Abstract
The uncontrolled increase in population and human activities has led to a significant rise in the demand for energy resources. The excessive use of limited fuel sources, unplanned deforestation, and greenhouse gas emissions have necessitated the search for and development of alternative, sustainable energy resources that cause minimal or no harm to the environment. The introduction of green and eco-friendly technologies offers a potential solution to address the growing demand in fields such as petroleum and hydrogen production, with the ultimate goal of promoting environmental sustainability. This review article highlights recent innovations in the field of heterogeneous catalysis, focusing on the development of various catalytic materials and processes, as well as their future prospects for both industrial and academic advancements. A brief discussion is presented on the efficient performance of solid acid and base catalysts, metal-organic frameworks (MOFs), electrocatalysts, and photocatalysts. The discussed catalytic systems have been explored for their potential applications, including biodiesel and hydrogen production as alternative energy sources, as well as CO2 reduction and the degradation of toxic dyes and organic pollutants for environmental remediation. Overall, the significance of heterogeneous catalysts has been explored, emphasizing their critical role in driving industrial progress and economic growth.
1 Introduction
Catalysis is a fundamental element of many chemical processes and hence it is a main strength of all chemical industries. 1 It is proven and now a day there is a growing concern about the catalysts that are environmentally friendly and safer technologies for the better industrial productions. Not less than 15 Nobel prizes have been awarded for studies on catalysis and many chemists around the world are repeatedly advancing the catalysts they have, and are motivated to discover new ones.
All the catalytic process can be applied either homogeneous or heterogeneous catalysis. Heterogeneous catalysis is a process in which catalyst being used is in a different phase than the reactants, i.e. the catalysis is usually in a solid phase and the reactant is in a liquid or in gaseous phase. As a part of catalysis, heterogeneous catalysis has prior importance in many areas of the chemical research for energy and environmental applications. 2 , 3 Although, there are many advantages of homogeneous catalysis which is pH dependent and can function with wide pH range. 3 Further, overall, most of (>80 %) chemical and bio-catalytic processes well established with heterogeneous catalysis and thus it is much successive catalytic process of vibrant branch of chemistry as well as pharmaceutical world. Figures 1–3 emphasizing the importance of the catalytic process and among all the process, how heterogeneous catalysis being a large part of chemistry itself can be found here. 4 , 5

Global catalyst market share 2018 (%) by applications 4

Involvement of catalytic and non-catalytic process to the chemical industries 5

Involvement of heterogeneous catalytic systems to various fields 5
The beginning era of heterogeneous catalysis, academic research and industrial processes were focusing on production of bulk chemicals and refining the oil as fuel on large scale requirement and it has been expanded rapidly in the recent decades. 6 , 7 Heterogeneous Catalysis is now become a key factor for many industries for the conversion of petroleum and natural gas into cleaner and efficient fuels, but now there is a demand for the production of alternative fuels such as hydrogen and biofuels. 6 , 7 , 8 Heterogeneous catalysis is still in use for the selective synthesis of special intermediates or products and fine chemicals in bulk production. Now a day it is possible to find the alternative route for the energy and environmental issues due to major advances in the design and development of catalyst at the molecular level.
In continuation, there are many efforts in progress to unlock energy sources that are much more difficult to use than fossil fuels, but are “greener”, where heterogeneous photocatalysis could make it economically feasible to produce hydrogen fuel from water splitting and leads to find novel methods to produce efficient energy sources from the raw resources such as CO2, 8 biomass 9 etc. Green technologies have included heterogeneous catalysis as being advantageous for regeneration and reusability, production of no waste or very less amount of waste, easy set up process with lower cost, high thermal and chemical stabilities and good selectivity of final product. Indeed, heterogeneous catalysis is being used for the environmental remediation for water, air and soil by applying an environmentally benign approach.
The conceptual platform for the practicable uses of catalytic systems can be defined by energy and mass dimensions. It consists of processes and materials being used with mass inputs in terms of raw materials to be converted into added-value ones directly or indirectly equal to the energy resources with or without emissions to be treated. 10 , 11 In this context, the consumed energy can be considered an input for endothermic reactions, while the energy generated serves as an output for exothermic reactions. Light, heat and electrons are often considered specific resources that promote catalytic systems by adding energy, leading to processes such as photocatalysis or photoelectrocatalysis. 10 , 12 , 13
The types of catalytic systems can be identified based on the structure and nature of the catalytic materials, which impart their catalytic functionality. Table 1, 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 and Figure 4 illustrate the most commonly used catalytic processes and materials.
Types of catalytic processes commonly used.
| No. | Catalytic processes | Ref. |
|---|---|---|
| A | Oxidation | |
| 1 | Advanced oxidation process
|
14 , 15 , 16 , 17 |
| 2 | Oxidation of SOx, NOx and COx | 18 |
| Oxidation of carbon monoxide to carbon dioxide (CO2) | 19 | |
| 3 | Oxidation of alkanes, alkenes and aromatics | 20 |
| 4 | Oxidation of alcohol (methanol to CO2) | 21 |
| B | Hydrogenation | |
| 5 | Hydrogenation process of vegetable oils | 22 |
| 6 | Hydrogenation of alkenes and aromatics | 23 |
| 7 | Formation of ammonia | 24 |
| 8 | Hydrogenation process of CO2 | 25 |
| 9 | Hydrogenation or hydrocracking of plastic and rubber waste | 26 |
| C | Dehydrogenation | |
| 10 | Dehydrogenation for H2 production | 27 |
| D | Reforming | |
| 11 | Catalytic dry reforming of methane | 28 |
| 12 | Catalytic reforming of biofuels | 29 |
| 13 | Catalytic reforming of oxygenates | 30 |
| 14 | Dry reforming processes for hydrogen production | 31 |
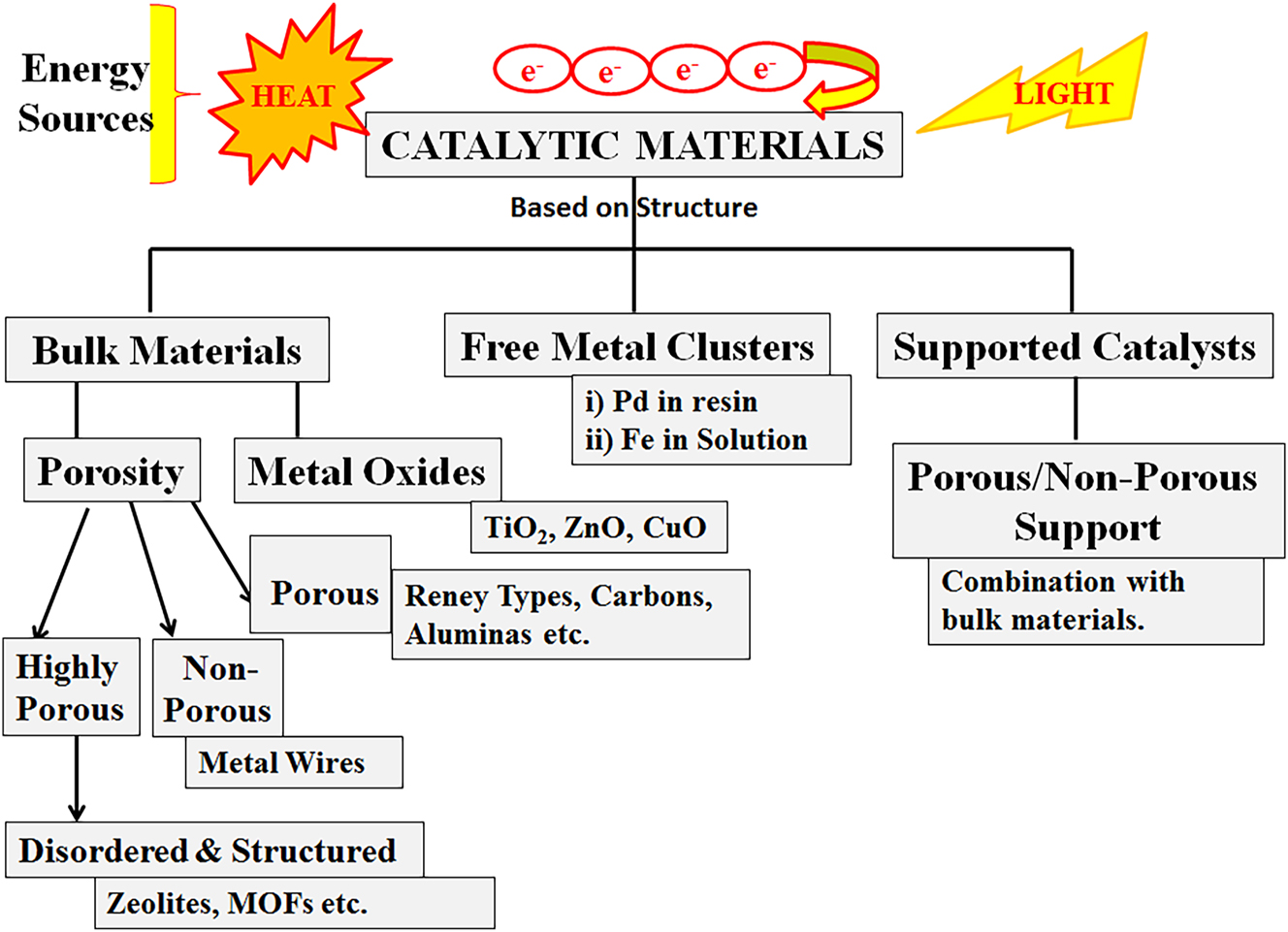
The types of catalytic systems based on structure.
There are also some drawbacks of heterogeneous catalysis such as it requires higher temperature and pressure for the reaction to occur, thus it requires higher energy consumption and therefore utility and energy related costs are very high. 2 To develop and design a highly efficient and environmentally-friendly catalytic materials and systems are very effective approaches to generate novel energy sources and remediation of contaminated soil, air and wastewater. 6 , 7b Thus, the bridging connection between a material structure and chemistry with efficient performance of heterogeneous catalyst would provide rational informations for catalyst design and addressing the challenges in promising use in the research area of energy resources.
The current review article shows recent development and progress in heterogeneous catalysis for energy and environmental applications. Heterogeneous catalysis consists of many subclasses depending on nature and structure of the catalyst used in solid phase such as solid acid catalyst, solid base catalyst and nano catalyst.
2 Energy applications
2.1 Biodiesel production
Currently, the use and storage of fuel energy for economic development and finding of an alternative fuel sources are in prior demand due to the ever-increasing world population. It is now an urgent worldwide issue to implement the possible all the efforts for the sustainable automotive fuels. 7 Since, last few decades, the biodiesel production has been promoted on the large scale as a safe and novel technology. Biodiesel belongs to the class of renewable and sustainable fuel source receiving more attention as rapidly growing field with high potential rather than other energy sources and evidently beneficial for a long-term investment. 32 , 33 , 34
Processes for biodiesel production can be classified as catalytically, chemically (base or acid catalysis), biologically (enzyme catalysis) and non-catalytic processes. Chemically, biodiesel (alkyl-ester derivatives) is usually produced by chemical catalytic transesterification of refined vegetable oils/waste cooking oils (long chain fatty acids) or animal fats. The chemical catalysts used earlier are strong acidic (HCl, H2SO4, HF, p-toluenesulfonic acid) or basic solutions (NaOH, CH3ONa and KOH).7b
Now days, a “green” approach for biodiesel production has been adopted the use of sustainable solid acid catalysts as replacements for such liquid acid catalysts so that the use of harmful/corrosive substances and unwanted formation of toxic wastes could be avoided. With remarkable developments and fruitful researches reported in the past few years for the industrial production, heterogeneous catalysis is growing up in the market for the biodiesel production.7b , 33a Heterogeneous catalytic transesterification is a process where the catalysts remain in different phase to that of the reactants and provides easier separation of biodiesel from glycerol. 35 , 36 , 37 The catalytic conversion of oil into biodiesel is slowly but the resulting biodiesel could be possible in an economical way. 38 , 39 Firstly, surface morphology characteristics, having large pore size to minimize diffusion problems is an ideal characteristic of a solid catalyst as being a part of heterogeneous catalysis. Another one, is deactivation, mainly achieved because of availability more active catalytic sites, high catalytic stability versus leaching and the probability to tune the hydrophobicity of the surface with the repulsion of highly polar compounds. 40 From the literature, solid base catalysts such as base zeolites, alkali earth metal oxides, hydrotalcites (or layered double hydroxides), alumina loaded with various compounds and mixed oxide have been quite successful with high conversion and yield of biodiesel obtained.33b
2.1.1 Solid acid catalyst (SAC)
SAC can be defined on the basis of nature and number of present active acid sites (Brønsted or Lewis acidity), arability of the acid sites and morphology of the support. Therefore, the acid catalysts could be used directly for biodiesel formation from low-grade, highly-acidic and water-containing oils. Thus, solid acid catalysis is now well established as benign alternatives to the unrecyclable-homogeneous acid catalysts and succeeds in the petrochemical industries. Many more benefits avail to this class of catalysts such as, (i) the catalysts could be modified and tunable, selective and easy regenerate and reuse, (ii) minimize product contamination through easy separation from target product, (iii) elimination of washing step particular for biodiesel production, (iv) replaced use of corrosive liquid acids and thus corrosion issue could be minimized, (v) could be employed in continuous flow process, (vi) minimum catalyst deactivation, (vii) catalyst could be rapidly prepared through economically and environmentally affordable process. 3 The surface morphology of the solid catalyst plays a key role for better catalytic performance through selective adsorption of reactant molecules and converts into the desired product. However, many more finding has to search out to connect and fill the knowledge gap between the fundamental concept of reaction pathway and the mechanisms of reactants on the solid catalysts.
They are mainly used for important acid catalyzed organic transformations such as Friedel-Craft alkylation/acylation, esterification/transesterification, acetal/ketal formation etc.7b Literature report highlighting the extensive use of solid acid catalysts for biodiesel production through esterification or transesterification processes. For example, the mechanism of the acid catalyzed transesterification reaction of triglycerides is well understood. 6 , 41 , 42 Still there are some challenges facing for biodiesel production such as to increase rate of reaction and to reduce unwanted side reaction. Thus, these challenges lead to develop a novel, efficient and stable catalytic system and more efforts for reduction in cost of preparation the catalyst and sequentially reduced biodiesel production costs. There is lots of research work in progress to overcome the said challenges through potential application of various solid acid catalysts for biodiesel production.
The ideal characteristics of a solid acid catalyst are strong Brønsted and/or Lewis acid properties, unique porosity or textural properties and a hydrophobic surface. Till date, the catalytic potential of solid acids have been reported for the biodiesel production such as acidic inorganic ion exchangers, acidic clays, metal oxides, mixed metal oxides, H-form zeolites, heteropolyacids (HPAs), modified silica, acidic ionic liquids, hybrid organo-inorganic materials and carbon based materials.
The Brønsted acid sites are highly polarized hydroxyl groups at the surface of the catalyst that serve as the H+-donor, while the Lewis acid sites are coordinatively unsaturated cationic sites, which leave the exposed M+ ion to interact with guest molecules and act as the acceptor of the electron-pair. With respect to base catalysts, biodiesel production processes based on the use of solid acid catalysts are effective alternatives to the conventional processes because of their easy workup and the simultaneous promotion of esterification and transesterification reactions without soap formation.
Zeolites and zeolite type materials : Zeolites are naturally abundant three dimensional frameworks with crystalline structures of aluminosilicates interlinked by oxygen atoms. They contain Al, Si and O in their framework, and cations, water and/or other molecules within their pores. 34 They are well established and robust materials because of having molecular porous structure with channel of uniform sizes gives materials to be ion exchange characteristic. The catalytic activity depends on replacement of Si4+ by Al3+ with crystalline SiO2 framework which generates negative charges in the catalyst framework and thus improves catalytic activity. Interestingly, the adsorption ability could be modified through synthesis methods as expected due to the strong electric field created by the loosely held cations within the catalyst framework. Thus, desired catalytic performance in term of good activity and selectivity could be obtained as it has ability to introduce active sites within the pores and channels. 3
Zeolite can accommodate number of cations such as Na+, K+, Ca2+, Mg2+ and many more from which it has its basic nature. H-ZSM-5, H-MOR, H-BETA and H-USY are H+ ion exchanged acid form zeolites found to be not much catalytic active for esterification or transesterification reactions of biodiesel production due to internal diffusion limitations of the bulky reactant molecules into the micro-pores of zeolites. 3 Zeolites Y are weak solid acid catalyst while Zeolites β are three-dimensional structure of 12-membered ring channels with high silica content having both Lewis and Brønsted-acid sites. Lewis-acid sites are mainly present in the micro-porous walls. Zeolite β does not exhibit high activity in transesterification, but it could be used for selective removal of FFAs in waste oil. Silicoalumino-phosphates, the recently developed Zeolite types materials foe examples, SAPO4, ALPO4, MeAPSO4 and MeAPO4 could be synthesized with coordinated Al, Si, P, transition metals and other elements. 3
The catalytic activity of the protonated form of H–Y zeolite (Faujasite with high Si/Al ratio) has been investigated as solid acid catalyst for esterification of oleic acid with methanol. 43 At optimized conditions, methyl esters production yield reaches up to ∼95 % and the used catalyst could be regenerated easily and reused for the same. Septiani et al. 44 have prepared Zeolite ZSM-5 and studied its catalytic activity for the transesterification of vegetable oil for biodiesel production. The results of the catalytic reaction show a maximum biodiesel yield of ∼92 % at the optimized reaction time and temperature. Zeolite and zeolite-based heterogeneous catalysts 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 for biodiesel production have been summarized in Table 2.
Zeolites and zeolite-based heterogeneous catalysts for biodiesel production using esterification or transesterification reactions.
| Sr. No. | Catalysts | Esterification/transesterification reactions molar ratio of oil: alcohol | Ref. |
|---|---|---|---|
| 1 | H–Y(80) zeolite (faujasite with high Si/Al ratio) | Esterification of soybean oil Oil: methanol = 1:3, 1:6 and 1:9 |
43 |
| 2 | Zeolite ZSM-5 prepared from rice husk ash | Transesterification of vegetable oil Oil: methanol = 1:3 |
44 |
| 3 | H-zeolite | Transesterification of vegetable oil Oil: methanol = 19.4:1 |
45 |
| 4 | Y-type zeolites with Al2O3 and Na2O | Transesterification sunflower and waste oil Oil: methanol = 12:1 to 48:1 |
46 |
| 5 | Faujasite (FAU)-type zeolite prepared using Irish shale rock | Esterification of vegetable oil Oleic acid: ethanol = 4:1–15:1 by mass |
47 |
| 6 | KOH/zeolite prepared by impregnation in KOH | Transesterification of waste sunflower oil Oil: methanol = 1:1.51 |
48 |
| 7 | KOH/Bentonite prepared by impregnation method | Transesterification of refined soybean oil Oil: methanol = 6:1 −14:1 |
49 |
| 8 | Y-type zeolites, with different Al2O3 and Na2O percentage | Transesterification of vegetable oil Oleic acid: methanol = 6:1 |
50 |
| 9 | ZSM-5 and beta hierarchical zeolites | Transesterification of algae oil Oil: methanol = 100:1 |
51 |
| 10 | Alkali loaded low-Al zeolite beta | Transesterification of triolein Oil: methanol = 1:15 (w/w) |
52 |
| 11 | Zeolite linde type a (LTA, dehydrate form) | Transesterification of triolein (methanol in excess) | 53 |
| 12 | KOH/Zeolite prepared by impregnation method | Transesterification of triglyceride with excess methanol | 54 |
| 13 | Natural zeolite bayah banten with KOH impregnation method | Transesterification using vegetable oil Jatropha oil: methanol = 1:1 | 55 |
| 14 | Zeolite based catalysts prepared from fine powder and kaolinite using pelletization method | Simultaneous transesterification and esterification of waste cooking oil Oil: methanol = 1:5 |
56 |
| 15 | Natural zeolite with potassium impregnation | Transesterification of rice bran oil Oil: methanol = 1:6 to 1:12 |
57 |
| 16 | Zirconia supported on Indonesia natural zeolite | Esterification of palm oil sludge Oil: methanol = 1:4 |
58 |
| 17 | KOH/NaY zeolite prepared by the impregnation method | Transesterification of palm oil Oil: methanol = 1:15 |
59 |
| 18 | KOH/Al2O3 with varying KOH loadings prepared by the impregnation of the Al2O3 support | Transesterification of vegetable oil Oleic acid: methanol = 1:15 |
60 |
| 19 | NaX zeolite impregnated with mixture of CaO nanoparticles/ethanol | Transesterification sunflower Oil: methanol = 1:6 |
61 |
| 20 | Sodic potassic clinoptilolite and sodic potassic calcic clinoptilolite zeolites | Transesterification of waste cooking oil Oil: methanol = 68–27 (vol%) |
62 |
| 21 | Lanthanum-natural zeolite (La/Zeolite) | Transesterification crude palm oil Oil: methanol = 1:9 |
63 |
| 22 | ZnO/Zeolite, PbO/Zeolite | Esterification of Jatropha oil Oil: methanol = 1:6 |
64 |
| 23 | Zeolite X and a from flyash using hydrothermal treatment | Transesterification of refined mustard oil Oil: methanol = 1:6 |
65 |
| 24 | Zeolite MCM-22 and zeolite beta | Transesterification of triolein in excess methanol | 66 |
| 25 | Zeolites loaded with CH3COOK | Transesterification of Jatropha curcas
Oil: methanol = 1:10 |
67 |
| 26 | HZSM-5 and HZSM-5 impregnated with sulfated lanthanum oxide (SO4 2−/La2O3) | Esterification of oleic acid Acid: methanol = 1:5, 1:10 and 1:20 |
68 |
| 27 | Commercial H–Y and ZSM-5 zeolite | Esterification of palmitic acid Acid: methanol = 1:2 |
69 |
Metal oxides: Metal oxides are materials of typical characteristics such as thermal and mechanical stability, high specific surface area, and large pore size and pore volume. Thus, they are extensively being used as catalyst supports for catalytic purpose.
The typical superacids such as SO4 2−/ZrO2, SO4 2−/TiO2, SO4 2−/Ta2O5, SO4 2−/Nb2O5 etc.7b They possess potential economic and green benefits for a number of hydrocarbon reactions under mild reaction condition and thus, interest has been generated to use of such materials for transesterification and esterification reactions to produce biodiesel. 70 Herein, an interesting factor about the metal oxides (ZrO2, TiO2, Ta2O5, Nb2O5), they possess the both Brønsted and Lewis acidity that can be improved by modifying its surface by sulfate groups which makes materials to be promising catalyst to promote protonation in the esterification process. 71 Castro et al. 72 have studied heterogeneous solid acid catalysis for esterification and transesterification processes for biodiesel production as renewable fuels to meet the future societal requirements. The authors have prepared sulfated niobium oxide (Nb2O5/SO4) impregnation method and used as solid acid catalyst for biodiesel production from macaw palm oil via high free fatty acid content transesterification with ethanol. As results, the catalyst Nb2O5/SO4, exhibits excellent catalytic activity towards biodiesel production (about ∼99 % ester content). The spent catalyst could be reused up to five consecutive catalytic cycles which shows excellent yield of the desired products and the reducing of the yield after fifth catalytic cycle could be due to the handling/physical loss of the catalyst during recycles and regeneration. 72 Sulfated zinc oxides SO4 2−-ZnO and SO4 2−/ZnO have been synthesized by co-precipitation and impregnation respectively and characterized as active solid acid catalyst. 73 The prepared catalysts have been used for trans-esterification of soybean oil with methanol to compare their performance for biodiesel production. As results at optimized reaction conditions, SO4 2–ZnO prepared by co-precipitation method exhibit highest catalytic activity and obtained FAME yields of ∼80 %.
Sulfonic ion exchange resins such as Amberlyst-15, –16, −35, −36 and −131, 74 , 75 Nafion resins 76 , 77 , 78 , 79 , 80 as SAC-13 and NR50 and EBD-100, -200 and −300, 81 were commonly used heterogeneous catalysts and found to be effective in liquid phase esterification and trans-esterification reactions. The protons bonded to the sulfonic groups are the responsible for the strong acidic active sites which are available on the cross-linked polymeric matrix of these ion exchange resins. The catalytic activity more depend on their surface properties which in turn effective populations of the acidic active sites for the trans-esterification and esterification reactions.
Mesoporous silica (SBA-15) supported ZnO/SBA-15 and MgO/SBA-15 have been prepared and characterized for solid acid catalytic activity. The results show that catalysts exhibit good catalytic activity and promote the esterification of lauric acid with 1-butanol to produce biodiesel (∼81 % yield of butyl ester) under atmospheric pressure and reflux conditions. 82 Tesser et al. have prepared Niobia supported catalyst by impregnation of silica pallets with loading of Nb and characterized for catalytic characteristics. The prepared solid acid catalyst has been used in both transesterification (free acid methyl ester conversion) and esterification (free fatty acid conversion) reactions of triglycerides for biodiesel production. The study, Nb supported on silica found to be much active in both the mentioned reactions with no loss of catalytic activity in the continuous run of 100 h but slow leaching of Nb observed which could be reduced the catalytic potential. 83 Xie et al. prepared lithium doped zinc oxide (Li/ZnO) using impregnation method followed by calcination at different temperatures and evaluated its catalytic activity for transesterification of soybean oil with methanol at optimized reaction conditions. 84 As results, Li/ZnO exhibits good catalytic activities towards soybean conversion to ester as obtained ∼96 % yields. The catalytic sites are in basic nature which is due to formation of Li2O from thermal decomposition of LiNO3 at higher calcination temperatures. The authors also have prepared CaO–MoO3-SBA-15 by incipient impregnation method with different calcination temperatures and tested for the similar transesterification reaction of soybean oil with methanol for biodiesel production. 85 The prepared solid catalysts, a catalyst with loading of 40 % and calcined at 550 °C shows highest catalytic activity (∼83 % yield of oil conversion) at the optimized the reaction conditions. The catalyst could be easily revered and reused without loss of its potential catalytic activity. 85 Viswanathan et al. 86 prepared sulfated Fe2O3–TiO2 from ilmenite and calcined for different temperatures. The prepared catalyst examined for its acidic active sites and used for conversion of vegetable oil to biodiesel via transesterification reaction with methanol. As results, the catalyst calcined below 500 °C shows higher conversion of vegetable oil with remarkable yield of biodiesel which is attributed to the high affinity of hydroxyl groups of methanol on Fe2O3–TiO2. The removal of sulfate groups has been observed during calcination on above 500 °C could reduce % yield of biodiesel production. 86
Mixture of strontium and nickel oxide has been synthesized by coprecipitation method followed by different calcination temperatures and characterized for catalyst properties. 87 The prepared catalysts have been used as heterogeneous catalysts for the transesterification of macaw palm oil with methanol for biodiesel production. The catalyst with the calcination temperature of 1,100 °C has highest catalytic activity as achieved ∼97 % conversion at the optimized reaction conditions as well as recycling studies also favoring the good % yield for the five catalytic runs. 87 Ong et al. 88 have studied catalytic activity of the carbon supported CuO catalyst in which the copper nanoparticles have been synthesized by sol-gel method and the nanoparticles impregnated into the pore network of the carbon supported. The prepared catalysts used in esterification of free fatty acid in the rubber seed oil and ∼95 % of the free fatty acid conversion was obtained at the optimized reaction conditions. The catalytic performance is comparable to the liquid acid H2SO4 as well as compared with other heterogeneous catalysts, the carbon supported-CuO could performed at lower temperature. The reusability study also shows about easy recovery and separation of the catalyst after process completion however it found with lower activity in the use of first catalytic cycle. Zhang et al. 89 have prepared sulfonic/carboxylic dual-acid catalyst based on sulfur-rich graphene oxide (GO-S) as cost effective SAC used for the esterification of the oleic acid with methanol to produce methyl-ester as biodiesel. The results show, GO-S exhibits good catalytic performance in term of activity and reusability which has high TON factor compared to other carbon based SACs. Having a two-dimensional layered structure and the strong synergetic effect between–SO3H and–COOH groups on the graphene nano-sheets, are the key factors for the catalytic activity of GO-S. 89 The study reveals the promising use of the GO-S in the esterification of the waste fatty acids to convert into the valuable biofuels.
2.1.2 Solid base catalysts
Metal oxides: Recently, alumina has been exhibited as a good support for catalytic material in catalytic process due to having good characteristics such as thermal and mechanical stabilities, high surface area, large pore size and pore volume. 90 Anisuzaman et al. have prepared CaO-supported basic alumina catalyst by impregnation method followed by calcination temperatures and characterized for their catalytic properties. 90
Alkali metal oxides (CaO, MgO and BaO) doped silica (SiO2) has been synthesized using sol-gel method followed by different calcination temperatures and used as base catalysts for the biodiesel production. 91 The catalytic transesterification reaction of corn oil with methanol shows that the catalyst CaO/SiO2 exhibits highest biodiesel production compared to other catalysts with the purity and yield of ∼97 % and ∼82 % respectively. 91 Ismail et al. have synthesized calcium oxide (CaO) from mud clam shell and used as potential heterogeneous catalyst for the biodiesel production. 92 The results of the catalytic transesterification reaction of castor oil with methanol show a good efficiency of CaO catalyst with the obtained ∼96 % yield of biodiesel formation using the optimum reaction conditions as well as the CaO catalyst from mud clam shell could be recycled and reused up to five catalytic runs. 92 Thitsartarn et al. also have reported calcium doped cerium-incorporated SBA-15 (Ca/CeS) synthesized by direct synthesis of Ca-incorporated-SBA-15 followed by impregnation of CaO. 93 The catalytic studies, the Ca/CeS shows excellent catalytic performance for biodiesel production trough catalytic transesterification of palm oil with methanol and also the highest catalytic activity found amongst the other supported catalyst such as CaO/CeO2 and CaO/CeO2-SBA-15. As being heterogeneous catalysis, negligible leaching of elements was observed due to strong interaction between CaO and CeS support. Furthermore, beneficially the catalyst could be reused for 15 catalytic runs with insignificant decrease in catalytic activity which reveals the very good efficiency of the catalyst for the biodiesel production. 93 Heterogeneous strontium oxide/calcium oxide (SrO–CaO) mixed metal oxides catalyst has been used in the ultrasonic-assisted transesterification of Jatropha oil into biodiesel. The catalyst characterization and the catalytic studies show that the prepared catalyst exhibits high efficiency for the conversion of Jatropha oil into biofuels. 94
Biodiesel production from non-edible Jatropha oil has been reported using acid-base bi-functionalized CaO–La2O3 heterogeneous catalyst. 95 The catalyst comprising CaO and La2O3 mixed metal oxides with different Ca/La ratios have been prepared by using co-precipitation method. As results, the highest biodiesel yield of ∼98 % has been achieved and binary catalyst system found to be more stable which was supported by recycling and reusability of the catalyst even after four catalytic cycles. The observed very good catalytic activity in the mixed metal-metal oxide is due to well dispersion of CaO on composite surface which enhance both surface acidic and basic sites as compared to that of bulk CaO and La2O3 metal oxide. 95 Wang et al. 96 have reported a series of acid-base bi-functional metal-boron catalysts prepared by sol-gel method followed by calcination temperatures and characterized by spectral and surface characteristics. Amongst the prepared catalysts, the Ca-Boron (700 °C) catalyst shows highest catalytic performance for the production of biodiesel with high acid value (yield of ∼98 %) through transesterification reaction of non-edible oil (Jatropha) with methanol using optimized reaction conditions. The catalyst exhibits a good stability and reusability which makes it promising acid-base bi-functional catalytic material for the biodiesel production. 96
Lee et al. 97 have studied the catalytic activity of mixed metal oxides (MMOs) (CaO–MgO, CaO–ZnO, CaO–La2O3 and MgO–ZnO) synthesized by co-precipitation method followed by calcination at different temperatures. MMOs contained high basicity and strong basic strength compared to single metal oxide which shows excellent catalytic performance in the transesterification process. As results of the studied catalytic reactions, the Ca-based MMOs catalysts exhibit highest catalytic activity in term of ∼90 % yields of biodiesel production and reusability with low metal leaching. Amongst three Ca-based MMOs, CaO–ZnO has comparative high conversion rate with ∼94 % yield of biodiesel and could be used for four catalytic cycles with negligible loss of activity. 97 Yigezu et al. 98 have studied catalytic activity of metal oxides such as Co3O4, KOH, MoO3, NiO, V2O5 and ZnO for catalytic conversion of oil into organic liquid products. As results, the catalysts are found to be suitable and active solid catalysts for the catalytic cracking of vegetable oil for bio-fuel production. 98
The review chapter more discussed on basic mixed-metal oxides (CaO and MgO based), acidic mixed-metal oxides (SiO2, SnO2 and Fe2O3 based) and acid-base bi-functional mixed-metal oxide (ZnO–La2O3, MnCeO2 and SrO–ZrO2). 60 Many of the catalysts studied are excellent in the catalytic performance for the biodiesel production through esterification or in-situ esterification and transesterification processes. 60 Refaat has briefly presented a review chapter on synthesis methods and characterization of various metal oxides commonly being used in the esterification and transesterification of oil with alcohol for the biodiesel production. 99 The chapter highlighting about the catalytic activity of alkali earth metal oxides, transition metal oxides, mixed metal oxides and supported metal oxides as heterogeneous catalysts in the transesterification reaction. The author has discussed about the catalyst selection and catalyst preparation for efficient transesterification process as well as explained the deactivation issue of the catalyst as important as catalyst activity. The author has concluded about the synergy between emerging technologies as ultra-sonication and microwave irradiation, on one side, and heterogeneous catalysis, on the other side, for the production of biodiesel appears to be very promising. 99 Cardoso et al. 100 have reported a review article about the importance characteristics related to the synthetic route, stability and activity of the tin catalysts in the esterification of triglycerides for biodiesel production with high free acid methyl ester content. They have explained the main structural features and physical properties of the solid tin catalysts such as SnO2, SO4 2−–SnO2, SO4 2−–SnO2–Al2O3, SO4 2−–SnO2–Fe2O3 with different Sn to Al and Fe molar ratio, for better understanding and significant contribution for the biodiesel production. 100
Vedrine has reported a review chapter on the fundamentals of heterogeneous catalysis and on metal oxides special members of heterogeneous catalyst family, acid-base catalyzed reactions, selective partial and total oxidation, de-pollution, biomass conversion and green chemistry including photocatalysis. 61 The main focus of the article is discussion on current industrial applications of supported and non-supported metal oxide catalysts. Chouhan et al. have presented a comprehensive review on a large number of modern heterogeneous catalysts for biodiesel production. 6 The report content discussed on alkali metal oxides, alkaline metal oxides and their derivatives, transition metal oxides, mixed-metal oxides (MMOs), ion exchange resin type oxides, sulfated oxides as solid acid catalysts, carbon and boron group base solids and enzyme based solid catalysts. The authors have discussed on the catalytic activity, selectivity, catalyst loading, catalyst reusability and summary of the future scopes. 6 Diamantopoulos et al. have introduced heterogeneous catalysts as important materials for the biodiesel production. 101 The authors have discussed on zeolite, mixed oxides, sulfonic acid group catalysts, sulfonic acid modified SiO2, HPAs and polyoxonetalates, supported and substituted HPAs and solid acid catalyst based in waste carbon. They have explained the catalytic activity of these catalysts in terms of their efficiency and reusability. The review revels the fact about the advantages of the solid acid catalysts although they are not much active than base catalysts, benefits such as procedure simplification and positive environmental impacts, support the utility of biodiesel production by using various efficient solid acid catalysts. The lower catalytic activity of the solid acids compared to other catalyst for transesterification reactions is usually compensated by operating at higher calcination temperatures (120–450 °C). 101
Table 3 summarizing the metal oxides and mixed-metal oxides (MMOs) 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 as heterogeneous catalysts for biodiesel production via esterification or/and transesterification reactions.
Metal-oxides and mixed-metal oxides as heterogeneous catalysts for biodiesel production using esterification or transesterification reactions.
| Sr. No. | Catalysts | Esterification/transesterification reactions molar ratio of oil: alcohol | Ref. |
|---|---|---|---|
| 1 | Sulphated zinc oxide (SO4 2−-ZnO and SO4 2−/ZnO prepared by coprecipitation and impregnation method respectively | Transesterification of soybean oil Oil: methanol = 6:1 |
73 |
| 2 | Lithium-doped ZnO prepared by using impregnation method followed by calcinations | Transesterification of vegetable oil Oil: methanol = 1:12 |
74 |
| 3 | CaO–MoO3-SBA-15 prepared by incipient impregnation method | Transesterification of soybean oil Oil: methanol = 1:50 |
75 |
| 4 | Sulfated Fe2O3–TiO2 using conc. H2SO4 | Esterification of vegetable oil Oil: methanol = 1:20 |
76 |
| 5 | Strontium and nickel- oxides prepared by using a co-precipitation method | Transesterification using macaw palm oil Oil: methanol = 1:9 |
77 |
| 6 | Cu-nanoparticles impregnated in porous carbon support | Transesterification of pail oil/animal fat Oil: methanol = 1:10 |
78 |
| 7 | Dual-acid catalyst sulfur-rich graphene oxide prepared by simple modified Hummer’s method | Esterification of oleic acid with methanol Acid: methanol = 20:50 (w/w; g) |
79 |
| 8 | Alkali earth metal oxides (CaO, MgO and BaO) doped SiO2 | Transesterification of vegetable oil/animal fat oil: methanol = 1:16 | 81 |
| 9 | Calcium oxide derived from mud clam shell | Transesterification of caster oil Oil: methanol = 1:14 |
92 |
| 10 | Ca-doped Ce-incorporated SBA-15 impregnated with CaO. (CaO/CeO2 and CaO–CeO2/SBA-15) | Transesterification of palm oil Oil: methanol = 1:20 |
93 |
| 11 | SrO–CaO mixed metal oxides | Transesterification of Jatropha oil Oil: methanol = 1:10 |
94 |
| 12 | CaO/La2O3 prepared by co-precipitation method | Transesterification of crude Jatropha oil Oil: methanol = 1:25 |
95 |
| 13 | Acid-base bi-functional metal-boron catalysts prepared by sol-gel method (metal = Ca2+,Al3+, Mg2+, Zr4+) | Transesterification of crude Jatropha oil Oil: methanol = 1:20 |
96 |
| 14 | Mixed metal oxides (CaO–MgO, CaO–ZnO, CaO–La2O3 and MgO–ZnO) | Transesterification of high acid Jatropha
Oil: methanol = 1:25 |
97 |
| 15 | Metal oxides (Co3O4, KOH, MoO3, NiO, V2O5 and ZnO) | Transesterification vegetable oil with excess methanol | 98 |
| 16 | NaY zeolite-supported La2O3 | Transesterification of castor oil. Oil: methanol = 1:25 |
102 |
| 17 | Alkaline earth metal oxides (BaO, CaO, MgO and SrO) | Transesterification of palm oil Oil: methanol = 1:3 −1:15 (% w/w) |
103 |
| 18 | CsHPW12O40, Nb2O5, sulfated titania (TiO2/SO4 2−), sulfated zirconia (ZrO2/SO4 2−) and SnO2/SO4 2− | Esterification of dodecanoic (lauric) acid with methanol, propanol, 2-ethylhexanol Acid: alcohol = 1:1 up to 1:5 |
104 |
| 19 | ZnO/zeolite and PbO/zeolite prepared by hydrothermal impregnation precipitation method | Transesterification of Jatropha curcas and Pongomia pinnata oil Oil: methanol (excess) = 1:>3 |
105 |
Hydrotalcites: The hydroxyl carbonates of magnesium and aluminum, hydrotalcites (HTs) are minerals belonging to the family of anionic clays, containing anionic species in the inter-lamellar space. HTs are being used as solid catalysts due to their physicochemical properties, such as thermal stability, porosity, specific large surface area (after calcination), memory effect, basicity or/and acidity, and anion exchange capacity. 106 , 107 Since last many years, researchers are developing Mg/Al as well as Ca/Al and mixed metal-based HTs materials using various synthesis routes and applied as heterogeneous catalytic materials for base catalyzed organic synthesis. 108 , 109 Herein, such studies have been noted about the performance of HTs for the biodiesel production.
Xie et al. have developed calcined Mg/Al hydrotalcites as heterogeneous base catalyst by using coprecipitation method for transesterification process of soybean oil and methanol to produce FAME through optimization of reaction conditions. 110 The catalyst calcined at 500 °C with Mg/Al ratio of 3.0 found to be most active having higher basicity and suitable for the reaction having ∼67 % FAME conversion. Yagiz et al. 111 have studied catalytic performances of the lipase enzyme immobilized hydrotalcites prepared by co-precipitation method by using commercial zeolites as immobilization materials. The obtained immobilized lipase materials have been used for transesterification reaction of oil. As results, HT was found to be the suitable supporting material for lipase than the other four types of zeolites used and it observed to be able to catalyze the transesterification of waste cooking oil with methanol to produce methyl esters. 111 Mg/Al hydrotalcites prepared by co-precipitation of Mg2+ and Al3+ ions in alkaline solution of NaOH followed by calcination at 400 °C. 112 The prepared catalyst used biodiesel production from pongamia oil with methanol through transesterification under optimized reaction conditions and resulted ∼90 % yield of pongamia oil methyl ester showing potential activity of the present catalyst. 112 The hydrotalcites catalyst with Mg/Al molar ratio of 3.0, calcined at 450 °C under argon (Ar) flow for 6 h has been characterized for spectral analysis. 113 The prepared material has been tested for transesterification reaction of soybean oil with methanol under mild conditions of temperature and pressure to produce biodiesel. The results show highest fatty acid methyl ester conversion of ∼94 % at optimized reaction conditions which makes material to be a promising heterogeneous base catalyst for biodiesel formation. 113 Chelladurai et al. 114 have studied an environmentally benign process for the transesterification reaction of neem oil with methanol to yield fatty acid methyl esters (FAME) using Zn–Mg–Al hydrotalcites as heterogeneous solid base catalysts thorough optimization of reaction conditions. The obtained highest triglyceride conversion rate was ∼92 % at optimized conditions and this could be attributed to the incorporation of Zn into the Mg–Al hydrotalcite material.
Diserio et al. 115 have reported catalytic performances of Mg/Al hydrotalcites (Pural©MG76) for biodiesel production through transesterification of soybean oil with methanol using both an autoclave and continuous packed bed reactor. The observations confirmed about the calcined Mg/Al hydrotalcites as effective heterogeneous catalyst for the transesterification of triglycerides with methanol where the used catalysts could be regenerated by washing with acetone and reused for the economical way without loss of significant performance. 115 Costarrosa et al. 116 have studied influences of the reaction parameters in terms of response surface methodology (RSM) for the heterogeneous transesterification of waste cooking oil with methanol to produce biodiesel using calcined Mg–Al hydrotalcite catalyst. The study has proved RSM as good indicative tool for predicting the biodiesel yield and also about the calcined Mg/Al hydrotalcites as an effective basic catalyst for the production of biodiesel from waste cooking oil. 116 Mg/Al hydrotalcites has been synthesized by urea hydrolysis and co-precipitation methods and characterized. 117 The prepared material tested for transesterification process of waste cooking oil (a triglyceride) with methanol and optimized the reaction conditions using hexane and glycerol as co-solvents. At optimized reaction condition, Mg/Al hydrotalcites as solid base catalyst gives maximum biodiesel yield and compared with the results of KOH as liquid homogeneous base catalyst. 117 A series of solid base catalysts, KF-doped three-metal mixed hydrotalcites (KF/Ca–Mg–Al) with different cation ratio have been synthesized and applied in transesterification process of palm oil with methanol to yield FAME. 118 The doping of KF and synergetic effect of Ca2+ and Mg2+ broadly improved the catalytic activity of the HT catalysts. As results, the prepared three-metal mixed hydrotalcites found to be highly active for biodiesel production and obtained ∼99 % FAME yield at optimized reaction conditions. 118 The spent catalysts could be regenerated and reused in the transesterification reaction where the FAME yield decreased apparently compared to fresh catalyst due to the fact that the layer structure of the catalyst helped raise the activity of the catalyst.
The Mg/Al based catalysts (double oxides of Mg and Al) prepared by coprecipitation method and characterized for structural properties. 119 The formed double lamellar hydroxides followed by calcination gives double oxides show interesting catalyst properties such as a large superficial area, synergic effect between its elements (which favors the development of the its basic characteristics) and also a shape memory effect, which allows the regeneration of the original structure. The prepared catalysts have been tested through heterogeneous catalytic transesterification reaction of vegetable oil with methanol and resulted in ∼97 % FAME formation. The study shows the potential use of the MgAl based hydrotalcites for the biodiesel production and suggesting further structural modification for the improved catalytic activity. 119 Mg/Al hydrotalcites doped with lanthanum and zinc cations with different amounts prepared by using co-precipitation method followed by calcination and the materials have been used as solid base catalyst in transesterification of soybean oil with methanol and n-octanol for biodiesel and biolubricants production respectively. 120 The transesterification reaction of canola oil with methanol has been reported using Mg/Al hydrotalcites heterogeneous solid base catalyst prepared by coprecipitation method followed by calcination. 121 The results of the catalytic reaction have been compared with KOH as conventional homogeneous catalyst where the present catalyst found to be less active than KOH.
The potential contribution of Mg/Al hydrotalcites prepared through layered double hydroxide has been reported for the biodiesel production using transesterification of triglycerides. 122 The interesting physico-chemical characteristics such as texture and surface morphology as well as basicity of the prepared materials could be modified through synthesis routes and chemical composition. The present report shows useful catalytic characteristic of the hydrotalcites as mixture of mixed oxides catalysts and as supports of active species like alkaline and alkaline earth metals compound. 122 Simonetti et al. have reported use of Ca/Al based hydrotalcites catalysts prepared by impregnation method using KOH solution followed by calcination for the transesterification reaction of soybean oil in methanol and ethanol to produce biodiesel. 108 The catalytic performance of impregnated and non-impregnated catalysts has been compared for the transesterification reaction and as a result, the non-impregnated exhibited with higher activity and hence better performances due to availability of active sites used in the transesterification process. 108 Mg/Al hydrotalcites prepared by co-precipitation followed by calcination and loaded with K2CO3. 123 The obtained material used as solid base catalyst for transesterification reaction of Jatropha curcas oil with methanol to produce FAME. In the study, spectral analysis confirmed the metal oxide formation which makes catalyst to be highly active and it could produce about ∼96 % yield of biodiesel (FAME) under optimized reaction condition. 123 Xue et al. 124 have synthesized activated Mg/Al hydrotalcites NPs by co-precipitation and hydrothermal activation with aqueous Ca(OH)2 solution and characterized its catalytic properties. 124 The prepared Ca-hydrotalcite contained both acidic and basic active sites due to which it could be used for esterification and transesterification of different oils with high acid value. The results show high catalytic activity for production of biodiesel (∼93 % yield) from crude oils with high acid value without pretreatment. 124
Table 4 highlighting the use of various HTs as heterogeneous base catalysts for biodiesel production through esterification or transesterification process of oil with methanol/ethanol using optimization of oil to alcohol molar ratio. 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124
Hydrotalcites (HTs) as heterogeneous base catalysts for biodiesel production using esterification or transesterification reactions.
| Sr. No. | HTs as base catalysts | Esterification/transesterification reactions molar ratio of oil: alcohol | Ref. |
|---|---|---|---|
| 1 | Ca/Al based HTs (Ca/Al ratio of 2:0) prepared by impregnation method using KOH solution | Transesterification of soybean oil Oil: methanol/Ethanol = 1:30 (∼90–95 % FAME conversion) |
108 |
| 2 | Calcined Mg/Al HTs prepared by coprecipitation using alkaline solution | Transesterification process of soybean oil Oil: methanol = 15:1 (∼67 % FAME conversion) |
110 |
| 3 | HT-immobilized and zeolites-immobilized lipase enzymatic materials | Transesterification waste cooking oil Oil: methanol = 1:4 (∼91 % FAME conversion |
111 |
| 4 | Calcined Mg/Al HTs prepared by coprecipitation using alkaline solution | Transesterification pongamia oil Oil: methanol = 1:6 (∼90 % yield of pongamia oil methyl ester) |
112 |
| 5 | Calcined Mg/Al HTs under Ar flow (Mg/Al ratio of 3.0) prepared by coprecipitation using alkaline solution | Transesterification of soybean oil Oil: methanol = 1:20 (∼94 % FAME conversion) |
113 |
| 6 | Calcined Zn–Mg–Al HTs | Transesterification reaction of neem oil Oil: methanol = 1:10 (∼91 % FAME conversion) |
114 |
| 7 | Mg/Al HTs (Pural©MG76) | Transesterification of soybean oil in autoclave and in a continuous packed bed reactor Oil: methanol = 160 g :160 g (w/w) (∼80–85 % FAME conversion) |
115 |
| 8 | Calcined Mg/Al HTs (Mg/Al ratio of 3.1) prepared by coprecipitation using alkaline solution | Transesterification of waste cooking oil Oil: methanol = 1:8 (∼95–98 % FAME conversion) |
116 |
| 9 | Calcined Mg/Al HTs (Mg/Al ratio of 3.1) prepared by urea hydrolysis and coprecipitation methods | Transesterification of waste cooking oil Oil: methanol = 1:6 (∼78–96 % FAME conversion) |
117 |
| 10 | KF-doped three-metal mixed HTs (KF/Ca–Mg–Al) | Transesterification process of palm oil Oil: methanol = 1:12 (∼99 % FAME conversion) |
118 |
| 11 | Calcined Mg/Al based double oxides of Mg and Al prepared by coprecipitation using NaOH solution | Transesterification of soybean oil Oil: methanol = 1:9 and 1:12 (∼97 % FAME conversion) |
119 |
| 12 | Mg/Al HTs (Mg/Al ratio of 2.0) doped with lanthanum (La3+) and zinc(Zn2+) cations | Transesterification of soybean oil Oil: methanol = 1:15 (∼97 % FAME conversion) |
120 |
| 13 | Calcined Mg/Al HTs prepared by coprecipitation using alkaline solution | Transesterification of canola oil Oil: methanol = 1:6 and 1:12 (∼71 % FAME conversion) |
121 |
| 14 | Mg/Al HTs (Mg/Al ratio of 2:3) prepared by layered double hydroxide | Transesterification of triglycerides Oil: methanol = 1:12 (∼96 % FAME conversion) |
122 |
| 15 | Calcined K2CO3 loaded Mg/Al HTs (Mg/Al ratio of 3:1) prepared by coprecipitation using alkaline solution | Transesterification of Jatropha curcas oil Oil: methanol = 1:6 and 1:12 (∼96 % FAME conversion) |
123 |
| 16 | Mg/Al hydrotalcites NPs by coprecipitation and hydrothermal activation with aqueous Ca(OH)2 | Transesterification of Jatropha curcas oil Oil: methanol = 1:30 (∼93 % FAME conversion) |
124 |
Metal Organic Frameworks (MOFs): MOFs are porous materials, composed of metal sites and organic linkers, have become a research hotspot due to their exceptional advantages, such as versatile synthetic strategies for controlled morphology, abundant pores, and high surface area. 125 These properties grant MOFs versatile functionalities, which have attracted significant interest from the scientific community in recent years. 125 Their crystalline-to-amorphous nature, consisting of metal ions or clusters linked to organic ligands, offers remarkable surface areas, a high degree of structural diversity, and tunable porosity, making them ideal for a wide range of applications. 125
With the advantageous character of polyporous materials, MOFs have gained the most suitability for biodiesel synthesis. 126 Molybdenum-MOFs has been synthesized by solvothermal method and applied as catalyst in the esterification reactions of oleic acid and palmitic acid to obtain biodiesel. 127 As a result, a 95 % yield was achieved with the fresh Mo-MOFs, and it was successfully recycled for four cycles, maintaining ∼92 % efficiency. A hydrothermal synthesis of chromium(III) nitrate nonahydrate salt with EDTA as an organic ligand has been reported to prepare Cr(III)-EDTA MOF, which demonstrates efficient and promising performance for the esterification of oleic and palmitic acids under mild reaction conditions. 128 Copper(II) based MOFs, Cu-benzenedicarboxylic acid (Cu-BDC), Cu-benzenetricarboxylic acid (Cu-BTC) and MOF-74, provide many active sides that act as Lewis acid species and therefore, used as catalysts in the esterification process of waste cooking oil. 129 The synthesis of 75 % and 50 % oil content with Cu-BDC catalyst converted in FFA content of 5.49 % and 4.826 %, respectively.
2.2 Hydrogen production
Hydrogen (H2), as a fuel and one of the most extensively studied substitute energy resources for available sources those are being consumed rapidly. 130 , 131 The combustion of hydrogen can be considered as environmentally benign because it produces only water as by-product, and its adoption as fuel in internal combustion engines, would lead to a remarkable reduction in atmospheric pollution. 132 Now days, hydrogen-based technologies are being proposed for having economically and environmentally efficient system to produce hydrogen such as photo-catalytic water-splitting, 133 , 134 , 135 , 136 , 137 hydrocarbon decomposition, 138 , 139 photo-reforming 140 , 141 , 142 , 143 , 144 aqueous phase reforming, 145 , 146 , 147 , 148 , 149 , 150 and auto-thermal/oxidative steam reforming. 151 , 152 , 153 In this view, mono-metallic, bi-metallic or poly-metallic heterogeneous catalysts based on NPs (oxide supported) are well established catalytic systems for hydrogen production. Several Pd, Pt, Ru, Rh-based heterogeneous catalysts with comparable hydrogen production rate have been reported.
Santo et al. 132 have reviewed the specific catalytic applications of reforming processes such as steam, auto-thermal/oxidative steam, aqueous phase and dry reforming, catalytic partial oxidations, decomposition, photocatalytic water-splitting and photo-reforming reactions. The authors have reported heterogeneous catalysis based on supported bimetallic NPs, to the reactions favorable for the hydrogen production from the renewable raw materials. 132 They have discussed on advantageous applications of bimetallic versus monometallic catalytic systems, as well as focused on characterizations of structural properties. The correlation between structure and catalytic properties has an influence on the promoter addition. The potential of bimetallic catalyst NPs could be seen from the number of examples of the industrial applications significantly such as hydrogen production using photo-reforming and photocatalytic water-splitting those are discussed in the present article. 132
Furthermore, metal-supported heterogeneous catalysts can induce changes in the electronic properties of the catalyst surface, offering numerous opportunities for developing advanced catalysts with enhanced catalytic performance. 26 The noble metal catalysts are also interesting aspects of catalysis for hydrogen production through HER because of their ΔGH* is closed to zero. 154 Zhou et al. have related the catalytic activity with the electronic structure and chemical composition of noble metal-based electrocatalysts. 154 In continuation, a series of lanthanum based heterogeneous catalysts of 1 wt% Pt/LaMO3 where M = Al, Cr, Mn, Fe, Co, Ni has been synthesized and used in the catalytic study of aqueous phase reforming of waste biomass, such as glycerol for sustainable hydrogen production. 155
Nagaoka et al. 156 have demonstrated hydrogen production by oxidative decomposition of ammonia at room temperature to an acidic RuO2/γ-Al2O3 catalyst. In the study ammonia chemisorbed onto RuO2/γ-Al2O3 catalyst which further increased the amount of the multilayer physisorbed ammonia which causes strong heat evolution that enables to initiate the oxidative decomposition of the ammonia onto large surface of RuO2 NPs. The authors have measured and compared the autoignition temperatures of the RuO2/γ-Al2O3 and RuO2/γ-La2O3 catalysts. 156 As results, on ammonia and oxygen supply to the catalyst at room temperature, it has produced hydrogen along with nitrogen and water vapor. The process requires neither an external energy source nor the use of any complex procedures. The present study briefly introduces a conceptual idea of self-heating of catalysts by adsorption of reactant molecules which is a novel strategy for the cold-start process for hydrogen production from ammonia and other reactions.
Recently, a study discussed the progress and challenges in using non-noble metal cobalt-based heterogeneous supported catalysts, particularly carbon materials, metal oxides, MOFs, and nickel foams, for hydrogen generation from ammonia borane. 157 It provides insights into how the preparation of the electronic and spatial structures of Co-based catalysts can significantly influence the decomposition of ammonia borane for hydrogen generation. 157 Brack et al. 158 have introduced heterogeneous catalysis for the hydrogen production from the hydrolysis process of sodium borohydride (NaBH4). The authors have discussed about the factors affecting the catalytic hydrolysis process of aqueous solution of NaBH4 such as catalyst role, reaction conditions (temperature, time, concentration and volume of NaBH4 and the used stabilizers). The article has brief discussion about the catalysts based on noble metals, mainly focus on Pt and Ru as most efficient at rapid production of hydrogen from the aqueous NaBH4 solution. As noble-metal based catalysts resulting much expensive, transition metal-based particularly Co and Ni-based catalytic system would be a desirable alternative and much more work has been done for the hydrogen generation. Cobalt-borides are found to be emerging catalyst of choice due to low cost and simple synthesis procedures with high catalytic activity leading to maximum hydrogen production yields. They also have explained other transition metal-based as well as metal free-based heterogeneous catalysts for practical applications of the hydrogen generation. 158 Srifa et al. 159 also have studied hydrogen generation by ammonia decomposition using Cs-modified Co3Mo3N catalysts prepared by a facile single-step decomposition of a mixture of hexamethylenetetramine and corresponding metal salts under the flow of nitrogen at 700 °C. The kinetic study of the catalytic process shows influence of the presence of the Cs species as it significantly enhanced the catalytic activity of Co3Mo3N, an excess amount of Cs could be reduced the activity and hence lower the H2 production rate. The observed enhancement could be due to electronic modification of Co3Mo3N by the electron donation of Cs promoter. 159
Yan et al. 160 have compared catalytic activities of Ni, Co and W with ZrO2 support for hydrogen production from PEG-contaminated wastewater by supercritical water gasification process. The results of the analysis show Ni/ZrO2 exhibits highest hydrogen yields which could be due to catalyst inhibition of toxic intermediate production. The study demonstrated the promising use of Ni/ZrO2 as heterogeneous catalyst for hydrogen production in a supercritical water gasification process with high activity and stability. 160 Yuranov et al. 161 have developed a heterogeneous catalytic reactor for continuous production of hydrogen via formic acid dehydrogenation. The catalyst support consisting of cross-linked polystyrene beads and polymer electrolyte fuel cell operated on H2 + CO2 mixtures to produce neat hydrogen. The model presented could help to use formic acid as hydrogen carrier medium and to make maximum industrial application for the sustainable technology. Yamashita et al. 162 have addressed most relevant research approaches in hydrogen generation from two of the most promising hydrogen storage materials such as formic acid and ammonia-borane over carbon supported metal NPs based catalysts. The authors have highlighted recent advancement in mono-metallic and bi-metallic Pd-based and Ru-based catalytic system, also discussed about the important features like nano-particle size, electronic features and composition, functionality of the support and influence of the used additives present in the medium. They have concluded about the prior meet of the high performance heterogeneous catalysts for the hydrogen production with efficiency, economic and reusability. 162 The authors also have reported design and development of efficient Pd-based heterogeneous catalysts supported on carbon or carbon containing materials for the formic acid dehydration process in liquid phase for hydrogen production. 163 They have described important features of metal active phase and catalytic support for finding the efficient catalyst under the optimum experimental conditions. The main issue has been noted about the stability of the catalyst which has to be improved for the practical applications. In this context, modulating the nanoparticle-support interaction could be of great significance in achieving catalysts with notable stability. The incorporation of an adequate heteroatom through doping in the carbon material could be an effective tool to form interesting catalytic system for the current demand through appropriate surface fictionalization. Another way of modification in the synthesis route so that porosity of the carbon material support could be utilized in anchoring and dispersing the metallic active phase would be effective work of interest through which active and durable catalytic system could be developed, and searching useful capping agent to inhibit the nano-particle sintering under using experimental conditions. Also, they have concluded about future investigation to be initiated the use of non-noble metallic NPs as cost effective and durable active catalysts for the hydrogen production from dehydrogenation of the formic acid. 163 Yamaguchi et al. 164 have reported gaseous fuel production from non-recyclable paper wastes such as shredded documents and paper sludge over supported metal catalysts. The gasification catalytic reactions show, a charcoal-supported ruthenium (Ru/C) catalyst found to most active and among to the metal used, the order of the catalytic activity was found to be Ru > Rh > Pt. The gas fuel yield increases by ball-milling treatment of the recycled paper and cellulose powder. 164 The present conceptual technique indicating the possibility of fuel gas production from paper wastes in high-temperature liquid water.
Sikander et al. 165 have introduced scientific approaches reported by researchers for the hydrogen production using HTs based catalysts through describing synthesis methods and structural properties. The authors have briefly explained co-precipitation, urea hydrolysis, microwave treatment and sol-gel method of synthesis for HTs. Various methods of hydrogen production have been discussed such as steam reforming of methane, methanol, ethanol and dry hydrocarbons and partial oxidation of methane. The catalysts, HTs are observed to be more active in almost all hydrogen production processes because of high surface area. Furthermore, the compositional ratios of active metallic cations along with their interaction with the interlayers are a key role for catalytic efficiency. 166 , 167 Doping of element is another way to enhance the catalytic performance where use of noble metals (Pd, 168 , 169 Pt, 170 Rh 171 ) have been reported successfully. The rigorous operating conditions of hydrogen production processes could damage structural properties of the catalyst, sintering of the cations and deactivation of the catalyst also affects the catalytic efficiency of the HTs. The authors also have developed double layered HT based Mg–Ni–Al catalyst using co-precipitation method by varying Ni content and studied its catalytic performance for the hydrogen production through methane decomposition. 172 The nickel concentration has not been effective to the overall methane decomposition reactions due to the sintering of active sites species leads to depriving of the overall rate of methane conversion. The used catalyst support have played a typical role in the diffusion of the deposited carbon, resulted improved the overall performance rate.
Three metal-based HTs (Ni/Zn/Al-HT) with different Ni/Zn ratios have been prepared by a coprecipitation method and characterized for structural properties where HT and ZnO phases observed along with Zn2+ into the layer. 145 The prepared material has been used for hydrogen production via aqueous phase reforming of ethylene glycol and resulting high H2 generation rate with high selectivity. 145 Ni–Fe mixed oxides obtained from reevesite, a HTs type material prepared by co-precipitation method and tested for steam reforming of ethanol for hydrogen production. 145 The comparative performance of hybrid materials comprising a Ni-based reforming catalyst and HT (Ni-HT) or calcium based sorbents (Ni–CaO/Al2O3) has been reported for sorption-enhanced steam methane reforming for H2 production. 173 The prepared hybrid material (Ni-HTs) exhibited with superior adsorption characteristics and stability compared to powdered mixtures of commercial Ni/Al2O3 catalyst and the respective sorbent (CaO). 174 He et al. 175 have studied catalytic performances of noble-metal free-catalyst obtained from Ni/Al HTs for hydrogen production through hydrous hydrazine (N2H4·H2O) decomposition and compared with the ∼78 wt % Ni/Al2O3-IMP sample. The results show that supported Ni catalyst with a high loading and good dispersion exhibits ∼100 % conversion and ∼93 % H2 selectivity for the N2H4·H2O decomposition at room temperature. The observed activity is due to the cooperation of Ni NPs and strong basic sites which make catalyst as a promising candidate to replace noble-metals for hydrogen production under ambient conditions. 174 Homsi et al. 176 have prepared Co-based HTs (Co6Al2 oxide) and thermally stabilized and impregnated using metal precursors of Cu and Ru followed by calcination. The characterization results show formation of CuO, RuO2, Co3O4 and CoAl2O4 after calcination of the impregnated materials. Both, impregnated and non-impregnated samples have been used for the steam-reforming of the ethanol for H2 production. 176 As results, Ru with impregnated samples exhibit high catalytic activity compared to Cu-impregnated sample which is attributed to the formation of easily reducible Ru and Co-oxide species at the surface of the support. 176 The authors also have studied catalytic performances of Cu/Co–Mg–Al-based catalysts in the ethanol steam reforming reaction under atmospheric pressure in a fixed catalytic bed reactor coupled to a micro-gas chromatography. 177 From the reaction, H2 and CO2 evolved and it increased with the cobalt content and 5Cu/Co6Al2 gives very good conversion of H2 and CO2. The presence of copper as an active phase has improved ethanol conversion and H2 production compared to the results obtained in the case of the supports alone. The study leads to develop a catalyst operate at low temperature with high selectivity to avoid formation of by-products. 177 Tuza et al. 178 have reported hydrogen production by aqueous-phase reforming of glycerol using Ni/Cu catalysts derived from HT precursors. The obtained materials have been characterized for structural and surface properties. A clear improvement in the orderliness of the layer has been noted with decreasing copper content and samples with higher amount of copper have larger BET surface areas. The Ni-catalyst without Cu shows high catalytic glycerol conversion observed with good H2 selectivity and Ni5Cu catalyst also shows high H2 selectivity with the smallest amount of CO and CH4. 178
The compositional variation and structural advantages such as highly ordered and tunable metal nodes and organic linkers, MOFs enable rapid ion transport and high capacitance. With their abundance of active energy storage sites, MOFs have emerged as attractive candidates to meet the demands of next-generation energy storage technologies in batteries and supercapacitors.125c MOFs and their nanocomposites have been widely synthesized, particularly for their potential applications as hydrogen storage materials in proton exchange membrane fuel cells (PEMFCs). 179 MOFs are also of interest as potential materials for use as electrode in lithium, zinc and sodium-ion batteries. 180
The catalysts used in the hydrogen production process have been summarized 26 , 132 , 145 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 in Table 5.
Hydrogen production using various catalysts.
| Sr. No. | Catalysts | Catalytic process | Ref. |
|---|---|---|---|
| 1 | Supported bimetallic NPs | Photo-reforming and photocatalytic water-splitting | 132 |
| 2 | Acidic RuO2/γ-Al2O3 catalyst | oxidative decomposition of ammonia | 156 |
| 2 | 1 wt% Pt/LaMO3 where M = Al, Cr, Mn, Fe, Co, Ni prepared by harsh hydrothermal conditions | Aqueous phase reforming of waste biomass | 157 |
| 3 | Noble-metal (Pt, Ru and Rh), transition metal (Ni and co) based catalysts | Hydrolysis process of sodium borohydride | 158 |
| 4 | Cs-modified Co3Mo3N | Ammonia decomposition | 159 |
| 5 | Ni, Co and W with ZrO2 support (Ni/ZrO2, Co/ZrO2, W/ZrO2) | PEG-contaminated wastewater by supercritical water gasification process. | 160 |
| 6 | Different active Ru complexes supports on zeolite ZSM-5, mesoporous MCM-41 silica and functionalized polystyrene beads | Formic acid dehydrogenation | 161 |
| 7 | Monometallic and bimetallic Pd-based catalytic systems, (Pd, Pd/Ag, Pd/Au) | Formic acid dehydrogenation | 162 |
| 8 | Carbon supported metal NPs | Dehydrogenation of formic acid and ammonia-borane | 163 |
| 9 | Charcoal-supported ruthenium (Ru/C) | Gasification catalytic reactions (gaseous fuel production from non-recyclable paper wastes) | 164 |
| 10 | Hydrotalcite (HTs) consisting of different metals like Fe, Ni, Cu, Pt | Steam reforming of methane, methanol, ethanol and dry hydrocarbons and partial oxidation of methane | 165 |
| 11 | Ce-promoted Ni/Mg–Al catalysts | Dry reforming of methane | 166 |
| 12 | HTs supported Pd2Ga and PdZn intermetallic NPs | Methanol steam reforming | 168 |
| 13 | PdZnAl-HTs supported catalyst | Oxidative methanol steam reforming | 169 |
| 14 | Ni, Co and pt/hydrotalcites-WO x | Ethanol steam reforming | 170 |
| 15 | Rh-doped Ni/Mg(Al)O catalysts | Methane steam reforming | 171 |
| 16 | Double layered HT based Mg–Ni–Al | Methane decomposition | 172 |
| 17 | Ni/Zn/Al derived HTs | Aqueous-phase reforming of ethylene glycol | 145 |
| 18 | HTs- and calcium-based hybrid materials | Methane steam reforming | 173 |
| 19 | Ni/Fe derived HTs | Ethanol steam reforming | 174 |
| 20 | Noble-metal-free catalyst derived from Ni–Al HTs | Hydrazine (N2H4·H2O) decomposition | 175 |
| 21 | Ru and Cu supported HTs | Methane steam reforming | 176 |
| 22 | Cu/Co–Mg–Al-based catalysts prepared by HTs route (5Cu/Co6Al2) | Ethanol steam reforming | 177 |
| 23 | Ni and Cu supported HTs | Aqueous-phase reforming of glycerol | 178 |
| 24 | Prussian blue analogues | Hydrolysis of NaBH4 | 181 |
| 25 | Fluorine-doped NiO and Ni@C prepared through organic-inorganic hybrid approach | Urea oxidation reaction | 182 |
3 Environmental applications
Currently, the problem associated to the energy and environment has no universal solution to solve it. Most of human activities and more, rapidly increasing population and thus, even faster increasing global energy consumption and environmental burdens lead to generate the said unsolved problems. Many more awareness and progressively efforts are being applied to improve the efficiency and accessibility of environmental remediation and Green Chemistry processes. Environmental catalysis certainly plays a typical role in the design and development of efficient processes and technologies to reduce or to minimize waste generation throughout saving energy. 183 Herein, different heterogeneous catalytic systems have been discussed to understand relation between the structure/chemistry and the performance in heterogeneous catalysis which would help to design novel efficient materials for addressing the challenges in potential applications for environmental remediation. These include metal-based and/or metal-free-based catalysts and carbon-based catalysts for green chemistry, semiconductors-based catalysts for photocatalysis and other catalytic systems for environmental technologies.
3.1 Carbon dioxide (CO2) reduction
Faster deforestation makes sudden increment of CO2 concentration in atmosphere and thus, global warming problem and climate change become the interest of attention. 150 Therefore, now it is become necessary to find out a significant alternative to capture CO2 and convert it into useful chemicals and sources of fuel. 183 The availability of the alternative energy source is connected to the source of hydrogen which is carbon neutral and economically feasible. In this view, the hydrogenation of CO2 to methanol synthesis is providing a renewable or sustainable source of hydrogen.
There has been focused research work on finding selective catalyst that can capture or convert through CO2 activation under mild reaction conditions with maximum selectivity and yield of methanol. Bahruji et al. 184 have studied structure activity relationship of two series of Pd/ZnO catalysts prepared by immobilization and impregnation methods for direct hydrogenation of CO2 to methanol. As results, different performances observed for both catalysts prepared by different methods. Pd-NPs deposited through sol-immobilization method which produced active Pd/ZnO NPs catalysts with controlled particle size. It is observed that, high Pd loadings for colloidal catalysts increases methanol formation due to Pd–Zn bimetallic formation and also, methanol production could be reduced at higher temperature due to some sintering effect. 184 A multicomponent Cs-doped CuO–Fe2O3 supported on Al2O3 (Fe–Cu–Cs/Al2O3) catalyst has been reported for chemical CO2 recycling by reverse water-gas shift (RWGS) reaction and compared its catalytic activity with that of Fe/Al2O3, Fe–Cu/Al2O3 and Fe–Cs/Al2O3 catalysts. 185 The addition of Cu to the bare Fe/Al2O3 catalyst enhances the activity and selectivity because of Cu–Fe effect and reduced sintering. Similarly, addition of Cs promoter affects the catalytic activity of Cu–Fe/Al2O3 and thus, multicomponent catalyst Cs–Fe–Cu/Al2O3 has highest level of CO2 conversion with full selectivity to CO formation at low temperature. The study reveals the role of promoter added to the catalysts, Cs helps in CO2 activation due to its basic character in nature through ease of electron transfer from the catalyst to the reactants and facilitation CO2 adsorption on the catalyst surface. Addition of promoter also helps to suppress the methanation process and it could improve selectivity towards desired product. 185 The study concluding about unexceptional stability and selectivity could be gained through Cs-doped heterogeneous catalytic materials and it suggests using of such materials in gas phase chemical CO2 recycling. The authors have reported multicomponent Ni-based catalysts supported on Al2O3 promoted by the addition of Sn and CeO2 for the catalytic conversion of CO2. 185 The results also favoring the earlier study as addition of Sn and CeO2 promoters have improved the catalytic activity of Ni/Al2O3 through enhancement of CO2 adsorption on the catalyst surface. The presence of CeO2 enhances the oxygen storage capacity and modified acid/base characteristics of the support lead to improve catalytic behavior over a range of temperatures and space velocities. The catalytic system has also been applied to bi-reforming of methane reaction and significant conversion with stability was observed. 186 The study conducted provides a strategy to design and develop efficient and economic catalyst for practical syngas production form CO2/CH4 mixtures. Mutz et al. 187 have prepared bimetallic Ni3Fe catalyst supported on γ-Al2O3 using homogeneous deposition-precipitation method for application in CO2 methanation and correlate performance and structure of the catalyst. Spectral analysis shows, formation of Ni3Fe alloy with high fraction of Ni and Fe resulting into small and well defined NPs with controlled particle size and good dispersion. The catalytic performance of the prepared Ni3Fe has been compared with that of mono-metallic catalyst of Ni by using a micro-channel packed bed reactor under industrially relevant reaction conditions. As results, Ni3Fe exhibited with improved performance to have conversion of CO2 about ∼71 % selectively for methanol production and most important about retained a high stability. The study indicating importance of synergetic effect of Ni and Fe due to which catalyst has excellent performance and stability. 187 Cobalt catalysts supported on ZrO2 (Co/ZrO2) and Al2O3 (Co/Al2O3) prepared by impregnation with different metal loadings show good catalytic activity for CO2 methanation. 188
Veselovskaya et al. 189 have studied direct air capture/methanation process over the commercial nickel methanation catalyst NKM-2V which is effectively combine CO2 capture from ambient air and convert it into methane by Sabatier reaction. The experimental analysis showing enhancement of CO2 to CH4 conversion with increase of temperature and decrease in CO2:H2 ratio. The Sabatier process also performed well in the two reactor system using K2CO3/Al2O3 material for direct capture of CO2 from ambient air and the commercial nickel catalyst for methanation of CO2 via Sabatier process. Wu et al. 190 have studied influence of formate (HCOO−) coverage on the reaction rate of methanol synthesis from CO2 hydrogenation over Cu (2 1 1) with the coverage-dependent adsorbate-adsorbate interactions. The study shows, increasing formate coverage would destabilize the adsorption of the reactant molecules/reaction intermediates and transition states. Turn over frequency varied at different coverage of surface species under the same reaction condition. The combination of HCOO and HCOOH hydrogenation steps could be a rate-determining step and thus, methanol formation could be controlled through surface coverage of formate and the effective free energy barriers. 190 Han et al. 191 have prepared Au nano-clusters with different particle size (average ∼2 nm) on various supports (ZnO, CeO2, TiO2, ZrO2) and studied their catalytic performance on direct hydrogenation of CO2 to methanol under solvent free condition. Among the all catalysts prepared, Au/ZrO2 with 1.6 nm particle size exhibits highest catalytic activity and selectivity towards methanol formation at low temperature, mainly because of small particle size and appropriate coupling between the Au particles and support in the catalyst. 191 Williams et al. 192 have prepared unsupported bimetallic Pd–In NPs by using thermal decomposition of Pd and In precursors in high boiling point solvent followed by reduction using dilute hydrogen gas. PdIn NPs catalyst has been tested for liquid phase methanol synthesis process and found to be most efficient catalyst showing ∼70 % higher methanol production rates compared to conventional catalysts such as Cu/ZnO/Al2O3. 192 Rao et al. 193 have studied fundamental physicochemical properties of CeO2 to improve its catalytic activity for energy and environmental applications. In the study, shuttle-shaped CeO2 prepared under hydrothermal condition by homogeneous precipitation method and the material shows good surface area and low temperature reducibility which show superior CO oxidation efficiency as environmental application. The linear sweep voltammetry, chronopotentiometry and CO stripping voltammetry studies show that CeO2 provides higher number of labile OH ads species through active triple-phase interfacial sites for facile oxidation of CO ads on Pt/C as energy application. 193 The study set a significant potential and anti-poisoning effect of CeO2 to Pt/C for electro-oxidation of ethanol in acidic media. Arena has reported the composite MnCeO x system having high redox activity, surface amphoteric behavior and good chemical stability as a viable alternative to highly-cost noble metals for a variety of environmental catalytic applications. 194 These include a range of applications such as the detoxification treatment of industrial wastewater; the abatement of pollutants in gas exhausts; and bio-15 fuel and fine-chemicals synthesis manufacture.
Jadhav et al. 195 have discussed about the recent advanced studies of last decade on catalytic CO2 hydrogenation to methanol. They have explained the importance of the various novel synthesis routes like solid-state reaction, ultra-nitrate combustion and reveres co-precipitation under ultrasound irradiation to prepare CuO/ZnO/ZrO2 heterogeneous catalysts. 195 The main focus was CuO/ZnO-based catalyst promoted with Pd and Ga for CO2 hydrogenation to methanol. As discussed earlier in the text, addition of Pd and Ga promoters enhances the catalytic activity, stability and selectivity of CuO/ZnO catalyst, i.e., Pd/ZnO 196 and Ga promoted Pd/SiO2, 197 show excellent catalytic behavior. Authors also have discussed two pathways of catalytic hydrogenation of CO2 to methanol; one is a RWGS and another, through mechanism of formate (HCOO−) intermediate formation. 195 They have concluded, among the available alternate catalytic techniques for methanol synthesis from CO2 dehydrogenation via RWGS reaction would be promising tool. Porosoff et al. 198 have demonstrated the possible use of a low-cost non-noble metallic material Mo2C (molybdenum carbide) as an active and selective heterogeneous catalyst for conversion of CO2 using hydrogen. The spectral analysis shows that carbide is preliminary active phase of Mo2C while oxide form was observed throughout the reaction. Mo2C catalyst has ability to break C=O bond of CO2 and to dissociate hydrogen to either perform hydrogenation of CO2 or remove oxygen from Mo2Cz–O intermediate, thus, this way catalyst could show its dual functionalities which makes it suitable for CO2 activation. 198 The present catalyst preparation is much lower cost make possible to set a large-scale catalytic CO2 conversion process. Waclawek et al. 2 have discussed the mechanism of heterogeneous catalysis and advances in its development for environmental applications. They also have introduced a heterogeneous catalysis as an important aspect of the chemical process, from the synthesis methods and characterization techniques through surface morphology. The authors have highlighted the challenges about to improve spectroscopic and microscopic techniques to provide structural information such as chemical composition, size and shape of metal-NPs and gas phase concentrations in a spatially and time-resolved manner. They have suggested, industrial chemistry needs to combine all of these things together to understand the solid handling, chemical reaction, energy engineering, and heat and mass transfer of these catalytic processes. Theoretical scientist and physicist would have good partnership to make possible to design and develop superior catalytic systems for inexpensive fuel-cell vehicles. 2
Yan et al. 199 have provided detailed overview of the development of nickel-based bimetallic catalysts for chemical and electrochemical processes such as catalytic reforming, dehydrogenation/hydrogenation and electro-catalysis for energy production and environmental remediation. The authors have discussed the role of bimetallic surface and each metal counterpart of the reactions, and the surface activity relationships which are typical part of catalytic performances. They have explained diffusion rate of Ni which is the major issues related to the catalyst activity and stability under the different reaction conditions like high temperature for reforming condition. On point of view, to develop cost effective catalysts without compromising their activity and stability, it could be possible by alternative way of introduction of non-metal elements such as boron and phosphorous and already nickel-borides and phosphides could further improves the catalytic behavior for the desired applications. 199 Labhasetwar et al. 200 have studied a number of perovskites based on Co, Mn, Ru, and Fe and their substituted compositions. The prepared perovskites catalysts show good redox properties, the promotional effect of co-ions, and the increased exposure of catalytically active transition metals in certain preparations. The catalysts exhibited enhanced catalytic activity for diesel soot oxidation, three-way catalysis, NO decomposition, low-temperature CO oxidation and oxidation of volatile organic compounds. 200 Wang et al. 201 studied the physicochemical properties of fly ash exhibiting strong thermal stability and characteristic of a catalyst support. The fly ash supported catalysts have shown a very good heterogeneous catalytic activity for a variety of catalytic reactions as H2 production, CO2 reforming of methane, NH3 decomposition, catalytic reduction of NO x and SO x , methane oxidation, dye decomposition, petroleum hydrocaracking. 201
MOFs have also been reported to be efficient photocatalyst 202 and electrocatalysts 203 for CO2 capture. Cobalt–porphyrin MOF has been examined for selective reduction of carbon dioxide to carbon monoxide in aqueous electrolytes with the 76 % yield and stability over 7 h with a per-site turnover number of ∼1,400.204b A case study on Bi-based MOFs has been reported for electrochemical CO2 reduction and as results excellent activity, selectivity, and durability for formate production.204c
Table 6 contains various catalytic systems for CO2 reduction 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 as environmental application.
Catalytic CO2 reduction using various catalysts.
| Sr. No. | Catalysts | Catalytic process | Ref. |
|---|---|---|---|
| 1 | Pd/ZnO | Direct hydrogenation of CO2 to methanol | 180 |
| 2 | Cs-doped CuO–Fe2O3 supported on Al2O3 (Fe–Cu–Cs/Al2O3) | Reverse water-gas shift (RWGS) reaction | 181 |
| 3 | Ni-based catalysts supported on Al2O3 | Catalytic conversion of CO2 and bi-reforming of methane, syngas production form CO2/CH4 mixtures | 182 |
| 4 | Ni3Fe catalyst supported on γ-Al2O3 | CO2 methanation | 183 |
| 5 | Cobalt catalysts supported on ZrO2 (Co/ZrO2) and Al2O3 (Co/Al2O3) | CO2 methanation | 184 |
| 6 | Commercial nickel methanation catalyst NKM-2V | Direct air capture/methanation process | 185 |
| 7 | Cu(211) | Hydrogenation of CO2 to methanol | 186 |
| 8 | Au nano-clusters with different particle size (average ∼2 nm) on various supports (ZnO, CeO2, TiO2, ZrO2) | Direct hydrogenation of CO2 to methanol | 187 |
| 9 | Unsupported bimetallic Pd–in NPs | Liquid phase methanol synthesis process | 188 |
| 10 | CeO2 (cerium oxide) | CO oxidation | 189 |
| 11 | Composite MnCeO x | Redox process | 190 |
| 12 | Carbon nanotube-supported Pd/ZnO | Hydrogenation of CO2 to methanol | 192 |
| 13 | Ga promoted Pd/SiO2 | Hydrogenation of CO2 to methanol | 193 |
| 14 | Mo2C (molybdenum carbide) | Hydrogenation of CO2 to methanol | 194 |
| 15 | Nickel-based bimetallic catalysts | Hydrogenation of CO2 to methanol | 195 |
| 16 | Fly ash supported catalysts | CO2 reforming of methane | 197 |
| 17 | Cobalt–porphyrin MOFs | Selective reduction of carbon dioxide to carbon monoxide in aqueous | 199 |
| 18 | Bi-based MOFs | Electrochemical CO2 reduction | 199 |
| 19 | NNU-29 (polyoxometalate-based MOFs) | Photoconversion CO2 to formate | 200 |
| 20 | Postsynthetic exchange (PSE) of Ti(iv) into a Zr(iv)-based MOF | Photocatalytic CO2 reduction to HCOOH | 201 |
| 21 | Bimetallic (Zr/Ti)-MOFs | Photocatalytic CO2 reduction to HCOOH | 202 |
3.2 Degradation of organic pollutants
Rapidly developing industrial activities produce huge amount of organics containing non-degradable solid wastes which are spread out in the open environment and further contaminates groundwater and surface water as well as air. The major contamination produces from petroleum refining, petrochemicals, pharmaceuticals, dye, paper, plastic, dairy production, fertilization and pesticide chemical industries etc. 207 , 208 , 209 Many reports are published related to develop effective process and technologies for waste-water treatment and oxidation process to decompose toxic and hazardous impurities at low cost and high efficiency. The literature on heterogeneous catalysis opens the ways about to use of novel catalytic systems for the decomposition of organic wastes from the aqueous solution as well as conversion of toxic gaseous pollutants to harmless products.
The most preferred catalytic systems are those based on photocatalysis with advanced oxidation processes (AOPs), as they offer the potential to utilize naturally available and renewable solar energy as a light source for photochemical waste remediation, making the process both green and sustainable. 209 Among AOPs, semiconductor-mediated photocatalysis has proven to be highly effective, as it completely mineralizes contaminants present in either the gas or liquid phase. 210 , 211 In these intriguing and effective fields of innovation, TiO2 photocatalysis and the photo-Fenton process are two well-established AOPs. As a result, there are a significant number of literatures on their environmental applications, such as water and air decontamination, as well as self-cleaning surfaces.
Merouani et al. have explored the use of AOPs, particularly for environmental remediation, followed by photoactivated processes. 14 , 15 , 16 , 17 In these studies, authors have developed a number of active radicals to facilitate the formation of reactive species for the degradation of toxic dyes such as chlorazol black,14a Allura Red AC,14b Safranin O,14c textile dyes and organic contamination. 15 The authors also applied the combined use of heterogeneous photocatalysis with high-frequency ultrasonic irradiation for dye and phenol degradation. 16 , 17 It was observed that the solar photocatalytic process operated efficiently, even when the pollutant was present at high concentration levels. It could be attributed to the occurrences of synergistic effect strongly dependent on the initial substrate concentration. 212
Duan et al. 213 have discussed AOPs as effective technique for activating superoxides radicals for degradation of organic pollutants in aqueous systems. Magnetic (Fe3O4) carbon supported manganese oxides (Fe3O4/C/Mn) have been used as an effective catalytic system for phenol degradation through peroxymonosulfate (PMS) activation. 214 In the catalytic system, radical generation takes place due to redox process of Mn4+/Mn3+ and magnetic support helps for catalyst separation. The catalyst CoFe2O4, prepared from Co–Fe alloys for the catalytic oxidation process via PMS activation to generate sulfate radicals. 215 A catalytic system, Ce–Mg/Al2O3/ozone has shown good efficiency for decomposition of resistant petroleum organic wastes. 216
Mesoporous CuO/TiO2 nanotubes and CuO nanorods-reduced graphene oxide prepared for catalytic oxidation of air pollutant CO to CO2. 217 Mixed metal oxides of Fe–W–Ce 218 and V2O5–WO3/TiO2, 219 used for conversion of NO x with NH3 into N2 and H2O. Oxidative dehydrogenation of ethane to ethylene and reduction of N2O could be simultaneously performed by using Cr/Al2O3, 220 as well as N2O has been directly decomposed on Cu–Zn/ZnAl2O4 and Zn/γ-Al2O4. 221
Wang et al. 222 have prepared red mud supported Co-oxide based catalysts as significantly effective for phenol decomposition via PMS activation. The authors also have reported natural zeolites supported Co oxide catalysts for phenol degradation through oxidation and adsorption process. 223 Sharma et al. 224 have demonstrated heterogeneous Fenton or Fenton-like reactions like H2O2–Fe3+ (solid)/nano-zero-valent iron/immobilized iron and electro-Fenton-pyrite for decomposition of dyes and phenols.
Wang et al. 225 have prepared MnO2 nanoparticles as α-, β- and γ–MnO2 obtained as nanowires, nanorods, and nanofibres respectively. The prepared catalysts were tested for phenol degradation and α–MnO2 was found to be more effective and stable in term of recyclability for phenol decomposition. They also have reported a comparative study of Mn3O4, Co3O4 and Fe3O4 nanoparticles for decomposition of phenolic contamination in aqueous solution. The catalysts are found to be effective in heterogeneous activation of PMS to produce sulfate radicals for phenol degradation and the order of catalytic activity was observed as Mn3O4 > Co3O4 > Fe3O4. 226 Powder activated carbon materials have been reported as green heterogeneous metal-free catalysts for the degradation of phenol through generation of sulfate radicals. 226 The degradation study shows that activated carbons were significantly effective with PMS to produce sulfate radicals compared to use with H2O2 and peroxydisulfate.
Galeano et al. 227 have briefly introduced mixed Al/metal-pillared clays as effective catalytic system for the degradation of organic pollutants in aqueous streams. The prepared catalytic system using Fe, Cu and Mn show metal pollution prevention and an effective performance under operative conditions. Al/Fe-pillared clay has been exhibited as most active catalyst for catalytic wet peroxide oxidation (CWPO) process and possesses desirable features of a catalyst able to operate the CWPO detoxification over a wide variety of real wastewaters. 227 Nair et al. have evaluated catalytic activity of Mn-substituted zinc nanoparticles (Mn x Zn1-x Fe2O4) prepared by sol-gel method. 228 As results, catalyst found to be very active wet peroxide oxidation of p-chlorophenol. Zhang et al. have evaluated catalytic performance of acid-modified coal fly ash catalyst for a Fenton-like process in p-nitrophenol wastewater treatment. 229 Zhen has briefly reviewed on cobalt oxide (Co3O4) 230 as environmental catalysis for catalytic reduction of NO by ammonia, hydro-desulfurization of COS, ozonation degradation of phenol, photocatalytic degradation of selective hydration of aromatic nitriles, 231 oxidative dehydrogenation of propane, 232 oxidative dehydrogenation of cyclohexane, 233 and hydrogen production by photo-catalytically. 234 Kumar et al. 235 have introduced various synthesis routes for aerogels and metal-organic framework as well as potential utilities as heterogeneous catalysts for environmental and energy applications. These include removal of heavy metals, CO2 capture and reduction, photodegradation of pollutants, air cleanup and water splitting as environmental and energy production through hydrogen, methane reforming, CO2 conversion and NO x removal as energy applications.
So far we have discussed on advanced oxidation processes (Fenton process, ozonation, sonolysis etc.) to reduce/control the organic pollutants from the aqueous and air, where active radicals involve in decomposition of large polluting molecules to small harmless molecules. Besides use of such heterogeneous catalysis, photocatalysis gaining huge attention with significant advancements being made in the synthesis of novel catalytic materials and nano-structures, and the design of efficient processes for the degradation of aqueous as well as atmospheric organic pollutants. Thus, photocatalysis is considered to be the most practical tool due to its usage of sunlight to decompose organic pollutants to convert them into valuable chemicals. A number of semiconductor-based (TiO2, CdS, CdSe, ZnO, SiO2, ZrO2 etc.) photocatalysis are now well established and are being employed as environment-friendly catalytic process.
Many researchers are engaged to design novel photocatalyst by considering the current environmental challenges and providing a clean and sustainable photocatalytic system. Dong et al. 236 , 237 , 238 have reported photocatalytic NO removal by using O/La co-functionalized amorphous graphitic carbon nitride (g–C3N4) which shows good efficiency. The incorporation of O and La form interlayer induce electron transportation which could reduce energy barriers and hence enhanced photocatalytic activity. The authors also have reported photocatalytic activity of Sr–intercalated g–C3N4 for oxidation of NO to convert non-toxic molecules selectively for air pollution control. 238 O/Ba co-functionalized amorphous carbon nitride system has exhibited enhanced photocatalytic NO removal rate with good selectivity to produces NO2 − and NO3 − while it also preventing the generation of toxic intermediate NO2. The authors also have prepared thermally and chemically stable Bi3WO6 tested for photocatalytic NO oxidization. 239 The presence of oxygen vacancy narrows the band gap and also promotes visible light absorption hence; the enhanced activity was observed.
Particularly, CdS as a photocatalyst has gained extensive interest due to its relatively narrow band-gap for visible-light absorption and sufficiently negative potential of the conduction band edge for the reduction of protons. 240 Generally, CdS is one of the most prominent semiconductors as a visible-light-responsive photocatalyst with a band gap of 2.4 eV among the other photocatalyst being used for hydrogen production. Cheng et al. 241 have introduced CdS and CdS–based photocatalyst that possess excellent photocatalytic activity in terms of solar-fuel generation and environmental cleaning through degradation of pollutants, hydrogen production and reduction of CO2 to hydrocarbon fuels. The monovalent CdS nanoparticles with different crystal forms (wurtzite phase and zinc blende phase) have been evaluated for the hydrogen production. 241 Zhang et al. have prepared CdS nanorods by a one-step solvothermal method and obtained an excellent photocatalytic activity for H2 production with a high dispersion of Pt. 242 There are many studies reported on binary CdS-based photocatalytic nano-composite materials such as CdS/ZnO, 243 CdS/TiO2, 244 , 245 and CdS/graphene, 246 CdS/sulfides (CdS/CuS), 247 CdS/ZnS, 248 Bi2S3/CdS 249 , 250 and, CdS/WS2, 251 MoS2/CdS, 252 for H2 evolution. The main advantage of the combination of such nano-composite construction is to improve photochemical properties and crystal forms of CdS and also increase the photogenerated electrons for photocatalytic H2 production. Recently, researchers have made efforts to combine CdS with other two co-catalysts to form ternary CdS-based photocatalysts such as CdS/WS2/graphene, 253 CdS/MoS2/graphene, 254 , 255 , 256 CdS/g–C3N4/CuS, 257 CdS/Ag2S/carbon-nano-tube (CNT), 258 CdS/WC/TiO2, 259 and CdS/MoS2/TiO2. 260 The photocatalytic hydrogen production was enhanced by using these ternary CdS-based photocatalysts and also preventing photo-corrosion.
Beside use of CdS for photocatalytic H2 production, it also makes significant contribution for environment cleaning as well as energy storage through conversion of carbon dioxide (CO2) to produce useful hydrocarbons such as methane, methanol, formic acid and formaldehyde etc. The reduced graphene oxide/CdS nano-rods composite exhibits very good photocatalytic activity for conversion of CO2 to methane where graphene oxide act as photoelectron acceptor and thus reduced recombination rate of photogenerated electron-hole pairs and hence enhanced photo-response. 261 CdS-based, CdS/ZnIn2S4, 262 nano-composite and CdS/TiO2, 263 nano-tube array has been exhibited with efficient dye degradation under visible irradiation and under sonication respectively.
TiO2, as an extensively studied and robust material in the field of photocatalysis research, as it was beginning from 1972 Fujishima and Honda et al., about the noble work of H2 production from water splitting. 264 TiO2 nanoparticles have wide range of applications and acts as back bone for many advanced process and devices such dye sensitized solar cells, sensors and rechargeable batteries/super capacitors etc. 265 , 266 Many more applications have already been well set for commercial productions like biomedical devices and drug delivery which has made TiO2 itself undivided part of photo-induced processes. Many researchers have reviewed about possible surface and structural modification for TiO2 and also about their catalytic mechanism revel more interesting facts about TiO2 photocatalysis. 244 , 266 , 267 , 268
Among all the possible applications of TiO2 as photocatalyst, it has been extensively used for photodegradation of various pollutants. It is very interesting to study about TiO2 semiconductor based photocatalytic mechanism upon absorption of photons and generation of reactive radical species through electron-hole pair recombination process. The pure mesoporous TiO2, TiO2 -nanorods and -nanotubes have been demonstrated for their excellent photocatalytic activity for degradation of organic pollutants under suitable conditions.
In addition, progressive performances continuing through surface modification into TiO2 by doping metal and non-metals which enhance visible light adsorption. Recently we have reviewed on the possible surface modification into TiO2 photocatalysts as well as including development of Plasmonic photocatalyst.245b
As part of environmental catalysts, MOFs also have been explored for air and water environmental remediation processes including removal of toxic metal ions, photocatalytic degradation of toxic dyes and other organic pollutants. 269 Recently MOFs of MIL-125(Ti)–NH2, MIL-125(Ti) and MIL-125(Ti)-(NH2)2, 270 UiO-66 and UiO-66-NH2, 271 MOF-5, 272 NH2–UiO-66(Zr) , 273 NH2-UiO-66(Hf) 274 and HKUST-1 275 are considered to have good to excellent gas adsorption capacity.
4 Major challenges for heterogeneous catalysis
Since over the many decades, catalysis researchers have been at the lead in addressing numerous challenges in generation of efficient energy resources, controlled on pollution, and sustainable development. The advanced strategies and approaches being developed by the catalysis community have led to significant changes, contributing to economic growth.
At the core of these efforts, heterogeneous catalysis offers low cost, high conversion efficiency, and product selectivity, enabling a wide range of industrial processes to proceed effectively. The recyclability of heterogeneous catalysts promotes green and sustainable manufacturing while preventing secondary pollution. From an economic perspective, the Heterogeneous Catalyst Market was valued at USD 24.6 billion in 2023 and is expected to register a compound annual growth rate (CAGR) of over 4.8 % between 2024 and 2032. 276 More than 80 % industries such as petrochemicals, chemicals, and refining continue to expand globally; the demand for heterogeneous catalysts is increasing.
However, applying heterogeneous catalysis on an industrial scale presents a exclusive set of challenges that can influence efficiency, cost, and sustainability. Therefore, its advancement requires the search for new materials with optimal catalytic activity and economic feasibility. In many important cases, catalyst deactivation often leads to a reduction in overall catalyst performance due to substantial or complete blocking of surface sites, carbon deposition, pore plugging, and the destruction of the catalyst. Catalyst deactivation (nonselective and selective deactivation) must be estimated in an industrial unit using temperature profiles, which can then guide decisions regarding improved process control and timely catalyst replacement.
The efficiency of a heterogeneous catalyst is strongly dependent on the ability of reactants to reach and interact with active sites on the catalyst surface. In the context of biodiesel production, the catalyst surface should exhibit hydrophobic properties, such as mesoporous surface reactivity, which helps facilitate the adsorption of oily hydrophobic species onto the catalyst’s surface. Therefore, the hydrophobicity of acid sites has become a key focus, as it minimizes poisoning by polar molecules. In case of high temperatures, catalyst may undergo structural changes leading to further loss of catalytic activity, compromising the process efficiency and leading to potential process failures. Developing and testing catalysts with sustainable thermal stability is essential for applications that involve high temperatures. Another important issue is translating a catalytic process from the laboratory to an industrial scale. Heat and mass transfer conditions are not identical at both scales, so scalability must be carefully considered for successful industrial application.
In general, variations or modifications in synthetic methods that controls the physico-chemical and thermal characteristics of heterogeneous catalysts that can lead to significant changes in reactivity and selectivity of the catalyst. Thus, we have to continue to design and modify new with the present characteristics to understand the interaction of the whole catalytic system. Additionally, understanding the effects of reaction conditions on the catalyst at a molecular level still requires the assistance of advanced, predetermined technologies. In this regard, computational screening technology can be a viable solution, providing acceptable estimations of surface reactivity with relatively low-cost information.
5 Conclusions
The present review article providing a prior literature on advancement of heterogeneous catalysis with its environmental and economic benefits in the field of energy generation and sustain the available feedstock as well. The varieties of materials have been discussed to explore theirs possible utilities as an emerging aspect of the materials science. Heterogeneous catalytic systems such as solid acids (H-form zeolites, sulfated-oxides, sulfonic ion exchangers, mesoporous silica materials, zirconia and titania supported materials), solid bases (β-zeolites, hydrotalcites, alkali metal- and mixed metal-oxides), transition metal based-MOFs catalysts are playing key role for biodiesel production as an alternative fuel source. As a part of heterogeneous catalysis, photocatalysis has major contribution for the environment remediation and exhibits as a promising green approach for the future demand with further investigation.
The present report could help to rational design a novel material by tuning the surface morphology and structural properties, and hence an efficient and non-expensive catalytic system as a part of eco-friendly way out to overcome the present challenges. The further progress in the heterogeneous catalytic systems will facilitate alternative fuel production and thus they can substitute the present expensive and hazardous catalytic systems.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The author has accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: Author declares no conflict of interest.
-
Research funding: No funding information.
-
Data availability: Not applicable.
References
1. (a)Bartholomew, C. H.; Farrauto, R. J. Fundamentals of Industrial Catalytic Processes, 2nd ed.; Wiley-Interscience: NJ, 2005.(b)Sheldon, R. A.; Arends, I.; Henefeld, U. Green Chemistry and Catalysis; Wiley VCH: Weinheim, Germany, 2007.(c)Gates, B. C. Catalytic Chemistry; Wiley: NY, 1991.10.1002/9780471730071Suche in Google Scholar
2. Waclawek, S.; Padil, V. V. T.; Cernik, M. Major Advances and Challenges in Heterogeneous Catalysis for Environmental Applications: A Review. Ecol. Chem. Eng. S. 2018, 25 (1), 9–34; https://doi.org/10.1515/eces-2018-0001.Suche in Google Scholar
3. Sani, Y. M.; Daud, W. M. A. W.; Aziz, A. R. A. Activity of Solid Acid Catalysts for Biodiesel Production: A Critical Review. Appl. Catal. A: Gen. 2014, 470, 140–161.10.1016/j.apcata.2013.10.052Suche in Google Scholar
4. Catalyst market size, share, trends, industry growth report 2019-2025; Grand View Research: San Francisco, CA, 2018. URL: https://www.grandviewresearch.com/industry-analysis/catalyst-market/request/rs1.Suche in Google Scholar
5. Thomas, J. M. Summarizing Comments on the Discussion and a Prospectus for Urgent Future Action. Phil. Trans. R. Soc. A. 2016, 374, 20150226; https://doi.org/10.1098/rsta.2015.0226.Suche in Google Scholar PubMed
6. Chouhan, A. P. S.; Sarma, A. K. Modern Heterogeneous Catalysts for Biodiesel Production: A Comprehensive Review. Renew. Sust. Energ. Rev. 2011, 15, 4378–4399; https://doi.org/10.1016/j.rser.2011.07.112.Suche in Google Scholar
7. (a)Sa, J. Fuel Production with Heterogeneous Catalysis, 1st ed.; CRC Press Taylor & Francis Group: FL, 2018.(b)Su, F.; Guo, Y. Advancements in Solid Acid Catalysts for Biodiesel Synthesis. Green Chem. 2014, 16, 2934–2957; https://doi.org/10.1039/c3gc42333f.(c)Parangi, T.; Mishra, M. K. Solid Acid Catalysts for Biodiesel Production. Comments Inorg. Chem. 2020, 40 (4), 176–216; https://doi.org/10.1080/02603594.2020.1755273.Suche in Google Scholar
8. Whang, H. S.; Lim, J.; Choi, M. S.; Lee, J.; Lee, H. Heterogeneous Catalysts for Catalytic CO2 Conversion into Value-Added Chemicals. BMC Chem. Eng. 2019, 1–9; https://doi.org/10.1186/s42480-019-0007-7.Suche in Google Scholar
9. Hara, M. Biomass Conversion by a Solid Acid Catalyst. Energ. Environ. Sci. 2010, 3, 601–607; https://doi.org/10.1039/b922917e.Suche in Google Scholar
10. Ignacio, M.-C. Catalytic Materials: Concepts to Understand the Pathway to Implementation. Ind. Eng. Chem. Res. 2021, 60 (51), 18543–18938.10.1021/acs.iecr.1c02681Suche in Google Scholar
11. Volker, H. Catalytic Process Development for Renewable Materials. Green Process. Synth. 2013, 2 (4), 375–376; https://doi.org/10.1515/gps-2013-0057.Suche in Google Scholar
12. Moulijn, J. A.; van Santen, R. A. History of Catalysis. In Contemporary Catalysis: Science, Technology, and Applications; Kamer, P. C. J.; Vogt, D.; Thybaut, J., Eds. The Royal Society of Chemistry: Croydon, UK, 2017; pp 1–28.10.1039/9781849739900-00001Suche in Google Scholar
13. Zhang, J.; Chen, H.; Duan, X.; Sun, H.; Wang, S. Photothermal Catalysis: From Fundamentals to Practical Applications. Mater. Today 2023, 68, 234–253; https://doi.org/10.1016/j.mattod.2023.06.017.Suche in Google Scholar
14. (a)Djaballah, M. L.; Merouani, S.; Bendjama, H.; Hamdaoui, O. Development of a Free Radical-Based Kinetics Model for the Oxidative Degradation of Chlorazol Black in Aqueous Solution Using Periodate Photoactivated Process. J. Photochem. Photobio. A: Chem. 2021, 408, 113102; https://doi.org/10.1016/j.jphotochem.2020.113102. (b)Hamdaoui, O.; Merouani, S.; Benmahmoud, H. C.; Ait Idir, M.; Ferkous, H.; Alghyamah, A. Ultrasound/Chlorine: A Novel Synergistic Sono-Hybrid Process for Allura Red AC Degradation. Catalysts 2022, 12, 1171; https://doi.org/10.3390/catal12101171. (c)Bekkouche, S.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M. Efficient Photocatalytic Degradation of Safranin O by Integrating Solar-UV/TiO2/persulfate Treatment: Implication of Sulfate Radical in the Oxidation Process and Effect of Various Water Matrix Components. J. Photochem. Photobio. A: Chem. 2017, 345, 80–91; https://doi.org/10.1016/j.jphotochem.2017.05.028.Suche in Google Scholar
15. (a)Meghlaoui, F. Z.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M.; Ashokkumar, M. Rapid Catalytic Degradation of Refractory Textile Dyes in Fe(II)/chlorine System at Near Neutral pH: Radical Mechanism Involving Dichlorine Radical Anion (Cl2●−)-Mediated Transformation Pathways and Impact of Environmental Matrices. Sep. Purif. Technol. 2019, 115685.(b)Meghlaoui, F. Z.; Merouani, S.; Hamdaoui, O.; Alghyamah, A.; Bouhelassa, M.; Ashokkumar, M. Fe(III)‐catalyzed Degradation of Persistent Textile Dyes by Chlorine at Slightly Acidic Conditions: the Crucial Role of Cl2●− Radical in the Degradation Process and Impacts of Mineral and Organic Competitors. Asia Pac. J. Chem. Eng. 2020, 16 (1), 2553; https://doi.org/10.1002/apj.2553.(c)Merouani, S.; Dehane, A.; Belghit, A.; Hamdaoui, O.; Tobba, Y. A.; Lahlou, C.; Shah, M. P. Protonated Hydroxylamine-Assisted Iron Catalytic Activation of Persulfate for the Rapid Removal of Persistent Organics from Wastewater. Clean – Soil, Air, Water 2022, 51 (3), 2100304; https://doi.org/10.1002/clen.202100304.Suche in Google Scholar
16. Bekkouche, S.; Bouhelassa, M.; Aissa, A. K.; Baup, S.; Gondrexon, N.; Petrier, C.; Merouani, S.; Hamdaoui, O. Synergy between Solar Photocatalysis and High Frequency Sonolysis toward the Degradation of Organic Pollutants in Aqueous Phase – Case of Phenol. Desalin. Water Treat. 2017, 62, 457–464; https://doi.org/10.5004/dwt.2017.20146.Suche in Google Scholar
17. Bouchareb, M. K.; Berkani, M.; Merouania, S.; Bouhelassa, M. Box-Wilson Design Modeling of Photocatalytic Degradation of Industrial Dye and Wastewater in Semi-pilot Solar Photoreactor. Water Sci. Technol. 2020, 82 (7), 1393–1403; https://doi.org/10.2166/wst.2020.405.Suche in Google Scholar PubMed
18. (a)Xu, Z.; Li, Y.; Shi, H.; Lin, Y.; Wang, Y.; Wang, Q.; Zhu, T. Application Prospect of K Used for Catalytic Removal of NOx, COx, and VOCs from Industrial Flue Gas: A Review. Catalysts 2021, 11 (4), 419; https://doi.org/10.3390/catal11040419.(b)Zhao, H.; Meng, P.; Gao, S.; Wang, Y.; Sun, P.; Wu, Z. Recent Advances in Simultaneous Removal of NOx and VOCs over Bifunctional Catalysts via SCR and Oxidation Reaction. Sci. Total Environ. 2024, 906; https://doi.org/10.1016/j.scitotenv.2023.167553.Suche in Google Scholar
19. (a)Dhal, G. C.; Dey, S. Catalytic Conversion of Carbon Monoxide into Carbon Dioxide over Spinel Catalysts: An Overview. Mater. Sci. Energy Technol. 2019, 2 (3), 575–588; https://doi.org/10.1016/j.mset.2019.06.003.(b)Kembo, J. P. N.; Wang, J.; Luo, N.; Gao, F.; Yi, H.; Zhao, S.; Zhou, Y.; Tang, X. A Review of Catalytic Oxidation of Carbon Monoxide over Different Catalysts with an Emphasis on Hopcalite Catalysts. New J. Chem. 2023, 47, 20222–20247; https://doi.org/10.1039/d3nj03074a.Suche in Google Scholar
20. (a)Khouw, C. B.; Dartt, C. B.; Labinger, J. A.; Davis, M. E. Studies on the Catalytic-Oxidation of Alkanes and Alkenes by Titanium Silicates. J. Catal. 1994, 149 (1), 195–205; https://doi.org/10.1006/jcat.1994.1285.(b)Fabrice, D.; Jacques, B.Jr.; Daniel, D.; Isabelle, G.; Gil, M. Catalytic Oxidation of Heavy Hydrocarbons over Pt/Al2O3. Influence of the Structure of the Molecule on its Reactivity. Appl. Catal. B Environ. 2010, 95 (3–4), 217–227; https://doi.org/10.1016/j.apcatb.2009.12.026.Suche in Google Scholar
21. Wang, L.; Zhang, X.; Yang, L.; Wang, C.; Wang, H. Photocatalytic Reduction of CO2 Coupled with Selective Alcohol Oxidation under Ambient Conditions. Catal. Sci. Technol. 2015, 5, 4800–4805; https://doi.org/10.1039/c5cy00772k.Suche in Google Scholar
22. Laverdura, U. P.; Rossi, L.; Ferella, F.; Courson, C.; Zarli, A.; Alhajyoussef, R.; Gallucci, K. Selective Catalytic Hydrogenation of Vegetable Oils on Lindlar Catalyst. ACS Omega 2020, 5 (36), 22901–22913; https://doi.org/10.1021/acsomega.0c02280.Suche in Google Scholar PubMed PubMed Central
23. Jing, Z.; Guo, Y.; Wang, Q.; Yan, X.; Yue, G.; Li, Z.; Liu, H.; Qin, R.; Zhong, C.; Li, M.; Xu, D.; Yao, X.; Shuai, X.; Shuai, M. Ambient Hydrogenation of Solid Aromatics Enabled by a High Entropy Alloy Nanocatalyst. Nat. Commun. 2024, 15, 5806–5816; https://doi.org/10.1038/s41467-024-50009-5.Suche in Google Scholar PubMed PubMed Central
24. (a)Ashida, Y.; Mizushima, T.; Arashiba, K.; Egi, A.; Tanaka, H.; Yoshizawa, K.; Nishibayashi, Y. Catalytic Production of Ammonia from Dinitrogen Employing Molybdenum Complexes Bearing N-Heterocyclic Carbene-Based PCP-type Pincer Ligands. Nat. Synth. 2023, 2, 635–644; https://doi.org/10.1038/s44160-023-00292-9.(b)Hattori, M.; Iijima, S.; Nakao, T.; Hosono, H.; Hara, M. Solid Solution for Catalytic Ammonia Synthesis from Nitrogen and Hydrogen Gases at 50 °C. Nat. Commun. 2020, 11, 2001; https://doi.org/10.1038/s41467-020-15868-8.Suche in Google Scholar
25. (a)Liu, X.-M.; Lu, G. Q.; Yan, Z.-F.; Beltramini, J. Recent Advances in Catalysts for Methanol Synthesis via Hydrogenation of CO and CO2. Ind. Eng. Chem. Res. 2003, 42 (25), 6518–6530; https://doi.org/10.1021/ie020979s.(b)Salami, R.; Zeng, Y.; Han, X.; Rohani, S.; Zheng, Y. Exploring Catalyst Developments in Heterogeneous CO2 Hydrogenation to Methanol and Ethanol: A Journey through Reaction Pathways. J. Energy Chem. 2025, 101, 345–384; https://doi.org/10.1016/j.jechem.2024.08.069.Suche in Google Scholar
26. Choi, Y.; Wu, X.; Lee, J.-W.; Na, K. Catalytic Dehydrogenation for Hydrogen Production Controlled by Metal-Supported Heterogeneous Catalysts. Catal. Sci. Technol. 2024, 14, 5784–5810; https://doi.org/10.1039/d4cy00875h.Suche in Google Scholar
27. (a)Meng, H.; Yang, Y.; Shen, T.; Yin, Z.; Zhang, J.; Yan, H.; Wei, M. Highly Efficient Hydrogen Production from Dehydrogenation Reaction of Nitrogen Heterocycles via Pd0–Pdδ+ Synergistic Catalysis. ACS Catal. 2023, 13 (13), 9234–9244; https://doi.org/10.1021/acscatal.3c01522.(b)Chulliyil, H. M.; Hamdani, I. R.; Ahmad, A.; Pillantakath, A. R.; Shoaibi, A. A.; Srinivasakannan, C.; Hossain, M. M. Catalytic Dehydrogenation of Methane to Hydrogen and Carbon Nanostructures with Fe–Mo Bimetallic Ctalysts. Ind. Eng. Chem. Res. 2024, 63 (9), 3853–3866; https://doi.org/10.1021/acs.iecr.3c03895.(c)Voskresenskaya, E. N.; Kirilets, V. M.; Taran, O. P.; Kuznetsov, B. N. Hydrogen Production by the Heterogeneous Catalytic Dehydrogenation of Formic Acid: A Review. Catal. Ind. 2024, 16, 339–349; https://doi.org/10.1134/s2070050424700181.Suche in Google Scholar
28. Wittich, K.; Kramer, M.; Bottke, N.; Schunk, S. A. Catalytic Dry Reforming of Methane: Insights from Model Systems. Chem.Cat.Chem. 2020, 12 (8), 2130–2147; https://doi.org/10.1002/cctc.201902142.Suche in Google Scholar
29. Frontera, P.; Antonucci, P. L.; Macario, A. Focus on Materials for Sulfur-Resistant Catalysts in the Reforming of Biofuels. Catalysts 2021, 11 (9), 1029; https://doi.org/10.3390/catal11091029.Suche in Google Scholar
30. Li, D.; Li, X.; Gong, J. Catalytic Reforming of Oxygenates: State of the Art and Future Prospects. Chem. Rev. 2016, 116 (19), 11529–11653; https://doi.org/10.1021/acs.chemrev.6b00099.Suche in Google Scholar PubMed
31. Aouad, S.; Labaki, M.; Ojala, S.; Seelam, P.; Turpeinen, E.; Gennequin, C.; Estephane, J.; Abi-Aad, E. A Review on the Dry Reforming Processes for Hydrogen Production: Catalytic Materials and Technologies. In Frontiers in Ceramic Science Catalytic Materials for Hydrogen Production and Electro-oxidation Reactions; Cesario, M. R.; Gennequin, C.; Abi-Aad, E.; De Macedo, D. A., Eds; Bentham Science Publisher: Portugal, Vol. 2, 2018.10.2174/9781681087580118020007Suche in Google Scholar
32. Thangaraj, B.; Solomon, R. P.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in Biodiesel Production- A Review. Clean Energ. 2019, 3 (1), 2–23; https://doi.org/10.1093/ce/zky020.Suche in Google Scholar
33. (a)Lee, A. F.; Bennett, J. A.; Manayil, J. C.; Wilson, K. Heterogeneous Catalysis for Sustainable Biodiesel Production via Esterification and Transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916; https://doi.org/10.1039/c4cs00189c.(b)Marwaha, A.; Dhir, A.; Mahla, S. K.; Mohapatra, S. K. An Overview of Solid Base Heterogeneous Catalysts for Biodiesel Production. Catal. Rev. 2018, 60, 594–628; https://doi.org/10.1080/01614940.2018.1494782.Suche in Google Scholar PubMed
34. Kiss, A. A.; Dimian, A. C.; Rothenberg, G. Solid Acid Catalysts for Biodiesel Production -Towards Sustainable Energy. Adv. Synth. Catal. 2006, 348, 75–81; https://doi.org/10.1002/adsc.200505160.Suche in Google Scholar
35. Helwani, Z.; Othman, M. R.; Aziz, N.; Fernando, W. J. N.; Kim, J. Technologies for Production of Biodiesel Focusing on Green Catalytic Techniques: A Review. Fuel Process. Technol. 2009, 90, 1502–1514; https://doi.org/10.1016/j.fuproc.2009.07.016.Suche in Google Scholar
36. Sharma, Y. C.; Singh, B. Development of Biodiesel: Current Scenario. Renew. Sustain. Energy Rev. 2009, 13, 1646–1651; https://doi.org/10.1016/j.rser.2008.08.009.Suche in Google Scholar
37. Okoronkwo, M. U.; Galadima, A.; Leke, L. Advances in Biodiesel Synthesis: From Past to Present. Elixir Appl. Chem. 2012, 43, 6924–6945.Suche in Google Scholar
38. West, A. H.; Posarac, D.; Ellis, N. Assessment of Four Biodiesel Production Processes Using HYSYS. Plant. Bioresour Technol 2008, 99, 6587–6601; https://doi.org/10.1016/j.biortech.2007.11.046.Suche in Google Scholar PubMed
39. Lourinho, G.; Brito, P. Advanced Biodiesel Production Technologies: Novel Developments. Rev. Environ. Sci. Biotechnol. 2014, 14 (2), 287–316; https://doi.org/10.1007/s11157-014-9359-x.Suche in Google Scholar
40. Miao, S.; Shanks, B. H. Esterification of Biomass Pyrolysis Model Acids over Sulfonic Acid-Functionalized Mesoporous Silicas. Appl. Catal. A: Gen. 2009, 359 (1–2), 113–120; https://doi.org/10.1016/j.apcata.2009.02.029.Suche in Google Scholar
41. Puna, J. F.; Gomes, J. F.; Correia, M. J.; Soares Dias, A. P.; Bordado, J. C. Advances on the Development of Novel Heterogeneous Catalysts for Transesterification of Triglycerides in Biodiesel. Fuel 2010, 89, 3602–3606; https://doi.org/10.1016/j.fuel.2010.05.035.Suche in Google Scholar
42. Zhang, Z. Branched Biodiesels. US 0045731 A1, 2008.Suche in Google Scholar
43. Pozzo, D. M. D.; dos Santos, J. A. A.; Junior, E. S.; Santos, R. F.; Feiden, A.; de Souza, S. N. M.; Burgardt, I. Free Fatty Acids Esterification Catalyzed by Acid Faujasite Type Zeolite. RSC Adv. 2019, 9, 4900–4907; https://doi.org/10.1039/c8ra10248a.Suche in Google Scholar PubMed PubMed Central
44. Septiani, U.; Putri, R. A.; Jamarun, N. Synthesis of Zeolite ZSM-5 from Rice Husk Ash as Cataliyst in Vegetable Oil Transesterification for Biodiesel Production. Der Pharm. Lett. 2016, 8 (19), 86–91.Suche in Google Scholar
45. Park, Y. M.; Lee, D. W.; Kim, D. K.; Lee, J. S.; Lee, K. Y. The Heterogeneous Catalyst System for the Continuous Conversion of Free Fatty Acids in Used Vegetable Oils for the Production of Biodiesel. Catal. Today 2008, 131, 238–243; https://doi.org/10.1016/j.cattod.2007.10.052.Suche in Google Scholar
46. Brito, A.; Borges, M.; Garin, M.; Hernandez, A. Biodiesel Production from Waste Oil Using Mg- Al Layered Double Hydroxide Catalysts. Energ. Fuel. 2009, 23, 2952–2958; https://doi.org/10.1021/ef801086p.Suche in Google Scholar
47. Doyle, A. M.; Alismaeel, Z. T.; Albayati, T. M.; Abbas, A. S. High Purity FAU-type Zeolite Catalysts from Shale Rock for Biodiesel Production. Fuel 2017, 199, 394–402; https://doi.org/10.1016/j.fuel.2017.02.098.Suche in Google Scholar
48. Noor Al-Jammal Zayed Al-Hamamre Mohammad Alnaief Manufacturing of Zeolite Based Catalyst from Zeolite Tuft for Biodiesel Production from Waste Sunflower Oil. Renew. Energ. 2016, 93, 449–459.10.1016/j.renene.2016.03.018Suche in Google Scholar
49. Fereidooni, L.; Tahvildari, K.; Abdollah, A.; Sharif, M. Biodiesel Production from Methanolysis of Refined Vegetable Oil through KOH/zeolite Catalyst. Der Pharma Chem. J 2017, 4 (4), 85–90.Suche in Google Scholar
50. Brito, A.; Borges, M. E.; Otero, N. Zeolite Y as a Heterogeneous Catalyst in Biodiesel Fuel Production from Used Vegetable Oil. Energ. Fuel. 2007, 21, 3280–3283; https://doi.org/10.1021/ef700455r.Suche in Google Scholar
51. Carrero, A.; Vicente, G.; Rodríguez, R.; Linares, M.; del Peso, G. L. Hierarchical Zeolites as Catalysts for Biodiesel Production from Nannochloropsis Microalga Oil. Catal. Today 2011, 167, 148–153; https://doi.org/10.1016/j.cattod.2010.11.058.Suche in Google Scholar
52. Wang, Y.-Y.; Lee, D. J.; Chen, B.-H. Low-Al Zeolite Beta as a Heterogeneous Catalyst in Biodiesel Production from Microwave-Assisted Transesterification of Triglycerides. Energy Proc. 2014, 61, 918–921; https://doi.org/10.1016/j.egypro.2014.11.995.Suche in Google Scholar
53. Dang, T.-H.; Chen, B.-H.; Lee, D.-J. Optimization of Biodiesel Production from Transesterification of Triolein Using Zeolite LTA Catalysts Synthesized from Kaolin Clay. J. Taiwan Inst. Chem. Eng. 2017, 79, 14–22.10.1016/j.jtice.2017.03.009Suche in Google Scholar
54. Dianursanti, D.; Hayati, S. J.; Putri, D. N. Biodiesel Synthesis via Transesterification of Lipid Chlorophyta Cultivated in Walne Rich Carbon Medium Using KOH/Zeolite Catalyst. AIP Conf. Proc. 2017, 1904, 020065; https://doi.org/10.1063/1.5011922.Suche in Google Scholar
55. Hartono, R.; Wijanarko, A.; Hermansyah, H. Synthesis of Biodiesel Using Local Natural Zeolite as Heterogeneous Anion Exchange Catalyst. IOP Conf. Ser. Mater. Sci. Eng. 2018, 345; https://doi.org/10.1088/1757-899x/345/1/012002.Suche in Google Scholar
56. Hassani, M.; Najafpour, G. D.; Mohammadi, M.; Rabiee, M. Preparation, Characterization and Application of Zeolite Based Catalyst for Production of Biodiesel from Waste Cooking Oil. J. Sci. Ind. Res. 2014, 73, 129–133.Suche in Google Scholar
57. Hidayat, A.; Mukti, M. I. F.; Handoko, B.; Sutrisno, B. Biodiesel Production from Rice Bran Oil over Modified Natural Zeolite Catalyst. Int. J Technol. 2018, 2, 400–411.10.14716/ijtech.v9i2.1084Suche in Google Scholar
58. Hidayat, A.; Sutrisno, B. Free Fatty Acids Esterification on Palm Oil Sludge Using Zirconia-Supported Indonesian Natural Zeolite as Heterogeneous Catalyst. Orient. J. Chem. 2018, 34 (5), 2464–2470; https://doi.org/10.13005/ojc/340531.MuktiSuche in Google Scholar
59. Intarapong, P.; Luengnaruemitchai, A.; Jai-In, S. Transesterification of Palm Oil over KOH/NaY Zeolite in a Packed-Bed Reactor. Int. J. Renew. Energ. Res 2011, 1 (4), 271–280.Suche in Google Scholar
60. Noiroj, K.; Intarapong, P.; Luengnaruemitchai, A.; JaiIn, S. A Comparative Study of KOH/Al2O3and KOH/NaY Catalysts for Biodiesel Production via Transesterification from Palmoil. Renew. Energy 2009, 34, 1145–1150; https://doi.org/10.1016/j.renene.2008.06.015.Suche in Google Scholar
61. Luz Martinez, S.; Romero, R.; Lopez, J. C.; Romero, A.; Sanchez Mendieta, V.; Natividad, R. Preparation and Characterization of CaO nanoparticles/NaX Zeolite Catalysts for the Transesterification of Sunflower Oil. Ind. Eng. Chem. Res. 2011, 50 (5), 2665–2670; https://doi.org/10.1021/ie1006867.Suche in Google Scholar
62. Mowla, D.; Rasti, N.; Keshavarz, P. Transesterification of Waste Cooking Oil for Biodiesel Production Using Modified Clinoptilolite Zeolite as a Heterogeneous Catalyst. Int. J Chem. Mol. Eng. 2016, 10 (9), 1201–1205.Suche in Google Scholar
63. Setianingsih, A.; Wisrayetti, K.; Bahri, S. Effect of Lanthanum-Natural Zeolite, La/NZA Catalyst on Biodiesel Production from Crude Palm Oil. IOP Conf. Ser. Mater. Sci. Eng. 2018, 345, 012007.10.1088/1757-899X/345/1/012007Suche in Google Scholar
64. Singh, D.; Ganesh, A.; Mahajani, S. Heterogeneous Catalysis for Biodiesel Synthesis and Valorization of Glycerol. Clean Techn. Environ. Policy. 2015, 17 (4), 1103–1110; https://doi.org/10.1007/s10098-014-0858-9.Suche in Google Scholar
65. Volli, V.; Purkait, M. K. Selective Preparation of Zeolite X and A from Flyash and its Use as Catalyst for Biodiesel Production. J. Hazard Mater. 2015, 297, 101–111; https://doi.org/10.1016/j.jhazmat.2015.04.066.Suche in Google Scholar PubMed
66. Wang, Y.-Y.; Wang, H.-H.; Chuang, T.-L.; Chen, B.-H.; Lee, D.-J. Biodiesel Produced from Catalyzed Transesterification of Triglycerides Using Ion-Exchanged Zeolite Beta and MCM-22. Energy Proc. 2014, 61, 933–936; https://doi.org/10.1016/j.egypro.2014.11.998.Suche in Google Scholar
67. Xue, W.; Zhou, Y.-C.; Song, B.-A.; Shi, X.; Wang, J.; Yin, S.-T.; Jin, L.-H.; Yang, S. Synthesis of Biodiesel from Jatropha Curcas L. Seed Oil Using Artificial Zeolites Loaded with CH3COOK as a Heterogeneous Catalyst. Nat. Sci. 2009, 1, 55–62; https://doi.org/10.4236/ns.2009.11010.Suche in Google Scholar
68. Vieira, S. S.; Magriotis, Z. M.; Santos, N. A. V.; Saczk, A. A.; Hori, C. E.; Arroyo, P. A. Biodiesel Production by Free Fatty Acid Esterification Using Lanthanum (La3+) and HZSM-5 Based Catalysts. Bioresour. Technol. 2013, 133, 248–255; https://doi.org/10.1016/j.biortech.2013.01.107.Suche in Google Scholar PubMed
69. Prinsen, P.; Luque, R.; Gonzalez-Arellano, C. Zeolite Catalyzed Palmitic Acid Esterification. Micropor. Mesopor. Mater. 2018, 262, 133–139; https://doi.org/10.1016/j.micromeso.2017.11.029.Suche in Google Scholar
70. Chang, F.; Zhou, Q.; Pan, H.; Liu, X.-F.; Zhang, H.; Yang, S. Solid Mixed-Metal-Oxide Catalysts for Biodiesel Production: A Review. Energy Technol. 2014, 2, 865–873; https://doi.org/10.1002/ente.201402089.Suche in Google Scholar
71. Vedrine, J. C. Heterogeneous Catalysis on Metal Oxides. Catalysts 2017, 7, 341; https://doi.org/10.3390/catal7110341.Suche in Google Scholar
72. da Conceicao, L. R. V.; Carneiro, L. M.; Rivaldi, J. D.; de Castro, H. F. Solid Acid as Catalyst for Biodiesel Production via Simultaneous Esterification and Transesterification of Macaw Palm Oil. Ind. Crops Prod. 2016, 89, 416–424, https://doi.org/10.1016/j.indcrop.2016.05.044.Suche in Google Scholar
73. Anggoro, I. D. D.; Buchori, L.; Rahmawati, D. A.; Intaningrum, D. Active Acid Catalyst of Sulphated Zinc Oxide for Transesterification of Soybean Oil with Methanol to Biodiesel. Proc. Environ. Sci. 2015, 23, 385–393; https://doi.org/10.1016/j.proenv.2015.01.055.Suche in Google Scholar
74. Peters, T. A.; Benes, N. E.; Holmen, A.; Keurentjes, J. T. F. Comparison of Commercial Solid Acid Catalysts for the Esterification of Acetic Acid with Butanol. Appl. Catal. A 2006, 297, 182–188; https://doi.org/10.1016/j.apcata.2005.09.006.Suche in Google Scholar
75. Park, J. Y.; Wang, Z. M.; Kim, D. K.; Lee, J. S. Effects of Water on the Esterification of Free Fatty Acids by Acid Catalyst. Renew. Energy 2010, 35 (3), 614–618; https://doi.org/10.1016/j.renene.2009.08.007.Suche in Google Scholar
76. Liu, Y. J.; Lotero, E.; Goodwin, J. G. A Comparison of the Esterification of Acetic Acid with Methanol Using Heterogeneous versus Homogeneous Acid Catalysis. J. Catal. 2006, 242, 278–286.10.1016/j.jcat.2006.05.026Suche in Google Scholar
77. Liu, Y. J.; Lotero, E.; Goodwin, J. G. Effect of Carbon Chain Length on Esterification of Carboxylic Acids with Methanol Using Acid Catalysis. J. Catal. 2006, 243, 221–228.10.1016/j.jcat.2006.07.013Suche in Google Scholar
78. Guerreiro, L.; Castanheiro, J. E.; Fonseca, I. M.; Aranda, R. M. M.; Ramos, A. M.; Vital, J. Transesterification of Soybean Oil over Sulfonic Acid Functionalized Polymeric Membranes. Catal. Today 2006, 118, 166–171; https://doi.org/10.1016/j.cattod.2005.12.012.Suche in Google Scholar
79. Lopez, D. E.; Goodwin, J. D.Jr.; Bruce, D. A.; Lotero, E. Transesterification of Triacetin with Methanol on Solid Acid and Base Catalysts. Appl. Catal. A 2005, 295, 97–105; https://doi.org/10.1016/j.apcata.2005.07.055.Suche in Google Scholar
80. Lopez, D. E.; Goodwin, J. D.Jr.; Bruce, D. A. Transesterification of Triacetin with Methanol on Nafion® Acid Resins. J. Catal. 2007, 245, 381–391.10.1016/j.jcat.2006.10.027Suche in Google Scholar
81. Russbueldt, B. M. E.; Hoelderich, W. F. New Sulfonic Acid Ion-Exchange Resins for the Preesterification of Different Oils and Fats with High Content of Free Fatty Acids. Appl. Catal. A 2009, 362, 47–57; https://doi.org/10.1016/j.apcata.2009.04.019.Suche in Google Scholar
82. Barros, S. D. T.; Coelho, A. V.; Lachter, E. R.; San Gil, R. A. S.; Dahmouche, K.; Pais da Silva, M. I.; Souza, A. L. F. Esterification of Lauric Acid with Butanol over Mesoporous Materials. Renew. Energ. 2013, 50, 585–589; https://doi.org/10.1016/j.renene.2012.06.059.Suche in Google Scholar
83. Serio, M. D.; Tesser, R.; Casale, L.; Dapos, A.; Trifuoggi, M.; Santacesaria, E. Heterogeneous Catalysis in Biodiesel Production: The Influence of Leaching. Top. Catal. 2010, 53, 811–819; https://doi.org/10.1007/s11244-010-9467-y.AngeloSuche in Google Scholar
84. Xie, W.; Yang, Z.; Chun, H. Catalytic Properties of Lithium-Doped ZnO Catalysts Used for Biodiesel Preparations. Ind. Eng. Chem. Res. 2007, 46, 7942–7949; https://doi.org/10.1021/ie070597s.Suche in Google Scholar
85. Xie, W.; Zhao, L. Heterogeneous CaO-MoO3 SBA-15 Catalysts for Biodiesel Production from Soybean Oil. Energ. Convers. Manage. 2014, 79, 34–42; https://doi.org/10.1016/j.enconman.2013.11.041.Suche in Google Scholar
86. Anuradha, S.; Antony, K. J. R.; Vijayaraghavan, V. R.; Viswanathan, B. Sulfated Fe2O3-TiO2 Catalyzed Transesterification of Soybean Oil to Biodiesel. Ind. J Chem. A. 2014, 53, 1493–1499.Suche in Google Scholar
87. de Abreu, W. C.; de Moura, C. V. R.; Costa, J. C. S.; de Moura, E. M. Strontium and Nickel Heterogeneous Catalysts for Biodiesel Production from Macaw Oil. J. Braz. Chem. Soc. 2017, 28 (2), 319–327.10.5935/0103-5053.20160181Suche in Google Scholar
88. Ong, H. R.; Khan, M. R.; Chowdhury, M. N. K.; Yousuf, A.; Cheng, C. K. Synthesis and Characterization of CuO/C Catalyst for the Esterification of Free Fatty Acid in Rubber Seed Oil. Fuel 2014, 120, 195–201; https://doi.org/10.1016/j.fuel.2013.12.015.Suche in Google Scholar
89. Zhang, H.; Luo, X.; Shi, K.; Wu, T.; He, F.; Zhou, S.; Chen, G.; Peng, C. Highly Efficient Sulfonic/carboxylic Dual-Acid Synergistic Catalysis for Esterification Enabled by Sulfur-Rich Graphene Oxide (GO-S). ChemSusChem 2017, 10 (17), 3352–3357; https://doi.org/10.1002/cssc.201700950.Suche in Google Scholar PubMed
90. Anisuzzaman, S. M.; Krishnaiah, D.; Bono, A.; Abang, S.; Sundang, M.; Suali, E.; Lahin, F. A.; Alawodeen, A. S. Synthesis and Characterization of Metal Oxide Promoted Alumina Catalyst for Biofuel Production. IOP Conf. Ser. Earth Environ. Sci. 2016, 36; https://doi.org/10.1088/1755-1315/36/1/012039.Suche in Google Scholar
91. Mohadesi, M.; Hojabri, Z.; Moradi, G. Biodiesel Production Using Alkali Earth Metal Oxides Catalysts Synthesized by Sol-Gel Method. Biofuel Res. J. 2014, 1, 30–33; https://doi.org/10.18331/brj2015.1.1.7.Suche in Google Scholar
92. Ismail, S.; Ahmed, A. S.; Anr, R.; Hamdan, S. Biodiesel Production from castor Oil by Using Calcium Oxide Derived from Mud Clam Shell. J Renew. Energ. 2016, 2016 (1), 5274917; https://doi.org/10.1155/2016/5274917.Suche in Google Scholar
93. Thitsartarn, W.; Maneerung, T.; Kawi, S. Highly Active and Durable Ca-Doped Ce-SBA-15 Catalyst for Biodiesel Production. Energy 2015, 89, 946–956; https://doi.org/10.1016/j.energy.2015.06.039.Suche in Google Scholar
94. Ali, S. D.; Javed, I. N.; Rana, U. L.; Nazar, M. F.; Ahmed, W.; Junaid, A.; Pasha, M.; Nazir, R.; Nazir, R. Novel SrO-CaO Mixed Metal Oxides Catalyst for Ultrasonic-Assisted Transesterification of Jatropha Oil into Biodiesel. Aust. J. Chem. 2017, 70 (3), 258; https://doi.org/10.1071/ch16236.Suche in Google Scholar
95. Lee, H. V.; Juan, J. C.; Taufiq-Yap, Y. H. Preparation and Application of Binary Acid-Base CaO-La2O3 Catalyst for Biodiesel Production. Renew. Energy. 2015, 74, 124–132; https://doi.org/10.1016/j.renene.2014.07.017.Suche in Google Scholar
96. Wang, A.; Li, H.; Zhang, H.; Pan, H.; Yang, S. Efficient Catalytic Production of Biodiesel with Acid-Base Bifunctional Rod-like Ca-B Oxides by the Sol-Gel Approach. Materials 2019, 12, 83; https://doi.org/10.3390/ma12010083.Suche in Google Scholar PubMed PubMed Central
97. Lee, H. V.; Juan, J. C.; Hin, T.-Y.; Ong, H. C. Environment-friendly Heterogeneous Alkaline-Based Mixed Metal Oxide Catalysts for Biodiesel Production. Energies 2016, 9, 611; https://doi.org/10.3390/en9080611.Suche in Google Scholar
98. Yigezu, Z. D.; Muthukumar, K. Catalytic Cracking of Vegetable Oil with Metal Oxides for Biofuels Production. Energ. Convers. Manage. 2014, 84, 326–333; https://doi.org/10.1016/j.enconman.2014.03.084.Suche in Google Scholar
99. Refaat, A. A. Biodiesel Production Using Solid Metal Oxide Catalysts. Int. J. Environ. Sci. Tech. 2011, 8 (1), 203–221; https://doi.org/10.1007/bf03326210.Suche in Google Scholar
100. Jose da Silva, M.; Cardoso, A. L. Heterogeneous Tin Catalysts Applied to the Esterification and Transesterification Reactions. J. Catal. 2013, 510509.10.1155/2013/510509Suche in Google Scholar
101. Diamantopoulos, N.; Panagiotaras, D.; Nikolopoulos, D. Comprehensive Review on the Biodiesel Production Using Solid Acid Heterogeneous Catalysts. J. Thermodyn. Catal. 2015, 6, 143.10.4172/2157-7544.1000143Suche in Google Scholar
102. Du, L.; Ding, S.; Li, Z.; Lv, E.; Lu, J.; Ding, J. Transesterification of castor Oil to Biodiesel Using NaY Zeolite-Supported La2O3 Catalysts. Energ. Convers. Manage. 2018, 173, 728–734; https://doi.org/10.1016/j.enconman.2018.07.053.Suche in Google Scholar
103. Salamatinia, B. Alkaline Earth Metal Oxide Catalysts for Biodiesel Production from Palm Oil: Elucidation of Process Behaviors and Modeling Using Response Surface Methodology. Iran. J. Chem. Chem. Eng. 2013, 32 (1), 113–126.Suche in Google Scholar
104. Kiss, A. A.; Dimian, A. C.; Rothenberg, G. Biodiesel by Catalytic Reactive Distillation Powered by Metal Oxides. Energ. Fuel. 2008, 22, 598–604; https://doi.org/10.1021/ef700265y.Suche in Google Scholar
105. Singh, D.; Bhoi, R.; Ganesh, A.; Mahajani, S. Synthesis of Biodiesel from Vegetable Oil Using Supported Metal Oxide Catalysts. Energ. Fuel. 2014, 28, 2743–2753; https://doi.org/10.1021/ef500045x.Suche in Google Scholar
106. Newman, S.; Jones, W. Synthesis, Characterization and Applications of Layered Double Hydroxides Containing Organic Guests. New J. Chem. 1998, 22, 105–115; https://doi.org/10.1039/a708319j.Suche in Google Scholar
107. Gomes, J. F. P.; Puna, J. F. B.; Goncalves, L. M.; Bordado, J. C. M. Study on the Use of MgAl Hydrotalcites as Solid Heterogeneous Catalysts for Biodiesel Production. Energy 2011, 36, 6770–6778; https://doi.org/10.1016/j.energy.2011.10.024.Suche in Google Scholar
108. Simonetti, E. A. N.; Thim, G. P.; Cortez, G. G. Ca-Al Hydrotalcites as Catalysts for Methanolysis and Ethanolysis of Soybean Oil. Mod. Res. Catal. 2014, 3, 117–127; https://doi.org/10.4236/mrc.2014.34015.Suche in Google Scholar
109. Baskaran, T.; Christopher, J.; Sakthivel, A. Progress on Layered Hydrotalcite (HT) Materials as Potential Support and Catalytic Materials. RSC Adv. 2015, 5, 98853–98875; https://doi.org/10.1039/c5ra19909c.Suche in Google Scholar
110. Xie, W.; Peng, H.; Chen, L. Calcined Mg-Al Hydrotalcites as Solid Base Catalysts for Methanolysis of Soybean Oil. J Mol. Catal. A: Chem. 2006, 246, 24–32; https://doi.org/10.1016/j.molcata.2005.10.008.Suche in Google Scholar
111. Yagiz, F.; Kazan, D.; Akin, A. N. Biodiesel Production from Waste Oils by Using Lipase Immobilized on Hydrotalcite and Zeolites. Chem. Eng. J. 2007, 134, 262–267; https://doi.org/10.1016/j.cej.2007.03.041.Suche in Google Scholar
112. Obadiah, A.; Kannan, R.; Ramasubbu, A.; Kumar, S. V. Mg-Al Hydrotalcites as Solid Base Catalysts for Biodiesel Production from Pongamia Oil. J Sci. Ind. Res. 2012, 71, 131–137.Suche in Google Scholar
113. Martins, M. I.; Pires, R. F.; Alves, M. J.; Hori, C. E.; Reis, M. H. M.; Cardoso, V. L. Transesterification of Soybean Oil for Biodiesel Production Using Hydrotalcite as Basic Catalyst. Chem. Eng. Trans. 2013, 32, 817–822.Suche in Google Scholar
114. Chelladurai, K.; Rajamanickam, M. Environmentally Benign Neem Biodiesel Synthesis Using Nano-Zn-Mg-Al Hydrotalcite as Solid Base Catalysts. J. Catal. 2014, 326575.10.1155/2014/326575Suche in Google Scholar
115. Dierio, M. D.; Mallardo, S.; Carotenuto, G.; Tesser, R.; Santacesaria, E. Mg/Al Hydrotalcite Catalyst for Biodiesel Production in Continuous Packed Bed Reactors. Catal. Today 2012, 195, 54–58; https://doi.org/10.1016/j.cattod.2012.01.013.Suche in Google Scholar
116. Costarrosa, L.; Leiva-Candia, D. E.; Cubero-Atienza, A. J.; Ruiz, J. J.; Dorado, M. P. Optimization of the Transesterification of Waste Cooking Oil with Mg-Al Hydrotalcite Using Response Surface Methodology. Energies 2018, 11, 302; https://doi.org/10.3390/en11020302.Suche in Google Scholar
117. Faiz, K.; Shuhaib, E.; Unni, N.; Simon, N.; Nagapadma, M.; Gopinath, N. Synthesis of Biodiesel Using Solid Heterogeneous Catalyst Mg-Al Hydrotalcite by Transesterification from Waste Cooking Oil. Asian J Appl. Sci. Technol. 2018, 2 (2), 1013–1020.Suche in Google Scholar
118. Gao, L.; Teng, G.; Lv, J.; Xiao, G. Biodiesel Synthesis Catalyzed by the KF/Ca-Mg-Al Hydrotalcite Base Catalyst. Energ. Fuel. 2010, 24, 646–651; https://doi.org/10.1021/ef900800d.Suche in Google Scholar
119. Gomes, J. F. P.; Puna, J. F. B.; Gonçalves, L. M.; Bordado, J. C. M. Study on the Use of MgAl Hydrotalcites as Solid Heterogeneous Catalysts for Biodiesel Production. Energy 2011, 36, 6770–6778; https://doi.org/10.1016/j.energy.2011.10.024.Suche in Google Scholar
120. Rahul, R.; Satyarthi, J. K.; Srinivas, D. Lanthanum and Zinc Incorporated Hydrotalcites as Solid Base Catalysts for Biodiesel and Biolubricants Production. Indian J. Chem. 2011, 50, 1117–1125.Suche in Google Scholar
121. Ilgen, O.; Incer, I. D.; Yildiz, M.; Alptekin, E.; Boz, N.; Canakci, M.; Akin, A. N. Investigation of Biodiesel Production from Canola Oil Using Mg-Al Hydrotalcite Catalysts. Turk. J. Chem. 2007, 31, 509–514.Suche in Google Scholar
122. Reyero, I.; Arzamendi, G.; Gandia, L. M. Hydrotalcites as Catalysts and Catalysts Precursors for the Synthesis of Biodiesel. Key Eng. Mater. 2013, 571, 1–26; https://doi.org/10.4028/www.scientific.net/kem.571.1.Suche in Google Scholar
123. Teng, G.; Gao, L.; Xiao, G.; Liu, H.; Lv, J. Biodiesel Preparation from Jatropha Curcas Oil Catalyzed. Appl. Biochem. Biotechnol. 2010, 162, 1725–1736; https://doi.org/10.1007/s12010-010-8953-9.Suche in Google Scholar PubMed
124. Wang, Y.-T.; Fang, Z.; Zhang, F.; Xue, B. J. One-step Production of Biodiesel from Oils with High Acid Value by Activated Mg-Al Hydrotalcite Nanoparticles. Bioresour. Technol. 2015, 93, 84–89; https://doi.org/10.1016/j.biortech.2015.06.059.Suche in Google Scholar PubMed
125. (a)Bhayana, S.; Nandal, R.; Khatri, S. Metal Organic Frameworks as Energy Storage Material: Their Contributions and Challenges. In Materials for Boosting Energy Storage. Vol. 1: Advances in Sustainable Energy Technologies; Kumar, S. S.; Sharma, P.; Kumar, T.; Kumar, V. Eds.; ACS Publication, USA, Vol. 1477, Ch. 6, 2024; pp 125–144, https://doi.org/10.1021/bk-2024-1477.ch006.(b)Sandhu, Z. A.; Raza, M. A.; Awwad, N. S.; Ibrahium, H. A.; Farwa, U.; Ashraf, S.; Dildar, A.; Fatima, E.; Ashraf, S.; Ali, F. Metal–organic Frameworks for Next-Generation Energy Storage Devices; a Systematic Review. Mater. Adv. 2024, 5, 30–50; https://doi.org/10.1039/d3ma00822c.(c)Pettinari, C.; Tombesi, A. MOFs for Electrochemical Energy Conversion and Storage. Inorganics 2023, 11, 65; https://doi.org/10.3390/inorganics11020065.(d)Silva, P.; Vilela, S. M. F.; Tome, J. P. C.; Almeida, P. F. A. Multifunctional Metal-Organic Frameworks: from Academia to Industrial Applications. Chem. Soc. Rev. 2015, 44, 6774–6803; https://doi.org/10.1039/c5cs00307e.Suche in Google Scholar
126. (a)Nguyen, V. G.; Sharma, P.; Dzida, M.; Hung, B. V.; Le, H. S.; El-Shafay, A. S.; Le, H. C.; Le, D. T. N.; Tran, V. D. A Review on Metal–Organic Framework as a Promising Catalyst for Biodiesel Production. Energy Fuel. 2024, 38 (4); https://doi.org/10.1021/acs.energyfuels.3c04203.(b)Taddeo, F.; Vitiello, R.; Russo, V.; Tesser, R.; Turco, R.; Di Serio, M. Biodiesel Production from Waste Oil Catalysed by Metal-Organic Framework (MOF-5): Insights on Activity and Mechanism. Catalysts 2023, 13, 503; https://doi.org/10.3390/catal13030503.(c)Basumatary, S. F.; Patir, K.; Das, B.; Saikia, P.; Brahma, S.; Basumatary, B.; Nath, B.; Basumatary, B.; Basumatary, S. Production of Renewable Biodiesel Using Metal Organic Frameworks Based Materials as Efficient Heterogeneous Catalysts. J. Clean. Prod. 2022, 358; https://doi.org/10.1016/j.jclepro.2022.131955.(d)Cong, W.-J.; Nanda, S.; Li, H.; Fang, Z.; Dalai, A. K.; Kozinskid, J. A. Metal–organic Framework-Based Functional Catalytic Materials for Biodiesel Production: a Review. Green Chem. 2021, 23, 2595–2618; https://doi.org/10.1039/d1gc00233c.(e)Zhang, Q.; Wang, J.; Zhang, S.; Ma, J.; Cheng, J.; Zhang, Y. Zr-Based Metal-Organic Frameworks for Green Biodiesel Synthesis: A Minireview. Bioeng (Basel) 2022, 9 (11), 700; https://doi.org/10.3390/bioengineering9110700.(f)Gouda, S. P.; Dhakshinamoorthy, A.; Rokhum, S. L. Metal-organic Framework as a Heterogeneous Catalyst for Biodiesel Production: A Review. Chem. Eng. J. Adv. 2022, 12; https://doi.org/10.1016/j.ceja.2022.100415.Suche in Google Scholar
127. Ghorbani-Choghamarani, A.; Taherinia, Z.; Tyula, Y. A. Efficient Biodiesel Production from Oleic and Palmitic Acid Using a Novel Molybdenum Metal–Organic Framework as Efficient and Reusable Catalyst. Sci. Rep. 2022, 12, 10338; https://doi.org/10.1038/s41598-022-14341-4.Suche in Google Scholar PubMed PubMed Central
128. Jafari, A.; Ghorbani-Choghamarani, A.; Aghavandi, A. Simple Synthesis of Novel Cr-EDTA-MOF: A Green, Reusable, and Versatile Catalyst for the Production of Biodiesel Fuel from Oleic Acid and Palmitic Acid. ACS Omega 2024, 9 (5), 5255–5264; https://doi.org/10.1021/acsomega.3c05294.Suche in Google Scholar PubMed PubMed Central
129. Berghuis, N. T.; Jaby, F. H.; Muttaqin, M. Potential of Copper-Based MOFs as Heterogeneous Catalysts in Biodiesel Production from Waste Cooking Oil (WCO). Resilience, EAI, 2024; https://doi.org/10.4108/eai.24-11-2023.2346497.Suche in Google Scholar
130. Lubitz, W.; Tumas, W. Hydrogen: An Overview. Chem. Rev. 2007, 107, 3900–3903; https://doi.org/10.1021/cr050200z.Suche in Google Scholar PubMed
131. Seh, Z. W.; Kibsgaard, J.; Dickens, C. F.; Chorkendorff, I.; Norskov, J. K.; Jaramillo, T. F. Combining Theory and Experiment in Electrocatalysis: Insights into Materials Design Sci. 2017, 355, https://doi.org/10.1126/science.aad4998.Suche in Google Scholar PubMed
132. Santo, V. D.; Gallo, A.; Naldoni, A.; Guidotti, M.; Psaro, R. Bimetallic Heterogeneous Catalysts for Hydrogen Production. Catal. Today 2012, 197, 190–205.10.1016/j.cattod.2012.07.037Suche in Google Scholar
133. Tentu, R. D.; Basu, S. Photocatalytic Water Splitting for Hydrogen Production. Curr. Opin. Electrochem. 2017, 5 (1), 56–62; https://doi.org/10.1016/j.coelec.2017.10.019.Suche in Google Scholar
134. Sultana, U. K.; Riches, J. D.; O’Mullane, A. P. Gold Doping in a Layered Co-ni Hydroxide System via Galvanic Replacement for Overall Electrochemical Water Splitting. Adv. Funct. Mater. 2018, 1804361; https://doi.org/10.1002/adfm.201804361.Suche in Google Scholar
135. Kamaroddin, M. F. A.; Sabli, N.; Abdullah, T. A. T. Hydrogen Production by Membrane Water Splitting Technologies. In Advances in hydrogen generation technologies; Eyvaz, M., Ed. IntechOpen: London, UK, 2018, Ch. 2.10.5772/intechopen.76727Suche in Google Scholar
136. Rajeshwar, K. Hydrogen Generation at Irradiated Oxide Semiconductor - Solution Interfaces. J. Appl. Electrochem. 2007, 37 (7), 765–787; https://doi.org/10.1007/s10800-007-9333-1.Suche in Google Scholar
137. Gallo, A.; Marelli, M.; Psaro, R.; Gombac, V.; Montini, T.; Fornasiero, P.; Pievo, R.; Santo, V. D. Bimetallic Au-Pt/TiO2 Photocatalysts Active under UV-A and Simulated Sunlight for H2 Production from Ethanol. Green Chem. 2012, 14, 330–333; https://doi.org/10.1039/c2gc16112e.Suche in Google Scholar
138. Lin, S.-Y.; Suzuki, Y.; Hatano, H.; Harada, M. Hydrogen Production from Hydrocarbon by Integration of Water-Carbon Reaction and Carbon Dioxide Removal (HyPr-RING Method). Energ. Fuel. 2001, 15 (2), 339–343; https://doi.org/10.1021/ef000089u.Suche in Google Scholar
139. Abbas, H. F.; Wan Daud, W. M. A. Hydrogen Production by Methane Decomposition: A Review. Int. J Hydrog. Energy 2010, 35 (3), 1160–1190; https://doi.org/10.1016/j.ijhydene.2009.11.036.Suche in Google Scholar
140. Rossetti, I. Hydrogen Production by Photoreforming of Renewable Substrates. ISRN Chem. Eng. 2012, 1–21; https://doi.org/10.5402/2012/964936.Suche in Google Scholar
141. Caravaca, A.; Jones, W.; Hardacre, C.; Bowker, M. H2 Production by the Photocatalytic Reforming of Cellulose and Raw Biomass Using Ni, Pd, Pt and Au on Titania. Proc. Math. Phys. Eng. Sci. 2016, 472, 2191; https://doi.org/10.1098/rspa.2016.0054.Suche in Google Scholar PubMed PubMed Central
142. Cai, X.; Wang, C.; Chen, Y.; Cheng, Z.; Shu, R.; Zhang, J.; Bu, E.; Liao, M.; Song, Q. A Novel Approach for Enhancing Hydrogen Production from Bio-Glycerol Photoreforming by Improving Colloidal Dispersion Stability. Sci. Total Environ. 2018, 627, 1464–1472; https://doi.org/10.1016/j.scitotenv.2018.02.009.Suche in Google Scholar PubMed
143. Zhang, L.; Chen, R.; Luo, J.; Miao, J.; Gao, J.; Liu, B. Sustainable Hydrogen and Chemical Production via Photo-Electrochemical Reforming of Biomass-Derived Alcohols. Nano Res. 2016, 9 (11), 3388–3393; https://doi.org/10.1007/s12274-016-1216-5.Suche in Google Scholar
144. Kondarides, D. I.; Daskalaki, V. M.; Patsoura, A.; Verykios, X. E. Hydrogen Production by Photo-Induced Reforming of Biomass Components and Derivatives at Ambient Conditions. Catal. Lett. 2008, 122 (1–2), 26–32; https://doi.org/10.1007/s10562-007-9330-3.Suche in Google Scholar
145. Chen, G.; Xu, N.; Li, X.; Liu, Q.; Yang, H.; Li, W. Hydrogen Production by Aqueous-phase Reforming of Ethylene Glycol over a Ni/Zn/Al Derived Hydrotalcite Catalyst. RSC Adv. 2015, 5, 60128–60134; https://doi.org/10.1039/c5ra07184d.Suche in Google Scholar
146. Meryemoglu, B.; Hasanoglu, A.; Kaya, B.; Irmak, S.; Erbatur, O. Hydrogen Production from Aqueous-phase Reforming of Sorghum Biomass: An Application of the Response Surface Methodology. Renew. Energ. 2014, 62, 535–541; https://doi.org/10.1016/j.renene.2013.08.018.Suche in Google Scholar
147. Rahman, M. M. H2 Production from Aqueous-phase Reforming of Glycerol over Cu-Ni Bimetallic Catalysts Supported on Carbon Nanotubes. Int. J Hydrog. Energy 2015, 40 (43), 14833–14844; https://doi.org/10.1016/j.ijhydene.2015.09.015.Suche in Google Scholar
148. Vaidya, P. D.; Lopez-Sanchez, J. A. Review of Hydrogen Production by Catalytic Aqueous-phase Reforming. Chem. Select. 2017, 2 (22), 6563–6567; https://doi.org/10.1002/slct.201700905.Suche in Google Scholar
149. Chen, G.-Y.; Li, W.-Q.; Chen, H.; Yan, B.-B. Progress in the Aqueous-phase Reforming of Different Biomass-Derived Alcohols for Hydrogen Production. J Zhejiang Uni.-Sci. A. 2015, 16 (6), 491–506; https://doi.org/10.1631/jzus.a1500023.Suche in Google Scholar
150. Cargnello, M.; Gasparotto, A.; Gombac, V.; Montini, T.; Barreca, D.; Fornasiero, P. Photocatalytic H2 and Added‐value By‐products – the Role of Metal Oxide Systems in Their Synthesis from Oxygenates. Eur. J. Inorg. Chem. 2011, 28, 4309–4323; https://doi.org/10.1002/ejic.201100532.Suche in Google Scholar
151. Vita, A.; Pino, L.; Italiano, C.; Palella, A. Steam Reforming, Partial Oxidation, and Autothermal Reforming of Ethanol for Hydrogen Production in Conventional Reactors. In Ethanol: Science and Engineering; Basile, A.; Iulianelli, A.; Dalena, F.; Veziroglu, T. N., Eds. Elsevier: Amsterdam, Netherlands, 2019; pp 159–191, Ch. 6.10.1016/B978-0-12-811458-2.00006-7Suche in Google Scholar
152. Patcharavorachot, Y.; Wasuleewan, M.; Assabumrungrat, S.; Arpornwichanop, A. Analysis of Hydrogen Production from Methane Autothermal Reformer with a Dual Catalyst-Bed Configuration. Theor. Found. Chem. Eng. 2012, 46 (6), 658–665; https://doi.org/10.1134/s0040579512060188.Suche in Google Scholar
153. Geissler, K.; Newson, E.; Vogel, F.; Truong, T.-B.; Hottinger, P.; Wokaun, A. Autothermal Methanol Reforming for Hydrogen Production in Fuel Cell Applications. Phys. Chem. Chem. Phys. 2001, 3, 289–293; https://doi.org/10.1039/b004881j.Suche in Google Scholar
154. Niu, H.; Wang, Q.; Huang, C.; Zhang, M.; Yan, Y.; Liu, T.; Zhou, W. Noble Metal-Based Heterogeneous Catalysts for Electrochemical Hydrogen Evolution Reaction. Appl. Sci. 2023, 13, 2177; https://doi.org/10.3390/app13042177.Suche in Google Scholar
155. Donald, I. Development of lanthanum-based heterogeneous catalysts for sustainable hydrogen production. Thesis, Loughborough University, Loughborough, U.K, 2023; https://doi.org/10.26174/thesis.lboro.24101373.v1.Suche in Google Scholar
156. Nagaoka, K.; Eboshi, K.; Takeishi, Y.; Tasaki, R.; Honda, K.; Imamura, K.; Sato, K. Carbon-free H2 Production from Ammonia Triggered at Room Temperature with an Acidic RuO2/γ-La2O3 Catalyst. Sci. Adv. 2017, 3; https://doi.org/10.1126/sciadv.1602747.Suche in Google Scholar PubMed PubMed Central
157. Li, Y.; Gao, X.; Iv, X.; Duan, Y.; Sui, D.; Chang, W.; Yang, Y. Recent Progress on Cobalt-Based Heterogeneous Catalysts for Hydrogen Production from Ammonia Borane. ChemCatChem 2024, e202401271; https://doi.org/10.1002/cctc.202401271.Suche in Google Scholar
158. Brack, P.; Dann, S. E.; Wijayantha, K. G. U. Heterogeneous and Homogenous Catalysts for Hydrogen Generation by Hydrolysis of Aqueous Sodium Borohydride (NaBH4) Solutions. Energy Sci. Eng. 2015, 3 (3), 174–188; https://doi.org/10.1002/ese3.67.Suche in Google Scholar
159. Srifa, A.; Okura, K.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. Hydrogen Production by Ammonia Decomposition over Cs-Modified Co3Mo3N Catalysts. Appl. Catal. B Environ. 2017, 218, 1–8; https://doi.org/10.1016/j.apcatb.2017.06.034.Suche in Google Scholar
160. Yan, B.; Wu, J.; Xie, C.; He, F.; Wei, C. Supercritical Water Gasification with Ni/ZrO2 Catalyst for Hydrogen Production from Model Wastewater of Polyethylene Glycol. J. Supercrit. Fluids 2009, 50, 155–161; https://doi.org/10.1016/j.supflu.2009.04.015.Suche in Google Scholar
161. Yuranov, I.; Autissier, N.; Sordakis, K.; Dalebrook, A.; Grasemann, M.; Orava, V.; Cendula, P.; Gubler, L.; Laurenczy, G. Heterogeneous Catalytic Reactor for Hydrogen Production from Formic Acid and its Use in Polymer Electrolyte Fuel Cells. ACS Sus. Chem. Eng. 2018, 6 (5), 6635–6643; https://doi.org/10.1021/acssuschemeng.8b00423.Suche in Google Scholar
162. Navlani-Garcia, M.; Mori, K.; Salinas-Torres, D.; Kuwahara, Y.; Yamashita, H. New Approaches toward the Hydrogen Production from Formic Acid Dehydrogenation over Pd-Based Heterogeneous Catalysts. Front. Mater. 2019, 6, 44; https://doi.org/10.3389/fmats.2019.00044.Suche in Google Scholar
163. Navlani-Garcia, M.; Mori, K.; Kuwahara, Y.; Yamashita, H. Recent Strategies Targeting Efficient Hydrogen Production from Chemical Hydrogen Storage Materials over Carbon-Supported Catalysts. NPG Asia Mater. 2018, 10, 277–292; https://doi.org/10.1038/s41427-018-0025-6.Suche in Google Scholar
164. Yamaguchi, A.; Hiyoshi, N.; Sato, O.; Bando, K. K.; Shirai, M. Gaseous Fuel Production from Nonrecyclable Paper Wastes by Using Supported Metal Catalysts in High Temperature Liquid Water. ChemSusChem 2010, 3, 737–741; https://doi.org/10.1002/cssc.201000082.Suche in Google Scholar PubMed
165. Sikander, U.; Sufian, S.; Salam, M. A. A Review of Hydrotalcite Based Catalysts for Hydrogen Production Systems. Int. J Hydrog. Energy 2017, 42, 19851–19868.10.1016/j.ijhydene.2017.06.089Suche in Google Scholar
166. Daza, C. E.; Gallego, J.; Mondragon, F.; Moreno, S.; Molina, R. High Stability of Ce-Promoted Ni/Mg-Al Catalysts Derived from Hydrotalcites in Dry Reforming of Methane. Fuel 2010, 89 (3), 592–603; https://doi.org/10.1016/j.fuel.2009.10.010.Suche in Google Scholar
167. Khan, A. I.; O’Hare, D. Intercalation Chemistry of Layered Double Hydroxides: Recent Developments and Applications. J Mater. Chem. 2002, 12 (11), 3191–3198; https://doi.org/10.1039/b204076j.Suche in Google Scholar
168. Ota, A.; Kunkes, E. L.; Kasatkin, I.; Groppo, E.; Ferri, D.; Poceiro, B.; Navarro Yerga, R. M.; Behrens, M. Comparative Study of Hydrotalcite-Derived Supported Pd2Ga and PdZn Intermetallic Nanoparticles as Methanol Synthesis and Methanol Steam Reforming Catalysts. J. Catal. 2012, 293 (0), 27–38; https://doi.org/10.1016/j.jcat.2012.05.020.Suche in Google Scholar
169. Lenarda, M.; Storaro, L.; Frattini, R.; Casagrande, M.; Marchiori, M.; Capannelli, G.; Uliana, C.; Ferrari, F.; Ganzerla, R. Oxidative Methanol Steam Reforming (OSRM) on a PdZnAl Hydrotalcite Derived Catalyst. Catal. Commun. 2007, 8 (3), 467–470; https://doi.org/10.1016/j.catcom.2006.07.023.Suche in Google Scholar
170. Contreras, J.; Tapia, C.; Fuentes, G.; Nuno, L.; Quintana, B.; Salmones, J.; Zeifert, B.; Córdova, I. Equilibrium Composition of Ethanol Steam Reforming Reaction to Produce H2 Applied to Ni, Co and Pt/hydrotalcites-WOx Catalysts. Int. J Hydrog. Energy 2014, 39 (29), 16608–16618; https://doi.org/10.1016/j.ijhydene.2014.04.080.Suche in Google Scholar
171. Li, D.; Shishido, T.; Oumi, Y.; Sano, T.; Takehira, K. Self-activation and Self-Regenerative Activity of Trace Rh-Doped Ni/Mg(Al)O Catalysts in Steam Reforming of Methane. Appl. Catal. Gen. 2007, 332 (1), 98–109; https://doi.org/10.1016/j.apcata.2007.08.008.Suche in Google Scholar
172. Sikander, U.; Samsudin, M. F.; Sufian, S.; KuShari, K.; Kait, C. F.; Naqvi, S. R.; Chen, W.-S. Tailored Hydrotalcite-Based Mg-Ni-Al Catalyst for Hydrogen Production via Methane Decomposition: Effect of Nickel Concentration and Spinel-like Structures. Int. J Hydrog. Energy 2018, 42, 19851–19868.Suche in Google Scholar
173. Dewoolkar, K. D.; Vaidya, P. D. Improved Hydrogen Production by Sorption-Enhanced Steam Methane Reforming over Hydrotalcite- and Calcium-Based Hybrid Materials. Energ. Fuel. 2015, 29, 3870–3878; https://doi.org/10.1021/acs.energyfuels.5b00584.Suche in Google Scholar
174. Abello, S.; Bolshak, E.; Montane, D. Ni-Fe Catalysts Derived from Hydrotalcite-like Precursors for Hydrogen Production by Ethanol Steam Reforming. Appl. Catal. A: Gen. 2013, 450, 261–274; https://doi.org/10.1016/j.apcata.2012.10.035.Suche in Google Scholar
175. He, L.; Huang, Y.; Wang, A.; Wang, X.; Chen, X.; Jose, J.; Delgado, Z. T. A Noble-metal-free Catalyst Derived from Ni-Al Hydrotalcite for Hydrogen Generation from N2H4·H2O Decomposition. Angew. Chem. Int. Ed. 2012, 51, 1–5; https://doi.org/10.1002/anie.201201737.Suche in Google Scholar PubMed
176. Homsi, D.; Aouad, S.; Gennequin, C.; Aboukais, A.; Abi-Aad, E. Hydrogen Production by Methane Steam Reforming over Ru and Cu Supported on Hydrotalcite Precursors. Adv. Mater. Res. 2011, 324, 453–456; https://doi.org/10.4028/www.scientific.net/amr.324.453.Suche in Google Scholar
177. Homsi, D.; Rached, J. A.; Aouad, S.; Gennequin, C.; Dahdah, E.; Estephane, J.; Tidahy, H. L.; Aboukais, A.; Abi-Aad, E. Steam Reforming of Ethanol for Hydrogen Production over Cu/Co-Mg-Al-Based Catalysts Prepared by Hydrotalcite Route. Environ. Sci. Pollut. Res. 2016, 24 (11), 9907–9913; https://doi.org/10.1007/s11356-016-7480-9.Suche in Google Scholar PubMed
178. Tuza, P. V.; Manfro, R. L.; Ribeiro, N. F. P.; Souza, M. M. V. M. Production of Renewable Hydrogen by Aqueous-phase Reforming of Glycerol over Ni-Cu Catalysts Derived from Hydrotalcite Precursors. Renew. Energ. 2013, 50, 408–414; https://doi.org/10.1016/j.renene.2012.07.006.Suche in Google Scholar
179. (a)Liang, X.; Wang, C.; Lei, Y.; Liu, Y.; Zhao, B.; Liu, F. Potential Applications of Metal Organic Framework-Based Materials for Proton Exchange Membrane Fuel Cells. Progr. Chem 2018, 30 (11), 1770–1783.(b)Si, G. R.; Yang, F.; He, T.; Kong, X. J.; Wu, W.; Li, T. C.; Wang, K.; Li, J. R. Enhancing Proton Conductivity in Zr-MOFs through Tuning Metal Cluster Connectivity. J. Mater. Chem. A 2022, 10, 1236–1240; https://doi.org/10.1039/d1ta09348g.(c)Feng, L.; Hou, H.-B.; Zhou, H. UiO-66 Derivatives and Their Composite Membranes for Effective Proton Conduction. Dalton Trans. 2020, 49, 17130–17139; https://doi.org/10.1039/d0dt03051a.(d)Choi, I.; Lim, J.; dos Reis, R.; Kim, E.; Lim, S. Y.; Dravid, V. P.; Kim, H.; Oh, K.-H.; Nam, K. W. Metal–organic Framework for High-Performance Catalyst Layers in Proton-Exchange Membrane Fuel Cells. J. Mater. Chem. A 2023, 11, 20583–20591; https://doi.org/10.1039/d3ta04377k.(e)Parangi, T. F.; Chudasama, U. V. Synthesis, Characterization, and Proton Conduction Behavior of Thorium and Cerium Phosphonates. ACS Omega 2019, 4 (2), 3716–3725; https://doi.org/10.1021/acsomega.8b03468.Suche in Google Scholar
180. (a)Jiang, Y.; Zhao, H.; Yue, L.; Liang, J.; Li, T.; Liu, Q.; Luo, Q.; Kong, X.; Lu, S.; Shi, X.; Zhou, K.; Sun, X. Recent Advances in Lithium-Based Batteries Using Metal Organic Frameworks as Electrode Materials. Electrochem. Commun. 2021, 122; https://doi.org/10.1016/j.elecom.2020.106881.(b)Zhao, R.; Liang, Z.; Zou, R.; Xu, Q. Metal-organic Frameworks for Batteries. Joule 2018, 2 (11), 2235–2259; https://doi.org/10.1016/j.joule.2018.09.019.(c)Nam, K. W.; Park, S. S.; dos Reis, R.; Vinayak, P.; Kim, H.; Mirkin, C. A.; Stoddart, J. F. Conductive 2D Metal-Organic Framework for High-Performance Cathodes in Aqueous Rechargeable Zinc Batteries. Nat. Commun. 2019, 10, 4948; https://doi.org/10.1038/s41467-019-12857-4.Dravid(d)Mehek, R.; Iqbal, N.; Noor, T.; Amjad, Z. B.; Ali, G.; Vignaroobanc, K.; Khan, M. A. Metal-organic Framework Based Electrode Materials for Lithium-Ion Batteries: A Review. RSC Adv. 2021, 11, 29247–29266; https://doi.org/10.1039/d1ra05073g.(e)Yin, C.; Xu, F.; Pan, Y.; Pan, C. Metal-organic Framework as Anode Materials for Lithium-Ion Batteries with High Capacity and Rate Performance. ACS Appl. Energy Mater. 2020, 3 (11), 10776–10786; https://doi.org/10.1021/acsaem.0c01822.(f)Tran, A.; Do, H. H.; Ha, D. C.; Ahn, S.; Kim, M.-G.; Kim, S. Y.; Lee, S.-W. Metal-organic Framework for Lithium and Sodium-Ion Batteries: Progress and Perspective. Fuel 2022, 319; https://doi.org/10.1016/j.fuel.2022.123856.Suche in Google Scholar
181. Muntean, M.; Guizzardi, D.; Schaaf, E.; Crippa, M.; Solazzo, E.; Olivier, J. G. J.; Vignati, E. Fossil CO2 emissions of all world countries – Report, EUR 29433 EN; Publications Office of the European Union: Luxembourg, 2018.Suche in Google Scholar
182. Perez-Fortes, M.; Tzimas, E. European Commission. In Carbon Capture Utilisation and Storage; SETIS, Luxemburg, U.K., 2016.Suche in Google Scholar
183. Juan, G.-S.; Raul, P.-H.; Desiree, D.-M. Inspirational Perspectives and Principles on the Use of Catalysts to Create Sustainability. Catal. Today 2022, 387, 237–243; https://doi.org/10.1016/j.cattod.2021.11.021.Suche in Google Scholar
184. Bahruji, H.; Bowker, M.; Hutchings, G.; Dimitratos, N.; Wells, P.; Gibson, E.; Jones, W.; Brookes, C.; Morgan, D.; Lalev, G. Pd/ZnO Catalysts for Direct CO2 Hydrogenation to Methanol. J. Catal. 2016, 343, 133–146; https://doi.org/10.1016/j.jcat.2016.03.017.Suche in Google Scholar
185. Pastor-Perez, L.; Baibars, F.; Sache, E. L.; Arellano-Garcia, H.; Gu, S.; Reina, T. R. CO2 Valorisation via Reverse Water-Gas Shift Reaction Using Advanced Cs Doped Fe-Doped Cu/Al2O3 Catalysts. J. CO2 Util. 2017, 21, 423–428.10.1016/j.jcou.2017.08.009Suche in Google Scholar
186. Stroud, T.; Smith, T.; Sache, S. L.; Santos, J. L.; Centeno, M. A.; Arellano-Garcia, H.; Odriozola, J. A.; Reina, T. R. Chemical CO2 Recycling via Dry and Bi Reforming of Methane Using Ni-Sn/Al2O3 and Ni-Sn/CeO2-Al2O3 Catalysts. Appl. Catal. B Environ. 2018, 225, 125–137.10.1016/j.apcatb.2017.10.047Suche in Google Scholar
187. Mutz, B.; Belimov, M.; Wang, W.; Sprenger, P.; Serrer, M.-A.; Wang, D.; Pfeifer, P.; Kleist, W.; Grunwaldt, J.-D. Potential of an Alumina-Supported Ni3Fe Catalyst in the Methanation of CO2: Impact of Alloy Formation on Activity and Stability. ACS Catal. 2017, 7, 6802–6814; https://doi.org/10.1021/acscatal.7b01896.Suche in Google Scholar
188. Li, W.; Nie, X.; Jiang, X.; Zhang, A.; Ding, F.; Liu, M.; Liu, Z.; Guo, X.; Song, C. ZrO2 Support Imparts Superior Activity and Stability of Co Catalysts for CO2 Methanation. Appl. Catal. B Environ. 2018, 220, 397–408; https://doi.org/10.1016/j.apcatb.2017.08.048.Suche in Google Scholar
189. Veselovskaya, J. V.; Parunin, P. D.; Okunev, A. G. Catalytic Process for Methane Production from Atmospheric Carbon Dioxide Utilizing Renewable Energy. Catal. Today 2017, 298, 117–123; https://doi.org/10.1016/j.cattod.2017.05.044.Suche in Google Scholar
190. Wu, P.; Yang, B. Significance of Surface Formate Coverage on the Reaction Kinetics of Methanol Synthesis from CO2 Hydrogenation over Cu. ACS Catal. 2017, 7 (10), 7187–7195; https://doi.org/10.1021/acscatal.7b01910.Suche in Google Scholar
191. Wu, C.; Zhang, P.; Zhang, Z.; Zhang, L.; Yang, G.; Han, B. Efficient Hydrogenation of CO to Methanol over Supported Subnanometric Gold Catalysts at Low Temperature. ChemCatChem 2017, 9 (19), 3691–3696; https://doi.org/10.1002/cctc.201700872.Suche in Google Scholar
192. Garcıa-Trenco, A.; Regoutz, A.; White, E. R.; Payne, D. J.; Shaffer, M. S. P.; Williams, C. K. PdIn Intermetallic Nanoparticles for the Hydrogenation of CO2 to Methanol. Appl. Catal. B Environ. 2018, 220, 9–18; https://doi.org/10.1016/j.apcatb.2017.07.069.Suche in Google Scholar
193. Meher, S. K.; Rao, G. R. Nanostructured CeO2 as Efficient Catalyst for Energy and Environmental Applications. J Chem. Sci. 2014, 126 (2), 361–372; https://doi.org/10.1007/s12039-014-0570-7.Suche in Google Scholar
194. Arena, F. 3Multipurpose Composite MnCeOx Catalysts for Environmental Applications. Cat. Sci. Technol. 2014, 4 (7), 1890–1898; https://doi.org/10.1039/c4cy00022f.Suche in Google Scholar
195. Jadhav, S. G.; Vaidya, P. D.; Bhanage, B. M.; Joshi, J. B. Catalytic Carbon Dioxide Hydrogenation to Methanol: A Review of Recent Studies. Chem. Eng. Res. Design. 2014, 92, 2557–2567; https://doi.org/10.1016/j.cherd.2014.03.005.Suche in Google Scholar
196. Liang, X. L.; Dong, X.; Lin, G. D.; Zhang, H. B. Carbon Nanotube-Supported Pd-ZnO Catalyst for Hydrogenation of CO2 to Methanol. Appl. Catal. B Environ. 2009, 88, 315–322; https://doi.org/10.1016/j.apcatb.2008.11.018.Suche in Google Scholar
197. Bonivardi, A. L.; Chiavassa, D. L.; Querini, C. A.; Baltanas, M. A. Enhancement of the Catalytic Performance to Methanol Synthesis from CO2/H2 by Gallium Addition to Palladium/silica Catalysts. Stud. Surf. Sci. Catal. 2000, 130, 3747–3752; https://doi.org/10.1016/s0167-2991(00)80606-0.Suche in Google Scholar
198. Porosoff, M. D.; Yang, X.; Boscoboinik, J. A.; Chen, J. G. Molybdenum Carbide as Alternative Catalysts to Precious Metals for Highly Selective Reduction of CO2 to CO. Angew. Chem. Int. Ed. 2014, 53 (26), 6705–6709; https://doi.org/10.1002/anie.201404109.Suche in Google Scholar PubMed
199. De©7, S.; Zhang, J.; Luque, R.; Yan, N. Ni-based Bimetallic Heterogeneous Catalysts for Energy and Environmental Applications. Energ. Environ. Sci. 2016, 9, 3314–3347; https://doi.org/10.1039/c6ee02002j.Suche in Google Scholar
200. Labhasetwar, N.; Saravanan, G.; Megarajan, S. K.; Manwar, N.; Khobragade, P. D.; Grasset, F.; Grasset, F. Perovskite-type Catalytic Materials for Environmental Applications. Sci. Technol. Adv. Mater. 2015, 16; https://doi.org/10.1088/1468-6996/16/3/036002.Suche in Google Scholar PubMed PubMed Central
201. Wang, S. Application of Solid Ash Based Catalysts in Heterogeneous Catalysis. Environ. Sci. Technol. 2008, 42, 7055–7063; https://doi.org/10.1021/es801312m.Suche in Google Scholar PubMed
202. Wang, N.; Zhao, Q.; Zhang, A. Catalytic Oxidation of Organic Pollutants in Wastewater via a Fenton-like Process under the Catalysis of HNO3-Modified Coal Fly Ash. RSC Adv. 2017, 7, 27619–27628; https://doi.org/10.1039/c7ra04451h.Suche in Google Scholar
203. (a)Stanley, P. M.; Ramm, V.; Fischer, R. A.; Warnan, J. Analysis of Metal–Organic Framework-Based Photosynthetic CO2 Reduction. Nat. Synth. 2024, 3, 307–318; https://doi.org/10.1038/s44160-024-00490-z.(b)Sumida, K.; Rogow, D. L.; Mason, J. A.; McDonald, T. M.; Bloch, E. D.; Herm, Z. R.; Bae, T.-H.; Long, J. R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112 (2), 724–781; https://doi.org/10.1021/cr2003272.(c)Trickett, C.; Helal, A.; Al-Maythalony, B.; Yamani, Z. H.; Cordova, K. E.; Yaghi, O. M. The Chemistry of Metal–Organic Frameworks for CO2 Capture, Regeneration and Conversion. Nat. Rev. Mater. 2017, 2, 17045; https://doi.org/10.1038/natrevmats.2017.45.(d)Mahajan, S.; Lahtinen, M. Recent Progress in Metal-Organic Frameworks (MOFs) for CO2 Capture at Different Pressures. J. Environ. Chem. Eng. 2022, 10 (6); https://doi.org/10.1016/j.jece.2022.108930.(e)Chen, Y.; Wang, D.; Deng, X.; Li, Z. Metal–organic Frameworks (MOFs) for Photocatalytic CO2 Reduction. Catal. Sci. Technol. 2017, 7, 4893–4904; https://doi.org/10.1039/c7cy01653k.Suche in Google Scholar
204. (a)Mtukula, A. C.; Zhang, X.-D.; Hou, S.-Z.; Huang, J.-M.; Xu, M.; Gu, Z.-Y. Metal-organic Frameworks for Efficient Electrochemical Reduction of Carbon Dioxide. Eur. J. Inorg. Chem. 2024, 26 (21), 202300170.(b)Kornienko, N.; Zhao, Y.; Kley, C. S.; Zhu, C.; Kim, D.; Lin, S.; Chang, C. J.; Yaghi, O. M.; Yang, P. Metal–organic Frameworks for Electrocatalytic Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2015, 137 (44), 14129–14135; https://doi.org/10.1021/jacs.5b08212.(c)Yao, D.; Tang, C.; Vasileff, A.; Zhi, X.; Jiao, Y.; Qiao, S.-Z. The Controllable Reconstruction of Bi-MOFs for Electrochemical CO2 through Electrolyte and Potential Mediation. Angew. Chem. Int. Ed. 2021, 60 (33), 18178–18184; https://doi.org/10.1002/anie.202104747.(d)Zhang, C.; Lin, Z.; Jiao, L.; Jiang, H.-L. Metal–organic Frameworks for Electrocatalytic CO2 Reduction: From Catalytic Site Design to Microenvironment Modulation. Angew. Chem. Int. Ed. 2024, 60 (33), 202414506; https://doi.org/10.1002/anie.202414506.Suche in Google Scholar PubMed
205. Li, X.-X.; Liu, J.; Zhang, L.; Dong, L.-Z.; Xin, Z.-F.; Li, S.-L.; Huang-Gu, X.-Q.; Huang, K.; Lan, Y.-Q. Hydrophobic Polyoxometalate-Based Metal-Organic Framework for Efficient CO2 Photoconversion. ACS Appl. Mater. Interfaces 2019, 11 (29), 25790–25795; https://doi.org/10.1021/acsami.9b03861.Suche in Google Scholar PubMed
206. Lee, Y.; Kim, S.; kang, J. K.; Cohen, S. M. Photocatalytic CO2 Reduction by a Mixed Metal (Zr/Ti), Mixed Ligand Metal-Organic Framework under Visible Light Irradiation. Chem. Commun. 2015, 51 (26), 5735–5738; https://doi.org/10.1039/c5cc00686d.Suche in Google Scholar PubMed
207. Acharya, R.; Naik, B.; Parida, K. Cr(VI) Remediation from Aqueous Environment through Modified-TiO2-Mediated Photocatalytic Reduction. Beilstein J. Nanotechnol. 2018, 9, 1448–1470; https://doi.org/10.3762/bjnano.9.137.Suche in Google Scholar PubMed PubMed Central
208. Mahlambi, M. M.; Ngila, C. J.; Mamba, B. B. Recent Developments in Environmental Photocatalytic Degradation of Organic Pollutants: the Case of Titanium Dioxide Nanoparticles – A Review. J. Nanomater. 2015, 29, 790173.10.1155/2015/790173Suche in Google Scholar
209. Byrne, C.; Subramanian, G.; Pillai, S. C. Recent Advances in Photocatalysis for Environmental Applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555; https://doi.org/10.1016/j.jece.2017.07.080.Suche in Google Scholar
210. Phonsy, P. D.; Anju, S. G.; Jyothi, K. P.; Yesodharan, S.; Yesodharan, E. P. Semiconductor Mediated Photocatalytic Degradation of Plastics and Recalcitrant Organic Pollutants in Water: Effect of Additives and Fate of Insitu Formed H2O2. J. Adv. Oxid. Technol. 2015, 18 (1); https://doi.org/10.1515/jaots-2015-0111.Suche in Google Scholar
211. Khader, E. M.; Muslim, S. A.; Saady, N. M. C.; Ali, N. S.; Salih, I. K.; Mohammed, T. J.; Albayati, T. M.; Zendehboudi, S. Recent Advances in Photocatalytic Advanced Oxidation Processes for Organic Compound Degradation: A Review. Desalination Water Treat. 2024, 318; https://doi.org/10.1016/j.dwt.2024.100384.Suche in Google Scholar
212. (a)Huang, N.; Wang, T.; Wang, W.; Wu, Q.; Li, A.; Hu, H. UV/chlorine as an Advanced Oxidation Process for the Degradation of Benzalkonium Chloride: Synergistic Effect, Transformation Products and Toxicity Evaluation. Water Res. 2017, 114, 246–253; https://doi.org/10.1016/j.watres.2017.02.015.(b)Ferkous, H.; Merouani, S.; Hamdaoui, O.; Petrier, C. Persulfate-enhanced Sonochemical Degradation of Naphthol Blue Black in Water: Evidence of Sulfate Radical Formation. Ultrason. Sonochem. 2017, 34, 580–587; https://doi.org/10.1016/j.ultsonch.2016.06.027.(c)Chadi, N. E.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M.; Ashokkumar, M. H2O2/Periodate (IO4-): A Novel Advanced Oxidation Technology for the Degradation of Refractory Organic Pollutants. Environ. Sci. Water Res. Technol. 2019, 5, 1113–1123; https://doi.org/10.1039/c9ew00147f.Suche in Google Scholar PubMed
213. Duan, X.; Wang, S. Heterogeneous Catalysis for Environmental Remediation. Catalysts 2017, 7, 236; https://doi.org/10.3390/catal7080236.Suche in Google Scholar
214. Wang, Y.; Xie, Y.; Chen, C.; Duan, X.; Sun, H.; Wang, S. Synthesis of Magnetic Carbon Supported Manganese Catalysts for Phenol Oxidation by Activation of Peroxymonosulfate. Catalysts 2017, 7, 3; https://doi.org/10.3390/catal7010003.Suche in Google Scholar
215. Zhu, K.; Jin, C.; Klencsar, Z.; Ganeshraja, A.; Wang, J. Cobalt-iron Oxide, Alloy and Nitride: Synthesis, Characterization and Application in Catalytic Peroxymonosulfate Activation for Orange II Degradation. Catalysts 2017, 7, 138; https://doi.org/10.3390/catal7050138.Suche in Google Scholar
216. Chen, C.; Chen, Y.; Yoza, B.; Du, Y.; Wang, Y.; Li, Q.; Yi, L.; Guo, S.; Wang, Q. Comparison of Efficiencies and Mechanisms of Catalytic Ozonation of Recalcitrant Petroleum Refinery Wastewater by Ce, Mg, and Ce-Mg Oxides Loaded Al2O3. Catalysts 2017, 7, 72; https://doi.org/10.3390/catal7030072.Suche in Google Scholar
217. Wang, Y.; Wen, Z.; Zhang, H.; Cao, G.; Sun, Q.; Cao, J. CuO Nanorods-Decorated Reduced Graphene Oxide Nanocatalysts for Catalytic Oxidation of CO. Catalysts 2016, 6, 214; https://doi.org/10.3390/catal6120214.Suche in Google Scholar
218. Stahl, A.; Wang, Z.; Schwammle, T.; Ke, J.; Li, X. Novel Fe-W-Ce Mixed Oxide for the Selective Catalytic Reduction of NOx with NH3 at Low Temperatures. Catalysts 2017, 7, 71; https://doi.org/10.3390/catal7020071.Suche in Google Scholar
219. Qi, C.; Bao, W.; Wang, L.; Li, H.; Wu, W. Study of the V2O5-WO3/TiO2 Catalyst Synthesized from Waste Catalyst on Selective Catalytic Reduction of NOx by NH3. Catalysts 2017, 7, 110; https://doi.org/10.3390/catal7040110.Suche in Google Scholar
220. Zhang, Y.; Kumar Megarajan, S.; Xu, X.; Lu, J.; Jiang, H. Catalytic Abatement of Nitrous Oxide Coupled with Ethane Oxydehydrogenation over Mesoporous Cr/Al2O3 Catalyst. Catalysts 2017, 7, 137; https://doi.org/10.3390/catal7050137.Suche in Google Scholar
221. Zheng, X.; Zhang, R.; Bai, F.; Hua, C. Catalytic Decomposition of N2O over Cu-Zn/ZnAl Catalysts. Catalysts 2017, 7, 166; https://doi.org/10.3390/catal7050166.Suche in Google Scholar
222. Muhammad, S.; Saputra, E.; Sun, H.; Ang, H. M.; Tade, M. O.; Wang, W. Heterogeneous Catalytic Oxidation of Aqueous Phenol on Red Mud Supported Cobalt Catalysts. Ind. Eng. Chem. Res. 2012, 51, 15351–15359; https://doi.org/10.1021/ie301639t.Suche in Google Scholar
223. Muhammad, S.; Saputra, E.; Sun, H.; Ang, H. M.; Tade, M. O.; Wang, W. Removal of Phenol Using Sulphate Radicals Activated by Natural Zeolite-Supported Cobalt Catalysts. Water Air Soil Pollut. 2013, 224, 1721; https://doi.org/10.1007/s11270-013-1721-z.Suche in Google Scholar
224. Jain, B.; Singh, A. K.; Kim, H.; Lichtfouse, E.; Sharma, V. K. Treatment of Organic Pollutants by Homogeneous and Heterogeneous Fenton Reaction Processes. Environ. Chem. Lett. 2018, 16 (3), 947–967; https://doi.org/10.1007/s10311-018-0738-3.Suche in Google Scholar
225. Saputra, E.; Muhammad, S.; Sun, H.; Wang, S.; Ang, H.-A.; Tade, M. O.; Wang, S. A Comparative Study of Spinel Structured Mn3O4, Co3O4 and Fe3O4 Nanoparticles in Catalytic Oxidation of Phenolic Contaminants in Aqueous Solutions. J Coll. Interface Sci 2013, 407, 467–473; https://doi.org/10.1016/j.jcis.2013.06.061.Suche in Google Scholar PubMed
226. Saputra, E.; Muhammad, S.; Sun, H.; Wang, S. Activated Carbons as Green and Effective Catalysts for Generation of Reactive Radicals in Degradation of Aqueous Phenol. RSC Adv. 2013, 3, 21905–21910; https://doi.org/10.1039/c3ra42455c.Suche in Google Scholar
227. Galeano, L.-A.; Vicente, M. A.; Gil, A. Catalytic Degradation of Organic Pollutants in Aqueous Streams by Mixed Al/M-Pillared Clays (M = Fe, Cu, Mn). Catal. Rev. Sci. Eng. 2014, 56 (3), 239–287; https://doi.org/10.1080/01614940.2014.904182.Suche in Google Scholar
228. Kurian, M.; Nair, D. Manganese Zinc Ferrite Nanoparticles as Efficient Catalysts for Wet Peroxide Oxidation of Organic Aqueous Wastes. J. Chem. Sci. 2015, 127 (3), 537–546; https://doi.org/10.1007/s12039-015-0806-1.Suche in Google Scholar
229. Wang, N.; Zhao, Q.; Zhang, A. Catalytic Oxidation of Organic Pollutants in Wastewater via a Fenton-like Process under the Catalysis of HNO3-Modified Coal Fly Ash. RSC Adv. 2017, 7, 27619–27628; https://doi.org/10.1039/c7ra04451h.Suche in Google Scholar
230. Zhen, M. Cobalt Oxide Catalysts for Environmental Remediation. Curr. Catal. 2014, 3, 15–26; https://doi.org/10.2174/22115447113029990017.Suche in Google Scholar
231. Gangarajula, Y.; Gopal, B. Spinel Cobalt Oxide Catalyzed Controlled Hydration of Aromatic Nitriles. Chem. Lett. 2012, 41, 101–103; https://doi.org/10.1246/cl.2012.101.Suche in Google Scholar
232. Davies, T. E.; García, T.; Solsona, B.; Taylor, S. H. Nanocrystalline Cobalt Oxide: A Catalyst for Selective Alkane Oxidation under Ambient Conditions. Chem. Commun. 2006, 3417–3419; https://doi.org/10.1039/b606973h.Suche in Google Scholar PubMed
233. Tyo, E. C.; Yin, C. R.; Vece, M. D.; Qian, Q.; Kwon, G.; Lee, S.; Lee, B.; DeBartolo, J. E.; Seifert, S.; Winans, R. E.; Si, R.; Ricks, B.; Goergen, S.; Rutter, M.; Zugic, B.; Flytzani-Stephanopoulos, M.; Wang, Z. W.; Palmer, R. E.; Neurock, M.; Vajda, S. Oxidative Dehydrogenation of Cyclohexane on Cobalt Oxide (Co3O4) Nanoparticles: The Effect of Particle Size on Activity and Selectivity. ACS Catal. 2012, 2, 2409–2423; https://doi.org/10.1021/cs300479a.Suche in Google Scholar
234. Mangrulkar, P. A.; Joshi, M. M.; Tijare, S. N.; Polshettiwar, V.; Labhsetwar, N. K.; Rayalu, S. S. Nano Cobalt Oxides for Photocatalytic Hydrogen Production. Int. J. Hydrogen Energ. 2012, 37, 10462–10466; https://doi.org/10.1016/j.ijhydene.2012.01.112.Suche in Google Scholar
235. Kumar, A.; Rana, A.; Sharma, G.; Sharma, S.; Naushad, M.; Mola, G. T.; Dhiman, P.; Stadler, F. J. Aerogels and Metal-Organic Frameworks for Environmental Remediation and Energy Production. Environ. Chem. Lett. 2018, 16 (3), 797–820; https://doi.org/10.1007/s10311-018-0723-x.Suche in Google Scholar
236. Chen, P.; Wang, H.; Liu, H.; Ni, Z.; Li, J.; Zhou, Y.; Dong, F. Directional Electron Delivery and Enhanced Reactants Activation Enable Efficient Photocatalytic Air Purification on Amorphous Carbon Nitride Co-functionalized with O/La. Appl. Catal. B Environ. 2019, 242, 19–30; https://doi.org/10.1016/j.apcatb.2018.09.078.Suche in Google Scholar
237. Dong, X.; Li, J.; Xing, Q.; Zhou, Y.; Huang, H.; Dong, F. The Activation of Reactants and Intermediates Promotes the Selective Photocatalytic NO Conversion on Electron-Localized Sr-Intercalated G-C3n4. Appl. Catal. B Environ. 2018, 232, 69–76; https://doi.org/10.1016/j.apcatb.2018.03.054.Suche in Google Scholar
238. Cui, W.; Li, J.; Sun, Y.; Wang, H.; Jiang, G.; Lee, S. C.; Dong, F. Enhancing ROS Generation and Suppressing Toxic Intermediate Production in Photocatalytic NO Oxidation on O/Ba Co-functionalized Amorphous Carbon Nitride. Appl. Catal. B Environ. 2018, 237, 938–946; https://doi.org/10.1016/j.apcatb.2018.06.071.Suche in Google Scholar
239. Huo, W. C.; Dong, X.; Li, J. Y.; Liu, M.; Liu, X. Y.; Zhang, Y. X.; Dong, F. Synthesis of Bi3WO6 with Gradient Oxygen Vacancies for Highly Photocatalytic NO Oxidation and Mechanism Study. Chem. Eng. J 2019, 361, 129–138; https://doi.org/10.1016/j.cej.2018.12.071.Suche in Google Scholar
240. Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-based Photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391; https://doi.org/10.1039/c7ee03640j.Suche in Google Scholar
241. Lang, D.; Xiang, Q.; Qiu, G.; Feng, X.; Liu, F. Effects of Crystalline Phase and Morphology on the Visible Light Photocatalytic H2-Production Activity of CdS Nanocrystals. Dalton Trans. 2014, 43, 7245–7253; https://doi.org/10.1039/c3dt53601g.Suche in Google Scholar PubMed
242. Zhang, L.; Fu, X.; Meng, S.; Jiang, X.; Wang, J.; Chen, S. Ultra-low Content of Pt Modified CdS Nanorods: One-Pot Synthesis and High Photocatalytic Activity for H2 Production under Visible Light. J. Mater. Chem. A. 2015, 3, 23732–23742; https://doi.org/10.1039/c5ta07459b.Suche in Google Scholar
243. Ma, D.; Shi, J. W.; Zou, Y.; Fan, Z.; Ji, X.; Niu, C. Highly Efficient Photocatalyst Based on a CdS Quantum dots/ZnO Nanosheets 0D/2D Heterojunction for Hydrogen Evolution from Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 25377–25386; https://doi.org/10.1021/acsami.7b08407.Suche in Google Scholar PubMed
244. (a)Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599; https://doi.org/10.1021/ie303468t.(b)Parangi, T.; Mishra, M. K. Titania Nanoparticles as Modified Photocatalysts: A Review on Design and Development. Comments Inorg. Chem. 2019, 39, 90–126; https://doi.org/10.1080/02603594.2019.1592751.Suche in Google Scholar
245. Kim, J. C.; Choi, J.; Lee, Y. B.; Hong, J. H.; Lee, J. I.; Yang, J. W.; Lee, W. I.; Hur, N. H. Enhanced Photocatalytic Activity in Composites of TiO2 Nanotubes and CdS Nanoparticles. Chem. Commun. 2006, 5024–5026; https://doi.org/10.1039/b612572g.Suche in Google Scholar PubMed
246. Cao, A.; Liu, Z.; Chu, S.; Wu, M.; Ye, Z.; Cai, Z.; Chang, Y.; Wang, S.; Gong, Q.; Liu, Y. A Facile One-step Method to Produce Graphene-CdS Quantum Dot Nanocomposites as Promising Optoelectronic Materials. Adv. Mater. 2010, 22 (1), 103–106; https://doi.org/10.1002/adma.200901920.Suche in Google Scholar PubMed
247. Cheng, F.; Xiang, Q. A Solid-State Approach to Fabricate a CdS/CuS Nano-Heterojunction with Promoted Visible-Light Photocatalytic H2-Evolution Activity. RSC Adv. 2016, 6, 76269–76272; https://doi.org/10.1039/c6ra16076j.Suche in Google Scholar
248. Jiang, D.; Sun, Z.; Jia, H.; Lu, D.; Du, P. A Cocatalyst-free CdS nanorod/ZnS Nanoparticle Composite for High-Performance Visible-Light-Driven Hydrogen Production from Water. J. Mater. Chem. A 2016, 4, 675–683; https://doi.org/10.1039/c5ta07420g.Suche in Google Scholar
249. Hao, L. X.; Chen, G.; Yu, Y. G.; Zhou, Y. S.; Han, Z. H.; Liu, Y. Sonochemistry Synthesis of Bi2S3/CdS Heterostructure with Enhanced Performance for Photocatalytic Hydrogen Evolution. Int. J. Hydrogen Energy 2014, 39, 14479–14486; https://doi.org/10.1016/j.ijhydene.2014.04.140.Suche in Google Scholar
250. Bankar, P. K.; Pawar, M. S.; Pawbake, A. S.; Warule, S. S.; Late, D. J.; More, M. A. Spatially Branched CdS-Bi2S3 Heteroarchitecture: Single Step Hydrothermal Synthesis Approach with Enhanced Field Emission Performance and Highly Responsive Broadband Photodetection. RSC Adv. 2016, 6, 95092–95100; https://doi.org/10.1039/c6ra21085f.Suche in Google Scholar
251. Zhong, Y.; Zhao, G.; Ma, F.; Wu, Y.; Hao, X. Utilizing Photocorrosion-Recrystallization to Prepare a Highly Stable and Efficient CdS/WS2 Nanocomposite Photocatalyst for Hydrogen Evolution. Appl. Catal. B Environ. 2016, 199, 466–472; https://doi.org/10.1016/j.apcatb.2016.06.065.Suche in Google Scholar
252. Yin, X. L.; Li, L. L.; Jiang, W. J.; Zhang, Y.; Zhang, X.; Wan, L. J.; Hu, J. S. MoS2/CdS Nanosheets-On-Nanorod Heterostructure for Highly Efficient Photocatalytic H2 Generation under Visible Light Irradiation. ACS Appl. Mater. Interfaces 2016, 8, 15258–15266; https://doi.org/10.1021/acsami.6b02687.Suche in Google Scholar PubMed
253. Xiang, Q. J.; Cheng, F. Y.; Lang, D. Hierarchical Layered WS2/graphene-Modified CdS Nanorods for Efficient Photocatalytic Hydrogen Evolution. ChemSusChem 2016, 9, 996–1002; https://doi.org/10.1002/cssc.201501702.Suche in Google Scholar PubMed
254. Jia, T.; Kolpin, A.; Ma, C.; Chan, R. C. T.; Kwok, W. M.; Tsang, S. C. E. A Graphene Dispersed CdS-MoS2 Nanocrystal Ensemble for Cooperative Photocatalytic Hydrogen Production from Water. Chem. Commun. 2014, 50, 1185–1188; https://doi.org/10.1039/c3cc47301e.Suche in Google Scholar PubMed
255. Chang, K.; Mei, Z.; Wang, T.; Kang, Q.; Ouyang, S.; Ye, J. MoS2/graphene Cocatalyst for Efficient Photocatalytic H2 Evolution under Visible Light Irradiation. ACS Nano 2014, 8, 7078–7087; https://doi.org/10.1021/nn5019945.Suche in Google Scholar PubMed
256. Yang, M.-Q.; Han, C.; Xu, Y.-J. Insight into the Effect of Highly Dispersed MoS2 versus Layer-Structured MoS2 on the Photocorrosion and Photoactivity of CdS in Graphene-CdS-MoS2 Composites. J. Phys. Chem. C 2015, 119, 27234–27246; https://doi.org/10.1021/acs.jpcc.5b08016.Suche in Google Scholar
257. Cheng, F.; Yin, H.; Xiang, Q. Low-temperature Solid-State Preparation of Ternary CdS/g-C3N4/CuS Nanocomposites for Enhanced Visible-Light Photocatalytic H2-Production Activity. Appl. Surf. Sci. 2017, 391, 432–439; https://doi.org/10.1016/j.apsusc.2016.06.169.Suche in Google Scholar
258. Neelgund, G. M.; Oki, A. Photocatalytic Activity of CdS and Ag2S Quantum Dots Deposited on Poly(amidoamine) Functionalized Carbon Nanotubes. Appl. Catal. B Environ. 2011, 110, 99–107; https://doi.org/10.1016/j.apcatb.2011.08.031.Suche in Google Scholar PubMed PubMed Central
259. Pan, Y. X.; Zhou, T.; Han, J.; Hong, J.; Wang, Y.; Zhang, W.; Xu, R. CdS Quantum Dots and Tungsten Carbide Supported on Anatase-Rutile Composite TiO2 for Highly Efficient Visible-Light-Driven Photocatalytic H2 Evolution from Water. Catal. Sci. Technol. 2016, 6, 2206–2213; https://doi.org/10.1039/c5cy01634g.Suche in Google Scholar
260. Wang, L.; Duan, X.; Wang, G.; Liu, C.; Luo, S.; Zhang, S.; Zeng, Y.; Xu, Y.; Liu, Y.; Duan, X. Omnidirectional Enhancement of Photocatalytic Hydrogen Evolution over Hierarchical “Cauline Leaf” Nanoarchitectures. Appl. Catal. B Environ. 2016, 186, 88–96; https://doi.org/10.1016/j.apcatb.2015.12.056.Suche in Google Scholar
261. Yu, J.; Jin, J.; Cheng, B.; Jaroniec, M. A Noble Metal-free Reduced Graphene Oxide-CdS Nanorod Composite for the Enhanced Visible-Light Photocatalytic Reduction of CO2 to Solar Fuel. J. Mater. Chem. A. 2014, 2, 3407–3416; https://doi.org/10.1039/c3ta14493c.Suche in Google Scholar
262. Tian, Q.; Wu, W.; Liu, J.; Wu, Z.; Yao, W.; Ding, J.; Jiang, C. Dimensional Heterostructures of 1D CdS/2D ZnIn2S4 Composited with 2D Graphene: Designed Synthesis and Superior Photocatalytic Performance. Dalton Trans. 2017, 46, 2770–2777; https://doi.org/10.1039/c7dt00018a.Suche in Google Scholar PubMed
263. Xie, Y.; Ali, G.; Yoo, S. H.; Cho, S. O. Sonication-assisted Synthesis of CdS Quantum-Dot-Sensitized TiO2 Nanotube Arrays with Enhanced Photoelectrochemical and Photocatalytic Activity. ACS Appl. Mater. Interfaces 2010, 2, 2910–2914; https://doi.org/10.1021/am100605a.Suche in Google Scholar PubMed
264. Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38; https://doi.org/10.1038/238037a0.Suche in Google Scholar PubMed
265. Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043; https://doi.org/10.1021/cr500008u.Suche in Google Scholar PubMed
266. Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852; https://doi.org/10.1021/cr5000738.Suche in Google Scholar PubMed
267. Dahl, M.; Liu, Y.; Yin, Y. Composite Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9853–9889; https://doi.org/10.1021/cr400634p.Suche in Google Scholar PubMed
268. Liu, L.; Chen, X. Titanium Dioxide Nanomaterials: Self-Structural Modifications. Chem. Rev. 2014, 114, 9890–9918; https://doi.org/10.1021/cr400624r.Suche in Google Scholar PubMed
269. (a)Yohannes, A.; Su, Y.; Yao, S. Emerging Applications of Metal−Organic Frameworks for Environmental Remediation. In: Metal−Organic Frameworks for Environmental Remediation; Ghosh, P.; Kumar, S. S.; Singh, L. Eds.; ACS, USA, Vol. 1395, Ch. 1, 2021; pp 1–23, https://doi.org/10.1021/bk-2021-1395.ch001.(b)Kumar, A.; Kumar, S.; Duhan, S.; Kumar, A.; Devi, S.; Kumar, P. Environmental Applications of Metal−organic Frameworks, In: Metal−organic frameworks for environmental remediation, Ghosh, P.; Kumar, S. S.; Singh, L. Eds.; ACS, USA, Vol. 1395, Ch. 10, 2021, pp. 247–255, https://doi.org/10.1021/bk-2021-1394.ch010.(c)Jang, S.; Song, S.; Lim, J. H.; Kim, H. S.; Phan, B. T.; Ha, K.-T.; Park, S.; Park, K. H. Application of Various Metal-Organic Frameworks (MOFs) as Catalysts for Air and Water Pollution Environmental Remediation. Catalysts 2020, 10 (2), 195; https://doi.org/10.3390/catal10020195.(d)Lopez, Y. C.; Viltres, H.; Gupta, N. K.; Acevedo-Pena, P.; Levya, C.; Ghaffari, Y.; Gupta, A.; Kim, S.; Bae, J.; Kim, K. S. Transition Metal-Based Metal–Organic Frameworks for Environmental Applications: A Review. Environ. Chem. Lett. 2021, 19, 1295–1334; https://doi.org/10.1007/s10311-020-01119-1.(e)Bhuyan, A.; Amaruzzaman, Md. Metal-organic Frameworks: A New Generation Potential Material for Aqueous Environmental Remediation. Inorg. Chem. Commun. 2022, 140, 109436.(f)Yang, C.; Xue, Z.; Wen, J. Recent Advances in MOF-Based Materials for Remediation of Heavy Metals and Organic Pollutants: Insights into Performance, Mechanisms, and Future Opportunities. Sustainability 2023, 15, 6686; https://doi.org/10.3390/su15086686.(g)Wen, Y.; Zhang, P.; Sharma, V. K.; Ma, X.; Zhou, H.-C. Metal-organic Frameworks for Environmental Applications. Cell Rep. Phys. Sci. 2021, 2 (2); https://doi.org/10.1016/j.xcrp.2021.100348.Suche in Google Scholar
270. Zhu, C.; Tian, Q.; Wan, S.; Xu, H.; Hu, J.; Jing, L. Recent Progress of NH2-MIL-125(Ti)-based Photocatalytic System in Energy Production and Environmental Purification. Chem. Eng. J. 2024, 497 (1); https://doi.org/10.1016/j.cej.2024.154689.Suche in Google Scholar
271. (a)Jrad, A.; Sabeh, G. A.; Hannouche, K.; Natour, R. A.; Haidar, O.; Sammoury, H.; Ahmad, M. A.; Hmade, M. Critical Role of Defects in UiO-66 Nanocrystals for Catalysis and Water Remediation. ACS Appl. Nano Mater. 2023, 6 (20), 18698–18720; https://doi.org/10.1021/acsanm.3c03787.(b)Liu, H.; Cheng, M.; Liu, Y.; Zhang, G.; Li, L.; Du, L.; Li, B.; Xiao, S.; Wang, G.; Yang, X. Modified UiO-66 as Photocatalysts for Boosting the Carbon-Neutral Energy Cycle and Solving Environmental Remediation Issues. Coord. Chem. Rev. 2022, 458; https://doi.org/10.1016/j.ccr.2022.214428.(c)Daliran, S.; Oveisi, A. R.; Kung, C.-W.; Sen, U.; Dhakshinamoorthy, A.; Chuang, C. H.; Khajeh, M.; Erkartal, M.; Hupp, J. T. Defect-enabling Zirconium-Based Metal–Organic Frameworks for Energy and Environmental Remediation Applications. Chem. Soc. Rev. 2024, 53, 6244–6294; https://doi.org/10.1039/d3cs01057k.Suche in Google Scholar
272. Razavi, L.; Raissi, H.; Hashemzehi, O.; Farzad, F. Significantly Enhanced Performance for Phenol Compounds Removal by MOF-5 Nano-Composite via its Surface Modification. npj Clean Water 2024, 7, 44; https://doi.org/10.1038/s41545-024-00338-1.(b)Lal, S.; Singh, P.; Singhal, A.; Kumar, S.; Gahlot, A. P. C.; Gandhi, N.; Kumari, P. Advances in Metal–Organic Frameworks for Water Remediation Applications. RSC Adv. 2024, 14, 3413–3446; https://doi.org/10.1039/d3ra07982a.Suche in Google Scholar
273. Kandiah, M.; Nilsen, M. H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E. A.; Bonino, F.; Lillerud, K. P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640; https://doi.org/10.1021/cm102601v.Suche in Google Scholar
274. Shen, L.; Liang, R.; Luo, M.; Jing, F.; Wu, L. Electronic Effects of Ligand Substitution on Metal-Organic Framework Photocatalysts: The Case Study of UiO-66. Phys. Chem. Chem. Phys. 2015, 17, 117–121; https://doi.org/10.1039/c4cp04162c.Suche in Google Scholar PubMed
275. (a)Bazzi, L.; Boukayouht, K.; Mansouri, S.; Hankari, S. A. Eco-friendly and Cost-Effective Synthesis of Hierarchical Porous HKUST-1 from Thin Waste Electric Cables for Enhanced Cationic Dye Removal. Proc. Safety Environ. Protect. 2024, 191A, 750–759; https://doi.org/10.1016/j.psep.2024.08.125.(b)Loera-Serna, S.; Beltran, H. I.; Mendoza-Sanchez, M.; Alvarez-Zeferino, J. C.; Almanza, F.; Fernandez-Luqueno, F. Effect of HKUST-1 Metal–Organic Framework in Root and Shoot Systems, as Well as Seed Germination. Environ. Sci. Pollut. Res. 2024, 31, 13270–13283; https://doi.org/10.1007/s11356-023-31728-6.(c)Kangra, B.; Jatrana, A.; Maan, S.; Chauhan, S.; Kumar, V. Effective Adsorption of Chlorpyrifos Pesticides by HKUST-1 Metalorganic Framework. J. Chem. Sci. 2022, 134, 104; https://doi.org/10.1007/s12039-022-02099-1.Suche in Google Scholar
276. Global Market Insights: URL: https://www.gminsights.com/industry-analysis/heterogeneous-catalyst-market. (Accessed on 20 November 2024)Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

