Abstract
Upregulation of defensive reflexes such as the nociceptive flexion reflex (NFR) has been attributed to sensitisation of peripheral and spinal nociceptors and is often considered biomarkers of pain. Experimental modulation of defensive reflexes raises the possibility that they might be better conceptualised as markers of descending cognitive control. Despite strongly held views on both sides and several narrative reviews, there has been no attempt to evaluate the evidence in a systematic manner. We undertook a meta-analytical systematic review of the extant English-language literature from inception. Thirty-six studies satisfied our a priori criteria. Seventeen were included in the meta-analysis. Reflexive threshold was lower in people with clinical pain than it was in pain-free controls, but reflex size, latency, and duration were unaffected. The pattern of difference was not consistent with sensitisation of nociceptive neurones, as these changes were not isolated to the affected body part but was more consistent with top-down cognitive control reflective of heightened protection of body tissue. The pattern of modulation is dependent on potentially complex evaluative mechanisms. We offer recommendations for future investigations and suggest that defensive reflex threshold may reflect a biomarker of a broader psychological construct related to bodily protection, rather than sensitisation of primary nociceptors, spinal nociceptors, or pain.
Introduction
Humans have a range of inbuilt protective mechanisms, from complex feelings such as pain, which is modulated by a potentially infinite number of factors, to arguably more simple responses such as reflexes. Generally, a reflex can be defined as an automatic and involuntary muscular response to an internal or external stimulus, mediated by neuronal pathways, often defensive in nature (Sherrington, 1910; Graziano and Cooke, 2006). The most obvious trigger of defensive reflexes is a sudden or noxious and unexpected stimulus. Noxious stimuli activate high threshold neurones, called nociceptors, which exist in all but a few of the tissue of the body. Activity in these nociceptors and their projections is clearly a potent modulator of pain, and nociceptive reflexes have been used as markers of pain relief or pain augmentation (Skljarevski and Ramadan, 2002). Measurement of reflex parameters holds an advantage in that they are not vulnerable to confounds of self-report, most notably responder bias and deceit (Skljarevski and Ramadan, 2002).
Some reflexes are clearly under top-down cognitive control, however. For example, the hand-blink reflex, triggered by electrical stimulation over the median nerve at the wrist, is larger when the stimulated hand is within the peripersonal space of the face (Sambo et al., 2012a) and this upregulation occurs in real time and, indeed, in a feedforward manner (Wallwork et al., 2016). The startle response can be modulated by factors such as the pleasantness/unpleasantness of odours (Ehrlichman et al., 1995) and by viewing emotionally salient images (Bradley et al., 1993). These reflexes, like the nociceptive flexion reflex (NFR), are clearly defensive in nature and upregulation in times of potential bodily threat would seem to offer some evolutionary advantage. This raises the possibility that defensive reflexes might offer a marker of ‘perceived need to protect’, a construct well established in the clinical literature (e.g. Moseley and Butler, 2015b) the assessment of which currently relies on self-report questionnaires (e.g. Symonds et al., 1996).
We undertook a systematic review and meta-analytical approach to comprehensively evaluate the quantifiable parameters of defensive reflexes in people with and without clinical (i.e. non-experimental) pain. We predicted that defensive reflexes would be augmented in people in pain, even when they do not involve the painful body part or the implicated nociceptive pathways. This augmentation would be reflected in several parameters – lower activation thresholds, greater size, shorter onset latencies, and longer duration. The advantage of evaluating several parameters that are mediated by different mechanisms is that, if differences emerged in some parameters and not in others, it might inform as to the likely mechanisms underpinning the effects.
Methods
We followed the recommendations set out in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009) and followed a protocol established a priori.
Defining terms and inclusion criteria
We defined a defensive reflex as being a transient, involuntary muscle response as a result of a detected external stimulus. Articles were included if they involved human participants with a non-neuropathic pain condition and a healthy control group and reported one or more of the following outcome measures for a defensive reflex for participants: stimulus threshold required to evoke an electromyographic (EMG) response (‘threshold’), peak amplitude of the EMG response to a standardised stimulus (‘peak amplitude’), area under the curve of the EMG response (AUC), latency between stimulus onset and the evoked EMG response (latency), or duration of the EMG response (‘duration’). Populations were included where pain was a significant complaint but were excluded if the integrity of the nervous system was compromised. That is, populations with an identified (or suspected) neurological lesion were excluded. We did not include studies testing the effect of experimentally induced pain because we wanted to capture the affective dimension of clinical pain, which includes a perceived degree of threat to health or life. The nature of experimental pain models is that they are required, by ethical guidelines, to be undertaken in a way that threat to the person is reduced and is short-lasting. The blink reflex [including corneal reflex, nociceptive blink reflex, somatosensory evoked blink response (R2 response only)], the nociceptive flexion reflex (also including nociceptive withdrawal response, RIII response), and the startle response (the eye-blink component) were included in the review on the basis that they satisfied our a priori definition of ‘defensive reflex’. Primary outcome measures from the reflexes were size (either peak amplitude or AUC), threshold, latency and duration of reflex response. The reflex was considered to be augmented if threshold was lower, latency was shorter, duration was longer, or if peak amplitude or AUC was greater.
Studies were excluded if the title or abstract stated that participants had a demonstrable lesion of the sensory neuraxis (e.g. stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, Parkinson’s disease, polio, diabetes, peripheral neuropathy or nerve injury, radiculopathy), if they were animal studies, if they investigated experimentally induced pain, or if they were not in the English language.
Search strategy and search screening
The following databases were searched, from their inception to June 2016: AMED (via Ovid SP), CINAHL (via EbscoHost), Cochrane Collaboration, Embase (via Ovid SP), Medline (via Ovid SP), and PsychInfo (via Ovid SP). Details of the search strategy, exemplified for Medline, can be found in the online Supplementary Material. Searches were limited to the English language. Grey literature was excluded. The reference lists of included studies and relevant reviews were screened for further relevant literature.
Two reviewers (S.B.W. and L.G.) independently screened the titles and abstracts (where available) of all studies for inclusion. Reviewers compared their results at the end of each round, and any discrepancies were resolved by discussion between the two reviewers, or if an agreement could not be reached, a third reviewer (M.J.C.) was consulted. Where it was clear from the study title or abstract that the study was not relevant or did not meet the selection criteria, it was excluded. Full texts of the remaining citations were then independently screened by two authors (S.B.W. and L.G.). Disagreement was resolved through discussion between the two review authors. Where resolution was not achieved, a third review author (M.J.C) considered the article(s) in question.
Risk of bias and data extraction
Risk of bias (ROB) and data extraction forms were developed, trialled, and adjusted as required prior to commencement of the study. The ROB assessment was based on the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (von Elm et al., 2007). Relevant items for case-control study designs were from the Cochrane Collaboration’s tool for assessing bias (see online Supplementary Material) (Higgins and Green, 2011). ROB was scored out of 12; one point was allocated per item that introduced bias. Studies with a score of 0–2 were considered ‘low risk’, those with a score of 3–5 were considered ‘medium risk’, those with a score of 6–8 were considered ‘high risk’, and those with a score of greater than 8 were considered ‘very high risk’. Two independent reviewers (S.B.W. and L.G.) identified ROB associated with each study and extracted data for inclusion in the meta-analysis. The following data were extracted from the study reports: demographics of both the pain group and control group, details of the painful condition including diagnosis where available, the defensive reflex(es) tested, and the reflex outcome measures. If outcome measures were incompletely reported, the authors of the study were contacted. The authors were contacted via e-mail where available, or post where e-mail was not available, a minimum of three times over a 2-month period, and if there was no response from the authors, the studies were excluded.
Data analysis
Data were pooled according to our a priori plan. We conducted separate meta-analyses for the effect of pain on the size of the reflex (including both peak amplitude and AUC), reflex threshold, latency, and duration. For meta-analysis, peak amplitude and AUC were grouped together as they both measure the size of the response.
Meta-analyses were performed using Review Manager 5 software [Review Manager (RevMan), 2011] using a random-effects and inverse variance approach. We chose a random effects model because we anticipated that there may be population differences between pain conditions. The standardised mean difference between the pain and control groups was calculated for each study and used for comparison between studies. Effect sizes were interpreted according to Cohen and Ebrary (1988) as follows: 0.2, small; 0.5, medium; 0.8, large. On occasions where there was more than one comparison from a single study included in the same meta-analysis (i.e. there was more than one pain group or more than one reflex tested), the number of participants in the participant group that was entered into the meta-analysis more than once was divided by the number of times it was entered. We used the χ2 test to measure a statistically significant amount of heterogeneity and the I2 test to measure the percentage of heterogeneity present.
Where significant heterogeneity was present (χ2p<0.05, I2≥40%) and there were adequate data, we conducted subgroup analysis of the effect of reflex type based on our a priori plan. Pre-planned subgroups were blink reflex, NFR/withdrawal reflex, and startle reflex. There was no subgrouping by pain condition. Sensitivity analyses were performed to investigate the impact of excluding studies with medium to high ROB on the pooled effect size and heterogeneity.
Results
Literature search
Figure 1 displays the results of the systematic review process (PRISMA flow chart). The main literature search yielded 1244 records; the initial title and abstract screening process excluded 1015 studies, leaving 229 full-text articles to screen. Of these, 33 articles met all inclusion criteria and were included in the review. The predominant reason for study exclusion was the lack of pain (or the reporting of) at the time of testing or the absence of a healthy control group. An additional three articles (Boureau et al., 1991; Leroux et al., 1995; Neziri et al., 2012) were found by searching the reference lists of included articles and relevant reviews. This left a total of 36 articles to be included in the review. Of these articles, only 17 were included in the meta-analysis (see Table 1). The remaining 19 were not included because the authors of studies were either not contactable or unable upon request to provide data from the outcome measures in question (means and standard deviations of the reflex response – see Table 2 for exclusion details).
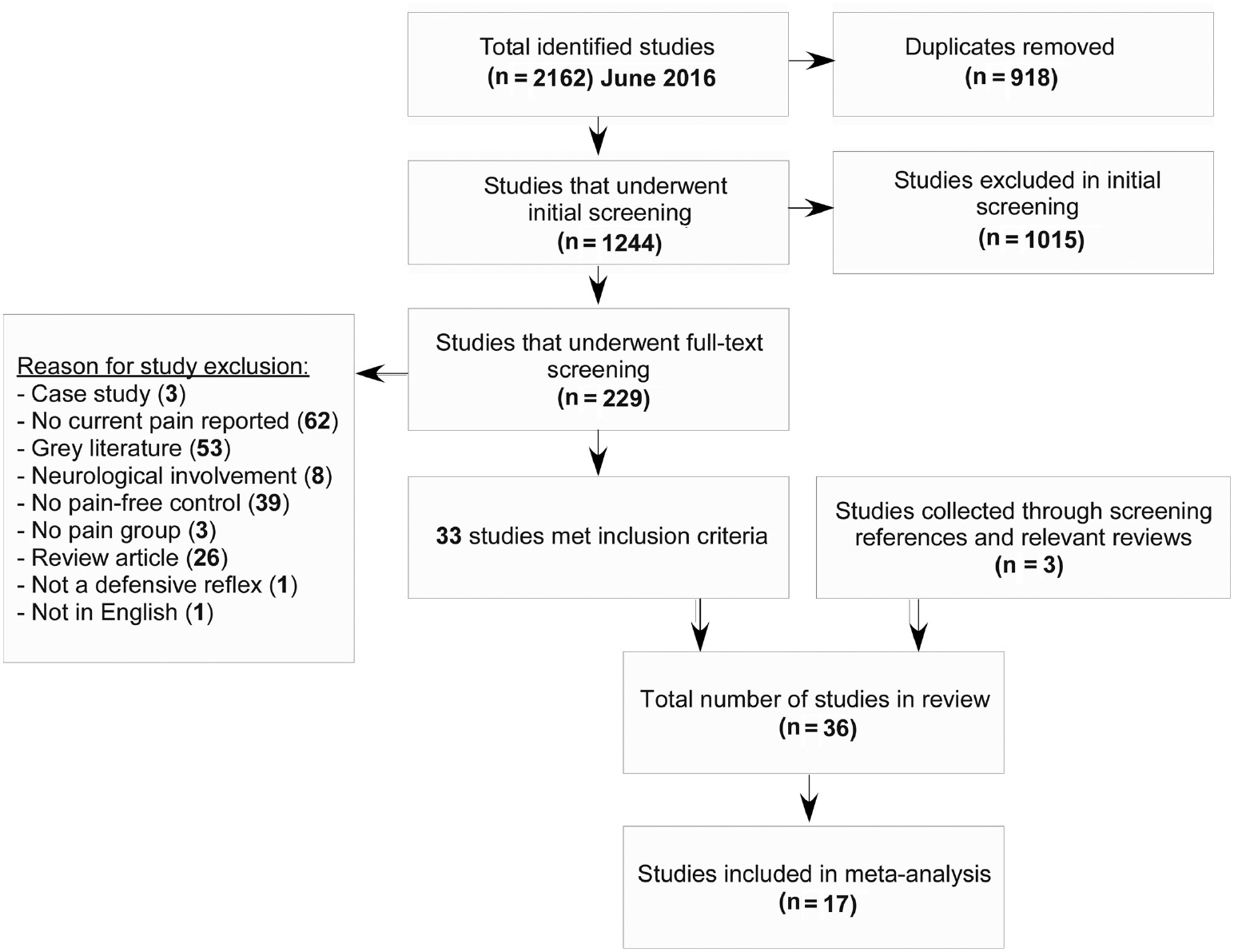
PRISMA flow-diagram outlining the study selection and review process.
Details of the 17 studies included in the meta-analyses.
| Study | Pain type | Reflex(s) tested | Sample size | Age – mean | Gender – males | ROB |
|---|---|---|---|---|---|---|
| Pain group:controls | Pain group:controls | Pain group:controls | ||||
| Avramidis et al. (1998) (a) | TTH | BR (size, latency) | 10:30 | 36: ‘matched’ | 4: ‘matched’ | Medium |
| Avramidis et al. (1998) (b) | Migraine | BR (size, latency) | 19:30 | 37.5: ‘matched’ | 9: ‘matched’ | Medium |
| Boureau et al. (1991) | Idiopathic, myofacial, headache pain | NFR (threshold) | 30:17 | Idiopathic (46.7) myofacial (50.8) headache (43) : 31.1 | 8:11 | High |
| Coffin et al. (2004) | IBS | NFR (threshold) | 17:10 | 51:37 | 3:3 | Low |
| Courtney et al. (2009) | Knee OA | NFR (threshold, duration, latency) | 16:16 | 59.6:60 | 4:8 | Low |
| Courtney et al. (2010) | Knee OA | FWR (nociceptive) (threshold) | 8:10 | 58.3:60 | 2:4 | Low |
| Curatolo et al. (2015) | Chronic pain | NFR (threshold) | 696:300 | 49.5:47 | 299:152 | Low |
| De Marinis et al. (2007) | Chronic migraine | BR (threshold, size, latency) | 35:35 | 37: ‘matched’ | 9: ‘matched’ | Medium |
| Flor et al. (1997) | Chronic upper back pain | SR (size) | 12:12 | 42.7:37.7 | 5:5 | Medium |
| Katsarava et al. (2004) | Migraine without aura | NBR (latency) | 28:30 | 27:25.7 | 7:11 | Low |
| Kofler and Halder (2014) | Fibromyalgia | BR (size, latency) | 10:26 | 48.2:45.2 | 0:0 | Low |
| Lim et al. (2012) | Chronic lateral epicondylalgia | NFR (threshold) | 30:31 | 52:48.7 | 21:19 | Low |
| Neziri et al. (2012) | CLBP | NFR (threshold) | 40:300 | 50.5:47.1 | 21:152 | Low |
| Peddireddy et al. (2009) | CTTH | NBR (size, duration, latency) | 30:30 | 46.6:47 | 15:15 | Low |
| Rhudy et al. (2013) (a) | RA | NFR (size, threshold) SR (size) | 17:19 | 44.7:46.7 | 3:4 | Low |
| Rhudy et al. (2013) (b) | Fibromyalgia | NFR (size, threshold) SR (size) | 17:19 | 49.4:46.7 | 2:4 | Low |
| Smith et al. (2013) (a) | Chronic WAD – did not respond to surgery | NFR (threshold) | 32 : 30 | 45.4 : 44.2 | 14 : 9 | Low |
| Smith et al. (2013) (b) | Chronic WAD – responded to surgery | NFR (threshold) | 58:30 | 44.9:44.2 | 18:9 | Low |
| Smith et al. (2014) | Chronic WAD | NFR (threshold) | 53:30 | 44.7:44.2 | 17:9 | Low |
| Sterling et al. (2008) | Chronic WAD | NFR (threshold) | 30:30 | 37.7:30.3 | 7:6 | Low |
TTH, Tension type headache; CTTH, chronic tension type headache; IBS, irritable bowel syndrome; OA, osteoarthritis; RA, rheumatoid arthritis; WAD, whiplash associated disorder; CLBP, chronic low back pain; BR, blink reflex; NBR, nociceptive blink reflex; NFR, nociceptive flexion reflex; FWR, flexor withdrawal response; SR, startle response. Text in italics indicates the reflex outcome(s) reported.
Details of the 19 studies that met all review inclusion criteria but were excluded from the meta-analyses.
| Author | Reason for exclusion | Pain type | Reflex tested | Outcome | Consistent with meta-analysis? | ROB |
|---|---|---|---|---|---|---|
| Al-Azzawi et al. (2008) | Not all have current pain. No response from authors | RA | BR (latency) | Prolonged BR latencies in patient group | No | Medium |
| Ayzenberg et al. (2006) | No response from author | Migraine groups | NBR (size, latency) | No difference between groups for either measure | Yes, yes | Medium |
| Banic et al. (2004) | No response from author | Fibromyalgia and whiplash | NWR (threshold) | Reduced reflex thresholds in both fibromyalgia and whiplash groups when compared to controls | Yes | Low |
| Biurrun Manresa et al. (2013a) | Incorrect format for meta-analysis | Musculoskeletal pain | NWR (threshold) | Pain groups had lower NWR thresholds than controls | Yes | Low |
| Biurrun Manresa et al. (2013b) | Author said inappropriate to use | Musculoskeletal pain | NWR | n/a | – | Low |
| Carleton et al. (2006) | Author said inappropriate to use | Musculoskeletal pain | SR | n/a | – | Medium |
| de Tommaso et al. (2000) | No response from author | Migraine without aura | BR (size) | No difference between groups | Yes | Medium |
| Desmeules et al. (2014) | No response from author | Fibromyalgia | NFR (threshold) | NFR threshold was reduced in patients compared to controls | Yes | Low |
| Desmeules et al. (2003) | No response from author | Fibromyalgia | NFR (threshold) | Decreased NFR threshold in patients compared to controls | Yes | Low |
| Filatova et al. (2008) | Did not record R2 response | Migraine and CTTH | BR | n/a | – | Medium |
| Katsarava et al. (2003) | Author unable to access data | Migraine | NBR | Not reported | – | Medium |
| Knost et al. (1997) | Author unable to access data | Prechronic pain | SR (size) | Not reported | – | Low |
| Lang et al. (2005) | No response from author | Persistent idiopathic facial pain | BR | Not reported | – | Low |
| Mendak et al. (2010) | No response from author | Burning mouth syndrome | BR | Not reported | – | Medium |
| Nappi et al. (2002) | Author unable to access data | Headache groups | NFR (threshold) | Reduced NFR threshold in patients with episodic cluster headache, compared to controls, but not chronic cluster headache | Yes, no | Medium |
| Neziri et al. (2010) | Incorrect format for meta-analysis | Chronic endometriosis | WR (size, threhsold) | Reflex amplitude was higher in pateints compared to controls. NFR thresholds were reduced in patients. | No, yes | Low |
| Peters et al. (1992) | Author unable to access data | CLBP and acute postoperative pain | NFR (threshold) | No difference in reflex thresholds between patient groups and control groups | No | Low |
| Sandrini et al. (2006) | Only six subjects in the pain group reported current pain. Author unable to access data | CTTH | NFR (threshold) | n/a | – | Medium |
| Leroux et al. (1995) | No response from author | Patellofemoral dysfunction | NFR (threshold) | Lower NFR threshold in pain group compared to controls | Yes | Medium |
Text in italics indicates the reflex outcome(s) reported.
Included studies
Of the included studies, 12 reported reflex threshold (Boureau et al., 1991; Coffin et al., 2004; De Marinis et al., 2007; Sterling et al., 2008; Courtney et al., 2009, 2010; Lim et al., 2012; Neziri et al., 2012; Rhudy et al., 2013; Smith et al., 2013, 2014; Curatolo et al., 2015), six reported reflex peak magnitude or AUC or both (Flor et al., 1997; Avramidis et al., 1998; De Marinis et al., 2007; Peddireddy et al., 2009; Rhudy et al., 2013; Kofler and Halder, 2014), six reported reflex latency (Avramidis et al., 1998; Katsarava et al., 2004; De Marinis et al., 2007; Courtney et al., 2009; Peddireddy et al., 2009; Kofler and Halder, 2014), and two reported reflex duration (Courtney et al., 2009; Peddireddy et al., 2009). Pain populations included ‘chronic pain’, migraine, migraine without aura, tension type headache, chronic tension type headache, fibromyalgia, rheumatoid arthritis, chronic upper back pain, chronic low back pain, knee osteoarthritis, chronic whiplash associated disorder, irritable bowel syndrome, chronic lateral epicondylalgia (‘tennis elbow’), and a combination of idiopathic pain, myofascial pain, and headache (see Table 1 for study details).
Risk of bias of included studies
Twelve studies were considered to be of ‘low risk’ of bias, four had ‘medium risk’, and only one study was considered to have a ‘high risk’ (see Table 1). Bias was introduced in three main ways: through a poor ‘representativeness of cases’ for participant recruitment, through low participant numbers in each group, through not specifically defining how sample size was determined. Of the studies not included in the meta-analysis but still meeting our inclusion criteria, nine had ‘low risk’, 10 had ‘medium risk’, and no studies were considered ‘high risk’ or ‘very high risk’.
Outcomes
Outcomes for studies included in the systematic review but not in the meta-analysis are presented in Table 2.
Reflex threshold
Twelve studies (Boureau et al., 1991; Coffin et al., 2004; De Marinis et al., 2007; Sterling et al., 2008; Courtney et al., 2009, 2010; Lim et al., 2012; Neziri et al., 2012; Rhudy et al., 2013; Smith et al., 2013, 2014; Curatolo et al., 2015) (pooled n=1906) were included in reflex threshold comparisons. Two studies investigated two patient groups (Rhudy et al., 2013; Smith et al., 2013), which increased our group comparisons to 15. Pain groups had an overall lower reflex threshold than controls, with a large effect size [−0.83 (95% CI, −1.18 to −0.47), p<0.0001] (see Figure 2). There was a large amount of heterogeneity between studies (I2=84%, p<0.001). A sensitivity analysis, including only those studies with a ‘low risk’ of bias, resulted in an increase in effect size [−0.9 (95% CI, −1.27 to −0.52), p<0.0001] and little influence on the heterogeneity between studies (I2=83%).
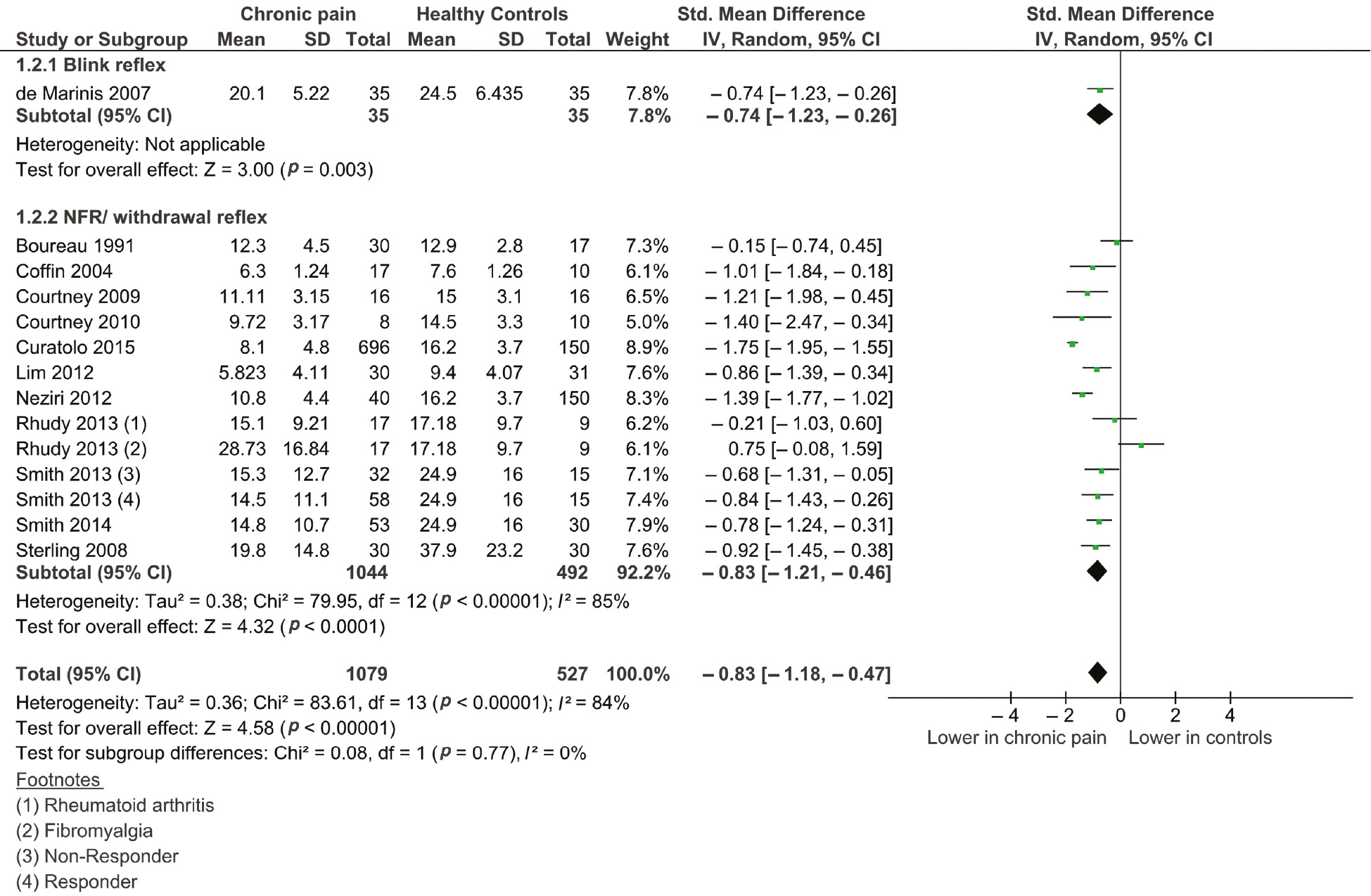
Forest plot of included studies measuring reflex thresholds.
Size of response
Six studies (Flor et al., 1997; Avramidis et al., 1998; De Marinis et al., 2007; Peddireddy et al., 2009; Rhudy et al., 2013; Kofler and Halder, 2014) (pooled n=297) were included in the main analysis for the size of the reflex. Two studies tested reflexes on two patient types (Avramidis et al., 1998; Rhudy et al., 2013), and one of these studies (Rhudy et al., 2013) tested two reflex types, increasing our group comparisons to 10. There was no difference between pain and control groups for the size of the reflex [overall effect size, −0.16 (95% CI, −0.65 to 0.33), p=0.52; see Figure 3], and there was a significant amount of heterogeneity between studies (I2=72%, p<0.001). One study (Avramidis et al., 1998) that found a smaller reflex size in the pain group than in the controls was responsible for heterogeneity between studies. A sensitivity analysis was conducted, including only studies with a ‘low risk’ of bias, which removed all heterogeneity (I2=0%). The sensitivity analysis removed the outlier (Avramidis et al., 1998), and the effect size became smaller [0.03 (95% CI −0.31 to 0.37)], confirming that there was no difference between those with clinical pain and the healthy controls.
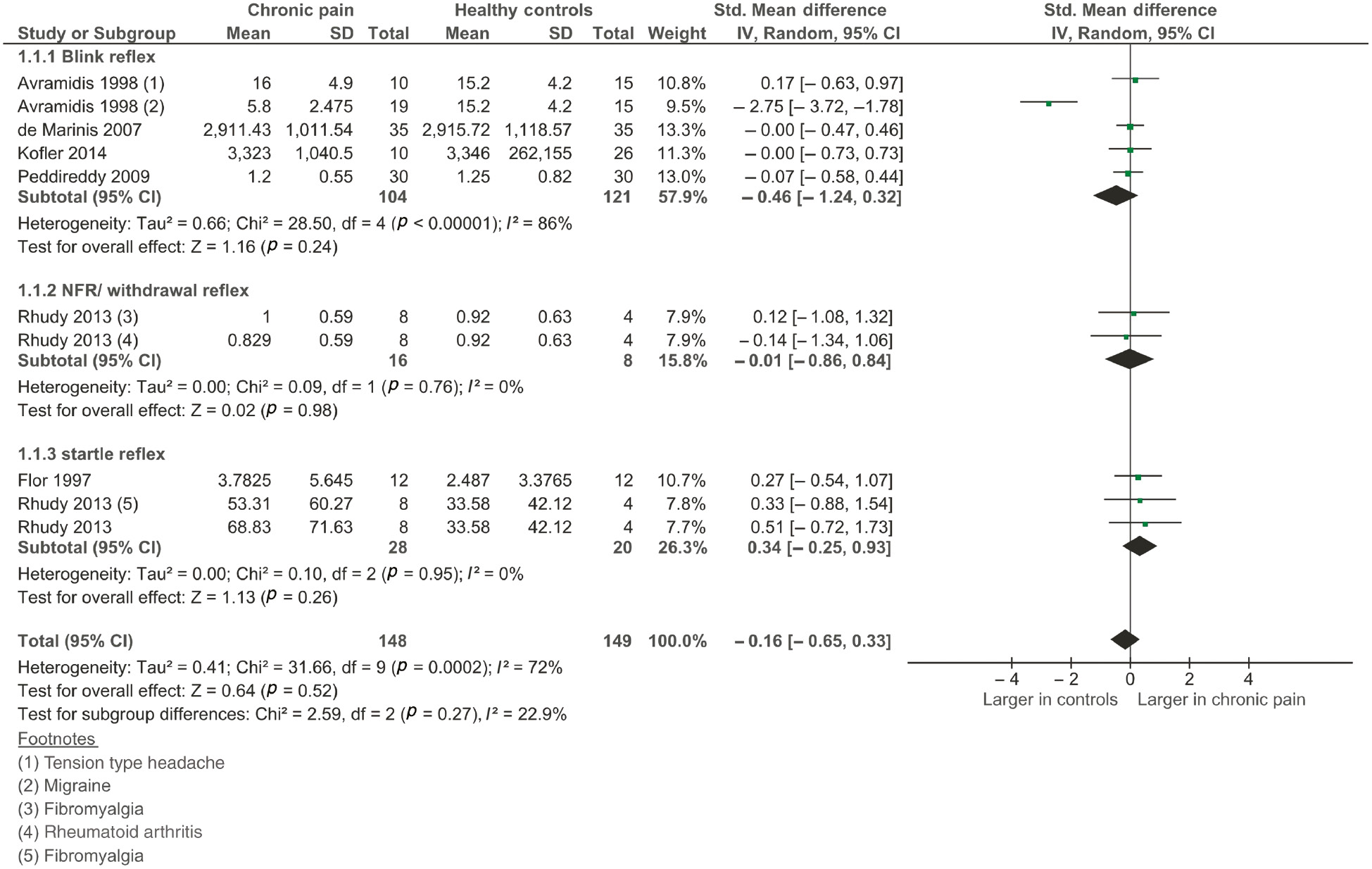
Forest plot of included studies measuring the size of the reflex.
Latency of response
Six studies (Avramidis et al., 1998; Katsarava et al., 2004; De Marinis et al., 2007; Courtney et al., 2009; Peddireddy et al., 2009; Kofler and Halder, 2014) (pooled n=319) were included in the main analysis for latency of the reflex. One study (Avramidis et al., 1998) investigated two patient groups raising the number of group comparisons to seven. There was no significant difference between groups [overall effect, −0.33 (95% CI, −0.94 to 0.28), p=0.28; see Figure 4] and a large amount of heterogeneity between studies (I2=85%, p<0.001). Subgroup analysis demonstrated a significantly reduced latency for the NFR/withdrawal reflex [−0.90 (95% CI, −1.59 to −0.20)] although the subgroup contained only a single study (n=36). A sensitivity analysis including only those studies with a ‘low risk’ of bias had little impact on heterogeneity (I2=88%, p<0.001), and the effect size remained non-significant.
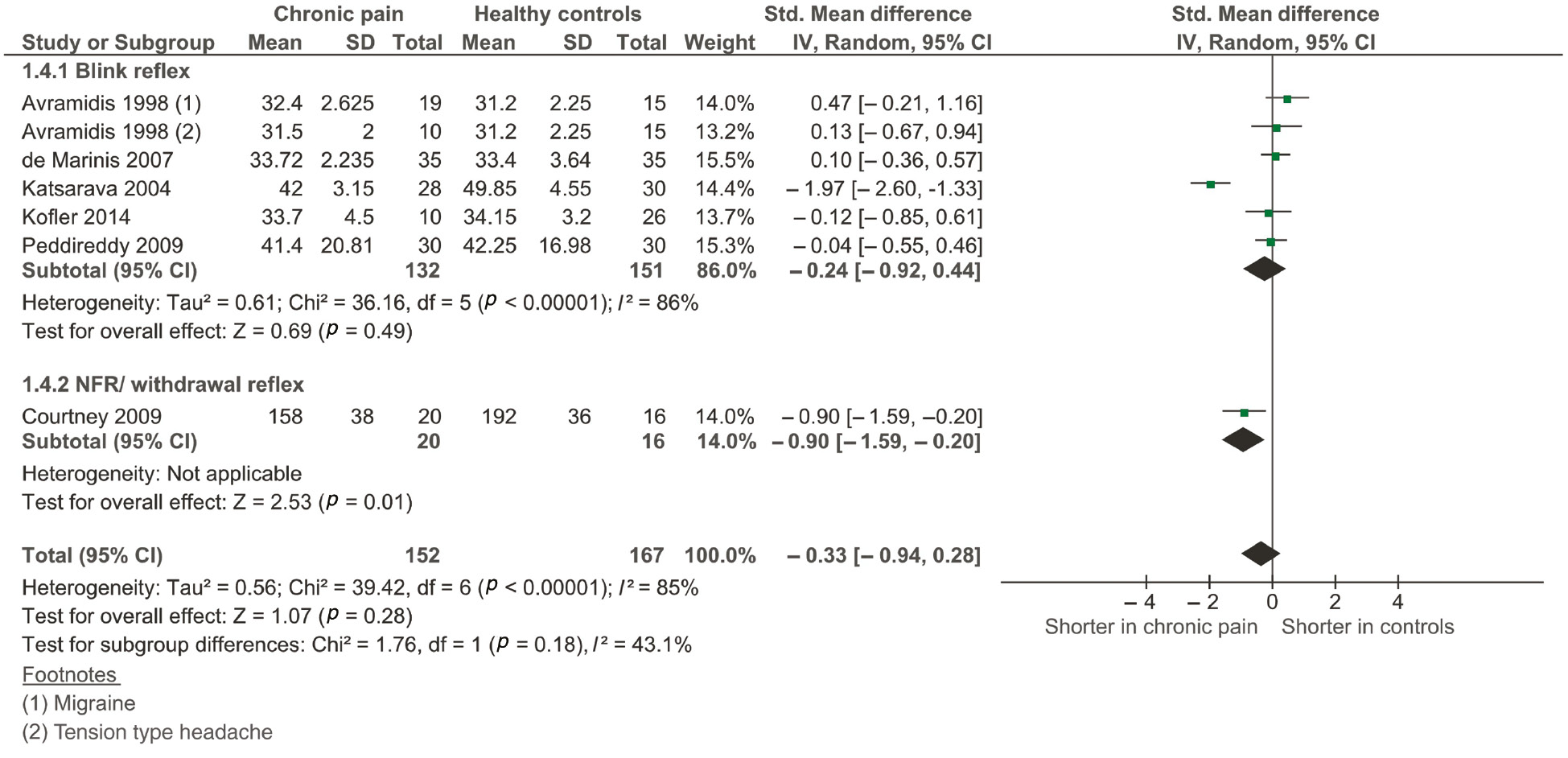
Forest plot of included studies measuring the latency of the reflex response.
Duration of response
Two studies (Courtney et al., 2009; Peddireddy et al., 2009) (pooled n=90) were included in the main analysis for duration of reflex. There was no difference between groups for either study, giving a small effect size [0.13 (95% CI, −0.29 to 0.55), p=0.55; see Figure 5] and low heterogeneity between studies (I2=0%). Both studies were considered to have ‘low risk’ of bias.
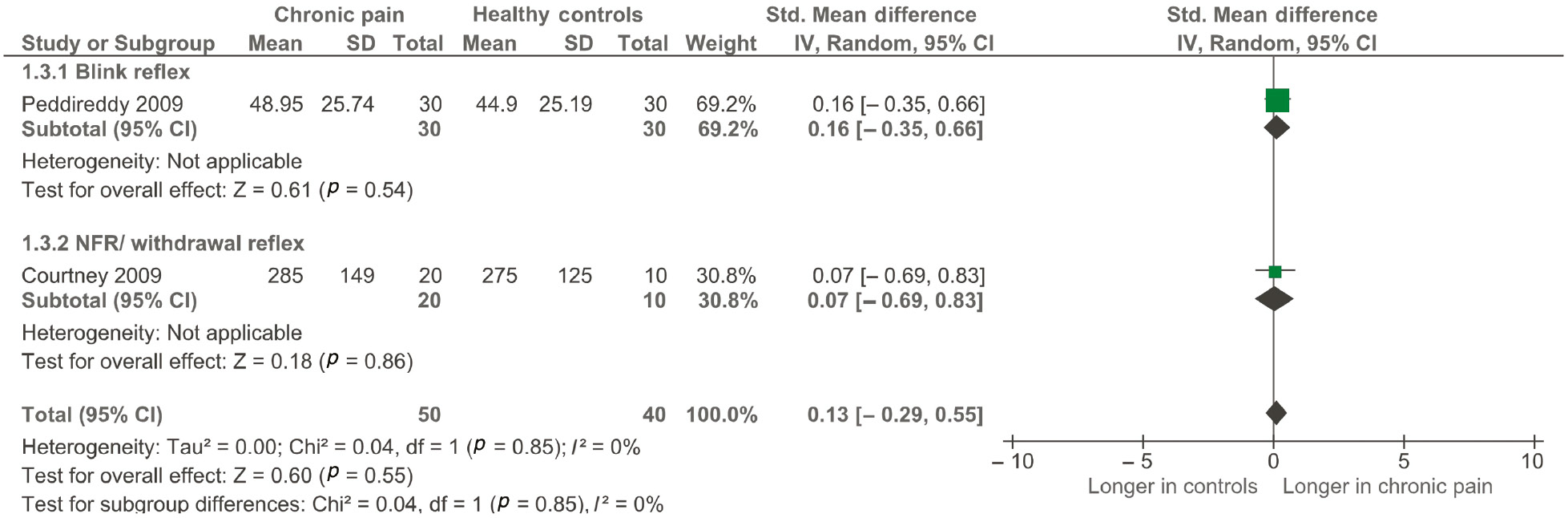
Forest plot of included studies measuring the duration of the reflex response.
Discussion
This meta-analytical systematic review aimed to comprehensively evaluate the quantifiable parameters of defensive reflexes in people with and without clinical (i.e. non-experimental) pain. We predicted that defensive reflexes would be augmented in people in pain, even when they do not involve the painful body part or the implicated nociceptive pathways. We found compelling evidence that the activation threshold is lower in people with pain than it is in healthy, pain-free controls. Contrary to our predictions, defensive reflex size, onset latency, and duration were no different between people with and without clinical pain. That lower reflex thresholds were not confined to the painful body part or the implicated nociceptive pathways supports our proposition. Moreover, the wider body of literature that is relevant to this issue but did not satisfy our a priori inclusion criteria is corroborative. Our results cast further doubt over the views that upregulated reflexes solely reflect sensitisation within the peripheral or spinal nociceptor or a biomarker of pain.
Reduced reflex threshold in people with pain
A reduced reflex activation threshold in people with pain might be attributed to a peripheral mechanism, a central mechanism, or both. Peripheral sensitisation refers to the shift in response profile of primary, or peripheral, nociceptors, such that they fire more readily (McMahon et al., 2006). Peripheral sensitisation results from the production and release of a swathe of chemical mediators, including those that are released when cells are damaged (Smolin, 1976; Neziri et al., 2012) and those that are released when nociceptors are active (Li et al., 2008). The latter process, called peptidergic or neurogenic inflammation, is triggered by the release of substance P and calcitonin gene related peptide at the peripheral terminals of nociceptors (Li et al., 2008). Although none of the constituent studies reported the presence or otherwise of peripheral sensitisation, it is reasonable to predict that peripheral sensitisation would have been present in at least some of them – for example, participants with rheumatoid arthritis (Rhudy et al., 2013). However, and critically, many studies did not evoke the reflex by delivering the stimulus to the body part that would be inflamed or peripherally sensitised. For example, participants with lateral epicondylalgia (elbow pain) received electrical stimuli to their ankle (Lim et al., 2012), yet it is their elbow that is likely to be inflamed and sensitised. The reduction in reflex threshold cannot therefore be solely attributed to sensitisation of the primary nociceptor.
Centrally driven augmentation could be mediated by sensitisation of the spinal nociceptor or descending facilitatory modulation. That central sensitisation can upregulate the NFR is not a new idea. In fact, this idea underpinned the use of the NFR in clinical and pharmacological studies as a supposedly ‘objective measure for pain’ (for a review, see Skljarevski and Ramadan, 2002). The process by which sensitisation occurs relates to a form of neuroplasticity whereby alterations in stimulus-response patterns occur over time by virtue of repeated exposure to a particular stimulus, in this case, to excitatory neurotransmitters at the spinal terminals (Woolf and Salter, 2000). The most obvious source of these neurotransmitters is the proximal terminals of primary nociceptors, but descending facilitatory neurones may also be involved (Roberts et al., 2009; Baron et al., 2013).
Withdrawal reflexes can clearly be modulated at the spinal nociceptor; people with complete spinal cord injury show signs of central sensitisation, which includes enlarge receptive fields and a lower reflex threshold after the application of topical capsaicin (Biurrun Manresa et al., 2014). Although spinal nociceptor sensitisation could explain why reflex thresholds to both noxious and non-noxious stimuli are lower in people with clinical pain, its effects would be expected to be confined to the painful body part and immediately surrounding tissue (Woolf et al., 1989). Perhaps this is relevant to the recent article by Woolf (2014), who revisits the definition of central sensitisation, moving away from a solely physiological construct – spinal nociceptor sensitisation – to a behavioural one driven by supraspinal input – where we see body-wide decreases in pain threshold. The neural substrate of this is necessarily top-down and therefore anatomically remote from the neural loops that subserve the defensive reflexes reviewed here. It can be seen then that changes in the state of nociceptive neurones that subserve the defensive reflex loops seem unlikely to completely explain the abnormally low reflex threshold that is evident at sites remote to the painful body part in people with clinical pain disorders. We contend that spinal sensitisation is unable to fully explain these lower reflex thresholds, particularly when evoked in regions remote to the painful area, and that the most obvious remaining explanation involves online descending facilitatory control.
There is evidence to suggest that cognitive factors can modulate top-down control of specific reflexes. For example, the presence of an unpleasant odour increases startle magnitude response (blink component) (Ehrlichman et al., 1995) and nociceptive withdrawal reflex (Bartolo et al., 2013); viewing an unpleasant picture increases the startle magnitude and viewing a pleasant one decreases it (Bradley et al., 1993); holding your hand near your face increases the hand-blink reflex triggered by median nerve stimulation at the wrist (Sambo et al., 2012a), an effect that is positively related to self-reported anxiety (Sambo and Iannetti, 2013) and that can be eliminated by placing a solid barrier, but not a fragile one, between the hand and the face (Sambo et al., 2012b). Moreover, this modulation of the hand-blink reflex according to how close the hand is to the face is modulated in a predictive fashion, being lower if the hand is moving away from the face than if it is moving towards the face (Wallwork et al., 2016). With respect to pain, De Marinis et al. (2007) tested blink reflexes in people with migraine and found that thresholds were lower during the migraine attack than not during an attack and were lower on the affected side of the head than on the unaffected side. Descending facilitatory control in line with a ‘greater need to protect’ is clearly a strong contender for explaining upregulated reflex activity in people with pain.
People with chronic pain often show reduced pain thresholds and elevated pain ratings in response to noxious stimuli beyond the site of their usual pain. Mechanical sensitivity immediately beyond the area of usual pain has been attributed to spinal sensitisation (Woolf, 1983), but when this mechanical sensitivity extends beyond the local area, it is attributed to a broader enhanced sensitivity within the central nervous system, possibly including changes in descending modulation (Woolf, 2014). Contemporary models of cortical contributions to enhanced sensitivity implicate altered stimulus response profiles in cortical networks and the ‘collaborative influence’ of other cues that signal tissue danger (Moseley and Butler, 2017), cues from across biological, psychological, and social domains. This is relevant to the current review because the literature clearly demonstrates that widespread decreased pain threshold and increased pain ratings are not entirely explained by peripheral and spinal sensitisation. Our results suggest that decreased reflex thresholds are also not entirely explained by peripheral and spinal sensitisation. Unlike pain ratings, however, reflex loops reviewed here do not involve cortical neurones, which implies that the effect must involve descending modulatory input.
No difference in size, latency or duration of reflexive response
Contrary to our predictions, people in pain did not have a larger reflex size, shorter latency, or longer duration of defensive reflexes than pain-free controls had. There are two possible explanations for this finding. The first relates to the possibility of behavioural advantage of earlier but not larger motor responses to threatening stimuli. The second relates to the methodological characteristics of the constituent studies and the possibilities that experimental approaches have not detected differences that may in fact exist.
Augmentation of the trigger, but not the latency or size of a defensive reflex, might imply that modulation involves recruitment of neurones independent from those that normally subserve the reflex. That is, if incumbent neuronal pools are upregulated, then the reflex would presumably occur at lower stimulus intensities (i.e. decreased reflex threshold) but would also occur earlier and be a larger response. In contrast, an independent interneuronal pathway to the alpha motor neurone that is functionally high threshold in normal state but becomes low threshold under conditions of upregulation would explain a lower threshold without latency or size effects (Figure 6). This functionality could be mediated via inhibition of descending inhibitory control, for example, where ‘off cells’ in the rostroventral medulla are inhibited, exerting a facilitatory effect on spinal nociceptive networks (Heinricher et al., 1989). It is also possible that the same effect could be evoked by activation of descending facilitatory neurones (Roberts et al., 2009). In either case, the low threshold pathway would be ‘brought online’ during situations of perceived vulnerability and need for more rapid protection should a threatening stimulus occur. Moreover, to mediate an augmentation of the trigger but not the size of the response would require collateral inhibitor connections between the pathways (Figure 6). Clearly, this is a proposition that remains to be tested, but it has some ecological value that would seem worthy of future investigations.
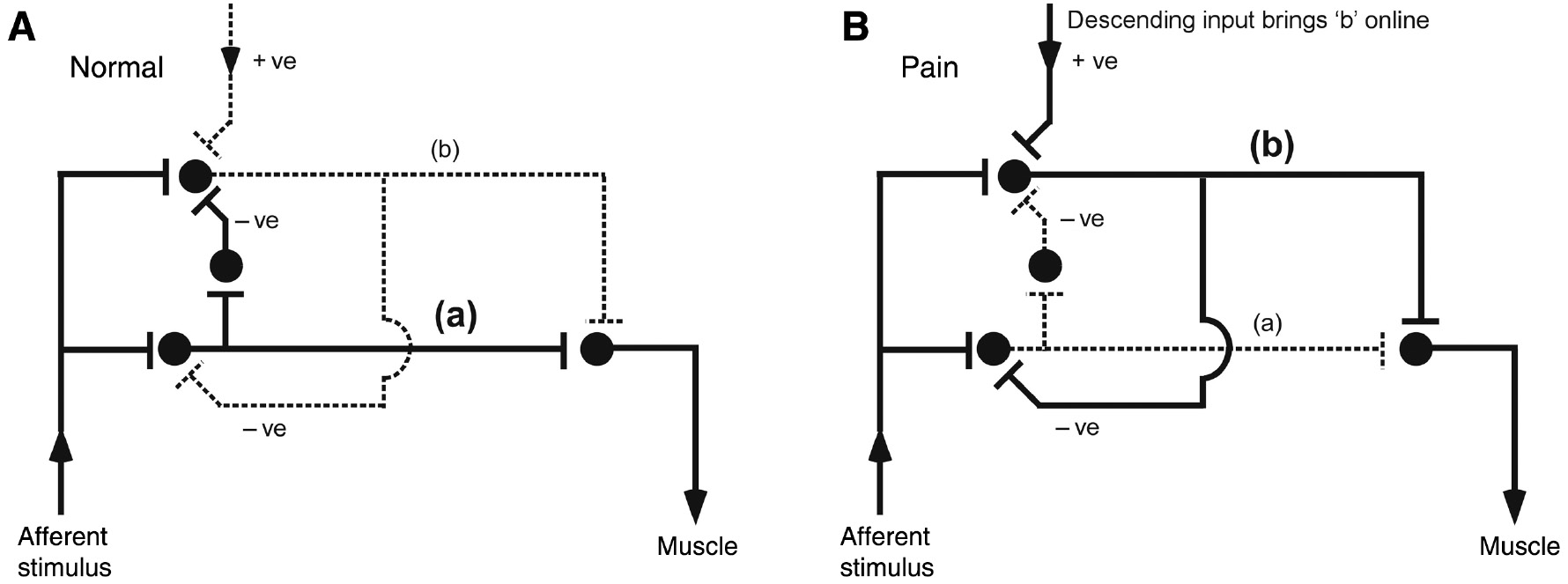
Possible neuronal pathways that would explain decreased reflex threshold in the absence of latency, duration or amplitude changes.
(A) In normal state, the stimulus activates pathway ‘a’ to elicit a motor response. (B) In people with chronic pain, descending facilitatory input brings an alternative pathway ‘online’. Dotted line reflects inactive and solid line active pathways.
The alternative possible explanation of null results for all but threshold data relates to methodological considerations and reasonably large signal to noise ratios within the constituent literature reviewed here. Experiments conducted using reflexes, such as many of those included in this review, are often undertaken at threshold intensities where the reflex size, latency, and duration are generally very similar. Therefore, it would be unlikely to expect differences in these outcomes – such as found in this review. Equally, that there are differences in stimulus thresholds between pain groups and healthy groups makes any comparisons difficult to interpret as any changes in size, latency, or duration may be reflective of a difference in stimulus threshold rather than any indication of a ‘need to protect’. Therefore, it would be more informative to perform comparisons between groups with all subjects receiving the same intensity stimulus. Furthermore, systematic differences between the two groups of people might be masked by large variability between participants in both groups. The experiments that are not confounded by these problems are those that investigate manipulations within participants – for example, the hand-blink reflex, modulations of which clearly involve magnitude (Sambo et al., 2012a,b).
There are indeed some data within the studies included here that seem consistent with the possibility that a null result in size data is a false negative. For example, the AUC for the nociceptive blink reflex was greater in migraineurs while they were suffering a migraine attack than while they were not, and it was greater on the affected side than on the unaffected side (Kaube et al., 2002). Furthermore, R2 latencies were also shorter during the migraine attack and, again, shorter on the affected side (Kaube et al., 2002). That is consistent with our initial predictions and suggests that within-subjects comparisons yield important information not captured by comparisons between groups, although such comparisons contain their own important sources of bias. Another possible explanation, which we were not able to clarify here, is that, because those in pain have a lower threshold for a response, then the size data reflect a response to a lower intensity stimulus. If so, any augmentation of the response would be counteracted by the lower stimulus intensity used. This is a speculative position but seems worthy of investigation. The most parsimonious conclusion to take from this review seems to be that, in order to verify that there is indeed no difference in size and latency data between those with and without pain, it may be necessary to use patients as their own controls – compare data obtained when they are in pain to data obtained when they are not.
Another method to investigate these reflexes may be to titrate reflex size to pain threshold. The advantage of this is that the percept is equal between pain patients and healthy controls, and therefore, the reflexive responses would be representative of a ‘need to protect’ for a given perceptual output. Despite the merit in this, it erroneously equates ‘pain’ to ‘nociception’ and provides no information about the level of input into the nociceptive system. There is now a large body of literature to show that nociception is a powerful influencer of pain but is neither sufficient nor necessary (see Moseley and Butler (2017), Melzack and Wall (1988), and Wall and McMahon (1986) for reviews on this). Therefore, to match stimuli to reported threat value is not addressing our primary interest of determining whether defensive reflexes are modulated by according to ‘a need to protect the self’.
Generalising across clinical pain conditions
This review included at least 13 different pain conditions: migraine, migraine without aura, tension-type headache, chronic tension-type headache, fibromyalgia, rheumatoid arthritis, chronic back pain, knee osteoarthritis, chronic whiplash, irritable bowel syndrome, chronic lateral epicondylalgia (‘tennis elbow’), a combination of idiopathic and myofascial pain, and a broad group with ‘chronic pain’. Each of these conditions is considered to be a chronic condition, often with unknown aetiology, although central sensitisation (conceived as generalised sensitivity as per Woolf (2014), not spinal nociceptor sensitisation as per Woolf et al. (1989)) is thought to play a significant role in the pathophysiology of each of these conditions (Woolf, 2011). We did not seek to test differences between specific clinical pain conditions, so we did not subgroup by condition. Clearly, this approach does not take into account variability that might be present between conditions, but the consistency of our results in each forest plot offers confidence in our a priori approach. The outliers within the data were scattered across a range of conditions (migraine, fibromyalgia, knee osteoarthritis), rather than one condition, which further supports our approach. It was beyond the scope of the current study to include healthy participants with experimental pain, although that would have allowed investigation of the effect of nociceptive stimulation, in a contrived and much less threatening context, on defensive reflexes locally and remote to the stimulated area. To iterate, we contend that the issue we highlighted earlier with respect to the difference between clinical pain and experimentally induced pain render this a different proposition, worthy of investigation but not to be conflated with the proposition investigated here.
It is important to note that lower reflex thresholds in people with pain do not imply a causative relationship between the two. Indeed, a modern conceptualisation of pain (e.g. Moseley and Butler, 2015b) holds that pain is the end product of a complex evaluative process that is usually, but not necessarily, triggered by noxious stimuli (see Harvie et al. (2015), Moseley (2004), and Moseley and Arntz (2007) for experimental evidence of the dissociation between nociception from pain and Madden et al. (2015) for a systematic review of associative learning of pain). Thus, pain and lower reflex thresholds are more likely to reflect epiphenomena in much the same way as has been proposed for pain and motor control (Moseley, 2013). However, it is certainly possible, and indeed suggested by this review, that decreased reflex threshold and the presence of pain share common contributing mechanisms, perhaps broadly captured by ‘the implicit need to protect body tissue’ (see Moseley and Butler (2015a) for coverage of this concept).
Studies not included in the meta-analyses
There were 19 studies that met the inclusion criteria for the review but could not be included in the meta-analyses because raw data could not be extracted (Peters et al., 1992; Leroux et al., 1995; Knost et al., 1997; de Tommaso et al., 2000; Nappi et al., 2002; Desmeules et al., 2003; Katsarava et al., 2003; Banic et al., 2004; Lang et al., 2005; Ayzenberg et al., 2006; Carleton et al., 2006; Sandrini et al., 2006; Al-Azzawi et al., 2008; Filatova et al., 2008; Mendak et al., 2010; Neziri et al., 2010; Biurrun Manresa et al., 2013a,b; Desmeules et al., 2014). We were unable to obtain a meaningful outcome relevant to our research question for eight studies (see Table 2 for details). Of the 11 studies that did report results, seven were consistent with the broader results of this review, two studies were opposing, and the remaining two were similar but with important albeit subtle differences (see Table 2 for details). The most prominent inconsistencies were those related to reflex thresholds: one study reported no difference in NFR thresholds between people with chronic low back pain or acute postoperative pain and pain-free controls (Peters et al., 1992), and the other reported no difference in NFR threshold between patients with chronic cluster headache and healthy controls (Nappi et al., 2002). The latter study (Nappi et al., 2002) had a medium risk of bias, which decreases our confidence in the results, but the former (Peters et al., 1992) had only a low risk of bias. Other studies reporting findings inconsistent with the meta-analysis include Al-Azzawi et al. (2008), who reported delayed blink reflex latencies in people with rheumatoid arthritis than in healthy controls and Neziri et al. (2010), who reported a higher reflex amplitude in people with chronic endometriosis than healthy controls.
Limitations
There were studies that were included in the systematic review and, had their data been available, may have affected the results of the meta-analysis and the overall conclusions. For example, for five studies, the authors were unable to access their data (Peters et al., 1992; Knost et al., 1997; Nappi et al., 2002; Katsarava et al., 2003; Sandrini et al., 2006), two considered that it was inappropriate to use their data and declined to share their results (Carleton et al., 2006; Biurrun Manresa et al., 2013a,b), and the authors of nine studies did not respond to several enquiries about their data (Leroux et al., 1995; de Tommaso et al., 2000; Desmeules et al., 2003, 2014; Banic et al., 2004; Lang et al., 2005; Ayzenberg et al., 2006; Al-Azzawi et al., 2008; Mendak et al., 2010). As noted in the peer review process of the current review, this does reflect a kind of bias – towards papers the authors of which are willing and able to share their data for the purposes of the meta-analysis. The gold standard approach to systematic review and meta-analysis, which we employed, serves to remove bias, for example, in literature selection and weighting of individual study outcomes. This allows much greater confidence in the results than narrative or non-systematic reviews would offer. It is common place in several fields to share data for the purposes of pooling and meta-analysis and making all data publically available after publication would improve meta-analysis robustness even further.
Non-English studies were excluded due to limited translational resources, and non-published studies were not sought. This may have introduced a publication bias; however, such biases are arguably more likely to give false positive effects, and the consistent lack of effect on most measures, across multiple studies and hundreds of participants, suggests against a publication bias here. The nature of a meta-analysis is that to integrate studies, all data need to be in a consistent format (means and standard deviations), which is not always suitable for all studies across the board. Unfortunately, when conducting a comprehensive review such as this, there will be inconsistencies between studies that will make direct comparisons difficult. Also affecting the homogeneity of the results is the differences in methodologies and underlying physiological hypotheses of all constituent studies. Many of the studies included did not set out to ask the question in which we were interested, which leaves open the possibility that, should more studies test our proposition with targeted designs, the null results on latency, size, and duration of the response may be countered. That said, inclusion of studies that obtain data on the question of interest without specifically targeting, and remaining consistent with the a priori inclusion and exclusion criteria, is clearly important for meta-analytical designs. It is important to acknowledge that this review focussed around the proposition that defensive reflexes would be upregulated in people with pain in a manner more consistent with notions of bodily protection than with sensitisation of spinal nociceptors or pain. However, we did not set out to quantify such a relationship between reflexes and perceived need to protect the body. This proposition was not mentioned in any of the constituent studies either. We contend that the current review clearly presents a trigger for a new line of enquiry into our proposition.
Concluding remarks
We found that the activation threshold to elicit a defensive reflex is lower in people with clinical pain than it is in pain-free controls. There appears to be no difference in size, latency, or duration of the reflex response between those with and without pain, but we remain cautious about this conclusion – it remains possible that a large signal to noise ratio in the constituent studies contributed to the lack of detectable effect. More studies with within-subject designs would be insightful. The pattern of reflex threshold shift cannot be explained solely by tissue-based augmentation consequent to inflammation or damage or spinal sensitisation because remote reflexes are clearly involved. We suggest that descending modulation with the primary goal of bodily protection underpins the augmentation, possibly by bringing independent intraspinal neuronal pathways online. The body of data seems consistent with the notion that augmented defensive reflexes reflect the need to protect, rather than a steady state of the nociceptive system. Finally, defensive reflex parameters do not provide a valid marker of usual pain intensity.
Conflicts of interest statement: In the last 5 years, G.L.M. has received support from Pfizer, Workers’ Compensation boards in Australia and Europe, Kaiser Permanente, Agile Physiotherapy, Results Physiotherapy, the International Olympic Committee, and Port Adelaide Football Club. He receives speaker fees for lectures on pain and rehabilitation and royalties for several books about pain and rehabilitation.
Funding sources:S.B.W. was supported by an Australian Postgraduate Award, Maurice de Rohan Scholarship, Ian Gould Scholarship, and an Australian Bicentennial Scholarship. L.G. was supported by the Swiss National Science Foundation (PBBEP1-144848). G.L.M. was supported by a Principal Research Fellowship from the National Health and Medical Research Council of Australia (ID 1061279); this work was supported by a project grant from the National Health and Medical Research Council of Australia to G.L.M. (ID 1008017).
References
Al-Azzawi, T.R., Hamdan, F.B., and Ali, A.K. (2008). Neurophysiologic evaluation of the temporomandibular joint and related masticatory muscles in rheumatoid arthritis patients. Neurosciences 13, 253–258.Search in Google Scholar
Avramidis, T.G., Podikoglou, D.G., Anastasopoulos, I.E., Koutroumanidis, M.A., and Papadimitriou, A.L. (1998). Blink reflex in migraine and tension-type headache. Headache 38, 691–696.10.1046/j.1526-4610.1998.3809691.xSearch in Google Scholar
Ayzenberg, I., Obermann, M., Nyhuis, P., Gastpar, M., Limmroth, V., Diener, H.C., Kaube, H., and Katsarava, Z. (2006). Central sensitization of the trigeminal and somatic nociceptive systems in medication overuse headache mainly involves cerebral supraspinal structures. Cephalalgia 26, 1106–1114.10.1111/j.1468-2982.2006.01183.xSearch in Google Scholar
Banic, B., Petersen-Felix, S., Andersen, O.K., Radanov, B.P., Villiger, P.M., Arendt-Nielsen, L., and Curatolo, M. (2004). Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain 107, 7–15.10.1016/j.pain.2003.05.001Search in Google Scholar
Baron, R., Hans, G., and Dickenson, A.H. (2013). Peripheral input and its importance for central sensitization. Ann. Neurol. 74, 630–636.10.1002/ana.24017Search in Google Scholar
Bartolo, M., Serrao, M., Gamgebeli, Z., Alpaidze, M., Perrotta, A., Padua, L., Pierelli, F., Nappi, G., and Sandrini, G. (2013). Modulation of the human nociceptive flexion reflex by pleasant and unpleasant odors. Pain 154, 2054–2059.10.1016/j.pain.2013.06.032Search in Google Scholar
Biurrun Manresa, J.A., Neziri, A.Y., Curatolo, M., Arendt-Nielsen, L., and Andersen, O.K. (2013a). Reflex receptive fields are enlarged in patients with musculoskeletal low back and neck pain. Pain 154, 1318–1324.10.1016/j.pain.2013.04.013Search in Google Scholar
Biurrun Manresa, J.A., Nguyen, G.P., Curatolo, M., Moeslund, T.B., and Andersen, O.K. (2013b). Probabilistic model for individual assessment of central hyperexcitability using the nociceptive withdrawal reflex: a biomarker for chronic low back and neck pain. BMC Neurosci. 14, 110.10.1186/1471-2202-14-110Search in Google Scholar
Biurrun Manresa, J.A., Finnerup, N.S., Johannesen, I.L., Biering-Sorensen, F., Jensen, T.S., Arendt-Nielsen, L., and Andersen, O.K. (2014). Central sensitization in spinal cord injured humans assessed by reflex receptive fields. Clin. Neurophysiol. 125, 352–362.10.1016/j.clinph.2013.06.186Search in Google Scholar
Boureau, F., Luu, M., and Doubrère, J.F. (1991). Study of experimental pain measures and nociceptive reflex in chronic pain patients and normal subjects. Pain 44, 131–138.10.1016/0304-3959(91)90126-ISearch in Google Scholar
Bradley, M.M., Lang, P.J., and Cuthbert, B.N. (1993). Emotion, novelty, and the startle reflex: Habituation in humans. Behav. Neurosci. 107, 970–980.10.1037/0735-7044.107.6.970Search in Google Scholar
Carleton, R.N., Asmundson, G.J., Collimore, K.C., and Ellwanger, J. (2006). Strategic and automatic threat processing in chronic musculoskeletal pain: a startle probe investigation. Cogn. Behav. Ther. 35, 236–247.10.1080/16506070600898504Search in Google Scholar
Coffin, B., Bouhassira, D., Sabate, J.M., Barbe, L., and Jian, R. (2004). Alteration of the spinal modulation of nociceptive processing in patients with irritable bowel syndrome. Gut 53, 1465–1470.10.1136/gut.2003.031310Search in Google Scholar
Cohen, J. and Ebrary, I. (1988). Statistical power analysis for the behavioural sciences, 2nd edn. (Hillsdale, NJ: L. Erlbaum Associates).Search in Google Scholar
Courtney, C.A., Lewek, M.D., Witte, P.O., Chmell, S.J., and Hornby, T.G. (2009). Heightened flexor withdrawal responses in subjects with knee osteoarthritis. J. Pain 10, 1242–1249.10.1016/j.jpain.2009.05.004Search in Google Scholar
Courtney, C.A., Witte, P.O., Chmell, S.J., and Hornby, T.G. (2010). Heightened flexor withdrawal response in individuals with knee osteoarthritis is modulated by joint compression and joint mobilization. J. Pain 11, 179–185.10.1016/j.jpain.2009.07.005Search in Google Scholar
Curatolo, M., Muller, M., Ashraf, A., Neziri, A.Y., Streitberger, K., Andersen, O.K., and Arendt-Nielsen, L. (2015). Pain hypersensitivity and spinal nociceptive hypersensitivity in chronic pain: prevalence and associated factors. Pain 156, 2373–2382.10.1097/j.pain.0000000000000289Search in Google Scholar
De Marinis, M., Pujia, A., Colaizzo, E., and Accornero, N. (2007). The blink reflex in ‘chronic migraine’. Clin. Neurophysiol. 118, 457–463.10.1016/j.clinph.2006.10.011Search in Google Scholar
de Tommaso, M., Guido, M., Libro, G., Sciruicchio, V., and Puca, F. (2000). Zolmitriptan reverses blink reflex changes induced during the migraine attack in humans. Neurosci. Lett. 289, 57–60.10.1016/S0304-3940(00)01255-6Search in Google Scholar
Desmeules, J.A., Cedraschi, C., Rapiti, E., Baumgartner, E., Finckh, A., Cohen, P., Dayer, P., and Vischer, T.L. (2003). Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 48, 1420–1429.10.1002/art.10893Search in Google Scholar PubMed
Desmeules, J., Chabert, J., Rebsamen, M., Rapiti, E., Piguet, V., Besson, M., Dayer, P., and Cedraschi, C. (2014). Central pain sensitization, COMT Val158Met polymorphism, and emotional factors in fibromyalgia. J. Pain 15, 129–135.10.1016/j.jpain.2013.10.004Search in Google Scholar PubMed
Ehrlichman, H., Brown, S., Zhu, J., and Warrenburg, S. (1995). Startle reflex modulation during exposure to pleasant and unpleasant odors. Psychophysiology 32, 150–154.10.1111/j.1469-8986.1995.tb03306.xSearch in Google Scholar PubMed
Filatova, E., Latysheva, N., and Kurenkov, A. (2008). Evidence of persistent central sensitization in chronic headaches: a multi-method study. J. Headache Pain 9, 295–300.10.1007/s10194-008-0061-7Search in Google Scholar
Flor, H., Knost, B., and Birbaumer, N. (1997). Processing of pain- and body-related verbal material in chronic pain patients: central and peripheral correlates. Pain 73, 413–421.10.1016/S0304-3959(97)00137-1Search in Google Scholar
Graziano, M.S.A. and Cooke, D.F. (2006). Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia 44, 845–859.10.1016/j.neuropsychologia.2005.09.009Search in Google Scholar PubMed
Harvie, D.S., Broecker, M., Smith, R.T., Meulders, A., Madden, V.J., and Moseley, G.L. (2015). Bogus visual feedback alters onset of movement-evoked pain in people with neck pain. Psychol. Sci. 26, 385–392.10.1177/0956797614563339Search in Google Scholar PubMed
Heinricher, M.M., Barbaro, N.M., and Fields, H.L. (1989). Putative nociceptive modulating nerons in the rostral centromedial medulla of the rat: firing of on- and off-cells is related to nociceptive responsiveness. Somatosens Motor Res 6, 427–439.10.3109/08990228909144685Search in Google Scholar PubMed
Higgins, J.P.T. and Green, S. (2011). Cochrane handbook for systematic reviews of interventions. Cochrane Collaboration, 5.1.0.Search in Google Scholar
Katsarava, Z., Giffin, N., Diener, H.C., and Kaube, H. (2003). Abnormal habituation of ‘nociceptive’ blink reflex in migraine – evidence for increased excitability of trigeminal nociception. Cephalalgia 23, 814–819.10.1046/j.1468-2982.2003.00591.xSearch in Google Scholar PubMed
Katsarava, Z., Limmroth, V., Baykal, O., Akguen, D., Diener, H.C., and Kaube, H. (2004). Differences of anti-nociceptive mechanisms of migraine drugs on the trigeminal pain processing during and outside acute migraine attacks. Cephalalgia 24, 657–662.10.1111/j.1468-2982.2004.00730.xSearch in Google Scholar PubMed
Kaube, H., Katsarava, Z., Przywara, S., Drepper, J., Ellrich, J., and Diener, H.C. (2002). Acute migraine headache: Possible sensitization of neurons in the spinal trigeminal nucleus? Neurology 58, 1234–1238.10.1212/WNL.58.8.1234Search in Google Scholar
Knost, B., Flor, H., Braun, C., and Birbaumer, N. (1997). Cerebral processing of words and the development of chronic pain. Psychophysiology 34, 474–481.10.1111/j.1469-8986.1997.tb02392.xSearch in Google Scholar PubMed
Kofler, M. and Halder, W. (2014). Alterations in excitatory and inhibitory brainstem interneuronal circuits in fibromyalgia: evidence of brainstem dysfunction. Clin. Neurophysiol. 125, 593–601.10.1016/j.clinph.2013.08.009Search in Google Scholar PubMed
Lang, E., Kaltenhauser, M., Seidler, S., Mattenklodt, P., and Neundorfer, B. (2005). Persistent idiopathic facial pain exists independent of somatosensory input from the painful region: findings from quantitative sensory functions and somatotopy of the primary somatosensory cortex. Pain 118, 80–91.10.1016/j.pain.2005.07.014Search in Google Scholar
Leroux, A., Bélanger, M., and Boucher, J.P. (1995). Pain effect on monosynaptic and polysynaptic reflex inhibition. Arch. Phys. Med. Rehabil. 76, 576–582.10.1016/S0003-9993(95)80514-1Search in Google Scholar
Li, D., Ren, Y., Xu, X., Zou, X., Fang, L., and Lin, Q. (2008). Sensitization of primary afferent nociceptors induced by intradermal capsaicin involves the peripheral release of calcitonin gene-related Peptide driven by dorsal root reflexes. J. Pain 9, 1155–1168.10.1016/j.jpain.2008.06.011Search in Google Scholar
Lim, E.C., Sterling, M., Pedler, A., Coombes, B.K., and Vicenzino, B. (2012). Evidence of spinal cord hyperexcitability as measured with nociceptive flexion reflex (NFR) threshold in chronic lateral epicondylalgia with or without a positive neurodynamic test. J. Pain 13, 676–684.10.1016/j.jpain.2012.04.005Search in Google Scholar
Madden, V.J., Harvie, D.S., Parker, R., Jensen, K.B., Vlaeyen, J.W.S., Moseley, G.L., and Stanton, T.R. (2015). Can pain or hyperalgesia be a classically conditioned response in humans? A systematic review and meta-analysis. Pain Med. 17, 1094–1111.10.1093/pm/pnv044Search in Google Scholar
McMahon, S.B., Bennett, D.L.H., and Bevan, S. (2006). Inflammatory mediators and modulators of pain. In: Wall and Melzack’s Textbook of Pain (5th Edition ed.), S.B. McMahon and M. Koltzenburg, eds. (London: Churchill-Livingstone), pp. 49–72.10.1016/B0-443-07287-6/50008-4Search in Google Scholar
Melzack, R. and Wall, P. (1988). The Challenge of Pain (2nd ed.) (New York, USA: Viking Penguin).Search in Google Scholar
Mendak, M., Konopka, T., Koszewicz, M., Koziorowska-Gawron, E., Ejma, M., and Budrewicz, S. (2010). Similarities between burning mouth syndrome and parkinson’s disease in selected electroneurophysiological studies. Adv. Clin. Exp. Med. 19, 731–738.Search in Google Scholar
Moher, D., Liberati, A., Tetzlaff, J., Altman, D.G., and Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 62, 1006–1012.10.1016/j.jclinepi.2009.06.005Search in Google Scholar
Moseley, G. L. (2004). Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic low back pain. Eur. J. Pain 8, 39–45.10.1016/S1090-3801(03)00063-6Search in Google Scholar
Moseley, G.L. (2013). Trunk muscle control and back pain: chicken, egg, neither or both? In: Spinal Control: the Rehabilitation of Back Pain, P.W. Hodges, J. Cholewicki, and J.H. van Dieen, eds. (Oxford, UK: Churchill Livingstone Elsevier).10.1016/B978-0-7020-4356-7.00011-2Search in Google Scholar
Moseley, G.L. and Arntz, A. (2007). The context of a noxious stimulus affects the pain it evokes. Pain 133, 64–71.10.1016/j.pain.2007.03.002Search in Google Scholar PubMed
Moseley, G. and Butler, D. (2015a). The Explain Pain Handbook: Protectometer. (Adelaide, Australia: Noigroup publications).Search in Google Scholar
Moseley, G. and Butler, D. (2015b). Fifteen years of explaining pain: the past, present, and future. J. Pain 16, 807–813.10.1016/j.jpain.2015.05.005Search in Google Scholar PubMed
Moseley, G.L. and Butler, D.S. (2017). Explain Pain Supercharged. (Adelaide, Australia: Noigroup publications).Search in Google Scholar
Nappi, G., Sandrini, G., Alfonsi, E., Cecchini, A.P., Micieli, G., and Moglia, A. (2002). Impaired circadian rhythmicity of nociceptive reflex threshold in cluster headache. Headache 42, 125–131.10.1046/j.1526-4610.2002.02028.xSearch in Google Scholar
Neziri, A.Y., Curatolo, M., Limacher, A., Nüesch, E., Radanov, B.P., Andersen, O.K., Arendt-Nielsen, L., and Jüni, P. (2012). Ranking of parameters of pain hypersensitivity according to their discriminative ability in chronic low back pain. Pain 153, 2083–2091.10.1016/j.pain.2012.06.025Search in Google Scholar
Neziri, A.Y., Haesler, S., Petersen-Felix, S., Muller, M., Arendt-Nielsen, L., Manresa, J.B., Andersen, O.K., and Curatolo, M. (2010). Generalized expansion of nociceptive reflex receptive fields in chronic pain patients. Pain 151, 798–805.10.1016/j.pain.2010.09.017Search in Google Scholar
Peddireddy, A., Wang, K., Svensson, P., and Arendt-Nielsen, L. (2009). Blink reflexes in chronic tension-type headache patients and healthy controls. Clin. Neurophysiol. 120, 1711–1716.10.1016/j.clinph.2009.06.024Search in Google Scholar
Peters, M.L., Schmidt, A.J., Van den Hout, M.A., Koopmans, R., and Sluijter, M.E. (1992). Chronic back pain, acute postoperative pain and the activation of diffuse noxious inhibitory controls (DNIC). Pain 50, 177–187.10.1016/0304-3959(92)90159-9Search in Google Scholar
Review Manager (RevMan) (Version Version 5.3). (2011): The Cochrane Collaboration.Search in Google Scholar
Rhudy, J.L., DelVentura, J.L., Terry, E.L., Bartley, E.J., Olech, E., Palit, S., and Kerr, K.L. (2013). Emotional modulation of pain and spinal nociception in fibromyalgia. Pain 154, 1045–1056.10.1016/j.pain.2013.03.025Search in Google Scholar PubMed PubMed Central
Roberts, J., Ossipov, M.H., and Porreca, F. (2009). Glial activation in the rostroventromedial medulla promotes descending facilitation to mediate inflammatory hypersensitivity. Eur. J. Neurosci. 30, 229–241.10.1111/j.1460-9568.2009.06813.xSearch in Google Scholar PubMed PubMed Central
Sambo, C.F. and Iannetti, G.D. (2013). Better safe than sorry? The safety margin surrounding the body is increased by anxiety. J. Neurosci. 33, 14225–14230.10.1523/JNEUROSCI.0706-13.2013Search in Google Scholar PubMed PubMed Central
Sambo, C.F., Liang, M., Cruccu, G., and Iannetti, G.D. (2012a). Defensive peripersonal space: the blink reflex evoked by hand stimulation is increased when the hand is near the face. J. Neurophysiol. 107, 880–889.10.1152/jn.00731.2011Search in Google Scholar PubMed
Sambo, C.F., Forster, B., Williams, S.C., and Iannetti, G.D. (2012b). To blink or not to blink: fine cognitive tuning of the defensive peripersonal space. J. Neurosci. 32, 12921–12927.10.1523/JNEUROSCI.0607-12.2012Search in Google Scholar PubMed PubMed Central
Sandrini, G., Rossi, P., Milanov, I., Serrao, M., Cecchini, A.P., and Nappi, G. (2006). Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension-type headache patients. Cephalalgia 26, 782–789.10.1111/j.1468-2982.2006.01130.xSearch in Google Scholar
Sherrington, C.S. (1910). Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J. Physiol. 40, 28–121.10.1113/jphysiol.1910.sp001362Search in Google Scholar
Skljarevski, V. and Ramadan, N.M. (2002). The nociceptive flexion reflex in humans – review article. Pain 96, 3–8.10.1016/S0304-3959(02)00018-0Search in Google Scholar
Smith, A.D., Jull, G., Schneider, G., Frizzell, B., Hooper, R.A., and Sterling, M. (2013). A comparison of physical and psychological features of responders and non-responders to cervical facet blocks in chronic whiplash. BMC Musculoskelet. Disord. 14, 313.10.1186/1471-2474-14-313Search in Google Scholar
Smith, A.D., Jull, G., Schneider, G., Frizzell, B., Hooper, R.A., and Sterling, M. (2014). Cervical radiofrequency neurotomy reduces central hyperexcitability and improves neck movement in individuals with chronic whiplash. Pain Med. (United States) 15, 128–141.10.1111/pme.12262Search in Google Scholar
Smolin, L.N. (1976). The peripheral mechanisms of sensitization of inflamed tissue. Prog. Brain Res. 43, 307–309.10.1016/S0079-6123(08)64362-9Search in Google Scholar
Sterling, M., Hodkinson, E., Pettiford, C., Souvlis, T., and Curatolo, M. (2008). Psychologic factors are related to some sensory pain thresholds but not nociceptive flexion reflex threshold in chronic whiplash. Clin. J. Pain 24, 124–130.10.1097/AJP.0b013e31815ca293Search in Google Scholar
Symonds, T.L., Burton, A.K., Tillotson, K.M., and Main, C.J. (1996). Do attitudes and beliefs influence work loss due to low back trouble? Occup. Med. (Lond.) 46, 25–32.10.1093/occmed/46.1.25Search in Google Scholar
von Elm, E., Altman, D.G., Egger, M., Pocock, S.J., Gøtzsche, P.C., and Vandenbroucke, J.P. (2007). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev. Med. 45, 247–251.10.1097/EDE.0b013e3181577654Search in Google Scholar
Wall, P. and McMahon, S. (1986). The relationship of perceived pain to afferent nerve impulses. Trends Neurosci. 9, 254–255.10.1016/0166-2236(86)90070-6Search in Google Scholar
Wallwork, S.B., Talbot, K., Camfferman, D., Moseley, G.L., and Iannetti, G.D. (2016). The blink reflex magnitude is continuously adjusted according to both current and predicted stimulus position with respect to the face. Cortex 81, 168–175.10.1016/j.cortex.2016.04.009Search in Google Scholar PubMed PubMed Central
Woolf, C.J. (1983). Evidence for a central component of post-injury pain hypersensitivity. Nature 306, 686–688.10.1038/306686a0Search in Google Scholar PubMed
Woolf, C.J. (2011). Central sensitization: implications for the diagnosis and treatment of pain. Pain 152 (Suppl. 3), S2–15.10.1016/j.pain.2010.09.030Search in Google Scholar PubMed PubMed Central
Woolf, C.J. (2014). What to call the amplification of nociceptive signals in the central nervous system that contribute to widespread pain? Pain Headache 155, 1911–1912.10.1016/j.pain.2014.07.021Search in Google Scholar PubMed
Woolf, C.J. and Salter, M.W. (2000). Neuroscience – neuronal plasticity: increasing the gain in pain. Science 288, 1765–1768.10.1126/science.288.5472.1765Search in Google Scholar PubMed
Woolf, C.J., Thompson, S.W., and King, A.E. (1989). Prolonged primary afferent induced alterations in dorsal horn neurones, an intracellular analysis in vivo and in vitro. J. Physiol. (Paris) 83, 255–266.Search in Google Scholar
Supplemental Material:
The online version of this article (DOI: https://doi.org/10.1515/revneuro-2016-0057) offers supplementary material, available to authorized users.
©2017, G. Lorimer Moseley et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Frontmatter
- Deciphering the modulatory role of oxytocin in human altruism
- How neuroscience can inform the study of individual differences in cognitive abilities
- Biogenetic and morphofunctional heterogeneity of mitochondria: the case of synaptic mitochondria
- Long non-coding RNAs: potential molecular biomarkers for gliomas diagnosis and prognosis
- Defensive reflexes in people with pain – a biomarker of the need to protect? A meta-analytical systematic review
- Blood-brain barrier-supported neurogenesis in healthy and diseased brain
- Mathematics, anxiety, and the brain
- Integrating neurobiology of emotion regulation and trauma therapy: reflections on EMDR therapy
- The potential of transcranial photobiomodulation therapy for treatment of major depressive disorder
Articles in the same Issue
- Frontmatter
- Deciphering the modulatory role of oxytocin in human altruism
- How neuroscience can inform the study of individual differences in cognitive abilities
- Biogenetic and morphofunctional heterogeneity of mitochondria: the case of synaptic mitochondria
- Long non-coding RNAs: potential molecular biomarkers for gliomas diagnosis and prognosis
- Defensive reflexes in people with pain – a biomarker of the need to protect? A meta-analytical systematic review
- Blood-brain barrier-supported neurogenesis in healthy and diseased brain
- Mathematics, anxiety, and the brain
- Integrating neurobiology of emotion regulation and trauma therapy: reflections on EMDR therapy
- The potential of transcranial photobiomodulation therapy for treatment of major depressive disorder

