Catalytic exploration metallic and nonmetallic nano-catalysts, properties, role in photoelectrochemistry for sustainable applications
-
Khaled Fahmi Fawy
Abstract
This article provides an overview of the photoelectrochemistry of nano-catalysts, their advantages, mechanisms, and the interactions between nanostructures and reactivity. The applications of nanomaterials in various processes, such as dye degradation, toxicity elimination, HER, CO2RR, and OER were found paramount. The types of catalysis, including homogeneous, heterogeneous, enzymatic, photocatalysis, and photo electrocatalysis, elucidate their significance, and unique applications are also included. Advanced catalysts, from semiconductor nanomaterials to cocatalysts and surface modifications, are explored for their ability to harness light energy and drive efficient redox reactions. The emerging trends in electrocatalyst design, such as metal-free carbon catalysts, carbon nanotubes, graphene, nanodiamond, porous carbon, metal and carbon composite catalysts, and other metal and carbon composite catalysts are very important for future perspectives, therefore their consideration in this review has been focused. It also briefly discusses the advantages and disadvantages of nano-catalysts, including advanced catalysis, photogenerated charge carriers, redox reactions, and cocatalysts and surface modifications.
1 Introduction
This review, explains the significance of nano photo/electrochemistry in catalysis and its relevance in various scientific and technological applications, and also a brief touch upon the challenges and opportunities in the field. Catalysis lies at the heart of numerous chemical processes, enabling the efficient transformation of reactants into products. As the demand for sustainable and energy-efficient technologies grows, the field of catalysis has expanded to explore novel approaches that leverage the unique properties of nanomaterials and the principles of photochemistry and electrochemistry. This review investigates into the fascinating realm of nano photo/electrochemistry for catalysis, where the combination of nanoscale structures, light energy, and electrochemical interfaces opens up new avenues for catalytic reactions with extraordinary efficiency and selectivity. The current study is structured to provide a comprehensive exploration of nano photo/electrochemistry for catalysis. It begins by elucidating the fundamental principles underlying photochemical and electrochemical reactions at the nanoscale. This foundational understanding sets the stage for the subsequent sections that delve into the various types of catalysis, essential catalyst features for electrocatalysis, the role of functional nanocatalysts, advancements in photocatalysis, and culminates in a reflective summary of the current state and future prospects of this burgeoning field. Intriguingly, the marriage of nanoscience, photochemistry, and electrochemistry has propelled catalysis into a new era of possibilities. As researchers continue to unravel the intricate mechanisms governing nanoscale catalytic processes, the applications of nano photo/electrochemistry are poised to revolutionize industries and contribute to sustainable technological advancements. This review embarks on a journey through the diverse landscapes of catalysis, shedding light on how the manipulation of matter at the nanoscale can inspire groundbreaking solutions to the challenges of a rapidly evolving world. 1 , 2 , 3
Nano photo/electrochemistry represents an innovative approach to catalysis that capitalizes on the intrinsic advantages of nanomaterials and the intricate interplay between light and electron transfer processes. The nanoscale dimension endows catalysts with enhanced surface area, modified electronic properties, and unique quantum effects, all of which synergistically influence catalytic behavior. Furthermore, the utilization of light energy in tandem with electrochemical driving forces offers the potential to overcome energy barriers and achieve transformations that were once deemed challenging or inefficient. 4 While the prospects of nano-photo/electrochemistry for catalysis are promising, several challenges must be navigated. The design and synthesis of functional nanocatalysts require precise control over size, shape, composition, and surface properties. Additionally, the integration of photochemical and electrochemical processes demands a deep understanding of the complex interplay between light absorption, charge separation, and catalytic reaction kinetics. However, these challenges are met with equally exciting opportunities to engineer catalysts with unparalleled activity, selectivity, and specificity, enabling applications across diverse domains including energy conversion, environmental remediation, and pharmaceutical synthesis. 5 Since the beginning of organic synthesis, visible light and their catalytic abilities has been considered as a versatile field of research, which attract chemists. 6 As light is the perfect source of renewable energy, resulting a strong link between photochemistry and sustainability. 7 , 8 Some chemical reactions are not possible by conventional ground state mixing, they can only be achieved by applying photons or radiations, this is due to the difference between the ground state and excited reactively of the molecules. 9 , 10 In this rapidly growing area of synthetic chemistry, photocatalysis leverages metal or organic catalysts that absorb visible light to accelerate reactions, enabling mild-condition access to highly reactive radicals through mechanisms like energy transfer, photo induced electron transfer, and atom transfer, with photoredox catalysis where light directly excites a catalytic intermediate driving significant innovation. Not only simple molecules but the 2D-nanomaterials are applied in the catalytic process. 11 , 12 , 13 These are efforts are done for generations of new idea in order to overcome energy crisis and develop renewable energy resources. 14 , 15 For example, Fujishima et al. developed the invention of TiO2 electrodes, for environmental remediation and sustainable energy resources. 16 The common form of catalysis is the use of semiconductors in photocatalysis which help in the designing of hybrid electrodes. 17 , 18 , 19 , 20
The nanomaterials epically 2D materials, are also applicable in synthesis chemistry and molecular design, but their performance in photocatalysis and electrocatalysis remains low, because the efficiency of kinetics and charge transfer are relatively low. Traditional bulk nanosheets, like g-C3N4, have low surface reactivity, poor solar abortively and charge recombination. Therefore, research focuses on developing the 2D nanomaterials catalysts which have ability of high mobility and charge carrier dynamics. Also, these 2D nanomaterials are consider as efficient catalysts for generation of 3D materials. 21 , 22 However, their photocatalytic and electrocatalytic has been lagged, by low transfer kinetics and in term of charge separation. 23 , 24 For instance, bulk nanosheets have been considered in the conventional design of materials based on graphitic carbon nitride (g-C3N4). 24 , 25 Therefore, it can be anticipated that the dimensionality and surface features are crucial in identifying the critical catalytic qualities for practical applications as well as the best method of producing the material. Consequently, research that tries to produce atomically thin 2D catalysts with improved mobility and charge mover dynamics. Due high electrical conductivity and larger surface area the 2D-nanomaterials are consider extremely effective in electrophotocatalysis. 26 The logical design and fabrication of effective nanomaterial’s catalyst, as well as the problems related to commercial applications, are still not sufficiently well understood. Because of this, a thorough examination is still required to offer fresh perspectives on the development and application of current breakthroughs, and basic research are required for unambiguous reaction pathways to increase catalytic performance for applications ready for commercial exploitation. Numerous top-notch reviews of nanomaterials for catalysis have been published which can cover various catalytical aspects of nanomaterials, 27 , 28 , 29 , 30 as in (Figure 1 27 ).
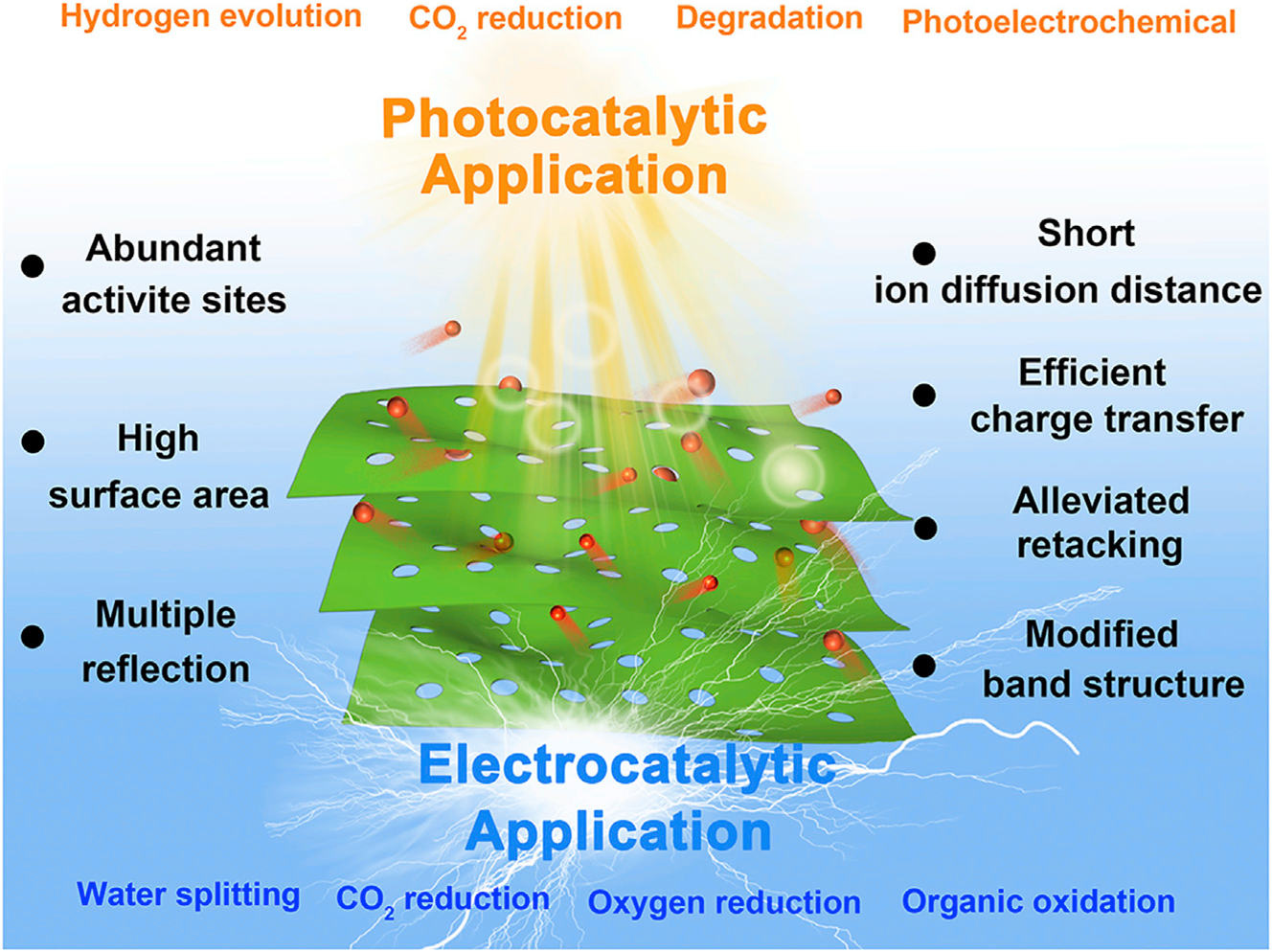
Photo-catalytical and electro-catalytical applications of nanomaterials, reproduced with permission from Elsevier, (Copy right 2020). 27
2 Photo/electrochemistry of catalyst at the nanoscale
The intricate interplay between photochemistry and electrochemistry at the nanoscale has paved the way for revolutionary advancements in catalysis. The fundamental principles underlying these processes highlight their applicability, and how nanomaterials’ unique properties influence their behavior as catalysts also play a very important role. Nanomaterials, under their small size, show properties distinct from their bulk complements. The high surface area-to-volume ratio enhances the exposure of active sites, augmenting catalytic reactivity. Quantum confinement and surface effects lead to discrete electronic energy levels, enabling the tuning of electronic properties for specific reactions. 31 These nanoscale effects drastically alter reaction kinetics, adsorption energies, and charge transfer mechanisms. Quantum confinement leads to discrete energy levels due to the confinement of electrons and holes within nanoscale dimensions. This influences band structures, leading to shifts in energy states and electronic transitions that affect reaction pathways. The dominance of surface atoms in nanomaterials results in enhanced surface reactivity. Catalytic sites located on the surface play a crucial role in reaction initiation, making surface engineering a critical aspect of catalyst design. 32
2.1 Photochemical reactions and mechanisms
Photochemistry involves the interaction of light with matter to induce chemical transformations. At the nanoscale, light–matter interactions become more pronounced due to increased surface area and confinement effects. Photochemical reactions offer the advantage of providing energy input directly from photons, allowing the activation of high-energy reactions at lower temperatures. Nanoscale catalysts efficiently absorb light due to their small dimensions and tailored electronic structures. This generates photogenerated electron-hole pairs, which can drive redox reactions by transferring charges to the catalyst’s surface. Photocatalysis involves a sequence of steps including light absorption, charge separation, surface reactions, and charge recombination. The nanoscale allows for efficient charge separation, minimizing recombination losses and enabling multiple redox cycles. 33
2.2 Electrochemical processes and interfaces
Electrochemistry deals with electron transfer reactions occurring at interfaces between electrodes and electrolytes. The nanoscale structure of catalysts significantly influences these interfaces, affecting charge transfer rates and electrocatalytic activity. Nanomaterials offer a high density of electrocatalytic sites due to their large surface area. The electrochemical double layer at the interface plays a critical role in facilitating charge transfer reactions and determining the overpotential required for catalysis. Common electrochemical reaction mechanisms involve steps such as adsorption, charge transfer, and desorption. Nanocatalysts provide abundant sites for these processes, enabling rapid reaction kinetics and lower energy barriers. 34
2.3 Interplay between nanostructures and reactivity
The combined effects of nanoscale size, shape, and composition create intricate nanostructures with diverse catalytic properties. These properties emerge from the synergy between photochemical and electrochemical phenomena. Nanostructures exhibit synergies between photochemical and electrochemical effects. Photoexcited charge carriers can be directed toward electrocatalytic sites, effectively utilizing light-induced energy to drive electrochemical reactions. Some nanomaterials exhibit plasmon resonances, where light interacts with free electrons on the nanoparticle surface. 35 Plasmonic effects can enhance light absorption and provide additional energy pathways for catalytic reactions. 36 In essence, the photo/electrochemistry of catalysts at the nanoscale represents a convergence of principles, where the distinct characteristics of nanomaterials synergize with photochemical and electrochemical processes. This intricate interplay opens up new horizons for tailoring catalysts’ behavior, enabling catalytic reactions with remarkable efficiency and selectivity.
3 Enhancement of catalytic performance for nano-catalytic materials
The most important concerns for enhancing photocatalysis and electrocatalysis performance are often carrier separation and transfer kinetics, 37 which can be strongly correlated with the structure-activity of catalysts. 38 , 39 The functionality and viability of 2D layered nanomaterials, such as graphene and graphite-like materials, are still limited in applications involving semiconductors, sensors, and catalysis. 40 To design the catalyst base on layered nanomaterials with improved electrocatalytic and photocatalytic potentials, a variety of numerous techniques must be investigated. In Figure 2 some basic terminologies are diagrammatically represented.

General routes for nano-catalysts used in photo/electro-catalysis, which cover certain characteristics like surface/interface, energy bands, number of reactive sides and electronic states, alongside with multiple advance approaches ie doping, phase transition, heterojunctions and defects, reproduced with permission from Springer link, (Copy right 2021). 41
4 Advantages of nano-catalysts
Structure-activity correlations in 2D nanocatalysts reveal the profound impact of geometric designs on catalytic performance. 38 This influence stems from the distinctive properties inherent to nanocatalysts. 39 Nanocatalysts possess several advantageous attributes, including expansive surface-active sites, elevated electron mobility, tunable band structures, electronic characteristics, and mechanical properties. Surface-active sites consist of spacious, exposed lattice planes with a high density, thereby augmenting catalytic reactions. The mechanical properties of nano-catalysts, encompassing robust catalyst durability and the potential for hybrid nanocatalysts to enhance catalytic performance, further bolster the catalytic activity of nanocatalysts. 41
4.1 Surface active sites
The distinctive geometric arrangements of nanocatalysts give rise to expansive specific surface areas, which are directly correlated to highly dense packing and lattice planes. These high-density surface-active sites on the material’s surface serve to enhance catalytic processes. Another avenue to enhance the exposure of surface-active sites involves reducing the lateral dimensions of nanocatalysts. 42 For instance, ultrasmall molybdenum disulfide (MoS2) demonstrates superior performance in hydrogen evolution reactions (HER) compared to bulk MoS2, ascribed to profusion of HER active sulfur edges. 43
4.2 Carrier mobility
The nanomaterials including black-phosphorus (BP), graphene, and transition metal-dichalcogenides (also called TMDs) exhibit profound electron mobilities or carrier mobilities. 44 For example, for the MoS2 and graphene reported mobilities falls within the range of 102–104 cm2 V−1 s−1 and around 101 cm2 V−1 s−1 respectively. 41 Owing to their ultrathin structure characterized by ultra-short transport paths and minimal intrinsic resistance, nanocatalysts facilitate rapid charge migration. Yu et al. observed a distinct decline in HER performance upon attaching an additional atomic layer to MoS2, which are perhaps due to the electronic mobilities along vertical direction, among material layers. 45
4.3 Energy band structures
The manipulation of the layers quantity in the crystal lattice enables the adjustment of the band gap across a range of 2D layered nanomaterials. This tunable band structure significantly influences the photocatalytic capabilities of the materials. MoS2’s band gap, for example, can be tailored by varying the cumulative layers; the band gaps for single and few layered are 1.85 ± 0.05 and 1 ± 0.20 eV respectively. 46 The reported band gap of g-C3N4 ranges between 1.6 and 1.1 eV when referenced to a normal hydrogen electrode (NHE). 47 By introducing different anions and cations into bismuth-based 2D layered nanomaterials, the band gap can be fine-tuned to span from 0.3 to 3.6 eV, resulting in light response spanning from ultraviolet to near-infrared. 48
4.4 Electronic properties
The manipulation of the electrical characteristics can be achieved by adjusting the thickness of nanocatalysts. 49 The electronic architectures of 2D nanomaterials play a pivotal role in controlling the binding improve among active sites and reactants, potentially reducing desorption kinetic barriers.
4.5 Mechanical properties
Evident mechanical features characterize nanomaterials imparting high catalyst durability and opening accesses to practical applications that benefit society. Moreover, the durability of nanomaterials paves the way for the conception of hybrid nanocatalysts aimed at catalytic enhancement.
5 Nanocatalysts
Nano catalysts play a pivotal role in enhancing electrochemical and photochemical reactions due to their unique properties and high surface area. They can be classified into different categories based on their composition, structure, and application. Some common classifications of nano catalysts for electrochemical and photochemical reactions are based upon composition, structure, applications, reactions, size etc.
Composition classification are based on metal, metal oxides or transition metal dichalcogenides. Metal-Based Nano Catalysts include noble metals (e.g., platinum, gold, silver) and non-noble metals (e.g., nickel, copper) that are often used for various electrochemical and photochemical reactions. Metal oxide nano catalysts have metal oxides (e.g., titanium dioxide, zinc oxide) which are frequently used in photocatalysis due to their ability to absorb light and generate electron-hole pairs for catalytic reactions. 50 , 51 According to structure the nano-catalyst can be nanoparcticles, nanowires, nanotubes, nonorods, nono-plate or even nono-composite. Nanoparticles are nanoscale particles of various shapes and sizes. They offer high surface area and can be tailored for specific reactions. One-dimensional nanostructures with high aspect ratios that provide efficient charge transport and catalytic activity are prominent properties of nanowire and nonotubes. Nanorods and nanoplates structures possess anisotropic properties and can be tuned for enhanced light absorption and charge separation in photocatalysis. Combination of different nanostructures or materials (nanocomposites) also synergistically enhance catalytic performance. Certain nano-catalyst are application base such as electronanocatalysts designed for OER, HER and PRR electrochemical reactions. Photonanocatalysts is used in photochemical reactions, particularly in applications like water splitting, pollutant degradation, and CO2 reduction. While Bifunctional Catalysts can perform both electrochemical and photochemical reactions, often used for integrated energy conversion systems. 52 , 53
Advance varieties of nono catalysts are design nowadays by incorporation multiple support for example carbon support nanocatalyst using carbon nanotubes, graphene etc that offer high conductivity and stability. Similarly, porous materials with metal nodes and organic linkers that can serve as supports for nano catalysts, offering tunable properties. These classifications demonstrate the diverse landscape of nano catalysts for electrochemical and photochemical reactions, reflecting the ongoing research and development in this field to harness their potential for sustainable energy conversion and environmental applications.
5.1 Classification of nanocatalysts
The acceleration of progress in the realm of ultrathin nanomaterials has been precipitated by the innovation surrounding atomically thin graphene nanomaterials. The majority of these nanomaterials can be broadly classified as layered materials. In these materials, the stacking of layers is driven by van der Waals interactions, while the atom layers within each stratum are frequently robustly interconnected through chemical bonds. 54 2D-materials normally applied for catalysis are graphene, g-CN, MOFs, COFs, Mxenes, LDHs, h-BN etc, where some classes of them are works as effective nanocatalyst. Graphene often conceptualized as an atomic monolayer of graphite. 55 Nanomaterials comprised of atomically thin graphene are alluring due to their superior performance in catalytic applications compared to traditional semiconductors. Given that graphene nanomaterials function as zero band-gap, semi-metals, they are generally employed as co-catalysts or as efficient catalyst supports, rather than standalone catalysts. 56
The g-C3N4 is graphene like 2D-materials 57 which possess chemical inertness in harsh acidic or alkaline environments, rendering them potential catalysts in various redox processes.
But still there are certain problems that limit their applicability in catalysis, some of these factors are limited surface area, high electric carrier recombination rates, and inadequate mass transfer. For the purpose of overcoming these issues surface defect engineering and elemental doping strategies has been employed. Transition metal dichalcogenides (TMDs) generally consist of layers of chalcogen atoms separated by layers of transition metal atoms, which can also act as nanocatalyst. 58 , 59 , 60 , 61 , 62 The band gap of TMDs can be tailored by adjusting the number of layers in the crystal. MoS2-based nanomaterials, a prototypical form of TMD, exhibit distinctive lattice vibration properties, notable catalytic activity, cost-effectiveness, and abundance. Owing to these unique qualities, 2D layered MoS2 nanoparticles have demonstrated considerable potential in a wide array of applications, potentially even replacing graphene nanomaterials. In a notable instance, Zhang et al. showcased impressive catalytic properties of MoS2 in N2 reduction, attaining high Faradaic efficiency and NH3 yield rates. 63 Graphene-like MXenes, including mono- and double-transition metal MXenes, are crafted through the arrangement of stacked scrolls and sheets. Monolayer MXenes, characterized by a significant concentration of electron states proximate to the Fermi level, exhibit metallic traits. By virtue of this electronic configuration, MXenes hold promise as layered materials for catalytic purposes. Due to their excellent electronic conductivity, high elastic moduli, and favorable hydrophilic attributes, MXenes have been employed in different catalytic reaction such as CO-oxidation, 64 Water gas shift reaction, 65 spanning hybrid electrochemical supercapacitors to Li-ion battery anodes. 41 Layered double hydroxides (LDHs) are crafted by juxtaposing positively charged host layers and interlayered structural water housing negatively charged anions. 66 Numerous reports underscore the catalytic potential of LDHs (Figure 3), especially those integrating transition metals, in various processes related to oxygen and hydrogen production. 67
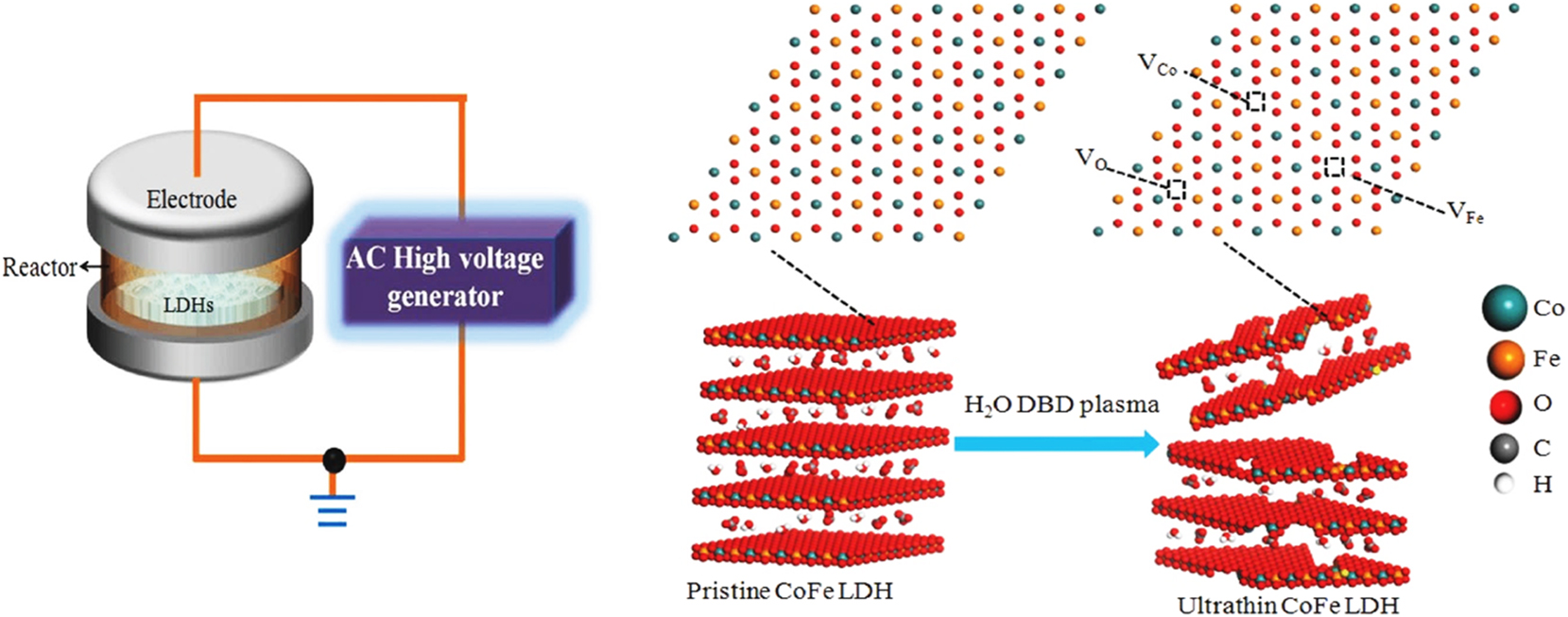
Water plasma enabled exfoliation for the synthesis of CoFe LDHs ultrathin nanosheet with multiple vacancies (OER electrolysis). The inter layered cations and anions interactions are destroyed which further enhance fast-exfoliation and resulting more vacancies. This route shows excellent kinetic and catalytic performance. The reactor for DBD were designed with plate to plate electrode at 50 V. 67
Bismuth, an environmentally benign metal, boasts a range of intriguing characteristics that render it applicable to diverse processes, including catalysis. The realm of 2D layered nanomaterials founded on bismuth has reported high performance outcomes in storage and energy conversion devices. 68 Manipulating the inherent structure by incorporating diverse cations and anions can modulate the band gap, yielding a light responsiveness spanning from ultraviolet to near-infrared wavelengths. This instrumentation also results in enhanced mass and mobility of photoexcited charge carriers, bolstering applications like photochemical catalysis, photodetection, and optoelectronic energy conversion. 69 Hexagonal boron nitride (h-BN) mirrors a hexagonal crystal structure reminiscent of graphite’s arrangement. 70 Owing to its exceptional resistance to high temperatures, noteworthy thermal conductivity (390 W m−1 K−1), robust chemical stability, resistance to acid corrosion, and superb electrical insulation, it has found utility as a catalyst carrier or catalyst itself. To address its intrinsic low electrical conductivity, efforts have been invested in functionalizing h-BN monolayers through combinations with materials that are electrically conductive in nature for example CNTs, rGO, etc. 71 MOFs possess single-layered lamellar structures, a mere atom thick, granting them high aspect ratios and the potential for post-synthesis adjustments to create tailored pores for catalysis and selective adsorption, including the incorporation of functional groups. The inclusion of transition metals in MOFs yields wide pore diameters, sufficient surface area, and a diverse spectrum of MOF structures, all advantageous for catalytic applications. 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 The formation of 2D metal nanomaterials, such as nanosheets, nanodisks, nanoplates, nanoribbons, nanorings, and nanobelts, hinges on the utilization of noble metal. 80 Owing to their intriguing electrical and structural characteristics, these 2D metal nanoparticles find use in a multitude of catalytic processes. Notably, Huang et al. demonstrated the remarkable catalytic improvement in the electrocatalysis in formic acid oxidation reaction. 81
5.2 Structures of nanocatalysts
Nanomaterials exhibit distinct crystal phases linked to atomic coordination, atomic arrangement, and layered stacking, 82 which can exert substantial control over their properties and catalytic behaviors. 83 Here, we will delve into the structures of the aforementioned nanocatalysts, while Figure 4 showcases a diverse array of catalytic nanomaterials with various structural attributes. Graphene, for instance, demonstrates a hexagonal or honeycomb-like shape owing to each carbon atom’s covalent bonding with the three neighboring atoms. 84 In contrast, g-C3N4 presents specific disparities in its planar structure compared to graphene. In g-C3N4, carbon and nitrogen atoms form N-substituted graphite frameworks in a sp2-hybridized configuration. There are two primary structural types within g-C3N4, namely tri-s-triazine units and s-triazine units. 85 The monolayer structure of tri-s-triazine-containing g-C3N4 becomes disordered at 900 K in a vacuum, disrupting hydrogen bonds among NH/NH2 groups and causing NH/NH2 groups to twist outward, as depicted in Figure 4a. 86 When exposed to visible light, these amorphous g-C3N4 nanomaterials may exhibit enhanced photocatalytic hydrogen production compared to crystalline g-C3N4. 86 Layered puckered honeycomb structural BP exhibits a Cmca space group and an orthorhombic crystal structure. Each phosphorus atom connects to three adjacent phosphorus atoms; three of these phosphorus atoms lie in the same plane, while the remaining one resides in a different plane. Figure 4b offers a top view of the typical BP structure. 87 Generally, van der Waals interactions enable monolayered Transition Metal Dichalcogenides (TMDs) to stack, forming layered TMDs. A single monolayered TMD comprises two chalcogen atomic layers and one interposed transition metal atomic layer. The top and side perspectives of a TMD’s structure are illustrated in Figure 4c, showcasing covalent bonds formed between the metal and chalcogen atoms via trigonal prismatic coordination. 88 Periodic porous Covalent Organic Frameworks (COFs) are systematically constructed using organic building blocks with covalent bonds. 89 , 90 Figure 4d 91 provides an overview of a typical COF structure, boasting inherent high porosity, adjustable pore size, excellent conjugation structure, extensive surface area, crystallinity, visible light response within broad range and limited secondary pollution. A type of ternary carbides “Mn+1AXn, MAX” is used as parent for the designing layered MXene “Mn+1AXn” via A-group selective etching. Geng et al. successfully catalyzed hydrogen evolution processes using a Mo2C-on-graphene MXene heterostructure, as illustrated in Figure 4e, 92 showcasing the crystal structure of Mo2C. Furthermore, a highly efficient oxygen evolution electrocatalyst, ultrathin CoFe LDH nanosheets were fabricated through Ar plasma exfoliation, as depicted in Figure 4f. 93 Bismuth-based 2D layered nanomaterials, possess a high-dispersion band, resulting in photogenerated carriers with low effective mass and high mobility. One prominent example is bismuth oxychloride (BiOCl), which exhibits a tetragonal structure with a P4/nmm space group. Figure 4g showcases the layered crystal structure, where the atoms of O and Bi are stacked in the form of sandwich between layers of Cl-atoms. 94 H-BN, belonging to the hexagonal crystal system, shares a hierarchical structure reminiscent of graphite, as illustrated in Figure 4h. 95 Typically, sp2 hybridized B and N atoms form a regular hexagonal ring network between separate layers, creating h-BN nanosheets. 95 The B and N atoms are strongly covalently bonded within the layers, akin to other layered nanomaterials. Moreover, weak van der Waals interactions between layers facilitate material exfoliation, yielding ultrathin nanosheets. 96 The strong coordination linkage is found among metal clusters (nodes) and organic ligands (linkers) in MOFs. These components self-assemble into compounds with periodic structures. Li and colleagues generated 2D layered MOFs from LDHs via a straightforward ligand-assisted approach, as depicted in Figure 4i. 97 Their findings indicated improved performance in water oxidation. However 2D-MOFs still have low electrical conductivity, falling below 10−14 S cm−1, primarily due to internal porosity resulting from the stacking of multiple atomic layers. 98

Some examples of 2D-dimensional nanomaterials. (a) Graphene and graphitic nitride structre. 86 (b) Structure of mono-elemental compound family. 87 (c) Structure of TMD. 88 (d) Structure of COF 91 (e) structure of MXene. Copyright 2017 reproduced with permission from Wiley–VCH 92 (f) structure of LDHs. 93 (g) Structure of BiOX. 94 (h) Structure of h-BN. 95 (i) Structure of MOFs. 97
5.3 Synthesis of nanocatalysts
Top-down and bottom-up approaches are the main techniques for creating layered nanomaterials. Top-down synthesis involves demixing stratified bulk materials, breaking bonds along the layer plane and breaking weak interlayer van der Waals interactions to produce 2D ultrathin nanosheets. 99 Techniques include liquid/gas exfoliation, 100 mechanical cleavage, 101 shaking treatment, 102 wet ball milling, 103 sonication, and chemical etching are used in top down synthesis. Liquid exfoliation introduces weakening of interlayers, resulting in space expansion and debonding. Mechanical cleavage prepares multiple 2D layered nanomaterials, such as graphene layers. The Scotch tape method is credited with discovering graphene and producing functional 2D monolayered nanomaterials.
It is observed that ultrathin layered MOFs with high crystalline nature, lateral area can be produce by wet ball milling grinds materials, with zirconia in proper solvent. The expected thickness will be about 1 nm for synthesized MOFs. However, top-down approaches have drawbacks, such as unstable nanosheet production, unpredictable layer counts, poor homogeneity, restricted use, low product yield, and stripped nanosheets that break apart and reassemble. 30 Further research on top-down synthesis of 2D nanomaterials is crucial, as ongoing work is expected to drive significant improvements. Alternatively, bottom-up synthesis of 2D layered nanomaterials depends on the directional assembly of small molecules, with growth limited to the vertical axis. Methods like chemical vapor deposition, surfactant-assisted synthesis, surfactant self-assembly, template-assisted synthesis, inorganic-organic lamellar, and solvothermal synthesis offer controlled synthesis and large-scale production for bottom up method (see Figure 5). Lang and colleagues used a bottom-up solvothermal approach to create atomic layered binary MOF nanosheets and achieved good oxygen generation. Chemical vapor deposition is a popular method for producing 2D materials on a large scale, offering superior control over material size and thickness. 104 However, conventional bottom-up synthesis methods often require substrates and surfactants, making it challenging to manufacture dispersed 2D nanomaterials and remove remaining surfactants, potentially limiting their applicability. 41

Comparison of top-down and bottom-up approaches in nanomaterial synthesis. Top-down techniques for 2D nanomaterial synthesis.
5.4 Catalytic applications of nanomaterials
Research shows the importance of nanomaterials, predominantly for, applications in biochemical and environmental technologies, including cancer treatment, toxicant removal, dye degradation, OER, and CO2RR. Table 1 also provides a comprehensive list of nanomaterial-based electrocatalysts utilized in practical applications. It includes information on synthesis procedures, environmental factors, unique electrocatalytic performance, and the fundamental catalysis mechanisms. 105 Consequently, there is an imperative need to exp cutting-edge technologies capable of effectively eliminating organic molecules from aquatic environments. In this context, the emerging catalytic applications of 2D nanomaterials for biochemical and environmental remediation were investigated.
List of some nanomaterials and their applications in electrocatalysis.
| S.No | Nanomaterials | Condition | Synthesis | Applications | Mechanism | Ref. |
|---|---|---|---|---|---|---|
| 1 | Co3S4 | Basic medium | Physical etching | HER | Abundant sulfur vacancies | 26 |
| 2 | Dg-MoS2 | Acidic | Electrodeposition | HER | Surface embellish for edge site explore | 106 |
| 3 | MoS2 | Acidic | Hydrothermal | HER | Facilitated ion diffusion by channel engineering | 25 |
| 4 | WSe2 | Acidic | Hydrothermal | HER | Many exposed sites | 107 |
| 5 | Co-N-GA | Acidic | Solvothermal | HER | Synergetic effect of N-doped C and inner Co | 108 |
| 6 | NiFe-LDH | Alkaline | OER | Ultrasonication | Metal and O-vacancies | 109 |
| 7 | NiFe-MOF | Alkaline | OER | Solvothermal | Fe constitutes the active sites | 67 |
| 8 | Ni(OH)2 | Alkaline | OER | Chemical etching | Inside sheet holes as a permeable channel | 110 |
| 9 | CoCo-LDH | Alkaline | OER | Soft template method | Active edges and low coordination numbers promote diffusion | 111 |
| 10 | BP | Alkaline | OER | Liquid phase exfoliation | Reduction in thickness promotes active sites for specific areas | 100 |
| 11 | Co-C3N4-CNT | Alkaline | OER, ORR | Polycondensation and leaching | M-N2 coordination | 55 |
5.5 Dye degradation
It is noteworthy that, nanomaterials are exceptionally compatible for photodegradation oxidative reactions of dyes, due to their optimal thickness and limited kinetic barriers. For instance, Zhang and collaborators employed a hydrothermal technique to synthesize BiOCl, which is a single-layered, highly crystallized material. 112 Figure 6a illustrates the process of photodegradation excitation of BiOCl nanosheet, along with the direct effect of semiconductor degradation efficiencies of BiOCl-001 and BiOCl-010 about 99 % and 59 % respectively. Intriguingly, the photocatalytic activity of direct semiconductors surpassed that of indirect semiconductors, and the photoexcitation performance of BiOCl nanomaterials was more pronounced under UV light than visible light. Additionally, as demonstrated in Figure 6b and c, the photocatalysts BiOCl and BiOCl–OH were developed for photocatalysis and degradation of dye named Rhodamine-B in wastewater, upon exposure to UV-radiation with wavelength equal to 365 nm. 113 The increase in oxygen vacancies induced by UV light was found to enhance the photocatalytic activity of BiOCl–OH in comparison to pure BiOCl. The OH-functional group in Rhodamine B dye, play a sufficient role in the photocatalytic degradation, as revealed by FT-IR analysis. 113 In the pursuit of effective photocatalysis for dye degradation and the generation of highly reactive oxygen species like H2O2, a heterostructure BP/CN nonmaterial (black porous: graphitic carbon nitride) was designed by Zheng et al. 114

5.6 Elimination of toxicants
Phenolic compounds constitute highly detrimental organic pollutants in water, stemming mainly from activities in the oil refining, printing, pesticide, and pharmaceutical industries. The presence of phenols in industrial wastewater poses a significant risk to surface water contamination. Consequently, the global pursuit of efficient and environmentally safe degradation methods is of utmost importance. 40 For instance, in a groundbreaking discovery, Liu and colleagues found that g-C3N4/Bi2WO6/rGO hetero-junctioned photocatalysts, containing 3 wt% of rGO, achieved an impressive 86 % reduction in ibuprofen through photocatalytic degradation under visible light and a remarkable 98 % reduction under solar light irradiation. 116 Considering the potential accumulation of antibiotics in the human body, posing a risk of irreversible harm, photocatalytic oxidation has emerged as a highly effective approach for removing antibiotics from wastewater. Despite potentially lower photodegradation rates in winter, Norvill and colleagues demonstrated a 93 % reduction of the antibiotic tetracycline under summer-like conditions, along with chemical oxygen demand and sufficient biomass. The studies highlight the superior removal capability of algal wastewater treatment compared to traditional biological wastewater treatment and mark a significant achievement in tetracycline removal from outdoor wastewater environments.
5.7 Hydrogen evolution reaction (HER)
In the light of escalating environmental pollution and the global energy crisis, the search for alternative energy sources has become imperative. 117 Hydrogen energy, a promising and clean energy source, offers the advantage of high energy density and minimal environmental impact. 118 The Hydrogen Evolution Reaction (HER), often referred to as the second half of water splitting, involves the cathodic reaction represented by the formula 2H+ + 2e− --- > H2. 119
The HER comprises two primary processes: proton adsorption and hydrogen desorption, which can be elaborated further in acidic solutions. These processes are rooted in the fundamental mechanisms known as the Tafel–mechanism, Heyrovsky–mechanism, and Volmer–mechanism. The sites of adsorptions of electrocatalysts, play an essential role in governing the HER. Extensive research is being conducted to harness the appealing physicochemical characteristics of 2D materials as potential catalysts for highly efficient HER activity. 120 For instance, Ma and colleagues utilized bulk black phosphorous to create ice-assisted exfoliated BP/g-C3N4 nanosheets, characterized by exceptional product quality, minimal structural flaws, and substantial lateral dimensions. Figure 7 illustrates the pertinent characteristics of the BP/g-C3N4 nanosheets. Upon taking absorption spectra of g-C3N4, BP, and g-C3N4/BP nanosheets, it was revealed that BP nanosheets exhibit a broad absorption band spanning the UV, visible, and NIR regions. In contrast, g-C3N4 and BP/g-C3N4 nanosheets possess absorption edges at 466 and 474 nm, respectively. Furthermore, various component ratios for BP/g-C3N4 nanosheets were examined, demonstrating their superior performance compared to individual BP and g-C3N4 catalysts. The presence of BP broadens the g-C3N4/BP absorption band, and the inclusion of g-C3N4 not only protects BP from oxidation but also creates a shallow interface with trapped charge sites, enhancing the separation of electric carriers in composite photocatalysts. This results in reduced limitations on fast carrier recombination in g-C3N4 or BP nanosheets.

Nanomaterial sheets of BP/C3N4 for high catalytic production of hydrogen with properties i.e. larger lateral size, high quality and lower anomalous structural defects. A nanosheet of BP/g-C3N4 for efficient catalytic hydrogen production due to properties like larger lateral size and limited structure defects. 121
5.8 Oxygen evolution reaction (OER)
The OER, which makes up the second half of water splitting, can be thought of as an oxidative process that needs four electrons and proton transfer. This reaction also has a high overpotential need and a slow kinetic response. Due to their huge specific area, high surface atom density, and atomic level thickness, 2D LDH nanosheets are able to significantly improve catalytic performance. 122 In order to improve catalytic OER, Song et al. 123 used layered LDH nanosheets that were subjected to liquid phase exfoliation. As observed in Figure 8, the exfoliated single layer LDH nanosheets perform better in the OER test than the bulk-layered LDHs. Additionally, Qin et al. created a 2D CoCo-LDH nanomesh as an OER electrocatalyst, which had a mesoporous structure and abundant high activity atoms with low ligancy, improving the diffusion of reactants and products as shown in Figure 8. 111 The CoCo-LDH nanomesh’s onset overpotential and overpotential (10) were reduced to 220 mV and 319 mV, respectively.

Catalytic enhancement of oxygen evaluation on CoCo-LDH nanomesh. 111
5.9 Carbon dioxide reduction reaction (CO2RR)
The CO2 content in the atmosphere has now surpassed the previous threshold of 23 million years and is rising at an unprecedented rate. One of the most powerful greenhouse gases is CO2, and an increase in CO2 levels is directly linked to climate change. An urgent worldwide issue is the capture and effective use of CO2. 124 In order to convert CO2 into non-toxic organics, 2D-layered nanomaterials have gained attention for applications in photocatalysis and electrocatalysis. 125 According to Figure 9a and b, 125 Ye and colleagues successfully developed a CO2RR for homogeneous Zn-MOF nanomaterials with a 4.7 nm layer thickness that has a 103.8 cm3 g−1 CO2 adsorption capacity. The synergistic effect of the increased lifespan of photogenerated electric carriers makes it possible to use 2D layered MOF nanosheets with optimal catalytic CO2RR activity in contrast to bulk MOFs with low efficiency. According to Zhao’s group, 2D ZnO was used for photocatalytic CO2RR, as depicted in Figure 9c–e. 126 The 2D ZnO nanosheets have larger surface catalytic active sites for CO2RR, a suitable bandgap, and optical absorbance when compared to their bulk equivalent.
6 Nanoscale effects on catalytic behavior
Nanomaterials, owing to their reduced dimensions and increased surface area, exhibit a plethora of unique properties that significantly influence their behavior as catalysts. These nanoscale effects play a pivotal role in dictating catalytic activity, selectivity, and overall performance. Understanding and harnessing these effects are crucial for designing and optimizing nanocatalysts with enhanced capabilities. Nanoscale materials are characterized by quantum size effects, where the confinement of electrons and holes within limited dimensions leads to quantized energy levels. This phenomenon is particularly relevant in nanocatalysts due to the discrete energy states that emerge. Quantum size effects influence electronic band structures, altering the density of states and the distribution of energy levels. These changes directly impact the catalyst’s electronic properties and reactivity. 127 Nanoscale materials can have band gaps that differ from bulk materials due to quantum size effects. Their tunability allows for the absorption of photons across a broader spectrum, enabling a wider range of photochemical reactions. 128 Quantum size effects also influence the energy landscape of catalytic reactions. As nanoparticle size changes, the availability of energy levels for adsorption, activation, and reaction intermediates varies, leading to size-specific catalytic behavior. 129 In nanocatalysts, a significant fraction of atoms resides on the surface due to the high surface area-to-volume ratio. Consequently, surface reactivity plays a pivotal role in catalytic processes. Active sites on the surface of nanomaterials often exhibit distinct electronic properties compared to bulk sites, enabling unique catalytic pathways. 130 Nanomaterials possess a larger proportion of atoms at or near the surface, providing more available sites for adsorption and reaction. This enhances catalytic activity by increasing the likelihood of reactant encounters. Surface atoms experience different coordination environments and interactions compared to bulk atoms. This results in modified bond strengths and reactivity, influencing catalytic selectivity and mechanisms. 131 Adsorption of reactants onto catalyst surfaces is a fundamental step in catalysis. In nanomaterials, adsorption energies can vary with particle size due to quantum size effects and changes in the distribution of active sites. Quantum size effects alter the electronic structure of surface atoms, leading to size-dependent variations in adsorption energies. This affects the strength of reactant binding and, consequently, reaction rates. 132 Surface atoms in nanomaterials may undergo reconstructions to minimize surface energy. These reconstructions can create unique catalytic sites that are absent in bulk materials, influencing reaction pathways. The reduced dimensions of nanomaterials facilitate faster diffusion of reactants and products to and from active sites. This enhanced mass transport accelerates reaction kinetics and reduces the likelihood of mass transfer limitations. 133 The shorter distance between the bulk and the catalyst’s surface allows for rapid diffusion of species. This minimizes diffusion limitations and ensures efficient utilization of catalytic sites. Nanoparticles with porous or hierarchical structures offer additional pathways for reactants to access active sites. This enhances catalytic efficiency and reduces the likelihood of surface saturation. In short words we can say that, nanoscale effects play a pivotal role in shaping catalytic behavior. The distinct electronic properties, altered surface reactivity, and enhanced mass transport of nanomaterials create a dynamic environment that significantly influences catalytic activity. By tailoring nanocatalyst properties to leverage these effects, researchers can design catalysts with enhanced performance for a wide range of applications. 134
7 Photochemical reactions and mechanisms
Photochemical reactions, involving the interaction of light with matter to initiate chemical transformations, are fundamental in nanocatalysis. The nanoscale dimension of catalysts introduces unique light–matter interactions and charge transfer mechanisms that lead to novel and enhanced photochemical processes. 135 Nanocatalysts possess a high surface area and unique electronic properties that facilitate efficient light absorption. The absorbed photons impart energy to the catalyst’s electrons, promoting them to higher energy states and generating photogenerated electron-hole pairs (excitons). These charge carriers are central to driving photochemical reactions. Nanomaterials exhibit enhanced light absorption due to their tunable electronic structures and high surface-to-volume ratio. This broadens the spectrum of light that can be utilized for catalysis. Upon striking photons on the valence band, its results excitation of electrons to the conducting band, generation pairs of electron-hole. These excitons are central to initiating redox reactions on the catalyst’s surface. 136 Nanophotochemistry has broad applications, ranging from solar fuel generation to environmental remediation. Nanocatalysts play a pivotal role in harnessing solar energy to drive reactions that were once thermodynamically unfavorable or kinetically challenging. Photocatalytic water splitting and carbon dioxide reduction hold promise for producing renewable hydrogen and hydrocarbons using solar energy. It has also the ability to break down pollutants, toxins, and contaminants in water and air, contributing to environmental remediation. Nano photochemistry enables controlled and selective synthesis of complex molecules, with applications in pharmaceuticals and fine chemicals. nanocatalysis has revolutionized photochemical reactions by providing an environment where light–matter interactions and charge transfer are tailored for enhanced catalytic performance. The understanding and manipulation of these mechanisms are paving the way for transformative applications that address energy, environment, and chemical synthesis challenges. 137
Photocatalysis involves a sequence of steps that enable light energy to drive chemical reactions. In nanocatalysts, efficient charge separation, minimized charge recombination, and enhanced surface interactions play critical roles in catalytic mechanisms. The photogenerated electron-hole pairs are separated at the catalyst’s interface. 138 The spatial confinement of charge carriers in nanomaterials reduces the probability of recombination. Photogenerated charges participate in surface reactions, where they interact with adsorbed reactants or intermediates. These reactions can lead to the formation of products with lower energy barriers. Charge carriers, if not efficiently utilized, can recombine and release energy as heat or light. The nanoscale dimensions allow for faster charge migration, minimizing recombination losses. 139
In many cases, nanocatalysts are paired with cocatalysts to enhance photocatalytic activity due the effect of cocatalyst and synergistic effect. Cocatalysts, often co-deposited on the nanocatalyst surface, provide additional reaction sites, facilitate charge transfer, and modify reaction pathways. Cocatalysts can provide active sites for specific reactions, promote charge separation, or facilitate the transfer of charge carriers to or from the catalyst’s surface. The combination of nanocatalysts and cocatalysts leads to synergistic effects where charge carriers generated by the nanocatalyst are efficiently utilized by the cocatalyst, leading to enhanced overall photocatalytic activity. 140 Baojun Ma et al. design hydrothermal method for water splitting with remarkable synergistic effect on the photocatalytic process by co-catalysis of noble metals. He prove that upon co-loded two cocatalyst (Pt and RuO2) on Zn2GeO4, the photocatalytic activity can be increased two to three times upon co-loading Pt–RuO2–Zn2GeO4 as compare to doping Pt–Zn2GeO4 or RuO2–Zn2GeO4 as shown in Figure 10. 140

The synergistic effect on photocatalytic water splitting reaction of two cocatalyst (Pt and RuO2) on Zn2GeO4 (a) Water splitting photocatalytic reaction scheme on Zn2GeO4 where the co coloaded materials are Pt and RuO2 (b) Water splitting reaction on Zn2GeO4 where the co-loaded materials are Pt and RuO2 (c–d) The photocatalytic activity of RuO2 hydrothermally loaded Zn2GeO4 at various temperature for 24 h where (a) at 190 °C and b for the O2 and H2 production. Reaction condition = 01 g catalyst; 50 mL water; 150 W Hg lamp. 140
7.1 Electrochemical processes and interfaces in nanocatalysis
Electrochemical processes and interfaces play a crucial role in nanocatalysis, where the combination of nanomaterials and electrochemistry leads to unique and efficient catalytic reactions. The fundamental principles of electrochemical processes in nanocatalysis, focusing on the electrocatalytic interfaces that govern charge transfer reactions are explored here. The interface between the nanocatalyst and the electrode, known as the electrocatalytic interface, is where charge transfer reactions take place. 141 The nanoscale dimensions of catalysts bring about changes in the electrocatalytic interface that influence reaction kinetics and efficiency. The electrocatalytic interface includes the electrical double layer, consisting of charged species near the electrode surface. The high surface area of nanocatalysts leads to an increased number of ions in the double layer, affecting charge transfer kinetics. Nanocatalysts offer a greater number of active sites due to their large surface area. These sites are crucial for adsorption of reactants and facilitating charge transfer processes. 142
Electrocatalysis involves charge transfer reactions at the electrocatalytic interface. Electrons are exchanged between the electrode and the nanocatalyst, leading to redox reactions that drive catalysis. In redox reactions, electrons are transferred between the nanocatalyst and the electrode. Nanocatalysts accelerate these electron transfer processes due to their high surface area and increased availability of active sites. Tafel slopes describe the relationship between current density and overpotential in electrocatalytic reactions. Nanocatalysts can exhibit different Tafel behavior compared to bulk materials, influencing reaction kinetics. 143 Nanoscale dimensions and composition significantly impact electrocatalytic behavior. These effects arise from the unique electronic and surface properties of nanomaterials. Nanocatalysts may exhibit lower overpotentials compared to bulk materials due to quantum size effects. 144 This reduces the energy required to initiate catalytic reactions. Quantum size effects and surface modifications influence the electronic structure of nanocatalysts. This affects charge transfer rates and the reactivity of catalytic sites. Nanocatalysts offer multiple pathways for charge transfer reactions, enhancing catalytic efficiency and providing opportunities for intricate reaction mechanisms. The presence of different sites and orientations on nanocatalysts allows for parallel reaction pathways, accommodating various reaction intermediates and enhancing overall catalytic performance. 145 Nanocatalysts may follow different reaction mechanisms compared to bulk materials due to the unique coordination environments of surface atoms. This can lead to the formation of unique reaction products. 146
7.2 Interplay between nanostructures and reactivity in nanocatalysis
The behavior of nanocatalysts is profoundly influenced by their nanostructures, which encompass size, shape, composition, and surface properties. This interplay between nanostructures and reactivity dictates catalytic performance, selectivity, and mechanistic pathways. Understanding and manipulating this relationship are paramount in tailoring nanocatalysts for specific applications. 147 This section explores into the complex influences between nanostructures and reactivity in nanocatalysis. Nanoparticles’ size dictates the distribution of surface atoms and quantum size effects, leading to distinctive reactivity profiles. Different sizes expose diverse facets and coordination environments, leading to size-specific active sites. These sites can exhibit varying binding affinities for reactants and intermediates. Quantum confinement modifies electronic energy levels, altering adsorption energies and reactivity. This size-dependent reactivity influences reaction kinetics and selectivity. 148 Nanoparticle shape profoundly influences catalytic behavior due to variations in exposed crystal facets and surface curvature. Different facets possess varying surface energies and arrangements of atoms. These facets can act as active sites for specific reactions, impacting catalytic activity. Curved surfaces on nanoparticles lead to higher surface atom density, potentially enhancing catalytic activity by providing more active sites. 149
7.2.1 Composition and alloying effects
The composition of nanocatalysts, including elemental composition and alloying, plays a pivotal role in modulating reactivity. Alloying different metals can create synergistic effects, altering electronic properties and surface reactivity. This can lead to enhanced catalytic activity and selectivity. Varying compositions enable the creation of multifunctional catalysts with diverse reactivity, allowing for simultaneous or sequential catalytic transformations. 150
7.2.2 Surface modifications and ligand effects
Surface modifications, such as functional groups and ligands, alter the catalytic environment by influencing adsorption and reaction pathways. Ligands attached to nanocatalyst surfaces can act as co-catalysts, modifying adsorption energies and reaction intermediates. This can lead to improved catalytic selectivity. Bulky ligands can hinder adsorption of specific reactants, influencing reaction pathways and favoring specific products. 151
7.2.3 Synergistic interactions
The interplay between different nanostructures, such as core–shell nanoparticles or heterostructures, can create synergistic effects that enhance catalytic reactivity. Core–shell nanoparticles combine the reactivity of different materials, offering complementary catalytic functionalities. This enhances catalytic performance by facilitating sequential reactions. Interfaces between different nanostructures enable charge transfer and create unique electronic environments, leading to enhanced catalytic activity. 152
8 Type of catalysis
Various types of catalysis, including homogeneous, heterogeneous, and enzymatic catalysis are available in literature. Here we will focus on how nanoscale catalysts contribute to enhancing these different types of catalytic reactions and their applications in industry and research. Catalysis is a cornerstone of modern chemical processes, and the integration of nanomaterials, photochemistry, and electrochemistry has given rise to a diverse range of catalytic types with unique applications. This section explores different types of catalysis in the context of nano photo/electrochemistry and highlights their significance and potential.
8.1 Homogeneous catalysis with nanocatalysts
Homogeneous catalysis involves catalysts and reactants in the same phase. Nanocatalysts offer advantages in this context due to their increased surface area and unique reactivity. Homogeneously dispersed nanocatalysts possess active sites throughout the reaction mixture, enhancing catalytic efficiency. Nanocatalysts in homogeneous systems enable rapid and efficient interactions between reactants and catalysts, resulting in higher reaction rates. Homogeneous catalysis with nanocatalysts finds applications in organic synthesis, polymerization, and other fine chemical processes. 153
Homogeneous catalysis involves catalysts and reactants in the same phase, often dissolved in a solvent. The integration of nanocatalysts into homogeneous catalytic systems has opened new avenues for efficient and selective reactions. This section explores the principles, advantages, and applications of homogeneous catalysis using nanocatalysts. Nanocatalysts, due to their high surface area and unique reactivity, exhibit distinct behavior in homogeneous catalytic systems. The increased surface area of nanocatalysts provides more active sites for reactant adsorption and reaction initiation, leading to higher catalytic activity. Nanoparticle size and shape influence catalytic behavior by modulating the distribution of active sites and quantum size effects. The choice of solvent in homogeneous catalysis with nanocatalysts can influence reaction rates, solubility, and coordination to the catalyst surface. 154
8.1.1 Advantages of homogeneous catalysis with nanocatalysts
The integration of nanocatalysts into homogeneous catalytic systems offers several advantages. Nanocatalysts enable rapid and efficient interaction between catalysts and reactants due to their high surface area and unique properties. It can facilitate reactions under milder conditions compared to traditional catalysts, leading to improved energy efficiency. The precise design of nanocatalysts allows for the tuning of selectivity by modifying their size, shape, and surface properties. Homogeneous catalysis with nanocatalysts finds applications across various fields. Nanocatalysts also facilitate selective C–C bond formation, cross-coupling reactions, and functional group transformations in organic synthesis. The production of specialty chemicals, pharmaceutical intermediates, and high-value compounds with high selectivity and enzyme-mimicking nanocatalysts offer efficient tools for biocatalytic transformations are also some best application of nanostructured enzymes. 155
8.1.2 Challenges and future directions
Despite its potential, homogeneous catalysis with nanocatalysts presents challenges that need to be addressed. Separating nanocatalysts from reaction mixtures can be challenging, requiring efficient recovery methods to avoid catalyst loss. Some nanocatalysts may undergo degradation under reaction conditions, necessitating strategies to improve catalyst stability. The translation of lab-scale homogeneous catalysis with nanocatalysts to industrial scale presents challenges related to cost, scalability, and catalyst recycling. Homogeneous catalysis with nanocatalysts represents a dynamic field that merges the advantages of both homogeneous and heterogeneous catalysis. 156 The high surface area, tunable properties, and enhanced reactivity of nanocatalysts enable efficient and selective reactions under milder conditions. As research progresses, overcoming challenges and developing practical strategies will unlock the full potential of homogeneous catalysis with nanocatalysts, leading to innovative applications across various chemical processes.
8.2 Heterogeneous catalysis on nanostructured surfaces
Heterogeneous catalysis involves catalysts in a different phase from the reactants. Nanostructured surfaces provide exceptional platforms for heterogeneous catalysis due to their high surface area and tunable properties. 157 , 158 , 159 Nanostructured surfaces offer increased surface area for reactant adsorption and catalytic reactions, leading to improved activity. 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 The specific arrangement of atoms on nanostructured surfaces can selectively expose certain active sites, enabling control over reaction pathways. Nanostructured surfaces allow easy separation of catalysts from the reaction mixture, enabling their reuse and contributing to sustainability. 168 , 169 Nanostructured surfaces introduce new dimensions to heterogeneous catalysis by offering a range of active sites and enhanced surface interactions. Nanostructured surfaces provide larger surface areas for reactant adsorption, leading to increased catalyst-substrate interactions for heterogenous catalysis. The specific arrangement of atoms on nanostructured allows for the exposure of different crystal facets and active sites, influencing catalytic surfaces activity. these surfaces exhibit size-dependent properties, where nanoscale features affect surface energy and reactivity which are very valuable in term of heterogenous catalysis. There are so many benefits of heterogeneous catalysis on nanostructured surfaces. The integration of nanostructured surfaces into heterogeneous catalytic systems offers numerous advantages including high activity, selective catalysis, recycling etc. 170 Nanostructured surfaces enhance catalytic activity by exposing more active sites, enabling efficient reactions with lower catalyst loadings. The controlled arrangement of atoms on nanostructured surfaces enables selectivity by favoring specific reaction pathways. Separation and reuse of nanostructured catalysts are facilitated due to their enhanced surface interactions and ease of recovery. The nanostructured surfaces drive reactions such as hydrogenation, oxidation, and hydrogenolysis, playing a pivotal role in industrial catalysis. These catalysts also contribute to environmental remediation by degrading pollutants, converting harmful gases, and purifying water. Energy-related catalytic processes like fuel cells, where they facilitate efficient energy conversion are also consider as their efficient applications. 85
Despite its advantages, heterogeneous catalysis on nanostructured surfaces poses challenges that require addressing. The exposure of high-energy facets on nanostructured surfaces can lead to catalyst degradation under harsh reaction conditions. Translating lab-scale synthesis of nanostructured catalysts to industrial-scale production involves challenges related to reproducibility and cost-effectiveness. Understanding the structure and dynamics of nanostructured surfaces during catalysis requires advanced characterization techniques. 171 Heterogeneous catalysis on nanostructured surfaces represents a frontier in catalytic science, blending the benefits of traditional heterogeneous catalysis with the unique properties of nanomaterials. The increased surface area, tailored reactivity, and efficient reactant interactions make nanostructured surfaces powerful tools for catalytic transformations across industries. As research advances and challenges are addressed, nanostructured surfaces will continue to redefine and innovate the field of heterogeneous catalysis.
8.3 Enzymatic catalysis and nanozymes
Enzymatic catalysis, using natural or engineered enzymes, has found synergy with nanomaterials, leading to the concept of nanozymes. Nanozymes mimic enzymatic activity and offer stability and reusability advantages over natural enzymes. Nanozymes exhibit high catalytic efficiency and can operate under a wide range of conditions, making them versatile tools for catalysis. Nanozymes find applications in biomedicine, including drug delivery, imaging, and therapeutic treatments, due to their catalytic and biocompatible properties. 172
Enzymatic catalysis, a biological process facilitated by enzymes, has been augmented through the development of nanozymes nanomaterials with enzyme-like catalytic properties. The synergy between enzymatic catalysis and nanozymes offers new avenues for versatile and efficient catalytic transformations. 173 Enzymes are biocatalysts that accelerate chemical reactions in living organisms. Enzymatic catalysis relies on the precise arrangement of active sites, substrates, and cofactors. Enzymes exhibit high substrate specificity, allowing for selective reactions in complex chemical mixtures. Enzymatic reactions often occur under mild conditions, making them suitable for biologically relevant transformations. Enzymes often demonstrate exquisite regio- and stereoselectivity, enabling the synthesis of complex molecules with high precision. 174 Nanozymes are nanomaterials that mimic enzyme-like catalytic activity, offering advantages such as stability, reusability, and ease of synthesis. Nanozymes can mimic a wide range of enzymatic reactions, from oxidations and reductions to hydrolysis and more. Nanozymes are often more stable than natural enzymes and can withstand a broader range of reaction conditions. Some nanozymes are biocompatible and can be used in biomedical applications, including drug delivery and diagnostics. The integration of nanozymes with enzymatic catalysis combines the strengths of both approaches. Nanozymes can match or even exceed the catalytic efficiency of natural enzymes, making them potent catalysts. Nanozymes are more robust and can be easily recovered and reused, reducing the need for enzyme regeneration. Nanozymes expand the scope of enzymatic reactions to non-biological substrates, broadening their synthetic applications. 175
The synergy between enzymatic catalysis and nanozymes has diverse applications. For example, the natural enzymes and nanozymes find applications in green chemistry, pharmaceutical synthesis, and the production of biofuels. Nanozymes are employed in biosensing, disease diagnosis, and drug delivery due to their biocompatibility and catalytic activity. Enzymes and nanozymes are used to degrade pollutants, detoxify environmental contaminants, and purify water. 176 Despite their potential, enzymatic catalysis and nanozymes face certain challenges. Expanding the substrate scope of nanozymes to match that of natural enzymes remains a challenge. Ensuring the biocompatibility of nanozymes for biomedical applications requires careful design and testing. The regulatory approval of nanozyme-based products requires comprehensive safety assessment and standardized testing. 177 Enzymatic catalysis and nanozymes present a harmonious blend of biological and nanotechnological approaches to catalysis. The combination of enzymatic selectivity with the robustness and versatility of nanozymes holds immense potential for revolutionizing catalytic transformations in both synthetic chemistry and biotechnology. As research advances and challenges are overcome, the collaboration between enzymatic catalysis and nanozymes will continue to shape the landscape of modern catalysis.
8.4 Photocatalysis and photoelectrocatalysis
Photocatalysis utilizes light energy to drive catalytic reactions, while photoelectrocatalysis combines light-induced charge transfer with electrocatalysis. Photocatalysis utilizes sunlight to initiate reactions that were previously energetically unfavorable, such as water splitting or pollutant degradation. Photoelectrocatalysis combines the advantages of both photochemistry and electrochemistry, allowing for efficient charge separation and reaction initiation. Photocatalysis and photoelectrocatalysis hold potential in renewable energy conversion processes, like hydrogen generation from water or carbon dioxide reduction. 178 The integration of nano photo/electrochemistry with different types of catalysis opens up a realm of possibilities in various fields, from energy to environmental remediation and beyond. Each type of catalysis leverages the unique properties of nanomaterials and the principles of photochemistry and electrochemistry, offering unprecedented control over reaction outcomes and catalytic efficiency. The synergy between these approaches is poised to redefine how we harness light and electron transfer processes for a sustainable and innovative future.
8.4.1 Essential features of catalyst for electrocatalysis
The fundamental characteristics that make a catalyst effective in electrocatalytic reactions are tried to explain here, like overpotential, electrocatalytic activity, and selectivity. Electrocatalysis involves using catalysts to facilitate and accelerate electrochemical reactions, such as those occurring in fuel cells, electrolyzers, and metal-air batteries. The design of effective 179 electrocatalysts requires careful consideration of several essential features that influence their performance and efficiency. 180
Catalytic Activity and Selectivity: The primary function of an electrocatalyst is to enhance the rates of desired electrochemical reactions while avoiding unwanted side reactions. High catalytic activity ensures rapid reaction kinetics, while selectivity ensures that the desired reaction pathway is favored over competing pathways. 181 Electrochemical Stability: Electrocatalysts must maintain their structural integrity and catalytic activity over extended periods under the often harsh conditions of electrochemical reactions. 179 Stability is crucial to ensure the longevity of the catalyst and prevent performance degradation. Efficient Charge Transfer: Electrocatalysts should facilitate efficient charge transfer between the electrode and the reactants. This requires optimal electron conductivity and facile pathways for charge transport. Large Surface Area: A higher surface area provides more active sites for reactions to occur, leading to increased catalytic activity. Nanostructured or porous catalysts often exhibit larger surface areas, enhancing their electrocatalytic performance. High Surface Reactivity: Active sites on the catalyst surface should be exposed and accessible for reactant adsorption and subsequent reaction. Enhancing surface reactivity ensures effective utilization of the catalyst’s active sites. 182 Tunable Surface Chemistry: Controlling the surface composition and properties of the catalyst allows tailoring of its catalytic behavior. Surface modifications can optimize adsorption energies, reaction intermediates, and selectivity. Electronic Structure: The electronic structure of the catalyst influences its interaction with reactants and charge transfer processes. A well-matched electronic structure between the catalyst and reactants can enhance reaction rates. 183 Catalyst-Substrate Compatibility: The catalyst should be compatible with the substrate and the reaction environment. This includes considerations of the electrode material, electrolyte composition, and temperature range. Availability and Cost: Commercial viability of electrocatalysts depends on their availability and cost-effectiveness. Developing catalysts that utilize abundant and low-cost materials is crucial for scalability. Catalyst Synthesis and Processing: The ease of synthesis and processing of the catalyst affects its reproducibility, scalability, and applicability. Catalysts should be producible with controlled properties for consistent performance. Catalyst-Substrate Attachment: In some cases, catalysts need to be securely attached to electrode surfaces to prevent detachment during operation. Strong and stable catalyst-substrate interaction is essential for long-term performance. Environmental Impact: Considering the environmental impact of catalyst synthesis and use is crucial for sustainable electrocatalysis. Developing greener and more eco-friendly synthesis routes is an important consideration. 184 The development of efficient electrocatalysts involves a delicate balance of multiple factors, from activity and stability to electronic structure and environmental impact. Successful catalyst design requires a multidisciplinary approach that combines materials science, surface chemistry, and electrochemistry. As researchers continue to explore and engineer catalysts with these essential features, electrocatalysis will play an increasingly pivotal role in advancing energy conversion and storage technologies.
8.4.2 Overpotential and faradaic efficiency in electrocatalysis
In electrocatalysis, understanding the concepts of overpotential and Faradaic efficiency is crucial for optimizing catalytic performance, energy efficiency, and reaction selectivity. These parameters provide insights into the efficiency of electrochemical reactions and the effectiveness of catalysts. 185 This section delves into the significance and implications of overpotential and Faradaic efficiency in electrocatalysis. Overpotential, often denoted as η, represents the additional potential applied to drive an electrochemical reaction beyond its thermodynamic equilibrium potential. 186 It’s the difference between the applied potential (Eapplied) and the thermodynamic equilibrium potential (Eeq) for a given reaction: η = Eapplied – Eeq. Overpotential serves as a measure of the energy required to initiate a reaction. It reflects the activation energy needed for charge transfer across the electrode-electrolyte interface and any kinetic barriers that might hinder the reaction.
Several factors that contribute to the magnitude of overpotential are catalyst activity, reaction kinetics, surface area of electrodes, and composition of electrolyte etc. The more active catalysts can achieve the same reaction rate at lower overpotentials, improving energy efficiency. Similarly, faster reaction kinetics often result in lower overpotentials since the energy barrier for charge transfer is reduced. A larger surface area provides more active sites, potentially reducing the overpotential, while ionic conductivity and reactants concentration in the electrolyte can also influence overpotential. 187
8.4.3 Faradaic efficiency
Faradaic efficiency (FE) measures the fraction of electrons involved in the desired electrochemical reaction compared to the total number of electrons transferred during the entire process. It is expressed as a percentage: [FE (%) = (Ndesired/Ntotal) × 100], Where Ndesired is the number of electrons involved in the desired reaction and Ntotal is the total number of electrons transferred during the process. Faradaic efficiency quantifies the selectivity of the electrochemical reaction and indicates the proportion of electron flow contributing to the desired product formation. Several factors that impact faradaic efficiency are catalyst selectivity, competing reactions, electrolyte composition etc. A selective catalyst favors the desired reaction pathway, leading to higher Faradaic efficiency for the target product. The unwanted side reactions can reduce the overall Faradaic efficiency by diverting electrons to undesired pathways and the electrolyte components can influence reaction selectivity and hence Faradaic efficiency. While the significance of overpotential and faradaic efficiency are measured in term of energy efficiency, yield of products, selective nature of reactions. Optimizing overpotential and Faradaic efficiency is crucial for various electrocatalytic applications. Minimizing overpotential reduces energy consumption in electrochemical processes. High Faradaic efficiency ensures a higher yield of the desired product and reduces waste. Faradaic efficiency provides insights into the selectivity of competing reaction pathways, aiding in catalyst design. Overpotential and Faradaic efficiency are fundamental concepts in electrocatalysis that impact the energy efficiency, selectivity, and overall performance of electrochemical reactions. Balancing these parameters through catalyst design, reaction conditions, and electrode engineering is essential for advancing the field and enabling sustainable and efficient electrochemical processes. 188
8.4.4 Active sites and surface reactivity in electrocatalysis
Active sites and surface reactivity are critical aspects of electrocatalysis that determine the efficiency and selectivity of electrochemical reactions. These concepts are central to understanding how catalysts interact with reactants at the electrode-electrolyte interface. This section explores the significance of active sites and surface reactivity in electrocatalysis and their influence on reaction mechanisms and performance. 189 Active sites are specific locations on a catalyst’s surface where reactants adsorb and undergo electrochemical reactions. These sites possess unique electronic, geometric, and chemical properties that facilitate charge transfer and reaction initiation. Active sites dictate the reactivity and selectivity of electrochemical reactions. Understanding and engineering these sites are essential for enhancing catalyst performance and designing efficient electrocatalysts. Identifying active sites involves various experimental and theoretical approaches, including: Surface characterization techniques such as scanning tunneling microscopy (STM) and X-ray spectroscopy provide insights into surface structure and composition and computational modeling density functional theory (DFT) calculations predict the energetics of adsorption and reaction pathways on different surface sites.
Surface reactivity refers to the propensity of a catalyst’s surface to interact with reactants, leading to adsorption, charge transfer, and reaction initiation. Several factors are their which impact surface reactivity in electrocatalysis process. For example, different crystallographic facets and sites on the catalyst surface can exhibit varying reactivity due to their distinct electronic and atomic arrangements. The availability of vacant or partially occupied surface sites affects the likelihood of reactant adsorption and reaction initiation. Variations in electron density at different surface sites influence adsorption energies and reaction kinetics. 190 Active sites and surface reactivity are fundamental considerations in electrocatalysis. Understanding how catalyst surfaces interact with reactants provides insights into reaction mechanisms and guides catalyst design for enhanced performance. The ability to engineer active sites and surface reactivity enables the development of efficient and selective electrocatalysts, with implications spanning energy conversion, environmental remediation, and beyond.
8.4.5 Nanocatalyst morphology and composition in electrocatalysis
The morphology and composition of nanocatalysts are pivotal factors influencing their catalytic performance in electrochemical reactions. Tailoring these attributes allows for precise control over surface properties, active site distribution, and charge transfer kinetics. This section explores the significance of nanocatalyst morphology and composition in electrocatalysis and their impact on reaction mechanisms and catalytic efficiency. 191 , 192
Engineering nanocatalyst morphology involves control synthesis, templating and shpe/size effect. Precise synthesis conditions can lead to specific nanoparticle sizes, shapes, and facets. While the templates and surfactants can guide the growth of nanoparticles, enabling control over morphology. Size-dependent properties and quantum effects become prominent at the nanoscale, influencing reactivity. Nanocatalyst composition refers to the elemental composition and chemical nature of the catalyst material. It dictates the electronic structure, surface energy, and interaction with reactants. Composition is very important because the composition significantly affects electrocatalytic performance, for example different elements and compositions lead to varying electronic configurations, influencing charge transfer kinetics. It also affects the strength of reactant adsorption, determining reaction rates and selectivity. Certain compositions can enhance catalyst stability under harsh electrochemical conditions. 193 Sometime we need certain Strategies for tuning nanocatalyst composition for efficiency like alloying, doping. The creation of bimetallic or multi-component nanoparticles by alloying different elements can enhance catalytic properties and introducing dopants into the catalyst lattice can modify its electronic structure and reactivity. Similarly, core–shell nanoparticles with different compositions offer unique catalytic synergies.
The combination of morphological and compositional control maximizes catalytic performance are very considerable. Morphological changes can influence the distribution and reactivity of active sites, while composition influences electronic properties. Tailoring both morphology and composition can lead to optimized active site distribution and reactivity. Morphology and composition engineering are key considerations in nanocatalyst design for electrocatalysis. These attributes directly influence surface properties, charge transfer kinetics, and reaction pathways. By precisely tailoring nanocatalyst morphology and composition, researchers can develop catalysts with enhanced activity, selectivity, and stability, paving the way for efficient and sustainable electrochemical processes across various applications. 194
8.4.6 Strategies to enhance electrocatalytic activity
Enhancing the electrocatalytic activity of catalysts is essential for improving the efficiency and performance of electrochemical reactions. Various strategies have been developed to optimize catalysts for faster reaction kinetics, higher selectivity, and improved stability. 195 The key approaches to boost electrocatalytic activity and their applications in diverse fields are morphology, composition engineering, surface medication or functionalization, supporting materials, alloying, heterostructures, substrate and computational screening.
Morphology and Composition Engineering: Tailoring the size, shape, and composition of catalyst nanoparticles can significantly impact their electrocatalytic activity. By optimizing these factors, researchers can increase the surface area, expose specific active sites, and fine-tune electronic properties for improved charge transfer kinetics. This strategy finds applications in fuel cells, electrolyzers, and metal-air batteries, where catalysts with enhanced activity lead to more efficient energy conversion and storage.
Surface Modifications and Functionalization: Introducing functional groups, ligands, or surface modifiers can modify the electronic structure and reactivity of catalysts. Surface functionalization enhances adsorption of reactants, stabilizes intermediates, and alters reaction pathways. 196 This approach is relevant in fuel cells and sensors, where controlled surface modifications improve catalyst performance and selectivity.
Nano structuring and Support Materials: Using nanostructured materials as catalyst supports or carriers can enhance electrocatalytic activity. Nanostructured supports provide higher surface area, while tailored interactions between the catalyst and support improve stability. Nanostructured catalysts find applications in water splitting, where efficient oxygen and hydrogen evolution reactions are crucial for renewable energy conversion.
Alloying and Bimetallic Catalysts: Creating bimetallic catalysts by alloying two different metals can induce synergistic effects, such as enhanced adsorption properties, modified electronic structure, and improved reactivity. Bimetallic catalysts are vital in electrochemical CO2 reduction, as they can facilitate the formation of specific products by optimizing adsorption energies. 197
Heterostructures and Interfaces: Combining different materials to form heterostructures or interfacial regions can lead to charge transfer, coupling of reactions, and localized electric fields, boosting catalytic activity. Heterostructures are applied in photoelectrochemical cells, where light-driven reactions are coupled with electrochemical processes for efficient solar fuel generation.
Catalyst-Substrate Interactions: Optimizing the interaction between the catalyst and substrate (electrode) enhances electron transfer, stabilizes intermediates, and reduces overpotential. This strategy is crucial in improving catalytic performance in electrochemical water splitting, where minimizing overpotential is essential for efficient energy conversion. 198
Rational Design and Computational Screening: Using computational methods, such as density functional theory (DFT), researchers can predict and design catalysts with specific properties, aiding in the discovery of high-performance materials. Rational design is widely employed in catalyst development for various applications, including CO2 reduction, water oxidation, and organic synthesis. Enhancing electrocatalytic activity is essential for advancing energy conversion, environmental remediation, and chemical synthesis. Employing strategies such as morphology engineering, surface modifications, and rational design enables the creation of efficient catalysts with improved performance and selectivity. As researchers continue to innovate in this field, electrocatalysis will play a central role in addressing critical challenges in sustainable energy and beyond. 96 , 199
9 Functional catalysis for electrocatalysis
This section will explore into specific examples of functional nanocatalysts designed for electrocatalytic reactions. We are tried to discuss the role of nanomaterials such as metal nanoparticles, metal-organic frameworks (MOFs), and carbon-based materials in electrocatalysis and to highlight recent advancements and breakthroughs in this area. Functional catalysis is a strategic approach that integrates catalysts with specific functional groups, ligands, or modifiers to enhance their electrocatalytic performance. This strategy leverages tailored interactions between the catalyst and reactants, optimizing charge transfer kinetics, selectivity, and stability. This section explores the concept of functional catalysis in electrocatalysis and its applications in various electrochemical reactions. 200 Functional groups or ligands are introduced to catalyst surfaces to alter their electronic properties, adsorption behavior, and reactivity. These modifications can enhance catalytic activity and selectivity by fine-tuning interactions with reactants and intermediates. Functional groups and ligands are commonly used in electrochemical reduction reactions, such as the electrocatalytic reduction of CO2 to valuable chemicals, where precise control over adsorption energies is essential for selective product formation. 201 Functional catalysis can stabilize catalysts by creating protective layers on their surfaces. These layers prevent degradation and enhance catalyst durability under harsh reaction conditions. Surface modification is crucial in oxygen evolution and reduction reactions in metal-air batteries, where catalyst stability is essential for prolonged device lifetime. 202
Incorporating redox-active ligands or mediators into catalyst designs enables electron transfer between the catalyst and reactants. These redox species facilitate charge transfer and can mediate multi-step reactions. Redox-active mediators are used in dye-sensitized solar cells and photoelectrochemical cells to enhance charge transfer between the photoactive material and the electrode, improving overall energy conversion efficiency. 203 Co-catalysts and co-reagents work in synergy with the main catalyst to enhance specific reaction steps. They can facilitate intermediate formation, increase reaction rates, and improve overall efficiency. Co-catalysts are employed in water splitting reactions to enhance the kinetics of hydrogen and oxygen evolution, enabling efficient and rapid electrochemical energy conversion. 204
Functional catalysis can be implemented in both heterogeneous and homogeneous systems. In heterogeneous catalysis, functional groups are attached to the catalyst surface, while in homogeneous catalysis, they are incorporated directly into the reaction solution. Homogeneous functional catalysis finds use in advanced oxidation processes for wastewater treatment, where specific functional molecules generate reactive species to degrade pollutants. 205 The advantages of functional catalysis can be seen in terms of tailored reactivity, selectivity, stability, and versatility. Functional groups allow precise control over catalyst-reactant interactions, enabling targeted reaction pathways. The functional modifications can guide reactants toward desired products, reducing unwanted side reactions. At the same time, layers and modifiers enhance catalyst stability, extending their lifespan in harsh electrochemical environments. Functional catalysis can be applied to various reactions and catalyst types, making it a versatile strategy. Functional catalysis offers a powerful tool for optimizing electrocatalytic performance by designing catalysts with tailored interactions and reactivity. Through the incorporation of functional groups, ligands, and modifiers, researchers can achieve enhanced selectivity, improved charge transfer kinetics, and prolonged catalyst lifetimes. This approach holds promise for addressing challenges in sustainable energy conversion, environmental protection, and chemical synthesis through efficient and controlled electrochemical reactions. 2
10 Noble metal nanoparticles in electrocatalysis
Noble metal nanoparticles, including platinum (Pt), gold (Au), and palladium (Pd), are widely utilized in electrocatalysis due to their unique properties that make them efficient catalysts for various electrochemical reactions. This section explores the significance of noble metal nanoparticles in electrocatalysis, their catalytic mechanisms, and their applications in different energy conversion and storage technologies. Noble metals exhibit remarkable electrocatalytic activity owing to their high conductivity, stability, and unique electronic structure. 206 These properties make them suitable for reactions involving charge transfer, such as oxygen reduction, hydrogen evolution, and other redox reactions. Pt-based nanoparticles are widely used as cathode catalysts in fuel cells and metal-air batteries due to their high ORR activity, which is essential for efficient energy conversion. Noble metal nanoparticles, particularly Pt and Pd, exhibit high HER activity, making them crucial for electrolyzers and photoelectrochemical cells. 207 The catalytic activity of noble metal nanoparticles stems from their ability to adsorb reactants and intermediates on their surfaces, allowing efficient charge transfer reactions. The interaction between reactants and metal surfaces, as well as the arrangement of atoms at the nanoparticle surface, play critical roles in catalytic mechanisms. 208 Noble metal nanoparticles are applicable as catalysts in both anode and cathode reactions, enhancing fuel cell efficiency and power output. Similarly, they facilitate water splitting by catalyzing both hydrogen evolution and oxygen evolution reactions. Noble metal nanoparticles serve as catalysts in oxygen reduction and evolution reactions in metal-air batteries, improving energy density and overall performance, and also employed in sensors for detecting various analytes due to their high sensitivity and selectivity.
Among the major challenges and future directions high cost of noble metals limits their widespread usage. Developing cost-effective alternatives and improving catalyst durability are ongoing challenges. Similarly, they can undergo degradation during prolonged electrochemical reactions, reducing their long-term stability. 209 Furthermore, transitioning from lab-scale synthesis to large-scale production of noble metal nanoparticles with controlled properties poses challenges. The emerging trends related to them includes their alloying, and surface engineering. Bimetallic and multimetallic nanoparticles are being explored to enhance catalytic activity and reduce the amount of expensive noble metals via alloying. Using non-noble metal supports, such as carbon or metal oxides, can improve stability and reduce catalyst loading. Tailoring the surface properties of noble metal nanoparticles through functionalization and engineering can further enhance their performance in surface engineering. Noble metal nanoparticles play a pivotal role in advancing electrocatalysis, offering high catalytic activity, stability, and versatility for various electrochemical reactions. While challenges related to cost, durability, and scalability remain, ongoing research is focused on developing innovative strategies to address these issues. With the integration of emerging trends such as alloying, support materials, and surface engineering, noble metal nanoparticles continue to drive progress in energy conversion, storage technologies, and electrochemical sensing applications. 210
10.1 Functionalized noble metal nanocrystals for electrocatalysis
The excessive utilization of fossil fuels leads to energy depletion, environmental degradation, and climate change, posing significant threats to both human security and the development of sustainable energy sources. 211 The foremost priority of nations worldwide is to accelerate the establishment of a dependable, enduring, and state-of-the-art energy supply system. Electrochemical technologies play a pivotal role in generating, converting, and storing clean, renewable energy, exemplified by technologies such as fuel cells and water electrolysis. These methods offer efficient solutions to reduce our dependence on fossil fuels and address the challenges of global energy scarcity and environmental pollution. 212 Nevertheless, there remains a critical need for highly efficient and dependable electrocatalysts to facilitate the sluggish cathode and anode reactions essential for fuel cell and water electrolysis applications. 213
Noble metal nanocrystals exhibit a unique electronic structure, characterized by incompletely filled d orbitals, and possess a high degree of chemical inertness. These attributes contribute to their remarkable electrocatalytic activity and stability. The electrocatalytic performance of noble metal nanocrystals is intricately linked to surface and interface parameters, in addition to their shape, composition, and crystal facets. In theory, the chemical functionalization of the electrocatalyst surface holds great significance as it can alter the structure of the electrode-electrolyte interface, resulting enhancing activity. 213 Various techniques, including electrochemical reduction, atmospheric heat treatment, and wet chemical procedures, have been developed to produce noble metal nanocrystals with controlled morphology and structure. Among these, the wet chemical approach stands out due to its distinct advantages, offering a homogeneous environment and greater tunability in the liquid phase. During wet chemical synthesis, surfactants play a dual role, acting as stabilizers to reduce nanoparticles to nanoscale sizes and preventing agglomeration through electrostatic stabilization and steric hindrance mechanisms. 213 , 214
Furthermore, surfactants exhibit varying adsorption states on different metal crystal planes, consequential in steady growth rates on several crystal planes, thereby foremost the favored alignment development of noble metal-nanocrystals. 215 Polyethyleneimine (PEI, Figure 11(a)) and polyallylamine (also known as polyallylamine hydrochloride, PA/PAA/PAH, Figure 11(b)) are two typical polymeric amine surfactants (PAM) frequently employed in our research. Their excellent hydrophilicity, strong coordination ability, and inherent electrostatic repulsion make them ideal surfactants and complexing agents. 216 In general, it is widely accepted that producing uniformly shaped noble metal nanocrystals in an aqueous solution presents greater challenges compared to oil phase synthesis. 213

Noble metal as nanococatalyst. Molecular structure of (a) = PEI and (b) PA/PAAPAH. (c) UV visible spectra of PAH, RhCl3 and their mixture. (d) LSV or linear sweep voltammetry curve of chemical reactions of RhCl3 with KCl and KCl + PAH at glassy carbon electrode. (e) Noble metal as nanococatalyst via PAM-assisted growth mechanism. 217
The PAMs can make coordination complexes with metals like Rh(III), Pt (II), Pd(II), and Ag(I). This is due to the existence of lone pairs on N-atom within NH2 and –HN– groups , 217 which help in nanocatalyst formulation. Consequently, anisotropic nanostructures such as nanocubes, nanowires, nanosheets, and nanonetworks are produced under varying reaction conditions, as nanospheres with low surface free energy are no longer favored in the final shape of noble metal nanocrystals. The UV–vis absorption peaks of a combined solution of PAH and Rh(III) differ from those of RhCl3 (Figure 11(c)), providing evidence of the formation of a RhIII-PAH complex. 217 Electrochemical measurements reveal that the formation of the RhIII-PAH complex substantially lowers the reduction potential of RhIII precursors, enabling kinetic control over the reduction and crystallization process (Figure 11(d)). 217 Initially, Rh(III)-PAH complexes are reduced to nearly spherical Rh seeds at the start of the reaction. The high adsorption of PAH prevents the formation of Rh (111) planes, ultimately resulting in ultrathin nanosheets exposing the Rh (111) plane. 217 Subsequent epitaxial growth of these nanosheets gives rise to three-dimensional Rh nanosheet nanoassemblies. Variations in atomic deposition and diffusion rates can significantly influence the ultimate morphology of the nanocrystals. While the rate of deposition can be controlled by varying the reduction rate of the metal precursor, the rate of diffusion is closely linked to nanocrystal surface characteristics. Hence, modifications in chemical kinetics parameters like temperature, reaction time, pH, reducing agent, metal ion precursor, PAM molecular weight, etc., can dramatically alter the rate of metal precursor reduction, thereby influencing the final nanocrystal morphology. 213 In recent years, an array of noble metal nanostructures has been synthesized by investigating the PAM-assisted synthesis mechanism of noble metal nanocrystals. Examples include Pt nanocubes, Pd icosahedrons, Pt tripods, and Pt/Pd nanodendrites 213 , 218 (Figure 11(e)). Importantly, these anisotropic noble metal nanocrystals have exhibited significantly improved catalytic activity across various electrochemical reactions.
11 Non-noble metal nanocatalysts in electrocatalysis
Non-noble metal nanocatalysts have gained significant attention in recent years as promising alternatives to expensive noble metals like platinum and palladium. These catalysts offer unique properties that make them attractive for various electrochemical reactions, addressing challenges related to cost, availability, and scalability. The significance of non-noble metal nanocatalysts in electrocatalysis, their catalytic mechanisms, and their applications in sustainable energy technologies are taking focus of research.
Global warming caused by greenhouse gas emissions and the depletion of nonrenewable fossil resources are two developing crises that can be directly linked to the expanding usage of fossil fuels as the primary source of energy in the globe. In order to achieve environmental sustainability, it is crucial to develop clean and renewable energy sources. 219 In this sense, the aforementioned problems might be resolved by capturing sunlight and subsequently turning solar energy into fuels (i.e., artificial photosynthesis). In this method, a semiconductor (SC) photocatalyst interacts with incident solar light that has photon energy equal to or greater than the bandgap of the semiconductor to form electron-hole pairs (Figure 14A, path a).
Examples include the conversion of water into hydrogen fuel and the conversion of CO2 into chemical feedstocks with added value such CO, CH4, and CH3OH (Figure 12A, path c). The holes in the valence band (VB), on the other hand, can cause water to oxidize to produce OH and O2, which are then used for the degradative oxidation of pathogens and pollutants in water or for other advantageous oxidation reactions (Figure 12A, path d). 220 Artificial photosynthesis that is powered by sunlight has some advantages for the sustainable generation of energy, but it also has some drawbacks. First, due to low absorption cross sections and the fact that most photocatalysts are only responsive to ultraviolet (UV) light, which accounts for only about 5 % of the total solar flux, their ability to absorb light is constrained.

Photocatalysis and Functionalized NPs. (A) The proposed mechanisms of semiconducting photocatalytic process under visible radiation. (B) Photocatalytic properties of functionalized plasmonic nanoparticles. 224
The strong Coulombic force between the photogenerated electrons and holes (Figure 12A, path b) and the lack of a specific driving force to drive their separation and delivery to the active sites where they are consumed are the causes of the photogenerated electrons and holes’ tendency to recombine immediately after their production, respectively. This obstructs their advantageous function in redox processes, evidently. Third, many of the processes that take place at the photocatalyst surface are thermodynamically unfavorable, and uphill energy barriers make it more difficult to drive these reactions without the right charge carriers and redox potentials. Fourth, the catalytic efficiency is made worse by the kinetically slow photocatalytic processes and other competitive surface side reactions. 221 , 222 The plasmonic nanostructures may serve as catalysts to speed up chemical reactions at the interface or cocatalysts to accommodate photogenerated electrons from the SC (electron sink function). All of these advantages give plasmon-based photocatalysts a promising future in terms of realistic grid integration for applications involving the conversion of sustainable energy and environmental cleanup. The function of plasmonic nanostructures in the hybrid metal/photocatalyst systems is summarized in Figure 12B. 223
Non-noble metal nanocatalysts offer several advantages for example non-noble metals are abundant and more cost-effective than noble metals, making them viable for large-scale applications similarly tailored nanostructures and compositions can achieve comparable or even superior catalytic activity to noble metals. Furthermore, non-noble metals are more readily available and can be synthesized in larger quantities, enabling scalable production. Non-noble metal nanocatalysts exhibit catalytic activity through mechanisms similar to noble metals. The arrangement of atoms on the nanoparticle surface, the interaction between reactants and metal sites, and the modification of electronic properties contribute to their activity. Their contribution in various chemical reactions like hydrogen evolution reactions, oxygen evaluation reaction, and electrocatalytic reduction are considerable. Nickel (Ni) and cobalt (Co), are used in electrolyzers and photoelectrochemical cells for HER due to their cost-effectiveness and reasonable activity. Cobalt oxide (Co3O4) and manganese oxide (MnOx), are utilized as catalysts for OER in water splitting and metal-air batteries. Copper (Cu) and other non-noble metals are explored for electrocatalytic CO2 reduction, aiming to convert CO2 into valuable fuels and chemicals. Iron (Fe) are investigated for nitrogen reduction reactions, which have potential applications in fertilizer production. 225
Achieving high activity and selectivity comparable to noble metals remains a challenge, requiring precise control over catalyst composition and structure. Some non-noble metal catalysts suffer from poor long-term stability due to corrosion and restructuring during electrochemical reactions. Non-noble metal catalysts may have slower kinetics and mass transport limitations that need to be addressed. Alloying non-noble metals with other elements or incorporating them into hybrid structures can enhance catalytic activity and stability. Combining non-noble metals with other catalysts, such as carbon materials, can exploit synergistic effects for improved performance. Similarly Utilizing advanced characterization methods helps understand the active sites and interactions in non-noble metal nanocatalysts. Non-noble metal nanocatalysts represent a promising avenue for advancing electrocatalysis, particularly in cost-sensitive applications such as renewable energy conversion and storage. As research continues to address challenges related to activity, selectivity, and durability, these catalysts have the potential to play a pivotal role in achieving sustainable and efficient electrochemical processes for a variety of energy and environmental applications. 226
12 Carbon-based nanomaterials as electrocatalysts
Carbon-based nanomaterials have emerged as versatile and promising electrocatalysts due to their exceptional properties, including high surface area, good electrical conductivity, chemical stability, and tunable surface functionalities. The significance of carbon-based nanomaterials in electrocatalysis, their catalytic mechanisms are nowadays center of research. For example, a single layer of carbon atoms arranged in a two-dimensional hexagonal lattice “Graphene” has high conductivity and large surface area make it an excellent electrocatalyst support. 227 Similarly the carbon nanotubes (CNTs), a type of cylindrical carbon structures with unique electronic properties and functionalized CNTs can also act as both catalysts and supports. The carbon nanofibers (CNFs) a more disordered structure is important for their high conductivity and mechanical strength. The carbon dots have fluorescent nature with tunable surface properties, offering potential for catalytic and sensing applications. 228 The catalytic activity of carbon-based nanomaterials is attributed to various mechanisms. Structural defects and edges on graphene, CNTs, and CNFs provide active sites for adsorption and reaction initiation. Introducing heteroatoms (e.g., nitrogen, sulfur) into the carbon lattice alters electronic properties, enhancing catalytic activity. Ordered graphitic regions on carbon-based materials facilitate charge transfer and electrochemical reactions. 229
Graphene-based materials and nitrogen-doped carbon catalysts exhibit ORR activity, making them potential alternatives to platinum in fuel cells and metal-air batteries. Carbon-based materials can serve as supports for non-noble metal catalysts in HER applications. Carbon-based nanomaterials functionalized with metal nanoparticles or co-catalysts show promise for electrochemical CO2 reduction to value-added products. These nanomaterials, when coupled with metal oxides or other electrocatalysts, can enhance water splitting efficiency. 229 , 230 There are various advantages of carbon base nanomaterials, as carbon is more abundant and inexpensive, making carbon-based nanomaterials a sustainable choice for electrocatalysis. Surface functionalization and doping allow tailoring of electronic and chemical properties for specific reactions. Carbon-based nanomaterials can serve as supports for other catalytic species, enhancing their stability and activity. Alongside with their advantages there are some challenges as well for example carbon-based nanomaterials may undergo degradation under harsh electrochemical conditions, affecting long-term stability, achieving high catalytic activity comparable to noble metals requires precise control over structure and defects, and diffusion limitations in porous carbon structures can impact reaction kinetics. Carbon-based nanomaterials offer a versatile platform for electrocatalysis, with applications ranging from energy conversion and storage to environmental remediation. By harnessing their tunable properties, researchers are working to enhance catalytic activity, stability, and selectivity. As advances in material synthesis, characterization, and functionalization continue, carbon-based nanomaterials are poised to play a pivotal role in enabling sustainable and efficient electrochemical processes. 231
The advantageous characteristics of carbon materials, including high electrical conductivity, cost-effectiveness, and robust chemical and mechanical durability, position them as excellent candidates for electrocatalytic reduction. 232 Carbonaceous materials such as graphite, graphene, carbon nanotubes, and porous carbon have emerged as effective options for electrocatalytic CO2 reduction, alongside other materials. 233 Notably, carbon-based materials exhibit a higher onset potential for the hydrogen evolution reaction (HER) compared to metal materials, which helps suppress H2 evolution and enhance CO2 reduction activity. Furthermore, in aqueous solutions at negative potentials, carbon materials exhibit chemical inertness. 234
12.1 Metal free carbon-based catalysts
Pure carbon compounds, due to their electroneutral nature with low CO2 adsorption capacity and high energy barriers for CO2 activation, are not considered effective for CO2 reduction. 233 To enhance a carbon framework’s ability to activate CO2, the incorporation of heteroatoms is a promising approach. The introduction of heteroatoms disrupts the electroneutrality of pure carbon structures, leading to charge redistribution and the creation of reactive sites for CO2 adsorption and activation. Heteroatoms like boron (B), nitrogen (N), and sulfur (S) exhibit distinct electronegativities compared to carbon atoms, influencing their reactivity. 235 In addition to heteroatom incorporation, the crystalline structures of carbon materials, characterized by unique binding energies of CO2-related intermediates, play a significant role in determining selectivity and activity in CO2 reduction. 234 Various carbon materials represent different dimensional structures, including OD, 1D, 2D, and 3D porous carbons (Figure 13). 233 , 234 , 236 Metal-free carbon materials can be modified to reduce CO2 into C2+ oxygenates through controlled heteroatom doping and structural engineering. The following section introduces several metal-free carbon materials suitable for converting CO2 into C2+ oxygenates. 234
Various carbon architectures, OD, 1D, 2D and 3D carbon base materials. 234
12.2 Carbon nanotubes
Carbon nanotubes (CNTs) consist of a graphitic honeycomb structure composed of sp2-hybridized carbon atoms, forming a cylindrical structure akin to a rolled-up graphite sheet. CNTs feature a unique architecture with layered inner and outer wall structures. 237 The introduction of oxygen through acid treatment can lead to the formation of oxygenated carbon nanotubes (O-CNTs), generating a variety of oxygen-containing groups. O-CNTs demonstrate the ability to convert CO2 into formic acid (HCOOH) and acetic acid (CH3COOH) at rates of 0.51 mol/h and 0.32 mol/h, respectively. CH3COOH production exhibits significantly higher Faradaic selectivity (71.5 %) compared to HCOOH (28.5 %). Oxygen functional groups on O-CNTs provide electron trapping sites, aiding in the reduction of adsorbed intermediates. However, the precise mechanism of CO2 reduction leading to CH3COOH production on O-CNTs remains uncertain. 238 CNTs, with their abundant functionalization sites, hold promise as electrocatalysts for CO2 reduction. 234
12.3 Graphene
Graphene, a two-dimensional (2D) material composed of sp2-hybridized carbon atoms arranged in a hexagonal lattice, boasts a high surface area, exceptional chemical stability, and excellent electrical conductivity. 239 Pristine graphene is typically inert towards CO2 reduction due to its ineffective CO2 activation, and ability to adsorb certain intermediates like COOH and CO. The undoped zigzag edge of graphene presents a significant energy barrier of approximately 1.3 eV for CO2 adsorption. Consequently, surface modification and heteroatom doping are employed to alter graphene’s electronic structure, enhancing its CO2 electroreduction activity. Nitrogen (N) doping is a common and effective method to modify graphene’s electronic structure and boost CO2 reduction activity for C2+ product synthesis. 58 Nitrogen-doped graphene quantum dots (NGQDs), featuring nanoscale dimensions, have been developed for the electrocatalytic conversion of CO2 into C2+ products (Figure 14). 240 NGQDs yield primarily formic acid (HCOOH) and carbon monoxide (CO) at an applied potential of 0.26 V versus reversible hydrogen electrode (RHE). However, when the applied potential shifts negatively, CO2 conversion extends to include oxygenates like CH3COO, C2H5OH, nC3H7OH, as well as hydrocarbons like CH4 and C2H4. The highest faradaic efficiency (FE) for C2+ oxygenates reaches 26 % at an applied potential of 0.78 V, with C2H5OH accounting for 16 % of the total. 22 , 234

NGQDs for electrocatalysis and their characterization. (a) Mechanism of electrocatalytic conversion of CO2 on nitrogen doped graphene quantum dots. 241 (b) TEM images of NGQDs. (c) The faradaic efficiency of reduced product on the surface of NGQDs. (d) The current densities of reduced products (C2H4, CH4, C2H5OH, CO, CH3COO−, C3H7OH) on the surface of NGQDs. 240
12.4 Nanodiamond
Diamond nanocrystals exhibit tetrahedral bonding units with sp3-hybridized carbon atoms. 242 When heteroatoms are introduced as dopants into diamonds, they experience a significant improvement in their electrical conductivity compared to their unaltered state. Heteroatom-doped diamonds offer several advantages, including a wide electrochemical potential window, a strong H2 evolution overpotential, and exceptional long-term stability, making them effective catalysts for electrochemical reactions. The unique sp3 bonding in diamonds plays a crucial role in facilitating C–C coupling and demonstrating remarkable selectivity in generating C2+ oxygenates through CO2 reduction. 234 , 243 Nanodiamond structures can enhance their electrochemical activity for CO2 by incorporating nitrogen (N). Our team achieved this by depositing N-doped nanodiamond (NDD/Si RA) on a Si rod array substrate using microwave plasma-enhanced chemical vapor deposition, as shown in Figure 15a–c. 243 NDD/Si RA exhibits an onset potential of 0.36 V versus reversible hydrogen electrode (RHE) for reducing CO2 to CH3COOH. At the potential range of 0.8–1.0 V, the total faradaic efficiency (FE) for CO2 reduction is impressively high, ranging from 91.2 % to 91.8 %, effectively suppressing the H2 evolution reaction. Specifically, at 0.8 V, the FE for CH3COOH production reaches approximately 77.3–77.6 %, surpassing the FE of HCOOH (13.6–14.6 %) under similar conditions. Notably, the CH3COOH generation rate peaks at 96.1 mg L−1 h−1 at a potential of 1.0 V. These findings underscore the advantages of NDD/Si RA in achieving C–C coupling and CH3COOH production, outweighing the challenges associated with low C2 selectivity in CO2 reduction. Our analysis suggests that CC coupling occurs when two CO2 radicals dimerize, forming OOCCOO, which subsequently undergoes hydrogenation to yield CH3COOH. The N-sp3-C species on NDD/Si RA serve as reactive sites for CO2 reduction and are positively correlated with CH3COOH formation. 234

Doped/undoped nanodiamonds (NDDs) and their role in catalytic reduction reactions. (a) NDD SEM images. (b) The rate of production of reduced products, on the surface of NDD. (c) The faradaic efficiencies (for formate and acetate) in the production on the surface of NDD. 243 (d) SEM images of boron and nitrogen doped nanodiamonds (BND). (e) The rate of production of reduced products, on the surface of BND. The faradaic efficiencies (for formate and acetate) in the production on the surface of BND. 244
Additionally, we employed a hot filament chemical vapor deposition method to create boron and nitrogen co-doped nanodiamond (BND) on a Si substrate, as depicted in Figure 15d–f. BND exhibits the ability to transform CO2 into HCOOH, HCHO, CH3OH, CH3COOH, and C2H5OH, with C2H5OH being the predominant product, boasting a maximum FE of 93.2 % at 1.0 V versus RHE. The increased presence of N in BND enables the synthesis of C2H5OH. Density functional theory (DFT) calculations indicate that the synergistic effect between B and N dopants significantly contributes to the enhanced capacity for C2H5OH production on BND. The connection of an oxygen (O) atom to a boron (B) atom enhances CO2 adsorption, while nitrogen (N) atoms serve as active sites for H transfer, expediting hydrogenation during CO2 reduction.
12.5 Porous carbon
In addition to traditional graphite and diamond structures, porous carbon materials featuring hierarchically structured 3D pores present an enticing option as electrocatalysts for CO2 reduction. These materials offer extra active sites for CO2 capture and reduction due to their large specific surface areas and diverse porous topologies. Porous carbon can be categorized based on pore size, such as microporous (pores smaller than 2 nm), mesoporous (pores between 2 and 50 nm), or macroporous (pores larger than 50 nm). The pore size, distribution, and hierarchically porous structure influence the accessibility of CO2 to reactive sites and the dynamics of electrolyte diffusion. Thus, tailoring the porous structure, in conjunction with heteroatom doping, plays a pivotal role in enhancing CO2 reduction capacity. 234 , 245 By manipulating the macro-, meso-, and microporous 3D carbon structures, we can create distinctive surfaces and electronic properties. Additionally, porous carbon typically contains both sp2 and sp3 hybridized carbon, benefiting from the unique characteristics of both carbon atom types. Therefore, by optimizing the concentrations of sp2/sp3 and porous distribution, the CO2 reduction activity of porous carbon can be finely tuned. The customization of porous structures, alongside heteroatom doping, represents a critical element in enhancing CO2 reduction capability. Song and colleagues, for instance, developed metal-free nitrogen-doped mesoporous carbon with an ordered cylindrical channel structure, as shown in Figure 16a–c. 246 The resulting catalysts efficiently and selectively convert CO2 into C2H5OH, with a FE of 77 % at 0.56 V versus RHE. The uniformly distributed pyridinic-N and pyrrolic-N in the inner layer of the cylindrical channel play a pivotal role in facilitating electron transfer for the dimerization of CO intermediates and the subsequent C–C coupling in electrochemical CO2 conversion. Furthermore, the cylindrical structural configuration of nitrogen-doped mesoporous carbon proves essential for electrochemical CO2 conversion. However, the rate of C2H5OH synthesis in N-doped mesoporous carbon remains limited due to significant kinetic barriers in C–C bond formation. 234 To alleviate these limitations and enhance important intermediate coupling, Song and colleagues introduced catalysts with carefully tailored designs, resulting in the creation of nitrogen-doped ordered mesoporous carbon (Figure 16d–f). 244 This modification led to an increased C2H5OH generation rate of up to 2.3 mmol g−1 h−1. The medium microporous structure, combined with high electron density in pyrrolic and pyridinic N, simplifies CO2 activation and C–C bond formation. The presence of medium micropores in the channel wall, along with the cylindrical structural configuration, enables the accumulation of electrolyte ions, resulting in high local electrical potentials that drive desolvation during electrochemical CO2 conversion. 246
Synthetic route and properties of N-doped mesopore/micropore porous carbon mateirals for catalytic CO2 reduction, resulting catalysts efficiently and selectively convert CO2 into C2H5OH (V versus RHE). (a) General scheme of mesoporous carbon and exchange of materials. (b) Various section TEM images of N-doped mesoporous carbon. (c) Faradaic efficiencies of CO2 reduction reactions on the surface of N-doped mesoporous carbon. 246 (d) General scheme of mesoporous, narrowpore, narrow micropore, madium micropore carbon. (e) TEM images of N-doped microporous carbon. (f) Faradaic efficiencies of CO2 reduction reactions on the surface of N-doped microporous carbon. 247
12.6 Metal and carbon composite catalysts
The development of metal and carbon composite electrocatalysts represents another design strategy for achieving CO2 reduction to C2+ oxygenates. These composite materials, comprising carbon and metal components (like Cu, Fe, Ni, Pt), that have ability to reduce CO2 to C2+ oxygenates at lower overpotentials compared to metal-free carbon catalysts and to CH3COOH, C2H5OH, CH3COCH3, and C3H7OH. The synergistic effects between metals and carbon materials appear to be responsible for the high catalytic activity and selectivity observed in these composite catalysts. 248 The activation and adsorption of CO2 are facilitated by metal particles, given their high CO binding energies and low H2 evolution overpotentials. The high surface area and flexible porous structure of carbon materials provide an extensive electrochemically active area for CO2 reduction, reducing resistance to reactant and product diffusion and, thereby, facilitating CO2 reduction.
13 Emerging trends in electrocatalyst design
The field of electrocatalyst design is constantly evolving, driven by the need for more efficient, cost-effective, and sustainable energy conversion and storage technologies. Several emerging trends are shaping the development of electrocatalysts, revolutionizing their performance and expanding their applications. This section explores some of these cutting-edge trends that are shaping the future of electrocatalyst design. Single-Atom Catalysis: Designing catalysts at the single-atom level offers unprecedented control over catalytic activity and selectivity. Single-atom catalysts (SACs) provide maximized atom utilization and minimized use of expensive materials. They can exhibit unique electronic and geometric properties that lead to enhanced reactivity and improved stability. 249 Hybrid and tandem catalysis are the results of combining multiple catalytic materials in a single system can achieve synergistic effects, enabling multi-step reactions or improving catalytic performance. Tandem catalysis involves sequential reactions within the same catalytic system, reducing intermediate separation and improving overall efficiency. 250 Similarly in cooperative catalysis, two or more catalysts work together to facilitate complex reactions that might not be achievable by a single catalyst. Cooperative electrocatalysis leverages interactions between different catalysts to enhance reaction rates and selectivity. 251 Advances in machine learning and computational techniques enable the rapid screening and design of potential catalysts. Data-driven approaches predict catalytic activity, selectivity, and stability based on known catalyst properties, accelerating the discovery of promising candidates. 252 The material structure also alter the rate of reactions, for example two-dimensional (2D) materials, such as transition metal dichalcogenides (TMDs), offer high surface area and unique electronic properties compare to other relevant materials. Combining different 2D materials into heterostructures allows tailoring of catalytic sites and electronic interactions. 253 Drawing inspiration from biological systems, biomimetic electrocatalysts mimic enzymatic processes to achieve high catalytic efficiency and selectivity. This trend explores the integration of natural motifs and synthetic catalysts. 254 Similarly the designing of electrocatalysts with dynamic or responsive properties allows adaptability to changing reaction conditions. Stimulus-responsive catalysts can enhance activity and selectivity under specific triggers. 255 Real-time in situ characterization techniques provide insights into catalyst behavior under working conditions. Operando studies offer a deeper understanding of catalytic mechanisms, aiding in catalyst optimization. 256 Expanding the applications of electrocatalysis beyond energy conversion and storage includes areas like electrochemical synthesis, environmental remediation, and electrochemical sensing. 257 Emerging trends in electrocatalyst design are reshaping the landscape of energy conversion, storage, and beyond. From advanced computational techniques to novel materials and innovative catalytic mechanisms, these trends are driving the development of more efficient, sustainable, and versatile electrocatalysts. As research progresses, these trends will continue to shape the future of electrochemistry, enabling breakthroughs in clean energy technologies and addressing global challenges.
14 Photocatalysis
14.1 Strategies for improving photocatalysis
Utilizing an innovative substance known as a nanocomposite, specifically designed to address certain shortcomings within the field of photocatalysis, offers an efficient solution. This approach is particularly promising for overcoming limitations associated with various substrates such as carbon-containing materials, 258 silica, 259 and primarily assisted polymers, 260 which have been previously reported as suitable for attaching nanoparticles. Among these substrates, polymer hosts stand out as a compelling choice for long-term applications due to their capacity to anchor nanoparticles, their virtually limitless architectural versatility, sophisticated and multifunctional surface chemistry, and robust mechanical strength, all of which are conducive to practical applications. The key driving force behind the growing interest in inorganic functional polymer composites, which combine novel optical, electrical, and catalytic capabilities of nanoparticles with the flexibility and transparency of the polymer matrix, lies in their ability to offer unique environmental processes, chemical inertness, excellent durability, and long-lasting stability. Additionally, these polymers facilitate the attachment of target organic molecules to their surfaces, leading to an overall enhancement in photocatalytic efficiency. 261 Photocatalysis, as a versatile technique, can facilitate various reactions, ranging from the mineralization of organic contaminants to complex organic processes. Its fundamental advantage lies in the conversion of light energy into chemical energy, thereby reducing energy consumption and providing a sustainable and cost-effective solution to pollution. Consequently, photocatalysis aligns with the principles of sustainable chemistry and green organic synthesis. However, it is worth noting that current photocatalytic materials predominantly rely on semiconductors, primarily TiO2. 262
14.2 Doping process
Doping is an important technique used to enlarge the photoactive zone, which prevents recombining of the electrons and holes produced during photogeneration, in addition to broadening the response spectrum range of the photocatalyst. 263 It is common practice to modify the photocatalyst by doping in order to improve its photocatalytic performance. An example of this is the use of nitrogen doping to alter and improve the performance of photocatalyst oxides. 264 Different from cation doping, which produced more unfavorable recombination locations for the electron-hole, is anion doping. It has been discovered that nitrogen doping has a greater visible light response and is more significant than carbon and sulfur doping. Due to its potent capacity to oxidize and significant reactivity to visible light. Since 2001, interest in the nitrogen-doped TiO2 photocatalyst has increased. In addition, vanadium and chromium doping of TiO2 lengthens its wavelength in the region of visible light. The photocatalytic effectiveness is nonetheless impacted by the recombination of the electrons and holes that occurs during the fusing of metal ions. 263 Doping, however, considerably lowers the bandgap, improving the photocatalytic characteristics. For instance, only absorption in the ultraviolet (UV) range (which includes 5 % of the solar spectrum) can result in the creation of electron-hole pairs. The observed band gap varies with the TiO2 polymorphs and is greater than 3.0 eV for rutile, 3.2 eV for anatase, and 3.13 eV for brookite, 265 indicating that the majority of solar energy cannot be converted into a reaction that uses photocatalysis. This restriction has been studied by narrowing the bandgap by doping pure TiO2 with an impurity element as N, S, or Fe. 266 TiO2 activity can be increased by visible light by absorbing more energy through doping. While the electron-hole recombination site may be trapped by intermediate energy states created by newly added atoms and defects, which reduces the effectiveness of the photocatalytic process. 267 Additionally, it has been noted that elemental doping of ZnO can compensate for the limited surface area and insufficient optical absorption of ZnO. 268
The bandgap energy of TiO2 can be reduced by synergistic co-doping, resulting functional visible light photocatalyst. Similarly, the co-doping of Fe3+ and graphene can also boost the photo-efficiency of semiconductors. 269 Therefore, according to Zhang et al. , 270 the co-doping of Ni2+ and Ti3+ caused a notable drop in the TiO2 anatase band, as demonstrated in Figure 17. 270 It is possible to increase photocatalytic activity in the visible light area by using a number of nonmetal atoms with high energies for ionization and electronegativity, such as I, Br, Cl, F, S, O, and N. 271 This is accomplished by narrowing the bandgap and shifting edge absorption. These heteroatoms with doped carbon skeletons have recently been employed to improve the catalytic process that led to the electron-induced regulation of carbon atoms. 272 Co-doping will undoubtedly increase the photocatalytic effectiveness by two or more atoms. By lowering the development of recombination centers and inactivating the impurity bands, it will increase the solubility of dopants.

General scheme of photocatalytic reaction for Ti3+ and Ni2+ co-doped TiO2, where organic contaminations can converted into CO2 and H2O. 270
14.3 Semiconductors immobilization on the surfaces of polymers
Due to the advantages of immobilizing TiO2, researchers worldwide are focusing on efficient methods to anchor TiO2 onto suitable substrates. The ideal polymer matrix should meet several criteria, including strong catalyst-substrate affinity, compatibility with the chosen linkage method, precise surface properties, good contaminant adsorption capacity, and prevention of photocatalyst leaching. One notable example is the use of 3,5-dinitrosalicylic acid/chitosan/MnFe2O4 (DNSA/Cs/MnFe2O4) nanocatalyst, which significantly enhances adsorption and photodecomposition. The addition of DNSA/Cs as an interface connector between MnFe2O4 and methylene blue (MB) accelerates photodegradation and enhances catalytic efficiency, providing a promising method for water purification as show in Figure 18. 273
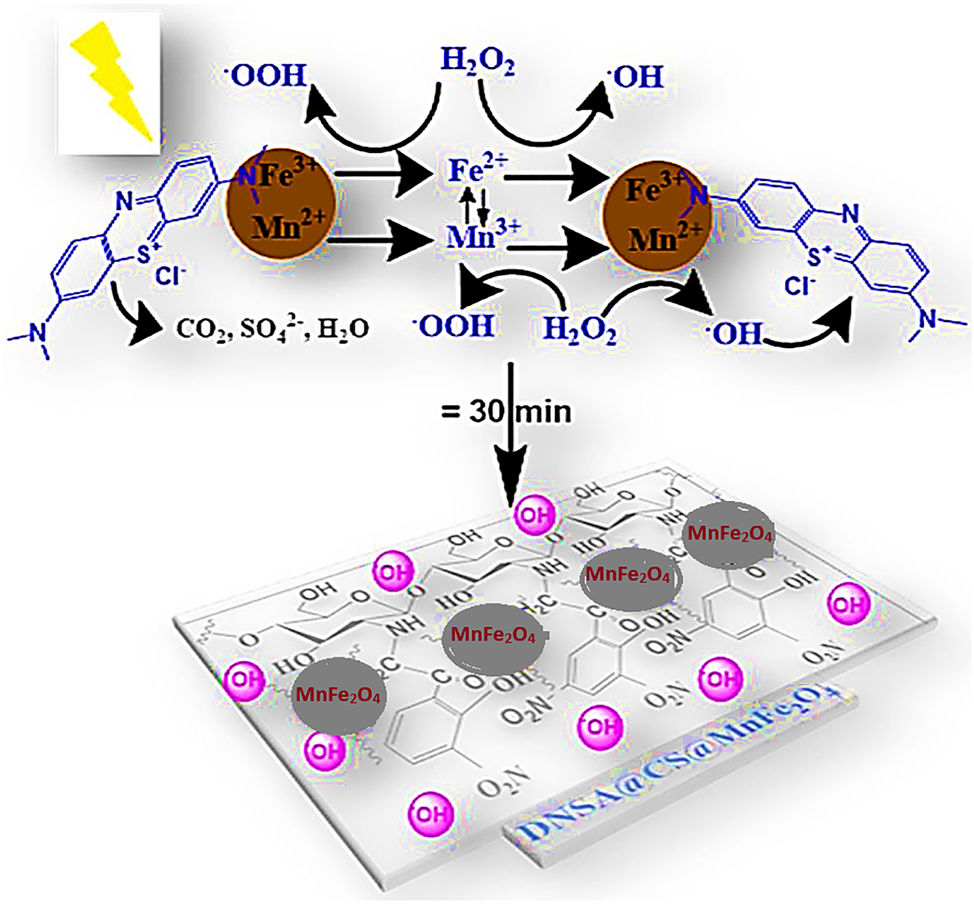
The proposed mechanism of MB decomposition a Fento-like activation, onto DNSA@Cs@MnFe2O4 magnetic as a special type of nanocatalyst. 273
15 Semiconductors conducting polymers hybrid photocatalysts
Conducting polymers like polypyrrole (PPy), polyaniline (PANI), and polythiophene (PTh) have high charge mobility and absorb light from visible to near-infrared regions. These polymers can be used as photosensitizers to enhance the photocatalytic activity of semiconductor materials. New composite materials, such as PANI and TiO2NPs, show significant catalytic activity for dye degradation, offering the potential for effective pollutant treatment. 274
16 Advantages and disadvantages of nanocatalysts
Nanocatalysts have demonstrated effectiveness in room temperature mineralization of harmful organic compounds, making them valuable for water purification. They are often cost-effective, environmentally friendly, chemically stable, and exhibit excellent photoactive properties at the nanoscale. However, some nanocatalysts may suffer from photo-corrosion, limiting their chemical stability. To overcome these issues, researchers are exploring alternative nanocomposites with reduced toxicity and improved stability, such as magnetic nanocatalysts that enable catalyst recovery and reuse. 261 Despite significant progress in nanocatalytic materials, there is a need for the development of reactors tailored to nanocatalyst modifications. 275 Microfluidic reactors, operating with small amounts of reactants, offer advantages such as high surface-to-volume ratios and enhanced mass transfer, promising improved catalytic performance. Additionally, the creation of unique nanomaterial structures with superior characteristics, such as nanoflowers, nanorods, nanocones, nanospheres, and nanofibers, opens new avenues for research. 276 The development of novel nanocatalysts with enhanced stability, cost-effectiveness, and efficiency remains a key focus for future studies in the field of photocatalysis. Co-doping, the integration of various thermodynamic processes, and the development of effective water treatment methods will continue to shape the future of nanocatalysis. 261
16.1 Advanced catalysts for photocatalysis
Photocatalysis, the use of light energy to drive chemical reactions, holds significant promise for sustainable energy production, environmental remediation, and synthesis of valuable compounds. Advanced catalysts play a crucial role in enhancing the efficiency and selectivity of photocatalytic processes. 277 Semiconductor photocatalysts, such as titanium dioxide (TiO2) and metal oxides, harness light energy to generate electron-hole pairs, initiating redox reactions. Advances in semiconductor design, including bandgap engineering and surface modifications, enhance their photocatalytic activity. 278 Plasmonic materials, like gold and silver nanoparticles, exhibit surface plasmon resonance, enabling localized electromagnetic fields that enhance light absorption and catalytic activity. Plasmonic nanoparticles can facilitate reactions that are not easily achievable with traditional catalysts. 279 Similarly the two-dimensional materials, such as graphene, transition metal dichalcogenides (TMDs), and black phosphorus, offer high surface area and tunable electronic properties. They can be used as photocatalysts themselves or as co-catalysts to enhance activity and selectivity. MOFs and COFs exhibit high porosity and tunable structures, making them ideal candidates for photocatalysis. Their pore structures can facilitate reactant adsorption, while metal nodes can act as active sites for redox reactions. 280 Integrating homogeneous catalysts into heterogeneous photocatalytic systems enables the exploitation of both light-induced charge carriers and solution-phase catalytic intermediates. This synergy enhances reaction efficiency and selectivity. 281 likewise the introduction of co-catalysts, such as noble metal nanoparticles or co-catalytic species, onto photocatalyst surfaces can also facilitate charge separation and enhance reaction kinetics. Rational design and precise loading are crucial for optimizing their effects. Hybrid systems can tailor light absorption and charge transfer processes. Using light-harvesting molecules, quantum dots, or dye-sensitized systems can expand the light absorption range of photocatalysts. These systems transfer energy to the catalyst, initiating photochemical reactions. 282 Developing catalysts that enable multi-electron transfer processes and cascade reactions can facilitate complex transformations, making photocatalysis a versatile tool for organic synthesis. 283 Beyond water splitting, advanced photocatalysts are being explored for carbon dioxide reduction, pollutant degradation, and fine chemical synthesis. Tailoring catalyst properties for specific reactions is a key focus. Advanced catalysts are pivotal in advancing the field of photocatalysis, unlocking its potential for sustainable energy conversion and environmental remediation. From innovative materials to synergistic co-catalysts and tailored structures, these catalysts enable more efficient light-driven reactions with applications ranging from hydrogen generation to pollutant removal and organic synthesis. As research continues, the development of advanced photocatalysts will contribute to addressing pressing global challenges and creating a greener and more sustainable future.
16.2 Semiconductor nanomaterials for photocatalysis
Semiconductor nanomaterials have revolutionized the field of photocatalysis by enabling the conversion of light energy into chemical reactions. These materials, through their unique electronic properties and tunable surfaces, have found applications in various domains, from environmental remediation to energy production. The significance of semiconductor nanomaterials in photocatalysis, their mechanisms, and their diverse applications are very valuable. 284
16.3 Photogenerated charge carriers and redox reactions in photocatalysis
Photogenerated charge carriers serve as the driving force for redox reactions in photocatalysis, enabling a wide range of applications from water splitting to environmental remediation. Understanding and optimizing charge carrier dynamics and their interaction with the catalyst’s surface are key factors in enhancing the efficiency and selectivity of photocatalytic processes. As researchers continue to unravel these intricate mechanisms, the field of photocatalysis holds promise for contributing to clean energy production and sustainable chemical transformations. The efficient utilization of photogenerated charge carriers is a fundamental aspect of photocatalysis, enabling redox reactions that drive various chemical transformations. This interplay between light-induced charge separation and subsequent redox processes is essential for the success of photocatalytic reactions. 285
16.4 Photogenerated charge carriers
When semiconductor nanomaterials absorb photons with energy greater than their bandgap, electron-hole pairs (e-h pairs) are generated. Upon promotion of electron from the valence band (VB) to the conduction band (CB), mobile charge carrier is generated, which result the corresponding positive charge, or holes, left in the VB (as shown in Figure 17). The spatial separation of these charge carriers is crucial for initiating redox reactions. The efficient charge carrier dynamics involve in the charge separation, transport and charge recombination. In the charge separation the photo-generated e-h pairs should be separated quickly, typically within femtoseconds to picoseconds, to prevent recombination. Then the separated carriers move towards the material surface or interface where redox reactions occur and lastly the undesirable e-h recombination can lead to energy loss and reduced photocatalytic activity. 286
16.5 Redox reactions in photocatalysis
The photogenerated charge carriers participate in redox reactions at the catalyst’s surface. In the oxidation reactions, the photogenerated holes (h+) can oxidize molecules adsorbed on the catalyst surface, leading to the removal of electrons and the generation of radical cations. While the photogenerated electrons (e−) reduce adsorbed species, leading to the formation of radical anions. But sometimes the radicals generated during oxidation and reduction can further react to form intermediate species crucial for the final products. The applicability photogenerated charge carrier is more prominent in water splitting, degradation of pollutants, reduction of carbon dioxide etc, In water splitting, photogenerated holes (h+) are involved in water oxidation, while photogenerated electrons (e−) reduce protons to form hydrogen. Charge carriers participate in the oxidation of organic pollutants adsorbed on the catalyst’s surface which help in the degradation of pollutants. Photogenerated charge carriers also reduce carbon dioxide to valuable chemicals such as hydrocarbons and alcohols. In some cases, the efficiency of charge carrier utilization is not very good, so we need to improve via various available techniques for example using cocatalysts, surface modifications and tandem catalysis. Co-catalysts can trap and transfer charge carriers more efficiently, preventing recombination and enhancing redox reactions. Tailoring the catalyst’s surface properties can also improve charge separation and favor certain redox reactions. In the same way combining multiple catalysts can facilitate charge carrier transfer between different materials, enhancing overall efficiency. 287
16.6 Cocatalysts and surface modifications in photocatalysis
Cocatalysts and surface modifications play a crucial role in enhancing the efficiency, selectivity, and overall performance of photocatalytic reactions. These strategies involve tailoring the surface properties of semiconductor nanomaterials to improve charge separation, redox reactions, and reactant adsorption. Cocatalysts are secondary materials integrated with semiconductor photocatalysts to facilitate charge transfer, prevent recombination, and enhance catalytic activity. Common types of cocatalysts include noble metal nanoparticles, co-catalytic metal oxides, and organic molecules. Various mode of mechanisms is available in literature including charge carrier transfer, surface reaction sites, and energy level alignment. Cocatalysts can act as electron or hole acceptors, preventing recombination and prolonging the lifetime of photogenerated charge carriers. Cocatalysts also provide additional active sites for reactant adsorption and redox reactions, accelerating overall reaction rates. When the energy levels of cocatalysts match with those of the semiconductor, it promote efficient charge transfer across the interface. 288
Cocatalysts like platinum (Pt) or nickel oxide (NiOx) enhance the efficiency of hydrogen evolution and oxygen evolution reactions in water splitting. They improve the degradation of organic pollutants by facilitating radical generation and surface reaction sites. Also they aid in the reduction of carbon dioxide to valuable chemicals by promoting specific reaction pathways. 289 Surface modifications involve altering the surface properties of semiconductor nanomaterials to enhance photocatalytic performance. Common surface modifications include doping, functionalization, and heterostructure. Surface modifications can alter the band structure of semiconductors, leading to improved light absorption and charge separation. Functional groups introduced through surface modifications can enhance the adsorption of reactants, facilitating redox reactions. Surface defects introduced by modifications can act as charge traps, reducing charge carrier recombination. 290 Surface-modified photocatalysts can efficiently produce hydrogen or other solar fuels by enhancing reaction rates and charge carrier dynamics. Modified photocatalysts can effectively remove pollutants from water and air by enhancing their adsorption and degradation. Tailored surface properties enable the synthesis of complex molecules, mimicking natural photosynthesis. Still the optimization of cocatalyst loading to achieve the desired effect without blocking active sites is challenging. Ensuring the stability of cocatalysts and surface modifications under harsh reaction conditions is crucial for long-term performance. Developing cocatalysts and surface modifications specific to each photocatalytic reaction requires a deep understanding of reaction mechanisms. Cocatalysts and surface modifications serve as effective strategies to enhance the performance of semiconductor photocatalysts. By improving charge transfer, reaction kinetics, and surface properties, these approaches unlock the full potential of photocatalysis for various applications. As researchers continue to innovate in cocatalyst design and surface modification techniques, the field of photocatalysis is poised to contribute significantly to sustainable energy production, environmental protection, and green chemistry. 291 A short comparison between photocatalysis and electrocatalysis is given in Table 2.
The correlation, comparisons and characteristics of photocatalysis and electrocatalysis.
| Photocatalysis | Electrocatalysis | ||
|---|---|---|---|
| Catalysts characteristics | Type | Nanomaterials powders or composites thin films | Thin films of nanomaterials or powder loaded electrodes |
| Hydrophilicity | Hydrophilic | Hydrophilic or hydrophobic | |
| Recyclability | Feasible for thin films, complex for powder | Feasible | |
| Condition of reaction system | Energy input | Solar energy | Externally applied voltage |
| configuration | Single reaction chamber | One or two reaction chambers with membrane | |
| Benefits | Pathway for charge transfer | Relatively short | Relatively long |
| Efficiency | Low | High | |
| Cost | Low | High | |
17 Nanostructured photoelectrodes for enhanced photocatalysis and photoelectrochemical reactions
Nanostructured photoelectrodes represent a pivotal advancement in the realm of photocatalysis and photoelectrochemistry. These engineered materials combine the benefits of high surface area, efficient charge transport, and light absorption to optimize light-induced reactions. 292 This section explores the significance of nanostructured photoelectrodes, their design principles, mechanisms, and applications in driving efficient photocatalytic and photoelectrochemical processes. The design of nanostructured photoelectrodes are also depend upon high surface area, effective charge transport, cocatalyst etc. Photoelectrodes that are nanostructures offer a large surface-to-volume ratio, maximizing the area available for reactant adsorption and catalytic reactions also they can enhance light absorption by trapping and guiding photons within the material, thereby increasing the efficiency of charge generation. Similarly provide efficient pathways for charge carriers to move to the electrode surface, minimizing recombination and can accommodate co-catalysts, promoting charge separation and facilitating surface reactions. 293 Nanostructured photoelectrodes exemplify the synergy between material engineering and light-driven reactions, offering significant advancements in both photocatalysis and photoelectrochemical applications. By leveraging high surface area, light absorption enhancement, and efficient charge transport, these innovative materials hold promise for sustainable energy conversion, environmental remediation, and the synthesis of valuable chemicals. As research continues to refine design strategies, address challenges, and uncover new possibilities, nanostructured photoelectrodes are poised to reshape the landscape of light-induced chemical processes. 294
18 Challenges and opportunities in nano photo/electrochemistry for catalysis
The integration of nanomaterials, photochemistry, and electrochemistry to build advanced catalysts introduces a realm of both challenges and opportunities. Navigating these aspects is essential for unlocking the full potential of nano photo/electrochemistry in catalysis.
The precise control of nanomaterial properties, such as size, shape, composition, and surface chemistry, remains a challenge. The development of reproducible and scalable synthesis methods is necessary to tailor nanocatalysts for specific reactions. Efficient utilization of photo-generated charge carriers requires a deep understanding of their behavior, including recombination pathways and transport mechanisms. Balancing charge separation and recombination is critical for achieving high catalytic activity. Many catalytic reactions involve harsh conditions that can degrade nanomaterials over time. Designing catalysts that withstand chemical and electrochemical stresses while maintaining their activity is a significant challenge. The efficient coupling of photochemical and electrochemical processes demands precise control over catalyst-electrode interfaces. Achieving optimal charge transfer and avoiding undesirable side reactions at these interfaces is nontrivial. While nanocatalysts offer increased surface area and reactivity, achieving high selectivity and specificity in complex reaction networks remains challenging. Developing catalysts that can selectively guide reactions to desired products is a key goal. Unraveling the intricate mechanisms of nano photo/electrocatalysis requires advanced experimental techniques and theoretical models. Bridging the gap between fundamental understanding and practical application is a continuous challenge. 295 The high surface area and unique electronic properties of nanomaterials offer opportunities to significantly boost catalytic activity. This is particularly relevant for energy-intensive reactions such as water splitting and carbon dioxide reduction. Nanocatalysts can be precisely engineered to exhibit specific properties, enabling fine-tuning of catalytic performance. Tailoring these properties allows researchers to optimize catalysts for desired reactions. The combination of photochemical and electrochemical pathways can lead to synergistic effects, where the strengths of both processes complement each other. This opens up new reaction pathways and facilitates the activation of otherwise inert molecules. Nano photo/electrocatalysis holds immense potential for renewable energy conversion, such as solar fuel production. By harnessing solar energy to drive catalytic reactions, these processes could contribute to a sustainable energy future. The photocatalytic degradation of pollutants and contaminants presents an environmentally friendly approach to water and air purification. Nano photo/electrocatalysts offer efficient ways to address pressing environmental challenges. The unique reactivity of nanocatalysts can enable precise control over reaction pathways, leading to highly selective synthesis of complex molecules. This has implications in pharmaceuticals and fine chemicals production. Advances in spectroscopy and microscopy techniques allow for real-time monitoring of catalytic processes at the nanoscale. This provides insights into reaction mechanisms and aids catalyst design. The complexity of nano photo/electrochemistry bridges disciplines, encouraging collaboration between chemists, physicists, material scientists, and engineers. This multidisciplinary approach fosters innovation and accelerates progress. In short, the challenges posed by nano photo/electrochemistry for catalysis are matched by the exciting opportunities it presents. Overcoming these challenges will require a concerted effort from researchers across different fields, driven by the potential to revolutionize how we approach energy conversion, environmental protection, and chemical synthesis. As advancements continue to unfold, the journey towards harnessing the power of nanoscale interactions for catalysis promises a transformative impact on science, technology, and society as a whole.
19 Conclusions
The integration of nano photo/electrochemistry with various types of catalysis has ushered in a new era of efficient and sustainable chemical transformations. The review introduced the convergence of nano photo/electrochemistry and catalysis, highlighting their importance in various applications. It set the stage for understanding the interplay between nanomaterials, photochemistry, and electrochemistry. The challenges and opportunities in the field were discussed, including the need for improved catalytic efficiency, selectivity, and understanding of complex reaction mechanisms. The potential to address global challenges through innovative catalytic processes was emphasized. The current study elucidated the intricate relationship between nanomaterials and catalytic behavior. It explored the ways nanoscale properties influence catalytic activity, selectivity, and mechanisms, emphasizing the importance of surface effects and electron transfer processes. The influence of nanoscale effects, such as size, shape, and surface structure, on catalytic behavior was examined. These effects were shown to modify reaction kinetics, adsorption properties, and the distribution of active sites. The mechanisms of photochemical reactions and their significance in initiating otherwise thermodynamically unfavorable transformations, the importance of excited states and energy transfer processes in catalysis, and the role of electrochemical processes in catalysis, including electron transfer reactions and double-layer effects, were explored. The interaction between nanostructures and reactivity was examined, emphasizing how nanoscale features influence reaction pathways and surface reactions. Various types of catalysis were found very serious, including homogeneous and heterogeneous catalysis, enzymatic catalysis, photocatalysis, and photoelectrocatalysis for nanoscale chemistry. Each type was presented with its significance and unique applications in chemical transformations. The role of advanced catalysts in enhancing photocatalysis was explored. Semiconductor nanomaterials, plasmonic nanoparticles, and 2D materials were discussed for their ability to harness light energy for efficient redox reactions. Cocatalysts and surface modifications were highlighted as strategies to improve photocatalytic performance. Their influence on charge transfer, surface reaction sites, and energy level alignment were presented as critical factors in optimizing catalytic efficiency. The design principles, mechanisms, and applications of nanostructured photoelectrodes were discussed. Their ability to enhance light absorption, charge transport, and reaction kinetics in both photocatalysis and photoelectrochemistry was emphasized. The integration of nano photo/electrochemistry and catalysis has opened up novel avenues for efficient and sustainable chemical transformations. By understanding the nanoscale effects, photochemical and electrochemical processes, and the interplay between different catalyst types, researchers can tailor catalytic systems for specific reactions and applications. Advanced catalysts, including semiconductor nanomaterials and cocatalysts, offer the potential to revolutionize energy conversion, environmental remediation, and chemical synthesis. Looking ahead, the continued exploration of advanced catalysts, the development of novel materials, and the optimization of surface modifications hold the promise of even greater achievements in nano photo/electrocatalysis. Emerging trends such as single-atom catalysis, machine learning-assisted catalyst design, and dynamic catalytic systems will likely drive breakthroughs in the field. As this interdisciplinary field advances, it will play an increasingly vital role in addressing global challenges and shaping a sustainable and innovative future. The fusion of nano photo/electrochemistry with catalysis represents a dynamic field where nanomaterials, light energy, and redox reactions converge to enable transformative chemical processes with far-reaching implications.
Funding source: King Khalid University
Award Identifier / Grant number: RGP-2/527/45
-
Research ethics: Not Applicable.
-
Informed consent: All authors have read and agreed to the published version of the manuscript.
-
Author contributions: Conceptualization and formal analysis; K.F.F. and G.R.O., Data curation and Figures sitting by; A.I., Y.J. and H.K., Formal analysis and funding; F.S.A., Investigation; P.K.P. and S.A., Methodology; J.U.R., F.S.A., Software; A.I. and H.K., Supervision and writing J.U.R., Writing, review, revision and editing; K.S.N., D.B., and J.U.R.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: Not Applicable.
-
Research funding: The authors express their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia, for this work through a research group program under grant number RGP-2/527/45.
-
Data availability: Not Applicable.
References
1. Pan, Y.; Tang, L.; Ding, M. Electrostatic Polarization in Single-Atom Catalysis. Cell Rep. Phy. Sci. 2023, 4; https://doi.org/10.1016/j.xcrp.2023.101417.Suche in Google Scholar
2. Xu, H.; Zhao, Y.; Wang, Q.; He, G.; Chen, H. Supports Promote Single-Atom Catalysts toward Advanced Electrocatalysis. Coord. Chem. Rev. 2022, 451, 214261; https://doi.org/10.1016/j.ccr.2021.214261.Suche in Google Scholar
3. Dou, S.; Wang, X.; Wang, S. Rational Design of Transition Metal-based Materials for Highly Efficient Electrocatalysis. Small Methods 2019, 3, 1800211; https://doi.org/10.1002/smtd.201800211.Suche in Google Scholar
4. Gür, T. M. Review of Electrical Energy Storage Technologies, Materials and Systems: Challenges and Prospects for Large-Scale Grid Storage. Energy Environ. Sci. 2018, 11, 2696–2767; https://doi.org/10.1039/c8ee01419a.Suche in Google Scholar
5. Lv, X. W.; Weng, C. C.; Zhu, Y. P.; Yuan, Z. Y. Nanoporous Metal Phosphonate Hybrid Materials as a Novel Platform for Emerging Applications: a Critical Review. Small 2021, 17, 2005304; https://doi.org/10.1002/smll.202005304.Suche in Google Scholar PubMed
6. Ciamician, G.; Silber, P. Chemische Lichtwirkungen. Ber. Dtsch. Chem. Ges. 1908, 41, 1071–1080; https://doi.org/10.1002/cber.190804101211.Suche in Google Scholar
7. Ciamician, G. Actions chimiques de la lumière. Bull. Soc. Chim. Fr. 1908, 4, 1–27.Suche in Google Scholar
8. Albini, A.; Fagnoni, M. Green Chemistry and Photochemistry Were Born at the Same Time. Green Chem. 2004, 6, 1–6; https://doi.org/10.1039/b309592d.Suche in Google Scholar
9. Turro, N. J.; Ramamurthy, V.; Scaiano, J. C. Modern Molecular Photochemistry of Organic Molecules; University Science Books: Sausalito, CA, 2010.Suche in Google Scholar
10. Shaw, M. H.; Twilton, J.; MacMillan, D. W. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926; https://doi.org/10.1021/acs.joc.6b01449.Suche in Google Scholar PubMed PubMed Central
11. Wu, L.; Li, Y.; Zhou, B.; Liu, J.; Cheng, D.; Guo, S.; Xu, K.; Yuan, C.; Wang, M.; Hong Melvin, G. J.; Ortiz-Medina, J.; Ali, S.; Yang, T.; Kim, Y. A.; Wang, Z. Vertical Graphene on Rice-Husk-Derived SiC/C Composite for Highly Selective Photocatalytic CO2 Reduction into CO. Carbon 2023, 207, 36–48; https://doi.org/10.1016/j.carbon.2023.03.003.Suche in Google Scholar
12. Yasin, G.; Ali, S.; Ibraheem, S.; Kumar, A.; Tabish, M.; Mushtaq, M. A.; Ajmal, S.; Arif, M.; Khan, M. A.; Saad, A.; Qiao, L.; Zhao, W. Simultaneously Engineering the Synergistic-Effects and Coordination-Environment of Dual-Single-Atomic Iron/Cobalt-Sites as a Bifunctional Oxygen Electrocatalyst for Rechargeable Zinc-Air Batteries. ACS Catal. 2023, 2313–2325; https://doi.org/10.1021/acscatal.2c05654.Suche in Google Scholar
13. Shah, R.; Ali, S.; Raziq, F.; Ali, S.; Ismail, P. M.; Shah, S.; Iqbal, R.; Wu, X.; He, W.; Zu, X.; Zada, A.; Adnan; Mabood, F.; Vinu, A.; Jhung, S. H.; Yi, J.; Qiao, L. Exploration of Metal Organic Frameworks and Covalent Organic Frameworks for Energy-Related Applications. Coord. Chem. Rev. 2023, 477, 214968; https://doi.org/10.1016/j.ccr.2022.214968.Suche in Google Scholar
14. Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2019, 120, 919–985; https://doi.org/10.1021/acs.chemrev.9b00201.Suche in Google Scholar PubMed
15. Pan, L.; Ai, M.; Huang, C.; Yin, L.; Liu, X.; Zhang, R.; Wang, S.; Jiang, Z.; Zhang, X.; Zou, J.-J.; Mi, W. Manipulating Spin Polarization of Titanium Dioxide for Efficient Photocatalysis. Nat. Commun. 2020, 11, 418; https://doi.org/10.1038/s41467-020-14333-w.Suche in Google Scholar PubMed PubMed Central
16. Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38; https://doi.org/10.1038/238037a0.Suche in Google Scholar PubMed
17. Raziq, F.; Aligayev, A.; Shen, H.; Ali, S.; Shah, R.; Ali, S.; Bakhtiar, S. H.; Ali, A.; Zarshad, N.; Zada, A.; Xia, X.; Zu, X.; Khan, M.; Wu, X.; Kong, Q.; Liu, C.; Qiao, L. Exceptional Photocatalytic Activities of rGO Modified (B,N) Co-doped WO3, Coupled with CdSe QDs for One Photon Z-Scheme System: A Joint Experimental and DFT Study. Adv. Sci. 2022, 9, 2102530; https://doi.org/10.1002/advs.202102530.Suche in Google Scholar PubMed PubMed Central
18. Raziq, F.; Khan, K.; Ali, S.; Ali, S.; Xu, H.; Ali, I.; Zada, A.; Muhammad Ismail, P.; Ali, A.; Khan, H.; Wu, X.; Kong, Q.; Zahoor, M.; Xiao, H.; Zu, X.; Li, S.; Qiao, L. Accelerating CO2 Reduction on Novel Double Perovskite Oxide with Sulfur, Carbon Incorporation: Synergistic Electronic and Chemical Engineering. Chem. Eng. J. 2022, 446, 137161; https://doi.org/10.1016/j.cej.2022.137161.Suche in Google Scholar
19. Wahid, F.; Ali, S.; Ismail, P. M.; Raziq, F.; Ali, S.; Yi, J.; Qiao, L. Metal Single Atom Doped 2D Materials for Photocatalysis: Current Status and Future Perspectives. Prog. Energy 2022, 5, 012001; https://doi.org/10.1088/2516-1083/ac9eff.Suche in Google Scholar
20. Bao, L.; Ren, X.; Liu, C.; Liu, X.; Dai, C.; Yang, Y.; Bououdina, M.; Ali, S.; Zeng, C. Modulating the Doping State of Transition Metal Ions in ZnS for Enhanced Photocatalytic Activity. Chem. Commun. 2023, 59, 11280–11283; https://doi.org/10.1039/d3cc03436d.Suche in Google Scholar PubMed
21. Chhowalla, M.; Shin, H. S.; Eda, G.; Li, L.-J.; Loh, K. P.; Zhang, H. The Chemistry of Two-Dimensional Layered Transition Metal Dichalcogenide Nanosheets. Nat. Chem. 2013, 5, 263–275; https://doi.org/10.1038/nchem.1589.Suche in Google Scholar PubMed
22. Wu, M.; Wu, T.; Sun, M.; Lu, L.; Li, N.; Zhang, C.; Huang, B.; Du, Y.; Yan, C.-H. General Synthesis of Large-Area Flexible Bi-atomic Subnano Thin Lanthanide Oxide Nanoscrolls. Nano Energy 2020, 78, 105318; https://doi.org/10.1016/j.nanoen.2020.105318.Suche in Google Scholar
23. Zhang, H. Ultrathin Two-Dimensional Nanomaterials. ACS Nano 2015, 9, 9451–9469; https://doi.org/10.1021/acsnano.5b05040.Suche in Google Scholar PubMed
24. Tang, J.-Y.; Kong, X. Y.; Ng, B.-J.; Chew, Y.-H.; Mohamed, A. R.; Chai, S.-P. Midgap-state-mediated Two-step Photoexcitation in Nitrogen Defect-Modified gC 3 N 4 Atomic Layers for Superior Photocatalytic CO2 Reduction. Catal. Sci. Technol. 2019, 9, 2335–2343; https://doi.org/10.1039/c9cy00449a.Suche in Google Scholar
25. Wang, G.; Tao, J.; Zhang, Y.; Wang, S.; Yan, X.; Liu, C.; Hu, F.; He, Z.; Zuo, Z.; Yang, X. Engineering Two-Dimensional Mass-Transport Channels of the MoS2 Nanocatalyst toward Improved Hydrogen Evolution Performance. ACS Appl. Mater. Interfaces 2018, 10, 25409–25414; https://doi.org/10.1021/acsami.8b07163.Suche in Google Scholar PubMed
26. Kibsgaard, J.; Chen, Z.; Reinecke, B. N.; Jaramillo, T. F. Engineering the Surface Structure of MoS2 to Preferentially Expose Active Edge Sites for Electrocatalysis. Nat. Mater. 2012, 11, 963–969; https://doi.org/10.1038/nmat3439.Suche in Google Scholar PubMed
27. Wang, H.; Liu, X.; Niu, P.; Wang, S.; Shi, J.; Li, L. Porous Two-Dimensional Materials for Photocatalytic and Electrocatalytic Applications. Matter 2020, 2, 1377–1413; https://doi.org/10.1016/j.matt.2020.04.002.Suche in Google Scholar
28. Qin, D.; Zhou, Y.; Wang, W.; Zhang, C.; Zeng, G.; Huang, D.; Wang, L.; Wang, H.; Yang, Y.; Lei, L.; Chen, S.; He, D. Recent Advances in Two-Dimensional Nanomaterials for Photocatalytic Reduction of CO 2: Insights into Performance, Theories and Perspective. J. Mater. Chem. A 2020, 8, 19156–19195; https://doi.org/10.1039/d0ta07460h.Suche in Google Scholar
29. Khan, K.; Tareen, A. K.; Aslam, M.; Sagar, R. U. R.; Zhang, B.; Huang, W.; Mahmood, A.; Mahmood, N.; Khan, K.; Zhang, H.; Guo, Z. Recent Progress, Challenges, and Prospects in Two-Dimensional Photo-Catalyst Materials and Environmental Remediation. Nano-Micro Lett. 2020, 12, 1–77; https://doi.org/10.1007/s40820-020-00504-3.Suche in Google Scholar PubMed PubMed Central
30. Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; Sindoro, M.; Zhang, H. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331; https://doi.org/10.1021/acs.chemrev.6b00558.Suche in Google Scholar PubMed
31. Fan, L.; Zhu, B.; Su, P.-C.; He, C. Nanomaterials and Technologies for Low Temperature Solid Oxide Fuel Cells: Recent Advances, Challenges and Opportunities. Nano Energy 2018, 45, 148–176; https://doi.org/10.1016/j.nanoen.2017.12.044.Suche in Google Scholar
32. Doyle, R. L.; Lyons, M. E. The Oxygen Evolution Reaction: Mechanistic Concepts and Catalyst Design. In Photoelectrochemical Solar Fuel Production; Springer, Cham: Switzerland, 2016; pp 41–104.10.1007/978-3-319-29641-8_2Suche in Google Scholar
33. Wang, Z.; Li, C.; Domen, K. Recent Developments in Heterogeneous Photocatalysts for Solar-Driven Overall Water Splitting. Chem. Soc. Rev. 2019, 48, 2109–2125; https://doi.org/10.1039/c8cs00542g.Suche in Google Scholar PubMed
34. Yang, M. Q.; Wang, J.; Wu, H.; Ho, G. W. Noble Metal-free Nanocatalysts with Vacancies for Electrochemical Water Splitting. Small 2018, 14, 1703323; https://doi.org/10.1002/smll.201703323.Suche in Google Scholar PubMed
35. Li, Z.; Rigor, J.; Large, N.; El-Khoury, P. Z.; Kurouski, D. Underlying Mechanisms of Hot Carrier-Driven Reactivity on Bimetallic Nanostructures. J. Phys. Chem. C 2021, 125, 2492–2501; https://doi.org/10.1021/acs.jpcc.1c00155.Suche in Google Scholar
36. Boriskina, S. V.; Ghasemi, H.; Chen, G. Plasmonic Materials for Energy: From Physics to Applications, Mater. Today Off. 2013, 16, 375–386; https://doi.org/10.1016/j.mattod.2013.09.003.Suche in Google Scholar
37. Pan, L.; Sun, S.; Chen, Y.; Wang, P.; Wang, J.; Zhang, X.; Zou, J. J.; Wang, Z. L. Advances in Piezo‐phototronic Effect Enhanced Photocatalysis and Photoelectrocatalysis. Adv. Energy Mater. 2020, 10, 2000214; https://doi.org/10.1002/aenm.202000214.Suche in Google Scholar
38. Qian, W.; Zhao, K.; Zhang, D.; Bowen, C. R.; Wang, Y.; Yang, Y. Piezoelectric Material-Polymer Composite Porous Foam for Efficient Dye Degradation via the Piezo-Catalytic Effect. ACS Appl. Mater. Interfaces 2019, 11, 27862–27869; https://doi.org/10.1021/acsami.9b07857.Suche in Google Scholar PubMed
39. Xu, S.; Qian, W.; Zhang, D.; Zhao, X.; Zhang, X.; Li, C.; Bowen, C. R.; Yang, Y. A Coupled Photo-Piezo-Catalytic Effect in a BST-PDMS Porous Foam for Enhanced Dye Wastewater Degradation. Nano Energy 2020, 77, 105305; https://doi.org/10.1016/j.nanoen.2020.105305.Suche in Google Scholar
40. Zhang, S.; Li, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wen, T.; Chen, J.; Wang, X. Recent Developments of Two-Dimensional Graphene-Based Composites in Visible-Light Photocatalysis for Eliminating Persistent Organic Pollutants from Wastewater. Chem. Eng. J. 2020, 390, 124642; https://doi.org/10.1016/j.cej.2020.124642.Suche in Google Scholar
41. Qian, W.; Xu, S.; Zhang, X.; Li, C.; Yang, W.; Bowen, C.; Yang, Y. Differences and Similarities of Photocatalysis and Electrocatalysis in Two-Dimensional Nanomaterials: Strategies, Traps, Applications and Challenges. Nano-Micro Lett. 2021, 13, 156; https://doi.org/10.1007/s40820-021-00681-9.Suche in Google Scholar PubMed PubMed Central
42. Zhou, M.; Zhang, Z.; Huang, K.; Shi, Z.; Xie, R.; Yang, W. Colloidal Preparation and Electrocatalytic Hydrogen Production of MoS 2 and WS 2 Nanosheets with Controllable Lateral Sizes and Layer Numbers. Nanoscale 2016, 8, 15262–15272; https://doi.org/10.1039/c6nr04775k.Suche in Google Scholar PubMed
43. Wang, T.; Liu, L.; Zhu, Z.; Papakonstantinou, P.; Hu, J.; Liu, H.; Li, M. Enhanced Electrocatalytic Activity for Hydrogen Evolution Reaction from Self-Assembled Monodispersed Molybdenum Sulfide Nanoparticles on an Au Electrode. Energy Environ. Sci. 2013, 6, 625–633; https://doi.org/10.1039/c2ee23513g.Suche in Google Scholar
44. Fiori, G.; Bonaccorso, F.; Iannaccone, G.; Palacios, T.; Neumaier, D.; Seabaugh, A.; Banerjee, S. K.; Colombo, L. Electronics Based on Two-Dimensional Materials. Nat. Nanotechnol. 2014, 9, 768–779; https://doi.org/10.1038/nnano.2014.207.Suche in Google Scholar PubMed
45. Yu, Y.; Huang, S.-Y.; Li, Y.; Steinmann, S. N.; Yang, W.; Cao, L. Layer-dependent Electrocatalysis of MoS2 for Hydrogen Evolution. Nano Lett. 2014, 14, 553–558; https://doi.org/10.1021/nl403620g.Suche in Google Scholar PubMed
46. Javaid, M.; Drumm, D. W.; Russo, S. P.; Greentree, A. D. A Study of Size-dependent Properties of MoS2 Monolayer Nanoflakes Using Density-Functional Theory. Sci. Rep. 2017, 7, 1–11; https://doi.org/10.1038/s41598-017-09305-y.Suche in Google Scholar PubMed PubMed Central
47. Li, Y.; Li, P.; Wang, J.; Yang, Y.; Yao, W.; Wei, Z.; Wu, J.; Yan, X.; Xu, X.; Liu, Y.; Zhu, Y. Water Soluble Graphitic Carbon Nitride with Tunable Fluorescence for Boosting Broad-Response Photocatalysis. Appl. Catal. B Environ. 2018, 225, 519–529; https://doi.org/10.1016/j.apcatb.2017.12.017.Suche in Google Scholar
48. He, R.; Xu, D.; Cheng, B.; Yu, J.; Ho, W. Review on Nanoscale Bi-based Photocatalysts. Nanoscale Horizons 2018, 3, 464–504; https://doi.org/10.1039/c8nh00062j.Suche in Google Scholar PubMed
49. Guo, Y.; Xu, K.; Wu, C.; Zhao, J.; Xie, Y. Surface Chemical-Modification for Engineering the Intrinsic Physical Properties of Inorganic Two-Dimensional Nanomaterials. Chem. Soc. Rev. 2015, 44, 637–646; https://doi.org/10.1039/c4cs00302k.Suche in Google Scholar PubMed
50. Tan, C.; Zhang, H. Two-dimensional Transition Metal Dichalcogenide Nanosheet-Based Composites. Chem. Soc. Rev. 2015, 44, 2713–2731; https://doi.org/10.1039/c4cs00182f.Suche in Google Scholar PubMed
51. Majidi, L.; Yasaei, P.; Warburton, R. E.; Fuladi, S.; Cavin, J.; Hu, X.; Hemmat, Z.; Cho, S. B.; Abbasi, P.; Vörös, M.; Cheng, L.; Sayahpour, B.; Bolotin, I. L.; Zapol, P.; Greeley, J.; Klie, R. F.; Mishra, R.; Khalili-Araghi, F.; Curtiss, L. A.; Salehi-Khojin, A. New Class of Electrocatalysts Based on 2D Transition Metal Dichalcogenides in Ionic Liquid. Adv. Mater. 2019, 31, 1804453; https://doi.org/10.1002/adma.201804453.Suche in Google Scholar PubMed
52. Jin, M.; Lu, S.-Y.; Zhong, X.; Liu, H.; Liu, H.; Gan, M.; Ma, L. Spindle-like MOF Derived TiO2@ NC–NCNTs Composite with Modulating Defect Site and Graphitization Nanoconfined Pt NPs as Superior Bifunctional Fuel Cell Electrocatalysts. ACS Sustain. Chem. Eng. 2020, 8, 1933–1942; https://doi.org/10.1021/acssuschemeng.9b06329.Suche in Google Scholar
53. Rana, A. K.; Mishra, Y. K.; Gupta, V. K.; Thakur, V. K. Sustainable Materials in the Removal of Pesticides from Contaminated Water: Perspective on Macro to Nanoscale Cellulose. Sci. Total Environ. 2021, 797, 149129; https://doi.org/10.1016/j.scitotenv.2021.149129.Suche in Google Scholar PubMed
54. Nicolosi, V.; Chhowalla, M.; Kanatzidis, M. G.; Strano, M. S.; Coleman, J. N. Liquid Exfoliation of Layered Materials. Science 2013, 340, 1226419; https://doi.org/10.1126/science.1226419.Suche in Google Scholar
55. Zheng, Y.; Jiao, Y.; Zhu, Y.; Cai, Q.; Vasileff, A.; Li, L. H.; Han, Y.; Chen, Y.; Qiao, S.-Z. Molecule-level G-C3n4 Coordinated Transition Metals as a New Class of Electrocatalysts for Oxygen Electrode Reactions. J. Am. Chem. Soc. 2017, 139, 3336–3339; https://doi.org/10.1021/jacs.6b13100.Suche in Google Scholar PubMed
56. Sturala, J.; Luxa, J.; Pumera, M.; Sofer, Z. Chemistry of Graphene Derivatives: Synthesis, Applications, and Perspectives. Chem.-Eur. J. 2018, 24, 5992–6006; https://doi.org/10.1002/chem.201704192.Suche in Google Scholar PubMed
57. Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J. M.; Domen, K.; Antonietti, M. A Metal-free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80; https://doi.org/10.1038/nmat2317.Suche in Google Scholar PubMed
58. Muhammad Ismail, P.; Ali, S.; Raziq, F.; Bououdina, M.; Abu-Farsakh, H.; Xia, P.; Wu, X.; Xiao, H.; Ali, S.; Qiao, L. Stable and Robust Single Transition-Metal Atom Catalyst for CO2 Reduction Supported on Defective WS2. Appl. Surf. Sci. 2023, 157073; https://doi.org/10.1016/j.apsusc.2023.157073.Suche in Google Scholar
59. Abid, A.; Haneef, M.; Ali, S.; Dahshan, A. Optoelectronic and Photocatalytic Properties of GaN, GeS and SiS Monolayers and Their vdW Heterostructures. J. Phys. Chem. Solid. 2022, 161, 110433; https://doi.org/10.1016/j.jpcs.2021.110433.Suche in Google Scholar
60. Xu, W.; Ali, S.; Jin, Y.; Wu, X.; Xu, H. Intrinsic Ferromagnetic Semiconductors in Two-Dimensional Alkali-Based Chromium Chalcogenides. ACS Appl. Electron. Mater. 2020, 2, 3853–3858; https://doi.org/10.1021/acsaelm.0c00686.Suche in Google Scholar
61. Zhang, Y.; Guo, H.; Sun, W.; Sun, H.; Ali, S.; Zhang, Z.; Saito, R.; Yang, T. Scaling Law for Strain Dependence of Raman Spectra in Transition-Metal Dichalcogenides. J. Raman Spectrosc. 2020, 51, 1353–1361; https://doi.org/10.1002/jrs.5908.Suche in Google Scholar
62. Ismail, P. M.; Ali, S.; Ali, S.; Li, J.; Liu, M.; Yan, D.; Raziq, F.; Wahid, F.; Li, G.; Yuan, S.; Wu, X.; Yi, J.; Chen, J. S.; Wang, Q.; Zhong, L.; Yang, Y.; Xia, P.; Qiao, L. Photoelectron “Bridge” in Van Der Waals Heterojunction for Enhanced Photocatalytic CO2 Conversion under Visible Light. Adv. Mater., n/a 2303047; https://doi.org/10.1002/adma.202303047.Suche in Google Scholar PubMed
63. Zhang, L.; Ji, X.; Ren, X.; Ma, Y.; Shi, X.; Tian, Z.; Asiri, A. M.; Chen, L.; Tang, B.; Sun, X. Electrochemical Ammonia Synthesis via Nitrogen Reduction Reaction on a MoS2 Catalyst: Theoretical and Experimental Studies. Adv. Mater. 2018, 30, 1800191; https://doi.org/10.1002/adma.201800191.Suche in Google Scholar PubMed
64. Ali, S.; Xie, Z.; Xu, H. Stability and Catalytic Performance of Single-Atom Supported on Ti2CO2 for Low-Temperature CO Oxidation: A First-Principles Study. ChemPhysChem 2021, 22, 2352–2361; https://doi.org/10.1002/cphc.202100436.Suche in Google Scholar PubMed
65. Zhang, Z.; Zheng, B.; Tian, H.; He, Y.; Huang, X.; Ali, S.; Xu, H. Rational Design of Highly Efficient MXene-Based Catalysts for Water-Gas-Shift Reaction. Phys. Chem. Chem. Phys. 2022, 24, 18265–18271; https://doi.org/10.1039/d1cp05789h.Suche in Google Scholar PubMed
66. Yang, H.; Li, Z.; Lu, B.; Gao, J.; Jin, X.; Sun, G.; Zhang, G.; Zhang, P.; Qu, L. Reconstruction of Inherent Graphene Oxide Liquid Crystals for Large-Scale Fabrication of Structure-Intact Graphene Aerogel Bulk toward Practical Applications. ACS Nano 2018, 12, 11407–11416; https://doi.org/10.1021/acsnano.8b06380.Suche in Google Scholar PubMed
67. Liu, R.; Wang, Y.; Liu, D.; Zou, Y.; Wang, S. Water-plasma-enabled Exfoliation of Ultrathin Layered Double Hydroxide Nanosheets with Multivacancies for Water Oxidation. Adv. Mater. 2017, 29, 1701546; https://doi.org/10.1002/adma.201701546.Suche in Google Scholar PubMed
68. Xu, K.; Wang, L.; Xu, X.; Dou, S. X.; Hao, W.; Du, Y. Two Dimensional Bismuth-Based Layered Materials for Energy-Related Applications. Energy Storage Mater. 2019, 19, 446–463; https://doi.org/10.1016/j.ensm.2019.03.021.Suche in Google Scholar
69. Pei, Y.; Wang, H.; Snyder, G. J. Band Engineering of Thermoelectric Materials. Adv. Mater. 2012, 24, 6125–6135; https://doi.org/10.1002/adma.201202919.Suche in Google Scholar PubMed
70. Ali, S.; Haneef, M.; Akbar, J.; Ullah, I.; Ullah, S.; Samad, A. Single Au Atom Supported Defect Mediated Boron Nitride Monolayer as an Efficient Catalyst for Acetylene Hydrochlorination: A First Principles Study. Mol. Catal. 2021, 511, 111753; https://doi.org/10.1016/j.mcat.2021.111753.Suche in Google Scholar
71. Saha, S.; Jana, M.; Khanra, P.; Samanta, P., Koo, H.; Murmu, N. C., Kuila, T., Band Gap Engineering of Boron Nitride by Graphene and its Application as Positive Electrode Material in Asymmetric Supercapacitor Device. ACS Appl. Mater. Interfaces 2015, 7, 14211–14222; https://doi.org/10.1021/acsami.5b03562.Suche in Google Scholar PubMed
72. Zuhra, Z.; Ali, S.; Ali, S.; Xu, H.; Wu, R.; Tang, Y. Exceptionally Amino-Quantitated 3D MOF@CNT-sponge Hybrid for Efficient and Selective Recovery of Au(III) and Pd(II). Chem. Eng. J. 2021, 133367; https://doi.org/10.1016/j.cej.2021.133367.Suche in Google Scholar
73. Iqbal, R.; Akbar, M. B.; Ahmad, A.; Hussain, A.; Altaf, N.; Ibraheem, S.; Yasin, G.; Khan, M. A.; Tabish, M.; Kumar, A.; Majeed, M. K.; Saleem, A.; Ali, S. Exploring the Synergistic Effect of Novel Ni-Fe in 2D Bimetallic Metal-Organic Frameworks for Enhanced Electrochemical Reduction of CO2. Adv. Mater. Interfac. 2022, 9, 2101505; https://doi.org/10.1002/admi.202101505.Suche in Google Scholar
74. Iqbal, R.; Ali, S.; Saleem, A.; Majeed, M. K.; Hussain, A.; Rauf, S.; Rehman Akbar, A.; Xu, H.; Qiao, L.; Zhao, W. Electrically Conductive Pt-MOFs for Acidic Oxygen Reduction: Optimized Performance via Altering Conjugated Ligands. Chem. Eng. J. 2022, 140799; https://doi.org/10.1016/j.cej.2022.140799.Suche in Google Scholar
75. Iqbal, R.; Ali, S.; Yasin, G.; Ibraheem, S.; Tabish, M.; Hamza, M.; Chen, H.; Xu, H.; Zeng, J.; Zhao, W. A Novel 2D Co3(HADQ)2 Metal-Organic Framework as a Highly Active and Stable Electrocatalyst for Acidic Oxygen Reduction. Chem. Eng. J. 2022, 430, 132642; https://doi.org/10.1016/j.cej.2021.132642.Suche in Google Scholar
76. Qadir, S.; Gu, Y.; Ali, S.; Li, D.; Zhao, S.; Wang, S.; Xu, H.; Wang, S. A Thermally Stable Isoquinoline Based Ultra-microporous Metal-Organic Framework for CH4 Separation from Coal Mine Methane. Chem. Eng. J. 2022, 428, 131136; https://doi.org/10.1016/j.cej.2021.131136.Suche in Google Scholar
77. Ali, S.; Ismail, P. M.; Wahid, F.; Kumar, A.; Haneef, M.; Raziq, F.; Ali, S.; Javed, M.; Khan, R. U.; Wu, X.; Xiao, H.; Yasin, G.; Qiao, L.; Xu, H. Benchmarking the Two-Dimensional Conductive Y3(C6X6)2 (Y = Co, Cu, Pd, Pt; X = NH, NHS, S) Metal-Organic Framework Nanosheets for CO2 Reduction Reaction with Tunable Performance. Fuel Process. Technol. 2022, 236, 107427; https://doi.org/10.1016/j.fuproc.2022.107427.Suche in Google Scholar
78. Iqbal, R.; Ali, S.; Saleem, A.; Majeed, M. K.; Hussain, A.; Rauf, S.; Rehman Akbar, A.; Xu, H.; Qiao, L.; Zhao, W. Electrically Conductive Pt-MOFs for Acidic Oxygen Reduction: Optimized Performance via Altering Conjugated Ligands. Chem. Eng. J. 2023, 455, 140799; https://doi.org/10.1016/j.cej.2022.140799.Suche in Google Scholar
79. Shah, R.; Ali, S.; Ali, S.; Xia, P.; Raziq, F.; Adnan; Mabood, F.; Shah, S.; Zada, A.; Ismail, P. M.; Hayat, A.; Rehman, A. U.; Wu, X.; Xiao, H.; Zu, X.; Li, S.; Qiao, L. Amino Functionalized Metal-Organic framework/rGO Composite Electrode for Flexible Li-Ion Batteries. J. Alloys Compd. 2023, 936, 168183; https://doi.org/10.1016/j.jallcom.2022.168183.Suche in Google Scholar
80. Liu, Z.; Yang, X.; Lu, B.; Shi, Z.; Sun, D.; Xu, L.; Tang, Y.; Sun, S. Delicate Topotactic Conversion of Coordination Polymers to Pd Porous Nanosheets for High-Efficiency Electrocatalysis. Appl. Catal. B Environ. 2019, 243, 86–93; https://doi.org/10.1016/j.apcatb.2018.10.028.Suche in Google Scholar
81. Huang, X.; Tang, S.; Mu, X.; Dai, Y.; Chen, G.; Zhou, Z.; Ruan, F.; Yang, Z.; Zheng, N. Freestanding Palladium Nanosheets with Plasmonic and Catalytic Properties. Nat. Nanotechnol. 2011, 6, 28–32; https://doi.org/10.1038/nnano.2010.235.Suche in Google Scholar PubMed
82. Chhowalla, M.; Voiry, D.; Yang, J.; Shin, H. S.; Loh, K. P. Phase-engineered Transition-Metal Dichalcogenides for Energy and Electronics. MRS Bull. 2015, 40, 585–591; https://doi.org/10.1557/mrs.2015.142.Suche in Google Scholar
83. Chang, K.; Hai, X.; Pang, H.; Zhang, H.; Shi, L.; Liu, G.; Liu, H.; Zhao, G.; Li, M.; Ye, J. Targeted Synthesis of 2H‐and 1T‐phase MoS2 Monolayers for Catalytic Hydrogen Evolution. Adv. Mater. 2016, 28, 10033–10041; https://doi.org/10.1002/adma.201603765.Suche in Google Scholar PubMed
84. Allen, M. J.; Tung, V. C.; Kaner, R. B. Honeycomb Carbon: a Review of Graphene. Chem. Rev. 2010, 110, 132–145; https://doi.org/10.1021/cr900070d.Suche in Google Scholar PubMed
85. Zheng, Y.; Liu, J.; Liang, J.; Jaroniec, M.; Qiao, S. Z. Graphitic Carbon Nitride Materials: Controllable Synthesis and Applications in Fuel Cells and Photocatalysis. Energy Environ. Sci. 2012, 5, 6717–6731; https://doi.org/10.1039/c2ee03479d.Suche in Google Scholar
86. Kang, Y.; Yang, Y.; Yin, L. C.; Kang, X.; Liu, G.; Cheng, H. M. An Amorphous Carbon Nitride Photocatalyst with Greatly Extended Visible-light-responsive Range for Photocatalytic Hydrogen Generation. Adv. Mater. 2015, 27, 4572–4577; https://doi.org/10.1002/adma.201501939.Suche in Google Scholar PubMed
87. You, H.; Jia, Y.; Wu, Z.; Wang, F.; Huang, H.; Wang, Y. Room-temperature Pyro-Catalytic Hydrogen Generation of 2D Few-Layer Black Phosphorene under Cold-Hot Alternation. Nat. Commun. 2018, 9, 2889; https://doi.org/10.1038/s41467-018-05343-w.Suche in Google Scholar PubMed PubMed Central
88. Yifan, S.; Kazunori, F.; Zhong, L.; Yu, L.; Mauricio, T.; Terrones, M.; Schaak, R. E. Low-Temperature Solution Synthesis of Transition Metal Dichalcogenide Alloys with Tunable Optical Properties. J. Am. Chem. 2017, 139, 11096–11105; https://doi.org/10.1021/jacs.7b04443.Suche in Google Scholar PubMed
89. Ali, S.; Iqbal, R.; Wahid, F.; Ismail, P. M.; Saleem, A.; Ali, S.; Raziq, F.; Ullah, S.; Ullah, I.; Tahir; Zahoor, M.; Wu, X.; Xiao, H.; Zu, X.; Qiao, L. Cobalt Coordinated Two-Dimensional Covalent Organic Framework a Sustainable and Robust Electrocatalyst for Selective CO2 Electrochemical Conversion to Formic Acid. Fuel Process. Technol. 2022, 237, 107451; https://doi.org/10.1016/j.fuproc.2022.107451.Suche in Google Scholar
90. Ali, S.; Yasin, G.; Iqbal, R.; Huang, X.; Su, J.; Ibraheem, S.; Zhang, Z.; Wu, X.; Wahid, F.; Ismail, P. M.; Qiao, L.; Xu, H. Porous Aza-Doped Graphene-Analogous 2D Material a Unique Catalyst for CO2 Conversion to Formic-Acid by Hydrogenation and Electroreduction Approaches. Mol. Catal. 2022, 524, 112285; https://doi.org/10.1016/j.mcat.2022.112285.Suche in Google Scholar
91. Tan, X.; Zeng, W.; Fan, Y.; Yan, J.; Zhao, G. Covalent Organic Frameworks Bearing Pillar [6] Arene-Reduced Au Nanoparticles for the Catalytic Reduction of Nitroaromatics. Nanotechnology 2020, 31, 135705; https://doi.org/10.1088/1361-6528/ab5ff5.Suche in Google Scholar PubMed
92. Geng, D.; Zhao, X.; Chen, Z.; Sun, W.; Fu, W.; Chen, J.; Liu, W.; Zhou, W.; Loh, K. P. Direct Synthesis of Large-area 2D Mo2C on In Situ Grown Graphene. Adv. Mater. 2017, 29, 1700072; https://doi.org/10.1002/adma.201700072.Suche in Google Scholar PubMed
93. Wang, Y.; Zhang, Y.; Liu, Z.; Xie, C.; Feng, S.; Liu, D.; Shao, M.; Wang, S. Layered Double Hydroxide Nanosheets with Multiple Vacancies Obtained by Dry Exfoliation as Highly Efficient Oxygen Evolution Electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 5867–5871; https://doi.org/10.1002/anie.201701477.Suche in Google Scholar PubMed
94. Lu, J.; Zhou, W.; Zhang, X.; Xiang, G. Electronic Structures and Lattice Dynamics of Layered BiOCl Single Crystals. Journal Phy. Chem. Lett. 2020, 11, 1038–1044; https://doi.org/10.1021/acs.jpclett.9b03575.Suche in Google Scholar PubMed
95. Weng, Q.; Wang, B.; Wang, X.; Hanagata, N.; Li, X.; Liu, D.; Wang, X.; Jiang, X.; Bando, Y.; Golberg, D. Highly Water-Soluble, Porous, and Biocompatible Boron Nitrides for Anticancer Drug Delivery. ACS Nano 2014, 8, 6123–6130; https://doi.org/10.1021/nn5014808.Suche in Google Scholar PubMed
96. Khan, S.; Zahoor, M.; Rahman, M. U.; Gul, Z. Cocrystals; Basic Concepts, Properties and Formation Strategies. Z. Phys. Chem. 2023, 237, 273–332; https://doi.org/10.1515/zpch-2022-0175.Suche in Google Scholar
97. Cai, M.; Liu, Q.; Xue, Z.; Li, Y.; Fan, Y.; Huang, A.; Li, M.-R.; Croft, M.; Tyson, T. A.; Ke, Z.; Li, G. Constructing 2D MOFs from 2D LDHs: A Highly Efficient and Durable Electrocatalyst for Water Oxidation. J. Mater. Chem. A 2020, 8, 190–195; https://doi.org/10.1039/c9ta09397d.Suche in Google Scholar
98. Givaja, G.; Amo-Ochoa, P.; Gomez-Garcia, C. J.; Zamora, F. Electrical Conductive Coordination Polymers. Chem. Soc. Rev. 2012, 41, 115–147; https://doi.org/10.1039/c1cs15092h.Suche in Google Scholar PubMed
99. Dhakshinamoorthy, A.; Asiri, A. M.; Garcia, H. 2D Metal–Organic Frameworks as Multifunctional Materials in Heterogeneous Catalysis and Electro/photocatalysis. Adv. Mater. 2019, 31, 1900617; https://doi.org/10.1002/adma.201900617.Suche in Google Scholar PubMed
100. Ren, X.; Zhou, J.; Qi, X.; Liu, Y.; Huang, Z.; Li, Z.; Ge, Y.; Dhanabalan, S. C.; Ponraj, J. S.; Wang, S.; Zhong, J.; Zhang, H. Few-layer Black Phosphorus Nanosheets as Electrocatalysts for Highly Efficient Oxygen Evolution Reaction. Adv. Energy Mater. 2017, 7, 1700396; https://doi.org/10.1002/aenm.201700396.Suche in Google Scholar
101. Zheng, C.; Yu, L.; Zhu, L.; Collins, J. L.; Kim, D.; Lou, Y.; Xu, C.; Li, M.; Wei, Z.; Zhang, Y.; Edmonds, M. T.; Li, S.; Seidel, J.; Liu, J. Z.; Tang, W. X.; Fuhrer, M. S. Room temperature in-plane ferroelectricity in van der Waals In2Se3. Sci. Adv. 2018, 4, eaar7720; https://doi.org/10.1126/sciadv.aar7720.Suche in Google Scholar PubMed PubMed Central
102. Kondo, A.; Tiew, C. C.; Moriguchi, F.; Maeda, K. Fabrication of Metal–Organic Framework Nanosheets and Nanorolls with N-Donor Type Bridging Ligands. Dalton Trans. 2013, 42, 15267–15270; https://doi.org/10.1039/c3dt52130c.Suche in Google Scholar PubMed
103. Peng, Y.; Li, Y.; Ban, Y.; Jin, H.; Jiao, W.; Liu, X.; Yang, W. Metal-organic Framework Nanosheets as Building Blocks for Molecular Sieving Membranes. Science 2014, 346, 1356–1359; https://doi.org/10.1126/science.1254227.Suche in Google Scholar PubMed
104. Cai, Z.; Liu, B.; Zou, X.; Cheng, H.-M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133; https://doi.org/10.1021/acs.chemrev.7b00536.Suche in Google Scholar PubMed
105. Qian, W.; Wu, Z.; Jia, Y.; Hong, Y.; Xu, X.; You, H.; Zheng, Y.; Xia, Y. Thermo-electrochemical Coupling for Room Temperature Thermocatalysis in Pyroelectric ZnO Nanorods. Electrochem. Commun. 2017, 81, 124–127; https://doi.org/10.1016/j.elecom.2017.06.017.Suche in Google Scholar
106. Kumar, P.; Singh, J.; Pandey, A. C. Rational Low Temperature Synthesis and Structural Investigations of Ultrathin Bismuth Nanosheets. RSC Adv. 2013, 3, 2313–2317; https://doi.org/10.1039/c2ra21907g.Suche in Google Scholar
107. Wang, X.; Chen, Y.; Zheng, B.; Qi, F.; He, J.; Li, Q.; Li, P.; Zhang, W. Graphene-like WSe2 Nanosheets for Efficient and Stable Hydrogen Evolution. J. Alloys Compd. 2017, 691, 698–704; https://doi.org/10.1016/j.jallcom.2016.08.305.Suche in Google Scholar
108. Zhu, Z.; Yang, Y.; Guan, Y.; Xue, J.; Cui, L. Construction of a Cobalt-Embedded Nitrogen-Doped Carbon Material with the Desired Porosity Derived from the Confined Growth of MOFs within Graphene Aerogels as a Superior Catalyst towards HER and ORR. J. Mater. Chem. A 2016, 4, 15536–15545; https://doi.org/10.1039/c6ta05196k.Suche in Google Scholar
109. Zhao, Y.; Zhang, X.; Jia, X.; Waterhouse, G. I.; Shi, R.; Zhang, X.; Zhan, F.; Tao, Y.; Wu, L. Z.; Tung, C. H.; O’Hare, D.; Zhang, T. Sub-3 Nm Ultrafine Monolayer Layered Double Hydroxide Nanosheets for Electrochemical Water Oxidation. Adv. Energy Mater. 2018, 8, 1703585; https://doi.org/10.1002/aenm.201703585.Suche in Google Scholar
110. Kong, X.; Zhang, C.; Hwang, S. Y.; Chen, Q.; Peng, Z. Free-standing Holey Ni (OH) 2 Nanosheets with Enhanced Activity for Water Oxidation. Small 2017, 13, 1700334; https://doi.org/10.1002/smll.201700334.Suche in Google Scholar PubMed
111. Qin, M.; Li, S.; Zhao, Y.; Lao, C. Y.; Zhang, Z.; Liu, L.; Fang, F.; Wu, H.; Jia, B.; Liu, Z.; Wang, W. A.; Liu, Y.; Qu, X. Unprecedented Synthesis of Holey 2D Layered Double Hydroxide Nanomesh for Enhanced Oxygen Evolution. Adv. Energy Mater. 2019, 9, 1803060; https://doi.org/10.1002/aenm.201803060.Suche in Google Scholar
112. Castellanos-Gomez, A.; Cappelluti, E.; Roldán, R.; Agraït, N.; Guinea, F.; Rubio-Bollinger, G. Electric-field Screening in Atomically Thin Layers of MoS2: the Role of Interlayer Coupling. Adv. Mater. 2013, 25, 899–903; https://doi.org/10.1002/adma.201203731.Suche in Google Scholar PubMed
113. Sujuan, W.; Jiawei, X.; Jianguo, S.; Wen, Z.; Zhenzhong, Y.; Lin, G.; Xixiang, Z.; Shi-Ze, Y.; Yang, S. Z. Hydroxyl-dependent Evolution of Oxygen Vacancies Enables the Regeneration of BiOCl Photocatalyst. ACS Appl. Mater. Interfaces 2017, 9, 16620–16626; https://doi.org/10.1021/acsami.7b01701.Suche in Google Scholar PubMed
114. Zheng, Y.; Yu, Z.; Ou, H.; Asiri, A. M.; Chen, Y.; Wang, X. Black Phosphorus and Polymeric Carbon Nitride Heterostructure for Photoinduced Molecular Oxygen Activation. Adv. Funct. Mater. 2018, 28, 1705407; https://doi.org/10.1002/adfm.201705407.Suche in Google Scholar
115. Jiang, J.; Zhao, K.; Xiao, X.; Zhang, L. Synthesis and Facet-dependent Photoreactivity of BiOCl Single-Crystalline Nanosheets. J. Am. Chem. Soc. 2012, 134, 4473–4476; https://doi.org/10.1021/ja210484t.Suche in Google Scholar PubMed
116. Liu, S.-H.; Tang, W.-T.; Chou, P.-H. Microwave-assisted Synthesis of Triple 2D G-C3N4/Bi2WO6/rGO Composites for Ibuprofen Photodegradation: Kinetics, Mechanism and Toxicity Evaluation of Degradation Products. Chem. Eng. J. 2020, 387, 124098; https://doi.org/10.1016/j.cej.2020.124098.Suche in Google Scholar
117. Zhao, X.; Zhang, D.; Xu, S.; Qian, W.; Han, W.; Wang, Z. L.; Yang, Y. Stretching-enhanced Triboelectric Nanogenerator for Efficient Wind Energy Scavenging and Ultrasensitive Strain Sensing. Nano Energy 2020, 75, 104920; https://doi.org/10.1016/j.nanoen.2020.104920.Suche in Google Scholar
118. Ali, S.; Ali, S.; Ismail, P. M.; Shen, H.; Zada, A.; Ali, A.; Ahmad, I.; Shah, R.; Khan, I.; Chen, J.; Cui, C.; Wu, X.; Kong, Q.; Yi, J.; Zu, X.; Xiao, H.; Raziq, F.; Qiao, L. Synthesis and Bader Analyzed Cobalt-Phthalocyanine Modified Solar UV-Blind β-Ga2O3 Quadrilateral Nanorods Photocatalysts for Wide-Visible-Light Driven H2 Evolution. Appl. Catal. B Environ. 2022, 307, 121149; https://doi.org/10.1016/j.apcatb.2022.121149.Suche in Google Scholar
119. Zhang, C.; Shi, Y.; Yu, Y.; Du, Y.; Zhang, B. Engineering Sulfur Defects, Atomic Thickness, and Porous Structures into Cobalt Sulfide Nanosheets for Efficient Electrocatalytic Alkaline Hydrogen Evolution. ACS Catal. 2018, 8, 8077–8083; https://doi.org/10.1021/acscatal.8b02056.Suche in Google Scholar
120. Hu, S.; Zhu, M. Ultrathin Two-Dimensional Semiconductors for Photocatalysis in Energy and Environment Applications. ChemCatChem 2019, 11, 6147–6165; https://doi.org/10.1002/cctc.201901597.Suche in Google Scholar
121. Zhang, Q.; Huang, S.; Deng, J.; Gangadharan, D. T.; Yang, F.; Xu, Z.; Giorgi, G.; Palummo, M.; Chaker, M.; Ma, D. Ice-assisted Synthesis of Black Phosphorus Nanosheets as a Metal-free Photocatalyst: 2D/2D Heterostructure for Broadband H2 Evolution. Adv. Funct. Mater. 2019, 29, 1902486; https://doi.org/10.1002/adfm.201902486.Suche in Google Scholar
122. Yin, H.; Tang, Z. Ultrathin Two-Dimensional Layered Metal Hydroxides: an Emerging Platform for Advanced Catalysis, Energy Conversion and Storage. Chem. Soc. Rev. 2016, 45, 4873–4891; https://doi.org/10.1039/c6cs00343e.Suche in Google Scholar PubMed
123. Song, F.; Hu, X. Exfoliation of Layered Double Hydroxides for Enhanced Oxygen Evolution Catalysis. Nat. Commun. 2014, 5, 4477; https://doi.org/10.1038/ncomms5477.Suche in Google Scholar PubMed
124. Asadi, M.; Kim, K.; Liu, C.; Addepalli, A. V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J. M.; Haasch, R.; Zapol, P.; Kumar, B.; Klie, R. F.; Abiade, J.; Curtiss, L. A.; Salehi-Khojin, A. Nanostructured Transition Metal Dichalcogenide Electrocatalysts for CO2 Reduction in Ionic Liquid. Science 2016, 353, 467–470; https://doi.org/10.1126/science.aaf4767.Suche in Google Scholar PubMed
125. Ye, L.; Gao, Y.; Cao, S.; Chen, H.; Yao, Y.; Hou, J.; Sun, L. Assembly of Highly Efficient Photocatalytic CO2 Conversion Systems with Ultrathin Two-Dimensional Metal–Organic Framework Nanosheets. Appl. Catal. B Environ. 2018, 227, 54–60; https://doi.org/10.1016/j.apcatb.2018.01.028.Suche in Google Scholar
126. Zhao, Y.; Liu, N.; Zhou, S.; Zhao, J. Two-dimensional ZnO for the Selective Photoreduction of CO 2. J. Mater. Chem. A 2019, 7, 16294–16303; https://doi.org/10.1039/c9ta04477a.Suche in Google Scholar
127. Mohamed, R.; McKinney, D.; Sigmund, W. Enhanced Nanocatalysts. Mater. Sci. Eng. R Rep. 2012, 73, 1–13; https://doi.org/10.1016/j.mser.2011.09.001.Suche in Google Scholar
128. Ning, C.-Z.; Dou, L.; Yang, P. Bandgap Engineering in Semiconductor Alloy Nanomaterials with Widely Tunable Compositions. Nat. Rev. Mater. 2017, 2, 1–14; https://doi.org/10.1038/natrevmats.2017.70.Suche in Google Scholar
129. Onuchic, J. N.; Kobayashi, C.; Miyashita, O.; Jennings, P.; Baldridge, K. K. Exploring Biomolecular Machines: Energy Landscape Control of Biological Reactions. Phil. Trans. Biol. Sci. 2006, 361, 1439–1443; https://doi.org/10.1098/rstb.2006.1876.Suche in Google Scholar PubMed PubMed Central
130. Zhang, L. H.; Shi, Y.; Wang, Y.; Shiju, N. R. Nanocarbon Catalysts: Recent Understanding Regarding the Active Sites. Adv. Sci. 2020, 7, 1902126; https://doi.org/10.1002/advs.201902126.Suche in Google Scholar PubMed PubMed Central
131. Mu, Y.; Wang, T.; Zhang, J.; Meng, C.; Zhang, Y.; Kou, Z. Single-atom Catalysts: Advances and Challenges in Metal-Support Interactions for Enhanced Electrocatalysis. Electrochem. Energy Rev. 2022, 1–42; https://doi.org/10.1007/s41918-021-00124-4.Suche in Google Scholar
132. Schauermann, S.; Nilius, N.; Shaikhutdinov, S.; Freund, H.-J. Nanoparticles for Heterogeneous Catalysis: New Mechanistic Insights. Acc. Chem. Res. 2013, 46, 1673–1681; https://doi.org/10.1021/ar300225s.Suche in Google Scholar PubMed
133. Sun, D.; Kim, D.-P. Hydrophobic MOFs@ Metal Nanoparticles@ COFs for Interfacially Confined Photocatalysis with High Efficiency. ACS Appl. Mater. Interfaces 2020, 12, 20589–20595; https://doi.org/10.1021/acsami.0c04537.Suche in Google Scholar PubMed
134. Chaturvedi, S.; Dave, P. N.; Shah, N. K. Applications of Nano-Catalyst in New Era. J. Saudi Chem. Soc. 2012, 16, 307–325; https://doi.org/10.1016/j.jscs.2011.01.015.Suche in Google Scholar
135. Cheng, H.; Fuku, K.; Kuwahara, Y.; Mori, K.; Yamashita, H. Harnessing Single-Active Plasmonic Nanostructures for Enhanced Photocatalysis under Visible Light. J. Mater. Chem. A 2015, 3, 5244–5258; https://doi.org/10.1039/c4ta06484d.Suche in Google Scholar
136. Jang, J. S.; Kim, H. G.; Lee, J. S. Heterojunction Semiconductors: A Strategy to Develop Efficient Photocatalytic Materials for Visible Light Water Splitting. Catal. Today 2012, 185, 270–277; https://doi.org/10.1016/j.cattod.2011.07.008.Suche in Google Scholar
137. Colmenares, J. C.; Varma, R. S.; Nair, V. Selective Photocatalysis of Lignin-Inspired Chemicals by Integrating Hybrid Nanocatalysis in Microfluidic Reactors. Chem. Soc. Rev. 2017, 46, 6675–6686; https://doi.org/10.1039/c7cs00257b.Suche in Google Scholar PubMed
138. Zhou, Y.; Wang, Z.; Huang, L.; Zaman, S.; Lei, K.; Yue, T.; Li, Z. a.; You, B.; Xia, B. Y. Engineering 2D Photocatalysts toward Carbon Dioxide Reduction. Adv. Energy Mater. 2021, 11, 2003159; https://doi.org/10.1002/aenm.202003159.Suche in Google Scholar
139. Tu, W.; Li, Y.; Kuai, L.; Zhou, Y.; Xu, Q.; Li, H.; Wang, X.; Xiao, M.; Zou, Z. Construction of Unique Two-Dimensional MoS 2–TiO 2 Hybrid Nanojunctions: MoS 2 as a Promising Cost-Effective Cocatalyst toward Improved Photocatalytic Reduction of CO 2 to Methanol. Nanoscale 2017, 9, 9065–9070; https://doi.org/10.1039/c7nr03238b.Suche in Google Scholar PubMed
140. Ma, B.; Wen, F.; Jiang, H.; Yang, J.; Ying, P.; Li, C. The Synergistic Effects of Two Co-catalysts on Zn 2 GeO 4 on Photocatalytic Water Splitting. Catal. Lett. 2010, 134, 78–86; https://doi.org/10.1007/s10562-009-0220-8.Suche in Google Scholar
141. Xu, Y.; Chen, L.; Wang, X.; Yao, W.; Zhang, Q. Recent Advances in Noble Metal Based Composite Nanocatalysts: Colloidal Synthesis, Properties, and Catalytic Applications. Nanoscale 2015, 7, 10559–10583; https://doi.org/10.1039/c5nr02216a.Suche in Google Scholar PubMed
142. Xu, W.; Zhang, Y.; Chen, T. Single Particle Nanocatalysis: Fundamentals and Applications; John Wiley & Sons: Changchun, 2019.10.1002/9783527809721Suche in Google Scholar
143. Agbo, P.; Danilovic, N. An Algorithm for the Extraction of Tafel Slopes. J. Phys. Chem. C 2019, 123, 30252–30264; https://doi.org/10.1021/acs.jpcc.9b06820.Suche in Google Scholar
144. Ren, X.; Pang, L.; Zhang, Y.; Ren, X.; Fan, H.; Liu, S. F. One-step Hydrothermal Synthesis of Monolayer MoS 2 Quantum Dots for Highly Efficient Electrocatalytic Hydrogen Evolution. J. Mater. Chem. A 2015, 3, 10693–10697; https://doi.org/10.1039/c5ta02198g.Suche in Google Scholar
145. Wu, K.; Sun, L. D.; Yan, C. H. Recent Progress in Well-controlled Synthesis of Ceria-based Nanocatalysts towards Enhanced Catalytic Performance. Adv. Energy Mater. 2016, 6, 1600501; https://doi.org/10.1002/aenm.201600501.Suche in Google Scholar
146. Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Accounts Chem. Res. 2013, 46, 1740–1748; https://doi.org/10.1021/ar300361m.Suche in Google Scholar PubMed
147. Xu, W.; Kong, J. S.; Yeh, Y.-T. E.; Chen, P. Single-molecule Nanocatalysis Reveals Heterogeneous Reaction Pathways and Catalytic Dynamics. Nat. Mater. 2008, 7, 992–996; https://doi.org/10.1038/nmat2319.Suche in Google Scholar PubMed
148. Jiang, Y.; Weiss, E. A. Colloidal Quantum Dots as Photocatalysts for Triplet Excited State Reactions of Organic Molecules. J. Am. Chem. Soc. 2020, 142, 15219–15229; https://doi.org/10.1021/jacs.0c07421.Suche in Google Scholar PubMed
149. Cui, C.-H.; Yu, S.-H. Engineering Interface and Surface of Noble Metal Nanoparticle Nanotubes toward Enhanced Catalytic Activity for Fuel Cell Applications. Acc. Chem. Res. 2013, 46, 1427–1437; https://doi.org/10.1021/ar300254b.Suche in Google Scholar PubMed
150. Regulacio, M. D.; Han, M.-Y. Composition-tunable Alloyed Semiconductor Nanocrystals. Acc. Chem. Res. 2010, 43, 621–630; https://doi.org/10.1021/ar900242r.Suche in Google Scholar PubMed
151. Mavrikakis, M.; Barteau, M. A. Oxygenate Reaction Pathways on Transition Metal Surfaces. J. Mol. Catal. A: Chem. 1998, 131, 135–147; https://doi.org/10.1016/s1381-1169(97)00261-6.Suche in Google Scholar
152. Walker, J. M.; Akbar, S. A.; Morris, P. A. Synergistic Effects in Gas Sensing Semiconducting Oxide Nano-Heterostructures: A Review. Sensor. Actuator. B Chem. 2019, 286, 624–640; https://doi.org/10.1016/j.snb.2019.01.049.Suche in Google Scholar
153. Zecchina, A.; Bordiga, S.; Groppo, E. Selective Nanocatalysts and Nanoscience: Concepts for Heterogeneous and Homogeneous Catalysis; John Wiley & Sons, 2011.10.1002/9783527635689Suche in Google Scholar
154. Zuliani, A.; Ivars, F.; Luque, R. Advances in Nanocatalyst Design for Biofuel Production. ChemCatChem 2018, 10, 1968–1981; https://doi.org/10.1002/cctc.201701712.Suche in Google Scholar
155. Nasrollahzadeh, M.; Sajjadi, M.; Shokouhimehr, M.; Varma, R. S. Recent Developments in Palladium (Nano) Catalysts Supported on Polymers for Selective and Sustainable Oxidation Processes. Coord. Chem. Rev. 2019, 397, 54–75; https://doi.org/10.1016/j.ccr.2019.06.010.Suche in Google Scholar
156. Shylesh, S.; Schünemann, V.; Thiel, W. R. Magnetically Separable Nanocatalysts: Bridges between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2010, 49, 3428–3459; https://doi.org/10.1002/anie.200905684.Suche in Google Scholar PubMed
157. Ali, S.; Fu Liu, T.; Lian, Z.; Li, B.; Sheng Su, D. The Effect of Defects on the Catalytic Activity of Single Au Atom Supported Carbon Nanotubes and Reaction Mechanism for CO Oxidation. Phys. Chem. Chem. Phys. 2017, 19, 22344–22354; https://doi.org/10.1039/c7cp03793g.Suche in Google Scholar PubMed
158. Song, J.; Liu, T.; Ali, S.; Li, B.; Su, D. The Synergy Effect and Reaction Pathway in the Oxygen Reduction Reaction on the Sulfur and Nitrogen Dual Doped Graphene Catalyst. Chem. Phys. Lett. 2017, 677, 65–69; https://doi.org/10.1016/j.cplett.2017.03.088.Suche in Google Scholar
159. Liu, T.; Ali, S.; Lian, Z.; Li, B.; Su, D. S. CO2 Electoreduction Reaction on Heteroatom-Doped Carbon Cathode Materials. J. Mater. Chem. A 2017, 5, 21596–21603; https://doi.org/10.1039/c7ta06674k.Suche in Google Scholar
160. Liu, T.; Ali, S.; Li, B.; Su, D. S. Revealing the Role of Sp2@sp3 Structure of Nanodiamond in Direct Dehydrogenation: Insight from DFT Study. ACS Catal. 2017, 7, 3779–3785; https://doi.org/10.1021/acscatal.6b03619.Suche in Google Scholar
161. Sun, X.; Han, P.; Li, B.; Mao, S.; Liu, T.; Ali, S.; Lian, Z.; Su, D. Oxidative Dehydrogenation Reaction of Short Alkanes on Nanostructured Carbon Catalysts: a Computational Account. Chem. Commun. 2018, 54, 864–875; https://doi.org/10.1039/c7cc06941c.Suche in Google Scholar PubMed
162. Ali, S.; Liu, T.; Lian, Z.; Sheng Su, D.; Li, B. The stability and reactivity of transition metal atoms supported mono and di vacancies defected carbon based materials revealed from first principles study. Appl. Surf. Sci. 2019, 473, 777–784; https://doi.org/10.1016/j.apsusc.2018.12.153.Suche in Google Scholar
163. Ali, S.; Olanrele, S.; Liu, T.; Lian, Z.; Si, C.; Yang, M.; Li, B. Single Au Anion Can Catalyze Acetylene Hydrochlorination: Tunable Catalytic Performance from Rational Doping. J. Phys. Chem. C 2019, 123, 29203–29208; https://doi.org/10.1021/acs.jpcc.9b07557.Suche in Google Scholar
164. Ali, S.; Qiu, Y.; Lian, Z.; Olanrele, S.; Lan, G.; Li, Y.; Su, D. S.; Li, B. Screening of Active Center and Reactivity Descriptor in Acetylene Hydrochlorination on Metal-free Doped Carbon Catalysts from First Principle Calculations. Appl. Surf. Sci. 2019, 478, 574–580; https://doi.org/10.1016/j.apsusc.2019.02.007.Suche in Google Scholar
165. Qiu, Y.; Ali, S.; Lan, G.; Tong, H.; Fan, J.; Liu, H.; Li, B.; Han, W.; Tang, H.; Liu, H.; Li, Y. Defect-rich Activated Carbons as Active and Stable Metal-free Catalyst for Acetylene Hydrochlorination. Carbon 2019, 146, 406–412; https://doi.org/10.1016/j.carbon.2019.01.102.Suche in Google Scholar
166. Ali, S.; Khan, M. B. A.; Khan, S. A.; Noora Noora, the Catalytic Performance of Metal-free Defected Carbon Catalyst towards Acetylene Hydrochlorination Revealed from First-Principles Calculation. Int. J. Quant. Chem. 2020, 120, e26418; https://doi.org/10.1002/qua.26418.Suche in Google Scholar
167. Ali, S.; Lian, Z.; Li, B. Density Functional Theory Study of a Graphdiyne-Supported Single Au Atom Catalyst for Highly Efficient Acetylene Hydrochlorination. ACS Appl. Nano Mater. 2021, 4, 6152–6159; https://doi.org/10.1021/acsanm.1c00945.Suche in Google Scholar
168. Zhao, K.; Wang, X.; He, D.; Wang, H.; Qian, B.; Shi, F. Recent Development towards Alkene Hydroformylation Catalysts Integrating Traditional Homo-And Heterogeneous Catalysis. Catal. Sci. Technol. 2022, 12, 4962–4982; https://doi.org/10.1039/d2cy00845a.Suche in Google Scholar
169. Zhang, J.; Wang, H.; Wang, L.; Ali, S.; Wang, C.; Wang, L.; Meng, X.; Li, B.; Su, D. S.; Xiao, F.-S. Wet-Chemistry Strong Metal–Support Interactions in Titania-Supported Au Catalysts. J. Am. Chem. Soc. 2019, 141, 2975–2983; https://doi.org/10.1021/jacs.8b10864.Suche in Google Scholar PubMed
170. Shokouhimehr, M. Magnetically Separable and Sustainable Nanostructured Catalysts for Heterogeneous Reduction of Nitroaromatics. Catalysts 2015, 5, 534–560; https://doi.org/10.3390/catal5020534.Suche in Google Scholar
171. Yu, S.; Liu, B.; Wang, Q.; Gao, Y.; Shi, Y.; Feng, X.; An, X.; Liu, L.; Zhang, J. Ionic Liquid Assisted Chemical Strategy to TiO2 Hollow Nanocube Assemblies with Surface-Fluorination and Nitridation and High Energy Crystal Facet Exposure for Enhanced Photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 10283–10295; https://doi.org/10.1021/am5016809.Suche in Google Scholar PubMed
172. Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412; https://doi.org/10.1021/acs.chemrev.8b00672.Suche in Google Scholar PubMed
173. von Hippel, P. H.; Berg, O. G. Facilitated Target Location in Biological Systems. J. Biol. Chem. 1989, 264, 675–678; https://doi.org/10.1016/s0021-9258(19)84994-3.Suche in Google Scholar
174. Busto, E.; Gotor-Fernández, V.; Gotor, V. Hydrolases in the Stereoselective Synthesis of N-Heterocyclic Amines and Amino Acid Derivatives. Chem. Rev. 2011, 111, 3998–4035; https://doi.org/10.1021/cr100287w.Suche in Google Scholar PubMed
175. Bilal, M.; Khaliq, N.; Ashraf, M.; Hussain, N.; Baqar, Z.; Zdarta, J.; Jesionowski, T.; Iqbal, H. M. Enzyme Mimic Nanomaterials as Nanozymes with Catalytic and Multifunctional Attributes. Colloids Surf. B Biointerfaces 2022, 112950; https://doi.org/10.1016/j.colsurfb.2022.112950.Suche in Google Scholar PubMed
176. Gu, C.; Kong, X.; Yan, S.; Gai, P.; Li, F. Glucose Dehydrogenase-like Nanozyme Based on Black Phosphorus Nanosheets for High-Performance Biofuel Cells. ACS Sustain. Chem. Eng. 2020, 8, 16549–16554; https://doi.org/10.1021/acssuschemeng.0c05743.Suche in Google Scholar
177. Alizadeh, N.; Salimi, A. Multienzymes Activity of Metals and Metal Oxide Nanomaterials: Applications from Biotechnology to Medicine and Environmental Engineering. J. Nanobiotechnol. 2021, 19, 1–31; https://doi.org/10.1186/s12951-021-00771-1.Suche in Google Scholar PubMed PubMed Central
178. Daghrir, R.; Drogui, P.; Robert, D. Photoelectrocatalytic Technologies for Environmental Applications. J. Photochem. Photobiol. Chem. 2012, 238, 41–52; https://doi.org/10.1016/j.jphotochem.2012.04.009.Suche in Google Scholar
179. Kimmel, Y. C.; Xu, X.; Yu, W.; Yang, X.; Chen, J. G. Trends in Electrochemical Stability of Transition Metal Carbides and Their Potential Use as Supports for Low-Cost Electrocatalysts. ACS Catal. 2014, 4, 1558–1562; https://doi.org/10.1021/cs500182h.Suche in Google Scholar
180. Hou, S.; Kluge, R. M.; Haid, R. W.; Gubanova, E. L.; Watzele, S. A.; Bandarenka, A. S.; Garlyyev, B. A Review on Experimental Identification of Active Sites in Model Bifunctional Electrocatalytic Systems for Oxygen Reduction and Evolution Reactions. Chemelectrochem 2021, 8, 3433–3456; https://doi.org/10.1002/celc.202100584.Suche in Google Scholar
181. Garcia-Segura, S.; Lanzarini-Lopes, M.; Hristovski, K.; Westerhoff, P. Electrocatalytic Reduction of Nitrate: Fundamentals to Full-Scale Water Treatment Applications. Appl. Catal. B Environ. 2018, 236, 546–568; https://doi.org/10.1016/j.apcatb.2018.05.041.Suche in Google Scholar
182. Vogt, C.; Weckhuysen, B. M. The Concept of Active Site in Heterogeneous Catalysis. Nat. Rev. Chem 2022, 6, 89–111; https://doi.org/10.1038/s41570-021-00340-y.Suche in Google Scholar PubMed
183. Giulimondi, V.; Mitchell, S.; Pérez-Ramírez, J. Challenges and Opportunities in Engineering the Electronic Structure of Single-Atom Catalysts. ACS Catal. 2023, 13, 2981–2997; https://doi.org/10.1021/acscatal.2c05992.Suche in Google Scholar PubMed PubMed Central
184. Chen, D.; Zhu, J.; Pu, Z.; Mu, S. Anion Modulation of Pt-group Metals and Electrocatalysis Applications. Chem.-Eur. J. 2021, 27, 12257–12271; https://doi.org/10.1002/chem.202101645.Suche in Google Scholar PubMed
185. Kim, C.; Jeon, H. S.; Eom, T.; Jee, M. S.; Kim, H.; Friend, C. M.; Min, B. K.; Hwang, Y. J. Achieving Selective and Efficient Electrocatalytic Activity for CO2 Reduction Using Immobilized Silver Nanoparticles. J. Am. Chem. Soc. 2015, 137, 13844–13850; https://doi.org/10.1021/jacs.5b06568.Suche in Google Scholar PubMed
186. Zhang, X.; Sun, X.; Guo, S.-X.; Bond, A. M.; Zhang, J. Formation of Lattice-Dislocated Bismuth Nanowires on Copper Foam for Enhanced Electrocatalytic CO 2 Reduction at Low Overpotential. Energy Environ. Sci. 2019, 12, 1334–1340; https://doi.org/10.1039/c9ee00018f.Suche in Google Scholar
187. Paulus, U.; Wokaun, A.; Scherer, G.; Schmidt, T.; Stamenkovic, V.; Markovic, N. M.; Ross, P. Oxygen Reduction on High Surface Area Pt-Based Alloy Catalysts in Comparison to Well Defined Smooth Bulk Alloy Electrodes. Electrochim. Acta 2002, 47, 3787–3798; https://doi.org/10.1016/s0013-4686(02)00349-3.Suche in Google Scholar
188. Liang, F.; Zhang, K.; Zhang, L.; Zhang, Y.; Lei, Y.; Sun, X. Recent Development of Electrocatalytic CO2 Reduction Application to Energy Conversion. Small 2021, 17, 2100323; https://doi.org/10.1002/smll.202100323.Suche in Google Scholar PubMed
189. Garlyyev, B.; Fichtner, J.; Piqué, O.; Schneider, O.; Bandarenka, A. S.; Calle-Vallejo, F. Revealing the Nature of Active Sites in Electrocatalysis. Chem. Sci. 2019, 10, 8060–8075; https://doi.org/10.1039/c9sc02654a.Suche in Google Scholar PubMed PubMed Central
190. Tian, G. L.; Zhang, Q.; Zhang, B.; Jin, Y. G.; Huang, J. Q.; Su, D. S.; Wei, F. Toward Full Exposure of “Active Sites”: Nanocarbon Electrocatalyst with Surface Enriched Nitrogen for Superior Oxygen Reduction and Evolution Reactivity. Adv. Funct. Mater. 2014, 24, 5956–5961; https://doi.org/10.1002/adfm.201401264.Suche in Google Scholar
191. Hu, Y.; Wu, P.; Yin, Y.; Zhang, H.; Cai, C. Effects of Structure, Composition, and Carbon Support Properties on the Electrocatalytic Activity of Pt-Ni-Graphene Nanocatalysts for the Methanol Oxidation. Appl. Catal. B Environ. 2012, 111, 208–217; https://doi.org/10.1016/j.apcatb.2011.10.001.Suche in Google Scholar
192. Wang, Y.-J.; Zhao, N.; Fang, B.; Li, H.; Bi, X. T.; Wang, H. Carbon-supported Pt-Based Alloy Electrocatalysts for the Oxygen Reduction Reaction in Polymer Electrolyte Membrane Fuel Cells: Particle Size, Shape, and Composition Manipulation and Their Impact to Activity. Chem. Rev. 2015, 115, 3433–3467; https://doi.org/10.1021/cr500519c.Suche in Google Scholar PubMed
193. Choi, S.-I.; Lee, S.-U.; Kim, W. Y.; Choi, R.; Hong, K.; Nam, K. M.; Han, S. W.; Park, J. T. Composition-controlled PtCo Alloy Nanocubes with Tuned Electrocatalytic Activity for Oxygen Reduction. ACS Appl. Mater. Interfaces 2012, 4, 6228–6234; https://doi.org/10.1021/am301824w.Suche in Google Scholar PubMed
194. Mistry, H.; Varela, A. S.; Kühl, S.; Strasser, P.; Cuenya, B. R. Nanostructured Electrocatalysts with Tunable Activity and Selectivity. Nat. Rev. Mater. 2016, 1, 1–14; https://doi.org/10.1038/natrevmats.2016.9.Suche in Google Scholar
195. Yang, B.; Ding, W.; Zhang, H.; Zhang, S. Recent Progress in Electrochemical Synthesis of Ammonia from Nitrogen: Strategies to Improve the Catalytic Activity and Selectivity. Energy Environ. Sci. 2021, 14, 672–687; https://doi.org/10.1039/d0ee02263b.Suche in Google Scholar
196. Lenne, Q.; Leroux, Y. R.; Lagrost, C. Surface Modification for Promoting Durable, Efficient, and Selective Electrocatalysts. Chemelectrochem 2020, 7, 2345–2363; https://doi.org/10.1002/celc.202000132.Suche in Google Scholar
197. Scaria, J.; Nidheesh, P.; Kumar, M. S. Synthesis and Applications of Various Bimetallic Nanomaterials in Water and Wastewater Treatment. J. Environ. Manag. 2020, 259, 110011; https://doi.org/10.1016/j.jenvman.2019.110011.Suche in Google Scholar PubMed
198. Costentin, C.; Savéant, J.-M. Towards an Intelligent Design of Molecular Electrocatalysts. Nat. Rev. Chem 2017, 1, 0087; https://doi.org/10.1038/s41570-017-0087.Suche in Google Scholar
199. Xiao, C.; Zhang, J. Architectural Design for Enhanced C2 Product Selectivity in Electrochemical CO2 Reduction Using Cu-Based Catalysts: a Review. ACS Nano 2021, 15, 7975–8000; https://doi.org/10.1021/acsnano.0c10697.Suche in Google Scholar PubMed
200. Aggarwal, P.; Sarkar, D.; Awasthi, K.; Menezes, P. W. Functional Role of Single-Atom Catalysts in Electrocatalytic Hydrogen Evolution: Current Developments and Future Challenges. Coord. Chem. Rev. 2022, 452, 214289; https://doi.org/10.1016/j.ccr.2021.214289.Suche in Google Scholar
201. Jin, S.; Hao, Z.; Zhang, K.; Yan, Z.; Chen, J. Advances and Challenges for the Electrochemical Reduction of CO2 to CO: from Fundamentals to Industrialization. Angew. Chem. 2021, 133, 20795–20816; https://doi.org/10.1002/ange.202101818.Suche in Google Scholar
202. Zheng, Y.; Vasileff, A.; Zhou, X.; Jiao, Y.; Jaroniec, M.; Qiao, S.-Z. Understanding the Roadmap for Electrochemical Reduction of CO2 to Multi-Carbon Oxygenates and Hydrocarbons on Copper-Based Catalysts. J. Am. Chem. Soc. 2019, 141, 7646–7659; https://doi.org/10.1021/jacs.9b02124.Suche in Google Scholar PubMed
203. Francke, R.; Little, R. D. Redox Catalysis in Organic Electrosynthesis: Basic Principles and Recent Developments. Chem. Soc. Rev. 2014, 43, 2492–2521; https://doi.org/10.1039/c3cs60464k.Suche in Google Scholar PubMed
204. Zhang, K.; Jin, B.; Park, C.; Cho, Y.; Song, X.; Shi, X.; Zhang, S.; Kim, W.; Zeng, H.; Park, J. H. Black Phosphorene as a Hole Extraction Layer Boosting Solar Water Splitting of Oxygen Evolution Catalysts. Nat. Commun. 2019, 10, 2001; https://doi.org/10.1038/s41467-019-10034-1.Suche in Google Scholar PubMed PubMed Central
205. Tufail, A.; Price, W. E.; Mohseni, M.; Pramanik, B. K.; Hai, F. I. A Critical Review of Advanced Oxidation Processes for Emerging Trace Organic Contaminant Degradation: Mechanisms, Factors, Degradation Products, and Effluent Toxicity. J. Water Proc. Eng. 2021, 40, 101778; https://doi.org/10.1016/j.jwpe.2020.101778.Suche in Google Scholar
206. Noor, T.; Yaqoob, L.; Iqbal, N. Recent Advances in Electrocatalysis of Oxygen Evolution Reaction Using Noble‐metal, Transition‐metal, and Carbon‐based Materials. Chemelectrochem 2021, 8, 447–483; https://doi.org/10.1002/celc.202001441.Suche in Google Scholar
207. Esposito, D. V.; Hunt, S. T.; Kimmel, Y. C.; Chen, J. G. A New Class of Electrocatalysts for Hydrogen Production from Water Electrolysis: Metal Monolayers Supported on Low-Cost Transition Metal Carbides. J. Am. Chem. Soc. 2012, 134, 3025–3033; https://doi.org/10.1021/ja208656v.Suche in Google Scholar PubMed
208. Li, H.; Lai, J.; Li, Z.; Wang, L. Multi-sites Electrocatalysis in High‐entropy Alloys. Adv. Funct. Mater. 2021, 31, 2106715; https://doi.org/10.1002/adfm.202106715.Suche in Google Scholar
209. Scofield, M. E.; Liu, H.; Wong, S. S. A Concise Guide to Sustainable PEMFCs: Recent Advances in Improving Both Oxygen Reduction Catalysts and Proton Exchange Membranes. Chem. Soc. Rev. 2015, 44, 5836–5860; https://doi.org/10.1039/c5cs00302d.Suche in Google Scholar PubMed
210. Zhang, J.; Li, C. M. Nanoporous Metals: Fabrication Strategies and Advanced Electrochemical Applications in Catalysis, Sensing and Energy Systems. Chem. Soc. Rev. 2012, 41, 7016–7031; https://doi.org/10.1039/c2cs35210a.Suche in Google Scholar PubMed
211. Fan, Y.; Wu, Y.; Clavel, G.; Raza, M. H.; Amsalem, P.; Koch, N.; Pinna, N. Optimization of the Activity of Ni-Based Nanostructures for the Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2018, 1, 4554–4563; https://doi.org/10.1021/acsaem.8b00666.Suche in Google Scholar
212. Zheng, X.; Li, B.; Wang, Q.; Wang, D.; Li, Y. Emerging Low-Nuclearity Supported Metal Catalysts with Atomic Level Precision for Efficient Heterogeneous Catalysis. Nano Res. 2022, 15, 7806–7839; https://doi.org/10.1007/s12274-022-4429-9.Suche in Google Scholar
213. Xue, Q.; Wang, Z.; Ding, Y.; Li, F.; Chen, Y. Chemical Functionalized Noble Metal Nanocrystals for Electrocatalysis. Chin. J. Catal. 2023, 45, 6–16; https://doi.org/10.1016/s1872-2067(22)64186-x.Suche in Google Scholar
214. Xu, G.-R.; Bai, J.; Yao, L.; Xue, Q.; Jiang, J.-X.; Zeng, J.-H.; Chen, Y.; Lee, J.-M. Polyallylamine-functionalized Platinum Tripods: Enhancement of Hydrogen Evolution Reaction by Proton Carriers. ACS Catal. 2017, 7, 452–458; https://doi.org/10.1021/acscatal.6b03049.Suche in Google Scholar
215. Fu, G.; Zhang, Q.; Wu, J.; Sun, D.; Xu, L.; Tang, Y.; Chen, Y. Arginine-mediated Synthesis of Cube-like Platinum Nanoassemblies as Efficient Electrocatalysts. Nano Res. 2015, 8, 3963–3971; https://doi.org/10.1007/s12274-015-0899-3.Suche in Google Scholar
216. Ge, Z. X.; Wang, T. J.; Ding, Y.; Yin, S. B.; Li, F. M.; Chen, P.; Chen, Y. Interfacial Engineering Enhances the Electroactivity of Frame-like Concave RhCu Bimetallic Nanocubes for Nitrate Reduction. Adv. Energy Mater. 2022, 12, 2103916; https://doi.org/10.1002/aenm.202103916.Suche in Google Scholar
217. Bai, J.; Xu, G.-R.; Xing, S.-H.; Zeng, J.-H.; Jiang, J.-X.; Chen, Y. Hydrothermal Synthesis and Catalytic Application of Ultrathin Rhodium Nanosheet Nanoassemblies. ACS Appl. Mater. Interfaces 2016, 8, 33635–33641; https://doi.org/10.1021/acsami.6b11210.Suche in Google Scholar PubMed
218. Xu, G.-R.; Han, S.-H.; Liu, Z.-H.; Chen, Y. The Chemical Functionalized Platinum Nanodendrites: The Effect of Chemical Molecular Weight on Electrocatalytic Property. J. Power Sources 2016, 306, 587–592; https://doi.org/10.1016/j.jpowsour.2015.12.039.Suche in Google Scholar
219. Guo, Q.; Ma, Z.; Zhou, C.; Ren, Z.; Yang, X. Single Molecule Photocatalysis on TiO2 Surfaces: Focus Review. Chem. Rev. 2019, 119, 11020–11041; https://doi.org/10.1021/acs.chemrev.9b00226.Suche in Google Scholar PubMed
220. Li, X.; Yu, J.; Jaroniec, M. Hierarchical Photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636; https://doi.org/10.1039/c5cs00838g.Suche in Google Scholar PubMed
221. Sayed, M.; Xu, F.; Kuang, P.; Low, J.; Wang, S.; Zhang, L.; Yu, J. Sustained CO2-photoreduction Activity and High Selectivity over Mn, C-Codoped ZnO Core-Triple Shell Hollow Spheres. Nat. Commun. 2021, 12, 4936; https://doi.org/10.1038/s41467-021-25007-6.Suche in Google Scholar PubMed PubMed Central
222. Low, J.; Cheng, B.; Yu, J.; Jaroniec, M. Carbon-based Two-Dimensional Layered Materials for Photocatalytic CO2 Reduction to Solar Fuels. Energy Storage Mater. 2016, 3, 24–35; https://doi.org/10.1016/j.ensm.2015.12.003.Suche in Google Scholar
223. Jiangtian, L.; Fanke, M.; Savan, S.; Mingjia, Z.; Ming, Z.; Nianqiang, W.; Li, M.; Bristow, A. D.; Wu, N. Photocatalytic Activity Enhanced by Plasmonic Resonant Energy Transfer from Metal to Semiconductor 2012; https://doi.org/10.1021/ja305603t.Suche in Google Scholar PubMed
224. Sayed, M.; Yu, J.; Liu, G.; Jaroniec, M. Non-noble Plasmonic Metal-Based Photocatalysts. Chem. Rev. 2022, 122, 10484–10537; https://doi.org/10.1021/acs.chemrev.1c00473.Suche in Google Scholar PubMed
225. Du, C.; Gao, X.; Chen, W. Recent Developments in Copper-Based, Non-noble Metal Electrocatalysts for the Oxygen Reduction Reaction. Chin. J. Catal. 2016, 37, 1049–1061; https://doi.org/10.1016/s1872-2067(15)61059-2.Suche in Google Scholar
226. Ribeiro, F. W. P.; de Souza Lucas, F. W.; Mascaro, L. H.; Morais, S.; da Silva Casciano, P. N.; de Lima-Neto, P.; Correia, A. N. Electroanalysis of Formetanate Hydrochloride by a Cobalt Phthalocyanine Functionalized Multiwalled Carbon Nanotubes Modified Electrode: Characterization and Application in Fruits. Electrochim. Acta 2016, 194, 187–198; https://doi.org/10.1016/j.electacta.2016.02.086.Suche in Google Scholar
227. Tang, H.; Hessel, C. M.; Wang, J.; Yang, N.; Yu, R.; Zhao, H.; Wang, D. Two-dimensional Carbon Leading to New Photoconversion Processes. Chem. Soc. Rev. 2014, 43, 4281–4299; https://doi.org/10.1039/c3cs60437c.Suche in Google Scholar PubMed
228. Centi, G.; Perathoner, S. Carbon Nanotubes for Sustainable Energy Applications. ChemSusChem 2011, 4, 913–925; https://doi.org/10.1002/cssc.201100084.Suche in Google Scholar PubMed
229. Cheng, X.; Guo, H.; Li, W.; Yang, B.; Wang, J.; Zhang, Y.; Du, E. Metal-free Carbocatalysis for Persulfate Activation toward Nonradical Oxidation: Enhanced Singlet Oxygen Generation Based on Active Sites and Electronic Property. Chem. Eng. J. 2020, 396, 125107; https://doi.org/10.1016/j.cej.2020.125107.Suche in Google Scholar
230. Wei, Q.; Tong, X.; Zhang, G.; Qiao, J.; Gong, Q.; Sun, S. Nitrogen-Doped Carbon Nanotube and Graphene Materials for Oxygen Reduction Reactions. Catalysts 2015, 5, 1574–1602; https://doi.org/10.3390/catal5031574.Suche in Google Scholar
231. Han, C.; Li, Y.-H.; Qi, M.-Y.; Zhang, F.; Tang, Z.-R.; Xu, Y.-J. Surface/Interface Engineering of Carbon‐Based Materials for Constructing Multidimensional Functional Hybrids. Sol. RRL 2020, 4, 1900577; https://doi.org/10.1002/solr.202070081.Suche in Google Scholar
232. Yang, N.; Waldvogel, S. R.; Jiang, X. Electrochemistry of Carbon Dioxide on Carbon Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 28357–28371; https://doi.org/10.1021/acsami.5b09825.Suche in Google Scholar PubMed
233. Jia, C.; Dastafkan, K.; Ren, W.; Yang, W.; Zhao, C. Carbon-based Catalysts for Electrochemical CO 2 Reduction. Sustain. Energy Fuels 2019, 3, 2890–2906; https://doi.org/10.1039/C9SE00527G.Suche in Google Scholar
234. Zhao, K.; Quan, X. Carbon-based Materials for Electrochemical Reduction of CO2 to C2+ Oxygenates: Recent Progress and Remaining Challenges. ACS Catal. 2021, 11, 2076–2097; https://doi.org/10.1021/acscatal.0c04714.Suche in Google Scholar
235. Duan, X.; Xu, J.; Wei, Z.; Ma, J.; Guo, S.; Wang, S.; Liu, H.; Dou, S. Metal‐free Carbon Materials for CO2 Electrochemical Reduction. Adv. Mater. 2017, 29, 1701784; https://doi.org/10.1002/adma.201701784.Suche in Google Scholar PubMed
236. Liu, X.; Dai, L. Carbon-based Metal-free Catalysts. Nat. Rev. Mater. 2016, 1, 1–12; https://doi.org/10.1038/natrevmats.2016.64.Suche in Google Scholar
237. Genovese, C.; Ampelli, C.; Perathoner, S.; Centi, G. Electrocatalytic Conversion of CO2 to Liquid Fuels Using Nanocarbon-Based Electrodes. J. Energy Chem. 2013, 22, 202–213; https://doi.org/10.1016/s2095-4956(13)60026-1.Suche in Google Scholar
238. Marepally, B. C.; Ampelli, C.; Genovese, C.; Tavella, F.; Veyre, L.; Quadrelli, E. A.; Perathoner, S.; Centi, G. Role of Small Cu Nanoparticles in the Behaviour of Nanocarbon-Based Electrodes for the Electrocatalytic Reduction of CO2. J. CO2 Util. 2017, 21, 534–542; https://doi.org/10.1016/j.jcou.2017.08.008.Suche in Google Scholar
239. Zhu, X.; Li, Y. Review of Two-dimensional Materials for Electrochemical CO2 Reduction from a Theoretical Perspective. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2019, 9, e1416; https://doi.org/10.1002/wcms.1416.Suche in Google Scholar
240. Zhao, K.; Nie, X.; Wang, H.; Chen, S.; Quan, X.; Yu, H.; Choi, W.; Zhang, G.; Kim, B.; Chen, J. G. Selective Electroreduction of CO2 to Acetone by Single Copper Atoms Anchored on N-Doped Porous Carbon. Nat. Commun. 2020, 11, 2455; https://doi.org/10.1038/s41467-020-16381-8.Suche in Google Scholar PubMed PubMed Central
241. Zou, X.; Liu, M.; Wu, J.; Ajayan, P. M.; Li, J.; Liu, B.; Yakobson, B. I. How Nitrogen-Doped Graphene Quantum Dots Catalyze Electroreduction of CO2 to Hydrocarbons and Oxygenates. ACS Catal. 2017, 7, 6245–6250; https://doi.org/10.1021/acscatal.7b01839.Suche in Google Scholar
242. Duan, X.; Tian, W.; Zhang, H.; Sun, H.; Ao, Z.; Shao, Z.; Wang, S. sp2/sp3 Framework from Diamond Nanocrystals: a Key Bridge of Carbonaceous Structure to Carbocatalysis. ACS Catal. 2019, 9, 7494–7519; https://doi.org/10.1021/acscatal.9b01565.Suche in Google Scholar
243. Liu, Y.; Chen, S.; Quan, X.; Yu, H. Efficient Electrochemical Reduction of Carbon Dioxide to Acetate on Nitrogen-Doped Nanodiamond. J. Am. Chem. Soc. 2015, 137, 11631–11636; https://doi.org/10.1021/jacs.5b02975.Suche in Google Scholar PubMed
244. Liu, Y.; Zhang, Y.; Cheng, K.; Quan, X.; Fan, X.; Su, Y.; Chen, S.; Zhao, H.; Zhang, Y.; Yu, H.; Hoffmann, M. R. Selective Electrochemical Reduction of Carbon Dioxide to Ethanol on a Boron‐and nitrogen‐Co‐doped Nanodiamond. Angew. Chem. 2017, 129, 15813–15817; https://doi.org/10.1002/ange.201706311.Suche in Google Scholar
245. Zhao, S.; Lu, X.; Wang, L.; Gale, J.; Amal, R. Carbon-based Metal-free Catalysts for Electrocatalytic Reduction of Nitrogen for Synthesis of Ammonia at Ambient Conditions. Adv. Mater. 2019, 31, 1805367; https://doi.org/10.1002/adma.201805367.Suche in Google Scholar PubMed
246. Song, Y.; Chen, W.; Zhao, C.; Li, S.; Wei, W.; Sun, Y. Metal-free Nitrogen-doped Mesoporous Carbon for Electroreduction of CO2 to Ethanol. Angew. Chem. Int. Ed. 2017, 56, 10840–10844; https://doi.org/10.1002/anie.201706777.Suche in Google Scholar PubMed
247. Song, Y.; Wang, S.; Chen, W.; Li, S.; Feng, G.; Wei, W.; Sun, Y. Enhanced Ethanol Production from CO2 Electroreduction at Micropores in Nitrogen-doped Mesoporous Carbon. ChemSusChem 2020, 13, 293–297; https://doi.org/10.1002/cssc.201902833.Suche in Google Scholar PubMed
248. Baturina, O. A.; Lu, Q.; Padilla, M. A.; Xin, L.; Li, W.; Serov, A.; Artyushkova, K.; Atanassov, P.; Xu, F.; Epshteyn, A.; Brintlinger, T.; Schuette, M.; Collins, G. E. CO2 Electroreduction to Hydrocarbons on Carbon-Supported Cu Nanoparticles. ACS Catal. 2014, 4, 3682–3695; https://doi.org/10.1021/cs500537y.Suche in Google Scholar
249. Mitchell, S.; Vorobyeva, E.; Pérez-Ramírez, J. The Multifaceted Reactivity of Single‐atom Heterogeneous Catalysts. Angew. Chem. Int. Ed. 2018, 57, 15316–15329; https://doi.org/10.1002/anie.201806936.Suche in Google Scholar PubMed
250. Xu, L.; Li, C.-g.; Zhang, K.; Wu, P. Bifunctional Tandem Catalysis on Multilamellar Organic–Inorganic Hybrid Zeolites. ACS Catal. 2014, 4, 2959–2968; https://doi.org/10.1021/cs500653p.Suche in Google Scholar
251. Li, M.; Huang, H.; Low, J.; Gao, C.; Long, R.; Xiong, Y. Recent Progress on Electrocatalyst and Photocatalyst Design for Nitrogen Reduction. Small Methods 2019, 3, 1800388; https://doi.org/10.1002/smtd.201800388.Suche in Google Scholar
252. Goldsmith, B. R.; Esterhuizen, J.; Liu, J. X.; Bartel, C. J.; Sutton, C. Machine Learning for Heterogeneous Catalyst Design and Discovery. AIChE 2018, 64, 2311–2323; https://doi.org/10.1002/aic.16198.Suche in Google Scholar
253. Gibertini, M.; Koperski, M.; Morpurgo, A. F.; Novoselov, K. S. Magnetic 2D Materials and Heterostructures. Nat. Nanotechnol. 2019, 14, 408–419; https://doi.org/10.1038/s41565-019-0438-6.Suche in Google Scholar PubMed
254. Xu, W.; Jiao, L.; Wu, Y.; Hu, L.; Gu, W.; Zhu, C. Metal–organic Frameworks Enhance Biomimetic Cascade Catalysis for Biosensing. Adv. Mater. 2021, 33, 2005172; https://doi.org/10.1002/adma.202005172.Suche in Google Scholar PubMed
255. Vlatković, M.; Collins, B. S.; Feringa, B. L. Dynamic Responsive Systems for Catalytic Function. Chem.--Eur. J. 2016, 22, 17080–17111; https://doi.org/10.1002/chem.201602453.Suche in Google Scholar PubMed
256. Chee, S. W.; Lunkenbein, T.; Schlögl, R.; Cuenya, B. R. In Situ And Operando Electron Microscopy in Heterogeneous Catalysis – Insights into Multi-Scale Chemical Dynamics. J. Phys. Condens. Matter 2021, 33, 153001; https://doi.org/10.1088/1361-648x/abddfd.Suche in Google Scholar
257. Paul, R.; Zhu, L.; Chen, H.; Qu, J.; Dai, L. Recent Advances in Carbon‐based Metal‐free Electrocatalysts. Adv. Mater. 2019, 31, 1806403; https://doi.org/10.1002/adma.201806403.Suche in Google Scholar PubMed
258. Köhler, M.; Fritzsche, W. Nanotechnology: An Introduction to Nanostructuring Techniques; John Wiley & Sons: Weinheim, 2008.10.1002/9783527621132Suche in Google Scholar
259. Tomalia, D. A. Birth of a New Macromolecular Architecture: Dendrimers as Quantized Building Blocks for Nanoscale Synthetic Polymer Chemistry. Prog. Polym. Sci. 2005, 30, 294–324; https://doi.org/10.1016/j.progpolymsci.2005.01.007.Suche in Google Scholar
260. Cerro-Lopez, M.; Méndez-Rojas, M. A. Application of Nanomaterials for Treatment of Wastewater Containing Pharmaceuticals, Ecopharmacovigilance; Springer, Cham: Mexico, 2017; pp 201–219.10.1007/698_2017_143Suche in Google Scholar
261. El-Naggar, M. E.; Shoueir, K. Recent Advances in Polymer/metal/metal Oxide Hybrid Nanostructures for Catalytic Applications: A Review. J. Environ. Chem. Eng. 2020, 8, 104175; https://doi.org/10.1016/j.jece.2020.104175.Suche in Google Scholar
262. Sajanlal, P. R.; Sreeprasad, T. S.; Samal, A. K.; Pradeep, T. Anisotropic Nanomaterials: Structure, Growth, Assembly, and Functions. Nano Rev. 2011, 2, 5883; https://doi.org/10.3402/nano.v2i0.5883.Suche in Google Scholar PubMed PubMed Central
263. Zhang, X.; Li, L.; Sun, Z.; Luo, J. Rational Chemical Doping of Metal Halide Perovskites. Chem. Soc. Rev. 2019, 48, 517–539; https://doi.org/10.1039/c8cs00563j.Suche in Google Scholar PubMed
264. Wang, H.; Bian, Y.; Hu, J.; Dai, L. Highly Crystalline Sulfur-Doped Carbon Nitride as Photocatalyst for Efficient Visible-Light Hydrogen Generation. Appl. Catal. B Environ. 2018, 238, 592–598; https://doi.org/10.1016/j.apcatb.2018.07.023.Suche in Google Scholar
265. Liu, J.; Han, L.; An, N.; Xing, L.; Ma, H.; Cheng, L.; Yang, J.; Zhang, Q. Enhanced Visible-Light Photocatalytic Activity of Carbonate-Doped Anatase TiO2 Based on the Electron-Withdrawing Bidentate Carboxylate Linkage. Appl. Catal. B Environ. 2017, 202, 642–652; https://doi.org/10.1016/j.apcatb.2016.09.057.Suche in Google Scholar
266. Xiao, Y.; Cheng, N.; Kondamareddy, K. K.; Wang, C.; Liu, P.; Guo, S.; Zhao, X.-Z. W-Doped TiO2 Mesoporous Electron Transport Layer for Efficient Hole Transport Material Free Perovskite Solar Cells Employing Carbon Counter Electrodes. J. Power Sources 2017, 342, 489–494; https://doi.org/10.1016/j.jpowsour.2016.12.079.Suche in Google Scholar
267. Lindgren, T.; Mwabora, J. M.; Avendaño, E.; Jonsson, J.; Hoel, A.; Granqvist, C.-G.; Lindquist, S.-E. Photoelectrochemical and Optical Properties of Nitrogen Doped Titanium Dioxide Films Prepared by Reactive DC Magnetron Sputtering. J. Phys. Chem. B 2003, 107, 5709–5716; https://doi.org/10.1021/jp027345j.Suche in Google Scholar
268. Wang, L.; Li, D.-B.; Li, K.; Chen, C.; Deng, H.-X.; Gao, L.; Zhao, Y.; Jiang, F.; Li, L.; Huang, F.; He, Y.; Song, H.; Niu, G.; Tang, J. Stable 6%-efficient Sb2Se3 Solar Cells with a ZnO Buffer Layer. Nat. Energy 2017, 2, 1–9; https://doi.org/10.1038/nenergy.2017.46.Suche in Google Scholar
269. Low, W.; Boonamnuayvitaya, V. Enhancing the Photocatalytic Activity of TiO2 Co-doping of Graphene–Fe3+ Ions for Formaldehyde Removal. J. Environ. Manag. 2013, 127, 142–149; https://doi.org/10.1016/j.jenvman.2013.04.029.Suche in Google Scholar PubMed
270. Zhang, H.; Xing, Z.; Zhang, Y.; Li, Z.; Wu, X.; Liu, C.; Zhu, Q.; Zhou, W. Ni 2+ and Ti 3+ Co-doped Porous Black Anatase TiO 2 with Unprecedented-High Visible-Light-Driven Photocatalytic Degradation Performance. RSC Adv. 2015, 5, 107150–107157; https://doi.org/10.1039/c5ra23743b.Suche in Google Scholar
271. Sun, Y.; Xiong, T.; Dong, F.; Huang, H.; Cen, W. Interlayer-I-doped BiOIO 3 Nanoplates with an Optimized Electronic Structure for Efficient Visible Light Photocatalysis. Chem. Commun. 2016, 52, 8243–8246; https://doi.org/10.1039/c6cc03630a.Suche in Google Scholar PubMed
272. Zhang, J.; Xia, Z.; Dai, L. Carbon-based Electrocatalysts for Advanced Energy Conversion and Storage. Sci. Adv. 2015, 1, e1500564; https://doi.org/10.1126/sciadv.1500564.Suche in Google Scholar PubMed PubMed Central
273. Shoueir, K.; El-Sheshtawy, H.; Misbah, M.; El-Hosainy, H.; El-Mehasseb, I.; El-Kemary, M. Fenton-Like Nanocatalyst for Photodegradation of Methylene Blue under Visible Light Activated by Hybrid Green DNSA@ Chitosan@ MnFe2O4. Carbohydr. Polym. 2018, 197, 17–28; https://doi.org/10.1016/j.carbpol.2018.05.076.Suche in Google Scholar PubMed
274. Hameed, T. A.; Wassel, A. R.; El Radaf, I. Investigating the Effect of Thickness on the Structural, Morphological, Optical and Electrical Properties of AgBiSe2 Thin Films. J. Alloys Compd. 2019, 805, 1–11; https://doi.org/10.1016/j.jallcom.2019.07.041.Suche in Google Scholar
275. Navia-Mendoza, J. M.; Filho, O. A. E.; Zambrano-Intriago, L. A.; Maddela, N. R.; Duarte, M. M. M. B.; Quiroz-Fernández, L. S.; Baquerizo-Crespo, R. J.; Rodríguez-Díaz, J. M. Advances in the Application of Nanocatalysts in Photocatalytic Processes for the Treatment of Food Dyes: A Review. Sustainability 2021, 13, 11676; https://doi.org/10.3390/su132111676.Suche in Google Scholar
276. Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; Fu, Y. Advances in Designs and Mechanisms of Semiconducting Metal Oxide Nanostructures for High-Precision Gas Sensors Operated at Room Temperature. Mater. Horiz. 2019, 6, 470–506; https://doi.org/10.1039/c8mh01365a.Suche in Google Scholar
277. Luis, N.; Wang, J.-A. Advanced Catalytic Materials: Photocatalysis and Other Current Trends; BoD–Books on Demand: Croatia, 2016.Suche in Google Scholar
278. Zamri, M. S. F. A.; Sapawe, N. Performance Studies of Electrobiosynthesis of Titanium Dioxide Nanoparticles (TiO2) for Phenol Degradation. Mater. Today: Proc. 2018, 5, 21797–21801; https://doi.org/10.1016/j.matpr.2018.07.034.Suche in Google Scholar
279. Harra, J.; Mäkitalo, J.; Siikanen, R.; Virkki, M.; Genty, G.; Kobayashi, T.; Kauranen, M.; Mäkelä, J. M. Size-controlled Aerosol Synthesis of Silver Nanoparticles for Plasmonic Materials. J. Nanopart. Res. 2012, 14, 1–10; https://doi.org/10.1007/s11051-012-0870-0.Suche in Google Scholar PubMed PubMed Central
280. Paitandi, R. P.; Wan, Y.; Aftab, W.; Zhong, R.; Zou, R. Pristine Metal–Organic Frameworks and Their Composites for Renewable Hydrogen Energy Applications. Adv. Funct. Mater. 2023, 33, 2203224; https://doi.org/10.1002/adfm.202203224.Suche in Google Scholar
281. Gao, C.; Wang, J.; Xu, H.; Xiong, Y. Coordination Chemistry in the Design of Heterogeneous Photocatalysts. Chem. Soc. Rev. 2017, 46, 2799–2823; https://doi.org/10.1039/c6cs00727a.Suche in Google Scholar PubMed
282. Yu, H.; Shi, R.; Zhao, Y.; Waterhouse, G. I.; Wu, L. Z.; Tung, C. H.; Zhang, T. Smart Utilization of Carbon Dots in Semiconductor Photocatalysis. Adv. Mater. 2016, 28, 9454–9477; https://doi.org/10.1002/adma.201602581.Suche in Google Scholar PubMed
283. Proppe, A. H.; Li, Y. C.; Aspuru-Guzik, A.; Berlinguette, C. P.; Chang, C. J.; Cogdell, R.; Doyle, A. G.; Flick, J.; Gabor, N. M.; van Grondelle, R.; Hammes-Schiffer, S.; Jaffer, S. A.; Kelley, S. O.; Leclerc, M.; Leo, K.; Mallouk, T. E.; Narang, P.; Schlau-Cohen, G. S.; Scholes, G. D.; Vojvodic, A.; Yam, V. W. W.; Yang, J. Y.; Sargent, E. H. Bioinspiration in Light Harvesting and Catalysis. Nat. Rev. Mater. 2020, 5, 828–846; https://doi.org/10.1038/s41578-020-0222-0.Suche in Google Scholar
284. Clarizia, L.; Russo, D.; Di Somma, I.; Andreozzi, R.; Marotta, R. Metal-Based Semiconductor Nanomaterials for Photocatalysis, Multifunctional Photocatalytic Materials for Energy; Elsevier Woodhead Publishing: Italy, 2018; pp 187–213.10.1016/B978-0-08-101977-1.00010-7Suche in Google Scholar
285. Shen, M.; Ding, T.; Rackers, W. H.; Tan, C.; Mahmood, K.; Lew, M. D.; Sadtler, B. Single-molecule Colocalization of Redox Reactions on Semiconductor Photocatalysts Connects Surface Heterogeneity and Charge-Carrier Separation in Bismuth Oxybromide. J. Am. Chem. Soc. 2021, 143, 11393–11403; https://doi.org/10.1021/jacs.1c02377.Suche in Google Scholar PubMed
286. Li, J.; Wu, N. Semiconductor-based Photocatalysts and Photoelectrochemical Cells for Solar Fuel Generation: a Review. Catal. Sci. Technol. 2015, 5, 1360–1384; https://doi.org/10.1039/c4cy00974f.Suche in Google Scholar
287. Khan, M.; Nadeem, M.; Idriss, H. Ferroelectric Polarization Effect on Surface Chemistry and Photo-Catalytic Activity: A Review. Surf. Sci. Rep. 2016, 71, 1–31; https://doi.org/10.1016/j.surfrep.2016.01.001.Suche in Google Scholar
288. Feng, C.; Wu, Z. P.; Huang, K. W.; Ye, J.; Zhang, H. Surface Modification of 2D Photocatalysts for Solar Energy Conversion. Adv. Mater. 2022, 34, 2200180; https://doi.org/10.1002/adma.202200180.Suche in Google Scholar PubMed
289. Kwofie, F.; Cheng, Y.; Zhang, R.; Tang, H. NiOx Boosted Pt-Shell for Efficient Hydrogen Evolution Reaction. Mater. Today Sustain. 2022, 19, 100170; https://doi.org/10.1016/j.mtsust.2022.100170.Suche in Google Scholar
290. Kumar, S. G.; Devi, L. G. Review on Modified TiO2 Photocatalysis under UV/visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. 2011, 115, 13211–13241; https://doi.org/10.1021/jp204364a.Suche in Google Scholar PubMed
291. Park, H.; Kim, H.-i.; Moon, G.-h.; Choi, W. Photoinduced Charge Transfer Processes in Solar Photocatalysis Based on Modified TiO 2. Energy Environ. Sci. 2016, 9, 411–433; https://doi.org/10.1039/c5ee02575c.Suche in Google Scholar
292. Bhattacharya, C.; Saji, S. E.; Mohan, A.; Madav, V.; Jia, G.; Yin, Z. Sustainable Nanoplasmon-Enhanced Photoredox Reactions: Synthesis, Characterization, and Applications. Adv. Energy Mater. 2020, 10, 2002402; https://doi.org/10.1002/aenm.202002402.Suche in Google Scholar
293. Corby, S.; Rao, R. R.; Steier, L.; Durrant, J. R. The Kinetics of Metal Oxide Photoanodes from Charge Generation to Catalysis. Nat. Rev. Mater. 2021, 6, 1136–1155; https://doi.org/10.1038/s41578-021-00343-7.Suche in Google Scholar
294. Zhang, X. Development of Copper-and Tungsten-Based Phosphide Nanostructures for Photocatalytic Reduction Reactions. PolyU Electronic Theses 2021, 2024, 130–143; https://theses.lib.polyu.edu.hk/handle/200/11630.Suche in Google Scholar
295. Jin, L.; Liu, B.; Duay, S. S.; He, J. Engineering Surface Ligands of Noble Metal Nanocatalysts in Tuning the Product Selectivity. Catalysts 2017, 7, 44; https://doi.org/10.3390/catal7020044.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.


