. 2015; St-Pierre et al. 2006). MiR-485-5p or miR-485-3p, as a suppressor of PGC1α, may play important roles in PGC1α-mediated inflammation and mitochondrial dysfunction in the pathogenesis of AD and PD.
Taken together, these studies demonstrated that miR-485 is able to regulate the expression of various inflammation- and apoptosis-related molecules, and they may play key roles in the regulation of inflammatory responses and apoptotic processes in the brain of AD and PD patients.
MiR-485-mediated synapse loss and synaptic dysfunction in neurodegenerative diseases
Synapses are functional units of the nervous system and are essential for the transmission of nervous impulses from one neuron (generally called a presynaptic neuron) to another neuron (generally called a postsynaptic neuron) (Hu and Li 2017). The development and structural plasticity of synapses, which are temporally and spatially controlled by gene expression, is required to regulate synaptic functions (Hu and Li 2017). Accumulating evidence suggests that synaptic impairments and synapse loss occur at the early stage of AD and PD and precede neuronal cell death, which causes a cognitive decline in AD and movement impairment in PD (Gcwensa et al. 2021; Selkoe 2002; Zolochevska and Taglialatela 2020).
Recent studies have shown that miR-485 regulates synaptic morphology and plasticity. One of the presynaptic proteins, synaptic vesicle glycoprotein 2A (SV2A), is located in synaptic vesicles at presynaptic terminals and is involved in neurotransmitter release such as dopamine (Bajjalieh et al. 1994; Dunn et al. 2017; Heurling et al. 2019). According to previous studies, SV2A expression in the brain has shown a significant loss in patients with AD and PD, which are associated with impaired global cognition and mild motor deficits, respectively (Dunn et al. 2017; Heurling et al. 2019; Mecca et al. 2020; Robinson et al. 2014). In hippocampal neurons, miR-485-5p can target SV2A and regulate SV2A transcript abundance and protein expression (Cohen et al. 2011). Especially, miR-485-5p regulated the number of SV2A proteins in individual presynaptic terminals (Cohen et al. 2011). These findings indicate that miR-485-5p can control neurotransmitter release in AD and PD patients by regulating a ubiquitous presynaptic protein SV2A.
In addition, miR-485-5p regulates dendritic spine density, postsynaptic density protein 95 (PSD-95) clustering, and surface expression of AMPA receptors containing the GluR2 subunit at the synaptic plasma membrane, and miniature excitatory postsynaptic current frequency in cultured hippocampal neurons (Cohen et al. 2011). MiR-485-5p also regulates nerve growth factor-dependent neurite outgrowth in PC12 cells, tau mRNA/protein concentrations, and the extent of axonal outgrowth in hippocampal neurons (Cohen et al. 2014). Furthermore, the levels of presynaptic synaptophysin and PSD-95 protein expression were reduced in miR-485-3p-transduced primary neurons (Koh et al. 2021). Taken together, these findings suggest that miR-485 is involved in structural and morphological changes in pre- and post-synaptic neurons, and it plays a crucial role in the synaptic functional alterations on behavioral impairment in AD or PD patients.
MiR-485 as potential biomarker and therapeutic target in NDDs
The etiology and pathogenesis of NDDs including AD are not completely clear, but it is certain that progressive neuronal degeneration causes irreversible damage to the brain of NDDs. Since treatment option and recovery are very limited in the late stage of NDDs, early diagnosis is very important. However, there are no effective early diagnostic methods in NDDs, yet (Cordell et al. 2013). It has been demonstrated that the dysregulation of miR-485-5p or miR-485-3p expression is commonly found in plasma, CSF, and postmortem tissues of the brain in patients with NDDs, including AD and PD (Figure 1 and Table 2). As demonstrated above, the concentrations of circulating miR-485-5p and miR-485-3p in the plasma are strongly associated with the symptoms of AD and PD (Chen et al. 2018; Khoo et al. 2012; Koh et al. 2021; Ravanidis et al. 2020; Yu et al. 2021). Therefore, miR-485-5p and miR-485-3p in peripheral circulation may serve as novel diagnostic biomarkers for NDDs. Since peripheral blood collection in repeatedly is clinically less invasive and more convenient than CSF collection, it is suitable for use as a sample specimen for early diagnosis of NDDs.
Currently, many of miRNA-based treatment strategies such as miRNA antisense technology and miRNA replacement therapy have been developed (Srivastava et al. 2016; Titze-de-Almeida et al. 2020). It is well-known that the antisense technology (e.g., antisense oligonucleotide) is widely used to inhibit expression of target miRNA and relevant proteins which involving in development of diseases (Lima et al. 2018). Conversely, the miRNA replacement therapy (e.g., miRNA mimic) has been developed to promote RNA-induced silencing complex and reduce target mRNA expression levels (Lykhmus et al. 2016). Based on these miRNA-based therapies, mimicking miR-485 alleviates the miR-485 target (e.g., AKT3 or PGC1α)-mediated neuroinflammation and apoptosis in NDDs, suggesting that miR-485-5p or miR-485-3p may be used as therapeutic targets for NDDs via improved miRNA delivery methods in clinical trials.
Conclusions and future directions
In this review, we summarized the 1) biological characteristics of miR-485, 2) clinical evidence of the involvement of abnormal miR-485 expression in NDDs, 3) novel roles of miR-485 in neuroinflammation, apoptosis, and synaptic dysfunction in the pathogenesis of NDDs, and 4) potential for used as biomarker and therapeutic target in NDDs. However, further studies are needed to clarify its biological properties in NDDs such as specific biogenesis pathway of miR-485, relative expression of miR-485-5p/miR-485-3p, and gene target network analysis by in silico approaches. Moreover, advanced clinical and molecular data based on a large cohort are needed to develop new strategies for prognostic, diagnostic, and therapeutic application of miR-485 for aging and age-related NDDs.
Acknowledgments
We thank members of the Biorchestra Corporation for their comments and suggestions, and we would like to thank Editage (www.editage.co.kr) for English language editing.
-
Author contributions: Conceptualization, H.-J.C. and J.-H.R.; writing – original draft preparation, I.S.R.; writing – review and editing, I.S.R., D.H.K., H.-J.C.; supervision, J.-H.R. All authors have read and agreed to responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare that there is no conflict of interest.
References
Aalaei-Andabili, S.H. and Rezaei, N. (2016). MicroRNAs (MiRs) precisely regulate immune system development and function in immunosenescence process. Int. Rev. Immunol. 35: 57–66, https://doi.org/10.3109/08830185.2015.1077828.Suche in Google Scholar PubMed
Aksoy-Aksel, A., Zampa, F., and Schratt, G. (2014). MicroRNAs and synaptic plasticity--a mutual relationship. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, https://doi.org/10.1098/rstb.2013.0515.Suche in Google Scholar PubMed PubMed Central
Bajjalieh, S.M., Frantz, G.D., Weimann, J.M., McConnell, S.K., and Scheller, R.H. (1994). Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J. Neurosci. 14: 5223–5235, https://doi.org/10.1523/jneurosci.14-09-05223.1994.Suche in Google Scholar PubMed PubMed Central
Bentwich, I., Avniel, A., Karov, Y., Aharonov, R., Gilad, S., Barad, O., Barzilai, A., Einat, P., Einav, U., Meiri, E., et al.. (2005). Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 37: 766–770, https://doi.org/10.1038/ng1590.Suche in Google Scholar PubMed
Briggs, C.E., Wang, Y., Kong, B., Woo, T.U., Iyer, L.K., and Sonntag, K.C. (2015). Midbrain dopamine neurons in Parkinson’s disease exhibit a dysregulated miRNA and target-gene network. Brain Res. 1618: 111–121, https://doi.org/10.1016/j.brainres.2015.05.021.Suche in Google Scholar PubMed PubMed Central
Burgert, T., Schmidt, V., Caglayan, S., Lin, F., Fuchtbauer, A., Fuchtbauer, E.M., Nykjaer, A., Carlo, A.S., and Willnow, T.E. (2013). SORLA-dependent and -independent functions for PACS1 in control of amyloidogenic processes. Mol. Cell Biol. 33: 4308–4320, https://doi.org/10.1128/mcb.00628-13.Suche in Google Scholar
Burgos, K., Malenica, I., Metpally, R., Courtright, A., Rakela, B., Beach, T., Shill, H., Adler, C., Sabbagh, M., Villa, S., et al.. (2014). Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer’s and Parkinson’s diseases correlate with disease status and features of pathology. PLoS One 9: e94839, https://doi.org/10.1371/journal.pone.0094839.Suche in Google Scholar PubMed PubMed Central
Busche, M.A., Chen, X., Henning, H.A., Reichwald, J., Staufenbiel, M., Sakmann, B., and Konnerth, A. (2012). Critical role of soluble amyloid-beta for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 109: 8740–8745, https://doi.org/10.1073/pnas.1206171109.Suche in Google Scholar PubMed PubMed Central
Busche, M.A., Grienberger, C., Keskin, A.D., Song, B., Neumann, U., Staufenbiel, M., Forstl, H., and Konnerth, A. (2015). Decreased amyloid-beta and increased neuronal hyperactivity by immunotherapy in Alzheimer’s models. Nat. Neurosci. 18: 1725–1727, https://doi.org/10.1038/nn.4163.Suche in Google Scholar PubMed
Cardo, L.F., Coto, E., Ribacoba, R., Menendez, M., Moris, G., Suarez, E., and Alvarez, V. (2014). MiRNA profile in the substantia nigra of Parkinson’s disease and healthy subjects. J. Mol. Neurosci. 54: 830–836, https://doi.org/10.1007/s12031-014-0428-y.Suche in Google Scholar PubMed
Chen, L., Yang, J., Lu, J., Cao, S., Zhao, Q., and Yu, Z. (2018). Identification of aberrant circulating miRNAs in Parkinson’s disease plasma samples. Brain Behav. 8: e00941, https://doi.org/10.1002/brb3.941.Suche in Google Scholar PubMed PubMed Central
Childs, B.G., Durik, M., Baker, D.J., and van Deursen, J.M. (2015). Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 21: 1424–1435, https://doi.org/10.1038/nm.4000.Suche in Google Scholar PubMed PubMed Central
Cho, K.H.T., Xu, B., Blenkiron, C., and Fraser, M. (2019). Emerging roles of miRNAs in brain development and perinatal brain injury. Front. Physiol. 10: 227, https://doi.org/10.3389/fphys.2019.00227.Suche in Google Scholar PubMed PubMed Central
Ciron, C., Zheng, L., Bobela, W., Knott, G.W., Leone, T.C., Kelly, D.P., and Schneider, B.L. (2015). PGC-1alpha activity in nigral dopamine neurons determines vulnerability to alpha-synuclein. Acta Neuropathol. Commun. 3: 16, https://doi.org/10.1186/s40478-015-0200-8.Suche in Google Scholar PubMed PubMed Central
Cogswell, J.P., Ward, J., Taylor, I.A., Waters, M., Shi, Y., Cannon, B., Kelnar, K., Kemppainen, J., Brown, D., Chen, C., et al.. (2008). Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J. Alzheim. Dis. 14: 27–41, https://doi.org/10.3233/jad-2008-14103.Suche in Google Scholar PubMed
Cohen, J.E., Lee, P.R., Chen, S., Li, W., and Fields, R.D. (2011). MicroRNA regulation of homeostatic synaptic plasticity. Proc. Natl. Acad. Sci. U. S. A. 108: 11650–11655, https://doi.org/10.1073/pnas.1017576108.Suche in Google Scholar PubMed PubMed Central
Cohen, J.E., Lee, P.R., and Fields, R.D. (2014). Systematic identification of 3’-UTR regulatory elements in activity-dependent mRNA stability in hippocampal neurons. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369: 20130509, https://doi.org/10.1098/rstb.2013.0509.Suche in Google Scholar PubMed PubMed Central
Coppede, F. (2012). Genetics and epigenetics of Parkinson’s disease. Sci. World J. 2012: 489830, https://doi.org/10.1100/2012/489830.Suche in Google Scholar PubMed PubMed Central
Cordell, C.B., Borson, S., Boustani, M., Chodosh, J., Reuben, D., Verghese, J., Thies, W., and Fried, L.B., and Medicare Detection of Cognitive Impairment, W. (2013). Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimer’s Dementia 9: 141–150, https://doi.org/10.1016/j.jalz.2012.09.011.Suche in Google Scholar PubMed
Crump, C.M., Hung, C.H., Thomas, L., Wan, L., and Thomas, G. (2003). Role of PACS-1 in trafficking of human cytomegalovirus glycoprotein B and virus production. J. Virol. 77: 11105–11113, https://doi.org/10.1128/jvi.77.20.11105-11113.2003.Suche in Google Scholar PubMed PubMed Central
Crump, C.M., Xiang, Y., Thomas, L., Gu, F., Austin, C., Tooze, S.A., and Thomas, G. (2001). PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 20: 2191–2201, https://doi.org/10.1093/emboj/20.9.2191.Suche in Google Scholar PubMed PubMed Central
de Lau, L.M.L. and Breteler, M.M.B. (2006). Epidemiology of Parkinson’s disease. Lancet Neurol. 5: 525–535, https://doi.org/10.1016/s1474-4422(06)70471-9.Suche in Google Scholar PubMed
Deng, Y., Ding, Y., and Hou, D. (2014). Research status of the regulation of miRNA on BACE1. Int. J. Neurosci. 124: 474–477, https://doi.org/10.3109/00207454.2013.858249.Suche in Google Scholar PubMed
Dickson, J.R., Kruse, C., Montagna, D.R., Finsen, B., and Wolfe, M.S. (2013). Alternative polyadenylation and miR-34 family members regulate tau expression. J. Neurochem. 127: 739–749, https://doi.org/10.1111/jnc.12437.Suche in Google Scholar PubMed PubMed Central
Dinamarca, M.C., Rios, J.A., and Inestrosa, N.C. (2012). Postsynaptic receptors for amyloid-beta oligomers as mediators of neuronal damage in Alzheimer’s disease. Front. Physiol. 3: 464, https://doi.org/10.3389/fphys.2012.00464.Suche in Google Scholar PubMed PubMed Central
Dobri, A.M., Dudau, M., Enciu, A.M., and Hinescu, M.E. (2021). CD36 in Alzheimer’s disease: an overview of molecular mechanisms and therapeutic targeting. Neuroscience 453: 301–311, https://doi.org/10.1016/j.neuroscience.2020.11.003.Suche in Google Scholar PubMed
DuBois, J.C., Ray, A.K., Gruber, R.C., Zhang, Y., Aflakpui, R., Macian-Juan, F., and Shafit-Zagardo, B. (2019). Akt3-Mediated protection against inflammatory Demyelinating disease. Front. Immunol. 10: 1738, https://doi.org/10.3389/fimmu.2019.01738.Suche in Google Scholar PubMed PubMed Central
Dunn, A.R., Stout, K.A., Ozawa, M., Lohr, K.M., Hoffman, C.A., Bernstein, A.I., Li, Y., Wang, M., Sgobio, C., Sastry, N., et al.. (2017). Synaptic vesicle glycoprotein 2C (SV2C) modulates dopamine release and is disrupted in Parkinson disease. Proc. Natl. Acad. Sci. U. S. A. 114: E2253–E2262, https://doi.org/10.1073/pnas.1616892114.Suche in Google Scholar PubMed PubMed Central
Ekshyyan, O. and Aw, T.Y. (2004). Apoptosis: a key in neurodegenerative disorders. Curr. Neurovascular Res. 1: 355–371, https://doi.org/10.2174/1567202043362018.Suche in Google Scholar PubMed
Faghihi, M.A., Zhang, M., Huang, J., Modarresi, F., Van der Brug, M.P., Nalls, M.A., Cookson, M.R., St-Laurent, G.3rd, and Wahlestedt, C. (2010). Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol. 11: R56, https://doi.org/10.1186/gb-2010-11-5-r56.Suche in Google Scholar PubMed PubMed Central
Fang, M., Wang, J., Zhang, X., Geng, Y., Hu, Z., Rudd, J.A., Ling, S., Chen, W., and Han, S. (2012). The miR-124 regulates the expression of BACE1/beta-secretase correlated with cell death in Alzheimer’s disease. Toxicol. Lett. 209: 94–105, https://doi.org/10.1016/j.toxlet.2011.11.032.Suche in Google Scholar PubMed
Fehlmann, T., Lehallier, B., Schaum, N., Hahn, O., Kahraman, M., Li, Y., Grammes, N., Geffers, L., Backes, C., Balling, R., et al.. (2020). Common diseases alter the physiological age-related blood microRNA profile. Nat. Commun. 11: 5958, https://doi.org/10.1038/s41467-020-19665-1.Suche in Google Scholar PubMed PubMed Central
Folstein, M.F., Folstein, S.E., and McHugh, P.R. (1975). Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12: 189–198, https://doi.org/10.1016/0022-3956(75)90026-6.Suche in Google Scholar PubMed
Formosa, A., Markert, E.K., Lena, A.M., Italiano, D., Finazzi-Agro, E., Levine, A.J., Bernardini, S., Garabadgiu, A.V., Melino, G., and Candi, E. (2014). MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c, miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p, mapped to the 14q32.31 locus, regulate proliferation, apoptosis, migration and invasion in metastatic prostate cancer cells. Oncogene 33: 5173–5182, https://doi.org/10.1038/onc.2013.451.Suche in Google Scholar PubMed
Forstl, H. and Kurz, A. (1999). Clinical features of Alzheimer’s disease. Eur. Arch. Psychiatr. Clin. Neurosci. 249: 288–290, https://doi.org/10.1007/s004060050101.Suche in Google Scholar PubMed
Freedman, J.E., Gerstein, M., Mick, E., Rozowsky, J., Levy, D., Kitchen, R., Das, S., Shah, R., Danielson, K., Beaulieu, L., et al.. (2016). Diverse human extracellular RNAs are widely detected in human plasma. Nat. Commun. 7: 11106, https://doi.org/10.1038/ncomms11106.Suche in Google Scholar PubMed PubMed Central
Gao, F., Wu, H., Wang, R., Guo, Y., Zhang, Z., Wang, T., Zhang, G., Liu, C., and Liu, J. (2019). MicroRNA-485-5p suppresses the proliferation, migration and invasion of small cell lung cancer cells by targeting flotillin-2. Bioengineered 10: 1–12, https://doi.org/10.1080/21655979.2019.1586056.Suche in Google Scholar PubMed PubMed Central
Gcwensa, N.Z., Russell, D.L., Cowell, R.M., and Volpicelli-Daley, L.A. (2021). Molecular mechanisms underlying synaptic and Axon degeneration in Parkinson’s disease. Front. Cell. Neurosci. 15: 626128, https://doi.org/10.3389/fncel.2021.626128.Suche in Google Scholar PubMed PubMed Central
Goh, S.Y., Chao, Y.X., Dheen, S.T., Tan, E.K., and Tay, S.S. (2019). Role of MicroRNAs in Parkinson’s disease. Int. J. Mol. Sci. 20: 5649, https://doi.org/10.3390/ijms20225649.Suche in Google Scholar PubMed PubMed Central
Gonzales, E.B. and Sumien, N. (2017). Acidity and acid-sensing ion channels in the normal and Alzheimer’s disease brain. J. Alzheim. Dis. 57: 1137–1144, https://doi.org/10.3233/jad-161131.Suche in Google Scholar PubMed
Grajchen, E., Wouters, E., van de Haterd, B., Haidar, M., Hardonniere, K., Dierckx, T., Van Broeckhoven, J., Erens, C., Hendrix, S., Kerdine-Romer, S., et al.. (2020). CD36-mediated uptake of myelin debris by macrophages and microglia reduces neuroinflammation. J. Neuroinflammation 17: 224, https://doi.org/10.1186/s12974-020-01899-x.Suche in Google Scholar PubMed PubMed Central
Grasso, M., Piscopo, P., Crestini, A., Confaloni, A., and Denti, M.A. (2015). Circulating microRNAs in neurodegenerative diseases. Exper. Suppl. 106: 151–169.10.1007/978-3-0348-0955-9_7Suche in Google Scholar PubMed
Gu, J., Shao, R., Li, M., Yan, Q., and Hu, H. (2020). MiR-485-3p modulates neural stem cell differentiation and proliferation via regulating TRIP6 expression. J. Cell Mol. Med. 24: 398–404, https://doi.org/10.1111/jcmm.14743.Suche in Google Scholar PubMed PubMed Central
Gui, Y., Liu, H., Zhang, L., Lv, W., and Hu, X. (2015). Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 6: 37043–37053, https://doi.org/10.18632/oncotarget.6158.Suche in Google Scholar PubMed PubMed Central
Hagan, J.P., O’Neill, B.L., Stewart, C.L., Kozlov, S.V., and Croce, C.M. (2009). At least ten genes define the imprinted Dlk1-Dio3 cluster on mouse chromosome 12qF1. PLoS One 4: e4352, https://doi.org/10.1371/journal.pone.0004352.Suche in Google Scholar PubMed PubMed Central
Hallett, P.J., Engelender, S., and Isacson, O. (2019). Lipid and immune abnormalities causing age-dependent neurodegeneration and Parkinson’s disease. J. Neuroinflammation 16: 153, https://doi.org/10.1186/s12974-019-1532-2.Suche in Google Scholar PubMed PubMed Central
He, C., Su, C., Zhang, W., and Wan, Q. (2021). miR-485-5p alleviates Alzheimer’s disease progression by targeting PACS1. Transl. Neurosci. 12: 335–345, https://doi.org/10.1515/tnsci-2020-0177.Suche in Google Scholar PubMed PubMed Central
Hebert, S.S., Horre, K., Nicolai, L., Papadopoulou, A.S., Mandemakers, W., Silahtaroglu, A.N., Kauppinen, S., Delacourte, A., and De Strooper, B. (2008). Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc. Natl. Acad. Sci. U. S. A. 105: 6415–6420, https://doi.org/10.1073/pnas.0710263105.Suche in Google Scholar PubMed PubMed Central
Hector, A. and Brouillette, J. (2020). Hyperactivity induced by soluble amyloid-beta oligomers in the early stages of Alzheimer’s disease. Front. Mol. Neurosci. 13: 600084, https://doi.org/10.3389/fnmol.2020.600084.Suche in Google Scholar PubMed PubMed Central
Henneges, C., Reed, C., Chen, Y.F., Dell’Agnello, G., and Lebrec, J. (2016). Describing the sequence of cognitive decline in Alzheimer’s disease patients: results from an observational study. J. Alzheim. Dis. 52: 1065–1080, https://doi.org/10.3233/jad-150852.Suche in Google Scholar PubMed PubMed Central
Heurling, K., Ashton, N.J., Leuzy, A., Zimmer, E.R., Blennow, K., Zetterberg, H., Eriksson, J., Lubberink, M., and Scholl, M. (2019). Synaptic vesicle protein 2A as a potential biomarker in synaptopathies. Mol. Cell. Neurosci. 97: 34–42, https://doi.org/10.1016/j.mcn.2019.02.001.Suche in Google Scholar PubMed
Hu, Z. and Li, Z. (2017). miRNAs in synapse development and synaptic plasticity. Curr. Opin. Neurobiol. 45: 24–31, https://doi.org/10.1016/j.conb.2017.02.014.Suche in Google Scholar PubMed PubMed Central
Huan, T., Chen, G., Liu, C., Bhattacharya, A., Rong, J., Chen, B.H., Seshadri, S., Tanriverdi, K., Freedman, J.E., Larson, M.G., et al.. (2018). Age-associated microRNA expression in human peripheral blood is associated with all-cause mortality and age-related traits. Aging Cell 17: e12687, https://doi.org/10.1111/acel.12687.Suche in Google Scholar PubMed PubMed Central
Hwang, H.W. and Mendell, J.T. (2006). MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br. J. Cancer 94: 776–780, https://doi.org/10.1038/sj.bjc.6603023.Suche in Google Scholar PubMed PubMed Central
Ibáñez, P., Bonnet, A.M., Débarges, B., Lohmann, E., Tison, F., Agid, Y., Dürr, A., Brice, A., and Pollak, P. (2004). Causal relation between α-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364: 1169–1171, https://doi.org/10.1016/s0140-6736(04)17104-3.Suche in Google Scholar PubMed
Jahn, H. (2013). Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci. 15: 445–454, https://doi.org/10.31887/dcns.2013.15.4/hjahn.Suche in Google Scholar
Jankovic, J. (2008). Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 79: 368–376, https://doi.org/10.1136/jnnp.2007.131045.Suche in Google Scholar PubMed
Joch, M., Ase, A.R., Chen, C.X., MacDonald, P.A., Kontogiannea, M., Corera, A.T., Brice, A., Seguela, P., and Fon, E.A. (2007). Parkin-mediated monoubiquitination of the PDZ protein PICK1 regulates the activity of acid-sensing ion channels. Mol. Biol. Cell 18: 3105–3118, https://doi.org/10.1091/mbc.e05-11-1027.Suche in Google Scholar PubMed PubMed Central
Jovanovic, M. and Hengartner, M.O. (2006). miRNAs and apoptosis: RNAs to die for. Oncogene 25: 6176–6187, https://doi.org/10.1038/sj.onc.1209912.Suche in Google Scholar PubMed
Khoo, S.K., Petillo, D., Kang, U.J., Resau, J.H., Berryhill, B., Linder, J., Forsgren, L., Neuman, L.A., and Tan, A.C. (2012). Plasma-based circulating MicroRNA biomarkers for Parkinson’s disease. J. Parkinsons Dis. 2: 321–331, https://doi.org/10.3233/jpd-012144.Suche in Google Scholar PubMed
Kim, G.H., Kim, J.E., Rhie, S.J., and Yoon, S. (2015). The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 24: 325–340, https://doi.org/10.5607/en.2015.24.4.325.Suche in Google Scholar PubMed PubMed Central
Koh, H.S., Lee, S., Lee, H.J., Min, J.W., Iwatsubo, T., Teunissen, C.E., Cho, H.J., and Ryu, J.H. (2021). Targeting MicroRNA-485-3p blocks Alzheimer’s disease progression. Int. J. Mol. Sci. 22: 3566, https://doi.org/10.3390/ijms222313136.Suche in Google Scholar PubMed PubMed Central
Komnig, D., Imgrund, S., Reich, A., Grunder, S., and Falkenburger, B.H. (2016). ASIC1a deficient mice show unaltered neurodegeneration in the subacute MPTP model of Parkinson disease. PLoS One 11: e0165235, https://doi.org/10.1371/journal.pone.0165235.Suche in Google Scholar PubMed PubMed Central
Kottgen, M., Benzing, T., Simmen, T., Tauber, R., Buchholz, B., Feliciangeli, S., Huber, T.B., Schermer, B., Kramer-Zucker, A., Hopker, K., et al.. (2005). Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J. 24: 705–716, https://doi.org/10.1038/sj.emboj.7600566.Suche in Google Scholar PubMed PubMed Central
Krokker, L., Patocs, A., and Butz, H. (2021). Essential role of the 14q32 encoded miRNAs in endocrine tumors. Genes 12: 698, https://doi.org/10.3390/genes12050698.Suche in Google Scholar PubMed PubMed Central
Kwon, H.S. and Koh, S.H. (2020). Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl. Neurodegener. 9: 42, https://doi.org/10.1186/s40035-020-00221-2.Suche in Google Scholar PubMed PubMed Central
Lackinger, M., Sungur, A.O., Daswani, R., Soutschek, M., Bicker, S., Stemmler, L., Wust, T., Fiore, R., Dieterich, C., Schwarting, R.K., et al.. (2019). A placental mammal-specific microRNA cluster acts as a natural brake for sociability in mice. EMBO Rep. 20: e46429, https://doi.org/10.15252/embr.201846429.Suche in Google Scholar PubMed PubMed Central
Lagana, A., Dirksen, W.P., Supsavhad, W., Yilmaz, A.S., Ozer, H.G., Feller, J.D., Vala, K.A., Croce, C.M., and Rosol, T.J. (2017). Discovery and characterization of the feline miRNAome. Sci. Rep. 7: 9263, https://doi.org/10.1038/s41598-017-10164-w.Suche in Google Scholar PubMed PubMed Central
Lagos-Quintana, M., Rauhut, R., Lendeckel, W., and Tuschl, T. (2001). Identification of novel genes coding for small expressed RNAs. Science 294: 853–858, https://doi.org/10.1126/science.1064921.Suche in Google Scholar PubMed
Landgraf, P., Rusu, M., Sheridan, R., Sewer, A., Iovino, N., Aravin, A., Pfeffer, S., Rice, A., Kamphorst, A.O., Landthaler, M., et al.. (2007). A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129: 1401–1414, https://doi.org/10.1016/j.cell.2007.04.040.Suche in Google Scholar PubMed PubMed Central
Lau, P., Bossers, K., Janky, R., Salta, E., Frigerio, C.S., Barbash, S., Rothman, R., Sierksma, A.S., Thathiah, A., Greenberg, D., et al.. (2013). Alteration of the microRNA network during the progression of Alzheimer’s disease. EMBO Mol. Med. 5: 1613–1634, https://doi.org/10.1002/emmm.201201974.Suche in Google Scholar PubMed PubMed Central
Lima, J.F., Cerqueira, L., Figueiredo, C., Oliveira, C., and Azevedo, N.F. (2018). Anti-miRNA oligonucleotides: a comprehensive guide for design. RNA Biol. 15: 338–352, https://doi.org/10.1080/15476286.2018.1445959.Suche in Google Scholar PubMed PubMed Central
Lin, X., Wang, R., Li, R., Tao, T., Zhang, D., and Qi, Y. (2022). Diagnostic performance of miR-485-3p in patients with Parkinson’s disease and its relationship with neuroinflammation. NeuroMolecular Med. 24: 195–201, https://doi.org/10.1007/s12017-021-08676-w.Suche in Google Scholar PubMed
Liu, Z., Xiao, H., Li, H., Zhao, Y., Lai, S., Yu, X., Cai, T., Du, C., Zhang, W., and Li, J. (2012). Identification of conserved and novel microRNAs in cashmere goat skin by deep sequencing. PLoS One 7: e50001, https://doi.org/10.1371/journal.pone.0050001.Suche in Google Scholar PubMed PubMed Central
Lou, C., Xiao, M., Cheng, S., Lu, X., Jia, S., Ren, Y., and Li, Z. (2016). MiR-485-3p and miR-485-5p suppress breast cancer cell metastasis by inhibiting PGC-1alpha expression. Cell Death Dis. 7: e2159, https://doi.org/10.1038/cddis.2016.27.Suche in Google Scholar PubMed PubMed Central
Lykhmus, O., Mishra, N., Koval, L., Kalashnyk, O., Gergalova, G., Uspenska, K., Komisarenko, S., Soreq, H., and Skok, M. (2016). Molecular mechanisms regulating LPS-induced inflammation in the brain. Front. Mol. Neurosci. 9: 19, https://doi.org/10.3389/fnmol.2016.00019.Suche in Google Scholar PubMed PubMed Central
Ma, W., Li, Y., Wang, C., Xu, F., Wang, M., and Liu, Y. (2016). Serum miR-221 serves as a biomarker for Parkinson’s disease. Cell Biochem. Funct. 34: 511–515, https://doi.org/10.1002/cbf.3224.Suche in Google Scholar PubMed
Maes, O.C., Sarojini, H., and Wang, E. (2009). Stepwise up-regulation of microRNA expression levels from replicating to reversible and irreversible growth arrest states in WI-38 human fibroblasts. J. Cell. Physiol. 221: 109–119, https://doi.org/10.1002/jcp.21834.Suche in Google Scholar PubMed
Mango, D. and Nistico, R. (2018). Role of ASIC1a in Abeta-induced synaptic alterations in the hippocampus. Pharmacol. Res. 131: 61–65, https://doi.org/10.1016/j.phrs.2018.03.016.Suche in Google Scholar PubMed
Mattson, M.P. (2000). Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 1: 120–129, https://doi.org/10.1038/35040009.Suche in Google Scholar PubMed
Mecca, A.P., Chen, M.K., O’Dell, R.S., Naganawa, M., Toyonaga, T., Godek, T.A., Harris, J.E., Bartlett, H.H., Zhao, W., Nabulsi, N.B., et al.. (2020). In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimer’s Dementia 16: 974–982, https://doi.org/10.1002/alz.12097.Suche in Google Scholar PubMed PubMed Central
Millecamps, S. and Julien, J.P. (2013). Axonal transport deficits and neurodegenerative diseases. Nat. Rev. Neurosci. 14: 161–176, https://doi.org/10.1038/nrn3380.Suche in Google Scholar PubMed
Muther, C., Jobeili, L., Garion, M., Heraud, S., Thepot, A., Damour, O., and Lamartine, J. (2017). An expression screen for aged-dependent microRNAs identifies miR-30a as a key regulator of aging features in human epidermis. Aging (Albany NY) 9: 2376–2396, https://doi.org/10.18632/aging.101326.Suche in Google Scholar PubMed PubMed Central
Nair, V.D. and Ge, Y. (2016). Alterations of miRNAs reveal a dysregulated molecular regulatory network in Parkinson’s disease striatum. Neurosci. Lett. 629: 99–104, https://doi.org/10.1016/j.neulet.2016.06.061.Suche in Google Scholar PubMed
Nixon, R.A. and Yang, D.S. (2012). Autophagy and neuronal cell death in neurological disorders. Cold Spring Harbor Perspect. Biol. 4: a008839, https://doi.org/10.1101/cshperspect.a008839.Suche in Google Scholar PubMed PubMed Central
Noren Hooten, N., Abdelmohsen, K., Gorospe, M., Ejiogu, N., Zonderman, A.B., and Evans, M.K. (2010). microRNA expression patterns reveal differential expression of target genes with age. PLoS One 5: e10724, https://doi.org/10.1371/journal.pone.0010724.Suche in Google Scholar PubMed PubMed Central
Noren Hooten, N., Fitzpatrick, M., Wood, W.H.3rd, De, S., Ejiogu, N., Zhang, Y., Mattison, J.A., Becker, K.G., Zonderman, A.B., and Evans, M.K. (2013). Age-related changes in microRNA levels in serum. Aging (Albany NY) 5: 725–740, https://doi.org/10.18632/aging.100603.Suche in Google Scholar PubMed PubMed Central
Ortega-Ramirez, A., Vega, R., and Soto, E. (2017). Acid-sensing ion channels as potential therapeutic targets in neurodegeneration and neuroinflammation. Mediat. Inflamm. 2017: 3728096, https://doi.org/10.1155/2017/3728096.Suche in Google Scholar PubMed PubMed Central
Otto, T., Candido, S.V., Pilarz, M.S., Sicinska, E., Bronson, R.T., Bowden, M., Lachowicz, I.A., Mulry, K., Fassl, A., Han, R.C., et al.. (2017). Cell cycle-targeting microRNAs promote differentiation by enforcing cell-cycle exit. Proc. Natl. Acad. Sci. U. S. A. 114: 10660–10665, https://doi.org/10.1073/pnas.1702914114.Suche in Google Scholar PubMed PubMed Central
Pasquinelli, A.E., Reinhart, B.J., Slack, F., Martindale, M.Q., Kuroda, M.I., Maller, B., Hayward, D.C., Ball, E.E., Degnan, B., Muller, P., et al.. (2000). Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 408: 86–89, https://doi.org/10.1038/35040556.Suche in Google Scholar PubMed
Paul, S., Bravo Vazquez, L.A., Perez Uribe, S., Roxana Reyes-Perez, P., and Sharma, A. (2020). Current status of microRNA-based therapeutic approaches in neurodegenerative disorders. Cells 9: 1698, https://doi.org/10.3390/cells9071698.Suche in Google Scholar PubMed PubMed Central
Qin, W., Haroutunian, V., Katsel, P., Cardozo, C.P., Ho, L., Buxbaum, J.D., and Pasinetti, G.M. (2009). PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch. Neurol. 66: 352–361.10.1001/archneurol.2008.588Suche in Google Scholar
Querfurth, H.W. and LaFerla, F.M. (2010). Alzheimer’s disease. N. Engl. J. Med. 362: 329–344, https://doi.org/10.1056/nejmra0909142.Suche in Google Scholar
Rani, A., O’Shea, A., Ianov, L., Cohen, R.A., Woods, A.J., and Foster, T.C. (2017). miRNA in circulating microvesicles as biomarkers for age-related cognitive decline. Front. Aging Neurosci. 9: 323, https://doi.org/10.3389/fnagi.2017.00323.Suche in Google Scholar PubMed PubMed Central
Ravanidis, S., Bougea, A., Papagiannakis, N., Maniati, M., Koros, C., Simitsi, A.M., Bozi, M., Pachi, I., Stamelou, M., Paraskevas, G.P., et al.. (2020). Circulating Brain-enriched MicroRNAs for detection and discrimination of idiopathic and genetic Parkinson’s disease. Mov. Disord. 35: 457–467, https://doi.org/10.1002/mds.27928.Suche in Google Scholar PubMed
Reitz, C. and Mayeux, R. (2014). Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 88: 640–651, https://doi.org/10.1016/j.bcp.2013.12.024.Suche in Google Scholar PubMed PubMed Central
Rhoades, J.L., Nelson, J.C., Nwabudike, I., Yu, S.K., McLachlan, I.G., Madan, G.K., Abebe, E., Powers, J.R., Colon-Ramos, D.A., and Flavell, S.W. (2019). ASICs mediate food responses in an enteric serotonergic neuron that controls foraging behaviors. Cell 176: 85–97 e14, https://doi.org/10.1016/j.cell.2018.11.023.Suche in Google Scholar PubMed PubMed Central
Robinson, J.L., Molina-Porcel, L., Corrada, M.M., Raible, K., Lee, E.B., Lee, V.M., Kawas, C.H., and Trojanowski, J.Q. (2014). Perforant path synaptic loss correlates with cognitive impairment and Alzheimer’s disease in the oldest-old. Brain 137: 2578–2587, https://doi.org/10.1093/brain/awu190.Suche in Google Scholar PubMed PubMed Central
Ruan, N., Tribble, J., Peterson, A.M., Jiang, Q., Wang, J.Q., and Chu, X.P. (2021). Acid-sensing ion channels and mechanosensation. Int. J. Mol. Sci. 22: 4810, https://doi.org/10.3390/ijms22094810.Suche in Google Scholar PubMed PubMed Central
Selkoe, D.J. (2002). Alzheimer’s disease is a synaptic failure. Science 298: 789–791, https://doi.org/10.1126/science.1074069.Suche in Google Scholar PubMed
Sharma, S. and Lu, H.C. (2018). microRNAs in neurodegeneration: current findings and potential impacts. J. Alzheimer’s Dis. Park. 8.10.4172/2161-0460.1000420Suche in Google Scholar PubMed PubMed Central
Sheinerman, K.S., Toledo, J.B., Tsivinsky, V.G., Irwin, D., Grossman, M., Weintraub, D., Hurtig, H.I., Chen-Plotkin, A., Wolk, D.A., McCluskey, L.F., et al.. (2017). Circulating brain-enriched microRNAs as novel biomarkers for detection and differentiation of neurodegenerative diseases. Alzheimer’s Res. Ther. 9: 89, https://doi.org/10.1186/s13195-017-0316-0.Suche in Google Scholar PubMed PubMed Central
Shigemizu, D., Akiyama, S., Asanomi, Y., Boroevich, K.A., Sharma, A., Tsunoda, T., Matsukuma, K., Ichikawa, M., Sudo, H., Takizawa, S., et al.. (2019). Risk prediction models for dementia constructed by supervised principal component analysis using miRNA expression data. Commun. Biol. 2: 77, https://doi.org/10.1038/s42003-019-0324-7.Suche in Google Scholar PubMed PubMed Central
Smith, L.M. and Strittmatter, S.M. (2017). Binding sites for amyloid-beta oligomers and synaptic toxicity. Cold Spring Harb. Perspect. Med. 7: a024075, https://doi.org/10.1101/cshperspect.a024075.Suche in Google Scholar PubMed PubMed Central
Spires-Jones, T.L. and Hyman, B.T. (2014). The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 82: 756–771, https://doi.org/10.1016/j.neuron.2014.05.004.Suche in Google Scholar PubMed PubMed Central
Srivastava, A., Dixit, A.B., Banerjee, J., Tripathi, M., and Sarat Chandra, P. (2016). Role of inflammation and its miRNA based regulation in epilepsy: implications for therapy. Clin. Chim. Acta 452: 1–9, https://doi.org/10.1016/j.cca.2015.10.023.Suche in Google Scholar PubMed
St-Pierre, J., Drori, S., Uldry, M., Silvaggi, J.M., Rhee, J., Jager, S., Handschin, C., Zheng, K., Lin, J., Yang, W., et al.. (2006). Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127: 397–408, https://doi.org/10.1016/j.cell.2006.09.024.Suche in Google Scholar PubMed
Suh, N. (2018). MicroRNA controls of cellular senescence. BMB Rep. 51: 493–499, https://doi.org/10.5483/bmbrep.2018.51.10.209.Suche in Google Scholar PubMed PubMed Central
Sweeney, G. and Song, J. (2016). The association between PGC-1alpha and Alzheimer’s disease. Anat. Cell Biol. 49: 1–6.10.5115/acb.2016.49.1.1Suche in Google Scholar PubMed PubMed Central
Sweeney, P., Park, H., Baumann, M., Dunlop, J., Frydman, J., Kopito, R., McCampbell, A., Leblanc, G., Venkateswaran, A., Nurmi, A., et al.. (2017). Protein misfolding in neurodegenerative diseases: implications and strategies. Transl. Neurodegener. 6: 6, https://doi.org/10.1186/s40035-017-0077-5.Suche in Google Scholar PubMed PubMed Central
Tan, L., Yu, J.T., Tan, M.S., Liu, Q.Y., Wang, H.F., Zhang, W., Jiang, T., and Tan, L. (2014). Genome-wide serum microRNA expression profiling identifies serum biomarkers for Alzheimer’s disease. J. Alzheim. Dis. 40: 1017–1027, https://doi.org/10.3233/jad-132144.Suche in Google Scholar
Tatura, R., Kraus, T., Giese, A., Arzberger, T., Buchholz, M., Hoglinger, G., and Muller, U. (2016). Parkinson’s disease: SNCA-, PARK2-, and LRRK2- targeting microRNAs elevated in cingulate gyrus. Park. Relat. Disord. 33: 115–121, https://doi.org/10.1016/j.parkreldis.2016.09.028.Suche in Google Scholar PubMed
Titze-de-Almeida, S.S., Soto-Sanchez, C., Fernandez, E., Koprich, J.B., Brotchie, J.M., and Titze-de-Almeida, R. (2020). The promise and challenges of developing miRNA-based therapeutics for Parkinson’s disease. Cells 9: 841, https://doi.org/10.3390/cells9040841.Suche in Google Scholar PubMed PubMed Central
Wang, L., Huang, D., Jiang, Z., Luo, Y., Norris, C., Zhang, M., Tian, X., and Tang, Y. (2017). Akt3 is responsible for the survival and proliferation of embryonic stem cells. Biol. Open 6: 850–861.10.1242/bio.024505Suche in Google Scholar PubMed PubMed Central
Wang, M., Qin, L., and Tang, B. (2019a). MicroRNAs in Alzheimer’s disease. Front. Genet. 10: 153, https://doi.org/10.3389/fgene.2019.00153.Suche in Google Scholar PubMed PubMed Central
Wang, R., Zuo, X., Wang, K., Han, Q., Zuo, J., Ni, H., Liu, W., Bao, H., Tu, Y., and Xie, P. (2018). MicroRNA-485-5p attenuates cell proliferation in glioma by directly targeting paired box 3. Am. J. Cancer Res. 8: 2507–2517.Suche in Google Scholar
Wang, W.X., Huang, Q., Hu, Y., Stromberg, A.J., and Nelson, P.T. (2011). Patterns of microRNA expression in normal and early Alzheimer’s disease human temporal cortex: white matter versus gray matter. Acta Neuropathol. 121: 193–205, https://doi.org/10.1007/s00401-010-0756-0.Suche in Google Scholar PubMed PubMed Central
Wang, W.X., Rajeev, B.W., Stromberg, A.J., Ren, N., Tang, G., Huang, Q., Rigoutsos, I., and Nelson, P.T. (2008). The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 28: 1213–1223, https://doi.org/10.1523/jneurosci.5065-07.2008.Suche in Google Scholar PubMed PubMed Central
Wang, Y., Xu, E., Musich, P.R., and Lin, F. (2019b). Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci. Ther. 25: 816–824, https://doi.org/10.1111/cns.13116.Suche in Google Scholar PubMed PubMed Central
Wang, Y.Z., Zeng, W.Z., Xiao, X., Huang, Y., Song, X.L., Yu, Z., Tang, D., Dong, X.P., Zhu, M.X., and Xu, T.L. (2013). Intracellular ASIC1a regulates mitochondrial permeability transition-dependent neuronal death. Cell Death Differ. 20: 1359–1369, https://doi.org/10.1038/cdd.2013.90.Suche in Google Scholar PubMed PubMed Central
Weinberg, R.B., Mufson, E.J., and Counts, S.E. (2015). Evidence for a neuroprotective microRNA pathway in amnestic mild cognitive impairment. Front. Neurosci. 9: 430, https://doi.org/10.3389/fnins.2015.00430.Suche in Google Scholar PubMed PubMed Central
Winter, J., Jung, S., Keller, S., Gregory, R.I., and Diederichs, S. (2009). Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 11: 228–234, https://doi.org/10.1038/ncb0309-228.Suche in Google Scholar PubMed
Xu, M., Wu, R., Zhang, L., Zhu, H.Y., Xu, G.Y., Qian, W., and Zhang, P.A. (2020). Decreased MiR-485-5p contributes to inflammatory pain through post-transcriptional upregulation of ASIC1 in rat Dorsal Root Ganglion. J. Pain Res. 13: 3013–3022, https://doi.org/10.2147/jpr.s279902.Suche in Google Scholar PubMed PubMed Central
Yamanaka, M., Ishikawa, T., Griep, A., Axt, D., Kummer, M.P., and Heneka, M.T. (2012). PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J. Neurosci. 32: 17321–17331, https://doi.org/10.1523/jneurosci.1569-12.2012.Suche in Google Scholar
Yao, L., Zhu, Z., Wu, J., Zhang, Y., Zhang, H., Sun, X., Qian, C., Wang, B., Xie, L., Zhang, S., et al.. (2019). MicroRNA-124 regulates the expression of p62/p38 and promotes autophagy in the inflammatory pathogenesis of Parkinson’s disease. Faseb. J. 33: 8648–8665, https://doi.org/10.1096/fj.201900363r.Suche in Google Scholar PubMed
Yu, L., Li, H., Liu, W., Zhang, L., Tian, Q., Li, H., and Li, M. (2021). MiR-485-3p serves as a biomarker and therapeutic target of Alzheimer’s disease via regulating neuronal cell viability and neuroinflammation by targeting AKT3. Mol. Genet. Genom. Med. 9: e1548, https://doi.org/10.1002/mgg3.1548.Suche in Google Scholar PubMed PubMed Central
Yu, X.W., Hu, Z.L., Ni, M., Fang, P., Zhang, P.W., Shu, Q., Fan, H., Zhou, H.Y., Ni, L., Zhu, L.Q., et al.. (2015). Acid-sensing ion channels promote the inflammation and migration of cultured rat microglia. Glia 63: 483–496, https://doi.org/10.1002/glia.22766.Suche in Google Scholar PubMed
Zhang, M. and Bian, Z. (2021). Alzheimer’s disease and microRNA-132: a widespread pathological factor and potential therapeutic target. Front. Neurosci. 15, https://doi.org/10.3389/fnins.2021.687973.Suche in Google Scholar PubMed PubMed Central
Zhao, X., Grotta, J., Gonzales, N., and Aronowski, J. (2009). Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke 40: S92–S94, https://doi.org/10.1161/strokeaha.108.533158.Suche in Google Scholar PubMed
Zheng, B., Liao, Z., Locascio, J.J., Lesniak, K.A., Roderick, S.S., Watt, M.L., Eklund, A.C., Zhang-James, Y., Kim, P.D., Hauser, M.A., et al.. (2010). PGC-1alpha, a potential therapeutic target for early intervention in Parkinson’s disease. Sci. Transl. Med. 2: 52ra73, https://doi.org/10.1126/scitranslmed.3001059.Suche in Google Scholar PubMed PubMed Central
Zolochevska, O. and Taglialatela, G. (2020). Selected microRNAs increase synaptic resilience to the damaging binding of the Alzheimer’s disease amyloid beta oligomers. Mol. Neurobiol. 57: 2232–2243, https://doi.org/10.1007/s12035-020-01868-8.Suche in Google Scholar PubMed PubMed Central
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- The many facets of CD26/dipeptidyl peptidase 4 and its inhibitors in disorders of the CNS – a critical overview
- Functional changes in brain oscillations in dementia: a review
- The role of microRNA-485 in neurodegenerative diseases
- Predictive models for the incidence of Parkinson’s disease: systematic review and critical appraisal
- Involvement of nerve growth factor (NGF) in chronic neuropathic pain – a systematic review
- Immunosenescence of brain accelerates Alzheimer’s disease progression
- Pathophysiological aspects of complex PTSD – a neurobiological account in comparison to classic posttraumatic stress disorder and borderline personality disorder
Artikel in diesem Heft
- Frontmatter
- The many facets of CD26/dipeptidyl peptidase 4 and its inhibitors in disorders of the CNS – a critical overview
- Functional changes in brain oscillations in dementia: a review
- The role of microRNA-485 in neurodegenerative diseases
- Predictive models for the incidence of Parkinson’s disease: systematic review and critical appraisal
- Involvement of nerve growth factor (NGF) in chronic neuropathic pain – a systematic review
- Immunosenescence of brain accelerates Alzheimer’s disease progression
- Pathophysiological aspects of complex PTSD – a neurobiological account in comparison to classic posttraumatic stress disorder and borderline personality disorder

