Maternal exposure to particulate matter and nitrogen oxides during pregnancy and attention deficit hyperactivity disorder in offspring: a systematic review and meta-analysis
-
Esther Abraham
Abstract
Objectives
Attention deficit hyperactivity disorder (ADHD) is the most prevalent neurodevelopmental condition in Australia, with one in 20 children diagnosed. Air pollutants have been associated with poorer child neurodevelopmental outcomes. This systematic review and meta-analysis aims to determine the association between particulate matter (PM2.5, PM10), and nitrogen oxide (NOx) exposure during pregnancy and the development of ADHD in offspring.
Content
We searched MEDLINE; CINAHL; EMBASE; PsycINFO; The Cochrane Library and Google Scholar (until March 2023), and included English language, human studies if they investigated the association between PM2.5, PM10, and/or NOx (NO and/or NO2) exposure during pregnancy and a clinician-based ADHD diagnosis up to 18 years of age. Three studies met these criteria, with two suitable for meta-analysis due to comparable NOx exposure data.
Summary
For every 10 μg/m3 increase in maternal NOx exposure during pregnancy, there was a 9 % increased odds of ADHD diagnosis in childhood (adjusted odds ratio 1.09; 95 % confidence interval: 1.01–1.17).
Outlook
Our findings add to the evidence for an association between air pollution exposure during pregnancy and alterations in offspring neurodevelopment. Since there were only two studies which could be meta-analysed and a very low certainty of the evidence, more research is needed to confirm these findings and inform future interventions and policy.
Introduction
The prenatal environment is known to be particularly susceptible to interferences in fetal development, particularly in the brain and spinal cord, with key aspects of central nervous system development, including refinement of neural connections and synapse formation occurring throughout pregnancy [1], 2]. Environmental factors, such as air pollution, exposure to cigarette smoke and exposure to pesticides and organic solvents have a known association with adverse health outcomes in the fetus and further into childhood [3]. These environmental exposures are also known to be associated with infant neurodevelopment, increasing the likelihood of impairments in cognition, emotional functioning, and social processing that may manifest as neurodevelopmental conditions such as Attention Deficit Hyperactivity Disorder (ADHD) [4]. Air pollution has become a global public health concern, with some of the most concerning pollutants including Particulate Matter (PM) (PM2.5, PM10 and diesel PM), carbon monoxide (CO), ozone (O3), sulfur dioxide (SO2), and nitrogen oxides (NOx) [5]. Sources of these pollutants include combustion of biofuel and fossil fuels, photochemical reactions with nitrogen oxides and combustion involved in heating, power generation and transport [5]. PM2.5 (particulate matter with a diameter less than 2.5 µM) is produced by bushfire smoke and vehicle exhaust. On the other hand, PM10 (particulate matter with diameters greater than 2.5 µM and less than 10 µM) includes black carbon, dust, and mechanically generated particles, as well as pollution from bushfires [6]. NOx which comprises of nitrogen oxide (NO) and nitrogen dioxide (NO2) has outdoor sources of traffic and burning fossil fuels, and indoor sources being the burning of natural gas and wood [7].
Current literature has explored the association between prenatal NO2 and PM exposure (PM2.5 and PM10) and offspring neurodevelopment. Positive associations between PM2.5 exposure during pregnancy and neurodevelopmental outcomes in children of various ages have been reported [8], including an increased risk of suspected developmental delay [9], and poorer cognitive development, particularly in girls [10]. These studies highlighted the critical windows of exposure as mid- or late pregnancy (weeks 18–34) [8], 10], as well as consistently throughout the entire pregnancy which suggests that chronic exposure may also be important [9]. Higher PM2.5 exposures from traffic and other sources during pregnancy have also been correlated with poorer neurodevelopmental measures including IQ, attention, and memory, with poorer executive function between ages 5–11 years [11].
Studies exploring ADHD as an outcome following maternal exposure to air pollutants are limited. ADHD is a neurodevelopmental condition characterized by behavioral issues, with impairments in attention and memory, emotional regulation and self-control [12]. It is the most prevalent neurodevelopmental condition in Australia, affecting one in every 20 children [13]. If not diagnosed and managed properly, the behavioral and social issues that are associated with ADHD can contribute to the development of significant long term adverse outcomes such as insomnia, and alcohol and substance abuse behaviors [14], [15], [16]. While ADHD is regarded as a highly heritable disorder, it is likely that environmental factors account for 10–40 % of the variance [17]. There is evidence of some associations between perinatal risk factors (poor maternal respiratory health, maternal allergic disease and prenatal exposure to cigarette smoke and air pollution) and ADHD [18], 19]. However, there is little knowledge on the effects of PM and NO2 exposure prenatally on ADHD outcomes specifically. Fuertes et al. [20] found that exposure to NO2 and PM2.5 during early life (first 5 years of life) was associated with a significantly increased likelihood of ADHD. Another systematic review found a positive association between PM exposure prenatally and postnatally, and behavioral problems related to attention (including ADHD) however it was unable to distinguish between the effects of prenatal and postnatal exposure [21]. The largest study to investigate the neurological effects of prenatal exposure to PM using Magnetic Resonance Imaging (MRI) is that from Peterson et al. [22]. They found that higher prenatal exposure to PM was linked in a dose-response relationship to reduced left hemisphere white matter volumes in children. This correlated with slower processing speed and greater externalizing problems which are important ADHD symptoms [22]. However, this study’s outcomes did not include a clinical diagnosis of ADHD and prenatal PM monitoring was limited to a short window during the 3rd trimester.
Numerous mechanisms have been proposed for potential pathways through which prenatal air pollutant exposure may impact neurodevelopmental outcomes. A birth cohort study by Saenen et al. [23] exploring the relationship between prenatal PM2.5 exposure and placental brain-derived neurotrophic factor (BDNF) expression, found that BNDF plays a significant role in developing the fetal nervous system, and air pollutants can decrease its production, thus affecting the trajectory of normal fetal neurodevelopment. PM exposures have also been associated with astrocyte and microglial activation, triggering neuroinflammation and oxidative stress [24]. Proteins linked to oxidative stress, in turn, have been implicated in ADHD [24], and neurons are particularly vulnerable to oxidative stress as they have a high oxygen consumption [25]. Maternal inflammation is another potential mechanism, as cytokines can cross the placenta and the blood-brain barrier [26], consequently activating microglia and influencing fetal brain development [25], 27]. Recent studies using a Mendelian Randomisation approach have provided evidence of a causal pathway between air pollution exposure (including PM2.5 and NO2) and ADHD [28] which may be mediated via changes to the gut microbiota [29].
There may be ways to mitigate the effects of prenatal air pollution exposure on infant neurodevelopment. Goodrich et al. [30] have explored the association of both periconceptional folic acid supplementation and air pollutant exposure with the development of autism. They found mothers with exposure to air pollutants above the median value, taking periconceptional folic acid above 800 μg in the first month of pregnancy, had a reduced likelihood of their offspring developing autism, as compared to those taking less than 800 μg of folic acid. This suggests that there may be potential to mitigate the effects of prenatal air pollutant exposure on neurodevelopment via folic acid supplementation. More research is needed to understand interventions that could be applied to improve health outcomes for mother and infant.
The knowledge gap in data relating to prenatal air pollution exposure and ADHD outcomes in the offspring requires further investigation. The focus of this review is therefore to synthesise the available evidence from the current studies investigating the relationship between prenatal air pollutant exposure to NOx, PM2.5 and PM10, and ADHD diagnosis in children.
Aims/Hypothesis
Our primary aim was to determine the association between PM2.5, PM10, and NOx exposure during pregnancy (all trimesters, and all child-bearing age groups) and the development of attention deficit hyperactivity disorder (ADHD) in offspring up to the age of 18 years.
We hypothesised there will be an increased risk of developing ADHD in the offspring associated with greater exposure to PM2.5, PM10, and NOx during the mother’s pregnancy.
Methods
The systematic review protocol was registered in Prospero on 15th August 2022 (registration number 343094). PRISMA 2020 [32] was used to report the findings of the systematic review.
Selection process
Electronic searches of MEDLINE; CINAHL; EMBASE; PsycINFO; and The Cochrane Library were conducted on the 21st February 2023 and included all studies up to the date of the search. The Google Scholar database was not used in the initial search and was later searched on the 6th March 2023. Only the first 300 results were included due to the exhaustive results that were generated.
The following search terms were used: (“pregnancy” OR “fetal”) AND (“particulate matter” OR air pollut*) AND (“attention deficit hyperactivity disorder” OR “ADHD” OR “Attention Deficit Disorders with Hyperactivity” OR “Attention Deficit Disorder”). The full list of search terms can be seen in Supplementary Table S1. Inclusion and exclusion criteria are shown in Table S2. We included any cohort studies, case control studies or randomised controlled trials in humans published in English which examined pregnancy exposure to the air pollutants PM2.5, PM10 or NO2/NOX and a clinical diagnosis of ADHD in the offspring.
After removal of duplicates, screening against our inclusion and exclusion criteria was conducted in Covidence, first with title and abstract, and then full text screening. This process was carried out independently by three reviewers (EA, RB, ROB) and each result was reviewed by an independent investigator. Any disagreements at either stage of screening were resolved through discussion with the senior author.
Data extraction
Data was extracted from each study by one reviewer, and the results were independently checked by a second reviewer. Extracted data included the study location, study design, participant population, pollutants assessed, exposure assessment methodology, exposure window during pregnancy, ADHD diagnosis methodology, confounding factors and main results. These results can be seen in Table 1.
Risk of bias assessment
The risk of bias quality assessment was conducted using the ‘Risk of Bias in Non-Randomised Studies – of Exposures (ROBINS-E)’ tool. Each study was assessed on seven domains: confounding, exposure assessment, selection bias, post-exposure interventions, missing data, outcome assessment and selective reporting. Each domain was scored as low risk, some concerns, high risk or very high risk and each study received an overall risk of bias score. The ROBINS-E template was completed by two independent reviewers (EA, ROB) and any discrepancies were resolved by consultation with a third reviewer (RB).
Meta analysis
Meta-analyses combining studies with the same exposure and reporting on the odds of children having an ADHD diagnosis were performed using Review Manager (RevMan Computer program, Version 5.4, The Cochrane Collaboration, 2020) utilising fixed effects models. Odds ratios (both adjusted and unadjusted) were pooled using the generic inverse variance method. Forest plots were generated and heterogeneity was tested for using the chi-square test and the I-square percentage. We used Grading of Recommendations, Assessment, Development and Evaluations (GRADE) to determine certainty of the meta-analysis results [31].
Results
Literature search and study selection
Our search strategy yielded 2,601 publications from the six databases Medline, CINAHL, EMBASE, PsycINFO, Google Scholar, and Cochrane Library database. After removal of duplicates in Endnote and Covidence 1,935 publications remained. These publications were screened by title and abstract, with 27 studies remaining for full text review. Following full text review, 24 studies were removed – 16 studies did not include ADHD diagnosis as the outcome, one was removed due to the exposure being perfluoroalkyl substances rather than PMs (Particulate Matter) or NOx (nitrogen oxides), and seven were removed as they were reviews or meta-analyses. This yielded a total of three studies for the final extraction and systematic review, of which two were included in the meta-analysis (Figure 1).
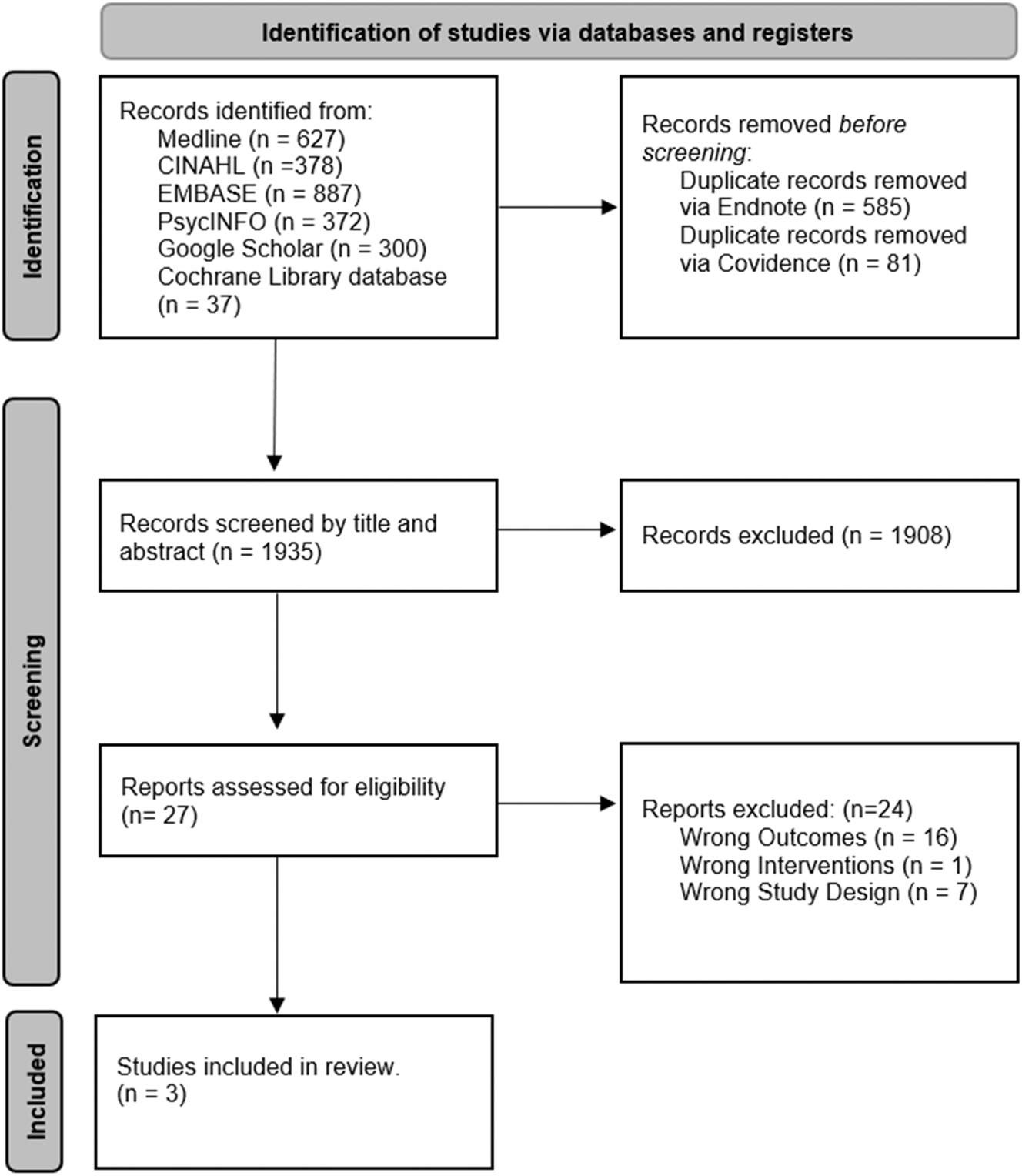
PRISMA flow diagram for search of database and registers.
Risk of bias and quality assessment
The analyzed studies were assessed for risk of bias using the ROBINS-E assessment tool, represented visually in Supplementary Figure 1. The overall risk of bias was low for two studies (Chang et al. [32] and Oudin et al. [33]) with direction of bias being towards harm of (higher) exposure. Shih et al. [34] had a higher risk of bias as the outcome of a clinician-based diagnosis was self-reported by the child’s parents, rather than using diagnostic tools or clinician review, possibly decreasing the validity and reliability of results. The study also mentions missing data in regard to exposure measurement, with missing data being supplemented with an estimation of exposure for that time period – this however is unlikely to majorly impact the outcomes of the study. The exposure measurement method was less reliable than the methods used in the other studies. The study otherwise showed low risk of bias in other domains.
Study characteristics
Table 1 provides a summary of the study characteristics of the three studies included in this review.
Summary of Study Characteristics on Prenatal Exposure to Air Pollutants and ADHD diagnosis.
| Study details | Participants | Pollutants | Exposure assessment | Exposure window | ADHD diagnosis | Confounding factors | Main results | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| Chang et al. (2022) Taiwan maternal and child health database |
425,736 infants born in central Taiwan between 2004 and 2015 Follow up at 5 years |
PM2.5
Mean prenatal exposure: 37.21 ± 14.65 μg/m3 |
Daily PM2.5 concentrations using 1 km satellite-based estimation model based on maternal residential address | Prenatal period (with trimester specific levels) and postnatally up to 5 years of life | Based on ICD-9-CM code 314 extracted from records of NHIRD Age at diagnosis: 5 years |
Child: Sex, birth weight, preterm birth, iron deficiency anaemia, asthma, atopic eczema, allergic rhinitis Mother: Age at delivery, anaemia, heart disease, chronic diabetes, gestational diabetes mellitus, polyhydramnios, oligohydramnios, chronic hypertension, gestational hypertension, preeclampsia, maternal smoking and drug use Family/environment: Socioeconomic status |
Proportion of population with ADHD diagnosis: 2.18 % The hazard ratio (HR) of ADHD was significantly associated with a 10 μg/m3 increase in PM2.5 during the first trimester and increased at PM2.5>16 μg/m3 |
Low |
| Oudin et al. (2019) maternal air pollution in Southern Sweden | 48,571 Skane children born between 1998 and 2006. Follow up between 1 and 17 years. |
NOx Mean prenatal exposure: 17.7 ± 10.3 μg/m3 |
Modelled NOx using Gaussian dispersion model based on maternal residency during pregnancy (AERMOD) | Pregnancy | ADHD diagnosis based on DSM made by a clinician at departments of child and adolescent psychiatry and ICD10 diagnosis Age at diagnosis: Between 1 and 17 years |
Child: Sex Mother: Country of birth, age, parity, smoking, BMI, distance from psychiatry unit Family/environment: Socioeconomic status |
Proportion of population with ADHD diagnosis: 1.48 % No associations were found between NOx exposure and the risk of developing ADHD |
Low |
| Shih et al. (2020) Taiwan birth cohort study |
16,376 Taiwanese children born in 2005 Follow up at 6 months and 8 years |
NOx (NO, NO2) SO2, CO, PM10 Mean prenatal exposure: NOx: 49.9 ± 10.88 μg/m3 SO2: 4.74 ± 1.55 ppb CO: 559 ± 96.7 ppb PM10: 61.8 ± 15.0 μg/m3 |
Air pollution data retrieved from fixed-site stations using chemiluminescence for NO, NO2, NOx, UV fluorescence for CO, infrared spectroscopy for SO2 and beta-ray attenuation for PM10 | Pregnancy | Physician or specialist diagnosis of hyperactivity reported by parent. Age at diagnosis: Between 1 and 8 years |
Child: Sex, delivery method, birth in summer (June-August) Mother: Age, residence (urban or rural) Family/environment: Annual household income |
Proportion of population with ADHD diagnosis: 2.3 % No association between PM10, or SO2 and hyperactivity. Statistically significant association with NO, NO2 and NOx |
Some concerns |
Chang et al. [32]
This study used birth record data from the Taiwan Maternal and Child Health Database (TMCHD), to examine the association between maternal exposure to PM2.5 and ADHD development in the infant. This is a nationwide birth cohort of all infants born in Taiwan between 2004 and 2011. After excluding stillbirths, multiple births, and infants with birth defects the final study population was 425,736 births. Participants were followed up for five years, with 0.2 % loss to follow-up. 9,294 children were diagnosed with ADHD during the study period (2.18 %). The outcome was identified using the ICD-9-CM (International Classification of Disease, Ninth Revision, Clinical Modification) diagnostic code for ADHD in the inpatient and outpatient records from the National Health Insurance Research Database (NHIRD) during the follow up period. The exposure measured in this study was solely PM2.5, and the exposure window was from the beginning of pregnancy until the end of the five-year follow up period. Results were presented separately for each trimester, the entire prenatal period and each postnatal year, allowing data extraction related to pregnancy exposure only. PM2.5 concentration was measured using a satellite-based estimation model using maternal address. The covariates analyzed included child sex, birth weight, preterm birth, comorbidities (e.g., iron deficiency anemia, asthma), maternal smoking, and drug use. A Cox proportional-hazards (PH) model and its time transform was used to evaluate the association between PM2.5 exposure and the incidence of ADHD in the offspring. This found that for every 10 μg/m3 increase in PM2.5 exposure during the first trimester, the hazards ratio for ADHD in the offspring was increased by 26 % (HR 1.26; 95 % confidence interval [CI]: 1.13–1.40). In addition, the hazard ratios of ADHD in the offspring significantly increased with PM2.5 exposure over 16 μg/m3 during the first trimester (HR range: 1.05–4.52).
Oudin et al. [33]
This longitudinal cohort study sourced data from the Maternal Air Pollution in Southern Sweden (MAPSS) database, which includes 99 % of all children born in Skåne between 1999 and 2009, to study the association between air pollutant exposure during pregnancy and diagnosed autism and ADHD in the child. For the purpose of this review, the focus was on ADHD as the outcome. The total study population was 48,571 children, of which 718 children were diagnosed with ADHD (1.48 %), excluding those with co-occurring autism (n=72). These participants were followed up between 2004 and 2016, with age of diagnosis between one and 17 years of age. The outcome was identified by clinician-diagnosis at the Department of Child and Adolescent Psychiatry of ADHD using the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria, 5th Edition, and followed by the International Classification of Mental and Behavioral Disorders version 10 (ICD10) diagnosis. The exposure measured in this study was solely NOx, the exposure window being during pregnancy. NOx concentration was measured using a Gaussian dispersion model, AERMOD, and an emission database on road networks. These levels were then geocoded to participants’ residential address and trimester-specific exposure quartiles, as well as an average exposure level throughout the entire pregnancy, and a continuous measure of NOx, were calculated for each participant. The covariates analyzed included child sex, distance from the psychiatric unit, maternal birth country, age, parity, smoking, BMI, education, and disposable income. Odds ratios were produced using logistic regression. Sensitivity analyses were run using Cox Proportional Hazard Regression. Results from logistic regression analysis did not find a significant association with prenatal NOx exposure for any trimester or linear estimates for all of pregnancy and ADHD diagnosis (OR 0.99, 95 % CI 0.91–1.09). This did not change with Cox proportional regression analysis nor with adjusting for confounders including infant sex, maternal age, parity, maternal smoking, BMI, education, disposable income and country of birth (OR 1.02, 95 % CI 0.92–1.13). Subgroup analysis considered children born to Swedish-born mothers and those living within the catchment areas, these results were also very similar to main results.
Shih et al. [34]
This prospective longitudinal cohort study used data from the Taiwan Birth Cohort Study (TBCS) which included 24,200 Taiwanese children born in 2005 (approximately 12 % of all Taiwanese births in 2005) to determine the association between air pollutant exposure and ADHD diagnosis. Infants with intrauterine growth restriction (IUGR), preterm birth, fetal distress, or low birth weight, or mothers with twin pregnancies, and those who smoke or drank alcohol during pregnancy were excluded. Participants were followed up at 6 months and 8-years. 1526 cases (8.1 %) were lost to follow up at the 8-year mark. The final study population was 16,376 infants, of which 374 (2.3 %) had received a hyperactivity disorder diagnosis by the age of 8 years old. The outcome was identified by parent self-reporting of a hyperactivity diagnosis made by a clinician. The exposures measured in this study included nitric oxides (NO, NO2), SO2, CO, and PM10 or less. These were measured using data from fixed site-stations for air quality monitoring. NOx was measured using chemiluminescence and PM10 was measured using the β-ray attenuation method. For each air pollutant a daily average was calculated based on >18 hourly measurements, otherwise data was considered missing and was estimated. The daily exposures were geocoded to each participants’ home address to the township level to estimate their personal exposure. Odds ratios for the association between maternal exposure to each air pollutant and the development of hyperactivity in the child were evaluated using logistic regression analysis. This found no significant association between hyperactivity and PM10 or SO2. However, a significant association was found between NOx and hyperactivity disorder (OR 1.36, 95 % CI 1.18–1.57). This remained statistically significant after adjusting for sex, urban residence, birth in summer, low annual household income, and average ambient temperature (aOR 1.28, 95 % CI 1.08–1.52). A significant association was found individually for NO (OR 1.31, 95 % CI 1.17–1.48), and NO2 (OR 1.22, 95 % CI 1.04–1.42). However, after adjusting for co-variates, only NO remained statistically significant (aOR 1.26, 95 % CI 1.09–1.46), while NO2 did not (aOR 1.10, 95 % CI 0.92–1.3).
Meta analysis
Both Shih et al. [34] and Oudin et al. [33] explored NOx as an exposure and thus were able to be meta-analyzed. The mathematical working can be found in Table S3.
The pooled OR estimates from these two studies showed that NOx exposure during pregnancy was associated with a significantly increased odds of ADHD [OR 1.09, 95 % CI 1.02–1.16] (Figure 2). The test for heterogeneity showed significant heterogeneity between studies [Chi2=10.47, df=1, I2=90 % (p=0.001)].

Meta-analysis of the association between maternal NOx exposure and infant ADHD development unadjusted for confounding factors.
These results remained consistent when combining data which was adjusted for confounding factors [OR 1.09, 95 % CI 1.01–1.17] (Figure 3). However, heterogeneity was reduced to a moderate level, and was statistically insignificant [Chi2=3.3, df=1, I2=70 % (p=0.07)].
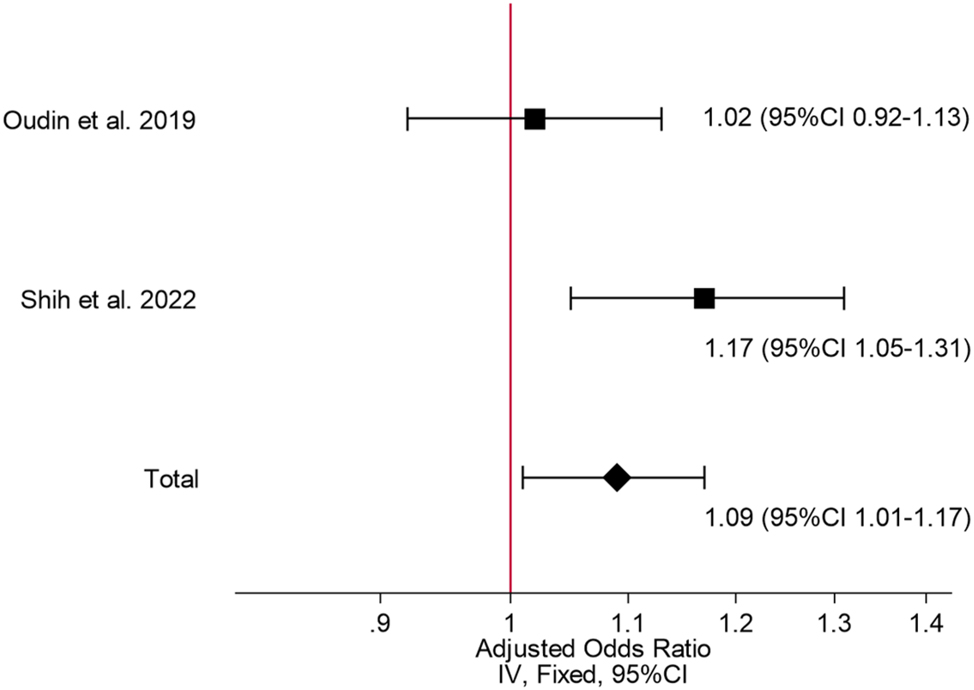
Meta-analysis of the association between maternal NOx exposure and infant ADHD development adjusted for confounding factors.
The certainty of evidence of these meta-analyses was rated as very low using GRADE.
Discussion
This meta-analysis demonstrated that for every 10 μg/m3 increase in exposure to NOx in pregnancy there was a 9 % increased odds of ADHD diagnosis in childhood. However, this finding should be interpreted with caution, given that only two studies could be combined, one of which had a high risk of bias in one domain and some concerns overall. In addition, applying GRADE criteria resulted in a very low certainty of the evidence, due to the included studies being observational, the risk of bias issues, imprecision (with data coming from only two studies) and inconsistency, with results driven by one study (Shih et al.) [34].
While multiple studies have delved into the relationship between PM2.5, PM10, NOx, and ADHD, particularly throughout early childhood, there has been limited attention directed towards understanding the association with exposure during pregnancy [18], 21], 22], [35], [36], [37], [38]. Similarly, studies have explored the connection between these air pollutants and other neurodevelopmental outcomes, yet few have examined ADHD diagnosis specifically [4], [8], [9], [10], [11, 39], 40]. Several reports examined ADHD symptoms in children in relation to prenatal exposure to these pollutants but did not specify a clinical ADHD diagnosis [41], [42], [43], [44].
Our systematic review identified three studies, and we conducted a meta-analysis on only two of these, which we acknowledge is a major limitation of this work. Chang et al. [32] explored PM2.5 effects, Shih et al. [34] investigated NOx and PM10, and Oudin et al. [33] focused on NOx. Chang et al. [32], a birth cohort study, assessed prenatal and early life exposure to PM2.5 and ADHD, over a period of 11 years. Their findings indicated that exposure to PM2.5 levels above 16 μg/m3 during pregnancy increased the likelihood of ADHD in children. Shih et al.’s [34] Taiwanese birth cohort study explored childhood hyperactivity disorder and prenatal traffic-based air pollutant exposure, finding a positive association. This study specifically addressed hyperactivity disorder; as such, it is important to note that ADHD encompasses three primary subtypes as per the DSM-5: Inattentive, Hyperactive-Impulsive, and Combined type, with the hyperactive-impulsive subtype being more common in pre-school aged children [45], 46]. However, Shih et al. [34] may have inadvertently excluded children with an ADHD diagnosis not involving hyperactivity, such as ADHD with inattention subtype, and as such, their findings may be understated. Given that their results already demonstrated a significant association between hyperactivity-impulsive type ADHD and NOx, it follows that if inattention-type ADHD were included in the analysis, the outcomes would likely remain significant. Also, this study may have not included all children with ADHD, as it only included children with a diagnosis up to 8 years of age. Oudin et al. [33] found the mean age of an ADHD diagnosis was 10 years, thus some children may have been missed. Oudin et al.’s Swedish study utilised an epidemiological database for participant selection, applying standardised diagnostic measures for autism and ADHD, and excluding ADHD cases who also had autism [33]. All three studies did not control for maternal ADHD diagnoses either, which may be a limitation.
Although postnatal exposure to air pollutants has been linked to a rise in ADHD prevalence, only one systematic review thus far has examined this prenatally, but without conducting a meta-analysis [47]. Our study addresses this gap. Secondly, given the increasing rates of ADHD in children, early detection, and earlier provision of targeted supports is crucial for improved well-being for children and families [48]. ADHD can greatly affect the child and family in a multitude of ways including financially, emotionally, and mentally [46], [47], [48], [49], [50], [51]. Identifying risk factors like air pollutant exposure aids in this effort of prevention and early detection. It has been shown that the closure of a coal burning power plant in China resulted in less polycyclic aromatic hydrocarbon exposure, leading to better neurodevelopmental outcomes for children [52]. The findings of future research in this area are needed to inform interventions to enhance air quality standards and minimise emission levels and exposure via policy implementations.
Exposure to NO2 and PM2.5 in early life significantly increases the likelihood of ADHD [20]. Chang et al. [32] has suggested that while prenatal exposure is important, early life exposure may be even more relevant to an ADHD diagnosis. Distinguishing prenatal effects from postnatal exposure however, remains challenging [21]. This also presents a two-hit scenario, and exploring the compounding effects of prenatal and postnatal exposure on outcomes is important for future research.
Research examining neurodevelopmental outcomes suggests sensitive time periods in pregnancy seem to differ based on the specific neurodevelopmental domain under consideration [11]. Chang et al.’s [32] paper involving PM2.5 concentration measurements during all three trimesters identified the first trimester as the most sensitive period. Notably, this study demonstrated sex-specific results, showing a greater effect of PM2.5 exposure on ADHD in boys during the first trimester compared to girls. However, both Oudin et al. [33] and Shih et al. [34] did not identify trimester-specific associations.
A limitation of our study lies in the incorporation of only two out of three studies from the systematic review into a meta-analysis, the inability to conduct a trimester specific meta-analysis, due to lack of data in the original studies, and the very low certainty of the evidence. Our inclusion criteria, focusing on clinician diagnosed ADHD, excluded several studies with different ADHD diagnostic measures, or which focused on symptoms. Although this strict approach ensured precision, a broader meta-analysis would have been possible if studies considering ADHD symptoms or non-clinician-based diagnoses had been incorporated, which would have required broader search terms.
Effects on ADHD-like symptoms could be important since ADHD can be considered dimensionally, with cerebral cortical thickness and its rate of thinning linked to the trajectory of ADHD symtoms from childhood to adulthood [53]. A recent Chinese study identified that exposure to PM2.5, PM10 and NO2 from the seventh month of pregnancy to 4 months after birth was a particularly important period in the association with the child’s hyperactivity behaviours at 3 years of age [44]. A Spanish study reported that prenatal exposure to PM2.5 between 16 and 22 weeks gestation was associated with more hyperactivity/impulsivity symptoms at age 5 years, as reported by teachers, but not at age 7 years, as reported by parents [54]. There was no association between PM2.5 exposure in utero and inattention symptoms [54]. Meanwhile, the study from Mooney et al. [55] showed no individual association between child PM2.5 or NO2 exposure on ADHD symptoms, measured with the Child Behavior Checklist or ADHD Rating Scale in 7–11 year old children across three cohorts. However, the ADHD polygenic risk score and family conflict were significantly associated, suggesting a gene-environment interaction.
A further limitation was that two of the three studies reviewed were based in Taiwan, thus it may be difficult to generalise our results to the global population, particularly as PM and NO2 concentrations can vary across regions [32]. PM2.5 can come from a variety of sources including residential, commercial, transportation, and industrial combustion and processing. Therefore, different geographical locations may have different levels of PM2.5 toxicity [32], 56]. Additionally, there was moderate (70 %) heterogeneity between the two studies in the meta-analysis, contributing to the difficulty drawing firm conclusions. Inclusion of only English-language studies, may have narrowed the search scope. Our review’s strength is that it focused on prenatal exposure to prevalent air pollutants and the likelihood of ADHD diagnosis, an avenue that had thus far remained relatively unexplored.
Conclusions
This systematic review investigated the relationship between prenatal air pollutant exposure – particularly PM2.5, PM10, NO2, and NOx – and the development of ADHD in the child. One study reported a positive association between PM2.5 and ADHD development [32], and meta-analysis found a significant positive association between NOx exposure in pregnancy and ADHD development in childhood. However, the very low certainty of this evidence must be acknowledged. These results suggest the need for further research into the possible mechanisms between maternal air pollution exposure and infant neurodevelopment, with research from a wider variety of locations required, and future studies should aim to standardise exposure and outcome assessment.
Funding source: Australian Medical Research Future Fund
Award Identifier / Grant number: 1196252
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission. EA, ROB, and RB conducted the review and meta-analysis and were supervised by OW, TB and VEM.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: Vanessa Murphy is funded by a fellowship (Investigator grant ID 1196252) from the Australian Medical Research Future Fund (MRFF).
-
Data availability: Not applicable.
References
1. White, TA, Miller, SL, Sutherland, AE, Allison, BJ, Camm, EJ. Perinatal compromise affects development, form, and function of the hippocampus part one; clinical studies. Pediatr Res 2024;95:1698–708. https://doi.org/10.1038/s41390-024-03105-7.Search in Google Scholar PubMed PubMed Central
2. Mottahedin, A, Ardalan, M, Chumak, T, Riebe, I, Ek, J, Mallard, C. Effect of neuroinflammation on synaptic organization and function in the developing brain: implications for neurodevelopmental and neurodegenerative disorders. Front Cell Neurosci 2017;11:190. https://doi.org/10.3389/fncel.2017.00190.Search in Google Scholar PubMed PubMed Central
3. Triche, EW, Hossain, N. Environmental factors implicated in the causation of adverse pregnancy outcome. Semin Perinatol 2007;31:240–2. https://doi.org/10.1053/j.semperi.2007.07.013.Search in Google Scholar PubMed PubMed Central
4. Volk, HE, Perera, F, Braun, JM, Kingsley, SL, Gray, K, Buckley, J, et al.. Prenatal air pollution exposure and neurodevelopment: a review and blueprint for a harmonized approach within ECHO. Environ Res 2021;196:110320. https://doi.org/10.1016/j.envres.2020.110320.Search in Google Scholar PubMed PubMed Central
5. Brunekreef, B. 6.2 Key new findings on health effects of air pollutants and impact of evidence for EU policies and WHO guidelines – the REVIHAAP and HRAPIE projects. ISEE Conf Abstr 2013;2013:5735. https://doi.org/10.1289/isee.2013.s-3-19-04.Search in Google Scholar
6. Bakulski, KM, Fisher, JD, Dou, JF, Gard, A, Schneper, L, Notterman, DA, et al.. Prenatal particulate matter exposure is associated with saliva DNA methylation at age 15: applying cumulative DNA methylation scores as an exposure biomarker. Toxics 2021;9. https://doi.org/10.3390/toxics9100262.Search in Google Scholar PubMed PubMed Central
7. American Lung Association. Nitrogen dioxide. Available from: https://www.lung.org/clean-air/outdoors/what-makes-air-unhealthy/nitrogen-dioxide.Search in Google Scholar
8. Tapia, VL, Vasquez, BV, Vu, B, Liu, Y, Steenland, K, Gonzales, GF. Association between maternal exposure to particulate matter (PM2.5) and adverse pregnancy outcomes in Lima, Peru. J Expo Sci Environ Epidemiol 2020;30:689–97. https://doi.org/10.1038/s41370-020-0223-5.Search in Google Scholar PubMed PubMed Central
9. Wang, P, Zhao, Y, Li, J, Zhou, Y, Luo, R, Meng, X, et al.. Prenatal exposure to ambient fine particulate matter and early childhood neurodevelopment: a population-based birth cohort study. Sci Total Environ 2021;785:147334. https://doi.org/10.1016/j.scitotenv.2021.147334.Search in Google Scholar PubMed
10. Ha, S, Yeung, E, Bell, E, Insaf, T, Ghassabian, A, Bell, G, et al.. Prenatal and early life exposures to ambient air pollution and development. Environ Res 2019;174:170–5. https://doi.org/10.1016/j.envres.2019.03.064.Search in Google Scholar PubMed PubMed Central
11. Chiu, YH, Hsu, HH, Coull, BA, Bellinger, DC, Kloog, I, Schwartz, J, et al.. Prenatal particulate air pollution and neurodevelopment in urban children: examining sensitive windows and sex-specific associations. Environ Int 2016;87:56–65. https://doi.org/10.1016/j.envint.2015.11.010.Search in Google Scholar PubMed PubMed Central
12. The Royal Childrens Hospital Melbourne. Kids health information: attention deficit hyperactivity disorder (ADHD). Available from: https://www.rch.org.au/kidsinfo/fact_sheets/Attention_deficit_hyperactivity_disorder_ADHD/#:∼:text=It.Search in Google Scholar
13. Lundervold, AJ, Jensen, DA, Haavik, J. Insomnia, alcohol consumption and ADHD symptoms in adults. Front Psychol 2020;11. https://doi.org/10.3389/fpsyg.2020.01150.Search in Google Scholar PubMed PubMed Central
14. Zulauf, CA, Sprich, SE, Safren, SA, Wilens, TE. The complicated relationship between attention deficit/hyperactivity disorder and substance use disorders. Curr Psychiatry Rep 2014;16:436. https://doi.org/10.1007/s11920-013-0436-6.Search in Google Scholar PubMed PubMed Central
15. Brown, TE, Romero, B, Sarocco, P, Atkins, N, Schwartz, EJ, Rhoten, S. The patient perspective: unmet treatment needs in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord 2019;21. https://doi.org/10.4088/pcc.18m02397.Search in Google Scholar
16. Liu, X, Dalsgaard, S, Munk-Olsen, T, Li, J, Wright, RJ, Momen, NC. Parental asthma occurrence, exacerbations and risk of attention-deficit/hyperactivity disorder. Brain Behav Immun 2019;82:302–8. https://doi.org/10.1016/j.bbi.2019.08.198.Search in Google Scholar PubMed PubMed Central
17. Sciberras, E, Mulraney, M, Silva, D, Coghill, D. Prenatal risk factors and the etiology of ADHD–review of existing evidence. Curr Psychiatry Rep 2017;19:1. https://doi.org/10.1007/s11920-017-0753-2.Search in Google Scholar PubMed
18. Thygesen, M, Holst, GJ, Hansen, B, Geels, C, Kalkbrenner, A, Schendel, D, et al.. Exposure to air pollution in early childhood and the association with Attention-Deficit hyperactivity disorder. Environ Res 2020;183:108930. https://doi.org/10.1016/j.envres.2019.108930.Search in Google Scholar PubMed PubMed Central
19. Straughen, JK, Sitarik, AR, Johnson, CC, Wegienka, G, Ownby, DR, Johnson-Hooper, TM, et al.. Prenatal IgE as a risk factor for the development of childhood neurodevelopmental disorders. Frontiers Pediatrics 2021;9. https://doi.org/10.3389/fped.2021.601092.Search in Google Scholar PubMed PubMed Central
20. Fuertes, E, Standl, M, Forns, J, Berdel, D, Garcia-Aymerich, J, Markevych, I, et al.. Traffic-related air pollution and hyperactivity/inattention, dyslexia and dyscalculia in adolescents of the German GINIplus and LISAplus birth cohorts. Environ Int 2016;97:85–92. https://doi.org/10.1016/j.envint.2016.10.017.Search in Google Scholar PubMed
21. Donzelli, G, Llopis-Gonzalez, A, Llopis-Morales, A, Cioni, L, Morales-Suárez-Varela, M. Particulate matter exposure and attention-deficit/hyperactivity disorder in children: a systematic review of epidemiological studies. Int J Environ Res Publ Health 2019;17. https://doi.org/10.3390/ijerph17010067.Search in Google Scholar PubMed PubMed Central
22. Peterson, BS, Rauh, VA, Bansal, R, Hao, X, Toth, Z, Nati, G, et al.. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry 2015;72:531–40. https://doi.org/10.1001/jamapsychiatry.2015.57.Search in Google Scholar PubMed PubMed Central
23. Saenen, ND, Plusquin, M, Bijnens, E, Janssen, BG, Gyselaers, W, Cox, B, et al.. In utero fine particle air pollution and placental expression of genes in the brain-derived neurotrophic factor signaling pathway: an ENVIRONAGE birth cohort study. Environ Health Perspect 2015;123:834–40. https://doi.org/10.1289/ehp.1408549.Search in Google Scholar PubMed PubMed Central
24. Myhre, O, Låg, M, Villanger, GD, Oftedal, B, Øvrevik, J, Holme, JA, et al.. Early life exposure to air pollution particulate matter (PM) as risk factor for attention deficit/hyperactivity disorder (ADHD): need for novel strategies for mechanisms and causalities. Toxicol Appl Pharmacol 2018;354:196–214. https://doi.org/10.1016/j.taap.2018.03.015.Search in Google Scholar PubMed
25. Kerekes, N, Sanchéz-Pérez, AM, Landry, M. Neuroinflammation as a possible link between attention-deficit/hyperactivity disorder (ADHD) and pain. Med Hypotheses 2021;157:110717. https://doi.org/10.1016/j.mehy.2021.110717.Search in Google Scholar PubMed
26. Goasdoué, K, Miller, SM, Colditz, PB, Björkman, ST. Review: the blood-brain barrier; protecting the developing fetal brain. Placenta 2017;54:111–6. https://doi.org/10.1016/j.placenta.2016.12.005.Search in Google Scholar PubMed
27. Ginsberg, Y, Khatib, N, Weiner, Z, Beloosesky, R. Maternal inflammation, fetal brain implications and suggested neuroprotection: a summary of 10 Years of research in animal models. Rambam Maimonides Med J 2017;8. https://doi.org/10.5041/rmmj.10305.Search in Google Scholar PubMed PubMed Central
28. Ma, YY, Li, QY, Shi, AY, Li, JL, Wang, YJ, Li, X. Association of air pollutants with psychiatric disorders: a two-sample Mendelian randomization. Ecotoxicol Environ Saf 2024;285:117105. https://doi.org/10.1016/j.ecoenv.2024.117105.Search in Google Scholar PubMed
29. Li, C, Chen, H, Gu, Y, Chen, W, Liu, M, Lei, Q, et al.. Causal effects of PM2.5 exposure on neuropsychiatric disorders and the mediation via gut microbiome: a Mendelian randomization study. Ecotoxicol Environ Saf 2024;275:116257. https://doi.org/10.1016/j.ecoenv.2024.116257.Search in Google Scholar PubMed
30. Goodrich, AJ, Volk, HE, Tancredi, DJ, McConnell, R, Lurmann, FW, Hansen, RL, et al.. Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder. Autism Res 2018;11:69–80. https://doi.org/10.1002/aur.1885.Search in Google Scholar PubMed PubMed Central
31. Guyatt, GH, Oxman, AD, Vist, GE, Kunz, R, Falck-Ytter, Y, Alonso-Coello, P, et al.. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924. https://doi.org/10.1136/bmj.39489.470347.ad.Search in Google Scholar PubMed PubMed Central
32. Chang, YC, Chen, WT, Su, SH, Jung, CR, Hwang, BF. PM(2.5) exposure and incident attention-deficit/hyperactivity disorder during the prenatal and postnatal periods: a birth cohort study. Environ Res 2022;214:113769. https://doi.org/10.1016/j.envres.2022.113769.Search in Google Scholar PubMed
33. Oudin, A, Frondelius, K, Haglund, N, Källén, K, Forsberg, B, Gustafsson, P, et al.. Prenatal exposure to air pollution as a potential risk factor for autism and ADHD. Environ Int 2019;133:105149. https://doi.org/10.1016/j.envint.2019.105149.Search in Google Scholar PubMed
34. Shih, P, Huang, CC, Pan, SC, Chiang, TL, Guo, YL. Hyperactivity disorder in children related to traffic-based air pollution during pregnancy. Environ Res 2020;188:109588. https://doi.org/10.1016/j.envres.2020.109588.Search in Google Scholar PubMed
35. Min, J-y, Min, K-b. Exposure to ambient PM10 and NO2 and the incidence of attention-deficit hyperactivity disorder in childhood. Environ Int 2017;99:221–7. https://doi.org/10.1016/j.envint.2016.11.022.Search in Google Scholar PubMed
36. Markevych, I, Tesch, F, Datzmann, T, Romanos, M, Schmitt, J, Heinrich, J. Outdoor air pollution, greenspace, and incidence of ADHD: a semi-individual study. Sci Total Environ 2018;642:1362–8. https://doi.org/10.1016/j.scitotenv.2018.06.167.Search in Google Scholar PubMed
37. Aghaei, M, Janjani, H, Yousefian, F, Jamal, A, Yunesian, M. Association between ambient gaseous and particulate air pollutants and attention deficit hyperactivity disorder (ADHD) in children; a systematic review. Environ Res 2019;173:135–56. https://doi.org/10.1016/j.envres.2019.03.030.Search in Google Scholar PubMed
38. Zhang, M, Wang, C, Zhang, X, Song, H, Li, Y. Association between exposure to air pollutants and attention-deficit hyperactivity disorder (ADHD) in children: a systematic review and meta-analysis. Int J Environ Health Res 2022;32:207–19. https://doi.org/10.1080/09603123.2020.1745764.Search in Google Scholar PubMed
39. Suades-González, E, Gascon, M, Guxens, M, Sunyer, J. Air pollution and neuropsychological development: a review of the latest evidence. Endocrinology 2015;156:3473–82. https://doi.org/10.1210/en.2015-1403.Search in Google Scholar PubMed PubMed Central
40. Castagna, A, Mascheroni, E, Fustinoni, S, Montirosso, R. Air pollution and neurodevelopmental skills in preschool- and school-aged children: a systematic review. Neurosci Biobehav Rev 2022;136:104623. https://doi.org/10.1016/j.neubiorev.2022.104623.Search in Google Scholar PubMed
41. Peterson, BS, Bansal, R, Sawardekar, S, Nati, C, Elgabalawy, ER, Hoepner, LA, et al.. Prenatal exposure to air pollution is associated with altered brain structure, function, and metabolism in childhood. J Child Psychol Psychiatry 2022;63:1316–31. https://doi.org/10.1111/jcpp.13578.Search in Google Scholar PubMed
42. Fang, XY, Strodl, E, Liu, BQ, Liu, L, Yin, XN, Wen, GM, et al.. Association between prenatal exposure to household inhalants exposure and ADHD-like behaviors at around 3 years of age: findings from Shenzhen Longhua Child Cohort Study. Environ Res 2019;177:108612. https://doi.org/10.1016/j.envres.2019.108612.Search in Google Scholar PubMed
43. Gong, T, Almqvist, C, Bölte, S, Lichtenstein, P, Anckarsäter, H, Lind, T, et al.. Exposure to air pollution from traffic and neurodevelopmental disorders in Swedish twins. Twin Res Hum Genet 2014;17:553–62. https://doi.org/10.1017/thg.2014.58.Search in Google Scholar PubMed
44. Liu, B, Fang, X, Strodl, E, He, G, Ruan, Z, Wang, X, et al.. Fetal exposure to air pollution in late pregnancy significantly increases ADHD-risk behavior in early childhood. Int J Environ Res Publ Health 2022;19. https://doi.org/10.3390/ijerph191710482.Search in Google Scholar PubMed PubMed Central
45. Willcutt, EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics 2012;9:490–9. https://doi.org/10.1007/s13311-012-0135-8.Search in Google Scholar PubMed PubMed Central
46. Addanki, SS, Chandrasekaran, V, Kandasamy, P. Attention deficit hyperactivity disorder in preschool children: a cross-sectional study of clinical profile and Co-morbidity. Indian J Psychol Med 2023;45:257–62. https://doi.org/10.1177/02537176221127642.Search in Google Scholar PubMed PubMed Central
47. Kaur, S, Morales-Hidalgo, P, Arija, V, Canals, J. Prenatal exposure to air pollutants and attentional deficit hyperactivity disorder development in children: a systematic review. Int J Environ Res Publ Health 2023;20. https://doi.org/10.3390/ijerph20085443.Search in Google Scholar PubMed PubMed Central
48. Abdelnour, E, Jansen, MO, Gold, JA. ADHD diagnostic trends: increased recognition or overdiagnosis? Mo Med 2022;119:467–73.Search in Google Scholar
49. Schein, J, Adler, LA, Childress, A, Cloutier, M, Gagnon-Sanschagrin, P, Davidson, M, et al.. Economic burden of attention-deficit/hyperactivity disorder among children and adolescents in the United States: a societal perspective. J Med Econ 2022;25:193–205. https://doi.org/10.1080/13696998.2022.2032097.Search in Google Scholar PubMed
50. Moen, ØL, Hedelin, B, Hall-Lord, ML. Parental perception of family functioning in everyday life with a child with ADHD. Scand J Public Health 2015;43:10–7. https://doi.org/10.1177/1403494814559803.Search in Google Scholar PubMed
51. Peasgood, T, Bhardwaj, A, Biggs, K, Brazier, JE, Coghill, D, Cooper, CL, et al.. The impact of ADHD on the health and well-being of ADHD children and their siblings. Eur Child Adolesc Psychiatr 2016;25:1217–31. https://doi.org/10.1007/s00787-016-0841-6.Search in Google Scholar PubMed PubMed Central
52. Kalia, V, Perera, F, Tang, D. Environmental pollutants and neurodevelopment: review of benefits from closure of a coal-burning power plant in tongliang, China. Glob Pediatr Health 2017;4:2333794x17721609. https://doi.org/10.1177/2333794x17721609.Search in Google Scholar PubMed PubMed Central
53. Shaw, P, Malek, M, Watson, B, Greenstein, D, de Rossi, P, Sharp, W. Trajectories of cerebral cortical development in childhood and adolescence and adult Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry 2013;74:599–606. https://doi.org/10.1016/j.biopsych.2013.04.007.Search in Google Scholar PubMed PubMed Central
54. Chen, WJ, Rector-Houze, AM, Guxens, M, Iniguez, C, Swartz, MD, Symanski, E, et al.. Susceptible windows of prenatal and postnatal fine particulate matter exposures and attention-deficit hyperactivity disorder symptoms in early childhood. Sci Total Environ 2024;912:168806. https://doi.org/10.1016/j.scitotenv.2023.168806.Search in Google Scholar PubMed PubMed Central
55. Mooney, MA, Ryabinin, P, Morton, H, Selah, K, Gonoud, R, Kozlowski, M, et al.. Joint polygenic and environmental risks for childhood attention-deficit/hyperactivity disorder (ADHD) and ADHD symptoms dimensions. JCPP Adv 2023;3:e12152. https://doi.org/10.1002/jcv2.12152.Search in Google Scholar PubMed PubMed Central
56. Tessum, MW, Anenberg, SC, Chafe, ZA, Henze, DK, Kleiman, G, Kheirbek, I, et al.. Sources of ambient PM(2.5) exposure in 96 global cities. Atmos Environ 19942022;286:119234. https://doi.org/10.1016/j.atmosenv.2022.119234.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/reveh-2024-0073).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Reviews
- The association of particulate matter PM2.5 and nitrogen oxides from ambient air pollution and mental health of children and young adults- a systematic review
- Plant endophytic bacteria reduce phthalates accumulation in soil-crop-body system: a review
- A review in analytical progress for house dust mite allergens
- Global research trends and emerging hotspots in acute high altitude illness: a bibliometric analysis and review (1937–2024)
- Sustainable materials and energy from pine needle waste – a review
- Interrelation between prenatal mercury-selenium exposure and glutathione gene polymorphism: impact on growth and development in children
- Connecting the dots: environmental pollution and Autism Spectrum Disorder
- Phthalates, bisphenols and per-and polyfluoroalkyl substances migration from food packaging into food: a systematic review
- Dietary intake of dioxins and cancer – where do we stand?
- Unfinished business: formaldehyde exposure from uniforms and the case for U.S. textile regulation
- A mini-review on the health risks associated with sodium p-perfluorous nonenoxybenzene sulfonate exposure
- Maternal exposure to particulate matter and nitrogen oxides during pregnancy and attention deficit hyperactivity disorder in offspring: a systematic review and meta-analysis
Articles in the same Issue
- Frontmatter
- Reviews
- The association of particulate matter PM2.5 and nitrogen oxides from ambient air pollution and mental health of children and young adults- a systematic review
- Plant endophytic bacteria reduce phthalates accumulation in soil-crop-body system: a review
- A review in analytical progress for house dust mite allergens
- Global research trends and emerging hotspots in acute high altitude illness: a bibliometric analysis and review (1937–2024)
- Sustainable materials and energy from pine needle waste – a review
- Interrelation between prenatal mercury-selenium exposure and glutathione gene polymorphism: impact on growth and development in children
- Connecting the dots: environmental pollution and Autism Spectrum Disorder
- Phthalates, bisphenols and per-and polyfluoroalkyl substances migration from food packaging into food: a systematic review
- Dietary intake of dioxins and cancer – where do we stand?
- Unfinished business: formaldehyde exposure from uniforms and the case for U.S. textile regulation
- A mini-review on the health risks associated with sodium p-perfluorous nonenoxybenzene sulfonate exposure
- Maternal exposure to particulate matter and nitrogen oxides during pregnancy and attention deficit hyperactivity disorder in offspring: a systematic review and meta-analysis

