Abstract
Organometallic complexes, which include ligands such as carbon monoxide (CO), carbenes, alkyls, phenyls, p-bound alkynes, alkenes, cyclopentadienyls, and arenes, have been extensively utilized in fields like materials chemistry and catalysis. These complexes also offer opportunities for the development of new medications with unique modes of action. Specifically, we are interested in anticancer drugs that can enhance the effectiveness of platinum treatments, broaden their range of action, reduce adverse effects, and prevent resistance. The distinct physiochemical properties of organometallic complexes have made them valuable in homogeneous catalysis, including the production of lead compounds and therapeutic possibilities. Over the past 20 years, a small group of researchers worldwide has explored the medical applications of these compounds’ unique characteristics, such as their structural diversity, potential for ligand exchange, and redox and catalytic properties. The results have been remarkable, and it is anticipated that numerous other organometallic compounds will undergo clinical trials in the coming years in addition to those already underway. In this brief study, we outline the advantages that organometallic metal complexes have over coordination compounds and pure organic molecules.
1 Introduction
Compounds that possess a polarized Mδ+ – Cδ-single sigma bond between carbon and metal are referred to as organometallic compounds. Usually, the complex environmental moiety that the organometallic fragment is attached to may not be easily identified. However, recent years have seen significant advancements in the field of speciation analysis. The organometallic fragment must be freed from its environmental binding and quantified in order to facilitate speciation analysis in the environment. Due to their formation or introduction, organometallic compounds can be found in the natural environment. When introduced to the environment, organometallic compounds can do so either directly through use as goods with environmentally related features (biocides, for example) or indirectly through extra use for a different primary purpose (polymer stabilizers, gasoline additives). As organometallic compounds, compounds including arsenic, mercury, tin, and lead play significant roles in the environment. 1 , 2 , 3 The alkyl and aryl derivatives of the major group elements predominate in organometallic compounds. Since most research has been done on these chemicals, the focus will be on them. An exceptionally significant type of organometallic compounds are metal carbonyl complexes; for structural and bonding reasons alone, they are interesting. As starting points for a variety of further metal complexes and clusters comprising carbonyls as well as catalysts. 4 Numerous significant conceptual breakthroughs, unexpected structures, and practical catalysts for industrial and organic synthesis processes have come from the organometallic sector. Asymmetric induction at very high levels can be achieved by many catalysts, selectively producing one enantiomer of a chiral product. The field is beginning to make links with biochemistry with the discovery of enzymes that carry out organometallic catalysis (e.g., acetyl CoA synthase). An understanding of the chemistry of metal and metal oxide surfaces – two important players in heterogeneous catalysis – has been aided by concepts from organometallic chemistry. Because metal-organic and organometallic compounds are increasingly preferred as the precursors for depositing materials on different substrates by thermal degradation of the metal compound, the sector is also forging connections with the chemistry of materials. 5 , 6
Without question, chemists and the molecules they create have made a huge contribution to the amazing advancements in medicine. There are 82 non-radioactive elements listed in the periodic table, of which more than 60 readily form covalent, air- and water-stable molecular compounds that may have applications in medical chemistry. However, just ten of those more than sixty components are present in the vast majority of medications. Remarkably, there are remarkably few medications or drug possibilities that don’t also contain platinum or another transition metal. 7 , 8 , 9 However, it is evident that the field of organometallic chemistry – which is defined as the study of chemical compounds with at least one metal-carbon bond, is currently far more well-known for its numerous uses in catalysis than for its use in medicine. While the subject of study pertaining to the application of organometallic compounds for therapeutic purposes – known as medicinal organometallic chemistry – has received increasing attention in recent years, When weighed against the scientific fields of biosensing or even catalysis, this one’s reputation and value are still insignificant. 10 , 11 Our goal in writing this paper is to show readers that organometallic compounds have amazing physicochemical properties, which, in the context of medicinal chemistry, are highly interesting and rather unique. Amazing progress in this area of study has lately been made, as will be discussed in more detail below, despite the pharmaceutical business and academia showing only “polite” curiosity. Certain organometallic drugs have even progressed to clinical trials, and recent research has shown metal-specific mechanisms of action. 12 , 13 Significantly, these substances may be used as antibacterial, anticancer, antimalarial, or diagnostic agents in a variety of medical fields. Since this has already been done recently, we won’t go into every recent advancement in medicinal organometallic chemistry in this contribution. 14 , 15 , 16 , 17 , 18 We would want to draw attention to the particular qualities or benefits (such as structural diversity, ligand exchange potential, redox and catalytic characteristics, etc.), that organometallics has over only organic molecules and provide one or two specific instances to illustrate them.
2 Structural diversity
A carbon atom can have four distinct substituents in two distinct enantiomers that cannot be superimposable. On the other hand, there are thirty distinct stereoisomers in an octahedral transition metal complex with six substituents! Clearly, let’s consider the core atom (carbon or transition metal) to be only a template for the orientation of its substituents in three-dimensional space. Typical transition metal complexes have a greater variety of structures than pure organic molecules. The racemization is unlikely to happen in kinetically inert transition metal complexes, similar to organic molecules; instead, the ligand placement around the metal center needs to be stereochemical stable. Not all transition metals satisfy this criterion to the same extent, and the metal’s oxidation state may also have an impact on conformational stability. The most appropriate transition metals are often those in the second and third rows with closed electron shells because they are more commonly utilized in medicinal inorganic chemistry when structurally stiff molecules are needed, such as Ru(II), Os(II), or Ir(III). Meggers and colleagues have designed protein kinase inhibitors using structurally inert Ru(II) complexes. 19 , 20 , 21 These proteins are ideal targets to change, such as signaling pathways within cancer cells, because they are known to have numerous critical activities in the body. The natural substance staurosporin, which binds to the ATP binding site competitively to inhibit kinases, served as the model for this work. Figure 1 shows the structure of one Ru-based metal complex 1 (FL-411) as an example and illustrates how metallo-pyridocarbazole complexes bind similarly to the ATP binding site. Ru-containing degradation products are generally thought to have low metal-based toxicity, and these complexes’ synthetic chemistry is well-established, providing access to a vast array of molecules. The creation of selective kinase inhibitors, which should target one of the 518 distinct kinases encoded in the human genome, is greatly facilitated by this. 22 The exceptional selectivity of the observed metal complexes – some of which have as low as pM affinity to one particular kinase in vitro – is well supported structurally by the nearly twelve co-crystal structures with metal complexes acting as inhibitors that have been discovered thus far. The Ru complexes mentioned above are easily investigated in vivo since they are typically 5 stable to air and water and can even tolerate exposure to mM concentrations of biological ligands like glutathione. For example, the formation of a hyperdorsalized phenotype in Xenopus laevis embryos demonstrates the effect of blocking the kinase GSK3 with complex 2, which in turn switches on the wnt signaling pathway in vivo. 23 Remarkably, the combination DW12(Ru/Os) exhibits nearly identical anti-proliferative action regardless of whether Os or Ru is the central atom. 24 This effectively highlights the metal centers’ sole structural function in these complexes.
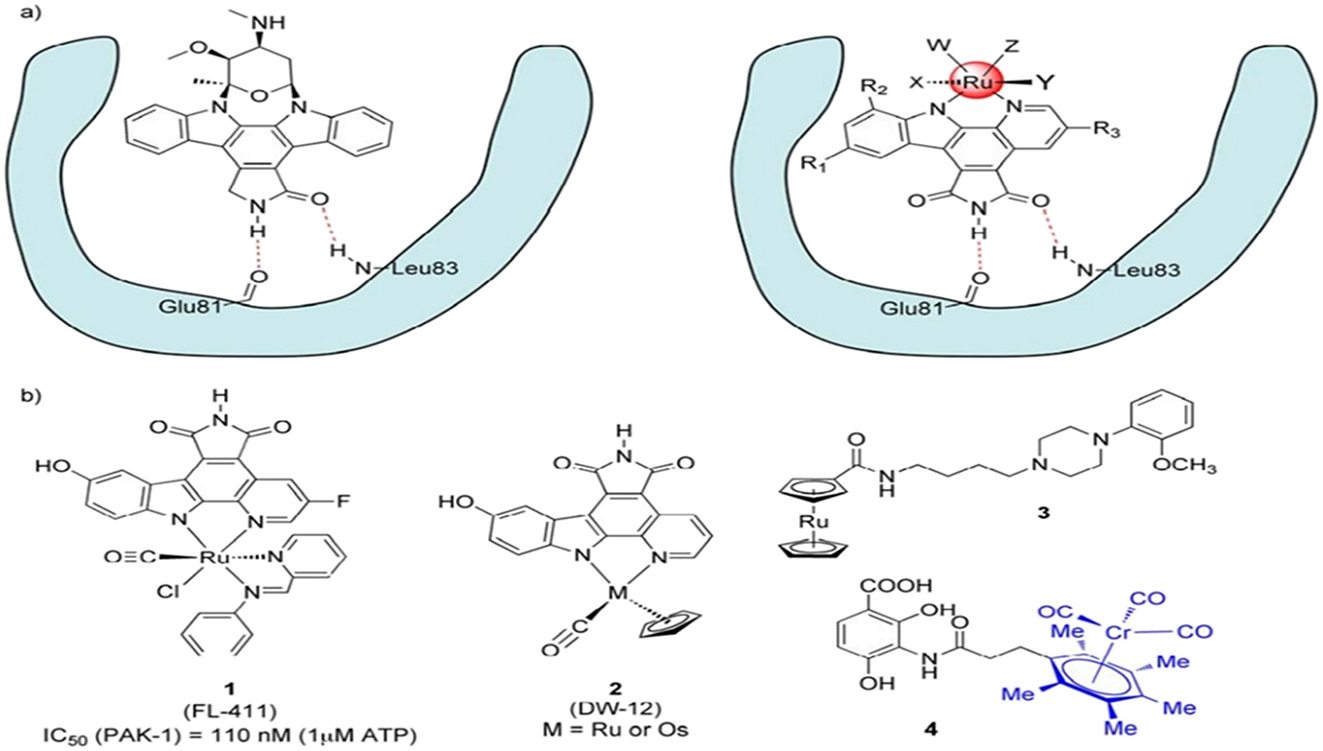
a) The binding motif of octahedral metal complexes and the natural product staurosporine to the ATP binding site in kinases (magenta). b) Two metal-based kinase inhibitors (1 and 2) from Meggers’ lab, an agonist that selectively targets the dopamine D4 subtype and contains a 6-ruthenocine (3), and a homolog of platensimycin that contains chromium (4). Six stereocenters are substituted for a multi-cyclic organic group in position 4 by the chromium tricarbonyl moiety in blue.
In other areas of medicinal organometallic chemistry, the idea of bio-isosteric replacement of organic groups with metal complexes has also been applied. Firstly, organometallic Ru complexes have been used by Gmeiner’s group to target dopamine receptors. They discovered that the ruthenium metallocene derivative (3, Figure 1) has special D4-selective agonist qualities and has a nM affinity for G-protein coupled receptors like dopamine and serotonin receptors. 25 Various organometallic complexes were employed in our research as organometallic counterparts of platensimycin, a fascinating naturally occurring substance possessing antibacterial properties. Platensimycin’s antibacterial action is linked to fatty acid biosynthesis suppression in bacteria, namely inhibition of the FabF and FabH enzymes. Although several ferrocene derivatives offered intriguing new perspectives on structure-activity correlations (SAR). 26 , 27 , 28 The most active substance was an arene chromium tricarbonyl derivative (4, Figure 1). 29 According to molecular docking studies, this substance fits the FabF enzyme’s active pocket perfectly. However, proteomic studies revealed a distinct mode of action for 4 that is not associated with a single molecular target and differs from the suppression of fatty acid production. 30
3 Redox properties
We covered fully inert metal complexes in the previous section, where the metal core served just as a structural component. However, as electron transfer processes are commonly carried out by a wide variety of enzymes and cofactors, including cytochromes and the Fe–S clusters, many metal complexes will easily undergo them. The possibility that this redox activity could be extremely advantageous for the metal complex’s therapeutic potential has only lately come to light. One well-known instance is from the group of Jaouen. By utilizing the concept of a “bio-isosteric” substitution of metallocene fragments for phenyl rings, They created several variations of the popular anticancer medication tamoxifen. 31 The ferrocene derivatives, later dubbed “ferrocifens” (see compound 5 in Figure 2 as an example), demonstrated certain additional, noteworthy properties in addition to an activity comparable to that of tamoxifen itself, but the majority of derivatives were very inactive. 32 The first-line treatment for breast cancer is tamoxifen. By binding to the estrogen receptor α subtype (ERα), the medication suppresses the transcription of DNA mediated by estradiol in tumor cells. It follows that only breast cancer types that overexpress the ER-will be susceptible to the effects of tamoxifen and related substances. Regretfully, around one-third of breast cancer patients do not meet this criterion, and those who do not have overexpression of ER-actually face a much worse prognosis. To the amazement of the researchers, some ferrocenes showed comparable efficacy against all strains of breast cancer cell lines, including those that were ER-negative. Extensive research demonstrated that the activity is associated with the iron center’s reversible redox behavior in these compounds. It was suggested that the oxidation of Fe(II) to Fe(III) in the ferrocene core starts the process of proton-coupled electron abstraction in the organic component, which in turn causes the phenolic part to oxidize to a quinone methide. 33 Quinone methides, which are believed to interact with thiols and nucleobases in vivo, are already formed in tamoxifen and has been suggested as essential to the drug’s action. The quick and reversible production of Fe(III) species (Figure 2) is assumed to function as a “redox antenna” for the metallocene, therefore providing ferrocenes with a different mechanism of action apart from binding to estrogen receptors. 34 Analogs of ferrocifen in Ru provide intriguing experimental support for this idea. Similar to tamoxifen, these compounds are inactive against ER-negative cell lines but do show tamoxifen-like action against ER-positive cancer cells. Although ruthenocene and ferrocene are extremely similar in terms of size and structure overall, there are significant differences in their electrochemistry, including a larger redox potential and – most importantly – no reversibility for the electron transfer reaction. Therefore, unlike the kinase inhibitors mentioned above, where the idea of a merely structural role for the metal ions is supported by swapping structurally comparable metal ions, The ferrocifen case study illustrates how important a metal’s redox activity is, and how changing a compound’s redox characteristics through isostructural replacement of metal ions can actually render it inactive. 35
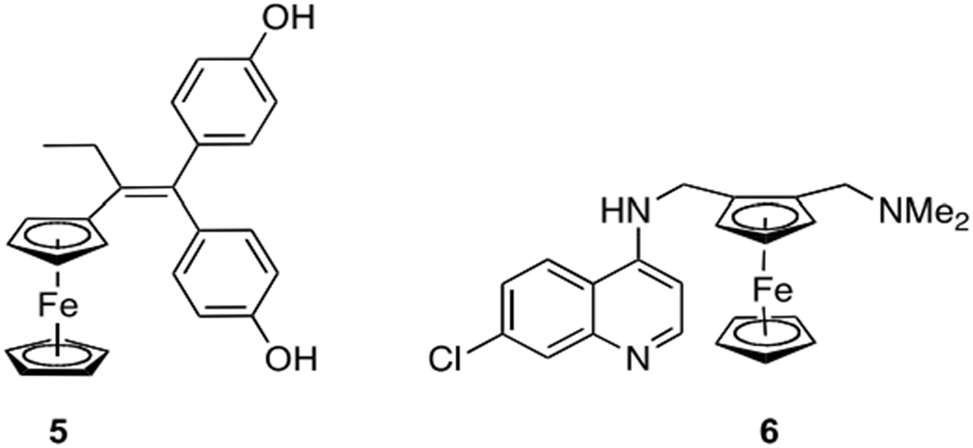
An effective and more adaptable substitute for ferroquine (6) and the anti-tumor medication tamoxifen (ferrocifen, 5), a malaria treatment option in clinical trials that doesn’t cause chloroquine resistance to develop cross-resistance.
It appears that ferroquine 6, an analogue of the well-known anti-malarial drug candidate chloroquine, is also affected by the redox activity of iron in a ferrocene derivative (Figure 2). yet it appears that other factors also come into play. The most advanced organometallic drug candidate at the moment, ferroquine, is about to begin phase III clinical trials. For more information on ferroquine, see this pretty extensive recent article. 36
4 Ligand exchange
Cisplatin is arguably the greatest illustration of how metal complexes can exchange ligands “for medicinal purposes,” as this capacity is well-known. 37 , 38 This ability to interchange ligands is not exclusive to coordination complexes. Ligand exchange is also possible in organometallic compounds. This has uses in many branches of medical chemistry, but mostly in the synthesis of new chemotherapeutic drugs, as will be seen below. In fact, it is believed that a ligand exchange mechanism is responsible for at least some of the antiproliferative activity of certain organometallic compounds. 21 , 39 , 40 , 41 Although the mode of action of both derivatives appears to utilize a similar ligand exchange process, we will only address two examples in this section where the organometallic anticancer drugs have distinct cellular targets. As of now, in addition to the ferrocifens already mentioned, organogold compounds, 42 Ru-arene derivatives, and titanocene derivatives are the most researched types of organometallic anticancer chemicals. 43 , 44 The research teams for Sadler, 45 (Scheme 1a) and Dyson, 46 (Scheme 1c) are developing these chemicals separately. It’s interesting to note that, although having similar structures and derivatives that can both (maybe) interchange ligands, these two kinds of half-sandwich “piano stool” Organometallics function through a completely distinct method. First, a water molecule is substituted for the chloride anion in compounds of the type [(6-arene)Ru (en) (Cl)]+ (en = ethylenediamine), which is similar to the procedure for cisplatin (Scheme 1a). The newly created aqua species then exhibits a strong affinity for the N7 location of guanine nucleotides when binding to nuclear DNA. 47 , 48 , 49 The resemblance to cisplatin’s method of action ends here since these derivatives only create monoadducts with DNA, whereas cisplatin forms both bifunctional adducts and DNA cross-links. It is noteworthy to emphasize that these [(6-arene)Ru (en) (Cl)]+ compounds exhibit activity against cell lines resistant to cisplatin, indicating that the detoxification process is distinct from that of cisplatin. 50 In contrast, there are significant differences in the in vivo behaviors of RAPTA-C and RAPTA-T (where PTA represents the 1,3,4-triaza-7-phosphatricyclo-[3.3.1.1] decane 10 ligand and RA stands for Ruthenium-Arene; refer to Scheme 1c). They can indeed lower the weight and quantity of lung metastases in CBA mice with MCa mammary cancer while having minimal impact on the main tumor. It’s interesting to note that these compounds still exhibit ligand exchange, which may be a factor in their cytotoxic activity, even in the absence of hydrolysis. It has been shown that these Ru complexes attach to serum protein even in blood plasma, where the chloride content is high enough to prevent the hydrolysis of the dichloride-containing RAPTA molecules. 40 There is compelling evidence from additional experimental observations that the hydrolysis phase is not necessary. RAPTA-containing substances, whose carboxylate ligands have taken the place of chloride ligands and which are resistant to hydrolysis (such as oxalo-RAPTA-C in Scheme 1c) yet exhibits comparable in vitro activity to that of the original molecule RAPTA-C! When all the data is considered collectively, It is now believed that RAPTA complexes connect to proteins, specifically cathepsin B (cat B), through several interactions rather than DNA. most notably by attaching itself to a cysteine’s sulfur atom in the enzyme’s active site (Scheme 1b). It appears that the unique characteristics of the PTA ligand also influence these significant variations. It is important to note that organometallics’ capacity for ligand exchange has applications outside of chemotherapeutic medicine. Therefore, certain metal carbene compounds (such as those of Au, Ag, Rh, and Ru) exhibit extremely promising antibacterial potential. 51 , 52 Overall, the examples above make it abundantly evident that organometallic compounds have exceptional qualities that make them valuable tools in the creation of medicinal chemistry. One such property is their capacity to firmly engage targets via coordinative bonds. 53 , 54
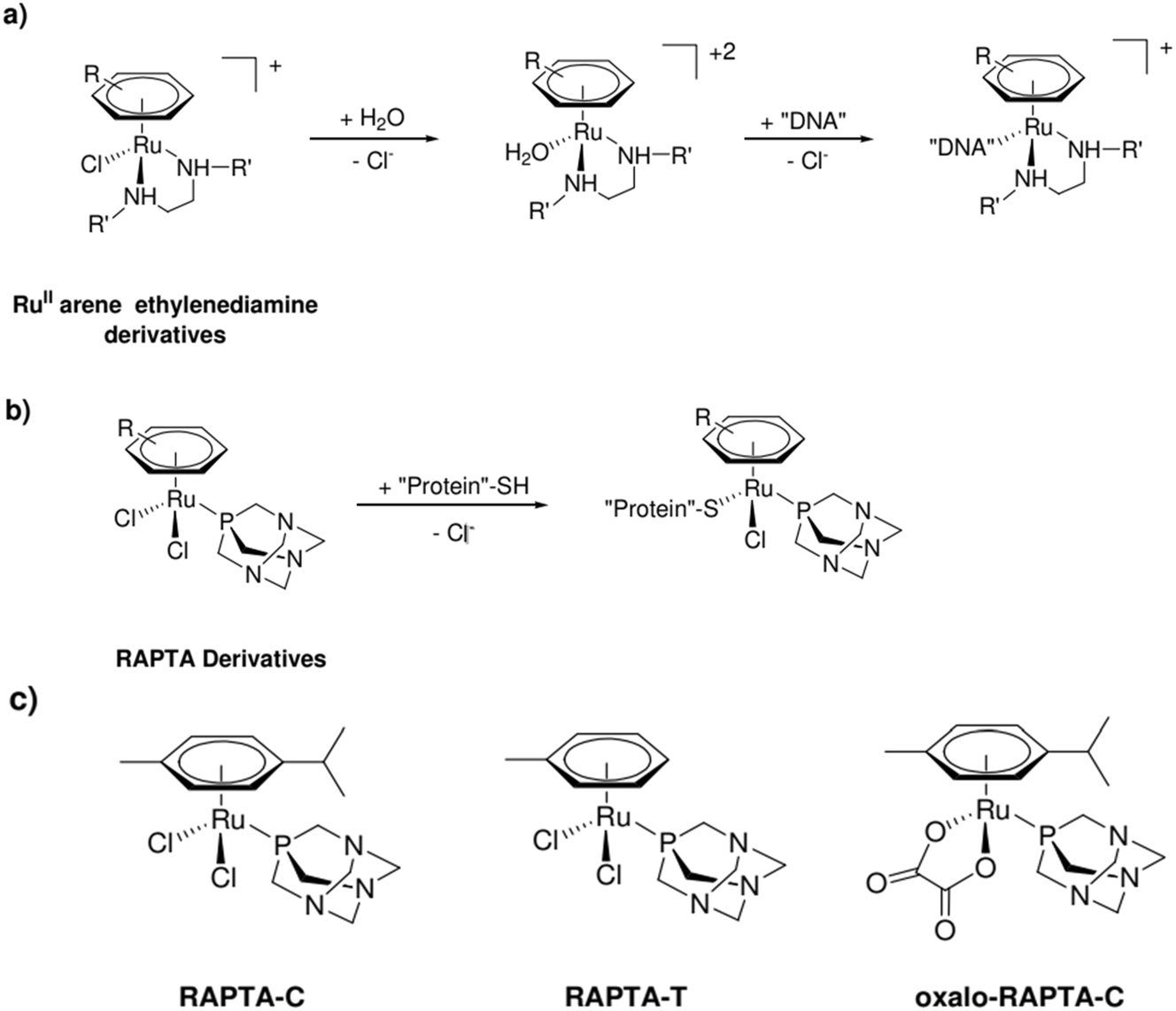
a) A simplified depiction of Ru II arene ethylenediamine’s mode of action and b) RAPTA derivatives, emphasizing how the two categories of chemicals have distinct biological objectives; c) RAPTA-C, RAPTA-T, and oxalo-RAPTA-C structures.
In addition to the above-mentioned instances, the capacity of organometallics to engage in ligand exchange may result in further intriguing medical uses. In fact, a biomolecule (such as DNA, protein, etc.) does not exchange a “useless” ligand (such as Cl-, etc.) with another biomolecule to produce a fresh metal adduct. Which will prevent this specific biomolecule from functioning properly. The released ligand may really be a molecule 12 with its therapeutic action, such as carbon monoxide (CO). 55 In fact, CO has a wide range of advantageous therapeutic effects as a tiny messenger molecule that is comparable to NO despite its unfavorable image as the “silent killer.” Examples of these include the prevention of organ graft rejection, the mitigation of inflammation, and the preservation of the heart after reperfusion following cardiopulmonary bypass surgery. 56 , 57 , 58 It goes without saying that the use of gaseous CO presents clear challenges for both its safe handling and its targeted, targeted administration to a particular area of the body. 58 Thus, CO-releasing molecules – often referred to as CORMs – offer an alluring substitute for the inhalation of CO gas. But as Motterlini and Otterbein (2010) pointed out, there is little likelihood that any of the CORMs that have been documented thus far will make it to the clinic. These substances don’t have the drug-like qualities required to be approved by pharmaceutical companies as medications given this, Zobi et al. 57 Intended to employ vitamin B12, or cyanocobalamine, as a biocompatible scaffold for CORMs that contain rhenium. One of the main justifications for using these kinds of organometallic complexes is that the oxidized product that remains after CO is released – rhenium pertechnetate ReO4 – is among the least hazardous of the rarest inorganic chemicals. 58 Their two Re-vitamin B12 bioconjugates (e.g., B12-ReBr2(CO)2H2O, Figure 3) were demonstrated to shield heart tissue from ischemia-reperfusion injury and to have favorable half-lives for biological/medical applications. All of the Re bioconjugates in this study had improved qualities over their parent complexes, as stated by the scientists. They are more biocompatible, more water soluble, and more stable in aqueous aerobic environments. 59
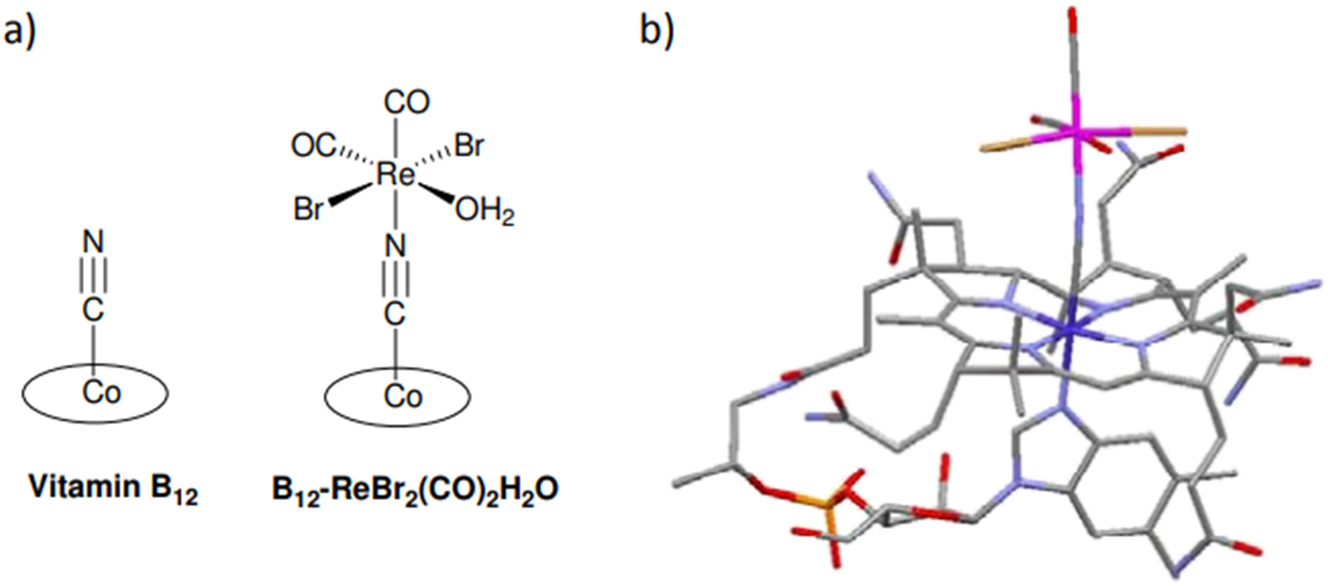
a) Vitamin B12’s and its rhenium complex, B12-ReBr2(CO)2H2O, schematic structures; b) The B12-ReBr2(CO)2H2O structure as determined by DFT. For clarity, H atoms are not included. Structured line drawing produced with data from Dr. Fabio Zobi with assistance from the Mercury Program.
Furthermore, these molecules appear to have a very broad therapeutic potential. Given that the liver stores and processes vitamin B12, one may believe that rhenium bioconjugates could be helpful in treating hepatic inflammation. Given that CO has been demonstrated to help prevent organ rejection, they might also be administered to patients receiving liver transplants. 57
5 Catalytic properties
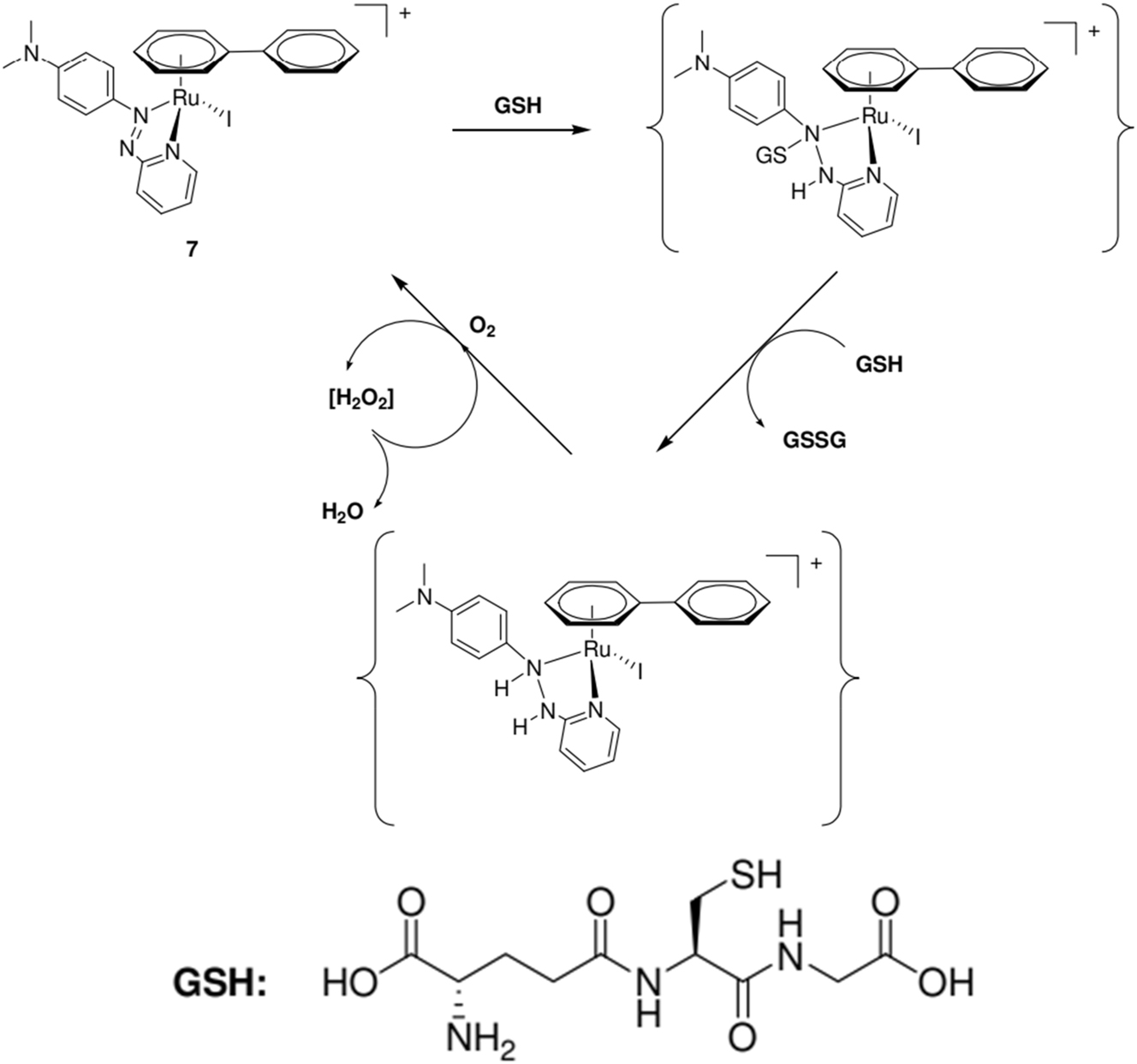
The capacity of these derivatives to catalyze chemical reactions is a physicochemical characteristic of organometallic compounds that have undoubtedly been underappreciated to date for its promise in medicinal chemistry and chemical biology. From this angle, the recent findings of Sadler and associates involving half-sandwich “piano-stool” ruthenium arene complexes unquestionably represent a breakthrough in the development of catalytic medications. In fact, they discovered that even Ru organometallic complexes like 7 (Scheme 2), which are comparatively hydrolytically inert, are extremely harmful to human lung A549 and ovarian A2780 cancer cells. 60 Reactive oxidative species (ROS) have been linked to the mechanism of action of these drugs. To explain the cytotoxic action of these Ru complexes, the authors have hypothesized a redox catalytic cycle involving glutathione attack on the azo link of coordinated azo pyridine (Scheme 2). Notably, reducing the azopyridine ligand by itself is challenging. However, the ligand’s reduction potential becomes biologically accessible upon coordination to the Ru center. It should be noted that the creation of ROS has also been linked to the ferrocenyl moiety of the antimalarial drug ferroquine (see above). Overall, we firmly believe that the creation of organometallic anticancer chemicals will enter a new frontier thanks to these instances. 60 , 61
6 Application of organometallic complex in medicine chemistry
Organometallic complexes have a broad range of possible medical uses and can comprise a variety of Cbound ligands (Table 1). even though there aren’t many instances of therapeutic application. Here, the emphasis is on anticancer organometallic compounds. It became clear that this complex works in a different way than the platinum medications and that DNA might not be the main target. Since then, interest in the emerging topic of bioorganometallic chemistry has grown quickly, owing to its potential for use in various biotechnological applications as well as in treatment and diagnostics. 46 , 70 , 77 , 78 The hydrophilicity and hydrophobicity of the coordination complex’s faces can be controlled by cyclopentadienyl ligands, carbon-bound arenes, and p-bound arenes (which affect cell absorption and targeting). 79 , 80
Here are a few instances of organometallic fragments and their possible uses in medicine.
| Hapticity/Fragment | Example | Application | Ref |
|---|---|---|---|
| h1/M-CO | [Ru(II) (Gly) (CO3)Cl] [Mn(CO)3X&(polymer)] (CORMs, Photo-CORMs) | Organ rejection, tissue ischemia, vascular dysfunction, and inflammation | 62 , 63 |
| M-CN | Na2 [Fe(CN) (NO)] | Vasodilator (“Nipride”), anti-hypertension | 64 |
| M-alkyl | Methylcobalamin Me2As(SG) (Darinaparsin) | Vasodilator (“Nipride”), anti-hypertension Anticancer |
65
66 |
| M-phenyl | Phenylmercuric nitrate | Antiseptic | 67 |
| M-carbene | Ag(I) (N-heterocyclic carbene) | Antibacterial, anticancer | 68 |
| M-alkynyl | [Pd(CˆNˆC) (CˆCPh)] PF6 [Pt (CˆNˆC) (CˆC-L)] CF3SO3 |
Photo-cytotoxic DNA binding |
69 |
| h4/M-diene | Ir(COD) (acac) | Anticancer | 70 |
| h5/M-Cp | Ferroquine Ferrocifens (Cp*)Rh(III)/Ir(III) |
Antimalarial Anticancer Anticancer |
67 , 71 , 72 |
| h6/M-arene | Ru(II), Os(II) arenes | Anticancer Antimalerial |
73 , 74 , 75 , 76 |
Recently, efforts have been made by our team and others to develop novel organometallic anticancer compounds that could potentially resolve clinical issues with platinum-based medications, particularly the restricted range of action, adverse effects, and resistance. 80 We have extended the design ideas for organometallic Rh(III) and Ir(III) Cp* complexes to Ru(II) and Os(II) arene complexes. For these organometallic complexes, investigations have been conducted on the rate of hydrolysis, acidity of the aqua adducts, interactions with nucleobases, hydrophobicity, and cell accumulation. 81 , 82 , 83
While metallodrugs present a promising avenue for novel mechanisms of action, achieving clinical approval for them will depend on improving our understanding of their target sites and mechanisms of action at the molecular level as well as minimizing the side effects that are frequently linked to heavy metals. Such advancements are anticipated to depend heavily on new techniques for tracking the temporal and spatial speciation of metallodrugs in cells at physiologically relevant concentrations, as are contemporary genomics and proteomics, which may ultimately direct the application of metallodrugs in personalized treatment. 46
6.1 Metallocenes
6.1.1 Ferrocenes
The ferrocenium cation [Fe (h5-C5H5)2]þ, is cytotoxic to several cancer cell lines, although ferrocene, Fe (h5-C5H5)2 (Figure 4a), is comparatively non-toxic. 84 Although the exact mechanism of action of [Fe (h5-C5H5)2] þ remains unknown, given that it has been demonstrated to produce hydroxyl radicals in a physiological setting, It is conceivable that it produces these radicals within cancer cells, causing damage to the cell membrane and DNA (Figure 4). 67
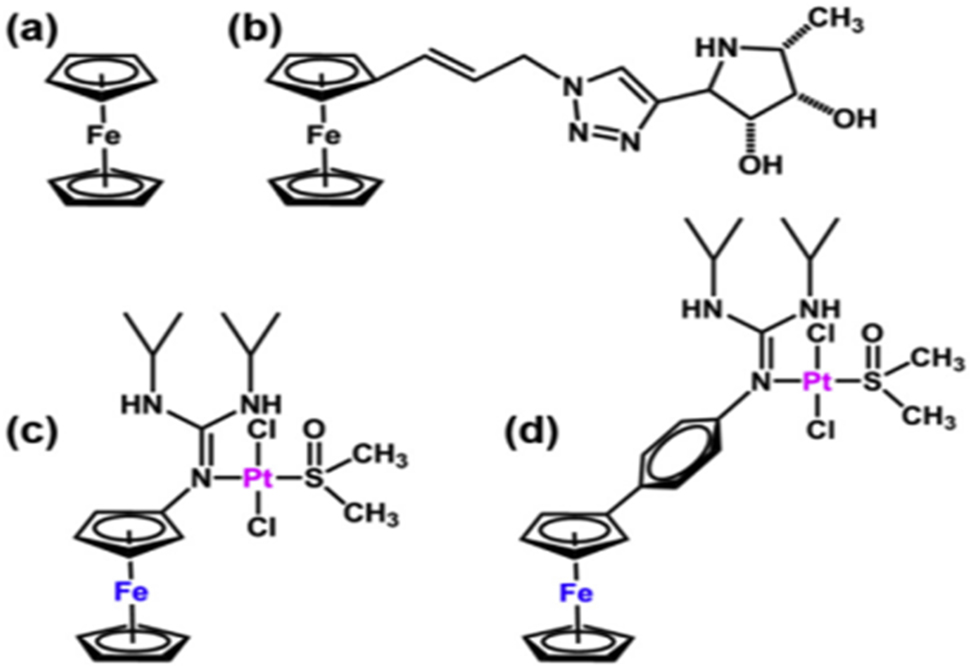
The chemical structures of Pt (II)eguanidine complexes functionalized with ferrocene, ferrocene, and a ferrocene-iminosugar conjugate complex (a, b, and c, d).
6.1.2 Titanocenes
Dichlorotitanocene [Ti (h5-C5H5)2Cl2] Since (Figure 5a) has a cisdichlorido motif, which was predicted to result in a GG crosslink akin to that created by cisplatin, it was initially investigated as an anticancer compound. 85 In contrast, it was discovered that the structure of the molybdenum analog linked to DNA was similar to that of cisplatin. 86 Little evidence suggests that DNA nucleotides are the target of [Ti (h5-C5H5)2Cl2]. CClinical examinations of After phase II trials and almost ten years, titanocene dichloride was finally discontinued. 87 Its weak hydrolytic stability and low water solubility led to formulation issues that ultimately impeded its development. Titanocene-based anti-cancer treatments are further restricted by their poor selectivity and ignorance of their mode(s) of action. Nonetheless, it is evident that the mechanism of action of commercial platinum medicines differs significantly from that of titanocene derivatives, and further investigation of this family of organometallic complexes may result in a valuable clinical therapeutic candidate (Scheme 3).
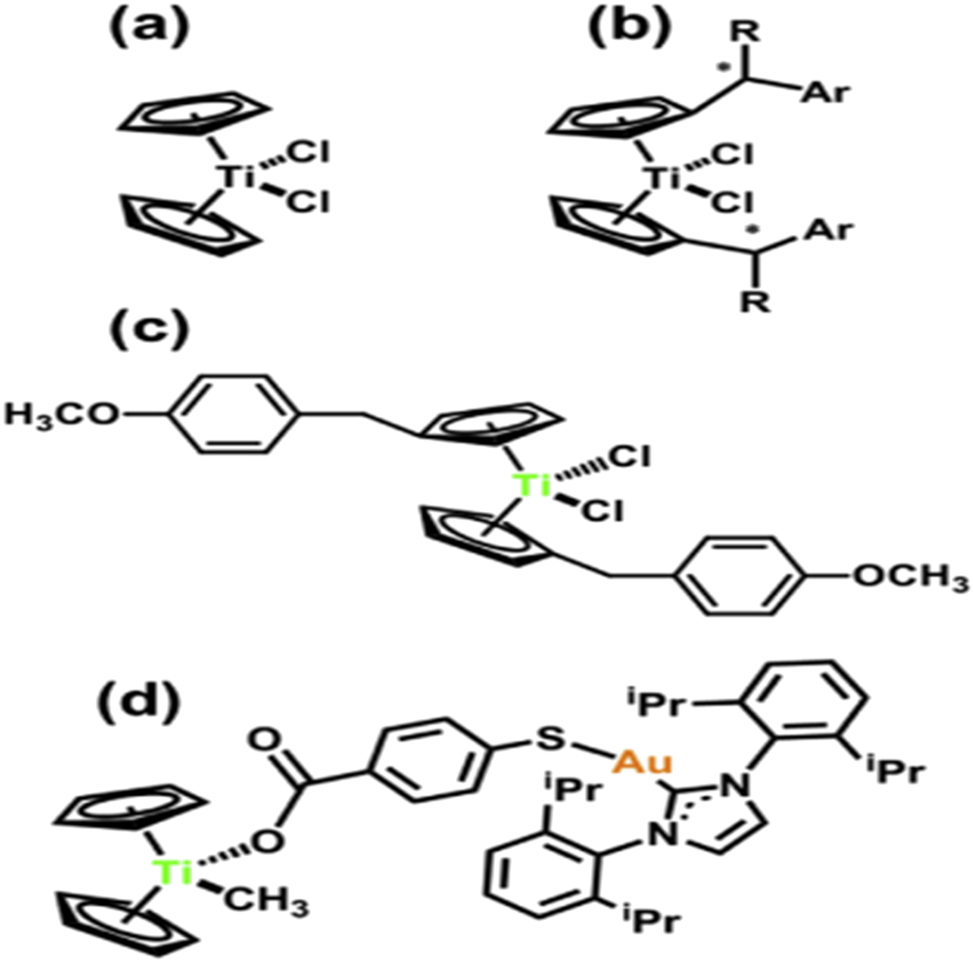
The compounds that inhibit TrRx are the titanocene (a), titanocene Y (b), enantiopure chiral titanocenes (R = Et, Ar = 2-MeOC6H4), and titanocenee-Au(I)-ecarbene complex (d).
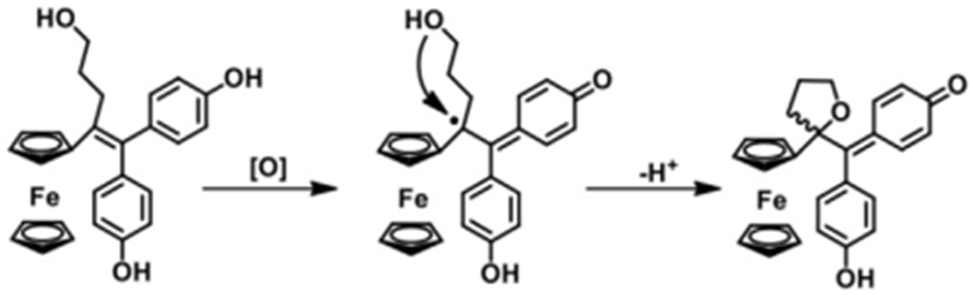
The creation mechanism of the new heterocyclic ferrocenyl QM species is proposed. 84
7 Conclusions
Though remarkable progress has been made lately in the field of therapeutic organometallic chemistry, we believe that these molecules’ full potential remains untapped. Metal-based radiopharmaceuticals are one topic that was left out of the previous discussion. All transition metals have many isotopes throughout the periodic table, some of which are useful for radiation and imaging. It appears that the unique qualities of organometallic medication prospects have, even outside the field of radiopharmaceuticals, not yet been completely utilized. Organometallics offer a significantly more varied stereochemistry than organic molecules, as well as a wide range of structural variations, from linear to octahedral and even beyond. The medicinal chemist has control over kinetic parameters, such the rate of ligand exchange, thanks to rational ligand design (see above). In addition, they are generally uncharged with the metal atom in a low oxidation state, kinetically stable, somewhat lipophilic, and susceptible to a wide range of common chemical transformations. When medicinal chemists have access to organometallics’ full potential, We anticipate more amazing findings that, when turned into medications, will eventually help patients as well. We have provided an overview of some recent developments in the field of organometallic anticancer compound design. The large range of target sites, which depends on the metal, its oxidation state, and the kinds and quantity of coordinated ligands, is noteworthy. Their anticancer properties stem from a variety of processes, including disruption of the redox equilibrium in cells, production of ROS, and assaults on proteins, enzymes, and DNA. It is crucial to identify correlations between structure and activity for organometallic compounds. Even little structural modifications can have a significant impact on activity.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Albrecht-Schmittve, T. E. Organometallic and Coordination Chemistry of the Actinides; Springer Science & Business Media: Verlag Berlin Heidelberg, Vol. 127, 2008.10.1007/978-3-540-77837-0Search in Google Scholar
2. Mamand, D. M.; Aziz, N. M.; Omer, R. A. Effect of Doping of Metal Salts on Polymers and Their Applications in Various Fields. Rev. Inorg. Chem. 2025, 45, 359–374. https://doi.org/10.1515/revic-2024-0034.Search in Google Scholar
3. Aziz, K. N.; Ahmed, K. M.; Omer, R. A.; Qader, A. F.; Abdulkareem, E. I. A Review of Coordination Compounds: Structure, Stability, and Biological Significance. Rev. Inorg. Chem. 2025, 45, 1–19. https://doi.org/10.1515/revic-2024-0035.Search in Google Scholar
4. Henderson, W.; McIndoe, J. S. Mass Spectrometry of Inorganic and Organometallic Compounds: Tools-Techniques-Tips; John Wiley & Sons: Chichester, 2005.10.1002/0470014318Search in Google Scholar
5. Crabtree, R. H. J. J. O. O. C. NHC Ligands versus Cyclopentadienyls and Phosphines as Spectator Ligands in Organometallic Catalysis. J. Organomet. Chem. 2005, 690 (24–25), 5451–5457. https://doi.org/10.1016/j.jorganchem.2005.07.099.Search in Google Scholar
6. Omer, P. K.; Abdulkareem, E. I.; Omer, R. A.; Faruq Rashid, R. A Review of Organometallic Compounds as Versatile Sensors in Environmental, Medical, and Industrial Applications. Rev. Inorg. Chem. 2025, 45, 397–409. https://doi.org/10.1515/revic-2024-0055.Search in Google Scholar
7. Gielen, M.; Tiekink, E. R. Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: the use of Metals in Medicine; John Wiley & Sons: Chichester, 2005.10.1002/0470864052Search in Google Scholar
8. Gasser, G. Inorganic Chemical Biology: Principles, Techniques and Applications; John Wiley & Sons: Chichester, 2014.10.1002/9781118682975Search in Google Scholar
9. Muhammed, F. S.; Salih, M. I.; Omer, R. A.; Qader, A. F.; RashidIman, R. F.; Abdulkareem, E. I. A Review: Evaluating Methods for Analyzing Kidney Stones and Investigating the Influence of Major and Trace Elements on Their Formation. Rev. Inorg. Chem. 2025, 45, 307–319. https://doi.org/10.1515/revic-2024-0045.Search in Google Scholar
10. Gasser, G.; Ott, I.; Metzler-Nolte, N. J. J. O. M. C. Organometallic Anticancer Compounds. J. Med. Chem. 2011, 54 (1), 3–25. https://doi.org/10.1021/jm100020w.Search in Google Scholar PubMed PubMed Central
11. Aziz, K.N.; Mahmood Ahmed, K.; Omer, R. A.; Qader, A. F.; Abdulkareem, E. I. Organometallic Complexes and Reaction Methods for Synthesis: a Review. Rev. Inorg. Chem. 2024, 45. https://doi.org/10.1515/revic-2024-0037.Search in Google Scholar
12. Gianferrara, T.; Bratsos, I.; Alessio, E. J. D. T. A Categorization of Metal Anticancer Compounds Based on Their Mode of Action. Dalton Trans. 2009, (37), 7588–7598. https://doi.org/10.1039/b905798f.Search in Google Scholar PubMed
13. Omer, P. K.; Aziz, N. M.; Omer, R. A. Comprehensive Review of Metal-Based Coordination Compounds in Cancer Therapy: from Design to Biochemical Reactivity. Rev. Inorg. Chem. 2024, 45. https://doi.org/10.1515/revic-2024-0030.Search in Google Scholar
14. Jaouen, G.; Metzler-Nolte, N. Medicinal organometallic chemistry; Springer: Paris, Vol. 32, 2010.10.1007/978-3-642-13185-1Search in Google Scholar
15. Hartinger, C. G.; Dyson, P. J. J. C. S. R. Bioorganometallic Chemistry – From Teaching Paradigms to Medicinal Applications. Chem. Soc. Rev. 2009, 38 (2), 391–401. https://doi.org/10.1039/b707077m.Search in Google Scholar PubMed
16. Ronconi, L.; Sadler, P. J. J. C. C. R. Using Coordination Chemistry to Design New Medicines. Coord. Chem. Rev. 2007, 251 (13–14), 1633–1648. https://doi.org/10.1016/j.ccr.2006.11.017.Search in Google Scholar
17. Smith, G. S.; Therrien, B. J. D. T. Targeted and Multifunctional Arene Ruthenium Chemotherapeutics. Dalton trans. 2011, 40 (41), 10793–10800. https://doi.org/10.1039/c1dt11007a.Search in Google Scholar PubMed
18. Crabtree, R.; Mingos, M. Comprehensive organometallic chemistry III: from fundamentals to applications; Elsevier Science: Amsterdam ;Tokyo, 2006.Search in Google Scholar
19. Meggers, E. J. C. C. Targeting Proteins with Metal Complexes. Chem. Commun. 2009, (9), 1001–1010.10.1039/b813568aSearch in Google Scholar PubMed
20. Mulcahy, S. P.; Meggers, E. J. M. O. C. Organometallics as Structural Scaffolds for Enzyme Inhibitor Design. Med. Organomet. Chem. 2010, 141–153.10.1007/978-3-642-13185-1_6Search in Google Scholar
21. Alessio, E. Bioinorganic medicinal chemistry; John Wiley & Sons: Wenheim, 2011.10.1002/9783527633104Search in Google Scholar
22. Meggers, E. J. C. O. I. C. B. Exploring Biologically Relevant Chemical Space with Metal Complexes. Curr. Opin. Chem. Biol. 2007, 11 (3), 287–292. https://doi.org/10.1016/j.cbpa.2007.05.013.Search in Google Scholar PubMed
23. Williams, D. S.; Atilla, G. E.; Bregman, H.; Arzoumanian, A.; Klein, P. S.; Meggers, E. Switching on a Signaling Pathway with an Organoruthenium Complex. Angew. Chem. 2005, 44 (13), 1984–1987. https://doi.org/10.1002/anie.200462501.Search in Google Scholar PubMed
24. Maksimoska, J.; Williams, D.; Atilla-Gokcumen, G.; Smalley, K.; Carroll, P.; Webster, R.; Filippakopoulos, P.; Knapp, S.; Herlyn, M.; Meggers, E. Similar Biological Activities of Two Isostructural Ruthenium and Osmium Complexes. Chem. Eur. J. 2008, 14 (16), 4816–4822. https://doi.org/10.1002/chem.200800294.Search in Google Scholar PubMed PubMed Central
25. Schlotter, K.; Boeckler, F.; Hübner, H.; Gmeiner, P. Fancy Bioisosteres: Metallocene-Derived G-Protein-Coupled Receptor Ligands with Subnanomolar Binding Affinity and Novel Selectivity Profiles. J. Med. Chem. 2005, 48 (11), 3696–3699. https://doi.org/10.1021/jm050170s.Search in Google Scholar PubMed
26. Patra, M.; Gasser, G.; Wenzel, M.; Merz, K.; Bandow, J. E.; Metzler-Nolte, N. Synthesis and Biological Evaluation of Ferrocene-Containing Bioorganometallics Inspired by the Antibiotic Platensimycin Lead Structure. Organometallics 2010, 29 (19), 4312–4319. https://doi.org/10.1021/om100614c.Search in Google Scholar
27. Patra, M.; Gasser, G.; Wenzel, M.; Merz, K.; Bandow, J. E.; Metzler-Nolte, N. Synthesis of Optically Active Ferrocene‐Containing Platensimycin Derivatives with a C6–C7 Substitution Pattern. Eur. J. Inorg. Chem. 2011, 22 (22) 3295–3302.10.1002/ejic.201100497Search in Google Scholar
28. Omar, R. A.; Koparir, P.; Sarac, K.; Koparir, M.; Safin, D. A. A Novel Coumarin-Triazole-Thiophene Hybrid: Synthesis, Characterization, ADMET Prediction, Molecular Docking and Molecular Dynamics Studies with a Series of SARS-CoV-2 Proteins. J. Chem. Sci. 2023, 135 (1), 6. https://doi.org/10.1007/s12039-022-02127-0.Search in Google Scholar PubMed PubMed Central
29. Patra, M.; Gasser, G.; Pinto, A.; Merz, K.; Ott, I.; Bandow, J.; Metzler-Nolte, N. Synthesis and Biological Evaluation of Chromium Bioorganometallics Based on the Antibiotic Platensimycin Lead Structure. ChemMedChem 2009, 4 (11), 1930–1938. https://doi.org/10.1002/cmdc.200900347.Search in Google Scholar PubMed
30. Wenzel, M.; Patra, M.; Albrecht, D.; Chen, D. Y. K.; Nicolaou, K. C.; Metzler-Nolte, N.; Bandow, J. E. Proteomic Signature of Fatty Acid Biosynthesis Inhibition Available for in Vivo Mechanism-of-Action Studies. AAC 2011, 55 (6), 2590–2596. https://doi.org/10.1128/aac.00078-11.Search in Google Scholar PubMed PubMed Central
31. Vessieres, A.; Top, S.; Pigeon, P.; Hillard, E.; Boubeker, L.; Spera, D.; Jaouen, G. Modification of the Estrogenic Properties of Diphenols by the Incorporation of Ferrocene. Generation of Antiproliferative Effects in Vitro. J. Med. Chem. 2005, 48 (12), 3937–3940. https://doi.org/10.1021/jm050251o.Search in Google Scholar PubMed
32. Hillard, E. A.; Vessieres, A.; Jaouen, G. J. M. O. C. Ferrocene Functionalized Endocrine Modulators as Anticancer Agents. Med. Organomet. Chem. 2010, 81–117. https://doi.org/10.1007/978-3-642-13185-1_4.Search in Google Scholar
33. Hillard, E.; Vessières, A.; Thouin, L.; Jaouen, G.; Amatore, C. Ferrocene-Mediated Proton-Coupled Electron Transfer in a Series of Ferrocifen-Type Breast-Cancer Drug Candidates. Angew. Chem. 2006, 118 (2), 291–296. https://doi.org/10.1002/ange.200502925.Search in Google Scholar
34. Herson, P.; Jaouen, G.; Mansuy, D. J. A. C. Ferrocenyl Quinone Methides as Strong Antiproliferative Agents: Formation by Metabolic and Chemical Oxidation of Ferrocenyl Phenols. Angew. Chem 2009, 121, 9288–9290.10.1002/ange.200903768Search in Google Scholar
35. Schatzschneider, U.; Metzler-Nolte, N. J. A. C. I. E. New Principles in Medicinal Organometallic Chemistry. Angew. Chem., Int. Ed. 2006, 45 (10), 1504–1507. https://doi.org/10.1002/chin.200622288.Search in Google Scholar
36. Biot, C.; Dive, D. J. M. O. C. Bioorganometallic Chemistry and Malaria. Med. Organomet. Chem. 2010, 155–193. https://doi.org/10.1007/978-3-642-13185-1_7.Search in Google Scholar
37. Wang, D.; Lippard, S. J. J. N. R. D. D. Cellular Processing of Platinum Anticancer Drugs. Nat. Rev. Drug Discov. 2005, 4 (4), 307–320. https://doi.org/10.1038/nrd1691.Search in Google Scholar PubMed
38. Ott, I. On the Medicinal Chemistry of Gold Complexes as Anticancer Drugs. Coord. Chem. Rev. 2009, 253, 1670–1681.10.1016/j.ccr.2009.02.019Search in Google Scholar
39. Pizarro, A.M.; Habtemariam, A.; Sadler, P. J. J. M. O. C. Activation Mechanisms for Organometallic Anticancer Complexes. Med. Organomet. Chem. 2010, 21–56. https://doi.org/10.1007/978-3-642-13185-1_2.Search in Google Scholar
40. Casini, A.; Hartinger, C. G.; Nazarov, A. A.; Dyson, P. J. Organometallic Antitumour Agents with Alternative Modes of Action. Med. Organomet. Chem. 2010, 57–80. https://doi.org/10.1007/978-3-642-13185-1_3.Search in Google Scholar
41. Bratsos, I.; Gianferrara, T.; Alessio, E.; Hartinger, C. G.; Jakupec, M. A.; Keppler, B. K. Ruthenium and Other Non-Platinum Anticancer Compounds. Bioinorg. Med. Chem. 2011, 151–174. https://doi.org/10.1002/9783527633104.ch5.Search in Google Scholar
42. Berners-Price, S. J. J. B. M. C. Gold-Based Therapeutic Agents: A New Perspective. Bioinorg. Med. Chem. 2011, 197–222. https://doi.org/10.1002/9783527633104.ch7.Search in Google Scholar
43. Hogan, M.; Tacke, M. J. M. O. C. Titanocenes: Cytotoxic and Anti-angiogenic Chemotherapy Against Advanced Renal-Cell Cancer. Med. Organomet. Chem. 2010, 119–140. https://doi.org/10.1007/978-3-642-13185-1_5.Search in Google Scholar
44. Koparir, P.; Parlak, A. E.; Karatepe, A.; Omar, R. A. Elucidation of Potential Anticancer, Antioxidant and Antimicrobial Properties of Some New Triazole Compounds Bearing Pyridine-4-Yl Moiety and Cyclobutane Ring. Arab. J. Chem. 2022, 15 (7), 103957. https://doi.org/10.1016/j.arabjc.2022.103957.Search in Google Scholar
45. Morris, R. E.; Aird, R. E.; del Socorro Murdoch, P.; Chen, H.; Cummings, J.; Hughes, N. D.; Parsons, S.; Parkin, A.; Boyd, G.; Jodrell, D. I.; Sadler, P. J. Inhibition of Cancer Cell Growth by Ruthenium (II) Arene Complexes. J. Med. Chem. 2001, 44 (22), 3616–3621. https://doi.org/10.1021/jm010051m.Search in Google Scholar PubMed
46. Gasser, G.; Metzler-Nolte, N. J. C. O. I. C. B. The Potential of Organometallic Complexes in Medicinal Chemistry. Curr Opin Chem Biol. 2012, 16 (1–2), 84–91. https://doi.org/10.1016/j.cbpa.2012.01.013.Search in Google Scholar PubMed
47. Novakova, O.; Chen, H.; Vrana, O.; Rodger, A.; Sadler, P. J.; Brabec, V. DNA Interactions of Monofunctional Organometallic Ruthenium (II) Antitumor Complexes in Cell-free Media. J. Med. Chem. 2003, 42 (39), 11544–11554. https://doi.org/10.1021/bi034933u.Search in Google Scholar PubMed
48. Liu, H. K.; Wang, F.; Parkinson, J. A.; Bella, J.; Sadler, P. J. Ruthenation of Duplex and Single-stranded D (CGGCCG) by Organometallic Anticancer Complexes. Chem. Eur. J. 2006, 12 (23), 6151–6165. https://doi.org/10.1002/chem.200600110.Search in Google Scholar PubMed
49. Wang, F.; Xu, J.; Habtemariam, A.; Bella, J.; Sadler, P. J. Competition between Glutathione and Guanine for a Ruthenium (II) Arene Anticancer Complex: Detection of a Sulfenato Intermediate. J. Am. Chem. Soc. 2005, 127 (50), 17734–17743. https://doi.org/10.1021/ja053387k.Search in Google Scholar PubMed
50. Aird, R.; Cummings, J.; Ritchie, A. A.; Muir, M.; Morris, R. E.; Chen, H.; Sadler, P. J.; Jodrell, D. I. In Vitro And in Vivo Activity and Cross Resistance Profiles of Novel Ruthenium (II) Organometallic Arene Complexes in Human Ovarian Cancer. Br. J. Cancer. 2002, 86 (10), 1652–1657. https://doi.org/10.1038/sj.bjc.6600290.Search in Google Scholar PubMed PubMed Central
51. Cannon, C. L.; Hogue, L. A.; Vajravelu, R. K.; Capps, G. H.; Ibricevic, A.; Hindi, K. M.; Kascatan-Nebioglu, A.; Walter, M. J.; Brody, S. L.; Youngs, W. J. Vitro and Murine Efficacy and Toxicity Studies of Nebulized SCC1, a Methylated Caffeine-Silver (I) Complex, for Treatment of Pulmonary Infections. Antimicrob. Agents. Chemother. 2009, 53 (8), 3285–3293. https://doi.org/10.1128/aac.00314-09.Search in Google Scholar
52. Youngs, W. J.; Knapp, A. R.; Wagers, P. O.; Tessier, C. A. Nanoparticle Encapsulated Silver Carbene Complexes and Their Antimicrobial and Anticancer Properties: a Perspective. Dalton. Trans. 2012, 41 (2), 327–336. https://doi.org/10.1039/c1dt11100k.Search in Google Scholar PubMed
53. Melaiye, A.; Sun, Z.; Hindi, K.; Milsted, A.; Ely, D.; Reneker, D. H.; Tessier, C. A.; Youngs, W. J. Silver (I)− Imidazole Cyclophane Gem-Diol Complexes Encapsulated by Electrospun Tecophilic Nanofibers: Formation of Nanosilver Particles and Antimicrobial Activity. J. Am. Chem. Soc. 2005, 127 (7), 2285–2291. https://doi.org/10.1021/ja040226s.Search in Google Scholar PubMed
54. Hindi, K. M.; Panzner, M. J.; Tessier, C. A.; Cannon, C. L.; Youngs, W. J. The Medicinal Applications of Imidazolium Carbene− Metal Complexes. Chem. Rev. 2009, 109 (8), 3859–3884. https://doi.org/10.1021/cr800500u.Search in Google Scholar PubMed PubMed Central
55. Mann, B. E.; Motterlini, R. J. C. C. CO and NO in Medicine. Chem. Commun. 2007, (41), 4197–4208. https://doi.org/10.1039/b704873d.Search in Google Scholar PubMed
56. Mann, B. E. J. M. O. C. Carbon Monoxide: An Essential Signalling Molecule. Med. Organomet. Chem. 2010, 247–285. https://doi.org/10.1007/978-3-642-13185-1_10.Search in Google Scholar
57. Motterlini, R.; Otterbein, L. E. J. N. R. D. D. The Therapeutic Potential of Carbon Monoxide. Nat. Rev. Drug Discov. 2010, 9 (9), 728–743. https://doi.org/10.1038/nrd3228.Search in Google Scholar PubMed
58. Zobi, F.; Blacque, O.; Jacobs, R. A.; Schaub, M. C.; Bogdanova, A. Y. 17 e− Rhenium Dicarbonyl CO-Releasing Molecules on a Cobalamin Scaffold for Biological Application. Dalton. Trans. 2012, 41 (2), 370–378. https://doi.org/10.1039/c1dt10649j.Search in Google Scholar PubMed
59. Zobi, F.; Degonda, A.; Schaub, M. C.; Bogdanova, A. Y. CO Releasing Properties and Cytoprotective Effect of Cis-Trans-[ReII (CO) 2Br2L2] N Complexes. Inorg. Chem. 2010, 49 (16), 7313–7322. https://doi.org/10.1021/ic100458j.Search in Google Scholar PubMed
60. Dougan, S. J.; Habtemariam, A.; McHale, S. E.; Parsons, S.; Sadler, P. J. Catalytic Organometallic Anticancer Complexes. Proc. Natl. Acad. Sci. 2008, 105 (33), 11628–11633. https://doi.org/10.1073/pnas.0800076105.Search in Google Scholar PubMed PubMed Central
61. Alberto, R. J. M. O. C. Organometallic Radiopharmaceuticals. Med. Organomet. Chem. 2010, 219–246. https://doi.org/10.1007/978-3-642-13185-1_9.Search in Google Scholar
62. Kautz, A. C.; Kunz, P. C.; Janiak, C. J. D. T. CO-Releasing Molecule (CORM) Conjugate Systems. J. Organomet. Chem. 2016, 45 (45), 18045–18063.10.1039/C6DT03515ASearch in Google Scholar PubMed
63. Wright, M. A.; Wright, J. A. J. D. T. PhotoCORMs: CO Release Moves into the Visible. Chem. Commun. 2016, 45 (16), 6801–6811.10.1039/C5DT04849DSearch in Google Scholar
64. Hottinger, D. G.; Beebe, D. S.; Kozhimannil, T.; Prielipp, R. C.; Belani, K. G. Sodium Nitroprusside in 2014: A Clinical Concepts Review. Chem. Rev. 2014, 30 (4), 462.10.4103/0970-9185.142799Search in Google Scholar PubMed PubMed Central
65. Gruber, K.; Puffer, B.; Kräutler, B. J. C. S. R. Vitamin B 12-Derivatives – Enzyme Cofactors and Ligands of Proteins and Nucleic Acids. Inorg. Chem. 2011, 40 (8), 4346–4363.10.1039/c1cs15118eSearch in Google Scholar PubMed
66. Mann, K. K.; Wallner, B.; Lossos, I. S.; Miller, W. H.Jr. Darinaparsin: a Novel Organic Arsenical with Promising Anticancer Activity. Acc. Chem. Res. 2009, 18 (11), 1727–1734.10.1517/13543780903282759Search in Google Scholar PubMed
67. Govender, P.; Riedel, T.; Dyson, P. J.; Smith, G. S. Regulating the Anticancer Properties of Organometallic Dendrimers Using Pyridylferrocene Entities: Synthesis, Cytotoxicity and DNA Binding Studies. Dalton. Trans. 2016, 45 (23), 9529–9539.10.1039/C6DT00849FSearch in Google Scholar PubMed
68. Ghdhayeb, M.Z.; Haque, R. A.; Budagumpi, S.; Ahamed, M. B.; Majid, A. M. Mono-and Bis-N-Heterocyclic Carbene Silver (I) and Palladium (II) Complexes: Synthesis, Characterization, Crystal Structure and In Vitro Anticancer Studies. J. Inorg. Biochem. 2017, 121, 222–230.10.1016/j.poly.2016.09.065Search in Google Scholar
69. Hung, F. F.; Wu, S. X.; To, W. P.; Kwong, W. L.; Guan, X.; Lu, W.; Low, K. H.; Che, C. M. Palladium (II) Acetylide Complexes with Pincer‐Type Ligands: Photophysical Properties, Intermolecular Interactions, and Photo‐Cytotoxicity. Curr. Med. Chem. 2017, 12 (1), 145–158.10.1002/asia.201601414Search in Google Scholar PubMed
70. Zhang, P.; Sadler, P. J. J. J. O. O. C. Advances in the design of organometallic anticancer complexes. Dalton. Trans. 2017, 839, 5–14.10.1016/j.jorganchem.2017.03.038Search in Google Scholar
71. Patra, M.; Gasser, G. J. N. R. C. The Medicinal Chemistry of Ferrocene and its Derivatives. Nat. Rev. Chem. 2017, 1 (9), 0066.10.1038/s41570-017-0066Search in Google Scholar
72. Jaouen, G. J. C. S. R. Sodium Nitroprusside in 2014: A Clinical Concepts Review. A. Vessi Res, S. Top. 2015, 44, 8802–8817.10.1039/C5CS00486ASearch in Google Scholar PubMed
73. Nazarov, A. A.; Gardini, D.; Baquié, M.; Juillerat-Jeanneret, L.; Serkova, T. P.; Shevtsova, E. P.; Scopelliti, R.; Dyson, P. J. Organometallic Anticancer Agents that Interfere with Cellular Energy Processes: a Subtle Approach to Inducing Cancer Cell Death. Chem. Soc. Rev. 2013, 42 (7), 2347–2350.10.1039/C2DT31936ESearch in Google Scholar PubMed
74. Ekengard, E.; Glans, L.; Cassells, I.; Fogeron, T.; Govender, P.; Stringer, T.; Chellan, P.; Lisensky, G. C.; Hersh, W. H.; Doverbratt, I.; Lidin, S. Antimalarial Activity of Ruthenium (II) and Osmium (II) Arene Complexes with Mono-And Bidentate Chloroquine Analogue Ligands. Expert. Opin. Investig. Drugs. 2015, 44 (44), 19314–19329.10.1039/C5DT02410BSearch in Google Scholar PubMed
75. Grozav, A.; Balacescu, O.; Balacescu, L.; Cheminel, T.; Berindan-Neagoe, I.; Therrien, B. Synthesis, Anticancer Activity, and Genome Profiling of Thiazolo Arene Ruthenium Complexes. Dalton. Trans. 2015, 58 (21), 8475–8490.10.1021/acs.jmedchem.5b00855Search in Google Scholar PubMed
76. Zeng, J.; Sun, G.; Yao, W.; Zhu, Y.; Wang, R.; Cai, L.; Liu, K.; Zhang, Q.; Liu, X. W.; Wan, Q. Cover Picture: 3‐Aminodeoxypyranoses in Glycosylation: Diversity‐Oriented Synthesis and Assembly in Oligosaccharides (Angew. Chem. Int. Ed. 19/2017). Polyhedron 2017, 56 (19), 5133.10.1002/anie.201702798Search in Google Scholar
77. Noffke, A.L.; Habtemariam, A.; Pizarro, A. M.; Sadler, P. J. Designing Organometallic Compounds for Catalysis and Therapy. Chem. Asian J. 2012, 48 (43), 5219–5246.10.1039/c2cc30678fSearch in Google Scholar PubMed
78. Albada, B.; Metzler-Nolte, N. J. C. R. Organometallic–peptide Bioconjugates: Synthetic Strategies and Medicinal Applications. Nat. Rev. Chem. 2016, 116 (19), 11797–11839.10.1021/acs.chemrev.6b00166Search in Google Scholar PubMed
79. Romero-Canelon, I.; Sadler, P. J. J. I. C. Next-generation Metal Anticancer Complexes: Multitargeting via Redox Modulation. Mem. Inst. Oswaldo. Cruz. 2013, 52 (21), 12276–12291.10.1021/ic400835nSearch in Google Scholar PubMed
80. Liu, Z.; Sadler, P. J. J. A. O. C. R. Organoiridium Complexes: Anticancer Agents and Catalysts. Dalton. Trans. 2014, 47 (4), 1174–1185.10.1021/ar400266cSearch in Google Scholar PubMed PubMed Central
81. Wang, F.; Xu, J.; Wu, K.; Weidt, S. K.; Mackay, C. L.; Langridge-Smith, P. R.; Sadler, P. J. Competition between Glutathione and DNA Oligonucleotides for Ruthenium (II) Arene Anticancer Complexes. Dalton. Trans. 2013, 42 (9), 3188–3195.10.1039/C2DT32091FSearch in Google Scholar PubMed
82. Lin, Y.; Huang, Y.; Zheng, W.; Wang, F.; Habtemariam, A.; Luo, Q.; Li, X.; Wu, K.; Sadler, P. J.; Xiong, S. Organometallic Ruthenium Anticancer Complexes Inhibit Human Glutathione-S-Transferase π. J. Med. Chem. 2013, 128, 77–84.10.1016/j.jinorgbio.2013.07.029Search in Google Scholar PubMed
83. Jurgens, S.; Kuhn, F. E.; Casini, A. J. C. M. C. Cyclometalated Complexes of Platinum and Gold with Biological Properties: State-Of-The-Art and Future Perspectives. Angew. Chem. 2018, 25 (4), 437–461.10.2174/0929867324666170529125229Search in Google Scholar PubMed
84. Wang, Y.; Pigeon, P.; Top, S.; McGlinchey, M. J.; Jaouen, G. Organometallic Antitumor Compounds: Ferrocifens as Precursors to Quinone Methides. Angew. Chem. 2015, 127 (35), 10368–10371. https://doi.org/10.1002/ange.201503048.Search in Google Scholar
85. Nieto, D.; Bruña, S.; González-Vadillo, A. M.; Perles, J.; Carrillo-Hermosilla, F.; Antiñolo, A.; Padrón, J. M.; Plata, G. B.; Cuadrado, I. Catalytically Generated Ferrocene-Containing Guanidines as Efficient Precursors for New Redox-Active Heterometallic Platinum (II) Complexes with Anticancer Activity. Organometallics 2015, 34 (22), 5407–5417. https://doi.org/10.1021/acs.organomet.5b00751.Search in Google Scholar
86. Kuo, L. Y.; Kanatzidis, M. G.; Sabat, M.; Tipton, A. L.; Marks, T. J. Metallocene Antitumor Agents. Solution and Solid-State Molybdenocene Coordination Chemistry of DNA Constituents. J. Am. Chem. Soc. 1991, 113 (24), 9027–9045. https://doi.org/10.1021/ja00024a002.Search in Google Scholar
87. Mross, K.; Robben-Bathe, P.; Edler, L.; Baumgart, J.; Berdel, W.; Fiebig, H.; Unger, C. Phase I Clinical Trial of a Day-1,-3,-5 Every 3 Weeks Schedule with Titanocene Dichloride (MKT 5) in Patients with Advanced Cancer-A Study of the Phase I Study Group of the Association for Medical Oncology (AIO) of the German Cancer Society. In Vitro Toxicol. 2000, 23 (6), 576–579. https://doi.org/10.1159/000055009.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Advancements in CNT-based materials for optimized pharmaceutical removal via adsorption and photocatalysis
- Review on removal of heavy metals from industrial effluents by adsorption
- Cutting-edge techniques in low-temperature electrochemical water splitting: advancements in hydrogen production
- Biological activities of metal complexes with Schiff base
- Potential of organometallic complexes in medicinal chemistry
- Advancement in schiff base complexes for treatment of colon cancer
- Magnetic nanoparticles for efficient heavy metal removal: synthesis, adsorption capacity, and key experimental parameters
- Coal-based carbon/graphene quantum dots: formation mechanisms and applications
- Advancements in transition metal-catalyzed 1,2,3-triazole synthesis via azide–alkyne cycloaddition
- Gold complexes: a new frontier in the battle against lung cancer
Articles in the same Issue
- Frontmatter
- Advancements in CNT-based materials for optimized pharmaceutical removal via adsorption and photocatalysis
- Review on removal of heavy metals from industrial effluents by adsorption
- Cutting-edge techniques in low-temperature electrochemical water splitting: advancements in hydrogen production
- Biological activities of metal complexes with Schiff base
- Potential of organometallic complexes in medicinal chemistry
- Advancement in schiff base complexes for treatment of colon cancer
- Magnetic nanoparticles for efficient heavy metal removal: synthesis, adsorption capacity, and key experimental parameters
- Coal-based carbon/graphene quantum dots: formation mechanisms and applications
- Advancements in transition metal-catalyzed 1,2,3-triazole synthesis via azide–alkyne cycloaddition
- Gold complexes: a new frontier in the battle against lung cancer