Concentration of Tetrabromobisphenol-A in fish: systematic review and meta-analysis and probabilistic health risk assessment
-
Trias Mahmudiono
, Negin Rezaeiarshad
Abstract
Tetrabromobisphenol A (TBBP-A) is an emerging pollutant that enters water resources and affects various marine organisms, such as fish. Consequently, numerous studies globally investigated TBBP-A concentrations in fish fillets of the current study were meta-analyze concentration of TBBP-A in fish fillets and estimate the associated health risks for consumers. The search encompassed international databases, including Science Direct, PubMed, Scopus, Embase, and Web of Science from January 1, 2005, to July 20, 2023. The ranking of countries based on the pooled (Mean) concentration of TBBP-A in fish was as follows: China (1.157 µg/kg-ww) > Czech Republic (1.027 µg/kg-ww) > France (0.500 µg/kg-ww) ∼ Switzerland (0.500 µg/kg-ww) > Netherlands (0.405 µg/kg-ww) > Germany (0.33 µg/kg-ww) > Sweden (0.165 µg/kg-ww)>UK (0.078 µg/kg-ww) > Belgium (0.065 µg/kg-ww) > South Korea (0.013 µg/kg-ww) ∼ Japan (0.013 µg/kg-ww) > Ireland (0.005 µg/kg-ww). The risk assessment showed that the carcinogenic and non-carcinogenic risks of TBBP-A in China and France are higher compared to other countries; however, within all countries, these risks were found to be within acceptable limits.
Introduction
Environment contamination [1], [2], [3], [4], including food [5], [6], [7], [8], [9], [10], has increased over decades [11], [12], [13]. These pollutants include microbes [14], [15], [16], mycotoxins, pesticides, and heavy metals, causing various diseases [17]. Brominated flame retardants (BFRs) constitute the largest group of flame retardants in the market due to their low cost and high efficiency, making them highly effective in increasing fire resistance [18], [19], [20]. BFRs are added either through by chemical bonding or as additives in the manufacture of various polymer materials and serve as raw materials for different industries [19], [20], [21], [22], [23]. Among the BFRs, Tetrabromobisphenol A (TBBP-A) is widely used as a reactive flame retardant (about 90 %) in microelectronic applications such as printed circuit boards, and is rarely used as an additive in the production of acrylonitrile butadiene styrene resins [20], [24], [25], [26].
The highest consumption of this compound occurs in Asia; primarily because most electronic items using circuit boards are produced in this region [24]. Long-term use has led to its presence in various environmental matrices including water, soil, air, dust, sediments, sewage sludge, marine and terrestrial organisms, albeit in low concentrations. Subsequently, it enters the food chain through different routes during production, use, and disposal [23], [24], [25], [26]. The European Commission (EU/118/2014) has recognized these compounds as potential food contaminants, prompting EU member states to investigate their occurrence in food [18]. However, due to its high lipophilicity, transferability to recovery products, bioaccumulation, and chemical stability, it is known as a stable emerging micropollutant in the environment [27]. High consumption of TBBPA can potentially have negative effects on humans and the environment, especially in aquatic environments [24].
Exposure to TBBP-A may result in endocrine disruption, immunotoxicity, neurotoxicity, carcinogenicity, negative reproductive and developmental effects, and long-term consequences, including effects on the second generation [20], 23], 25], 27], 28]. Consequently, TBBP-A has been classified as a carcinogen (Group 2A) by the World Health Organization [29].
The two primary modes of human exposure to TBBP-A are through diet and dust inhalation [19], 21], 23], 28]. Diet, especially seafood consumption, is the main route of human exposure to some BFRs, whose metabolites have also been found in marine organisms and human breast milk. TBBP-A has been detected in low concentrations in various marine organisms, including mollusks, crabs, fish, and porpoises collected from the North Sea [25].
Wang et al. conducted a study in China in 2013 regarding the presence of TBBP-A in food including fish/seafood. This pollutant was detected in only 39 % of all analyzed foods. In fish and seafood, TBBP-A was found in only 5 of 18 samples, with an average concentration of 6.01 ng/g [23].
In 2016, TBBP-A levels were evaluated in 115 samples across 9 food categories in Korean markets, indicating higher contamination levels in fish and cephalopods compared to other food items [30]. Similarly, in 2019, the levels of TBBP-A were evaluated in 24 samples of wild fish and seafood and 16 samples of farmed bivalve mollusks from the western Mediterranean Sea, all registering levels were below the LOQ of 0.05 ng/g fw [31]. Another study in European markets in 2016 evaluated the concentration of halogenated flame retardants, including TBBP-A, in commercial seafood, with 90.5 % of the products showing contamination. The maximum concentration of TBBP-A was reported as 48.6 ng/g lw [32].
In the UK, among 156 food samples collected, TBBP-A was detected above the LOD only in a few processed food samples with low contamination levels (0.01–0.15 ng/g whole weight) [33].
A study in the coastal areas of Bohai Sea, China, studied the distribution patterns and nutritional transfer properties of TBBP-A/S and its analogs in 97 biological samples (including 5 species of phycophyta, 2 species of zooplankton, 14 species of invertebrates, and 13 species of fish). The concentration of TBBP-A and analogs ranged from not detected (ND) to 2782.8 ng/g lipid weight (LW) [25]. In recent years, several review articles have been published on the pollutant TBBP-A, encompassing its presence in consumer goods, various environments (such as sediments, surface water, soil, sewage sludge, indoor and outdoor air, and indoor dust), as well as in a variety of biological samples and the food chain (including plants, fish, humans, birds, and invertebrates) [34], [35], [36], [37], [38].
He et al. conducted a comprehensive investigation into environmental occurrences and bioremediation methods for emerging organohalogens that TBBP-A and fish were a small part of their study [38].
Although several studies have evaluated TBBP-A concentrations in fish fillets, no systematic review study has been conducted [18], 19], 22], 26], 28], 33], 39], 40]. Therefore, the main aims of the current study were to meta-analyze the concentration of TBBP-A in fish fillets and conduct a probabilistic health risk assessment.
Materials and methods
Search strategy
The search was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol [41], 42]. Two authors (F.SA and Y.FA) searched international databases, including Science Direct, PubMed, Scopus, Embase, and Web of Science, from January 1, 2005, to July, 20, 2023. Keywords were retrieved according to medical subject headings (MeSH Terms) and published preliminary papers. Keywords included “(Tetrabromobisphenol-A)” OR “TBBP-A “OR flame retardant” OR “brominated flame retardant” OR “4,4′-(Propane-2,2-diyl)bis(2,6-dibromophenol)” AND “fish” OR “Shrimp” OR “Marine Foods” OR “Sea Foods”.
Duplicate papers were removed, and papers were screened based on title and abstract in EndNote software version 8. After reading the full text of the papers, those meeting the inclusion criteria were included in the data extraction stage. In cases of disagreement on paper selection between the authors (N.RE and Y.FA), the corresponding author (F.SA) made the final decision. The references of papers were checked to retrieve additional relevant papers.
Inclusion/exclusion criteria and data extraction
Our inclusion criteria were included English full texts; analysis of TBBP-A in fish fillets, reliable detection methods, and the presentation of concentration statistics (average, range, standard deviation).
Books, book chapters, review papers, letters to editors, thesis, and conferences were excluded. Data extracted included country, statistics on TBBP-A concentration in fish fillets (mean, standard deviation, minimum and maximum values, limit of detection [LOD], and method of detection). The concentration unit of TBBP-A was converted to µg/kg-ww.
Meta-analysis of data
Meta-analysis of TBBP-A concentration in fish fillets was performed using mean and standard error statistics. The I2 index was used to detect the heterogeneity; if the I2 index was >50 %, a random effects model was used to calculate the pooled (mean) effect size [43], [44], [45]. The meta-analysis of data was performed by Stata Version 14.0 (College Station, TX, USA).
Health risk assessment
The chronic daily intake (CDI) resulting from the ingestion of TBBP-A content in fish fillets was estimated using the following equation [46], [47]:
Where, CDI is chronic daily intake; C, concentration of TBBP-A in fish; IR, ingestion rate (IR of fish per capital based on country was presented in Appendix 1); ED, exposure duration (adults: 70 y); EF, exposure frequency (350 d/y); BW, body weight (adults: 70 kg) and AT, average lifetime (adults: 25,550 d).
The non-carcinogenic risk of TBBP-A was estimated using the following equation [48]:
Where, THQ is the target hazard quotient and RfD, oral reference dose. RfD of TBBP-A based on uterine hyperplasia is equal to 0.6 mg/bw.kg-d. THQ≤1 value indicates acceptable non-carcinogenic risk.
The carcinogenic risk of TBBP-A in fish was calculated by the following equation [48], [49], [50]:
Where, CR is carcinogenic risk and CSF, cancer slope factor. CSF of TBBP-A is equal to 0.00315 (mg/kg-d)−1. CR≤1.00E−6 value indicates an acceptable carcinogenic risk [48].
Uncertainty analysis
To minimize uncertainty in the risk assessment, the Monte Carlo Simulation (MCS) was employed to identify an uncertain event’s possible outcomes [51]. Based on this method, distribution type included variables such as concentration and ingestion rate were selected as log normal and body weight as normal distribution [8], 52], 53]. The cut-point of health risk was set at 5,000 repetitions and 95 % of THQ and CR. The MCS method was conducted by the Oracle Crystal Ball software (version 11.1.2.4.600).
Results
Number of studies in countries
Fourteen papers comprising 26 data-reports (sample size: 466) were included in our meta-analysis (Figure 1). The ranking of countries based on study count was South Korea (5)∼UK (5)>Czech Republic (3)∼China (3)∼Japan (3)>Belgium (1) Ireland (1)∼Netherlands (1)∼Sweden (1)∼France (1)∼Switzerland (1)∼Germany (1) (Table 1 and Figure 2).

Process of section paper based on PRISMA.
Main characteristic included in our study.
| Country | Sample number | Mean (µg/kg-ww) | SD (µg/kg-ww) | Min (µg/kg-ww) | Max (µg/kg-ww) | LOD | Method of detection | References |
|---|---|---|---|---|---|---|---|---|
| Czech | 32 | 5.50E−01 | 1.13E−01 | 0.00E+00 | 0.00E+00 | 0.1–1 μg/kg | UHPLC/MS–MS | [22] |
| Czech | 48 | 1.29E+00 | 1.07E+00 | 1.40E−01 | 4.43E+00 | LC–MS/MS | [39] | |
| South Korean | 11 | 1.40E−01 | 4.58E−01 | ND | 1.78E+00 | 0.043 μg/kg | LC–MS/MS | [19] |
| South Korean | 11 | 1.60E−02 | 3.30E−02 | ND | 8.90E−02 | 0.043 μg/kg | LC–MS/MS | [19] |
| South Korean | 11 | 2.60E−02 | 6.70E−02 | ND | 2.51E−01 | 0.043 μg/kg | LC–MS/MS | [19] |
| South Korean | 11 | 4.00E−03 | 1.40E−02 | ND | 5.30E−02 | 0.036 μg/kg | LC–MS/MS | [19] |
| South Korean | 12 | 1.80E−02 | 1.08E−02 | 0.036 μg/kg | LC–MS/MS | [19] | ||
| Belgium | 61 | 6.50E−02 | 3.00E−02 | NM | UHPLC/MS–MS | [28] | ||
| Ireland | 10 | 5.00E−03 | 3.00E−03 | 0.01 μg/kg | LC–MS/MS | [18] | ||
| UK | 36 | 1.00E−02 | 1.00E−02 | <0.01 | <0.03 | 0.01 | HPLC-MS/MS | [33] |
| China | 13 | 2.10E−02 | 1.26E−02 | 100 pg/g Dw | HPLC-MS/MS | [25] | ||
| UK | 30 | 9.23E−01 | 3.89E−01 | LC-ESI-MS/MS | [40] | |||
| UK | 22 | 2.00E−05 | 1.20E−05 | 0.00003–0.00005 µg/kg | LC–MS/MS | [21] | ||
| Japan | 15 | 1.00E−02 | 6.00E−03 | HRGC/HRMS | [54] | |||
| Japan | 15 | 1.00E−02 | 6.00E−03 | HRGC/HRMS | [54] | |||
| Japan | 15 | 2.00E−02 | 1.20E−02 | HRGC/HRMS | [54] | |||
| China | 34 | 3.30E+00 | 6.60E−01 | 1.20E+00 | 9.00E+00 | 0.015 μg/kg | LC-MS | [24] |
| China | 18 | 1.62E−01 | 3.30E−01 | 0.00E+00 | 1.14E+00 | NM | UHPLC/MS–MS | [23] |
| Czech | 15 | 1.39E+00 | 1.48E+00 | 1.60E−01 | 6.06E+00 | UHPLC/MS–MS | [20] | |
| Netherlands | 7 | 4.05E−01 | 2.25E−02 | 3.60E−01 | 4.50E−01 | 0.1 µg/kg | LC–MS/MS | [26] |
| UK | 4 | 7.05E−01 | 2.43E−01 | 2.20E−01 | 1.19E+00 | 0.1 µg/kg | LC–MS/MS | [26] |
| UK | 7 | 6.65E−01 | 1.78E−01 | 3.10E−01 | 1.02E+00 | 0.1 µg/kg | LC–MS/MS | [26] |
| Sweden | 7 | 1.65E−01 | 8.25E−02 | 0.00E+00 | 3.30E−01 | 0.1 µg/kg | LC–MS/MS | [26] |
| France | 7 | 5.00E−01 | 1.30E−01 | 2.40E−01 | 7.60E−01 | 0.1 µg/kg | LC–MS/MS | [26] |
| Switzerland | 7 | 5.00E−01 | 1.30E−01 | 2.40E−01 | 7.60E−01 | 0.1 µg/kg | LC–MS/MS | [26] |
| Germany | 7 | 3.35E−01 | 5.75E−02 | 2.20E−01 | 4.50E−01 | 0.1 µg/kg | LC–MS/MS | [26] |
-
SD, standard deviation; LOD, limit of detection; ND, not detected; NM, not mentioned.

The number study on TBBP-A in fish based on countries.
Method of detection
The ranking of detection methods based on study count was LC–MS/MS (15) > UHPLC/MS–MS (4) > HRGC/HRMS (3) > HPLC-MS/MS (2) > LC-MS (1) ∼ LC-ESI-MS/MS (1) (Table 1 and Figure 3).

The number study based on method of detection TBBP-A.
Concentration of TBBP-A in fish
Countries ranked by the pooled concentration of TBBP-A in fish were: China (1.157 µg/kg-ww) > Czech Republic (1.027 µg/kg-ww) > France (0.500 µg/kg-ww) ∼ Switzerland (0.500 µg/kg-ww) > Netherlands (0.405 µg/kg-ww) > Germany (0.33 µg/kg-ww 5) > Sweden (0.165 µg/kg-ww)> UK (0.078 µg/kg-ww) > Belgium (0.065 µg/kg-ww) > South Korean (0.013 µg/kg-ww) ∼ Japan (0.013 µg/kg-ww) > Ireland (0.005 µg/kg-ww) (Table 2).
Meta-analysis concentration (µg/kg-ww) of Tetrabromobisphenol in fish.
| Country | Study number | ES | Lower | Upper | Weight (%) | Heterogeneity statistic | Degrees of freedom | p-Value | I2 index |
|---|---|---|---|---|---|---|---|---|---|
| Czech | 3 | 1.027 | 0.398 | 1.655 | 4.83 | 27.19 | 2 | 0 | 92.60 % |
| South Korean | 5 | 0.013 | 0.003 | 0.023 | 23.25 | 8.43 | 4 | 0.077 | 52.60 % |
| Belgium | 1 | 0.065 | 0.057 | 0.073 | 6.31 | 0 | 0 | – | |
| Ireland | 1 | 0.005 | 0.003 | 0.007 | 6.4 | 0 | 0 | – | |
| UK | 5 | 0.078 | 0.049 | 0.106 | 15.34 | 336.82 | 4 | 0 | 98.80 % |
| China | 3 | 1.157 | 0.000 | 2.648 | 7.65 | 841.55 | 2 | 0 | 99.80 % |
| Japan | 3 | 0.013 | 0.008 | 0.017 | 19.14 | 9.26 | 2 | 0.01 | 78.40 % |
| Netherlands | 1 | 0.405 | 0.388 | 0.422 | 5.96 | 0 | 0 | – | |
| Sweden | 1 | 0.165 | 0.104 | 0.226 | 3.18 | 0 | 0 | – | |
| France | 1 | 0.500 | 0.404 | 0.596 | 1.82 | 0 | 0 | – | |
| Switzerland | 1 | 0.500 | 0.404 | 0.596 | 1.82 | 0 | 0 | – | |
| Germany | 1 | 0.335 | 0.292 | 0.378 | 4.29 | 0 | 0 | – | |
| Overall | 26 | 0.139 | 0.124 | 0.155 | 100 | 5293.03 | 25 | 0 | 99.50 % |
-
Effect Size (ES): Pooled concentration (µg/kg-ww); I2 index of heterogeneity.
Probabilistic health risk assessment
Maximum THQ values for TBBP-A in fish were observed in China (6.69E−6), France (1.77E−6), and Czech Republic (1.24E–6) (Figure 4). Maximum CR values for TBBP-A in fish were noted in China (1.26E−8), France (3.35E−9), and Czech Republic (2.33E−9) (Figure 5). Across all countries, CR values were below 1E−6, indicating acceptable carcinogenic risk for consumers.






Non-carcinogenic risk due to TBBP-A in fish based on countries subgroups.THQ shows non-carcinogenic risk (THQ=CDI/RfD).


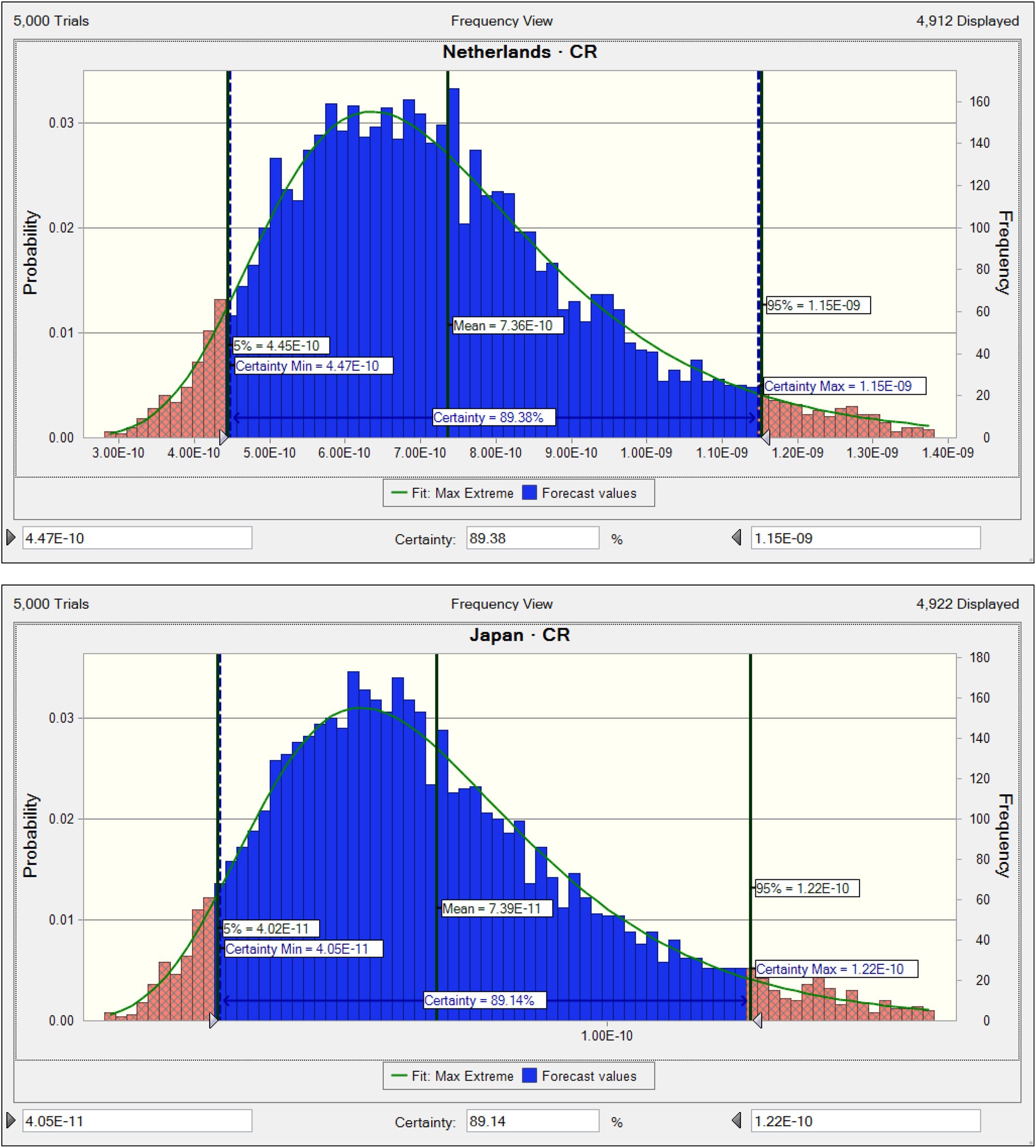



Carcinogenic risk due to TBBP-A in fish based on countries subgroups. CR shows carcinogenic risk (CR=CDI × CSF).
Discussion
It is worth noting that TBBP-A is classified as an emerging contaminant, meaning its potential environmental impacts and accumulation pathways might not be fully understood or recognized by the scientific community [24]. The absence of specific regulatory recognition or guidelines for TBBP-A in fish can lead to lower research priority [31].
Regulatory guidelines shape research priorities and allocate resources to address substance-related risks. Restricted funding or budgetary limitations can impede extensive research. Consequently, there might be relatively less emphasis on studying TBBP-A presence in fish compared to more well-established contaminants [55], [56], [57], [58]. The limited number of studies on TBBP-A in fish can indeed be attributed to factors such as low familiarity with the chemical, the newness of the pollutant, cost considerations, and the lack of recognition of its accumulation chain [59]. These factors, individually or collectively, contribute to a shortage of research specifically focusing on TBBP-A in fish.
According to research findings, the main sources of TBBP-A entering the environment are associated with the use and disposal of products that contain this flame retardant [60], 61]. Brominated flame retardants (BFRs) have diverse applications across various industries, including textiles, wiring, furniture, industrial paints, plastics, and foams [62]. Moreover, their use is prevalent in electronic products, playing a crucial role in improving fire resistance [63]. Over time, TBBP-A can migrate from these products into the surrounding environment through pathways such as wastewater discharges, landfill leachate, or atmospheric deposition [64]. Once released, TBBP-A can enter aquatic habitats through runoff or direct contact with water sources. TBBP-A can enter the aquatic environment through effluent from wastewater treatment plants (WWTPs) [65]. TBBP-A-containing wastewater, which may originate from industrial processes, residential sources, or even landfill leachate, can be discharged into aquatic habitats after treatment [66]. While WWTPs remove many contaminants, including TBBP-A, certain amounts can still be present in the effluent and enter the environment. Landfills can be another source of TBBP-A discharge into the aquatic environment [67]. TBBP-A can be present in waste materials, such as electronic waste, disposed of in landfills. Over time, rainfall or leachate from the landfill can carry TBBP-A into surrounding soil and water sources, potentially leading to its entry into aquatic ecosystems [68]. Regarding the accumulation of TBBP-A in fish tissues, certain flame retardants, including TBBP-A, have the potential to bioaccumulate in organisms. Fish can be exposed to TBBP-A through water or by ingesting contaminated prey. Once absorbed, TBBP-A can accumulate in fish tissues, particularly in fatty tissues, over time. However, as reported by Ashizuka et al., there was no correlation between TBBP-A and fat content in any region. This lack of correlation may be attributed to the chemical characteristics of TBBP-A, which has a phenolic structure, and its rapid metabolic conversion within the body [54]. The study of Driffield et al. found no detectable levels of TBBP-A above the limit of detection, which was as low as 0.05 mg kg–1. This is likely because TBBP-A forms strong chemical bonds with the polymer matrix during the manufacturing process, minimizing its potential for leaching. Moreover, since the levels of these flame retardants in the shellfish samples were similar, it was concluded that exposure to these brominated flame retardants (BFRs) through shellfish consumption is not considered significant compared to exposure from other dietary sources [21]. Chen et al. and Gong et al. conducted studies revealing significant bioaccumulation of TBBP-A in lower trophic levels, specifically in Tigriopus japonicus, a type of zooplankton. The bioaccumulation factor was high, indicating substantial accumulation of TBBP-A in these organisms [69], 70].
The majority of analytical methods for halogenated flame retardants, such as TBBP-A, involve liquid chromatographic (LC) separation combined with mass spectrometric (MS) or optical detection techniques [71]. LC–MS can easily detect polar analytes such as TBBP-A and/or hydroxylated BFRs, which typically necessitate derivatization prior to GC analysis [72]. LC-MS is more commonly used than conventional methods for measuring TBBP-A concentrations in fish due to its higher sensitivity, selectivity, structural confirmation capabilities, and established methods [26]. These advantages make LC-MS a reliable and efficient technique for assessing TBBP-A contamination in fish and understanding associated risks. LC-ESI-MS/MS, a powerful analytical technique combining liquid chromatography with electrospray ionization and tandem mass spectrometry, is relatively less commonly used for TBBP-A analysis in fish [22]. As Fernandes et al., have mentioned, analyzing compounds such as BDE-209 and DBDPE necessitates meticulous attention to ensuring clean contact surfaces throughout the entire process, including the injector, GC column, transfer lines, and MS sources [33]. Additionally, it is crucial to have a thorough understanding and precise control of the temperatures at which degradation of these compounds may initiate. In the ICP-MS system employed for bromine measurement, the internal transfer lines are not typically under the direct control of the operator, which may lead to the possibility of adsorption occurring [33].
Liu et al. attempted to analyze TBBP-A-BAE and TBBP-A-BDBPE using Agilent Technologies (6460 Triple Quadrupole MS/MS) and Waters (Quattro Ultima Triple Quadrupole MS/MS) with APCI-MS. However, the MS signals obtained were very low. To address this issue, the pretreatment method was optimized. In previous studies, HPLC coupled with a UV detector was utilized for the analysis of TBBP-A-BAE and TBBP-A-BDBPE. In Liu et al. study, HPLC-DAD (High-Performance Liquid Chromatography with Diode Array Detection) was employed, enabling the complete separation of 209 TBBP-A-BAE and TBBP-A-BDBPE from other interfering peaks [25].
This finding suggests a significant presence of TBBP-A contamination in fish from Chinese waters, likely due to industrial activities and potential improper waste management practices. China has become the largest importer of e-waste in the world, which has led to heightened public concerns regarding the significant release of hazardous materials, including brominated flame retardants, into the environment [73]. This release occurs during the dismantling of e-waste, improper disposal practices, and inadequate recycling processes. As mentioned by Okeke et al., the available data suggests that the main sources of TBBP-A in China are associated with primitive e-waste dismantling methods, TBBP-A manufacturing, and the processing of TBBP-A-based materials [66]. For example, the Pearl River Delta (PRD) region in China is recognized as a prominent center for the electronics industry, where chemicals like DBDPE and TBBP-A-DBPE are extensively utilized [74]. Consequently, As reported by He et al., the high concentrations of these substances found in the region are consistent with their widespread usage in electronic products [24]. Similarly, another study identified elevated TBBP-A concentrations in fish samples obtained from the Bohai Sea, an important fishing region in China. The study suggested that the high TBBP-A levels in fish were linked to industrial and municipal wastewater discharges, as well as atmospheric deposition [25]. These findings emphasize the possibility of TBBP-A contamination in fish from specific regions in China where industrial activities and inadequate waste management practices may contribute to the presence of this flame retardant in aquatic environments. The concentration of TBBP-A can vary among different fish species. This variation can be influenced by factors such as the species’ feeding habits, habitat, and position in the food chain [75]. Some fish species may have higher levels of TBBP-A due to their feeding patterns or their exposure to contaminated environments, while others may have lower levels. For example, predatory fish higher up in the food chain, such as shark, swordfish, and large tuna species (e.g., bluefin tuna), have been found to have higher levels of TBBP-A. This is because they consume other fish containing TBBP-A, and the contaminants can accumulate and biomagnify as they move up the food chain.
The findings from the study conducted by Ashizuka et al. in Japan support the idea that TBBP-A has low bioavailability and can be easily metabolized within the organism. In a separate investigation by the Ministry of Health, Labor and Welfare of Japan, it was estimated that the average daily fish consumption per person in Japan is approximately 82.2 g. Based on this estimation, the study calculated the daily intake from fish to be 0.58 ng per kilogram of body weight (ng/kg bw/day) for TBBP-A, assuming a body weight of 50 kg. Therefore, the contamination level in fish was generally not regarded as a significant issue [54].
THQ in the all countries was below than 1, hence consumers are at an acceptable non-carcinogenic risk. In the case of TBBP-A in fish from China, the maximum THQ value indicates that the estimated exposure dose of TBBP-A through fish consumption in China exceeded the reference dose, raising potential health concerns. This suggests that the levels of TBBP-A in fish from China may pose a greater risk to human health compared to other countries where TBBP-A contamination in fish has been assessed. The risk of TBBP-A exposure in China can potentially be influenced by two factors: per capita consumption of fish and the concentration of TBBP-A in fish. China has a notable tradition of consuming fish due to its large population and cultural dietary preferences [76]. Chinese cuisine has a long-standing tradition of incorporating fish as a prominent source of protein, and the per capita consumption of fish varies across different regions in China. It is worth noting that THQ values are influenced by various factors, including the concentration of the contaminant, the consumption rate of fish, and the specific reference dose used in the assessment [76].
The markedly lower levels of carcinogenic and non-carcinogenic risks compared to the standard limits suggest that the low concentration of TBBP-A, coupled with its low toxicity, is the primary reason behind this observation. In addition to the low concentration of TBBP-A and its low toxicity, several other factors can contribute to the decrease in carcinogenic and non-carcinogenic risks associated with it. These factors include the various pathways of exposure to TBBP-A, such as inhalation or dermal contact. Effective risk management practices, such as the implementation of regulatory controls and monitoring programs, also play a crucial role in reducing risks. Moreover, individuals’ dietary habits and consumption patterns, such as opting for fish with lower TBBP-A levels or reducing fish consumption frequency, can impact their exposure levels. Additionally, environmental factors, such as pollution control measures and stringent regulations, have the potential to influence the levels of TBBP-A in the environment and consequently in fish.
Conclusions
This study aimed to gather and analyze existing research on TBBP-A in fish through a systematic search. The concentration of TBBP-A in fish was meta-analyzed and health risk assessments were estimated by the MCS method. The studies conducted in China and the Czech Republic indicated significantly higher TBBP-A levels compared to other countries. Consequently, it is imperative to conduct more studies and implement programs aimed at reducing emission sources in these specific regions.
The risk assessment revealed that both carcinogenic and non-carcinogenic risks associated with TBBP-A in China and France are higher compared to other countries. However, overall, the carcinogenic and non-carcinogenic risks across all countries fall within acceptable ranges. Hence, it is recommended to conduct similar studies in diverse countries to comprehensively determine the health implications of TBBP-A in marine foods.
Acknowledgment
The authors would like to thank all those participated in this study.
-
Research ethics: The conducted research is not related to either human or animal use.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Research funding: Not applicable.
-
Data availability: All data inside the manuscript have been specified clearly in the manuscript.
References
1. Lu, G, Duan, L, Meng, S, Cai, P, Ding, S, Wang, X. Development of a colorimetric and turn-on fluorescent probe with large stokes shift for H2S detection and its multiple applications in environmental, food analysis and biological imaging. Dyes Pigments 2023;220:111687. https://doi.org/10.1016/j.dyepig.2023.111687.Search in Google Scholar
2. Tang, Y, Yang, G, Liu, X, Qin, L, Zhai, W, Fodjo, EK, et al.. Rapid sample enrichment, novel derivatization, and high sensitivity for determination of 3-chloropropane-1, 2-diol in soy sauce via high-performance liquid chromatography–tandem mass spectrometry. J Agric Food Chem 2023;71:15388–97. https://doi.org/10.1021/acs.jafc.3c05230.Search in Google Scholar PubMed
3. Guo, Q, Li, T, Qu, Y, Liang, M, Ha, Y, Zhang, Y, et al.. New research development on trans fatty acids in food: biological effects, analytical methods, formation mechanism, and mitigating measures. Prog Lipid Res 2023;89:101199. https://doi.org/10.1016/j.plipres.2022.101199.Search in Google Scholar PubMed
4. Zhang, G, Zhao, Z, Zhu, Y. Changes in abiotic dissipation rates and bound fractions of antibiotics in biochar-amended soil. J Clean Prod 2020;256:120314. https://doi.org/10.1016/j.jclepro.2020.120314.Search in Google Scholar
5. Sahni, P, Sharma, S. Quality characteristics, amino acid composition, and bioactive potential of wheat cookies protein-enriched with unconventional legume protein isolates. Qual Assur Saf Crop Foods 2023;15:1–10. https://doi.org/10.15586/qas.v15i2.1160.Search in Google Scholar
6. Özlü, H, Çevik, B, Atasever, M, Sarıalioğlu, MF, Alkan Polat, B. Investigation of meat species adulteration in beef-based meat products via real-time PCR in Türkiye. Qual Assur Saf Crop Foods 2023;15:42–8. https://doi.org/10.15586/qas.v15i4.1374.Search in Google Scholar
7. Liang, M, Yang, Z, Xu, K, Chen, X, Yang, J, Liu, W, et al.. Release behavior and kinetic analysis of eugenol from clove particles using P&T–GC-MS method. Ital J Food Sci 2023;35:69–78. https://doi.org/10.15586/ijfs.v35i4.2400.Search in Google Scholar
8. Pasalari, N, Ghaffari, HR, Borzoei, M, Kamari, Z, Mehri, F, Fakhri, Y, et al.. Potentially toxic elements (PTES) concentration in anchovy fish sauce from Hormozgan province, Iran: a probabilistic health risk study. Int J Environ Anal Chem 2023:6:1–16. https://doi.org/10.1080/03067319.2023.2207458.Search in Google Scholar
9. Mahmudiono, T, Hoseinvandtabar, S, Mehri, F, Borzoei, M, Heidarinejad, Z, Amin Nakoozadeh, M, et al.. Potentially toxic elements (PTEs) in coastal sediments of Bandar Abbas city, North of Persian Gulf: an ecological risk assessment. Int J Environ Health Res 2023;9:1–15. https://doi.org/10.1080/09603123.2023.2173154.Search in Google Scholar PubMed
10. Nematollahi, A, Abdi, L, Abdi-Moghadam, Z, Fakhri, Y, Borzoei, M, Tajdar-oranj, B, et al.. The concentration of potentially toxic elements (PTEs) in sausages: a systematic review and meta-analysis study. Environ Sci Pollut Control Ser 2021;28:55186–201. https://doi.org/10.1007/s11356-021-14879-2.Search in Google Scholar PubMed
11. Amiri, S, Motalebi Moghanjougi, Z, Rezazadeh Bari, M, Mousavi Khaneghah, A. Natural protective agents and their applications as bio-preservatives in the food industry: an overview of current and future applications. Ital J Food Sci 2021;33:55–68. https://doi.org/10.15586/ijfs.v33isp1.2045.Search in Google Scholar
12. Bangar, SP, Sharma, N, Bhardwaj, A, Phimolsiripol, Y. Lactic acid bacteria: a bio-green preservative against mycotoxins for food safety and shelf-life extension. Qual Assur Saf Crop Foods 2022;14:13–31. https://doi.org/10.15586/qas.v14i2.1014.Search in Google Scholar
13. Mirmahdi, RS, Zoghi, A, Mohammadi, F, Khosravi-Darani, K, Jazaiery, S, Mohammadi, R, et al.. Biodecontamination of milk and dairy products by probiotics: boon for bane. Ital J Food Sci 2021;33:78–91. https://doi.org/10.15586/ijfs.v33isp2.2053.Search in Google Scholar
14. Ahmad, HA, Ahmad, S, Gao, L, Ismail, S, Wang, Z, El-Baz, A, et al.. Multi-omics analysis revealed the selective enrichment of partial denitrifying bacteria for the stable coupling of partial-denitrification and anammox process under the influence of low strength magnetic field. Water Res 2023;245:120619. https://doi.org/10.1016/j.watres.2023.120619.Search in Google Scholar PubMed
15. Zhao, Y, Dong, Y, Chen, X, Wang, Z, Cui, Z. Using sulfide as nitrite oxidizing bacteria inhibitor for the successful coupling of partial nitrification-anammox and sulfur autotrophic denitrification in one reactor. Chem Eng J 2023;475:146286. https://doi.org/10.1016/j.cej.2023.146286.Search in Google Scholar
16. Liu, J, Li, H, Harvey, J, Airey, G, Lin, S, Lee, SLJ, et al.. Study on leaching characteristics and biotoxicity of porous asphalt with biochar fillers. Transport Res Transport Environ 2023;122:103855. https://doi.org/10.1016/j.trd.2023.103855.Search in Google Scholar
17. Zeng, Y, Ma, W, Xue, H, Ren, X, Zhu, G, Xiao, K, et al.. Exploring the efficacy of Shexiang Tongxin extract pills in severe heart failure. Qual Assur Saf Crop Foods 2023;15:49–59. https://doi.org/10.15586/qas.v15i4.1340.Search in Google Scholar
18. Lopez, MG, Driffield, M, Fernandes, AR, Smith, F, Tarbin, J, Lloyd, AS, et al.. Occurrence of polybrominated diphenylethers, hexabromocyclododecanes, bromophenols and tetrabromobisphenols A and S in Irish foods. Chemosphere 2018;197:709–15. https://doi.org/10.1016/j.chemosphere.2018.01.089.Search in Google Scholar PubMed
19. Lee, J-G, Anh, J, Kang, GJ, Kim, D, Kang, Y. Development of an analytical method for simultaneously determining TBBPA and HBCDs in various foods. Food Chem 2020;313:126027. https://doi.org/10.1016/j.foodchem.2019.126027.Search in Google Scholar PubMed
20. Svihlikova, V, Lankova, D, Poustka, J, Tomaniova, M, Hajslova, J, Pulkrabova, J. Perfluoroalkyl substances (PFASs) and other halogenated compounds in fish from the upper Labe River basin. Chemosphere 2015;129:170–8. https://doi.org/10.1016/j.chemosphere.2014.09.096.Search in Google Scholar PubMed
21. Driffield, M, Harmer, N, Bradley, E, Fernandes, AR, Rose, M, Mortimer, D, et al.. Determination of brominated flame retardants in food by LC–MS/MS: diastereoisomer-specific hexabromocyclododecane and tetrabromobisphenol A. Food Addit Contam 2008;25:895–903. https://doi.org/10.1080/02652030701882999.Search in Google Scholar PubMed
22. Lankova, D, Kockovska, M, Lacina, O, Kalachova, K, Pulkrabova, J, Hajslova, J. Rapid and simple method for determination of hexabromocyclododecanes and other LC–MS–MS-amenable brominated flame retardants in fish. Anal Bioanal Chem 2013;405:7829–39. https://doi.org/10.1007/s00216-013-7076-x.Search in Google Scholar PubMed
23. Wang, J, Zhao, X, Wang, Y, Shi, Z. Tetrabromobisphenol A, hexabromocyclododecane isomers and polybrominated diphenyl ethers in foodstuffs from Beijing, China: contamination levels, dietary exposure and risk assessment. Sci Total Environ 2019;666:812–20. https://doi.org/10.1016/j.scitotenv.2019.02.324.Search in Google Scholar PubMed
24. He, M-J, Luo, XJ, Yu, LH, Wu, JP, Chen, SJ, Mai, BX. Diasteroisomer and enantiomer-specific profiles of hexabromocyclododecane and tetrabromobisphenol A in an aquatic environment in a highly industrialized area, South China: vertical profile, phase partition, and bioaccumulation. Environ Pollut 2013;179:105–10. https://doi.org/10.1016/j.envpol.2013.04.016.Search in Google Scholar PubMed
25. Liu, A-F., Qu, G, Yu, M, Liu, Y, Shi, J, Jiang, G. Tetrabromobisphenol-A/S and nine novel analogs in biological samples from the Chinese Bohai Sea: implications for trophic transfer. Environ Sci Technol 2016;50:4203–11. https://doi.org/10.1021/acs.est.5b06378.Search in Google Scholar PubMed
26. Kotthoff, M, Rüdel, H, Jürling, H. Detection of tetrabromobisphenol A and its mono-and dimethyl derivatives in fish, sediment and suspended particulate matter from European freshwaters and estuaries. Anal Bioanal Chem 2017;409:3685–94. https://doi.org/10.1007/s00216-017-0312-z.Search in Google Scholar PubMed PubMed Central
27. Sahlabadi, F, Eslami, A, Alavi, N, Sadani, M, Torabbeigi, M, Arshadi, M. High-efficient removal of tetrabromobisphenol A from waste mobile phone printed circuit boards leached solution by micellar enhanced ultrafiltration. Environ Health Eng Manag 2023;10:97–105.10.34172/EHEM.2023.11Search in Google Scholar
28. Poma, G, Malysheva, SV, Goscinny, S, Malarvannan, G, Voorspoels, S, Covaci, A, et al.. Occurrence of selected halogenated flame retardants in Belgian foodstuff. Chemosphere 2018;194:256–65. https://doi.org/10.1016/j.chemosphere.2017.11.179.Search in Google Scholar PubMed
29. Pei, S, Shi, H, Zhang, J, Wang, S, Ren, N, You, S. Electrochemical removal of tetrabromobisphenol A by fluorine-doped titanium suboxide electrochemically reactive membrane. J Hazard Mater 2021;419:126434. https://doi.org/10.1016/j.jhazmat.2021.126434.Search in Google Scholar PubMed
30. Lee, J-G, Jeong, Y, Kim, D, Kang, GJ, Kang, Y. Assessment of tetrabromobisphenol and hexabromocyclododecanes exposure and risk characterization using occurrence data in foods. Food Chem Toxicol 2020;137:111121. https://doi.org/10.1016/j.fct.2020.111121.Search in Google Scholar PubMed
31. Chessa, G, Cossu, M, Fiori, G, Ledda, G, Piras, P, Sanna, A, et al.. Occurrence of hexabromocyclododecanes and tetrabromobisphenol A in fish and seafood from the sea of Sardinia–FAO 37.1. 3 area: their impact on human health within the European Union marine framework strategy directive. Chemosphere 2019;228:249–57. https://doi.org/10.1016/j.chemosphere.2019.04.046.Search in Google Scholar PubMed
32. Aznar-Alemany, O, Trabalón, L, Jacobs, S, Barbosa, VL, Tejedor, MF, Granby, K, et al.. Occurrence of halogenated flame retardants in commercial seafood species available in European markets. Food Chem Toxicol 2017;104:35–47. https://doi.org/10.1016/j.fct.2016.12.034.Search in Google Scholar PubMed
33. Fernandes, A, Mortimer, D, Rose, M, Smith, F, Panton, S, Garcia-Lopez, M. Bromine content and brominated flame retardants in food and animal feed from the UK. Chemosphere 2016;150:472–8. https://doi.org/10.1016/j.chemosphere.2015.12.042.Search in Google Scholar PubMed
34. Zuiderveen, EAR, Slootweg, JC, de Boer, J. Novel brominated flame retardants - a review of their occurrence in indoor air, dust, consumer goods and food. Chemosphere 2020;255:126816. https://doi.org/10.1016/j.chemosphere.2020.126816.Search in Google Scholar PubMed
35. Shi, Z, Zhang, L, Li, J, Wu, Y. Legacy and emerging brominated flame retardants in China: a review on food and human milk contamination, human dietary exposure and risk assessment. Chemosphere 2018;198:522–36. https://doi.org/10.1016/j.chemosphere.2018.01.161.Search in Google Scholar PubMed
36. Yu, G, Bu, Q, Cao, Z, Du, X, Xia, J, Wu, M, et al.. Brominated flame retardants (BFRs): a review on environmental contamination in China. Chemosphere 2016;150:479–90. https://doi.org/10.1016/j.chemosphere.2015.12.034.Search in Google Scholar PubMed
37. Law, RJ, Herzke, D, Harrad, S, Morris, S, Bersuder, P, Allchin, CR. Levels and trends of HBCD and BDEs in the European and Asian environments, with some information for other BFRs. Chemosphere 2008;73:223–41. https://doi.org/10.1016/j.chemosphere.2008.02.066.Search in Google Scholar PubMed
38. He, H, Li, Y, Shen, R, Shim, H, Zeng, Y, Zhao, S, et al.. Environmental occurrence and remediation of emerging organohalides: a review. Environ Pollut 2021;290:118060. https://doi.org/10.1016/j.envpol.2021.118060.Search in Google Scholar PubMed
39. Hloušková, V, Lanková, D, Kalachová, K, Hrádková, P, Poustka, J, Hajšlová, J, et al.. Occurrence of brominated flame retardants and perfluoroalkyl substances in fish from the Czech aquatic ecosystem. Sci Total Environ 2013;461:88–98. https://doi.org/10.1016/j.scitotenv.2013.04.081.Search in Google Scholar PubMed
40. Harrad, S, Abdallah, MAE, Rose, NL, Turner, SD, Davidson, TA. Current-use brominated flame retardants in water, sediment, and fish from English lakes. Environ Sci Technol 2009;43:9077–83. https://doi.org/10.1021/es902185u.Search in Google Scholar PubMed
41. Moher, D, Shamseer, L, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al.. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1–9. https://doi.org/10.1186/2046-4053-4-1.Search in Google Scholar PubMed PubMed Central
42. Khanverdiluo, S, Talebi-Ghane, E, Heshmati, A, Mehri, F. The concentration of polycyclic aromatic hydrocarbons (PAHs) in mother milk: a global systematic review, meta-analysis and health risk assessment of infants. Saudi J Biol Sci 2021;28:6869–75. https://doi.org/10.1016/j.sjbs.2021.07.066.Search in Google Scholar PubMed PubMed Central
43. Zeng, J, Luo, Y, Yu, M, Li, J, Liu, Z. CCDC26 rs4295627 polymorphisms associated with an increased risk of glioma: a meta-analysis. Adv Clin Exp Med 2017;26:1275–81. https://doi.org/10.17219/acem/68067.Search in Google Scholar PubMed
44. Li, J, Chen, Y, Xiang, Q, Xiang, J, Tang, Y, Tang, L. 5HTTLPR polymorphism and postpartum depression risk: a meta-analysis. Medicine 2020;99:e22319. https://doi.org/10.1097/md.0000000000022319.Search in Google Scholar PubMed PubMed Central
45. Fu, S, Duan, L, Zhong, Y, Zeng, Y. Comparison of surgical excision followed by adjuvant radiotherapy and laser combined with steroids for the treatment of keloids: a systematic review and meta-analysis. Int Wound J 2023;12:15–23. https://doi.org/10.1111/iwj.14449.Search in Google Scholar PubMed PubMed Central
46. Einolghozati, M, Talebi-Ghane, E, Khazaei, M, Mehri, F. The level of heavy metal in fresh and processed fruits: a study meta-analysis, systematic review, and health risk assessment. Biol Trace Elem Res 2022;201:2582–96.10.1007/s12011-022-03332-1Search in Google Scholar PubMed
47. Qin, Y, Huang, C, Huang, G, Li, H, Shohag, M, Gu, M, et al.. Relative bioavailability of selenium in rice using a rat model and its application to human health risk assessment. Environ Pollut 2023;338:122675. https://doi.org/10.1016/j.envpol.2023.122675.Search in Google Scholar PubMed
48. Ghane, ET, Poormohammadi, A, Khazaei, S, Mehri, F. Concentration of potentially toxic elements in vegetable oils and health risk assessment: a systematic review and meta-analysis. Biol Trace Elem Res 2022;200:437–46. https://doi.org/10.1007/s12011-021-02645-x.Search in Google Scholar PubMed
49. Xiong, J, Wen, D, Zhou, H, Chen, R, Wang, H, Wang, C, et al.. Occurrence of aflatoxin M1 in yogurt and milk in central-eastern China and the risk of exposure in milk consumers. Food Control 2022;137:108928. https://doi.org/10.1016/j.foodcont.2022.108928.Search in Google Scholar
50. Xiong, J, Chen, F, Zhang, J, Ao, W, Zhou, X, Yang, H, et al.. Occurrence of aflatoxin M1 in three types of milk from Xinjiang, China, and the risk of exposure for milk consumers in different age-sex groups. Foods 2022;11:3922. https://doi.org/10.3390/foods11233922.Search in Google Scholar PubMed PubMed Central
51. Harrison, RL. Introduction to Monte Carlo simulation. AIP Conf Proc 2010;1204:17–21.10.1063/1.3295638Search in Google Scholar PubMed PubMed Central
52. Asadi, A, Fakhri, Y, Salimi, Y, Daglioglu, N, Tahmasebifard, M, Aghajarinezhad, M. Nicotine consumption rate through wastewater-based epidemiology: a systematic review, meta-analysis and probabilistic risk assessment. Environ Sci Pollut Control Ser 2023:30:63416–26. https://doi.org/10.1007/s11356-023-27017-x.Search in Google Scholar PubMed PubMed Central
53. Fakhri, Y, Nematollahi, A, Bafandeh Tiz, P, Alipour, M, Shahmohammadi, S, Soleymannejad, F, et al.. The concentration of potentially hazardous trace elements (PHTEs) among tap drinking water samples from Ilam city, Iran: a probabilistic non-carcinogenic risk study. Int J Environ Anal Chem 2022;102:5122–35. https://doi.org/10.1080/03067319.2020.1791331.Search in Google Scholar
54. Ashizuka, Y, Nakagawa, R, Hori, T, Yasutake, D, Tobiishi, K, Sasaki, K. Determination of brominated flame retardants and brominated dioxins in fish collected from three regions of Japan. Mol Nutr Food Res 2008;52:273–83. https://doi.org/10.1002/mnfr.200700110.Search in Google Scholar PubMed
55. Thomsen, C, Lundanes, E, Becher, G. Brominated flame retardants in plasma samples from three different occupational groups in Norway. J Environ Monit 2001;3:366–70. https://doi.org/10.1039/b104304h.Search in Google Scholar PubMed
56. Fakhri, Y, Mahmudiono, T, Ranaei, V, Sarafraz, M, Nematollahi, A, Mousavi Khaneghah, A. The concentration of radionuclides (Lead-210, Polonium-210, and Cesium-137) in the muscle of sardine fish: a global systematic review, meta-analysis, and exposure assessment. Biol Trace Elem Res 2023;201:2011–21. https://doi.org/10.1007/s12011-022-03289-1.Search in Google Scholar PubMed
57. Ghajarbeygi, P, Ranaei, V, Pilevar, Z, Nematollahi, A, Ghanbari, S, Rahimi, H, et al.. The concentration of radioisotopes (Potassium-40, Polonium-210, Radium-226, and Thorium-230) in fillet tissue carp fishes: a systematic review and probabilistic exposure assessment. Int J Environ Health Res 2022;34:273–294.10.1080/09603123.2022.2147905Search in Google Scholar PubMed
58. Fakhri, Y, Sarafraz, M, Pilevar, Z, Daraei, H, Rahimizadeh, A, Kazemi, S, et al.. The concentration and health risk assessment of radionuclides in the muscle of tuna fish: a worldwide systematic review and meta-analysis. Chemosphere 2022;289:133149. https://doi.org/10.1016/j.chemosphere.2021.133149.Search in Google Scholar PubMed
59. He, M-J, Luo, XJ, Yu, LH, Liu, J, Zhang, XL, Chen, SJ, et al.. Tetrabromobisphenol-A and hexabromocyclododecane in birds from an e-waste region in South China: influence of diet on diastereoisomer-and enantiomer-specific distribution and trophodynamics. Environ Sci Technol 2010;44:5748–54. https://doi.org/10.1021/es101503r.Search in Google Scholar PubMed
60. Sjödin, A, Carlsson, H, Thuresson, K, Sjölin, S, Bergman, Å, Östman, C. Flame retardants in indoor air at an electronics recycling plant and at other work environments. Environ Sci Technol 2001;35:448–54. https://doi.org/10.1021/es000077n.Search in Google Scholar PubMed
61. Tao, L, Wu, JP, Zhi, H, Zhang, Y, Ren, ZH, Luo, XJ, et al.. Aquatic bioaccumulation and trophic transfer of tetrabromobisphenol-A flame retardant introduced from a typical e-waste recycling site. Environ Sci Pollut Control Ser 2016;23:14663–70. https://doi.org/10.1007/s11356-016-6940-6.Search in Google Scholar PubMed
62. Ekpe, OD, Choo, G, Barceló, D, Oh, J-E. Introduction of emerging halogenated flame retardants in the environment. In: Comprehensive analytical chemistry, Ahuja S, editor. Amsterdam, The Netherlands: Elsevier; 2020:1–39 pp.10.1016/bs.coac.2019.11.002Search in Google Scholar
63. Oomen, AG, Janssen, PJCM, Dusseldorp, A, Noorlander, CW. Exposure to chemicals via house dust. RIVM report 609021064. Bilthoven (NL): National Institute for Public Health and the Environment;2008.Search in Google Scholar
64. Wang, J, Liu, L, Wang, J, Pan, B, Fu, X, Zhang, G, et al.. Distribution of metals and brominated flame retardants (BFRs) in sediments, soils and plants from an informal e-waste dismantling site, South China. Environ Sci Pollut Control Ser 2015;22:1020–33. https://doi.org/10.1007/s11356-014-3399-1.Search in Google Scholar PubMed
65. Kim, U-J, Lee, I-S, Oh, J-E. Occurrence, removal and release characteristics of dissolved brominated flame retardants and their potential metabolites in various kinds of wastewater. Environ Pollut 2016;218:551–7. https://doi.org/10.1016/j.envpol.2016.07.037.Search in Google Scholar PubMed
66. Okeke, ES, Huang, B, Mao, G, Chen, Y, Zhengjia, Z, Qian, X, et al.. Review of the environmental occurrence, analytical techniques, degradation and toxicity of TBBPA and its derivatives. Environ Res 2022;206:112594. https://doi.org/10.1016/j.envres.2021.112594.Search in Google Scholar PubMed
67. Gallen, C, Drage, D, Kaserzon, S, Baduel, C, Gallen, M, Banks, A, et al.. Occurrence and distribution of brominated flame retardants and perfluoroalkyl substances in Australian landfill leachate and biosolids. J Hazard Mater 2016;312:55–64. https://doi.org/10.1016/j.jhazmat.2016.03.031.Search in Google Scholar PubMed
68. Morris, S, Allchin, CR, Zegers, BN, Haftka, JJH, Boon, JP, Belpaire, C, et al.. Distribution and fate of HBCD and TBBPA brominated flame retardants in North Sea estuaries and aquatic food webs. Environ Sci Technol 2004;38:5497–504. https://doi.org/10.1021/es049640i.Search in Google Scholar PubMed
69. Gong, W, Wang, J, Cui, W, Zhu, L. Distribution characteristics and risk assessment of TBBPA in seawater and zooplankton in northern sea areas, China. Environ Geochem Health 2021;43:4759–69. https://doi.org/10.1007/s10653-021-00948-5.Search in Google Scholar PubMed
70. Chen, X, Zhu, LY, Huang, Y, Gong, WJ, Hao, Y. Community structure of the zooplankton in the Southern Yellow sea. Mar Sci 2017;41:41–9.Search in Google Scholar
71. Guerra, P, Eljarrat, E, Barceló, D. Determination of halogenated flame retardants by liquid chromatography coupled to mass spectrometry. TrAC Trends in Analytical Chemistry 2011;30:842–55. https://doi.org/10.1016/j.trac.2011.01.018.Search in Google Scholar
72. Covaci, A, Voorspoels, S, Abdallah, MAE, Geens, T, Harrad, S, Law, RJ. Analytical and environmental aspects of the flame retardant tetrabromobisphenol-A and its derivatives. J Chromatogr A 2009;1216:346–63. https://doi.org/10.1016/j.chroma.2008.08.035.Search in Google Scholar PubMed
73. Lu, C, Zhang, L, Zhong, Y, Ren, W, Tobias, M, Mu, Z, et al.. An overview of e-waste management in China. J Mater Cycles Waste Manag 2015;17:1–12. https://doi.org/10.1007/s10163-014-0256-8.Search in Google Scholar
74. Ali, N, Ismail, IMI, Eqani, SAMAS, Kadi, MW, Covaci, A. Environmental exposure of emerging brominated flame retardants (BFRs) in developing conutries: their significance for human exposure. Organohalogen Compd 2014;76:788–91.Search in Google Scholar
75. Law, RJ, Allchin, CR, de Boer, J, Covaci, A, Herzke, D, Lepom, P, et al.. Levels and trends of brominated flame retardants in the European environment. Chemosphere 2006;64:187–208. https://doi.org/10.1016/j.chemosphere.2005.12.007.Search in Google Scholar PubMed
76. Zhou, L, Jin, S, Zhang, B, Cheng, G, Zeng, Q, Wang, D. Determinants of fish consumption by household type in China. Br Food J 2015;117:1273–88. https://doi.org/10.1108/bfj-05-2014-0182.Search in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/reveh-2023-0157).
© 2024 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Reviews
- Mercury and cadmium-induced inflammatory cytokines activation and its effect on the risk of preeclampsia: a review
- Prevalence of chronic obstructive pulmonary disease in Indian nonsmokers: a systematic review & meta-analysis
- Beyond the outdoors: indoor air quality guidelines and standards – challenges, inequalities, and the path forward
- Cadmium exposure and thyroid hormone disruption: a systematic review and meta-analysis
- New generation sequencing: molecular approaches for the detection and monitoring of bioaerosols in an indoor environment: a systematic review
- Concentration of Tetrabromobisphenol-A in fish: systematic review and meta-analysis and probabilistic health risk assessment
- The association between indoor air pollution from solid fuels and cognitive impairment: a systematic review and meta-analysis
- Phthalates and uterine disorders
- Effectiveness of educational interventions for the prevention of lead poisoning in children: a systematic review
- Association between exposure to per- and polyfluoroalkyl substances and levels of lipid profile based on human studies
- Summary of seven Swedish case reports on the microwave syndrome associated with 5G radiofrequency radiation
- Expanding the focus of the One Health concept: links between the Earth-system processes of the planetary boundaries framework and antibiotic resistance
- Exploring the link between ambient PM2.5 concentrations and respiratory diseases in the elderly: a study in the Muang district of Khon Kaen, Thailand
- Standards for levels of lead in soil and dust around the world
- Tributyltin induces apoptosis in mammalian cells in vivo: a scoping review
- The influence of geology on the quality of groundwater for domestic use: a Kenyan review
- Biological concentrations of DDT metabolites and breast cancer risk: an updated systematic review and meta-analysis
- Letter to the Editor
- Ancient medicine and famous iranian physicians
Articles in the same Issue
- Frontmatter
- Reviews
- Mercury and cadmium-induced inflammatory cytokines activation and its effect on the risk of preeclampsia: a review
- Prevalence of chronic obstructive pulmonary disease in Indian nonsmokers: a systematic review & meta-analysis
- Beyond the outdoors: indoor air quality guidelines and standards – challenges, inequalities, and the path forward
- Cadmium exposure and thyroid hormone disruption: a systematic review and meta-analysis
- New generation sequencing: molecular approaches for the detection and monitoring of bioaerosols in an indoor environment: a systematic review
- Concentration of Tetrabromobisphenol-A in fish: systematic review and meta-analysis and probabilistic health risk assessment
- The association between indoor air pollution from solid fuels and cognitive impairment: a systematic review and meta-analysis
- Phthalates and uterine disorders
- Effectiveness of educational interventions for the prevention of lead poisoning in children: a systematic review
- Association between exposure to per- and polyfluoroalkyl substances and levels of lipid profile based on human studies
- Summary of seven Swedish case reports on the microwave syndrome associated with 5G radiofrequency radiation
- Expanding the focus of the One Health concept: links between the Earth-system processes of the planetary boundaries framework and antibiotic resistance
- Exploring the link between ambient PM2.5 concentrations and respiratory diseases in the elderly: a study in the Muang district of Khon Kaen, Thailand
- Standards for levels of lead in soil and dust around the world
- Tributyltin induces apoptosis in mammalian cells in vivo: a scoping review
- The influence of geology on the quality of groundwater for domestic use: a Kenyan review
- Biological concentrations of DDT metabolites and breast cancer risk: an updated systematic review and meta-analysis
- Letter to the Editor
- Ancient medicine and famous iranian physicians

