Abstract
The microtubule-associated protein (MAP) TAU is mainly sorted into the axon of healthy brain neurons. Somatodendritic missorting of TAU is a pathological hallmark of many neurodegenerative diseases, including Alzheimer’s disease (AD). Cause, consequence and (patho)physiological mechanisms of TAU sorting and missorting are understudied, in part also because of the lack of readily available human neuronal model systems. The human neuroblastoma cell line SH-SY5Y is widely used for studying TAU physiology and TAU-related pathology in AD and related tauopathies. SH-SY5Y cells can be differentiated into neuron-like cells (SH-SY5Y-derived neurons) using various substances. This review evaluates whether SH-SY5Y-derived neurons are a suitable model for (i) investigating intracellular TAU sorting in general, and (ii) with respect to neuron subtype-specific TAU vulnerability. (I) SH-SY5Y-derived neurons show pronounced axodendritic polarity, high levels of axonally localized TAU protein, expression of all six human brain isoforms and TAU phosphorylation similar to the human brain. As SH-SY5Y cells are highly proliferative and readily accessible for genetic engineering, stable transgene integration and leading-edge genome editing are feasible. (II) SH-SY5Y-derived neurons display features of subcortical neurons early affected in many tauopathies. This allows analyzing brain region-specific differences in TAU physiology, also in the context of differential vulnerability to TAU pathology. However, several limitations should be considered when using SH-SY5Y-derived neurons, e.g., the lack of clearly defined neuronal subtypes, or the difficulty of mimicking age-related tauopathy risk factors in vitro. In brief, this review discusses the suitability of SH-SY5Y-derived neurons for investigating TAU (mis)sorting mechanisms and neuron-specific TAU vulnerability in disease paradigms.
Introduction
Alzheimer’s disease (AD) and related neurodegenerative diseases constitute a major scourge of modern healthcare due to their tremendously high and increasing prevalence (Korolev 2014). One key player in AD and related so-called tauopathies is the microtubule-associated protein (MAP) TAU. Under healthy conditions, TAU is predominantly sorted to the axonal compartment of brain neurons (Binder et al. 1985; Kempf et al. 1996) where it regulates the assembly of microtubule filaments (Cleveland et al. 1977a; Weingarten et al. 1975). TAU missorting into the somatodendritic compartment, site-specific hyperphosphorylation and formation of TAU-containing neurofibrillary tangles (NFT) are typical pathological hallmarks of AD and other tauopathies (Brion et al. 1985; Wischik et al. 1988; Zempel and Mandelkow 2014). In the last decades, much effort has been invested in unraveling the physiological functions and pathomechanisms linked to TAU sorting and missorting.
Mouse models or rodent-derived neuronal cultures are commonly used for TAU studies, including research on TAU sorting. However, these models have several limitations as (i) they require the killing of animals, (ii) they suffer from limitations in translatability, including different isoform expression patterns and species-dependent differences regarding the cellular machinery and interaction partners (Janke et al. 1999), and (iii) in case of ‘humanized’ mouse models, they exhibit artificial genetic settings because of overexpression of (multiple) human transgenes (Andorfer et al. 2003; Duff et al. 2000; Hashimoto et al. 2019; He et al. 2020) (see Table 1 for summary). Another cellular model, human induced pluripotent stem cell (hiPSC)-derived neurons overcome many of these obstacles and constitute a powerful tool for TAU-related research (Muratore et al. 2014; Silva et al. 2016; Sohn et al. 2019; Verheyen et al. 2015; Wang et al. 2017). However, differentiation of hiPSC-derived neurons is expensive, time-consuming and results in cultures with variable homogeneity and differentiation efficiency (Gunhanlar et al. 2018; Hu et al. 2010; Lin et al. 2020) (see Table 1 for summary).
Comparison of neuronal model systems for TAU sorting research.
| Model system | SH-SY5Y-derived neurons | Rodent primary neurons | ‘Humanized’ mouse models | hiPSC-derived neurons |
|---|---|---|---|---|
| Feature | ||||
| Human-derived | + | − | − | + |
| No animal need | + | − | − | + |
| Proliferative | + | − | − | + |
| Low cultivation cost | + | (+) | − | − |
| Accessible for | ||||
| Genetic manipulation | + | (+) | (+) | + |
| Fast differentiation | + | + | + | − |
| Culture homogeneity | (+) | (+) | + | (+) |
| Neuronal maturity | (+) | + | + | (+) |
| Expression of six | ||||
| TAU isoforms | + | − | (+) | + |
| Human brain-like | ||||
| Phosphorylation state | + | (+) | + | + |
| Efficient sorting of | ||||
| Endogenous TAU | + | + | + | + |
| Efficient sorting of | ||||
| Transfected TAU | + | (+) | n/a | + |
-
+ = feature is present/available in this cell model, (+) = feature is partially present/available or dependent on the experimental (e.g., cultivation, differentiation, (trans) genetic setup) conditions, − = feature is not or almost not present/available for this model, n/a = no data available or not commonly used.
The human neuroblastoma cell line SH-SY5Y, subcloned from the SK-N-SH line (Biedler and Schachner 1978), is an easy-to-handle and proliferative cell line with well-established differentiation methods for generating stable neuronal cultures. SH-SY5Y-derived neurons have been widely used for TAU-related research as they yield homogeneous and reproducible human-derived neuronal cultures with robust expression and axonal distribution of TAU, thereby suitable also for addressing axonal TAU sorting (Kovalevich and Langford 2013) (see Table 1 for summary). Interestingly, the neuronal identity of SH-SY5Y-derived neurons depends on the used differentiation procedure (Kovalevich and Langford 2013), which bears potential for neuronal subtype-specific TAU studies.
The current review aims to evaluate the suitability of SH-SY5Y-derived neurons for TAU sorting research. Moreover, the features and challenges of the drug-dependent identity of SH-SY5Y-derived neurons will be discussed regarding their use to mimic neuronal subtypes of brain regions early affected in AD and other tauopathies.
Suitability of SH-SY5Y-derived neurons for investigating TAU sorting
Neuronal maturity
Neuronal maturity is an important prerequisite for TAU sorting-related research as TAU is specifically enriched in the axon of mature neurons (Binder et al. 1985; Kempf et al. 1996). The human SH-SY5Y neuroblastoma cells can be differentiated into neuronal cells with several substances, including the vitamin A derivative retinoic acid (RA), different phorbol esters, dibutyryl-cAMP, or the brain-derived neurotrophic factor (BDNF) (Kovalevich and Langford 2013). The maturation of SH-SY5Y-derived neurons is well characterized, especially for RA-based and BDNF-based differentiation.
Some observations question the neuronal maturity of SH-SY5Y-derived neurons, such as the moderate outgrowth of dendritic processes (Bell et al. 2021; Encinas et al. 2000) or the lack of spontaneous activity after RA-driven differentiation (Jahn et al. 2017). Jahn and colleagues (2017) argue, however, that spontaneous activity, seen e.g., in rodent primary cultures, might not be mandatory to prove neuronal maturity. Further, ankyrin G (ANKG) is weakly expressed in SH-SY5Y-derived neurons without enrichment at the proximal axon (Bell et al. 2021). ANKG is known to be a key player for the development of the axon initial segment (AIS), a specialized region at the proximal axon, involved in the generation of action potentials and anterograde cargo transport (Leterrier 2016; Rasband 2010).
Nevertheless, SH-SY5Y-derived neurons express classical neuronal maturation markers including neuronal nuclei (NeuN), high-weight neurofilament (NF-H), the microtubule-associated protein 2 (MAP2) or growth-associated protein 43 (GAP43) (Bell et al. 2021; Cheung et al. 2009; Encinas et al. 2000; Lopes et al. 2010; Påhlman et al. 1984; Paik et al. 2019; Shipley et al. 2016). They are excitable due to the expression of voltage-gated sodium (e.g., Nav1.1, Nav1.2), calcium and potassium channels (Hill et al. 2014; Johansson 1994; Park et al. 2010; Sun et al. 2020; Tosetti et al. 1998), and they exhibit activity-dependent synapse and vesicle formation (Cheung et al. 2009; Jahn et al. 2017; Sarkanen et al. 2007), suggesting the presence of functional synaptic networks. Morphologically, SH-SY5Y-derived neurons exhibit pronounced axonal outgrowth (Figure 1A) (Bell et al. 2021; Encinas et al. 2000; Kovalevich and Langford 2013; Shipley et al. 2016). Taken together, there is strong evidence for the neuronal maturity and function of SH-SY5Y-derived neurons.
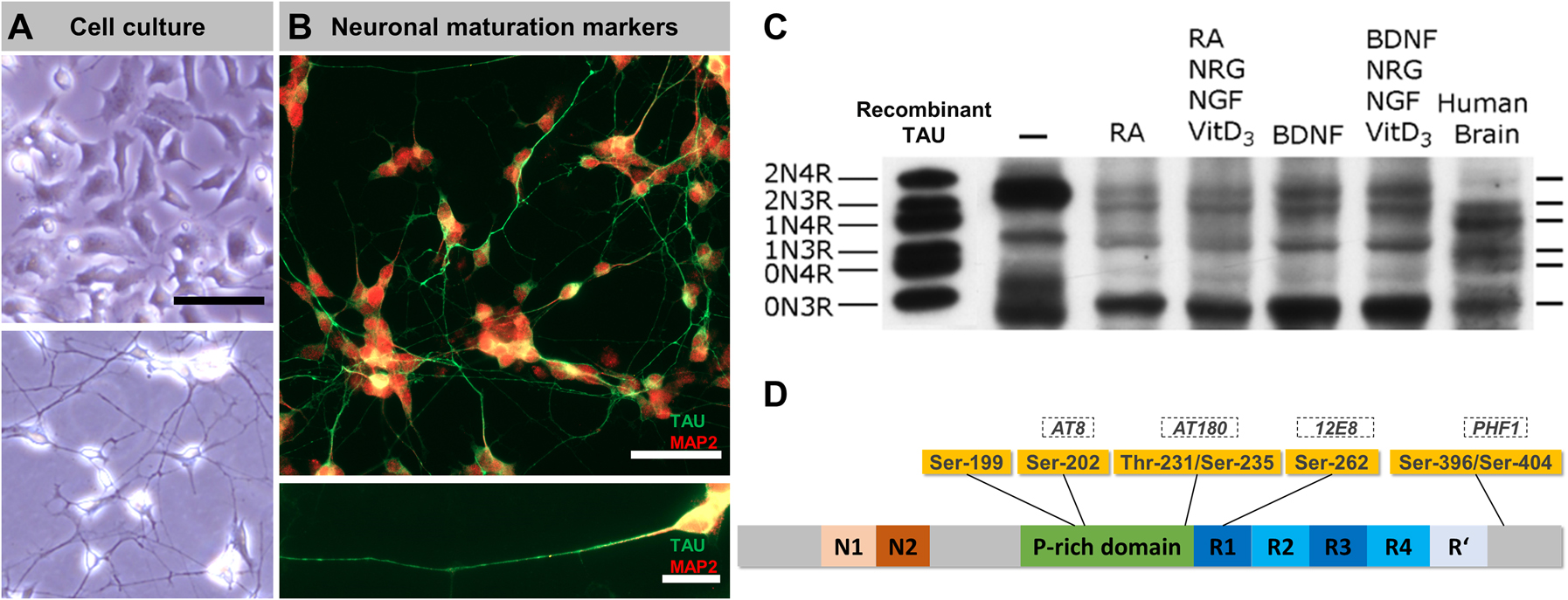
SH-SY5Y-derived neurons show axonal TAU enrichment and expression of all six human isoforms.
(A) Representative images of undifferentiated SH-SY5Y cells (top panel) and SH-SY5Y-derived neurons (bottom panel) in culture (cultures were grown on Poly-d-Lysine (20 μg/mL)-coated glass coverslips in Dulbecco's modified eagle medium (DMEM)/F12 (#10565018, Thermo Fisher) and 10% fetal bovine serum (BioChrom AG)); for differentiation, cells were grown for seven days in DMEM/F12, 10% fetal bovine serum and 10 µM retinoic acid (RA), followed by seven days in serum-free DMEM/F12 and 10 ng/mL brain-derived neurotrophic factor (BDNF). Note the altered morphology and pronounced neurite outgrowth upon differentiation. Scale bar: 50 µm. (B) Immunostainings of SH-SY5Y-derived neurons (cells were fixed with 3.7% FA for 1 h, blocked with 5% bovine serum albumin (BSA) and 0.1% Triton X-100 for 5 min, immunostained with polyclonal anti-TAU (K9JA, 1:1000 in phosphate-buffered saline (PBS), A0024, Dako, second AB: donkey anti-rabbit + AlexaFluor488, 1:1000 in PBS, A21202, Thermo Fisher) and chicken anti-MAP2 (1:2000, ab5392, Abcam, second AB: goat anti-chicken AF647, 1:1000 in PBS, A21449, Thermo Fisher) antibodies, and mounted (PolyMount, Polysciences), procedure adapted from Zempel and Mandelkow (2017)) demonstrate the strong expression and polarized distribution of neuronal maturation markers TAU (green, mainly axonal) and MAP2 (red, mainly somatic). Scale bar (top): 50 µm, scale bar (bottom): 20 µm. (C) Western blot analysis of TAU isoform expression (de-phosphorylated lysates) in undifferentiated SH-SY5Y cells (lane 1), differently treated SH-SY5Y-derived neurons (lanes 2–5) and human brain lysate (lane 6). The very left lane shows a recombinant TAU standard. Note the abundance of all six isoforms upon differentiation with varying ratios compared to the human brain. The blot was adapted and modified from Agholme et al. (2010). (D) Overview of the TAU protein (grey bar, colored sections indicate distinct TAU domains) and common tauopathy-associated TAU hyperphosphorylation sites (yellow boxes, corresponding epitopes recognized by specific antibodies are indicated in dashed boxes) that appear highly phosphorylated in SH-SY5Y cells.
TAU expression and subcellular localization
Little amounts of TAU protein are detectable in undifferentiated SH-SY5Y cells, where it is present in the cytoplasm and the nucleus (Uberti et al. 1997). Differentiation of SH-SY5Y cells with RA or the phorbol ester TPA results in a substantial increase of overall TAU protein levels (Encinas et al. 2000; Smith et al. 1995) with a neuron-like subcellular distribution, i.e., increased axonal and decreased somatic TAU levels (Figure 1A, B) (Agholme et al. 2010; Encinas et al. 2000; Uberti et al. 1997). The use of combinatorial treatments, e.g., RA and BDNF or BDNF and the neuronal growth factor (NGF), further enhances the axonal outgrowth and the total TAU expression to levels (Agholme et al. 2010; Bell et al. 2021; Encinas et al. 2000; Shipley et al. 2016), comparable to those of the human brain (Agholme et al. 2010). The observed separation of axonal TAU and somatodendritic MAP2 (Figure 1B) (Bell et al. 2021; Encinas et al. 2000; Shipley et al. 2016) indicates the establishment of neuronal cell polarity (Goedert et al. 1991).
In this context, it would be worth examining whether SH-SY5Y-derived neurons properly distribute also transfected TAU, an often-faced challenge in experiments with rodent primary cultures (Bachmann et al. 2020; Bell et al. 2021; Xia et al. 2016; Zempel, Luedtke, et al. 2017). Indeed, recent data suggest that SH-SY5Y-derived neurons tolerate overexpression of transfected hemagglutinin (HA)-tagged TAU better than primary cultures (at least for 9 days), and that they sort transfected TAU with endogenous-like efficiency. In contrast, a C-terminally truncated TAU construct is missorted to the soma in SH-SY5Y-derived neurons similar to mouse primary neurons (Bell et al. 2021) (see Table 1 for comparison). It is remarkable that efficient sorting of endogenous and transfected TAU apparently happens without classical ANKG-mediated AIS formation in SH-SY5Y-derived neurons (Bell et al. 2021). Former studies claimed that ANKG-mediated AIS formation is critical for the process of neuronal polarization (Leterrier 2016, 2018; Rasband 2010), while more recent studies, indeed, question the necessity of ANKG for proper TAU sorting (Van Beuningen et al. 2015; Kneynsberg et al. 2019), in agreement with a putative redundancy of several mechanisms responsible for anterograde trafficking of TAU (Zempel and Mandelkow 2019). More studies are necessary to clarify the role of ANKG and other AIS proteins, e.g., the tripartite motif-containing protein 46 (TRIM46) or end-binding proteins (EBs), in the context of axonal TAU sorting.
Expression pattern of TAU isoforms
Alternative splicing of the exons 2 and 3 (either 0N, 1N, or 2N isoforms) and exon 10 (3R or 4R isoforms) results in six major TAU isoforms in the mature human brain (Cleveland et al. 1977a,b; Goedert, Spillantini, Jakes, et al. 1989; Goedert, Spillantini, Potier, et al. 1989), compared to only three isoforms in the adult rodent brain (Götz et al. 1995). Thus, it is clear that a suitable model for studying TAU sorting should be human-derived and display the expression of all six brain-specific human TAU isoforms. The isoform expression pattern in the human brain depends on the developmental stage and the analyzed brain region (Couchie et al. 1992; Goedert, Spillantini, Jakes, et al. 1989; Goedert, Spillantini, Potier, et al. 1989; Nunez and Fischer 1997). Moreover, the axodendritic distribution is markedly different between the six major TAU isoforms (Zempel, Dennissen, et al. 2017).
Early studies on TAU isoform expression in SH-SY5Y cells showed consistently that undifferentiated cells express only the shortest TAU isoform 0N3R (Dupont-Wallois et al. 1995; Smith et al. 1995; Tanaka et al. 1995; Uberti et al. 1997). More recently, TAU mRNA containing exon 2 (1N) or exon 10 (4R) was found in untreated SH-SY5Y cells, suggesting at least basal expression of larger isoforms (Figure 1C) (Agholme et al. 2010). Reports about differentiated SH-SY5Y-derived neurons vary in their described isoform expression pattern. Former studies detected either no shift in isoform expression upon RA treatment (Smith et al. 1995), weak expression of an additional 64 kDa-sized isoform (probably representing the 2N4R isoform) (Dupont-Wallois et al. 1995), or low levels of 4R isoform mRNA upon three weeks of RA treatment (Uberti et al. 1997). More recent findings, however, found robust expression of all six major isoforms in SH-SY5Y-derived neurons (Figure 1C) (Agholme et al. 2010).
The isoform ratio in SH-SY5Y-derived neurons differs notably from the human brain (Trabzuni et al. 2012), with more 3R than 4R TAU, less 2N isoforms and more 0N3R-TAU (Agholme et al. 2010). This may suggest that a cultivation time of up to three weeks produces SH-SY5Y-derived neurons displaying an intermediate stage of maturity. Later studies, however, found roughly equal amounts of 3R and 4R isoforms, as typically seen in the adult human brain (Arendt et al. 2016; Goedert and Jakes 1990; Lee Virginia et al. 1998; Spillantini and Goedert 1998), after RA treatment (Lee et al. 2015). Despite this discrepancy regarding the isoform ratio, these studies demonstrate the principal presence of all six TAU isoforms in SH-SY5Y-derived neurons.
AD animal models that express all six human TAU isoforms while the endogenous mouse Mapt expression is knocked out (Andorfer et al. 2003; Duff et al. 2000) have already been available for years. However, one bottleneck for these AD animal models is to achieve a human-like isoform ratio of 3R and 4R isoforms. Recent mouse lines could overcome this issue by introducing multiple, partially mutagenized human MAPT transgenes into a Mapt-KO mouse background (Hashimoto et al. 2019; He et al. 2020). However, these mice harbour a highly artificial genetic MAPT setup, and they still lack a human cellular environment, making e.g., isoform-specific interaction studies difficult to interpret.
SH-SY5Y-derived neurons can serve to clarify whether certain TAU isoforms contribute differently to cellular TAU functions under physiological conditions and possibly convey tauopathy-related toxicity, e.g., by being more susceptible to mislocalization, hyperphosphorylation or aggregation. Taken together, the TAU isoform ratio of SH-SY5Y-derived neurons differs from that in the mature human brain, but the strong expression of all six major isoforms already upon brief differentiation periods allows investigating TAU isoform-specific localization and disease-associated mislocalization.
TAU phosphorylation state
More than 90 reported phosphorylation sites illustrate the striking importance of these posttranslational modifications for TAU functionality (Arendt et al. 2016). The phosphorylation state of TAU directly influences the microtubule-binding affinity and thereby its mobility and intracellular localization (Biernat et al. 1993; Morris et al. 2015; Noble et al. 2013; Reynolds et al. 2008; Usardi et al. 2011). Hyperphosphorylation correlates with somatodendritic missorting and aggregation of TAU (Alavi Naini and Soussi-Yanicostas 2015; Buée et al. 2000; Hasegawa et al. 1996; Kopke et al. 1993; Noble et al. 2013; Šimić et al. 2016; Zheng-Fischhöfer et al. 1998).
Consequently, early TAU studies with SH-SY5Y cells put great effort into analyzing the phosphorylation state of TAU in SH-SY5Y cells. They revealed that many TAU residues, including Ser-199, Ser-202 (AT8 epitope), Thr-231/Ser-235 (AT180), Ser-262 (12E8) and Ser-396/Ser-404 (PHF1) are phosphorylated in undifferentiated SH-SY5Y cells (Figure 1D) (Majd, Koblar, et al. 2018; Smith et al. 1995; Tanaka et al. 1995). As many of these residues are hyperphosphorylated in AD, TAU was considered as phosphorylated in an AD-like manner (Tanaka et al. 1995). The phosphorylation state can be explained by the high levels of 0N3R-TAU in undifferentiated SH-SY5Y cells. In early developmental stages, when 0N3R-TAU is the predominant isoform, TAU phosphorylation is increased (Buée et al. 2000; Goedert, Spillantini, Jakes, et al. 1989). Interestingly, no substantial change in TAU phosphorylation was seen upon differentiation with RA (Smith et al. 1995) despite the expression of larger TAU isoforms (Agholme et al. 2010; Lee et al. 2015). This might be due to the fact that 0N3R-TAU appears as the major isoform also in differentiating SH-SY5Y cells (Agholme et al. 2010).
It was first shown in SH-SY5Y cells that okadaic acid and other phosphatase inhibitors can evoke AD-like TAU hyperphosphorylation and oligomerization, followed by microtubule disassembly and cell death, via inactivation of protein phosphatase 1 (PP1), PP2A, and other phosphatases, and activation of mitogen-activated protein kinase (MAPK), cyclin-dependent kinase 1 (CDK1) and CDK5 kinases (Boban et al. 2019; Dupont-Wallois et al. 1995; Tanaka et al. 1998). These findings in SH-SY5Y cells provided a direct link between the phosphorylation state, the microtubule stability, and cell death as postulated from previous in vitro interaction assays (Arendt et al. 2016). The wide-ranging cellular effects of okadaic acid (Valdiglesias et al. 2013), however, make it difficult to claim a direct causal link between the pathological TAU oligomerization and the observed enzyme (in)activation without further research (Boban et al. 2019).
Many recent TAU studies in SH-SY5Y cells focused on TAU (hyper)phosphorylation, including the role of kinases/phosphatases and cellular pathways in misbalancing the TAU phosphorylation state (Hernandez et al. 2009; Majd, Koblar, et al. 2018; Majd, Majd, et al. 2018; Sayas et al. 1999; Wang et al. 2018), the influence of microglia-mediated neuroinflammation (Chan et al. 2019; Chen et al. 2018; Lee et al. 2015; Yeo et al. 2018), the link between hyperglycaemia and TAU phosphorylation (Chen et al. 2019; Nie et al. 2018; Santa-Catalina et al. 2016, 2017), or the correlation of TAU phosphorylation and sleep disorders in AD patients (Liu et al. 2019).
Taken together, the TAU phosphorylation state in SH-SY5Y cells is similar to that of human brain neurons (Arendt et al. 2016; Tanaka et al. 1995), and its regulation involves known TAU-interacting kinases and phosphatases (Hernandez et al. 2009; Sayas et al. 1999; Tanaka et al. 1998). These remarkable similarities in TAU phosphorylation are critical for the suitability of SH-SY5Y-derived neurons for the investigation of TAU sorting since TAU phosphorylation and localization are closely linked.
Genetic engineering of SH-SY5Y cells
SH-SY5Y-derived neurons display many features of matured neuronal cells, including the post-mitotic character. Post-mitotic cells are inaccessible for most stable genetic engineering approaches. However, in the undifferentiated state, SH-SY5Y cells are rapidly dividing and can be used for the stable integration of transgenes, including variants of the TAU-encoding MAPT gene. In the past, transfection and stable integration of linearized 1N3R- and 1N4R-MAPT cDNA into SH-SY5Y cells was used to mimic the misbalance of 3R/4R isoform ratios (Delobel et al. 2003; Mailliot et al. 2000). Several pathological MAPT variants affect the alternative splicing of exon 10, which can result in tauopathies, such as progressive supranuclear palsy (PSP) or frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17) (Lee et al. 2001).
Other studies generated SH-SY5Y cell lines with stable overexpression of only 4R isoforms (David et al. 2002), a exon 6-containing isoform (Luo et al. 2004) or a pro-aggregant TAU variant (Chang et al. 2016; Chen et al. 2018). These transgenic TAU isoforms or mutants are, however, lacking the features of endogenous regulation of MAPT expression. Recent genome editing techniques, such as clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9, were shown to be applicable in SH-SY5Y cells (Bao et al. 2016; Kim et al. 2017, 2018; Prasuhn et al. 2018; Xu et al. 2018). This allows the generation of complete or isoform-specific TAU knock-out lines or the introduction of gene edits on a single base level, e.g., by using base editor enzymes (Rees and Liu 2018) or the recently described prime editing technique (Anzalone et al. 2019).
However, one has to consider the genetic predispositions of SH-SY5Y cells, as large-scale chromosomal abnormalities and imbalances are reported for neuroblastoma cell lines in general (Ambros et al. 2009; George et al. 2007; Schleiermacher et al. 2010, 2011; Stallings 2007). Accordingly, SH-SY5Y cells show trisomy of chromosome (chr) 7, a duplication of the q-arm of chr1, and further complex rearrangements on most chromosomes leading to both copy number gains and losses (Yusuf et al. 2013). Besides other loci of neurobiological interest, a copy number gain of the MAPT locus on chr17 was confirmed in different studies (Cohen et al. 2003; Do et al. 2009; Kryh et al. 2011; Spengler et al. 2002; Yusuf et al. 2013). This genetic arrangement of SH-SY5Y cells complicates the generation of homozygous MAPT mutant knock-out (KO) or knock-in cell lines, as it requires successful editing of presumably three MAPT gene copies, and it also impedes heterozygous edits, which usually lead to roughly 50% of affected proteins. The successful generation of a MAPT-KO SH-SY5Y cell line recently demonstrated that in fact CRISPR/Cas9-based homozygous MAPT editing is possible in SH-SY5Y cells (Sola et al. 2020).
Neuronal identity of SH-SY5Y-derived neurons
The susceptibility to TAU pathology varies drastically among different brain regions, neuronal subtypes and depending on the type of disease (Braak and Braak 1991; Braak and Del Tredici 2011; Dickson et al. 2011), as well as TAU expression levels, the subcellular TAU distribution, or the TAU isoform ratio (Goedert, Spillantini, Potier, et al. 1989). This raises the question whether TAU properties per se are crucial for the different susceptibility of different brain regions being affected by TAU pathology and TAU-mediated neurodegeneration. Thus, a cell model that mimics features of early affected brain regions would bear great potential for future research.
Undifferentiated SH-SY5Y cells are considered immature catecholaminergic neurons since they express markers of immature neurons (Lopes et al. 2010; Xie et al. 2010) and key proteins of the catecholaminergic metabolism (Cuende et al. 2008; Kovalevich and Langford 2013; Lopes et al. 2010; Oyarce and Fleming 1991; Xie et al. 2010). Interestingly, the reports about the neuronal identity of mature SH-SY5Y-derived neurons vary depending on the substances used for differentiation protocols (Kovalevich and Langford 2013) (see Figure 2 for summary). The most common and often-used substance, the vitamin A derivative RA, was shown to elevate the levels of activated choline acetyltransferase, which is typical for cholinergic neurons (Adem et al. 1987; Lopes et al. 2010; Presgraves et al. 2003). However, the cholinergic character of RA-treated cells is under debate, as the expression of noradrenaline (Påhlman et al. 1984) and of the vesicular monoamine transporter, a key enzyme of catecholaminergic neurons, was reported in some studies (Lopes et al. 2010; Presgraves et al. 2003), but not in others (Encinas et al. 2000). Another common differentiation procedure, the combinatorial treatment of RA and the BDNF, results in extensively branched neurons that are categorized based on the expression of marker proteins either as noradrenergic (Encinas et al. 2000), dopaminergic (Neuhaus et al. 2014) or cholinergic (de Medeiros et al. 2019). Besides RA and BDNF, phorbol esters (e.g., 12-O-Tetradecanoyl-phorbol-13-acetate (TPA)) (Murphy et al. 1991; Påhlman et al. 1981, 1984; Scott et al. 1986), dibutyryl-cAMP (db-cAMP) (Itano et al. 1996; Kume et al. 2008; Sánchez et al. 2004) or other drugs are used alone or in combination (Påhlman et al. 1984; Simpson et al. 2001) to generate SH-SY5Y-derived neurons with varying neuronal identity, e.g., noradrenergic (TPA, db-cAMP) or dopaminergic (RA+TPA).
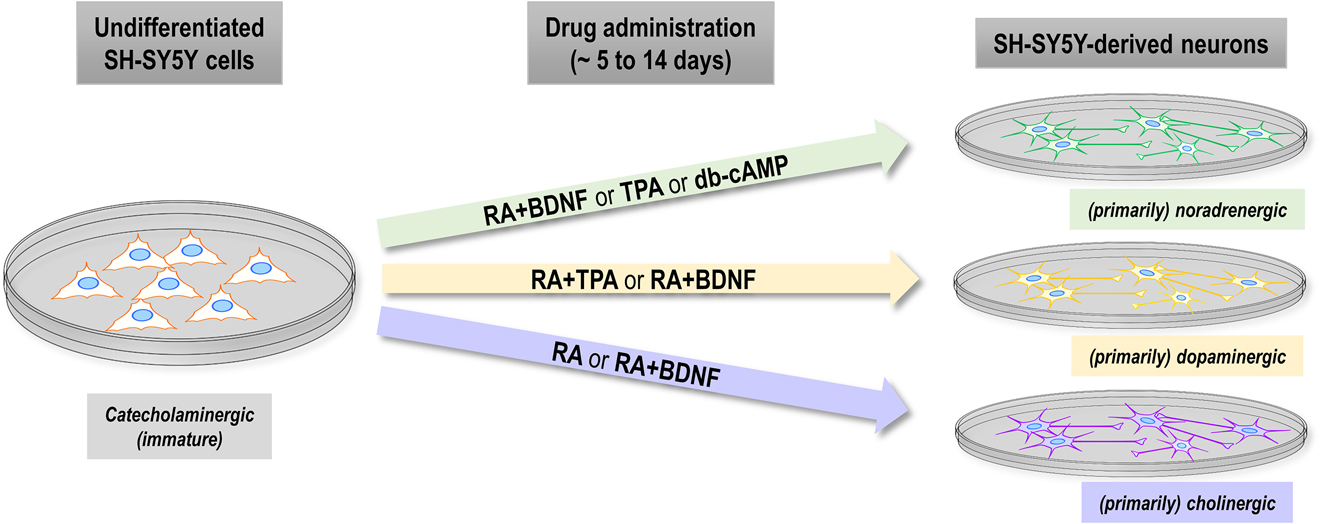
Drug-dependent neuronal identity of SH-SY5Y-derived neurons mimics features of noradrenergic, dopaminergic or cholinergic neurons.
Overview of the reported neuronal identity for undifferentiated SH-SY5Y cells (left) and SH-SY5Y-derived neurons (right) with respect to commonly administered substances (middle) for differentiation. Undifferentiated SH-SY5Y display features of immature catecholaminergic neurons. Primarily noradrenergic neurons are reported after treatment with RA+BDNF, TPA or db-cAMP, primarily dopaminergic neurons after treatment with RA+TPA or RA+BDNF. Neurons with a primarily cholinergic identity result from differentiation with RA or with RA+BDNF. Administration with two drugs always refers to sequential treatment in the order of appearance. Duration of drug administration varies between protocols but is usually between 5 and 14 days. Please note: The reports about the neuronal identity of SH-SY5Y cells upon differentiation, which is visualized here, are partially inconsistent and question the generation of clearly distinct neuronal subtypes (see Chapter ‘Neuronal identity of SH-SY5Y-derived neurons’ for discussion).
Taken together, the classification of SH-SY5Y-derived neurons may depend on the applied substances and be influenced by the focuses of the actual study. It is, however, certain that SH-SY5Y-derived neurons display some key features of noradrenergic, dopaminergic and cholinergic neurons. This gives rise to both (i) the potential of SH-SY5Y-derived neurons for studies on neuronal subtype-specific AD/tauopathy susceptibility and (ii) the accompanying challenges, including the resemblance of age-related risk factors, as summarized and discussed below (see Table 2 for summary).
Features and challenges of the neuronal identity of SH-SY5Y-derived neurons.
| Features |
|
| Challenges |
|
Features
In the progression of AD and other neurodegenerative diseases, certain brain regions are typically early affected while other regions show pathological alterations only in late disease stages. In several subcortical nuclei, considerable neuronal loss can be observed in initial disease stages or even pre-clinically (Arendt et al. 2016). These subcortical nuclei are, amongst others, the nucleus basalis of Meynert (NBM, containing mainly cholinergic neurons), the substantia nigra pars compacta (SNc, dopaminergic neurons) and the locus coeruleus (LC, noradrenergic neurons) (Lyness et al. 2003). The formation of TAU-containing NFT’s in NBM neurons and massive depletion of acetylcholine within cortical and hippocampal regions, resulting from a loss of NBM cholinergic projections, coincide with early clinical symptoms of AD (Bowen et al. 1976; Davies and Maloney 1976; Lyness et al. 2003; Mesulam et al. 2004; Mufson et al. 2000, 2002; Sassin et al. 2000). Within the SNc, TAU-NFT formation, pigmented neuronal loss and other pathological alterations are found in AD (Burns et al. 2005; Ditter and Mirra 1987; Forstl et al. 1994; Gibb et al. 1989; Storga et al. 1996; Tabaton et al. 1985) and other tauopathies (Spillantini et al. 1997, 1998). The noradrenergic neurons of the LC complex are early affected by NFT formation and degeneration in AD patients (Aston-Jones and Cohen 2005; Baker et al. 1989; Braak and Del Tredici 2004; Busch et al. 1997; German et al. 1992; Grudzien et al. 2007), and seem to become compromised even in young adults without any clinical phenotype (Braak and Del Tredici 2011).
While comprehensive descriptions of TAU-NFT formation and neuronal loss in these subcortical nuclei are available, the pathomechanisms underlying their vulnerability are still elusive (Satoh and Iijima 2019). Since SH-SY5Y-derived neurons share properties of LC, NBM or SNc neurons, they may be a powerful tool for the evaluation of subtype-dependent vulnerability. This is particularly true as the TAU physiology specific for these neurons may contribute to their increased vulnerability. Several aspects mimicking neuronal TAU physiology are observable in SH-SY5Y-derived neurons: polarized subcellular distribution, isoform expression levels and ratios comparable to the human brain, and the possibility to induce NFT-like TAU hyperphosphorylation and oligomerization by phosphatase inhibition (see Chapter ‘TAU phosphorylation state’).
Challenges
Besides the advantages of SH-SY5Y-derived neurons for studying neuron subtype-specific TAU vulnerability, some limitations that have to be considered. As for all cellular models of AD or related tauopathies, which are mainly age-dependent disorders (Fjell et al. 2014; Lindemer et al. 2017; Tarantini et al. 2017; West et al. 1994), it is questionable whether up to three-week-old neuronal cultures can resemble the cellular properties of subcortical neurons in the brain of aged AD patients. Furthermore, the expression profiles, e.g., of RA- and RA/BDNF-treated cells, appear inconsistent among different studies, and it remains questionable whether the neuronal subtype can be clearly defined. This is a non-negligible issue since a more comprehensive biochemical characterization of the generated neurons would be expensive and time-consuming, without the guarantee of a conclusive outcome. Indeed, the available data rather suggest that SH-SY5Y-derived neurons do not resemble segregated and distinct neuronal subtypes, which can be separated by protein expression or transmitter release, but rather exhibit different manifestations of a gradual neuronal entity.
Another obstacle of using SH-SY5Y-derived neurons may be the general problem of transferring findings from (mostly 2-D) cell cultures to the brain or living organisms. Cell culture systems lack the direct (non-neuronal) cellular environment and also synaptic input from other brain regions. Besides that, major intrinsic features of LC, NBM and SNc neurons are difficult to recapitulate in cell culture. Several characteristics of subcortical neurons, which are hard to model in vitro, are thought to have a massive impact on the susceptibility for TAU pathology of those neurons. For the noradrenergic LC neurons (Satoh and Iijima 2019), this includes (i) the up to several cm-long, thin and poorly myelinated, heavily branched axons spanning throughout the cortex without relay, which leads to high energy demand and oxidative stress (Braak and Braak 1996; Braak et al. 2003; German et al. 1987) (SH-SY5Y-derived neurons: axons range roughly between 50 and 150 µm for RA, TPA or db-cAMP treatment (Kume et al. 2008; Påhlman et al. 1981; Presgraves et al. 2003; Simpson et al. 2001) and up to 200 µm and more for RA/BDNF (Encinas et al. 2000; Shipley et al. 2016), and show only moderate branching), (ii) increased energy demand and reactive oxygen species (ROS) production because of the tonic activity (Sanchez-Padilla et al. 2014) (SH-SY5Y-derived neurons: increased excitability and membrane potentials (Åkerman et al. 1984; Brown et al. 2005; Tosetti et al. 1998) but no tonic activity), and (iii) elevated exposure to toxins and pathogens as LC neurons innervate the brain capillary system and associated astrocyte end feet (Cohen et al. 1997; Kisler et al. 2017; Pamphlett 2014). As all subcortical nuclei share great similarities regarding morphology and innervation (Ramón-Moliner and Nauta 1966; Rossor 1981), the mentioned risk factors may be largely true for NBM and SNc neurons, as well (Braak and Braak 1996; Braak et al. 2003).
In brief, the targeted differentiation of SH-SY5Y cells into neurons with features of noradrenergic, dopaminergic or cholinergic neurons bears great potential for research on AD-selective vulnerability since the mimicked subcortical nuclei are early affected in AD patients. However, the generation of distinct neuronal subtypes does not appear clearly defined with current differentiation procedures, and SH-SY5Y-derived neurons lack major characteristics of their in vivo correlates, which might be crucial for tauopathy-related vulnerability.
Conclusion
Human-derived SH-SY5Y neuroblastoma cells are robust, cheap, highly proliferative, and can be differentiated into neuronal cells with straight-forward protocols. Although the maturity of SH-SY5Y-derived neurons is debatable, they meet several requirements for TAU sorting research: SH-SY5Y-derived neurons exhibit (i) pronounced neuronal polarity after several days of differentiation, (ii) high levels of total TAU protein, (iii) expression of all major human isoforms, (iv) efficient axonal targeting of TAU protein, and (v) a human brain-like TAU phosphorylation state. Further, SH-SY5Y cells are accessible for genetic manipulation, i.e., stable integration of recombinant TAU transgenes and the editing of the MAPT locus through recent CRISPR/Cas9-based methods prior to neuronal differentiation.
SH-SY5Y-derived neurons resemble, depending on the used treatment, neuron subtypes of distinct subcortical LC, NBM and SNc nuclei that are severely affected in AD and other tauopathies. This steerable differentiation bears great potential for comparative studies of neuron-specific TAU expression patterns, intracellular localization and vulnerability to TAU pathology. However, there are inherent limitations regarding the translatability from SH-SY5Y-derived to subcortical neurons, e.g., the lack of age-dependent risk factors, the difficulty of defining the exact neuronal subtype or the lack of brain-spanning projections (on a cm-scale) leading to high energy demands and oxidative stress. These caveats have to be considered when addressing cell type-specific vulnerability in SH-SY5Y-derived neurons.
Taken together, the properties of SH-SY5Y-derived neurons discussed in this review make them a powerful neuronal cell model for investigating at least certain mechanisms of and requirements for axonal TAU sorting under human-like conditions.
Funding source: Else Kröner-Fresenius-Stiftung
Funding source: Köln Fortune
Funding source: Studienstiftung des Deutschen Volkes
Acknowledgements
We thank Sarah Bachmann and others for critical manuscript revision.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was funded by Else-Kröner-Fresenius Stiftung, Köln Fortune, and supported by a doctoral fellowship of the Studienstiftung des deutschen Volkes.
-
Conflict of interest statement: The authors declare that they have no competing interests.
References
Adem, A., Mattsson, M.E.K., Nordberg, A., and Påhlman, S. (1987). Muscarinic receptors in human SH-SY5Y neuroblastoma cell line: regulation by phorbol ester and retinoic acid-induced differentiation. Dev. Brain Res. 33: 235–242, https://doi.org/10.1016/0165-3806(87)90156-8.Suche in Google Scholar
Agholme, L., Lindström, T., Kgedal, K., Marcusson, J., and Hallbeck, M. (2010). An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheim. Dis. 20: 1069–1082.10.3233/JAD-2010-091363Suche in Google Scholar
Åkerman, K.E.O., Scott, I.G., and Andersson, L.C. (1984). Functional differentiation of a human ganglion cell derived neuroblastoma cell line SH-SY5Y induced by a phorbol ester (TPA). Neurochem. Int. 6: 77–80.10.1016/0197-0186(84)90029-9Suche in Google Scholar
Alavi Naini, S.M., and Soussi-Yanicostas, N. (2015). Tau hyperphosphorylation and oxidative stress, a critical vicious circle in neurodegenerative tauopathies? Oxid. Med. Cell. Longev. 2015: 151979.10.1155/2015/151979Suche in Google Scholar PubMed PubMed Central
Ambros, P.F., Ambros, I.M., Brodeur, G.M., Haber, M., Khan, J., Nakagawara, A., Schleiermacher, G., Speleman, F., Spitz, R., London, W.B., et al. (2009). International consensus for neuroblastoma molecular diagnostics: report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br. J. Canc. 100: 1471–1482, https://doi.org/10.1038/sj.bjc.6605014.Suche in Google Scholar PubMed PubMed Central
Andorfer, C., Kress, Y., Espinoza, M., De Silva, R., Tucker, K.L., Barde, Y.A., Duff, K., and Davies, P. (2003). Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 86: 582–590, https://doi.org/10.1046/j.1471-4159.2003.01879.x.Suche in Google Scholar PubMed
Anzalone, A.V., Randolph, P.B., Davis, J.R., Sousa, A.A., Koblan, L.W., Levy, J.M., Chen, P.J., Wilson, C., Newby, G.A., Raguram, A., et al. (2019). Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576: 149–157, https://doi.org/10.1038/s41586-019-1711-4.Suche in Google Scholar PubMed PubMed Central
Arendt, T., Stieler, J.T., and Holzer, M. (2016). Tau and tauopathies. Brain Res. Bull. 126: 238–292, https://doi.org/10.1016/j.brainresbull.2016.08.018.Suche in Google Scholar PubMed
Aston-Jones, G., and Cohen, J.D. (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28: 403–450.10.1146/annurev.neuro.28.061604.135709Suche in Google Scholar PubMed
Bachmann, S., Bell, M., Klimek, J., and Zempel, H. (2020). Subcellular localization of TAU isoforms and their influence on microtubule dynamics. bioRxiv: 2020.06.16.154757.Suche in Google Scholar
Baker, K.G., Törk, I., Hornung, J.P., and Halasz, P. (1989). The human locus coeruleus complex: an immunohistochemical and three dimensional reconstruction study. Exp. Brain Res. 77: 257–270, https://doi.org/10.1007/bf00274983.Suche in Google Scholar
Bao, L., Chen, S.J., Conrad, K., Keefer, K., Abraham, T., Lee, J.P., Wang, J.F., Zhang, X.Q., Hirschler-Laszkiewicz, I., Wang, H.G., et al. (2016). Depletion of the human ion channel TRPM2 in neuroblastoma demonstrates its key role in cell survival through modulation of mitochondrial reactive oxygen species and bioenergetics. J. Biol. Chem. 291: 24449–24464, https://doi.org/10.1074/jbc.m116.747147.Suche in Google Scholar
Bell, M., Bachmann, S., Klimek, J., Langerscheidt, F., and Zempel, H. (2021). Axonal TAU sorting is independent of ANKG and TRIM46 enrichment at the AIS in SH-SY5Y-derived neurons. Neuroscience, 461: 155–171, https://doi.org/10.1016/j.neuroscience.2021.01.041.Suche in Google Scholar
Biedler, J.L., and Schachner, M. (1978). Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 38: 3751–3757.Suche in Google Scholar
Biernat, J., Gustke, N., Drewes, G., Mandelkow, E., and Mandelkow, E. (1993). Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11: 153–163, https://doi.org/10.1016/0896-6273(93)90279-z.Suche in Google Scholar
Binder, L.I., Frankfurter, A., and Rebhun, L.I. (1985). The distribution of tau in the mammalian central nervous central nervous. J. Cell Biol. 101: 1371–1378, https://doi.org/10.1083/jcb.101.4.1371.Suche in Google Scholar PubMed PubMed Central
Boban, M., Babić Leko, M., Miškić, T., Hof, P.R., and Šimić, G. (2019). Human neuroblastoma SH-SY5Y cells treated with okadaic acid express phosphorylated high molecular weight tau-immunoreactive protein species. J. Neurosci. Methods 319: 60–68, https://doi.org/10.1016/j.jneumeth.2018.09.030.Suche in Google Scholar PubMed PubMed Central
Bowen, D.M., Smith, C.B., White, P., and Davison, A.N. (1976). Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain 99: 459–496, https://doi.org/10.1093/brain/99.3.459.Suche in Google Scholar PubMed
Braak, H., and Braak, E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82: 239–259, https://doi.org/10.1007/bf00308809.Suche in Google Scholar PubMed
Braak, H., and Braak, E. (1996). Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol. 92: 197–201, https://doi.org/10.1007/s004010050508.Suche in Google Scholar PubMed
Braak, H., Rüb, U., Gai, W.P., and Del Tredici, K. (2003). Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural. Transm. 110: 517–536, https://doi.org/10.1007/s00702-002-0808-2.Suche in Google Scholar PubMed
Braak, H., and Del Tredici, K. (2004). Alzheimer’s disease: intraneuronal alterations precede insoluble amyloid-β formation. Neurobiol. Aging 25: 713–718, doi:https://doi.org/10.1016/j.neurobiolaging.2003.12.015.Suche in Google Scholar PubMed
Braak, H., and Del Tredici, K. (2011). The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 121: 171–181, https://doi.org/10.1007/s00401-010-0789-4.Suche in Google Scholar
Brion, J.P., Passareiro, H., Nunez, J., and Flament-Durand, J. (1985). Mise En Evidence Immunologique De La Proteine Tau Au Niveau Des Lesions De Degenerescence Neurofibrillaire De La Maladie D Alzheimer. Arch. Biol. 96: 229–235.Suche in Google Scholar
Brown, A.M., Riddoch, F.C., Robson, A., Redfern, C.P.F., and Cheek, T.R. (2005). Mechanistic and functional changes in Ca2+ entry after retinoic acid-induced differentiation of neuroblastoma cells. Biochem. J. 388: 941–948, https://doi.org/10.1042/bj20042127.Suche in Google Scholar
Buée, L., Bussière, T., Buée-Scherrer, V., Delacourte, A., and Hof, P.R. (2000). Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev. 33: 95–130, doi:https://doi.org/10.1016/s0165-0173(00)00019-9.Suche in Google Scholar
Burns, J.M., Galvin, J.E., Roe, C.M., Morris, J.C., and McKeel, D.W. (2005). The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology 64: 1397–1403, https://doi.org/10.1212/01.wnl.0000158423.05224.7f.Suche in Google Scholar
Busch, C., Bohl, J., and Ohm, T.G. (1997). Spatial, temporal and numeric analysis of Alzheimer changes in the nucleus coeruleus. Neurobiol. Aging 18: 401–406, https://doi.org/10.1016/s0197-4580(97)00035-3.Suche in Google Scholar
Chan, E.W.L., Krishnansamy, S., Wong, C., and Gan, S.Y. (2019). The NLRP3 inflammasome is involved in the neuroprotective mechanism of neural stem cells against microglia-mediated toxicity in SH-SY5Y cells via the attenuation of tau hyperphosphorylation and amyloidogenesis. Neurotoxicology 70: 91–98, https://doi.org/10.1016/j.neuro.2018.11.001.Suche in Google Scholar PubMed
Chang, K.H., Chen, I.C., Lin, H.Y., Chen, H.C., Lin, C.H., Lin, T.H., Weng, Y.T., Chao, C.Y., Wu, Y.R., Lin, J.Y., et al. (2016). The aqueous extract of Glycyrrhiza inflata can upregulate unfolded protein response-mediated chaperones to reduce tau misfolding in cell models of Alzheimer’s disease. Drug Des. Dev. Ther. 10: 885–896.10.2147/DDDT.S96454Suche in Google Scholar PubMed PubMed Central
Chen, I.C., Lin, T.H., Hsieh, Y.H., Chao, C.Y., Wu, Y.R., Chang, K.H., Lee, M.C., Lee-Chen, G.J., and Chen, C.M. (2018). Formulated Chinese medicine shaoyao gancao tang reduces tau aggregation and exerts neuroprotection through anti-oxidation and anti-inflammation. Oxid. Med. Cell. Longev. 2018: 9595741. https://doi.org/10.1155/2018/9595741.Suche in Google Scholar PubMed PubMed Central
Chen, L., Zhou, L., Yu, P., Fang, F., Jiang, L., Fei, J., Xiao, H., and Wang, J. (2019). Methamphetamine exposure upregulates the amyloid precursor protein and hyperphosphorylated tau expression: the roles of insulin signaling in sh-sy5y cell line. J. Toxicol. Sci. 44: 493–503, https://doi.org/10.2131/jts.44.493.Suche in Google Scholar PubMed
Cheung, Y.T., Lau, W.K.W., Yu, M.S., Lai, C.S.W., Yeung, S.C., So, K.F., and Chang, R.C.C. (2009). Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 30: 127–135, https://doi.org/10.1016/j.neuro.2008.11.001.Suche in Google Scholar PubMed
Cleveland, D.W., Hwo, S.Y., and Kirschner, M.W. (1977a). Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J. Mol. Biol. 116: 227–247, https://doi.org/10.1016/0022-2836(77)90214-5.Suche in Google Scholar
Cleveland, D.W., Hwo, S.Y., and Kirschner, M.W. (1977b). Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J. Mol. Biol. 116: 207–225, https://doi.org/10.1016/0022-2836(77)90213-3.Suche in Google Scholar
Cohen, N., Betts, D.R., Rechavi, G., Amariglio, N., and Trakhtenbrot, L. (2003). Clonal expansion and not cell interconversion is the basis for the neuroblast and nonneuronal types of the SK-N-SH neuroblastoma cell line. Canc. Genet. Cytogenet. 143: 80–84, https://doi.org/10.1016/s0165-4608(02)00835-x.Suche in Google Scholar
Cohen, Z., Molinatti, G., and Hamel, E. (1997). Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J. Cerebr. Blood Flow Metabol. 17: 894–904, https://doi.org/10.1097/00004647-199708000-00008.Suche in Google Scholar
Couchie, D., Mavilia, C., Georgieff, I.S., Liem, R.K.H., Shelanski, M.L., and Nunez, J. (1992). Primary structure of high molecular weight tau present in the peripheral nervous system. Proc. Natl. Acad. Sci. U.S.A. 89: 4378–4381, https://doi.org/10.1073/pnas.89.10.4378.Suche in Google Scholar
Cuende, J., Moreno, S., Bolaños, J.P., and Almeida, A. (2008). Retinoic acid downregulates Rae1 leading to APCCdh1 activation and neuroblastoma SH-SY5Y differentiation. Oncogene 27: 3339–3344, https://doi.org/10.1038/sj.onc.1210987.Suche in Google Scholar
David, D.C., Layfield, R., Serpell, L., Narain, Y., Goedert, M., and Spillantini, M.G. (2002). Proteasomal degradation of tau protein. J. Neurochem. 83: 176–185, https://doi.org/10.1046/j.1471-4159.2002.01137.x.Suche in Google Scholar
Davies, P., and Maloney, A.J.F. (1976). Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 308: 1403, https://doi.org/10.1016/s0140-6736(76)91936-x.Suche in Google Scholar
Delobel, P., Mailliot, C., Hamdane, M., Sambo, A.V., Bégard, S., Violleau, A., Delacourte, A., and Buée, L. (2003). Stable-tau overexpression in human neuroblastoma cells: an open door for explaining neuronal death in tauopathies. Ann. N. Y. Acad. Sci. 1010: 623–634, doi:https://doi.org/10.1196/annals.1299.115.Suche in Google Scholar PubMed
Dickson, D.W., Kouri, N., Murray, M.E., and Josephs, K.A. (2011). Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J. Mol. Neurosci. 45: 384–389, doi:https://doi.org/10.1007/s12031-011-9589-0.Suche in Google Scholar PubMed PubMed Central
Ditter, S.M., and Mirra, S.S. (1987). Neuropathologic and clinical features of Parkinson’s disease in Alzheimer’s disease patients. Neurology 37: 754–760, https://doi.org/10.1212/wnl.37.5.754.Suche in Google Scholar
Do, J.H., Ko, H.M., Suk, K., Park, E.J., and Choi, D.K. (2009). Genome-wide inspection of chromosomal aberrations in microglia BV-2 cells by array-based comparative genomic hybridization. Biochip J. 3: 28–36.Suche in Google Scholar
Duff, K., Knight, H., Refolo, L.M., Sanders, S., Yu, X., Picciano, M., Malester, B., Hutton, M., Adamson, J., Goedert, M., et al. (2000). Characterization of pathology in transgenic mice over-expressing human genomic and cDNA tau transgenes. Neurobiol. Dis. 7: 87–98, https://doi.org/10.1006/nbdi.1999.0279.Suche in Google Scholar
Dupont-Wallois, L., Sautière, P.E., Cocquerelle, C., Bailleul, B., Delacourte, A., and Caillet-Boudin, M.L. (1995). Shift from fetal-type to Alzheimer-type phosphorylated tau proteins in SKNSH-SY 5Y cells treated with okadaic acid. FEBS Lett. 357: 197–201, https://doi.org/10.1016/0014-5793(94)01361-4.Suche in Google Scholar
Encinas, M., Iglesias, M., Liu, Y., Wang, H., Muhaisen, A., Ceña, V., Gallego, C., and Comella, J.X. (2000). Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J. Neurochem. 75: 991–1003, https://doi.org/10.1046/j.1471-4159.2000.0750991.x.Suche in Google Scholar
Fjell, A.M., McEvoy, L., Holland, D., Dale, A.M., and Walhovd, K.B. (2014). What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog. Neurobiol. 117: 20–40, doi:https://doi.org/10.1016/j.pneurobio.2014.02.004.Suche in Google Scholar
Forstl, H., Levy, R., Burns, A., Luthert, P., and Cairns, N. (1994). Disproportionate loss of noradrenergic and cholinergic neurons as cause of depression in Alzheimer’s disease – a hypothesis. Pharmacopsychiatry 27: 11–15, https://doi.org/10.1055/s-2007-1014267.Suche in Google Scholar
George, R.E., Attiyeh, E.F., Li, S., Moreau, L.A., Neuberg, D., Li, C., Fox, E.A., Meyerson, M., Diller, L., and Fortina, P., et al. (2007). Genome-wide analysis of neuroblastomas using high-density single nucleotide polymorphism arrays. PLoS One 2: e255, doi:https://doi.org/10.1371/journal.pone.0000255.Suche in Google Scholar
German, D.C., Manaye, K.F., White, C.L., Woodward, D.J., McIntire, D.D., Smith, W.K., Kalaria, R.N., and Mann, D.M.A. (1992). Disease‐specific patterns of locus coeruleus cell loss. Ann. Neurol. 32: 667–676, https://doi.org/10.1002/ana.410320510.Suche in Google Scholar
German, D.C., White, C.L., and Sparkman, D.R. (1987). Alzheimer’s disease: neurofibrillary tangles in nuclei that project to the cerebral cortex. Neuroscience 21: 305–312, https://doi.org/10.1016/0306-4522(87)90123-0.Suche in Google Scholar
Gibb, W.R.G., Mountjoy, C.Q., Mann, D.M.A., and Lees, A.J. (1989). The substantia nigra and ventral tegmental area in Alzheimer’s disease and Down’s syndrome. J. Neurol. Neurosurg. Psychiatry 52: 193–200, https://doi.org/10.1136/jnnp.52.2.193.Suche in Google Scholar PubMed PubMed Central
Goedert, M., Crowther, R.A., and Garner, C.C. (1991). Molecular characterization of microtubule-associated proteins tau and map2. Trends Neurosci. 14: 193–199, doi:https://doi.org/10.1016/0166-2236(91)90105-4.Suche in Google Scholar
Goedert, M., and Jakes, R. (1990). Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J. 9: 4225–4230, https://doi.org/10.1002/j.1460-2075.1990.tb07870.x.Suche in Google Scholar
Goedert, M., Spillantini, M.G., Jakes, R., Rutherford, D., and Crowther, R.A. (1989). Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3: 519–526, https://doi.org/10.1016/0896-6273(89)90210-9.Suche in Google Scholar
Goedert, M., Spillantini, M.G., Potier, M.C., Ulrich, J., and Crowther, R.A. (1989). Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J. 8: 393–399, https://doi.org/10.1002/j.1460-2075.1989.tb03390.x.Suche in Google Scholar
Götz, J., Probst, A., Spillantini, M.G., Schäfer, T., Jakes, R., Bürki, K., and Goedert, M. (1995). Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J. 14: 1304–1313, https://doi.org/10.1002/j.1460-2075.1995.tb07116.x.Suche in Google Scholar
Grudzien, A., Shaw, P., Weintraub, S., Bigio, E., Mash, D.C., and Mesulam, M.M. (2007). Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol. Aging 28: 327–335, https://doi.org/10.1016/j.neurobiolaging.2006.02.007.Suche in Google Scholar
Gunhanlar, N., Shpak, G., van der Kroeg, M., Gouty-Colomer, L.A., Munshi, S.T., Lendemeijer, B., Ghazvini, M., Dupont, C., Hoogendijk, W.J.G., Gribnau, J., et al. (2018). A simplified protocol for differentiation of electrophysiologically mature neuronal networks from human induced pluripotent stem cells. Mol. Psychiatr. 23: 1336–1344, https://doi.org/10.1038/mp.2017.56.Suche in Google Scholar
Hasegawa, M., Jakes, R., Crowther, R.A., Lee, V.M.Y., Ihara, Y., and Goedert, M. (1996). Characterization of mAb AP422, a novel phosphorylation-dependent monoclonal antibody against tau protein. FEBS Lett. 384: 25–30, https://doi.org/10.1016/0014-5793(96)00271-2.Suche in Google Scholar
Hashimoto, S., Matsuba, Y., Kamano, N., Mihira, N., Sahara, N., Takano, J., Muramatsu, S.I., Saido, T.C., and Saito, T. (2019). Tau binding protein CAPON induces tau aggregation and neurodegeneration. Nat. Commun. 10: 2394, https://doi.org/10.1038/s41467-019-10278-x.Suche in Google Scholar PubMed PubMed Central
He, Z., McBride, J.D., Xu, H., Changolkar, L., Kim, S.J., Zhang, B., Narasimhan, S., Gibbons, G.S., Guo, J.L., Kozak, M., et al. (2020). Transmission of tauopathy strains is independent of their isoform composition. Nat. Commun. 11: 7, https://doi.org/10.1038/s41467-019-13787-x.Suche in Google Scholar PubMed PubMed Central
Hernandez, P., Lee, G., Sjoberg, M., and MacCioni, R.B. (2009). Tau phosphorylation by cdk5 and Fyn in response to amyloid peptide Aβ25-35: involvement of lipid rafts. J. Alzheim. Dis. 16: 149–156, https://doi.org/10.3233/jad-2009-0933.Suche in Google Scholar
Hill, A.J., Jones, N.A., Smith, I., Hill, C.L., Williams, C.M., Stephens, G.J., and Whalley, B.J. (2014). Voltage-gated sodium (NaV) channel blockade by plant cannabinoids does not confer anticonvulsant effects per se. Neurosci. Lett. 566: 269–274, https://doi.org/10.1016/j.neulet.2014.03.013.Suche in Google Scholar
Hu, B.Y., Weick, J.P., Yu, J., Ma, L.X., Zhang, X.Q., Thomson, J.A., and Zhang, S.C. (2010). Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. U.S.A. 107: 4335–4340, https://doi.org/10.1073/pnas.0910012107.Suche in Google Scholar
Itano, Y., Ito, A., Uehara, T., and Nomura, Y. (1996). Regulation of Bcl-2 protein expression in human neuroblastoma SH-SY5Y cells: positive and negative effects of protein kinases C and A, respectively. J. Neurochem. 67: 131–137, https://doi.org/10.1046/j.1471-4159.1996.67010131.x.Suche in Google Scholar
Jahn, K., Wieltsch, C., Blumer, N., Mehlich, M., Pathak, H., Khan, A.Q., Hildebrandt, H., and Frieling, H. (2017). A cell culture model for investigation of synapse influenceability: epigenetics, expression and function of gene targets important for synapse formation and preservation in SH-SY5Y neuroblastoma cells differentiated by retinoic acid. J. Neural. Transm. 124: 1341–1367, https://doi.org/10.1007/s00702-017-1769-9.Suche in Google Scholar
Janke, C., Beck, M., Stahl, T., Holzer, M., Brauer, K., Bigl, V., and Arendt, T. (1999). Phylogenetic diversity of the expression of the microtubule-associated protein tau: implications for neurodegenerative disorders. Mol. Brain Res. 68: 119–128, https://doi.org/10.1016/s0169-328x(99)00079-0.Suche in Google Scholar
Johansson, S. (1994). Graded action potentials generated by differentiated human neuroblastoma cells. Acta Physiol. Scand. 151: 331–341, https://doi.org/10.1111/j.1748-1716.1994.tb09752.x.Suche in Google Scholar PubMed
Kempf, M., Clement, A., Faissner, A., Lee, G., and Brandt, R. (1996). Tau binds to the distal axon early in development of polarity in a microtubule- and microfilament-dependent manner. J. Neurosci. 16: 5583–5592, https://doi.org/10.1523/jneurosci.16-18-05583.1996.Suche in Google Scholar
Kim, H., Ham, S., Jo, M., Lee, G.H., Lee, Y.S., Shin, J.H., and Lee, Y. (2017). CRISPR-Cas9 mediated telomere removal leads to mitochondrial stress and protein aggregation. Int. J. Mol. Sci. 18: 2093, doi:https://doi.org/10.3390/ijms18102093.Suche in Google Scholar PubMed PubMed Central
Kim, S., Yun, S.P., Lee, S., Umanah, G.E., Bandaru, V.V.R., Yin, X., Rhee, P., Karuppagounder, S.S., Kwon, S.H., Lee, H., et al. (2018). GBA1 deficiency negatively affects physiological α-synuclein tetramers and related multimers. Proc. Natl. Acad. Sci. U.S.A. 115: 798–803, https://doi.org/10.1073/pnas.1700465115.Suche in Google Scholar PubMed PubMed Central
Kisler, K., Nelson, A.R., Montagne, A., and Zlokovic, B.V. (2017). Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 18: 419–434, doi:https://doi.org/10.1038/nrn.2017.48.Suche in Google Scholar
Kneynsberg, A., Vega, I., and Kanaan, N.M. (2019). TRIM46 knockdown causes neuronal tau redistribution and increases axosomatic tau diffusion. Alzheimer’s Dement. 15: 1622.10.1016/j.jalz.2019.06.4852Suche in Google Scholar
Kopke, E., Tung, Y.C., Shaikh, S., Del Alonso, C.A., Iqbal, K., and Grundke-Iqbal, I. (1993). Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J. Biol. Chem. 268: 24374–24384, https://doi.org/10.1016/s0021-9258(20)80536-5.Suche in Google Scholar
Korolev, I.O. (2014). Alzheimer’s disease : a clinical and basic science review. Med. Stud. Res. J. 4: 24–33.Suche in Google Scholar
Kovalevich, J., and Langford, D. (2013). Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 1078: 9–21, https://doi.org/10.1007/978-1-62703-640-5_2.Suche in Google Scholar PubMed PubMed Central
Kryh, H., Carén, H., Erichsen, J., Sjöberg, R.M., Abrahamsson, J., Kogner, P., and Martinsson, T. (2011). Comprehensive SNP array study of frequently used neuroblastoma cell lines; copy neutral loss of heterozygosity is common in the cell lines but uncommon in primary tumors. BMC Genom. 12: 443, doi:https://doi.org/10.1186/1471-2164-12-443.Suche in Google Scholar PubMed PubMed Central
Kume, T., Kawato, Y., Osakada, F., Izumi, Y., Katsuki, H., Nakagawa, T., Kaneko, S., Niidome, T., Takada-Takatori, Y., and Akaike, A. (2008). Dibutyryl cyclic AMP induces differentiation of human neuroblastoma SH-SY5Y cells into a noradrenergic phenotype. Neurosci. Lett. 443: 199–203, https://doi.org/10.1016/j.neulet.2008.07.079.Suche in Google Scholar PubMed
Lee, M., McGeer, E., and McGeer, P.L. (2015). Activated human microglia stimulate neuroblastoma cells to upregulate production of beta amyloid protein and tau: implications for Alzheimer’s disease pathogenesis. Neurobiol. Aging 36: 42–52, https://doi.org/10.1016/j.neurobiolaging.2014.07.024.Suche in Google Scholar PubMed
Lee, V.M.Y., Goedert, M., and Trojanowski, J.Q. (2001). Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24: 1121–1159, https://doi.org/10.1146/annurev.neuro.24.1.1121.Suche in Google Scholar PubMed
Lee Virginia, M.Y., Zhukareva, V., Vogelsberg-Ragaglia, V., Wszolek, Z., Reed, L., Miller, B.I., Geschwind, D.H., Bird, T.D., McKeel, D., Coate, A., et al. (1998). Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 282: 1914–1917.10.1126/science.282.5395.1914Suche in Google Scholar PubMed
Leterrier, C. (2016). The axon initial segment, 50 Years later: a nexus for neuronal organization and function. Curr. Top. Membr. 77: 185–233, https://doi.org/10.1016/bs.ctm.2015.10.005.Suche in Google Scholar PubMed
Leterrier, C. (2018). The axon initial segment: an updated viewpoint. J. Neurosci. 38: 2135–2145.10.1523/JNEUROSCI.1922-17.2018Suche in Google Scholar
Lin, H.C., He, Z., Ebert, S., Schörnig, M., Santel, M., Weigert, A., Hevers, W., Kasri, N.N., Taverna, E., Camp, J.G., et al. (2020). Ngn2 induces diverse neuronal lineages from human pluripotency. bioRxiv, https://doi.org/10.1101/2020.11.19.389445.Suche in Google Scholar
Lindemer, E.R., Greve, D.N., Fischl, B.R., Augustinack, J.C., and Salat, D.H. (2017). Regional staging of white matter signal abnormalities in aging and Alzheimer’s disease. NeuroImage Clin. 14: 156–165, https://doi.org/10.1016/j.nicl.2017.01.022.Suche in Google Scholar
Liu, Z., Wang, F., Tang, M., Zhao, Y., and Wang, X. (2019). Amyloid β and tau are involved in sleep disorder in Alzheimer’s disease by orexin A and adenosine A(1) receptor. Int. J. Mol. Med. 43: 435–442, https://doi.org/10.3892/ijmm.2018.3935.Suche in Google Scholar
Lopes, F.M., Schröder, R., da Frota Júnior, M.L.C., Zanotto-Filho, A., Müller, C.B., Pires, A.S., Meurer, R.T., Colpo, G.D., Gelain, D.P., Kapczinski, F., et al. (2010). Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res. 1337: 85–94, https://doi.org/10.1016/j.brainres.2010.03.102.Suche in Google Scholar
Luo, M.H., Tse, S.W., Memmott, J., and Andreadis, A. (2004). Novel isoforms of tau that lack the microtubule-binding domain. J. Neurochem. 90: 340–351, https://doi.org/10.1111/j.1471-4159.2004.02508.x.Suche in Google Scholar
Lyness, S.A., Zarow, C., and Chui, H.C. (2003). Neuron loss in key cholinergic and aminergic nuclei in Alzheimer disease: a meta-analysis. Neurobiol. Aging 24: 1–23, https://doi.org/10.1016/s0197-4580(02)00057-x.Suche in Google Scholar
Mailliot, C., Bussiére, T., Hamdane, M., Sergeant, N., Caillet, M.L., Delacourte, A., and Buée, L.U.C. (2000). Pathological tau phenotypes: the weight of mutations, polymorphisms, and differential neuronal vulnerabilities. Ann. N. Y. Acad. Sci. 920: 107–114, https://doi.org/10.1111/j.1749-6632.2000.tb06911.x.Suche in Google Scholar PubMed
Majd, S., Koblar, S., and Power, J. (2018). Compound C enhances tau phosphorylation at Serine396 via PI3K activation in an AMPK and rapamycin independent way in differentiated SH-SY5Y cells. Neurosci. Lett. 670: 53–61.10.1016/j.neulet.2018.01.049Suche in Google Scholar PubMed
Majd, S., Majd, Z., Koblar, S., and Power, J. (2018). Beta estradiol and norepinephrine treatment of differentiated SH-SY5Y cells enhances tau phosphorylation at (Ser396) and (Ser262) via AMPK but not mTOR signaling pathway. Mol. Cell. Neurosci. 88: 201–211, https://doi.org/10.1016/j.mcn.2018.02.004.Suche in Google Scholar PubMed
de Medeiros, L.M., De Bastiani, M.A., Rico, E.P., Schonhofen, P., Pfaffenseller, B., Wollenhaupt-Aguiar, B., Grun, L., Barbé-Tuana, F., Zimmer, E.R., Castro, M.A.A., et al. (2019). Cholinergic differentiation of human neuroblastoma SH-SY5Y cell line and its potential use as an in vitro model for alzheimer’s disease studies. Mol. Neurobiol. 56: 7355–7367, https://doi.org/10.1007/s12035-019-1605-3.Suche in Google Scholar
Mesulam, M., Shaw, P., Mash, D., and Weintraub, S. (2004). Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann. Neurol. 55: 815–828.10.1002/ana.20100Suche in Google Scholar
Morris, M., Knudsen, G.M., Maeda, S., Trinidad, J.C., Ioanoviciu, A., Burlingame, A.L., and Mucke, L. (2015). Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat. Neurosci. 18: 1183–1189.10.1038/nn.4067Suche in Google Scholar
Mufson, E.J., Ma, S.Y., Cochran, E.J., Bennett, D.A., Beckett, L.A., Jaffar, S., Saragovi, H.U., and Kordower, J.H. (2000). Loss of nucleus basalis neurons containing trkA immunoreactivity in individuals with mild cognitive impairment and early Alzheimer’s diseases. J. Comp. Neurol. 427: 19–30.10.1002/1096-9861(20001106)427:1<19::AID-CNE2>3.0.CO;2-ASuche in Google Scholar
Mufson, E.J., Ma, S.Y., Dills, J., Cochran, E.J., Leurgans, S., Wuu, J., Bennett, D.A., Jaffar, S., Gilmor, M.L., and Levey, A.I., et al. (2002). Loss of basal forebrain p75NTR immunoreactivity in subjects with mild cognitive impairment and Alzheimer’s disease. J. Comp. Neurol. 443: 136–153.10.1002/cne.10122Suche in Google Scholar
Muratore, C.R., Rice, H.C., Srikanth, P., Callahan, D.G., Shin, T., Benjamin, L.N.P., Walsh, D.M., Selkoe, D.J., and Young-Pearse, T.L. (2014). The familial Alzheimer’s disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum. Mol. Genet. 23: 3523–3536.10.1093/hmg/ddu064Suche in Google Scholar
Murphy, N.P., Ball, S.G., and Vaughan, P.F.T. (1991). Potassium‐ and carbachol‐evoked release of [3H]noradrenaline from human neuroblastoma cells, SH‐SY5Y. J. Neurochem. 56: 1810–1815.10.1111/j.1471-4159.1991.tb02085.xSuche in Google Scholar
Neuhaus, J.F.G., Baris, O.R., Hess, S., Moser, N., Schröder, H., Chinta, S.J., Andersen, J.K., Kloppenburg, P., and Wiesner, R.J. (2014). Catecholamine metabolism drives generation of mitochondrial DNA deletions in dopaminergic neurons. Brain 137: 354–365.10.1093/brain/awt291Suche in Google Scholar
Nie, S.D., Li, X., Tang, C.E., Min, F.Y., Shi, X.J., Wu, L.Y., Zhou, S.L., Chen, Z., Wu, J., and Song, T., et al. (2018). High glucose forces a positive feedback loop connecting ErbB4 expression and mTOR/S6K pathway to aggravate the formation of tau hyperphosphorylation in differentiated SH-SY5Y cells. Neurobiol. Aging 67: 171–180.10.1016/j.neurobiolaging.2018.03.023Suche in Google Scholar
Noble, W., Hanger, D.P., Miller, C.C.J., and Lovestone, S. (2013). The importance of tau phosphorylation for neurodegenerative diseases. Front. Neurol. 4: 83.10.3389/fneur.2013.00083Suche in Google Scholar
Nunez, J., and Fischer, I. (1997). Microtubule-associated proteins (MAPs). In the peripheral nervous system during development and regeneration. J. Mol. Neurosci. 8: 207–222.10.1007/BF02736834Suche in Google Scholar
Oyarce, A.M., and Fleming, P.J. (1991). Multiple forms of human dopamine β-hydroxylase in SH-SY5Y neuroblastoma cells. Arch. Biochem. Biophys. 290: 503–510, https://doi.org/10.1016/0003-9861(91)90573-2.Suche in Google Scholar
Påhlman, S., Odelstad, L., Larsson, E., Grotte, G., and Nilsson, K. (1981). Phenotypic changes of human neuroblastoma cells in culture induced by 12‐O‐tetradecanoyl‐phorbol‐13‐acetate. Int. J. Canc. 28: 583–589.10.1002/ijc.2910280509Suche in Google Scholar
Påhlman, S., Ruusala, A.I., Abrahamsson, L., Mattsson, M.E.K., and Esscher, T. (1984). Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ. 14: 135–144.10.1016/0045-6039(84)90038-1Suche in Google Scholar
Paik, S., Somvanshi, R.K., and Kumar, U. (2019). Somatostatin-mediated changes in microtubule-associated proteins and retinoic acid–induced neurite outgrowth in SH-SY5Y cells. J. Mol. Neurosci. 68: 120–134, https://doi.org/10.1007/s12031-019-01291-2.Suche in Google Scholar PubMed
Pamphlett, R. (2014). Uptake of environmental toxicants by the locus ceruleus: a potential trigger for neurodegenerative, demyelinating and psychiatric disorders. Med. Hypotheses 82: 97–104.10.1016/j.mehy.2013.11.016Suche in Google Scholar PubMed
Park, J.H., Park, S.J., Chung, M.K., Jung, K.H., Choi, M.R., Kim, Y., Chai, Y.G., Kim, S.J., and Park, K.S. (2010). High expression of large-conductance Ca2+-activated K+ channel in the CD133+ subpopulation of SH-SY5Y neuroblastoma cells. Biochem. Biophys. Res. Commun. 396: 637–642, https://doi.org/10.1016/j.bbrc.2010.04.142.Suche in Google Scholar PubMed
Prasuhn, J., Mårtensson, C.U., Krajka, V., Klein, C., and Rakovic, A. (2018). Genome-edited, TH-expressing neuroblastoma cells as a disease model for dopamine-related disorders: a proof-of-concept study on DJ-1-deficient parkinsonism. Front. Cell. Neurosci. 11: 426.10.3389/fncel.2017.00426Suche in Google Scholar PubMed PubMed Central
Presgraves, S.P., Ahmed, T., Borwege, S., and Joyce, J.N. (2003). Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox. Res. 5: 579–598.10.1007/BF03033178Suche in Google Scholar PubMed
Ramón‐Moliner, E., and Nauta, W.J.H. (1966). The isodendritic core of the brain stem. J. Comp. Neurol. 126: 311–335.10.1007/978-1-4684-7920-1_14Suche in Google Scholar
Rasband, M.N. (2010). The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 11: 552–562.10.1038/nrn2852Suche in Google Scholar
Rees, H.A., and Liu, D.R. (2018). Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 19: 770–788.10.1038/s41576-018-0059-1Suche in Google Scholar
Reynolds, C.H., Garwood, C.J., Wray, S., Price, C., Kellie, S., Perera, T., Zvelebil, M., Yang, A., Sheppard, P.W., and Varndell, I.M., et al. (2008). Phosphorylation regulates tau interactions with Src homology 3 domains of phosphatidylinositol 3-kinase, phospholipase Cγ1, Grb2, and Src family kinases. J. Biol. Chem. 283: 18177–18186.10.1074/jbc.M709715200Suche in Google Scholar
Rossor, M.N. (1981). Parkinson’s disease and Alzheimer’s disease as disorders of the isodendritic core. Br. Med. J. (Clin. Res. Ed.) 283: 1588–1590.10.1136/bmj.283.6306.1588Suche in Google Scholar
Sánchez, S., Jiménez, C., Carrera, A.C., Diaz-Nido, J., Avila, J., and Wandosell, F. (2004). A cAMP-activated pathway, including PKA and PI3K, regulates neuronal differentiation. Neurochem. Int. 44: 231–242.10.1016/S0197-0186(03)00150-5Suche in Google Scholar
Santa-Catalina, M.O., Caballero Bermejo, M., Argent, R., Alonso, J.C., Centeno, F., and Lorenzo, M.J. (2017). JNK signaling pathway regulates sorbitol-induced Tau proteolysis and apoptosis in SH-SY5Y cells by targeting caspase-3. Arch. Biochem. Biophys. 10.1016/j.abb.2017.11.004Suche in Google Scholar PubMed
Sanchez-Padilla, J., Guzman, J.N., Ilijic, E., Kondapalli, J., Galtieri, D.J., Yang, B., Schieber, S., Oertel, W., Wokosin, D., and Schumacker, P.T., et al. (2014). Mitochondrial oxidant stress in locus coeruleus is regulated by activity and nitric oxide synthase. Nat. Neurosci. 17: 832–840.10.1038/nn.3717Suche in Google Scholar PubMed PubMed Central
Santa-Catalina, M.O., Caballero-Bermejo, M., Argent, R., Alonso, J.C., Cuenda, A., Lorenzo, M.J., and Centeno, F. (2016). Hyperosmotic stress induces tau proteolysis by caspase-3 activation in SH-SY5Y cells. J. Cell. Biochem. 117: 2781–2790.10.1002/jcb.25579Suche in Google Scholar PubMed
Sarkanen, J.R., Nykky, J., Siikanen, J., Selinummi, J., Ylikomi, T., and Jalonen, T.O. (2007). Cholesterol supports the retinoic acid-induced synaptic vesicle formation in differentiating human SH-SY5Y neuroblastoma cells. J. Neurochem. 102: 1941–1952, https://doi.org/10.1111/j.1471-4159.2007.04676.x.Suche in Google Scholar PubMed
Sassin, I., Schultz, C., Thal, D.R., Rüb, U., Arai, K., Braak, E., and Braak, H. (2000). Evolution of Alzheimer’s disease-related cytoskeletal changes in the basal nucleus of Meynert. Acta Neuropathol. 100: 259–269.10.1007/s004019900178Suche in Google Scholar PubMed
Satoh, A., and Iijima, K.M. (2019). Roles of tau pathology in the locus coeruleus (LC) in age-associated pathophysiology and Alzheimer’s disease pathogenesis: potential strategies to protect the LC against aging. Brain Res. 1702: 17–28.10.1016/j.brainres.2017.12.027Suche in Google Scholar
Sayas, C.L., Moreno-Flores, M.T., Avila, J., and Wandosell, F. (1999). The neurite retraction induced by lysophosphatidic acid increases Alzheimer’s disease-like Tau phosphorylation. J. Biol. Chem. 274: 37046–37052.10.1074/jbc.274.52.37046Suche in Google Scholar
Schleiermacher, G., Janoueix-Lerosey, I., Ribeiro, A., Klijanienko, J., Couturier, J., Pierron, G., Mosseri, V., Valent, A., Auger, N., and Plantaz, D., et al. (2010). Accumulation of segmental alterations determines progression in neuroblastoma. J. Clin. Oncol. 28: 3122–3130.10.1200/JCO.2009.26.7955Suche in Google Scholar
Schleiermacher, G., Michon, J., Ribeiro, A., Pierron, G., Mosseri, V., Rubie, H., Munzer, C., Bénard, J., Auger, N., and Combaret, V., et al. (2011). Segmental chromosomal alterations lead to a higher risk of relapse in infants with MYCN-non-amplified localised unresectable/disseminated neuroblastoma (a SIOPEN collaborative study). Br. J. Canc. 105: 1940–1948.10.1038/bjc.2011.472Suche in Google Scholar
Scott, I.G., Åkerman, K.E.O., Heikkilä, J.E., Kaila, K., and Andersson, L.C. (1986). Development of a neural phenotype in differentiating ganglion cell‐derived human neuroblastoma cells. J. Cell. Physiol. 128: 285–292.10.1002/jcp.1041280221Suche in Google Scholar
Shipley, M.M., Mangold, C.A., and Szpara, M.L. (2016). Differentiation of the SH-SY5Y human neuroblastoma cell line. J. Vis. Exp. 2016: 53193.10.3791/53193Suche in Google Scholar
Silva, M.C., Cheng, C., Mair, W., Almeida, S., Fong, H., Biswas, M.H.U., Zhang, Z., Huang, Y., Temple, S., and Coppola, G., et al. (2016). Human iPSC-derived neuronal model of tau-A152T frontotemporal dementia reveals tau-mediated mechanisms of neuronal vulnerability. Stem Cell Rep. 7: 325–340.10.1016/j.stemcr.2016.08.001Suche in Google Scholar
Šimić, G., Babić Leko, M., Wray, S., Harrington, C., Delalle, I., Jovanov-Milošević, N., Bažadona, D., Buée, L., de Silva, R., and Giovanni, G. Di, et al. (2016). Tau protein hyperphosphorylation and aggregation in Alzheimer’s disease and other tauopathies, and possible neuroprotective strategies. Biomolecules 6: 2–28.10.3390/biom6010006Suche in Google Scholar
Simpson, P.B., Bacha, J.I., Palfreyman, E.L., Woollacott, A.J., McKernan, R.M., and Kerby, J. (2001). Retinoic acid-evoked differentiation of neuroblastoma cells predominates over growth factor stimulation: an automated image capture and quantitation approach to neuritogenesis. Anal. Biochem. 298: 163–169.10.1006/abio.2001.5346Suche in Google Scholar
Smith, C.J., Anderton, B.H., Davis, D.R., and Gallo, J.M. (1995). Tau isoform expression and phosphorylation state during differentiation of cultured neuronal cells. FEBS Lett. 375: 243–248.10.1016/0014-5793(95)01221-YSuche in Google Scholar
Sohn, P.D., Huang, C.T.L., Yan, R., Fan, L., Tracy, T.E., Camargo, C.M., Montgomery, K.M., Arhar, T., Mok, S.A., and Freilich, R., et al. (2019). Pathogenic tau impairs axon initial segment plasticity and excitability homeostasis. Neuron 104: 458–470.10.1016/j.neuron.2019.08.008Suche in Google Scholar
Sola, M., Magrin, C., Pedrioli, G., Pinton, S., Salvadè, A., Papin, S., and Paganettoi, P. (2020). Tau affects P53 function and cell fate during the DNA damage response. Nat. Commun. Biol. 3: 245.10.1038/s42003-020-0975-4Suche in Google Scholar
Spengler, B.A., Biedler, J.L., and Ross, R.A. (2002). A corrected karyotype for the SH-SY5Y human neuroblastoma cell line. Canc. Genet. Cytogenet. 138: 177–178.10.1016/S0165-4608(02)00523-XSuche in Google Scholar
Spillantini, M.G., Bird, T.D., and Ghetti, B. (1998). Frontotemporal dementia and Parkinsonism linked to chromosome 17: a new group of tauopathies. Brain Pathol. 8: 387–402.10.1111/j.1750-3639.1998.tb00162.xSuche in Google Scholar
Spillantini, M.G., and Goedert, M. (1998). Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 21: 428–433.10.1016/S0166-2236(98)01337-XSuche in Google Scholar
Spillantini, M.G., Goedert, M., Crowther, R.A., Murrell, J.R., Farlow, M.R., and Ghetti, B. (1997). Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc. Natl. Acad. Sci. U.S.A. 94: 4113–4118.10.1073/pnas.94.8.4113Suche in Google Scholar
Stallings, R.L. (2007). Origin and functional significance of large-scale chromosomal imbalances in neuroblastoma. Cytogenet. Genome Res. 118: 110–115.10.1159/000108291Suche in Google Scholar
Storga, D., Vrecko, K., Birkmayer, J.G.D., and Reibnegger, G. (1996). Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci. Lett. 203: 29–32.10.1016/0304-3940(95)12256-7Suche in Google Scholar
Sun, J.F., Zhao, M.Y., Xu, Y.J., Su, Y., Kong, X.H., and Wang, Z.Y. (2020). Fenamates inhibit human sodium channel Nav1.2 and protect glutamate-induced injury in SH-SY5Y cells. Cell. Mol. Neurobiol. 40: 1405–1416, https://doi.org/10.1007/s10571-020-00826-1.Suche in Google Scholar PubMed
Tabaton, M., Schenone, A., Romagnoli, P., and Mancardi, G.L. (1985). A quantitative and ultrastructural study of substantia nigra and nucleus centralis superior in Alzheimer’s disease. Acta Neuropathol. 68: 218–223.10.1007/BF00690198Suche in Google Scholar PubMed
Tanaka, T., Iqbal, K., Trenkner, E., Liu, D.J., and Grundke-Iqbal, I. (1995). Abnormally phosphorylated tau in SY5Y human neuroblastoma cells. FEBS Lett. 360: 5–9.10.1016/0014-5793(95)00061-DSuche in Google Scholar
Tanaka, T., Zhong, J., Iqbal, K., Trenkner, E., and Grundke-Iqbal, I. (1998). The regulation of phosphorylation of τ in SY5Y neuroblastoma cells: the role of protein phosphatases. FEBS Lett. 426: 248–254.10.1016/S0014-5793(98)00346-9Suche in Google Scholar
Tarantini, S., Tran, C.H.T., Gordon, G.R., Ungvari, Z., and Csiszar, A. (2017). Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp. Gerontol. 94: 52–58.10.1016/j.exger.2016.11.004Suche in Google Scholar
Tosetti, P., Taglietti, V., and Toselli, M. (1998). Functional changes in potassium conductances of the human neuroblastoma cell line SH-SY5Y during in vitro differentiation. J. Neurophysiol. 79: 648–658, https://doi.org/10.1152/jn.1998.79.2.648.Suche in Google Scholar
Trabzuni, D., Wray, S., Vandrovcova, J., Ramasamy, A., Walker, R., Smith, C., Luk, C., Gibbs, J.R., Dillman, A., and Hernandez, D.G., et al. (2012). MAPT expression and splicing is differentially regulated by brain region: relation to genotype and implication for tauopathies. Hum. Mol. Genet. 21: 4094–4103.10.1093/hmg/dds238Suche in Google Scholar
Uberti, D., Rizzini, C., Spano, P.F., and Memo, M. (1997). Characterization of tau proteins in human neuroblastoma SH-SY5Y cell line. Neurosci. Lett. 235: 149–153.10.1016/S0304-3940(97)00715-5Suche in Google Scholar
Usardi, A., Pooler, A.M., Seereeram, A., Reynolds, C.H., Derkinderen, P., Anderton, B., Hanger, D.P., Noble, W., and Williamson, R. (2011). Tyrosine phosphorylation of tau regulates its interactions with Fyn SH2 domains, but not SH3 domains, altering the cellular localization of tau. FEBS J. 278: 2927–2937.10.1111/j.1742-4658.2011.08218.xSuche in Google Scholar PubMed
Valdiglesias, V., Prego-Faraldo, M.V., Paśaro, E., Meńdez, J., and Laffon, B. (2013). Okadaic acid: more than a diarrheic toxin. Mar. Drugs 11: 4328–4349.10.3390/md11114328Suche in Google Scholar PubMed PubMed Central
Van Beuningen, S.F.B., Will, L., Harterink, M., Chazeau, A., Van Battum, E.Y., Frias, C.P., Franker, M.A.M., Katrukha, E.A., Stucchi, R., and Vocking, K., et al. (2015). TRIM46 controls neuronal polarity and axon specification by driving the formation of parallel microtubule arrays. Neuron 88: 1208–1226.10.1016/j.neuron.2015.11.012Suche in Google Scholar PubMed
Verheyen, A., Diels, A., Dijkmans, J., Oyelami, T., Meneghello, G., Mertens, L., Versweyveld, S., Borgers, M., Buist, A., and Peeters, P., et al. (2015). Using human iPSC-derived neurons to model TAU aggregation. PLoS One 10: e0146127, https://doi.org/10.1371/journal.pone.0146127.Suche in Google Scholar PubMed PubMed Central
Wang, C., Ward, M.E., Chen, R., Liu, K., Tracy, T.E., Chen, X., Xie, M., Sohn, P.D., Ludwig, C., and Meyer-Franke, A., et al. (2017). Scalable production of iPSC-derived human neurons to identify tau-lowering compounds by high-content screening. Stem Cell Rep. 9: 1221–1233.10.1016/j.stemcr.2017.08.019Suche in Google Scholar
Wang, H.B., Li, T., Ma, D.Z., and Zhi, H. (2018). ERa36 gene silencing promotes tau protein phosphorylation, inhibits cell proliferation, and induces apoptosis in human neuroblastoma SH-SY5Y cells. FASEB J. 32: 6456–6468.10.1096/fj.201701386Suche in Google Scholar
Weingarten, M.D., Lockwood, A.H., Hwo, S.Y., and Kirschner, M.W. (1975). A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. U.S.A. 72: 1858–1862.10.1073/pnas.72.5.1858Suche in Google Scholar
West, M.J., Coleman, P.D., Flood, D.G., and Troncoso, J.C. (1994). Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 344: 769–772.10.1016/S0140-6736(94)92338-8Suche in Google Scholar
Wischik, C.M., Novak, M., Thogersen, H.C., Edwards, P.C., Runswick, M.J., Jakes, R., Walker, J.E., Milstein, C., Roth, M., and Klug, A. (1988). Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 85: 4506–4510.10.1073/pnas.85.12.4506Suche in Google Scholar PubMed PubMed Central
Xia, D., Gutmann, J.M., and Gotz, J. (2016). Mobility and subcellular localization of endogenous, gene-edited Tau differs from that of over-expressed human wild-type and P301L mutant Tau. Sci. Rep. 1852: 913–924.10.1038/srep29074Suche in Google Scholar PubMed PubMed Central
Xie, H.R., Hu, L. Sen, and Li, G.Y. (2010). SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. 123: 1086–1092.Suche in Google Scholar
Xu, Y., Gao, Y.W., and Yang, Y. (2018). SC79 protects dopaminergic neurons from oxidative stress. Oncotarget 9: 12639–12648.10.18632/oncotarget.23538Suche in Google Scholar PubMed PubMed Central
Yeo, E.T.Y., Wong, K.W.L., See, M.L., Wong, K.Y., Gan, S.Y., and Chan, E.W.L. (2018). Piper sarmentosum Roxb. confers neuroprotection on beta-amyloid (Aβ)-induced microglia-mediated neuroinflammation and attenuates tau hyperphosphorylation in SH-SY5Y cells. J. Ethnopharmacol. 217: 187–194.10.1016/j.jep.2018.02.025Suche in Google Scholar PubMed
Yusuf, M., Leung, K., Morris, K.J., and Volpi, E.V. (2013). Comprehensive cytogenomic profile of the in vitro neuronal model SH-SY5Y. Neurogenetics 14: 63–70.10.1007/s10048-012-0350-9Suche in Google Scholar PubMed PubMed Central
Zempel, H., Dennissen, F.J.A., Kumar, Y., Luedtke, J., Biernat, J., Mandelkow, E.M., and Mandelkow, E. (2017). Axodendritic sorting and pathological missorting of Tau are isoform-specific and determined by axon initial segment architecture. J. Biol. Chem. 292: 12192–12207.10.1074/jbc.M117.784702Suche in Google Scholar PubMed PubMed Central
Zempel, H., Luedtke, J., and Mandelkow, E. (2017). Tracking Tau in neurons: How to transfect and track exogenous tau into primary neurons. Methods in Molecular Biology 1523: 335–340.10.1007/978-1-4939-6598-4_21Suche in Google Scholar PubMed
Zempel, H., and Mandelkow, E. (2014). Lost after translation: missorting of Tau protein and consequences for Alzheimer disease. Trends Neurosci. 37: 721–732.10.1016/j.tins.2014.08.004Suche in Google Scholar PubMed
Zempel, H., and Mandelkow, E.M. (2017). Tracking Tau in neurons: how to grow, fix, and stain primary neurons for the investigation of Tau in all developmental stages. Methods Mol. Biol. 1523: 327–334.10.1007/978-1-4939-6598-4_20Suche in Google Scholar PubMed
Zempel, H., and Mandelkow, E. (2019). Mechanisms of Axonal Sorting of Tau and Influence of the Axon Initial Segment on Tau Cell Polarity. Advances in Experimental Medicine and Biology 1184: 69–77.10.1007/978-981-32-9358-8_6Suche in Google Scholar PubMed
Zheng-Fischhöfer, Q., Biernat, J., Mandelkow, E.M., Illenberger, S., Godemann, R., and Mandelkow, E. (1998). Sequential phosphorylation of Tau by glycogen synthase kinase-3β and protein kinase A at Thr212 and Ser214 generates the Alzheimer-specific epitope of antibody AT100 and requires a paired-helical-filament-like conformation. Eur. J. Biochem. 252: 542–552.10.1046/j.1432-1327.1998.2520542.xSuche in Google Scholar PubMed
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- SH-SY5Y-derived neurons: a human neuronal model system for investigating TAU sorting and neuronal subtype-specific TAU vulnerability
- Auditory mismatch negativity in bipolar disorder: a focused review
- Information seeking criteria: artificial intelligence, economics, psychology, and neuroscience
- The influence of rs53576 polymorphism in the oxytocin receptor (OXTR) gene on empathy in healthy adults by subtype and ethnicity: a systematic review and meta-analysis
- The association of oxytocin with major depressive disorder: role of confounding effects of antidepressants
- The impact of COVID-19 on diagnostic biomarkers in neuropsychiatric and neuroimmunological diseases: a review
- Disruption of circadian rhythm and risk of autism spectrum disorder: role of immune-inflammatory, oxidative stress, metabolic and neurotransmitter pathways
Artikel in diesem Heft
- Frontmatter
- SH-SY5Y-derived neurons: a human neuronal model system for investigating TAU sorting and neuronal subtype-specific TAU vulnerability
- Auditory mismatch negativity in bipolar disorder: a focused review
- Information seeking criteria: artificial intelligence, economics, psychology, and neuroscience
- The influence of rs53576 polymorphism in the oxytocin receptor (OXTR) gene on empathy in healthy adults by subtype and ethnicity: a systematic review and meta-analysis
- The association of oxytocin with major depressive disorder: role of confounding effects of antidepressants
- The impact of COVID-19 on diagnostic biomarkers in neuropsychiatric and neuroimmunological diseases: a review
- Disruption of circadian rhythm and risk of autism spectrum disorder: role of immune-inflammatory, oxidative stress, metabolic and neurotransmitter pathways

