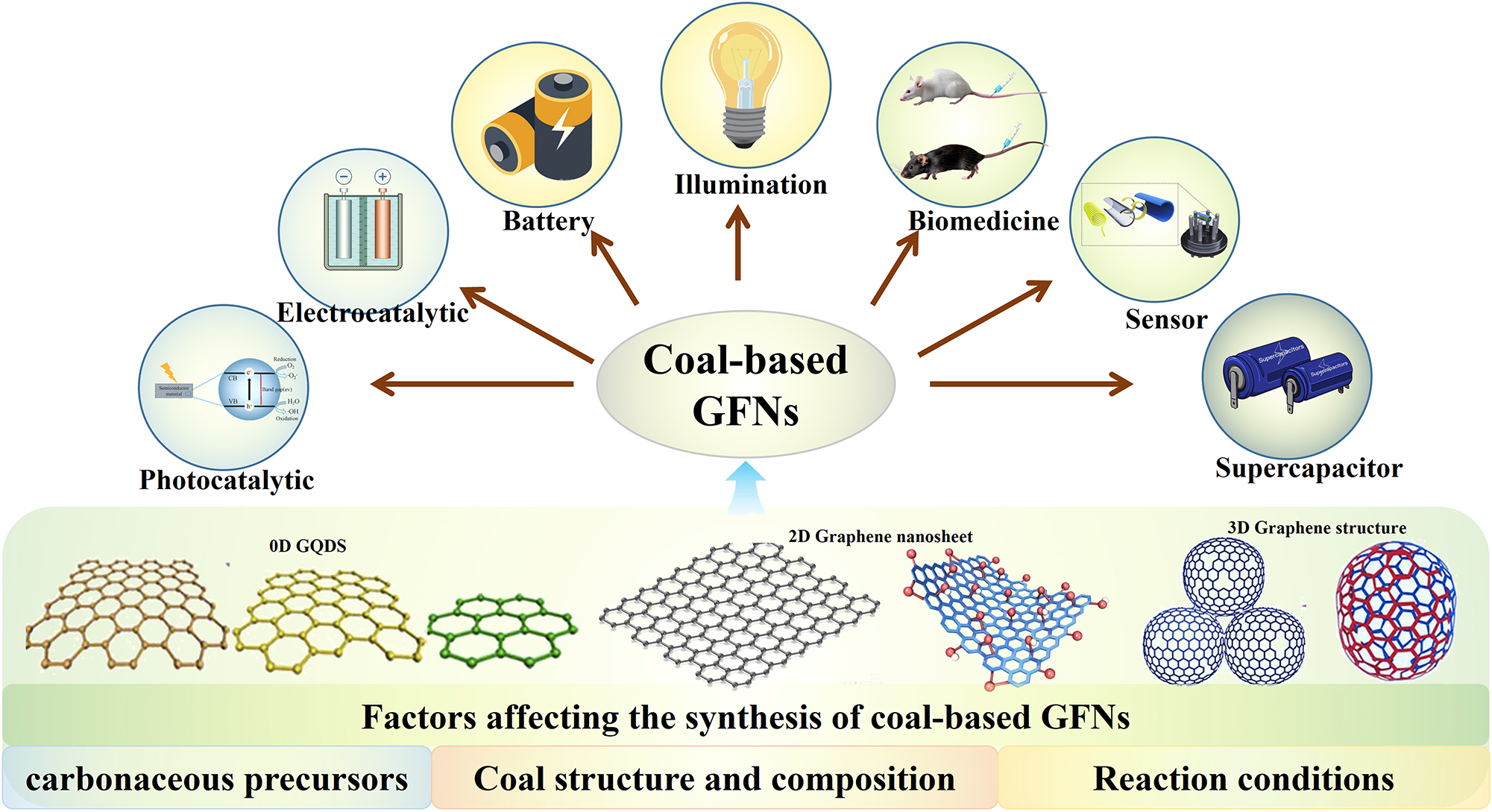
Abstract
Coal, a natural non-homogeneous polymer material, boasts a complex three-dimensional cross-linked network structure. Developing low-carbon processing methods and achieving clean utilization of coal are crucial challenges in today’s research landscape. In recent years, graphene and its derivatives, collectively known as graphene family nanomaterials (GFNs), have emerged as revolutionary multifunctional carbon nanomaterials due to their exceptional properties and vast application potential. The unique molecular characteristics of coal and its derivatives present a significant opportunity for the synthesis of GFNs. Therefore, it is necessary to summarize the research progress made on coal-based GFNs to clarify their future development trajectory. This paper reviews the synthesis methods employed for coal-based GFNs and discusses the factors influencing this process. Moreover, the applications of coal-based GFNs in the fields of biomedicine, sensing, photo/electrocatalysis and energy storage are summarized and the challenges of coal-based GFNs are presented. Hopefully, this review can spark new ideas for clean production of coal and the design of coal-based functional materials.
1 Introduction
Coal, the most widely distributed and abundant fossil fuels on earth, is not only the main energy source for some countries, but also was widely applied as chemical raw materials. 1 , 2 , 3 Driven by ‘Peak Carbon and Carbon Neutral’ targets, the world is undergoing a green transformation, presenting both opportunities and challenges for coal utilization. The traditional downstream coal processing industry is inefficient, environmentally destructive and limited in its potential utilization. Therefore, in-depth research on clean and functional applications for coal is an important strategy to overcome the current bottleneck of coal application. 4 Coal is essentially a natural non-homogeneous polymer material with a three-dimensional (3D) cross-linked network structure, consisting of a certain number of hydroaryl and aryl units linked by macromolecular structures and various chemical bonds. 5 , 6 Depending on the degree of coalification, coal can be simply divided into anthracite, bituminous coal and lignite. Coalification, essentially the ‘maturation’ of coal, leads to two key trends: larger aromatic structures and a higher overall carbon content. 7 Therefore, This inherent aromatic structure makes coal an ideal precursor for engineering valuable carbon-based materials through a process called carbon molecular engineering. Beyond direct use, coal can be transformed into various valuable chemical products and fuels through catalytic processing or pyrolysis. These products include coal tar, coal asphalt, coke, and liquid fuels. These derivatives inherit the compositional and structural properties of coal, making them ideal precursors for the preparation of advanced carbon materials. In 1991, preparation of the fullerenes using coal was firstly demonstrated by Pang et al. 8 Subsequently, more researchers focused on the development and exploration of coal-based carbon materials, such as carbon nanotubes, carbon nanofibers, carbon quantum dots and graphene. These coal-based carbon materials were widely used in the fields of biomedicine, photocatalysis, electrochemistry and energy storage. 9
Graphene is considered as a revolutionary material and one of the most important advanced functional carbon materials available. Andre Geim and Konstantin Novoselov won the “2010 Nobel Prize” in Physics, as the first isolation of graphene by mechanical exfoliation in 2004. 10 , 11 , 12 The discovery of graphene marked a new era in material science. 13 , 14 Graphene, a single-layer honeycomb lattice, is composed of sp2 hybridized carbon atoms that tightly aligned with excellent optical properties and remarkable electronic properties, thereby exhibiting excellent optical transparency, specific surface area, tensile strength, stiffness, and thermal conductivity of electrons. 15 , 16 , 17 As the increase of the global production of graphene, a significant amount of related research was carried out, resulting in numbers of major breakthroughs in innovative application and basic science. Graphene and its derivatives are known as graphene family nanomaterials (GFNs), including graphene quantum dots (GQDs), graphene oxide (GO), reduced graphene oxide (RGO), few layer graphene (FLG), graphene nanosheets, and 3D graphene nanomaterials. The GFNs have shown great potential for application in scientific and industrial fields, such as biomedicine, 18 , 19 electronics, 20 , 21 energy storage, 22 , 23 , 24 , 25 catalysis, 26 , 27 , 28 , 29 optical detection, 30 , 31 and sensing, 32 , 33 etc., due to their excellent optical, electronic, chemical, and mechanical properties. The development of economically and commercially viable large-scale synthesis of GFNs without compromising the properties and quality of carbon materials is an urgent need for current research. Currently, the GFNs have been successfully synthesized from several precursors, such as graphite, biomass materials, chemical fuels, carbon nanotubes, liquids, or gases. 34 , 35 , 36 , 37 High carbon content, low cost, and high availability are the main features of the synthesized GFNs precursors.
As is reported, the utilization of coal and its derivatives (natural carbonaceous materials) as precursors for the production of the GFNs has become an emerging trend in the field of graphene research (Figure 1). Therefore, to unlock the full potential of coal-based GFNs, a critical review summarizing the progress on their synthesis and application is necessary. This will provide invaluable insights for guiding future development efforts. However, to date, the current research landscape lacks such comprehensive summaries, creating a gap in our understanding of this promising field. This paper presents a review on the synthesis approaches of coal-based GFNs and the influencing factors of synthesis. Moreover, the applications of coal-based GFNs in the fields of biomedicine, sensing, photo/electrocatalysis and energy storage are also summarized. Finally, the challenges and prospects of the coal-based GFNs are presented.
Summary on the synthesis and application of coal-based GFNs.
2 Synthesis of coal-based GFNs
In order to further develop their excellent properties in various applications, several reliable synthetic routes have been developed for the preparation of GFNs. In general, they can be categorized into “bottom-up” and “top-down” approaches. Currently, the most common high-quality GFNs are usually prepared by top-down methods, such as chemical exfoliation, thermal exfoliation and deposition of graphite. In the bottom-up approach, GFNs are synthesized from atoms or molecules by chemical reactions. However, the bottom-up synthesis process is not widely used due to its complexity and limited scale. Here, coal-based GFNs are categorized into different classes based on type and size, and their synthesis processes are explored separately.
2.1 Graphene
Chemical vapor deposition (CVD) is a promising technique for graphene synthesis, but the choice of carbon precursor is crucial. While coal offers a readily available source, its impurities like fatty chains and heteroatoms hinder the formation of high-quality, pristine graphene layers. Rane et al. explored this challenge by utilizing aromatic fractions derived from coal for CVD on nickel and copper foils. Nickel’s defect-healing properties and high carbon solubility allowed both aromatic fractions (with or without heteroatoms) to form defect-free, multi-layered graphene (around 11 layers). 38 In contrast, copper’s high hydrogen solubility and competition for carbon resulted in primarily amorphous carbon formation for both fractions. Notably, the heteroatom-free aromatic fraction still contains alkyl pendant substituents and does not have symmetry and the same planarity, thus preventing the formation of defect-free graphene on copper. The quality of the precursor on copper and the substrate on nickel determine the quality of graphene.
Due to its inherent advantages, direct laser inscription presents an attractive alternative for modifying coal. This environmentally friendly and cost-effective technique avoids complex procedures and harsh conditions. This is particularly relevant when considering coal’s structure. Coal can be simplified as a network of aromatic domains (rich in desirable carbon rings) linked by weaker ether bonds and aliphatic chains. Laser inscription could potentially target these weaker bonds, manipulating the coal’s structure for various applications. The photothermal effect during laser scribing causes the defects between aromatic carbons in coal to be repaired and the fatty chains to be evaporated, thus facilitating the formation of ordered graphitic materials. 39 Coal-based graphene can also be obtained by combining physical and chemical methods. Products with nearly 11 graphene layers can be obtained from coke using thermal stripping combined with solvent stripping techniques. 40 Despite the structural defects in the coke, the prepared graphene has properties similar to those of conventional graphite.
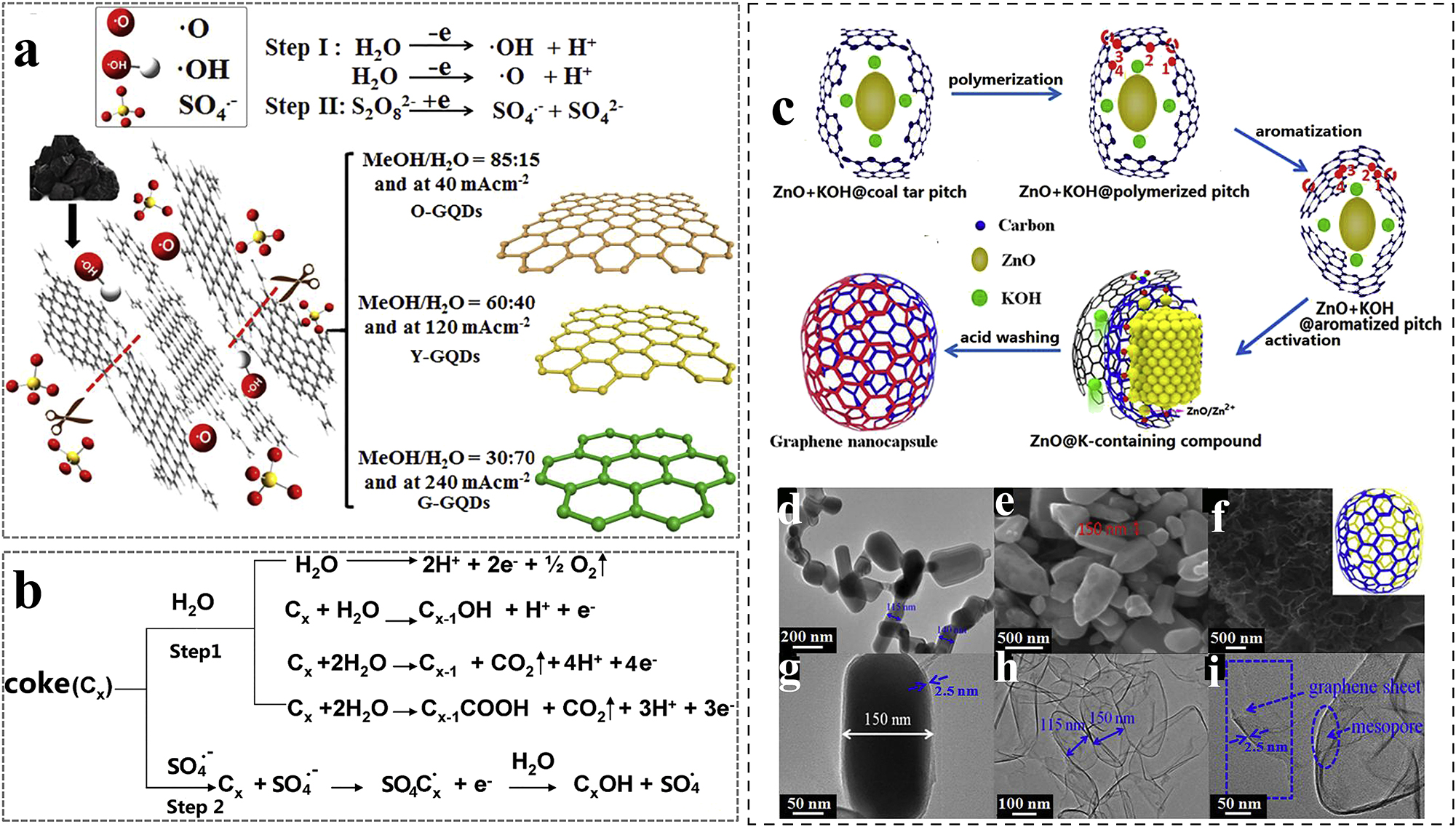
Graphene produced from a carbon source in less than 1 s by flash joule heating (FJH) is called “flash joule graphene” (FG). 41 , 42 , 43 FJH utilizes the action of an electric field under the material’s own resistive exotherm and has a very short reaction time. The graphene yield of this process depends on the carbon content of the feedstock. Coal tar pitch (CTP)-based graphene can be synthesized using FJH at coupled electric field and temperature. 44 Joule heat provides energy for the thermodynamics and kinetics of the reaction (Figure 2a–d). High temperatures during coal tar conversion facilitate the dissociation of branched hydrocarbon chains into smaller, highly reactive alkane and olefin fragments (radicals). The number of these radicals generally increases with the energy input, potentially influencing the quality of the resulting graphene. However, the mass of the final graphene product is primarily determined by the cooling rate. Faster cooling can lead to a disordered, turbostratic structure, while slower cooling promotes the formation of well-defined layers and potentially thicker, multilayer graphene. Compared to conventional graphene production processes, FJH does not require large amounts of solvents and can be used for large-scale production of graphene. However, due to the complexity of the FJH process, its exact mechanism is still an understudied issue.
In graphene synthesis, molten metals play a critical role. They act as a solvent, allowing carbon atoms to dissolve and cluster together to form graphene nuclei. Additionally, they function as catalyst, promoting the decomposition of carbon source materials into individual carbon atoms and volatile byproducts. The possible mechanisms for growing large multilayer graphene on molten Ce may be as follows. 46 At elevated temperatures exceeding cerium’s melting point, anthracite decomposes into carbon atoms and polycyclic aromatic hydrocarbons (PAHs). These carbon atoms can dissolve (saturate) into the molten cerium, potentially acting as nucleation sites for subsequent graphene growth. Additionally, cerium might facilitate the alignment of existing graphite flakes. The dissolved carbon atoms and PAHs can then combine to form larger graphene sheets. These sheets may further stack to create multilayer graphene.
Metal-loaded FLG materials can be prepared from bituminous coal over Fe catalysts using a microwave-assisted catalytic graphitization process. 47 Carbothermal reduction transforms iron oxide into iron particles. Subsequently, excess carbon in the system precipitates onto these iron particles. This initially forms amorphous carbon, which can then transform into more stable graphite over time or under specific conditions. Subsequently, bituminous coal was dissolved into the iron droplets. When the Fe droplet and carbon concentration are supersaturated, the change in Gibbs free energy causes the FLG to grow layer by layer on the surface of Fe atoms, and amorphous C is transformed into ordered C. The thickness of carbon layers is inversely proportional to the heating rate. Slower heating promotes thicker layers, while faster heating results in thinner structures. Encouragingly, the team discovered that KOH activation significantly accelerated the synthesis of FLG. Moreover, the KOH-activated coal yielded FLG flakes with greater regularity and continuity compared to the unmodified process. 48 Lin et al. employed a combined theoretical and experimental approach to investigate the synthesis process and optimal growth environment for CTP-based graphene nanosheets (GNs) using Al4C3 as a pyrolysis precursor. 49 , 50 The formation of Al4C3 leads to a diversity of thermal stresses in the carbon material after pyrolysis, thus favoring the exfoliation of GNs. Theoretical analyses show different bond breaking mechanisms for Al4C3 under shear and tensile strains. The shear strain mechanism is the breaking of C1–C2 and C1–C3 bonds, and the tensile deformation mechanism can be considered to be the breaking of C2–C3 bonds. The team also prepared GNs with crystalline structure using nano Al2O3 as a filler. 51 The conversion of Al2O3 to Al4C3 by reacting with carbon in CTP at a temperature of 1,600 °C may be responsible for the formation of GNs.
2.2 GO and RGO
Studies have shown that coal and its derivatives hold promise as a cost-effective alternative to graphite for the production of GO. This finding could significantly impact the scalability and affordability of GO-based technologies. 45 , 52 , 53 , 54 , 55 During the formation of coal-based GO synthesized by the solvothermal method, the edges of the graphene surface are mainly introduced with carboxyl groups. 56 Therefore, the damage on the graphene surface basal plane is minimized, which is conducive to keeping the graphene domains intact in coal. Thermal reduction transforms coal-based GO into coal-based RGO. This process typically involves a loss of mass due to the removal of oxygen-containing functional groups. Additionally, the graphitic character of the RGO increases, signified by a higher sp2 carbon content within the graphene domains.
Different sources of coke materials can also be used as precursors for graphene oxides, and the obtained GO are characterized by similarities in the type and content of oxygen functional groups and defects to conventional graphite oxides. 57 The increase in the degree of orientation of the parent coke crystal structure improves the yield of GO with a consequent increase in flake size. The preparation of coal-based GO nanomaterials by nitric acid substitution method using coal and coal coke as carbon sources revealed that the oxygen content of coal GO was slightly higher, and the graphitization of coal coke GO was higher. 58 The conductivity depends on the carrier concentration and the mobility of the electrons produced after thermal reduction. High-temperature thermal reduction not only increases the sp2 clustering of coal coke GO and orders the formation of rings or chains, but also generates structural defects at the deoxygenation sites. The increase in sp2 structural domains favors the increase of charge carriers, but the structural defects are not conducive to electron migration. These defects may provide a good compromise between irreversible capacity and storage capacity, thus giving the material great potential for battery electrode applications.
2.3 GQDs
The coal-based GQDs exhibit a fascinating dependence of their properties on the synthesis method. Different preparation processes can tailor the microstructure, electron transfer capabilities, and photoluminescence (PL) properties of the GQDs. Studies using UV/Vis absorption spectroscopy reveal two key absorption peaks. These peaks are generally attributed to electronic transitions within the sp2 carbon domains (π–π* transitions) and oxygen-containing functional groups (n–π* transitions). The oxygen-containing functional groups such as hydroxyl, carboxyl, and epoxy groups on the aromatic surface of GQDs make them exhibit excellent dispersibility in different solvents. Interestingly, the PL spectra of GQDs are related to the excitation wavelength. The shift of the PL peak with excitation wavelength may be due to the quantum size effect due to the degree of graphitization and may also be attributed to the surface defects formed by the functionalization of surface functional groups.
The electrochemical stripping method has the advantages of high yield, high efficiency, low cost and easy operation. Multicolor fluorescent GQDs can be synthesized by electrochemical stripping method using coke as carbon source. 59 The possible formation mechanism of GQDs can be divided into two key steps (Figure 3a and b). Firstly, ⋅O and ⋅OH radicals generated from H2O decomposition attack the edge surface of coke to accelerate its hydroxylation or oxidation process, which promotes the edge surface to carry out more edge sheets. 60 Secondly, the specific electrolyte (NH4)2S2O8 forms ⋅SO4− radicals with strong oxidizing properties through electrochemical reactions. The opening of the edge sheets makes it easier for the ⋅SO4− radicals to be successfully inserted into the lamellae. Subsequently, the products obtained from the splitting of the lamellae by the radicals expand along the stacking direction, favoring the destruction of the weaker aliphatic C–C bonds. The stronger aromatic C–C bonds will mostly remain intact and integrate to form aromatic crystalline carbon structures to form nanoscale GQDs. 61
The pulsed laser ablation liquid (PLAL) technique can also be used for the synthesis of coal-based graphene oxide quantum dots (GOQDs). 63 The possible mechanism for the formation of GOQDs from coal begins with a Coulomb explosion on the surface of the solid target during laser pulse injection. The plasma plume first forms around the target coal due to the absorption ionization of multi-photons. 64 , 65 Subsequently, its expansion and cooling occur leading to the creation of cavitation bubbles in solution. When the temperature and pressure inside the bubble are lower than the surrounding solution, the carbon clusters in the cavitation bubble are burned away from the target while still having high surface energy, tending to aggregate and form lamellar graphene sheets. 66 , 67 Due to the inherent hexagonal monolayer structure of graphite, carbon clusters produced during synthesis tend to adopt a two-dimensional form. A laser beam then breaks these graphene sheets into nanoscale GOQDs. However, the formation and size of GOQDs are influenced by several factors. Lower laser power densities can hinder GOQD formation, and variations in the cooling rate within the laser-induced plasma plume can also affect their size.
Ultrasound physical shearing method can be used for the preparation of coal-based GQDs. 68 The ultrasonic irradiation effect broke the bridge bonds in the coal, resulting in a nanoscale sp2 carbon crystal structure with many edge defects. A study has synthesized S, P, and N co-doped GQDs (NPS-GQDs) using one-step wet chemistry and dialysis using anthracite coal as a carbon source. 69 The S, P, and N introduced more defects and disorder at the edges and bottom of the coal-based GQDs. During the formation of GQDs in coal, the oxidant inserts into the coal and oxidizes the interlayers of the graphitic structure in the coal. As the oxidant penetrates deeper, the spacing between graphene layers increases. This weakens the bonds between the layers, causing them to break at specific points and ultimately forming GQDs.
Coal-based GQDs can also be obtained in the presence of an acid-free oxidizer. Uniformly distributed CTP-based GQDs can be synthesized using mild oxidation with H2O2. 70 A possible formation mechanism involves H2O2 selectively oxidizing the alkyl chains at the edges of the CTP molecule, converting them into oxygen-containing functional groups. This leaves the aromatic core intact, allowing it to form the graphitic domain structure of the resulting GQDs. Ghorai et al. successfully synthesized blue-emitting GQDs using coal solvothermal technique assisted by acid-free and oxyketone oxidants. 71 The reaction mechanism involves a combination of free radical oxidation and solvent thermal redox reaction. The oxygen ketone oxidizer in the solvent thermal redox reaction generates ⋅SO4− or ⋅OH will cleave successive graphite segments of coal. A combination of oxidative solvation and solvent thermal redox-driven chemical functionalization enables the efficient conversion of coal into ultra-small GQDs.
2.4 3D graphene structure
Coal emerges as a promising precursor for synthesizing 3D porous graphene materials. These 3D structures, typically composed of graphitized coal-derived graphene, boast a high concentration of mesopores, resulting in larger pore volume and greater surface area. 40 Xing et al. prepared coal-based porous graphene (CPG) by graphitization-liquid-phase oxidation-rapid thermal reduction method using anthracite coal as a carbon source. 72 During liquid oxidation and thermal reduction, the strong bonding between adjacent graphite layers in coal hinders the complete separation of graphene nanosheets and GO. This limited separation allows the individual sheets to remain interconnected, forming a highly continuous 3D porous skeleton (Figure 2d). A large number of oxygen-containing functional groups in CPG are removed and form nanopores during rapid thermal reduction. Additionally, the CPG possesses numerous structural defects within the graphene skeleton. These defects come in the form of tiny nanopores and active oxygen-containing functional groups.
Since CTP is formed from a large number of aromatic organic compounds by a polymerization process that promotes double bond rearrangement, it can be used as a feedstock for the production of 3D graphene structures. The preparation of 3D interconnected graphene nanocapsules (GNCs) 62 and hollow porous graphene balls (HPGBs) 73 can be achieved by using CTP as a carbon source in combination with an in situ KOH activation technique using a ZnO/MgO template strategy. The formation of GNCs was attributed to CTP liquefaction to form 3D interconnected films containing the polycyclic aromatic molecule ZnO + KOH@CTP (Figure 3c–i). The C–H bonds on the aromatic rings and some weak C–C bonds on the single chains between the aromatic rings will break with the heat treatment time, generating some PAH molecules and reactive radicals. The reactive radicals undergo polymerization reactions via individual carbon atoms on adjacent aromatic rings. The formed polymer film is further heated to undergo graphitization which is again activated by the embedded KOH at high temperature to form some oxygen-containing groups and an abundance of layered short pores. The formation of HPGB is attributed to the CTP liquid coated on the surface of MgO nanoparticles acting as templates and forming a thin coating made of CTP-derived aromatics. The hydrocarbons were decomposed on heating and some gaseous products were formed in the thin carbonaceous coatings. The coating is gradually transformed into a thin carbon layer with a spherical structure by a combination of the MgO template effect, decomposition, and reorganization of hydrocarbons and internal gas pressure. Simultaneously, KOH mixed in bitumen and embedded in the thin coating undergoes melting and diffusion in the reactants, followed by activation of aromatics at high temperatures and formation of pores of different sizes in the spherical shell of the thin carbon layer. Finally, different 3D graphene structures were obtained separately by acid washing.
Open-shelled hollow multilayer graphene spheres can be prepared at lower temperatures using anthracite and semi-coke as carbon sources and nickel as catalyst. 74 The growth process of Ni begins with the NiO particles obtained by NiAc2 decomposition being reduced by amorphous carbon to metallic Ni and forming molten Ni droplets on the surfaces of anthracite and semi-coke particles as the temperature increases. Meanwhile, the carbon atoms dissolve into the Ni droplets, and when the carbon concentration reaches supersaturation, graphene nucleates and grows on the surface of the Ni droplets. Finally, the graphene sheets grow along the lateral direction and nucleate layer by layer on the surface of Ni droplets to form multilayer graphene.
2.5 Graphene film
Vijapur et al. synthesized large-area oligolayer graphene films using coal as a carbon source using CVD technique and investigated the growth mechanism. 75 , 76 Hydrocarbons released during coal pyrolysis can be used as precursors for the synthesis of graphene films. First, the carbon atoms in various hydrocarbon gases released from coal are adsorbed by copper, a copper-catalyzed reaction occurs, and an amorphous carbon film is formed on the copper substrate through catalytic dissociation and resolution reactions. Subsequently, hydrogen-catalyzed dehydrogenation and graphitization of amorphous carbon occurs and graphene domains are formed. The graphene domains increase in size over time and fuse to form a continuous graphene film on a copper substrate. Different hydrocarbon precursor gases affect the formation of the graphene layer. Singh’s research on coal-derived graphene thin films (CDG) revealed several promising properties for electrochemical applications. These films exhibited reduced corrosion, enhanced safety, increased current response, a wider potential window, and remarkable long-term stability when tested in a Na2SO4 electrolyte. 77
2.6 Graphene-like derived materials
A study has prepared coal-derived carbon nanomaterials (CNMs) consisting of onion-like fullerenes and chemically transformed graphene-like nanosheets from low-quality coal by an oxidation-extraction process. 78 Polyaromatic and irregular hydrocarbons in coal were first preferentially oxidized to form partially ordered and defective carbon layers. Subsequently, Na+ formed during the extraction process may be inserted between the disordered and ordered layers of the coal structure and undergo interlayer rearrangement. A large number of aromatic fragments including C1 or C2 carbon units in coal underwent rearrangement and aggregation to form chemically transformed graphene-like nanosheets and onion-like fullerenes. Interestingly, the higher sulfur content found in lower-quality coal can actually be beneficial. During the formation of metal-sulfur compounds (eutectics), this sulfur helps create active sites on the metal catalyst. These active sites then play a crucial role in the growth of coal-derived CNMs.
Few-layer graphene derivatives with layer spacing of 0.348 nm can be synthesized from low-grade coal by ultrasound-assisted chemical method. 79 Most of the surface functional groups of coal were further removed during the reduction process, thus its aromatic C network was restored to obtain coal-based graphene derivatives. The coal-based GO exhibited characteristic absorption bands similar to those of standard GO at 240 nm and 300 nm due to π–π* electron leaps and n–π* electron leaps, respectively. In contrast, the coal-based graphene derivatives show only 264 absorption bands attributed to π–π* electron leaps of the conjugated aromatic π system. Sierra et al. prepared oligolayer graphene and graphene-like derivatives by stearic acid-assisted planetary milling of graphite and coke materials. 80 The process resulted in materials with no oxygen incorporation, leading to reduced thermal stability, smaller particle size, and a significantly increased surface area. The morphology of the obtained graphene products showed stacked flakes with transverse dimensions between 1 µm and 3 µm.
The preparation of coal-based defective graphene-like (DGLC) nanomaterials can also be achieved using the KOH activation method. 81 KOH reacts violently with carbon during high-temperature treatment, and the resulting metal K is embedded in the carbon matrix and diffuses between the carbon layers, resulting in the formation of graphene-like products. Moreover, the vigorous reaction and reconfiguration that occurs with K2CO3 will release gases such as CO2, H2, and H2O thereby causing the product to form a large number of defects/edges and nanopores. This process will also occur with the retention of abundant oxygen-containing groups and in situ doping of O heteroatoms. Thus, the prepared DGLC materials exhibit graphene-like morphology, abundant oxygen-containing functional groups and defects/fringes.
3 Factors affecting the synthesis of coal-based GFNs
3.1 Differences in carbonaceous precursors
The study of colloidal solutions of graphene-like structures prepared from different grades of coals revealed that thermal oxidative destruction of anthracite and low volatile bituminous coals led mainly to the formation of nanoparticles with d = 122–190 nm, and thermal oxidative destruction of bituminous coals formed nanoparticles with d = 50 nm (V = 5.2 %) and d = 164 nm (V = 16 %). 82 Lignite thermal oxidative damage formed nanoparticles of d = 32 nm (V = 17 %) and d = 122 nm (V = 11 %). Differences in coal sources used as carbonaceous precursors can lead to differences in products. Lower order coals may produce more oxygenated functional groups, while higher order coals may provide more graphitized frameworks. Coal derivatives that have been pretreated at high temperatures, such as coke and coal tar, form larger graphitic domains during the heating process, tending to produce coal-based GQDs of relatively large size. Coal’s unique structure makes it more efficient than graphite (pure sp2-carbon) for creating tiny GQDs through a process called oxidative stripping. This process breaks down the coal’s inherent mix of ordered (crystalline) and disordered carbon regions more readily, yielding nano-sized GQDs. Bituminous coal has smaller polyaromatic domains and more fatty carbon, while coke and anthracite have a certain amount of graphite-like stacked domains. 83 The shapes and sizes of GQDs prepared from different coal sources showed significant differences.
The basic units of coal are connected to each other by bridge bonds such as –O–, –S–, –CH2–O– and –CH2–CH2–. Their edges are usually capped with alkyl side chains and other functional groups. As the coal rank increases (indicating a higher degree of coalification), the content of functional groups and side chains decreases. Dong et al. showed that the yield of GQDs decreased with increasing coal rank. 84 Higher rank coals contained higher yields of other carbon-based nanomaterials, such as agglomerated carbon nanocrystals, CQDs, graphene oxide, and agglomerated GQDs. Xu et al. discovered that certain types of raw coal are not ideal for producing high-quality GQDs. Coals with either very low or very high levels of graphitization were found to be unsuitable for this process. 69 The formation of coal-based GQDs is explored using four different coal rank coals, namely, coal ash, anthracite, coking coal and lignite, as carbon sources. During the formation of GQDs from coal, the oxidant inserts into the coal and oxidizes the interlayers of graphite structure in the coal. The layer spacing increases with the degree of oxidant penetration, and bridge bonds break at weak points and form GQDs. Therefore, raw coals with either too low or too high graphitization cannot be successfully synthesized into high-quality GQDs. Conversely, coals with an optimal degree of graphitization readily yield uniform GQDs exhibiting short-range order but long-range disorder.
The synthesis of GO is achievable using various coal tars as a starting material. 85 Among them, for anthracene oil and impregnated tar, the size and yield of the prepared flakes depended on the size of their parent graphite crystal domains. Whereas, binder grade tar reduced the yield of oxidation and flaking due to the presence of quinoline insoluble particles (QIs). Upon further investigation, it was found that this result was due to the high reactivity of QIs to oxidation that altered the distribution of functional groups in GO and prevented them from exfoliation. 86 The presence of QIs in the parent graphite favors the production of FLGs with reduced size. Whereas the higher crystallinity of the parent graphite without QIs favors the generation of larger GO flakes.
3.2 Differences in coal structure and composition
The main components of coal are the specular and inert groups, with a lesser amount of the chitin group. 87 , 88 The specular group is easy to graphitize with larger coherent domains and higher H/C ratios, whereas the inert group is less graphitizable with lower H/C and higher O/C. 89 , 90 The microcomposition of coal is crucial for the graphitization and utilization of coal. Graphene nanosheets (GNs) can be prepared using different fractions of inertia-rich coal as a carbon source. 91 Studies have shown that graphene nanostructures (GNs) derived from coal rich in inert macerals exhibit greater resistance to oxidation, leading to enhanced stability. Interestingly, a higher oxygen-to-carbon (O/C) ratio in these GNs is associated with larger and more developed structures. Furthermore, electrochemical experiments reveal that these inert-derived GNs possess superior specific capacitance and resistance.
Li et al. prepared coal-based graphene using 13 types of anthracite coals collected from Qinshui Coalfield as carbon sources. 92 The results showed that the degree of graphitization of coal-based graphite was positively correlated with the reflectivity of the raw coal, negatively correlated with the content of the specular group of the raw coal, and positively correlated with the content of the inert group. The crystal height (Lc) and crystal width (La) of coal-based graphite and graphene were linearly and positively correlated with the reflectance of raw coal. Unlike raw coal, demineralized coal, and coal-based graphite, coal-based graphene boasts a thin, transparent structure composed solely of carbon and oxygen. However, it comes with a trade-off, exhibiting a higher degree of defects (ID/IG) compared to its less processed counterparts. Furthermore, the higher the reflectivity of the raw coal, the more ideal the prepared coal-based graphene micro-morphology and the fewer the number of layers characterized by HRTEM. The team also prepared coal-based GQDs and coal-based graphene sheets (GS) symbionts by using three different reflectance raw coals from this coalfield as the carbon source. 93 Coal-based GQDs and GS are found to be distributed throughout the different layers within the coal-derived composite structure. This stable arrangement allows them to coexist harmoniously. The coal-based GS is the main part of the symbiosis, and the GQDs are mainly distributed at the edges and folds of the GS layer. The elements of the symbiotic body are only C and O, and the morphology and crystal parameters are similar to those of graphene. A positive correlation exists between the reflectivity of raw coal and the characteristics of GQDs within the symbionts. Higher reflectivity corresponds to larger diameters of the GQDs’ six-membered rings, increased density, fewer vortex layer structures, and a smoother lattice skeleton. Coal-based products prepared from this process exhibit similar functional groups overall. However, the content of C–C/C=C increases as the reflectivity of the raw coal increases. Conversely, the content of C=O decreases with higher reflectivity. The coal-based products all emit blue light under UV irradiation, and the absorption of UV light increases with the increase of coal reflectivity, resulting in the enhancement of fluorescence intensity.
Coal contains a variety of minerals with large differences in their types, contents and distribution. The preparation of coal-based graphene is also affected by the minerals in coal. The minerals in anthracite physically inhibited the oriented development of graphene sheets. 94 The high-temperature transformation products of minerals lead to the formation of irregular pore defects on the graphene surface, which seriously affects the performance of coal-based graphene materials. Whereas, the demineralization of anthracite reduces the number of oxygen-containing functional groups and defects on the surface of coal-based graphene, favoring the improvement of its morphology and properties.
The properties of carbon materials are influenced by the structure of the carbon source. Directional pressure is an important factor affecting the graphitization of carbonaceous precursors. There are few studies on the effect of pressure on the properties of carbon sources in coal, in which tectonically deformed coal (TDC) is a product of coal seams that are extruded by shear and geologic tectonics. Coal-based graphene products were prepared from normal structural coal (NSC) and TDC as carbon sources, and it was found that tectonic stresses resulted in slightly higher ratios of long aromatic grains, multilayer stacking, and preferred orientation degree (POD) in TDC carbon sources than in NSC. 95 TDC-derived coal-based microcrystalline graphite exhibits superior graphitization (G) and vertical height (d) compared to NSC at the same temperature. This is attributed to TDC’s increased pore structure, higher degree of ordering, and larger microcrystalline size. Therefore, the tectonic stress promotes the increase of graphitized structure of carbon sources in coal, and it is easier for TDC to prepare well-ordered coal-based graphite.
3.3 Differences in reaction conditions
The reaction solvent can have a great influence on the electronic properties and morphology of the resulting graphitic materials. It has been studied that coal-based graphitic materials with different aspect ratios have been prepared in a reducing or oxidizing environment provided by supercritical EtOH (scEtOH) and supercritical H2O (scH2O), respectively. 96 The high reactivity of scH2O and the accompanying hydrothermolysis reaction may lead to coal depolymerization via C–C bond fission, resulting in a high yield (55 wt%) of monolayer oxidized GQD. The aspect ratio, size and number of layers of the prepared GQDs decrease with the increase of supercritical reaction duration. While scEtOH has a weaker basicity and more pronounced hydrogen bonding acidity than scH2O, which causes coal cutting mainly by means of its line defects and heteroatom doping sites. Notably, the reduction environment facilitated by scEtOH predominantly yields ribbon-shaped graphene materials with exceptionally high aspect ratios.
The structure of coal-based GFNs can be adjusted by rationally changing the reaction parameters such as synthesis method, reaction temperature, pH and reagent composition/ratio. Based on the synthesis strategy for GCNs reported by He et al., Puente-Siller et al. evaluated the effect of working temperature on the formation of 3D GNCs nanostructures. 97 At temperatures of 600 °C and 700 °C, impurities are difficult to remove and the formation of GNCs are incomplete; at temperatures of 800–850 °C the particles obtained have the best properties and the size of the GNCs decrease with increasing temperature, which promotes the generation of GNCs; and at 900 °C, the experimental results do not show a significant amount of synthesized material, even though solids morphologically similar to the formed GNCs can be obtained.
The GQDs synthesized by the different methods have different bandgaps and sizes and exhibit different photophysical properties. 98 Furthermore, the stability of GQDs was investigated by studying the effect of pH on the fluorescence properties of coal-based GQDs. 99 , 100 The PL emissions of coal-based GQDs were all pH-dependent, and were maximal when the pH was neutral. Under alkaline conditions, the PL emission of the non-aggregated GQDs displayed a blue shift. This is attributed to the deprotonation of the carboxyl groups of the GQDs in alkaline solution which enhances the electrostatic repulsion between them and hence overcomes the tendency of aggregation in layer-to-layer superposition. A red shift occurs when the pH changes to acidic, which is attributed to the aggregation of GQDs in acidic/neutral solutions reducing the band gap. The latter also observed to observe a decrease in the PL intensity of the GQDs with increasing temperature. The observed decrease in quantum efficiency and PL intensity with increasing temperature can be attributed to an enhanced non-radiative decay rate. This means that at higher temperatures, excited electrons in the GQDs lose their energy through non-radiative pathways (e.g., heat) more readily, leading to a weaker PL signal.
Coal-derived volatile monocyclic aromatic hydrocarbons (VMAHs) emerge as promising high-quality feedstocks for the CVD synthesis of graphene. Notably, this approach enables graphene production at significantly lower temperatures compared to traditional small-molecule gas feedstocks. Zhao et al. 101 and Wu et al. 102 investigated the mechanism of graphene CVD growth at different temperatures by using benzene from coal tar and benzene from coal distillation and refining as carbon sources, respectively. The former found that the benzene ring was deposited on the surface of the copper foil as a complete six-membered ring structure in a gas-phase reaction at low temperatures, with little or no disintegration. When the temperature reaches 700 °C, the benzene ring begins to decompose into small gaseous molecules such as C2H6 and CH4. When the dummy is more completely transformed into tiny molecules and deposited on the surface of copper foil at higher temperatures (700 °C–900 °C), graphene is formed from a complex mixture of carbon sources, including benzene, and gaseous small molecules (methane and ethane). The latter, however, gave homogeneous monolayer graphene by CVD at high temperature (1,060 °C) due to the decomposition of the benzene ring into carbon atoms obtained by a catalytic reaction on a copper substrate. Relatively homogeneous but defective monolayer graphene structures were also obtained by low-temperature (500 °C) CVD due to the fact that the benzene rings can be directly removed from the hydrogen atoms and generated by re-bonding to each other at low temperatures. It was found that it is difficult to grow into uniform monolayer graphene at temperatures of 600 °C–900 °C. This is due to the fact that the benzene molecule is cleaved into a number of disordered macromolecular chains, which are not completely decomposed into atoms. These macromolecular chains are interconnected to form a graphene film, which reduces the mass of the monolayer graphene due to the low migration and diffusion of benzene molecules at low temperatures.
SiO2 nanoparticles can be used as carbon precursors for the modification of CTP and the presence of SiO2 affects the formation of catalytic graphene in CTP-derived carbon structures. 103 The results show that SiO2 in the CTP-SiO2 system undergoes carbon reduction and forms SiC upon heat treatment to 2,000 °C. The surface of SiC particles is the site of graphene formation. The SiC dissociates upon annealing at 2,800 °C and silicon is released. These reaction processes will affect the structure and microstructure of the CTP-based carbon matrix. Nanofillers inhibit crystal growth in the carbon matrix during low-temperature treatment of CTP (up to 1,000 °C). At temperatures of 2,000 °C and above, the presence of SiC favors crystal growth toward the a-axis graphite direction and inhibits crystal growth toward the c-axis, resulting in crystals consisting of graphene with significantly larger grain sizes in the a-axis than in the c-axis.
4 Application of coal-based GFNs
4.1 Energy storage
Driven by the urgency of addressing global environmental problems and the dwindling supplies of fossil fuels, the demand for clean energy has surged. With short charging time and long cycle life, supercapacitors have gained great attention as a new type of sustainable energy storage device. 104 The electrode materials mainly determine the electrochemical performance of supercapacitors. However, commercial carbon-based supercapacitors have poor rate performance and low capacitance. 105 , 106 A critical challenge in current energy storage research is the development of carbon-based capacitors that offer both high rate performance and high capacitance. It has been demonstrated that coal-based graphene derivative-based supercapacitors have excellent capacitive performance in both organic and aqueous electrolytes. 79 Table 1 summarizes the studies of coal-based GFNs electrode materials applied to supercapacitors. Wang et al. used large-size RGO extracted from lignite coal using a high-temperature and high-pressure method for the electrode material of supercapacitors, which exhibited high conductivity and porosity, improving the contact area between the electrolyte and the electrode and facilitating electron/ion transfer. 107
A summary of research on coal-based GFNs electrode materials for supercapacitor applications.
| Electrode material | Carbon source | Electrolyte | Synthesis method | Capacitance | Reference |
|---|---|---|---|---|---|
| Coal-based graphene derivative | Subbituminous | KOH; TEABF4/ACN | Ultrasonic-assisted chemical oxidation method, followed by chemical reduction | 95.65 F g−1 (0.5 A g−1); 50.89 F g−1 (0.5 A g−1) | 79] |
| RGO | Lignite | PVA/H3PO4 | High temperature–high pressure method | 30.6 F cm−3 (0.05 A cm−3) | 107 |
| 3D HPGBs | Coal tar pitch | KOH | Nano-MgO template strategy coupled with KOH activation | 321 F g−1 (0.05 A g−1) | 73] |
| 3D GNCs | Diverse aromatic hydrocarbons | KOH | Nano-ZnO-template strategy coupled with in-situ KOH activation technique | 277 F g−1 (0.05 A g−1) | 62 |
| 3D interconnected porous graphene | Graphitized coal | KOH | Combination of liquid intercalation/oxidation, thermal exfoliation and chemical activation | 225 F g−1 (0.5 A g−1) | 40] |
| A-NSPC | Coal tar pitch | KOH | An ammonium sulfate-assisted chemical blowing strategy | 368 F g−1 (0.5 A g−1) | 108 |
| HPCNs | Coal-based GQDs | KOH | Templateassisted assembly strategy | 230 F g−1 (1 A g−1) | 109 |
| RCDGO/Mn3O4 | Coal-derived graphite | K2SO4 | Reduction with hydrazine | 260 F g−1 (100 mA g−1) | 110 |
Both of the 3D coal-based HPGBs 73 and 3D GNCs 62 exhibit high capacitance, excellent cycling stability and good multiplicity performance as electrode materials for supercapacitors. The 3D carbon shell layer formed by the polycyclic aromatic hydrocarbon molecules of CTP in HPGB improves the electrical conductivity of the electrons. The rich pore structure of HPGB provides abundant active sites and channels for the storage and transportation of ions, and its 3D carbon shell structure contributes to the improvement of electron conductivity and the reduction of electron transfer resistance. The abundant active sites and porous channels in 3D GNCs are favorable to increase the capacitance of GNC electrodes and reduce the ion transfer resistance. Moreover, the graphene network of 3D GNCs helps to shorten the diffusion distance of electrolyte ions and improve the electron conduction. Sun et al. prepared 3D hierarchical porous coal-based graphene supercapacitors exhibited high power density, high energy density and excellent cycling stability in both EMIMBF4 ionic liquid electrolyte and aqueous solution (Figure 4a–j). 40 This may be attributed to its hierarchical void structure and large specific surface area that facilitates rapid ion migration and charge storage.
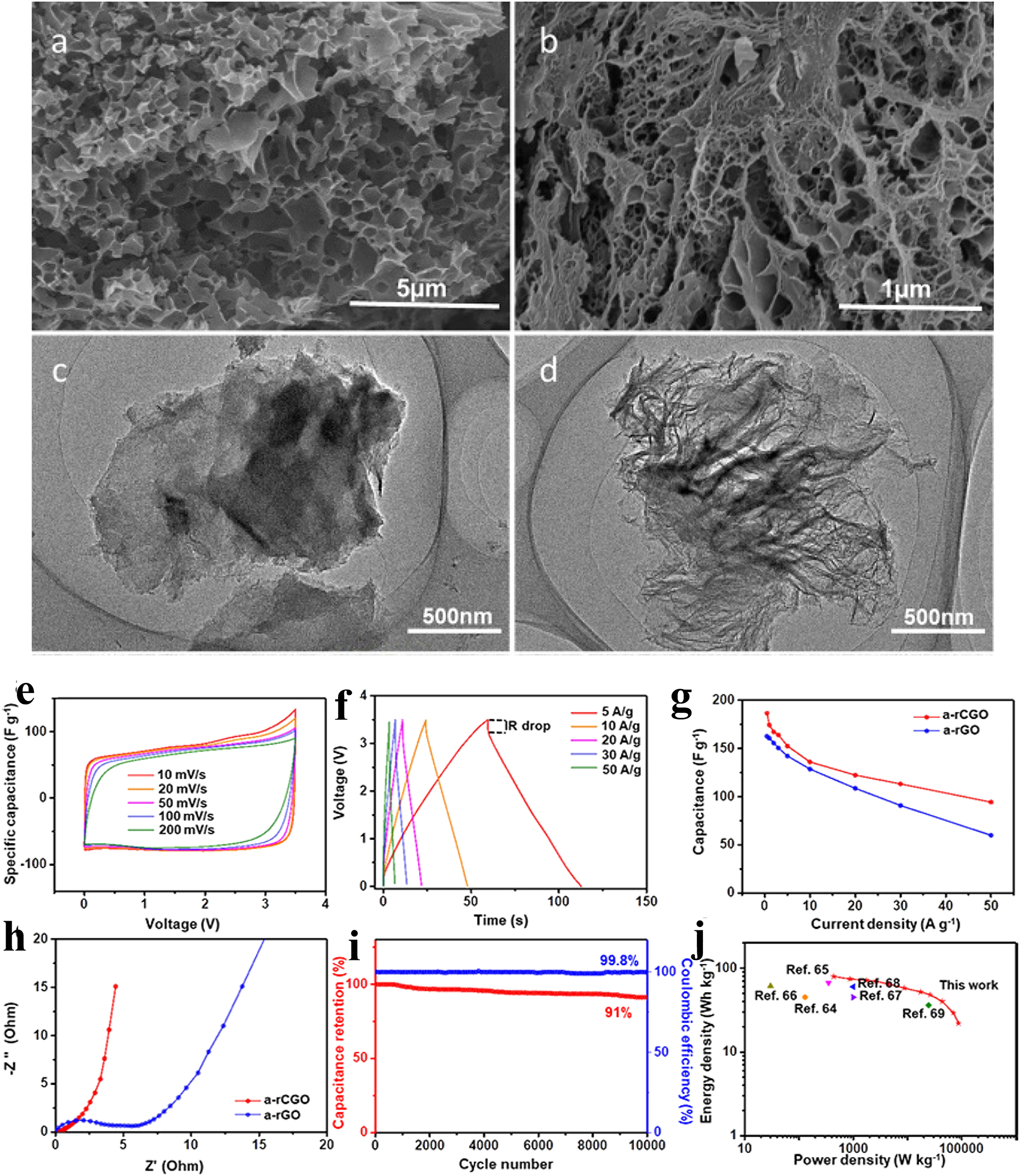
Morphology and applications of 3D hierarchical porous coal-based graphene. (a–d) SEM images of the as-prepared materials, (e–j) electrochemical performance of the materials measured in EMIMBF4 ionic liquid electrolyte. 40
KOH activation of N, S co-doped graphene-like porous carbon (NSPC) prepared from CTP leads to A-NSPC. 108 Notably, the A-NSPC material remarkably retains its interconnected 3D porous network structure. This, coupled with its high specific surface area and rich medium-to-high-scale pores, translates to excellent cycling stability and superior electrochemical performance compared to NSPC. Moreover, the bonds between heteroatoms and C atoms were broken during KOH activation, resulting in a decrease in N and S content and an increase in O content. A proper and higher ratio of oxygens (both C=O and C–O) is favorable for capacitance enhancement. 111 Hierarchical porous carbon nanosheets (HPCNs) prepared from coal-based GQDs as a cornerstone were in situ activated by a small amount of KOH to obtain AHHPCNs exhibiting higher capacitance and good cycling stability than HPCNs. 67 The enhanced performance stems from two key factors: the enlarged mesopore volume facilitates faster ion transport through increased channels, while the increased microporosity and larger specific surface area offer more abundant sites for energy storage.
Coal-based GO/Mn3O4 (RCDGO/Mn3O4) can be used as an electrode material for supercapacitors. 110 Graphene as a conductive substrate not only provides electron transfer channels for Mn3O4, but also provides double-layer capacitance for the overall energy storage (Figure 5f–j). Mn3O4 is tightly immobilized on the highly conductive graphene sheet through strong interactions not only to enable rapid electron transfer, but also to prevent the buildup of the graphene sheet so that its surface area can be used for storing charge. Therefore, the electrode material exhibits high reversibility and good chemical stability.
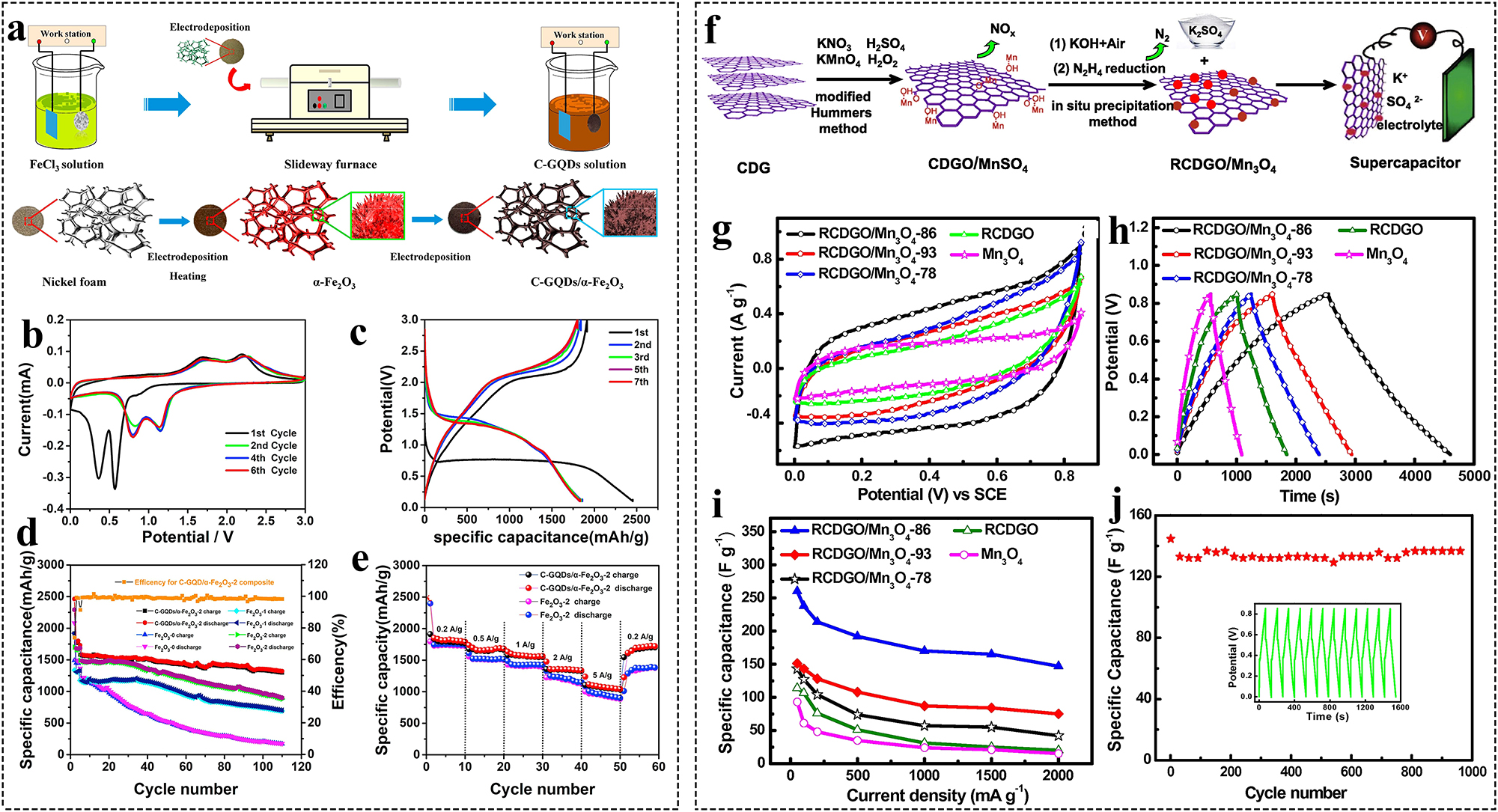
Preparation and application of various types of coal-based GFNs. (a) Preparation of C-GQDs/α-Fe2O3 composites, (b–e) electrochemical performance of C-GQDs/α-Fe2O3 composites; 68 (f) schematic illustration of a by-product free strategy to synthesis of RCDGO/Mn3O4 composite and K2SO4 compound using CDG as the carbon source, (g–j) electrochemical performance of RCDGO/Mn3O4 composites. 110
Rechargeable lithium batteries (LiB) are considered as the most promising application for electric vehicles, electronics and even grid-level electrical energy storage due to their excellent electrochemical properties and high energy density. 112 , 113 , 114 Current LiBs have already reached the upper limit of energy density. 115 , 116 Therefore, the development and exploration of LiB with longer lifetime and higher energy density is crucial for its application market. Table 2 summarizes the studies of coal-based GFNs electrode materials applied to LiB. The large specific surface area and multilayer structure of coal-based multilayer graphene allow it to exhibit excellent Li+ storage capacity and Li+ diffusion, and thus show superior multiplication capacity as an anode material for Li-ion batteries. 46 Xing et al. prepared coal-based CPG for use as an anode material for LiB showed relatively low resistance, excellent cycling performance, excellent multiplication capability ability and high reversible capacity. 45 CPG’s excellent performance for lithium-ion storage can be attributed to several key factors. Firstly, its unique structure of continuous, corrugated nanosheets helps to prevent volume changes during charging and discharging, while also allowing for fast movement of electrons within the material. Secondly, the interconnected network of pores at different size scales provides a large surface area for lithium ions to interact with the carbon and efficient pathways for them to move throughout the electrode. Finally, the defects within CPG’s structure, along with its large pore volume and high surface area, create abundant sites for storing lithium ions, leading to a high storage capacity.
A summary of research on coal-based GFNs electrode materials for LiB applications.
| Electrode material | Carbon source | Synthesis method | Electrochemical properties | Reference |
|---|---|---|---|---|
| Multilayer graphene sheets | Anthracite | Molten metal solvent growth method | 605 mAh g−1 | 46] |
| Porous graphene | Anthracite | Graphitization coupled with liquid oxidation-rapid thermal reduction | 770 mAh g−1 (0.1 C) | 72] |
| Few-layer graphene | Bituminous coal | Microwave-assisted catalytic graphitization process | 287.91 mAh g−1 (0.1 C) | 117 |
| Multilayer graphene spheres | Anthracite; semi-coke | Nickel-catalyzed graphitization strategy | 389.8 mAh g−1; 401.4 mAh g−1 (0.1 A g−1) | 74] |
| Graphene foam | Humic acid derived from coal | Freeze-drying operation | 656 mAh g−1 (50 mA g−1) | 118 |
| C-GQDs/α-Fe2O3 | Anthracite | Second-step electrodeposition | 1,582.5 mAh g−1 (1 A g−1) | 119 |
| α-Fe2O3/CG | Taixi coal | Hydrothermal synthesis strategy | 1,000 mAh g−1 (0.2 A g−1) | 68] |
| FeS@GQDs | Coal tar pitch | Solvothermal method | 718.7 mAh g−1 (100 mA g−1) | 120 |
| Graphene/MoS2 | Humic acid derived from lignite | In situ hydrothermal approach followed by high-temperature calcination | 929 mAh g−1 (100 mA g−1) | 121 |
Coal-based FLGs with Fe and Ni dual-catalytic graphitization prepared using highly ordered graphite layers can promote rapid lithium ion migration. 47 The electrochemical performance of the S5 % Fe–Ni catalyst was superior to that of a single S10 % Fe catalyst. Zhang et al. prepared TDC-based graphene (TRGO) exhibited excellent lithium storage performance, multiplicity performance and cycling stability (Figure 6a–f). 95 Its high pore specific surface area provides more active sites for Li+ migration, the rich mesoporous and microporous structures can shorten the Li+ diffusion path and improve the Li+ storage sites, respectively, and the excellent microcrystalline structure orientation morphology reduces the surface charge transfer resistance and facilitates the efficient Li+ conductivity.
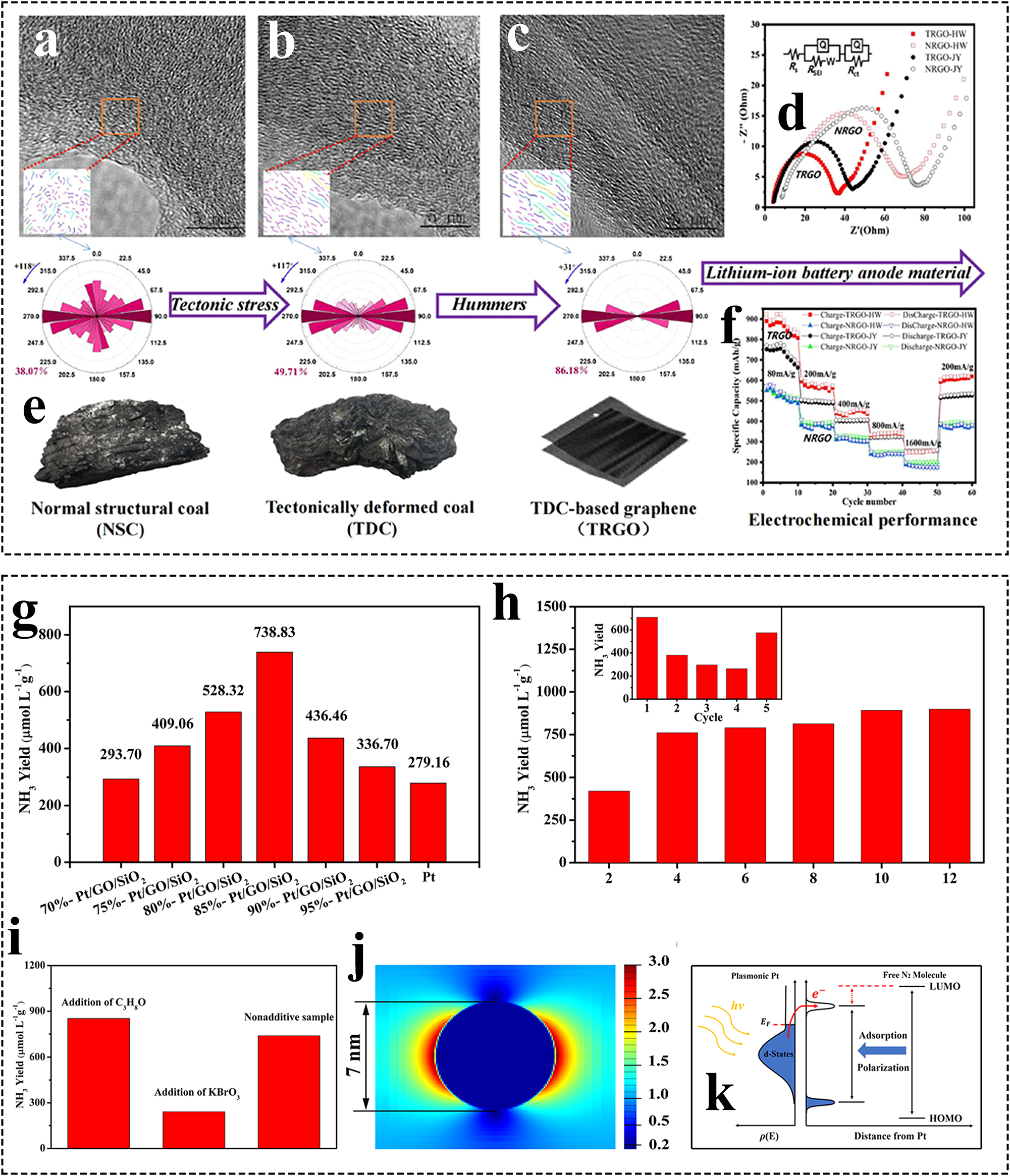
Preparation and application of various types of coal-based GFNs. (a–c) Distribution characteristics of aromatic fringes of anthracite, graphite, and graphene series samples, (e) macro coal sample characteristics of TDCs/NSCs; (d) EIS behavior and (f) rate capability of TRGO/NRGO; 95 (g–h) photocatalytic synthesis of NH3 by Pt/GO/SiO2, (i–k) mechanistic investigation of NH3 synthesis by Pt/GO/SiO2 photocatalysis. 122
It has been found that hollow multilayer graphene spheres as an anode material for LiB also exhibit excellent cycling stability, excellent multiplicity performance and enhanced reversible capacity. 74 Porous 3D graphene and micron-sized silicon composite anode (Si@G foam) using humic acid (HA) extracted from coal as the carbon source exhibited high electrochemical performance with high reversible capacity and remarkable capacity retention. 123 Silicon particles were loaded on a graphene backbone that served as a matrix for the electrical conductors. This 3D graphene network structure not only provides sufficient internal voids to accommodate the volume expansion of the silicon particles, but also shortens the diffusion path of Li+ ions.
Zhang et al. prepared antler-shaped α-Fe2O3 nanoparticles by electrodeposition, and C-GQDs/α-Fe2O3 composites were prepared by a two-step electrodeposition method using coal-based GQDs as the electrolyte. 124 The C-GQD loading formed a “bridge effect” between the antler skeletons and protected the numerous pores on the skeletons (Figure 5a–e). This porous structure facilitates the rapid Li+ transport and improves the conductivity of the material. Meanwhile, the continuous C-GQD network structure maintains the structural integrity of the electrodes and buffers the volume expansion during charging and discharging. Therefore, the composites exhibit good multiplicity and cycling performance. The team also prepared 3D nanoflower-like α-Fe2O3/coal-based graphene (α-Fe2O3/CG) composites by hydrothermal synthesis and used them as an anode for LiB exhibiting high multiplicity performance and excellent cycling stability. 39 The CG as a support structure facilitates the mitigation of the structural collapse of Fe2O3 and acts as a conductive network providing a fast electron transfer rate at the material surface. The nanoflower structure of the composite shortens the diffusion distance of lithium ions and adapts to the volume change during charging and discharging.
The ability of FeS@GQD composites, synthesized using CTP as a carbon source, to store lithium ions was investigated. 120 The bud-like morphology of FeS@ GQD can be observed with many folds at larger magnification. These folds act as lithium storage sites and channels, and their surfaces can flexibly inhibit the volume expansion during electrochemical processes and thus organize to limit the rapid degradation of battery capacity. The successful coupling between GQD and FeS, evidenced by the formation of C–S–C bonds, leads to a synergistic effect, resulting in a material with good porosity and a large specific surface area. Thus, the composite exhibits higher initial coulombic efficiency and better rate performance than pure FeS. Coal-derived graphene embedded with molybdenum disulfide (MoS2-G) can also be used as anode material for high performance LiBs. 121 MoS2-G provides a larger interlayer space and more active sites for accommodating more lithium ions. Therefore, the synergistic effect of graphene and MoS2 improves its lithium storage performance.
Sodium ion batteries (SIBs) are considered to be an alternative to LiB due to their low price, wide distribution, and natural abundance, and show superior potential in energy storage. 3D coal-based graphite microcrystalline/graphene composites (3D-CGC/G) prepared by hydrothermal method showed good electrochemical stability as SIB anodes. 125 The 3D graphene framework of the composite ensured a fast transfer path for electrolyte ions and electrons, and the CGC with abundant defects and various oxygen functional groups provided more reaction sites on the carbon surface for adsorption of Na+.
Chalcogenide solar cells have emerged as a promising photovoltaic technology, attracting significant research interest in recent years. However, optimizing the interface between the carbon electrode and the chalcogenide material remains a critical challenge for maximizing the efficiency of carbon-based chalcogenide solar cells. Coal-based N-GQDs can improve the photovoltaic performance of chalcogenide compounds. 126 In this case, Pb2+ with electron deficient structure can be used as Lewis acid and N-GQD can be used as Lewis base. The electron-rich pyridine nitrogen atoms in N-GQDs establish coordination bonds with uncoordinated lead (Pb2⁺) ions by sharing electron pairs. This interaction effectively reduces the density of hole traps and passivates Pb2⁺ defects on the perovskite surface (Figure 7a). Pb0 disappears after passivation treatment. The non-radiative composite of the chalcogenide films is greatly reduced due to the passivation of N-GQD and the long-term stability is improved.
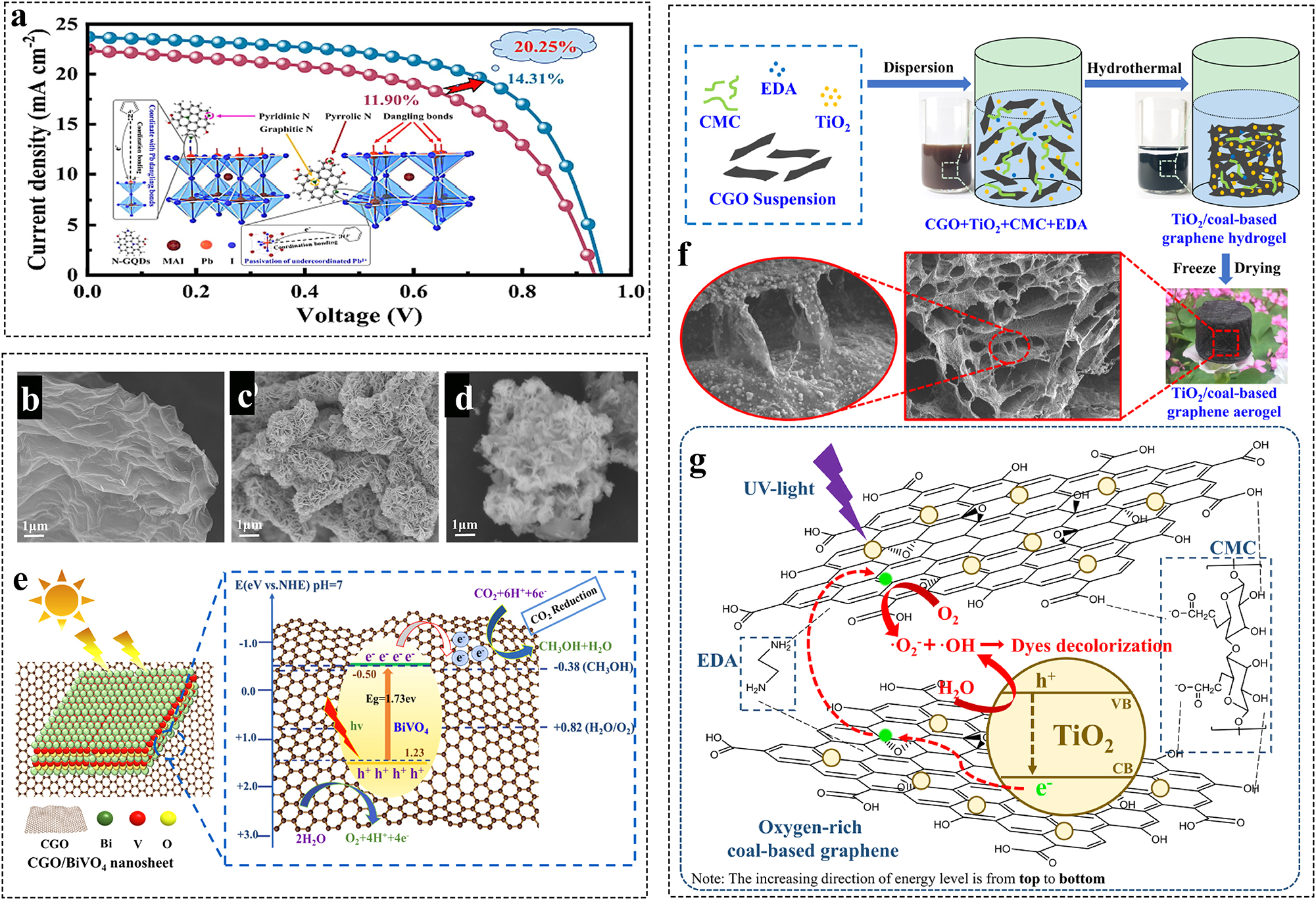
Preparation and application of various types of coal-based GFNs. (a) Schematic illustration of the passivation mechanism of N-GQDs for the MAPbI3 perovskite; 126 (b–d) SEM images for CGO, BiVO4 and CGO/BiVO4 samples, respectively, (e) a schematic of the method of charge separation and transportation in a CGO/BiVO4 combination; 123 (f) schematic illustration of the synthesis route for preparing TiO2/coal-based graphene aerogels, (g) the adsorption-photocatalysis synergy mechanism of TCGA for dyes in aqueous solution under UV light irradiation. 127
Electrospun carbon nanofiber fabrics (ECNFs) show great potential in many aspects such as electronic devices and flexible energy storage materials, but their limited flexibility and strength restrict practical applications. Incorporation of coal-based GQDs (CGQDs) into the spinning solution significantly improves the hydrophobicity, flexibility and strength of ECNFs. 128 This may be attributed to the fact that CGQDs with high chemical reactivity and abundant oxygen-containing functional groups act as cross-linking agents to form a flexible, robust and dense carbon skeleton. The increase in carbon nanofibers was accompanied by an increase in the hydrophobicity of ECNFs.
Incorporation of quantum dots into polymer matrices is also an effective means to improve their application in fields such as photovoltaics. The polymers on the one hand can slow down the agglomeration of QDs and thus ensure their emission properties, and on the other hand can act as a matrix to provide chemical and mechanical stability to the composites. Kovalchuk et al. prepared polymer luminescent composite PVA/GQD by aqueous solution casting method using coal-based GQD as a substrate and polyvinyl alcohol (PVA) as a matrix polymer. 129 The solid-state fluorescence exhibited by the polymer luminescent film was attributed to the fluorescent properties of coal-based GQD. The PL spectrum of the PVA/GQD nanocomposite is broad and covers most of the visible light range, so its application as a luminescent body for white light LEDs has great potential.
4.2 Electrocatalysis
A highly catalytically active oxygen removal reaction (OER) electrocatalyst FeNi/C-LSG can be obtained by non-in situ electrochemical deposition of hydroxide FeNi on the prepared coal-based laser-etched graphene C-LSG. 119 The surface roughened C-LSG exposed more active sites of FeNi hydroxide and improved the reaction kinetics. Silicon hydroxide as active material also contributed to the high activity of FeNi/C-LSG. A recent study by Maung et al. investigated the dielectric properties of co-doped Ag/Ni nano GO composites derived from coal-extracted GO. 130 The study found that the dielectric constant of these composites exhibits a dual response: it decreases with increasing frequency due to charge orientation and dipole rotation, and increases with increasing temperature due to higher electrical conductivity.
Nitrogen and Boron emerge as a novel double dopant pair, offering exciting possibilities for material development. Fei et al. self-assembled coal-based GQDs on graphene to form nanosheets, and formed nitrogen- and boron-co-doped carbon nanomaterials (BN-GQD/G) by high-temperature annealing. 131 The strong interactions between the carbonyl and hydroxyl functional groups of GQD and GO ensured a tight stacking between them and the formation of GQD/G hybrid nanosheets. The abundance of oxygen-containing functional groups and exposed edges of GQD facilitates doping with dopants. BN-GQD/G exhibits excellent ORR activity, but it is affected by dopant concentration and doping time. The ORR activity decreases with increasing doping time. Lower BN doping content leads to a lower number of electrocatalytic sites, which reduces the ORR activity. While higher BN doping content will produce B and N doping pairs with no promoting effect on ORR activity.
Porous n-doped carbon nanosheets (PNCNs) with defects and high graphitic nitrogen content can be synthesized using coal-based GQDs as nitrogen and carbon sources, and their ORR properties can be explored. 132 The abundance of edge defects in coal-based GQDs contributes to the generation of large specific surface area PNCNs. The loose laminated structure of PNNs can also provide additional active sites and improve the mass transfer process of ORR. PCNs-900 (obtained at 900 °C) exhibited excellent ORR activity, which was attributed to the synergistic effect of its high graphitic nitrogen content and high defect density. Watermelon-like metallic Co/graphene hybrids (Co@CEG) can be obtained by electrochemical stripping and in situ functionalization using graphitized coal as a carbon source. 133 The in-situ modification of nitrogen heteroatoms and watermelon-like metallic Co resulted in a perfect combination of conductive graphene-like networks and a greatly enhanced surface area of uniformly dispersed metallic Co. The active Co clusters not only protect the surrounding carbon from corrosion, but also promote electron transfer at the metal/carbon interface. This unique watermelon-like layered structure allows Co@CEG to exhibit superior ORR activity to Pt–C with long-term stability, superior methanol tolerance, efficient four-electron pathways, higher current density and excellent half-wave potential.
Electrochemical reduction of O2 to generate H2O2 is currently a promising approach in the field of H2O2 synthesis, in which the choice of electrocatalyst is crucial. Zhang et al. used the prepared DGLC materials for the electrochemical synthesis of H2O2 and found that they possessed excellent H2O2 selectivity, ORR activity, two-electron selectivity, and electrocatalytic stability. 81 This may be attributed to the synergistic effect of oxygen-containing groups (especially ether groups) and defects/fringes.
4.3 Sensor
Coal-based GQDs can be used as fluorescent probes to detect various metal ions present in water. It has been shown that Cu2+ exhibits the highest selectivity for coal-based GQDs with significant bursting effect. This is attributed to the multisite coordination of oxygen-containing groups in GQD with Cu2+. 124 The Cu2+ concentration showed a linear relationship with the GQD fluorescence intensity ratio of the burst in the range of 0–8 μM. Xu et al. used the prepared coal-based NPS-GQDs for high-sensitivity detection of Pb2+ and found that Pb2+ had a significant bursting effect on its fluorescence. 69 The sulfhydryl and hydroxyl groups of NPS-GQDs have fast chelation kinetics and strong binding affinity with Pb2+, which can change the exciton distribution and electronic structure of NPS-GQDs. The addition of Pb facilitates the nonradiative complexation of excitons through an efficient electron transfer process within the GQDs. The excited state electrons are transferred from the NPS-GQDs to the LUMO of the Pb2+ cation, while the electrons return to the ground state of the NPS-GQDs with a radiationless transfer, leading to their fluorescence burst. Therefore, NPS-GQD can be used as a fluorescent probe for the detection of Pb2+ with an absolute detection limit of 0.75 μM in the range of 1–20 μM.
Particularly, coal-based GQDs can be used as a tool for the detection of glutathione (GSH). Kundu et al. used two different coal-based GQDs synthesized for the detection of GSH and Mn ions (2+ and 7+). 100 The fluorescence of the synthesized GQDs was burst in the presence of Mnn+. Interestingly, the prepared GQDs showed significant changes in both fluorescence intensity and steady-state absorption, but the excited state lifetime values remained constant in the presence of different Mnn+ bursting agents. Moreover, the fluorescence of GQDs recovered in the presence of GSH. Therefore, the synthesized GQDs-Mn nanoprobe system can be effectively applied to the sensitive and selective analysis of GSH through the fluorescence “off-on” mechanism. In the presence of GSH, the detection limits of the two coal-based GQDs were 36 mM and 27 mM for Mn7+ and 96 mM and 99 mM for Mn2+, respectively.
4.4 Photocatalysis
Ammonia (NH3) plays a crucial role in various industries, serving as a key raw material for numerous daily chemical products. Beyond its established applications, NH3 is gaining significant interest as a potential hydrogen storage medium due to its ability to be stored as a liquid and its high hydrogen content. 134 Unique GO-SiO2-Pt sandwich structure was prepared by loading Pt nanoparticles on coal-based GO/SiO2 surface for photocatalytic N2/H2O synthesis of NH3. 122 The Pt nanoparticles were deposited on the SiO2 surface and well dispersed on the coal-based GO/SiO2, which increased the visible light-accepting area of Pt and avoided re-agglomeration (Figure 6g–k). Both the SPR effect of Pt and the electric field induced by photoexcited electrons are involved in the photocatalytic synthesis of NH3. The photoexcited electrons play an important role in the reduction of N2; whereas the electric field induced by the SPR effect of the Pt component on the GO/SiO2 surface may be involved in the polarization of N2, which improves the efficiency of the photocatalytic NH3 synthesis by weakening the N≡N bond.
Zhang et al. prepared Pt/coal-based graphene (Pt/CBG) composites and their good catalytic activity in CO2 photocatalytic reduction process. 135 The Pt nanoparticles were highly dispersed and homogeneous on the CBG surface. The scholar also synthesized 2D/2D nanocomposite structures of coal-based GO/BiVO4 (CGO/BiVO4) by a hydrothermal method and exhibited high catalytic activity for the photoreduction of CO2 to CH3OH. 118 The enhanced photocatalytic activity was firstly attributed to the abundant surface active sites, unique ultrathin structure and wide visible light absorption range of the CGO/BiVO4 composite (Figure 7b–e). Secondly, the simultaneous use of CGO as a carrier and a redox intermediate can improve the separation efficiency of the photogenerated carriers at the heterogeneous interface and reduce the interfacial resistance.
Loading ZnO nanorods onto coal-based GO/SiO2 can obtain ZnO/GO/SiO2 photocatalysts. 136 GO/SiO2 can promote the separation and transfer of photogenerated carriers and act as a carrier of ZnO to promote the generation of active radicals from H2O2. Therefore, ZnO/GO/SiO2 photocatalytic fenton-like oxidative degradation of methylene blue showed excellent catalytic activity. The photocatalytic degradation of 2-nitrophenol by coal-derived CNMs prepared by the OCE method could reach 93 % under natural light. 78
The photocatalytic degradation of methyl orange and rhodamine B was investigated using different coal-based graphene/TiO2 composite photocatalysts prepared by two methods: wet mixing and hydrothermal mixing. 137 The results showed that the composites prepared by hydrothermal method exhibited higher catalytic activity and TiO2 was uniformly distributed on the surface of GO. The incorporation of graphene offers a significant enhancement in the photocatalytic activity of TiO2. Graphene’s exceptional conductivity and large surface area create efficient pathways for charge transfer, thereby significantly suppressing the recombination rate of electron-hole pairs within the photocatalyst. TiO2/coal-based graphene aerogels (TCGAs) also exhibited significantly enhanced photocatalytic activity for the photocatalytic removal of dyes from aqueous solutions. 127 The enhanced photocatalytic performance can be attributed to several factors. Firstly, the crosslinked OCG carbon skeleton offers an efficient framework for transporting electrons during dye removal. Secondly, the abundant structural defects and heteroatoms within the TCGAs composites contribute by providing numerous active sites. Finally, the well-developed 3D structure ensures sufficient space for fuel adsorption (Figure 7f and g).
4.5 Biomedical
The increasing prevalence of chronic diseases and the continuous emergence of new health threats have fueled a surge in the importance of biomedical research. Coal-based GFNs have received attention in biomedical fields such as cancer therapy, biosensing, and bioimaging due to stable photoluminescence, high selectivity, high water solubility, low cytotoxicity, and biocompatibility. Some studies have verified the anticancer properties of synthesized coal-based GQDs by DAPI-stained images and cell morphology analysis of breast cancer cells and neuroblastoma cells. 71 Kang et al. found that PanC-1 cells incubated with coal-based GOQD effectively maintained their original morphology and exhibited green PL. 63 When the GOQD concentration was increased from 0.1 mg mL−1 to 5 mg mL−1, PanC-1 cell survival remained greater than 85 %. Thus, GOQD has low cytotoxicity and high biocompatibility, and has great potential for bioimaging and its related applications.
Biosensing can detect various biomolecules such as nucleic acids, enzymes and proteins. Coal-based GFNs are expected to be a promising material for the development of nanobiosensors capable of selective and highly sensitive detection of DNA. Detection of DNA using coke-based GQD as a fluorescence resonance energy transfer receptor was found to have a low detection limit of 0.004 nM. 138 The wide linear detection range spanned three orders of magnitude and was determined to be 0.004–4 nM. Lee et al. found that the prepared magnetic Fe3O4-loaded coal-based GO nanoparticles have enough space to bind to single-stranded DNA (ssDNA) aptamers and be separated by a magnetic separator for detection of ssDNA, and that the ability of Fe3O4-Coal-GO to capture ssDNA aptamers is about 1.5-fold stronger than that of Fe3O4-Graphite-GO. 56
Synthetic oxidants hold promise as a novel therapeutic approach, offering effects comparable to metalloenzymes in treating diseases associated with excessive oxidative stress. Nilewski et al. reported coal-based GQDs and their poly (ethylene glycol) functionalized derivatives (PEG-GQDs) as potent antioxidants. 139 Furthermore, H2O2 reduction experiments performed by bEnd to evaluate the protective ability of PEG-GQDs found that they have better biostability and solubility, and exhibit in vitro cytoprotective effects against H2O2 even with delayed administration.
Pakhira et al. developed an efficient method for obtaining GO from low-grade coal via cold nitric acid leaching. Coal-based GO can be folded into a closed fist form and was shown to trap tetraphenylporphyrin (TPP). 140 The GO – TPP composites retained the properties of GO, were soluble in ethanol, and were soluble and intact in phosphate buffered saline (PBS, pH, 6.8). GO-TPP releases all trapped TPP in higher pH PBS buffer by opening the clenched fist. Similarly, GO traps donepezil, a drug for Alzheimer’s disease, and releases it at a higher pH of 7.4. This pH-dependent interconversion allows GO to act as a Trojan horse for carrying and releasing drug molecules in a physically compatible environment.
Viral infections are a serious threat to human health while causing a huge social and economic burden. Therefore, the development of novel reusable and functional personal protective equipment is essential to improve public health safety. Galante et al. coated coal-based nano GO functionalized with octadecylamine amine on polyethylene terephthalate fabrics for virus repellency studies. 141 Furthermore, the fabric surface was coated with a polydimethylsiloxane (PDMS) layer and RGO enabling it to exhibit superhydrophobicity and excellent durability. It was shown that the functionalized nano GO/PDMS coating reduced the titers of coronavirus B, herpes simplex virus type 1 and human adenovirus type 5 by 2.4 log(99.6 %), 2.2 log(99.4 %) and 1.8 log(98.6 %), respectively. Therefore, the coating can be used for the development and utilization of antiviral personal protective equipment.
4.6 Others
Doping of coke-derived flash graphene (MCFG) into diglycidyl ether bisphenol A (DGEBA) epoxy resin yielded DGEBA epoxy composites prepared at high loading ratios ranging from 20 % to 50 %. 142 The low-cost-produced MCFG became a filler substitute for the epoxy resin, with a density increase of only 0.06–0.23 g cm−3. MCFG was used as a reinforcing additive to improve the mechanical properties of the composites. The optimized toughness, hardness, Young’s modulus, maximum strain, and maximum compressive strength were increased by 496 %, 140 %, 92 %, 61 %, and 145 %, respectively, and energy consumption and greenhouse gas emissions were reduced.
Coal-based GO aerogel loaded with carboxymethyl cellulose has a 3D interconnected porous structure, abundant oxygen-containing functional groups and beneficial graphene framework defects, which provides effective adsorption sites and good diffusion channels for the adsorption and transport of dye molecules. 143 Large-size graphene (GO-NC) using needle coke as a carbon source contains oxygen-containing groups such as carboxyl and epoxy/hydroxyl groups, which can provide more active sites. 72 The adsorption of MG was found to be superior to GO prepared from natural graphite by the preparation of GO-NC/PAM hydrogel. H₂-DBD plasma technology enables the one-step preparation of coal-based graphene materials (NP/GS) decorated with highly dispersed noble metal nanoparticles through the simultaneous reduction of noble metal salts and GO. These NP/GS composites exhibit high catalytic activity for NOₓ reduction at atmospheric pressure via the ammonia-catalyzed SCR reaction. 144
5 Conclusions
The abundance of coal resources globally necessitates the development of new technologies for low-carbon utilization strategies. This is crucial for mitigating the environmental impact of coal dependence. The development and exploration of coal-based GFNs provide new prospects for the functional application of coal, which can help to realize the strategic goal of “carbon peaking and carbon neutrality”. This article reviews the diverse synthesis methods of coal-based GFNs including graphene, GO, RGO, GQDs, graphene films and 3D graphene nanomaterials. Factors affecting the synthesis of coal-based GFNs include differences in carbonaceous precursors, differences in coal structures and components and differences in reaction conditions. Moreover, coal-based GFNs play an important application in the fields of energy storage, biomedicine, photo-/electrocatalysis and sensing. While significant progress has been made, the control over the structural and functional properties of coal-based GFNs remains limited due to the complexity of the coal molecular structure. Further research is crucial to address this challenge and unlock the full potential of these materials:
Large-scale production of coal-based GFNs faces significant challenge in terms of cost, quality, and environmental impact. Furthermore, many synthesis methods remain unexplored, limiting the ability to precisely tailor GFN properties like morphology, thickness, size, and surface defects. Bridging the gap between promising applications and industrial-scale production necessitates substantial advancements in these areas.
Currently, A key challenge for coal-based GFNs lies in achieving optimal performance for specific applications. In the future, researchers can modify the functionality of the prepared coal-based GFNs through different synthesis strategies or post-processing strategies, and also optimize their performance by constructing heterojunctions, doping elements, and constructing complexes, so as to meet the needs of different fields.
Unveiling the formation mechanism of coal-based GFNs is crucial for achieving controlled preparation of high-quality materials. Therefore, the structures of coal and coal-based GFNs need to be further analyzed through theoretical calculations, simulations, and in situ characterization to provide a theoretical basis and validation methods for the mechanism study.
Limited research on the potential environmental and biological toxicity of coal-based GFNs presents a significant hurdle to their wider application in fields like biomedicine, fluorescent devices, and sensors. These concerns necessitate a comprehensive evaluation of their safety profile. The development of robust toxicity assessment methods is crucial for a more informed understanding of potential risks associated with coal-based GFNs.
In summary, this paper reviews the conversion of coal and its derivatives into diverse GFNs with tailorable morphologies and sizes through various preparation strategies. Unraveling the structure-property relationships in these coal-derived carbon materials remains a crucial challenge for future research. Despite this, the development of coal-based GFNs presents a promising avenue for advancing carbon and coal chemistry. This approach has the potential to not only enrich these scientific fields but also promote low-carbon utilization and clean coal production, ultimately breaking free from the limitations of traditional coal applications. The future of the coal industry may very well be illuminated by the transformative potential of coal-based GFNs.
Funding source: China Postdoctoral Science Foundation
Award Identifier / Grant number: 2022M712881
Funding source: Joint Fund for Science and Technology R&D Programs of Henan Province
Award Identifier / Grant number: 222301420036
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 52174262, 52304299
Funding source: National Key R&D Program of China
Award Identifier / Grant number: 2021YFC2902604
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission. Linjing Hao: Investigation, Methodology, Writing the original draft. Jing-He Yang: Writing-review & editing. Guosheng Li: Writing-review & editing. Peng Li: Methodology, Supervision, Resources, Conceptualization, Writing-review & editing. Yijun Cao: Methodology, Conceptualization, Supervision.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: The financial supports by the National Natural Science Foundation of China (Grant No. 52174262, and 52304299), National Key R&D Program of China (2021YFC2902604), Joint Fund for Science and Technology R&D Programs of Henan Province (222301420036), China Postdoctoral Science Foundation (2022M712881).
-
Data availability: Not applicable.
References
1. Höök, M.; Zittel, W.; Schindler, J.; Aleklett, K. Global Coal Production Outlooks Based on a Logistic Model. Fuel 2010, 89 (11), 3546–3558. https://doi.org/10.1016/j.fuel.2010.06.013.Suche in Google Scholar
2. Li, H.; He, X.; Wu, T.; Jin, B.; Yang, L.; Qiu, J. Synthesis, Modification Strategies and Applications of Coal-Based Carbon Materials. Fuel Process. Technol. 2022, 230, 107203. https://doi.org/10.1016/j.fuproc.2022.107203.Suche in Google Scholar
3. Du, M.; Advincula, P. A.; Ding, X.; Tour, J. M.; Xiang, C. Coal‐Based Carbon Nanomaterials: En Route to Clean Coal Conversion Toward Net Zero CO2. Adv. Mater. 2023, 35 (25), 2300129. https://doi.org/10.1002/adma.202300129.Suche in Google Scholar PubMed
4. Cai, T.; Liu, B.; Pang, E.; Ren, W.; Li, S.; Hu, S. A Review on the Preparation and Applications of Coal-Based Fluorescent Carbon Dots. N. Carbon Mater. 2020, 35 (6), 646–666. https://doi.org/10.1016/S1872-5805(20)60520-0.Suche in Google Scholar
5. Lingam, K.; Podila, R.; Qian, H.; Serkiz, S.; Rao, A. M. Evidence for Edge-State Photoluminescence in Graphene Quantum Dots. Adv. Funct. Mater. 2013, 23 (40), 5062–5065. https://doi.org/10.1002/adfm.201203441.Suche in Google Scholar
6. Chen, Y.; Zheng, M.; Xiao, Y.; Dong, H.; Zhang, H.; Zhuang, J.; Hu, H.; Lei, B.; Liu, Y. A Self‐Quenching-Resistant Carbon-Dot Powder with Tunable Solid-State Fluorescence and Construction of Dual-Fluorescence Morphologies for White Light-Emission. Adv. Mater. 2016, 28 (2), 312–318. https://doi.org/10.1002/adma.201503380.Suche in Google Scholar PubMed
7. Mathews, J. P.; Chaffee, A. L. The Molecular Representations of Coal-A Review. Fuel 2012, 96, 1–14. https://doi.org/10.1016/j.fuel.2011.11.025.Suche in Google Scholar
8. Pang, L. S. K.; Vassallo, A. M.; Wilson, M. A. Fullerenes from Coal. Nature 1991, 352 (6335), 480; https://doi.org/10.1038/352480a0.Suche in Google Scholar
9. Li, K.; Liu, G.; Zheng, L.; Jia, J.; Zhu, Y.; Zhang, Y. Coal-Derived Carbon Nanomaterials for Sustainable Energy Storage Applications. N. Carbon Mater. 2021, 36 (1), 133–154. https://doi.org/10.1016/S1872-5805(21)60010-0.Suche in Google Scholar
10. Novoselov, K. S.; Geim, A. K.; Morozov, S. V.; Jiang, D.; Katsnelson, M. I.; Grigorieva, I. V.; Dubonos, S. V.; Firsov, A. A. Two-Dimensional Gas of Massless Dirac Fermions in Graphene. Nature 2005, 438 (7065), 197–200. https://doi.org/10.1038/nature04233.Suche in Google Scholar PubMed
11. Geim, A. K.; Novoselov, K. S. The Rise of Graphene. Nat. Mater. 2007, 6 (3), 183–191. https://doi.org/10.1038/nmat1849.Suche in Google Scholar PubMed
12. Neto, A. H. C.; Guinea, F.; Peres, N. M. R.; Novoselov, K. S.; Geim, A. K. The Electronic Properties of Graphene. Rev. Mod. Phys. 2009, 81 (1), 109–162. https://doi.org/10.1103/RevModPhys.81.109.Suche in Google Scholar
13. Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A Review of Graphene and Graphene Oxide Sponge: Material Synthesis and Applications to Energy and the Environment. Energy Environ. Sci. 2014, 7 (5), 1564–1596. https://doi.org/10.1039/c3ee43385d.Suche in Google Scholar
14. Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S. H. M.; Li, H. Review on Graphene-Graphene Oxide-Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15 (3), 1012. https://doi.org/10.3390/ma15031012.Suche in Google Scholar PubMed PubMed Central
15. Novoselov, K. S.; Geim, A. K.; Morozov, S. V.; Jiang, D.; Zhang, Y.; Dubonos, S. V.; Grigorieva, I. V.; Firsov, A. A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306 (5696), 666–669. https://doi.org/10.1126/science.1102896.Suche in Google Scholar PubMed
16. Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S. I.; Seal, S. Graphene Based Materials: Past, Present and Future. Prog. Mater. Sci. 2011, 56 (8), 1178–1271. https://doi.org/10.1016/j.pmatsci.2011.03.003.Suche in Google Scholar
17. Latif, U.; Dickert, F. L. Graphene Hybrid Materials in Gas Sensing Applications. Sensors 2015, 15 (12), 30504–30524. https://doi.org/10.3390/s151229814.Suche in Google Scholar PubMed PubMed Central
18. Feng, L.; Liu, Z. Graphene in Biomedicine: Opportunities and Challenges. Nanomedicine 2011, 6 (2), 317–324. https://doi.org/10.2217/NNM.10.158.Suche in Google Scholar PubMed
19. Novoselov, K. S.; Fal’Ko, V. I.; Colombo, L.; Gellert, P. R.; Schwab, M. G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490 (7419), 192–200. https://doi.org/10.1038/nature11458.Suche in Google Scholar PubMed
20. Avouris, P. Graphene: Electronic and Photonic Properties and Devices. Nano Lett. 2010, 10 (11), 4285–4294. https://doi.org/10.1021/nl102824h.Suche in Google Scholar PubMed
21. Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-Graphene in Biomedicine: Theranostic Applications. Chem. Soc. Rev. 2013, 42 (2), 530–547. https://doi.org/10.1039/c2cs35342c.Suche in Google Scholar PubMed
22. Li, F.; Song, J.; Yang, H.; Gan, S.; Zhang, Q.; Han, D.; Ivaska, A.; Niu, L. One-Step Synthesis of graphene/SnO2 Nanocomposites and its Application in Electrochemical Supercapacitors. Nanotechnology 2009, 20 (45), 455602. https://doi.org/10.1088/0957-4484/20/45/455602.Suche in Google Scholar PubMed
23. Kim, F.; Luo, J.; Cruz-Silva, R.; Cote, L. J.; Sohn, K.; Huang, J. Self-Propagating Domino-Like Reactions in Oxidized Graphite. Adv. Funct. Mater. 2010, 20 (17), 2867–2873. https://doi.org/10.1002/adfm.201000736.Suche in Google Scholar
24. Wu, Z.; Ren, W.; Wen, L.; Gao, L.; Zhao, J.; Chen, Z.; Zhou, G.; Li, F.; Cheng, H. Graphene Anchored with Co3O4 Nanoparticles as Anode of Lithium Ion Batteries with Enhanced Reversible Capacity and Cyclic Performance. ACS Nano 2010, 4 (6), 3187–3194. https://doi.org/10.1021/nn100740x.Suche in Google Scholar PubMed
25. Yang, S.; Feng, X.; Ivanovici, S.; Muellen, K. Fabrication of Graphene-Encapsulated Oxide Nanoparticles: Towards High-Performance Anode Materials for Lithium Storage. Angew. Chem. Int. Ed. 2010, 49 (45), 8408–8411. https://doi.org/10.1002/anie.201003485.Suche in Google Scholar PubMed
26. Liu, H.; Ryu, S.; Chen, Z.; Steigerwald, M. L.; Nuckolls, C.; Brus, L. E. Photochemical Reactivity of Graphene. J. Am. Chem. Soc. 2009, 131 (47), 17099. https://doi.org/10.1021/ja9043906.Suche in Google Scholar PubMed
27. Liu, J.; Bai, H.; Wang, Y.; Liu, Z.; Zhang, X.; Sun, D. D. Self-Assembling TiO2 Nanorods on Large Graphene Oxide Sheets at a Two-Phase Interface and Their Anti-Recombination in Photocatalytic Applications. Adv. Funct. Mater. 2010, 20 (23), 4175–4181. https://doi.org/10.1002/adfm.201001391.Suche in Google Scholar
28. Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-Graphene Composite as a High Performance Photocatalyst. ACS Nano 2010, 4 (1), 380–386. https://doi.org/10.1021/nn901221k.Suche in Google Scholar PubMed
29. Du, J.; Lai, X.; Yang, N.; Zhai, J.; Kisailus, D.; Su, F.; Wang, D.; Jiang, L. Hierarchically Ordered Macro-Mesoporous Tio 2-Graphene Composite Films: Improved Mass Transfer, Reduced Charge Recombination, and Their Enhanced Photocatalytic Activities. ACS Nano 2011, 5 (1), 590–596. https://doi.org/10.1021/nn102767d.Suche in Google Scholar PubMed
30. Gray, A.; Balooch, M.; Allegret, S.; De Gendt, S.; Wang, W. Optical Detection and Characterization of Graphene by Broadband Spectrophotornetry. J. Appl. Phys. 2008, 104 (5), 053109. https://doi.org/10.1063/1.2974096.Suche in Google Scholar
31. Mueller, T.; Xia, F.; Avouris, P. Graphene Photodetectors for High-Speed Optical Communications. Nat. Photonics 2010, 4 (5), 297–301. https://doi.org/10.1038/NPHOTON.2010.40.Suche in Google Scholar
32. Arsat, R.; Breedon, M.; Shafiei, M.; Spizziri, P. G.; Gilje, S.; Kaner, R. B.; Kalantar-Zadeh, K.; Wlodarski, W. Graphene-Like Nano-Sheets for Surface Acoustic Wave Gas Sensor Applications. Chem. Phys. Lett. 2009, 467 (4–6), 344–347. https://doi.org/10.1016/j.cplett.2008.11.039.Suche in Google Scholar
33. He, Q.; Sudibya, H. G.; Yin, Z.; Wu, S.; Li, H.; Boey, F.; Huang, W.; Chen, P.; Zhang, H. Centimeter-Long and Large-Scale Micropatterns of Reduced Graphene Oxide Films: Fabrication and Sensing Applications. ACS Nano 2010, 4 (6), 3201–3208. https://doi.org/10.1021/nn100780v.Suche in Google Scholar PubMed
34. Yang, D.; Li, Z.; Liu, M.; Zhang, X.; Chen, Y.; Xue, H.; Ye, E.; Luque, R. Biomass-Derived Carbonaceous Materials: Recent Progress in Synthetic Approaches, Advantages, and Applications. ACS Sustain. Chem. Eng. 2019, 7 (5), 4564–4585. https://doi.org/10.1021/acssuschemeng.8b06030.Suche in Google Scholar
35. Hussain, A.; Mehdi, S. M.; Abbas, N.; Hussain, M.; Naqvi, R. A. Synthesis of Graphene from Solid Carbon Sources: A Focused Review. Mater. Chem. Phys. 2020, 248, 122924. https://doi.org/10.1016/j.matchemphys.2020.122924.Suche in Google Scholar
36. Yan, Y.; Nashath, F. Z.; Chen, S.; Manickam, S.; Lim, S. S.; Zhao, H.; Lester, E.; Wu, T.; Pang, C. H. Synthesis of Graphene: Potential Carbon Precursors and Approaches. Nanotechnol. Rev. 2020, 9 (1), 1284–1314. https://doi.org/10.1515/ntrev-2020-0100.Suche in Google Scholar
37. Saha, J. K.; Dutta, A. A Review of Graphene: Material Synthesis from Biomass Sources. Waste Biomass Valori. 2022, 13 (3), 1385–1429. https://doi.org/10.1007/s12649-021-01577-w.Suche in Google Scholar PubMed PubMed Central
38. Rane, K.; Adams, J. J.; Thode, J. M.; Leonard, B. M.; Huo, J.; Goual, L. Multistep Fractionation of Coal and Application for Graphene Synthesis. ACS Omega 2021, 6 (25), 16573–16583. https://doi.org/10.1021/acsomega.1c01614.Suche in Google Scholar PubMed PubMed Central
39. Zhang, C.; Xie, Y.; Zhang, C.; Lin, J. Upgrading Coal to Multifunctional Graphene-Based Materials by Direct Laser Scribing. Carbon 2019, 153, 585–591. https://doi.org/10.1016/j.carbon.2019.07.070.Suche in Google Scholar
40. Sun, L.; Zhao, Z.; Sun, Y.; Wang, X.; Liu, X.; Yang, Y.; Qiu, J. Activated Coal-Based Graphene with Hierarchical Porous Structures for Ultra-High Energy Density Supercapacitors. Diam. Relat. Mater. 2020, 106, 107827. https://doi.org/10.1016/j.diamond.2020.107827.Suche in Google Scholar
41. Algozeeb, W. A.; Savas, P. E.; Luong, D. X.; Chen, W. Y.; Kittrell, C.; Bhat, M.; Shahsavari, R.; Tour, J. M. Flash Graphene from Plastic Waste. ACS Nano 2020, 14 (11), 15595–15604. https://doi.org/10.1021/acsnano.0c06328.Suche in Google Scholar PubMed
42. Luong, D. X.; Bets, K. V.; Algozeeb, W. A.; Stanford, M. G.; Kittrell, C.; Chen, W.; Salvatierra, R. V.; Ren, M.; Mchugh, E. A.; Advincula, P. A.; Wang, Z.; Bhatt, M.; Guo, H.; Mancevski, V.; Shahsavari, R.; Yakobson, B. I.; Tour, J. M. Gram-Scale Bottom-Up Flash Graphene Synthesis. Nature 2020, 577 (7792), 647–651. https://doi.org/10.1038/s41586-020-1938-0.Suche in Google Scholar PubMed
43. Advincula, P. A.; Luong, D. X.; Chen, W. Y.; Raghuraman, S.; Shahsavari, R.; Tour, J. M. Flash Graphene from Rubber Waste. Carbon 2021, 178, 649–656. https://doi.org/10.1016/j.carbon.2021.03.020.Suche in Google Scholar
44. Huang, P.; Zhu, R.; Zhang, X.; Zhang, W. Effect of Free Radicals and Electric Field on Preparation of Coal Pitch-Derived Graphene Using Flash Joule Heating. Chem. Eng. J. 2022, 450, 137999. https://doi.org/10.1016/j.cej.2022.137999.Suche in Google Scholar
45. Xing, B.; Zeng, H.; Huang, G.; Zhang, C.; Yuan, R.; Cao, Y.; Chen, Z.; Yu, J. Porous Graphene Prepared from Anthracite as High Performance Anode Materials for Lithium-Ion Battery Applications. J. Alloys Compd. 2019, 779, 202–211. https://doi.org/10.1016/j.jallcom.2018.11.288.Suche in Google Scholar
46. Yu, C.; Shen, W.; Yan, J.; Zhong, M.; Zhang, J.; Li, L.; Hao, Q.; Gao, F.; Tian, Y.; Huang, Y. Growing Large-Area Multilayer Graphene Sheets on Molten Cerium via Anthracite as Carbon Source. Inorg. Chem. Commun. 2020, 112, 107729. https://doi.org/10.1016/j.inoche.2019.107729.Suche in Google Scholar
47. Islam, F.; Tahmasebi, A.; Wang, R.; Yu, J. Structure of Coal-Derived Metal-Supported Few-Layer Graphene Composite Materials Synthesized Using a Microwave-Assisted Catalytic Graphitization Process. Nanomaterials 2021, 11 (7), 1672. https://doi.org/10.3390/nano11071672.Suche in Google Scholar PubMed PubMed Central
48. Islam, F.; Tahmasebi, A.; Moghtaderi, B.; Yu, J. Structural Investigation of the Synthesized Few-Layer Graphene from Coal under Microwave. Nanomaterials 2022, 12 (1), 57. https://doi.org/10.3390/nano12010057.Suche in Google Scholar PubMed PubMed Central
49. Xu, H.; Lin, Q.; Zhou, T.; Chen, T.; Lin, S.; Dong, S. Facile Preparation of Graphene Nanosheets by Pyrolysis of Coal-Tar Pitch with the Presence of Aluminum. J. Anal. Appl. Pyrol. 2014, 110, 481–485. https://doi.org/10.1016/j.jaap.2014.10.017.Suche in Google Scholar
50. Lin, P.; Zhang, Y.; Tan, X.; Xiong, R.; Sa, B.; Lin, Q. Microscopic Origin of Graphene Nanosheets Derived from Coal-Tar Pitch by Treating Al4C3 as the Intermediate. Phys. Chem. Chem. Phys. 2021, 23 (21), 12449–12455. https://doi.org/10.1039/D1CP01575C.Suche in Google Scholar PubMed
51. Wang, K.; Zhang, X.; Zhang, X.; Lin, Q.; Huang, X. Preparation of Fluffy Graphene Nanosheets from Coal-Tar Pitch with Nano-Al2O3 as Filler. J. Anal. Appl. Pyrol. 2016, 117, 354–356. https://doi.org/10.1016/j.jaap.2015.12.009.Suche in Google Scholar
52. Purwandari, V.; Gea, S.; Wirjosentono, B.; Haryono, A.; Kusumawati, Y.; Fatmawati, S.; Purnomo, A. S.; Kurniawan, F.; Juwono, H. Synthesis of Graphene Oxide from the Sawahlunto-Sijunjung Coal via Modified Hummers Method. AIP Conf. Proc. 2018, 2049 (1), 020065. https://doi.org/10.1063/1.5082470.Suche in Google Scholar
53. Liu, S.; Xue, J.; Liu, X.; Chen, H.; Li, X. Pitch Derived Graphene Oxides: Characterization and Effect on Pyrolysis and Carbonization of Coal Tar Pitch. J. Anal. Appl. Pyrol. 2020, 145, 104746. https://doi.org/10.1016/j.jaap.2019.104746.Suche in Google Scholar
54. Das, B.; Kundu, R.; Chakravarty, S. Preparation and Characterization of Graphene Oxide from Coal. Mater. Chem. Phys. 2022, 290, 126597. https://doi.org/10.1016/j.matchemphys.2022.126597.Suche in Google Scholar
55. Sahoo, P.; Shubhadarshinee, L.; Jali, B. R.; Mohapatra, P.; Barick, A. K. Synthesis and Characterization of Graphene Oxide and Graphene from Coal. Mater. Today: Proc. 2022, 56, 2421–2427. https://doi.org/10.1016/j.matpr.2021.08.206.Suche in Google Scholar
56. Lee, S.; Mahajan, R. L. A Facile Method for Coal to Graphene Oxide and its Application to a Biosensor. Carbon 2021, 181, 408–420. https://doi.org/10.1016/j.carbon.2021.05.007.Suche in Google Scholar
57. Sierra, U.; Álvarez, P.; Blanco, C.; Granda, M.; Santamaría, R.; Menéndez, R. Cokes of Different Origin as Precursors of Graphene Oxide. Fuel 2016, 166, 400–403. https://doi.org/10.1016/j.fuel.2015.10.112.Suche in Google Scholar
58. Leandro, A. P. M.; Seas, M. A.; Vap, K.; Tyrrell, A. S.; Jain, V.; Wahab, H.; Johnson, P. A. Evolution of Structural and Electrical Properties in Coal-Derived Graphene Oxide Nanomaterials During High-Temperature Annealing. Diam. Relat. Mater. 2021, 112, 108244. https://doi.org/10.1016/j.diamond.2021.108244.Suche in Google Scholar
59. He, M.; Guo, X.; Huang, J.; Shen, H.; Zeng, Q.; Wang, L. Mass Production of Tunable Multicolor Graphene Quantum Dots from an Energy Resource of Coke by a One-Step Electrochemical Exfoliation. Carbon 2018, 140, 508–520. https://doi.org/10.1016/j.carbon.2018.08.067.Suche in Google Scholar
60. Seel, J. A.; Dahn, J. R. Electrochemical Intercalation of Pf6 into Graphite. J. Electrochem. Soc. 2000, 147 (3), 892–898. https://doi.org/10.1149/1.1393288.Suche in Google Scholar
61. Katinonkul, W.; Lerner, M. M. Graphite Intercalation Compounds with Large Fluoroanions. J. Fluor. Chem. 2007, 128 (4), 332–335. https://doi.org/10.1016/j.jfluchem.2006.10.004.Suche in Google Scholar
62. He, X.; Li, X.; Ma, H.; Han, J.; Zhang, H.; Yu, C.; Xiao, N.; Qiu, J. Zno Template Strategy for the Synthesis of 3d Interconnected Graphene Nanocapsules from Coal Tar Pitch as Supercapacitor Electrode Materials. J. Power Sources 2017, 340, 183–191. https://doi.org/10.1016/j.jpowsour.2016.11.073.Suche in Google Scholar
63. Kang, S.; Kim, K. M.; Jung, K.; Son, Y.; Mhin, S.; Ryu, J. H.; Shim, K. B.; Lee, B.; Han, H.; Song, T. Publisher Correction: Graphene Oxide Quantum Dots Derived from Coal for Bioimaging: Facile and Green Approach. Sci. Rep. 2020, 10 (1), 4101. https://doi.org/10.1038/s41598-020-60499-0.Suche in Google Scholar PubMed PubMed Central
64. Yang, G. W. Laser Ablation in Liquids: Applications in the Synthesis of Nanocrystals. Prog. Mater. Sci. 2007, 52 (4), 648–698. https://doi.org/10.1016/j.pmatsci.2006.10.016.Suche in Google Scholar
65. Amendola, V.; Meneghetti, M. Laser Ablation Synthesis in Solution and Size Manipulation of Noble Metal Nanoparticles. Phys. Chem. Chem. Phys. 2009, 11 (20), 3805–3821. https://doi.org/10.1039/b900654k.Suche in Google Scholar PubMed
66. Amendola, V.; Meneghetti, M. What Controls the Composition and the Structure of Nanomaterials Generated by Laser Ablation in Liquid Solution? Phys. Chem. Chem. Phys. 2013, 15 (9), 3027–3046. https://doi.org/10.1039/c2cp42895d.Suche in Google Scholar PubMed
67. Zhang, D.; Goekce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117 (5), 3990–4103. https://doi.org/10.1021/acs.chemrev.6b00468.Suche in Google Scholar PubMed
68. Zhang, Y.; Li, K.; Ren, S.; Dang, Y.; Liu, G.; Zhang, R.; Zhang, K.; Long, X.; Jia, K. Coal-Derived Graphene Quantum Dots Produced by Ultrasonic Physical Tailoring and Their Capacity for Cu(ii) Detection. ACS Sustain. Chem. Eng. 2019, 7 (11), 9793–9799. https://doi.org/10.1021/acssuschemeng.8b06792.Suche in Google Scholar
69. Xu, Y.; Wang, S.; Hou, X.; Sun, Z.; Jiang, Y.; Dong, Z.; Tao, Q.; Man, J.; Cao, Y. Coal-Derived Nitrogen, Phosphorus and Sulfur Co-Doped Graphene Quantum Dots: A Promising Ion Fluorescent Probe. Appl. Surf. Sci. 2018, 445, 519–526. https://doi.org/10.1016/j.apsusc.2018.03.156.Suche in Google Scholar
70. Liu, Q.; Zhang, J.; He, H.; Huang, G.; Xing, B.; Jia, J.; Zhang, C. Green Preparation of High Yield Fluorescent Graphene Quantum Dots from Coal-Tar-Pitch by Mild Oxidation. Nanomaterials 2018, 8 (10), 844. https://doi.org/10.3390/nano8100844.Suche in Google Scholar PubMed PubMed Central
71. Ghorai, S.; Roy, I.; De, S.; Dash, P. S.; Basu, A.; Chattopadhyay, D. Exploration of the Potential Efficacy of Natural Resource-Derived Blue-Emitting Graphene Quantum Dots in Cancer Therapeutic Applications. New J. Chem. 2020, 44 (14), 5366–5376. https://doi.org/10.1039/C9NJ06239D.Suche in Google Scholar
72. Xing, X.; Zhang, X.; Zhang, K.; Jin, L.; Cao, Q. Preparation of Large-Sized Graphene from Needle Coke and the Adsorption for Malachite Green with its Graphene Oxide. Fullerenes, Nanotub. Carbon Nanostruct. 2019, 27 (2), 97–105. https://doi.org/10.1080/1536383X.2018.1512099.Suche in Google Scholar
73. He, X.; Zhang, H.; Zhang, H.; Li, X.; Xiao, N.; Qiu, J. Direct Synthesis of 3d Hollow Porous Graphene Balls from Coal Tar Pitch for High Performance Supercapacitors. J. Mater. Chem. A 2014, 2 (46), 19633–19640. https://doi.org/10.1039/C4TA03323J.Suche in Google Scholar
74. Zhong, M.; Yan, J.; Wu, H.; Shen, W.; Zhang, J.; Yu, C.; Li, L.; Hao, Q.; Gao, F.; Tian, Y.; Huang, Y.; Guo, S. Multilayer Graphene Spheres Generated from Anthracite and Semi-Coke as Anode Materials for Lithium-Ion Batteries. Fuel Process. Technol. 2020, 198, 106241. https://doi.org/10.1016/j.fuproc.2019.106241.Suche in Google Scholar
75. Vijapur, S. H.; Wang, D.; Botte, G. G. Raw Coal Derived Large Area and Transparent Graphene Films. ECS Solid State Lett. 2013, 2 (7), M45–M47. https://doi.org/10.1149/2.005307ssl.Suche in Google Scholar
76. Vijapur, S. H.; Wang, D.; Ingram, D. C.; Botte, G. G. An Investigation of Growth Mechanism of Coal Derived Graphene Films. Mater. Today Commun. 2017, 11, 147–155. https://doi.org/10.1016/j.mtcomm.2017.04.003.Suche in Google Scholar
77. Singh, A.; Ojha, A. K. Coal Derived Graphene as an Efficient Supercapacitor Electrode Material. Chem. Phys. 2020, 530, 110607. https://doi.org/10.1016/j.chemphys.2019.110607.Suche in Google Scholar
78. Das, T.; Boruah, P. K.; Das, M. R.; Saikia, B. K. Formation of Onion-Like Fullerene and Chemically Converted Graphene-Like Nanosheets from Low-Quality Coals: Application in Photocatalytic Degradation of 2-Nitrophenol. RSC Adv. 2016, 6 (42), 35177–35190. https://doi.org/10.1039/C6RA04392E.Suche in Google Scholar
79. Tamuly, J.; Bhattacharjya, D.; Saikia, B. K. Low-Grade Coal Feedstock for the Synthesis of High-Valued Graphene Derivatives for Energy Storage Application. Energy Fuels 2023, 37 (4), 3260–3271. https://doi.org/10.1021/acs.energyfuels.2c04086.Suche in Google Scholar
80. Sierra, U.; Mercado, A.; Cuara, E.; Barriga-Castro, E. D.; Cortés, A.; Gallardo-Vega, C.; Fernández, S. Coke-Derived Few Layer Graphene-Like Materials by Mild Planetary Milling Exfoliation. Fuel 2020, 262, 116455. https://doi.org/10.1016/j.fuel.2019.116455.Suche in Google Scholar
81. Zhang, C.; Zhang, J.; Zhang, J.; Song, M.; Huang, X.; Liu, W.; Xiong, M.; Chen, Y.; Xia, S.; Yang, H.; Wang, D. Tuning Coal into Graphene-Like Nanocarbon for Electrochemical H2O2 Production with Nearly 100% Faraday Efficiency. ACS Sustain. Chem. Eng. 2021, 9 (28), 9369–9375. https://doi.org/10.1021/acssuschemeng.1c02357.Suche in Google Scholar
82. Savitskii, D. P. Preparation and Characterization of Colloidal Dispersions of Graphene-Like Structures from Different Ranks of Coals. J. Fuel Chem. Technol. 2017, 45 (8), 897–907.10.1016/S1872-5813(17)30043-9Suche in Google Scholar
83. Ye, R.; Peng, Z.; Metzger, A.; Lin, J.; Mann, J. A.; Huang, K.; Xiang, C.; Fan, X.; Samuel, E. L. G.; Alemany, L. B.; Martí, A. A.; Tour, J. M. Bandgap Engineering of Coal-Derived Graphene Quantum Dots. ACS Appl. Mater. Interfaces 2015, 7 (12), 7041–7048. https://doi.org/10.1021/acsami.5b01419.Suche in Google Scholar PubMed
84. Dong, Y.; Lin, J.; Chen, Y.; Fu, F.; Chi, Y.; Chen, G. Graphene Quantum Dots, Graphene Oxide, Carbon Quantum Dots and Graphite Nanocrystals in Coals. Nanoscale 2014, 6 (13), 7410–7415. https://doi.org/10.1039/C4NR01482K.Suche in Google Scholar
85. Fernández-García, L.; Álvarez, P.; Pérez-Mas, A. M.; Blanco, C.; Santamaría, R.; Menéndez, R.; Granda, M. Peculiarities of the Production of Graphene Oxides with Controlled Properties from Industrial Coal Liquids. Fuel 2017, 203, 253–260. https://doi.org/10.1016/j.fuel.2017.04.130.Suche in Google Scholar
86. Fernández-García, L.; Álvarez, P.; Pérez-Mas, A. M.; Blanco, C.; Santamaría, R.; Menéndez, R.; Granda, M. Role of Quinoline Insoluble Particles During the Processing of Coal Tars to Produce Graphene Materials. Fuel 2017, 206, 99–106. https://doi.org/10.1016/j.fuel.2017.05.099.Suche in Google Scholar
87. Marques, M.; Suarez-Ruiz, I.; Flores, D.; Guedes, A.; Rodrigues, S. Correlation Between Optical, Chemical and Micro-Structural Parameters of High-Rank Coals and Graphite. Int. J. Coal Geol. 2009, 77 (3–4), 377–382. https://doi.org/10.1016/j.coal.2008.06.002.Suche in Google Scholar
88. Yusuf, M.; Kumar, M.; Khan, M. A.; Sillanpaa, M.; Arafat, H. A Review on Exfoliation, Characterization, Environmental and Energy Applications of Graphene and Graphene-Based Composites. Adv. Colloid Interface Sci. 2019, 273, 102036. https://doi.org/10.1016/j.cis.2019.102036.Suche in Google Scholar PubMed
89. Van Niekerk, D.; Mathews, J. P. Molecular Representations of Permian-Aged Vitrinite-Rich and Inertinite-Rich South African Coals. Fuel 2010, 89 (1), 73–82. https://doi.org/10.1016/j.fuel.2009.07.020.Suche in Google Scholar
90. Rodrigues, S.; Suarez-Ruiz, I.; Marques, M.; Camean, I.; Flores, D. Microstructural Evolution of High Temperature Treated Anthracites of Different Rank. Int. J. Coal Geol. 2011, 87 (3–4), 204–211. https://doi.org/10.1016/j.coal.2011.06.009.Suche in Google Scholar
91. Wang, L.; Zhang, H.; Li, Y. On the Difference of Characterization and Supercapacitive Performance of Graphene Nanosheets from Precursors of Inertinite- and Vitrinite-Rich Coal. J. Alloys Compd. 2020, 815, 152502. https://doi.org/10.1016/j.jallcom.2019.152502.Suche in Google Scholar
92. Li, R.; Tang, Y.; Che, Q.; Huan, X.; Ma, P.; Luo, P.; Mao, X. Study on the Microstructure of the Symbiosis of Coal-Based Graphene and Coal-Based Graphene Quantum Dots: Preparation and Characterization. Nanotechnology 2022, 33 (45), 455702. https://doi.org/10.1088/1361-6528/ac842e.Suche in Google Scholar PubMed
93. Li, R.; Tang, Y.; Che, Q.; Ma, P.; Luo, P.; Lu, X.; Dong, M. Effects of Coal Rank and Macerals on the Structure Characteristics of Coal-Based Graphene Materials from Anthracite in Qinshui Coalfield. Minerals 2022, 12 (5), 588. https://doi.org/10.3390/min12050588.Suche in Google Scholar
94. Lan, C.; Tang, Y.; Huan, X.; Che, Q. Effects of Minerals in Anthracite on the Formation of Coal-Based Graphene. ChemistrySelect 2019, 4 (19), 5937–5944. https://doi.org/10.1002/slct.201900669.Suche in Google Scholar
95. Zhang, H.; Zhang, Y.; Li, J.; Ma, Z. Advantages of Structure and Electrochemical Properties of Graphene Prepared from Tectonically Deformed Coal. ACS Omega 2023, 8 (28), 25142–25154. https://doi.org/10.1021/acsomega.3c02073.Suche in Google Scholar PubMed PubMed Central
96. Sasikala, S. P.; Henry, L.; Yesilbag Tonga, G.; Huang, K.; Das, R.; Giroire, B.; Marre, S.; Rotello, V. M.; Penicaud, A.; Poulin, P.; Aymonier, C. High Yield Synthesis of Aspect Ratio Controlled Graphenic Materials from Anthracite Coal in Supercritical Fluids. ACS Nano 2016, 10 (5), 5293–5303. https://doi.org/10.1021/acsnano.6b01298.Suche in Google Scholar PubMed
97. Puente-Siller, D. M.; García-Castillo, A. E.; López-Corpus, J. A.; Perea-Garduño, A. Evolution in the Formation of Graphene Nanocapsules from Coal Tar Pitch. Int. J. Coal Sci. Technol. 2020, 7 (4), 816–824.10.1007/s40789-020-00311-6Suche in Google Scholar
98. Ye, R.; Xiang, C.; Lin, J.; Peng, Z.; Huang, K.; Yan, Z.; Cook, N. P.; Samuel, E. L. G.; Hwang, C.; Ruan, G.; Ceriotti, G.; Raji, A. O.; Martí, A. A.; Tour, J. M. Correction: Corrigendum: Coal as an Abundant Source of Graphene Quantum Dots. Nat. Commun. 2015, 6 (1), 2943. https://doi.org/10.1038/ncomms8063.Suche in Google Scholar PubMed
99. Ye, R.; Xiang, C.; Lin, J.; Peng, Z.; Huang, K.; Yan, Z.; Cook, N. P.; Samuel, E. L. G.; Hwang, C.; Ruan, G.; Ceriotti, G.; Raji, A. O.; Martí, A. A.; Tour, J. M. Coal as an Abundant Source of Graphene Quantum Dots. Nat. Commun. 2013, 4 (1), 2943. https://doi.org/10.1038/ncomms3943.Suche in Google Scholar PubMed
100. Kundu, A.; Maity, B.; Basu, S. Coal-Derived Graphene Quantum Dots with a Mn2+/Mn7+ Nanosensor for Selective Detection of Glutathione by a Fluorescence Switch-Off-On Assay. New J. Chem. 2022, 46 (16), 7545–7556. https://doi.org/10.1039/D2NJ00220E.Suche in Google Scholar
101. Zhao, S.; Luo, Z.; Fang, M.; Wang, Q.; Cen, J. Characteristics of Graphene Growth at Different Temperatures from the Benzene Ring Structure in Coal Tar. Processes 2023, 11 (2), 593. https://doi.org/10.3390/pr11020593.Suche in Google Scholar
102. Wu, D.; Wang, M.; Zeng, J.; Yao, J.; Jia, C.; Zhang, H.; Li, J. Preparation and Characterization of Graphene from Refined Benzene Extracted from Low-Rank Coal: Based on the Cvd Technology. Molecules 2021, 26 (7), 1900. https://doi.org/10.3390/molecules26071900.Suche in Google Scholar PubMed PubMed Central
103. Gubernata, M.; Fraczek-Szczyptaa, A.; Tomalab, J.; Blazewicza, S. Catalytic Graphene Formation in Coal Tar Pitchderived Carbon Structure in the Presence of SiO2 Nanoparticles. Ceram. Int. 2017, 44 (3), 3085–3091.10.1016/j.ceramint.2017.11.072Suche in Google Scholar
104. Simon, P.; Gogotsi, Y. Materials for Electrochemical Capacitors. Nat. Mater. 2008, 7 (11), 845–854. https://doi.org/10.1038/nmat2297.Suche in Google Scholar PubMed
105. Kondrat, S.; Perez, C. R.; Presser, V.; Gogotsi, Y.; Kornyshev, A. A. Effect of Pore Size and its Dispersity on the Energy Storage in Nanoporous Supercapacitors. Energy Environ. Sci. 2012, 5 (4), 6474–6479. https://doi.org/10.1039/c2ee03092f.Suche in Google Scholar
106. Lukatskaya, M. R.; Mashtalir, O.; Ren, C. E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P. L.; Naguib, M.; Simon, P.; Barsoum, M. W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341 (6153), 1502–1505. https://doi.org/10.1126/science.1241488.Suche in Google Scholar PubMed
107. Wang, Y.; Zhang, C. Reduced Graphene Oxide Derived from Low-Grade Coal for High-Performance Flexible Supercapacitors with Ultrahigh Cyclability. Nanomaterials 2022, 12 (17), 2989. https://doi.org/10.3390/nano12172989.Suche in Google Scholar PubMed PubMed Central
108. Li, G.; Chen, S.; Wang, Y.; Wang, G.; Wu, Y.; Xu, Y. N, S Co-Doped Porous Graphene-like Carbon Synthesized by a Facile Coal Tar Pitch-Blowing Strategy for High-Performance Supercapacitors. Chem. Phys. Lett. 2023, 827, 140712. https://doi.org/10.1016/j.cplett.2023.140712.Suche in Google Scholar
109. Zhang, S.; Zhu, J.; Qing, Y.; Fan, C.; Wang, L.; Huang, Y.; Sheng, R.; Guo, Y.; Wang, T.; Pan, Y.; Lv, Y.; Song, H.; Jia, D. Construction of Hierarchical Porous Carbon Nanosheets from Template-Assisted Assembly of Coal-Based Graphene Quantum Dots for High Performance Supercapacitor Electrodes. Mater. Today Energy 2017, 6, 36–45. https://doi.org/10.1016/j.mtener.2017.08.003.Suche in Google Scholar
110. Gao, F.; Qu, J.; Zhao, Z.; Zhou, Q.; Li, B.; Qiu, J. A Green Strategy for the Synthesis of Graphene Supported Mn3O4 Nanocomposites from Graphitized Coal and Their Supercapacitor Application. Carbon 2014, 80, 640–650. https://doi.org/10.1016/j.carbon.2014.09.008.Suche in Google Scholar
111. Razmjooei, F.; Singh, K.; Kang, T. H.; Chaudhari, N.; Yuan, J.; Yu, J. Urine to Highly Porous Heteroatom-Doped Carbons for Supercapacitor: A Value Added Journey for Human Waste. Sci. Rep. 2017, 7, 10910. https://doi.org/10.1038/s41598-017-11229-6.Suche in Google Scholar PubMed PubMed Central
112. Jaguemont, J.; Boulon, L.; Dube, Y. A Comprehensive Review of Lithium-Ion Batteries Used in Hybrid and Electric Vehicles at Cold Temperatures. Appl. Energy 2016, 164, 99–114. https://doi.org/10.1016/j.apenergy.2015.11.034.Suche in Google Scholar
113. Schmidt, T. S.; Beuse, M.; Zhang, X.; Steffen, B.; Schneider, S. F.; Pena-Bello, A.; Bauer, C.; Parra, D. Additional Emissions and Cost from Storing Electricity in Stationary Battery Systems. Environ. Sci. Technol. 2019, 53 (7), 3379–3390. https://doi.org/10.1021/acs.est.8b05313.Suche in Google Scholar PubMed
114. Chen, T.; Jin, Y.; Lv, H.; Yang, A.; Liu, M.; Chen, B.; Xie, Y.; Chen, Q. Applications of Lithium-Ion Batteries in Grid-Scale Energy Storage Systems. Trans. Tianjin Univ. 2020, 26 (3), 208–217. https://doi.org/10.1007/s12209-020-00236-w.Suche in Google Scholar
115. Flamary-Mespoulie, F.; Boulineau, A.; Martinez, H.; Suchomel, M. R.; Delmas, C.; Pecquenard, B.; Le Cras, F. Lithium-Rich Layered Titanium Sulfides: Cobalt- and Nickel-Free High Capacity Cathode Materials for Lithium-Ion Batteries. Energy Storage Mater. 2020, 26, 213–222. https://doi.org/10.1016/j.ensm.2019.12.033.Suche in Google Scholar
116. Shang, Y.; Sun, X.; Chen, Z.; Xiong, K.; Zhou, Y.; Cai, S.; Zheng, C. Carbon-Doped Surface Unsaturated Sulfur Enriched CoS2@RGO Aerogel Pseudocapacitive Anode and Biomass-Derived Porous Carbon Cathode for Advanced Lithium-Ion Capacitors. Front. Chem. Sci. Eng. 2021, 15 (6), 1500–1513. https://doi.org/10.1007/s11705-021-2086-2.Suche in Google Scholar
117. Islam, F.; Wang, J.; Tahmasebi, A.; Wang, R.; Moghtaderi, B.; Yu, J. Microwave-Assisted Coal-Derived Few-Layer Graphene as an Anode Material for Lithium-Ion Batteries. Materials 2021, 14 (21), 6468. https://doi.org/10.3390/ma14216468.Suche in Google Scholar PubMed PubMed Central
118. Zhang, X.; Wang, H.; Pushparaj, R. I.; Mann, M.; Hou, X. Coal-Derived Graphene Foam and Micron-Sized Silicon Composite Anodes for Lithium-Ion Batteries. Electrochim. Acta 2022, 434, 141329. https://doi.org/10.1016/j.electacta.2022.141329.Suche in Google Scholar
119. Zhang, Y.; Zhang, K.; Jia, K.; Liu, G.; Ren, S.; Li, K.; Long, X.; Li, M.; Qiu, J. Preparation of Coal-Based Graphene Quantum Dots/α-Fe2O3 Nanocomposites and Their Lithium-Ion Storage Properties. Fuel 2019, 241, 646–652. https://doi.org/10.1016/j.fuel.2018.12.030.Suche in Google Scholar
120. Shi, F.; Liu, Q.; Zhang, J.; Xing, B.; Huang, G.; Jia, J.; Zhang, C. One-Pot Synthesis of an Fes@gqds Composite for Lithium Storage with Coal Tar Pitch as “Natural gqds”. Energy Fuels 2022, 36 (4), 2130–2139. https://doi.org/10.1021/acs.energyfuels.1c03598.Suche in Google Scholar
121. Pushparaj, R. I.; Cakir, D.; Zhang, X.; Xu, S.; Mann, M.; Hou, X. Coal-Derived Graphene/MoS2 Heterostructure Electrodes for Li-Ion Batteries: Experiment and Simulation Study. ACS Appl. Mater. Interfaces 2021, 13 (50), 59950–59961. https://doi.org/10.1021/acsami.1c18993.Suche in Google Scholar PubMed
122. Xu, B.; Maimaiti, H.; Wang, S.; Zhai, P.; Zhang, H. Direct Photocatalytic Synthesis of N2/H2O to Ammonia by Plasmonic Metal Pt Supported on Coal Based Graphene Oxide/Silica Dioxide. React. Kinet. Mech. Catal. 2020, 130 (2), 1155–1170. https://doi.org/10.1007/s11144-020-01802-y.Suche in Google Scholar
123. Zhang, Y.; Zheng, L.; Jia, J.; Li, K.; Zhang, T.; Yu, H. Construction of 2d-Coal-Based Graphene/2d-Bismuth Vanadate Compound for Effective Photocatalytic CO2 Reduction to CH3OH. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128321. https://doi.org/10.1016/j.colsurfa.2022.128321.Suche in Google Scholar
124. Zhang, Y.; Zhang, K.; Ren, S.; Jia, K.; Dang, Y.; Liu, G.; Li, K.; Long, X.; Qiu, J 3d Nanoflower-Like Composite Anode of α-Fe2O3/Coal-Based Graphene for Lithium-Ion Batteries. J. Alloys Compd. 2019, 792, 828–834. https://doi.org/10.1016/j.jallcom.2019.04.011.Suche in Google Scholar
125. Kang, M.; Zhao, H.; Ye, J.; Song, W.; Shen, H.; Mi, J.; Li, Z. Adsorption Dominant Sodium Storage in Three-Dimensional Coal-Based Graphite Microcrystal/Graphene Composites. J. Mater. Chem. A 2019, 7 (13), 7565–7572. https://doi.org/10.1039/C8TA12062E.Suche in Google Scholar
126. Hu, Q.; Yang, X.; Qi, Y.; Wei, P.; Cheng, J.; Xie, Y. Optimization of Perovskite/Carbon Interface Performance Using N-Doped Coal-Based Graphene Quantum Dots and its Mechanism Analysis. J. Energy Chem. 2023, 79, 242–252. https://doi.org/10.1016/j.jechem.2022.12.014.Suche in Google Scholar
127. Lv, Y.; Xing, B.; Yi, G.; Guo, H.; Huang, G.; Zhang, C.; Liu, Q.; Zhang, X.; Cao, Y.; Li, L. Synthesis of Oxygen-Rich TiO2/Coal-Based Graphene Aerogel for Enhanced Photocatalytic Activities. Mater. Sci. Semicond. Process. 2020, 117, 105169. https://doi.org/10.1016/j.mssp.2020.105169.Suche in Google Scholar
128. Zhu, J.; Zhang, S.; Wang, L.; Jia, D.; Xu, M.; Zhao, Z.; Qiu, J.; Jia, L. Engineering Cross-Linking by Coal-Based Graphene Quantum Dots Toward Tough, Flexible, and Hydrophobic Electrospun Carbon Nanofiber Fabrics. Carbon 2018, 129, 54–62. https://doi.org/10.1016/j.carbon.2017.11.071.Suche in Google Scholar
129. Kovalchuk, A.; Huang, K.; Xiang, C.; Martí, A. A.; Tour, J. M. Luminescent Polymer Composite Films Containing Coal-Derived Graphene Quantum Dots. ACS Appl. Mater. Interfaces 2015, 7 (47), 26063–26068. https://doi.org/10.1021/acsami.5b06057.Suche in Google Scholar PubMed
130. Maung, M. M.; Aung, C. N.; Panomsuwan, G.; Win, K. K. Dielectric Properties and Electrochemical Behavior of Graphene Oxide Derived from Myanmar Coal Minerals. J. Met. Mater. Miner. 2023, 33 (2), 148–155.10.55713/jmmm.v33i2.1705Suche in Google Scholar
131. Fei, H.; Ye, R.; Ye, G.; Gong, Y.; Peng, Z.; Fan, X.; Samuel, E. L. G.; Ajayan, P. M.; Tour, J. M. Boron- and Nitrogen-Doped Graphene Quantum Dots/Graphene Hybrid Nanoplatelets as Efficient Electrocatalysts for Oxygen Reduction. ACS Nano 2014, 8 (10), 10837–10843. https://doi.org/10.1021/nn504637y.Suche in Google Scholar PubMed
132. Lei, J.; Wang, K.; Deng, B.; Li, Y.; Zhang, S.; Cao, Y. Enhanced Oxygen Reduction of Porous N-Doped Carbon Nanosheets with Graphitic N and Defects Obtained from Coal-Based Graphene Quantum Dots. J. Alloys Compd. 2022, 914, 165359. https://doi.org/10.1016/j.jallcom.2022.165359.Suche in Google Scholar
133. Wang, J.; Wang, C. Watermelon-Like Metallic Co/Graphene-Like Nanohybrids from Electrochemical Exfoliation of Anthracite Coal as Superior Oxygen Reduction Reaction Electrocatalyst. ACS Sustain. Chem. Eng. 2019, 7, 12457–12463. https://doi.org/10.1021/acssuschemeng.9b02029.Suche in Google Scholar
134. Boggs, B. K.; Botte, G. G. On-Board Hydrogen Storage and Production: An Application of Ammonia Electrolysis. J. Power Sources 2009, 192 (2), 573–581. https://doi.org/10.1016/j.jpowsour.2009.03.018.Suche in Google Scholar
135. Zhang, Y.; Liu, G.; Cai, J.; Zhou, A.; Zhang, X.; Qiu, J. Preparation of Graphene from Taixi Anthracite and its Photocatalyst Performance for CO2 Conversion. Proc. Inst. Mech. Eng. - Part N J. Nanoeng. Nanosyst. 2014, 228 (1), 61–64. https://doi.org/10.1177/1740349913489278.Suche in Google Scholar
136. Xu, B.; Maimaiti, H.; Wang, S.; Awati, A.; Wang, Y.; Zhang, J.; Chen, T. Preparation of Coal-Based Graphene Oxide/SiO2 Nanosheet and Loading ZnO Nanorod for Photocatalytic Fenton-Like Reaction. Appl. Surf. Sci. 2019, 498, 143835. https://doi.org/10.1016/j.apsusc.2019.143835.Suche in Google Scholar
137. Liu, G.; Li, K.; Jia, J.; Zhang, Y. Coal-Based Graphene as a Promoter of TiO2 Catalytic Activity for the Photocatalytic Degradation of Organic Dyes. N. Carbon Mater. 2022, 37 (6), 1172–1180. https://doi.org/10.1016/S1872-5805(21)60047-1.Suche in Google Scholar
138. Yew, Y. T.; Loo, A. H.; Sofer, Z.; Klímová, K.; Pumera, M. Coke-Derived Graphene Quantum Dots as Fluorescence Nanoquencher in Dna Detection. Appl. Mater. Today 2017, 7, 138–143. https://doi.org/10.1016/j.apmt.2017.01.002.Suche in Google Scholar
139. Nilewski, L.; Mendoza, K.; Jalilov, A. S.; Berka, V.; Wu, G.; Sikkema, W. K. A.; Metzger, A.; Ye, R.; Zhang, R.; Luong, D. X.; Wang, T.; Mchugh, E.; Derry, P. J.; Samuel, E. L.; Kent, T. A.; Tsai, A.; Tour, J. M. Highly Oxidized Graphene Quantum Dots from Coal as Efficient Antioxidants. ACS Appl. Mater. Interfaces 2019, 11 (18), 16815–16821. https://doi.org/10.1021/acsami.9b01082.Suche in Google Scholar PubMed
140. Pakhira, B.; Ghosh, S.; Maity, S.; Sangeetha, D. N.; Laha, A.; Allam, A.; Sarkar, S. Extraction of Preformed Graphene Oxide from Coal: Its Clenched Fist Form Entrapping Large Molecules. RSC Adv. 2015, 5 (108), 89076–89082. https://doi.org/10.1039/C5RA15699H.Suche in Google Scholar
141. Galante, A. J.; Yates, K. A.; Romanowski, E. G.; Shanks, R. M. Q.; Leu, P. W. Coal-Derived Functionalized Nano-Graphene Oxide for Bleach Washable, Durable Antiviral Fabric Coatings. ACS Appl. Nano Mater. 2022, 5 (1), 718–728. https://doi.org/10.1021/acsanm.1c03448.Suche in Google Scholar
142. Advincula, P. A.; Meng, W.; Eddy, L. J.; Beckham, J. L.; Siqueira, I. R.; Luong, D. X.; Chen, W.; Pasquali, M.; Nagarajaiah, S.; Tour, J. M. Ultra-High Loading of Coal-Derived Flash Graphene Additives in Epoxy Composites. Macromol. Mater. Eng. 2023, 308 (6), 2200640. https://doi.org/10.1002/mame.202200640.Suche in Google Scholar
143. Lv, Y.; Xing, B.; Zheng, M.; Yi, G.; Huang, G.; Zhang, C.; Yuan, R.; Chen, Z.; Cao, Y. Hydrothermal Synthesis of Ultra-Light Coal-Based Graphene Oxide Aerogel for Efficient Removal of Dyes from Aqueous Solutions. Nanomaterials 2018, 8 (9), 670. https://doi.org/10.3390/nano8090670.Suche in Google Scholar PubMed PubMed Central
144. Zhou, Q.; Zhao, Z.; Zhang, Y.; Meng, B.; Zhou, A.; Qiu, J. Graphene Sheets from Graphitized Anthracite Coal: Preparation, Decoration, and Application. Energy Fuels 2012, 26 (8), 5186–5192. https://doi.org/10.1021/ef300919d.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.